Abstract
Background:
There have been no comprehensive large-scale studies that have evaluated the benefits of chemotherapy-based regimens in addressing HER2-altered advanced non-small-cell lung cancer (NSCLC) in a first-line setting. Data on HER2 alteration subtypes and concomitant alterations are also limited. Accordingly, our retrospective, real-world POLISH study assesses the efficacy of first-line chemotherapy alone (C) as well as combinations with immune checkpoint inhibitors (C + I) or angiogenesis inhibitors (C + A) for HER2-altered NSCLC; molecular features are also reported.
Methods:
HER2-altered NSCLC patients who received a first-line treatment between November 2015 and September 2021 were screened. Patients treated with C, C + I, or C + A were included in our final efficacy analysis. Progression-free survival (PFS) was compared between the subgroups. A Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed to evaluate concomitant alterations.
Results:
A total of 293 patients were screened, with an identification of HER2 amplification and 37 distinct HER2 mutations, and 210 cases treated with C, C + I, or C + A were ultimately included. C + A achieved longer PFS than C (5.63 vs 4.03 months, hazard ratio: 0.64, 95% confidence interval [CI]: 0.46–0.88, p = 0.006). C + I did not improve median PFS compared to C + A or C (both p > 0.05), despite the programmed cell death ligand-1 (PD-L1) expression or tumor mutational burden. KEGG analysis revealed that concomitant upregulation of PI3 K/AKT pathway signaling was common in HER2-altered NSCLC.
Conclusion:
Chemotherapy plus angiogenesis inhibitors may yield a greater survival benefit than chemotherapy alone in a first-line setting for HER2-altered NSCLC, whereas an immune-based combination therapy may not be superior to a sole chemotherapy regimen. Activation of PI3 K/AKT signaling may mediate immunosuppression in HER2-altered NSCLC.
Keywords: angiogenesis inhibitor, chemotherapy, HER2 alteration, immunotherapy, non-small-cell lung cancer, real-world study
Introduction
Lung cancer remains the most fatal malignant disease worldwide, with a 5-year relative survival rate of 5% when metastasis is diagnosed. 1 Recent approval of various targeted agents have greatly benefited the prognoses of patients carrying driver genes; these include epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and c-ros oncogene 1 receptor tyrosine kinase (ROS-1). Further research into pathogenic mechanisms has revealed other oncogenic drivers in lung cancer, such as human epidermal growth factor receptor 2 (HER2, also known as ERBB2). 2
As member of the ERBB receptor tyrosine kinase family, HER2 presents three primary oncogenic-activation mechanisms: HER2 mutation, amplification, and protein overexpression. 3 In non-small-cell lung cancer (NSCLC), HER2 mutations and amplification are uncommon, accounting for only 1.6–3% and 2–4%, respectively. The most common mutational subtype is A775_G776insYVMA, a 12-base pair (bp) in-frame insertion in exon 20.4–6 Numerous studies have demonstrated that post-chemotherapy prognoses of NSCLC patients with HER2 alterations are unfavorable, with a median progression-free survival (PFS) of 4.9–5.9 months and median overall survival (OS) of 9.9–10.7 months in first-line or second-line settings.7,8
In the past five years, immune checkpoint inhibitors (ICIs) of anti-programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) antibodies have emerged as a standard first-line treatment for oncogene-naïve advanced NSCLC, as it has demonstrated greater benefits than chemotherapy. Nevertheless, several large randomized clinical trials have linked oncogenic drivers such as EGFR and ALK with poor responses to immunotherapy.9,10 Currently, there is no available comprehensive study evaluating the efficacy of first-line combination strategies using chemotherapy plus angiogenesis inhibitors or ICIs for advanced NSCLC patients with HER2 alterations. Little is known about how these therapies interact with the heterogeneous landscape of HER2 alterations in NSCLC, or why these patients have a poor response to ICIs. Therefore, we conducted this large cohort real-world study to investigate the clinical outcomes of chemotherapy alone, chemotherapy plus ICIs, or angiogenesis inhibitors in advanced NSCLC patients with HER2 alterations in a first-line setting. We also performed a molecular analysis to explore the possible mechanisms underlying the minimal response to ICIs in this subset population.
Patients and methods
Study design and population
Data of metastatic NSCLC patients harboring HER2 alterations who received first-line systemic therapies at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences between November 30, 2015, and September 1, 2021, were retrospectively reviewed. The inclusion criteria were as follows: (1) ⩾18 years of age, (2) histologically or cytologically confirmed metastatic NSCLC, (3) confirmed HER2 mutations or HER2 amplification (defined as HER2 copy number ⩾3.6211) at primary diagnosis detected via next-generation sequencing (NGS) testing with tumor tissues or liquid biopsy samples, and (4) pretreatment with first-line therapies. In addition, patients treated with HER2-targeted therapy, an angiogenesis inhibitor or ICI monotherapy, or an angiogenesis inhibitor plus ICI combined with or without chemotherapy, as well as those without available data for efficacy evaluation, were excluded from the efficacy analysis. PD-L1 expressions were detected using a Dako 22 C3 pharmDx test kit. The PD-L1 tumor proportion score (TPS) was calculated as the percentage of ⩾100 viable tumor cells with complete or partial membrane staining. Tumor mutational burden (TMB), defined as the number of somatic, coding base substitutions, and short insertions and deletions per megabase of the genome examined, was assessed using formalin-fixed, paraffin-embedded tissue samples or blood using the NGS method covering various gene panels. NGS testing was performed in institutional laboratories or qualified third-party genetic testing companies that had acquired the national quality system certification, and all NGS testing was performed based on the Illumina sequencing system. Lesions were identified according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. All clinical data were extracted from electronic records. Informed consent was exempted from this retrospective observational study. The study was approved by the Ethics Committee of the National Cancer Center (approval number: 18-070/1648) and conducted in accordance with the Declaration of Helsinki.
Treatment and assessment
Patients who met the above inclusion criteria were included in the HER2 molecular analysis (HMA) set, and those who received first-line chemotherapy alone (C), chemotherapy plus angiogenesis inhibitors (C + A), or chemotherapy plus ICIs (C + I) were included in the HER2 efficacy analysis (HEA) set. Patients received chemotherapy, ICIs, and/or angiogenesis inhibitors at a standard dose according to the guidelines in clinical practice. Baseline images of measurable target lesions were obtained with computed tomography of the chest and abdomen and magnetic resonance imaging of the brain. Guidelines from the RECIST version 1.1 were used to classify a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). PFS was defined as the duration from the initiation of first-line therapy to the date of documented disease progression by the investigator or death from any cause. The objective response rate (ORR) was defined as the percentage of confirmed CR and PR. The disease control rate (DCR) was defined as the percentage of patients with CR, PR, and SD. OS was defined as the time from the initiation of first-line therapy to death. Both current and former smokers were classified as ‘smokers’, whereas non-smokers were those who smoked fewer than 100 cigarettes in their lifetime.
Statistical analysis
Statistical analyses were conducted using SPSS (version 23.0, SPSS Inc., Chicago, IL, USA). Patients in the HEA set were divided into three subgroups according to their first-line regimen. Continuous variables were summarized using medians and ranges, and categorical variables were described using frequency and percentage. Comparisons among the subgroups were accordingly performed using analysis of variance or the chi-square test. PFS and OS were analyzed using the Kaplan–Meier method. PFS values between different subgroups were compared using the log-rank test (two-sided), and the corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional regression model. P < 0.05 was considered statistically significant. The p values for these analyses are nominal, and all are two-sided. Survival curves were plotted using GraphPad Prism version 5.0. A Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed in R version 4.0.5 with DAVID version 6.8 to reveal differentially expressed concomitant mutations with HER2 alterations.
Results
Patient characteristics
In total, 293 metastatic NSCLC patients harboring HER2 mutations or HER2 amplification were included in the HMA set. All patients had adenocarcinoma, and no eligible squamous disease was found. As shown in the flow chart (Supplementary Figure 1), 76 patients received first-line HER2-targeted therapy, two patients received angiogenesis inhibitors alone, and four received PD-1 inhibitors alone or plus angiogenesis inhibitors. Except for one patient with no available data for the efficacy evaluation owing to a loss from follow-up after the first cycle of C + A, the remaining 210 patients in the HEA set were all treated with first-line chemotherapy-based regimens, including C (n = 83, 39.5%), C + A (n = 81, 38.6%), and C + I (n = 46, 21.9%). In the HEA set, the PD-L1 expression was observed in 71 patients (33.8%). Thirty-seven patients (17.6%) had a TPS of <1%. Twenty-three patients (11.0%) had 1 ⩽ TPS < 50%, and 11 patients (5.2%) had TPS < 50%. In addition, 48 cases (22.9%) were available to assess the TMB values. Thirty-two cases (15.2%) had TMB values of less than 10 Muts/Mb, and only 6 cases (2.9%) were greater than 10. There was no difference in baseline characteristics among the subgroups, except for the PD-L1 expression (p = 0.01; Table 1).
Table 1.
Clinicopathological characteristics of HER2-altered patients in first-line treatment.
| Characteristics | Total (n = 210) | C (n = 83) | C + A (n = 81) | C + I (n = 46) | p value |
|---|---|---|---|---|---|
| Age (years) | 54.7 ± 9.92 | 54.6 ± 8.94 | 54.4 ± 10.82 | 55.4 ± 10.13 | 0.61 |
| Gender | 0.51 | ||||
| Female | 112 (53.3%) | 46 (55.4%) | 45 (55.6%) | 21 (45.7%) | |
| Male | 98 (46.7%) | 37 (44.6%) | 36 (44.4%) | 25 (54.3%) | |
| Smoking history | 0.88 | ||||
| Never | 139 (66.2%) | 56 (67.5%) | 54 (66.7%) | 29 (63.0%) | |
| Current/former | 71 (33.8%) | 27 (32.5%) | 27 (33.3%) | 17 (37.0%) | |
| CNS metastases | 0.61 | ||||
| Presence | 28 (13.3%) | 11 (13.3%) | 9 (11.1%) | 8 (17.4%) | |
| Absence | 182 (86.7%) | 72 (86.7%) | 72 (88.9%) | 38 (82.6%) | |
| NGS specimen | 0.80 | ||||
| Tumor tissue | 194 (92.4%) | 78 (94.0%) | 74 (91.4%) | 42 (91.3%) | |
| Plasma | 16 (7.6%) | 5 (6.0%) | 7 (8.6%) | 4 (8.7%) | |
| HER2 variants | 0.50 | ||||
| Ex20ins | 178 (84.8%) | 72 (86.7%) | 70 (86.4%) | 36 (78.3%) | |
| Missense | 20 a (9.5%) | 6 (7.3%) | 8 (9.7%) | 6 (13.0%) | |
| Amplification | 13 b (6.2%) | 5 (6.0%) | 3 (3.7%) | 5 (10.9%) | |
| Deletion | 1 (0.5%) | 0 | 1 (1.2%) | 0 | |
| PD-L1 expression | 0.01 | ||||
| Negative | 37 (17.6%) | 11 (13.3%) | 21 (25.9%) | 5 (10.9%) | |
| 1 ⩽ TPS < 50% | 23 (11.0%) | 5 (6.0%) | 7 (8.6%) | 11 (23.9%) | |
| TPS ⩾ 50% | 11 (5.2%) | 3 (3.6%) | 5 (6.2%) | 3 (6.5%) | |
| NA | 139 (66.2%) | 64 (77.1%) | 48 (59.3%) | 27 (58.7%) | |
| TMB value (Mb/Muts) | 0.30 | ||||
| <10 | 32 (15.2%) | 9 (10.8%) | 17 (21.0%) | 6 (13.0%) | |
| ⩾10 | 6 (2.9%) | 4 (4.8%) | 1 (1.2%) | 1 (2.2%) | |
| NA | 172 (81.9%) | 70 (84.4%) | 63 (77.8%) | 39 (84.8%) | |
| Chemotherapy regimens | 0.13 | ||||
| Platinum/pemetrexed | 185 (88.1%) | 70 (84.3%) | 71 (87.7%) | 44 (95.7%) | |
| Other platinum-based regimens | 25 (11.9%) | 13 (15.7%) | 10 (12.3%) | 2 (4.3%) | |
C, chemotherapy alone; C + A, chemotherapy plus angiogenesis inhibitors; C + I, chemotherapy plus immune checkpoint inhibitors; Ex20ins, exon 20 insertion; NA, not available; TMB, tumor mutational burden; TPS, tumor proportion score.
One patient was detected to be exon 20 insertion G778dup with G776 S missense mutation.
One patient was detected to be exon 20 insertion A775_G776insYVMA with HER2 amplification.
Molecular landscape of HER2 alterations
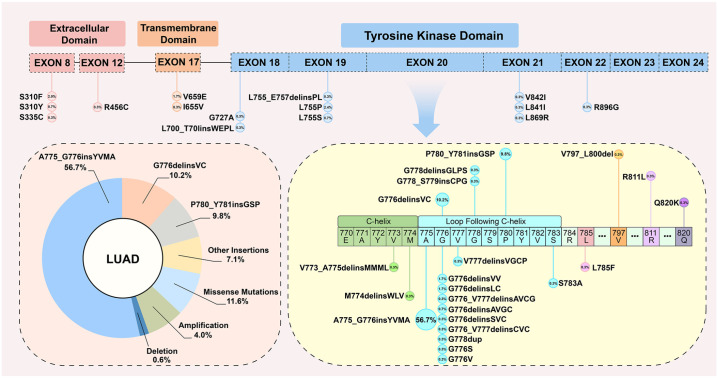
The HER2 mutation or amplification statuses were determined using tumor tissues in 194 (92.4%) patients and from plasma in 16 (7.6%) patients. In the HMA set, apart from the HER2 amplification (n = 12, 4.0%), NGS testing identified 37 distinct HER2 mutations, including 16 types of insertions, 19 types of missense mutations (n = 34, 11.6%), and 2 types of deletions (Figure 1). The most common alteration subtype was the exon 20 insertion (ex20ins, n = 246, 83.9%); the 12-bp in-frame insertion A775_G776insYVMA (n = 166, 56.7%) was most dominant, followed by the 6-bp insertion G776delinsVC (n = 30, 10.2%), 9-bp insertion P780_Y781insGSP (n = 29, 9.8%), and other ex20ins variants (n = 21, 7.2%). In addition, three patients were identified with concomitant ex20ins and missense mutations, including G778dup with G776 S, P780_Y781insGSP with L785 F, and A775_G776insYVMA with S783A. The other three cases were deemed A775_G776insYVMA with HER2 amplification.
Figure 1.
Proportion and detailed molecular landscape of NSCLC patients with distinct HER2-activating alterations.
LUAD, lung adenocarcinoma.
KEGG analysis for concomitant alterations
The Venn plot demonstrates the top-10 concomitant genes together with HER2 detected by NGS testing among HEA-set patients at primary diagnosis (n = 101, 48.1%); TP53 (n = 71, 70.3%) aberrations were the most frequent co-mutations observed in this study (Figure 2(a)). In addition, 27 cases (26.7%) presented concomitant alterations in the cell cycle pathway signaling, including CDKN2A, MDM2, MDM4, CDK4, CDK12, CCNE1, CCND1, and RB1. Fourteen patients (13.9%) had concomitant alterations in the PI3 K/AKT signaling pathway, including AKT1, AKT2, PIK3CA, and PTEN. KEGG analysis revealed differentially expressed concomitant alterations in patients with HER2 heterogeneous mutations or amplification. KEGG analysis was performed for the 15 significant signaling pathways. Among these, pathways in cancer, PI3 K/AKT, and the cell cycle comprised the majority, at 39, 25, and 26 counts, respectively. In addition, other concomitant signaling pathways that might mediate drug resistance in HER2-altered NSCLC have also been identified and reported, including microRNAs in cancer, MAPK, HIF-1, and ERBB signaling pathways (Figure 2(b)).
Figure 2.
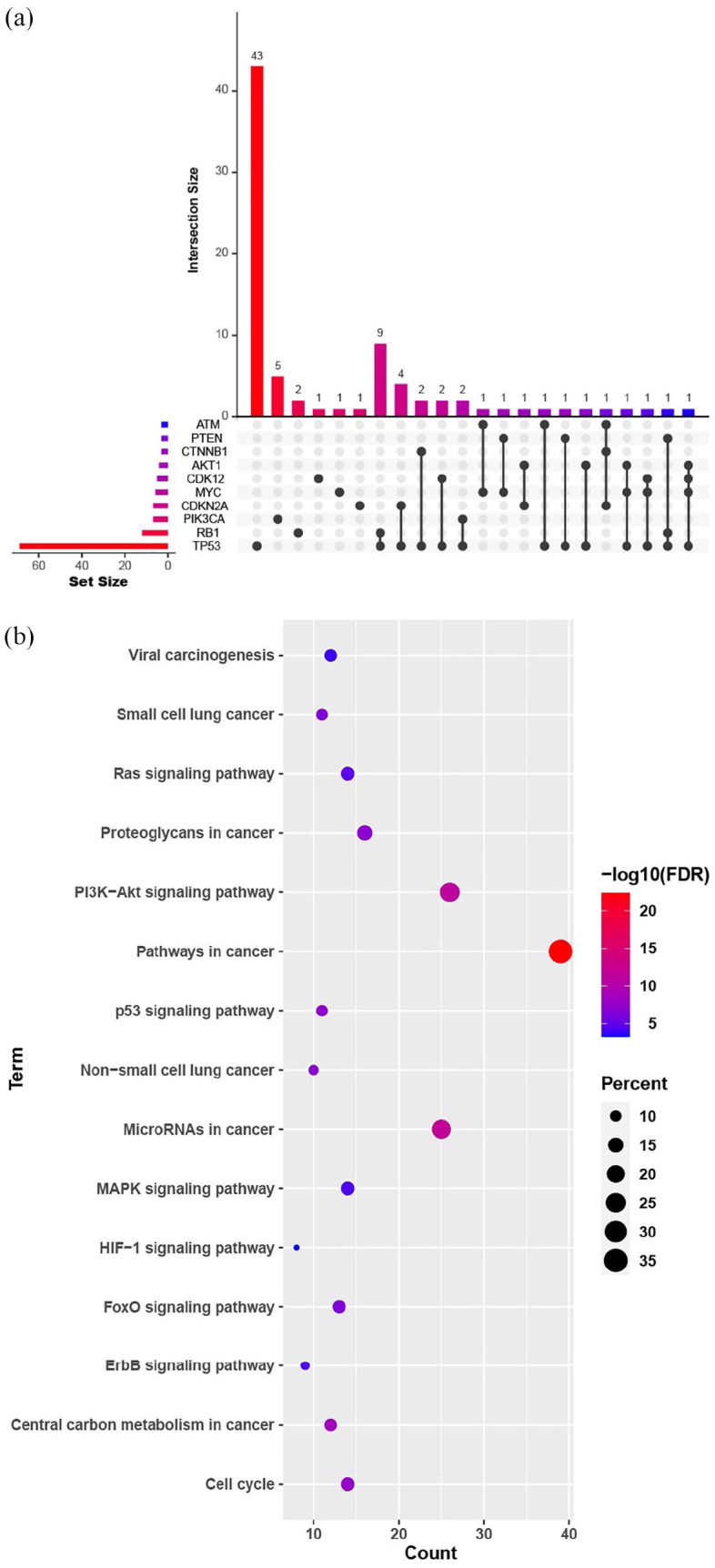
(a) Top 10 concomitant altered genes together in NSCLC patients with HER2 alterations detected using next-generation sequencing among patients at primary diagnosis as per Venn plot and (b) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of concomitant signaling pathways in patients with HER2 mutations and HER2 amplification.
Regimens and efficacy
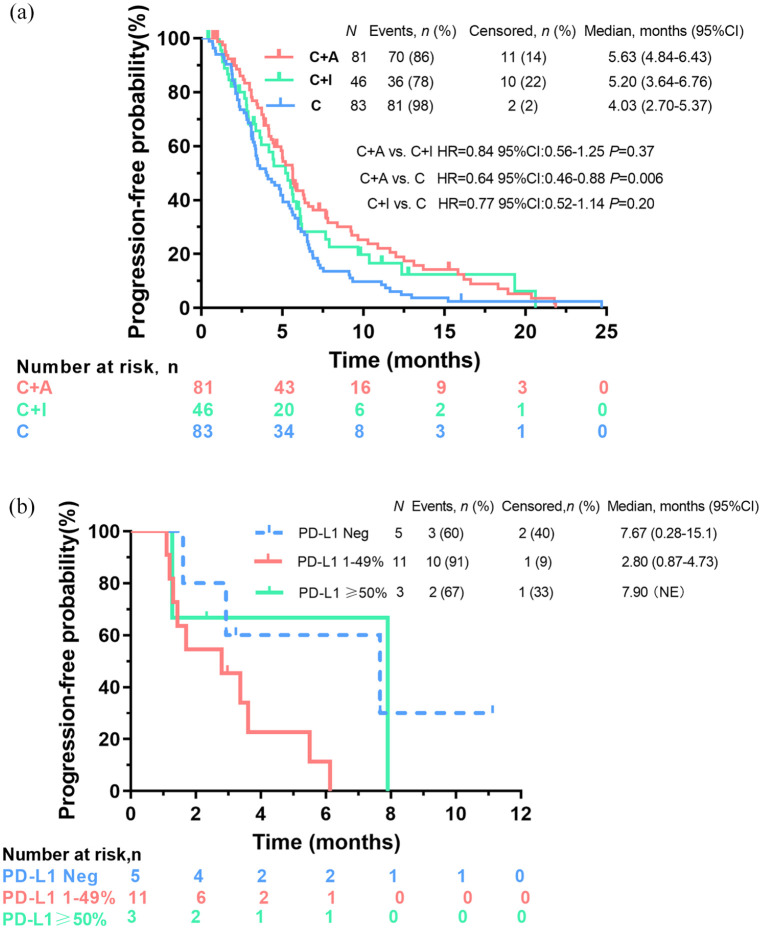
Among the 210 patients available for first-line treatment efficacy analysis, 187 had PFS events. Eighty-three patients (39.5%) received C, and the majority (n = 71, 85.5%) were treated with platinum/pemetrexed. The ORR, DCR, median PFS, and median OS of C were 16.9% (14/83), 89.2% (74/83), 4.03 months (95% CI: 2.70–5.37), and 31.67 months (95% CI: 29.63–33.71), respectively. Eighty-one patients (38.6%) were treated with C + A, and most received platinum/pemetrexed plus bevacizumab (n = 69, 85.2%). The ORR, DCR, median PFS, and median OS of C + A were 23.8% (19/80), 91.3% (73/80), 5.63 months (95% CI: 4.84–6.43), and 36.27 months (95% CI: 28.71–42.83), respectively. Forty-six patients (21.9%) were administered C + I, and nearly all (n = 45, 97.8%) received platinum/pemetrexed chemotherapy plus PD-1 inhibitors. One patient received nedaplatin/pemetrexed plus the PD-L1 inhibitor atezolizumab. The ORR, DCR, and median PFS of C + I were 28.9% (13/45), 80.0% (36/45), and 5.20 months (95% CI: 3.64–6.76), respectively. At the cutoff time, the median OS for C + I was immature because only two deaths had occurred. The 6-month and 12-month survival rates of C + I were 75.6% (34/45) and 53.3% (24/45), respectively. The PFS based on the above three treatment patterns is listed in Figure 3(a).
Figure 3.
(a) Kaplan–Meier curves of PFS in patients harboring HER2-activating alterations with chemotherapy alone (C) vs chemotherapy plus angiogenesis inhibitors (C + A) vs chemotherapy plus ICIs (C + I) in a first-line setting and (b) PFS outcomes among subgroups with different programmed cell death ligand 1 (PD-L1) tumor proportion score.
HR, hazard ratio.
Significant PFS differences were observed between subgroups C + A and C (median, 5.63 vs4.03 months, HR = 0.64, 95% CI: 0.46–0.88, p = 0.006). No difference in PFS was observed between subgroups C + I and C (median, 5.20 vs 4.03 months, HR = 0.77, 95% CI: 0.52–1.14, p = 0.20), or C + A and C + I (median, 5.63 vs 5.20 months, HR = 0.84, 95% CI: 0.56–1.25, p = 0.37). There was no difference in OS between C + A and C (median, 36.27 vs 31.67 months, HR = 1.07, 95% CI: 0.64–1.78, p = 0.80). Similarly, no statistical differences in PFS were observed between HER2 alterations (amplification vs ex20ins mutations vs missense mutations) and between patients with or without brain metastases (Supplementary Figures 2 and 3).
PD-L1 and TMB as biomarkers for ICI efficacy evaluation
Among the 45 patients receiving C + I and available for efficacy evaluation, 19 had molecular data for PD-L1 expression. For five patients with TPS negativity, the ORR was 20% (1/5), and the median PFS was 7.67 months (95% CI: 0.28–15.1). Among the 11 patients who had 1 ⩽ TPS < 50%, ORR was 18.2% (2/11), with a median PFS of 2.80 months (95% CI: 0.87–4.73). Of note, for three patients with TPS ⩾ 50% (two with TPS 50%, and one with TPS 95%), only one achieved PR, with a PFS of 2.33 months. One patient achieved SD with a PFS of 7.90 months, and the other developed PD at the initial response evaluation, with a PFS of 1.27 months (Figure 3(b)).
Among seven patients who had detected TMB per NGS, one with a TMB of 12.7 Muts/Mb showed PD to PD-1 inhibitors with pemetrexed chemotherapy, with a short PFS of 1.27 months. For two patients who responded to PR and with a PFS of 5.97 and 7.67 months, their TMB values were 0 and 4.8 Muts/Mb, respectively.
Discussion
Our study provides comprehensive real-world evidence regarding the clinical efficacy of various therapies for advanced NSCLC with heterogeneous HER2 alterations in a first-line setting. We examined full-scale heterogeneous genetic alterations of HER2, including 16 types of insertions, 19 types of missense mutations, 2 types of deletions and amplification. In accordance with previous studies, ex20ins was the most prevalent variant, and the predominant variant was A775_G776insYVMA, followed by G776delinsVC and P780_Y781insGSP.12,13
In this study, the overall median PFS values of the first-line treatments were 4.03 months for C, 5.63 months for C + A, and 5.20 months for C + I, respectively. These outcomes are similar to previous studies that have indicated a PFS of 1.9–7.5 months for HER2-mutant NSCLC.8,13–23 The outcomes of chemotherapy-based regimens and PD-1/PD-L1 inhibitors alone as a first-line treatment for advanced NSCLC with HER2 alterations in a real-life setting are listed in Table 2. Notably, ours is one of the few studies to analyze the clinical outcomes of chemotherapy in combination with angiogenesis inhibitors for NSCLC carrying HER2 alterations. Additional angiogenesis inhibitors can enhance drug delivery to tumor sites via ‘vessel normalization’. 24 Thus, a synergistic effect might result in increased anti-tumor activity. Recently, Offin et al. 25 reported that HER2-mutated NSCLCs were associated with an increased risk of brain metastases development, and that HER2-mutant patients with brain metastases had a worse prognosis than those without brain metastases. In this study, 13.3% of patients had brain metastases at the baseline, and their mPFS was 3.97 months; these results are numerically inferior to those without brain metastases (5.03 months, HR: 0.80, 95% CI: 0.53–1.21).
Table 2.
Reported retrospective studies on efficacy of first-line chemotherapy or ICIs for advanced NSCLC with HER2 alterations.
| Author | Year | Sample size a | Therapeutics | ORR (%) | DCR (%) | Median PFS (months) |
|---|---|---|---|---|---|---|
| Eng et al. 14 | 2016 | 38 | Chemotherapy | NA | NA | 7.5 |
| Mazières et al. 15 | 2016 | 93 (101) | Chemotherapy | 43.5 | 70.7 | 6 |
| Song et al. 16 | 2016 | 14 (21) | Platinum-based chemotherapy | NA | NA | 4.6 |
| Wang et al. 17 | 2018 | 25 (29) | Pemetrexed-based chemotherapy | 36 | 92 | 5.1 |
| Auliac et al. 8 | 2019 | 20 (23) | Platinum-based chemotherapy + bevacizumab | 61.5 | 84.6 | 6.7 |
| Mazieres et al. 18 | 2019 | 29 | ICIs: PD-1/PD-L1 inhibitors alone | 7 | 37 | 2.5 |
| Guisier et al. 19 | 2020 | 23 | ICIs: PD-1/PD-L1 inhibitors alone | 27.3 | 50 | 2.2 |
| Xu et al. 13 | 2020 | 75 | Chemotherapy | NA | NA | 5.5 |
| Chen et al. 20 | 2021 | 21 | ICIs: PD-1/PD-L1 inhibitors alone | 0 | NA | 1.9 |
| Lau et al. 21 | 2021 | 14 | ICIs: PD-1 inhibitors alone or plus CTLA-4 inhibitor | 29 | 57 | 3.6 |
| Saalfeld et al. 22 | 2021 | 27 (61) | 5 cases with PD-1 inhibitors alone 22 with PD-1 inhibitors + platinum-based chemotherapy |
PD-1 inhibitors alone: 20 PD-1 inhibitors + platinum-based chemotherapy: 52 |
NA | PD-1 inhibitors + platinum-based chemotherapy: 6.0 |
| Yang et al. 23 | 2021 | 82 (98) | Chemotherapy | 37.8 | 84.1 | 5.77 |
DCR, disease control rate; ICI, immune checkpoint inhibitor; NA, not available; ORR, overall response rate; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PFS, progression-free survival.
Expressed as number in the first-line setting (total number).
In the pre-immunotherapy era, HER2 amplification was considered as both predictive in NSCLC patients treated with gefitinib as well as a mechanism of resistance to EGFR tyrosine kinase inhibitors (such as afatinib and lapatinib) in 12% of non-T790M mutant NSCLC patients.26,27 In recent years, immunotherapy has revolutionized the therapeutic paradigms for advanced NSCLC patients. However, increasing evidence has shown that HER2-altered NSCLC patients appear to be insensitive to ICIs. 28 Similarly, our study has also demonstrated that ICIs in combination with chemotherapy might not improve survival outcomes over chemotherapy alone in patients with HER2-altered disease, including HER2 amplification. However, these results should be interpreted with caution owing to a small sample size. In addition, PD-L1 expressions and TMB values have reportedly impacted immunotherapy efficacy in many clinical trials.29,30 In this study, we also analyzed this relationship. Unfortunately, patients with high PD-L1 expression and TMB did not show a significant improvement in PFS compared to other patients. Our observation was consistent with another study that demonstrated no obvious difference in PFS between HER2-mutant NSCLC with different PD-L1 TPS. 22
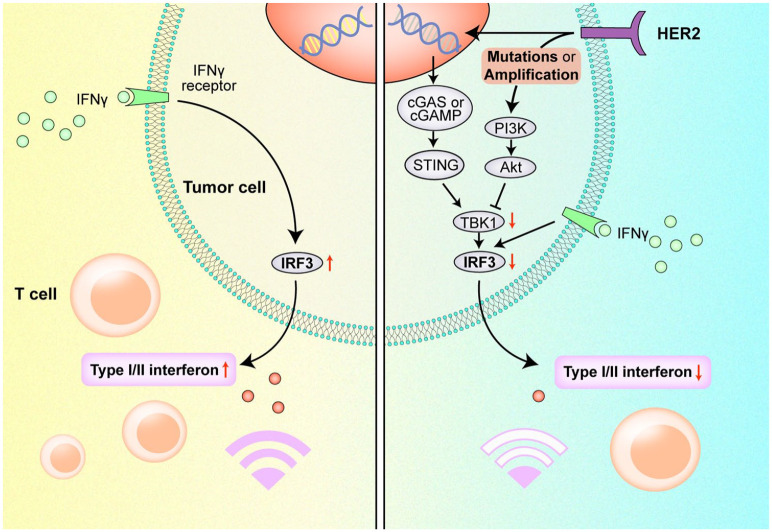
Preclinical data showed that HER2 amplification may abolish phosphorylation of TANK-binding kinase 1 (TBK1) and attenuate stimulator of interferon genes (STING) signaling, thus suppressing type-I and -II interferon and antitumor immune responses.31,32 Our KEGG analysis revealed that upregulation of PI3 K/AKT signaling was common in NSCLC patients with HER2 mutations or amplification, which has also been reported in other HER2-positive solid tumors.33–35 We supposed that the PI3K/AKT signaling, together with other signaling pathways related to the cell cycle and microRNA, might influence drug resistance to ICIs in HER2-altered NSCLC. Regarding possible mechanisms explaining the immunosuppressive microenvironment in HER2-altered NSCLC, we propose that HER2 mutations or amplification may activate the downstream MAPK and PI3K/AKT signaling pathways. Specifically, AKT1 recruitment may impair TBK1 phosphorylation and suppress interferon regulatory factor 3 (IRF3), thereby disrupting STING signaling in antitumor immune responses (Figure 4). Therefore, novel treatment strategies targeting immunosuppressive signaling pathways together with HER2 should be developed to optimize targeted immunotherapy in this subset of patients.
Figure 4.
Possible mechanism for immunosuppression in HER2-altered NSCLC mediated by the PI3 K/AKT signaling pathway.
In addition, we identified and reported other concomitant alterations in several signaling pathways that likely mediate drug resistance to anti-HER2 therapeutics. TP53 aberrations were the most common co-mutations in this study, thereby corroborating previous studies showing that concomitant TP53 mutations were predominant among HER2 ex20ins.36,37 In addition, KEGG analyses may provide additional details on drug resistance in NSCLC patients with HER2 heterogeneous alterations. Recent studies have found that microRNAs strongly influence chemotherapy resistance to cancer, including NSCLC. 38 The dysregulation of microRNAs is closely related to remodeling of tumors and the microenvironment. 39 In addition to the pathway mentioned above, other signaling pathways, including the cell cycle, MAPK, HIF-1, and ERBB were also reportedly involved in drug resistance in NSCLC.40–43
HER2-mutated NSCLCs have reportedly represented a distinct molecular subgroup of NSCLC. 44 Ninomiya et al. 45 reported that, compared to EGFR- and ALK-positive NSCLC, HER2-aberrant diseases were less responsive to targeted therapies. In addition, HER2 ex20ins seems to have distinct characteristics from other HER2 alterations (mainly non-ex20ins), with less sensitivity to conventional HER2-targeted TKIs (such as neratinib, afatinib, and dacomitinib), having only reached ORRs of 7.7–12% and median PFS of 2.9–5.5 months. 44 Recently, other novel HER2-targeted agents have been evaluated in HER2-altered NSCLC. A ZENITH20-2 study of poziotinib showed that the mPFS was 5.5 months and the ORR was 27% in patients with advanced HER2 exon 20 mutant NSCLC. 46 Zhou et al. 12 reported a mPFS of 6.9 months and an ORR of 30% for pyrotinib as second-line or above therapy in patients with HER2-mutant advanced lung adenocarcinoma. In addition, ado-trastuzumab emtansine showed a mPFS of 5 months and an ORR of 44% for advanced NSCLC with HER2 alterations. 47 Similarly, a DESTINY-Lung01 study reported a mPFS of 8.2 months and an ORR of 55% for trastuzumab deruxtecan in metastatic HER2-mutated NSCLC. 48 Although HER2-targeted therapies have greatly improved the prognoses of HER2-overexpressing breast cancer patients, they appear to be not ideal in patients with HER2-overexpressing NSCLC. A HER2-CS STUDY reported that patients with HER2-overexpressing NSCLC were insensitive to anti-HER2 agents, with a response rate of 0%. 45 A HER2 overexpressing cohort of DESTINY-Lung01 showed an ORR of 24.5% and an estimated mPFS of 5.4 months for trastuzumab deruxtecan. 49 However, the above studies on anti-HER2 therapies were mainly focused on previously treated HER2-altered NSCLC. Further studies are needed to assess the effectiveness of anti-HER2 agents in a first-line setting.
This study had several limitations. First, biases were inevitable owing to the retrospective nature of the study, and the treatment regimens were highly heterogeneous. In addition, although this study provided comprehensive HER2 molecular subtypes and concomitant mutations in other signaling pathways, only half of the patients had available NGS data, and we did not have genetic information on other co-existing alterations or data on whole exome sequencing. Another limitation was the lack of in vitro evidence of tumor immunohistochemistry or flow cytometry data of the PI3 K/AKT pathway and other immunosuppressive genes. Finally, we included a relatively limited number of patients with HER2 missense mutations and amplification, which prevented us from providing corroborative data on the subgroups.
In conclusion, this comparative POLISH study provides a novel insight into the outcomes of advanced HER2-altered NSCLC patients receiving a chemotherapy-based regimen as a first-line treatment in a real-world setting. First-line chemotherapy plus angiogenesis inhibitors might confer additional survival chances in patients with NSCLC with HER2 alterations, as compared to using chemotherapy alone. The addition of ICIs to chemotherapy did not yield an improved survival benefit. Future prospective studies are required to verify these perspectives in first-line treatment settings for HER2-altered NSCLC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221082339 for First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2-altered NSCLC: a retrospective real-world POLISH study by Guangjian Yang, Yaning Yang, Runze Liu, Weihua Li, Haiyan Xu, Xuezhi Hao, Junling Li, Puyuan Xing, Shuyang Zhang, Xin Ai, Fei Xu and Yan Wang in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank all the patients and their families who participated in this study. They thank Liping Sun, Yanhua Xu, Li Zhang (a former employee) and Yunjie Yu (a former employee) from Jiangsu Hengrui Pharmaceuticals Co., Ltd. for providing the study design and medical writing support.
Footnotes
Author Contributions: Guang-Jian Yang: Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Writing – original draft; Writing – review & editing
Ya-Ning Yang: Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Writing – original draft; Writing – review & editing
Run-Ze Liu: Formal analysis; Methodology; Software; Validation; Visualization; Writing – original draft; Writing – review & editing
Wei-Hua Li: Data curation; Project administration; Resources; Validation
Hai-Yan Xu: Data curation; Formal analysis; Project administration; Resources
Xue-Zhi Hao: Data curation; Project administration; Resources; Validation
Jun-Ling Li: Data curation; Investigation; Project administration; Resources; Validation
Pu-Yuan Xing: Data curation; Project administration; Resources
Shu-Yang Zhang: Data curation; Investigation; Project administration; Resources; Validation
Xin Ai: Data curation; Project administration
Fei Xu: Project administration; Software
Yan Wang: Conceptualization; Funding acquisition; Supervision; Writing – review & editing
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Beijing Health Promotion Association (grant no. 2021-053-ZZ).
Data availability: Data supporting the results presented in this study are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Guangjian Yang, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yaning Yang, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Runze Liu, Guangxi Medical University, Nanning, China.
Weihua Li, Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Haiyan Xu, Department of Comprehensive Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xuezhi Hao, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Junling Li, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Puyuan Xing, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Shuyang Zhang, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xin Ai, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Fei Xu, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yan Wang, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Street South, Chaoyang District, Beijing 100021, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. NCCN clinical practice guidelines in oncology (NCCN Guidelines®), non-small cell lung cancer. Version 5, 2021. www.nccn.org/patients
- 3. Peters S, Zimmermann S. Targeted therapy in NSCLC driven by HER2 insertions. Transl Lung Cancer Res 2014; 3: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012; 18: 4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005; 65: 1642–1646. [DOI] [PubMed] [Google Scholar]
- 6. Mazieres J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013; 31: 1997–2003. [DOI] [PubMed] [Google Scholar]
- 7. Zhou J, Ding N, Xu X, et al. Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies. Ther Adv Med Oncol 2020; 12: 1758835920936090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auliac JB, Dô P, Bayle S, et al. Non-small cell lung cancer patients harboring HER2 mutations: clinical characteristics and management in a real-life setting. Cohort HER2 EXPLORE GFPC 02-14. Adv Ther 2019; 36: 2161–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 11. Zhang G-C, Liao N, Chen B, et al. Next-generation sequencing (NGS) identifies a new breast cancer subtype with HER2 low-amplification status as a candidate for targeted therapy. J Clin Oncol 2020; 38: 553–553. [Google Scholar]
- 12. Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J Clin Oncol 2020; 38: 2753–2761. [DOI] [PubMed] [Google Scholar]
- 13. Xu F, Yang G, Xu H, et al. Treatment outcome and clinical characteristics of HER2 mutated advanced non-small cell lung cancer patients in China. Thorac Cancer 2020; 11: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eng J, Hsu M, Chaft JE, et al. Outcomes of chemotherapies and HER2 directed therapies in advanced HER2-mutant lung cancers. Lung Cancer 2016; 99: 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016; 27: 281–286. [DOI] [PubMed] [Google Scholar]
- 16. Song Z, Yu X, Shi Z, et al. HER2 mutations in Chinese patients with non-small cell lung cancer. Oncotarget 2016; 7: 78152–78158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Zhang S, Wu F, et al. Outcomes of pemetrexed-based chemotherapies in HER2-mutant lung cancers. BMC Cancer 2018; 18: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019; 30: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guisier F, Dubos-Arvis C, Vinas F, et al. Efficacy and safety of anti-PD-1 immunotherapy in patients with advanced NSCLC With BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol 2020; 15: 628–636. [DOI] [PubMed] [Google Scholar]
- 20. Chen K, Pan G, Cheng G, et al. Immune microenvironment features and efficacy of PD-1/PD-L1 blockade in non-small cell lung cancer patients with EGFR or HER2 exon 20 insertions. Thorac Cancer 2021; 12: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau SCM, Fares AF, Le LW, et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin Lung Cancer 2021; 22: 253–259. [DOI] [PubMed] [Google Scholar]
- 22. Saalfeld FC, Wenzel C, Christopoulos P, et al. Efficacy of immune checkpoint inhibitors alone or in combination with chemotherapy in NSCLC harboring ERBB2 mutations. J Thorac Oncol 2021; 16: 1952–1958. [DOI] [PubMed] [Google Scholar]
- 23. Yang S, Wang Y, Zhao C, et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl Lung Cancer Res 2021; 10: 753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001; 7: 987–989. [DOI] [PubMed] [Google Scholar]
- 25. Offin M, Feldman D, Ni A, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer 2019; 125: 4380–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross HJ, Blumenschein GR, Jr, Aisner J, et al. Randomized phase II multicenter trial of two schedules of lapatinib as first- or second-line monotherapy in patients with advanced or metastatic non-small cell lung cancer. Clin Cancer Res 2010; 16: 1938–1949. [DOI] [PubMed] [Google Scholar]
- 27. Ricciardi GR, Russo A, Franchina T, et al. NSCLC and HER2: between lights and shadows. J Thorac Oncol 2014; 9: 1750–1762. [DOI] [PubMed] [Google Scholar]
- 28. Riudavets M, Sullivan I, Abdayem P, et al. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021; 6: 100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score >/= 50. J Clin Oncol 2021; 39: 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu S, Zhang Q, Zhang F, et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol 2019; 21: 1027–1040. [DOI] [PubMed] [Google Scholar]
- 32. Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer 2021; 21: 181–197. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Pan C, Guo L, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol 2016; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanker AB, Pfefferle AD, Balko JM, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci U S A 2013; 110: 14372–14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007; 12: 395–402. [DOI] [PubMed] [Google Scholar]
- 36. Liu Z, Wu L, Cao J, et al. Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients. Onco Targets Ther 2018; 11: 7323–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang W, Zhao S, Liang Y, et al. Mutation variants and co-mutations as genomic modifiers of response to afatinib in HER2-mutant lung adenocarcinoma. Oncologist 2020; 25: e545–e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang F, Ning Z, Ma L, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer 2017; 16: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu MX, Zhou KC, Cao Y. MCRS1 overexpression, which is specifically inhibited by miR-129*, promotes the epithelial-mesenchymal transition and metastasis in non-small cell lung cancer. Mol Cancer 2014; 13: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katayama K, Yoshioka S, Tsukahara S, et al. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther 2007; 6: 2092–2102. [DOI] [PubMed] [Google Scholar]
- 41. Ma J, Lyu H, Huang J, et al. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 2014; 13: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin SC, Chien CW, Lee JC, et al. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J Clin Invest 2011; 121: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirchner M, Kluck K, Brandt R, et al. The immune microenvironment in EGFR- and ERBB2-mutated lung adenocarcinoma. ESMO Open 2021; 6: 100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friedlaender A, Subbiah V, Russo A, et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol 2022; 19: 51–69. [DOI] [PubMed] [Google Scholar]
- 45. Ninomiya K, Hata T, Yoshioka H, et al. A prospective cohort study to define the clinical features and outcome of lung cancers harboring HER2 aberration in Japan (HER2-CS STUDY). Chest 2019; 156: 357–366. [DOI] [PubMed] [Google Scholar]
- 46. Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. Epub ahead of print 22 September 2021. DOI: 10.1200/JCO.21.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol 2018; 36: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 2022; 386: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakagawa K, Nagasaka M, Felip E, et al. OA04.05 trastuzumab deruxtecan in HER2-overexpressing metastatic non-small cell lung cancer: interim results of DESTINY-lung01. J Thorac Oncol 2021; 16: S109–S110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221082339 for First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2-altered NSCLC: a retrospective real-world POLISH study by Guangjian Yang, Yaning Yang, Runze Liu, Weihua Li, Haiyan Xu, Xuezhi Hao, Junling Li, Puyuan Xing, Shuyang Zhang, Xin Ai, Fei Xu and Yan Wang in Therapeutic Advances in Medical Oncology