Abstract
Background:
Increasing evidence suggests improved time metrics leading to better clinical outcomes when stroke patients with suspected large vessel occlusion (LVO) are transferred directly to the angiography suite (DTAS) compared with cross-sectional imaging followed by transfer to the angiography suite. We performed a systematic review and meta-analysis on the efficacy and safety of DTAS approaches.
Methods:
We searched Embase, Medline, Scopus, and clinicaltrials.gov for studies comparing outcomes of DTAS and conventional triage. Eligible studies were assessed for risk of bias. We performed a random-effects meta-analysis on the differences of median door-to-groin and door-to-reperfusion times between intervention and control group. Secondary outcomes included good outcome at 90 days (modified Rankin Scale ⩽ 2) rate of symptomatic intracranial hemorrhage (sICH) and mortality within 90 days.
Results:
Eight studies (one randomized, one cluster-randomized trial and six observational studies) with 1938 patients were included. Door-to-groin and door-to-reperfusion times in the intervention group were on median 29.0 min [95% confidence interval (CI): 14.3–43.6; p < 0.001] and 32.1 min (95% CI: 15.1–49.1; p < 0.001) shorter compared with controls. Prespecified subgroup analyses for transfer (n = 1753) and mothership patients (n = 185) showed similar reductions of the door-to-groin and door-to-reperfusion times in response to the intervention. The odds of good outcome did not differ significantly between both groups but were numerically higher in the intervention group (odds ratio: 1.38, 95% CI: 0.97–1.95; p = 0.07). There was no significant difference for mortality and sICH between the groups.
Conclusion:
DTAS approaches for the triage of suspected LVO patients led to a significant reduction in door-to-groin and door-to-reperfusion times but an effect on functional outcome was not detected. The subgroup analysis showed similar results for transfer and mothership patients.
Registration: This study was registered in PROSPERO (CRD42020213621).
Keywords: cone beam CT, direct to angiography approaches, mechanical thrombectomy, stroke, triage
Introduction
Mechanical thrombectomy (MT) after intravenous thrombolytic treatment with recombinant tissue plasminogen activator (iv-rtPA), which has been shown to be superior to iv-rtPA alone, 1 is now standard of care for acute ischemic stroke due to large vessel occlusion (LVO). Clinical outcome is highly dependent on fast restoration of blood flow, and as such, the benefit of MT rapidly decreases with treatment delays. 2 Hence, current guidelines emphasize workflow speed as a key component of acute stroke care.3,4 The Stroke Treatment Academic Industry Roundtable identified the shortening of time to reperfusion to the minimum possible as an important variable in stroke treatment and as a priority target in stroke research. 5 While the time from symptom onset to admission can only be influenced at a policy level, door-to-groin and door-to-reperfusion times are highly dependent on intra-hospital procedures and structures. 6 Standard procedure in most hospitals is to triage suspected stroke patients by multidetector computed tomography (MDCT) or magnetic resonance imaging (MRI). If a target occlusion for MT is identified, the patient is then transported to the angiography suite for emergent MT. One possible approach to reduce door-to-groin and door-to-reperfusion times are so-called ‘one-stop management’ or ‘direct to angiography suite’ (DTAS) approaches, with diagnostic imaging and MT both performed in the angiography suite and bypassing an extra diagnostic imaging stop. 7 However, the effect of DTAS approaches on reducing time to treatment and clinical outcomes is a matter of debate, and results of recent randomized trials have shown conflicting results.8,9 We therefore performed a systematic review and meta-analysis in order to examine the efficacy and safety of DTAS approaches for rapid initiation of MT.
Methods
This systematic review and meta-analysis was registered at PROSPERO (CRD42020213621). All analyses are reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (PRISMA). 10 All data and supporting materials are available within the article and an online-only Data Supplement.
Search strategy
We developed the search strategies in collaboration with an information specialist (C.A.-H.). It was peer-reviewed by a second information specialist. We searched the bibliographic databases Embase (via embase.com excluding conference abstracts), Medline (via Ovid), Scopus, and clinicaltrials.gov (Primary search 31 August 2020; last update 18 August 2021). Search strings around the concepts stroke, MT, and DTAS were composed of database-specific subject headings (where applicable) and text word synonyms. The complete search strategies are deposited in the online-only Data Supplement. To complement the results of direct database searching, we screened the bibliographic references of all included articles and the citing articles of those indexed in Scopus or the Web of Science.
Eligibility
Studies were included if they compared DTAS triage approaches with conventional triage approaches of suspected acute ischemic stroke patients and reported effect on door-to-groin and door-to-reperfusion times. Studies had to report either the median and interquartile range (IQR) or the mean and standard deviation (SD) of door-to-groin and door-to-reperfusion times for a DTAS workflow and for a control group. All types of studies (including observational and case-matched studies) were included. Only studies from peer-reviewed journals were included to safeguard the quality of data. Reviews, conference abstracts, editorials, and guidelines were excluded. We included all articles that had an English title and abstract. For manuscripts written in other languages, we contacted the corresponding author, asking for the results in English. We restricted our search to studies published in 2010 or later, since DTAS approaches were not feasible in earlier years (the first patient triaged via a DTAS approach was reported in 2017 11 ) nor was MT established as routine care before 2010.
Screening, data extraction, and outcomes
The search results were exported to Endnote X9 and de-duplicated using the Bramer method. 12 Two reviewers (A.B. and I.T.) screened references based on titles and abstracts. Selected references were retrieved in full-text. Two authors (A.B. and I.T.) independently assessed the eligibility of all retrieved studies. In case of disagreement, a third author (M.N.P.) made the final judgment. Data were extracted by two authors (A.B. and I.T.). In case of publications from the same study with overlapping time periods, the publication with the larger number of patients was chosen. The filled-in data extraction forms can be found in the supplement. In case of missing data, the corresponding authors were contacted at least twice by e-mail and missing data were obtained for all studies.
The primary outcome variables of interest were the median differences (including IQR) of door-to-groin and door-to-reperfusion times between the intervention and control group. The secondary outcome variables of interest were the rates of 72-h symptomatic intracranial hemorrhage (sICH), 13 90-day good functional outcome [i.e. modified Rankin Scale (mRS) ⩽ 2], and 90-day mortality for both groups. Predictors of interest were prehospital screening methods, type of preinterventional imaging, and study characteristics such as study design, the year of the study, and sample size.
Risk of bias assessment
Risk of bias assessment was done independently by two authors (A.B. and I.T.). Risk of bias of nonrandomized studies was assessed with the ROBINS-I tool, which was developed by the Cochrane Collaboration and categorizes risk of systematic bias as low, moderate, serious, and critical. 14 If a study was rated critical, that is, did not provide useful data in at least one domain, it was excluded from the meta-analysis. 14 For randomized studies, the ROB 2 tool was used. The risk categories were low risk, some concerns, and high risk. 15
Statistical analyses
All analyses were performed in R version 4.0.3 (2020-10-10). To perform a meta-analysis on the primary outcomes of interest, we used the quantile estimation method proposed by McGrath et al. 16 as implemented in the R package ‘metamedian’. After estimating the variance of the difference in medians in each study, studies were meta-analyzed using random-effects model per the inverse variance method. For the binary secondary outcome measures of good functional outcome (mRS ⩽ 2), 72-h sICH, and 90-day mortality, we calculated odds ratio estimates using random-effects models with the R package ‘metafor’ and the DerSimonian-Laird estimator for the amount of heterogeneity. 17
A prespecified subgroup analysis on the primary outcome measures was performed for mothership patients (patients presenting directly to the comprehensive stroke center) and transfer patients (patients presenting first to a primary stroke center from which they were transferred for MT to a comprehensive stroke center).
Results
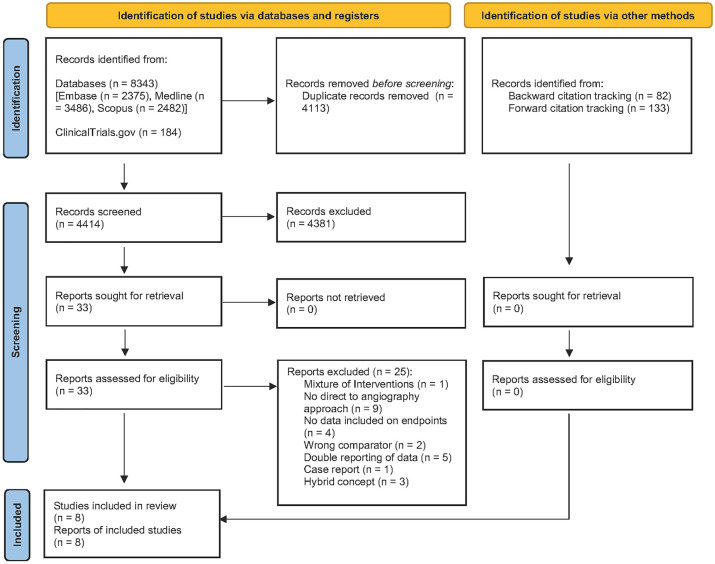
Our literature search identified 4414 potentially relevant unique articles out of which 33 were retained for full-text review (Figure 1; a detailed overview of excluded studies can be found in the Supplemental Material under part 5). Five studies had to be excluded because of potentially overlapping patients.7,18 –21 All of these studies were from the same author groups and contained overlapping time periods. Out of these studies, we chose those with the longest time periods to include the largest number of patients possible. As Sarraj et al. 22 included for the endpoint door to groin the data from the transfer patients from Requena et al., 23 we chose to only include Sarraj et al. in this analysis. However, the mothership patients (n = 79) from Requena et al. 23 were used in all other analyses and the transfer patients for the analysis of door-to-reperfusion times.
Figure 1.
Flowchart of included and excluded articles, following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
A total of eight studies met our inclusion criteria and were included in the meta-analysis on effects of DTAS approaches (Table 1). Additional data were obtained for five of these eight studies to perform meta-analysis on subgroups.8,9,23 –25 In addition, two ongoing randomized controlled studies (NCT03969511 and NCT04701684) were identified on clinicaltrials.gov, but results were not available at the time of analysis.
Table 1.
Overview over studies included in systematic review/meta-analysis.
| Author | Country | Study design | Study period | Intervention group (n) | Control group (n) | Mothership / transfer | Included in meta-analysis |
|---|---|---|---|---|---|---|---|
| Aoki et al. 24 | Japan | Retrospective, consecutive patients, single center | 2012–2018 | 40 | 27 | Only transfer | Yes |
| Bouslama et al. 26 | USA | Retrospective, case-control, single center | 2016–2017 | 49 | 49 | Only transfer | Yes |
| Jadhav et al. 27 | USA | Retrospective, consecutive patients, single center | 2013–2016 | 111 | 150 | Only transfer | Yes |
| Pfaff et al. 8 | Germany | Prospective, cluster-randomized, nonblinded, per-protocol analysis | 2017–2019 | 26 | 34 | Mothership (30%) / transfer (70%) | Yes |
| Psychogios et al. 25 | Germany | Retrospective, case-control, single center | 2016 -2018 | 43 | 43 | Mothership (56%) / transfer (44%) | Yes |
| Requena et al. 23 | Spain | Retrospective, case-control, single center | 2016–2019 | 174 | 175 | Mothership (23%) / transfer (77%) | Yes |
| Requena et al. 9 | Spain | Randomized, blinded endpoint evaluation, single center | 2018–2020 | 74 | 64 | Mothership (29%)/ transfer (71%) | Yes |
| Sarraj et al. 22 | USA, Spain | Retrospective, cohort study, multicenter | 2014–2020 | 327 | 813 | Only transfer | Yes |
Eight studies with 1938 patients (704 intervention group, 1234 control group) reported the effect of DTAS approaches on door to groin and seven studies with 1068 patients (517 intervention group, 551 control group) on door-to-reperfusion times.
We were able to perform subgroup analysis for transfer patients in seven studies (n = 1753; 626 intervention and 1127 control) on door-to-groin times and in seven studies (n = 883, 439 intervention and 444 control) on door-to-reperfusion times. Subgroup analysis for mothership patients was done in all four studies, which included mothership patients (n = 185 mothership patients; 78 intervention group and 107 control group).
Studies differed in design with one randomized study, one cluster-randomized study, two studies that reported results from case-matched patients [with different criteria although all used baseline National Institute of Health Stroke Scale (NIHSS) and age] and three studies that reported on consecutive patients. Seven of eight studies were monocentric. Detailed assessment of risk of bias is available in the online-only supplement. The most common source of bias was selection bias or possible confounding due to the retrospective nature of most studies. Risk of Bias was rated serious in three of eight (37.5%) studies.
Primary analysis: time from door to groin and door to reperfusion
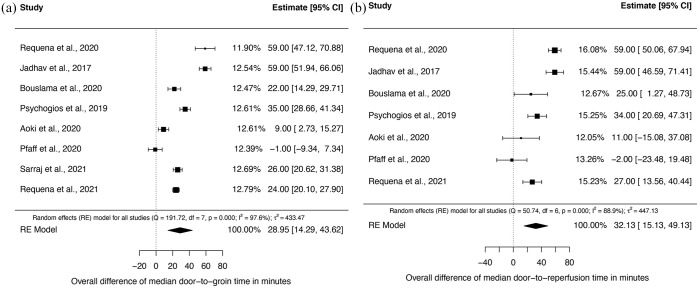
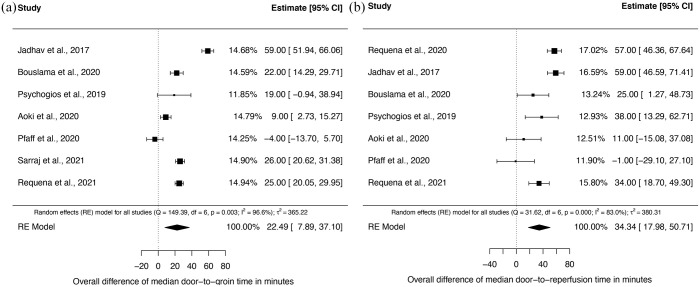
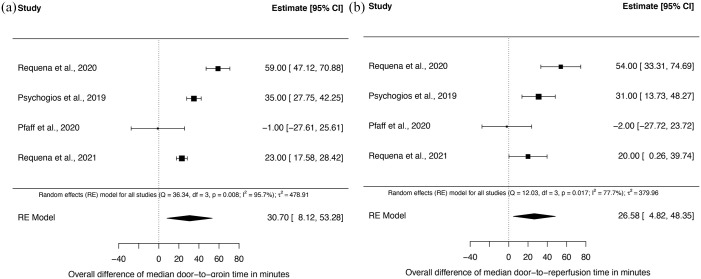
Time from door to groin and door to reperfusion was significantly shorter in the DTAS group in seven of eight (87.5%) studies. Random-effects meta-analysis of eight studies showed a significant difference of median door-to-groin times of 29.0 min [95% confidence interval (CI): 14.3–43.6; p < 0.001] and of median door-to-reperfusion times of 32.1 min (95% CI: 15.1–49.1; p < 0.001) in favor of DTAS (Figure 2; Table 2). High I 2 values indicated considerable heterogeneity among studies for both primary endpoints (door to groin and door to reperfusion). As prespecified (PROSPERO entry CRD42020213621), we performed subgroup analysis for both primary endpoints for transfer and mothership patients. In transfer patients, both door-to-groin and door-to-reperfusion times were significantly shorter. The median difference of door-to-groin times was 22.5 min (95% CI: 7.9–37.1) and of door-to-reperfusion times was 34.3 min (95% CI: 18.0–50.7) in favor of the DTAS group (Figure 3(a) and (b)). Also, in mothership patients both door-to-groin and door-to-reperfusion times were significantly shorter in DTAS patients with a median difference of 30.7 min (95% CI: 8.1–53.3) and 26.6 min (95% CI: 4.8–48.4) (Figure 4(a) and (b)).
Figure 2.
Forest plots of the median differences on door-to-groin times (a) and door-to-perfusion times (b) between DTAS and traditional triaged patients.
Table 2.
Random-effects meta-analysis of differences in median door-to-groin and door-to-reperfusion times.
| No. of studies | No. of patients (intervention/control group) | Weighted median difference, min (95% CI) | |
|---|---|---|---|
| Door-to-groin time | |||
| All patients | 8 | 1938 (704 / 1234) | 29.0 (14.3–43.6) |
| Transfer only | 7 | 1753 (626 / 1127) | 22.5 (7.9–37.1) |
| Mothership patients only | 4 | 185 (107 / 78) | 30.7 (8.1–52.3) |
| Door-to-reperfusion time | |||
| All patients | 7 | 1068 (517 / 551) | 32.1 (15.1–49.1) |
| Transfer only | 7 | 883 (439 / 444) | 34.3 (18.0–50.7) |
| Mothership patients only | 4 | 185 (78 / 107) | 26.6 (4.8–48.4) |
CI, confidence interval.
Figure 3.
Forest plots for the subgroup analysis of transfer patients of the median differences of door-to-groin times (a) and door-to-perfusion times (b) between DTAS and traditional triaged patients.
Figure 4.
Forest plot for the subgroup analysis of mothership patients of the median differences of door-to-groin times (a) and door-to-perfusion times (b) between DTAS and traditional triaged patients.
Secondary analysis: clinical outcomes and safety endpoints
Random-effects meta-analysis on secondary endpoints did not detect any significant differences (Table 3). Numerically the odds of a good functional outcome were higher in the intervention group than in the control group [odds ratio (OR): 1.38, 95% CI: 0.97–1.95; eFigure 1 in the Supplemental Material]. However, the difference did not reach statistical significance, and overall, the evidence for an effect of the intervention on the probability of a good outcome is only moderate. The incidence of 72-h sICH (OR: 0.84; 95% CI: 0.58–1.24) and 90-day mortality (OR: 0.74; 95% CI: 0.48–1.15) appeared not to differ between groups (eFigure 2 and 3).
Table 3.
Random-effects meta-analysis of secondary outcomes.
| No. of studies | No. of patients (intervention/control group) | Odds ratio (95% CI) | |
|---|---|---|---|
| Good functional outcome (mRS ⩽ 2 at 90 days | 8 | 1938 (704 / 1234) | 1.38 (0.97–1.95) |
| Symptomatic intracranial hemorrhage | 8 | 1938 (704 / 1234) | 0.84 (0.58–1.24) |
| Mortality at 90 days | 8 | 1938 (704 / 1234) | 0.74 (0.48–1.15) |
CI, confidence interval.
Discussion
This systematic review and meta-analysis included eight studies with a total of 704 patients in the DTAS and 1234 patients in the control group. Our findings show that DTAS approaches for the triage of acute stroke patients with a suspected LVO lead to a significant reduction in both door-to-groin and door-to-reperfusion times [median reduction of 29.0 min (95% CI: 14.3–43.6) and 32.1 min (95% CI: 15.5–49.1)]. Although we are uncertain about the impact on functional outcome at 90 days, the pooled estimates favored the intervention. We did not find any difference in mortality within 90 days and the occurrence of sICH. These findings should be interpreted with caution due to the small number of studies and the different design approaches, which have resulted in substantial heterogeneity in both the intervention and control group.
All eight studies in this meta-analysis included transfer patients, in whom DTAS was associated with shorter door-to-groin and door-to-reperfusion times. A possible explanation might be that staff and other resources could be prepared prior to patient’s arrival. Furthermore, in these patients the LVO was confirmed at the referring hospital in most cases, leading to a clear indication for performing MT. These processes can be further optimized if the primary stroke centers and comprehensive stroke centers are integrated in a network and use tools such as teleconsulting and teleradiology. 28 Our results support a recent expert statement, recommending repeated imaging in transfer patients only in cases of clinical deterioration or improvement, 29 as this can significantly reduce door-to-treatment times in these patients.
In mothership patients (extracted from four studies), we observed similar effects. The lower certainty of the effect might be attributable to low statistical power due to a substantially lower number of patients (145 mothership versus 1753 transfer patients). Since there are often long distances between the emergency department, CT/MRI suite, and angiography suite, 30 one would expect greater time savings with a DTAS approach in mothership patients. However, there might be other structural factors limiting the effect of DTAS in mothership patients. Due to the absence of a reliable prehospital screening tool in stroke patients, the first focused neurological exam in mothership patients is often done in the emergency room, possibly leading to a later activation of the angiography team. This is especially problematic during off-hours when interventionalists are on call and have to reach the hospital from home. One possible approach to overcome this limitation is the utilization of prehospital scales for in the field detection of LVOs, such as the Rapid Arterial Occlusion Evaluation (RACE) scale, 31 the Los Angeles Motor Score (LAMS), 32 the Prehospital Acute Stroke Severity (PASS) scale, 33 and the Field Assessment Stroke Triage for Emergency Department (FAST-ED) scale.19,20,23 All these scales have limited accuracy for the identification of LVOs, with sensitivity ranging from 38% to 62% and specificity ranging from 80% to 93%.34,35 A further validation of the RACE scale with a threshold of ⩾5 in a sample of 1822 patients showed that 35% of the patients presented with an LVO and 20% were eligible for MT. 36 As recent literature suggests that an LVO can be detected with very high sensitivity with a flat detector CT (FDCT) angiography, the detection within the angiosuite should not pose a problem. 37 Furthermore, advances with FDCT perfusion enable the physician to also detect smaller, distal occlusions such as M2 or M3 occlusions. 38 However, even under optimal circumstances the workload of the angiography team will increase if DTAS is adopted in mothership patients. 29 Another strategy to prevent angiosuite overload might be to use a specialized vascular neurologist team on admission for the selection of patients with high probability of LVO. A recent study even found the largest effect of DTAS on functional outcome in patients presenting in the very early (1–3 h) time-window, 23 which might be explained by a faster stroke lesion growth in patients with hyperacute stroke. 39 As mothership patients routinely present earlier than transfer patients, future trials on DTAS in mothership patients should be a primary focus. This is underscored by a recent analysis showing that every 10 min of earlier treatment initiation in patients undergoing MT increases the net monetary benefit of the intervention by $10,915. 40
Our study could not show an effect on functional outcome (albeit a trend in favor of DTAS was apparent). Interestingly, the results of the two included randomized studies were conflicted with Pfaff et al. finding no effect on functional outcome, while Requena et al. found significantly better odds for functional independence in patients triaged with a DTAS approach.8,9 This might be attributable to specific factors associated with a certain center, for example, a specially trained ambulance services or stroke network and highlights the need for well-designed multicenter trials to examine the benefit of DTAS with more certainty. The fact that the rate of sICH was not higher in the DTAS arm is promising, since reliable hemorrhage exclusion on FDCT is still a topic of discussion. Our results are in line with a prior analysis indicating that hemorrhage detection can be done with adequate safety on FDCT. 41 As recent studies showed the value of bridging therapy in LVO patients, this should be further addressed in adequately powered studies.42,43
This study has several limitations. The number of studies is small, and we observed considerable heterogeneity of the study results. Heterogeneity could be attributed to the following reasons: (a) While in three studies8,25,26 noncontrast FDCT and FDCT angiography were performed prior to groin puncture, the remaining studies performed only minimal (noncontrast FDCT) or no imaging prior to groin puncture, (b) the distances between emergency room, MDCT suite and angiography suite varied in all studies, 30 (c) the usage of prehospital scales was heterogeneous among studies and might have influenced door-to-groin and door-to-reperfusion times as they allow to skip the emergency department completely, and (d) studies differed in designs. However, as these variations were consistent within the studies, we do not think they influenced the direction of the effect. In addition, most data were collected retrospectively in a single-center design without blinding of personnel and participants, possibly leading to performance bias. Furthermore, due to missing data we were not able to adjust our meta-analysis for parameters, which also influence clinical outcome such as metrics of salvageable tissue, intravenous thrombolysis rates, and the success of MT (e.g. final modified thrombolysis in cerebral infarction scores). As these parameters significantly influence clinical outcome, our inability to adjust for them might potentially account for the lack of statistical differences in the present meta-analysis. However, since all but one study 8 showed a similar effect direction, these results can provide valuable insight on the possible effects of DTAS approaches. Finally, given that the number of included studies was small, and it is a rapid evolving topic, regular updates are warranted.
Conclusion
Direct to angiography suite approaches for the triage of suspected LVO patients lead to a significant improvement of in-hospital workflow time metrics. However, in our meta-analysis, they did not translate into improved clinical outcomes. This highlights the need for well-designed randomized, multicenter trials to evaluate the effect of DTAS approaches in different hospital settings.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221078177 for Direct to angiography suite approaches for the triage of suspected acute stroke patients: a systematic review and meta-analysis by Alex Brehm, Ioannis Tsogkas, Johanna M. Ospel, Christian Appenzeller-Herzog, Junya Aoki, Kazumi Kimura, Johannes A.R. Pfaff, Markus A. Möhlenbruch, Manuel Requena, Marc J. Ribo, Amrou Sarraj, Alejandro M. Spiotta, Peter Sporns and Marios-Nikos Psychogios in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank Hannah Ewald, PhD (University Medical Library Basel), for her support in peer-reviewing the search strategy.
Footnotes
Author contributions: Alex Brehm: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing – original draft; Writing – review & editing.
Ioannis Tsogkas: Data curation; Investigation; Writing – review & editing.
Johanna M. Ospel: Data curation; Investigation; Writing – review & editing.
Christian Appenzeller-Herzog: Data curation; Investigation; Writing – review & editing.
Junya Aoki: Data curation; Investigation; Writing – review & editing.
Kazumi Kimura: Data curation; Investigation; Writing – review & editing.
Johannes A.R. Pfaff: Data curation; Investigation; Writing – review & editing.
Markus A. Möhlenbruch: Data curation; Investigation; Writing – review & editing.
Manuel Requena: Data curation; Investigation; Writing – review & editing.
Marc J. Ribo: Data curation; Investigation; Writing – review & editing.
Amrou Sarraj: Data curation; Investigation; Writing – review & editing.
Alejandro M. Spiotta: Formal analysis; Methodology; Writing – review & editing.
Peter Sporns: Data curation; Formal analysis; Investigation; Methodology; Writing – review & editing.
Marios-Nikos Psychogios: Conceptualization; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alex Brehm  https://orcid.org/0000-0002-1630-6210
https://orcid.org/0000-0002-1630-6210
Manuel Requena  https://orcid.org/0000-0002-5671-6484
https://orcid.org/0000-0002-5671-6484
Peter Sporns  https://orcid.org/0000-0002-3028-0539
https://orcid.org/0000-0002-3028-0539
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alex Brehm, Department of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, 4031 Basel, Switzerland.
Ioannis Tsogkas, Department of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, Basel, Switzerland.
Johanna M. Ospel, Department of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, Basel, Switzerland
Christian Appenzeller-Herzog, University Medical Library Basel, University Basel, Basel, Switzerland.
Junya Aoki, Department of Neurology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan.
Kazumi Kimura, Department of Neurology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan.
Johannes A.R. Pfaff, Department of Neuroradiology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria
Markus A. Möhlenbruch, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany
Manuel Requena, Department of Neurology, Hospital Universitari Vall d’Hebron, Barcelona, Spain.
Marc J. Ribo, Department of Neurology, Hospital Universitari Vall d’Hebron, Barcelona, Spain
Amrou Sarraj, Department of Neurology, University of Texas McGovern Medical School, Houston, TX, USA.
Alejandro M. Spiotta, Department of Neurosurgery, Medical University of South Carolina, Charleston, SC, USA
Peter Sporns, Department of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, Basel, SwitzerlandDepartment of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Marios-Nikos Psychogios, Department of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, Basel, Switzerland.
References
- 1. Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals From the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 4. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2019; 11: 535–538. [DOI] [PubMed] [Google Scholar]
- 5. Jovin TG, Albers GW, Liebeskind DS, et al. Stroke treatment academic industry roundtable: the next generation of endovascular trials. Stroke 2016; 47: 2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janssen PM, Venema E, Dippel DWJ. Effect of workflow improvements in endovascular stroke treatment: a systematic review and meta-analysis. Stroke 2019; 50: 665–674. [DOI] [PubMed] [Google Scholar]
- 7. Brehm A, Tsogkas I, Maier IL, et al. One-stop management with perfusion for transfer patients with stroke due to a large-vessel occlusion: feasibility and effects on in-hospital times. Am J Neuroradiol 2019; 40: 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfaff JAR, Schönenberger S, Herweh C, et al. Direct transfer to angio-suite versus computed tomography-transit in patients receiving mechanical thrombectomy: a randomized trial. Stroke 2020; 51: 2630–2638. [DOI] [PubMed] [Google Scholar]
- 9. Requena M, Olivé-Gadea M, Muchada M, et al. Direct to angiography suite without stopping for computed tomography imaging for patients with acute stroke: a randomized clinical trial. JAMA Neurol 2021; 78: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Psychogios MN, Bähr M, Liman J, et al. One stop management in acute stroke: first mothership patient transported directly to the angiography suite. Clin Neuroradiol 2017; 27: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104: 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao NM, Levine SR, Gornbein JA, et al. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke 2014; 45: 2728–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 16. McGrath S, Sohn H, Steele R, et al. Meta-analysis of the difference of medians. Biom J 2020; 62: 69–98. [DOI] [PubMed] [Google Scholar]
- 17. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 18. Psychogios MN, Behme D, Schregel K, et al. One-stop management of acute stroke patients minimizing door-to-reperfusion times. Stroke 2017; 48: 3152–3155. [DOI] [PubMed] [Google Scholar]
- 19. Ribo M, Boned S, Rubiera M, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J Neurointerv Surg 2018; 10: 221–224. [DOI] [PubMed] [Google Scholar]
- 20. Mendez B, Requena M, Aires A, et al. Direct transfer to angio-suite to reduce workflow times and increase favorable clinical outcome a case-control study. Stroke 2018; 49: 2723–2727. [DOI] [PubMed] [Google Scholar]
- 21. Sanjuan Menéndez E, Girón Espot P, Calleja Macho L, et al. Implementation of a protocol for direct stroke patient transfer and mobilization of a stroke team to reduce times to reperfusion. Emergencias 2019; 31: 385–390. [PubMed] [Google Scholar]
- 22. Sarraj A, Goyal N, Chen M, et al. Direct to angiography vs repeated imaging approaches in transferred patients undergoing endovascular thrombectomy. JAMA Neurol 2021; 78: 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Requena M, Olivé M, García-Tornel Á, et al. Time matters: adjusted analysis of the influence of direct transfer to angiography-suite protocol in functional outcome. Stroke 2020; 51: 1766–1771. [DOI] [PubMed] [Google Scholar]
- 24. Aoki J, Suzuki K, Kanamaru T, et al. Direct transfer to the angiography suite from outside hospitals to shorten the door to groin puncture time. Clin Neurol 2020; 60: 289–292. [DOI] [PubMed] [Google Scholar]
- 25. Psychogios MN, Maier IL, Tsogkas I, et al. One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med 2019; 8: 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouslama M, Haussen DC, Grossberg JA, et al. Flat-panel detector CT assessment in stroke to reduce times to intra-arterial treatment: a study of multiphase computed tomography angiography in the angiography suite to bypass conventional imaging. Int J Stroke 2021; 16: 63–72. [DOI] [PubMed] [Google Scholar]
- 27. Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke 2017; 48: 1884–1889. [DOI] [PubMed] [Google Scholar]
- 28. Stefanou MI, Stadler V, Baku D, et al. Optimizing patient selection for interhospital transfer and endovascular therapy in acute ischemic stroke: real-world data from a supraregional, hub-and-spoke neurovascular network in Germany. Front Neurol 2020; 11: 600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McTaggart RA, Holodinsky JK, Ospel JM, et al. Leaving no large vessel occlusion stroke behind: reorganizing stroke systems of care to improve timely access to endovascular therapy. Stroke 2020; 51: 1951–1960. [DOI] [PubMed] [Google Scholar]
- 30. Ospel JM, Almekhlafi MA, Menon BK, et al. Workflow patterns and potential for optimization in endovascular stroke treatment across the world: results from a multinational survey. J NeuroInterv Surg 2020; 12: 1194–1198. [DOI] [PubMed] [Google Scholar]
- 31. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion. Stroke 2014; 45: 87–91. [DOI] [PubMed] [Google Scholar]
- 32. Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke 2008; 39: 2264–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hastrup S, Damgaard D, Johnsen Søren P, et al. Prehospital acute stroke severity scale to predict large artery occlusion. Stroke 2016; 47: 1772–1776. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen TTM, van den Wijngaard IR, Bosch J, et al. Comparison of prehospital scales for predicting large anterior vessel occlusion in the ambulance setting. JAMA Neurol 2021; 78: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith EE, Kent DM, Bulsara KR, et al. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018; 49: e111–e122. [DOI] [PubMed] [Google Scholar]
- 36. Carrera D, Gorchs M, Querol M, et al. Revalidation of the RACE scale after its regional implementation in Catalonia: a triage tool for large vessel occlusion. J Neurointerv Surg 2019; 11: 751–756. [DOI] [PubMed] [Google Scholar]
- 37. Hoelter P, Goelitz P, Lang S, et al. Visualization of large vessel occlusion, clot extent, and collateral supply using volume perfusion flat detector computed tomography in acute stroke patients. Acta Radiol 2019; 60: 1504–1511. [DOI] [PubMed] [Google Scholar]
- 38. Mueller A, Wagner M, Hattingen E, et al. Flat panel computed tomography pooled blood volume and infarct prediction in endovascular stroke treatment. Stroke 2019; 50: 3274–3276. [DOI] [PubMed] [Google Scholar]
- 39. Broocks G, Rajput F, Hanning U, et al. Highest lesion growth rates in patients with hyperacute stroke: when time is brain particularly matters. Stroke 2019; 50: 189–192. [DOI] [PubMed] [Google Scholar]
- 40. Kunz WG, Hunink MG, Almekhlafi MA, et al. Public health and cost consequences of time delays to thrombectomy for acute ischemic stroke. Neurology 2020; 95: e2465. [DOI] [PubMed] [Google Scholar]
- 41. Leyhe JR, Tsogkas I, Hesse AC, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. J Neurointerv Surg 2017; 9: 1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katsanos AH, Turc G, Psychogios M, et al. Utility of intravenous alteplase prior to endovascular stroke treatment: a systematic review and meta-analysis of RCTs. Neurology 2021; 97: e777–e784. [DOI] [PubMed] [Google Scholar]
- 43. Rossi R, Fitzgerald S, Molina S, et al. The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. J Thromb Thrombolysis 2021; 51: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221078177 for Direct to angiography suite approaches for the triage of suspected acute stroke patients: a systematic review and meta-analysis by Alex Brehm, Ioannis Tsogkas, Johanna M. Ospel, Christian Appenzeller-Herzog, Junya Aoki, Kazumi Kimura, Johannes A.R. Pfaff, Markus A. Möhlenbruch, Manuel Requena, Marc J. Ribo, Amrou Sarraj, Alejandro M. Spiotta, Peter Sporns and Marios-Nikos Psychogios in Therapeutic Advances in Neurological Disorders