Abstract
Background:
Estimates of detectable antinuclear antibodies (ANA) prevalence vary widely, from 6% in healthy populations to 50–80% in patients with autoimmune disease. However, there is a lack of evidence about the overall prevalence in inflammatory bowel disease (IBD) and ANA seroconversion after the beginning of biological therapy.
Objectives:
The aim of the study was to investigate the overall prevalence of ANA in IBD patients, their relationship with different treatments, clinical outcomes and the seroconversion rate of ANA in patients treated with biological therapy.
Methods:
Ambispective observational study including all consecutive IBD patients was carried out. Information about the presence of ANA, disease phenotype, duration, activity, complications, and past and current treatments were transversally collected. Retrospectively, in patients with detectable ANA, data regarding previous ANA detection and the diagnosis of lupus-like syndrome (LLS) was gathered.
Results:
A total of 879 IBD patients were included. We observed a detectable ANA prevalence of 13.6%. The presence of ANA was frequently associated with biological therapy (36/118) and decreased when immunomodulators were combined to this therapy (7/32). Of 78 patients with ANA prior to the beginning of biological therapy, a seroconversion rate of 28.8% was observed after a mean of 3.14 years. Only 1 patient suffered LLS.
Conclusion:
Our study showed a prevalence of detectable ANA higher than the expected in healthy population. The presence of ANA was lower when immunomodulator therapy is associated. The ANA seroconversion rate is relevant after the initiation of biological treatment nevertheless, the risk of LLS appeared to be marginal.
Keywords: antinuclear antibodies, anti-TNF, ustekinumab, Crohn’s disease, inflammatory bowel disease, lupus-like syndrome, seroconversion, ulcerative colitis, vedolizumab
Introduction
Antinuclear antibodies (ANA) are autoantibodies able to recognize self-proteins within cell nucleus structures. 1 These antibodies are classified according to the structure recognized against DNA, histones or against extractable nuclear antigens. 2 Recently, the International Consensus on ANA Patterns (ICAP) has recognized that the worldwide-accepted indirect immunofluorescence test for ANA study recognized other structures besides nucleus. 3 Low titers of ANA were found physiologically in healthy population with no relevance for the future appearance of any autoimmune disease. 4 However, the presence of high titers of ANA could indicate the existence of autoimmune diseases such as systemic lupus erythematosus (SLE) or autoimmune hepatitis (AIH).5,6 Thus, reported prevalence of ANA in autoimmune diseases range from 50% to 80%. 7
Inflammatory bowel disease (IBD) is an immunomediated disease that associate gastrointestinal inflammation and extraintestinal manifestations in 10–40% of the patients.8–10 To reduce the inflammatory burden, mesalamine, immunomodulators and biological treatments are frequently used.11,12 Anti-tumour necrosis factor (anti-TNF) are known to be associated to positive ANA in a percentage of 20–45%. 13 Moreover, an incidence of 5% of induced lupus-like syndrome (LLS) has been reported in those patients. 14 However, there is less data available concerning the global prevalence of ANA in IBD and their relationship with clinical characteristics of the disease. Only a few articles including small sample size have studied the modification of ANA after the prescription of biological therapy. For this reason, we designed a study with two aims. First, we investigated the overall prevalence of ANA in IBD patients, their relationship with the treatments and the incidence of LLS in our cohort of patients. Second, the seroconversion rate of ANA in those patients with positive ANA has been analysed regarding the different type of biological therapy.
Methods
Study design
We performed an ambispective study according to the STROBE statement (Supplementary Material). 15 Our study included a cohort of consecutive IBD patients above 18 years old who attended the IBD outpatient clinic of the University Hospital Marques de Valdecilla in Santander between December 2018 and December 2019. A local database for IBD patients treated in our tertiary referral hospital was used to identify the cohort. Patients were classified in two groups: IBD patients with positive ANA test and IBD patients with negative ANA test. ANA titers above 1:160 dilution were considered positive meanwhile ANA dilution above 1:320 was deemed high titers. Patients who suffered any autoimmune disorder, such as Sjögren syndrome, AIH, scleroderma or SLE, or cancer related to positive ANA, were excluded from the study. Clinical characteristics, demographic records and disease evolution of both categories were compared. The patients were grouped in immunomodulator therapy when azathioprine, methotrexate or 6-mercaptopurine were administered; biological therapy when infliximab, golimumab, adalimumab, ustekinumab, vedolizumab were prescribed or combination therapy when one therapy of each group was taken at the time of the interview. Afterwards, a retrospective study was conducted to record ANA seroconversion in patients whose ANA had been previously analysed. LLS was diagnosed based on clinical criteria established by European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR). 16
Ethical considerations
Study participants stem from the INSTInCT study, a prospective cohort of immunomediated diseases (Immunomediated Non-alcoholic SteaTohepatitis; Prevalence and Characterization; ClinicalTrials.gov Identifier: NCT03760172). This study was conducted in accordance with the Declaration of Helsinki principles, the European General Data Protection Regulation (GDPR) 2016/679 and the Spanish Data Protection Organic Law 3/2018. The protocol was approved on 21 September 2018 by the Ethical Committee of Cantabria (code 2018.139) and the informed consent was signed for every patient before the inclusion in INSTInCT study.
Data collection
All patients were diagnosed of IBD and categorized in Crohn’s disease (CD), ulcerative colitis (UC) or IBD-unclassified (IBD-U) according to the recommendation set by the European Crohn and Colitis Organization (ECCO).17,18 The location and behaviour of IBD were classified according to the Montreal Classification. 19 Demographic characteristics, clinical characteristics, treatments and surgeries and autoimmune disorders were recorded for the transversal study. Current and past IBD therapies as well as IBD activity indexes (Mayo Partial Score for UC and Harvey-Bradshaw index for CD) were collected by clinical interview. Bleeding was defined when patient required blood transfusion. Biochemical parameters including kidney function, liver test, reactive C protein (RCP) and hemogram were analysed at the same time than the interview. Total immunoglobulin levels, ANA, anti-mitochondrial (AMAs) and anti-smooth muscle antibodies (SMA) were also evaluated to exclude AIH as a cause of positive ANA.
Results of ANA in medical history were researched in a retrospective way in those patients with valid ANA results in the transversal cohort after the beginning of biological therapy. We also assessed the side effects of all biological therapies prescribed to our patients. ANA were analysed by indirect immunofluorescence on HEp-2 cells (Biosystems, Barcelona, Spain).
Statistical analysis
A statistical analysis was performed with median and standard deviation for continuous variables and percentages for qualitative variables. Chi-square test was used for qualitative variables while quantitative variables between two groups were analysed by Student t test. A multivariate analysis through logistic regression was used to calculate odds ratio (OR) in order to compare the risk of every variable with respect to the reference group choosing a confidence interval (CI) of 95% and an alpha error of 5%. Analysis of variance test was selected by multivariate variables. The analysis was performed separately for each variable and afterwards, a multivariate analysis was done to evaluate confounder factors for those variables which were clinically or statistically significant in univariate analysis. A significant result was considered when the p value was <0.05. Seroconversion rate was calculated by the proportion of patients during the study who developed detectable levels in blood after the beginning of biological therapy. All statistical analyses were performed with STATA Statistical Software: Release 14 (StataCorp LP, College Station, Texas, USA).
Results
Eight hundred seventy-nine IBD patients were initially evaluated. After exclusion criteria, 852 patients were included for final analysis. The study scheme is presented in Figure 1. The average age of patients was 51.09 years [standard deviation (SD) = 12.54]. The percentage of women was slightly higher (51.3%, n = 437). Mean age at IBD diagnosis was 38.37 years (SD = 13.4) while the mean duration of IBD was 13.22 years (SD = 9.55). Regarding the type of IBD, UC was diagnosed in 50.7% (n = 432) of patients, CD in 46.5% (n = 396) and 2.8% (n = 24) of IBD-U. Clinical and demographic characteristics of our cohort are described in Table 1.
Figure 1.
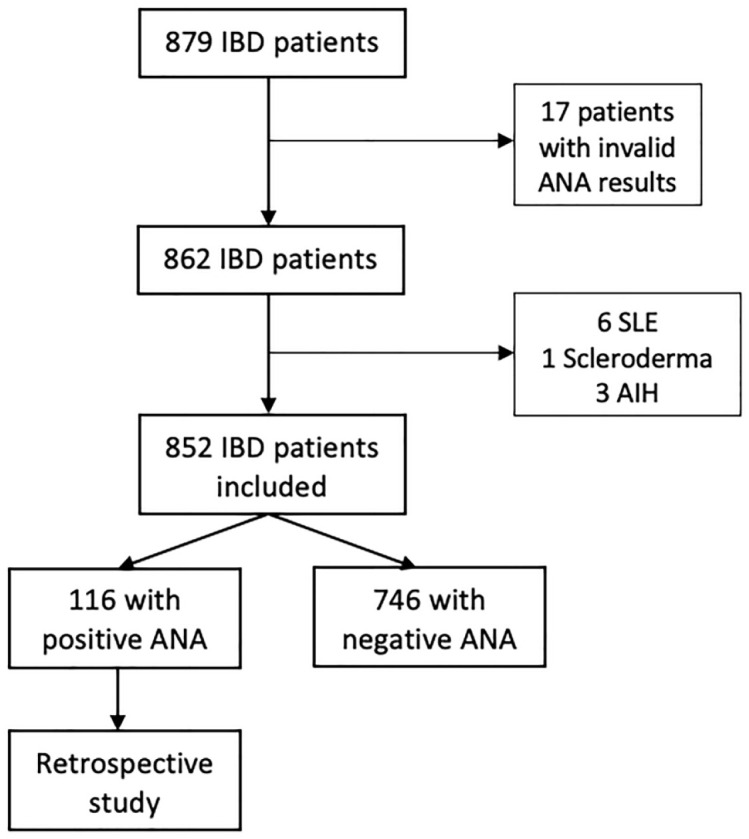
Study scheme shows the selection of the patients included in the study.
AIH, autoimmune hepatitis; ANA, antinuclear antibodies; IBD, inflammatory bowel disease; SLE, systemic lupus erythematosus.
Table 1.
Baseline characteristics of the patients.
| Total (n = 852) | Ulcerative colitis (n = 432) | Crohn`s disease (n = 396) | |
|---|---|---|---|
| Gender: men, n (%) | 415 (48.7) | 202 (46.8) | 199 (50.2) |
| Age (years), mean (SD) | 51.5 (12.54) | 51.83 (11.93) | 50.23 (13.12) |
| Onset age (years), mean (SD) | 38.37 (13.40) | 40.22 (12.05) | 35.89 (14.25) |
| Duration of IBD (years), mean (SD) | 13.22 (9.55) | 12.10 (9.46) | 14.85 (10.45) |
| Tobacco, n (%) | |||
| • Current | 196 (23) | 74 (17.1) | 114 (28.8) |
| • Non-smoker | 258 (30.3) | 143 (33.1) | 108 (27.3) |
| • Former | 398 (47.7) | 215 (49.8) | 174 (44.9) |
| Familiar history of IBD (yes), n (%) | 162 (19.0) | 68 (15.74) | 90 (22.7) |
| Complications (intraabdominal abscess, megacolon, bleeding), n (%) |
138 (16.2) | 33 (7.7) | 104 (26.3) |
| Location, n (%): | |||
| • Proctitis | 166 (38.6) | ||
| • Left-side colitis | 140 (32.6) | ||
| • Extensive colitis | 124 (28.8) | ||
| • Ileal | 246 (62.1) | ||
| • Colonic | 46 (11.6) | ||
| • Ileocolonic | 103 (26.0) | ||
| Behaviour, n (%): | |||
| • Inflammatory | 212 (53.5) | ||
| • Stricturing | 145 (36.6) | ||
| • Penetrating | 39 (9.9) | ||
| Perianal disease, n (%) | 54 (6.3) | 5 (1.2) | 49 (12.4) |
| Extraintestinal | 152 (17.8) | 63 (14.6) | 87 (22.0) |
| Manifestations, n (%) | |||
| • Axial arthritis | 46 (5.4) | 22 (5.1) | 23 (5.8) |
| • Peripheral arthritis | 51 (6.0) | 20 (4.6) | 31 (7.8) |
| • Skin manifestations | 34 (4.0) | 13 (3.0) | 20 (5.1) |
| • Ocular manifestations | 14 (1.6) | 4 (0.9) | 10 (2.5) |
| • Liver manifestations | 7 (0.8) | 4 (0.9) | 3 (0.8) |
| Treatment, n (%) | |||
| • Mesalamine | 588 (69.0) | 348 (80.6) | 222 (56.1) |
| • Corticosteroids | 25 (2.9) | 10 (2.3) | 14 (3.5) |
| • Thiopurines | 170 (20.0) | 53 (12.3) | 116 (29.9) |
| • Anti-TNF | 126 (14.8) | 42 (9.7) | 83 (21.0) |
| • Vedolizumab | 6 (0.7) | 4 (0.9) | 1 (0.3) |
| • Ustekinumab | 18 (2.1) | 1 (0.2) | 17 (4.3) |
| Surgery, n (%) | 179 (1.9) | 21 (4.9) | 157 (39.7) |
| Haemoglobin (g/dl), mean (SD) | 13.9 (1.4) | 14.1 (1.3) | 13.8 (1.5) |
| Leukocytes (×109/l), mean (SD) | 7.2 (2.1) | 7.0 (1.9) | 7.4 (2.3) |
| Platelets (×109/l), mean (SD) | 242.1 (66.5) | 239.0 (65.4) | 247.0 (66.9) |
| Albumin (g/dl), mean (SD) | 4.5 (0.3) | 4.5 (0.2) | 4.4 (0.3) |
| C reactive protein (g/dl), mean (SD) | 0.5 (0.8) | 0.5 (0.5) | 0.6 (1.0) |
| Immunoglobulin G (mg/dl), mean (SD) | 1165.9 (290.3) | 1190.0 (261.0) | 1139.1 (317.9) |
| Immunoglobulin A (mg/dl), mean (SD) | 252.4 (115.4) | 242.8 (98.4) | 263.7 (131.8) |
| Immunoglobulin M (mg/dl), mean (SD) | 119.2 (75.7) | 116.1 (69.5) | 122.3 (80.3) |
IBD, inflammatory bowel disease; SD, standard deviation.
Prevalence of ANA in IBD patients
A prevalence of positive ANA was found in 116 (13.6%) IBD patients. Women had a higher frequency of ANA compared with men (64.7%, n = 75 versus 35.3%, n = 41) (p = 0.02). A significant statistical association was observed between positive ANA and age. The mean age of patients was older for positive ANA than negative ANA (53.86 years, SD = 12.05 versus 50.7 years, SD = 12.6) (p = 0.01) although no association with the evolution time of IBD was described (p = 0.6). Higher onset age of IBD was observed in positive ANA (41.55 years, SD = 1.32) than negative ANA (37.87 years, SD = 13.2) (p = 0.005). The results according to age showed a percentage of 2.5% for those below 29 years, 32.8% between 30 and 49 years, 54.3% between 50 and 69 years and 9.5% in those patients above 70 years. Although no statistical significance was obtained in our cohort, our data showed a tendency to more positive ANA in those former smokers (56.9%) (p = 0.06). No more positive ANA in patients with extraintestinal manifestations or autoimmune diseases were found.
Factors associated with positive ANA
In the univariate analysis, being female, higher average age and onset age of IBD, lower haemoglobin and albumin levels and higher immunoglobulin G (IgG) and immunoglobulin M (IgM) levels were observed for patients with positive ANA (p < 0.05). No association was observed for inflammatory parameters such as Harvey-Bradshaw index, Mayo partial index, C reactive protein or extraintestinal manifestations. Clinical characteristics and biochemical parameters analysed in univariate analysis are detailed in Table 2.
Table 2.
Clinical and therapeutic factors associated with positive ANA in univariate analysis.
| Positive ANA (n = 116). |
Negative ANA (n = 736) |
OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Gender, n (%) | Men | 41 (35.3) | 374 (50.8) | Ref. | 0.001* |
| Women | 75 (64.7) | 362 (49.2) | 1.89 (1.26 – 2.83) | ||
| Age (years), mean (SD) | 53.9 (12.0) | 50.7 (12.6) | 1.02 (1.00–1.04) | < 0.05* | |
| IBD onset age (years), mean (SD) | 41.6 (14.3) | 37.9 (13.2) | 1.02 (1.01–1.04) | < 0.001* | |
| Duration of IBD (years), mean (SD) | 12.8 (9.6) | 13.3 (9.5) | 0.99 (0.97–1.02) | 0.6 | |
| Familiar history of IBD, n (%) | Yes | 28 (24.1) | 134 (18.2) | 1.43 (0.9–2.27) | 0.1 |
| No | 88 (75.9) | 602 (81.8) | Ref. | ||
| Tobacco, n (%) | Current | 21 (18.1) | 175 (23.8) | Ref. | 0.06 |
| Non-smoker | 29 (25.0) | 229 (31.1) | 1.06 (0.59–1.9) | ||
| Former | 66 (56.9) | 332 (45.1) | 1. 66 (0.98–2.78) | ||
| Type of disease, n (%) | Crohn’s disease | 59 (51.8) | 337 (47.2) | 1.2 (0.81–1.78) | 0.4 |
| Ulcerative colitis | 55 (48.3) | 377 (52.1) | Ref. | ||
| Complications, n (%) | Yes | 22 (20.0) | 116 (15.8) | 1.25 (0.76–2.06) | 0.4 |
| No | 94 (81.0) | 619 (84.2) | Ref. | ||
| Extraintestinal manifestations, n (%) | Yes | 19 (16.4) | 133 (18.0) | 0.89 (0.53–1.5) | 0.7 |
| No | 97 (83.6) | 609 (81.9) | Ref. | ||
| Treatment, n (%) | Mesalamine | 65 (56.0) | 498 (67.7) | Ref. | < 0.001 |
| Immunomodulator | 8 (6.9) | 131 (17.8) | 0.47 (0.19–1.01) | ||
| Biological therapy | 36 (31.0) | 82 (11.1) | 3.36 (2.03–5.50) | ||
| Combo therapy | 7 (6.0) | 25 (3.4) | 2.14 (0.75–5.36) | ||
| Surgery, n (%) | Yes | 32 (27.6) | 147 (20.0) | 1.53 (0.98–2.38) | 0.06 |
| No | 84 (72.4) | 589 (80.0) | Ref. | ||
| Haemoglobin (g/dl), mean (SD) | 13.7 (1.2) | 14.0 (1.4) | 0.88 (0.77–1.01) | 0.07 | |
| Leukocytes (109/l), mean (SD) | 7.3 (2.2) | 7.2 (2.1) | 1.01 (0.92–1.11) | 0.7 | |
| Platelets (109/l), mean (SD) | 242.1 (59.5) | 242.1 (67.6) | 1.00 (0.99–1.00) | 0.9 | |
| Albumin (g/dl), mean (SD) | 4.41 (0.3) | 4.47 (0.3) | 0.44 (0.22–0.88) | < 0.05* | |
| C reactive protein (g/dl), mean (SD) | 0.55 (0.9) | 0.54 (0.7) | 1.02 (0.79–1.31) | 0.8 | |
| Immunoglobulin G (mg/dl), mean (SD) | 1243.2 (321.0) | 1153.8 (283.6) | 1.00 (1.00–1.00) | < 0.05* | |
| Immunoglobulin A (mg/dl), mean (SD) | 264.03 (117.4) | 250.6 (115.1) | 1.00 (0.99–1.00) | 0.2 | |
| Immunoglobulin M (mg/dl), mean (SD) | 146.4 (98.5) | 115.0 (70.7) | 1.00 (1.00–1.01) | < 0.001* | |
| Harvey-Bradshaw Index, mean (SD) | 1.05 (1.9) | 1.10 (2.1) | 0.99 (0.86–1.13) | 0.8 | |
| Mayo score, mean (SD) | 0.46 (1.0) | 0.46 (1.1) | 1.00 (0.77–1.29) | 0.9 |
ANA, antinuclear antibodies; CI, confidence interval; IBD, inflammatory bowel disease; OR, odds ratio; SD, standard deviation; * p-value ⩽ 0.05
The gender, IBD onset age, mesalamine, immunomodulator therapy, IgG and IgM levels remained significant in the multivariate analysis (Table 3). As a result, being woman was a risk factor for positive ANA as well its presence was related to elderly patients. Immunomodulator therapy was a factor that reduces ANA presence.
Table 3.
Univariate and multivariate analyses show the associated factors with the presence of ANA.
| Unadjusted odds ratio | Confidence interval | Adjusted odds ratio | Confidence interval | |
|---|---|---|---|---|
| Gender: women | 1.89 | 1.26–2.84 | 2.20 | 1.42–3.42 |
| IBD onset age | 1.02 | 1.01–1.04 | 1.032 | 1.02–1.05 |
| Average age | 0.2 | 0.00–0.04 | ||
| Tobacco: smoker | 1.28 | 1.01–1.63 | ||
| Mesalamine | 0.61 | 0.41–0.91 | 0.27 | 0.17–0.44 |
| Immunomodulator | 0.34 | 0.16–0.72 | 0.17 | 0.07–0.38 |
| Biological therapy | 3.59 | 2.28–5.66 | ||
| Combo therapy | 1.83 | 0.77–4.32 | ||
| Surgery | 1.53 | 0.98–2.38 | ||
| Haemoglobin | 0.88 | 0.77–1.01 | ||
| Albumin | 0.44 | 0.22–0.88 | ||
| Immunoglobulin G | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 |
| Immunoglobulin M | 1.00 | 1.00–1.01 | 1.00 | 1.00–1.01 |
IBD, inflammatory bowel disease.
ANA presence according to the type of therapy
At blood sampling, lower rates of positive ANA were found in patients under immunomodulator with or without biological therapy compared with patients on biological treatment (p = 0.001) (Figure 2). For those treated with biological therapy, anti-TNF was administered in 84% (n = 126/150), vedolizumab in 4% (n = 6/150) and ustekinumab in 12% (n = 18/150). Positive ANA were observed in 28.6% (n = 36) patients with anti-TNF therapy, 33.3% (n = 2) with vedolizumab and 27.8% (n = 5) with ustekinumab (p = 0.96).
Figure 2.
Prevalence of ANA according to therapeutic approach. Combination therapy was defined as the simultaneous administration of an immunomodulator and a biological therapy.
Titers of ANA and their relationship with the treatment
All the patients with positive ANA had titers above 1:160 dilution. The titers of positive ANA in the cohort were 28.5% for 1:160 dilution and 23.3% for 1:320 dilution. It is important to consider that a percentage of 48.3% of the patients presented titers above 1:640. ANA titers according to the type of treatment and dilution are exposed in Figure 3.
Figure 3.
Titers of ANA concerning the treatment. Combination therapy was defined as the simultaneous administration of an immunomodulator and a biological therapy.
ANA, antinuclear antibodies.
ANA seroconversion and LLS after the beginning of biological therapy
An ANA result was collected in 78 of 150 patients treated with biological therapy with a mean of 3.14 years (SD = 5.58) before the beginning of biological treatment. Mean age of this group was 48.33 years (SD = 13.91) while the evolution time of the disease was 13.94 years (SD = 8.41). The overall seroconversion rate of 25.4% (16/63) was observed for patients with negative ANA prior to the beginning of biological therapy. A percentage of 28% (14/59) had a seroconversion with anti-TNF while 18.2% (2/11) were observed with ustekinumab (p = 0.5). Any patient on vedolizumab treatment experienced seroconversion. In our cohort, 15 patients had positive ANA previous to the biological therapy and positive ANA were maintained in 33.3% (n = 5) after the treatment. Of 298 biological treatments prescribed to our cohort, only an LLS was diagnosed due to the diagnosis of nephropathy with proteinuria, arthritis and positive ANA after the commencement of infliximab. Renal biopsy showed mesangial expansion of the kidney glomerulus.
Discussion
Our study shows a prevalence of positive ANA of 13% in patients with IBD, higher than the percentage described in healthy population and represents the largest cohort of patients published to date. In healthy population, 2–8% of positive ANA were described considering 1:100 dilution titers.20,21 This prevalence depends on the gender, the age or the ethnicity. A ratio 2:1 between women and men was observed in our study in agreement with other cohorts. 22 Moreover, positive ANA were increased in consonance with age, 23 finding a percentage of 54.3% of positive ANA in patients whose age was between 50 and 69. However, most of the studies described the presence of these antibodies associated with rheumatological diseases, cancer or infections belonging to the diagnostic criteria in SLE or AIH.24–26 Regarding IBD, a prevalence of 18–32% of ANA was recorded at 1:40 dilutions albeit no recent studies have measured their frequency at significant titers. 27 Our prevalence remains higher compared with healthy population after considering all the previous factors. A great variability in the prevalence of ANA has been observed in the three previously published articles; therefore, the frequency of positive ANA in IBD depends on the selected titre.28,29
Nowadays, no study has investigated the link between the presence of ANA and the type of treatment in such a large cohort. In our cohort, a 66.8% of patients treated with mesalamine showed positive ANA without any rheumatoid or other associated disease that justified it. In contrast to that, immunomodulator therapy reduced the formation of antibodies by themselves and in combination with biological therapy in the 170 patients of our study (OR = 0.5; 95% CI: 0.31–0.97), similarly to Beigel et al. 30 that described a reduction in the presence of ANA when the immunomodulator was combined with anti-TNF. This decrement was also observed in the production of antibodies against anti-TNF. 31 The mechanism of action of immunomodulators in ANA formation is unknown albeit similar effect was detected with other drugs that inhibit DNA synthesis like hydroxychloroquine.32,33 A possible explanation would be a reduction in memory T cells through Rac-1. 34 This suggests that immunomodulators could be a treatment for induced LLS.
Anti-TNF treatment has revolutionized the therapy in IBD. 35 Various side effects of these treatments have been described since their approval in 1999 like induced LLS. 36 A prevalence of 20–45% of ANA formation after the beginning of anti-TNF has been proven.36,37 A possible mechanisms behind this production might have to be the induction of T-cell apoptosis by anti-TNF therapy through mTNF/TNFR2 pathway. 38 However, LLS only developed in 1–5% of the patients.14,39 In our cohort, the incidence of induced LLS was less than 1%. After the revision of published literature, the frequency of LLS is highly variable because there is not a consensus in the definition of this syndrome. Yanai et al. considered LLS when a patient had a compatible serology and at least one clinical manifestation meanwhile other studies contemplated the time-effect and the combination of clinical manifestation exclusively.40,41 Paradoxical manifestations of anti-TNF therapy trigger dermatological and joint symptoms similar to lupus so both syndromes could be difficult to differentiate. 42 We have considered serological criteria and ⩾10 points in clinical criteria according to the classification that was established in the 2019 consensus. 16 LLS diagnosis was more constrained in our study than in the previous definitions and enabling us to differentiate between this syndrome and paradoxical reactions of anti-TNF.
Concerning recently approved biologicals, our study is the first one that reports the prevalence of ANA in patients treated with vedolizumab. Only an article by Rodríguez-Jiménez et al. 43 described the absence of association between ustekinumab and ANA in 76 patients with skin psoriasis. On our study, 24 patients were treated with vedolizumab or ustekinumab reaching an ANA prevalence of 27.8% for the ustekinumab group and 33.3% for the vedolizumab group. However, no reliable conclusions could be ascertained in both biologics due to previous anti-TNF treatment in those patients.
A seroconversion rate of 25.4% in our cohort was comparable to the seroconversion rate of 25% described in a prospective study by Santos-Antunes et al. 37 of a group of 68 patients during an average time of 4 years. A previous report about suppurative hidradenitis indicated that only 1 in 31 anti-TNF treated patients had a seroconversion after 0.9 years of treatment. 44 A proportional relationship between the time of the treatment and the seroconversion rate could be explained by the differences in seroconversion rates. 45 Only 3 out of 11 patients with previous negative ANA suffered a seroconversion in the ustekinumab group and no patient had a seroconversion in the vedolizumab group. No conclusion can be drawn as both groups of patients had been treated with anti-TNF previously. Despite the fact that no LLS reports were described with anti-interleukin (IL)-12 or IL-23, paradoxical reactions have been published with this treatment so the future presentation of LLS could not be discarded.46,47
An interesting fact in our study was the statistical association between ANA and the levels of albumin, IgG and IgM. Those antibodies have been associated with inflammatory activity in autoimmune diseases.48,49 Hence, ANA formation could trigger an inflammatory response through activation of T helper lymphocytes type 17 (Th17), IL-12 and the reduction of IL-10, all of them involved in the physiopathology of IBD. 50 Moreover, bowel inflammation is known to increase IgG levels and reduce serum albumin levels.51,52 An immune activation to recruit innate cells during autoimmune diseases release the production of IgG. 53 Elevated levels of IgG, a reduction in serum albumin and positive ANA could operate as reactants of chronic inflammation in patients who have required more biological therapy and surgeries. However, no correlation has been observed between the presence of ANA and acute inflammatory parameters such as RCP, erythrocyte sedimental rate, haemoglobin, Harvey-Bradshaw index or Mayo Partial Score.
The limitations of our study include the retrospective data collection regarding the detection of ANA before the anti-TNF treatment. Prospective studies with serial measurements at set intervals are necessary to calculate the real conversion rate of ANA after the beginning of different type of treatments. Furthermore, the type of ANA was not available in our cohort. Another limitation is the influence in the prevalence and seroconversion rate in those patients treated with ustekinumab or vedolizumab that has been previously treated by anti-TNF therapy. In addition, other diseases that have been associated with positive ANA had not been excluded such as suppurative hidradenitis or Helicobacter pylori infections. 52
In conclusion, our study shows a higher prevalence of positive ANA than the one found in healthy populations. The presence of ANA is high in IBD patients and decreases with immunomodulator therapy associated to biological therapy. A high proportion of patients experience seroconversion after the beginning of biological with a slight risk of suffering LLS.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221077837 for Prevalence of antinuclear antibodies in inflammatory bowel disease and seroconversion after biological therapy by María José García, Juan Carlos Rodríguez-Duque, Marta Pascual, Coral Rivas, Beatriz Castro, Sandra Raso, Marcos López-Hoyos, María Teresa Arias-Loste and Montserrat Rivero in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: María José García: Formal analysis; Methodology; Writing – original draft.
Juan Carlos Rodríguez-Duque: Data curation; Investigation; Writing – review & editing.
Marta Pascual: Data curation; Writing – review & editing.
Coral Rivas: Data curation; Writing – review & editing.
Beatriz Castro: Investigation; Writing – review & editing.
Sandra Raso: Investigation; Writing – review & editing.
Marcos López-Hoyos: Investigation; Resources; Writing – review & editing.
María Teresa Arias-Loste: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Montserrat Rivero: Conceptualization; Supervision; Validation; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCRD, MTAL, MP, CR, BC, SR and MLP have no conflict of interest to declare. MJG has received financial support for travelling and educational activities from MSD, Janssen, Abbvie, Takeda and Ferring. MR has served as a speaker and advisory member for Abbvie, MSD and Janssen.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors report funding support from the Spanish Instituto de Salud Carlos III Grant (FIS–PI18/01304) related to this article.
ORCID iD: María José García  https://orcid.org/0000-0002-6517-7005
https://orcid.org/0000-0002-6517-7005
Availability of data and materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
María José García, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Avenida Valdecilla s/n., CP 39008 Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain; University of Cantabria, Santander, Spain.
Juan Carlos Rodríguez-Duque, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain; University of Cantabria, Santander, Spain.
Marta Pascual, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
Coral Rivas, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
Beatriz Castro, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
Sandra Raso, Immunology Department, Hospital Universitario Marqués de Valdecilla, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
Marcos López-Hoyos, Immunology Department, Hospital Universitario Marqués de Valdecilla, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
María Teresa Arias-Loste, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain; University of Cantabria, Santander, Spain.
Montserrat Rivero, Gastroenterology and Hepatology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Group of Clinical and Translational Research in Digestive Diseases Infection, Immunity and Digestive Pathology Group, Instituto de Investigación Sanitaria de Valdecilla (IDIVAL), Santander, Spain.
References
- 1. Sur LM, Floca E, Sur DG, et al. Antinuclear antibodies: marker of diagnosis and evolution in autoimmune diseases. Lab Med 2018; 49: e62–e73. [DOI] [PubMed] [Google Scholar]
- 2. Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited. Diagn Pathol 2009; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan EKL, Damoiseaux J, Carballo OG, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns (ICAP) 2014-2015. Front Immunol 2015; 6: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Allaf AW, Ottewell L, Pullar T. The prevalence and significance of positive antinuclear antibodies in patients with fibromyalgia syndrome: 2-4 years’ follow-up. Clin Rheumatol 2002; 21: 472–477. [DOI] [PubMed] [Google Scholar]
- 5. Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity – does it no longer exist? QJM 2004; 97: 303–308. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015; 63: 971–1004. [DOI] [PubMed] [Google Scholar]
- 7. Didier K, Bolko L, Giusti D, et al. Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol 2018; 9: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 2001; 96: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 9. Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navrátil V, Cveková S, Slodicˇka P, et al. Extraintestinal complications of inflammatory bowel diseases. Vnitr Lek 2021; 67: 92–96. [PubMed] [Google Scholar]
- 11. Wang Y, Parker CE, Feagan BG, et al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016; 5: CD000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bots S, Gecse K, Barclay M, et al. Combination immunosuppression in IBD. Inflamm Bowel Dis 2018; 24: 539–545. [DOI] [PubMed] [Google Scholar]
- 13. Vaglio A, Grayson PC, Fenaroli P, et al. Drug-induced lupus: traditional and new concepts. Autoimmun Rev 2018; 17: 912–918. [DOI] [PubMed] [Google Scholar]
- 14. Aghdashi MA, Khadir M, Dinparasti-Saleh R. Relationship between antinuclear antibodies and lupus erythematous manifestations in rheumatoid arthritis and ankylosing spondylitis patients following treatment with infliximab and etanercept. Curr Rheumatol Rev 2019; 15: 61–66. [DOI] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 16. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019; 71: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 18. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 19. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Racoubian E, Zubaid RM, Shareef MA, et al. Prevalence of antinuclear antibodies in healthy Lebanese subjects, 2008–2015: a cross-sectional study involving 10,814 subjects. Rheumatol Int 2016; 36: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 21. Marin GG, Cardiel MH, Cornejo H, et al. Prevalence of antinuclear antibodies in 3 groups of healthy individuals: blood donors, hospital personnel, and relatives of patients with autoimmune diseases. J Clin Rheumatol 2009; 15: 325–329. [DOI] [PubMed] [Google Scholar]
- 22. Li X, Liu X, Cui J, et al. Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J Clin Lab Anal 2019; 33: e22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannouli E, Chatzidimitriou D, Gerou S, et al. Frequency and specificity of antibodies against nuclear and cytoplasmic antigens in healthy individuals by classic and new methods. Clin Rheumatol 2013; 32: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 24. Vlagea A, Falagan S, Gutiérrez-Gutiérrez G, et al. Antinuclear antibodies and cancer: a literature review. Crit Rev Oncol Hematol 2018; 127: 42–49. [DOI] [PubMed] [Google Scholar]
- 25. Leuchten N, Hoyer A, Brinks R, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res 2018; 70: 428–438. [DOI] [PubMed] [Google Scholar]
- 26. Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Serology in autoimmune hepatitis: a clinical-practice approach. Eur J Intern Med 2018; 48: 35–43. [DOI] [PubMed] [Google Scholar]
- 27. Folwaczny C, Noehl N, Endres SP, et al. Antinuclear autoantibodies in patients with inflammatory bowel disease. Dig Dis Sci 1997; 42: 1593–1597. [DOI] [PubMed] [Google Scholar]
- 28. Barahona-Garrido J, Camacho-Escobedo J, García-Martínez CI, et al. Antinuclear antibodies: a marker associated with steroid dependence in patients with ulcerative colitis. Inflamm Bowel Dis 2009; 15: 1039–1043. [DOI] [PubMed] [Google Scholar]
- 29. Zauli D, Crespi C, Dall’Amore P, et al. Antibodies to the cytoskeleton components and other autoantibodies in inflammatory bowel disease. Digestion 1985; 32: 140–144. [DOI] [PubMed] [Google Scholar]
- 30. Beigel F, Schnitzler F, Paul Laubender R, et al. Formation of antinuclear and double-strand DNA antibodies and frequency of lupus-like syndrome in anti-TNF-α antibody-treated patients with inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 91–98. [DOI] [PubMed] [Google Scholar]
- 31. Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019; 4: 341–353. [DOI] [PubMed] [Google Scholar]
- 32. Ciak J, Hahn FE. Chloroquine: mode of action. Science 1966; 151: 347–349. [DOI] [PubMed] [Google Scholar]
- 33. Dinse GE, Parks CG, Meier HCS, et al. Prescription medication use and antinuclear antibodies in the United States, 1999–2004. J Autoimmun 2018; 92: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003; 111: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mandel MD, Miheller P, Müllner K, et al. Have biologics changed the natural history of Crohn’s disease? Dig Dis 2014; 32: 351–359. [DOI] [PubMed] [Google Scholar]
- 36. Quezada SM, McLean LP, Cross RK. Adverse events in IBD therapy: the 2018 update. Expert Rev Gastroenterol Hepatol 2018; 12: 1183–1191. [DOI] [PubMed] [Google Scholar]
- 37. Santos-Antunes J, Nunes ACR, Lopes S, et al. The relevance of vitamin D and antinuclear antibodies in patients with inflammatory bowel disease under anti-TNF treatment: a prospective study. Inflamm Bowel Dis 2016; 22: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 38. Atreya R, Zimmer M, Bartsch B, et al. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14+ macrophages. Gastroenterology 2011; 141: 2026–2038. [DOI] [PubMed] [Google Scholar]
- 39. Macaluso FS, Sapienza C, Ventimiglia M, et al. Lupus-like reactions in patients with inflammatory bowel disease treated with anti-TNFs are insidious adverse events: data from a large single-center cohort. Scand J Gastroenterol 2019; 54: 1102–1106. [DOI] [PubMed] [Google Scholar]
- 40. Yanai H, Shuster D, Calabrese E, et al. The incidence and predictors of lupus-like reaction in patients with IBD treated with anti-TNF therapies. Inflamm Bowel Dis 2013; 19: 2778–2786. [DOI] [PubMed] [Google Scholar]
- 41. Verma HD, Scherl EJ, Jacob VE, et al. Anti-nuclear antibody positivity and the use of certolizumab in inflammatory bowel disease patients who have had arthralgias or lupus-like reactions from infliximab or adalimumab. J Dig Dis 2011; 12: 379–383. [DOI] [PubMed] [Google Scholar]
- 42. Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012; 9: 496–503. [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez-Jiménez P, Chicharro P, Godoy A, et al. No evidence for induction of autoantibodies or autoimmunity during treatment of psoriasis with ustekinumab. Br J Dermatol 2017; 177: 862–863. [DOI] [PubMed] [Google Scholar]
- 44. Mulani S, McNish S, Jones D, et al. Prevalence of antinuclear antibodies in hidradenitis suppurativa. Int J Rheum Dis 2018; 21: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nancey S, Blanvillain E, Parmentier B, et al. Infliximab treatment does not induce organ-specific or nonorgan-specific autoantibodies other than antinuclear and anti-double-stranded DNA autoantibodies in Crohn’s disease. Inflamm Bowel Dis 2005; 11: 986–991. [DOI] [PubMed] [Google Scholar]
- 46. García García MJ, Rivero Tirado M, Callizo MEP. Paradoxical arthritis in inflammatory bowel disease patients on ustekinumab treatment. Inflamm Bowel Dis 2019; 25: e89. [DOI] [PubMed] [Google Scholar]
- 47. Benzaquen M, Flachaire B, Rouby F, et al. Paradoxical pustular psoriasis induced by ustekinumab in a patient with Crohn’s disease-associated spondyloarthropathy. Rheumatol Int 2018; 38: 1297–1299. [DOI] [PubMed] [Google Scholar]
- 48. Solow EB, Vongpatanasin W, Skaug B, et al. Antinuclear antibodies in the general population: positive association with inflammatory and vascular biomarkers but not traditional cardiovascular risk factors. Clin Exp Rheumatol 2018; 36: 1031–1037. [PubMed] [Google Scholar]
- 49. Villegas-Zambrano N, Martínez-Taboada VM, Bolívar A, et al. Correlation between clinical activity and serological markers in a wide cohort of patients with systemic lupus erythematosus: an eight-year prospective study. Ann N Y Acad Sci 2009; 1173: 60–66. [DOI] [PubMed] [Google Scholar]
- 50. Hu J, Zhang Y, Liu X, et al. Relationship between certain T helper cytokines and ANA staining: who is the helper? Int Immunopharmacol 2015; 24: 208–210. [DOI] [PubMed] [Google Scholar]
- 51. Powell-Tuck J. Protein metabolism in inflammatory bowel disease. Gut 1986; 27: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gouni-Berthold I, Baumeister B, Berthold HK, et al. Immunoglobulins and IgG subclasses in patients with inflammatory bowel disease. Hepatogastroenterology 1999; 46: 1720–1723. [PubMed] [Google Scholar]
- 53. Aschermann S, Lux A, Baerenwaldt A, et al. The other side of immunoglobulin G: suppressor of inflammation. Clin Exp Immunol 2010; 160: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221077837 for Prevalence of antinuclear antibodies in inflammatory bowel disease and seroconversion after biological therapy by María José García, Juan Carlos Rodríguez-Duque, Marta Pascual, Coral Rivas, Beatriz Castro, Sandra Raso, Marcos López-Hoyos, María Teresa Arias-Loste and Montserrat Rivero in Therapeutic Advances in Gastroenterology




