Abstract
Objective
To determine whether the methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism is linked to the risk of ischaemic stroke and circulating homocysteine (Hcy) levels in a Chinese population.
Methods
This case–control study recruited angiogram-diagnosed patients with ischaemic stroke and healthy control subjects. The plasma Hcy concentrations were measured and the MTHFR C677T gene polymorphism was genotyped. The National Institutes of Health Stroke Scale (NIHSS) was used to assess the severity of the ischaemic stroke.
Results
This study recruited 198 patients with ischaemic stroke and 168 controls. The TT genotype conferred a higher risk for ischaemic stroke than the CC genotype (odds ratio of 3.563; 95% confidence interval [CI] 1.412, 4.350). The T allele was the predisposing allele for ischaemic stroke. Hcy had an area under the receiver operating characteristic (ROC) curve of 0.624 (95% CI 0.530, 0.758). The ROC for Hcy demonstrated its usefulness in predicting ischaemic stroke. Hcy levels were not associated with ischaemic stroke severity as measured by the NIHSS.
Conclusion
The MTHFR C677T gene polymorphism affects circulating Hcy levels. The MTHFR C677T gene polymorphism and hyperhomocysteinaemia may play important roles in predicting the risk of ischaemic stroke.
Keywords: Ischaemic stroke, MTHFR gene polymorphisms, C677T mutation, homocysteine, case–control study
Introduction
Stroke is a serious threat to health and a leading cause of disability and death worldwide. 1 The most common type of stroke is ischaemic stroke. 2 According to the World Health Organization, approximately 15 million people are diagnosed with ischaemic stroke each year, with approximately 10 million dying or becoming permanently disabled. 3 As stroke can result in long-term disability, early detection of risk factors and active control are critical to effectively prevent the occurrence of stroke.
Extensive research in recent years has revealed that high homocysteine (Hcy) levels are a new and distinct risk factor for stroke.4,5 Hcy is a nonprotein amino acid containing sulphur that is produced during the metabolism of methionine. 6 Hcy has been identified as an independent risk factor for cerebrovascular disease in patients of any age with very high serum Hcy concentrations. 7 In older patients, hyperhomocysteinaemia (HHcy) may be attributed to the lack of enzymes involved in the metabolism of Hcy and B vitamins, nutritional deficiencies of vitamin cofactors, age, medication use or other factors, such as living conditions. 8 There are two major pathways for Hcy metabolism: (i) remethylation; and (ii) transsulfuration. 9 Folic acid provides a methyl group for Hcy in the remethylation pathway, which forms tetrahydrofolate in the presence of vitamin B12–dependent methionine synthase. 9 Hcy is then converted to methionine. 9 The transsulfuration pathway occurs in the reaction catalysed by Hcy on serine via cystathionine b-synthase, with vitamin B6 as a cofactor. 10 Cystathionine is formed as a result of this reaction and is then converted to cysteine. 10 Cysteine is converted into sulphates. 10 Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that participates in the metabolism of methionine to produce Hcy. 11
The alteration of MTHFR activity is caused by several mutations in the MTHFR gene. 12 The MTHFR gene, which is found on chromosome 1 (1p36.3), encodes a 77 kDa dimeric protein that is the rate-limiting enzyme in the methyl cycle. 13 MTHFR catalyses only one biochemical reaction: the formation of 5-methylenetetrahydrofolate, a cosubstrate for the remethylation of Hcy to methionine. 12 MTHFR C677T (C substitution at base pair 677) is a common MTHFR gene mutation that causes alanine to be replaced by valine at position 222 of the encoded protein product. 14 Previous research has linked TT variants of the MTHFR C677T polymorphism to elevated serum Hcy concentrations and the severity of ischaemic stroke, implying an important role as a marker of ischaemic stroke. 15 It has been proposed that the MTHFR C677T polymorphism alters the methylation state of DNA, thereby altering lipid metabolism, which is involved in the process of ischaemic stroke. 14 As a result of its central role, MTHFR gene polymorphism is associated with a variety of medical conditions, including as Alzheimer's disease, brain diseases and severe vascular disease. 8 Although several studies have suggested that HHcy can cause ischaemic stroke,15–17 the relationship between Hcy and the severity of ischaemic stroke has rarely been reported. 15 It has been proposed that the MTHFR C677T gene polymorphism may influence the relationship between Hcy levels and the risk of ischaemic stroke. 18
The objective of this current study was to determine if there was a correlation between the severity of ischaemic stroke, the MTHFR C677T polymorphism and plasma Hcy levels in a patient population for southwest China.
Patients and methods
Study population
This case–control study enrolled consecutive stroke patients at the outpatient clinic of Suining Central Hospital, Suining, Sichuan Province, China between July 2018 and October 2020. The inclusion criteria were as follows: (i) age ≥18 years; (ii) patients suffering from an ischaemic stroke in accordance with diagnostic criteria in China's 2010 guidelines for the diagnosis and treatment of acute ischaemic stroke. 2 The exclusion criteria were as follows: (i) patients suffering from a cerebral haemorrhage; (ii) pregnant or breastfeeding women; (iii) patients with malignant tumours. In addition, a control group of healthy subjects aged ≥18 years were recruited from the check-up centre of Suining Central Hospital. Individuals in the control group had no history of acute ischaemic stroke or cardiovascular disease.
The Research Ethics Committee of Suining Central Hospital approved the study protocol (no. 20171104). All research was carried out under the applicable guidelines and regulations. All participants provided verbal informed consent for this research.
Baseline data collection
A standardized questionnaire was designed to obtain information on age, sex, smoking, alcohol intake, hypertension, diabetes mellitus and hyperlipidaemia.
Lipid profile test
Venous blood samples (5 ml) were collected after a 12-h fast and placed in 3.8 mg/ml ethylenediaminetetra-acetic (EDTA) acid tubes (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). The samples were centrifuged at 2810 g for 10 min at room temperature (Labtrip RZ-50; Guangzhou SimplyLab Scientific Instrument, Guangzhou, China), after which the upper plasma layer was separated immediately and stored at −70°C until analysis. The lipid profile, which included total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), was determined using a photometric method on a Dade Behring Dimension® RxL Max® Integrated Chemistry System (Siemens Healthcare, Erlangen, Germany).
Assessment of ICAS
To confirm the diagnosis of intracranial atherosclerotic stenosis (ICAS), all patients underwent computerized tomography angiography brain scans on a multi-slice spiral computerized tomography scanner (SOMATOM Definition AS; Siemens Healthcare). ICAS of the three arteries that were evaluated (bilateral middle cerebral artery, bilateral intracranial internal carotid artery and basilar artery) was classified as mild (<50% signal reduction), moderate (50% to <70% signal reduction), severe (≥70% signal reduction) or occluded. A neurologist specialist in stroke (L.H. or J.L.) and a clinical neuroradiologist performed the stenosis diagnosis and grading. Disagreements in their assessments were resolved through consensus.
Evaluation of stroke severity
The National Institutes of Health Stroke Scale (NIHSS) was used to assess the severity of stroke at the time of admission. 19 The NIHSS consists of a 11-item neurological examination that assesses consciousness, speech, myodynamia, visual field deficits, pupillary, facial palsy, ataxia and sensory loss. Each item is scored individually and a score of 0 is considered normal. The overall NIHSS score is 0 for no stroke, 1–4 for a minor stroke, 5–15 for a moderate stroke, 16–20 for a moderate-to-severe stroke and 21–42 for a severe stroke. 19 In this current study, an overall NIHSS score ≤15 points was considered a minor-moderate stroke and a score >16 points was a moderate-severe stroke.
Detection of Hcy and C677T polymorphism of the MTHFR gene
The plasma Hcy levels and MTHFR C677T gene polymorphisms were measured in the Laboratory Department, Suining Central Hospital and KingMed Diagnostics, Chengdu, Sichuan Province, China, respectively. Two venous blood samples (5 ml) were collected after a 12-h fast and placed in 3.8 mg/ml EDTA tubes (Becton, Dickinson and Co.). The samples were centrifuged at 2810 g for 10 min at room temperature (Labtrip RZ-50; Guangzhou SimplyLab Scientific Instrument), after which the upper plasma layer was separated immediately and stored at −70°C until analysis. The plasma Hcy concentration was measured on a Hitachi 7600 automatic biochemical analyser (Hitachi, Tokyo, Japan) using an Hcy enzyme-linked immunosorbent assay kit (Shenzhen Mindray Bio-medical Electronics, Shenzhen, China). HHcy was defined as a plasma Hcy concentration >15 mol/l.
On the morning of the second day after admission, each participant provided a fasting venous blood sample (2 ml) into heparin anticoagulation tubes, which was stored at −70°C until analysis. Polymerase chain reaction (PCR) restriction fragment length polymorphism methods were used to determine the MTHFR C677T gene polymorphism. Briefly, Primer 5.0 software (Premier Biosoft, San Francisco, CA, USA) was used for primer design and the primers were synthesized by Shanghai Bioengineering Co. Ltd. (Shanghai, China). The upstream primer sequence of MTHFR C677T was 5′-CATCCCTATTGGCAGGTTAC-3′ and the downstream primer sequence was 5′-GACGGTGCGGTGAGAGTG-3′. According to the manufacturer's instructions, genomic DNA samples were extracted using a blood genomic extraction kit (Tiangen Bio, Beijing, China). The reaction system included 2.5 µl × 10 buffer (Sangon Biotech [Shanghai], Shanghai, China), 0.5 µl magnesium chloride solution, 2 µl deoxynucleotide triphosphates (Proteinbio, Nanjing, China), 1 µl upstream and 1 µl downstream primers, 1 µl DNA template (50 ng/µl), 0.2 IU Taq enzyme (Takara Biomedical Technology [Beijing], Beijing, China) and double-distilled H2O to a total volume of 25 µl. The PCR was undertaken on a S1000 thermal cycler (Bio-Rad, Hercules, CA, USA) and the cycling programme involved preliminary denaturation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 15 s and elongation at 72°C for 30 s, followed by a final elongation step at 72°C for 3 min. After HinfI endonuclease digestion (Takara Biomedical Technology [Beijing]), the fragment lengths were as follows: 246 base pairs (bp) for the CC genotype; 246, 174 and 72 bp for the CT genotype; and 174 and 72 bp for the TT genotype. Following the PCR, the specificity of the PCR product was determined using a gel imager following 2% agarose gel electrophoresis and the PCR product was purified using PCR Purification Reagent (Qiagen, Hilden, Germany).
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA). Normally distributed continuous data are presented as mean ± SD and compared using Student's t-test. Continuous data that were not normally distributed are presented as median (interquartile range) and compared using Mann–Whitney U-test. Categorical data are presented as n of patients (%) and compared made using χ2-test. To compare multiple groups, Kruskal–Wallis tests or variance tests were used. 20 χ2-test analysis was used to determine genotype/allele frequencies and differences in genetic and allelic frequencies when the Hardy–Weinberg equilibrium was considered. The predictive power was evaluated using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The odds ratio (OR) was calculated using 2 × 2 cross-tabulation to analyse the correlations between each polymorphism. A P-value ≤0.05 was considered statistically significant.
Results
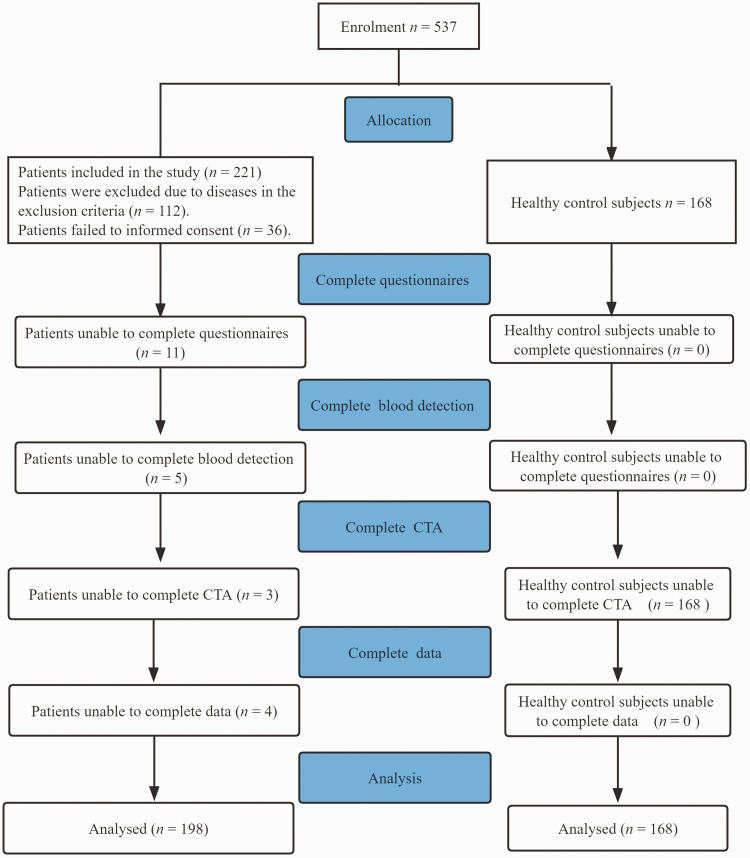
Of the 537 people enrolled in this current study, 366 had complete baseline information and agreed to participate (Figure 1). Of these, 198 were patients with ischaemic stroke and 168 were healthy control subjects. The baseline characteristics of the study participants are shown in Table 1. The median plasma Hcy concentration was significantly higher in the patient group compared with the control group (P = 0.041). BMI was significantly higher in patients compared with controls (P = 0.032). Patients had a significantly higher prevalence of hypertension, diabetes mellitus, smoking and dyslipidaemia than control subjects (P < 0.05 for all comparisons).
Figure 1.
Flow diagram showing the progress of patients with ischaemic stroke and healthy control subjects through the selection process in a study that aimed to determine if there was a correlation between the severity of ischaemic stroke, the methylenetetrahydrofolate reductase C677T gene polymorphism and plasma homocysteine levels. CTA, computed tomography angiography.
Table 1.
Baseline demographic and clinical characteristics of patients with ischaemic stroke and healthy control subjects that were included a study that aimed to determine if there was a correlation between the severity of ischaemic stroke, the methylenetetrahydrofolate reductase C677T gene polymorphism and plasma homocysteine levels.
| Characteristic | Control group n =168 | Patient group n = 198 | Z/t/χ2 | Statistical analysesa |
|---|---|---|---|---|
| Homocysteine, mmol/l | 14.64 (12.40, 15.80) | 19.31 (16.18, 20.83) | 4.85 | P = 0.041 |
| Total cholesterol, mmol/l | 5.14 (4.22, 5.71) | 5.03 (4.05, 5.81) | 0.43 | NS |
| Triglyceride, mmol/l | 1.55 (0.99, 1.80) | 2.00 (1.08, 2.39) | 2.06 | NS |
| LDL-C, mmol/l | 2.66 (2.17, 3.07) | 2.62 (2.04, 3.17) | 0.02 | NS |
| HDL-C, mmol/l | 1.46 (1.23, 1.59) | 1.38 (1.16, 1.51) | 1.44 | NS |
| BMI, kg/m² | 23.33 ± 2.60 | 24.41 ± 2.50 | 2.39 | P = 0.032 |
| Sex, female | 78 (46.43%) | 70 (35.35%) | 1.54 | NS |
| Hypertension | 106 (63.10%) | 150 (75.76%) | 6.12 | P = 0.013 |
| Diabetes mellitus | 26 (15.48%) | 64 (32.32%) | 8.10 | P = 0.004 |
| Smoker | 38 (22.62%) | 62 (31.31%) | 2.58 | P = 0.025 |
| Alcohol drinker | 36 (21.43%) | 50 (25.25%) | 0.65 | NS |
| Carotid artery stenosis | 138 (82.14%) | 162 (81.82%) | 0.84 | NS |
| Dyslipidaemia | 64 (38.10%) | 108 (54.55%) | 3.47 | P = 0.021 |
Data presented as mean ± SD, median (interquartile range) or n of patients (%).
aNormally distributed continuous data are presented as mean ± SD and compared using Student's t-test; continuous data that were not normally distributed are presented as median (interquartile range) and compared using Mann–Whitney U-test; and categorical data are presented as n of patients (%) and compared made using χ2-test; NS, no significant between-group difference (P > 0.05).
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index.
The patients were stratified based on their MTHFR C677T genotypes. The highest levels of Hcy were demonstrated in patients with the TT genotype compared with the CT and CC genotypes (P = 0.027) (Table 2). A total of 58 of 66 (87.88%) patients with the TT genotype had high levels of Hcy (≥15 µmol/l). A total of 43 of 66 (65.15%) patients with the TT genotype had an NIHSS ≥15. The highest NIHSS scores were shown in patients with the TT genotype compared with the CT and CC genotypes, although the between-group differences were not significant. TT genotype carriers had higher lipid concentrations compared with the CT and CC genotype carries, the between-group differences were not significant.
Table 2.
Baseline demographic, clinical and biochemical characteristics of patients (n = 198) with ischaemic stroke stratified according to their methylenetetrahydrofolate reductase C677T genotype.
| Characteristic | Patient group n = 198 |
||||
|---|---|---|---|---|---|
| CC genotype n = 72 | CT genotype n = 60 | TT genotype n = 66 | Z/t/χ2 | Statistical analysesa | |
| Triglyceride, mg/dl | 1.99 ± 1.10 | 1.66 ± 0.74 | 2.19 ± 2.23 | 0.67 | NS |
| Total cholesterol, mg/dl | 4.88 ± 1.08 | 5.07 ± 1.23 | 5.26 ± 1.17 | 0.74 | NS |
| LDL-C, mg/dl | 2.55 ± 0.80 | 2.60 ± 0.70 | 2.86 ± 0.84 | 1.11 | NS |
| HDL-C, mg/dl | 1.32 ± 0.21 | 1.39 ± 0.35 | 1.50 ± 0.30* | 2.26 | NS |
| Age, years | 69.73 ± 8.17 | 66.34 ± 10.02 | 69.72 ± 10.67 | 1.43 | NS |
| BMI, kg/m² | 24.56 ± 2.45 | 24.71 ± 2.73 | 23.51 ± 2.46 | 1.51 | NS |
| Homocysteine, µmol/l | 13.74 ± 4.32 | 14.06 ± 4.26 | 18.07 ± 6.00 | 1.65 | P = 0.027 |
| NIHSS | 13.16 ± 5.23 | 12.46 ± 4.72 | 14.18 ± 5.03 | NS | |
| Carotid artery stenosis | 2.16 | NS | |||
| None | 7 (9.72%) | 8 (13.33%) | 6 (9.09%) | ||
| <30% | 44 (61.11%) | 34 (56.67%) | 22 (33.33%) | ||
| 30–69% | 9 (12.50%) | 8 (13.33%) | 12 (18.18%) | ||
| ≥70% | 12 (16.67%) | 10 (16.67%) | 26 (39.39%) | ||
| Homocysteine | 2.89 | P = 0.013 | |||
| <15 µmol/l | 54 (75.00%) | 34 (56.67%) | 8 (12.12%) | ||
| ≥15 µmol/l | 18 (25.00%) | 26 (43.33%) | 58 (87.88%)*# | ||
| NIHSS score | NS | ||||
| <15 | 41 (56.94%) | 28 (46.67%) | 23 (34.85%) | ||
| ≥15 | 31 (43.06%) | 32 (53.33%) | 43 (65.15%) | ||
Data presented as mean ± SD or n of patients (%).
aNormally distributed continuous data are presented as mean ± SD and compared using Student's t-test; and categorical data are presented as n of patients (%) and compared made using χ2-test; *P < 0.05 compared with the CT genotype; #P = 0.017 compared with the CC genotype; NS, no significant between-group difference (P > 0.05).
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale.
Regarding genotypic distribution, both patients and controls were in Hardy–Weinberg equilibrium for the polymorphism. Compared with the control subjects, a lower proportion of patients had the CC and CT genotypes and a higher proportion of patients had the TT genotype (P = 0.031) (Table 3). The OR for ischaemic stroke was 1.716 (95% confidence interval [CI] 0.998, 2.918; P = 0.024) for the CT genotype and 3.563 (95% CI 1.412, 4.350; P < 0.001) for the TT genotype. Patients with ischaemic stroke carrying the minor T allele were significantly associated with a higher risk of ischaemic stroke compared with those carrying the C allele (OR 2.136, 95% CI 1.724, 2.871; P = 0.012).
Table 3.
Genotypic and allelic distribution and association of the polymorphism of the methylenetetrahydrofolate reductase (MTHFR) C677T gene with ischaemic stroke.
| MTHFR C677T | Control group n =168 | Patient group n = 198 | P-value | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Genotype | P = 0.031 | |||||
| CC | 101 (60.12%) | 94 (47.47%) | 1 | |||
| CT | 62 (36.90%) | 72 (36.36%) | 1.716 | 0.998, 2.918 | P = 0.024 | |
| TT | 5 (2.98%) | 32 (16.16%) | 3.563 | 1.412, 4.350 | P < 0.001 | |
| Allele | ||||||
| C | 264 | 260 | P = 0.012 | P = 0.012 | ||
| T | 72 | 136 | 2.136 | 1.724, 2.871 | ||
OR, odds ratio; CI, confidence interval.
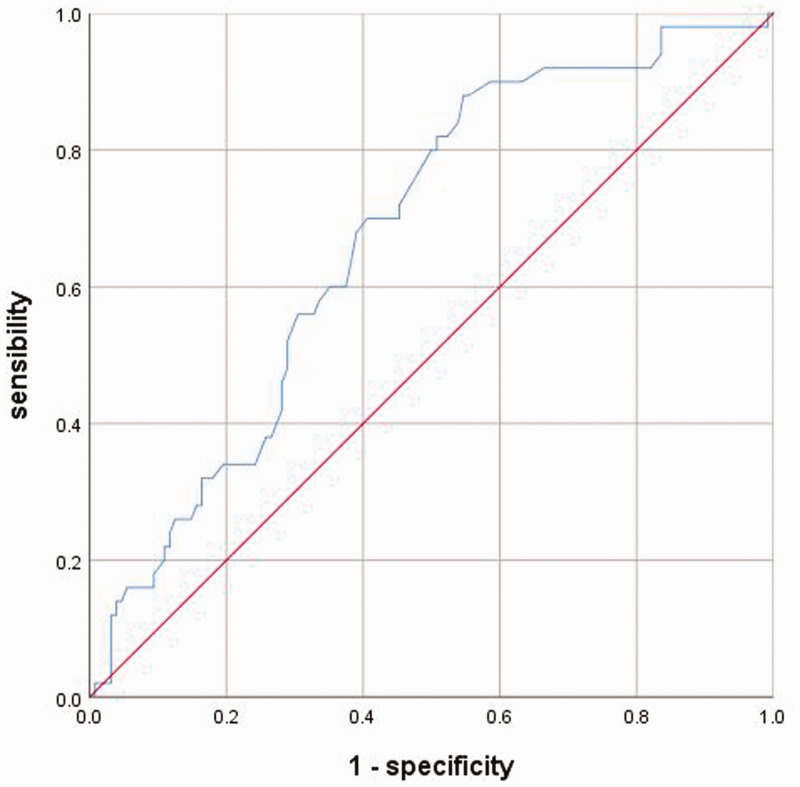
Plasma Hcy levels were significantly higher in patients with ischaemic stroke compared with the control subjects (P = 0.041) (Table 1). The ROC curve for Hcy as an indicator of the presence of ischaemic stroke is shown in Figure 2. The AUC of the ROC curve for Hcy was 0.624 (95% CI 0.530, 0.758, P = 0.024).
Figure 2.
The receiver operating characteristic curve (ROC) analysis for homocysteine (Hcy) levels in predicting ischaemic stroke. The area under the ROC curve for Hcy was 0.624 (95% confidence interval 0.530, 0.758, P = 0.024).
Based on the median of NIHSS score, patients with ischaemic stroke were stratified into two groups: low NIHSS score (<15) and high NIHSS score (≥15). The median Hcy levels were higher in the high NIHSS score group (median [interquartile range] 21.1 µmol/l [7.9–50.0]) compared with the low NIHSS score group (median [interquartile range] 14.2 µmol/l [3.7–50.0]), but the difference was not significant.
Discussion
The main finding of this current study was that there was an increase in Hcy levels in patients with ischaemic stroke due to the C677T polymorphism of the MTHFR gene. Hcy levels, as depicted in the ROC curve, may be a useful tool in predicting ischaemic stroke. When compared with control subjects, patients with ischaemic stroke had a higher prevalence of the TT genotype and the T allele. These findings suggest that the MTHFR C677T polymorphism may be linked to increased susceptibility to ischaemic stroke in a southwest Chinese population. High levels of Hcy and C677T polymorphism frequencies in the MTHFR gene of patients with ischaemic stroke, in contrast, were not associated with the severity of ischaemic stroke as measured by the NIHSS.
The MTHFR gene C677T polymorphism, which is associated with Hcy metabolism, has been studied in a variety of stroke pathologies, hypertension and other immune diseases.15,21 MTHFR is a folate-dependent enzyme that participates in the conversion of Hcy to methionine by converting folate to its active cofactor. 22 In terms of genotype frequencies, studies of this polymorphism have revealed a significant correlation between patients and control groups.20,23,24 According to previous research findings, the distribution of the MTHFR gene C677T polymorphism seems to be very heterogeneous, with genotypic frequencies varying from study to study. For example, in 71 patients with ischaemic stroke, an Indonesian study demonstrated a genotypic distribution for the CC:CT:TT genotypes of 81.9%:13.9%:4.2%. 23 In a study in Thailand, the genotypic distribution in 106 patients for the CC:CT:TT genotypes was 73.1%:20.6%:6.4%. 25 The genotypic distribution in this current study for the CC:CT:TT genotypes was 47.47%: 36.36%:16.16%, which was consistent with previous studies.25,26
Given the apparent metabolic link observed in the current patients between high levels of Hcy and the MTHFR enzyme, the current study analysed the effect of the MTHFR gene C677T polymorphism on plasma Hcy concentrations. Hcy levels were found to be significantly related to the genotype (TT) and allele (T) of the MTHFR C677T gene polymorphism. However, contradictory findings have been reported previously. A previous investigated 71 post-ischaemic stroke patients and discovered that the MTHFR C677T allele polymorphism was not related to hyperhomocysteinaemia. 23 Other research demonstrated that the C677T polymorphism of the MTHFR gene was associated with ischaemic stroke and increased Hcy levels.15,27 It has also been suggested that this polymorphism could be an underlying genetic cerebrovascular risk factor for HHcy.15,21Thermolability of MTHFR increases Hcy concentrations by inactivating the resulting active dimer, which interferes with the binding of the flavin adenine dinucleotide, which varies the Hcy methylation pathway and MTHFR activity. 28 The C677TT polymorphism of the MTHFR gene has been related to endothelial dysfunction and vascular oxidative stress caused by high levels of 5-methylenetetrahydrofolate (5-MTHRF).29,30 MTHFR-linked hyperhomocysteinaemia has previously been regarded as the main cause of endothelial dysfunction and atherogenesis. 31 The MTHFR 677TT variant has a stronger influence on 5-MTHF levels than on Hcy levels. 24
In the current study, the TT genotype was associated with a higher risk of ischaemic stroke than the CC genotype, with an OR of 3.563 (95% CI 1.412, 4.350). This demonstrates that the T allele is a susceptibility allele for ischaemic stroke and that it may be related to the severity of the disease based on the NIHSS scores. A previous study demonstrated in a relatively small cohort (n = 165) the link between the C677T polymorphism (both homozygous and heterozygous) and the risk for ischaemic stroke in patients with type 2 diabetes mellitus. 16 Moreover, the findings suggested that the MTHFR C677T mutation confers a higher risk for stroke to both homozygous and heterozygous T allele carriers. 16 Another study demonstrated no detectable correlation between MTHFR gene polymorphisms and stroke in a Zambian population. 32 The MTHFR C667T gene polymorphism was not associated with ischaemic stroke in a Chinese population. 33 Different racial and ethnic groups, different intakes of folic acid and vitamin B and small sample sizes could explain the discrepancy between these studies.16,32,33 Previous research has shown the MTHFR C677 T allele increased susceptibility to ischaemic stroke and elevated blood lipid levels. 34 It has been proposed that the dyslipidaemia caused by the MTHFR C667T gene polymorphism is one of the major factors in the progression of ischaemic stroke. 35
A previous study demonstrated that the MTHFR C677T polymorphism, in conjunction with traditional risk factors such as LDL-C, HDL-C and smoking, was a risk factor for atherosclerosis. 36 The C667T polymorphism in the MTHFR gene influences carotid atherosclerosis pathogenesis by destroying endothelial cells and promoting smooth muscle cell proliferation. 37 In addition, a link has been found between plasma Hcy, the MTHFR C677T gene polymorphism and carotid intimal thickness in South Asian, Chinese and European Canadians. 38 This current study found no significant differences in the MTHFR C677T gene polymorphism based on the extent of carotid artery stenosis, which is consistent with a previous study. 25
The association between the MTHFR C677T gene polymorphism and the severity of cerebral infarction was significant in a previous report. 34 In that study, stroke severity was higher in patients with the C677T mutation, who had higher NIHSS and modified Rankin Score scores. 34 A large prospective cohort study in a Chinese population with a median follow-up of 4.5 years discovered no significant association between the MTHFR C677T gene polymorphism and stroke mortality. 39 A previous study was unable to demonstrate an association between MTHFR gene polymorphisms and ischaemic stroke, possibly as a consequence of the study's small sample size (67 patients and 60 controls). 17 The current study recruited more people than this previous study (198 patients and 168 controls). 17 Higher levels of Hcy were found in the NIHSS ≥15 group in the current study, but there was no significant difference between the two groups based on disease severity. The lack of a correlation between Hcy levels and stroke severity in the current study could be attributed to the multifactorial nature of ischaemic stroke, unclassified cerebral infarcts and the probable connection between Hcy and other cerebrovascular risk factors. The nutritional intake of folate and a deficiency of vitamins B12 and B6 could be factors influencing Hcy concentrations, given their contribution as cofactors in the control of its metabolism. 40 Nutritional deficiencies in folic acid and vitamin B may affect Hcy concentrations because they act as cofactors in controlling Hcy metabolism, as may the presence of other mutations and the role of traditional cerebrovascular risk factors such as hypertension and smoking. 41
The relationship between HHcy, the MTHFR C677T gene polymorphism and the severity of ischaemic stroke has rarely been studied. For example, the findings of three previous studies demonstrated a significant correlation between high levels of Hcy and the severity of ischaemic stroke as measured using the NIHSS, independent of MTHFR polymorphism.18,42,43 A previous study found that the TT genotype of the MTHFR C677T gene polymorphism was related to a higher incidence of vulnerable plaque and a high Hcy level, both of which were thought to be predictors of atherosclerotic plaque instability. 44 In 2005, a Chinese-based population study found a significant link between stroke severity (high NIHSS scores) and disturbance of MTHFR genetic activity (the A222V amino acid substitution mutation raises Hcy levels even further). 45 A meta-analysis of 15 000 patients of different ethnicities from Northern and Central Europe, North America and Italy reported that the MTHFR C677T gene polymorphism was associated with an increased risk of stroke. 46 It has been confirmed that the MTHFR C677T gene polymorphism influences Hcy levels in a variety of populations, including Americans, Asians and Europeans. 47 A previous study demonstrated that the TT genotype of the MTHFR C677T gene polymorphism had an effect on Hcy levels but was not related to ischaemic stroke in a Chinese population. 21 Other studies in Tunisian, Romanian and British populations found that the MTHFR C677T gene polymorphism was linked to both ischaemic stroke and Hcy.15,16,48
This current study had several limitations. First, because all of the controls were from the same hospital, selection bias could not be avoided. Secondly, the size of the sample was small and the crude OR was calculated without adjusting for potential confounders. Therefore, the low statistical power may have contributed to inconsistent results. Thirdly, the controls in this current study were blood donors that did not have cerebral angiography. Furthermore, hyperhomocysteinaemia could promote an ischaemic stroke via different pathogenic mechanisms, including cardioembolic mechanisms that can be due to the effects of Hcy on specific cardiac channels, increasing the risk of covert atrial fibrillation. 49 The current study did not analyse the association between different subtypes of stroke, the presence of MTHFR C677T polymorphism and high serum levels of Hcy, which will be studied in the future.
In conclusion, the MTHFR C677T gene polymorphism influenced circulating levels of Hcy, with the TT genotype having the highest levels. The findings showed that the MTHFR C667T gene polymorphism and HHcy play a major role in the prediction of ischaemic stroke. However, there was no significant relationship between Hcy levels, the MTHFR C677T gene polymorphism and the severity of ischaemic stroke as measured by the NIHSS.
Acknowledgements
We thank all study staff and participants for their important contributions.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Lu-Wen Huang https://orcid.org/0000-0002-4258-4863
References
- 1.Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008; 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 2.The 2010 Guidelines for the diagnosis and treatment of acute ischemic stroke in China. Chinese J Clinic 2011; 39: 227–233. [Google Scholar]
- 3.Ziganshina LE, Abakumova T, Kuchaeva A. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev 2010: CD007026. [DOI] [PubMed] [Google Scholar]
- 4.Spence JD: Homocysteine lowering for stroke prevention: Unravelling the complexity of the evidence. Int J Stroke 2016; 11: 744–747. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Jiang S, Zhang Y, et al. H-type hypertension and risk of stroke in Chinese adults: A prospective, nested case-control study. J Transl Int Med 2015; 3: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 1998; 338: 1042.–. [DOI] [PubMed] [Google Scholar]
- 7.Tu W, Yan F, Chao B, et al. Status of hyperhomocysteinemia in China: results from the China Stroke High-risk Population Screening Program, 2018. Front Med 2021; 15: 903.–. [DOI] [PubMed] [Google Scholar]
- 8.Ostrakhovitch EA, Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res Rev 2019; 49: 144–164. [DOI] [PubMed] [Google Scholar]
- 9.Piazzolla G, Candigliota M, Fanelli M, et al. Hyperhomocysteinemia is an independent risk factor of atherosclerosis in patients with metabolic syndrome. Diabetol Metab Syndr 2019; 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadithi M, Mulder CJ, Stam F, et al. Effect of B vitamin supplementation on plasma homocysteine levels in celiac disease. World J Gastroenterol 2009; 15: 955.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CJ, Yang TC, Chang C, et al. Homocysteine is a bystander for ST-segment elevation myocardial infarction: a case-control study. BMC Cardiovasc Disord 2018; 18: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H, Huang S, Yang Y, et al. Association Between MTHFR Polymorphisms and the Risk of Essential Hypertension: An Updated Meta-analysis. Front Genet 2021; 12: 698590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyette P, Pai A, Milos R, et al. Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome 1998; 9: 652.–. [DOI] [PubMed] [Google Scholar]
- 14.Abhinand PA, Manikandan M, Mahalakshmi R, et al. Meta-analysis study to evaluate the association of MTHFR C677T polymorphism with risk of ischemic stroke. Bioinformation 2017; 13: 214.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chita DS, Tudor A, Christodorescu R, et al. MTHFR Gene Polymorphisms Prevalence and Cardiovascular Risk Factors Involved in Cardioembolic Stroke Type and Severity. Brain Sci 2020; 10: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fekih-Mrissa N, Mrad M, Klai S, et al. Methylenetetrahydrofolate reductase (C677T and A1298C) polymorphisms, hyperhomocysteinemia, and ischemic stroke in Tunisian patients. J Stroke Cerebrovasc Dis 2013; 22: 465–469. [DOI] [PubMed] [Google Scholar]
- 17.Hermans MP, Gala JL, Buysschaert M. The MTHFR CT polymorphism confers a high risk for stroke in both homozygous and heterozygous T allele carriers with Type 2 diabetes. Diabet Med 2006; 23: 529–536. [DOI] [PubMed] [Google Scholar]
- 18.Arsene D, Gaina G, Balescu C, et al. C677T and A1298C methylenetetrahydropholate reductase (MTHFR) polymorphisms as factors involved in ischemic stroke. Rom J Morphol Embryol 2011; 52: 1203–1207. [PubMed] [Google Scholar]
- 19.Ortiz GA and Sacco RL. National Institutes of Health Stroke Scale(NIHSS). In Wiley StatsRef: Statistics Reference Online. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118445112.stat06823 (accessed on 10 February 2019).
- 20.Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis 2018; 17: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang S, Li J, Zhang Y, et al. Methylenetetrahydrofolate reductase C677T polymorphism, hypertension and risk of stroke: a prospective, nested case-control study. Int J Neurosci 2017; 127: 253.–. [DOI] [PubMed] [Google Scholar]
- 22.Shahzad K, Hai A, Ahmed A, et al. A Structured-based Model for the Decreased Activity of Ala222Val and Glu429Ala Methylenetetrahydrofolate Reductase (MTHFR) Mutants. Bioinformation 2013; 9: 929.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramukarso DT, Faradz SM, Sari S, et al. Association between methylenetetrahydrofolate reductase (MTHFR) polymorphism and carotid intima medial thickness progression in post ischaemic stroke patient. Ann Transl Med 2015; 3: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakkakula BVKS. Association between MTHFR 677C>T polymorphism and vascular complications in sickle cell disease: A meta-analysis. Transfus Clin Biol 2019; 26: 284–288. [DOI] [PubMed] [Google Scholar]
- 25.Chutinet A, Suwanwela NC, Snabboon T, et al. Association between genetic polymorphisms and sites of cervicocerebral artery atherosclerosis. J Stroke Cerebrovasc Dis 2012; 21: 379–385. [DOI] [PubMed] [Google Scholar]
- 26.Bouzidi N, Hassine M, Fodha H, et al . Association of the methylene-tetrahydrofolate reductase gene rs1801133 C677T variant with serum homocysteine levels, and the severity of coronary artery disease. Sci Rep 2020; 10: 10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casas JP, Bautista LE, Smeeth L, et al. Homocysteine and stroke: evidence on a causal link from mendelian randomization. Lancet 2005; 365: 224–232. [DOI] [PubMed] [Google Scholar]
- 28.Cohen E, Margalit I, Shochat T, et al. Gender differences in homocysteine concentrations, a population-based cross-sectional study. Nutr Metab Cardiovasc Dis 2019; 29: 9–14. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Gutierrez J. . Genetic Risk Factors of Intracranial Atherosclerosis. Curr Atheroscler Rep 2020; 22: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guldener CV, and Stehouwer CDA. Homocysteine and large arteries. Adv Cardiol 2007; 44: 278.–. [DOI] [PubMed] [Google Scholar]
- 31.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 32.Atadzhanov M, Mwaba MH, Mukomena PN, et al. Association of the APOE, MTHFR and ACE Genes Polymorphisms and Stroke in Zambian Patients. Neurol Int 2013; 5: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou W, Liu X, Shen Y, et al. Association of CVD candidate gene polymorphisms with ischemic stroke and cerebral hemorrhage in Chinese individuals. PLoS One 2014; 9: e105516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitsavos C, Panagiotakos D, Trichopoulou A, et al. Interaction between the Mediterranean diet and methylenetetrahydrofolate reductase C677T mutation on oxidized low-density lipoprotein concentrations: The ATTICA study. Nutr Metab Cardiovasc Dis 2006; 16: 91–99. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Xu L, Xia H, et al. Association of MTHFR C677T gene polymorphism with metabolic syndrome in a Chinese population: a case-control study. J Int Med Res 2018; 46: 2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponomarenko IV, Sukmanova IA. Thrombosis risk factors and gene mutations in young age patients with acute coronary syndrome. Kardiologiia 2019; 59: 19–24. [DOI] [PubMed] [Google Scholar]
- 37.Li A, Huang W, Yang Q, et al. Expression of the C677T Polymorphism of the 5, 10-Methylenetetrahydrofolate Reductase (MTHFR) Gene in Patients with Carotid Artery Atherosclerosis. Med Sci Monit 2020; 26: e920320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelemen LE, Anand SS, Hegele RA, et al. Associations of plasma homocysteine and the methylenetetrahydrofolate reductase C677T polymorphism with carotid intima media thickness among South Asian, Chinese and European Canadians. Atherosclerosis 2004; 176: 361–370. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Sun K, Chen J, et al. High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci (Lond) 2009; 118: 187–194. [DOI] [PubMed] [Google Scholar]
- 40.Manolescu BN, Oprea E, Farcasanu IC, et al. Homocysteine and vitamin therapy in stroke prevention and treatment: a review. Acta Biochim Pol 2010; 57: 467.–. [PubMed] [Google Scholar]
- 41.Abd El-Aziz TA, Mohamed RH. Influence of MTHFR C677T gene polymorphism in the development of cardiovascular disease in Egyptian patients with rheumatoid arthritis. Gene 2017; 610: 127–132. [DOI] [PubMed] [Google Scholar]
- 42.Soriente L, Coppola A, Madonna P, et al. Homozygous C677T mutation of the 5,10 methylenetetrahydrofolate reductase gene and hyperhomocysteinemia in Italian patients with a history of early-onset ischemic stroke. Stroke 1998; 29: 869–871. [DOI] [PubMed] [Google Scholar]
- 43.Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr 2019; 19: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Y, Liu Z, Li L, et al. OLR1, PON1 and MTHFR gene polymorphisms, conventional risk factors and the severity of coronary atherosclerosis in a Chinese Han population. Cell Physiol Biochem 2013; 31: 143–152. [DOI] [PubMed] [Google Scholar]
- 45.Baum L, Wong KS, Ng HK, et al. Methylenetetrahydrofolate reductase gene A222V polymorphism and risk of ischemic stroke. Clin Chem Lab Med 2004; 42: 1370–1376. [DOI] [PubMed] [Google Scholar]
- 46.Cronin S, Furie KL, Kelly PJ. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke 2005; 36: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 47.Cui T. MTHFR C677T mutation increased the risk of Ischemic Stroke, especially in large-artery atherosclerosis in adults: an updated meta-analysis from 38 researches. Int J Neurosci 2016; 126: 10–19. [DOI] [PubMed] [Google Scholar]
- 48.Rutten-Jacobs LCA, Traylor M, Adib-Samii P, et al. Association of MTHFR C677T Genotype With Ischemic Stroke Is Confined to Cerebral Small Vessel Disease Subtype. Stroke 2016; 47: 646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acampa M, Lazzerini PE, Guideri F, et al. Homocysteine and P wave dispersion in patients with heart transplantation. Clin Transplant 2011; 25: 119–125. [DOI] [PubMed] [Google Scholar]