Abstract
Severe hypoxia results in complete loss of central nervous system (CNS) function in mammals, while several other vertebrates, such as zebrafish, can regenerate after hypoxia-induced injury of CNS. Since the cellular mechanism involved in this remarkable feature of other vertebrates is still unclear, we studied the cellular regeneration of zebrafish brain, employing zebrafish embryos from transgenic line huORFZ exposed to hypoxia and then oxygen recovery. GFP-expressing cells, identified in some cells of the CNS, including some brain cells, were termed as hypoxia-responsive recovering cells (HrRCs). After hypoxia, HrRCs did not undergo apoptosis, while most non-GFP-expressing cells, including neurons, did. Major cell types of HrRCs found in the brain of zebrafish embryos induced by hypoxic stress were neural stem/progenitor cells (NSPCs) and radial glia cells (RGs), that is, subtypes of NSPCs (NSPCs-HrRCs) and RGs (RGs-HrRCs) that were induced by and sensitively responded to hypoxic stress. Interestingly, among HrRCs, subtypes of NSPCs- or RGs-HrRCs could proliferate and differentiate into early neurons during oxygen recovery, suggesting that these subtype cells might play a critical role in brain regeneration of zebrafish embryos after hypoxic stress.
Keywords: central nervous system, neural stem/progenitor cells, brain injury, zebrafish, neurogenesis
Introduction
Regeneration of the central nervous system (CNS) in the mammalian system is quite limited. However, zebrafish are capable of neurogenesis and regeneration of adult brain1,2. Traumatic injury in the adult zebrafish brain leads to increased proliferation of neural stem cells without inducing glial scars 3 . Moreover, Gan et al 4 found that microglia can accumulate rapidly in response to neuronal injuries and promote neuronal proliferation around the traumatic brain injury (TBI) in zebrafish larvae. Li et al 5 found that increased concentration of macrophages can improve neurological function in the brain injury of zebrafish larvae. More importantly, Ohnmacht et al 6 demonstrated that the motor neuron regeneration of zebrafish at larval stage is similar to that of zebrafish at the adult stage as a result of the plasticity of larval progenitor/stem cells. Studies have already shown the contribution of this well-established genetic model fish to our understanding of the stem and progenitor cells involved in brain regeneration7,8. Consequently, the zebrafish has become the preferred vertebrate model for the in vivo study of neuronal regeneration of brain.
The her4.1-positive ventricular radial glia progenitor cells are involved in neuronal regeneration in the stab lesion of zebrafish brain 9 . In addition to radial glia cells (RGs), nonglia progenitors are also involved in nerve repair after external brain injury. Zebrafish brain injury occurs both externally and internally. Upon external brain injury, neuronal progenitors are recruited to the injuried site, resulting in generating additional neurons during regeneration10,11. This report suggests that neuronal progenitors and her4.1-positive ventricular radial glia progenitor cells are involved in neuronal regeneration after external brain injury. Most regenerative cells are derived from the regions of constitutive neurogenesis12,13. Alzheimer’s disease is an example of internal brain injury. The Alzheimer’s disease zebrafish model revealed that amyloid toxicity, which causes symptoms of Alzheimer’s disease in fish brain, is similar to that in human brain 14 . In the adult zebrafish, neural stem cells proliferate, form new neurons, and integrate into the remaining circuitry 15 . Thus, a group of neural stem cells and glia cells are induced in the damaged brain to promote the repair and regeneration of internal brain injury.
A zebrafish transgenic line huORFZ harbors a DNA construct in which the upstream open reading frame (uORF) sequence from human CCAAT/enhancer-binding protein homologous protein gene (chop; huORFchop) was fused with GFP reporter at downstream (huORFchop–gfp) driven by a cytomegalovirus promoter 16 . The translation of transgenic huORFchop–gfp mRNA is mediated by the huORFchop motif. At the normal condition, the translation of downstream GFP is completely repressed by the inhibitory uORF motif. However, at the stress condition, increased Endouc ribonuclease disrupts the inhibitory structure of huORFchop motif in a manner that allows the translation of downstream GFP reporter 17 . In this study, when huORFZ embryos were exposed to hypoxic stress, we found that some cell subtypes in the CNS could sense and respond to this stress such that the downstream GFP was, in turn, only apparent in these specific subtype cell populations.
When huORFZ embryos were exposed to hypoxic stress, Zeng et al 18 previously reported that a population of GFP-expressing cells, named as hypoxia-responsive recovering cells (HrRCs), was also found in the spinal cord. However, the biological characteristics of HrRCs found in the spinal cord may not be similar to those found in the brain. Furthermore, the functional role of HrRCs induced by hypoxic stress in the brain remains to be fully characterized. In this study, we determined that neural stem/progenitor cells (NSPCs) and RGs comprise the major cell type among HrRCs present in the brain after CNS injury. It was also determined that the NSPCs-HrRCs and RGs-HrRCs subtypes play a major role in neuronal regeneration in the zebrafish brain after hypoxic stress.
Materials and Methods
Ethics Statement
The animal protocol, which was strictly followed in this study, was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), National Taiwan University, Taiwan, with approval number NTU-102-EL-19.
Zebrafish
Zebrafish (Dario rerio) wild-type (WT) AB strain and transgenic line huORFZ 16 were maintained in 28.5°C water and exposed to a 14/10-h light/dark cycle. Embryo medium (EM) was replaced by fresh medium containing 0.003% 1-phenyl-2-thiourea at 24 hpf to reduce pigmentation.
Hypoxic Exposure of Zebrafish Embryos From Transgenic Line huORFZ
A hypoxic environment was established in the medium used to incubate zebrafish embryos. Following the method previously described by Zeng et al 18 , an influx of nitrogen gas was forced into embryonic medium to generate the final O2/N2 ratio that reached to 5% O2 and 95% N2. The same strategy of this approach was also reported by Khaliullina-Skultety et al. Under the specific 5% O2 hypoxic stress, we studied the response of zebrafish brain 19 . Briefly, 80 ml of EM at pH 5.8 was placed in a 100-ml serum bottle capped with a rubber stopper having a glass tube and kept at 28°C for 10 min, followed by pumping 99% nitrogen for 5 min to make deoxygenated EM. Plastic wrapping was affixed to prevent water spillage. When 100 zebrafish embryos had developed at 72 hpf, they were transferred to a 3-cm Petri dish filled with freshly deoxygenated EM, sealed tightly with three layers of paraffin, and kept at 28°C for 2.5 h. After that, the hypoxia-exposed larvae were collected, along with replacement of fresh EM, and kept at 28°C for the following experiments.
Immunostaining of Embryos
The immunostaining pattern of embryos was observed under a Zeiss LSM 780 confocal microscope. Primary antibodies were prepared from mouse, including anti-Gfap (RG marker) at 1:200 (Millipore), anti-HuC/HuD (early neuron marker) at 1:500 (Invitrogen), and rabbit polyclonal primary antibody against Phospho-Histone H3 Ser10 (Millipore) at 1:200 dilution. Secondary antibodies were goat anti-rabbit or anti-mouse Cy3-conjugated fluorescence at 1:500 (Millipore). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) at 1:1000 (Sigma).
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
Whole-mount TUNEL staining in whole zebrafish embryos (Roche) was used following the manufacturer’s instructions. Briefly, zebrafish larvae were fixed in 4% paraformaldehyde at 4°C overnight and then rinsed twice with PBS. Endogenous peroxidases were blocked by incubation in 3% hydrogen peroxide in methanol for 15 min at room temperature. After washing with Phosphate-buffered saline (PBS), the treated larvae were incubated with 90-µl labeling solution plus 10-µl enzyme solution at 37°C for 2 h. After washing with Phosphate-buffered saline with 0.1% Tween 20 detergent (PBST) for 5 min, images were examined by confocal microscopy.
Labeling and Tracing of 5-Bromo-2′-Deoxyuridine (BrdU)
We followed the method described previously by Huang et al 20 . The larvae were maintained for 12 h in aquarium water containing 10-mM BrdU (Sigma-Aldrich). Immunoblot analysis we used was monoclonal mouse anti-BrdU antibody (Abcam) at a dilution of 1:400 in blocking solution and Cy3-conjugated anti-mouse secondary antibody (Invitrogen).
Confocal Microscopy and Image Processing
Fluorescence signals were captured by a Zeiss LSM 780 confocal microscope. Images were analyzed using ZEISS ZEN imaging software.
Whole-Mount In Situ Hybridization (WISH)
The procedures were described by Zeng et al 21 . Images were captured using a light stereomicroscope with CCD camera (MZ FLIII, Leica).
Dissociation of Embryos and Immunostaining of Embryonic Cells
The procedures for dissociation were described by Lee et al 16 . After dissociation of embryonic cells, the suspended cells were fixed with 2.5% paraformaldehyde for 1 h, followed by incubation with 0.2% Triton X-100 for 30 min, and then treatment with blocking buffer (5% bovine serum albumin) for another 1 h. Primary antibodies were rabbit anti-Sox2 at 1:200, mouse anti-HuC/D at 1:500, and DAPI at 1:800.
Fluorescence-Activated Cell Sorting
A FACSAria cell sorting system (BD Biosciences, FACSVerse™) was used to perform fluorescence-activated cell sorting (FACS) to sort single cells under sterilized condition according to the protocols described by Zeng et al 18 and Vitak et al 22 . Briefly, after embryonic tissue dissociation and cell fixation, we performed immunostaining via specific antibody against either Sox2 or GFAP, using red fluorescent signal. Red- and GFAP-expressing cells were sorted from the cell suspension by FACS and presented by several gates: (1) total analytic cells gated at P1; (2) GFP-expressing GFAP cells gated at P2; (3) red-labeled Sox2 cells gated at P3; and (4) cells with red signal overlapping cells with GFP signal gated at P4 and labeled in blue. Therefore, the percentage of NSPCs or RGs among HrRCs was calculated by cell number in P4 among 3 × 105 examined cells.
Western Blot Analysis
Total proteins extracted from embryos were analyzed on a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis according to the procedures described by Zeng et al 18 , except that the antibodies against zebrafish Chop and α-tubulin (Sigma) with a dilution of 1:200 and 1:1000, respectively, were used.
Results
The Increased Levels of Chop mRNA and Its Encoded Protein Chop Corresponded to the Number of GFP-Expressing Cells Present in the Brain of Hypoxia-Exposed huORFZ Embryos During Recovery
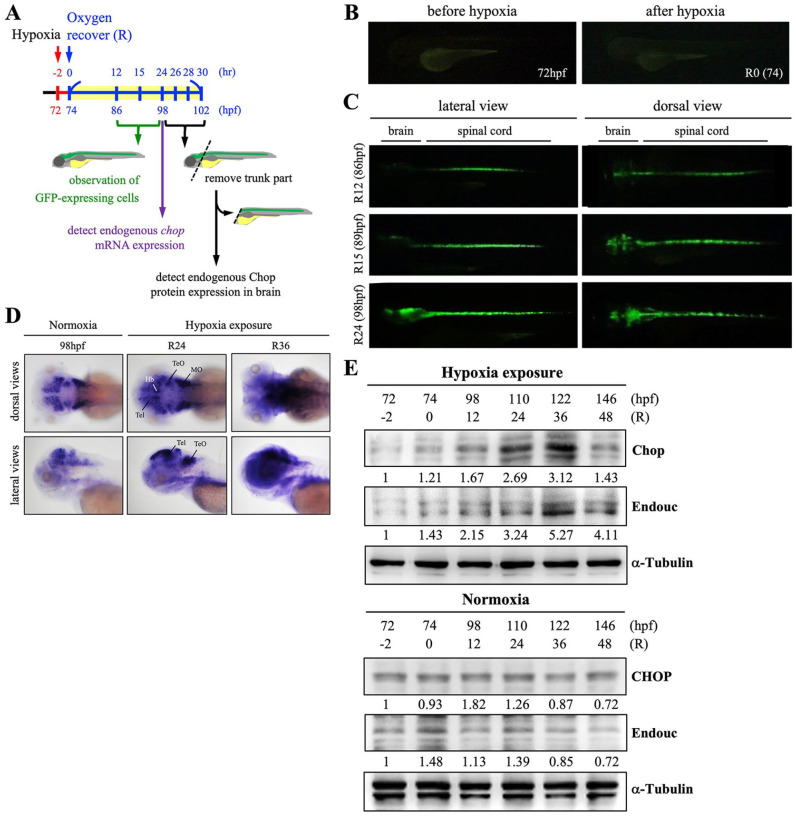
The huORFZ embryos were exposed to hypoxia for 2 h, but after recovery for 12 h (R12) (Fig. 1A), GFP-expressing cells were observed in the spinal cord of these same hypoxia-exposed huORFZ embryos. These results were consistent with those described by Zeng et al 18 who named these cells as HrRCs. We found that HrRCs also appeared in the brain of hypoxia-exposed huORFZ embryos at R15 and reached maximal level at R24 (Fig. 1C). In contrast, since GFP was not expressed in the mock control groups, no signal appeared in the images, resulting in a black background under fluorescence microscopy (Fig. 1B). These data indicated that hypoxic stress could induce huORFZ embryos to generate a specific cell subtype population of HrRCs in the CNS.
Figure 1.
Expression of exogenous GFP and endogenous chop transcripts and the appearance of HrRCs in the brain of hypoxia-exposed huORFZ embryos. (A) Schematic illustration of experimental workflow. Zebrafish embryos from transgenic line huORFZ developed at 72 hpf, 2 h prior to oxygen recovery (R-2), were exposed to hypoxia for 2 h, followed by the start of oxygen recovery (R0, 74 hpf). (B) No GFP signal was presented in the huORFZ embryos served as mock control groups at 72 hpf and R0 (74 hpf). (C) The GFP signal started to appear in embryos undergoing oxygen recovery for 12 h (R12), while GFP was observed in the brain at R15. (D) Dorsal and lateral views of the temporospatial expression of chop transcript in the nonhypoxia-exposure huORFZ (normoxia; positive control group) embryos at 98 hpf (equivalent to R24) and hypoxia-exposure huORFZ embryos at R24 (equivalent to 98 hpf) and R36 (equivalent to 110 hpf) using whole-mount in situ hybridization (WISH). The chop mRNA was mainly expressed in the telencephalon (Tel), habenula (Hb), optic tectum (TeO), ventricular zone (VZ), and medulla oblongata (MO). Western blot analyses of (E) CHOP and Endouc proteins presented at various stages of normoxia- and hypoxia-exposure huORFZ embryos. Total proteins were extracted from the heads of embryos at the start of recovery (74 hpf; R0) through 146 hpf (R72), as indicated. The control group consisted of untreated huORFZ embryos (normoxia) after extraction of total proteins from the head at the corresponding hpf of treated embryos. α-Tubulin served as a loading control. HrRCs: hypoxia-responsive recovering cells; hpf: hours postfertilization.
Furthermore, we employed WISH to detect the amount of endogenous chop mRNA expression levels in nonhypoxia-exposure huORFZ embryos at 98 hpf (corresponding to R24 stage of hypoxia-exposure huORFZ embryos) and in the hypoxia-exposure huORFZ embryos at R36 (corresponding to nonhypoxia-exposure huORFZ embryos at 108 hpf). The result showed a very low level of endogenous chop mRNA expressed in the nonhypoxia-exposure huORFZ embryos at 98 hpf compared with hypoxia-exposure huORFZ embryos at R24. The chop mRNA was sensitively translated in the telencephalon (Tel), tectum opticum (TeO), and medulla oblongata (MO) at R24 and highly expressed in the Tel and ventricular zone (VZ) at R36 (Fig. 1D). Since the VZ functions as a neural precursor to generate neurons in the telencephalon of zebrafish10,23, we speculate that HrRCs may possess neuronal regeneration capability after hypoxic stress.
Since HrRCs were present in the brain of hypoxia-exposed huORFZ, we further determined any correspondingly increased expression level of endogenous Chop protein in the huORFZ brain. To accomplish this, brains of embryos were collected at R-2, R0, R24 R26, R28, and R30, corresponding to 72, 74, 96, 98, 100, and 102 hpf in the embryonic development of nonhypoxia-exposed embryos, respectively, and detected the expression level of endogenous Chop protein. Results showed that the expression level of Chop protein was expressed at low level in head of normoxic WT embryos. However, Chop expression was greatly increased in the head of hypoxia-exposure WT embryos starting at R24 and decreasing at R48 (Fig. 1E). Since the Chop level in normoxic WT embryos was not different between 74-hpf-embryos and 98–146-hpf-embryos, suggesting that the increase of Chop in hypoxia-exposure embryos was positively dependent on the duration of oxygen recovery (OR) until R48 (Fig. 1E). Taken together, we conclude that the zebrafish brain responded hypoxia stress at R24, starting actively regenerative processes and then going back to normal state at R48.
In addition to the dynamic change of Chop protein, we also examined Endouc protein which was a novel poly(U)-specific endoribonuclease to induce chop mRNA translation 16 . To confirm that this endogenous stress-related protein could be induced by hypoxia, we performed the Western blot experiment to detect the protein level of Endouc in zebrafish brain. The result demonstrated that Endouc protein was expressed at a very low level in the head of untreated WT embryos, while Endouc was starting to greatly increase at R24 but decreasing at R48 in the head of hypoxia-exposure WT embryos (Fig. 1D). This result was consistent with the dynamic change of Chop protein, suggesting that again the GFP expression observed in huORFZ embryos corresponds to stress-related proteins expressed in WT embryos during recovery time after hypoxia.
Based on these results, we conclude that (1) the amount of HrRCs present in the brain of hypoxia-exposed huORFZ embryos corresponds with the increase of endogenous chop transcript and CHOP protein levels in the zebrafish brain and (2) the increased level of CHOP protein expressed in the brain was positively dependent on the duration of OR time up to R36, but returned to normal state at R48.
HrRCs in the Brain of Hypoxia-Exposed Embryos Did Not Undergo Apoptosis During Recovery
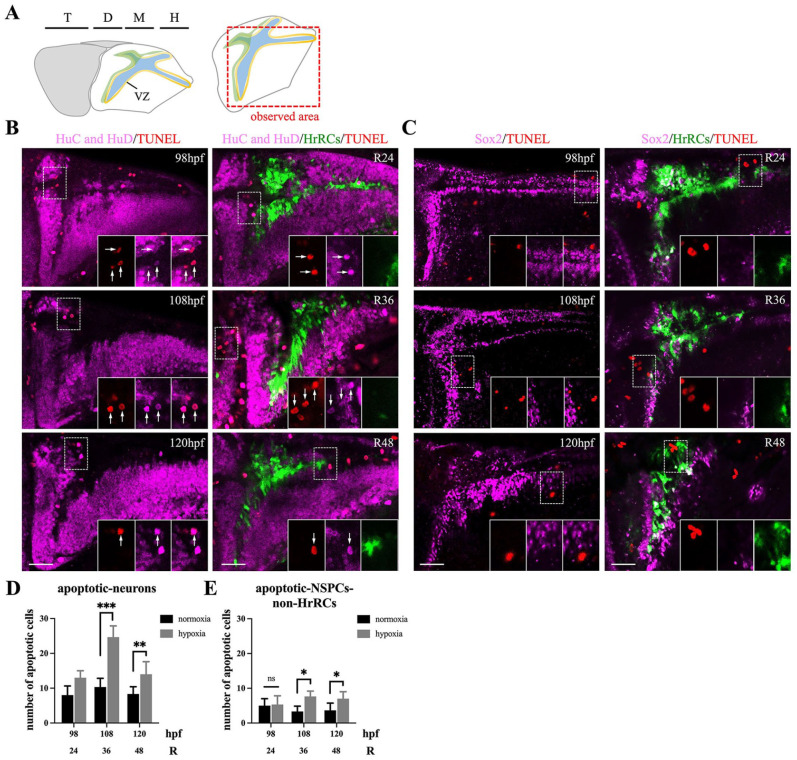
We next asked if HrRCs in the brain of hypoxia-exposed huORFZ embryos would undergo apoptotic cell death. To address this, we employed TUNEL to label apoptotic cells shown on the VZ of brain with red fluorescence, followed by whole-mount immunofluorescence staining (Fig. 2A), after exposure to hypoxia for 2 h. No NSPCs-HrRCs (quiescent proregenerative NSPCs belonging to group of HrRCs population in the brain of zebrafish embryos exposed to hypoxia; Table 1) underwent apoptosis at R24, R36, and R48, but only a few NSPCs-non-HrRCs (NSPCs that are insensitive to hypoxia and remain inactive without responding to hypoxia; Table 1) did undergo apoptosis. TUNEL-red signal was present in HuC/HuD-marked early neurons in the brain of huORFZ embryos after exposure to hypoxia. The number of early neurons, as indicated by the TUNEL-red signal, increased from R24 to R36, but decreased at R48 (Figs. 2B, D), suggesting that most early neurons underwent apoptosis after hypoxic stress. Next, we determined whether Sox2-marked NSPCs in the brain of huORFZ embryos underwent apoptosis after hypoxia exposure. While no NSPCs-HrRCs underwent apoptosis at R24, R36, and R48, a few NSPCs-non-HrRCs did undergo apoptosis (Fig. 2C, E). These results indicated that the NSPCs in zebrafish brain can be characterized by the heterogeneity of subtypes, accordingly, presenting different cell fate.
Figure 2.
HrRCs in the brain of hypoxia-exposed huORFZ embryos did not undergo apoptosis during oxygen recovery. TUNEL staining as a quantitative assay was performed to determine the hypoxia-induced zebrafish embryos apoptosis. (A) Schematic representation of the embryonic brain (72 hpf), showing the forebrain (in yellow), midbrain (m), and hindbrain (h). Forebrain is subdivided in the telencephalon (t) and the diencephalon (d). The red dotted line box indicated the observation area. Apoptotic cells were marked by red after TUNEL staining with (B) early neuron marker, HuC/HuD, and (C) NSPC marker, Sox2, were observed under a fluorescent confocal microscopy in the normoxic huORFZ embryos at 98, 108, and 120 hpf (equivalent to R24, R36, and R48, respectively) and hypoxic huORFZ embryos at R24, R36, and R48 (equivalent to 98, 108, and 120 hpf, respectively). Each figure in the lower right three panels was amplified from the area indicated by the dotted line box. Arrow indicated that the cells expressed red-colored TUNEL signals. Quantitative analyses of (D) apoptotic early neurons (HuC/HuD marker) and (E) apoptotic NSPCs-non-HrRCs (Sox2 marker) by TUNEL assay. Black and gray bars indicated the normoxic and hypoxic huORFZ embryos, respectively. Data were averaged from five examined embryos at the same position of brain. The unpaired t test showed the significant difference of apoptotic cells between two studied groups (Statistical analysis was based on t test at **P < 0.05, ***P < 0.001 significance). Error bar indicates SEM. The scale bar is 40 μm. HrRCs: hypoxia-responsive recovering cells; TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling; hpf: hours postfertilization; NSPCs: neural stem/progenitor cells; VZ: ventricular zone; SEM: standard error of the mean; HuC and HuD: Neural Hu proteins.
Table 1.
Summary of the Markers Expressed in the Different HrRC Subpopulations.
| HrRC subpopulations | Full name | Markers | Expressed signals |
|---|---|---|---|
| NSPCs-HrRCs | Neural stem/progenitor cells of HrRCs-subtype | Sox2, GFP | Sox2-red and GFP colocalization |
| RGs-HrRCs | Radial glia cells of HrRCs-subtype | Gfap, GFP | Gfap-red and GFP colocalized signals |
| NSPCs/RGs-HrRCs | Neural stem/progenitor and radial glia cells of HrRCs-subtype | Sox2, Gfap, GFP | Gfap-red, Sox2-pink, and GFP triple colocalized signals |
| NSPCs-non-HrRCs | Neural stem/progenitor cells of non-HrRCs-subtype | Sox2 | Sox2-red signal only (no GFP signal) |
| RGs-non-HrRCs | Radial glia cells of non-HrRCs-subtype | Gfap | Gfap-red signal only |
| Proliferated HrRCs-neurons | Proliferated HrRCs directly differentiated into neurons | BrdU(+), HuC/HuD, GFP | BrdU-red, HuC/HuD-pink, and GFP triple colocalized signals |
HrRCs: hypoxia-responsive recovering cells; NSPCs: neural stem/progenitor cells; RGs: radial glia cells.
Since increased CHOP protein promotes the survival of neuronal cells against hypoxia stress-induced death 24 and increased Endouc/ENDOU poly(U)-endoribonuclease disrupts the inhibitory structure of the huORFchop motif in a manner that allows the translation of downstream chop mRNA 17 , we speculate that the biological characteristics which make HrRCs different from other subtype cells could be attributed to the ability of HrRCs to sensitively increase Endouc/ENDOU at the stress condition, resulting in the increased resistance of CHOP to apoptosis.
The chop is only minimally expressed under normal conditions, whereas under stress, chop is substantially induced, accumulating in the nucleus to suppress transcription of the Bcl-2 gene, finally inducing cellular apoptosis25,26. Maytin et al 27 also demonstrated that overexpression of CHOP causes cell cycle arrest and/or apoptosis. On the other hand, Halterman et al 24 reported on administering hypoxic stress to mouse primary neuronal cells and then removing it. Under these conditions, CHOP combined with a brain-derived neurotrophic factor to form a complex. This caused a reduction in the cleavage of PARP activities and, finally, promoted neuronal cell survival. This line of evidence suggests that CHOP can promote cell survival at the initial stage of stress. It is only when CHOP is highly accumulated in cells, such as induction by excessive and prolonged stress, that cell apoptosis occurs.
In this study, the GFP signal shown on zebrafish transgenic line huORFZ served as a reference marker for the translation of endogenous chop mRNA. It has been reported that the increase of CHOP is time-dependent in hypoxic cells 28 . Indeed, our in vivo study showed that the amount of CHOP protein in embryos increased from R12 to R36, consistent with the report published by Dong et al 28 . This evidence supports our hypothesis that increased CHOP protein could promote the survival of neural cells during recovery, after removal of stress, precisely at the R36 time point, which, therefore, appears to be critical for the increased resistance of CHOP to apoptosis. Therefore, brain HrRCs represent specific hypoxia-activated cells able to promote regenerative neurogenesis instead of merely stochastic activation and a bias in the detection and analysis. This line of evidence suggested that HrRCs did not undergo apoptosis during recovery time at R24, while most non-HrRCs did.
HrRCs in the Brain of Hypoxia-Exposed Embryos Were Not Neurons
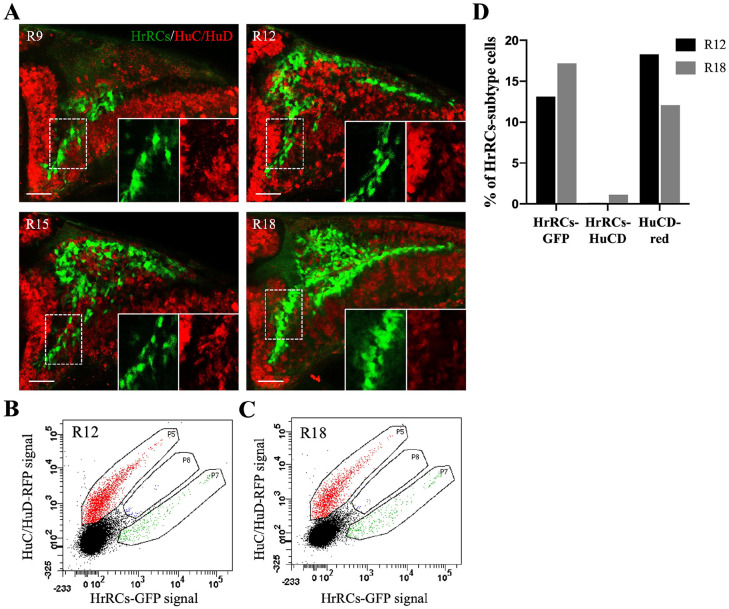
To further elucidate whether HrRCs in the brain are neurons, we used immunofluorescence analysis of the HuC/HuD marker shown on the brain. HrRCs in the brain were not colocalized with the HuC/HuD marker at R9, R12, R15, and R18 (Fig. 3; Supplemental Fig. S1; movies 1 and 2). In addition, we used FACS analysis to determine the percentages of HrRCs colocalized with the HuC/HuD marker at R12 and R18. In addition, we used FACS analysis to determine the percentages of HrRCs colocalized with the HuC/HuD marker at R12 and R18. The results demonstrated that they were 0.1% and 0.03% at R12 and R18 (Fig. 3B–D), respectively, suggesting that the number of HuC/HuD-expressing HrRCs was extremely low at R12 and R18. We also noticed that the percentages of HuC/HuD cells were decreased from R12 to R18 (Fig. 3B–D), suggesting that neurons underwent apoptosis in hypoxia-exposure huORFZ embryos. These results were consistent with the TUNEL assay which showed that neurons could undergo apoptosis, resulting in the decreased number of HuC/HuD cells from R12 to R18 (Fig. 2).
Figure 3.
HrRCs in the brain did not express the early neuronal marker HuC/HuD. The images of fluorescence confocal microscopy were obtained from the hypoxia-exposure huORFZ embryos during recovery at different stages. (A) GFP-expressing HrRCs were observed under fluorescence microscopy at R9, R12, R15, and R18. The lower right two panels were amplified from the area indicated by the white dotted line box. (B–C) Data displayed the percentage of red-labeled HuC/HuD neurons overlapped with GFP-expressing HrRCs (at P8 gate) at R12 and R18 obtained through FACS sorting and immunostaining. (D) Calculation of the percentages of three different subtype cell populations isolated by FACS at R12 and R18. The scale bar is 20 µm. HrRCs: hypoxia-responsive recovering cells; FACS: fluorescence-activated cell sorting.
Subtypes of NSPCs and RGs Are Major Constituents Among HrRCs in the Brain of Hypoxia-Exposed Embryos
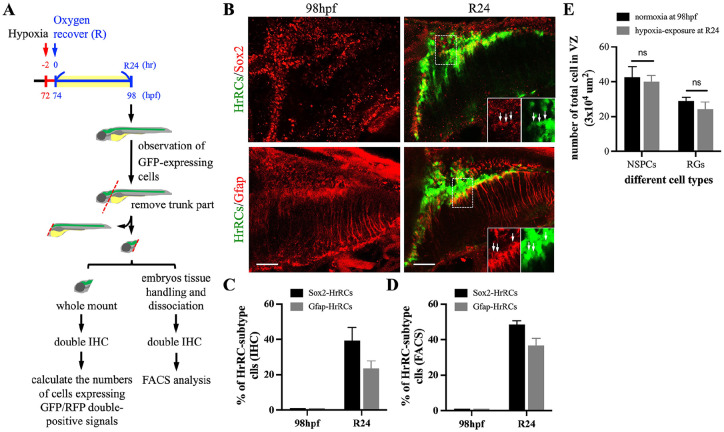
We carried out immunofluorescence staining using antibody against sex determining region Y (SRY)-box 2 (Sox2) to determine whether NSPCs comprise a cell type within HrRCs in the brain of hypoxia-exposed zebrafish embryos (Fig. 4A). We found that no HrRCs appeared in the normoxia group (Fig. 4B, left panels and Fig. 4C). However, the percentages of NSPCs-HrRCs, RGs-HrRCs, and NSPCs/RGs-HrRCs (Table 1) were 39.33 ± 7.5, 23.67 ± 4.16, and 6.33 ± 3.51%, respectively (Fig. 4B, right panels and Fig. 4C; Supplemental Fig. S2), suggesting that the major subtype cells of HrRCs were those expressing Sox2 and Gfap, but with a minor percentage of Sox2/Gfap coexpressing HrRCs at R24. Taken together, we conclude that Sox2- and Gfap-expressing cells overlapped with a large portion of GFP-expressing HrRCs, suggesting that NSPCs and RGs consist of the major subtype cells among HrRCs at R24.
Figure 4.
The HrRC population mostly comprised neural stem/progenitor cells and radial glia cells. (A) Schematic illustration of experimental workflow. Zebrafish embryos from transgenic line huORFZ developed at 72 hpf, 2 h prior to oxygen recovery (R-2), were exposed to hypoxia for 2 h, followed by the start of oxygen recovery (R0, 74 hpf). The heads of huORFZ embryos at R24 were collected. Immunostaining for FACS sorting was performed and the number of specific cell types calculated. To distinguish NSPCs among all HrRCs, antiserum against SRY (sex determining region Y)-box 2 (Sox2) labeled with red fluorescent signal was used. Subtype markers of (B) RGs (Gfap, red) and NSPCs (Sox2, red) that colocalized with HrRCs (green) were indicated by yellow signals. The lower right three panels were amplified from the area indicated by the box. Arrows indicated that GFP-expressing HrRCs were coexpressed with either RG (Gfap, pink) or NSPC (Sox2, pink) signal. (C) The ratio of HrRCs with either Sox2- or Gfap-expressing in the normoxic huORFZ embryos at 98 hpf and hypoxia-exposure huORFZ embryos at R24 were counted. The diagram exhibited the percentages of NSPC and RG subtypes displaying yellow color among total GFP-expressing HrRCs. Data were averaged from counting five embryos at the same position of brain. (D) Calculating the ratio of two cell types among GFP-expressing cell populations isolated by FACS. Error bars indicate SEM. The scale bar is 20 µm. (E) Calculating the number of NSPCs and RGs among total examined cells in the VZ (area was 3 × 104 μm2). HrRCs: hypoxia-responsive recovering cells; hpf: hours postfertilization; FACS: fluorescence-activated cell sorting; NSPCs: neural stem/progenitor cells; RGs: radial glia cells; VZ: ventricular zone; n.s.: no significant difference; SEM: standard error of the mean.
We further defined the proportion of NSPCs-HrRCs among the entire cell population of HrRCs in the brain of hypoxia-exposed embryos using FACS combined with cell immunofluorescence staining (Supplemental Fig. S3). According to our results, NSPCs-HrRCs and RGs-HrRCs accounted for 48.3 ± 2.5% and 36.3 ± 4.7% (Fig. 4D) of HrRCs induced in the brain. After counting the number of Sox2(+) and Gfap(+) cells in normoxia group and hypoxia group, we found that there was no significant difference between normoxia and hypoxia groups (Fig. 4E). The line of evidence suggested that the quiescent pro-regenerative subtypes of NSPCs and RGs cells existed in the brain. However, in case of hypoxic stress, these subtypes of NSPCs and RGs, such as NSPCs-HrRCs and RGs-HrRCs, respectively, became the proregenerative subtypes which were responsible for neuronal regeneration. Taken together, (1) only around one third of NSPCs are NSPCs-HrRCs which are able to be quickly induced by and sensitively responds to hypoxia exposure; (2) two thirds of NSPCs are quiescent NSPCs-non-HrRCs that do not sensitively respond to hypoxic stress; (3) the subtypes of NSPCs-HrRCs and RGs-HrRCs are the major constituents of HrRCs in the zebrafish hypoxia-brain, suggesting that they play a major role during neuronal regeneration after stress; and (4) the quiescent pro-regenerative subtypes of NSPCs and RGs are original existence in the brain, whereas these quiescent subtypes could be induced by hypoxic stress to become active subtypes of NSPCs- and RGs-HrRCs, respectively.
HrRCs Are Able to Proliferate In Vivo
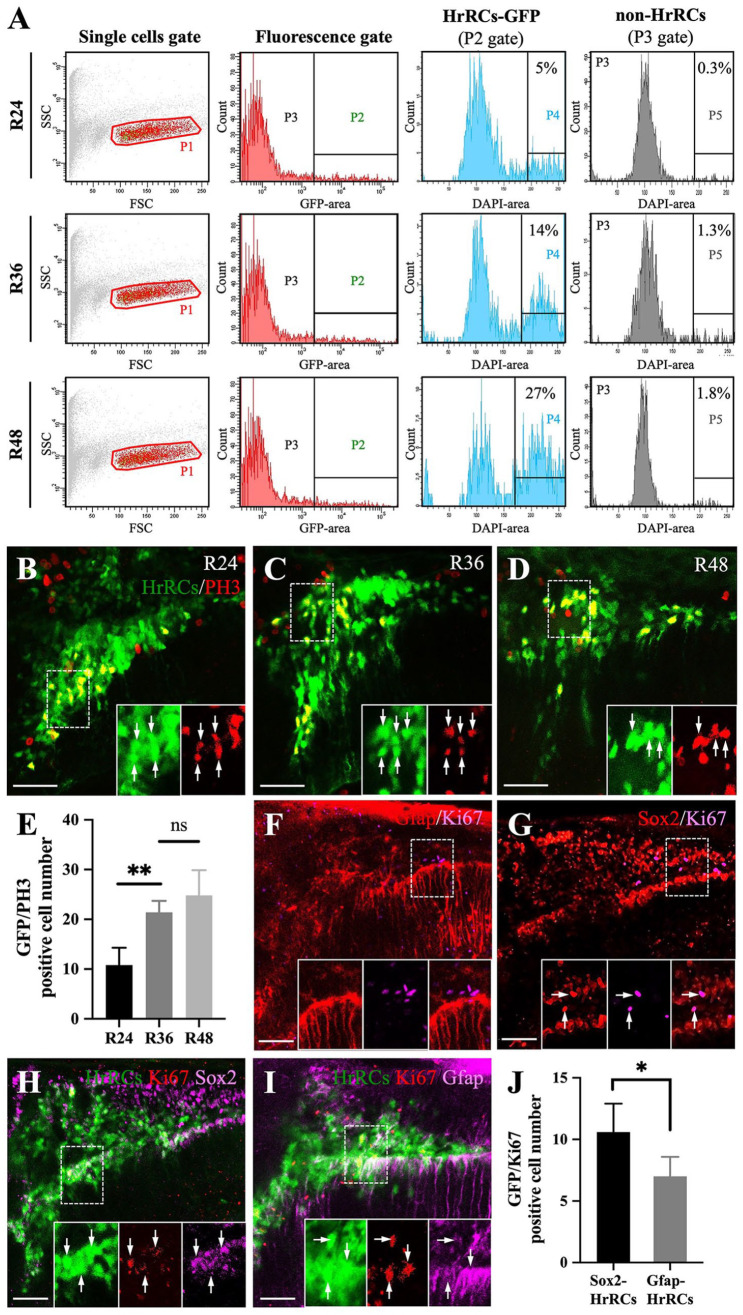
We next examined the ability of HrRCs in the brain of hypoxia-exposed embryos to proliferate during the recovery stage. Here, we employed FACS combined with immunofluorescence staining. First, a fluorescence gate was set, and signal cells (P1) collected by FACS were divided into two populations: green fluorescence cells selected in the P2 gate (HrRCs-GFP) and non-green fluorescence cells selected in the P3 gate (non-HrRCs; Table 1). Subsequently, a DAPI-area histogram (calculated as G2 m) was used for cell cycle analysis (P4 and P5 gates). These results showed that the proportions of HrRCs-GFP G2 m cells at R24, R36, and R48 were 5, 14, and 27%, respectively (Fig. 5A) In contrast, the proportion of non-HrRCs G2 m cells at R24, R36, and R48 were 0.3, 1.3, and 1.8%, respectively (Fig. 5A). These results suggested that GFP-expressing HrRCs induced by hypoxic stress could proliferate, while non-HrRCs could not. Furthermore, we used immunofluorescence staining to detect the mitosis marker phospho-Histone 3 (PH3). The expression of exogenous GFP was mainly apparent in the VZ of brain and entire spinal cord at R24 (dorsal view of Fig. 1B, R24), while endogenous chop mRNA was mainly expressed in the telencephalon, habenula, optic tectum, and medulla oblongata, but expressed to a lesser degree in the VZ at R24 (Fig. 1C). Therefore, we focused on the specific brain area in the VZ. Most GFP-positive HrRCs could express a PH3 signal at R24, R36, and R48 (Fig. 5B–D). Importantly, the number of PH3-expressing HrRCs and the percentages were 10.80 ± 3.49%, 21.40 ± 4.16%, and 24.80 ± 5.07% at R24, R36, and R48, respectively (Fig. 5E), suggesting that GFP-positive HrRCs are major cells undergoing proliferation and that the increased number of proliferated cells was positively dependent on the duration of OR time after hypoxia. On the other hand, we also observed that a small number of non-HrRCs could express a PH3 signal in the VZ at R24, but these PH3-expressing non-HrRCs dramatically lessened in number at R48 (Fig. 5B–D), suggesting that the non-GFP-positive stem cells contributed least among proliferation subtypes. Overall, we conclude that (1) the in vitro FACS data were consistent with the in vivo data and (2) the number of proliferating HrRCs at R36 and R48 was higher than the number of proliferating HrRCs at R24.
Figure 5.
Using an in vivo system to demonstrate that GFP-expressing HrRCs could proliferate. The heads of hypoxia-exposed huORFZ embryos during recovery time, as indicated, were collected, subjected to cell suspension, and then FACS sorting combined with immunostaining. (A) Scatter profiles of brain cells obtained from treated huORFZ embryos. Single-cell fraction was gated (red gate; P1). Cells expressing GFP, such as GFP-expressing HrRCs, were located at P2 gate, while cells not expressing GFP, such as non-HrRCs, were located at P3 gate. After selecting P2 and P3 gates, single cells were gated based on the DAPI-area signal. Cell cycle analysis was calculated G2 m from a DAPI-area histogram shown on P4 and P5 gates which were calculated from P2 and P3 gates, respectively. The number shown in the right corner represents the percentage of the number of proliferated cells among the total examined cells. (B–D) Hypoxia-exposure huORFZ embryos were stained for M phase marker (PH3, red) at R24, R36, and R48. The lower right three panels were amplified from the area indicated by the box, and the arrows indicated that the HrRCs could express the PH3-red signal. (E) The average number of HrRCs, both GFP-positive HrRCs and PH3-positive cells per embryos (n = 5), was calculated and graphed with error bars (two-way ANOVA with multiple comparisons test, **P < 0.01; t test: **P < 0.01). Error bars indicate SEM. (F–G) Zebrafish embryos were stained for proliferation marker (Ki67, red) together with (F) NSPCs (Sox2, pink) and (G) RGs (Gfap, pink) in the normoxic huORFZ embryos at 98 hpf. The lower right three panels were amplified from the area indicated by the box, and the arrows indicated that HrRCs could coexpress Ki67-red, together with either NSPCs (Sox2, pink) or RGs (Gfap, pink) signal. (H–I) Zebrafish embryos were stained for markers of (H) Sox2 and (I) Gfap combined with proliferate marker (Ki67, red) in the hypoxic huORFZ embryos at R48. The lower right three panels were amplified from the area indicated by box, and the arrow indicated the NSPCs-HrRCs and RGs-HrRCs subtypes’ cell population could coexpress Ki67-red signal. (J) The number of NSPCs-HrRCs and RGs-HrRCs that were also PH3-positive in an embryo was calculated and graphed with error bars. Data were averaged from five embryos. The unpaired t test showed the different degree between proliferated-NSPCs-HrRCs and proliferated-RGs-HrRCs subtype cells (Statistical analysis was based on t test at *P < 0.05 significance). Error bars indicate SEM. The scale bar in (B–C) and (F–I) represents 20 and 15 µm, respectively. HrRCs: hypoxia-responsive recovering cells; FACS: fluorescence-activated cell sorting; DAPI: 4′,6-diamidino-2-phenylindole; NSPCs: neural stem/progenitor cells; RGs: radial glia cells; hpf: hours postfertilization; n.s.: not significant; SEM: standard error of the mean; ANOVA: analysis of variance.
To determine which subtype cells in the population of HrRCs could proliferate, we carried out a new triple immunostaining experiment using proliferation marker Ki67 (RFP signal, red color) and either neural stem cell marker Sox2 (Alexa 633 signal, pink color) or GFAP-marked glia cell (Alexa 633 signal, pink color). We have already shown the absence of GFP signal in embryos from transgenic line huORFZ at normoxia, but its presence in some subtypes of Sox2(+) and GFAP(+) cells after hypoxic stress (Fig. 1B). When we studied the dynamic change in cell number of GFP-expressing HrRCs after hypoxic stress, we found that (1) the number of Ki67-expressing NSPCs-HrRCs and RGs-HrRCs (Fig. 5H, I) was higher than that of control normoxic Ki67-expressing NSPCs and RGs, respectively (Fig. 5F, G), and (2) some HrRCs were colocalized according to the appearance of BrdU-red and HuC/HuD-pink signals. Thus, based on the number of NSPCs-HrRCs and RGs-HrRCs cell subtypes induced by hypoxic stress, we can infer that most of these cells will eventually undergo proliferation and differentiation into neurons. In addition, we also found that the cell number of Ki67-expressing NSPCs-HrRCs was higher than that of Ki67-expressing RGs-HrRCs (Fig. 5J), suggesting that NSPCs-HrRCs subtype cells might play more contributions on neurogenesis compared with RGs-HrRCs subtype cells. Taken together, we suggest that NSPCs-HrRCs and RGs-HrRCs subtypes could proliferate after hypoxia exposure, but that neither NSPCs-non-HrRCs nor most Gfap-non-HrRCs could. In summary, although only a few non-GFP cells could proliferate, it is unlikely that the GFP-expressing HrRCs cells would be positive for proliferation markers by chance as the entire stem cell population is activated.
Taken together, it was concluded that (1) the number of proliferative HrRCs was increased after exposure to hypoxia; (2) hypoxia-exposure huORFZ embryos normally undergo developmental proliferation up to R24, but actively undergo regenerative processes at R48 after hypoxic stress; (3) HrRCs were the major regenerative cells, given their ability to proliferate and participate in neurogenesis; and (4) the major proliferated HrRCs were subtypes of NSPCs-HrRCs and RGs-HrRCs.
HrRCs Are Also Able to Differentiate Into Neurons In Vivo
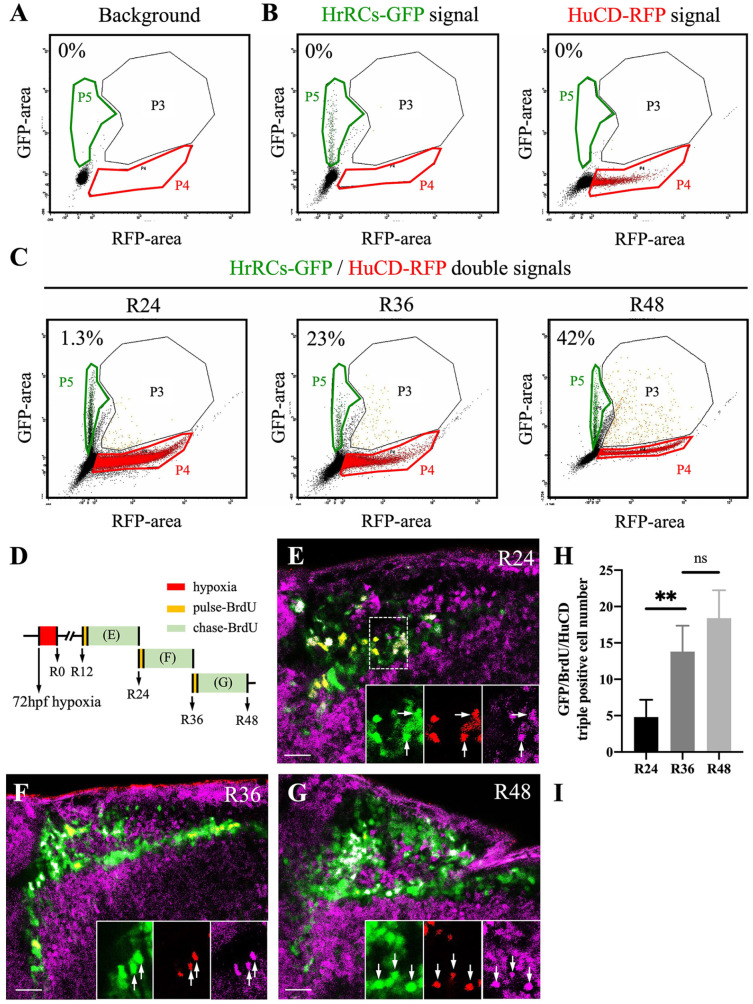
To examine whether proliferating HrRCs can undergo further differentiation into neurons, we again employed the combination of FACS and immunofluorescence staining. We dissociated the cells from the head part of the 96-hpf WT zebrafish embryos prior to employing FACS to define the background (Fig. 6A). Subsequently, we collected cells from the hypoxia-exposed huORFZ embryos which exhibited HrRCs-GFP signal and HuC/HuD-RFP signal after immunofluorescence staining. After cell dissociation, FACS was used to gate cells exhibiting HrRCs-GFP and HuC/HuD-RFP signals which were in the P5- and P4-gates, respectively, while cells that simultaneously expressed both GFP and RFP were in the P3-gate (Fig. 6B). Results showed that the proportion of cells displaying the colocalization of GFP and RFP signals at R24, R36, and R48 was 1.3%, 23%, and 42%, respectively (Fig. 6C, I). These data indicated that HrRCs can further develop and differentiate into neurons in the hypoxia-exposed huORFZ embryos during recovery time.
Figure 6.
HrRCs can differentiate into neurons in vivo. Brain samples were obtained from nontreated huORFZ and hypoxia-exposed huORFZ embryos during recovery time and then subjected to cell suspension, followed by flow cytometry and immunostaining with HuC/HuD antibody. (A) To serve as background control, a single-cell fraction was obtained after suspending the brain of nontreated embryos. (B) Cells expressing GFP signal were located at P5 gate (green color gate). After immunostaining, Sox2 cells labeled with red were located at P4 gate (red color gate). Cells coexpressing green and red signals were located at P3 gate (black color gate). (C) After suspending the brain cells of hypoxia-exposed huORFZ embryos during recovery at R24, R36, and R48, cells expressing green, red, and coexpressing green and red signals were located at P5, P4, and P3 gates, respectively. The number shown in the upper left corner of each panel represents the percentage of neurons differentiated from HrRCs among total examined HrRCs. (D) Experimental design to depict the pulse-chase BrdU of HrRCs to determine if proliferative HrRCs could directly differentiate into neurons. (E–G) Immunostaining patterns against BrdU for hypoxia-exposure huORFZ embryos. Pulse BrdU: huORFZ embryos were incubated with BrdU for 20 min. Chase BrdU: embryos were incubated in medium without BrdU for 12 h. Embryos were collected and observed at R24, R36, and R48. BrdU-labeled cells were the proliferated cells. (H) Calculating the percentages of GFP/BrdU-red/HuCD-pink overlapped signals among HrRCs at R24, R36, and R48 (two-way ANOVA with multiple comparisons test, **P < 0.01; t test: **P < 0.01), while there was no significant difference between R36 and R48 groups. Error bars indicated SEM. (I) Calculating the percentages of HuCD-expressing HrRCs at R24, R36, and R48 by FACS. The scale bar is 10 μm. HrRCs: hypoxia-responsive recovering cells; BrdU: 5-bromo-2′-deoxyuridine; FACS: fluorescence-activated cell sorting; n.s.: not significant; SEM: standard error of the mean; ANOVA: analysis of variance.
Next, to determine whether the proliferative HrRCs could be directly differentiated into neurons, we performed a new experiment in which we used a pulse-chase BrdU-labeled incorporation assay (Fig. 6D), followed by triple staining of BrdU, HuC/HuD, and HrRCs to determine whether proliferated HrRCs could directly differentiate into neurons (proliferated HrRCs-neurons) during recovery. Some GFP(+)-HrRCs overlapped with red-fluorescent BrdU. After embryos were continuously incubated in BrdU medium for 20 min and interrupted by non-BrdU treatment for another 12 h (R12–R24, R24–R36, and R36–R48), we found that BrdU-red and HuC/HuD-pink signals overlapped with some GFP-HrRCs, suggesting that proliferation could be taking place from R12 to R24, R24 to R36, and R36 to R48 (Fig. 6E–G). Moreover, the number of proliferated HrRCs-neurons at R24 to R36 and R36 to R48 was found to be significantly higher than the number of proliferated HrRCs-neurons at R12 to R24 (Fig. 6H). In addition, we found that HrRCs can differentiate into neuron at late neuronal regeneration, using flow cytometry analysis. We set up a new experiment to perform double immunostaining for GFP and HuC/HuD at R60. The result demonstrated that only a few HuC- and HuD-expressing HrRCs were still observed at R60 (Supplemental Fig. S3), suggesting that HrRCs could differentiate into early neurons until R60. Furthermore, we noticed that the total number of HrRCs was significantly decreased at R60 compared with that the total number of HrRCs at R24 since the hypoxic stress for embryos was no longer presented at R60, causing the microenvironment of the brain to return back to normoxia condition. Therefore, the subtypes of quiescent proregenerative NSPCs-HrRCs and RGs-HrRCs were back to normal types of NSPCs and RGs, respectively.
This conclusion was supported by the results obtained from in vivo PH3 immunostaining and in vitro FACS data. This line of evidence suggests that the proliferation and differentiation of HrRCs are products of regeneration, not normal development.
Discussion
A Subtype Cell Population That Sensitively Responds to Hypoxic Stress Can Be Identified in the Zebrafish Transgenic Line huORFZ
By using transgenic line huORFZ which harbors a huORFchop–gfp DNA construct controlled by human uORFchop cassette 16 , we revealed a unique HrRC population existing in the zebrafish brain. Zeng et al 18 reported that HrRCs also exist in the spinal cord of hypoxia-exposed zebrafish embryos, but that they comprise a different percentage of different subtype cells, that is, Sox(+)-NSPCs, 36.3 ± 1.0%; Gfap(+)-RGs, 26.1 ± 1.2%; OLG(+)-OLPs, 13.2 ± 0.6%; and O4(+)-OLs, 3.4 ± 0.6% of total HrRCs. The presence of HrRCs in other species is an open question. With the exception of HrRCs per se, scientists have reported a significant difference in the proliferation pattern was found in the brain’s ventricular cells of newts, leading the activation of neural stem cells putatively involved in regeneration after injury29–31. Similar to salamanders and teleost fish, the source of regenerated cells in Xenopus larvae lies within the VZ of the respective brain region 32 . In both the telencephalon and optic tectum of Xenopus larvae, injury induces the increased proliferation of Sox2-expressing neural progenitors, which, in turn, ultimately differentiate into N-β-tubulin-positive neurons 33 . This line of evidence suggests that neural stem cells, as well as Sox2-expressing neural progenitors, are involved in regeneration of brain after injury. Yet, it is still unknown whether all Sox2-expressing neural progenitors are totally induced to take part in regeneration after brain injury. In this study, we only found that some HrRC-specific subtype cells are sensitively induced by hypoxia to play a role in regeneration after brain injury. Thus, based on still sparse evidence, it could be speculated that a cell population like HrRCs might be found in other species, and it remains a question to be addressed in the future.
Hypoxia-Induced GFP Expression Pattern and Endogenous CHOP Protein Level Found in huORFZ During Neural Regeneration
Lee et al 34 demonstrated that GFP expression pattern shown in huORFZ embryos is tissue-specific response to various stresses. The intensity of GFP signal in huORFZ embryos is positively correlated with the strength or duration of stresses. In addition, we found that most HrRCs in brain, such as NSPCs-HrRCs and RGs-HrRCs, can survive, proliferate, and differentiate into neurons, while other non-HrRCs cannot. The line of evidence suggests that a particular cell population composed of several subtype of stress-responsive cells is able to sensitively respond to hypoxic stress. We employed transgenic huORFZ embryos to label proregenerative Sox2- and Gfap-HrRC subtype cells in brain that faithfully reflect hypoxic stress because all stress-responsive cells are able to quickly respond to hypoxic stress, increasing Endouc to abolish the inhibitory RNA structure formed by huORFchop transcript, resulting in GFP expression17,18. The C/EBP homologous protein (chop) gene is responsive to endoplasmic reticulum (ER) stress. Chop protein frequently serves as an apoptotic indicator 35 . Nonetheless, the increase of Chop translation after having blocked the translation inhibition mediated by the uORF of chop transcript could also facilitate the survival of stressed cells at the initial time of stress24,36 and could thus be considered as a cell survival marker 37 . This suggests that Chop is a bifunctional protein. We also found that Chop is permissive for the survival of HrRCs during OR following hypoxic stress. Using a zebrafish-specific Chop antibody to examine the expression of endogenous Chop protein, we found it to be higher at R24 than that in embryos before treatment (R-2), or at the beginning of OR (R0). Therefore, we suggest that Chop can facilitate the survival of HrRCs during OR after stress.
HrRCs Sensitively Respond to ER Stress and Play a Proregenerative Role in CNS Neurogenesis
Brain injury, as a consequence of hypoxic stress, would result in ER stress to brain cells. At the initial stage, the induction of unfolded protein response (UPR) combined with the phosphorylation of eukaryotic initiation factor α (eIF2α) causes a reduction in global protein synthesis 38 . Meanwhile, the gene encoding Chop is activated by inositol-requiring protein 1 and ATF6 transcription factor, resulting in extensive translation of chop mRNA 38 . Although chop translation is inhibited by the uORF at the 5′-region of chop mRNA (uORFchop) 35 under normal condition, UPR regulates gene expression of downstream genes, including chop, to overcome ER stress before apoptosis of affected cells under the stress condition 39 . Consequently, uORFchop-mediated translation inhibition of chop mRNA is suppressed, resulting the translation of Chop protein. Such increased Chop protein then promotes the survival of neuronal cells against hypoxia stress-induced death 24 . This confirms Chop as a reliable protein marking the presence of ER stress within cells. Lee et al 16 generated a zebrafish transgenic line huORFZ which harbors a human uORFchop (huORFchop) motif to inhibit the translation of downstream GFP reporter in the absence of stress. However, GFP is exclusively expressed in the CNS of huORFZ embryos encountering stresses. Specifically, when huORFZ embryos are exposed to hypoxia, GFP becomes apparent in a specific subtype cell population, termed HrRCs, in the spinal cord 18 . These HrRCs consist of multiple subtype cells that contribute to neuronal regeneration after hypoxia. Similarly, when the spinal cord of huORFZ embryos is mechanically injured [spinal cord injury (SCI)], GFP is only expressed in a specific subtype cell population, termed SCI stress-responsive regenerating cells (SrRCs), located at both sides of the lesion 40 . Interestingly, this GFP-expressing subtype cells, such as HrRCs and SrRCs, can survive and play a regenerative role in the postlesion (injury) microenvironment. In this study, we employed the same strategy to perform hypoxia stress to zebrafish embryos, but we focused on brain injury instead of SCI. We found that HrRCs are also observed in the brain. This GFP-expressing subtype cells not only sensitively respond to ER stress but also play a regenerative role in a manner similar to that of hypoxic stress that occurs in the spinal cord. Therefore, the transgenic zebrafish line huORFZ may also be an excellent model to study the characteristics of major cell subtypes participating in neuronal regeneration in brain after hypoxic stress.
We also noticed that the expression of exogenous GFP was mainly apparent in the VZ of brain and entire spinal cord at R24 (dorsal view of Fig. 1B, R24), while the endogenous chop mRNA was mainly expressed in the telencephalon, habenula, optic tectum, and medulla oblongata, but expressed to a lesser degree in the VZ at R24 (Fig. 1C). Interestingly, our results demonstrated that the GFP-negative cells (non-HrRCs), expressing a TUNEL-apoptotic signal, were in the regions of brain other than VZ where chop mRNA was highly translated (Fig. 2). Therefore, these GFP-negative (non-HrRCs) cells containing a high expression of chop mRNA do not sensitively respond to stress, resulting in the absence of Chop protein while undergoing apoptosis. In contrast, the cells containing a lesser degree of chop mRNA expression in the VZ survived and showed a GFP signal. This should provide the basis for understanding why the results, as observed in Fig. 1C, D, show that the GFP expression pattern did not perfectly correspond with the in situ pattern of endogenous chop mRNA found in huORFZ embryos at R24.
The Proregenerative HrRCs Play Role in Regenerative Neurogenesis, Which Is a Different Role From Playing in Normal Development
Hypoxia causes apoptosis of neurons and induces apoptotic response 41 . However, different from cells during normal development, HrRCs proliferate and differentiate into neurons during OR after hypoxic stress. More specifically, in the hypoxic condition in zebrafish brain, some cells cannot resist hypoxic stress, resulting in apoptosis (Fig. 2B), whereas a specific cell subtype, HrRCs (Fig. 2C), can resist hypoxic stress and undergo proliferation and differentiation into neurons (Fig. 7). To demonstrate the phenomenon, Zeng et al 18 reported a distinct cell population induced by hypoxia and involved in neuronal regeneration of zebrafish spinal cord after hypoxia. These quiescent proregenerative subtype cells that exist in the brain, namely hypoxia-induced NSPCs- and RGs-HrRCs, can proliferate and differentiate into functional neurons in the hypoxia-reoxygenation microenvironment. This conclusion is supported by Sawahata et al 42 who demonstrated that hypoxia-reoxygenation treatment of zebrafish larvae could induce neuronal dysfunction on one hand, but glial activation on the other hand.
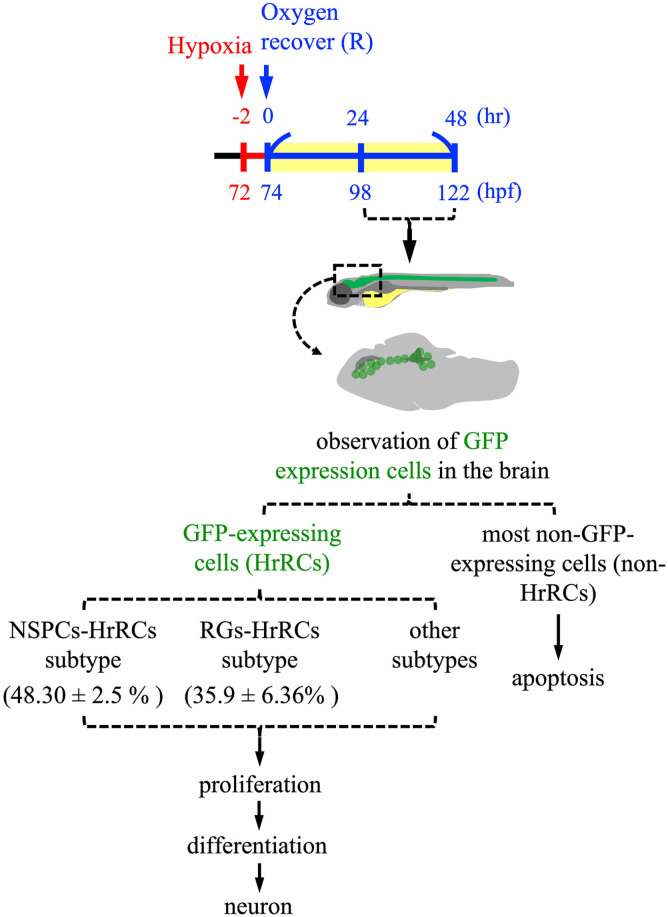
Figure 7.
Cell fate of NSPC subtype cells which are highly sensitive to hypoxia stress in the brain of hypoxia-exposed zebrafish embryos during oxygen recovery. The embryos developed at 72 hpf from zebrafish transgenic line huORFZ were exposure to hypoxia for 2 h, followed by recovery with oxygen for 9 h (R9) to R48. During oxygen recovery within 48 h, the GFP-expressing cells [GFP(+)], which are cells highly sensitive to hypoxia, termed as HrRCs, are found in the brain at R9. Brain HrRCs are mostly composed of NSPCs and RGs. While most negative GFP-expressing cells [GFP(−)] are apoptotic during recovery from stress, GFP-expressing HrRCs are not. Instead, some HrRCs, such as a group of NSPC subtype cells (NSPCs-HrRCs subtype) and RG subtype cells (RGs-HrRCs subtype), could undergo proliferation and differentiation into neurons during regeneration. NSPCs: neural stem/progenitor cells; hpf: hours postfertilization; HrRCs: hypoxia-responsive recovering cells; RGs: radial glia cells.
At R24, HrRCs Start Proliferating and Differentiating Into Neurons
We demonstrated that HrRCs-GFP signal could be observed at R9 (Fig. 3A). Some specific cells from different cell types, such as NSPCs and RGs, would continuously respond to stress and become HrRC subtype cells until at R24 (Fig. 3A). Thus, the number of HrRCs reached to maximum at R24. However, these HrRCs did not overlap with early neuron marker HuC/HuD at least until at R18. Only a few of HuC/HuD-expressing HrRCs could be occasionally observable at R24 (Fig. 6C). We noticed that, although all HrRCs are not formed synchronously during R9 through R24, most HrRCs start to undergo neurogenesis at R24 and reached to the greatest number of neurons at R48. In the future, it would be worthwhile to reveal why all HrRCs start to proliferate and differentiate at R24, even some HrRCs are present at an earlier stage such as R9.
Cell Heterogeneity Exists in the CNS of Zebrafish After Different Stress
A green fluorescence signal can be apparent in some cells and tissues in the embryos derived from the transgenic line huORFZ. For instance, a green fluorescence signal could be induced for subsequent observation in the CNS and skin cells after heat-shock treatment 34 , and green fluorescence signal was present along both sides of the injury site after mechanical SCI. It has been demonstrated that this GFP-expressing cell population, termed as SrRCs, in the spinal cord plays a major role in neuronal regeneration after SCI 40 . Nevertheless, GFP-expressing HrRCs in embryos display not only in the spinal cord but also in the brain. Although spinal cord HrRCs were previously studied by Zeng et al18,40, the biological characteristics of GFP-expressing HrRCs appearing in the brain remain unknown. Therefore, in this study, we characterized a cell population of HrRCs appearing in the brain of hypoxia-exposed huORFZ transgenic zebrafish during OR. Like the HrRCs reported in the spinal cord, NSPCs also comprise the major cell type among the specific cell populations of HrRCs in hypoxia-exposed zebrafish embryos 17 . However, we found that NSPCs-HrRCs in the hypoxia-stressed brain can proliferate after stress, while NSPCs-non-HrRCs cannot. Moreover, we found that brain-HrRCs do not undergo apoptosis, but rather express an early neuron marker, HuC/HuD, eventually differentiating into neurons possibly involved in neuronal regeneration after hypoxic stress.
Relationship Among Subtypes of her4.1-Radial Glia Progenitor Cells, Neurog1-Neuronal Precursor Cells, RGs-HrRCs, and NSPCs-HrRCs
Kawauchi et al 43 reported that self-renewing Sox2+ neural stem cells give rise to transit amplifying progenitors that express Mash1 followed by immediate neuronal precursors (INPs) that express neurogenin1 (neurog1). These neurog1-positive neuronal precursor cells in the telencephalon can proliferate, migrate, and differentiate into neurog1+ neuronal precursors after brain injury, suggesting that this cell subtype is involved in neuronal regeneration 1 . Kroehne et al 9 also found that traumatic lesion in the brain of adult zebrafish could induce a group of her4.1-positive ventricular radial glia progenitor cells able to react to injury, proliferate, generate neuroblasts, and migrate to the lesion site. Interestingly, taking advantage of zebrafish transgenic line huORFZ, we discovered in this study a cell population in the CNS composed of subtypes of different neural cell types. These GFP-expressing HrRCs in the CNS turned out to be the most sensitive responders to hypoxia-exposed zebrafish embryos and could thus serve as the “first responders” to hypoxic stress during GFP translation. Specifically, we found that hypoxia can induce subtypes of NSPCs and RGs, termed NSPCs-HrRCs and RGs-HrRCs subtypes, in the brain able to sensitively respond to hypoxic stress. These NSPCs-HrRCs and RGs-HrRCs subtypes, two major constitutes among HrRCs, can then proliferate into neurons during OR after hypoxic stress. Therefore, it is highly likely that these NSPCs-HrRCs and RGs-HrRCs subtypes are involved in neuronal regeneration similar to NSPCs-HrRCs and RGs-HrRCs subtypes found in the hypoxia-exposure spinal cord, as reported by Zeng et al 18 . In contrast, another cell subtype population, NSPCs-non-HrRCs and RGs-non-HrRCs, is not involved in neuronal regeneration because they are subject to apoptosis after hypoxic stress. In this study, we found that HrRCs in embryonic brain can proliferate and differentiate into early neurons during recovery after hypoxia exposure. This evidence leads us to conclude high heterogeneity of the same cell type in zebrafish brain, including subtypes of her4.1-positive ventricular radial glia progenitor cells, neurog1-positive neuronal precursor cells, RGs-HrRCs, and NSPCs-HrRCs highly sensitive to hypoxic stress, as reported in this study.
März et al 12 demonstrated that NSPCs are more aggregated in the VZ. Interestingly, as shown in Figs. 3A and 4A in this study, we demonstrated that HrRCs are located in the VZ of zebrafish brain. We also noticed that 48% of HrRCs composed of NSPCs in the brain (NSPCs-HrRCs) are able to differentiate into neurons during recovery 12 . Meanwhile, Hui et al 13 reported that proliferating NSPCs are particularly located in the VZ around the central canal of the spinal cord. When zebrafish embryos were exposed to hypoxia, Zeng et al 18 also found that 36% of HrRCs were composed of NSPCs located in the VZ of the spinal cord and that they contributed to neuronal regeneration. Our results show that NSPCs-HrRCs are induced and presented in the VZ of brain and spinal cord after hypoxic stress in the zebrafish embryos and that NSPCs-HrRCs comprise the cell subtype mostly involved in neuronal regeneration of brain and spinal cord after hypoxia. Taken together, it is likely that neurog1-positive neuronal precursor cells, her4.1-positive ventricular radial glia progenitor cells, and NSPCs-HrRCs constitute the three important progenitors involved in brain neuronal regeneration. We hypothesize the contribution of these three progenitors to neuronal regeneration in brain after hypoxic stress following a model whereby (1) neurons in the parenchyma of the injured brain undergo cell death; (2) NSPCs-HrRCs start self-renewing and give rise to INPs which express neurog1; (3) NSPCs-HrRCs and INPs differentiate into neurons and finally contribute to neural tissue recovery; and (4) her4.1-positive ventricular radial glia progenitor cells also generate mature neurons. However, marking and understanding the cooperativity among these three progenitors involved in brain neuronal regeneration remain to be elucidated, for example, by establishing transgenic zebrafish with tricolor labeling and long-term observation in vivo.
Conclusions
In this study, we demonstrated that a subtype cell population, termed as HrRCs, exists in zebrafish brain and can respond sensitively to hypoxic stress. HrRCs are mostly composed of NSPC and RG subtypes, the major proliferating cells after hypoxic stress. They can further differentiate into neurons, contributing to neural regeneration in zebrafish brain.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897221077930 for Hypoxia-Responsive Subtype Cells Differentiate Into Neurons in the Brain of Zebrafish Embryos Exposed to Hypoxic Stress by Chih-Wei Zeng, Jin-Chuan Sheu and Huai-Jen Tsai in Cell Transplantation
Acknowledgments
We thank TC3 and TC5 of National Taiwan University (NTU) for helping with the BD FACSAria cell sorting system and providing confocal microscopy. Flow Cytometry and Cell Sorting was performed on a BD FACSAria III system in Technology Commons in College of Life Science and the Instrumentation Center sponsored by Ministry of Science and Technology, NTU, with the technical assistance provided by Ms Wan-Shu Yao. We are very grateful for the support of President Spencer Lee and Dr Hsiao-Ching Nien, the Liver Disease Prevention and Treatment Research Foundation, Taipei, Taiwan.
Footnotes
Author Contributions: C.-W.Z. contributed to the study design, carrying out experiments, data analysis, and interpretation, as well as writing the draft. Being a PI, H.-J.T. conceived and designed the research, performed data interpretation, and contributed to edit the article. All authors read and approved the final article.
Ethical Approval: This study was approved by the Institute of Biomedical Science, Mackay Medical College, New Taipei City, Taiwan.
Statement of Human and Animal Rights: All the experimental procedures involving animals were conducted and approved by the Institute of Biomedical Science, Mackay Medical College, New Taipei City, Taiwan.
Statement of Informed Consent: There are no human subjects in the article in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology, Taiwan (Grant Number 108-2811-B-715-500), and partially supported by the Liver Disease Prevention and Treatment Research Foundation (to J.-C.S.)
ORCID iD: Huai-Jen Tsai  https://orcid.org/0000-0001-8242-4939
https://orcid.org/0000-0001-8242-4939
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kishimoto N, Shimizu K, Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech. 2012;5:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alunni A, Bally-Cuif L. A comparative view of regenerative neurogenesis in vertebrates. Development. 2016;143:741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silver J, Miller J. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. [DOI] [PubMed] [Google Scholar]
- 4. Gan D, Wu S, Chen B, Zhang J. Application of the zebrafish traumatic brain injury model in assessing cerebral inflammation. Zebrafish. 2020;17:73–82. [DOI] [PubMed] [Google Scholar]
- 5. Li Z, Xiao J, Xu X, Li W, Zhong R, Qi L, Chen J, Cui G, Wang S, Zheng Y, Qiu Y, et al. M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci Adv. 2021;7:eabb6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohnmacht J, Yang Y, Maurer G, Barreiro-Iglesias A, Tsarouchas T, Wehner D, Sieger D, Becker C, Becker T. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Development. 2016;143:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kettleborough R, Busch-Nentwich E, Harvey S, Dooley C, de Bruijn E, van Eeden F, Sealy I, White R, Herd C, Nijman I, Fényes F, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138(22):4831–41. [DOI] [PubMed] [Google Scholar]
- 10. Lam C, März M, Strähle U. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev Dyn. 2009;238(2):475–86. [DOI] [PubMed] [Google Scholar]
- 11. Barbosa J, Sanchez-Gonzalez R, Di Giaimo R, Baumgart E, Theis F, Gotz M, Ninkovic J. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science. 2015;15:789–93. [DOI] [PubMed] [Google Scholar]
- 12. März M, Chapouton P, Diotel N, Vaillant C, Hesl B, Takamiya M, Lam C, Kah O, Bally-Cuif L, Strähle U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia. 2010;58:870–88. [DOI] [PubMed] [Google Scholar]
- 13. Hui S, Nag T, Ghosh S. Characterization of proliferating neural progenitors after spinal cord injury in adult zebrafish. PLoS ONE. 2015;10:e0143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cosacak M, Bhattarai P, Reinhardt S, Petzold A, Dahl A, Zhang Y, Kizil C. Single-cell transcriptomics analyses of neural stem cell heterogeneity and contextual plasticity in a zebrafish brain model of amyloid toxicity. Cell Rep. 2019;27:1307–18. [DOI] [PubMed] [Google Scholar]
- 15. Bhattarai P, Thomas A, Zhang Y, Kizil C. The effects of aging on Amyloid-β42-induced neurodegeneration and regeneration in adult zebrafish brain. Neurogenesis. 2017;4:e1322666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H, Chen Y, Liu Y, Lin K, Chen S, Lin C, Lu Y, Hsu P, Lee S, Tsai H. Transgenic zebrafish model to study translational control mediated by upstream open reading frame of human chop gene. Nucleic Acids Res. 2011;39:139–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee H, Fu C, Lin C, Hu J, Huang T, Lo K, Tsai H, Sheu J, Tsai H. Poly(U)-specific endoribonuclease ENDOU promotes translation of human CHOP mRNA by releasing uORF element-mediated inhibition. EMBO J. 2021;40:e104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng C, Kamei Y, Wang C, Tsai H. Subtypes of hypoxia-responsive cells differentiate into neurons in spinal cord of zebrafish embryos after hypoxic stress. Biol Cell. 2016;108: 357–77. [DOI] [PubMed] [Google Scholar]
- 19. Khaliullina-Skultety H, Zi Chao N, Harris W. Induction of hypoxia in living frog and zebrafish embryos. J Vis Exp. 2017;124:55710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang HY, Dai ES, Liu JT, Tu CT, Yang TC, Tsai HJ. The embryonic expression patterns and the knockdown phenotypes of zebrafish ADP-ribosylation factor-like 6 interacting protein gene. Dev Dyn. 2009;238:232–40. [DOI] [PubMed] [Google Scholar]
- 21. Zeng C, Sheu J, Tsai H. The neuronal regeneration of adult zebrafish after spinal cord injury is enhanced by transplanting optimized number of neural progenitor cells. Cell Transplant. 2020. doi: 10.1177/0963689720903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vitak SA, Torkenczy KA, Rosenkrantz JL, Fields AJ, Christiansen L, Wong MH, Carbone L, Steemers FJ, Adey A. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat Methods. 2017;14:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adolf B, Chapouton P, Lam C, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;29:278–93. [DOI] [PubMed] [Google Scholar]
- 24. Halterman M, Gill M, DeJesus C, Ogihara M, Schor N, Federoff H. The endoplasmic reticulum stress response factor CHOP-10 protects against hypoxia-induced neuronal death. J Biol Chem. 2010;285:21329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–53. [DOI] [PubMed] [Google Scholar]
- 26. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maytin E, Ubeda M, Lin J, Habener J. Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 Kinase-Dependent and-Independent Mechanisms. Exp Cell Res. 2001;267:193–204. [DOI] [PubMed] [Google Scholar]
- 28. Dong B, Zhou H, Han C, Yao J, Xu L, Zhang M, Fu Y, Xia Q. Ischemia/reperfusion-induced CHOP expression promotes apoptosis and impairs renal function recovery: the role of acidosis and GPR4. PLoS ONE. 2014;9:e110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg C, Oeffner A, Gerbert B, Weber M, Brabant G, Schumm-Draeger P, Mann K, Herrmann B. Prevalence of anterior pituitary dysfunction in patients following traumatic brain injury (TBI) in a German multi-centre screening program. Exp Clin Endocrinol Diabetes. 2010;118:139–44. [DOI] [PubMed] [Google Scholar]
- 30. Madden L, DeVon H. A systematic review of the effects of body temperature on outcome after adult traumatic brain injury. J Neurosci Nurs. 2015;47:190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joven A, Simon A. Homeostatic and regenerative neurogenesis in salamanders. Prog Neurobiol. 2018;170:81–98. [DOI] [PubMed] [Google Scholar]
- 32. Lust K, Tanaka E. A comparative perspective on brain regeneration in amphibians and teleost fish. Dev Neurobiol. 2019;79:424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKeown C, Sharma P, Sharipov H, Shen W, Cline H. Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis. J Comp Neurol. 2013;521: 2262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee H, Lu P, Huang H, Chu C, Li H, Tsai H. Zebrafish transgenic line huORFZ is an effective living bioindicator for detecting environmental toxicants. PLoS ONE. 2014;9:e90160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skalet A, Isler J, King L, Harding H, Ron D, Monroe J. Rapid B cell receptor-induced unfolded protein response in nonsecretory B cells correlates with pro- versus antiapoptotic cell fate. J Biol Chem. 2005;280:39762–71. [DOI] [PubMed] [Google Scholar]
- 37. Kroeger H, Grandjean J, Chiang W, Bindels D, Mastey R, Okalova J, Nguyen A, Powers E, Kelly J, Grimsey N, Michaelides M, et al. Proteostasis modulation prevents photoreceptor pathology in retinal organoids. Cell Stem Cell. SSRN Electron J. Published online December 10, 2020. doi: 10.2139/ssrn.3746623 [DOI] [Google Scholar]
- 38. Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–55. [DOI] [PubMed] [Google Scholar]
- 39. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. [DOI] [PubMed] [Google Scholar]
- 40. Zeng C, Kamei Y, Shigenobu S, Sheu J, Tsai H. Injury-induced Cavl-expressing cells at lesion rostral side play major roles in spinal cord regeneration. Open Biol. 2021;11:200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vogelstein D, Lane AJ. Levine surfing the p53 network. Nature. 2000;408:307–10. [DOI] [PubMed] [Google Scholar]
- 42. Sawahata M, Izumi Y, Akaike A, Kume T. In vivo brain ischemia-reperfusion model induced by hypoxia-reoxygenation using zebrafish larvae. Brain Res Bull. 2021;173:45–52. [DOI] [PubMed] [Google Scholar]
- 43. Kawauchi S, Santos R, Kim J, Hollenbeck P, Murray R, Calof A. The role of foxg1 in the development of neural stem cells of the olfactory epithelium. Ann N Y Acad Sci. 2009;1170:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897221077930 for Hypoxia-Responsive Subtype Cells Differentiate Into Neurons in the Brain of Zebrafish Embryos Exposed to Hypoxic Stress by Chih-Wei Zeng, Jin-Chuan Sheu and Huai-Jen Tsai in Cell Transplantation