Abstract
Hypoxic myoclonus, also known as Lance Adams syndrome, is a rare syndrome that results from the serious brain damage caused by cerebral hypoxia that often follows cardiopulmonary resuscitation. This current case report describes two patients with post-hypoxic myoclonus, both of whom received cardiopulmonary resuscitation. The neurological symptoms of these two patients were significantly improved by the administration of clonazepam and sodium valproate sustained-release tablets. The report presents a literature review detailing the pathogenesis, diagnosis and treatment of Lance Adams syndrome. The timely diagnosis and treatment of Lance Adams syndrome can significantly improve the quality of life of patients. Valproic acid, clonazepam and other antiepileptic drugs can be used. Whether levetiracetam is effective for cortical myoclonus requires further clinical study.
Keywords: Lance Adams syndrome, levetiracetam, case report
Introduction
With increasing knowledge of emergency medicine, improved intensive care and rescue treatments have resulted in ever increasing numbers of patients surviving cerebral ischaemia and hypoxic events. 1 There is a group of clinical syndromes that are characterized by action myoclonus as the main manifestation, which is known as Lance Adams syndrome or chronic post-hypoxia myoclonus. 2 These syndromes have attracted increasing attention in recent years. Hypoxic myoclonus was first reported by Lance and Adams in 1963. 3 It is more common in patients undergoing cardiopulmonary resuscitation. 4 Electroencephalogram (EEG) examination often shows multisource and multifocal lesions, with approximately half of them showing epileptic discharges. 5 Brain imaging shows no specific lesions. 6 The causes of hypoxic myoclonus include cardiac arrest, respiratory obstruction, anaesthesia accident, carbon monoxide poisoning, haemorrhagic shock, hanging and drowning. 4 The theoretical pathological mechanisms involved in the development of hypoxic myoclonus include the following: (i) abnormal cortical discharge theory; (ii) abnormal cerebellum brain stem and thalamus cortical circuit theory; (iii) neurotransmitter imbalance.7,8 The main diagnostic basis for Lance Adams syndrome is the patient's medical history and typical symptoms. 9 Early diagnosis and treatment are very important to improve the quality of life of patients. 10
This case report describes two patients with hypoxic myoclonus and provides a review of the current literature. The reporting of these two cases conforms to CARE guidelines. 11
Case report 1
A 59-year-old female was admitted to the Department of Neurology, Second Affiliated Hospital of Army Medical University, Chongqing, China on 30 October 2015 due to an ‘involuntary facial twitch for more than 7 years, aggravated in February 2015’. Seven years previously, due to asthma and dyspnoea, she suffered from respiratory and cardiac arrest in a hospital in Chongqing. She was in a coma after successful cardiopulmonary resuscitation. After 1 week, consciousness gradually returned, but the next day, her limbs and face twitched involuntarily. The attacks were frequent, occurring dozens of times a day, lasting for several seconds to minutes each time. The patient was fully conscious at the time of the attacks, and it became obvious that they occurred at the time of emotional tension and excitement, which affected the patient’s life and work. Assistance was required for eating, walking, urinating and defecating. There was no dysphagia or cough when drinking water. There was no disturbance of consciousness, incontinence of urine or faeces, numbness and weakness of limbs. The hospital diagnosed ‘secondary epilepsy’. The patient was administered 2 mg clonazepam orally twice a day + 60 mg phenobarbital orally twice a day, but symptom control was poor and the condition did not improve significantly. On 6 May 2009, the patient felt that the existing symptoms had worsened and the frequency of the involuntary twitching of her limbs and face had increased. In the Department of Neurology, Second Affiliated Hospital of Army Medical University, Chongqing, China, an EEG showed moderate-to-severe abnormalities. The diagnosis of ‘extrapyramidal lesions, sequelae of hypoxic–ischaemic encephalopathy, symptomatic epilepsy’ was made and the patient was administered 0.1 g carbamazepine orally three times a day + 0.5 g amlodipine orally three times a day + 0.2 g magnesium valproate orally three times a day. After treatment, the facial twitch improved, the number of limb-twitching attacks decreased and the patient was discharged. In 2011, the patient had developed shaking hands, which meant that she was unstable to hold chopsticks and a bowl, so needed help with feeding and walking. The patient went to a hospital in Chongqing and they gradually adjusted the drug administration to 1 g levetiracetam orally twice a day for 1 month + 500 mg sodium valproate sustained-release tablets orally twice a day for 1 month. In September 2015, because there was no reduction in the number of lower limb-twitching attacks, the hospital changed the sodium valproate sustained-release tablets to 300 mg oxcarbazepine orally once a day each morning for 2 weeks + 450 mg oxcarbazepine orally once a day each evening for 2 weeks, followed by 450 mg oxcarbazepine orally twice a day to the present time. However, 2 months ago, the patient's lower limb twitching increased significantly, which resulted in the patient being unable to walk on their own or with support. When the patient experienced emotional excitement or a noisy environment, this easily induced lower limb twitching, which lasted for a few seconds each time, without conscious disturbance and faecal incontinence. A physical examination upon admission to the Department of Neurology, Second Affiliated Hospital of Army Medical University, Chongqing, China on 30 October 2015 showed clear consciousness and involuntary twitching of the lower limbs. Magnetic resonance imaging (MRI) showed a few lacunar foci in the bilateral lateral ventricles and brain atrophy (Figure 1). Amplitude-integrated electroencephalography (aEEG) demonstrated the following: (i) irregular slow waves and synchronous slow wave activities in the right frontal and temporal areas; (ii) irregular slow wave activities of Zhongfu in the short-term paroxysmal whole lead and the back head was protruding; (iii) involuntary limb twitching during the detection was a non-epileptic event; (iv) no epileptic waves were found (Figure 2). Considering ‘myoclonus after hypoxia’, oxcarbazepine and levetiracetam were gradually stopped; and 1 mg clonazepam orally twice a day was administered for 2 months. The patient's lower limb twitching was gradually relieved and the patient was able to walk slowly with some help. At present, rehabilitation exercises of both lower limbs are in progress.
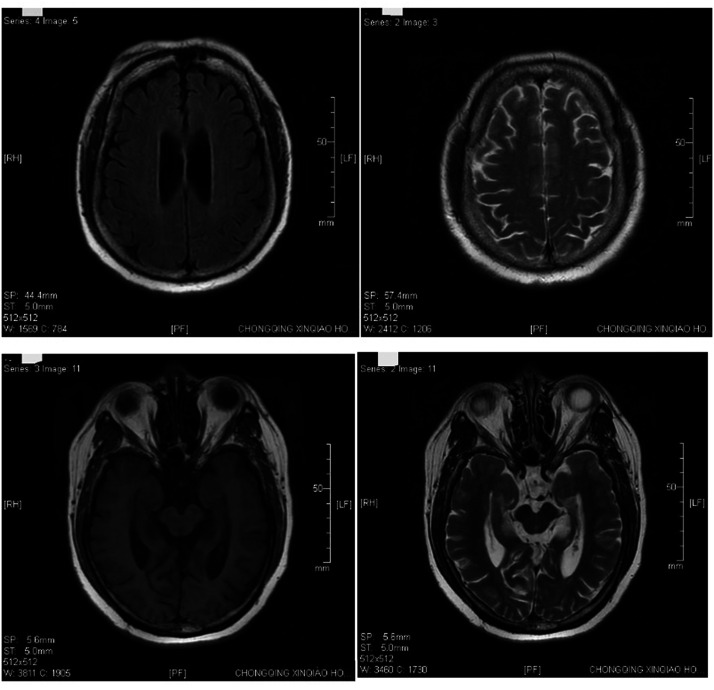
Figure1.
Brain magnetic resonance imaging of a 59-year-old female patient that presented with involuntary facial twitch for more than 7 years that was a complication of cardiopulmonary resuscitation following surgery. The imaging shows a few lacunar foci in the bilateral lateral ventricles and brain atrophy.
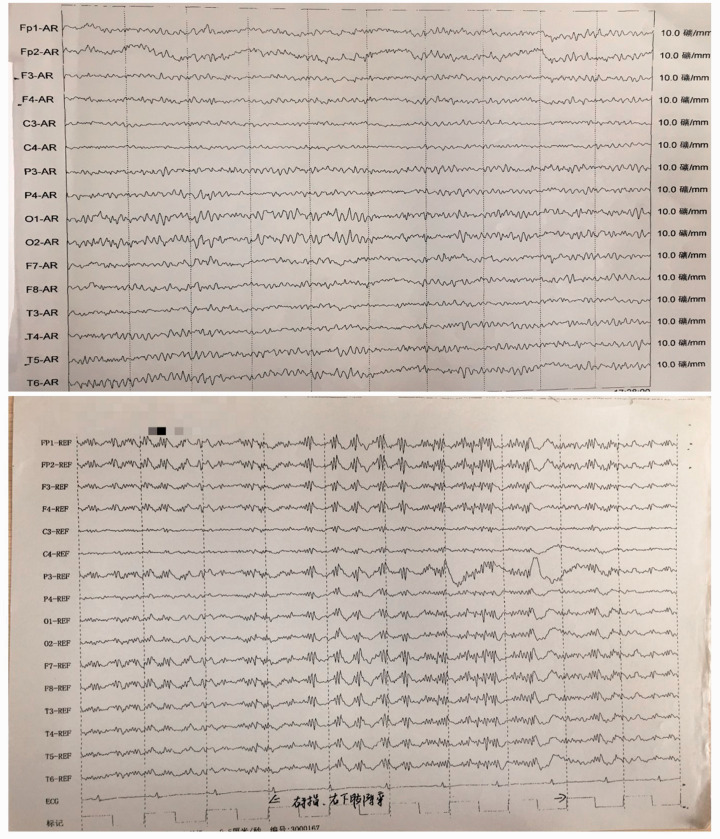
Figure 2.
Amplitude-integrated electroencephalography of a 59-year-old female patient that presented with involuntary facial twitch for more than 7 years that was a complication of cardiopulmonary resuscitation following surgery. No epileptic wave was found.
Case report 2
A 48-year-old female was admitted to the Department of Orthopaedics, Second Affiliated Hospital of Army Medical University, Chongqing, China on 16 March 2017 due to radiating pain in her right lower limb. On 21 March 2017, lumbar spine surgery was performed under epidural anaesthesia. At 1.5 h after the operation, the patient experienced respiratory failure, her heart rate disappeared, respiration stopped, the great artery pulsation disappeared, lips and limbs became cyanosed, she had a bilateral pupil diameter of 5 mm and her light reflex disappeared. Cardiopulmonary resuscitation was given immediately. After 19 min, the patient's heart rate recovered, but there was no spontaneous breathing and she remained in a coma. On 24 March 2017, 3 days after cardiopulmonary resuscitation, the patient was transferred to the Department of Neurology and developed clonus of the head, face and limbs. Physical examination after the transfer showed the following: coma, bilateral pupil diameter of 3 mm, slow light reflex, no pathological reflex. Computed tomography angiography of the neck showed no abnormalities. An aEEG showed the following: (i) sharp waves; (ii) sharp slow complex waves were occasionally seen in the bilateral frontal and temporal areas, and these were obvious in the left temporal area; (iii) clonic seizures were detected. The patient was treated as follows: (i) maintenance of vital signs; (ii) treatment for electrolyte acid-base balance disorder; (iii) anti-infection medications; (iv) 1 g levetiracetam orally twice a day for 2 months + 500 mg valproic acid orally twice a day for 2 months + 1 mg clonazepam orally every evening for 2 months; (v) and other symptomatic treatments. The patient’s spontaneous breathing and consciousness disorder gradually recovered. The patient was drowsy for 16 days after cardiopulmonary resuscitation (6 April 2017) and clear consciousness returned 18 days after cardiopulmonary resuscitation (8 April 2017). After stable vital signs and clear consciousness, she was hospitalized for 31 days (17 April 2017) and transferred to the Department of Rehabilitation, First Affiliated Hospital of Army Medical University, Chongqing, China for further hyperbaric oxygen rehabilitation treatment. After 10 days, the patient could pronounce single syllables without being able to speak completely; after more than 10 days, both upper limbs could be lifted up and both lower limbs could be lifted off the bed. In May 2017, after 1 month, the patient could walk without help. On 9 June 2017, the patient's family made the decision to stop administering the patient levetiracetam, valproic acid and clonazepam; and they treated her with Madopar (levodopa and benserazide) and Antam (piracetam) orally for 9 days (dose and frequency unknown). When the patient started to experience limb twitching again, her family stopped the treatment with Madopar and Antam, and reinstated treatment with 1 g levetiracetam orally twice a day for 2 months + 500 mg valproic acid orally twice a day for 2 months + 1 mg clonazepam orally every evening for 2 months. She was admitted to the Department of Neurology, Second Affiliated Hospital of Army Medical University, Chongqing, China again between 17 July 2016 and 16 August 2017. Physical examination on admission demonstrated the following: clear consciousness, slightly high muscle tension of the limbs, unstable and accurate bilateral finger nose test and heel knee tibia test, positive left Pap's sign. Brain MRI showed a right frontal lobe lacuna focus and the T2-weighted-fluid-attenuated inversion recovery white matter signal was slightly higher (Figure 3).
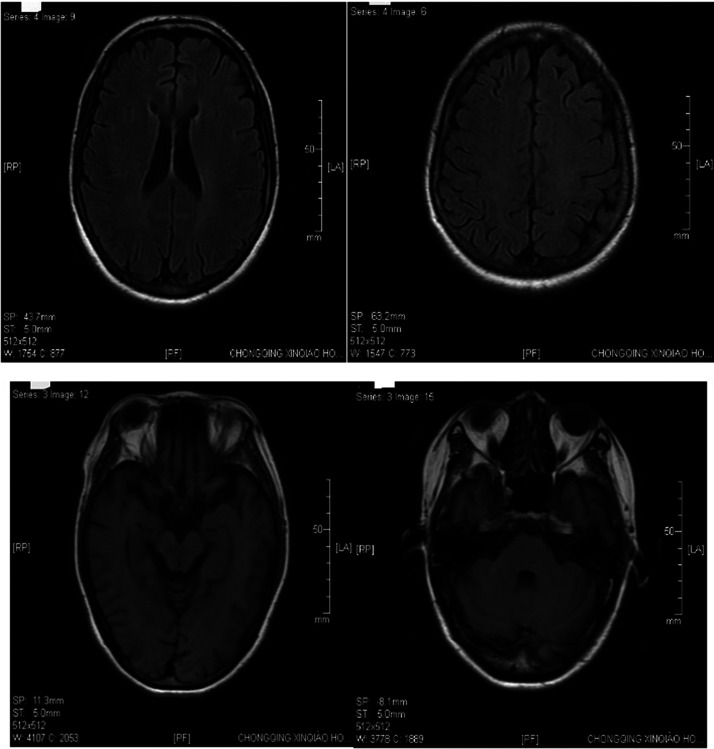
Figure 3.
Brain magnetic resonance imaging of a 48-year-old female patient that presented with radiating pain in her right lower limb. The imaging shows a right frontal lobe lacuna focus and the T2-weighted-fluid-attenuated inversion recovery white matter signal was slightly higher.
Dynamic video electroencephalogram demonstrated the following: several lower limb clonic seizures were detected in the patient, but an epileptiform discharge of the EEG was not obvious at the same time; the hand shaking was obvious when the patient started to hold an object and did the targeted action, but the EEG at the same time was mainly electromyography (EMG) and shaking artifacts (Figure 4). In consideration of ‘myoclonus after hypoxia’, levetiracetam was gradually stopped, and the patient was administered 1 mg clonazepam orally twice a day for 6 months + 500 mg valproate acid orally twice a day for 6 months + 30 mg idebenone orally three times a day for 3 months. The patient could walk for a certain distance independently when discharged.
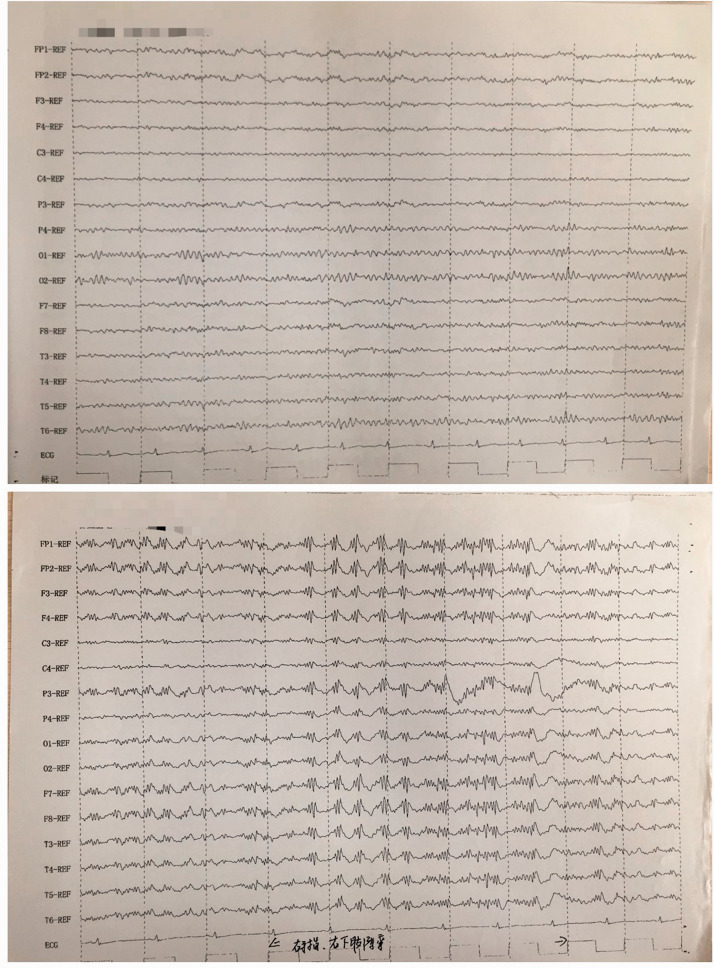
Figure 4.
Amplitude-integrated electroencephalography of a 48-year-old female patient that presented with radiating pain in her right lower limb. The findings were mainly electromyography and shaking artifacts.
Both of the patients described in this report were treated in accordance with the Human Ethics Committee of the Second Affiliated Hospital of Army Medical University. Both patients provided verbal informed consent for publication of their data.
Discussion
With the improvements made in emergency medicine, the survival rate of patients with cardiopulmonary arrest has increased significantly, but survivors are prone to complications of the nervous system, including myoclonus after hypoxia. 12 Post-hypoxic myoclonus is due to the brain damage caused by the hypoxia and 19–37% of patients with cardiopulmonary resuscitation that are in a coma develop post-hypoxic myoclonus. 2 According to the aetiology, myoclonus can be divided into physiological, idiopathic and symptomatic. 13 Idiopathic myoclonus refers to myoclonus without other neurological symptoms and signs. 14 Symptomatic myoclonus is easily induced by external stimulation, such as auditory, tactile and visual stimulation. 3 Myoclonus after hypoxia can also be divided into acute and chronic depending on the time of occurrence after the hypoxia. 15 Myoclonus after acute hypoxia occurs rapidly within a few hours of the hypoxic event and is characterized by transient and rapid twitching of the face, limbs and trunk. 16 Chronic post-hypoxic myoclonus, also known as Lance Adams syndrome, occurs slowly within a few days or months of the hypoxia. 2 Lance Adams syndrome was first reported by Lance and Adams in 1963. 3 Their report described four patients with myoclonus after cardiopulmonary resuscitation. 3 The symptoms were gradually resolved over time, but were easily induced by muscle stretch and were accompanied by other neurological deficits.3,17 Lance Adams syndrome is a rare syndrome characterized by motor and intentional myoclonus. 18 Myoclonus after hypoxia can be divided into cortical origin and subcortical origin. 7 Corticogenesis can be triggered by actions and thoughts, and is often manifested as non-rhythmicity of the upper limbs, lower limbs and face, stimulus sensitivity and action induction. 19 Subcorticogenic myoclonus is a type of myoclonus that originates from the subcortical structure, brain stem and spinal cord. 20 Subcorticogenic myoclonus is often rhythmic and insensitive to stimulation, but occasionally sensitive to stimulation. 7 Therefore, it is sometimes difficult to distinguish between the two origins. For each patient, it may be cortical, subcortical or both.4–8 This current case report describes two patients that underwent cardiopulmonary resuscitation and subsequently developed myoclonus several days later, which was consistent with Lance Adams syndrome. In the first case, the onset was associated with emotional tension and excitement, so the cortical origin was considered. In the second case, the origin was not clear based on their symptoms.
Post-hypoxic myoclonus and myoclonic epilepsy can occur after hypoxic encephalopathy. 21 Their clinical manifestations are similar, but the treatment is different. 22 Electrophysiological examination can distinguish between them. 19 The typical multi-spike slow wave of myoclonic epilepsy after hypoxia is different from that of myoclonic epilepsy in morphology. 23 ECG monitoring shows the artifact that appears synchronously with the wave, suggesting that it may be the action artifact caused by the patient's body twitching. EEG, EMG synchronous recording or spasmodic locking inverse averaging technique are the most commonly used identification methods. 23 There is no epileptic wave in the EEG of myoclonus after hypoxia. 24 EMG can record the very short and explosive EMG activity of the affected muscle. 23 The amplitude of the somatosensory-evoked potential and visual-evoked potential in patients with myoclonus after hypoxia increase significantly. 25 Sometimes a coma pattern can be seen in the EEG of patients with myoclonus after hypoxia, suggesting that myoclonus originates from the brain stem and is the result of cortical inhibition or brain stem release. 26 Myoclonus appeared in both of the current patients after cardiopulmonary resuscitation. The course of the disease in the first patient was 7 years, with the face and lower limbs obviously twitching. The dynamic EEG showed that there was no epileptic wave, so myoclonic epilepsy could be ruled out and the diagnosis of Lance Adams syndrome was clear. The second patient's aEEG showed that sharp waves and sharp slow complex waves were occasionally seen in the bilateral frontal and temporal areas, and these were obvious in the left temporal area. The morphology of sharp slow complex waves and typical multi-spike slow wave myoclonic epilepsy were carefully analysed. Different from the shape of multi-spike slow waves, the synchronous artifacts of sharp slow complex waves can be seen in EEG monitoring. Therefore, it is speculated that this may be synchronous artifacts caused by the patient's body shaking.
At present, the pathogenesis of myoclonus after hypoxia remains unclear. According to research, it may be related to the loss of 5-hydroxytryptamine (5-HT) in the inferior olivary nucleus. 9 The actions of 5-HT from the brain stem are mediated by the postsynaptic 5-HT receptor, which acts on the inferior olivary nucleus and reduces the threshold calcium conductance, thus inhibiting its rhythmic discharge. 8 Once 5-HT is absent in the inferior olivary nucleus, the neurons in the inferior olivary nucleus are in a state of irritability for a long time. 8 Research has shown that the γ-aminobutyric acid (GABA) type A receptors are involved in the pathogenesis of myoclonus after hypoxia. 10 GABA directly inhibits the release of neurotransmitters (including glutamate) by acting on presynaptic receptors, or acts on postsynaptic receptors to open chloride channels and cause hyperpolarization, thereby inhibiting neuronal excitability. 27 Therefore, the main treatment of Lance Adams syndrome is to increase the level of 5-HT and reduce the level of excitatory amino acids. At present, the main treatment of Lance Adams syndrome includes drug therapy and non-drug therapy. According to the above two potential mechanisms of action, drug therapy can be divided into: (i) drugs related to 5-HT, such as 5-HT, with clonazepam and sodium valproate being considered first-line drugs to control myoclonic seizures because they can increase the levels of 5-HT in the brain; and (ii) drugs related to amino acid neurotransmitters such as lamotrigine, riluzole, tetrahydronicotinic acid or hexahydrofolate.28,29 Research has found that lamotrigine can inhibit sodium influx, affect depolarization, affect glutamatergic neurotransmission and reduce the excitability of nerve cells, so as to alleviate the symptoms of myoclonus after hypoxia. 11 The dose of 25 mg lamotrigine may be related to the abnormal behaviour. 30 The lamotrigine treatment time window is generally within 8 h after hypoxia, which can reduce myoclonus after hypoxia. 31 In an experimental model of post-hypoxia myoclonus, riluzole significantly reduced myoclonic seizures compared with the control group and the reason might be that riluzole interferes with target molecules and blocks glutamatergic neurotransmission in the central nervous system. 12 Some studies suggest that cortical myoclonus is often treated with levetiracetam or piracetam, and subcortical myoclonus is usually treated with clonazepam.13–16 It has been reported that myoclonic seizures are sometimes triggered by the use of drugs to control myoclonus (such as carbamazepine, oxcarbazepine, phenytoin sodium), but the mechanism is unclear. 31 The first patient in the current report was unable to walk on their own due to increased shaking of the lower limbs after replacing sodium valproate sustained-release tablets with oxcarbazepine, which was consistent with a previous report. 31 It is thought that carbamazepine, oxcarbazepine and phenytoin sodium reduce the conductance of sodium and potassium ions, because ion exchange increases the number of calcium ions, which makes the calcium ions in the synaptic space activated, resulting in the muscle having sustained rhythmic impulses. 31 The first case in the current report had a long disease course and was treated with magnesium valproate, carbamazepine and oxcarbazepine in other hospitals. The twitching of the lower limbs was not relieved and she could not walk. Magnesium valproate, carbamazepine and oxcarbazepine were gradually stopped. After treatment with clonazepam, the twitching of the lower limbs gradually eased and the patient walked slowly with some support. Because the patient's lower limbs had been twitching for 7 years, they had developed muscle atrophy of both lower limbs with both limbs having a muscle strength of IV–. At present, the first case cannot walk on their own and they are undergoing rehabilitation exercises for their lower limbs. In the second case in the current report, a good understanding of Lance Adams syndrome by the medical team resulted in their timely treatment with valproic acid and clonazepam. When the patient was discharged, she could walk for a certain distance independently and had clonic seizures of the lower limbs.
In conclusion, the diagnosis of Lance Adams syndrome mainly depends on a thorough medical history, typical symptoms and signs and the EEG findings. Timely diagnosis and treatment can significantly improve the quality of life of patients. Valproic acid, clonazepam and other antiepileptic drugs can be used. The rarity of the syndrome means that it is not clear whether valproic acid and clonazepam should be the first choice for Lance Adams syndrome. Whether levetiracetam is effective for cortical myoclonus needs to be confirmed by larger clinical studies.
Acknowledgement
We sincerely thank Professor Zheng Jian (currently the director of the Department of Neurology, Guiqian International General Hospital, Guiyang, Guizhou Province, China) for his careful ongoing guidance and help with our clinical work.
Footnotes
Author contributions: Ruo-Dan Wang designed the project; Yan Xiao initiated the project; Li-Fa Chen collected and analysed the data; Yu Guo wrote the manuscript; and De-Hui Yin supervised all aspects of the project. All authors read and approved the final manuscript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ruo-Dan Wang https://orcid.org/0000-0001-8476-3328
References
- 1.Shi X, Bao J, Zhang H, et al. Emergency medicine in China: A review of the history of progress and current and future challenges after 40 years of reform. Am J Emerg Med 2020; 38: 662–669. [DOI] [PubMed] [Google Scholar]
- 2.Shin JH, Park JM, Kim AR, et al. Lance-adams syndrome. Ann Rehabil Med 2012; 36: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lance JM, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain 1963; 86: 111–136. [DOI] [PubMed] [Google Scholar]
- 4.Scheibe F, Neumann WJ, Lange C, et al. Movement disorders after hypoxic brain injury following cardiac arrest in adults. Eur J Neurol 2020; 27: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 5.van Zijl JC, Beudel M, vd Hoeven HJ, et al. Electroencephalographic Findings in Posthypoxic Myoclonus. J Intensive Care Med 2016; 31: 270–275. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo E, Gasparini S, Cianci V, et al. Serial MRI findings in brain anoxia leading to Lance-Adams syndrome: a case report. Neurol Sci 2013; 34: 2047–2050. [DOI] [PubMed] [Google Scholar]
- 7.Moreira Filho PF, Freitas MR, Hahn MD, et al. Encefalopatia mioclônica pós-anóxica (síndrome de Lance-Adams): estudo anatomopatológico de dois casos [Post-anoxic myoclonic encephalopathy (Lance-Adams syndrome): anatomopathological study of 2 cases]. Arq Neuropsiquiatr 1982; 40: 146–155 [Article in Portuguese, English abstract]. [DOI] [PubMed] [Google Scholar]
- 8.Welsh JP, Placantonakis DG, Warsetsky SI, et al. The serotonin hypothesis of myoclonus from the perspective of neuronal rhythmicity. Adv Neurol 2002; 89: 307–329. [PubMed] [Google Scholar]
- 9.English WA, Giffin NJ, Nolan JP. Myoclonus after cardiac arrest: pitfalls in diagnosis and prognosis. Anaesthesia 2009; 64: 908–911. [DOI] [PubMed] [Google Scholar]
- 10.Werhahn KJ, Brown P, Thompson PD, et al. The clinical features and prognosis of chronic posthypoxic myoclonus. Mov Disord 1997; 12: 216–220. [DOI] [PubMed] [Google Scholar]
- 11.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014; 67: 46–51. [DOI] [PubMed] [Google Scholar]
- 12.Mulder M, Geocadin RG. Neurology of cardiopulmonary resuscitation. Handb Clin Neurol 2017; 141: 593–617. [DOI] [PubMed] [Google Scholar]
- 13.Caviness JN. Myoclonus. Continuum (Minneap Minn) 2019; 25: 1055–1080. [DOI] [PubMed] [Google Scholar]
- 14.Yokota T, Hirashima F, Ito Y, et al. Idiopathic palatal myoclonus. Acta Neurol Scand 1990; 81: 239–242. [DOI] [PubMed] [Google Scholar]
- 15.Gupta HV, Caviness JN. Post-hypoxic Myoclonus: Current Concepts, Neurophysiology, and Treatment. Tremor Other Hyperkinet Mov (N Y) 2016; 6: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouwes A, van Poppelen D, Koelman JH, et al. Acute posthypoxic myoclonus after cardiopulmonary resuscitation. BMC Neurol 2012; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesan A, Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurol Clin 2006; 24: 123–132. [DOI] [PubMed] [Google Scholar]
- 18.Shugan A. A Case Study of Lance-Adams Syndrome. Neurodiagn J 2021; 61: 144–149. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YX, Liu JR, Jiang B, et al. Lance-Adams syndrome: a report of two cases. J Zhejiang Univ Sci B 2007; 8: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr J. Classifying myoclonus: a riddle, wrapped in a mystery, inside an enigma. Parkinsonism Relat Disord 2012; 18(Suppl 1): S174–S176. [DOI] [PubMed] [Google Scholar]
- 21.Freund B, Kaplan PW. Post-hypoxic myoclonus: Differentiating benign and malignant etiologies in diagnosis and prognosis. Clin Neurophysiol Pract 2017; 2: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy A, Chen R. Myoclonus: Pathophysiology and Treatment Options. Curr Treat Options Neurol 2016; 18: 21. [DOI] [PubMed] [Google Scholar]
- 23.Hui AC, Cheng C, Lam A, et al. Prognosis following Postanoxic Myoclonus Status epilepticus. Eur Neurol 2005; 54: 10–13. [DOI] [PubMed] [Google Scholar]
- 24.Pivik RT, Broughton RJ, Coppola R, et al. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology 1993; 30: 547–558. [DOI] [PubMed] [Google Scholar]
- 25.Szczepańska A, Dziadkowiak E, Bladowska J, et al. The Usefulness of Quantitative EEG and Advanced MR Techniques in the Monitoring and Long-Term Prognosis of Lance-Adams Syndrome. Front Neurol 2019; 10: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology 2012; 78: 796–802. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto RR, Truong DD, Nguyen KD, et al. Involvement of GABA(A) receptors in myoclonus. Mov Disord 2000; 15(Suppl 1): 47–52. [DOI] [PubMed] [Google Scholar]
- 28.Lim LL and, Ahmed A. Limited efficacy of levetiracetam on myoclonus of different etiologies. Parkinsonism Relat Disord 2005; 11: 135–137. [DOI] [PubMed] [Google Scholar]
- 29.Frucht S and, Fahn S. The clinical spectrum of posthypoxic myoclonus. Mov Disord 2000; 15(Suppl 1): 2–7. [DOI] [PubMed] [Google Scholar]
- 30.Crawford MJ, Sanatinia R, Barrett B, et al. Lamotrigine versus inert placebo in the treatment of borderline personality disorder: study protocol for a randomized controlled trial and economic evaluation. Trials 2015; 16: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zivkovic SA and, Brenner RP. A case of area-specific stimulus-sensitive postanoxic myoclonus. J Clin Neurophysiol 2003; 20: 111–116. [DOI] [PubMed] [Google Scholar]