ABSTRACT
Background
Different 25-hydroxyvitamin D [25(OH)D] thresholds for treatment with vitamin D supplementation have been suggested and are derived almost exclusively from observational studies. Whether other characteristics, including race/ethnicity, BMI, and estimated glomerular filtration rate (eGFR), should also influence the threshold for treatment is unknown.
Objectives
The aim was to identify clinical and biomarker characteristics that modify the response to vitamin D supplementation.
Methods
A total of 666 older adults in the Multi-Ethnic Study of Atherosclerosis (MESA) were randomly assigned to 16 wk of oral vitamin D3 (2000 IU/d; n = 499) or placebo (n = 167). Primary outcomes were changes in serum parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations from baseline to 16 wk.
Results
Among 666 participants randomly assigned (mean age: 72 y; 53% female; 66% racial/ethnic minority), 611 (92%) completed the study. The mean (SD) change in PTH was −3 (16) pg/mL with vitamin D3 compared with 2 (18) pg/mL with placebo (estimated mean difference: –5; 95% CI: –8, –2 pg/mL). Within the vitamin D3 group, lower baseline 25-hydroxyvitamin D [25(OH)D] was associated with a larger decline in PTH in a nonlinear fashion. With baseline 25(OH)D ≥30 ng/mL as the reference, 25(OH)D <20 ng/mL was associated with a larger decline in PTH with vitamin D3 supplementation (–10; 95% CI: –15, –6 pg/mL), whereas 25(OH)D of 20–30 ng/mL was not (–2; 95% CI: –6, 1 pg/mL). A segmented threshold model identified a baseline 25(OH)D concentration of 21 (95% CI: 13, 31) ng/mL as an inflection point for difference in change in PTH. Race/ethnicity, BMI, and eGFR did not modify vitamin D treatment response. There was no significant change in 1,25(OH)2D in either treatment group.
Conclusions
Of characteristics most commonly associated with vitamin D metabolism, only baseline 25(OH)D <20 ng/mL modified the PTH response to vitamin D supplementation, providing support from a clinical trial to use this threshold to define insufficiency. This trial was registered at clinicaltrials.gov as NCT02925195.
Keywords: vitamin D, cholecalciferol, vitamin D deficiency, vitamin D insufficiency, randomized clinical trial, standardization, HbA1c harmonization program, vitamin D standardization program
Introduction
Vitamin D and its metabolites regulate hundreds of genes related to bone metabolism, cell growth and differentiation, inflammatory cell function, and other basic cellular processes essential to human health (1–3). While vitamin D is effective for treating nutritional rickets and osteomalacia (4, 5), recent clinical trials of predominantly vitamin D–sufficient individuals from the general population demonstrate small to insignificant effects of supplementation on numerous chronic illnesses (6–9). However, response to supplementation is likely to vary across individuals, such that vitamin D supplementation offers clinical benefits for appropriate subsets of people.
Low circulating concentrations of total 25-hydroxyvitamin D [25(OH)D], the sum of 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3], are typically used to define “vitamin D insufficiency” and guide treatment. However, 25(OH)D thresholds used to define insufficiency are derived almost exclusively from observational studies of serum 25(OH)D concentrations (10, 11). Data from intervention studies are optimal to determine characteristics, such as 25(OH)D concentration, that modify the benefits and risks of vitamin D supplementation. The paucity of such data contributes to conflicting recommendations to define 25(OH)D insufficiency and to institute vitamin D supplementation from professional societies.
We conducted a randomized clinical trial of vitamin D to identify key clinical and biomarker characteristics that modify response to vitamin D treatment, assessed primarily by changes in serum concentrations of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D [1,25(OH)2D]. PTH is a classic response marker for vitamin D treatment, as its synthesis and secretion are directly suppressed by vitamin D receptor activation by 1,25(OH)2D, the vitamin D metabolite that mediates most of the biological effects of vitamin D (12, 13). In addition to baseline 25(OH)D concentration, we examined BMI, estimated glomerular filtration rate (eGFR), and race/ethnicity as potential effect modifiers, given the role of adiposity in vitamin D bioavailability (14), metabolism of 25(OH)D by the kidney (15), and observational data suggesting heterogeneity in associations of 25(OH)D with clinical outcomes by race/ethnicity (16–18). The knowledge gained from this study may impact patient-oriented care and health policy.
Methods
Study design
The Multi-Ethnic Study of Atherosclerosis (MESA) Individualized Response to Vitamin D (INVITe) study was a randomized, double-blind, placebo-controlled trial of vitamin D designed to examine individual-level characteristics that modify the response to vitamin D treatment (19). The INVITe trial was nested within MESA, an ongoing, community-based prospective cohort study of clinical and subclinical cardiovascular disease (20) and approved by the Institutional Review Board of each participating MESA INVITe study site.
Study population
Between 2000 and 2002, MESA recruited 6814 adults between the ages of 45 and 84 y without clinically apparent cardiovascular disease. For this study, participants were recruited from the MESA at year 15 examination or from 2 parallel MESA ancillary studies (MESA Family and MESA Air) (21) at 1 of 5 field centers located across the United States: Los Angeles County, CA; Chicago, IL; Baltimore and Baltimore County, MD; Forsyth County, NC; and Northern Manhattan and the Bronx, NY. We excluded MESA participants with a clinical history of primary hyperparathyroidism or sarcoidosis, a kidney stone within the last 5 y, a history of kidney dialysis or transplant, serum calcium >11 mg/dL at the baseline MESA examination, a self-reported history of hypercalcemia, current use of vitamin D >1000 IU/d or any activated vitamin D product, and an inability to provide informed consent.
Intervention
Participants were randomly assigned to 16 wk of oral vitamin D3 at a dose of 2000 IU/d or masked placebo in a 3:1 ratio because the short-term variability in response was expected to be substantially larger in the vitamin D3 group than in the control group. Assignments were computer-generated in blocks of 4, stratified by MESA study site. Treatment assignments were concealed to both participants and investigators. Blood and urine samples were collected at the baseline visit and at a single trial-specific study visit 16 wk after randomization for ascertainment of outcomes. Adherence by pill count and adverse events were also ascertained at the 16-wk study visit.
Outcomes
Two co-primary outcomes were the changes in serum PTH and total 1,25(OH)2D concentrations from baseline to 16 wk. Secondary outcomes were changes in serum total 25(OH)D and urine calcium excretion (as a marker of intestinal calcium absorption) (22, 23), quantified using spot urine calcium-to-creatinine ratios from a single-void urine sample. PTH was measured with the Beckman-Coulter DxI 800 automated 2-site immunoassay (Beckman-Coulter, Inc.). Serum 1,25-dihydroxyvitamin D2, 1,25-dihydroxyvitamin D3, 25(OH)D2, and 25(OH)D3 were measured by immunoaffinity and LC–tandem MS (LC-MS/MS) (24, 25). Total 1,25(OH)2D and 25(OH)D were calculated by the sum of their respective vitamin D2 and D3 concentrations. Interassay CVs (CV%) were calculated using repeat measurements of quality-control specimens (19). Our laboratory has been active participants in the Vitamin D Standardization Program, and our method for 25(OH)D is traceable to the relevant National Institute of Standards and Technology (NIST) and CDC reference measurement procedures (26). Specifically, using our in-house matrix-matched quality-control materials, the total mean CV% for total 25(OH)D across the entire study (June 2017 to April 2019) was 4.3%. Analysis of the NIST Standard Reference Materials (SRM) 972a by our immunoaffinity enrichment assay for total 25(OH)D demonstrated a mean bias of 2.0%; our accuracy-based vitamin D (ABVD) survey results for the same assay had a mean bias of 4.5%. Urine calcium, creatinine, and albumin were measured on a Beckman-Coulter DxC 600.
Covariates
Baseline demographics, including race/ethnicity, smoking status, and comorbidities, were ascertained at the baseline MESA INVITe examination through self-administered questionnaires, interviewer-administered standardized interviews, extensive in-person examinations, and laboratory data. Participants identified themselves as belonging to 1 of 4 racial/ethnic groups: Black, Chinese, Hispanic, or White. Diabetes status was defined by the use of an oral hypoglycemic medication or insulin, fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, or glycated hemoglobin (HbA1c) ≥6.5% (27). Weight and height were measured and used to calculate BMI in units of kg/m2. eGFR was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation (28). HbA1c and serum creatinine were analyzed at the Collaborative Studies Clinical Laboratory at the University of Minnesota Medical Center, Fairview (Minneapolis, MN), a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory. HbA1c values are harmonized to the National Glycohemoglobin Standardization Program (29), and serum creatinine is standardized with an analytical CV% of 2.2%.
1,25(OH)2D induces the metabolic clearance of 25(OH)D into 24,25-dihydroxyvitamin D [24,25(OH)2D] (15, 30), such that the 24,25(OH)2D-to-25(OH)D ratio [also known as the vitamin D metabolic ratio (VDMR)] has been used as a novel marker of functional vitamin D activity, and was evaluated as a potential modifier of vitamin D treatment response (31, 32). Serum concentrations of 24,25-dihydroxyvitamin D3 [24,25(OH)2D3] and vitamin D binding protein (VDBP) were measured by LC-MS/MS (24, 25, 33). Using our in-house matrix-matched quality-control materials, the total mean CV% for 24,25(OH)2D3 and VDBP across the entire study was 7.5% and 3.6%, respectively. As there was no spectroscopic evidence of 24,25-dihydroxyvitamin D2, the VDMR was calculated by dividing the baseline concentrations of 24,25(OH)2D3 by 25(OH)D3, and then multiplying by 1000, such that its units are in picograms per nanogram (16). Free 25(OH)D was calculated using the methods of Bikle et al. (34) and was used to calculate bioavailable 25(OH)D using equations developed by Vermeulen et al. (35). Serum calcium, phosphorous, and albumin were measured using the Beckman-Coulter AU 5812.
Statistical analysis
A sample size of 682 was calculated using a simulation study and linear regression to provide 80% power to detect clinically meaningful associations of individual factors with the co-primary outcomes, at a 2-sided α = 0.05 (19). Specifically, a sample size of 682 provided 80% power to detect a difference of 2.5 pg/mL in the effect of vitamin D treatment on change in PTH per each 10-ng/mL increment in baseline 25(OH)D concentration. This assumed an SD of 32 pg/mL for PTH. All participants were analyzed according to their randomized treatment group, regardless of adherence or follow-up. To assess potential modifiers of the vitamin D treatment response, we first restricted analyses to participants who were assigned to vitamin D3. The within-treatment approach was prespecified prior to the analysis, as were all potential modifiers of treatment response, which included age, sex, race/ethnicity, BMI, eGFR, the VDMR, and baseline concentrations of bioavailable 25(OH)D, total 25(OH)D, and VDBP. We performed linear regression with change in outcome as the dependent variable and potential modifiers as independent variables. For each potential modifier, an initial model was unadjusted, whereas a second model adjusted for age, sex, race/ethnicity, BMI, eGFR, and season at the baseline INVITe blood draw (as a categorical variable), selected a priori because their inclusion would make the primary inference more precise. All assumptions for the linear regression models were tested and there were no violations. We utilized the placebo group in sensitivity analyses of the full study population to confirm that associations between baseline characteristics with change in the outcomes were specific to treatment—that is, that they could not be explained by differences in change in the outcomes that occur with the simple passage of time. Specifically, we tested for interactions of potential modifiers with treatment assignment using Wald tests with Huber-White robust SEs. Multiple imputation (M = 20) with chained equations was used to impute missing data. We included modifiers of interest, model covariates, and follow-up outcomes in the imputation models. The resulting estimates were combined across imputations using Rubin's rules (36). Sensitivity analyses restricted to participants with complete data were also performed.
Among vitamin D3–treated participants, we used a threshold linear regression model to estimate the baseline 25(OH)D concentration where a segmented threshold effect occurred in its relation with the 16-wk change in PTH concentration, which has been previously shown to give well-calibrated inference in settings similar to this study (37). The CI for the change point used robust SEs and statistical significance for the change in association at the change point was based on a maximum likelihood ratio statistic. We used a Monte Carlo procedure with 10,000 samples from a multivariate normal distribution corresponding to model parameters to determine a reference distribution for the test statistic. Multiple imputation was not used for this analysis. Two-sided P < 0.05 was considered statistically significant. All analyses were conducted with R version 3.6.1 (R Foundation for Statistical Computing).
Results
Participant characteristics
The mean age of participants was 72 ± 8 y, 53% were female, and 66% were of racial or ethnic minority. Baseline characteristics were similar between the treatment groups, except that hypertension was more prevalent in the vitamin D3 group (67% vs. 57%; Table 1). The mean concentrations of vitamin D metabolism measures at baseline were 30 ± 11 ng/mL for total 25(OH)D, 48 ± 27 pg/mL for PTH, and 50 ± 18 pg/mL for total 1,25(OH)2D, and were similar between the treatment groups (Table 2).
TABLE 1.
Baseline characteristics of participants in the MESA INVITe1
| Placebo (n = 167) | Vitamin D3 (n = 499) | |
|---|---|---|
| Age, mean (SD), y | 72 (8) | 72 (8) |
| Female, n (%) | 81 (49) | 272 (55) |
| Race/ethnicity, n (%) | ||
| White | 58 (35) | 169 (34) |
| Black | 61 (37) | 184 (37) |
| Chinese | 19 (11) | 64 (13) |
| Hispanic | 29 (17) | 82 (16) |
| Study site, n (%) | ||
| Forsyth County, NC | 19 (11) | 57 (11) |
| Baltimore and Baltimore County, MD | 32 (19) | 96 (19) |
| Northern Manhattan and the Bronx, NY | 46 (28) | 140 (28) |
| Chicago, IL | 45 (27) | 130 (26) |
| Los Angeles County, CA | 25 (15) | 76 (15) |
| Gross annual family income ($), n (%) | ||
| <25,000 | 39 (23) | 110 (22) |
| 25,000–49,999 | 39 (23) | 132 (27) |
| 50,000–74,999 | 47 (28) | 151 (30) |
| 75,000–100,000 | 28 (17) | 56 (11) |
| >100,000 | 14 (8) | 50 (10) |
| Season at MESA INVITe baseline exam, n (%) | ||
| January–March | 44 (26) | 126 (25) |
| April–June | 53 (32) | 167 (34) |
| July–September | 45 (27) | 129 (26) |
| October–December | 25 (15) | 77 (15) |
| Ever smoker, n (%) | 91 (55) | 254 (51) |
| Antihypertensive medication use, n (%) | 94 (56) | 306 (61) |
| Nonstudy vitamin D supplements,2n (%) | ||
| None | 112 (67) | 303 (61) |
| 1–400 IU/d | 13 (8) | 26 (5) |
| 401–1000 IU/d | 40 (24) | 167 (34) |
| Prevalent CVD, n (%) | 5 (3) | 29 (6) |
| Hypertension, n (%) | 95 (57) | 332 (67) |
| Diabetes, n (%) | 33 (20) | 105 (21) |
| Systolic BP, mean (SD), mm Hg | 127 (20) | 126 (19) |
| BMI, mean (SD), kg/m2 | 28.4 (5.3) | 29.1 (5.9) |
| Creatinine, median (IQR), mg/dL | 0.87 (0.77, 1.00) | 0.90 (0.75, 1.04) |
| eGFR, mean (SD), mL/min/1.73 m2 | 80 (15) | 77 (18) |
| UACR, median (IQR), mg/g | 4 (3, 14) | 5 (3, 14) |
BP, blood pressure; CVD, cardiovascular disease; eGFR, estimate glomerular filtration rate; MESA INVITe, Multi-Ethnic Study of Atherosclerosis Individualized Response to Vitamin D Treatment Trial; UACR, urine albumin to creatinine ratio.
Mean daily intake from all reported supplements.
TABLE 2.
Effects of vitamin D3 compared with placebo on change in primary study outcomes and related measures of vitamin D metabolism1
| Placebo (n = 167) | Vitamin D3 (n = 499) | Difference in change from baseline2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline3 | After treatment3 | Change from baseline, mean (SD)4 | Baseline3 | After treatment3 | Change from baseline, mean (SD)4 | Mean difference (95% CI) | P 5 | |
| Primary study outcomes | ||||||||
| PTH, pg/mL | 44 (20) | 46 (24) | 2 (18) | 49 (29) | 46 (26) | –3 (16) | –5 (–8, –2) | <0.001 |
| Total 1,25(OH)2D, pg/mL | 50 (17) | 50 (15) | –1 (17) | 50 (19) | 52 (19) | 2 (16) | 2 (–1, 5) | 0.133 |
| Related measures of vitamin D metabolism | ||||||||
| Total 25(OH)D, ng/mL | 29 (10) | 28 (10) | –2 (6) | 30 (11) | 41 (11) | 11 (10) | 12 (10, 14) | <0.001 |
| Bioavailable 25(OH)D, ng/mL | 3.6 (1.2) | 3.3 (1.2) | –0.2 (0.7) | 3.7 (1.3) | 5.0 (1.4) | 1.3 (1.3) | 1.5 (1.3, 1.7) | <0.001 |
| 24,25(OH)2D3, pg/mL | 1.8 (1.0) | 1.6 (0.9) | –0.2 (0.6) | 1.9 (1.1) | 2.9 (1.6) | 1.1 (1.3) | 1.2 (1.0, 1.4) | <0.001 |
| VDMR, pg/ng | 62 (21) | 58 (21) | −3 (11) | 60 (23) | 72 (22) | 12 (16) | 15 (12, 17) | <0.001 |
| VDBP, μg/mL | 226 (31) | 227 (33) | 1 (19) | 223 (30) | 223 (36) | 0 (25) | 0 (-4, 4) | 0.965 |
| Albumin, mg/dL | 4.2 (0.3) | 4.1 (0.3) | 0 (0.2) | 4.2 (0.2) | 4.1 (0.2) | 0 (0.2) | 0 (0, 0) | 0.503 |
| Calcium, mg/dL | 9.4 (0.4) | 9.5 (0.4) | 0 (0.3) | 9.5 (0.4) | 9.5 (0.4) | 0 (0.3) | 0 (-0.1, 0.1) | 0.898 |
| Phosphorus, mg/dL | 3.3 (0.5) | 3.4 (0.5) | 0 (0.5) | 3.4 (0.4) | 3.4 (0.6) | 0.1 (0.5) | 0 (-0.1, 0.1) | 0.763 |
| Urine calcium/creatinine, mg/g | 76 (39, 114) | 68 (35, 126) | 1 (70) | 64 (32, 119) | 72 (34, 137) | 8 (83) | 7 (-8, 21) | 0.358 |
PTH, parathyroid hormone; VDBP, vitamin D binding protein; VDMR, vitamin D metabolite ratio [24,25(OH)2D3 to 25(OH)D3]; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
Modeled differences compare participants randomly assigned to vitamin D3 with those randomly assigned to placebo, and account for missing data using multiple imputation.
Calculated using participants with complete case data and are shown as mean (SD) except for urine calcium/creatinine, which is median (IQR).
Change from baseline after 16 wk of treatment calculated using participants with complete case data.
Test of the difference in change in vitamin D metabolism measured from baseline after 16 wk of treatment.
Retention and adherence
Of the 666 participants who were enrolled, 499 were randomly assigned to vitamin D3 and 167 to placebo. Six-hundred and eleven (92%) completed the study (Supplemental Figure 1). Among 600 participants with completed pill counts, the median (IQR) adherence to study medications was 98% (90, 100) and adherence was within 20% of expected (80% to 120% adherence) for 515 participants (86%). The mean serum 25(OH)D concentrations at the final study visit were 41 ± 11 ng/mL and 28 ± 10 ng/mL for the vitamin D3 and placebo groups, respectively (Table 2).
Change in vitamin D metabolism measures
The mean change in PTH concentration after 16 wk of treatment was −3 ± 16 pg/mL with vitamin D3 compared with 2 ± 18 pg/mL with placebo (estimated mean difference: –5; 95% CI: –8, –2 pg/mL; Table 2). The mean change in total 25(OH)D concentration was 11 ± 10 ng/mL with vitamin D3 compared with −2 ± 6 ng/mL with placebo (estimated mean difference: 12; 95% CI: 10, 14 ng/mL). There was no significant change in 1,25(OH)2D concentration or urine calcium excretion in either treatment group.
Modifiers of change in PTH
Within the vitamin D3 group, lower baseline total and bioavailable 25(OH)D concentrations and lower VDMR were associated with larger declines in PTH after adjusting for age, sex, race/ethnicity, BMI, eGFR, and season at the baseline exam (Table 3). Participants with baseline 25(OH)D <20 ng/mL had the largest decline in PTH (–11; 95% CI: –15, –6 pg/mL). In categorical analysis with baseline 25(OH)D ≥30 ng/mL as the reference, 25(OH)D <20 ng/mL was associated with a larger decline in PTH (–10; 95% CI: –15, –6 pg/mL), whereas 25(OH)D in the 20–30-ng/mL range was not (–2; 95% CI: –6, 1 pg/mL; Table 3 and Figure 1). Consistent results were obtained in placebo-controlled analyses using the full study population, where the probability that a participant will have the largest decline in PTH was seen in vitamin D3–treated participants with 25(OH)D <20 ng/mL (Supplemental Table 1 and Figure 1). Results remained consistent in sensitivity analysis excluding an outlier participant with change in PTH of –151 pg/mL. Concordantly, we identified a segmented threshold effect at a 25(OH)D concentration of 21 (95% CI: 13, 31) ng/mL in its association with the change in PTH (Figure 2). The difference in change in PTH comparing 25(OH)D <20 ng/mL with ≥20 ng/mL was largest among White individuals, but the test for 25(OH)D category–race interaction did not reach nominal statistical significance (P-interaction = 0.134; Table 4). In exploratory analysis that included an additional baseline 25(OH)D category of <12 ng/mL, we found a graded PTH response in those with 25(OH)D <20 ng/mL; specifically, the 20 participants with 25(OH)D <12 ng/mL had a greater reduction in PTH (–19; 95% CI: –34, –5 pg/mL) compared with participants with 25(OH)D between 12 and 20 ng/mL (−8; 95% CI: –11, –4 pg/mL; Supplemental Table 2).
TABLE 3.
Associations of baseline characteristics with change in PTH concentration (pg/mL) among participants assigned to vitamin D31
| Change in PTH from baseline, mean (95% CI)3 | Difference in change in PTH (95% CI)2 | ||||
|---|---|---|---|---|---|
| Variable | n | Unadjusted model | Adjusted model4 | P 5 | |
| Age (per decade) | 499 | 0 (–1, 2) | 0 (–2, 2) | 0.785 | |
| Sex | |||||
| Female | 272 | –3 (–5, –1) | Ref | Ref | 0.666 |
| Male | 227 | –3 (–5, –2) | –1 (–4, 2) | 0 (–2, 2) | |
| Race/ethnicity | |||||
| White | 169 | –3 (–6, 0) | Ref | Ref | 0.591 |
| Black | 184 | –4 (–6, –2) | –2 (–5, 2) | –1 (–5, 2) | |
| Hispanic | 82 | –1 (–4, 2) | 2 (–3, 6) | 2 (–3, 6) | |
| Chinese | 64 | –3 (–5, 0) | 0 (–5, 5) | 0 (–5, 5) | |
| BMI (kg/m2) | |||||
| <25 | 127 | –3 (–5, –1) | Ref | Ref | 0.307 |
| 25 to <30 | 189 | –1 (–4, 1) | 2 (–2, 6) | 2 (–2, 6) | |
| 30 to <35 | 107 | –5 (–9, –2) | –2 (–6, 2) | –2 (–6, 3) | |
| ≥35 | 76 | –4 (–7, 0) | 0 (–5, 4) | 0 (–5, 5) | |
| Creatinine (per 0.5 mg/dL) | 499 | –1 (–4, 2) | 0 (–3, 3) | 0.985 | |
| eGFR (per 10 mL/min/1.73m2) | 499 | 0 (–1, 1) | 0 (–1, 1) | 0.643 | |
| Bioavailable 25(OH)D (per 1-ng/mL decrement) | 499 | –3 (–4, –2) | –3 (–5, –2) | <0.001 | |
| 25(OH)D (ng/mL) | |||||
| <20 | 79 | –11 (-15, –6) | –10 (–14, –6) | –10 (–15, –6) | |
| 20 to <30 | 144 | –3 (–5, –1) | –2 (–5, 1) | –2 (–6, 1) | |
| ≥30 | 230 | –1 (–2, 1) | Ref | Ref | <0.001 |
| Per 10-ng/mL decrement | 499 | –4 (–5, –2) | –4 (–5, –3) | <0.001 | |
| VDBP (per 1-SD increment) | 499 | –1 (–2, 1) | –1 (–2, 1) | 0.380 | |
| VDMR tertiles6 | |||||
| Tertile 1 | 149 | –6 (–9, –3) | –4 (–8, –1) | –5 (–8, –1) | |
| Tertile 2 | 155 | –2 (–4, 0) | –1 (–4, 3) | –1 (–5, 3) | |
| Tertile 3 | 149 | –1 (–3, 1) | Ref | Ref | 0.030 |
| Per 1-SD decrement | 499 | –2 (–3, –1) | –2 (–4, –1) | 0.007 | |
eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; Ref, reference; VDBP, vitamin D binding protein; VDMR, vitamin D metabolite ratio [24,25-dihydroxyvitamin D3 to 25(OH)D3]; 25(OH)D, 25-hydroxyvitamin D.
Modeled estimates account for missing data using multiple imputation.
Change from baseline after 16 wk of vitamin D3 summarized over all participants (n = 499) using multiple imputation. The mean (95% CI) among all vitamin D3 participants was −3 (−4, −2) pg/mL.
Adjusted for age, sex, race/ethnicity, BMI, eGFR, and season at baseline exam, except for the model assessing serum creatinine, which was adjusted for age, sex, race/ethnicity, BMI, and season at baseline exam.
Test statistic for the covariate-adjusted model.
Tertile cutoffs are based on the full study population.
FIGURE 1.
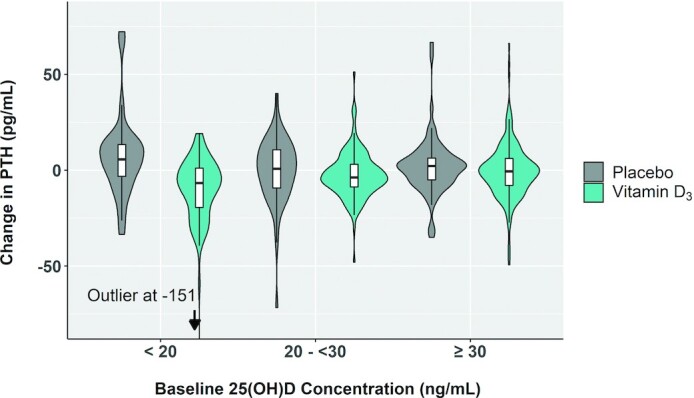
Change in PTH by baseline 25(OH)D. The horizontal line in each white box indicates the median; top and bottom box borders indicate the first and third quartiles, respectively. The vertical whiskers extending from the boxes depict the most extreme observation within 1.5 times the IQR of the nearest quartile. On each side of the white boxes are kernel density estimations that show the distribution shape of the data. Wider sections represent a higher probability that participants of the population (n = 453) will take on the given value; the thinner sections represent a lower probability. PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
FIGURE 2.
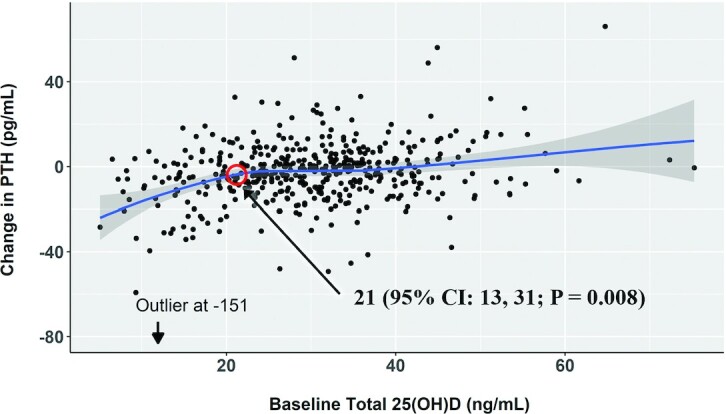
Loess curve showing change in PTH by baseline 25(OH)D among participants assigned to vitamin D3 (n = 453). The shaded area represents the pointwise 95% CI around the values on the fitted Loess curve. The red circle at 21 ng/mL represents the 25(OH)D concentration where a segmented threshold effect was seen using a threshold linear regression model with robust SEs (37). The test for statistical significance is based on a maximum likelihood ratio statistic. PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
TABLE 4.
Associations of baseline 25(OH)D and change in parathyroid hormone by race/ethnicity among participants assigned to vitamin D31
| Parathyroid hormone, pg/mL | |||||
|---|---|---|---|---|---|
| Baseline 25(OH)D (ng/mL) | n | Baseline, mean (SD) | After treatment, mean (SD) | Change from baseline, mean (95% CI) | Difference in change,2 mean (95% CI) |
| White | |||||
| <20 | 17 | 85 (42) | 63 (24) | –22 (–41, -3) | –22 (–30, –13) |
| ≥20 | 141 | 45 (31) | 45 (30) | 0 (–3, 2) | Ref |
| Black | |||||
| <20 | 42 | 63 (32) | 53 (26) | –10 (–15, –5) | –8 (–13, –3) |
| ≥20 | 117 | 51 (26) | 49 (26) | –2 (–5, 0) | Ref |
| Hispanic | |||||
| <20 | 12 | 59 (19) | 57 (16) | –2 (–9, 5) | –1 (–10, 8) |
| ≥20 | 64 | 46 (21) | 45 (26) | –1 (–4, 3) | Ref |
| Chinese | |||||
| <20 | 8 | 44 (14) | 38 (14) | –7 (–15, 2) | –5 (–13, 3) |
| ≥20 | 52 | 36 (18) | 34 (16) | –2 (–5, 1) | Ref |
Ref, reference; 25(OH)D, 25-hydroxyvitamin D.
Differences compare participants with baseline 25(OH)D <20 ng/mL with those with 25(OH)D ≥20 ng/mL [P = 0.134 for 25(OH)D category–race interaction].
Within the vitamin D3 group, race/ethnicity, BMI, eGFR, and serum creatinine were not associated with change in PTH (Table 3), and there was no significant association between any participant characteristic with the change in 1,25(OH)2D (Supplemental Table 3). However, in placebo-controlled analyses using the full study population, BMI and race/ethnicity were associated with change in 1,25(OH)2D at nominal levels of statistical significance (Supplemental Table 1).
Secondary outcomes
Within the vitamin D3 group, the mean change in 25(OH)D concentration was greatest among those with baseline 25(OH)D concentration <20 ng/mL (17; 95% CI: 15, 19 ng/mL; Supplemental Table 4). As continuous measures, lower total and bioavailable 25(OH)D and VDMR were each individually associated with a greater increase in 25(OH)D concentration. There was no significant association between any participant characteristic with the change in urine calcium excretion (Supplemental Table 5).
Adverse events
Adverse events were similar comparing the vitamin D3 with placebo group (Supplemental Table 6).
Discussion
In this randomized trial of vitamin D in a multi-ethnic cohort of generally healthy, older adults, we assessed a broad group of potential clinical and biomarker modifiers of treatment response to vitamin D. We found that lower total and bioavailable 25(OH)D concentrations and lower VDMR were associated with a greater biological response, assessed using change in PTH concentrations from baseline to 16 wk. Our primary results and threshold regression analysis support a 25(OH)D insufficiency threshold of 20 ng/mL (50 nmol/L), although this threshold may differ by race/ethnicity. Importantly, race/ethnicity, BMI, and eGFR did not significantly modify the response to vitamin D treatment.
To our knowledge, this is the first randomized trial to identify a threshold value for 25(OH)D insufficiency based on a biological response to vitamin D treatment. In a subset of 1660 participants in the Vitamin D and Omega-3 Trial (VITAL), Luttmann-Gibson et al. (38) found that baseline 25(OH)D concentration <20 ng/mL modified the 1-y change in 25(OH)D with vitamin D supplementation. 25(OH)D, however, is an inactive metabolite that may not reliably inform tissue-level vitamin D activity, hence our decision to use change in PTH as a primary outcome. A smaller study did not find a significant modification of PTH response by baseline 25(OH)D concentration, but it was limited by small sample size (n = 112) (39). A meta-analysis of vitamin D supplementation trials did report larger decreases in PTH concentration when baseline mean 25(OH)D concentration was <20 ng/mL, compared with ≥20 ng/mL, but this analysis was performed on the trial rather than the individual level (40). Subgroup analyses of large clinical trials, such as VITAL (6) and the Vitamin D Assessment Study (ViDA) (8) have not found significant effect modification by baseline 25(OH)D concentration on clinical outcomes, but these trials were not adequately powered to test for interactions, whereas the current study was specifically designed with quantitative biochemical outcomes to provide such power.
Altogether, limited trial data exist to guide recommendations for 25(OH)D thresholds to define insufficiency and trigger treatment, which vary across professional societies. The National Academy of Medicine (formerly the Institute of Medicine) has concluded that 20 ng/mL is the maximal 25(OH)D concentration needed to optimize bone health (11). Yet others, such as the Endocrine Society, have advocated for more aggressive treatment to a 25(OH)D concentration of 30 ng/mL to achieve maximal PTH suppression and calcium absorption (10, 41), which comes with potential risks (42–44). In this debate, our study provides additional data supporting the lower threshold of 20 ng/mL. Individuals with 25(OH)D <12 ng/mL are considered by some, including the National Academy of Medicine, to be at the highest risk of the adverse health consequences of vitamin D deficiency. This is also supported by our exploratory analysis, which saw the greatest change in PTH with vitamin D treatment in this group. However, the CIs for mean PTH reduction in those with 25(OH)D <12 and 12–20 ng/mL overlap; given the small number of participants with 25(OH)D <12 ng/mL, the observed differences in PTH reduction between these 2 groups are unlikely to be statistically significant. Larger studies with more individuals with 25(OH)D <12 ng/mL are needed, including future clinical trials that use disease endpoints. Our study used the individual change in PTH as a relative measure of treatment response and does not imply that specific PTH values or the absolute change in PTH observed should be used as treatment targets for the general population.
There was no difference in change in 1,25(OH)2D comparing the 2 treatment groups, which is consistent with other vitamin D supplementation studies (45, 46). This suggests that 1,25(OH)2D concentrations are tightly regulated and, in retrospect, was not a good outcome for this study. Given the observed PTH response, the effects of vitamin D treatment on PTH do not seem to be mediated by circulating 1,25(OH)2D. Prior trials of vitamin D also show no changes in urine calcium excretion that are attributable to the supplementation (47–50). Future studies should consider using more precise measures of intestinal calcium absorption.
Prior studies evaluating whether the VDMR modifies vitamin D treatment response have been inconclusive. A post hoc analysis of a randomized trial of oral vitamin D3 showed that lower VDMR predicted a greater 6-wk increase in 25(OH)D concentrations in the vitamin D treatment group (n = 60) (51). On the contrary, Francic et al. (52) did not find an association between baseline VDMR and the 8-wk change in 25(OH)D3 concentrations among vitamin D–treated participants (n = 52) in their analysis of a separate randomized trial. Our study was comparatively much larger in size and longer in duration and suggests that the VDMR may have clinical utility in predicting vitamin D treatment response, although whether it offers incremental value over baseline 25(OH)D is unclear.
We report a few pertinent negative findings. Studies assessing BMI as a modifier of vitamin D treatment response have yielded mixed results (38, 39, 53–55). We did not observe effect modification by BMI among participants treated with vitamin D, similar to 2 randomized trials assessing this relation (n = 1600 and n = 392) (38, 53). Racial differences in vitamin D metabolism have been well documented, and self-described Black participants in VITAL were reported to have larger increases in 25(OH)D and decreases in PTH than White participants with vitamin D supplementation (38). However, 2 other randomized trials, and now our study, did not find race/ethnicity to be a significant effect modifier of change in 25(OH)D or PTH concentration (56, 57). This discrepancy may be attributable to variation in African ancestry proportions across the different geographic locations in the United States from which participants were recruited. Both Black and White participants with 25(OH)D <20 ng/mL had a greater decline in PTH than those with 25(OH)D ≥20 ng/mL, and we did not find a statistically significant interaction between race/ethnicity with baseline 25(OH)D on vitamin D treatment response. However, our study was underpowered for this interaction. Because the largest estimate for change in PTH was observed among White participants with 25(OH)D <20 ng/mL, we believe there is still uncertainty about applying this threshold to people other than those who self-describe as White. In placebo-controlled analyses, associations between BMI and race/ethnicity with change in 1,25(OH)D reached nominal statistical significance, but are likely false positives given that they were not observed using the other outcome measures. Last, we hypothesized effect modification by eGFR but did not observe this.
Strengths of this study include the rigorous trial design used to assess modifiers of the vitamin D treatment response and to identify a 25(OH)D sufficiency threshold using proximate biological measures of treatment response, such as the change in PTH. Other strengths include good study retention, adherence, and consistent results in sensitivity analyses. This study also has important limitations. First, our study duration was short and the study lacks clinical endpoints. We selected this approach to most effectively interrogate individual characteristics modifying the response to vitamin D treatment, with the intent that this knowledge may be applied to subgroup analyses of longer trials with clinical outcomes. Next, our primary analysis examined only those treated with vitamin D3, where modifying characteristics were not randomized and may be confounded. Next, our results reflect a study performed in generally healthy, older adults and may not apply to other populations. Next, with 9 predictor variables (counting serum creatinine and eGFR as one) and 2 primary outcomes, multiple testing is also a concern. However, our main finding of effect modification by baseline 25(OH)D concentration would remain significant using a Bonferroni-corrected P-value threshold of 0.0028 (0.05/18). Last, race/ethnicity was self-identified rather than genetically estimated.
In conclusion, of clinical and biomarker characteristics commonly associated with circulating vitamin D concentrations and metabolism, 25(OH)D <20 ng/mL most clearly modified the PTH response to vitamin D supplementation and may be the most appropriate threshold to define vitamin D insufficiency. These findings provide a clearer and more robust definition of vitamin D insufficiency and may help guide appropriate clinical care. They may also focus post hoc analyses of completed trials on participants who are vitamin D insufficient, as some have already done, while new trials target this population.
Supplementary Material
Acknowledgments
We thank the INVITe trial Data Safety Monitoring Board [Kelley Branch, MD (chair), Edward Lipkin, MD, and Adam Szpiro, PhD] for their valuable contributions.
The authors’ responsibilities were as follows—BRK and IHdB: study oversight and data acquisition; SH, DKP, and KMR: statistical analysis; SH, DKP, DS, BRK, and IHdB: wrote the manuscript; IHdB: primary responsibility for final content; and all authors: research design, contributed to the interpretation of data, and critically read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The Multi-Ethnic Study of Atherosclerosis Individualized Response to Vitamin D (MESA INVITe) trial was funded by R01HL096875 from the National Heart, Lung, and Blood Institute (NHLBI). Additional support for MESA came from contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI; grants UL1-TR-000040 and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS); and UL1-RR-025005 from the National Center for Research Resources (NCRR). Funding for MESA Family was provided by grants R01-HL-071051, R01-HL-071205, R01-HL-071250, R01-HL-071251, R01-HL-071252, R01-HL-071258, and R01-HL-071259 from the NHLBI; UL1-TR-001079 and UL1-TR-001881 from NCATS; and grant UL1-RR-033176 from NCRR. Funding for MESA Air was provided by grants RD831697 and RD83830001 awarded by the US Environmental Protection Agency. Additional support came from grants R01DK088762, R01DK099199, 2T32DK007467-36, and 1F32DK128986-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and grant DK063491 from the NIDDK Diabetes Research Center to the Southern California Diabetes Endocrinology Research Center. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Environmental Protection Agency; or the US Department of Health and Human Services. None of the sources of support were involved in the study's design, implementation, analysis, interpretation of the data, or writing of the report. There are no restrictions regarding publication.
Supplemental Figure 1 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations used: eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; INVITe, Individualized Response to Vitamin D; MESA, Multi-Ethnic Study of Atherosclerosis; NIST, National Institute of Standards and Technology; PTH, parathyroid hormone; VDBP, vitamin D binding protein; VDMR, vitamin D metabolite ratio; VITAL, Vitamin D and Omega-3 Trial; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
Contributor Information
Simon Hsu, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA, USA.
David K Prince, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA, USA.
Kayleen Williams, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Norrina B Allen, Department of Internal Medicine, Northwestern University, Chicago, IL, USA.
Gregory L Burke, Division of Public Health Sciences Wake Forest School of Medicine, Winston-Salem, NC, USA.
Andrew N Hoofnagle, Department of Laboratory Medicine, University of Washington, Seattle, WA, USA.
Xiaohui Li, The Institute for Translational Genomics and Population Sciences, Department of Pediatrics, The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, USA.
Kiang J Liu, Department of Preventive Medicine, Northwestern University, Chicago, IL, USA.
Robyn L McClelland, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Erin D Michos, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA; Department of Epidemiology and the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Bruce M Psaty, Cardiovascular Health Research Unit, Departments of Medicine, Epidemiology, and Health Services, University of Washington, Seattle, WA, USA.
Steven J Shea, Department of Medicine, Columbia University College of Physicians and Surgeons, New York, NY, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA.
Kenneth M Rice, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Jerome I Rotter, The Institute for Translational Genomics and Population Sciences, Department of Pediatrics, The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, USA.
David Siscovick, New York Academy of Medicine, New York, NY, USA.
Russell P Tracy, Department of Biochemistry, University of Vermont, Burlington, VT, USA.
Karol E Watson, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Bryan R Kestenbaum, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA, USA.
Ian H de Boer, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval in accordance with established MESA policies.
References
- 1. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54. [DOI] [PubMed] [Google Scholar]
- 2. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 3. Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhambri R, Naik V, Malhotra N, Taneja S, Rastogi S, Ravishanker U, Mithal A. Changes in bone mineral density following treatment of osteomalacia. J Clin Densitom. 2006;9(1):120–7. [DOI] [PubMed] [Google Scholar]
- 5. Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM.. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med. 1999;341(8):563–8. [DOI] [PubMed] [Google Scholar]
- 6. Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino Det al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Boer IH, Zelnick LR, Ruzinski J, Friedenberg G, Duszlak J, Bubes VY, Hoofnagle AN, Thadhani R, Glynn RJ, Buring JEet al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2019;322(19):1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw K-T, Camargo CA Jr. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study : a randomized clinical trial. JAMA Cardiol. 2017;2(6):608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee Ret al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 11. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press, National Academy of Sciences; 2011. [Google Scholar]
- 12. Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78(5):1296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russell J, Lettieri D, Sherwood LM. Suppression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinology. 1986;119(6):2864–6. [DOI] [PubMed] [Google Scholar]
- 14. Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43(3):413–31. [DOI] [PubMed] [Google Scholar]
- 15. Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013;33(2):158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu S, Hoofnagle AN, Gupta DK, Gutierrez OM, Peralta CA, Shea S, Allen NB, Burke G, Michos ED, Ix JHet al. Race, ancestry, and vitamin D metabolism: the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2020;105(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Boer IH, Prince DK, Williams K, Allen NB, Burke GL, Hoofnagle AN, Hsu S, Li X, Liu KJ, McClelland RLet al. The Multi-Ethnic Study of Atherosclerosis individual response to vitamin D trial: building a randomized clinical trial into an observational cohort study. Contemp Clin Trials. 2021;103:106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu Ket al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 21. Kaufman JD, Spalt EW, Curl CL, Hajat A, Jones MR, Kim S-Y, Vedal S, Szpiro AA, Gassett A, Sheppard Let al. Advances in understanding air pollution and CVD. Global Heart. 2016;11(3):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devine A, Prince RL, Kerr DA, Dick IM, Criddle RA, Kent GN, Price RI, Webb PG. Correlates of intestinal calcium absorption in women 10 years past the menopause. Calcif Tissue Int. 1993;52(5):358–60. [DOI] [PubMed] [Google Scholar]
- 23. Scopacasa F, Wishart JM, Horowitz M, Morris HA, Need AG. Relation between calcium absorption and serum calcitriol in normal men: evidence for age-related intestinal resistance to calcitriol. Eur J Clin Nutr. 2004;58(2):264–9. [DOI] [PubMed] [Google Scholar]
- 24. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography–tandem mass spectrometry. Clin Chem. 2012;58(12):1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography–tandem mass spectrometry. Clin Chem. 2011;57(9):1279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sempos CT, Betz JM, Camara JE, Carter GD, Cavalier E, Clarke MW, Dowling KG, Durazo-Arvizu RA, Hoofnagle AN, Liu Aet al. General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J AOAC Int. 2017;100(5):1230–33. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37–42. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer H-M, Rohlfing C, England J, Bucksa J, Nowicki M. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Clin Chem. 2005;51(4):753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18. [DOI] [PubMed] [Google Scholar]
- 31. Ginsberg C, Katz R, de Boer IH, Kestenbaum BR, Chonchol M, Shlipak MG, Sarnak MJ, Hoofnagle AN, Rifkin DE, Garimella PSet al. The 24,25 to 25-hydroxyvitamin D ratio and fracture risk in older adults: the Cardiovascular Health Study. Bone. 2018;107:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bansal N, Katz R, Appel L, Denburg M, Feldman H, Go AS, He J, Hoofnagle A, Isakova T, Kestenbaum Bet al. Vitamin D metabolic ratio and risks of death and CKD progression. Kidney Int Rep. 2019;4(11):1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D–binding protein in Blacks and Whites. Clin Chem. 2016;62(1):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin d in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–9. [DOI] [PubMed] [Google Scholar]
- 35. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72. [DOI] [PubMed] [Google Scholar]
- 36. Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 37. Fong Y, Huang Y, Gilbert PB, Permar SR. chngpt: threshold regression model estimation and inference. BMC Bioinformatics. 2017;18(1):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luttmann-Gibson H, Mora S, Camargo CA, Cook NR, Demler OV, Ghoshal A, Wohlgemuth J, Kulkarni K, Larsen J, Prentice Jet al. Serum 25-hydroxyvitamin D in the VITamin D and Omega-3 TriaL (VITAL): clinical and demographic characteristics associated with baseline and change with randomized vitamin D treatment. Contemp Clin Trials. 2019;87:105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. Supplements of 20 μg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J Nutr. 2009;139(3):540–6. [DOI] [PubMed] [Google Scholar]
- 40. Moslehi N, Shab-Bidar S, Mirmiran P, Hosseinpanah F, Azizi F. Determinants of parathyroid hormone response to vitamin D supplementation: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2015;114(9):1360–74. [DOI] [PubMed] [Google Scholar]
- 41. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–6. [DOI] [PubMed] [Google Scholar]
- 42. Dror Y, Giveon SM, Hoshen M, Feldhamer I, Balicer RD, Feldman BS. Vitamin D levels for preventing acute coronary syndrome and mortality: evidence of a nonlinear association. J Clin Endocrinol Metab. 2013;98(5):2160–7. [DOI] [PubMed] [Google Scholar]
- 43. Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. JAMA Intern Med. 2008;168(15):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, Cao G, Burt V, Kramer H, Bailey RLet al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013;98(7):3001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batacchi Z, Robinson-Cohen C, Hoofnagle AN, Isakova T, Kestenbaum B, Martin KJ, Wolf MS, de Boer IH. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12(9):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu S, Zelnick LR, Lin YS, Best CM, Kestenbaum B, Thummel KE, Rose LM, Hoofnagle AN, de Boer IH. Differences in 25-hydroxyvitamin D clearance by eGFR and race: a pharmacokinetic study. J Am Soc Nephrol. 2021;32(1):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leaf DE, Korets R, Taylor EN, Tang J, Asplin JR, Goldfarb DS, Gupta M, Curhan GC. Effect of vitamin D repletion on urinary calcium excretion among kidney stone formers. Clin J Am Soc Nephrol. 2012;7(5):829–34. [DOI] [PubMed] [Google Scholar]
- 48. Taheri M, Tavasoli S, Shokrzadeh F, Amiri FB, Basiri A. Effect of vitamin D supplementation on 24-hour urine calcium in patients with calcium urolithiasis and vitamin D deficiency. Int Braz J Urol. 2019;45(2):340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferroni MC, Rycyna KJ, Averch TD, Semins MJ. Vitamin D repletion in kidney stone formers: a randomized controlled trial. J Urol. 2017;197(4):1079–83. [DOI] [PubMed] [Google Scholar]
- 50. Hesswani C, Noureldin YA, Elkoushy MA, Andonian S. Combined vitamin D and calcium supplementation in vitamin D inadequate patients with urolithiasis: impact on hypercalciuria and de novo stone formation. Can Urological Assoc J. 2015;9(11-12):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R.. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3-5):72–7. [DOI] [PubMed] [Google Scholar]
- 52. Francic V, Ursem SR, Dirks NF, Keppel MH, Theiler-Schwetz V, Trummer C, Pandis M, Borzan V, Grübler MR, Verheyen NDet al. The effect of vitamin D supplementation on its metabolism and the vitamin D metabolite ratio. Nutrients. 2019;11(10):2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao LJ, Zhou Y, Bu F, Travers-Gustafson D, Ye A, Xu X, Hamm L, Gorsage DM, Fang X, Deng HWet al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. J Clin Endocrinol Metab. 2012;97(8):2699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27(2):274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Canto-Costa MH, Kunii I, Hauache OM. Body fat and cholecalciferol supplementation in elderly homebound individuals. Braz J Med Biol Res. 2006;39(1):91–8. [DOI] [PubMed] [Google Scholar]
- 56. Aloia JF, Patel M, DiMaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. Vitamin D intake to attain a desired serum 25-hydroxyvitamin d concentration. Am J Clin Nutr. 2008;87(6):1952–8. [DOI] [PubMed] [Google Scholar]
- 57. Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98(3):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval in accordance with established MESA policies.


