ABSTRACT
Background
Prenatal multiple micronutrient supplementation (MMS) and lipid-based nutrient supplementation (LNS) can improve birth outcomes relative to iron-folic acid supplementation (IFA); however, effects on child postnatal growth remain unclear.
Objectives
The aim was to compare the effect of prenatal MMS, medium-quantity LNS (MQ-LNS), and IFA on child growth up to 2 y of age.
Methods
We conducted a cluster randomized controlled trial of prenatal nutritional supplementation in Madarounfa, Niger. Villages were randomly assigned for pregnant women to receive IFA (17 villages, 1105 women), MMS (18 villages, 1083 women) or MQ-LNS (18 villages, 1144 women). Pregnant women received nutritional supplements weekly until delivery, and children were followed up monthly from 6-8 wk to 24 mo of age. We assessed the effect of prenatal MMS and MQ-LNS compared with IFA and the effect of prenatal MMS compared with MQ-LNS on child length-for-age z scores (LAZ), weight-for-age z scores (WAZ), and weight-for-length z scores (WLZ) at 24 mo of age using generalized linear models. In secondary analyses, we used mixed-effects models to assess the trajectories of anthropometric z scores longitudinally from 6–8 wk to 24 mo.
Results
Compared with IFA, MMS and MQ-LNS had no effect on child LAZ, WAZ, or WLZ at 24 mo of age (P > 0.05). Children in the MQ-LNS arm had significantly higher mid-upper arm circumference at 24 mo than children in the MMS arm: mean difference 0.50 cm (95% CI 0.10, 0.91 cm). WAZ and WLZ trajectories were more negative in the MQ-LNS arm compared with IFA and MMS, with lower z scores from 14 to 20 mo of age. However, WAZ and WLZ trajectories converged after 20 mo of age, and there were no differences by 24 mo of age.
Conclusions
Prenatal MMS and MQ-LNS had limited effect on anthropometric measures of child growth up to 24 mo of age as compared with IFA in rural Niger.
Keywords: child growth, multiple micronutrient supplementation, prenatal supplementation, lipid-based nutrient supplements, Niger
Introduction
Maternal undernutrition in pregnancy is a major risk factor for poor child growth (1–3). In low- and middle-income countries (LMICs), 14% of child stunting is attributable to fetal growth restriction (4), and low maternal body mass index (BMI) in pregnancy is a leading risk factor for child wasting (5). Multiple concurrent micronutrient deficiencies and macronutrient deficiency are common among pregnant women in LMICs due to low dietary intake and increased nutrient demands in pregnancy (6, 7). Although data on micronutrient deficiencies among pregnant women are sparse, data on women of reproductive age in Africa suggest that ∼40% are folate or zinc deficient, 30% are iodine deficient, and 20% are vitamin B-12 deficient (6). In addition, 14% of pregnant women in LMICs are macronutrient deficient, as defined by low BMI (in kg/m2; <18.5) (3). Supplementation with multiple micro- and/or macronutrients could improve maternal nutrition in pregnancy in LMICs where pregnant women are at risk of undernutrition.
Prenatal multiple micronutrient supplementation (MMS) is one strategy to address maternal micronutrient deficiencies during pregnancy and improve birth outcomes (8, 9). Compared with iron and folic acid supplementation (IFA), MMS reduces the risk of stillbirth, low birth weight (LBW), small-for-gestational age, and preterm birth (6, 10–12). Although MMS has been shown to reduce adverse birth outcomes, which may contribute to poor postnatal child growth outcomes (4), evidence on the effect of MMS on postnatal child growth remains limited. Meta-analyses and systematic reviews suggest no overall effect of prenatal MMS on child stunting, wasting, or underweight as compared with IFA; however, only 7 trials were identified in the most recent meta-analysis (8, 12–14). In certain contexts, individual trials have demonstrated small-to-medium–sized benefits on anthropometric z scores and large reductions in child stunting (15–17), suggesting heterogeneity of effects across settings.
Another strategy to address both macro- and micronutrient deficiencies during pregnancy is prenatal supplementation with lipid-based nutrient supplements (LNS). LNS are ready-to-use supplements, which typically contain many of the same vitamins and minerals as MMS, but also provide energy, protein, and fatty acids (18). Prenatal LNS can improve birth outcomes, such as birth weight, birth length, and newborn stunting, as compared with IFA (19); however, effects on postnatal child linear or ponderal growth have not been demonstrated compared with IFA (19) or MMS (20).
Given the high burden of maternal undernutrition in LMICs and its potential consequences for child outcomes, more evidence is needed on the effect of prenatal nutritional supplementation on improving child outcomes in infancy. In this study, we assessed the effect of prenatal supplementation with MMS or LNS as compared with routine IFA on child growth outcomes in the first 2 y of life in rural Niger. In this setting, MMS and LNS supplementation had no effect on LBW and had a limited effect on gestational weight gain (Rattigan SM, Garba S, Plikaytis B, Sudfeld CR, Guindo O, Soumana I, Langendorf C, Grais RF, Isanaka S; unpublished data).
Methods
Study setting
This study was conducted in the Madarounfa Health District, Maradi region of south-central Niger. Maternal and child nutritional status in the region was poor, with 54% of children <5 y being stunted, 19% being wasted, and 43% being underweight (21). Approximately 20% of women of reproductive age were underweight (BMI <18.5) and 43% were anemic (21).
Study design, participants, and enrollment procedures
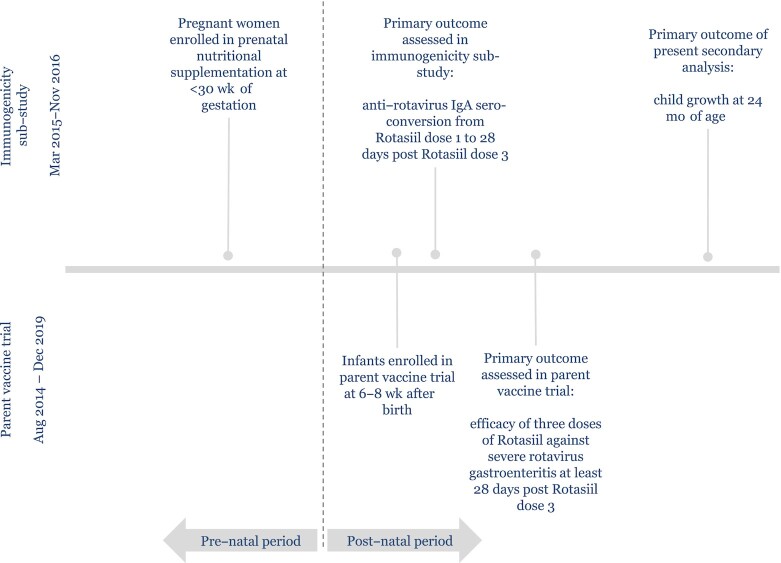
We conducted a double-blind, placebo-controlled randomized phase III clinical trial to assess the efficacy of Rotasiil (Serum Institute of India, Pvt Limited), a live, oral rotavirus vaccine against severe rotavirus gastroenteritis (22). Details on the study design, participants, and procedures of the vaccine efficacy trial have been previously published (22). Given evidence of lower efficacy of oral vaccines in high-mortality settings and the potential of nutritional supplementation to boost immunogenicity (23, 24), we nested a cluster randomized controlled trial (RCT) within the parent vaccine trial to test the effect of the type of prenatal nutritional supplementation on infant immune response to 3 doses of a live, oral rotavirus vaccine (immunogenicity trial) (25). By design, the nested immunogenicity trial was conducted concurrently with the parent vaccine trial, drawing from the same population but with separate enrollment and outcome assessment (Figure 1). The unit of randomization in the immunogenicity trial was the village (n = 53). Randomization of village clusters in the immunogenicity trial was stratified by village size (<100, 100–249, ≥250 nonpregnant women of reproductive age), and block randomization with permutated blocks of random sizes was used to allocate villages in a 1:1:1 ratio to 1 of 3 prenatal supplementation arms: 1) IFA, 2) MMS, and 3) LNS. After providing consent for village participation, the head of each village selected the name of 1 of the 3 supplements from a jar, which served as randomized village assignment. Nonpregnant women of reproductive age in participating villages provided informed consent for monthly pregnancy surveillance. Women with a confirmed pregnancy (based on a urine test) were screened for eligibility to enroll in the immunogenicity trial and begin prenatal supplementation. Inclusion criteria for pregnant women in the immunogenicity trial were as follows: <30 weeks’ gestation at the time of enrollment, intended to remain in the study area through delivery and for 2 y thereafter, and did not have a chronic health condition, severe illness, evident pregnancy complications (moderate to severe edema, hemoglobin (Hb) <7 g/dL, or diastolic blood pressure >90 mmHg), or known peanut allergy at the time of enrollment. Eligible women who provided informed consent were enrolled and received the supplement until pregnancy outcome. Women were enrolled into the immunogenicity trial from March 2015 to November 2016.
FIGURE 1.
Sequence of events in the parent vaccine trial and the immunogenicity substudy.
At 6–8 wk after birth, infants were screened for eligibility for enrollment in the parent double-blind, placebo-controlled phase III vaccine trial (22). Infant inclusion criteria for the vaccine trial were 6–8 wk of age, able to swallow and no history of vomiting within the past 24 h, parent/guardian intended to remain in the study area for 2 y, and parent/guardian provided written informed consent. Infants enrolled in the vaccine trial were followed up monthly until they reached 24 mo of age.
Study supplements
Women in the IFA arm received tablets containing 60 mg iron and 400 μg folic acid (Remedica Ltd) as the standard of care. Women were instructed to take 1 tablet daily. Women in the MMS arm received capsules containing a daily dose of 30 mg iron, 400 μg folic acid, and 20 other micronutrients (DSM Nutritional Products). The capsules provided 2 times the RDA for each micronutrient, except for iron, folic acid, calcium, phosphorous, potassium, and magnesium. This dose was more effective in improving birth weight in Guinea Bissau as compared with routine IFA relative to 1 time the RDA compared with IFA (26). Women in the LNS arm received a daily 40-g sachet of fortified, ready-to-use food made of peanuts, oil, dried skimmed-milk powder, and sugar (Nutriset S.A.S.). The LNS contained the same 22 micronutrients as the MMS. Due to its size, the product is classified as a medium-quantity LNS (MQ-LNS) (18). Detailed nutritional composition of the 3 study supplements is shown in Supplemental Table 1. Formative work conducted prior to the start of the trial showed that the 3 supplements were well accepted by the communities and pregnant women (27).
A study midwife provided the first package of supplements and instructions for use and storage at the time of enrollment. Community health assistants thereafter conducted weekly home visits to distribute a 10-d supply of the supplements: 7 days’ supply to be consumed until the next scheduled weekly home visit and 3 days’ extra supply. The extra supply was provided in case of loss, damage, or unexpected delay until the next home visit and was returned to the community health assistant at the next home visit if unused. Each week, the community health assistants reviewed supplement adherence, discussed health events and concerns since the last distribution, and provided the next 10-d supply. Since supplements were not identical in appearance, participants and study staff were not blinded to intervention allocation. Data analysts remained blinded to intervention allocation until the analysis was completed.
Data collection and measures
At enrollment, a study midwife collected data on maternal and household socioeconomic and demographic characteristics, conducted a physical and obstetric examination, and assessed maternal anthropometry, Hb, and malaria infection. Maternal weight was assessed using an electronic scale, and underweight defined as a BMI <18.5. Maternal Hb concentration was assessed from a finger-prick sample using a HemoCue machine (HemoCue Hb 301), and anemia was defined as Hb <11 g/dL. Malaria infection was assessed using a rapid diagnostic test [SD Bioline Malaria Ag Pf (HRP-2)]. Adherence to the supplementation regimen was defined as the mean percentage of supplements consumed by the woman, based on used supplement counts made by community health assistants during each home visit divided by the total number of supplements that should have been consumed from enrollment into the trial until delivery. A household wealth index was constructed using principal components analysis of 10 items describing asset and livestock ownership and housing quality. Food security was assessed using the household hunger scale (28). Improved sanitation was defined as household having access to a flush toilet, improved pit latrine, or slab latrine. Improved water source was defined as household using a covered or protected ground well for drinking water.
At infant enrollment in the vaccine trial at 6–8 wk of age, study staff assessed child growth at the health facilities. From 3 to 24 mo of age, community health assistants conducted monthly home visits to assess child growth, health, and nutrition. Child weight, length, and mid-upper arm circumference (MUAC) were assessed using standard protocols (29). Child weight was measured to the nearest 0.1 kg using a SECA scale until 6 mo and Salter scale thereafter. Recumbent length was measured to the nearest 0.1 cm using a wooden height board. We calculated anthropometric z scores according to the 2006 WHO child growth standards (30): length-for-age z score (LAZ), length-for-weight z score (WLZ), and weight-for-age z score (WAZ). Extreme values (< -6 z or > 6 z) for all anthropometric z scores were excluded. Stunting was defined as LAZ < -1, underweight as WAZ < −2, and wasting as WLZ < -2 (30).
Ethics
The study was approved by the Comité Consultatif National d'Ethique in Niger, the Comité de Protection des Personnes in France, the Commission d'Ethique de la Recherche sur l'Etre Humain, Hôpitaux Universitaires de Genève in Switzerland, the Research Ethics Review Committee of the WHO in Switzerland, and the Western Institutional Review Board in Olympia, WA, USA. An independent Data Safety and Monitoring Board, established prior to the start of the parent trial, conducted safety reviews for adverse and serious adverse events after half the pregnancies were enrolled and every 6 mo thereafter. The parent trial was registered with ClinicalTrials.gov, identifier NCT02145000.
Sample size
The primary endpoint of the immunogenicity trial designed to test the effect of prenatal nutritional supplementation on infant immune response was anti-rotavirus IgA seroconversion, defined as a ≥3-fold rise in serum titer of anti-rotavirus IgA from Rotasiil dose 1 to 28 d post–Rotasiil dose 3. The immunogenicity trial's sample size was based on power calculations to detect a 20% absolute difference in the proportion of children that seroconvert between nutritional supplements with 90% power and 0.05 α, assuming a 30% seroconversion rate in the IFA arm, 20% nonaccessibility, and 30% exclusion due to detection of rotavirus disease between Rotasiil doses (25).
Statistical analysis
In our primary analysis, we evaluated the effect of prenatal MMS and MQ-LNS compared with IFA and the effect of prenatal MQ-LNS compared with MMS on postnatal growth of singleton children at 24 mo of age. Multiple births were excluded from the analysis. We used generalized linear models to assess differences in continuous LAZ, WLZ, and WAZ at 24 mo of age and log-binomial models to assess the relative risk of stunting, wasting, and underweight at 24 mo of age. We present unadjusted mean differences (MDs) for continuous outcomes and relative risks (RRs) for binary outcomes with their 95% CIs. All models accounted for clustering at the village level using cluster-robust SEs.
In secondary analyses, we examined differences in LAZ, WAZ, and WLZ trajectories from 6–8 wk to 24 mo of age using linear mixed-effects models. Trajectory analyses included all singleton live births with at least 1 anthropometric measurement after birth. The models included the intervention arm, month of assessment, and an interaction term between these variables. The trajectory models accounted for clustering by village and a compound symmetry correlation structure for within-subject correlation. We tested for difference in trajectory over time for each group comparison: MMS vs. IFA, MQ-LNS vs. IFA, and MQ-LNS vs. MMS. If the test for difference in trajectory for a group comparison (interaction of intervention arm and assessment month) was statistically significant, we presented differences in mean z scores at each month of assessment from 6–8 wk to 24 mo. Differences were tested applying a Tukey-Kramer adjustment for multiple comparisons.
As a sensitivity analysis of the primary and secondary analyses, we estimated multivariate models to account for potential imbalance between randomized arms at enrollment and to potentially increase precision (31). We controlled for the following prespecified covariates, which are known predictors of child growth: household wealth, size, and food security; maternal age, education, and underweight; and child age and sex. We also controlled for maternal anemia, malaria infection, and whether the woman was enrolled into the immunogenicity trial during the hunger season (May–September). Multivariate models also accounted for whether the child was randomly assigned to the vaccine or placebo group of the parent vaccine trial.
Finally, we explored potential effect modification of MMS and MQ-LNS, relative to IFA, on postnatal growth outcomes by prespecified enrollment factors: maternal education, anemia, underweight, and season of enrollment into the trial; household wealth, food security, improved sanitation, and improved water source; child sex; and adherence to supplements. Interactions were considered statistically significant at P < 0.10 based on a Wald test. All analyses used the intention-to-treat principle and were conducted in Stata version 16 (StataCorp LP) (32).
Results
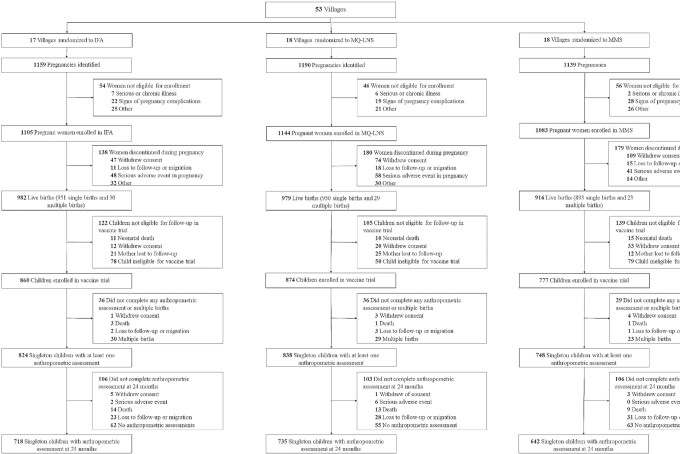
A total of 3332 pregnant women were enrolled in the immunogenicity trial (Figure 2). At enrollment, mothers’ socioeconomic and demographic characteristics were similar across intervention arms (Table 1). The current analysis of child growth outcomes included 2409 children with at least 1 anthropometric measurement, of whom 2095 (87%) had a measurement at 24 mo of age. Median adherence (Quintile1, Quintile 3) to the supplementation regimen was 84% (70%, 93%) in the IFA arm, 86% (73%, 93%) in the MMS arm, and 88% (76%, 94%) in the MQ-LNS arm (P value for differences across arms = 0.395). Results showed per-protocol efficacy of 66.7% (95% CI: 49.9%, 77.9%) (22).
FIGURE 2.
Study profile of the randomized trial of prenatal supplementation with IFA, MMS, and MQ-LNS. IFA, iron and folic acid; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements.
TABLE 1.
Socioeconomic and demographic characteristics of women enrolled in the trial at the time of enrollment1
| IFA (n = 1105) | MMS (n = 1083) | MQ-LNS (n = 1144) | |
|---|---|---|---|
| Household characteristics | |||
| Wealth index2 | 0.1 ± 1.6 | 0.2 ± 1.8 | −0.3 ± 1.6 |
| Number of children <5 y | 2.2 ± 1.9 | 2.4 ± 2.0 | 2.5 ± 1.9 |
| Number of household members | 10.5 ± 6.8 | 10.0 ± 6.5 | 10.1 ± 6.6 |
| Access to an improved latrine3 | 694 (63.3) | 605 (56.2) | 585 (51.3) |
| Access to an improved water source4 | 258 (23.4) | 267 (24.7) | 312 (27.3) |
| Little-to-no hunger in the past month5 | 974 (88.4) | 985 (91.2) | 1091 (95.8) |
| Maternal characteristics | |||
| Age, y | 26.5 ± 6.8 | 26.8 ± 6.8 | 27.0 ± 7.2 |
| Completed primary or higher education (≥6 y) | 61 (5.5) | 80 (7.4) | 66 (5.8) |
| Married or cohabitating | 1081 (97.9) | 1068 (98.6) | 1128 (98.6) |
| Anemic6 | 320 (32.5) | 317 (32.7) | 397 (38.4) |
| Underweight7 | 39 (3.9) | 37 (3.7) | 62 (5.9) |
| Malaria infection8 | 162 (16.2) | 150 (15.3) | 229 (21.9) |
| Enrolled in the hunger season (May–September) | 482 (43.7) | 363 (33.5) | 573 (50.1) |
| Gestational age, wk | 18.1 ± 3.9 | 18.3 ± 4.1 | 17.7 ± 3.9 |
Values are means ± SDs or n (%) unless otherwise specified. IFA, iron and folic acid; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements.
Constructed using principal components analysis of 10 items describing asset and livestock ownership, and housing quality.
Improved sanitation was defined as household having access to a flush toilet, improved pit latrine, or slab latrine.
Improved water source was defined as household using a covered or protected ground well for drinking water.
Based on the Household Hunger Scale (33).
Anemia defined as hemoglobin <11 g/dL.
Underweight defined as BMI <18.5 kg/m2.
Malaria infection based on a positive rapid diagnostic test.
Overall, at 24 mo of age, 69% of children were stunted, 41% were underweight, and 12% were wasted. MMS and MQ-LNS had no effect on any of the child anthropometric outcomes at 24 mo of age, relative to IFA (Tables 2 and 3). When comparing MQ-LNS with MMS, children in the MQ-LNS arm had significantly higher MUAC at 24 mo of age (MD: 0.50 cm; 95% CI: 0.10, 0.91 cm). Multivariate-adjusted estimates showed similar intervention effects (Supplemental Table 2).
TABLE 2.
Unadjusted effects of prenatal MMS, MQ-LNS, and IFA on continuous child growth outcomes at 24 mo of age1
| IFA (n = 718)2 | MQ-LNS (n = 735)2 | MMS (n = 642)2 | MQ-LNS vs. IFA3 | MMS vs. IFA3 | MQ-LNS vs. MMS3 | |
|---|---|---|---|---|---|---|
| LAZ | –2.53 ± 0.99 | –2.49 ± 0.96 | –2.60 ± 1.06 | 0.04 (–0.22, 0.30) | –0.07 (–0.33, 0.18) | 0.11 (–0.15, 0.38) |
| WAZ | –1.75 ± 1.06 | –1.87 ± 1.09 | –1.80 ± 1.08 | –0.12 (–0.44, 0.19) | –0.05 (–0.29, 0.18) | –0.07 (–0.38, 0.25) |
| WLZ | –0.59 ± 1.08 | –0.80 ± 1.32 | –0.64 ± 1.14 | –0.21 (–0.69, 0.26) | –0.05 (–0.25, 0.15) | –0.16 (–0.64, 0.32) |
| MUAC, cm | 13.69 ± 1.13 | 14.03 ± 1.12 | 13.52 ± 1.10 | 0.34 (–0.05, 0.73) | –0.16 (–0.48, 0.15) | 0.50 (0.10, 0.91) |
IFA, iron and folic acid; LAZ, length-for-age z score; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements; MUAC, midupper arm circumference; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Values are means ± SDs.
Values are mean differences (95% CIs) derived from generalized linear models. All estimates were unadjusted. SEs were clustered at the village level.
TABLE 3.
Unadjusted effects of prenatal MMS, MQ-LNS, and IFA on binary child growth outcomes at 24 mo of age1
| IFA (n = 718)2 | MQ-LNS (n = 735)2 | MMS (n = 642)2 | MQ-LNS vs. IFA3 | MMS vs. IFA3 | MQ-LNS vs. MMS3 | |
|---|---|---|---|---|---|---|
| Stunted, LAZ < –2 | 499 (69.6) | 498 (67.9) | 447 (70.0) | 0.98 (0.84, 1.14) | 1.01 (0.88, 1.14) | 0.97 (0.83, 1.13) |
| Underweight, WAZ < –2 | 277 (38.6) | 311 (42.4) | 268 (41.8) | 1.10 (0.86, 1.41) | 1.08 (0.86, 1.36) | 1.01 (0.78, 1.32) |
| Wasted, WLZ < –2 | 67 (9.3) | 113 (15.4) | 67 (10.5) | 1.65 (0.67, 4.05) | 1.12 (0.71, 1.77) | 1.47 (0.62, 3.47) |
IFA, iron and folic acid; LAZ, length-for-age z score; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Values are n (%).
Values are RRs (95% CI) derived from log-binomial models. All estimates were unadjusted. SEs were clustered at the village level.
We examined trajectories in anthropometric z scores from 6–8 wk to 24 mo of age (Figures 3 –5). Mean LAZ and WAZ over time were significantly lower in the MQ-LNS arm relative to IFA (P value for difference in trajectory <0.001, Figure 3, and P value for difference in trajectory <0.001, Figure 4, respectively), although we observed no statistically significant differences in LAZ or WAZ between intervention arms at individual time points (Supplemental Tables 3 and 4). The mean change in WLZ was significantly different in both the MMS and MQ-LNS arms (both P values for difference in trajectory <0.001), relative to IFA (Figure 5). Although children in the MQ-LNS arm had significantly higher WLZ at 1 mo of age compared with children in the IFA arm, they had lower WLZ from 16 to 19 mo of age (Supplemental Table 5). In addition, we found significant differences in mean LAZ, WAZ, and WLZ trajectories when comparing the MQ-LNS with MMS arms (all 3 P values for difference in trajectory <0.001). Relative to MMS, children in the MQ-LNS arm had significantly lower WAZ from 16 to 18 mo of age and lower WLZ from 14 to 20 mo of age (Supplemental Tables 4 and 5).
FIGURE 3.
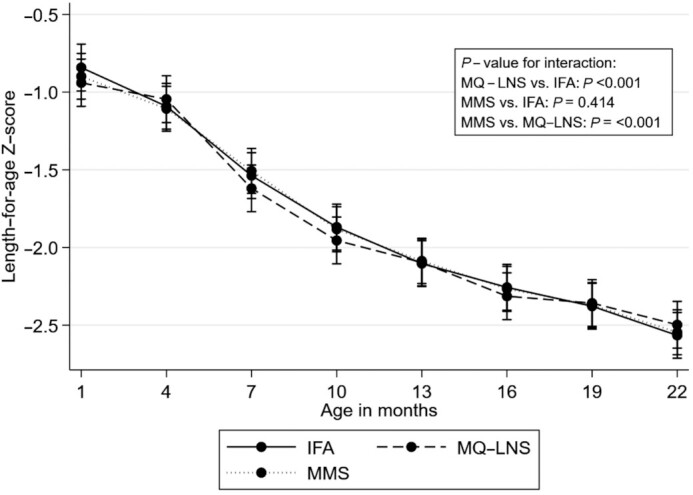
Unadjusted effect of daily prenatal supplementation with MMS or MQ-LNS as compared with IFA on child length-for-age z score from 6–8 wk to 24 mo of age. n = 824 in the IFA arm, n = 748 in the MMS arm, and n = 838 in MQ-LNS arm. Curves were derived from a linear mixed-effects model. Bars represent 95% CIs for each intervention arm at each time point. P values were derived from the interaction term between intervention arm and child age. IFA, iron and folic acid; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements.
FIGURE 5.
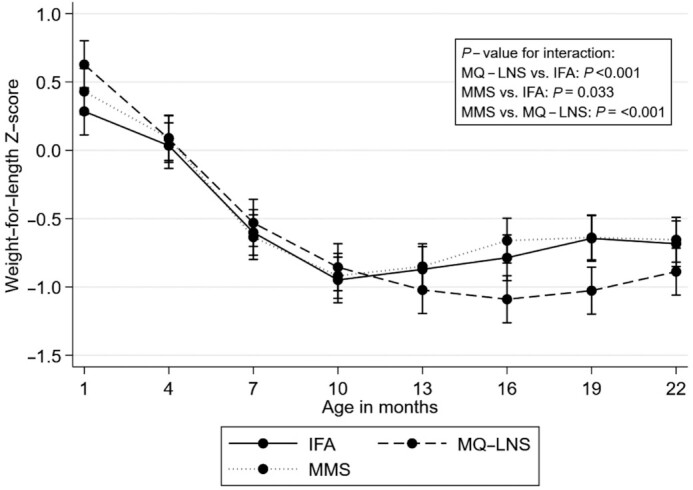
Unadjusted effect of daily prenatal supplementation with MMS or MQ-LNS as compared with IFA on child weight-for-length z score from 6–8 wk to 24 mo of age. n = 824 in the IFA arm, n = 748 in the MMS arm, and n = 838 in the MQ-LNS arm. Curves were derived from a linear mixed-effects model. Bars represent 95% CIs for each intervention arm at each time point. P values were derived from the interaction term between intervention arm and child age. IFA, iron and folic acid; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements.
FIGURE 4.
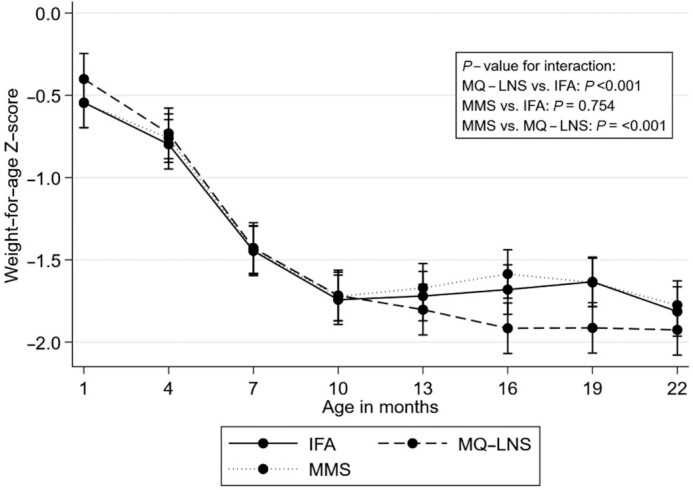
Unadjusted effect of daily prenatal supplementation with MMS or MQ-LNS as compared with IFA on child weight-for-age z score from 6–8 wk to 24 mo of age. n = 824 in the IFA arm, n = 748 in the MMS arm, and n = 838 in the MQ-LNS arm. Curves were derived from a linear mixed-effects model. Bars represent 95% CIs for each intervention arm at each time point. P values were derived from the interaction term between intervention arm and child age. IFA, iron and folic acid; MMS, multiple micronutrient supplements; MQ-LNS, medium-quantity lipid-based nutrient supplements.
In exploratory analyses to assess potential effect modifiers, we found that maternal anemia at enrollment modified the effect of MQ-LNS relative to IFA on LAZ and stunting at 24 mo of age. Specifically, the effect of MQ-LNS on LAZ as compared with IFA was MD = –0.11 (95% CI: –0.38, 0.16) among children of pregnant women who were anemic at enrollment and MD = 0.12 (95% CI: –0.16, 0.41) among children of pregnant women who were not anemic at enrollment (P-interaction = 0.02). The effect of MQ-LNS on stunting as compared with IFA was RR = 1.05 (95% CI: 0.89, 1.25) among children of pregnant women who were anemic at enrollment and RR = 0.94 (95% CI: 0.80, 1.10) among children of pregnant women who were not anemic at enrollment (P-interaction = 0.08). In addition, we found that enrollment during the hunger season modified the effect of MQ-LNS relative to IFA on wasting at 24 mo of age: RR = 2.13 (95% CI: 0.79, 5.77) among children of pregnant women enrolled during the non-hunger season (October–April); and RR = 1.12 (95% CI: 0.49, 2.55) among children of pregnant women enrolled during the hunger season (P-interaction = 0.08). Last, we found that child sex and household sanitation modified the effect of MMS relative to IFA on wasting at 24 mo. Specifically, the effect of MMS on wasting as compared with IFA was RR = 0.74 (95% CI: 0.37, 1.48) among boys and RR = 1.50 (95% CI: 0.90, 2.49) among girls (P-interaction = 0.05). The effect of MMS on wasting as compared with IFA was RR = 1.48 (95% CI: 0.91, 2.41) among children in households without an improved latrine at enrollment and RR = 0.85 (95% CI: 0.48, 1.49) among children in households with an improved latrine at enrollment (P-interaction = 0.05).
Discussion
In this cluster RCT conducted in rural Niger, prenatal MMS and MQ-LNS supplementation had no effect on child anthropometric measures at 24 mo of age relative to IFA. MQ-LNS improved MUAC at 24 mo as compared with MMS. MQ-LNS also improved WLZ at 1 mo of age relative to IFA. However, relative to both IFA and MMS, MQ-LNS led to more negative WAZ and WLZ trajectories in the second year of life, although differences in trajectories did not persist to 24 mo of age.
Prior studies to test the effect of prenatal MMS relative to IFA are few and have shown limited effect on child growth outcomes up to 24 mo of age. Several meta-analyses suggest that prenatal MMS does not improve child growth up to 9 y of age as compared with routine IFA (8, 12–14). However, individual trials in Burkina Faso, Nepal, and Vietnam have demonstrated small-to-medium–sized benefits on anthropometric z scores and large reductions in child stunting (15–17). Likewise, prior trials suggest that prenatal LNS supplementation appears to have no effect on postnatal child growth as compared with IFA, although the latest meta-analysis pooled only 4 studies (19). In undernourished populations, balanced energy and protein (BEP) supplementation, which typically provides more calories and protein than MQ-LNS, is recommended for improved birth outcomes (34). Although positive effects on small-for-gestational age and birth weight were found in 8 RCTs that used BEP supplements containing 417 to 1017 kcal with 7 to 40 g protein/d (35, 36), 1 meta-analysis showed that BEP supplementation did not increase child weight or length at 1 y of age, relative to IFA, based on 1 RCT that used supplements containing 470 kcal with 40 g protein/d (35). However, the varying macronutrient composition of individual BEP supplements used in these RCTs and this study's MQ-LNS formulation, as well as differences in the study populations, make the evidence difficult to compare.
Several explanations for the lack of effect of MMS and MQ-LNS as compared with IFA on LAZ, WLZ, and WAZ at 24 mo are plausible. First, findings from the immunogenicity trial suggested that MMS and MQ-LNS reduced LBW risk by 17% and 7%, respectively, relative to IFA; however, these findings were not statistically significant. There was also a limited effect on gestational weight gain (Rattigan SM, Garba S, Plikaytis B, Sudfeld CR, Guindo O, Soumana I, Langendorf C, Grais RF, Isanaka S; unpublished data). These results suggest that MMS and MQ-LNS in this study population did not meaningfully improve maternal undernutrition or fetal growth, which may have been expected to be on the pathway to improved child growth assessed in this analysis (4). Second, given the poor nutrient status in the region, it was hypothesized that the prenatal supplements may correct pre-existing micronutrient deficiencies (37). Although 1 time the RDA may be sufficient to improve micronutrient status but insufficient to eliminate micronutrient deficiencies as shown in 1 trial among pregnant women in rural Bangladesh (38), it is possible that 2 times the RDA provided by the MMS and MQ-LNS in this study were sufficient to reduce underlying deficiencies in the mother but may have still been insufficient to channel nutrients to the fetus needed for sustained improvements in child growth. Third, frequent acute or chronic infections during pregnancy, which can be prevalent in LMICs (39), can lead to nutrient losses and nutrient sequestration in the mother (26), which, in turn, may have limited the quantity of nutrients available to the fetus. Our study did not have complete data on maternal infection throughout pregnancy, but if prevalent in this setting, acute or chronic infection may have reduced the effective micronutrient dose provided to the mother through the study supplements. Likewise, acute or chronic infection in the child could have limited the potential benefits from the micronutrients received by the fetus during pregnancy. Last, IFA supplementation (60 mg Fe and 400 μg folic acid) has been shown to be effective to reduce LBW and is associated with reduced risk of stunting and higher LAZ (8, 40). However, the iron content of the MMS and MQ-LNS supplements in our study was lower (30 mg), such that we were simultaneously testing a reduced iron dose with the addition of macro- and micronutrients. Several trials have compared prenatal MMS and IFA with equal iron content (60 mg) and also showed no difference in child growth (41–43), indicating that the difference in iron content between nutritional supplements in our study might not explain the lack of effects on child growth observed later in infancy.
While we found no effect on indicators of child growth at 24 mo of age, except for MUAC when comparing MQ-LNS with MMS, we unexpectedly found that MQ-LNS led to transiently lower WAZ and WLZ from 14 to 20 mo of age as compared with IFA and MMS. One potential explanation for the observed transient deficit in WAZ and WLZ in late infancy is that infants exposed to MQ-LNS in utero had better nutritional conditions and may have been more sensitive to suboptimal postnatal environmental and nutrition factors. In a trial in Burkina Faso, which found that prenatal LNS led to greater declines in LAZ in the first year of life, as compared with MMS, Lanou et al. (20) hypothesized that these results were due to a mismatch between a better nutritional environment in utero (as evidenced by larger placentas in pregnant women who received LNS) and a poorer nutritional environment postnatally. Our findings that MQ-LNS improved WLZ at 1 mo of age relative to IFA are consistent with this hypothesis of a better nutritional environment in utero. Another potential explanation is that there was a higher propensity to share MQ-LNS with other household members or substituting it for food (27) and therefore the actual micronutrient intake in the MQ-LNS arm may have been lower than planned. Additional research is needed to understand the mechanisms that may lead to transient differences in anthropometric outcomes in children whose mothers receive prenatal MQ-LNS as compared with MMS or IFA.
Finally, we found evidence that maternal anemia at enrollment modified the effect of prenatal MQ-LNS relative to IFA on LAZ and stunting at 24 mo of age. Other studies have also demonstrated that maternal anemia during pregnancy modified the effect of MQ-LNS and MMS on birth outcomes, with larger benefits among anemic women (11, 44, 45). In this study, although we found statistical evidence of effect modification, we could not determine whether benefits were larger among anemic or nonanemic women given our wide CIs. Apart from direct improvements in maternal Hb, non-Hb pathways of impact of the supplements on child growth may include reductions in maternal and child inflammation and improvements in oxidative metabolism (11). In addition, we also found evidence of modification of the effect of MQ-LNS relative to IFA on wasting by season of enrollment into the trial, and of the effect of MMS relative to IFA on wasting by household sanitation and child sex. However, similar to maternal anemia at enrollment, we observed wide CIs and could not determine which subgroups may have benefited more from MQ-LNS or MMS. Previous studies have also shown significant interactions of the effect of prenatal LNS with other maternal characteristics at enrollment, such as maternal underweight (BMI <18.5), age, parity, and short stature (44, 45). Together with ours, these findings suggest that future interventions may target specific subgroups of pregnant women who might benefit most from prenatal LNS.
Our trial and the existing evidence suggest that, in the context of LMICs, prenatal supplementation alone is not sufficient to prevent child growth faltering (8, 12–14, 19). In our trial, nutritional supplementation started in pregnancy and therefore it is possible that preconception nutritional supplementation may confer greater benefits on child growth. Nevertheless, 1 trial conducted in 4 LMICs found that starting MQ-LNS prior to conception did not yield additional benefits on child linear growth at birth relative to starting MQ-LNS in pregnancy (46). As a result, evidence on preconception nutrition interventions in LMIC settings remains limited and it is not clear if initiation of MMS and MQ-LNS prior to conception would provide greater effects. In contrast, evidence suggests that combined pre- and postnatal supplementation might be an alternative strategy to improve child growth (47–52). Evidence on combining child micronutrient supplementation with water, sanitation, and hygiene (WASH) interventions is growing, but effects on child growth have been limited (53) and no trials to date have assessed the combination of prenatal micronutrient supplementation and WASH on postnatal child growth. For potentially more durable effects on child growth, future studies could therefore assess the effectiveness of interventions that combine prenatal micronutrient supplementation with postnatal interventions that improve child nutrition (through improved breast-milk quality or complementary feeding) and reduce environmental stressors such as WASH.
Our trial is subject to several limitations. First, we lacked data on maternal biomarkers and were therefore unable to directly assess intermediate effects of the study supplements on maternal micronutrient status. However, we observed high adherence (84% in the IFA arm, 88% in the MQ-LNS arm, and 86% in the MMS arm) and acceptability of the supplements (27), indicating improvements in micronutrient status were possible. Second, we lacked data on maternal infection and could not determine the extent to which maternal infection may have influenced nutrient availability. Third, child growth was only assessed through anthropometry. Data on child body composition might have provided a more complete picture of the effect of prenatal supplementation on child growth than anthropometry alone.
In conclusion, we found a limited effect of prenatal MMS and MQ-LNS on child growth at 24 mo of age in rural Niger. MQ-LNS increased MUAC at 24 mo as compared with MMS but appeared to lead to temporary, but more negative, WAZ and WLZ trajectories relative to IFA and MMS during the period of 14 and 20 mo of age, despite improving WLZ at 1 mo of age relative to IFA. It is important to note that, while we identified some transient differences in child growth between the prenatal supplementation arms, this study suggests that MMS and MQ-LNS were insufficient to prevent growth faltering for children in rural Niger at 24 mo of age. Future research should evaluate alternative nutritional support strategies that may improve child growth, such as combined pre- and postnatal supplementation and/or combining prenatal nutritional supplementation with interventions that reduce infections, environmental stressors, and other factors that may influence child growth in rural Niger for a more durable impact.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SI, CL, and RFG: designed the research; SG, OG, and IS: conducted the research; LB and CRS: analyzed the data; LB: wrote the first draft of the manuscript; and all authors provided important intellectual contributions, edited the manuscript, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Funding for this study was provided to Epicentre by Médecins Sans Frontières–Operational Center Geneva and the Kavli Foundation, Norway. The funders had no role in the study implementation, data collection, data analysis, data interpretation, or writing of the manuscript.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BEP, balanced energy and protein; Hb, hemoglobin; IFA, iron-folic acid supplementation; LAZ, length-for-age z score(s); LBW, low birth weight; LMIC, low- and middle-income country; LNS, lipid-based nutrient supplementation/supplements; MD, mean difference; MMS, multiple micronutrient supplements/supplementation; MQ-LNS medium-quantity lipid-based nutrient supplements/supplementation; MUAC, midupper arm circumference; RCT, randomized controlled trial; WASH, water, sanitation, and hygiene; WAZ, weight-for-age z score(s); WLZ, weight-for-length z score(s).
Contributor Information
Lilia Bliznashka, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Christopher R Sudfeld, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Souna Garba, Epicentre, Niamey, Niger.
Ousmane Guindo, Epicentre, Niamey, Niger.
Issaka Soumana, Epicentre, Niamey, Niger.
Irène Adehossi, National Pediatric Hospital, Niamey, Niger.
Céline Langendorf, Department of Research, Epicentre, Paris, France.
Rebecca F Grais, Department of Research, Epicentre, Paris, France.
Sheila Isanaka, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Research, Epicentre, Paris, France.
Data Availability
The deidentified dataset supporting this research can be made available following a submitted request as per Epicentre and General Data Protection Regulation (EU) 2016/679 data sharing policy. Additional information is available at https://epicentre.msf.org/en/data-access-request.
References
- 1. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet North Am Ed. 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 2. Black RE, Victora CG, Walker SP, Bhutta Z, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell Ret al. . Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 3. Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet North Am Ed. 2021;397(10282):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, Sania A, Smith Fawzi MC, Ezzati M, Fawzi WW. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Med. 2016;13(11):e1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Z, Kim R, Vollmer S, Subramanian SV. Factors associated with child stunting, wasting, and underweight in 35 low- and middle-income countries. JAMA Network Open. 2020;3(4):e203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourassa MW, Osendarp SJM, Adu-Afarwuah S, Ahmed S, Ajello C, Bergeron G, Black R, Christian P, Cousens S, Pee Set al. . Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low- and middle-income countries. Ann NY Acad Sci. 2019;1444(1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 8. Keats EC, Das JK, Salam RA, Lassi ZS, Imdad A, Black RE, Bhutta ZA. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc Health. 2021;5(5):367–84. [DOI] [PubMed] [Google Scholar]
- 9. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet North Am Ed. 2013;382(9890):452. [DOI] [PubMed] [Google Scholar]
- 10. Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3:(3):CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith ER, Shankar AH, Wu LS-F, Aboud S, Adu-Afarwuah S, Ali H, Agustina R, Arifeen S, Ashorn P, Bhutta ZAet al. . Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Global Health. 2017;5(11):e1090–100. [DOI] [PubMed] [Google Scholar]
- 12. Oh C, Keats EC, Bhutta ZA. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low- and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12(2):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devakumar D, Fall CHD, Sachdev HS, Margetts BM, Osmond C, Wells JCK, Costello A, Osrin D. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med. 2016;14(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu W-P, Lu M-S, Li Z-H, Zhang C-X. Effects of multimicronutrient supplementation during pregnancy on postnatal growth of children under 5 years of age: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberfroid D, Huybregts L, Lanou H, Ouedraogo L, Henry M-C, Meda N, Kolsteren P. Impact of prenatal multiple micronutrients on survival and growth during infancy: a randomized controlled trial. Am J Clin Nutr. 2012;95(4):916–24. [DOI] [PubMed] [Google Scholar]
- 16. Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet North Am Ed. 2008;371(9611):492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huy ND, Le Hop T, Shrimpton R, Hoa CV. An effectiveness trial of multiple micronutrient supplementation during pregnancy in Vietnam: impact on birthweight and on stunting in children at around 2 years of age. Food Nutr Bull. 2009;30:S506–16. [DOI] [PubMed] [Google Scholar]
- 18. Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, Dewey KG. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: Experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern Child Nutr. 2015;11:31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, Bhutta ZA. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev. 2018;8:(8):CD012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lanou H, Huybregts L, Roberfroid D, Nikièma L, Kouanda S, Van Camp J, Kolsteren P, Nikiema L, Kouanda S, Van Camp Jet al. . Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics. 2014;133(4):e1001–8. [DOI] [PubMed] [Google Scholar]
- 21. Institut National de la Statistique (INS); ICF International . Enquête Démographique et de Santé et à Indicateurs Multiples du Niger 2012. Calverton (MD): INS et ICF International; 2013. [Google Scholar]
- 22. Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo Aet al. . Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121–30. [DOI] [PubMed] [Google Scholar]
- 23. Church JA, Parker EP, Kirkpatrick BD, Grassly NC, Prendergast AJ. Interventions to improve oral vaccine performance: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(2):203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NAet al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet North Am Ed. 2010;376(9741):606–14. [DOI] [PubMed] [Google Scholar]
- 25. Isanaka S, Garba S, Plikaytis B, Malone McNeal M, Guindo O, Langendorf C, Adehossi E, Ciglenecki I, Grais RF. Immunogenicity of an oral rotavirus vaccine administered with prenatal nutritional support in Niger: a cluster randomized clinical trial. PLoS Med. 2021;18(8):e1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kæstel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr. 2005;59(9):1081–9. [DOI] [PubMed] [Google Scholar]
- 27. Clermont A, Kodish S, Matar Seck A, Salifou A, Rosen J, Grais R, Isanaka S. Acceptability and utilization of three nutritional supplements during pregnancy: findings from a longitudinal, mixed-methods study in Niger. Nutrients. 2018;10(8):1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ballard T, Coates J, Swindale A, Deitchler M. Household Hunger Scale (HHS): indicator definition and measurement guide. Washington, DC: Food and Nutrition Technical Assistance II Project, FHI 360; 2011. [Google Scholar]
- 29. Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Books; 1988. [Google Scholar]
- 30. WHO Multicentre Growth Reference Study Group . WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland); WHO; 2006. [Google Scholar]
- 31. Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity—whether and how to correct for many statistical tests. Am J Clin Nutr. 2015;102(4):721–8. [DOI] [PubMed] [Google Scholar]
- 32. StataCorp LP . Stata Statistical Software: release 16. College Station (TX): StataCorp LP; 2019. [Google Scholar]
- 33. Ballard T, Coates J, Swindale A, Deitchler M. Household Hunger Scale: indicator definition and measurement guide. Vol. 360.:Washington (DC): Food and Nutrition Technical Assistance II Project, FHI; 2011. [Google Scholar]
- 34. World Health Organization . WHO recommendations on antenatal care for a positive pregnancy experience. Geneva (Switzerland): World Health Organization; 2016. [PubMed] [Google Scholar]
- 35. Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015. [DOI] [PubMed] [Google Scholar]
- 36. Stevens B, Buettner P, Watt K, Clough A, Brimblecombe J, Judd J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: a systematic review and meta-analysis. Matern Child Nutr. 2015;11(4):415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr. 2006;83(4):788–94. [DOI] [PubMed] [Google Scholar]
- 38. Schulze KJ, Mehra S, Shaikh S, Ali H, Shamim AA, Wu LS-F, Mitra M, Arguello MA, Kmush B, Sungpuag Pet al. . Antenatal multiple micronutrient supplementation compared to iron–folic acid affects micronutrient status but does not eliminate deficiencies in a randomized controlled trial among pregnant women of rural Bangladesh. J Nutr. 2019;149(7):1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12(4):330–40. [DOI] [PubMed] [Google Scholar]
- 40. Nisar Y B, Aguayo VM, Billah SM, Dibley MJ. Antenatal iron-folic acid supplementation is associated with improved linear growth and reduced risk of stunting or severe stunting in South Asian children less than two years of age: a pooled analysis from seven countries. Nutrients. 2020;12(9):2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gernand AD, Schulze KJ, Nanayakkara-Bind A, Arguello M, Shamim AA, Ali H, Wu L, West KP, Christian P. Effects of prenatal multiple micronutrient supplementation on fetal growth factors: a cluster-randomized, controlled trial in rural Bangladesh. PLoS One. 2015;10(10):e0137269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christian P. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326(7389):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson W, Darboe MK, Sosseh F, Nshe P, Prentice AM, Moore SE. Association of prenatal lipid-based nutritional supplementation with fetal growth in rural Gambia. Matern Child Nutr. 2017;13(2):e12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, Arimond M, Vosti S, Dewey KG. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr. 2015;101(4):835–46. [DOI] [PubMed] [Google Scholar]
- 45. Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Van Camp J, Kolsteren P. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2009;90:1593–600. [DOI] [PubMed] [Google Scholar]
- 46. Hambidge KM, Westcott JE, Garcés A, Figueroa L, Goudar SS, Dhaded SM, Pasha O, Ali SA, Tshefu A, Lokangaka Aet al. . A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am J Clin Nutr. 2019;109(2):457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christian P, Kim J, Mehra S, Shaikh S, Ali H, Shamim AA, Wu L, Klemm R, Labrique AB, West KP. Effects of prenatal multiple micronutrient supplementation on growth and cognition through 2 y of age in rural Bangladesh: the JiVitA-3 Trial. Am J Clin Nutr. 2016;104(4):1175–82. [DOI] [PubMed] [Google Scholar]
- 48. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, Ashorn U, Zeilani M, Vosti S, Dewey KG. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr. 2016;104(3):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, Harjunmaa U, Lartey A, Phiri N, Phiri TEet al. . Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J Nutr. 2015;145(6):1345–53. [DOI] [PubMed] [Google Scholar]
- 50. Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr. 2005;81(4):880–8. [DOI] [PubMed] [Google Scholar]
- 51. Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, Harding KL, Cummins JR, Day LT, Saha SLet al. . Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr. 2016;103(1):236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Galasso E, Weber AM, Stewart CP, Ratsifandrihamanana L, Fernald LCH. Effects of nutritional supplementation and home visiting on growth and development in young children in Madagascar: a cluster-randomised controlled trial. Lancet Global Health. 2019;7(9):e1257–68. [DOI] [PubMed] [Google Scholar]
- 53. Pickering AJ, Null C, Winch PJ, Mangwadu G, Arnold BF, Prendergast AJ, Njenga SM, Rahman M, Ntozini R, Benjamin-Chung Jet al. . The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Global Health. 2019;7(8):e1139–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified dataset supporting this research can be made available following a submitted request as per Epicentre and General Data Protection Regulation (EU) 2016/679 data sharing policy. Additional information is available at https://epicentre.msf.org/en/data-access-request.