ABSTRACT
Background
Male obesity has been related to poor semen quality and may also have a negative effect on assisted reproductive technologies (ART) outcomes. Whether male waist circumference (WC), as a measure of central obesity, impacts a couple's fertility independently of BMI is unclear.
Objectives
To examine the associations of male WC with semen quality and couples’ outcomes of infertility treatment with ART.
Methods
Couples presenting to the Massachusetts General Hospital Fertility Center were invited to participate in the study. Between 2009 and 2019, 269 males provided 671 semen samples and 176 couples underwent 317 ART cycles. Height, weight, and WC were measured on site. We analyzed the association of male WC with semen quality and pregnancy outcomes using cluster-weighted regression models to account for repeated observations while adjusting for potential confounders. Models were also stratified by male BMI (<25 kg/m2 compared with ≥25 kg/m2).
Results
The median male age, WC, and BMI were 36.1 years, 96.0 cm, and 26.8 kg/m2, respectively. A 5-cm increase in WC was associated with a 6.3% (95% CI, 2.1–10.5%) lower sperm concentration after adjustment for potential confounders, including BMI. Male WC was also inversely related to the probability of achieving a live birth. For each 5-cm increase in male WC, the odds of a live birth per initiated cycle decreased by 9.0% (95% CI, 1.1%–16.4%) after accounting for several anthropometric and demographic characteristics of both partners. These associations were stronger among males in the normal BMI category (<25 kg/m2) than among overweight or obese males.
Conclusions
A higher male WC may be an additional risk factor for poor outcomes of infertility treatment, even after accounting for male and female partner BMIs, particularly in couples where the male partner has a normal BMI.
Keywords: central obesity, fat distribution, in vitro fertilization, male infertility, paternal factor, semen quality
Introduction
The age-adjusted prevalence of obesity among adults in the United States is 42.4% (1), and the prevalence of central obesity—defined by waist circumference (WC) cutoffs (2)—is 58.9% (3). A high WC is a risk factor for noncommunicable diseases, independently of BMI (4–6). Emerging evidence suggests that the excess accumulation of visceral fat plays an important role in the deleterious effects of central obesity (7, 8). The utility of WC as a marker of adiposity appears to be most salient in subgroups of the population in which BMI is less useful in discriminating between fat mass and overall mass, such as in elderly individuals and among younger adults within the normal BMI range (9, 10). WC in combination with BMI could differentiate obesity phenotypes related to visceral fat accumulation (11).
The role of overall adiposity on reproduction and fertility has been extensively evaluated. Female obesity has been related to a higher risk of ovulation disorders and anovulation, delayed time to pregnancy, a higher risk of infertility, and lower success rates of infertility treatment with assisted reproductive technologies (ART) (12). Likewise, male obesity has been related to lower circulating testosterone and other changes in reproductive hormones (13, 14), erectile dysfunction (15, 16), poor semen quality (17), and male factor infertility (18, 19), and may also have a negative effect on ART outcomes (20). Central obesity, however, has not received much attention on its potential role in reproduction. Female central obesity has been inversely related to reduced fecundity (21, 22) and lower success in infertility treatment with ART (23). Among males, some studies have reported inverse associations between WC and semen quality, but none have accounted for overall obesity (24–29). Moreover, whether male WC impacts a couple's fertility independently of their and their partner's BMI is not clear. Given that semen quality parameters are poor predictors of fertility, there is a need to directly evaluate the impact of male WC on treatment outcomes of ART. Therefore, we investigated the relationship of male WC with semen quality and couples’ outcomes of infertility treatment with ART in a cohort of subfertile couples presenting to an academic fertility center in Boston, Massachusetts. We hypothesized that men's WC would be inversely related to live birth rates after accounting for male and female partner BMIs.
Methods
Study population
Participants were subfertile couples participating in the Environment and Reproductive Health (EARTH) Study (30), which started in 2004 to assess the impacts of environmental, nutritional, and lifestyle factors on human fertility. Couples seeking infertility evaluation and treatment at Massachusetts General Hospital Fertility Center, Boston, MA, were invited to participate either independently or as a couple. Males were eligible if they were 18 to 55 years old, had no history of vasectomy, and were not taking anabolic steroids at enrollment. Approximately 60% of males approached were eligible and agreed to participate. The anthropometry protocol was modified in October 2009 to add the measurement of WC. Males were eligible to be included in this analysis if they had a measurement of WC along with at least 1 complete semen analysis (n = 276) or if their partner had completed at least 1 infertility treatment cycle with ART by January 2019 (n = 180). For semen quality analyses, azoospermic males (n = 6) and a man with implausible anthropometric information were excluded. For analyses of ART outcomes, cycles using donor sperm (n = 3) or donor oocytes (n = 6) were excluded. After exclusions, 269 males (671 semen samples) were included in the semen quality analyses and 176 couples (317 cycles) were included in the ART outcomes analyses (Figure 1). Participants included in the analyses had similar characteristics compared to those excluded (Supplemental Table 1). Written informed consent was obtained from all participants. The study was approved by the Institutional Review Boards of Massachusetts General Hospital and the Harvard T.H. Chan School of Public Health.
FIGURE 1.
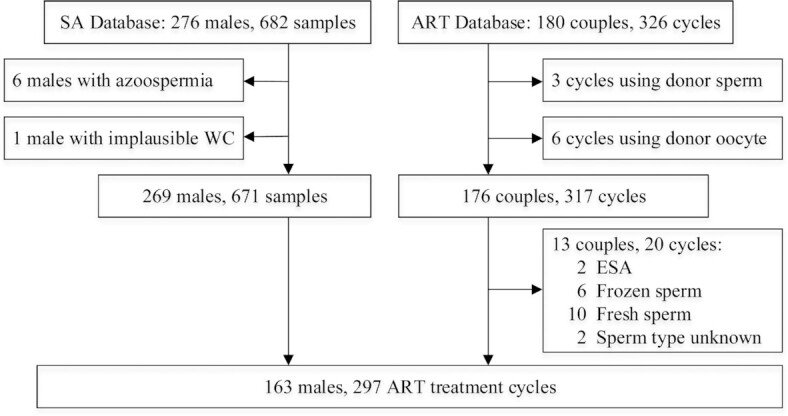
Flowchart of the study participants. ART, assisted reproductive technology; ESA, epididymal sperm aspiration; SA, semen quality analysis; WC, waist circumference.
Anthropometry and other key measurements
At enrollment, weight, height, and WC were measured on site by trained personnel. For measurement of WC, participants were instructed to stand and hold their shirts above the abdomen with their arms crossed. A Gullick II Plus Measuring Tape was horizontally placed at the level of the umbilicus and tied snugly around the bare midriff by reference to the tape's pressure indicator. The measurement results were recorded to the nearest 0.1 cm. During the same visit, participants completed a staff-administered questionnaire regarding demographics, medical history, and lifestyle factors. Participants were also asked to complete a questionnaire to provide more detailed information on medical, occupational, and reproductive history; diet; and lifestyle. Time spent in physical activities (including walking) and diet were assessed using validated questionnaires (31, 32). All relevant clinical information, including the infertility diagnosis, was extracted from the electronic medical records and described elsewhere (30).
Semen analysis, clinical management, and assessment of outcomes
The primary outcome in this study was live birth and the secondary outcomes were semen quality and other ART outcomes (fertilization, implantation, and clinical pregnancy). Males were asked to abstain from ejaculation for at least 48 hours prior to producing a semen sample. Males produced semen samples on site by masturbation. Of these 269 males, 34.2% provided 1 semen sample, 29.4% provided 2 samples, and 36.4% provided 3 or more samples (a maximum of 9). Samples were analyzed and reported according to the 2010 WHO manual procedures (33). Briefly, samples were placed at 37°C for 20 minutes to liquefy before analysis. Ejaculate volume was measured using a graduated serological pipet. Sperm concentration and motility were evaluated with a computer-aided semen analysis system (CASA; Hamilton-Thorne Biosciences Ceros, version 14), as previously described (34). Sperm motility was classified as total (progressive + nonprogressive) and progressive motilities. Sperm morphology was determined using the strict criteria proposed by Kruger et al. (35). Results of motility and morphology were expressed as the percentages of motile sperm and normal spermatozoa, respectively.
Female partners underwent 1 of 3 ovarian stimulation protocols as clinically indicated: 1) long-phase gonadotropin-releasing hormone (GnRH)–agonist protocol; 2) follicular phase GnRH-agonist flare protocol; and 3) GnRH-antagonist protocol. During gonadotropin stimulation, clinical staff monitored serum estradiol, follicle size and counts, and endometrial thickness before oocyte retrieval. For triggering final oocyte maturation, human chorionic gonadotropin was administered approximately 36 hours before oocyte retrieval. Embryologists categorized oocytes as germinal vesicle, metaphase I, metaphase II, or degenerated. The fertilization rate was calculated as the number of oocytes with 2 pronuclei divided by the number of metaphase II oocytes at 17 to 20 hours after insemination. Either programmed estrogen and progesterone replacement or natural cycle monitoring was performed for cryo-thaw cycles. Clinical outcomes were assessed in all cycles following embryo transfer. Successful implantation was defined as an elevation in human chorionic gonadotropin levels > 6 mIU/mL, typically measured 2 weeks after embryo transfer. Clinical pregnancy was defined as the presence of intrauterine pregnancy, confirmed by ultrasound at 6 weeks. Live birth was defined as the birth of a neonate at ≥24 weeks of gestation.
Statistical analysis
Males/couples were classified into tertiles according to the male WC. Baseline demographic and reproductive characteristics across tertiles of male WC were compared using Fisher exact tests for categorical variables and Kruskal-Wallis tests for continuous variables. Sperm concentrations and counts were log-transformed to more closely approximate a normal distribution.
To account for within-person correlations in repeated observations within individuals/couples and the possibility that the number of observations per subject/couple was informative, we used cluster-weighted generalized estimating equation (GEE) models (36) with a first-order autoregressive correlation structure to examine the association of male WC, as a continuous variable and also divided in tertiles, with semen quality parameters and ART treatment outcomes. Specifically, each semen sample or initiated cycle was weighted by the inverse of the total number of the observations per individual (semen analyses) or couple (ART cycles). For semen quality outcomes, we used linear GEE models. To facilitate clinical interpretation, we present the results of models for semen parameters as multivariable-adjusted marginal means (37). For fertilization rate and clinical outcomes, we used logistic GEE models. Linear trend tests were performed by including the continuous male WC in the models, and the effects of each 5-cm WC increase on outcomes of interest were estimated by ORs.
Confounding was evaluated by directed acyclic graphs based on prior knowledge and descriptive statistics in the study population (Supplemental Figure 1). Multiple sets of regression models were fit. For semen analysis, the first set of models adjusted for male age, race, education, smoking status, history of varicocele, and abstinence time; the second included an additional term for BMI; and the third was further adjusted for height. For the analysis of ART outcomes, the first set of models included male age and BMI; the second included additional terms for male height, race, education, smoking status, and history of varicocele; and the third included all covariates in the second set of models plus female age, BMI, WC, height, education, and primary infertility diagnosis. We also evaluated whether the associations of WC and study outcomes differed across strata of BMI. Tests for interaction were conducted using cross-product terms in the multivariable-adjusted models. Stratum-specific estimates were derived from separate models fit in each stratum. We also assessed the effects of male WC on clinical outcomes using WHO-suggested cutoff points (94 and 102 cm) (2). To test the robustness of the findings, we conducted sensitivity analyses, stratifying by mode of insemination (conventional in vitro fertilization compared with intracytoplasmic sperm injection) for fertilization rate. We also conducted analyses restricted to fresh embryo cycles, to the first treatment cycle of each couple, and to males with available information on both semen quality parameters and ART outcomes. Last, to examine the impact of our choice of covariance structure, we fit models for our main findings using an unstructured and a compound symmetry covariance structure instead of a first-order autoregressive structure. Analyses were performed using SAS (version 9.4; SAS Institute).
Results
The male median age, WC, and BMI were 36.1 years (IQR, 32.9–39.6 years), 96 cm (IQR, 89–105 cm) and 26.8 kg/m2 (IQR, 24.3–30.0 kg/m2), respectively. Most males were White (86%), had a graduate degree (63%), and had never smoked (67%). None of the males were underweight (BMI < 18.5 kg/m2). Females had a median age of 34.5 years (IQR, 32.0–38.0 years), median BMI of 22.8 kg/m2 (20.8–25.5 kg/m2) and median WC of 80 cm (IQR, 75–88.5 cm). Males’ WCs were positively correlated with their BMIs (r = 0.57) and height (r = 0.26) and with their partner's WC (r = 0.40). BMI was also positively correlated within couples (r = 0.29). No statistically significant difference was found in other baseline demographic or reproductive characteristics between the tertiles of male WC (Table 1).
TABLE 1.
Study sample characteristics by tertile of male waist circumference1
| Male waist circumference, cm | |||||
|---|---|---|---|---|---|
| Characteristics | All participants | ≤90 | 90.1–100.5 | >100.5 | P value2 |
| Males | |||||
| n | 269 | 85 | 91 | 93 | |
| Waist circumference, cm | 96.0 (89.0–105.0) | 85.5 (81.0–89.0) | 95.0 (92.5–98.5) | 109.0 (104.0–116.0) | <0.001 |
| Age, years | 36.1 (32.9–39.6) | 35.8 (32.6–38.4) | 35.7 (32.9–39.6) | 37.4 (33.5–40.6) | 0.27 |
| BMI, kg/m2 | 26.8 (24.3–30.0) | 24.0 (22.6–24.9) | 26.2 (24.9–27.8) | 31.0 (29.1–34.1) | <0.001 |
| Height, cm | 180.0 (175.0–184.0) | 178.0 (173.0–182.0) | 181.0 (175.0–185.0) | 180.0 (176.0–185.0) | 0.01 |
| White/Caucasian, n (%) | 231 (86) | 68 (80) | 81 (89) | 82 (88) | 0.20 |
| Educational level, n (%) | — | — | — | — | 0.10 |
| High school or some college | 29 (11) | 8 (9) | 5 (6) | 16 (17) | |
| College degree | 72 (27) | 20 (24) | 26 (29) | 26 (28) | |
| Graduate degree | 168 (63) | 57 (67) | 60 (66) | 51 (55) | |
| Abstinence time, days | 2.4 (2.0–3.0) | 2.2 (2.0–3.0) | 2.4 (2.1–3.1) | 2.2 (2.0–3.3) | 0.36 |
| Ever smoker, n (%) | 88 (33) | 31 (37) | 25 (28) | 32 (34) | 0.41 |
| Total energy intake, kcal/d | 1843.1 (1456.8–2254.4) | 1877.9 (1633.9–2227.8) | 1879.3 (1483.4–2381.8) | 1779.8 (1428.2–2214.9) | 0.74 |
| Prudent pattern score | −0.3 (−0.7 to 0.3) | −0.3 (−0.8 to 0.1) | −0.3 (−0.8 to 0.3) | −0.1 (−0.6 to 0.5) | 0.16 |
| Western pattern score | −0.2 (−0.7 to 0.6) | −0.2 (−0.6 to 0.3) | 0 (−0.6 to 0.8) | −0.4 (−0.9 to 0.4) | 0.20 |
| Total exercise, including walking, h/wk | 4.5 (0.3–10.0) | 5.0 (0.8–10.0) | 5.2 (0.3–11.5) | 3.0 (0–7.3) | 0.13 |
| Moderate-to-vigorous physical activity, h/wk | 2.0 (0–5.7) | 2.5 (0–5.9) | 2.0 (0–6.5) | 2.0 (0–5.0) | 0.40 |
| History of varicocele, n (%) | 20 (7) | 9 (11) | 5 (6) | 6 (7) | 0.43 |
| History of cryptorchidism, n (%) | 10 (4) | 2 (2) | 6 (7) | 2 (2) | 0.28 |
| Females | |||||
| n | 176 | 58 | 59 | 59 | |
| Age, y | 34.5 (32.0–38.0) | 34.5 (31.0–37.0) | 34.0 (31.0–38.0) | 35.0 (33.0–38.0) | 0.40 |
| BMI, kg/m2 | 22.8 (20.8–25.5) | 22.0 (20.0–24.2) | 23.0 (20.7–25.4) | 23.9 (21.5–28.4) | 0.002 |
| Waist circumference, cm | 80.5 (75.0–88.5) | 77.5 (71.0–82.0) | 80.0 (75.5–87.0) | 87.0 (79.0–98.0) | <0.001 |
| Height, cm | 165.0 (160.0–170.0) | 163.5 (158.0–169.0) | 165.0 (161.0–169.0) | 167.5 (163.0–172.0) | 0.01 |
| White/Caucasian, n (%) | 145 (82) | 45 (78) | 47 (80) | 53 (90) | 0.18 |
| Educational level, n (%) | — | — | — | — | 0.06 |
| High school or some college | 9 (5) | 2 (4) | 2 (3) | 5 (9) | |
| College degree | 54 (31) | 15 (26) | 14 (24) | 25 (42) | |
| Graduate degree | 113 (64) | 41 (71) | 43 (73) | 29 (49) | |
| Day 3 FSH, IU/L | 6.7 (5.9–8.0) | 6.7 (5.9–7.9) | 6.5 (5.7–8.3) | 6.7 (6.0–8.1) | 0.81 |
| Ever smoker, n (%) | 43 (24) | 14 (24) | 13 (22) | 16 (27) | 0.82 |
| Ever pregnant, n (%) | 73 (42) | 28 (48) | 20 (34) | 25 (42) | 0.29 |
| Total energy intake, kcal/d | 1682.7 (1317.8–1999.0) | 1660.4 (1334.2–1765.8) | 1682.7 (1318.9–2007.5) | 1682.7 (1190.1–2158.1) | 0.75 |
| Prudent pattern score | −0.2 (−0.6 to 0.4) | −0.4 (−0.8 to -0.2) | −0.2 (−0.5 to 0.4) | −0.2 (−0.6 to 0.8) | 0.04 |
| Western pattern score | −0.2 (−0.6 to 0.4) | −0.2 (−0.5 to 0.5) | −0.2 (−0.5 to 0.3) | −0.2 (−0.6 to 0.5) | 0.93 |
| Initial treatment protocol, n (%) | — | — | — | — | 0.06 |
| Antagonist | 33 (19) | 6 (10) | 12 (20) | 15 (25) | |
| Flare | 15 (9) | 5 (9) | 4 (9) | 6 (10) | |
| Luteal phase agonist | 117 (67) | 40 (69) | 39 (66) | 38 (64) | |
| Endometrial preparation | 11 (6) | 7 (12) | 4 (7) | 0 (0) | |
| Couples | |||||
| Primary infertility diagnosis, n (%) | — | — | — | — | 0.93 |
| Male factor | 67 (25) | 19 (22) | 22 (24) | 26 (28) | |
| Female factor | 83 (31) | 28 (33) | 28 (31) | 27 (29) | |
| Unexplained | 119 (44) | 38 (45) | 41 (45) | 40 (43) | |
Data are medians (IQRs) or n (%). FSH, follicle-stimulating hormone.
From Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables.
A higher male WC was related to a lower sperm concentration (Table 2). In models adjusting for BMI and demographic and reproductive characteristics, a 5-cm increase in WC was associated with a 6.3% (95% CI, 2.1%–10.5%) lower sperm concentration. Results were similar after a further adjustment for height (Table 2). Males with a WC larger than 100.5 cm had a 33.5% (95% CI, 3.9%–54.0%) lower total sperm count than males with a WC of 90 cm or less after accounting for BMI and demographic and reproductive characteristics. WC was unrelated to sperm motility or morphology.
TABLE 2.
Semen quality parameters in relation to male WC1
| Difference (95% CI) per 5 cm increase in male waist circumference2 | ||||
|---|---|---|---|---|
| Male WC, cm | ||||
| Semen quality parameters | ≤90 | 90.1–100.5 | >100.5 | |
| Number of semen samples | 205 | 231 | 235 | — |
| Number of males | 85 | 91 | 93 | — |
| Sperm concentration (% difference) | ||||
| Model 1 | 39.9 (28.6–55.8) | 31.9 (22.4–45.5) | 28.8 (20.7–40.1)3 | −4.07 (−7.54 to −0.60) |
| Model 2 | 41.5 (29.6–58.2) | 31.8 (22.4–45.0) | 26.7 (19.0–37.6)3 | −6.33 (−10.54 to −2.12) |
| Model 3 | 40.8 (29.1–57.2) | 31.9 (22.5–45.2) | 27.5 (19.4–38.8) 3 | −5.58 (−10.21 to −0.96) |
| Total sperm count (% difference) | ||||
| Model 1 | 91.4 (63.9–130.7) | 78.8 (54.5–114.1) | 65.7 (46.8–92.3)3 | −3.37 (−7.19 to 0.44) |
| Model 2 | 93.9 (65.9–134.0) | 78.6 (54.4–113.6) | 62.4 (43.2–90.2)3 | −4.37 (−8.90 to 0.15) |
| Model 3 | 91.5 (64.8–129.4) | 78.9 (54.7–113.8) | 64.9 (44.7–94.1) | −2.86 (−7.47 to 1.76) |
| Sperm motility (% motile) | ||||
| Model 1 | 42.1 (34.8–49.3) | 37.1 (29.8–44.5) | 37.0 (29.3–44.7) | −0.69 (−1.56 to 0.18) |
| Model 2 | 42.7 (35.0–50.4) | 37.1 (29.8–44.3) | 35.9 (28.1–43.7) | −1.08 (−2.35 to 0.20) |
| Model 3 | 42.6 (34.8–50.4) | 37.1 (29.9–44.3) | 36.0 (28.0–44.0) | −1.10 (−2.57 to 0.38) |
| Progressive motility (% progressively motile)4 | ||||
| Model 1 | 23.9 (19.3–28.4) | 20.7 (16.0–25.3) | 21.2 (16.0–26.3) | −0.43 (−1.08 to 0.21) |
| Model 2 | 24.2 (19.3–29.1) | 20.7 (16.1–25.3) | 20.6 (15.4–25.7) | −0.69 (−1.55 to 0.18) |
| Model 3 | 24.3 (19.2–29.3) | 20.7 (16.1–25.2) | 20.5 (15.3–25.7) | −0.78 (−1.77 to 0.20) |
| Sperm morphology (% normal)5 | ||||
| Model 1 | 5.9 (4.8–7.0) | 5.2 (3.9–6.6) | 5.4 (4.3–6.6) | −0.07 (−0.22 to 0.07) |
| Model 2 | 5.9 (4.7–7.0) | 5.2 (3.9–6.6) | 5.4 (4.3–6.6) | −0.09 (−0.27 to 0.09) |
| Model 3 | 5.9 (4.7–7.0) | 5.2 (3.9–6.6) | 5.5 (4.3–6.7) | −0.08 (−0.30 to 0.14) |
All analyses were conducted using cluster-weighted generalized estimating equations. Estimates under the columns for tertiles of WC are multivariable-adjusted marginal means for each semen parameter for the specified model. Estimates under the last column are multivariable-adjusted differences in each semen parameter for the specified model. Model 1 was adjusted for age (continuous), race (White, non-White), education (high school or some college, college, graduate), smoking status (ever, never), history of varicocele (yes, no) and abstinence time (<2 days, 2–3 days, 3–4 days, and ≥4 days). Model 2 was adjusted for the variables in Model 1 plus BMI (continuous). Model 3 was adjusted for the variables in Model 2 plus height (continuous). WC, waist circumference.
Sperm concentration and total count data were log-transformed to more closely approximate normal distribution. Thus, estimates represent percentage differences for concentration and total count and absolute differences for all other semen parameters.
P < 0.05 when compared with tertile 1.
Contains data from 658 samples because an evaluation on progressive motility was not performed for all samples.
Contains data from 639 samples because a morphologic evaluation was not performed for all samples.
Since WC may be more useful in differentiating lean mass from fat mass within the normal range of BMI than in the extremes of the BMI distribution, we then evaluated the association of WC with semen parameters within categories of BMI (Table 3). In these analyses, WC was inversely related to all semen quality parameters among males in the normal BMI category but not among overweight/obese males, although tests for effect modification suggested that these differences were not statistically significant (Table 3).
TABLE 3.
Adjusted difference of semen quality parameters per 5 cm increase in male waist circumference within categories of BMI1
| BMI, kg/m2 | P value for interaction | ||
|---|---|---|---|
| Semen quality parameters | 18.5 ≤ BMI < 25 | ≥25 | |
| Number of semen samples | 229 | 442 | |
| Number of males | 89 | 180 | |
| Sperm concentration (%) | — | — | 0.24 |
| Model 1 | −13.06 (−27.12 to 1.01) | −2.30 (−5.79 to 1.19) | |
| Model 2 | −24.76 (−41.20 to −8.33) | −4.16 (−8.06 to −0.26) | |
| Model 3 | −22.01 (−40.18 to -3.85) | −3.54 (−7.93 to 0.85) | |
| Total sperm count (%) | — | — | 0.16 |
| Model 1 | −11.29 (−23.84 to 1.27) | −1.65 (−5.96 to 2.66) | |
| Model 2 | −23.12 (−38.77 to -7.47) | −2.45 (−7.33 to 2.43) | |
| Model 3 | −22.05 (−37.04 to -7.06) | −0.65 (−5.69 to 4.38) | |
| Sperm motility (% motile) | 0.47 | ||
| Model 1 | −2.41 (−5.48 to 0.67) | −0.43 (−1.43 to 0.57) | |
| Model 2 | −4.39 (−8.12 to −0.66) | −0.72 (−1.96 to 0.51) | |
| Model 3 | −5.15 (−8.96 to −1.34) | −0.58 (−2.02 to 0.86) | |
| Progressive motility (%)2 | — | — | 0.61 |
| Model 1 | −1.42 (−3.48 to 0.64) | −0.27 (−1.05 to 0.51) | |
| Model 2 | −2.21 (−4.77 to 0.35) | −0.48 (−1.36 to 0.40) | |
| Model 3 | −3.09 (−5.68 to −0.5) | −0.43 (−1.43 to 0.57) | |
| Sperm morphology (% normal)3 | — | — | 0.85 |
| Model 1 | −0.30 (−0.73 to 0.14) | −0.03 (−0.21 to 0.15) | |
| Model 2 | −0.62 (−1.15 to −0.09) | −0.05 (−0.24 to 0.15) | |
| Model 3 | −0.74 (−1.32 to −0.16) | −0.02 (−0.25 to 0.22) | |
Sperm concentration and total count data were log-transformed to achieve normality. Thus, estimates represent percentage differences for total count and concentration and absolute differences for all other semen parameters. All analyses were conducted using cluster-weighted generalized estimating equations. Model 1 was adjusted for age (continuous), race (White, non-White), education (high school or some college, college, graduate), smoking status (ever, never), history of varicocele (yes, no) and abstinence time (<2 days, 2–3 days, 3–4 days, and ≥4 days). Model 2 was adjusted for the variables in Model 1 plus BMI (continuous). Model 3 was adjusted for the variables in Model 2 plus height (continuous).
Contains data from 658 samples because an evaluation on progressive motility was not performed for all samples.
Contains data from 639 samples because a morphologic evaluation was not performed for all samples.
We then investigated the relations between WC and ART outcomes. Male WC was unrelated to fertilization rates overall. However, for couples undergoing ART with conventional in vitro fertilization, the odds of fertilization decreased by 21.8% (95% CI, 4.0%–36.4%) per 5-cm increase in male WC when accounting for male and female demographic, reproductive, and anthropometric characteristics (Supplemental Table 1, Model 3). Nevertheless, WC was inversely related to the probabilities of implantation, clinical pregnancy, and a live birth during the course of infertility treatment with ART (Figure 2; Supplemental Table 2). In models adjusted for male and female demographic, reproductive, and anthropometric characteristics, a 5-cm increase in male WC was associated with 14.2% (95% CI, 2.8%–24.2%) lower odds of implantation, 12.1% (95% CI, 3.0%–20.3%) lower odds of clinical pregnancy, and 9.0% (95% CI, 1.1%–16.4%) lower odds of a live birth (Supplemental Table 2). When we assessed the associations of male WC with these outcomes within strata of BMI, the inverse relations of WC and ART outcomes were stronger among couples with leaner males (Table 4).
FIGURE 2.
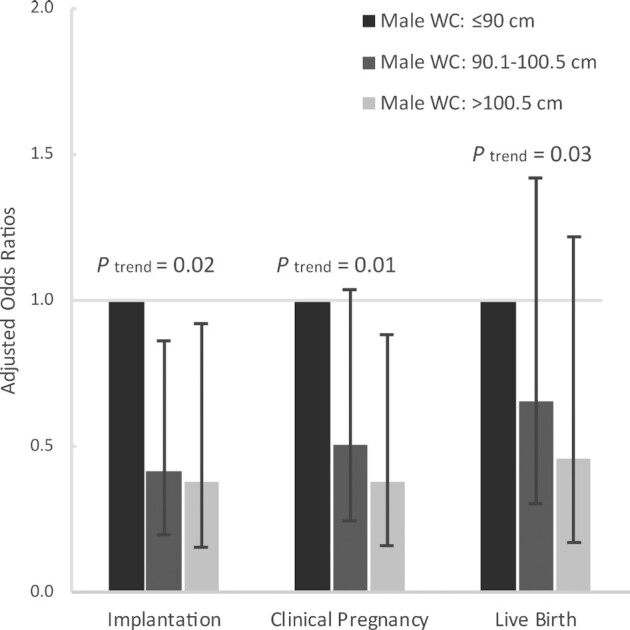
Associations between male WC and infertility treatment outcomes in 176 couples (317 initiated cycles) from the Environment and Reproductive Health Study (2009–2019). The analyses were conducted using cluster-weighted generalized estimating equations with a binomial distribution and log link function with adjustment for male age (continuous) and BMI (continuous), height (continuous), race (White, non-White), education (high school or some college, college, graduate), smoking status (ever, never), and history of varicocele (yes, no), and their partner's age (continuous) and BMI (continuous), height (continuous), education (high school or some college, college, graduate), and primary infertility diagnosis (male factor, female factor, unexplained). WC, waist circumference.
TABLE 4.
ORs (95% CIs) per 5 cm increase in male waist circumference for clinical outcomes per initiated treatment cycle within categories of BMI1
| BMI, kg/m2 | P value for interaction | ||
|---|---|---|---|
| Clinical outcomes | 18.5 ≤ BMI < 25 | ≥ 25 | |
| Number of cycles | 113 | 204 | |
| Number of couples | 58 | 118 | |
| Implantation | — | — | 0.05 |
| Model 1 | 0.58 (0.35–0.95) | 0.95 (0.87–1.04) | |
| Model 2 | 0.54 (0.32–0.89) | 0.91 (0.84–1.00) | |
| Model 3 | 0.31 (0.17–0.56) | 0.90 (0.82–0.99) | |
| Clinical pregnancy | — | — | 0.04 |
| Model 1 | 0.58 (0.34–1.00) | 0.96 (0.87–1.05) | |
| Model 2 | 0.60 (0.35–1.03) | 0.90 (0.81–0.99) | |
| Model 3 | 0.31 (0.18–0.51) | 0.90 (0.82–0.98) | |
| Live birth | — | — | 0.10 |
| Model 1 | 0.60 (0.33–1.09) | 0.99 (0.90–1.08) | |
| Model 2 | 0.63 (0.33–1.19) | 0.89 (0.81–0.98) | |
| Model 3 | 0.29 (0.14–0.59) | 0.89 (0.82–0.97) | |
All analyses were conducted by using cluster-weighted generalized estimating equations. Model 1 was adjusted for male age (continuous) and BMI (continuous). Model 2 was adjusted for the variables in Model 1 plus male height (continuous), race (White, non-White), education (high school or some college, college, graduate), smoking status (ever, never), and history of varicocele (yes, no). Model 3 was adjusted for the variables in Model 2 plus partner's age (continuous) and BMI (continuous), height (continuous), education (high school or some college, college, graduate), and primary infertility diagnosis (male factor, female factor, unexplained).
Adjustments for dietary patterns and physical activity did not have any impact on the relations between WC and ART outcomes (Supplementary Table 2, Models 4–6). The inverse relations between WC and ART outcomes were similar when using WHO-suggested cutoff points for male WC (Supplemental Table 3). Results were also comparable when restricting analyses to fresh embryo cycles (Supplemental Table 4), to the first treatment cycle of each couple (Supplemental Table 5), and to males with available information on both semen quality and ART outcomes (Supplemental Table 6). Of note, in this last subgroup of men, the associations between WC and ART outcomes persisted after adjustment for semen quality parameters (Supplemental Table 6). Last, results did not change when using different correlation structures to model the within-person correlations in outcomes (Supplemental Tables 7 and 8).
Discussion
We examined the associations of male WC with semen quality and clinical outcomes of ART among couples seeking infertility treatment. WC was inversely related to the sperm concentration independently of key potential confounders, including BMI, dietary patterns, and physical activity. Although male WC was not associated with the fertilization rate, it was negatively associated with clinical outcomes, including implantation, clinical pregnancy, and live birth, even after accounting for both partners’ BMIs. Notably, results were stronger among males within the normal BMI range than among overweight/obese males. Moreover, the associations of male WC with clinical ART outcomes were independent of the effects on semen quality. These findings suggest that male WC may provide independent and additional information to BMI for the prediction of achieving fatherhood with ART, particularly in couples with normal-weight males.
Our findings of inverse associations of WC with semen quality and worse ART outcomes are in agreement with the scant literature in this area. In line with our findings of an inverse relation between WC and sperm concentrations, previous studies carried in males from subfertile couples (24, 25) and males without known infertility (26–29) also report this relation. However, previous studies did not adjust for BMI, raising concerns that this relation may partially reflect well-described associations with BMI. In our analysis, we observed stronger associations between WC and semen quality parameters after adjusting for BMI and in analyses restricted to males with a normal BMI (18.5–25 kg/m2), suggesting that the relation of abdominal adiposity and semen quality may not only be independent of overall adiposity but may also be of concern among males not considered obese based on BMI criteria. We also found that male WC was associated with worse outcomes of infertility treatment with ART. This finding is consistent with the growing literature showing that a higher male BMI is related to ART failure (20). Data regarding male central obesity and fertility specifically are sparse. In fact, to our knowledge, this is the first study to assess the association between male WC and outcomes on infertility treatment with ART. The Longitudinal Investigation of Fertility and the Environment (LIFE) Study did not find an association between male central obesity and fecundability among pregnancy planners without a known history of infertility (22). Reasons for discordant findings may include the fact that couples presenting to fertility centers may be more sensitive to the effects of male central obesity than pregnancy planners in the general population. It should be noted, however, that differences in BMI and WC distribution could also account for the differences. In the LIFE Study, 83% of male participants were overweight or obese, while the corresponding figure in our study was 67%. Given that the associations between WC and ART outcomes were more pronounced among males with normal BMIs, if this were also true in the general population, the high prevalence of obesity in the LIFE study may have masked this true relation. Clearly, additional research on the relation between male WC and reproductive fitness is warranted, particularly among males within the normal BMI range, since findings from this study, as well as our previous findings among females in this cohort (23), suggest that WC may be an important factor influencing fertility above and beyond overall adiposity.
The relation of WC with worse semen quality and ART outcomes is biologically plausible. As a marker of visceral adipose tissue depots (38), central obesity has been linked with chronic inflammation and metabolic disorders, including insulin resistance and dyslipidemia, independently of overall obesity (39–41). The high load of systemic oxidative stress may drive changes in testicular and epididymal environments, affect the sperm membrane integrity, and induce genetic or epigenetic alterations of gametes (42, 43). Previous work has shown restoration of the reproductive hormonal profile and improved semen quality following abdominal fat loss (44). The inverse associations with semen quality parameters are likely due to factors previously attributed to the relation between obesity and semen quality, including an altered reproductive hormonal profile, as well as physical factors, such as an elevated scrotal temperature (45, 46). These factors, however, may not necessarily explain the inverse relation with chances of successful treatment outcomes with ART. In fact, our finding that further adjustment for semen parameters has little to no impact on the association of WC with ART outcomes, as well previous findings showing that pre-processing semen parameters do not predict ART outcomes (47), support this interpretation. Since in the setting of ART sperm is concentrated and selected to achieve better treatment outcomes, damage to the sperm in other ways, including damage to the sperm DNA integrity or epigenetic changes, as we and others have documented in relation to obesity (14, 48, 49), could be the mechanism underlying these relations. As the sperm genome is not expressed until 2–3 days of embryo development, exposures affecting the integrity of sperm DNA could result in no effects on the fertilization rate but rather be manifested as a failure of implantation or pregnancy loss (50, 51). This mechanism is consistent with our results.
Limitations of our study include its relatively small sample size. While the study was sufficiently large to document relations with clinically relevant outcomes, larger studies could evaluate these relations with more granularity, as well as possible interactions with female anthropometric factors. Second, WC was measured at the level of the umbilicus, which may result in loss of accuracy compared with the measurement at the level of the iliac crest or the midpoint between the iliac crest and last rib. However, as most of the participants’ BMIs were less than 35 kg/m2, there might be little difference between measurement results with the umbilicus or other anatomical landmarks. Moreover, previous work has shown that the measurement site for WC has little impact on its relation to direct measurements of abdominal fat mass or markers of cardiometabolic risk (52–56). Third, it is unknown whether these findings are generalizable to the couples attempting conception without medical assistance. Nevertheless, this cohort is comparable to couples presenting to infertility practices nationwide. Strengths of the study include the collection of anthropometric information by trained personnel using standardized protocols, the availability of repeated semen samples, and the availability of complete follow-ups for ART outcomes across multiple treatment cycles. Last, having enrolled men into a study of ART outcomes per se is a major strength of the study, not only because it allowed us to address the specific hypothesis of central obesity in the male partner, but also because it allows more generally for a comprehensive evaluation of prognostic factors of ART outcomes.
In summary, we found that a higher male WC was associated with a lower sperm concentration and a lower probability of achieving a live birth among couples undergoing infertility treatment. These associations were present even in men within the normal BMI range. These results suggest that central obesity may be an independent risk factor for male factor infertility, and highlight the need for infertility care providers to assess male partner factors in their clinical management of infertile couples.
Supplementary Material
Acknowledgments
We thank all members of the EARTH Study team, especially research nurses Jennifer B Ford and Myra Keller and physicians, fellows, and clinical staff at the Massachusetts General Hospital Fertility Center, as well as all the study participants. Members of the EARTH Study Team are: Russ Hauser, MD, ScD; Paige L Williams, PhD; Jorge E Chavarro, MD, ScD; Lidia Mínguez-Alarcón, PhD; Jennifer Ford, RN; Myra Keller, RN; Ramace Dadd, BA; Irene Souter, MD; John Petrozza, MD; Thomas L Toth, MD; Diane L Wright, PhD, HCLD; and Charles Bormann, PhD, HCLD.
The authors’ responsibilities were as follows – JEC: was involved in the study concept and design; HB, JEC: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; HB, LM-A, PLW, JEC: interpreted the data; LM-A, AS-H, DB: contributed to statistical analyses; JEC, PLW, IS, JA: were involved in acquisition of the data; and all authors: were involved in the critical revision of the manuscript and read and approved the final manuscript. HB was supported by the China Scholarship Council. All other authors report no conflicts of interest.
Notes
This study was supported by the National Institute of Environmental Health Sciences (grants R01ES022955, R01ES009718, and P30ES000002) and National Institute of Diabetes and Digestive and Kidney Diseases grant (P30DK046200). HB was supported by the China Scholarship Council.
Supplemental Tables 1–8 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article at https://academic.oup.com/ajcn/.
Abbreviations used: ART, assisted reproductive technologies; GEE, generalized estimating equation; GnRH, gonadotropin-releasing hormone; LIFE, Longitudinal Investigation of Fertility and the Environment; WC, waist circumference.
Contributor Information
Haiyang Bian, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Institute of Reproductive and Child Health and Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing, China.
Lidia Mínguez-Alarcón, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Albert Salas-Huetos, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
David Bauer, Harvard Extension School, Cambridge, MA, USA.
Paige L Williams, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Irene Souter, Vincent Obstetrics and Gynecology, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Jill Attaman, Vincent Obstetrics and Gynecology, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Jorge E Chavarro, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Harvard Medical School & Brigham and Women's Hospital, Boston, MA, USA.
EARTH Study Team:
Russ Hauser, Paige L Williams, Jorge E Chavarro, Lidia Mínguez-Alarcón, Jennifer Ford, Myra Keller, Ramace Dadd, Irene Souter, John Petrozza, L Toth Thomas, Diane L Wright, and Charles Bormann
Data Availability
Data described in the manuscript and analytic code will be made available upon request pending IRB approval and establishment of institutional data sharing agreements.
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 2. World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. World Health Organization Document Production Services; 2011. [Google Scholar]
- 3. Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States [Internet] . Available from: http://www.cdc.gov/ckd.
- 4. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–9. [DOI] [PubMed] [Google Scholar]
- 5. Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–8. [DOI] [PubMed] [Google Scholar]
- 6. Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality. Circulation. 2008;117(13):1658–67. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–8. [DOI] [PubMed] [Google Scholar]
- 8. Neeland IJ, Ross R, Després J-P, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25. [DOI] [PubMed] [Google Scholar]
- 9. Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, Willett WC. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–27. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, Thun MJ, Gapstur SM. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170(15):1293–301. [DOI] [PubMed] [Google Scholar]
- 11. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FBet al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chavarro JE, Toth TL. Obesity and Fertility. In: Gillman MW.Poston Leds. Maternal Obesity. New York, NY: Cambridge University Press; 2012. p. 20–30. [Google Scholar]
- 13. MacDonald A, Herbison G, Showell M, Farquhar C. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293–311. [DOI] [PubMed] [Google Scholar]
- 14. Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93(7):2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–84. [DOI] [PubMed] [Google Scholar]
- 16. Cheng JYW, Ng EM. Body mass index, physical activity and erectile dysfunction: an U-shaped relationship from population-based study. Int J Obes. 2007;31(10):1571–8. [DOI] [PubMed] [Google Scholar]
- 17. Salas-Huetos A, Maghsoumi-Norouzabad L, James ER, Carrell DT, Aston KI, Jenkins TG, Becerra-Tomas N, Javid AZ, Abed R, Torres PJet al. Male adiposity, sperm parameters and reproductive hormones: an updated systematic review and collaborative meta-analysis. Obes Rev. 2021;22(1):e13082. doi:10.1111/obr.13082. [DOI] [PubMed] [Google Scholar]
- 18. Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17(5):520–3. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen RHN, Wilcox AJ, Rolv S, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;(9):9. [DOI] [PubMed] [Google Scholar]
- 20. Mushtaq R, Pundir J, Achilli C, Naji O, Khalaf Y, El-Toukhy T. Effect of male body mass index on assisted reproduction treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2018;36(4):459–71. [DOI] [PubMed] [Google Scholar]
- 21. Wise LA, Palmer JR, Lynn R. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28(10):2856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sundaram R, Mumford SL, Buck Louis GM. Couples’ body composition and time-to-pregnancy. Hum Reprod. 2017;32(3):662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M-C, Mínguez-Alarcón L, Arvizu M, Chiu Y-H, Ford JB, Williams PL, Attaman J, Hauser R, Chavarro JE, Team ES. Waist circumference in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Obstet Gynecol. 2019;220(6):578.e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fejes I, Koloszár S, Szöllo˝si J, Závaczki Z, Pál A. Is semen quality affected by male body fat distribution?. Andrologia. 2010;37(5):155–9. [DOI] [PubMed] [Google Scholar]
- 25. Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27(8):2365–72. [DOI] [PubMed] [Google Scholar]
- 26. Ehala-Aleksejev K, Punab M. The different surrogate measures of adiposity in relation to semen quality and serum reproductive hormone levels among Estonian fertile men. Andrology. 2015;3(2):225–34. [DOI] [PubMed] [Google Scholar]
- 27. Eisenberg M L, Kim S, Chen Z, Sundaram R, Schisterman EF. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsao C-W, Liu C-Y, Chou Y-C, Cha T-L, Chen S-C, Hsu C-Y. Exploration of the association between obesity and semen quality in a 7630 male population. PLoS One. 2015;10(3):e0119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen YY, Kao TW, Peng TC, Yang HF, CJ WU, Chen WL. Metabolic syndrome and semen quality in adult population. J Diabetes. 2020;12(4):294–304. [DOI] [PubMed] [Google Scholar]
- 30. Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T. The environment and reproductive health (EARTH) study: a prospective preconception cohort. Hum Reprod Open. 2018;2018(2):hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 32. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 34. Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–91. [DOI] [PubMed] [Google Scholar]
- 35. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Oehninger SC. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49(1):112–7. [DOI] [PubMed] [Google Scholar]
- 36. Yland J, Messerlian C, Mínguez-Alarcón L, Ford JB, Hauser R, Williams PL, Team ES. Methodological approaches to analyzing IVF data with multiple cycles. Hum Reprod. 2019;34(3):549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34(4):216–21. [Google Scholar]
- 38. Pouliot M. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73(7):460–8. [DOI] [PubMed] [Google Scholar]
- 39. Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. [DOI] [PubMed] [Google Scholar]
- 40. Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183(2):308–15. [DOI] [PubMed] [Google Scholar]
- 41. Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330(7504):1363–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129(4):357. [PubMed] [Google Scholar]
- 43. Saez F, Drevet JR. Dietary cholesterol and lipid overload: impact on male fertility. Oxid Med Cell Longev. 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Faure C, Dupont C, Baraibar MA, Ladouce R, Cedrin-Durnerin I, Wolf JP, Levy R. In subfertile couple, abdominal fat loss in men is associated with improvement of sperm quality and pregnancy: a case-series. PLoS One. 2014;9(2):e86300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He Z, Rankinen T, Leon AS, Skinner JS, Tchernof A, Bouchard C. Plasma steroids, body composition, and fat distribution: effects of age, sex, and exercise training. Int J Obes. 2018;42(7):1366–77. [DOI] [PubMed] [Google Scholar]
- 46. Jo J, Kim H. The relationship between body mass index and scrotal temperature among male partners of subfertile couples. J Therm Biol. 2016;56:55–8. [DOI] [PubMed] [Google Scholar]
- 47. Harris AL, Vanegas JC, Hariton E, Bortoletto P, Palmor M, Humphries LA, Tanrikut C, Chavarro JE, Styer AK. Semen parameters on the day of oocyte retrieval predict low fertilization during conventional insemination IVF cycles. J Assist Reprod Genet. 2019;36(2):291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Hawula W, Ulańska A, Hanke W. Lifestyle factors and sperm aneuploidy. Reprod Biol. 2014;14(3):190–9. [DOI] [PubMed] [Google Scholar]
- 49. Donkin I, Versteyhe S, Ingerslev Lars R, Qian K, Barrès R. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23(2):369–78. [DOI] [PubMed] [Google Scholar]
- 50. Braude P, Bolton V, Moore SJN. Human gene expression first occurs between the four-and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–61. [DOI] [PubMed] [Google Scholar]
- 51. Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4(1):2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bosy-Westphal A, Booke CA, Blocker T, Kossel E, Goele K, Later W, Hitze B, Heller M, Gluer CC, Muller MJ. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J Nutr. 2010;140(5):954–61. [DOI] [PubMed] [Google Scholar]
- 53. Mason C, Katzmarzyk PT. Effect of the site of measurement of waist circumference on the prevalence of the metabolic syndrome. Am J Cardiol. 2009;103(12):1716–20. [DOI] [PubMed] [Google Scholar]
- 54. Brown RE, Randhawa AK, Canning KL, Fung M, Jiandani D, Wharton S, Kuk JL. Waist circumference at five common measurement sites in normal weight and overweight adults: which site is most optimal?. Clin Obes. 2018;8(1):21–9. [DOI] [PubMed] [Google Scholar]
- 55. Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Monig H, Muller MJ. Measurement of waist circumference at four different sites in children, adolescents, and young adults: concordance and correlation with nutritional status as well as cardiometabolic risk factors. Obes Facts. 2008;1(5):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willis LH, Slentz CA, Houmard JA, Johnson JL, Duscha BD, Aiken LB, Kraus WE. Minimal versus umbilical waist circumference measures as indicators of cardiovascular disease risk. Obesity (Silver Spring). 2007;15(3):753–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript and analytic code will be made available upon request pending IRB approval and establishment of institutional data sharing agreements.


