ABSTRACT
Background
Diet quality may be protective of physical function and muscle strength during aging.
Objectives
We aimed to investigate associations of the Mediterranean-Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet with physical function and grip strength.
Methods
Data were obtained from men and women in the Baltimore Longitudinal Study of Aging (mean ± SD age: 68 ± 14 y at first diet visit; n = 1358). Diet was assessed by FFQ. MIND diet score was calculated from 15 food groups, with a higher score indicating better diet quality; tertile categories of averaged MIND score across visits were used. Physical function was assessed using the Short Physical Performance Battery (SPPB), with a score < 10 indicative of impaired function, and the Health, Aging and Body Composition Physical Performance Battery (HABCPPB). The highest value of grip strength over 3 trials was used. Multivariable logistic and linear mixed-effects models were examined with repeated measurements of physical function and grip strength, respectively.
Results
MIND score was inversely associated with physical function impairment (per 1-point increment: OR: 0.81; 95% CI: 0.71, 0.93; P < 0.01), and with each SPPB component, over a median 6 y of follow-up. Participants in the highest compared with the lowest tertile of MIND diet score had 57% lower odds of functional impairment (OR: 0.43; 95% CI: 0.25, 0.73; P < 0.01), and slower decline by the HABCPPB. Men and women in the highest compared with the lowest tertiles of MIND score had 1.86-kg (95% CI: 0.33, 3.40 kg; P < 0.05) and 1.24-kg (95% CI: 0.04, 2.45 kg; P < 0.05) greater grip strength, respectively.
Conclusions
Adherence to the MIND dietary pattern was associated with lower odds of physical function impairment and decline, and with better muscle strength, indicating that the MIND dietary pattern may be protective of physical functional health in older adults.
Keywords: diet patterns, MIND diet, physical function, muscle strength, older individuals, BLSA
Introduction
Life expectancy worldwide, including the United States, has increased with improved access to health care and medicine, better management of chronic diseases, better protection from injury, and decreased child and infectious diseases mortality (1, 2). In the United States, the number of individuals aged 65 y and older increased from 35 million in 2000 to 49.2 million in 2016, and this upward trajectory is projected to continue (3). Physical function and muscle strength are important indicators of healthy aging and independent living in older populations. Declines in physical function and muscle strength in older individuals have been linked with loss of independence in performing activities of daily living (4), risk of hospitalization and increased length of hospital stay (5, 6), frailty (7), and mortality (8, 9). It is, therefore, important to investigate factors that may protect against physical function and muscle strength decline, to improve and maintain overall wellness in older individuals.
A Mediterranean-type dietary pattern, characterized by high intake of vegetables, fruit, olive oil, legumes, and cereals and relatively lower intake of red and processed meats, dairy, and sweets, has been associated with lower risk of mortality (10), cardiovascular disease (CVD) (10), diabetes (11), frailty (12), and disability (13). Similarly to the Mediterranean-type diet, the Dietary Approaches to Stop Hypertension (DASH) diet also has been found to have beneficial effects on health and chronic disease outcomes. The DASH diet emphasizes higher consumption of vegetables, fruit, nuts, whole grains, and beans, and lower consumption of sodium, and has been associated with reduced risk of hypertension (14) and CVD (15) and better maintenance of muscle strength (16). Previous studies have demonstrated that both the Mediterranean and DASH diets are associated with lower risk of Alzheimer disease and slower cognitive decline, chief concerns of older individuals (17, 18). The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet combines features of the Mediterranean and DASH dietary patterns, emphasizing foods and nutrients shown to benefit cognitive health and dementia prevention (19). Components of the MIND diet include a focus on 1) phytonutrient-rich foods, such as berries and green leafy vegetables, which have been demonstrated to have neuroprotective benefits; and 2) fish, a main source of omega-3 fatty acids whose higher intake has been associated with lower risk of dementia (20, 21).
Physical function and muscle strength have been shown to be associated with cognitive health (22). Although the protective association of the MIND diet with brain health has been reported in previous studies (20, 23–25), no study, to our knowledge, has investigated the associations between the MIND diet and physical function or muscle strength. If protective associations are found for physical function, this would further endorse dietary pattern–based recommendations for healthy aging.
Therefore, we examined associations of MIND diet adherence with physical function, measured using the Short Physical Performance Battery (SPPB) and the Health, Aging and Body Composition Physical Performance Battery (HABCPPB), and with muscle strength, assessed by grip strength. We hypothesized that higher adherence to the MIND dietary pattern would be associated with better physical function and greater muscle strength.
Methods
Study population
Data are from the Baltimore Longitudinal Study of Aging (BLSA), an ongoing prospective open cohort initiated in 1958, currently conducted by the Intramural Research Program of the National Institute on Aging (IRP-NIA). Study protocols and detailed information on the BLSA cohort have been provided elsewhere (26). Briefly, individuals who lived independently in the Washington–Baltimore area were enrolled, and follow-up visits were scheduled every 4 y for those <60 y old, every 2 y for those 60–79 y old, and annually for participants 80 y of age and older. During each visit, study staff administered interviews and conducted clinical examinations and laboratory assessments, either at the IRP-NIA Clinic Research Unit, or at a home visit for the most debilitated. Beginning in 2005, the BLSA initiated collection of dietary intake data using an FFQ which has been validated against diet records (27). For these analyses, we used data collected between 2005 and 2018, based on the availability of dietary (2005–2015) and physical function (2005–2018) data. The data used in this study will be made available upon request pending application to and approval by the BLSA cohort investigators adn research tem at the NIA. The study protocol was approved by the Institutional Review Board (IRB) of the National Institute of Environmental Health Sciences, and informed consent was obtained from participants at each visit. This project does not meet the definition of human subject research, as confirmed by the George Washington University IRB.
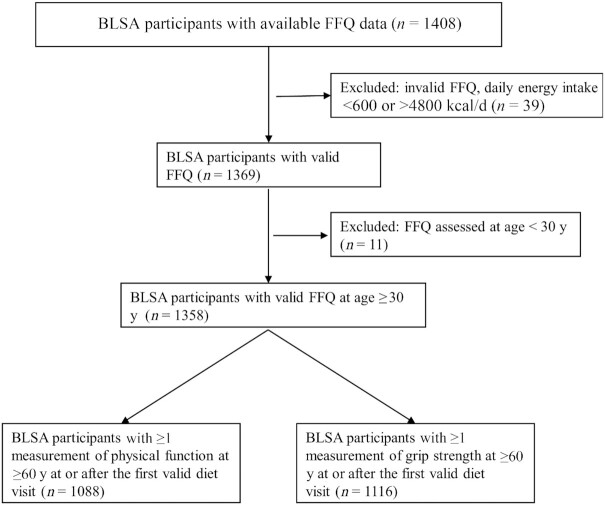
A total of 1402 participants had available FFQ data (Figure 1). We excluded participants with invalid dietary intake (energy intake < 600 or >4800 kcal/d, n = 39) and dietary intake assessed at <30 y old (n = 11) (27). We restricted regression analyses to those who had ≥1 measurement of physical function and grip strength at 60 y and older at or after the first valid diet visit date. The final sample sizes were 1084 for physical function and 1112 for grip strength.
FIGURE 1.
Participant flowchart. BLSA, Baltimore Longitudinal Study of Aging.
Dietary assessment
Self-reported dietary data in the past year were collected through a semiquantitative 100-food-item FFQ at each visit (27). The FFQ is a self-administrated questionnaire which was developed for the general US population. All FFQs were checked for completeness before being scanned and further processed at the Human Nutrition Research Center on Aging at Tufts University, Boston, MA. The University of Minnesota Nutrient Data System for Research program (28) was used to generate energy and nutrient estimates.
The MIND diet score is derived from consumption of 15 food groups: green leafy vegetables, other vegetables (excluding potatoes and potato products for these analyses), berries, nuts, olive oil, butter and margarine, cheese, whole grains, nonfried fish, beans, nonfried poultry, red meat and products, fast/fried foods, pastries and sweets, and wine. Individuals received a score of 0, 0.5, or 1 based on servings or frequency of consumption of each food group per week. Supplemental Table 1 shows detailed scoring criteria. Scores for the MIND pattern ranged from 0 to 15, with higher scores indicative of better diet quality. The MIND score was calculated for every visit with valid dietary intake data, and the average MIND score across all visits was used for each participant as the overall exposure, because the MIND scores over time did not vary significantly based on a generalized estimating equation model with repeated measures (age- and sex-adjusted P-trend of MIND score across visits = 0.287). The MIND score was analyzed as both a continuous scale and tertile categories.
Assessment of physical function and muscle strength
Physical function assessment
The SPPB was administrated at each follow-up visit to assess physical function (29). The SPPB consists of 3 tests of lower body performance: repeated chair stands (time used for standing up from a chair 5 times), progressive standing balance (ability to stand in side-by-side, semi-tandem, and full-tandem for 10 s each), and usual gait speed (over 6 m), and each test was scored from 0 to 4, with 4 indicating the best performance. The scores of the 3 tests were summed to derive the overall SPPB score, ranging from 0 to 12. Physical function impairment was defined as an SPPB score < 10 (30).
We also used the HABCPPB, an expanded measure of physical function with a higher measurement ceiling, which was derived based on the SPPB (31, 32) to evaluate functional decline. The HABCPPB consists of the 3 SPPB components supplemented with a narrow 20-cm-wide usual-pace balance walk over 6 m. The standing balance component expands the hold time to 30 s for the semi- and full-tandem stands and adds a single leg stand. A ratio was constructed for each component, in the form of actual performance divided by best possible performance. The HABCPPB was then calculated by summing up the 4 ratio scores for a possible score of 0–4, with a higher score indicating better physical function (31).
Grip strength assessment
Grip strength was measured at each follow-up visit with a Smedley Hand Dynamometer (Stoelting). During measurement, the dynamometer was adjusted to fit each grip and calibrated to weight. Grip strength was measured on both hands, and the highest grip strength value (kg) was used for analysis.
Covariates
Covariates were selected based on previous investigation (33) and included age, sex, race/ethnicity, education years, smoking status, BMI, physical activity, number of chronic diseases at first diet visit, time since first diet visit, and average energy intake across all valid diet visits. All covariates were assessed by questionnaire except for BMI. Race/ethnicity was self-reported by study participants and categorized into non-Hispanic white, non-Hispanic black, or other. Smoking status was categorized as never, former, or current smoker. Weight and height were measured without wearing shoes with a Detecto medical beam scale, and BMI (in kg/m2) was calculated as weight divided by square of height. Physical activity was evaluated by a validated leisure-time physical activity questionnaire (34) which assessed the duration and frequency of various sports and recreational activities and categorized individuals into 4 groups according to Metabolic Equivalent of Task minutes per week: sedentary (<50), low (50–249), moderate (250–499), and high (≥500). Number of chronic diseases, including chronic obstructive pulmonary disease, diabetes, cancer, Parkinson disease, bone disease, dyslipidemia, renal disease, and CVDs, was identified based on International Classification of Diseases ninth revision (ICD-9) codes and summed for analysis. Energy intake was calculated from the FFQ at each visit and averaged across all available visits.
Statistical analysis
Sociodemographic characteristics were reported as means ± SDs or percentages. t Test and chi-square tests were used to compare sociodemographic characteristics across tertiles of the averaged MIND diet score for continuous and categorical variables, respectively. Repeated-measures logistic or linear mixed-effects models were implemented with the SAS GLIMMIX or MIXED procedure to examine longitudinal associations between the averaged MIND diet score and physical function impairment, or grip strength, among participants with available functional measures at age 60 y and older. These were adjusted for age, sex, and race in base models, and in addition for years of education, smoking status, physical activity, number of chronic diseases, BMI, years since first diet visit, and mean energy intake in fully adjusted models. Physical function decline over time across MIND diet score tertiles was assessed by including an interaction term for MIND diet score tertile and time since first diet visit. For both models, the random intercept and slope of time since first diet visit were used, and models were adjusted for covariates as aforementioned. For grip strength, analyses were conducted by sex. We also performed sensitivity analyses on associations of averaged MIND diet score with repeated measures of SPPB and grip strength, by only including participants who were free of physical function impairment (SPPB score < 10) or low grip strength [<30 kg for men and <20 kg for women (35)] at their first diet visit, to examine associations of MIND diet with physical function and muscle strength among participants with better function at baseline. Participants with missing data for any variable were excluded from the regression analyses. All analyses were performed using SAS version 9.4 (SAS Institute, Inc), with a 2-tailed type I error α = 0.05 to assess statistical significance.
Results
The length of follow-up since first diet visit ranged from 0 to 13 y, with a median of 6 y. At first diet visit, the mean ± SD age of BLSA cohort participants was 68 ± 14 y (age range: 30–101 y), with 48% of participants male (Table 1). More than two-thirds were non-Hispanic white, and a majority had high educational attainment. Compared with participants in MIND diet score tertiles 1 and 2, those in the highest tertile tended to be younger, female, never smokers, and to have lower BMI, more years of education, higher physical activity, and fewer chronic diseases (P for all < 0.05).
TABLE 1.
Sociodemographic characteristics by averaged MIND diet score tertile among participants of the Baltimore Longitudinal Study of Aging1
| MIND score | |||||
|---|---|---|---|---|---|
| Overall | 1st tertile (3.5–7.8) | 2nd tertile (7.8–9.3) | 3rd tertile (9.3–13.0) | P | |
| n | 1358 | 463 | 447 | 448 | |
| Age at first diet visit, y | 68.0 ± 13.6 | 70.2 ± 14.3 | 67.7 ± 12.9 | 66.2 ± 13.2 | <0.001 |
| Males, % | 47.6 | 63.3 | 45.6 | 33.3 | <0.001 |
| Race/ethnicity, % | 0.417 | ||||
| Non-Hispanic white | 71.9 | 71.5 | 70.7 | 73.7 | |
| Non-Hispanic black | 22.1 | 23.5 | 23.3 | 19.4 | |
| Other | 6.0 | 5.0 | 6.0 | 6.9 | |
| Education years (n = 1357) | 16.7 ± 2.5 | 16.2 ± 2.6 | 16.7 ± 2.2 | 17.1 ± 2.5 | <0.001 |
| Smoking status at first diet visit, % (n = 1347) | <0.001 | ||||
| Never | 56.9 | 50.8 | 57.5 | 62.7 | |
| Former | 39.2 | 43.1 | 39.3 | 35.1 | |
| Current | 3.9 | 6.1 | 3.1 | 2.2 | |
| Physical activity at first diet visit, % (n = 1347) | <0.001 | ||||
| Sedentary | 9.7 | 14.0 | 9.7 | 5.1 | |
| Low | 38.5 | 42.7 | 39.7 | 33.1 | |
| Moderate | 28.4 | 22.8 | 28.4 | 34.0 | |
| High | 23.5 | 20.6 | 22.1 | 27.7 | |
| Chronic diseases at first diet visit, n (n = 1204) | 1.6 ± 1.3 | 1.8 ± 1.4 | 1.6 ± 1.3 | 1.3 ± 1.2 | <0.001 |
| BMI at first diet visit, kg/m2 (n = 1347) | 27.1 ± 5.0 | 27.9 ± 5.2 | 27.1 ± 4.8 | 26.4 ± 5.0 | <0.001 |
| Energy intake, kcal/d | 2088 ± 740 | 2068 ± 750 | 2118 ± 775 | 2080 ± 693 | 0.570 |
Values are means ± SDs or percentages unless indicated otherwise. MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay.
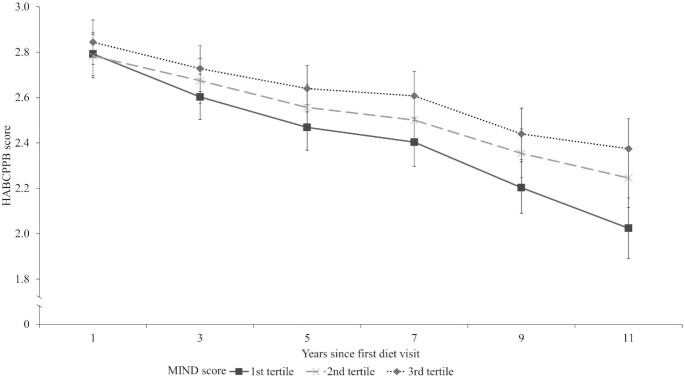
Using fully adjusted logistic mixed-effects models, participants with higher averaged MIND score on a continuous scale had lower odds of physical function impairment (SPPB < 10) over the follow-up time (per 1-point increment in MIND score: OR: 0.81; 95% CI: 0.71, 0.93; P = 0.003) (Table 2, Supplemental Table 2A–D). Compared with the lowest tertile of MIND score, participants in the third tertile had 57% (OR: 0.43; 95% CI: 0.25, 0.73; P = 0.002) lower odds of physical function impairment over time. Further, MIND score was associated with significantly lower odds of functional impairment for each component defined as not having a perfect score. Participants in the highest tertile had 46% (OR: 0.54; 95% CI: 0.36, 0.82; P = 0.003), 51% (OR: 0.49; 95% CI: 0.30, 0.80; P < 0.004), and 49% (OR: 0.51; 95% CI: 0.30, 0.87; P = 0.013) lower odds of being physically impaired in repeated chair stands, standing balance, and gait speed, respectively. In addition, better MIND score was associated with slower physical function decline as indicated by the HABCPPB (Figure 2). Compared with the lowest tertile of MIND score, physical function decline was significantly slower for both the second (P = 0.007) and third (P < 0.001) tertiles.
TABLE 2.
Associations of averaged MIND diet score with SPPB score and its components, among participants, aged 60 y and older, of the Baltimore Longitudinal Study of Aging1
| Model 12 (n = 1088) | Model 23 (n = 981) | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Odds of SPPB score ≤ 10 | ||||
| MIND score/1-point increment | 0.71 (0.63, 0.80) | <0.001 | 0.81 (0.71, 0.93) | 0.003 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.51 (0.33, 0.77) | 0.001 | 0.64 (0.41, 1.01) | 0.054 |
| 3rd vs. 1st tertile | 0.26 (0.16, 0.41) | <0.001 | 0.43 (0.25, 0.73) | 0.002 |
| Odds of SPPB component score ≤ 4 | ||||
| Chair stand | ||||
| MIND score/1-point increment | 0.77 (0.70, 0.85) | <0.001 | 0.87 (0.78, 0.96) | 0.008 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.51 (0.33, 0.77) | 0.001 | 0.73 (0.50, 1.05) | 0.092 |
| 3rd vs. 1st tertile | 0.26 (0.16, 0.41) | <0.001 | 0.54 (0.36, 0.82) | 0.003 |
| Standing balance | ||||
| MIND score/1-point increment | 0.75 (0.68, 0.84) | <0.001 | 0.85 (0.75, 0.96) | 0.009 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.55 (0.37, 0.81) | 0.003 | 0.65 (0.42, 0.99) | 0.046 |
| 3rd vs. 1st tertile | 0.33 (0.22, 0.52) | <0.001 | 0.49 (0.30, 0.80) | 0.004 |
| Gait speed | ||||
| MIND score/1-point increment | 0.77 (0.69, 0.86) | <0.001 | 0.86 (0.75, 0.99) | 0.032 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.63 (0.41, 0.95) | 0.026 | 0.71 (0.45, 1.12) | 0.138 |
| 3rd vs. 1st tertile | 0.35 (0.22, 0.55) | <0.001 | 0.51 (0.30, 0.87) | 0.013 |
Values are adjusted ORs and 95% CIs of physical function impairment assessed by the SPPB and its components per 1-point increment and tertile of MIND score. MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; SPPB, Short Physical Performance Battery.
Model 1 adjusted for age, sex, race, and years since first diet visit.
Model 2 adjusted for age, sex, race, years since first diet visit, years of education, smoking status, physical activity, number of chronic diseases, BMI, and average energy intake; n is smaller due to missingness on smoking status, physical activity, number of chronic diseases, and BMI.
FIGURE 2.
HABCPPB decline over follow-up time, stratified by MIND diet score tertile, adjusting for age, race, years since first diet visit, years of education, smoking status, physical activity, number of chronic diseases, BMI, and mean energy intake. Slower physical function declines were shown for the second (P = 0.007) and third (P < 0.001) MIND diet score tertiles than for the first tertile. HABCPPB, Health, Aging and Body Composition Physical Performance Battery; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay.
Protective associations were observed between averaged MIND score and grip strength in both men and women aged 60 y or older (Table 3, Supplemental Table 3A, B). For men, grip strength over time was 0.44 kg higher per averaged MIND score point (β: 0.44 kg; 95% CI: 0.06, 0.82 kg; P = 0.023), and participants in the highest compared with the lowest tertile had 1.86-kg higher grip strength (β: 1.86 kg; 95% CI: 0.33, 3.40 kg; P = 0.017) in the fully adjusted model. For women, grip strength over time was 0.37 kg higher per averaged MIND score point (β: 0.37 kg; 95% CI: 0.06, 0.68 kg; P = 0.020). Women in the highest compared with the lowest tertile had 1.24-kg higher grip strength (β: 1.24 kg; 95% CI: 0.04, 2.45 kg; P = 0.044).
TABLE 3.
Associations between MIND diet score and grip strength among participants, aged 60 y and older, in the Baltimore Longitudinal Study of Aging1
| Model 12 | Model 23 | |||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| Men (n = 545) | ||||
| MIND score/1-point increment | 0.52 (0.16, 0.87) | 0.004 | 0.44 (0.06, 0.82) | 0.023 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.82 (−0.52, 2.16) | 0.229 | 0.62 (−0.77, 2.01) | 0.381 |
| 3rd vs. 1st tertile | 2.10 (0.62, 3.58) | 0.005 | 1.86 (0.33, 3.40) | 0.017 |
| Women (n = 571) | ||||
| MIND score/1-point increment | 0.44 (0.16, 0.72) | 0.002 | 0.37 (0.06, 0.68) | 0.020 |
| MIND score tertile | ||||
| 2nd vs. 1st tertile | 0.51 (−0.59, 1.62) | 0.363 | 0.34 (−0.84, 1.51) | 0.572 |
| 3rd vs. 1st tertile | 1.49 (0.39, 2.58) | 0.008 | 1.24 (0.04, 2.45) | 0.044 |
Values are adjusted β coefficients and 95% CIs of grip strength per 1-point increment and tertile of MIND score. Grip strength was measured on both hands and the highest value was used. MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay.
Model 1 adjusted for age, race, and years since first diet visit.
Model 2 adjusted for age, race, years since first diet visit, years of education, smoking status, physical activity, number of chronic diseases, BMI, and average energy intake.
When including only participants with no physical function impairment and without low grip strength at first diet visit, the averaged MIND score remained associated with lower odds of physical function impairment (n = 754; per 1-point increment in MIND score: OR: 0.78; 95% CI: 0.65, 0.94; P = 0.008). Compared with the lowest MIND diet tertile, the highest tertile showed 58% lower odds of physical function impairment, as assessed by the SPPB (OR: 0.42; 95% CI: 0.20, 0.87; P = 0.019). For grip strength (n = 814), no significant results were found for the association with averaged MIND diet score (Supplemental Table 4).
Discussion
The MIND dietary pattern was designed for, and has shown protective associations with, cognitive health. This study examined associations of adherence to the MIND diet with physical function and muscle strength. Results demonstrate that intake patterns most consistent with the MIND diet are associated with better physical function and slower functional decline over the follow-up time, as assessed with the SPPB and the HABCPPB, and with better grip strength over time, in men and women aged 60 y and older.
The role of dietary patterns in the functional decline associated with aging has been investigated by other studies. In the Nurses’ Health Study, better diet quality, indicated by higher scores on the Alternative Healthy Eating Index (AHEI), was associated with lower self-reported physical function impairment in middle- to older-aged women over 18 y of follow-up (36). Further, in the Health Professionals Follow-up Study, middle- to older-aged men with higher AHEI scores had lower odds of impairment of physical function over 4 y of follow-up (37). Using data from individuals 65 y and older from the InCHIANTI (Invecchiare in Chianti) study, we demonstrated that a Mediterranean-type diet was associated with slower decline of physical function and lower incidence of mobility disability, as assessed by the SPPB over 9 y of follow-up (38). In addition, positive associations have been reported between a Mediterranean-type diet and grip strength among nonhospitalized older women aged 60–85 y in the Personalized ICT Supported Services for Independent Living and Active Ageing project (39).
The MIND diet is a primarily plant-based dietary pattern derived from components of the DASH and Mediterranean-type diets. Although it was developed for prevention of cognitive decline, its dietary components may also benefit physical functional well-being. For example, green leafy vegetables and other orange/yellow vegetables contribute to carotenoid intake, and lower concentrations of plasma carotenoids have been associated with lower upper- and lower-extremity strength (40). Hagan et al. (36, 37) demonstrated that lower intakes of romaine or leaf lettuce, broccoli, blueberries, and nuts and higher intakes of processed foods such as hamburger and bacon were associated with higher risk of self-reported physical function impairment in middle- to older-aged individuals. Vegetables, especially green leafy vegetables, are rich in inorganic nitrate, and higher dietary nitrate has been associated with better grip strength and physical function, as assessed by the timed-up-and-go test in older women in the Perth Longitudinal Study of Ageing (41). Berries, particularly emphasized by the MIND diet, contain polyphenols and phytonutrients. Urinary polyphenols, a biomarker of dietary polyphenols, have been associated with lower risk of physical function decline in older individuals after 9 y of follow-up in the InCHIANTI study (42). In the Seniors-ENRICA cohort, higher consumption of nuts was associated with lower risk of agility and mobility impairment among older men, and with lower risk of physical function impairment among older women, after 7 y of follow-up (43). Similarly, fatty fish intake has been associated with higher grip strength in older individuals (44), and n–3 fatty acids have shown beneficial effects on muscle volume and strength (45).
Strengths of this study include objectively assessed measures of physical function and grip strength in a longitudinal cohort. The use of the average of multiple measures of diet across younger and older ages accommodates changes in dietary intake and minimizes the impact of short-term alterations in diet. This study also has limitations. The BLSA cohort includes mostly non-Hispanic white individuals with relatively high education levels, and results may not be generalizable to the general US population. Dietary assessment in the cohort was conducted using a validated FFQ, a method which poses limitations in accurately estimating absolute nutrient values and suffers from general imprecision. However, these limitations were minimized in this investigation by the use of dietary patterns as the dietary exposures (as opposed to absolute nutrients) and by examining diet as a cumulative measure across the various assessment points to minimize potential misclassification. Another limitation is the potential for reverse causality, because the main analyses used all available valid dietary data, and when restricting to only dietary data collected before the onset of impaired function/functional decline/low grip strength, the associations for the SPPB were attenuated and nonsignificant results were shown for grip strength (Supplemental Table 5). Lastly, being observational in design, this investigation cannot establish causality, and residual confounding cannot be completely ruled out.
In conclusion, higher adherence to the MIND diet was associated with better physical function and grip strength. Further investigations are warranted on implementation of the MIND diet and its effects on slowing aging-related functional decline and maintaining muscle strength.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—YJ: conducted the statistical analyses and drafted the manuscript; TT and SAT: supported the statistical analyses and interpretation, and edited the manuscript; TT, SAT, EMS, KLT, and LF: critically revised the manuscript; EMS, KLT, and LF: provided guidance on analysis and interpretation; SAT: was responsible for study concept and design; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Institute on Aging grants R01AG051752 and R03AG065861 (to SAT). The sponsoring institution did not interfere with the collection, analysis, presentation, or interpretation of the data reported here.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; BLSA, Baltimore Longitudinal Study of Aging; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HABCPPB, Health, Aging and Body Composition Physical Performance Battery; InCHIANTI, Invecchiare in Chianti; IRB, Institutional Review Board; IRP-NIA, Intramural Research Program of the National Institute on Aging; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; SPPB, Short Physical Performance Battery.
Contributor Information
Sameera A Talegawkar, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Yichen Jin, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Eleanor M Simonsick, Translational Gerontology Branch, National Institute on Aging, Baltimore, MD, USA.
Katherine L Tucker, Department of Biomedical & Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA; Center for Population Health, University of Massachusetts Lowell, Lowell, MA, USA.
Luigi Ferrucci, Translational Gerontology Branch, National Institute on Aging, Baltimore, MD, USA.
Toshiko Tanaka, Translational Gerontology Branch, National Institute on Aging, Baltimore, MD, USA.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application to and approval by the Baltimore Longitudinal Study of Aging cohort investigators and research team at the National Institute on Aging.
References
- 1. Roser M, Ortiz-Ospina E, Ritchie H. Life expectancy. [Internet]. Our World in Data. Oxford, United Kingdom: Global Change Data Lab; 2013; [revised October 2019]. [Accessed Jan 12, 2021].Available from: https://ourworldindata.org/life-expectancy. [Google Scholar]
- 2. UN, Department of Economic and Social Affairs, Population Division. World population ageing 2019. ST/ESA/SER.A/444. New York: United Nations; 2020. [Google Scholar]
- 3. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. American Community Survey Reports, ACS-38. Suitland, MD: US Census Bureau; 2018. [Google Scholar]
- 4. Chou C-H, Hwang C-L, Wu Y-T. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93(2):237–44. [DOI] [PubMed] [Google Scholar]
- 5. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–8. [DOI] [PubMed] [Google Scholar]
- 6. Kelley AS, Ettner SL, Morrison RS, Du Q, Sarkisian CA. Disability and decline in physical function associated with hospital use at end of life. J Gen Intern Med. 2012;27(7):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dayhoff NE, Suhrheinrich J, Wigglesworth J, Topp R, Moore S. Balance and muscle strength as predictors of frailty among older adults. J Gerontol Nurs. 1998;24(7):18–27. [DOI] [PubMed] [Google Scholar]
- 8. Narain P, Rubenstein LZ, Wieland GD, Rosbrook B, Strome LS, Pietruszka F, Morley JE. Predictors of immediate and 6-month outcomes in hospitalized elderly patients: the importance of functional status. J Am Geriatr Soc. 1988;36(9):775–83. [DOI] [PubMed] [Google Scholar]
- 9. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi Ret al. . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73. [DOI] [PubMed] [Google Scholar]
- 10. Tong TY, Wareham NJ, Khaw K-T, Imamura F, Forouhi NG. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-Mediterranean population: the EPIC-Norfolk study. BMC Med. 2016;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koloverou E, Panagiotakos D, Pitsavos C, Chrysohoou C, Georgousopoulou E, Grekas A, Christou A, Chatzigeorgiou M, Skoumas I, Tousoulis Det al. . Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32(1):73–81. [DOI] [PubMed] [Google Scholar]
- 12. Ntanasi E, Yannakoulia M, Kosmidis M-H, Anastasiou CA, Dardiotis E, Hadjigeorgiou G, Sakka P, Scarmeas N. Adherence to Mediterranean diet and frailty. J Am Med Dir Assoc. 2018;19(4):315–22.e2. [DOI] [PubMed] [Google Scholar]
- 13. Silva R, Pizato N, da Mata F, Figueiredo A, Ito M, Pereira MG. Mediterranean diet and musculoskeletal-functional outcomes in community-dwelling older people: a systematic review and meta-analysis. J Nutr Health Aging. 2018;22(6):655–63. [DOI] [PubMed] [Google Scholar]
- 14. Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103(2):341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones NR, Forouhi NG, Khaw K-T, Wareham NJ, Monsivais P. Accordance to the Dietary Approaches to Stop Hypertension diet pattern and cardiovascular disease in a British, population-based cohort. Eur J Epidemiol. 2018;33(2):235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perry CA, Van Guilder GP, Kauffman A, Hossain M. A calorie-restricted DASH diet reduces body fat and maintains muscle strength in obese older adults. Nutrients. 2020;12(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–89. [DOI] [PubMed] [Google Scholar]
- 18. Chen X, Maguire B, Brodaty H, O'Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis. 2019;67(2):583–619. [DOI] [PubMed] [Google Scholar]
- 19. Morris MC, Tangney CC, Wang Y, Barnes LL, Bennett D, Aggarwal N. O2-02-04: MIND diet score more predictive than DASH or Mediterranean diet scores. Alzheimers Dement. 2014;10(4S Part 2):P166. [Google Scholar]
- 20. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barberger-Gateau P, Letenneur L, Deschamps V, Pérès K, Dartigues J-F, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325(7370):932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clouston SA, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, Rubin MS, Hofer SM. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35(1):33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15(4):581–9. [DOI] [PubMed] [Google Scholar]
- 25. Berendsen AM, Kang JH, Feskens EJ, de Groot C, Grodstein F, van de Rest O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. 2018;22(2):222–9. [DOI] [PubMed] [Google Scholar]
- 26. Shock NW, Greulich R, Andres R, Arrenberg D, Costa P, Lakatta E, Tobin J. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington (DC): US Government Printing Office; 1984. [Google Scholar]
- 27. Talegawkar SA, Tanaka T, Maras JE, Ferrucci L, Tucker KL. Validation of nutrient intake estimates derived using a semi-quantitative FFQ against 3 day diet records in the Baltimore Longitudinal Study of Aging. J Nutr Health Aging. 2015;19(10):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schakel S, Sievert Y, Buzzard I. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 29. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, Guralnik JM. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64A(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–9. [DOI] [PubMed] [Google Scholar]
- 32. Chiles Shaffer N, Fabbri E, Ferrucci L, Shardell M, Simonsick EM, Studenski S. Muscle quality, strength, and lower extremity physical performance in the Baltimore Longitudinal Study of Aging. J Frailty Aging. 2017;6(4):183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talegawkar SA, Jin Y, Xue Q-L, Tanaka T, Simonsick EM, Tucker KL, Ferrucci L. Dietary pattern trajectories in middle age and physical function in older age. J Gerontol A Biol Sci Med Sci. 2021;76(3):513–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talbot LA, Fleg JL, Metter EJ. Secular trends in leisure-time physical activity in men and women across four decades. Prev Med. 2003;37(1):52–60. [DOI] [PubMed] [Google Scholar]
- 35. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SMet al. . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagan KA, Chiuve SE, Stampfer MJ, Katz JN, Grodstein F. Greater adherence to the Alternative Healthy Eating Index is associated with lower incidence of physical function impairment in the Nurses’ Health Study. J Nutr. 2016;146(7):1341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hagan KA, Grodstein F. The Alternative Healthy Eating Index and physical function impairment in men. J Nutr Health Aging. 2019;23(5):459–65. [DOI] [PubMed] [Google Scholar]
- 38. Milaneschi Y, Bandinelli S, Corsi AM, Lauretani F, Paolisso G, Dominguez LJ, Semba RD, Tanaka T, Abbatecola AM, Talegawkar SAet al. . Mediterranean diet and mobility decline in older persons. Exp Gerontol. 2011;46(4):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrea L, Muscogiuri G, Di Somma C, Tramontano G, De Luca V, Illario M, Colao A, Savastano S. Association between Mediterranean diet and hand grip strength in older adult women. Clin Nutr. 2019;38(2):721–9. [DOI] [PubMed] [Google Scholar]
- 40. Semba RD, Blaum C, Guralnik JM, Moncrief DT, Ricks MO, Fried LP. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15(6):482–7. [DOI] [PubMed] [Google Scholar]
- 41. Sim M, Lewis JR, Blekkenhorst LC, Bondonno CP, Devine A, Zhu K, Peeling P, Prince RL, Hodgson JM. Dietary nitrate intake is associated with muscle function in older women. J Cachexia Sarcopenia Muscle. 2019;10(3):601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rabassa M, Zamora-Ros R, Andres-Lacueva C, Urpi-Sarda M, Bandinelli S, Ferrucci L, Cherubini A. Association between both total baseline urinary and dietary polyphenols and substantial physical performance decline risk in older adults: a 9-year follow-up of the InCHIANTI study. J Nutr Health Aging. 2016;20(5):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arias-Fernández L, Machado-Fragua MD, Graciani A, Guallar-Castillón P, Banegas JR, Rodríguez-Artalejo F, Lana A, Lopez-Garcia E. Prospective association between nut consumption and physical function in older men and women. J Gerontol A Biol Sci Med Sci. 2019;74(7):1091–7. [DOI] [PubMed] [Google Scholar]
- 44. Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA, Hertfordshire Cohort Study Group . Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire Cohort Study. J Am Geriatr Soc. 2008;56(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application to and approval by the Baltimore Longitudinal Study of Aging cohort investigators and research team at the National Institute on Aging.