Abstract
Approximately 500,000 dialysis patients in America are at a high risk of hyperkalemia, a condition where blood potassium becomes elevated above normal levels. Hyperkalemia is extremely dangerous, as it can result in severe cardiac complications if untreated. Hyperkalemia may be silent or present vague symptoms until those complications develop, at which point patients require emergency medical care. However, if patients have the ability to measure their potassium levels at home, they could detect hyperkalemia before it reaches a dangerous stage, and seek preventative medical care to avoid severe complications.
Therefore, we have designed a novel device allowing patients to measure their blood potassium levels at home. The workflow of our solution is as follows: (1) patients obtain a blood sample from a finger prick, (2) potassium concentration is measured with an ion specific electrode (ISE), and (3) the device displays their potassium levels and a recommended course of action based on their hyperkalemic risk.
We validate our solution with three major tests. First, our portable ISE technology must accurately measure potassium concentration in blood samples. Second, appropriate lancet parameters (gauge and depth) to minimize hemolysis in capillary blood samples must be found to minimize falsely elevated readings. Third, device portability and ease of use must be evaluated using patient input, as these factors will affect patient compliance. We have validated the use of portable ISE technology to feasibly measure potassium, and we continue to collect data for our second and third tests.
INTRODUCTION
About 20 million Americans suffer from chronic kidney disease (CKD), and of these, over half a million have impaired kidney function and require frequent dialysis treatment. Dialysis patients are at high risk of hyperkalemia, a condition where serum potassium levels are elevated above 3.5–5.5 mmol/L [1]. Hyperkalemia is known to be a silent killer, as symptoms often do not present themselves until potassium levels are severely elevated [2], resulting in heart palpitations, muscle pain and weakness, and potentially cardiac arrhythmia and death [3].
The risk that hyperkalemia poses to dialysis patients is evident when based on mortality trends for each day of the week. The current standard of care consists of three dialysis treatments per week, Monday-Wednesday-Friday (MWF) or Tuesday-Thursday-Saturday (TTS) cycle [4]. On the two-day intervals (either Sat/Sun or Sun/Mon depending on the schedule) during which patients receive no treatment, the instance of sudden cardiac death increases three-fold, as patients are unable to remove toxic substances from their blood themselves [5]. This problem is further exacerbated by the fact that 24% of dialysis patients miss their scheduled treatments, prolonging the time spent between treatments and increasing the risk for cardiac complications [6].
The increased mortality rate of patients due to cardiac complications strongly suggests that hyperkalemia is a potential major cause of death [7]. According to the United States Renal Data System Annual Report (2017), over 42% of deaths of dialysis patients are cardiovascular in origin, amounting to nearly 40,000 deaths per year [4]. This is markedly greater than the 25% of deaths that are cardiovascular in origin for the general population [7]. Past nephrologic studies further emphasize that hyperkalemia as the major predictor of mortality and sudden death for dialysis patients [2][3].
As illustrated in Fig. 1, there currently exists a missing step of intervention, as patients remain unaware of their condition until severe cardiac symptoms arise. However, if patients had the ability to monitor blood potassium levels between dialysis sessions, they would be able to take appropriate actions to reduce their potassium levels, such as seeking emergency dialysis treatment. The ability to monitor blood potassium levels at home would thus fill this missing point of intervention, and reduce the risk for dialysis patients.
Figure 1.
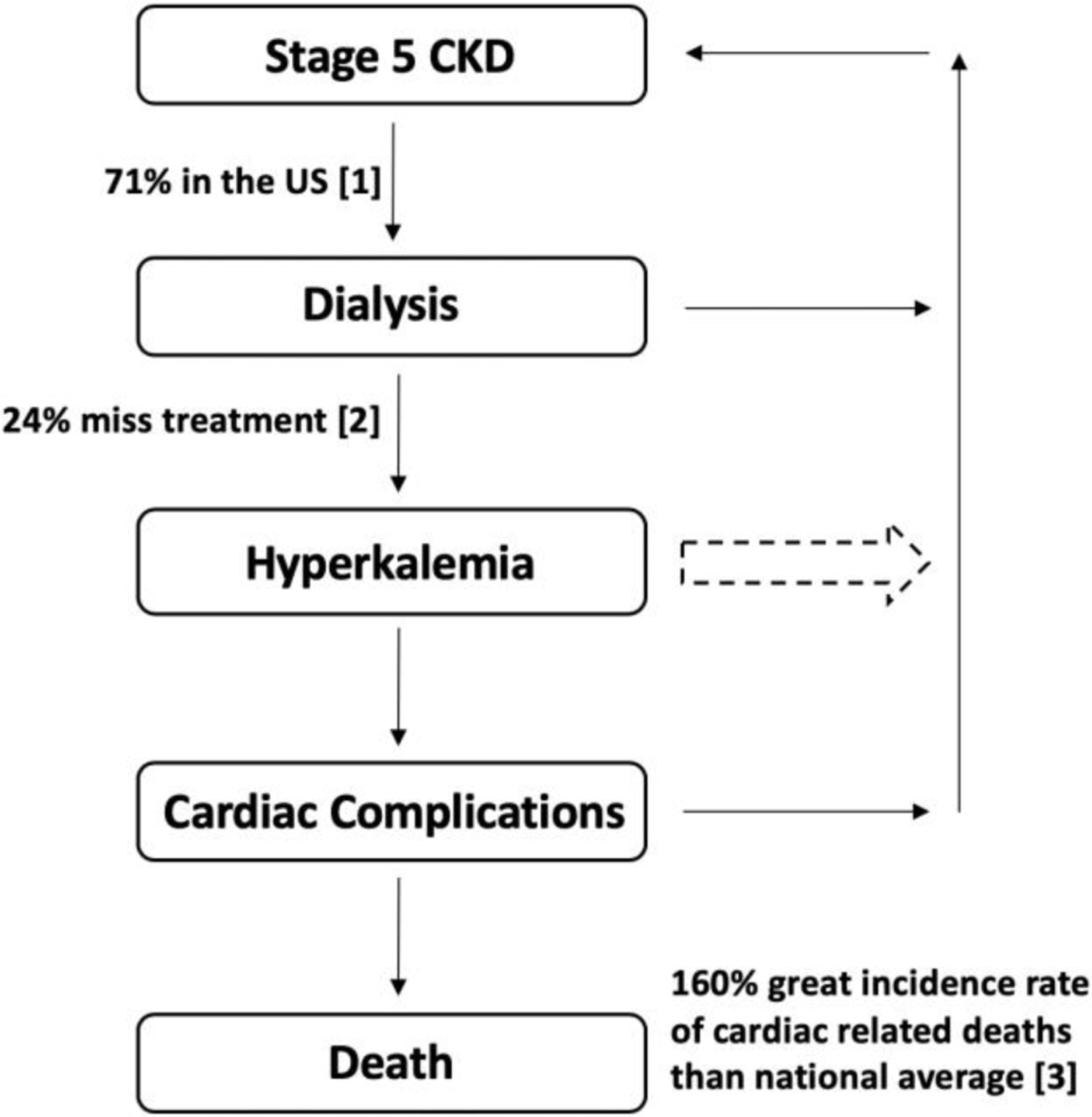
The current workflow of dialysis patients with undetected hyperkalemia. As a result, dialysis patients need a way to measure their blood potassium levels in an out of clinic setting in order to quantify their risk of hyperkalemia related complications.
1.1. ISE Development: Sample to Measurement
The current clinical standard for potassium measurement is a blood test, which is neither portable nor easily accessible for dialysis patients when they are not in a clinical setting. Therefore, with the patient in mind, we based our device on two driving principles. The first is a blood potassium sensor that is miniature, enabling portable, point of care use. The second is a simple, intuitive user interface that patients can easily navigate. With these principles at the core, we have developed a portable, handheld device with the following workflow: (1) patients prick their finger with a lancet to obtain a blood sample, (2) an ion specific electrode (ISE) measures the potassium concentration, and (3) a user interface outputs blood potassium concentration and recommended course of action. We envision that this device will work akin to current glucometers, with the workflow reflecting such as well. By having this design be easily accessible and portable for dialysis patients, they will be able to assess their current status with regards to blood potassium levels, allowing them to take preventative measures to avoid the consequences of an unchecked increase in blood potassium levels.
Based on feedback from pathologists at the Johns Hopkins Medical Institute, we have determined that whole blood is the most appropriate sample to measure potassium in, as whole blood is most analogous to blood serum, the sample used by blood labs for potassium measurement. Whole blood samples will be obtained by pricking the finger with a lancet. However, measuring whole blood comes with a risk of hemolysis, the rupturing of red blood cells from external forces, which can result in false positive measurements due to the high intracellular concentration of potassium. We aim to mitigate the risk of hemolysis by finding the depth and gauge of the lancet at which hemolysis in finger prick blood samples is minimized.
To measure potassium in the blood sample, we will use a potassium ion specific electrode (ISE), as it is the gold standard in clinical potassium measurements and has potential to be miniaturized for small blood volumes. The process to construct the ISE is shown in Fig. 2. The ISE contains a membrane laced with valinomycin, a ringed molecule that binds to potassium with high specificity. Once contacted with a potassium containing solution, potassium binds to valinomycin, creating a buildup of charge and generating a potential difference with respect to a reference. Therefore, by measuring this voltage, we will be able to calculate potassium concentration in sample solutions. Due to the relatively small concentrations of potassium present at clinically relevant levels, the signal will need to be amplified to accurately detect it. The complete process from sample to measurement is illustrated in Fig. 3. Circuitware shown in Fig. 4 enables integration of components.
Figure 2:
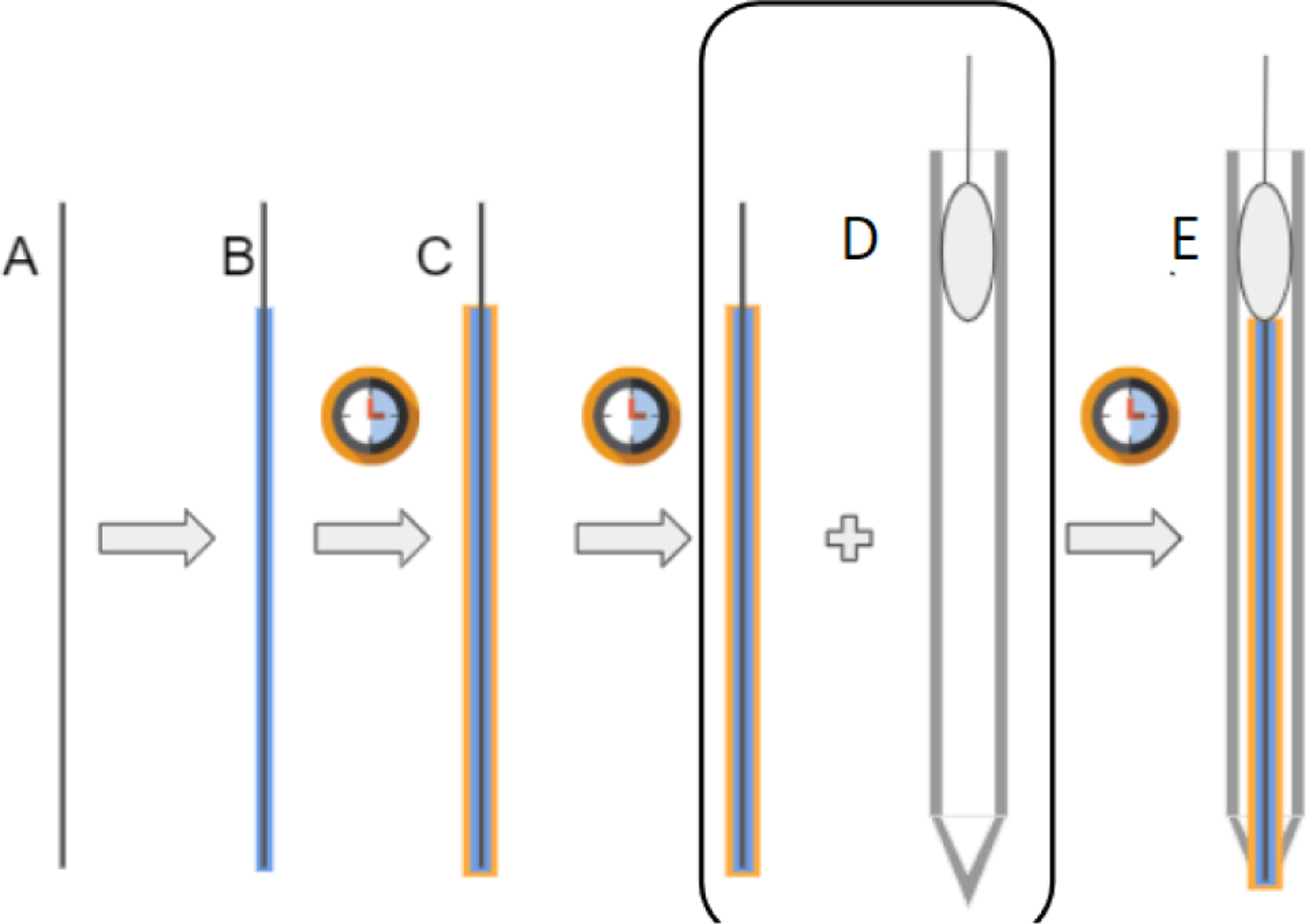
A) Filament of copper/graphite wire. B) Electrode is coated in Poly(vinyl-ferrocene) electrolyte, cured. C) Electrode is double dipped in potassium specific membrane (Valinomycin in PVC), cured again. D) Electrode is inserted into glass microcapillary tube, and secured with epoxy. E) Final result: an electrode double dipped in a potassium specific membrane and an electrolyte.
Figure 3.
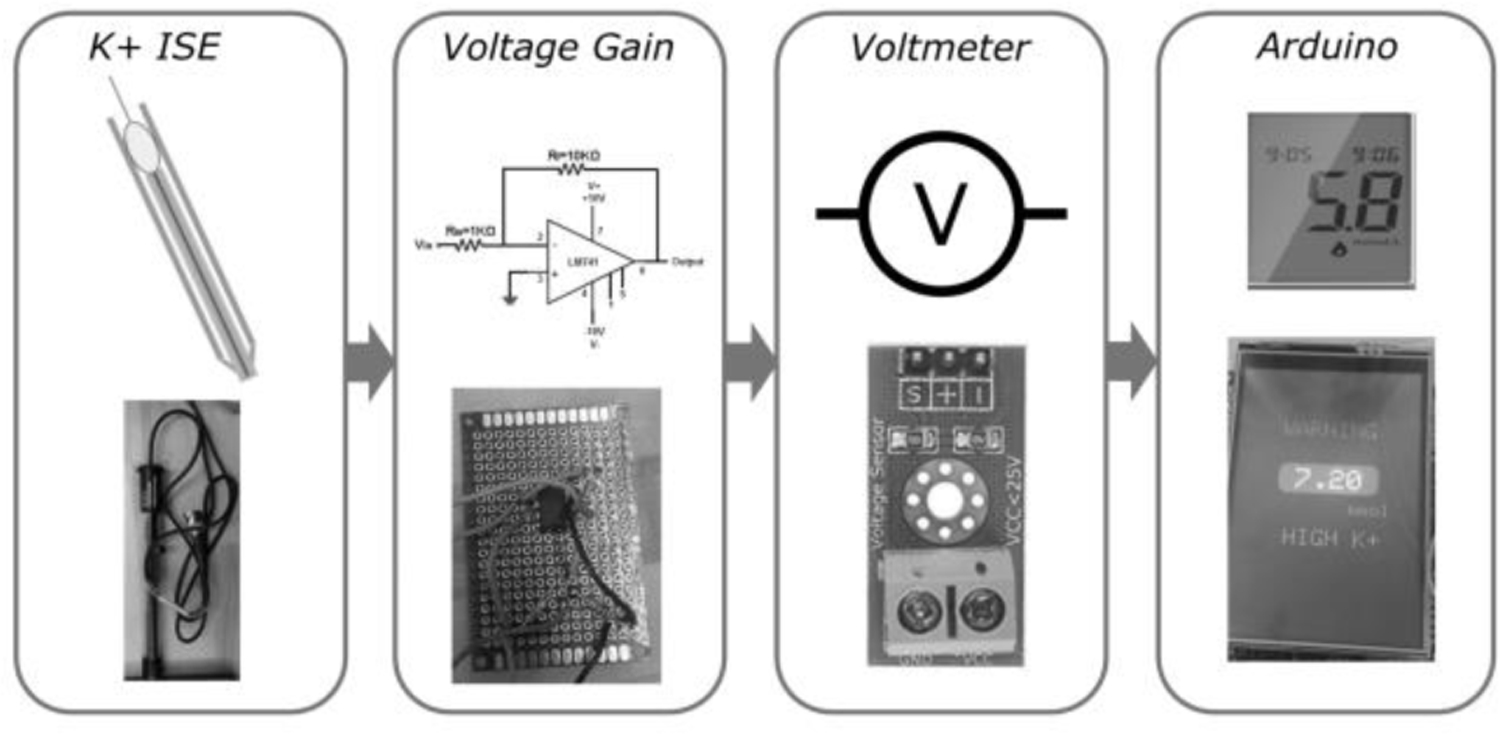
Schematic of the general components of our device. A) Sample blood potassium level is measured by in house ion selective electrode. B) Signal from the ion selective electrode goes to the voltage gain which amplifies the signal. C) Signal is read by a voltmeter which converts the signal into a voltage. D) Voltage is converted into a blood potassium level as there is a linear model between voltage generated and blood potassium level.
Figure 4.
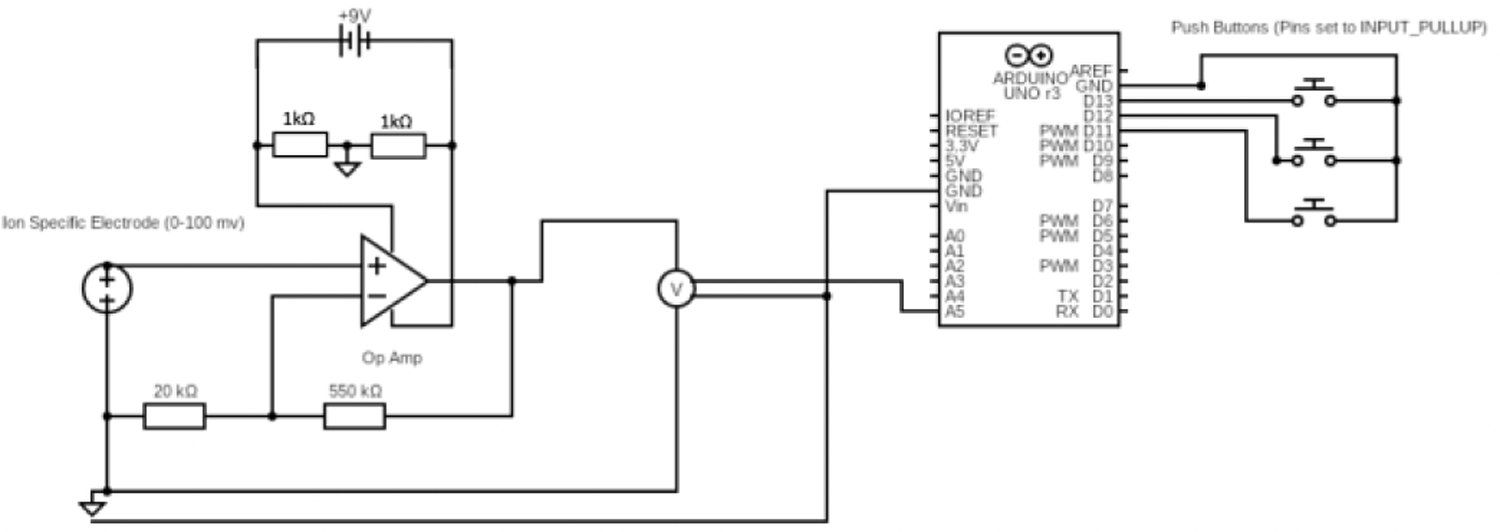
Planned circuit board for portable potassium monitor for dialysis patients.
1.3. Product in the Current Clinical Workflow
Dialysis patients currently have no method of monitoring their blood potassium levels at an out-of-clinic setting. Our device will be able to provide this information to the patient at a moment’s notice. Patients will be able to prick their own finger and analyze blood from their finger prick to obtain real time blood potassium concentration. In order to maximize patient compliance, our device is designed to be portable and easy to use for the patient. The pocket-sized hardware interface allows users to be able to take this device wherever they go. Once they have provided the blood sample, the user interface, as illustrated by Fig. 5, guarantees informativity by displaying potassium concentration, coloured feedback graded to level of risk (green, orange and red), and an advised course of action. This provides clear directives for patients to follow to minimize their risk of hyperkalemia. To maximize clinical utility, the device stores three months of data at a time, empowering users to discuss their potassium levels over time with their healthcare providers and to customize their treatment to better suit them. Future iterations of the device will provide higher-resolution information by continuously monitoring and providing feedback on patients’ potassium levels.
Figure 5.
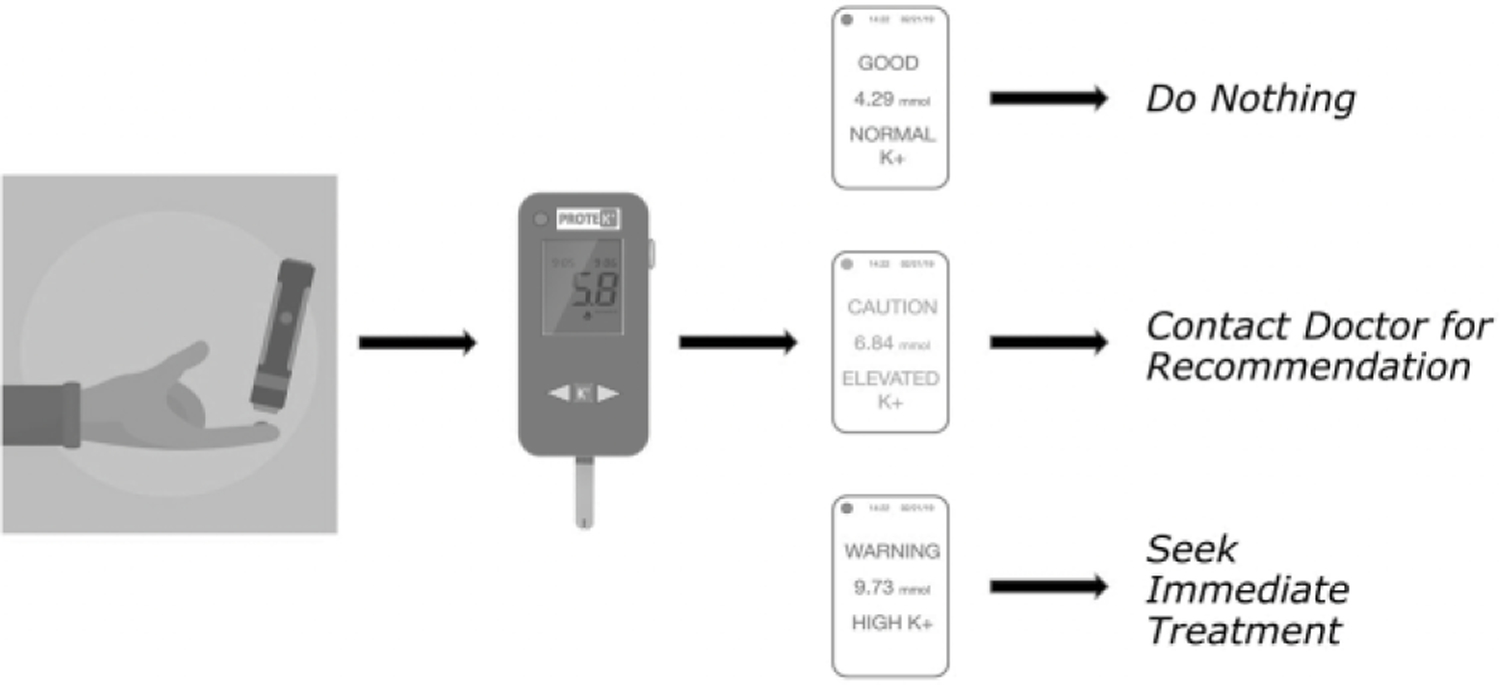
Schematic of the general workflow of our device. A) The patient will prick their finger with a disposable lancet and have the blood sample be uptaken into the test cartridge via capillary action. B) Test cartridge is inserted into the device. C) Blood potassium levels are displayed to the patient with clear color coordinated levels. D) Suggested actions are then presented to the patient depending on their blood potassium levels green = no treatment needed, orange = at risk/treatment recommended, red = seek immediate treatment
1.4. Testing of ISE Technology
There is some concern that the presence of interfering blood analytes might affect the results of a small ISE sensor in a portable potassium measuring device. In order to validate the ability of portable ISE technology to accurately measure potassium in blood samples, we have conducted a study comparing measurements obtained using a commercially-available soil potassium monitor, the HORIBA LAQUAtwin K-11 Potassium meter (hereafter referred to as the Horiba), to laboratory blood tests, the current gold standard. Though this potassium monitor is not FDA approved for this purpose and requires a large (200 μL) sample volume, it nevertheless can be used to validate the feasibility of portable ISE technology.
30 de-identified, minimally processed whole blood samples were obtained from the Johns Hopkins pathology laboratory. Following Horiba device calibration, a fixed quantity of each of the samples was applied to the Horiba ISE, and the resulting potassium was recorded. These same samples were then immediately returned to the Johns Hopkins Pathology lab for potassium testing. The discrepancy between the Horiba and pathology lab values were assessed using a paired T-test.
The results of the study are shown in Fig. 6. A linear relationship was revealed between the Horiba soil monitor readings on whole blood and the measurements made by the JHMI blood laboratory. Horiba measurements were off by a constant factor from the blood lab, suggesting that, with appropriate calibration, portable ISE technology could be used for blood potassium measurements as portable technologies.
Figure 6.
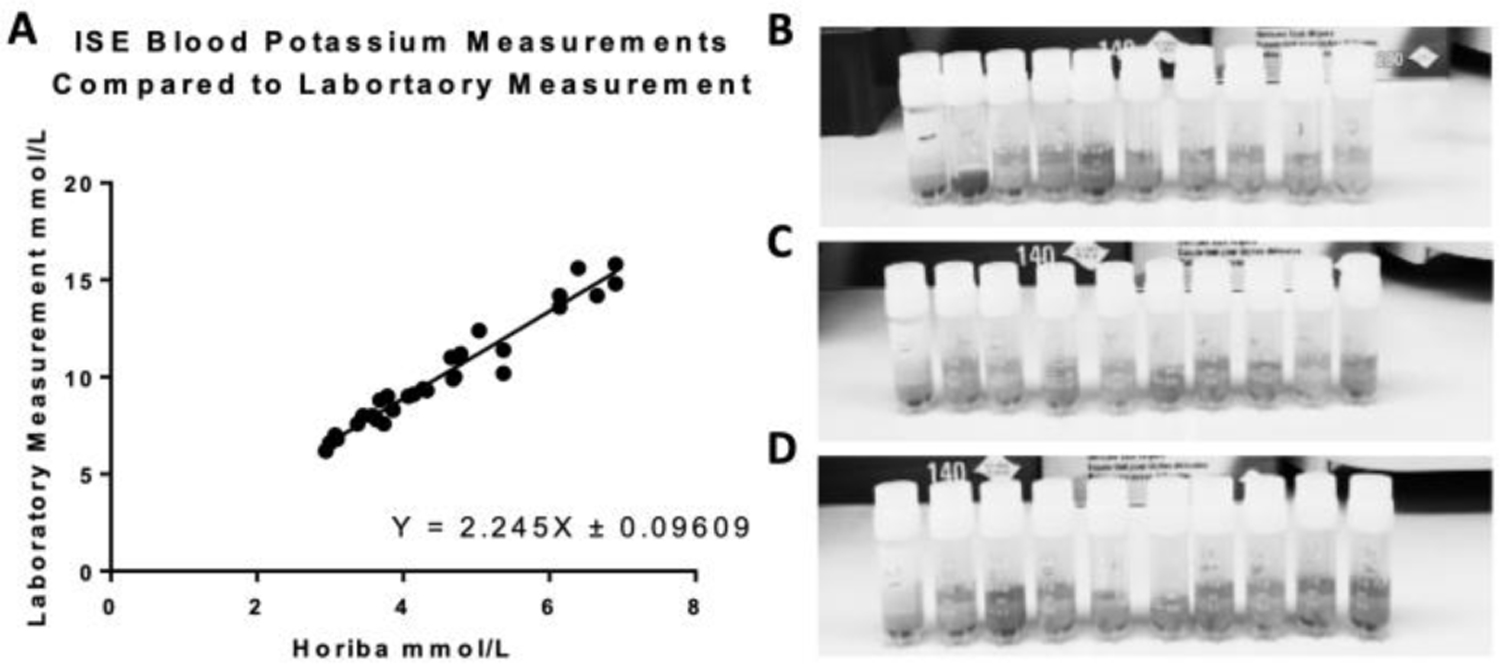
Results of an ISE technology verification. A) A HORIBA potassium soil monitor is used as the ISE with 200uL of whole blood used as the sample. Each blood sample was measured by the HORIBA potassium soil monitor and was also sent to the Johns Hopkins Medical Institute Blood Laboratory. The results were graphed, resulting in a linear relationship. It was found that ISEs are indeed effective at clinically relevant levels. B) Qualitative analysis of blood samples 1–10, left to right (the darker and redder the sample is, the more hemolysis is within the sample). C) Qualitative analysis of blood samples 11–20, left to right. D) Qualitative analysis of blood samples 21–30, left to right.
The majority of the samples displayed slightly elevated levels of potassium (which has a normal clinical range 3.5 mmol/L – 5.5 mmol/L), according to the laboratory measurements; this is indicative of hemolysis, which was confirmed using quantitative visual test of the plasma samples. This is likely because the whole blood samples used were reportedly a couple of days old, having been stored at 4 degrees Celsius. Some measurements, however, were within normal clinical levels. Thus, this verified that a valinomycin ion-selective electrode was a viable method for measuring blood potassium concentrations both at clinical normal levels and also at hyperkalemic levels. Building on these results, we will construct our own potassium specific ISEs, as previously described in Fig. 2.
1.5. Testing of Lancet Parameters
As mentioned previously, our potassium test runs the risk of false positives due to hemolysis. In order to assess the validity of our sample acquisition method, we are currently evaluating the degree of hemolysis in samples acquired from finger-pricking using lancets of varying depths and gauge sizes. It is crucial to the accuracy of our device that we ensure that the blood analyzed is within clinically acceptable degrees of hemolysis. To do so, we are currently evaluating different combinations of gauge sizes and lancet depths common among commercially available lancets. Five gauge sizes (25, 28, 30, 33, 36 gauge) and five lancet depths (1.3, 1.5, 1.8, 2.0, 2.2 mm) are being investigated, yielding a total of 25 unique gauge and lancet combinations. For each of these 25 settings, five healthy individuals will prick their fingers to obtain a blood sample. Hemolysis for each sample will be measured using a HemoCue plasma hemoglobin analyser, which provides qualitative hemolysis information, and will be averaged for each combination in order to assess the effect of each variable on observed hemolysis. A two-way ANOVA will be performed in order to evaluate the relationship between lancet parameters and degree of hemolyzed blood, thereby informing our method of accurate sample acquisition. Per clinical standards, we will be defining acceptable hemolysis as less than 100 mg/dL [8, 9].
We have recently achieved IRB approval for our study, and are currently collecting data for this portion of our testing.
1.6. Testing of User Interface and Preferences
We are currently conducting workflow usability tests on patients to ensure that patients can effectively use our device. This will help de-risk patient non-compliance, as patients are more likely to use a device that is portable and easy to use. To assess this, we are providing 30 subjects with example tasks, such as drawing blood and preparing it for analysis, determining risk of hyperkalemia, and navigating to previous data entries. For each task, we will count the number of mis-steps or mistakes a user makes, defining a successful outcome as two or fewer errors. Tasks failing this threshold for a significant fraction of users will be redesigned or clarified in the UI. The prototype given to volunteer testers can be seen in Fig. 7. Results from this test will be used to further inform and optimize our device’s user interface. In addition to user interface testing, we are testing for user preferences as well. In particular we are interested in finding out the optimal font, font size, and data placement on the screen in order to maximize the usability of our device. A sample UI test sheet is shown in Fig. 8 where font, font size, word placement, and color of text are experimented with in order to maximize user viewing of information from the out of clinic portable potassium monitor.
Figure 7.
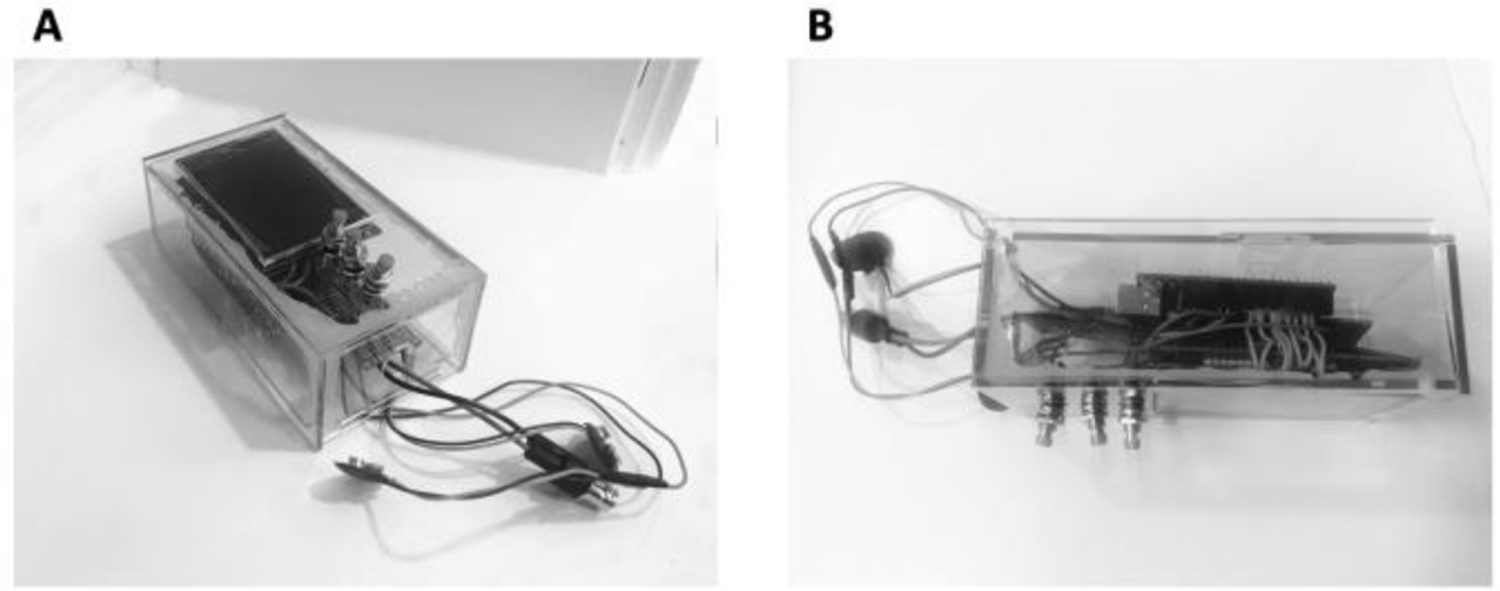
A) Isometric view of the final prototype - acrylic box with Arduino UNO LED screen; up, down, and select buttons; battery' clips; and a BNC connector for an ion selective electrode (ISE). B) Side view of the final prototype in A. This prototype was handed to volunteer testers who were asked to evaluate the weight, ergonomics, and dimensions.
Figure 8.
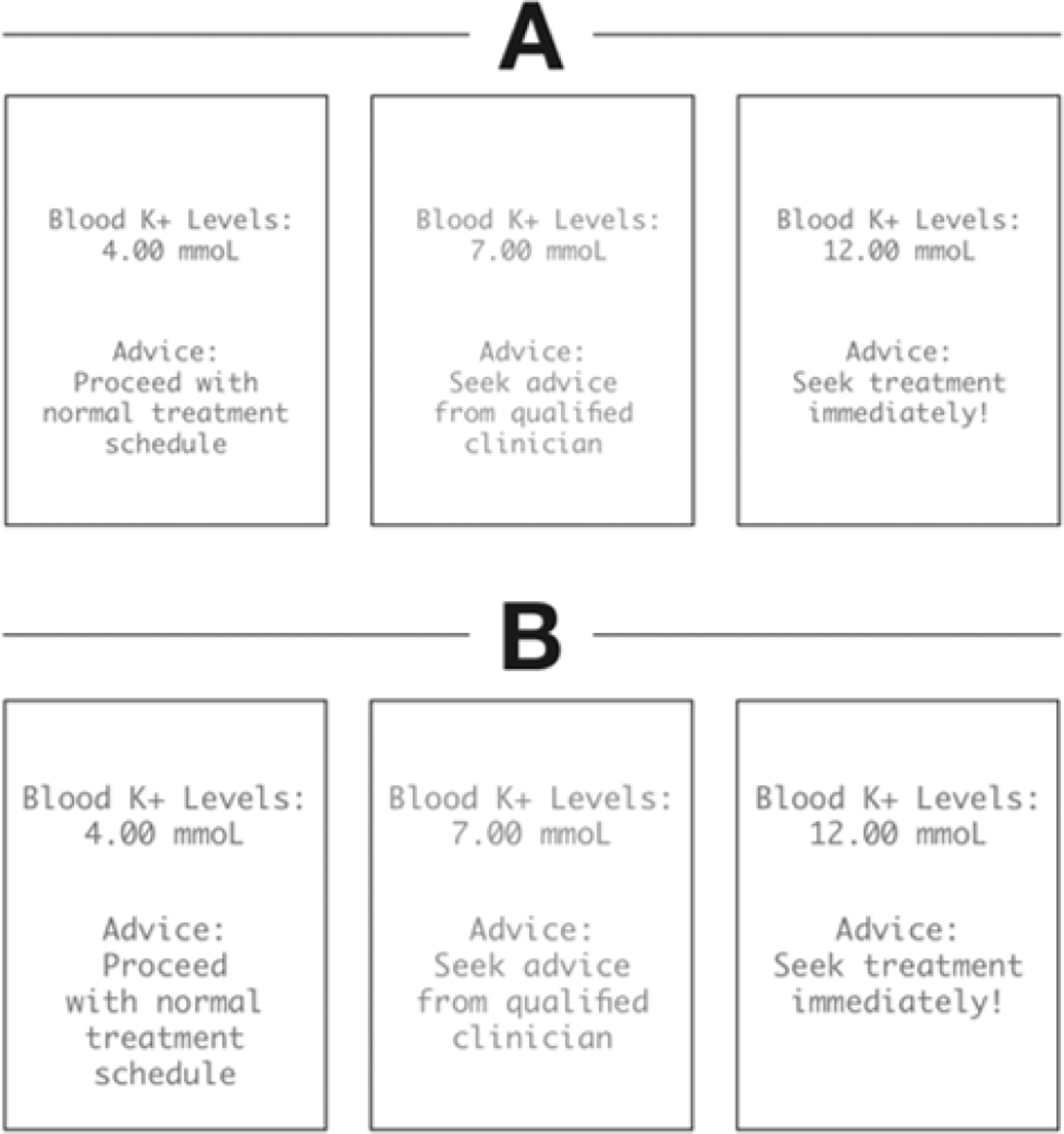
A sample UI preferences sheet that would be presented to a volunteer tester. The left-most panel of each choice would be presented in green to signify normal levels. The middle panel of each choice would be represented in orange to signify that while they do not need immediate treatment, they would be wary. The right most panel of each device would be presented in red to signify that they need immediate treatment. Different UI options varied by font size and text placement.
1.7. Conclusion and Future Considerations
Our results comparing the Horiba potassium soil meter to a laboratory blood test validate the use of portable ISE technology for accurate measurement of potassium in whole blood samples outside a clinical setting. This result indicates the feasibility of successfully developing an at-home blood potassium measurement device, which is one step towards meeting the need for early detection of hyperkalemia. Our initial prototyping also indicates that a simple system of a voltmeter, display, and microcomputer is sufficient for our device. However, subsequent prototypes must be miniaturized to maximize ease of use.
In the future, we aim to manufacture our ISEs in house and miniaturize their design while maintaining accuracy of 90% and precision of 2% as per our design parameters. We will continue to collect data from our lancet study to find the depth and gauge size that minimize hemolysis, and therefore sample error, in blood samples collected by finger prick. Once these goals are met and our device’s design is finalized, we will test our final prototype on dialysis patients to quantify accuracy and ensure our device is user friendly to ensure patient compliance.
Figure 2.
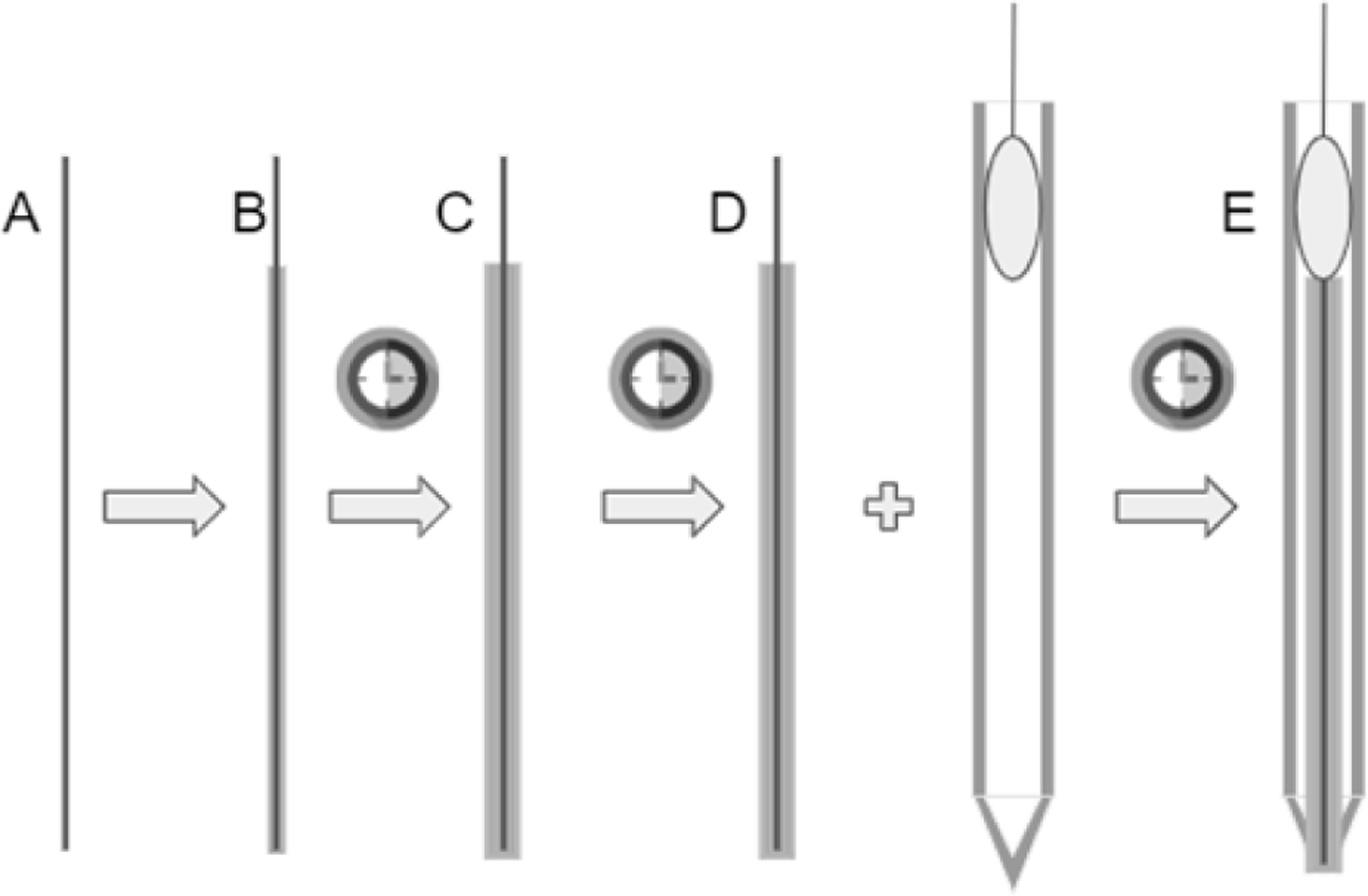
Construction of the ISE using a graphite core and valiuomycin as the ion-selective membrane. 4) Filament of copper/granite wire. B) Electrode is coated in Poly(vinyl-ferrocene) electrolyte, cured. C) Electrode is double dipped in potassium specific membrane (Valinomycin in PVC) and cured again. D) Electrode is inserted into glass micro capillary tube and secured with epoiy. E) Final result: an electrode double dipped in a potassium specific membrane and an electrolyte.
1.8. Acknowledgements
This work was supported by funding from the Johns Hopkins University Department of Biomedical Engineering’s Center for Bioengineering Innovation and Design.
NOMENCLATURE
- CKD
chronic kidney disease
- ISE
ion selective electrode
1.9 References
- [1].Wouters OJ, O'Donoghue DJ, Ritchie J, Kanavos PG, and Narva AS, 2015, “Early chronic kidney disease: diagnosis, management and models of care,” Nature reviews. Nephrology, 11(8), pp. 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, and Vincenti A, 2009, “Sudden death and associated factors in a historical cohort of chronic haemodialysis patients,” Nephrol Dial Transplant 24(8), pp. 2529–2536. [DOI] [PubMed] [Google Scholar]
- [3].Brunelli SM, Du Mond C, Oestreicher N, Rakov V, and Spiegel DM, 2017, “Serum potassium and short-term clinical outcomes among hemodialysis patients: Impact of the long interdialytic interval,” Am J Kidney Dis, 70(1), pp. 21–29. [DOI] [PubMed] [Google Scholar]
- [4].National Institutes of Health, 2017, “USRDS annual data report: Epidemiology of kidney disease in the United States,” from https://www.usrds.org/2016/view/Default.aspx.
- [5].Vespa J, Armstrong DM, and Medina L, 2018, “Demographic Turning Points for the United States: Population Projections for 2020 to 2060 Population Estimates and Projections Current Population Reports,” from www.census.gov/programs-surveys/popproj.
- [6].Salmi IA, Larkina M, Wang M, Subramanian L, Morgenstein H, Jacobsen SH, and Pisoni RL, 2018, “Missed Hemodialysis Treatments: International Variation, Predictors, and Outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS),” Am J Kidney Dis, 72(5), pp. 634–643. [DOI] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention, 2017, “Heart Disease Facts & Statistics,” from www.cdc.gov/heartdisease/facts.htm.
- [8].Heyer NJ, Derzon JH, Winges L, Shaw C, Mass D, Snyder SR, Epner P, Nichols JH, Gayken JA, Ernst D, and Liebow EB, 2012, “Effectiveness of practices to reduce blood sample hemolysis in EDs: a laboratory medicine best practices systematic review and meta-analysis,” Clinical biochemistry, 45(13–14), pp. 1012–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Knezevic C, 2018, Assistant Professor at The Johns Hopkins School of Medicine Department of Pathology, email communication. [Google Scholar]


