Abstract
Professional phagocytes (polymorphonuclear neutrophils and monocytes/macrophages) are a main component of the immune system. These cells are involved in both host defenses and various pathological settings characterized by excessive inflammation. Accordingly, they are key targets for immunomodulatory drugs, among which antibacterial agents are promising candidates. The basic and historical concepts of immunomodulation will first be briefly reviewed. Phagocyte complexity will then be unravelled (at least in terms of what we know about the origin, subsets, ambivalent roles, functional capacities, and transductional pathways of this cell and how to explore them). The core subject of this review will be the many possible interactions between antibacterial agents and phagocytes, classified according to demonstrated or potential clinical relevance (e.g., neutropenia, intracellular accumulation, and modulation of bacterial virulence). A detailed review of direct in vitro effects will be provided for the various antibacterial drug families, followed by a discussion of the clinical relevance of these effects in two particular settings: immune deficiency and inflammatory diseases. The prophylactic and therapeutic use of immunomodulatory antibiotics will be considered before conclusions are drawn about the emerging (optimistic) vision of future therapeutic prospects to deal with largely unknown new diseases and new pathogens by using new agents, new techniques, and a better understanding of the phagocyte in particular and the immune system in general.
When preparing this overview of a field in which I have been working for 15 years, I thought it would be easy to summarize the main data on the immunomodulatory potential of antibacterial agents on phagocytes. Since the understanding of the possible interferences of these bacterium-targeting agents with host cells (and their clinical impact) requires some knowledge of the main actor in the play, namely, the phagocyte, and the way in which the potential therapeutic value of immunomodulation has come to the forefront, I intended to present a brief overlook of a century's research on immunology and infection and then discuss the phagocyte itself. However, when I started to address the question of the complexity of this cell at the functional, transductional, and regulatory levels, I soon realized that, despite a substantial amount of published material in this field, we have so far only seen the tip of an iceberg. Consequently, the following two sections, which address the therapeutic relevance of the observed effects and future research prospects, will certainly raise more questions than answers.
Immunomodulation, a therapeutic need for the third millennium, is still in its infancy, and antibiotic therapy itself is only now approaching maturity. Many current antibacterial agents have not revealed all their facets, and new antimicrobial agents are forthcoming. The microbial world, the phagocyte, and the host still have tricks up their sleeves, holding the promise of a new and exciting research enterprise in years to come. I hope this review will provide a basic framework for those interested in this field.
BRIEF HISTORY OF IMMUNOMODULATION
The main data reported in this section have been taken from a number of excellent books and papers (10, 76, 79, 105, 111, 145, 146, 190, 234, 252, 293, 294, 415).
It is generally agreed that the concept of immunomodulation emerged in 1796 when Jenner undertook the first “vaccination”. Since then, many attempts have been made to help the immune system face external (bacteria, viruses, etc.) or internal (cancer and autoimmunity) attacks. These new therapeutic strategies (“prohost” treatment) have been made possible by about a century of fundamental discoveries and the recognition of immunology and microbiology as distinct scientific disciplines.
Hopes and Enthusiasm in the Preantibiotic Era: Phagocytes and Bacteria
The roots of immunology and microbiology date back to the last decades of the nineteenth century. In 1879, Pasteur discovered, largely by accident, that an attenuated culture of chicken cholera bacteria could immunize against subsequent challenge. The Pasteur Institute was opened in the fall of 1888. After years of patient observation, the first concept of a true host defense mechanism was forwarded by Metchnikoff in December 1882. Space is lacking here to list the names of all these passionate pioneers who, in the short period from the 1850s to the 1880s, amidst great excitement and confusion, established the theoretical and methodological bases for the new science of microbiology. Some notables include Koch, the founder of laboratory bacteriology; Behring, Kitasato, and Ehrlich (1890 and 1891), who developed the theory of humoral immunity; Wright (Sir Almroth “Almost” Wright, 1902), who reconciled the humoral and cellular aspects of immune defense with the concept of opsonins (humoral components aimed at preparing for and activating phagocytosis), and Ivanovski and Beijerinck (1892 and 1899), who found the first filterable agent (virus). These are but some of the many microbe hunters who lent their names to almost all bacterial genera. By the end of the nineteenth century, microbiology was a well-established discipline which had split into several specialized branches. Textbooks, journals, institutes, and courses on microbiology sprang up almost as quickly as newly discovered bacteria. In 1879, Pasteur's associate E. Duclaux established a course in microbiology at the Sorbonne. In 1884, Koch introduced a comprehensive course in medical microbiology at the University of Berlin. Microbiology techniques were sufficiently advanced for scientists to name many diseases caused by a specific bacterium or protozoan. The definition of viruses (as we know them) would take almost 50 years, from 1892 up to the first portrait of a tobacco mosaic virus obtained by an electron microscope in 1939. In the same short period (1884 to 1895), the three great discoveries relating to host defense mechanisms (phagocytes, antibodies, and complement) provided the foundations for host resistance and immunology. Immunomodulation was then thought of as an induction of immunity to pathogens. Several methods were devised to counteract infectious agents: vaccination with laboratory-modified pathogens (to create specific protection, although the immune participants were largely unknown); induction of active and passive immunity by transfer of humoral factors (serum therapy, replacement therapy), which seemed to deal the final blow to infectious diseases; and, by the followers of Metchikoff's cellular theory of immunity, some somewhat adventurous therapies to create beneficial inflammation. “Stimulation of the phagocyte” and the concept of “stimulins” were a great hope in the early nineteenth century (415). However, enthusiasm soon vanished when potentially immune-mediated diseases started to emerge; for example, tuberculin not only failed to cure tuberculosis but even worsened it (although it proved a wonderful diagnosic tool); the experiments by Richet and Portier in the 1900s demonstrated life-threatening hypersensitivity and anaphylactic reactions; Metchnikoff himself, after first refuting the principles of noxious inflammation of Conhein and Helmholz, turned his interest to a possible role of phagocytes in senility and the way in which bacterial toxins could transform the friendly phagocyte into a fearsome foe, and he suggested disinfection of the digestive tract to increase life expectancy; and P. Ehrlich recognized the limitations of serum therapy. Within 10 years, immunologic euphoria was replaced by profound frustration and a period that has been called the Dark Ages of Immunology (234).
Scientists then shifted from immunologic stimulation to the creation of chemotherapeutic “magic bullets,” which culminated with Preparation 606 (Salvarsan) by Ehrlich in Hoechst's laboratory in 1912. After the interest in antibiosis in the late nineteenth century and the discovery by Twort (1915) of bacteriophages (the possible “microbes of immunity”), the scientific community was ready to acknowledge the birth of chemotherapy.
Midcentury: “Miracle Drugs” and the End of Infectious Diseases?
In the latter decades of the nineteenth century, many observations on microbial antagonism and attempts to apply this phenomenon to treating diseases created a favorable climate for the advent of antibiotics. The birth of chemotherapy is officially assigned to 1928, with the discovery by Fleming of the potent lytic effect of a mold contaminant, Penicillium notatum, on a staphylococcal culture. It took almost 10 years before the therapeutic activity of penicillin G was demonstrated, thanks to Florey and Chain, among others (294), and a few more years to elucidate its chemical structure and produce it industrially by fermentation. The isolation of tyrothricin in 1939 and the demonstration of its powerful therapeutic effect greatly stimulated the development of antibiotic research. The golden age of antibiotics was starting, stimulated by the needs of World War II. Antibiotic screening and the search for “miracle molds” all around the world resulted in the discovery of almost all the main classes of these therapeutic agents within about 10 years. The term “antibiotics” was coined by Waksman and defined as “compounds produced by microorganisms that can inhibit the growth of other microorganisms or even destroy them.” Streptomycin was found in 1944, and gramicidin S was found in 1942. Chloramphenicol, erythromycin, neomycin, cephalosporins, and many others were found in the 1940s and 1950s. The natural backbones were chemically modified to improve stability, efficacy, pharmacokinetics, or toxicity from the 1960s up to the present, and chemical research gave birth to the modern fluoroquinolones. However, the use of these miracle drugs failed to take account of the fact that microorganisms have an extreme capacity to evolve resistance strategies and that creating new antibacterial weapons is an endless effort. Could the sentence of Sir Almroth Wright, “The physician of the future will be the immunisator,” (60) be a premonition?
The “Immunologic Burst”: Expanding Complexity of the Immune System
By the 1920s, the only thing which was perfectly clear in immunology was that “immunity, whether innate or acquired, is extremely complex in character” (363). Later, as more scientists have become involved, the evolution of immunology has been so dynamic that it has become a fundamental discipline of medicine and biology. Parallel technical advances have made it possible to identify and explore the various interconnected cellular and humoral components of the immune system. The first breakthrough came in the 1960s with the clonal selection theory (44) and the elucidation of the primary structure of the antigen receptor (82). The 1970s and 1980s saw remarkable theoretical and practical contributions to our understanding of the immune network, its cellular subsets and mediators (cytokines), and its involvement in cancer, autoimmunity, and organ transplantation. The development of new technologies (hybridomas and monoclonal antibodies [186] and PCR [293]), the birth of molecular biology, and the identification of intracellular messengers (cyclic AMP to heterotrimeric G proteins, the low-molecular-weight G proteins, and other intracellular biochemical cascades) illustrate the burst of immunology that has now “phagocytized” almost all fundamental disciplines from histology to chemistry, genetics, and even mathematics. New generations of genetically engineered drugs (“poison arrows”) are being proposed, targeted not only to microbes but also to chronic diseases, and will open a new era centered on recombinant DNA technology to design proteins with specific desirable functions that act on specific receptors or specific enzyme isoforms.
Hopes and Wisdom at the Dawn of the New Millennium
Technicians and theoreticians have given us a tremendous potential armamentarium against pathogenic microorganisms. However, lessons from the past have shown that announcements of medical miracles have been highly exaggerated. In 1976, it was widely believed that infectious diseases had been conquered; diseases like tuberculosis, cholera, and smallpox were of little concern to people in wealthy industrialized nations. Only the threat of AIDS from 1983, the discovery of infectious proteins (prions) in 1982, and the possibility of microbial terrorism, not to mention the potential nonterrestrial (space-borne) pathogens of tomorrow, have tempered our enthusiasm. The gospel of specific etiology from Koch's postulate, “a bacteria, a disease, a treatment,” has furnished the guidelines for medical research for about a century. The development of immunology has resulted in further complexity by combining external (environment and pathogens) and internal (neuro-endocrine-immune system) factors in the pathophysiological scenario of infectious diseases. With the advent of powerful new techniques and drugs, the challenge is to learn how to modulate the immune response to external conditions. Immunopharmacology is still a young science, and the immune system has not yet unveiled its molecular complexity. Nevertheless, along with Metchnikoff, we can say, “we therefore have the right to hope that in the future, medicine will find more than one way to bring phagocytes into play for the benefit of health.” Let us now approach this multifaceted cell.
PHAGOCYTES: DEFENDERS OR OFFENDERS?
Phagocytes, etymologically “devouring cells,” are characterized by the process of engulfing relatively large particles (phagocytosis) into vacuoles by a clathrin-independent process that generally requires actin polymerization (reviewed in reference 86a). This property is essential for their role in host defenses and is conserved throughout the evolutionary tree.
Phagocyte Lineages: from Amoebae to Diversity
“Phagocytes are merely the remnants of the digestive system of primitive beings” (252). Amoebae can be considered as a model of the primitive phagocyte and indeed possess all the functional characteristics and transductional systems of the “civilized” phagocytes present in metazoans (8, 9, 258, 334). A general overview of the defense system from primitive invertebrates to mammals reveals a constant role of hemocytes/phagocytes, which are derived from cells of the mesoderm when they were freed from nutritional duties in advanced multicellular invertebrates. Production of reactive oxygen intermediates and possibly of cytokine/cytokine-like molecules (maybe the remnants of a pheromone system in single-celled protozoa) is observed in hemocytes of invertebrates (5, 27). However, at the upper end of the evolutionary scale (mammals), phagocytes have evolved to an extreme diversity. Not only can phagocytes from different species possess peculiar antigenic markers, functional molecules, and activities, but also different phagocytic cell lineages and subsets can be identified in a given species. Roughly speaking, two main lineages exist: polymorphonuclear cells (polymorphonuclear neutrophils [PMNs] and polymorphonuclear eosinophils [PMEs]) and mononucleated cells, referred to as professional phagocytes (the subject of this review). Other cells (such as fibroblasts and epithelial cells) can occasionally phagocytose a more limited range of particles, but in general they do not possess bactericidal mechanisms (oxidants and antibiotics) or opsonin-binding receptors. The professional phagocytic lineages also show an extreme diversity. For instance, PMNs from healthy adults have a heterogenous response to the chemotaxin formyl-methionyl-leucyl-phenylalanine (fMLP) that correlates with the oxidative responsiveness of the cells; stable intersubject differences can also be detected (94). Technologic advances in flow cytometry that allowed the rapid evaluation of PMN membrane responses have shown intrinsic antigenic heterogeneity among PMNs (349). Functional heterogeneity has also been demonstrated between the various PMN pools, i.e., the bone marrow reserve (released by corticosteroids), the circulating granulocyte pool (the most commonly studied compartment), the marginated pool (cells adherent to the endothelium, released by epinephrine), and the tissue pool (245, 365). The second phagocytic lineage in mammals, the monocyte/macrophage system, has an even greater functional and morphological heterogeneity (124). The monocyte/macrophage system consists of bone marrow precursor cells, blood monocytes, and both mobile and fixed tissue macrophages (420). In the late 1960s, the term “mononucleated cell system” replaced the earlier term, reticuloendothelial system” (17), which also encompassed vascular endothelial cells, reticular cells, and dentritic cells of lymphoid germinal centers. Tissue macrophages are derived from blood monocytes which differentiate into specialized cell types according to their location (for example, the Kuppfer cells in the liver and synovial macrophages in the joint capsule). Some macrophages may pass through epithelia and become, for instance, alveolar macrophages or milk macrophages. Each subset of specialized macrophages possesses specific functional and morphological characteristics, but monocyte/macrophage heterogeneity is further amplified by the possibility of other subsets arising under specific pathological conditions (infection or inflammation), such as the “elicited” monocyte-derived macrophage or the epithelioid multinucleated giant cell derived from monocytes under the inflammatory conditions present in granulomas. Like PMNs, macrophages have interspecies and interindividual functional heterogeneity (4, 405).
The initial hypothesis that phagocytic activity was the hallmark of all myelomonocytic offspring rapidly turned into a dogma but is now increasingly rejected. An extended definition of the phagocytic system to some nonphagocytosing cells could open new horizons in the future, since recent work has shown that macrophages can be converted into potent dendritic cells devoid of classical phagocytic activities. The concept of developmental plasticity may have large implications for immune defenses (300).
Phagocyte Life and Functions: the Old and the New
Origin and fate of phagocytes.
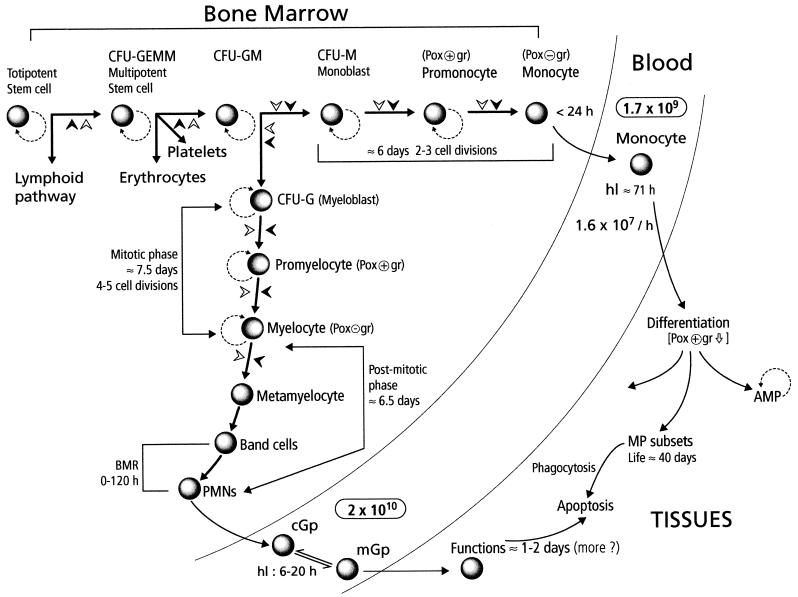
Phylogenetically and ontogenically, hematopoiesis does not occur in the bone marrow; however, at birth, hemopoietic activity is distributed throughout the skeleton in humans, and in adult life it is found almost exclusively in the bone marrow of the sternum and pelvis. The gradual maturation and differentiation of myelomonocytic cells is an incredibly complex process which involves many regulatory factors produced locally or systemically, as well as cell-cell and matrix-cell adhesion mechanisms (Fig. 1). All mature blood cell lineages are derived from a totipotent stem cell which gives rise to the multipotent stem cell and further to the phagocytic cell precursor CFU-GM (colony-forming unit for granulocytes and monocytes). Under specific influences, this cell gives rise to the two specialized phagocyte precursors (CFU-G-myeloblasts and CFU-M-monoblasts). The production of PMNs in bone marrow takes approximately 2 weeks and involves a first compartment of proliferating and differentiating cells (myeloblasts, promyelocytes, and myelocytes) and a second compartment of maturing, nondividing cells (metamyelocytes, band cells, and mature cells [the bone marrow reserve]). PMNs are released into the blood, where their half-life is about 6 to 20 h, and subsequently migrate into tissues, where they live for 1 to 2 days before becoming apoptotic and phagocytosed by resident macrophages. The overall production of PMNs is about 109 cells/kg/day. The number of circulating leukocytes (for example, PMNs) can be markedly increased by administration of foreign protein, in particular bacterial products, a phenomenon already observed by Lowit in 1892 and Metchnikoff and associates from 1905 to 1914. In 1949, Menkin expanded their observations on the striking leukocytosis that occurred during infection and inflammation. The source of these new circulating leukocytes was unknown. In 1955, Menkin proposed endogenous regulation by factors released from sites of inflammation. The bone marrow was recognized as the primary source of PMNs by Perry et al. in 1957 (299). The homeostatic and pathological mechanisms which control leukocytosis have been reviewed recently (162, 287). Details on neutrophil production and differentiation can be found in references 115, 123, and 347.
FIG. 1.
Origin and fate of phagocytes: a brief overview of the differentiation pathways of phagocytes and their fate. Details are given in the text. Abbreviations: CFU-GEMM/GM/G/M: colony-forming unit granulocyte-erythrocyte-monocyte-megakaryocyte/granulocyte-monocyte/granulocyte/monocyte; AMP/MP alveolar macrophage/macrophage; Pox+/− gr, peroxidase-positive/negative granules; hl, half-life; BMR, bone marrow reserve; cGp/mGp, circulating/marginated granulocyte pool; 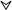 , decrease; solid arrowheads, factors involved in cell maturation (for example, CSF and cytokines); open arrowheads, influence of the microenvironment in cell maturation (cell receptors and matrix proteins); dashed curved lines, self-renewal potential of phagocyte precursors.
, decrease; solid arrowheads, factors involved in cell maturation (for example, CSF and cytokines); open arrowheads, influence of the microenvironment in cell maturation (cell receptors and matrix proteins); dashed curved lines, self-renewal potential of phagocyte precursors.
Monocyte-macrophage development follows a similar circuit in the bone marrow (116, 420). CFU-GM gives rise to the committed monoblast (CFU-M), a cell with high self-renewal capacity. The next transitional maturation stage is the promonocyte, which divides and matures into monocytes. The promonocyte cell cycle time in humans is approximately 2 days, and their is a minimum of three (probably more) generations between the precursor and the mature circulating monocyte. Monocytes leave the bone marrow within 24 h of completing their last division. There is no marrow reserve of monocytes. Circulating monocytes have a half-life of 71 h, and there does not seem to be a substantial marginated pool. When entering the tissues, monocytes undergo morphologic and functional differentiation into macrophages. The life span of macrophages may extend from weeks to months or even years.
In adults, circulating granulocytes represent about 50 to 60% of all blood leukocytes, and similar numbers (not directly available) are represented by the marginated pool. Monocytes represent less than 10% of total leukocytes.
Senescent monocytes, granulocytes, and probably macrophages undergo morphological and biochemical modification referred to as apoptosis. This phenomenon was discovered in 1972 by Kerr et al. (174), who distinguished it from necrosis or accidental cell death. Apoptosis is regulated by a fixed genetic program known as programmed cell death. Constitutive apoptosis observed in vitro can be amplified by various factors (interleukin-4 [IL-4], endotoxin, and tumor necrosis factor [TNF]), whereas some cytokines (granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage CSF [GM-CSF], and M-CSF) and other proinflammatory mediators can abrogate it. In general, macrophages resist apoptosis more than monocytes do. CD14, the receptor for lipopolysaccharide (LPS), plays a role in both the regulation of apoptosis and apoptotic cell recognition (142).
Phagocyte functions.
Since the historical experiment by Metchnikoff, phagocytes (“microphages” [PMNs] and macrophages) have been assigned a central role in immediate, nonspecific defenses against external aggression (mainly pathogens and their products). In 1908, in his talk after receiving the Nobel prize (252), Metchnikoff presented a visionary approach of the extreme complexity of these microscopic organisms as well as of their pleiotropic role. He not only pointed out the direct microbicidal mechanism of these cells but also suggested other possible functions which were recognized later, such as the secretion of substances “the complement in the humour originates in the white corpuscles,” “endolysins of Petterson and leukins of Schneider do exist,” the transfer of immunity by white corpuscles, their destruction of microbial toxins, the existence of “certain elements in the organism that promote phagocytosis, the secretins” (cytokines?), the resistance of microorganisms to phagocytes via “agressins,” the role of amboreceptors in increasing phagocytosis, etc.
A dichotomic presentation of phagocyte functions between PMNs and monocytes/macrophages has prevailed until recently. PMNs, which are short-lived but extremely abundant cells, were recognized as playing the fundamental role of destroying extracellular pathogens and some of their toxins, whereas monocyte-derived macrophages, long-lived cells, were thought to have (in addition to their phagocyte microbicidal potency) other important functions such as limiting the growth of obligate intracellular pathogens, producing many bioactive molecules important in regulating other cellular functions (complement components, prostaglandins, cytokines, etc.), controlling neoplasia, removing damaged and senescent cells, controlling wound repair, and processing antigens and transmitting the information to lymphocytes, thus directing and targeting the humoral and cellular specific immune responses. This simplistic scheme has been substantially modified by a revisited approach to PMN capabilities and function (68, 320).
(i) Classical view: PMNs and macrophages as warriors cooperating in the battle against foreign invaders.
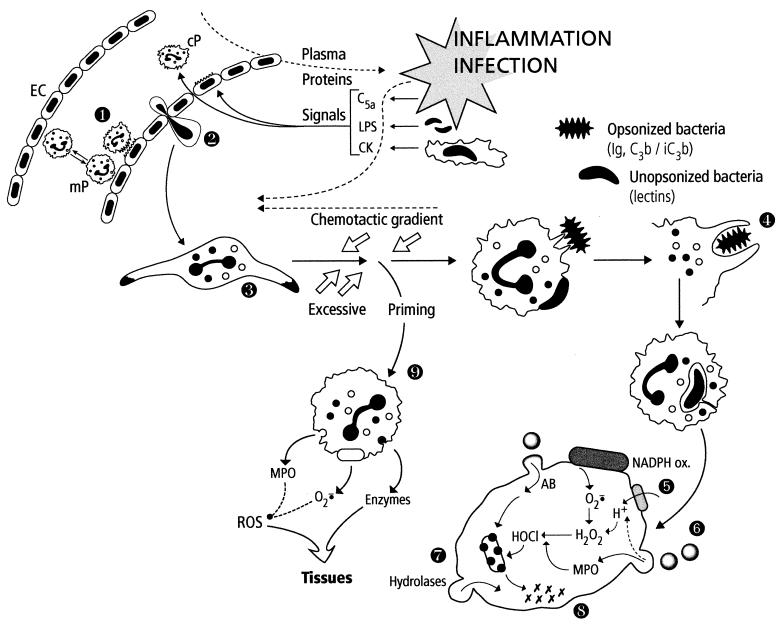
The two phagocytic lineages possess similar means of controlling external aggression, by a sequential multistep process including oriented motility (chemotaxis), recognition of foreign particles by membrane lectins and receptors, engulfment into a vacuole (phagosome), degranulation of intracellular secretory pools (granules) and release of natural antibiotics and enzymes into the phagosome (now a phagolysosome), production of reactive oxygen species by a complex enzymatic system (NADPH oxidase) located on the phagocyte membrane and/or reactive nitrogen species by an inducible nitric oxide synthase, and killing and digestion of engulfed material in the complex phagolysosomal medium. Owing to their abundance, rapidity, and more destructive bactericidal equipment, PMNs are the first line of defense (Fig. 2). As soon as a microbial pathogen enters the host, a localized, beneficial inflammatory response is generated by local resident macrophages, necrotized cells and tissues, plasma factors, and microbial products. The locally produced factors of inflammation (cytokines, activated complement protein, kinins, etc.) and microbial factors generate chemotactic gradients, modify endothelial cell membrane receptors, and promote a slowing of the blood flow. PMNs that are rolling along the endothelial surface (weak adhesion mediated by lectin-like molecules, the selectins) respond to the chemotactic and cell-mediated signals and are first activated to firmly adhere to the endothelium via their membrane integrins; the second step is transendothelial migration, referred to as diapedesis, followed by oriented migration (chemotaxis) toward the inflammatory site, a phenomenon which involves recognition of chemoattractants (complement factor C5a, IL-8, bacterial chemotaxins, platelet activating factor, leukotriene B4, etc.) by specialized receptors (serpentins [seven-transmembrane-domain G-protein-linked receptors]) followed by integrin-mediated attachment to the extracellular matrix and changes in cell shape by rearrangement of the actin cytoskeleton. This step can be observed within minutes after an inflammatory signal is generated. During this migratory phase, PMNs continue to receive information which will further modify their state of responsiveness (a phenomenon known as priming). Once they have arrived at the inflammatory site, PMNs can recognize pathogens via their membrane receptors for opsonins (e.g., complement factors C3b and iC3b, and the Fc component of immunoglobulins) which are present on the microbial surface or via microbial and phagocyte lectins (opsonin-independent phagocytosis). Lectin- or receptor-mediated activation of PMNs triggers phagocytosis, classically by a zipper mechanism of sequential recognition of the pathogen by phagocyte extensions (pseudopods) which finally engulf the microbe in a vacuole. Coiling phagocytosis is the most frequent unusual uptake: unilateral pseudopods wrap around the microorganism in multiple turns, giving rise to largely self-apposed pseudopodial surfaces. This phenomenon has been observed with Legionella pneumophila, Trypanosoma spp., Leishmania promastigotes, and occasionally with Staphylococcus aureus, Haemophilus influenzae, and Escherichia coli (323). In parallel, two phagocyte functions are activated: the release of granule contents into the phagosome and the oxidative burst. The oxidative burst was first described in 1933 by Baldridge and Gerard and consists of explosive oxygen consumption (50- to 100-fold increase) that is unrelated to mitochondrial respiration and reflects the activity of the NADPH oxidase system. This enzymatic complex is made up of cytosolic and membrane constituents, which are separated in resting PMN and are reassembled upon PMN activation. The primary constituents are a membrane flavocytochrome b (cytochrome b 558), which comprises two subunits (gp-91phox [phox for “phagocyte oxidase”], a glycoprotein of 91 kDa, and p22phox), and three cytosolic components, p47phox, p67phox, and p40phox. Other cofactors of this complex are the two 22-kDa low-molecular-mass GTP-binding proteins, Rac-2 (located in the cytosol) and Rap-1A (present in the plasma membrane and specific granule membranes). Recent insights into the various components of this system, their assembly, and molecular interactions are presented in various overviews (20, 67).
FIG. 2.
Summary of PMN functions. The localized inflammation following pathogen invasion generates many activating signals for endothelial cells (EC) and circulating PMNs (cP). Concomittant activation of these cells results in strong binding of PMNs to EC (step 1) and later in their transendothelial migration (diapedesis) (step 2). PMNs are attracted to the infected area (oriented migration, chemotaxis) along the chemotactic gradient (step 3). During their voyage, PMNs are primed by various signals (cytokines [CK], LPS, etc.) and are thus prepared to perform their bactericidal function. PMNs recognize pathogens via their membrane receptors for the Fc of immunoglobulins (Ig) or complement proteins (C3b/iC3b) or via lectins. The adherent pathogen is engulfed (phagocytosis) (step 4) in a vacuole. The contents of specific and azurophilic granules are released into the phagosome, which becomes a phagolysosome (degranulation, exocytosis) (step 6), parallel to the activation of the NADPH oxidase system initiating the oxidative burst (step 5). Oxygen-dependent and -independent (natural protein and peptide antibiotics [AB]) bactericidal systems cooperate to destroy the pathogen (step 7). The last step corresponds to the digestion of the bacterial debris by hydrolases and other lytic enzymes released in the phagolysosome (step 8). In the setting of excessive priming, PMNs stop migrating and the activation (degranulation, production of reactive oxygen species [ROS]) takes place in the tissues, which can be injured (step 9).
Upon activation, this system catalyzes the production of superoxide anion (O2·−) by the one-electron reduction of oxygen using NADPH as the electron donor: 2O2 + NADPH → 2O2·− + NADP+ + H+·. Superoxide anion is further dismutated into hydrogen peroxide (H2O2), which, in the presence of myeloperoxidase (MPO) released from PMN azurophilic (primary) granules and a halide, generates very potent oxidizing agents such as hypochlorous acid (HOCl) and chloramines. Other oxidative species such as singlet oxygen, the hydroxyl radical, and reactive nitrogen species can theoretically be produced by activated PMNs. Their relevance to bacterial killing inside the phagosome has recently been examined (133). The other way in which PMNs kill bacteria, known as the oxygen-independent system, is dependent on protein and peptide antibiotics, which are among the most phylogenetically conserved bactericidal molecules (103, 104, 147, 239). Most of these proteins (bactericidal permeability-increasing protein, cationic antimicrobial protein 37, and defensins) are stored in peroxidase-positive (azurophilic, primary) granules, where they colocalize with active proteases such as elastase, cathepsin G, and proteinase 3 (408). Bactericidal-permeability-increasing protein (BPI) is selectively active against gram-negative bacteria and has LPS-binding properties. Defensins are small peptides (3 to 4 kDa) that are active against gram-positive and gram-negative bacteria, fungi, some viruses, and tumor cells; there are three major human defensins: human neutrophil peptides 1 to 3 (H-NP 1 to H-NP 3) and a minor one (H-NP 4). (Some epithelial cells also contain defensins, but human monocytes do not.) Peroxidase-negative granules (specific granules) are noted for their membrane, which contains cytochrome b 558, and also for a variety of receptors for adhesion and phagocytosis; various metalloproteinases that are stored as zymogens; and a family of endotoxin-binding proteins (the cathelicidins) that was recently identified, one member of which (h-CAP 18) has been demonstrated in humans (360). The synergistic interaction of oxygen-dependent and -independent microbicidal PMN systems generally results in pathogen killing (98). However, pathogens have developed ways of avoiding PMN phagocytosis or even deactivating or destroying these cells. A few pathogens, such as some Ehrlichia spp., can multiply within PMNs. Since the first description in 1994, 400 cases of human ehrlichiosis (a tick-borne zoonosis) have been reported. Some other microorganisms can survive and persist within PMNs. For instance, PMNs have been suggested as a possible reservoir of intracellular S. aureus in recurrent human infections and chronic staphylococcal mastitis in dairy cows (416). By contrast, although macrophages potentially display bactericidal mechanisms, they represent safe harbors for many intracellular pathogens (129, 170, 263). Differences in the bactericidal systems which may account for this decreased potency of macrophages compared to PMNs are a less potent oxidative burst, the absence of MPO in differentiated macrophages, which prevents the terminal phases of oxidant-generating systems, and the absence of numerous antibacterial peptides and proteins. It has also been suggested that macrophages are unable to produce oxidants inside the phagosome because of the lack of the granule pool of NADPH oxidase (164). This defective bactericidal function can be boosted by cytokine stimulation. In particular, proinflammatory cytokines, interferon (IFN), bacteria, and their products synergistically induce NO synthase, which could be the major pathway of macrophage bactericidal activity. Among the major functions attributed to macrophages in host defenses is the triggering of a specific, antigen-driven immune response, both through the synthesis and release of various cytokines regulating T-lymphocyte functions and through the antigen-processing mechanisms which take place in late endosomal or phagosomal structures (370). Also, macrophages orchestrate the complex processes of cell proliferation and functional tissue regeneration within wounds through the generation of bioactive substances (72). Among the macrophage factors involved in this function are chemoattractants, which recruit and activate additional phagocytes; growth factors, which promote angiogenesis, cell proliferation, and protein synthesis; proteases and extracellular matrix protein; and factors that restrain tissue growth once repair is completed.
Complementary information on the classical role of PMNs and monocytes/macrophages in host defense can be obtained in references 98, 102, 143, and 314.
Eosinophils and neutrophils have similar life cycles, morphology, many lysosomal enzymes, and most chemotactic, phagocytic, and oxidative responses to membrane stimuli. Their role, however, is directed mainly at controlling metazoan parasite infections. Like neutrophils, eosinophils may be both beneficial and detrimental for the host. Their transduction pathways involve various phospholipases, kinases, and second messengers. Eosinophils will not be reviewed here, but details concerning their functions can be found in reference 102.
(ii) New aspects of phagocyte functions.
Whereas the perception of macrophages as primitive cells involved in host defense has shifted rapidly to the recognition of their role in regulating homeostasis and participating in multiple stages of the complex immune response, PMNs have long been considered important (see the consequences of neutropenia) but simple first-line defenders against infection. New insights were obtained in the late 1980s (320), including a complex metabolism, perhaps a longer half-life (particularly in inflammation and infection), interaction with other cells, a key role in many pathological processes, and the presence of receptors able to respond to immunomodulation. In particular, PMNs produce and synthesize a variety of proteins involved in self-regulation and regulation of other cells, such as cytokines (TNF, IL-1, IL-3, IL-6, IL-8, G-CSF, and GM-CSF), complement protein and receptors, major histocompatibility complex (MHC) class I, heat shock protein, and antiproteases, despite minimal protein synthesis equipment. An expression profile of active genes in granulocytes has recently been published (158). PMNs can regulate gene expression constitutively and inducibly by transcriptional and posttransductional events. Their role in limiting the infectious process of various intracellular pathogens (Listeria, Legionella pneumophila, Shigella, Chlamydia, and even mycobacteria), viruses, and some parasites (Entamoeba histolytica and Plasmodium falciparum) and tumor cells has been demonstrated or is strongly suspected. Novel ways in which PMNs phagocytose peculiar pathogens such as Borrelia burgdorferi have been observed (366). By contrast, a negative role of phagocytes in the dissemination of intracellular Listeria monocytogenes and phagocytosis-facilitated invasion has been suggested in central nervous system infection in vivo (74).
Other possible new functions of PMNs are related to their role in the specific immune response. In particular, PMNs can cooperate with professional antigen-presenting cells, enhancing the uptake and proteolysis of antigens; they can be induced to express MHC class II molecules and can present antigens to virus-specific cytolytic memory T lymphocytes. These properties seem to rely on the potent MPO-H2O2-Cl− pathway and chlorination activity of PMNs, whose products, acting as immunomodulators, provide a further link between innate and adaptive immunity (238). An immunoregulatory function has also been assigned to lactoferrin, an iron-binding protein present in specific granules which possesses antimicrobial properties (41). Other regulatory aspects of PMNs include the production (release) of various factors which modulate lymphocyte, monocyte, and eosinophil functions, thereby giving this cell a central role in host homeostasis. The presence of the proenkephalin system in PMNs also indicates a possible role in local analgesia.
Other advances in our understanding of phagocyte functions concern the recognition of their active role in hemostasis and thrombosis (reviewed in reference 84), their detrimental activities in many pathological settings (see below), and new insights into the complexity of cell-cell interactions and intracellular messages (see below). This knowledge is linked to the newer approaches and characterization of various cell constituents such as the many lectin-like macrophage receptors (362) which target highly specific interactions with the environment, other membrane receptors which mediate adhesion (361), intricate and redundant intracellular kinases, phosphatase and phospholipase activities, and the way in which extracellular signals (cytokines, microbial and inflammatory products, neuro and endocrine mediators) may regulate the functional properties of macrophages.
Phagocyte-Speak: Cell-Cell Communication and Intracellular Messages
Phagocytes respond to the variable conditions in the environment through selective recognition of other cell surface antigens or humoral mediators via a myriad of membrane receptors. The nomenclature of these receptors is often confusing to nonimmunologists, since various names are used interchangeably. An international committee meets periodically to reach a consensus on official names and CD (cluster differentiation) numbers. Here, a more practical designation is used, linking receptors to a specialized function or ligand and/or to a specific signaling mechanism, although there is some crossover between these categories. Roughly speaking, phagocyte membrane receptors include adhesion molecules (three families, i.e., integrins, selectins, and molecules belonging to the immunoglobulin superfamily), chemoattractant receptors (the serpentins), the opsonin protein (for the Fc of immunoglobulins and C3b/inactivated C3b complement protein), multiple receptors for other humoral mediators (including cytokine receptors, separated into five families, i.e., hematopoietic receptors, IFN receptors, TNF receptor, G-protein-coupled receptors, and those belonging to the immunoglobulin superfamily), LPS-binding receptors, adenosine receptors, neuromediator receptors, and lectin-like receptors (mannose-R, mannose-6-P-R, advanced glycosylation end-product R, etc.). None of these receptors is engaged in only one strictly defined function, and cross talk with synergistic or antagonistic effects occurs between the different molecules. For instance, adhesion receptors will not only trigger rolling, adhesion, and diapedesis of phagocytes but will also participate in chemotaxis, phagocytosis, and activation of the oxidative burst; similarly, chemoattractants can generally trigger degranulation and the oxidative burst, while opsonin-engaged receptors also activate these functions. An interesting phenomenon concerns ligands which do not directly stimulate a functional response but modulate phagocyte behaviour after a second stimulus. This is referred to as priming and is observed with some cytokines, endotoxin, and suboptimal concentrations of directly activating stimuli (292). Engagement of its ligand by a receptor molecule triggers a sequence of events known as a biochemical signaling pathway. The first, proximal event, related to the structure of the receptor, directs the main signaling pathways. Various receptor subgroups are defined according to the primary signal, including heterotrimeric G-protein-coupled receptors (serpentins and some cytokine receptors), glycosylphosphatidylinositol-anchored proteins (CD14, Fcγ-RIII, and urokinase-type plasminogen activator receptor), and tyrosine kinase receptors.
The emerging view of signal transduction is that cell pathways are regulated by the organization in macromolecular assemblies (55) involving not only a cascade of enzymatic activities and their corresponding products but also adapter proteins, which permit close association of effector enzymes and products or their translocation, and molecular switches such as the low-molecular-weight G proteins (Ras, Rho, Rab, Arf, and Ran), whose activity is regulated by their association with guanine nucleotides and by exogenous proteins involved in the GDP/GTP-bound form cycle (35).
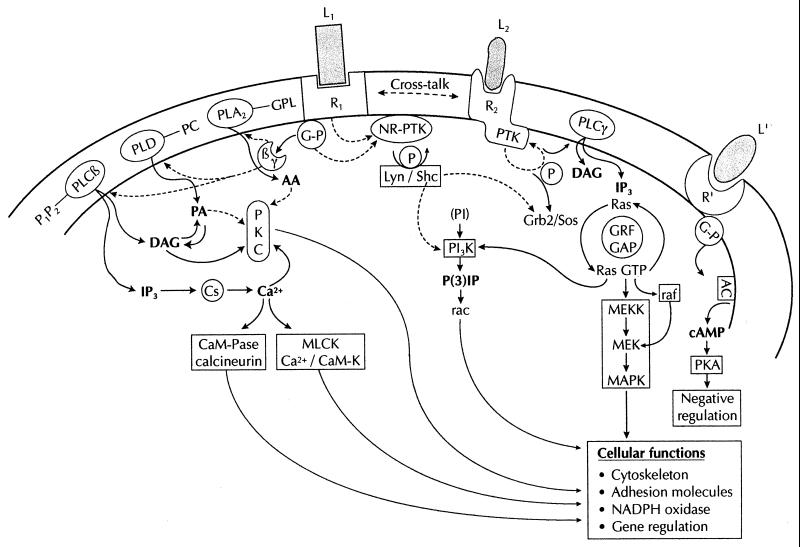
Under most normal and pathophysiological conditions, information received by phagocytes is mediated by one or several receptors. In vitro (and possibly occasionally in vivo), some phagocyte stimuli may bypass this step and directly activate an intracellular effector. For instance, extracellular Ca2+ and the most widely used phagocyte activator, phorbol myristate acetate (PMA), directly stimulate some intracellular protein kinase C isoforms (PKCs). It is beyond the scope of this review to deal in depth with the many cellular participants in signal transduction, but a schematic approach to the main groups of enzymes and mediators involved in signaling may help the reader understand the possible sites of interference of antibacterial agents with phagocyte function. I will attempt to roughly summarize the most important pathways. An extremely simplified diagram of neutrophil activation is given in Fig. 3. Chemoattractant binding to its receptor activates one (or three?) associated pertussis toxin-sensitive G-protein which further dissociates into subunits that interact with three phospholipases, PLC, PLD, and PLA2. The source of lipids used by these lipases is the phagocyte membrane. The first wave of lipid messengers includes inositol 3-phosphate (IP3) and diacylglycerol (DAG) derived from phosphatidylinositol-4,5-diphosphate by activation of membrane-bound PLC-β, phosphatidic acid (PA) and choline derived from phosphatidylcholine by PLD activity, and arachidonic acid and lysophospholipids derived from glycurophospholipids by PLA2 activity. These second messengers act on various cellular targets to deliver second-wave signals: IP3 releases Ca2+ from intracellular pools (IP3-sensitive calciosomes), and DAG with Ca2+ activates various PKC isoforms to phosphorylate important targets such as p47phox; Ca2+ may also activate Ca2+-dependent PKs to regulate the actin cytoskeleton. PA can either directly activate various kinases and phosphatases or be hydrolyzed by PA phosphohydrolase to give rise to DAG. Arachidonic acid serves mainly as a substrate for the synthesis of eicosanoids via the cyclooxygenase or lipoxygenase pathway, but it can also act as a second messenger in activating several kinases. Chemoattractant signaling also involves G-protein-coupled activation of various tyrosine kinases of the Src family, including the tyrosine kinase Lyn, which phosphorylates various adapter proteins such as Shc; the Shc-P-Lyn complex may serve to activate phosphatidylinositol 3-kinase (PI3-K), an enzyme which catalyzes the addition of a phosphate group to the D3 position of phosphatidylinositol lipids. Its importance in the regulation of various phagocyte functions is well documented. Another adapter protein linked to ShC is Grb2, which is generally associated with the Ras guanine nucleotide exchange regulator Sos, thus mediating the activation of the monomeric G protein Ras (a possible mechanism activating the mitogen-activated protein [MAP] kinase cascade). Ras GTP may also favor the membrane translocation and further activation of Raf serine/threonine kinase, another possible way of activating the dual Threo/Tyr-dual function kinase MEK (MAP kinase kinase). Cross-regulatory pathways involving receptor tyrosine kinases, receptors linked to soluble tyrosine kinase, and the low-molecular-weight G proteins (Ras, Rho, Rab, Arf, and Ran) to amplify or downregulate phospholipase and kinase action are also involved in phagocyte stimulation, not to mention the negative feedback involved in turning off the system. Not all the pathways are yet correctly placed hierarchically. In particular, whether PLC is upstream of PLD activation or whether both lipases are activated in divergent pathways is still unresolved. Other classes of receptors may involve similar pathways. In particular, receptor tyrosine kinases may undergo autophosphorylation followed by binding to adapter protein and recruitment of Sos with prolin-rich SH3 domains to the membrane, close to the small G protein Ras, which binds and activates the protein kinase Raf-1 and the MAP kinase cascade. Phagocytosis (classical or coiling phagocytosis) also involves tyrosine phosphorylation and PKC activation (59). Integrin-mediated signaling through similar pathways involves many other adapter proteins such as actin-binding proteins (vinculin, talin, and paxillin) between the cytoskeleton and signaling effectors. Focal adhesion kinase is central to this pathway: tyrosine autophosphorylation provides docking sites by SH2 domains for other kinases such as PI3-K and Src kinase, which amplify the activating signals (phosphorylation of paxillin, p130Cas, and focal adhesion kinase initiating the canonical Grb2-Sos-Ras-Raf pathway). The redundancy of signals may explain why Mac-1 (an integrin) serves as a signaling partner for several other receptors. Lastly, it is also important to note that these pathways are under the influence of posttranscriptional events such as prenylation, farnesylation, and carboxymethylation, which may promote or facilitate the interaction of Ras-related proteins with specific membrane targets (301). The multiple isoforms of phospholipases and protein kinases, and the recognized MAP kinase pathways (at least three) are not presented here, although they are certainly important for refining the phagocyte response to external stimuli. Distorsion of intracellular signaling pathways by various microorganisms has also been recognized recently (157, 272).
FIG. 3.
Schematic presentation of the main transductional pathways in phagocytes. Due to space limitation, not all routes and regulatory signals are shown. The main abbreviations are given in the text. Additional abbreviations: R1, R2, R′, receptors; L1, L2, L′, ligands; Ac, adenyl cyclase; cAMP, cyclic AMP; Cs, calciosome; CaM-K/CaM-Pase, calmodulin-dependent kinase/phosphatase; GAP, GTPase-activating protein; GRF, guanine regulatory exchange factor; GP, heterotrimeric G protein; GPL, glycurophospholipids; MLCK, myosin light-chain kinase; NR-PTK/PTK, nonreceptor/receptor protein tyrosine kinase; P, phosphorylation; PC, phosphatidylcholine.
Detailed insights into the transduction pathways of phagocytes are given in references 143 (a complete approach to the PMN), 348 (G-protein-coupled receptors), 61, 230, and 412 (selectins, integrins, and signal transduction), 35 (chemoattractant signaling), and many other reviews dealing with specific participants: Ca2+ (237, 356), PKC (226, 227), PI3-K (220), PLD (121), and MAP kinases (54, 106).
Phagocytes and the Host: “Trick or Treat”
The beneficial role of phagocytes in host defense is widely acknowledged (see above). Deficiencies in neutrophil numbers or function are substantial risk factors for developing potentially fatal bacterial and fungal infections (350). Gram-negative bacilli and S. aureus are the most common pathogens in patients with neutrophil defects; chronic granulomatous disease and leukocyte adhesion deficiency are the most frequent (although rare) congenital forms (235). There are detailed reviews that deal with the main inherited and acquired defects in neutrophil numbers and function (37, 91, 223, 235, 246, 350, 375, 409). Deficiencies in neutrophil function can be accompanied by defects in monocyte/macrophage function, but no defect strictly targeting the mononucleated phagocyte system has been identified. However, new immune deficiencies continue to be described (parallel to the development of techniques and better-coordinated analysis of rare inherited defects), such as the recently published defects in the IL-12–IFN-γ–TNF-α “circuit,” which is accompanied by severe atypical mycobacteriosis (350). Other potential beneficial effects of phagocytes include tissue repair and healing, and it is obvious that the secretion of many regulatory factors, including cytokines, is part of their role in the maintenance of host homeostasis.
In contrast to this beneficial role, phagocytes appear to be very fine-tuned cells which, by uncontrolled use of the same mechanisms as those used to destroy pathogens (i.e., oxidative species, enzymes, and mediators), can have detrimental effects on the host. These cells, which are considered a double-edged sword, play a fundamental role in the pathogenesis of exaggerated inflammatory responses (19, 359, 413). Neutrophils can defend themselves against the oxidant they produce through a potent antioxidant system (for example superoxide dismutase, catalase, glutathione-dependent H2O2-detoxifying system, α-tocopherol, and ascorbic acid). However, when produced in excess (particularly after priming by cytokines or endotoxin) in the extracellular medium, oxidative species can damage host tissue (139). The imbalance between proteinases and antiproteinases (which may be inactivated by oxidants), interaction with platelets, phagocyte-induced thrombosis (by plugging microvessels) and expression of procoagulant activity by monocytes also contribute to vascular injury (84). Release of chemotactic mediators (leukotrienes, platelet-activating factor, and IL-8) recruits new, elicited phagocytes that maintain the detrimental inflammatory response (221). Recently, it was shown that mammalian mitochondria produce N-formylpeptides (bacterial chemotaxins), raising the possibility that tissue injury or anoxia leads to the release of such mitochondrial contents, providing another mechanism for recruiting PMNs. In addition to causing vascular injury, PMNs can transmigrate and attack parenchymal cells (161). Disease conditions in which phagocyte-inflicted tissue damage plays an important role include acute events such as ischemia-reperfusion injury, shock, acute respiratory distress syndrome, acute allograft rejection, inflammatory bowel diseases and the Arthus reaction, and chronic diseases such as bronchiolitis, bronchiectasis, cystic fibrosis, diffuse panbronchiolitis, gastric ulceration, rheumatoid arthritis, and asthma. Dermatopathic and autoimmune diseases are often associated with neutrophil infiltration (246, 402). Vasculitis (63) and almost all diseases for which no etiology has been identified may potentially be related to abnormal phagocyte functions. Other possible deleterious consequences of phagocyte activities include a potential effect of MPO-mediated oxidation of anticancer drugs such as vincristine (343). Material, such as defensin, released by biomaterial-activated PMNs may contribute to creating an environment hostile to host defenses at the biomaterial surface by cell deactivation. Indeed, in most cases, the detrimental effect of phagocytes seems to originate in the uncontrolled development of pathogens (either demonstrated or suspected) which subvert phagocytic functions to their own needs. Extreme activation of inflammatory responses or deactivation of defence mechanisms will result in acute or chronic disease. For instance, the role of Mycoplasma or Chlamydia persistence in triggering chronic diseases such as asthma and unstable angor is under the spotlight. Intraphagocytic pathogens protected from the milieu (and possibly from therapeutics) are better able to survive. Altered cell signaling and phagocyte deactivation are frequently observed during intracellular infection (318). Some pathogens tip the cytokine balance in their favor to induce phagocytes to produce anti-inflammatory cytokines that render cells refractory to other activating signals (262).
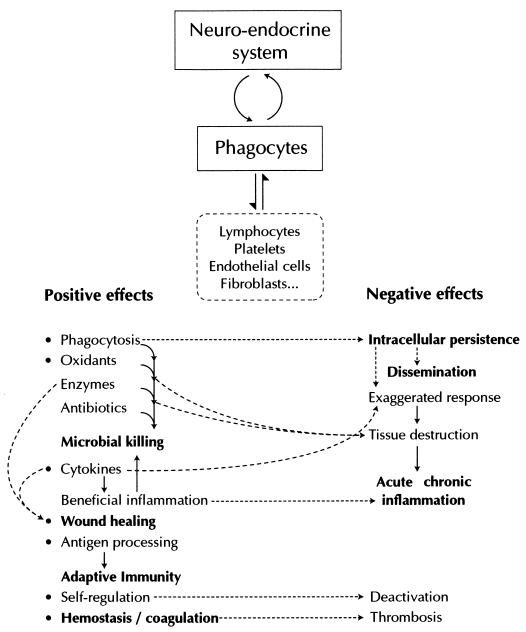
The concept of phagocytes as crucial for both host defense and pathogenicity (Fig. 4) supports the new approach to virulence and pathogenicity, depending on the initial status of the host (51). This concept forms the fundamental basis for future therapeutic guidelines in immunomodulation as proposed by Repine and Beehler (319), i.e., that “every effort be made to develop highly reversible and targeted drugs that decrease the harmful while retaining (or even enhancing) the beneficial effects of neutrophils.”
FIG. 4.
The phagocyte: a key effector and regulator in host homeostasis, defense, and disease.
Adventureland: How To Explore Phagocyte Functions
To analyze drug-mediated immunomodulating properties of phagocytes, one must be familiar with the main techniques used to analyze their functions. It is outside the scope of this review to describe all available techniques, but the main steps in the analysis of drug-phagocyte interactions, with emphasis on the problems encountered in such studies and the overall evaluation of the results, will be presented in this section. A practical and critical description of techniques routinely employed to study neutrophils (and sometimes monocytes and macrophages) is presented in references 153, 165, 251, 346, and 364.
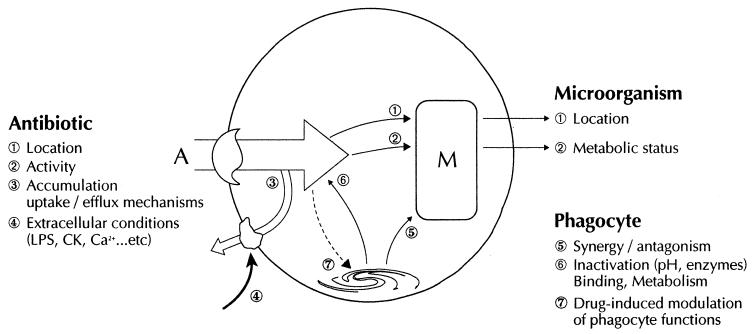
A summary of the advantages and disadvantages of techniques used to study drug-induced modulation of phagocyte functions is given in Table 1. One of the most widely employed approaches used to explore drug-induced modulation of phagocyte functions is the use of animal models of infection or inflammation. In some cases, the immunomodulatory activity of a compound can also be assessed in humans. These in vivo studies provide a view of the global efficacy of a drug in a specific clinical setting, and modifications of immune parameters such as blood leukocyte counts, the number of infiltrating neutrophils and monocytes/macrophages in infected or inflammatory sites, phagocyte morphology, and levels of immune mediators present in serum and other extracellular fluids. These studies are also the first step toward analyzing the functional properties of phagocytes ex vivo. The problems of extrapolating the data to the immunomodulatory potential of a drug are related first to the chosen model: animals differ from humans in many ways, such as susceptibility to different pathogens, drug metabolism, phagocyte receptors and functions, and chronobiology. Interspecies differences exist, and interindividual variability or chronobiological variations are also well documented in humans and animals (4, 94, 249, 302, 383). All these observations highlight the need for caution when extrapolating data across species barriers. Evaluation in humans is also restricted by ethical considerations and by the need for sufficient healthy individuals and patients (357). Monitoring of immune therapies is neither simple nor straightforward. It requires “familiarity with principles of immunologic assays, a great deal of judgment and considerable understanding of biologic, immunologic and therapeutic effects induced by biological response modifiers” (411). Defining the administration schedule (i.e., dosage, time, and duration of the protocol) and the survey protocol (e.g., sampling times and parameters assessed) is the most difficult but also the most important aspect of in vivo and ex vivo studies. Mention must be made of a rare in vivo method for evaluating neutrophil function (chemotaxis) by the Rebuck window assay (neutrophils migrate into a dermal abrasion and adhere to a glass slide), but this semiquantitative approach (dependent on blood counts) is poorly standardized and poorly reproducible. Ex vivo analyses can provide information on how phagocyte functions are modified by therapeutic concentrations under host conditions (mediators, cytokines, cell contacts, proteins, enzymes, etc.). In addition to the above-mentioned problems, problems inherent to these studies concern the isolation procedures (which, by separating the phagocyte from its context, may also suppress a drug-induced factor necessary for phagocyte modulation) and the pools that will be analyzed (405). The different functional capabilities of the various granulocyte pools and monocyte/macrophage subsets have been mentioned above. Easily available neutrophils are circulating cells (about 50% of blood PMNs), whereas monocytes are the most readily available mononucleated phagocytes. Monocyte-derived macrophages obtained by in vitro culture can also be assessed. Depending on the culture conditions, these cells can exhibit different morphologic, phenotypic, or functional characteristics. Alveolar and peritoneal macrophages and those present in other extracellular fluids are less easily studied (at least in humans). Ex vivo studies also have many of the problems inherent to in vitro techniques. In vitro studies analyze a theoretical question, outside the host context. Various phagocyte functions can be routinely assessed, such as adhesion, chemotaxis (under agarose or in Boyden chambers), phagocytosis (by techniques using adherent or nonadherent cells, radiolabeled bacteria, or staining), bactericidal activity (CFU counts or bacterial staining), degranulation (release of various enzymes present in different granule subsets, spontaneously or following stimulation), and oxidative burst (either in global assays such as oxygen consumption and luminol-amplified chemiluminescence or by measuring specific oxygen species, mainly superoxide anion-superoxide dismutase-inhibitable cytochrome c reduction and lucigenin-amplified chemiluminescence). Stimulation of phagocyte functions is generally studied with agents that mimic bacterial chemotaxins (formylated peptides such as fMLP) or that directly activate intracellular enzymes (phorbol esters such as PMA) or increase Ca2+ flux (calcium ionophores such as ionomycin and A23187). Phagocyte activity can be boosted by priming agents such as cytokines, before stimulation. Fluorescence-activated cell sorter analysis is a recent technique which provides information on many phagocyte functions and membrane antigens and permits rapid evaluation of individual phagocyte responses (33). Measurement of cytokine production by various specific immunoassays has also become routine (69). Lastly, although not directly an immunomodulatory effect, phagocytic uptake of drugs is currently measured by using either fluorescence-labeled or radiolabeled drugs to determine the amount of cell-associated drug or by directly assessing their cellular bioactivity (in the case of antibiotics, for instance). At the frontier between routine clinical studies and research is the study of bone marrow progenitors and in vitro differentiation by culture in semisolid agar medium. The main problems encountered in vitro are due to nonstandardization of techniques in different laboratories and sometimes artifacts introduced by the technique itself (407), separation of phagocytes from their context (a phenomenon already stressed by Metchnikoff: “this method [glass test tubes] cannot account satisfactorily for events that take place in living organisms”), and, as mentioned above, intra- and interspecies differences and chronobiology that often generate an unsubstantiated extrapolation of results. Various tools, mostly used in the research setting, are available for in-depth analysis of possible immunomodulatory effects and are becoming more refined as our knowledge of phagocyte functions progresses. Almost all transductional pathways can be measured in terms of production of specific messengers, enzyme activities, or cellular target modifications. Unfortunately, not all the pathways and their hierarchical organization have been fully elucidated, clearly hindering analytical studies. Novel pathways cannot be ruled out, and there are no strictly specific activators or inhibitors of a given pathway or enzyme. Research techniques include the detection of transcriptional activity, mRNA isolation, etc. Technological advances in molecular biology have truly revolutionized our approach to phagocyte behavior under the influence of various drugs. It should be noted that while in vivo and ex vivo studies deal with true phagocyte populations, phagocytic cell lines (HL-60, PLB-985, J774, and U937) are commonly used in vitro. These standardized cell lines theoretically avoid the problem of intra- and interspecies variability and heterogeneity. They are derived from human or animal cells, and some can be induced to differentiate into more mature forms. However, although they are convenient, the functional and secretory properties of these cells differ from those of true phagocytes (34).
TABLE 1.
Summary of the analytical approach to drug-phagocyte interactions
| Type of analysis | Parameters of interest | Problems |
|---|---|---|
| In vivo | ||
| Animal models (infection and inflammation) Humans (health, infection, and inflammation) | Global therapeutic efficacy in one specific system, cell morphology and number in blood and tissues, levels of mediators (in serum and other fluids), samples for ex vivo studies | Ethics, administration schedule, statistical analysis, interspecies differences, interindividual variability |
| Rebuk skin window | PMN chemotaxis | Inconsistent reproducibility |
| Ex vivo | ||
| Animal and human, PMN (blood and tissues), monocytes (blood), macrophages (fluids, monocyte derived) | Cell functions after complex in vivo interactions (drugs, mediators, cell-cell contacts) | Isolation procedures, phagocyte pools and subsets, phagocyte status |
| In vitro | ||
| Animal and human phagocytes, cell lines | Cell functions in precisely defined conditions, antigens, phagocyte progenitor maturation | Various techniques and stimuli (various activation pathways) standardization, artifactual milieu and conditions, extrapolation of results |
| Specific research on cells and cell lines | Transduction pathways, cellular targets, gene transcription | Insufficient knowledge, no highly specific activators and inhibitors |
ANTIBACTERIAL AGENTS AND PHAGOCYTES
On the basis of Metchnikoff's concept of stimulins, the possibility of strengthening phagocyte-mediated antibacterial defenses by antibiotic administration was explored very early after their discovery (26, 144, 267). Unfortunately, inconsistent data meant that these analyses become merely laboratory curiosities due to an insufficient knowledge of immune effectors, inadequate techniques, and blind faith in the potency of antibacterial drugs. Rapidly, however, the recognized side effects of some antibacterial agents on the immune response (particularly neutropenia, anaphylaxis, and allergy) reactivated the search for immune consequences of antibiotic use. The explosive interest in the knowledge of the interactions between these drugs and immunity, which began in the late 1970s, has come in successive waves, following three unrelated pathophysiological events: (i) the acknowledged increasing importance of intracellular pathogens resistant to classical β-lactams and aminoglycosides; (ii) the growing numbers of new categories of so-called immunocompromised patients, owing to medical and surgical progress, for whom even the most effective antibacterial combinations prove ineffective (exemplified by the AIDS pandemic); and (iii) the emergence of antibiotic-resistant bacteria, stimulating the search for new anti-infective approaches.
In addition, observations that various noninfectious diseases were improved by antibiotic therapy administered for concomitant infections and observations of the benefit of various antibiotics on certain inflammatory diseases reinforced interest in the immunomodulatory activity of antimicrobial drugs. A parallel change in our understanding of the functioning of the immune system with improved technology made it easier to conduct such investigations. Although the immunomodulatory profile of any drug encompasses its effect on specific and nonspecific immune mediators, owing to the key role of the phagocyte in innate and adaptive defenses and homeostasis, this cell is a major target for immunomodulation. The relevant literature has been periodically reviewed (16, 25, 113, 140, 197, 199, 202, 203, 205, 208, 236, 330, 386, 417), reflecting a gradual change from basic, fundamental, and sometimes controversial observations (considered epiphenomena of antibacterial activity) to serious, well-founded tests of the effects of some antibacterial agents in noninfectious diseases.
Complex Game for Two or More Players with High Stakes
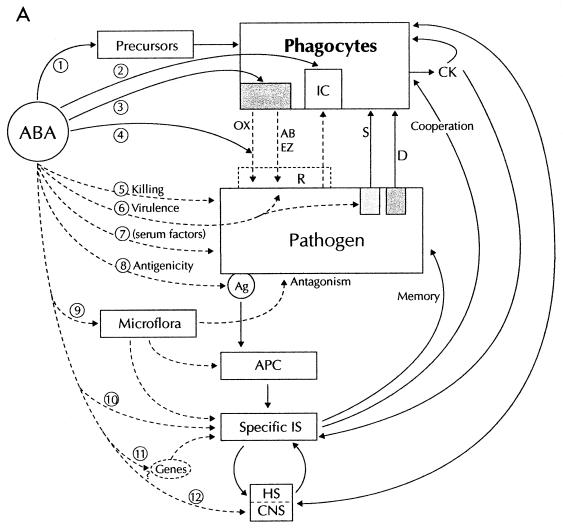
The steps which may theoretically be modified in the host-(microbe)-antibiotic interplay are summarized in Fig. 5. There are two main possibilities: antibacterial agents may directly or indirectly modulate the natural phagocyte-bacterium interaction (Fig. 5A), or phagocytes may alter the activity or structure of antibacterial agents with consequences for drug activity. Direct alteration of phagocyte functions may be a consequence of interference with myelopoiesis leading to detrimental effects such as neutropenia (step 1 in Fig. 5A); intracellular uptake and bioactivity (step 2); modification of a receptor or cellular effector, leading to altered functional activities (step 3); scavenging or inhibition of phagocyte products (step 4); indirect alterations of phagocyte activities due to direct antibacterial activity, with decreased bacterial load (step 5); alteration of virulence (pathogen structure and/or metabolism) (step 6) or antigenic structure (step 8); alteration of serum factors (for example opsonic or chemoattractant activity (step 7); modification of phagocyte regulatory factors such as the endogenous microflora (step 9), or effector functions of the specific immune system (step 10), or postulated regulatory genes (step 11) and the neuro-endocrino-immune axis (step 12).
FIG. 5.
Interactions between phagocytes and antibacterial agents. (A) Effects of antibacterial agents (ABA) on phagocyte activities. Detailed explanations are given in the text. Solid lines indicate the direct effects of ABA on phagocytes, and broken lines indicate indirect effects. Abbreviations: IC, intracellular; CK, cytokines; OX, oxidants; AB, natural antibiotics; EZ, enzymes; S, stimulation; D, deactivation; R, resistance; Ag, antigenic structure; APC, antigen-presenting cells; IS, immune system; HS, hormonal system; CNS, central nervous system. (B) Effects of phagocytes on ABA. Full explanations are given in the text. ABA∗, ABA modified by phagocyte products.
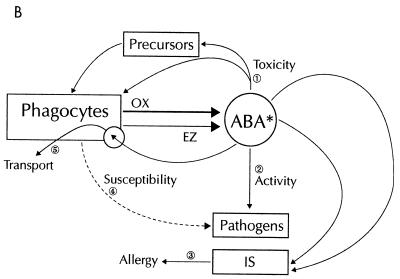
In the second scenario (Fig. 5B), phagocytes may directly or indirectly (through their products such as oxidants and enzymes) alter the structure of antibacterial agents, which may then become either more toxic for phagocytes or their bone marrow precursors (step 1) or trigger the immune system to initiate allergic phenomena (step 3) or have enhanced or decreased antibacterial activity (step 2). Phagocyte-mediated alterations of pathogen metabolism or structure may result in increased or decreased susceptibility of the pathogen to the antibacterial effect of the drug (step 4). Lastly, intracellular antibiotics may use phagocytes as taxis to get to the infected or inflammatory site (the “Battle of the Marne” scenario) (step 5). The problem with this simplistic categorization (often based on in vitro observations) is that it overlooks the fact that the phagocyte-drug interplay is a dynamic process in vivo. Both direct and indirect effects may operate sequentially or simultaneously, and the final outcome is often difficult to link to one or other phenomenon.
Two clinically relevant categories of antibiotic-induced effects are acknowledged: antibacterial drug-induced toxic and immunotoxic effects and intracellular bioactivity. Other effects with a potential clinical impact are phenomena usually observed in vitro, such as modulation of bacterial virulence, leading to antibacterial synergy or a proinflammatory effect; antibiotic activation or inactivation by phagocyte functions; and modulation of phagocyte functions or phagocyte products by antibiotics resulting either in immunodepression or anti-inflammatory activity. Lastly, miscellaneous effects such as modulation of the specific immune response and the impact on the microflora have also been described.
These general aspects will be discussed schematically, leading to a rough classification of antibacterial agents according to their interference with phagocyte functions. Peculiar aspects related to a given antibiotic or class of antibiotics will be dealt with in the following section.
Clinically relevant effects.
(i) Antibiotic-induced toxic and immunotoxic effects.
The most prominent toxic and immunotoxic reactions secondary to antibiotic administration (11, 70, 112, 125, 202, 275, 367) are listed in Table 2 (only adverse effects related to the immune system are envisaged here). Antibacterial agents are leading causes of neutropenia and agranulocytosis and, to a lesser extent, other immunotoxic effects. Neutropenia may be secondary to direct toxicity or immunologic mechanisms. Toxic reactions affect committed stem cells and/or proliferating precursor cells. Marrow damage is usually dose dependent and is more likely to occur in patients receiving high doses for long periods. The effect of drugs on granulopoiesis can be studied by in vitro marrow culture techniques. Chloramphenicol and β-lactams are examples of neutropenia-inducing drugs. Immunologic mechanisms of neutropenia usually take 1 to 2 weeks to be expressed and are not dose dependent. In susceptible patients, onset may occur within 24 to 48 h of starting the therapy. Immunologic toxicity is diagnosed by adding the patient's serum and the drug to bone marrow cultures. Penicillins, cephalosporins, and sulfonamides are frequently involved in such reactions.
TABLE 2.
Toxica and immunotoxic adverse events mediated by antibacterial agents
| Side effect (frequency) | Mechanism |
|---|---|
| Neutropenia and agranulocytosis | |
| Chloramphenicol (1/20,000; 50% mortality) | Toxicity (nitrosoderivative, dehydrochloramphenicol) |
| β-Lactams (5–15%) | Toxicity |
| Semisynthetic penicillins, high dose, long term Methicillin (8%) Cephalosporins (0.1%) | (in vitro: cephalosporins, imipenem > penicillins > monobactams; in vivo: penicillins > cephalosporins) Antibodies Individual susceptibility |
| Sulfonamides (0.1%) | Toxicity plus antibodies (phagocyte-mediated drug alteration) plus genetic defect (detoxifying enzymes) |
| Co-trimoxazole (10%) | |
| Dapsone (0.01%) | |
| Maloprim (pyrimethamine + dapsone) (0.5%) | |
| Isoniazid, clindamycin, PAS, rifampin, ethambutol, aminoglycosides, ciprofloxacin (in vitro + TNF) (0.1 to 0.5%) | Toxicity for progenitor cells |
| Autoimmune diseases | |
| Systemic lupus erythematosus antinuclear antibodies: isoniazid (20%) | Immune complexes (PMN-mediated oxidation of isoniazid) |
| Lupus syndromes: sulfonamides, nitrofurantoin | |
| Autoimmune anemia, thrombocytopenia; penicillins, cyclines, cephalosporins, streptomycin, sulfonamides, nitrofurantoin, etc. | Antibodies |
| Hypersensitivity | |
| Anaphylaxis: penicillin G (2%) | Allergy, pseudoallergy |
| Immediate hypersensitivity: penicillin G (0.7–10%), clindamycin (10%), co-trimoxazole (6%), gentamicin, streptomycin, isoniazid (2%), amikacin, chloramphenicol, p-aminosalicylic acid, rifampin, trimethoprim (0.5–2%), erythromycin, tetracycline, vancomycin (0.1–0.5%) (streptomycin: peripheral PMN) | Drug metabolism by phagocytes |
Note that only adverse effects related to the immune system are presented here.
It must be noted that there are no reports of monocyte/macrophage-depletion during antibacterial chemotherapy, suggesting either that no clinical syndrome is induced or that mononucleated cells are more resistant to toxic damage. Other allergic reactions to antibacterial agents are caused by specific interactions between drugs or their metabolites and components of the immune system. The sensitizing capacity of a drug (low-molecular-weight molecules) depends on its ability to combine or react irreversibly with a tissue protein. After initial exposure to the drug, there is a latency period of 10 to 20 days during which the drug- or metabolite-protein complex stimulates production of activated immune effector cells. On reexposure to the drug, the latency period may be short (anaphylaxis may occur immediately after initiation of treatment). Immunologic adverse reactions may involve IgE-mediated hypersensitivity (e.g., anaphylaxis with penicillin), cytotoxic antibodies with participation of complement (neutropenia, hemolysis, or thrombocytopenia with penicillin), immune complexes (serum sickness and drug-induced systemic lupus erythematosus with isoniazid, penicillins, sulfonamides, and streptomycins), or cell-mediated hypersensitivity (contact dermatitis with neomycin, penicillin, and nitrofurantoin) (11). As a rule, β-lactams, particularly penicillin G, are the antibiotics most frequently involved in these deleterious events. A substantial proportion (3 to 10%) of the population is at risk of anaphylactic reactions to penicillin. Up to 10% of these allergic reactions are life-threatening, and 2% are fatal. Cross-reactions may occur with other semisynthetic penicillins and, to a lesser extent, cephalosporins. Other possible drug-mediated allergies include skin eruptions, febrile mucocutaneous syndrome, fever, and pulmonary, renal, or hepatic hypersensitivity. Penicillin, sulfonamides, nitrofurantoin, isoniazid, and erythromycin have all been implicated (11).
Particular stress must be placed on the capacity of phagocytes to metabolize xenobiotics, including antibacterial agents (125, 202). Myeloperoxidase, prostaglandin synthase, and various cytochrome P450 isoenzymes, along with reactive oxidative species, can all be involved in the generation of haptens (125). Macrophages and PMNs appear to serve as a relay between the preimmunological phase (regional antibiotic bioactivation—sometimes to form directly toxic compounds—and neoantigen formation) and the specific immune response (sensitization) to these neoantigens. Genetic factors determine individual sensitization to a given drug, in terms of both antibody production and synthesis of enzymes which participate in drug metabolism and the formation of reactive metabolites (70, 125).
(ii) Intracellular bioactivity.
Following the initial observation by Rous and Jones in 1911 that the intraphagocytic environment afforded protection from extracellular factors such as serum, it was rapidly demonstrated that various intracellular pathogens were protected from the activity of penicillin or streptomycin (21, 26, 152, 232, 351). It was unclear whether this protection was derived from a failure of the antibiotic to enter the cell or from a particular metabolic state of the pathogen. Repeated experiments using various phagocytized organisms confirmed the inability of various β-lactams and other non-cell-penetrating drugs to destroy intracellular pathogens. The identification of Legionella pneumophila in the 1970s contributed to renewing interest in the macrolide family and other cell-penetrating agents. Extensive studies on the cellular penetration, location, and bioactivity of antibacterial agents now provide a simple classification for these drugs (reviewed in reference 45, 136, 185, 201, 202, 203, 247, 296, 355, 390, and 417). The cells most widely studied are blood phagocytes (particularly PMNs) alveolar or peritoneal macrophages, and some phagocytic cell lines (HL-60 and J774). Two types of methods are used which measure the overall cell-associated drug amount (radiolabeled or fluorescent drugs or high-performance liquid chromatography) or global antibacterial activity by using various models of infected cells or which directly measure the activity of a drug-treated phagocyte extract on susceptible pathogens. Each technique has specific advantages and pitfalls (201, 296); these must be taken into account before any conclusions are reached on the possible clinical relevance of the results. An important question raised by these experiments is the impact of cellular accumulation on intracellular bioactivity. Although a necessary condition for bioactivity, it is now widely acknowledged that intracellular uptake is not the sole factor involved (Fig. 6). The respective cellular locations of the drug and microorganism, the susceptibility of the pathogen (and its metabolic state), the overall accumulation of the drug (which depends on both uptake and efflux mechanisms and their inhibition or boosting by extracellular factors present at the sites of infection), the effects of phagocyte-derived products or constituents on the antibiotic or the microorganism, and the functional modulation of phagocytes by the drug are all important when considering discrepancies between cellular/extracellular concentration ratios and intracellular activity (135). Penicillin G is ineffective on S. aureus ingested by PMNs (150) but has significant activity against this bacterium when phagocytosed by monocytes. This is because these cells produce a factor which synergizes with penicillin G (387). Likewise, gentamicin kills intracellular Listeria monocytogenes within bactericidal and nonbactericidal peritoneal mouse macrophages, probably owing to its internalization by cells through pinocytosis (75). A similar mechanism has been proposed to explain the activity of ampicillin and ceftriaxone against Salmonella enterica serovar Typhi ingested by human monocyte-derived macrophages (52). The recognized importance of cellular accumulation for treating infections caused by intracellular pathogens has triggered novel strategies such as associating antibiotics with colloidal particulate carriers (liposomes or nanoparticles) to promote endocytosis (22, 58). Recently, an antimicrobial drug (primaquine) in a liposomal formulation was found to directly activate macrophages and also proved effective in a Leishmania infection model (23). Antibiotic accumulation is also the basis of tissue-directed pharmacokinetics (342) and the direct modulation of phagocyte functions or metabolism by high drug concentrations (see below). Tissue-directed pharmacokinetics has been studied mainly with macrolides (particularly azithromycin) and may explain the low level of these agents in serum relative to their high concentrations in tissue. This concept is based on rapid and massive accumulation by blood PMNs, which are rapidly attracted to the infected site, and supposes that the intracellular drug is slowly released in bioactive form along the migration route up to its final destination. Elegant in vitro experiments have lent weight to this concept (95, 114), but no consensus has yet been reached. Another point of importance relative to the intracellular accumulation of antibiotics, although it appears to belong primarily to a more fundamental approach, involves understanding the mechanisms which underlie the entry and efflux of drugs. Passive diffusion seems to be the predominant mechanism for most penicillin derivatives, at least in their ionized forms (weak organic acids). However, a probenecid/gemfibrozil-inhibitable organic anion transporter may be involved in the efflux of penicillin G and some quinolones (48, 332). Most quinolones seem to enter and exit loaded phagocytes by passive diffusion (78, 396). The extracellular Ca2+-induced decrease in levofloxacin uptake could be related to an alteration in the structural conformation of this drug (396). However, one active transport system (or possibly two) has been recognized recently for pefloxacin, ciprofloxacin, norfloxacin, and lomefloxacin (228, 250, 406). In particular, it was proposed that PMNs continuously transport ciprofloxacin via a transport pathway shared by adenine (406). Activation by PMA induces a higher-affinity transport pathway shared by a broad range of amino acids. Structure-activity relationships indicate the importance of the substituent at position 7 for the velocity of fluoroquinolone transport by quiescent cells, while fluorine or cyclic structures linked to position 8 appear to impair fluoroquinolone transport by PMA-activated cells (406).
FIG. 6.
Factors influencing the intracellular bioactivity of antibacterial agents. Full explanations are given in the text. Abbreviations: M, microorganism; A, antibacterial agent.
Lipid-soluble antibiotics (rifampin, chloramphenicol, trimethroprim, etc.) readily cross phagocyte membranes (296, 355). Aminoglycosides accumulate slowly (over days in macrophages) by fluid-phase pinocytosis (296, 355). Several specific membrane carriers for amino acids, nucleosides, and dipeptides have been suggested to play a role in the transport of ofloxacin (297) (although other authors did not confirm these results [291]), clindamycin (134), and ceftibuten in a nonphagocytic cell line (268). Similarly, glycoprotein 330 (gp330, megalin) mediates the uptake of various polybasic drugs including polymyxin B and aminoglycosides in epithelial cells (257). Megalin belongs to the low-density-lipoprotein receptor family, which includes the α2-macroglobulin receptor, a Ca2+-binding protein expressed in several cell types such as monocytes. Ca2+-dependent uptake of polymyxin B in macrophages (47) and of various macrolides in neutrophils (264) has been reported. Whether megalin mediates the uptake of these weak-base antibiotics in phagocytes has not been investigated. Indeed, macrolides have been extensively studied in this context owing to their extreme ability to concentrate within phagocytes (and other cells) (201). Lipophilicity or intragranular trapping by protonation has often been suggested as the essential passive mechanism responsible for drug uptake. Binding to intracellular targets in PMNs has also been proposed for erythromycin A (311). Some authors have proposed the nucleoside transport system for josamycin uptake (216) and another (unidentified) carrier for roxithromycin (138). Results from our group have confirmed the existence on the PMN membrane of an active saturable transport system common to all macrolides (and their recently synthesized derivatives, ketolides) which displays different affinities depending on the macrolide structure (398, 399). Complete identification of this carrier has not yet been achieved, but there are data suggesting a link with a putative receptor belonging to the P-glycoprotein (P-gP) family (148, 277, 278). This P-gP-like receptor could operate in different ways by controlling entry and/or efflux depending on the cell type. This hypothesis is further reinforced by the fact that FK-506, which belongs to the extended family of macrolides (206), is transported via P-gP in some cellular models (333). Some quinolones such as difloxacin, ciprofloxacin, and ofloxacin could also use this efflux carrier (118). In conclusion, even if class-specific characteristics of antibiotic accumulation exist, considerable differences sometimes occur within a given class because of chemical peculiarities; also, a phenomenon or mechanism described in one cell type (phagocyte, phagocyte subset, cell line, etc.) may not extend to another type; lastly, external factors (bacterial products, cytokines, etc.) which are present locally at inflammatory or infected sites, phagocytosis itself, and even smoking can modify the phagocytic activation state and thus the drug accumulation process. In addition, the pharmacokinetic properties of the drug (free or protein bound, ionization, local concentration, duration of exposure, etc.) influence its cellular uptake. Despite these difficulties, a simplified but arbitrary presentation of the accumulation of most antibacterial agents within human phagocytes can be derived (Table 3).
TABLE 3.
Schematic classification of the phagocytic uptake of antibacterial agents
| Drug | Cellular/extracellular concn ratio
|
Characteristics, location | Intracellular activitya | |
|---|---|---|---|---|
| PMN | Monocyte/macrophage | |||
| Poor accumulation | ||||
| β-Lactams | <1 | <1 | Passive diffusion | +/− |
| Meropenem | 2–10 | 3–12 | ||
| Imipenem | 3–0.3 | 3.7–0.8 | Transient accumulation (hydrolysis?) | |
| Aminoglycosides | <1 | 2–4 | Slow accumulation, pinocytosis (macrophage) (megalin?) | +/− |
| Daptomycin | 0.6 | |||
| Moderate accumulation | Passive diffusion, lipophilicity | |||
| Cyclines | 1–3 | 2–4 | Cytoplasm, nucleus | + |
| Fosfomycin | 1.8–2.2 | + | ||
| Isoniazid | 1–1.5 | 0.6–0.9 | Cytosol | + |
| Ethambutol | 1.4–4.8 | 0.1–9.5 | Cytosol | + |
| Chloramphenicol | 2.6–9.6 | 2–4 | Cytosol | + |
| Sulfamethoxazole | 1.7 | Cytosol | + | |
| Metronidazole | 1 | 0.5 | Cytosol | + |
| Lincomycin | 1.6–3 | 0.6–2 | Cytosol | + |
| Trimethoprim | 6–21 | 3 | Cytosol | + |
| Quinolones (most) | 3–10 | 4–10 | Cytosol, rapid efflux | + |
| Grepafloxacin | 66 | ? | ||
| Pefloxacin | 4–10 | Active transport system? | + | |
| Ciprofloxacin | 4–10 | Two active transport systems | ||
| Lomefloxacin ofloxacin, norfloxacin (others?) | Two active transport systems | |||
| Fusidic acid | 2–4 | |||
| Rifampin, rifabutin | 2.4–9.2 | + | ||
| Strong accumulation | Active mechanisms | |||
| Macrolides | >10–100 | Active transport (uptake and efflux) | +/− | |
| Erythromycin A derivatives | ||||
| Dibasic molecules (azithromycin, dirithromycin) | 80–>300 (at 180 min) | 100–<600 | Not saturable over 3 h, granules ++, slow efflux | |
| Monobasic molecules (erythromycin, roxithromycin, clarithromycin) | 10–34 (91)c | 38–61 (46–190)c | Plateau 30–60 min Fast efflux, granules + cytosol | |
| Ketolides | ||||
| HMR 3004 | >250 | Plateau, slow efflux | ||
| HMR 3647 | 250–300 | Progressive uptake and efflux | ||
| Clindamycin | 11–15 | 8–24 | Nucleoside transport system? | |
| Coumermycin | 11–17b | |||
| Teicoplanin | 52 (13)b | 41 | Membrane associated | +/− |
| Brodimoprim | 74 | |||
| Rifapentin | 88 | 62 | ||
| Streptogramins | ||||
| RP 54476 | 34 (120 min)d | |||
| RP 57669 | 50 (120 min) | |||
Intracellular bioactivity may differ according to bacterial species.
Measured by bioactivity.
Measured in cells from smokers.
Measured in J774 cells.
Effects with a potential clinical impact.
(i) Modulation of bacterial virulence.
A historical approach to the concept of antibiotic-induced modulation of virulence factors has been given elsewhere (306). It stresses the early observation by Parker and Marsh (295) of the “post-penicillin stationary phase” and that by Rammelkamp and Keefer (313) in an ex vivo experiment demonstrating enhanced killing of beta-hemolytic streptococci by subinhibitory (undetectable) concentrations of penicillin in whole human blood. Very soon thereafter, the deleterious consequences of the rapid bactericidal activity of some antibiotics (e.g., penicillin G and chloramphenicol) were acknowledged (317). Since then, observations of antibiotic effects in vitro and in vivo (animal models) and many clinical observations have unambiguously demonstrated the necessary cooperation between host defenses and antibiotics for successful therapy (400, 418). Indirect alterations of phagocyte activity by modulation of bacterial pathogenicity can be obtained in five ways. (i) A direct antibiotic (bacteriostatic or bactericidal) effect can be sufficient in a host with normal immune status by decreasing, even temporarily, the bacterial load and thereby permitting the host to build up the host defenses and eradicate the pathogen without excessive inflammation. However, too rapid a destruction of pathogens can have deleterious consequences by triggering excessive inflammation (46, 317). (ii) More interesting are the effects obtained with subinhibitory concentrations of antibiotics, which can alter the morphology, metabolism, and/or various constituents in such a way that the altered pathogen is rendered more susceptible to leukocyte action, a phenomenon globally referred to as post-antibiotic leukocyte enhancement (248). (iii) Sub- or suprainhibitory concentrations of antibiotics may also alter the production of various virulence factors released by bacteria (endotoxin, lipoteichoic acid, DNA, or enzymes), which either deactivate the phagocyte or exaggerate its response. (iv) In rare cases, antibiotics also combine with or directly inhibit some of these bacterial products. (v) Lastly, antibiotic-mediated destruction of the pathogen or modification of its antigenicity may further impair the development of specific protective immunity (a potential cause of relapse and carriage) or may lead to abnormal immunity (neoantigens cross-reacting with self-antigens), a possible source of autoimmune or chronic inflammatory disease.
Almost all classes of antibiotics are able to promote the first three responses (to various extents) to susceptible pathogens and, surprisingly, to some resistant pathogens (354, 371). Since most experiments are performed in vitro with selected, broth-cultured pathogens differing largely from those present at infected sites in terms of chemical, physical, and biological properties and with defined antibiotic conditions (concentration, exposure time, etc.), it is difficult to extend these results beyond the bench, despite having permitted dosage regimens to be established. Many reviews attempt to classify antibacterial agents according to their modulation of virulence factors (6, 108, 109, 137, 229, 386, 417, 418), depending on their mechanism of action (protein synthesis or cell wall inhibition), or bacterial species. Among the potential beneficial effects are those which favor phagocyte recruitment (generation of chemoattractants) and phagocytic killing (decreased production of antiphagocytic structures such as capsule, protein A, protein M; increased susceptibility to oxidants, enzymes, or natural antibiotics; increased opsonization by complement or antibody deposition for better phagocyte stimulation and engulfment). In contrast, deleterious potential has been attributed to agents which promote the release of proinflammatory bacterial mediators. The agents mainly involved in such effects are the β-lactams (with carbapenems being less effective due to their binding to penicillin-binding protein 2) (352, 379, 391). The clinical relevance of this effect has been reviewed (304). A mechanism for aminoglycoside-mediated toxicity has been suggested by the potential of aminoglycosides to increase the release of membrane vesicles containing various virulence factors (167). By contrast, polymyxin B displays an antiendotoxin effect by binding to the lipid A moiety of bacterial LPS and neutralizing its activity. The nephrotoxic activity of polymyxin B precludes its therapeutic use as an antiendotoxin, but a covalent conjugate of this drug with immunoglobulin G was recently found to be beneficial in the prevention of septic shock (73). Another interesting proposal concerns the use of clindamycin to suppress endotoxin release by subsequent administration of cephalosporins (180).
Another impact of antibiotics on the immune response is the possible shortening of infection, resulting in a reduction in a protective specific response and immunologic memory (283), which enables the host to resist subsequent challenge. This possibility has rarely been considered in general reviews dealing with the immunomodulatory properties of antibacterial agents (16). Some clinical data suggest a better immunoreactivity of patients after erythromycin (bacteriostatic) than penicillin (bactericidal) therapy (16). This has not generally been taken into consideration when the causes of early reinfection or relapses after apparently successful antimicrobial chemotherapy have been examined.
(ii) Modulation of antibiotic activity by phagocytes.
The possibility that antibiotics are inactivated by phagocytes, their products, or the intracellular medium has rarely been investigated (417). Part of this question has been dealt with in the section on intracellular bioactivity. There are no data clearly demonstrating a loss of activity due to intraphagosomal pH, enzymatic destruction, or binding to cellular constituents. For instance, hydrolysis of dirithromycin into erythromycylamine by PMNs has been suggested in various publications, but no assay of the respective compounds was presented (107). In particular, Geerdes-Fenge et al. (107) have assessed the in vivo uptake of dirithromycin by PMNs from volunteers by measuring erythromycylamine production. These authors used a bioassay, and it is recognized that the antibacterial activity of dirithromycin is less than 10% that of its hydrolysis product, erythromycylamine (179). However, when the uptake of these two drugs was assessed in vitro by a radiolabeling technique, the uptake of dirithromycin was significantly higher than that of its metabolite (265). Cephalosporins and β-lactams are inactivated by human pus, but this resulted from the accumulation of bacterial β-lactamases. Also, gentamicin can be inactivated by reversibly binding to DNA released from lysed neutrophils, and the bioinactivation of netilmicin and amikacin by disrupted (not intact) leukocytes (377) has been reported. No clear correlation of these data with therapeutic efficacy has been demonstrated. Also, the opposite scenario, i.e., modification of antibacterial agents leading to increased activity inside the phagosome or in the vicinity of phagocytes, has not been investigated. There are reports that optimal intracellular efficacy of various quinolones is obtained when PMNs have an intact oxidant-generating system (392), but this does not exclude the possibility that oxidants act first on bacteria to increase their susceptibility to the bactericidal action of the drugs. An interesting report concerned the inactivation of Escherichia coli penicillin-binding protein by human neutrophils (312). Whether this effect can optimize the bactericidal action of β-lactams has not been studied.
(iii) Modulation of phagocyte antibacterial activity by antibiotics.
The direct effect of antibiotics on phagocyte antibacterial activity can be obtained in two ways: direct interference with phagocyte functions and modulation of phagocyte weapons. With regard to the possible modulation (stimulation or inhibition) of phagocyte activities—the most widely investigated and controversial area—this review will only take into account the effects obtained in vitro, since ex vivo and in vivo results are due to global phenomena in which both direct and indirect effects play a role. A schematic presentation is given in Table 4. Details on the mechanisms involved (when data are available) will be provided in the discussions of individual drugs (see below). This scheme was summarized from references 16, 140, 199, 213, 236, 281, 282, and 417. Some antibiotics may either increase or decrease a given function depending on the cell type (e.g., cefotaxime), the technique used (e.g., chemotaxis) and other variables. Artifactual conditions such as scavenging of oxidant species (see below) may also lead to incorrect assumptions about the actual effect of an antibiotic on phagocyte activity (213). The modification of oxidant production by phagocytes in the presence of antimicrobial agents in vitro cannot always be ascribed to a direct effect of the drug on cell metabolism. The use of appropriate controls using cell-free oxidant-generating systems can help to characterize the target of antibiotic action. Among the drugs which have been recognized to display such effects are rifampin, which quenches superoxide anion (151), and cyclines, which scavenge hypochlorous acid (HOCl) (132), as does clofazimine (394) and various aminothiazolyl cephalosporins (215a). Penicillin G and ampicillin inhibit the chemiluminescence of PMNs and cell-free systems by scavenging HOCl and hydrogen peroxide (40, 126), whereas chloramphenicol increases it (40). Ampicillin has also been reported to act as an electron donor and/or superoxide generator (384). Dapsone and isoniazid directly interfere with MPO and impair the production of HOCl by the MPO-H2O2-halide system: dapsone converts MPO into its inactive (ferryl) form (394), while isoniazid serves as a suicide substrate for MPO (395). Cefdinir, a hydroxy-imino-aminothiazolyl cephalosporin, impairs MPO activity in the external medium but not in the phagolysosome, probably because it does not enter neutrophils (215). The major question arising from these results is the impact on bactericidal function and the tissue-destructive potential of neutrophils. Various in vitro experiments suggest, for instance, that some β-lactams may play a cytoprotective role (241, 288) or prevent antiprotease inactivation by activated neutrophils (65). The anti-inflammatory potential of dapsone, isoniazid clofazimine, and cyclines may be due to their impact on HOCl generation (132, 394), and various anti-inflammatory drugs also impair HOCl generation by the MPO-H2O2-halide system. However, the clinical relevance of these results has been questioned recently, since superoxide anion limits the potency of dapsone and various anti-inflammatory drugs (175).
TABLE 4.
In vitro effects of antibacterial agents on phagocyte functions
| Function | Drugs that increase function | Drugs that decrease function | Drugs with no effect on function |
|---|---|---|---|
| Chemotaxis | Nafcillin, clindamycin, erythromycin, bacitracin, chloramphenicol, dapsone, lincomycin | Tetracycline, clindamycin, erythromycin, gentamicin, fusidic acid, clofazimine | Tetracycline, clindamycin, erythromycin, gentamicin, chloramphenicol, quinolones, rifampin, β-lactams |
| Phagocytosis | Erythromycin, chloramphenicol, nafcillin | Tetracycline, tobramycin, polymyxin B | Tetracycline, gentamicin, polymyxin B, rifampin |
| Oxidative burst | Clindamycina, cefotaxime, quinolones, cefpimizole, josamycin | Cyclines, cefotaximeb, trimethoprim, rifampin, fusidic acidc, sulfonamides, chloramphenicol, erythromycin A (and derivatives) | Cyclines, clindamycin, quinolones, rifampin, fusidic acid, sulfonamides, chloramphenicol, spiramycin, glycopeptides, aminoglycosides |
| Bacterial killingd | Cefotaxime, cefodizime, roxithromycin | Sulfonamides, aminoglycosides | Rifampin, polymyxin |
| Cytokinese | Cefotaxime (IL-1), cefaclor (IL-1), cefodizimef (IL-1, IFN), erythromycin A derivatives (IL-10), ofloxacin (IL-2), spiramycin (IL-6) | Cefoxitin, cefodizime (IL-1, -8, TNF), erythromycin A derivatives (IL-1, IL-6, IL-8, TNF), ofloxacin, ciprofloxacin (IL-1, TNF) |
Depends on concentrations.
In monocytes.
In PMN.
Depends on bacterial species.
Depends on cytokines.
Restoration in immunocompromised animals.
Miscellaneous effects.
(i) Modulation of the specific immune response.
Indirect modulation of phagocyte functions may be obtained by alteration of various humoral factors which originate from specific immune effectors (e.g., immunoglobulins or cytokines) or from bacterial interaction with serum proteins (e.g., complement activation). The various effects reported in the literature are briefly summarized in Table 5. The detailed in vitro, ex vivo, and in vivo results can be found in references 16, 25, 109, 140, 199, and 331. The consequences of these effects may occur at the level of phagocyte bactericidal function (increased or decreased production of opsonins) or phagocyte activation. Complex in vivo systems (graft survival or tumor growth) cannot be used to identify the cellular effector involved (phagocytes, NK cells, or T lymphocytes).
TABLE 5.
Effects of antibacterial agents on the specific immune system and complement activation
| Function | Drugs that increase function | Drugs that decrease function |
|---|---|---|
| In vitro | ||
| Complement inactivation | Sulfonamides, tetracycline, ampicillin, streptomycin, gentamicin | |
| T and B lymphocytes | Cefotaxime, cefodizime, erythromycin A, fosfomycin, dapsone, ciprofloxacin | Cefmenoxime, josamycin, fusidic acid, cyclines, chloramphenicol, rifampin, clofazimine, co-trimoxazole, ampicillin |
| In vivo, ex vivo | ||
| T and B responses | Cefotaxime, cefodizime, dapsone, erythromycin A | Cyclines, ampicillin, mezlocillin, clofaximine |
| Graft survival | Chloramphenicol, fusidic acid, doxycycline, rifampin | Trimethoprim, rifampin |
| Tumor growth | Mezlocillin, doxycycline, rifampin | Trimethoprim, rifampin |
| Antibody protection | Cefotaxime, cefodizime | Chloramphenicol, co-trimoxazole, rifampin, josamycin, doxycycline |
| Delayed-type hypersensitivity | Cefodizime | Mezlocillin, cyclines, metronidazole, rifampin, erythromycin A |
Restoration in immunocompromised animals.
Depends on the administration schedule.
(ii) Impact on the host microflora.
A possible indirect modulation of phagocyte function can be produced by antibiotic-mediated destruction of the normal microbial flora, which directly (bacterial products) or indirectly (effect on T/B lymphocyte activity) interferes with phagocyte activation. The role of the gut microflora in the maturation of the mucosal immune barrier is well recognized and is best seen in newborns: the intestinal tract, which is sterile at birth, gradually becomes populated by bacteria (maternal and food bacterial flora), and after about a year the immune system starts to establish tolerance to these antigens. Various members of the physiological microflora release low-molecular-weight peptides which seem to be essential for adequate immune responses (310). The secretory immune system of the mucosa (gut-associated lymphoid tissue and bronchus-associated lymphoid tissue) is the transmission system between the endogenous or exogenous microflora and the host immune response. Antimicrobial therapy may cause pronounced disturbance in the normal microflora, leading to undesired effects such as overgrowth and superinfections by commensal microorganisms or toxin-mediated diseases (e.g., Clostridium difficile-induced pseudomembranous colitis) (224). In addition, decreased antigenic stimulation may lead to a defective immune response. Animal models have shown that intestinal decontamination with mezlocillin, for instance, results in reproducible immunosuppression including decreased macrophage activity (309). A recent provocative paper questioned whether microbes and infections might in fact be beneficial (329). As a putative cause of increased allergies and autoimmune diseases, the authors discussed the input deprivation syndrome in the immune system created by obsession with hygiene and vaccination (what about excessive antibiotic use?), which failed to maintain a correct cytokine balance and fine-tune T-cell regulation. By contrast, as already suspected by Metchnikoff, the normal intestinal flora (and its products) may have deleterious consequences; in particular, its role in the induction and development of colon cancer in elderly patients has been proposed, whereas excessive bacterial stimulation with subsequent imbalance in local production of proinflammatory and anti-inflammatory cytokines could generate intestinal inflammation, food allergy, or other atopic diseases (80). The use of “probiotics” such as lactobacilli (or selected antibiotics?) could thus be beneficial in many ways (74).
In conclusion, a simple classification of the immunoregulatory properties of antibacterial agents is presented in Fig. 7. A preliminary classification had been proposed earlier (199) and slightly modified (205). Here, the phagocyte is presented as the central element, and the potential modulatory effects of antibacterial agents are given in terms of class, ignoring peculiar, structure-related properties, which will be dealt with in the next section. Some effects which are obtained with most antibiotics (for example, their effects on pathogens) are not presented, and neither are effects on the specific immune system, which would excessively complicate the scheme. Special mention is made of a special group of drugs which interfere at almost all levels of the immune response: the immune response modifiers (as defined in reference 199), whose main (only?) example (cefodizime) will be dealt with in a later section.
FIG. 7.
Attempts to schematically classify antibacterial agents according to their effects on phagocyte activities. Abbreviations: R, restoration of a deficient function; IC, intracellular; INH, isoniazid.
Lexicon of Immunomodulatory Antibacterial Agents
The main in vitro effects of the various families of antibacterial agents (arbitrarily classified in alphabetical order) will be presented in this section. Whenever possible, the underlying mechanisms and structure-activity relationships will be given. Intracellular bioactivity, toxicity, and modulation of bacterial virulence have been reviewed in the preceding section. Ex vivo and in vivo effects will be discussed in the following section, along with their potential clinical relevance. Essential data on all antibacterial families have been reviewed in references 197, 236, and 388. Specific reviews of macrolides can be found in references 198, 207, and 209. General information on quinolones and macrolides can be found in reference 200.
Aminoglycosides.
Aminoglycosides interfere with bacterial protein synthesis by acting on the 30S ribosomal subunit. There are controversial data on the inhibitory effect of aminoglycosides at therapeutic concentrations on PMN chemotaxis, oxidative metabolism, and yeast killing (16, 42, 197, 236). Various mechanisms have been advanced, based on analyses performed in cell-free or nonphagocyte systems; they include binding to negatively charged membrane phospholipids leading to membrane disturbances, specific binding to inositol biphosphate resulting in inhibition of PLC, and inhibition of PKC. The bioactivity of streptomycin on intracellular E. coli has been suggested to rely on stimulation of (synergy with?) O2·−-dependent bactericidal mechanisms in macrophages (385), although drug uptake was not studied in this model. The effect of neomycin on leukotriene generation by PMNs was inhibitory or stimulatory depending on the concentration, as was its effect on the GTPase activity of crude membrane fractions. The clinical relevance of aminoglycoside interference with phagocytic functions is thus improbable; rather, these drugs (particularly neomycin) appear to be useful (although not specific) tools for the study of transduction pathways (42).
Ansamycins.
Antibacterial ansamycins (rifamycins) comprise a group of macrocyclic antibiotics containing a chromophoric naphthohydroquinone system spanned by a long aliphatic bridge. They are mainly effective against mycobacteria and alter RNA biosynthesis by interfering with RNA polymerase activity. Rifampin, the most important representative of this group, impairs various PMN functions such as chemotaxis and the oxidative burst (although light-absorbing activity and superoxide anion scavenging can artifactually interact with the detection method). Studies have shown that rifampin reduces humoral and cell-mediated immunity (140). A possible effect offsetting drug-induced immune depression was shown in a recent study in which rifampin increased GM-CSF- and IL-4-induced expression of CD1b (a human antigen-presenting molecule belonging to the nonclassical MHC-independent system involved in the presentation of nonpeptide antigens) thereby favoring the lipid/glycolipid antigen presentation mediated by CD1b on peripheral blood monocytes (373). A potential anti-inflammatory effect of rifampin and other derivatives has been suggested (see below).
Benzylpyrimidines (trimethoprim and analogs).
Benzylpyrimidines (dimethoxy benzylpyrimidines) include trimethoprim (TMP), tetroxoprim, epiroprim, and brodimoprim, which all inhibit dihydrofolate reductase. TMP is generally used in combination with another antifolate drug (sulfamethoxazole). In most studies, TMP, alone or in combination, had an inhibitory effect on PMN functions. In a single study, PMN chemotaxis and chemiluminescence were increased, and this effect was also observed with PMNs with defective functions (286). Recently, brodimoprim, in which the methoxy group in position 4 of the benzyl ring of the TMP molecule is replaced by a bromine atom, was shown to display greater lipophilicity and cellular uptake than TMP and had no inhibitory effect on PMN function, whereas TMP impaired the chemiluminescence of these cells (39). An inhibitory effect of TMP on the PLD-phosphatidate phosphohydrolase (PPH) pathway, leading to decreased generation of diradylglycerol, has been proposed as the mechanism underlying TMP-induced inhibition of PMN oxidative metabolism. However, the concentration which impaired the PMN oxidative burst by about 50% was far higher (about 1 mM) than therapeutic concentrations (298).
β-Lactams.
β-Lactam antibiotics represent more than half of all antimicrobial drugs. Structurally, they comprise five groups of compounds: penams (penicillins and β-lactamase inhibitors), penems (faropenem), carbapenems (imipenem, meropenem), cephems (cephalosporins, cephamycins, oxacephens, and carbacephems), and monobactams (aztreonam, etc.). All groups have a common antibacterial mechanism involving inhibition of various enzymes (PBP, penicillin-binding protein) involved in the synthesis of peptidoglycan. Many data are available on the in vitro effects of these drugs on phagocyte functions (and specific immune effectors), but no class- or subgroup-related effect has been demonstrated. Rather, particular aspects linked to chemical features have been identified. With the exception of cefodizime, whose immune response modifier (IRM) activity is described below, β-lactam-induced modulation of immune responses does not appear to be of major clinical relevance. As discussed in a previous section, some β-lactams can decrease the PMN oxidative burst by scavenging oxidative species or inhibiting MPO. The majority of experiments conducted in vitro have shown no major direct interference of β-lactam with phagocytes. The rare significant in vitro effects have been described. An overview of the effects of cephalosporin is provided in reference 204. A decrease in bacterial killing has been reported with carbenicillin (not ampicillin) and cephalothin, whereas cefotaxime has been shown to potentiate it, probably owing to an enhancement of the oxidative response of PMNs stimulated with complement-opsonized particles (212). High concentrations of meropenem have been reported to decrease superoxide anion production by PMNs (240). However, this drug potentiated phagocytosis and bacterial killing by human macrophages (49). The three chemically unrelated β-lactams (cefmetazole, imipenem, and cefoxitin) had similar stimulatory effects on various PMN functions (phagocytosis, oxidative burst, and antibody-dependent toxicity) and displayed chemoattractant activity (326–328): these antibiotics also significantly stimulated protein carboxy methylation, increased intracellular cyclic GMP levels, and decreased ascorbate content; cefaclor and cefetamet increased phagocytosis and bactericidal activity and decreased LTB4 production by PMNs (341). Faropenem enhanced superoxide anion production by PMNs, and the authors suggested interference at a site where Ca2+ regulates NADPH oxidase activation (338). An extensive evaluation of the effect of 26 different β-lactam antibiotics on murine PMN cytokinesis (random migration and chemotaxis) has led to a classification into six groups (173), but no structure-activity relationships have been demonstrated. Similarly, scarce and often controversial data have been reported on the effect of β-lactams on cytokine release. Recently, the amoxicillin-clavulanic acid combination, which increases the phagocytic and microbicidal activity of PMNs, was shown also to elicit the production of IL-1β and IL-8 by LPS- and Klebsiella-stimulated PMNs (315).
Chloramphenicol.
Chloramphenicol impairs bacterial protein biosynthesis by acting on the 50S ribosomal subunit. Controversial data exist on the potential drug-induced reduction in phagocyte functions (197). It seems unlikely that this drug displays any significant direct interference with phagocyte activities at therapeutic concentrations.
Cyclines.
Cyclines also interfere with bacterial protein synthesis by acting on the 30S ribosomal subunit. The first report of a depressive effect of cyclines on phagocytosis dates back to the early 1950s. Since then, these drugs have been widely studied in this context, with most reports confirming an inhibitory action on various phagocyte functions at therapeutic concentrations. Also, these drugs impair collagenase and gelatinase activity, an effect that appears to be specific for neutrophil or tumor cell-derived enzymes (89, 368). Few studies have investigated the effect of cyclines on cytokine production: paradoxically, minocycline and, to a lesser extent, tetracycline, increased IL-1β secretion by LPS-stimulated human monocytes (156). Various mechanisms have been proposed to explain the inhibitory action of cyclines, including chelation of Ca2+ (a property used to analyze Ca2+ fluxes and mobilization in activated PMNs), binding of intracellular Mg2+, photodamage of PMNs, and artifactual scavenging of hypochlorous acid. Structure-activity relationships indicate a parallel increase in lipid solubility (possibly cellular accumulation) and inhibitory properties (for example, doxycycline > chlortetracycline > tetracycline > oxytetracycline) (99, 100). However, other studies stress the different chemical reactivities of the various molecules under UV exposure. The clinical relevance of the inhibitory properties of cyclines on phagocyte functions is widely acknowledged (see below).
Fosfomycin.
Fosfomycin (1-cis-1,2-epoxypropylphosphoric acid) is a broad-spectrum bactericidal antibiotic, not related to any other known antibacterial agents, that interferes with bacterial cell wall biosynthesis by inhibiting the pyruvate–uridine diphosphate–N-acetylglucosamine transferase. In vitro, fosfomycin has immunomodulatory activity on B- and T-lymphocyte function, and also inhibits histamine release from basophils (218, 259, 260). With regard to phagocytes, it was recently reported that fosfomycin decreased the rate of synthesis of TNF-α and IL-1 but increased that of IL-6 (261). The possible therapeutic relevance of these effects is under evaluation (see below).
Fusidic acid.
Fusidic acid, a tetracyclic triterpenoic molecule used mainly as an antistaphylococcal agent, interferes with protein biosynthesis factors. This agent decreases PMN functions in vitro without markedly altering those of monocytes. Its possible value as an immunosuppressive agent has been promoted in human immunodeficiency virus infection, although direct antiviral activity has also been suggested.
Gyrase B inhibitors.
Gyrase B inhibitors consist of novobiocin and coumermycin, which impair bacterial DNA replication by inhibiting gyrase B activity. Few studies have been performed with these compounds. At therapeutic concentrations, coumermycin has been reported to impair chemotaxis, superoxide anion production, and intracellular killing of PMNs (389). Novobiocin interferes with metabolic processes in eukaryotic cells and, in particular, is a potent inhibitor of ADP ribosylation. It effectively suppresses the production of proinflammatory cytokines (TNF-α, IL-1, and IL-6), as well as the anti-inflammatory cytokine IL-10, by LPS-stimulated human monocytes (231). It also induces the shedding of CD14 and modulates the expression of other surface antigens. The cytosolic protein phosphorylation pattern was altered by novobiocin and other inhibitors of ADP ribosylation, pointing to a role of this process in monocyte transductional pathways. A species dependence with novobiocin was shown, since mouse macrophages were far less susceptible to the inhibitory effect of novobiocin on TNF production than were human monocytes (231). Also, although the drug had hepatoprotective properties in vivo, elevated TNF-α levels in mice treated with d-galactosamine were not reduced by novobiocin administration (231).
Isoniazid.
Isoniazid (an isonicotinic acid hydrazide) is an antituberculous agent. Its antimycobacterial activity has been attributed to its oxidative metabolism by mycobacterial peroxidases. This chemical reactivity explains its inhibition of the MPO-H2O2-halide system and also its potential toxicity after oxidation by activated leukocytes (149).
Lincosamides.
Lincomycin and clindamycin interact with bacterial protein synthesis at the level of the 50S ribosomal subunit. Clindamycin has long appeared as a forerunner in antimicrobial chemotherapy and was suggested as a possible immunomodulator in infection in the early 1980s (285). Since then, controversial data (enhancement, decrease, or no effect) on phagocyte functions have been reported with various techniques, depending on drug concentrations (in addition to other side effects and development of more active intracellular agents), and have tempered enthusiasm. Renewed interest in this drug has been generated by its potential prophylactic effect in LPS-induced septic shock (see above) (180).
Macrolides.
According to Woodward's chemical definition, macrolide antibiotics are characterized by a macrocyclic lactone ring, with few or no double bonds, linked to one or several amino or neutral sugars. Extensive chemical modifications of the natural compounds, particularly erythromycin A, have led to the development of semisynthetic molecules which can escape the strict chemical definition by containing a nitrogen atom in the lactone ring (azalides). Recent developments in macrolide chemistry have led to the advent of the ketolide family, derived from erythromycin A by withdrawing the l-cladinose at position 3 of the lactone ring and oxidizing it into a 3-keto function. All macrolide antibiotics impair bacterial protein synthesis by acting on the 50S bacterial ribosomal subunit. An extended definition of the macrolide family includes the complex structures of the nonantibacterial immunosuppressive agents FK-506 and rapamycin and the true macrolidic structures not in therapeutic use (owing to their toxicity) such as the concannamycins and bafilomycins (206). It is important to keep in mind this chemical continuum when considering the potential immunosuppressive effects of classical antibacterial macrolides (see below). Several reviews have highlighted the potential immunomodulating properties of macrolides (198, 206, 207, 209). The most important findings with regard to their interactions with phagocytes concern the inhibitory effect on oxidant production by stimulated cells and modulation of proinflammatory and anti-inflammatory cytokine release by these cells (206, 207, 209). Structure-activity studies have shown that only erythromycin A derivatives, including the azalide azithromycin, impair the phagocyte oxidative burst in a time- and concentration-dependent manner (2, 13, 14, 214, 222). In addition, these drugs directly stimulate exocytosis by human neutrophils (1–3). The chemical entity responsible for these effects was shown to be the l-cladinose at position 3 of the lactone ring, but this does not rule out the possibility that other structures may also interfere with phagocytic transductional targets (M. T. Labro, H. Abdelghaffar, and H. Kirst, Program Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-208, p. 191, 1997). In particular, we have observed that two ketolides (RU 64 004 [HMR 3004] and HMR 3647) also impair oxidant production by neutrophils (397, 398; M. T. Labro and H. Abdelghaffar, Program Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother. abstr. F-225, p. 139, 1996). With HMR 3004, the structure involved in this inhibitory effect could be the quinoline linked by a butyl chain to the C-11–C-12 carbazate, since this structure is also present in the immunosuppressive 4-amino quinoline antimalarials (397; Labro and Abdelghaffar, 36th ICAAC). With HMR 3647, the inhibitory structure has not been identified. The transductional pathway by which erythromycin A derivatives interfere with neutrophils seems to be the PLD-PPH pathway (2), which is crucial for the activation of exocytosis and oxidant production. We have demonstrated that these drugs directly stimulate PLD activity in resting PMNs, which results in the accumulation of PA, a messenger important for triggering exocytosis (2). In stimulated PMNs, these drugs impair PPH activity, resulting in a decrease in diradylglycerol production (2). Unpublished observations by our group also show that HMR 3004 and HMR3647 impair the activity of PPH (a target common to other antibiotics which impair oxidant production [298]), but that only HMR 3004 and chloroquine also stimulate PLD activity (and exocytosis). These studies suggest that macrolide-induced inhibition is linked to the cellular accumulation of these drugs. In vitro conditions which modify cellular uptake can thus interfere with the inhibitory effect of these drugs: for instance, PKA inhibitors, which impair macrolide uptake, also decrease the inhibitory effect of these drugs (256). In contrast, Kadota et al. (166) observed marked suppression of superoxide anion generation by G-CSF-primed neutrophils by therapeutic concentrations of erythromycin A, but the uptake of this drug was not investigated. Other authors have observed that proinflammatory cytokines (TNF-α and IFN-γ) increase macrolide uptake by macrophages or monocytic cell lines and favor the intracellular bactericidal activity of these drugs (30, 31, 290). Results from our group show a small (about 20%) but significant decrease in the uptake of roxithromycin and ketolides in TNF-α- and GM-CSF-primed PMNs (397). In addition, these two cytokines decreased the inhibitory effect of HMR 3647 only on oxidant production by PMNs (397). The clinical relevance of the antioxidant properties of macrolides is difficult to establish. Recently, Feldman et al. suggested that the antioxidant properties of macrolides could be beneficial in airway inflammation by protecting the ciliated epithelium against damage inflicted by bioactive phospholipid-sensitized phagocytes (88).
Macrolides interfere with cytokine production in vitro (reviewed in reference 207), generally decreasing proinflammatory cytokine production by stimulated phagocytes while increasing that of the anti-inflammatory cytokine IL-10 (97, 154, 172, 178, 261, 345). It is interesting that individual susceptibility to the immunomodulating activity of macrolides has been shown for both cytokine production (178) and oxidant production (214). The underlying mechanism (antibiotic uptake, cellular target, etc.) is not known. Modulation of proinflammatory cytokine production has also been observed in eosinophils (187) and in nonphagocytic cells (176, 369). The mechanisms underlying the inhibitory effect of macrolides on cytokine production have not been elucidated. In general, the suppression of cytokine release is accompanied by a parallel decrease in mRNA expression. Other macrolide-induced modifications of mammalian cell functions or metabolism have been described (accelerated apoptosis of neutrophils, inhibition of HL-60 cell growth, decreased glycoconjugate secretion by cultured human airway cells, etc.), which raise the possible existence of a common (multiple?) cellular targets of macrolide action. It is tempting to link these effects to those of the macrolidic immunosuppressant FK-506 or rapamycin and, by analogy to the mechanism of action of these drugs, to look for a putative macrolide-specific immunophilin governing the cellular action of these drugs.
Peptides.
Peptide antibiotics are a broad family comprising the polypeptides tyrocidins, gramicidins, and bacitracin (predominantly active against gram-positive bacteria but only suitable for local application), the polymyxins (active against gram-negative bacteria), the streptogramins, the antistaphylococcal glycopeptide vancomycin, the lipopeptide daptomycin, and the lipoglycopeptide teicoplanin. The mechanisms underlying the antibacterial activity of these drugs differ, with polymyxins acting by increasing the permeability of the cytoplasmic membrane, glycopeptides interfering with cell wall biosynthesis, and streptogramins impairing bacterial protein biosynthesis by acting on the 50S ribosomal subunit. In general, peptide antibiotics do not significantly alter phagocyte functions at therapeutic concentrations. The drug most extensively studied in this respect is polymyxin B, one of the first recognized inhibitors of PKC; this latter property has made it a useful tool for the study of transductional pathways (7). The ability of polymyxin B to bind the lipid A portion of LPS is unfortunately associated with toxicity, which contraindicates its general use in septic shock (this drug is used in vitro to neutralize possible LPS contamination). However, polymyxin B has a stimulatory effect on monocyte function and, in particular, stimulates the production of IL-1, IL-6, GM-CSF, and complement components. These effects may therefore lead to false interpretation when used in in vitro monocyte culture (150). Bacitracin binds Ca2+ and Mg2+, a property that has been held responsible for the inhibitory effect of these drugs on phagocytosis. Vancomycin and teicoplanin have been reported to depress some PMN functions but only at very high, clinically irrelevant, concentrations. At a concentration of 50 mg/liter, teicoplanin also increased the production of TNF-α, IL-1, and IL-6 by concanavalin A-stimulated human monocytes (382). There are no published data on the effect of streptogramin derivatives.
Quinolones.
Quinolones are synthetic antibacterial compounds, whose first representative (nalidixic acid) was synthesized in 1962. Since then, thousands of compounds have been made, of which the 6-fluorinated molecules (fluroquinolones) represent a breakthrough in 4-quinolone research. The antibacterial activity of 4-quinolones stems from their inhibitory effect on bacterial DNA gyrase (topoisomerase II) and thus on DNA replication. 4-Quinolones might also affect mammalian DNA metabolism, since mammalian cells also contain an essential type II DNA topoisomerase. Most in vitro data indicate no significant effect on phagocyte functions (chemotaxis, oxidative metabolism, or phagocytosis) by quinolones at therapeutic concentrations (200, 388, 389); significant synergy with oxidative species for intracellular bactericidal activity has been reported with various molecules (392). A synergistic effect has been observed between G-CSF and ofloxacin for the bactericidal activity of PMNs (192). The underlying mechanisms (G-CSF-induced increase in ofloxacin uptake or in superoxide anion production) were not investigated. Some authors have observed that high concentrations of ofloxacin and fleroxacin (25 to 100 mg/liter) potentiate the chemiluminescence response of PMNs, whereas other quinolones (sparfloxacin, lomefloxacin, grepafloxacin, AM-1155, etc.) significantly decrease it (193). Complementary studies from the same group suggested that the ofloxacin-induced increase in the PMN oxidative response was due to the enhancement of PKC (244, 271). A similar transient potentiating effect on the oxidative burst has been reported with ofloxacin, fleroxacin, sparfloxacin, and levofloxacin in rat macrophages (18). The lower concentrations (0.5 mg/liter) were more effective than the higher ones (50 mg/liter). All quinolones modestly but significantly impaired rat macrophage chemotaxis in a concentration-dependent manner. The effects of 4-quinolones on mediator (cytokine) production by monocytes are widely documented (200). At high concentrations, pefloxacin and ciprofloxacin decrease IL-1 production by LPS-stimulated human monocytes, and ciprofloxacin and ofloxacin (>25 mg/liter) decrease TNF-α production. These depressive effects were suggested to be linked to cyclic AMP accumulation. A suppressive effect of therapeutically achievable concentrations of trovafloxacin on the synthesis of IL-1α and β, IL-6, IL-10, GM-CSF, and TNF-α by LPS-stimulated human monocytes has also been reported (177). In addition, various authors have observed that 4-quinolones alter T- and B-lymphocyte functions and delay or suppress the proliferative response of human mononuclear cells (117, 119, 321, 322). Taken together, these data support an immunomodulatory effect of some 4-quinolones, at least in vitro. The clinical relevance of these results remains to be established, although there are some data on the potential in vivo suppressive effects of some quinolones in animal models (163). Induction of DNA damage and a stress response in mammalian cells has been proposed as the underlying mechanisms, at least for ciprofloxacin (321).
Riminophenazines.
Riminophenazines are structurally phenazine compounds in which a substituent (R) is included in the imino part of the molecule. Historically, they were derived from lichens and targeted Mycobacterium tuberculosis. The first clinically developed compound was clofazimine (lamprene), whose activity has been extended to other mycobacterial diseases (316). Several hundred analogs have now been synthesized, but they do not have better activity than clofazimine in animal models. The antimycobacterial mechanism of these drugs has not been found, but stimulation of PLA2 activity and lysophospholipid accumulation seem to be a common mechanism of action in prokaryotes and eukaryotes (393). The intracellular (phagocytic) accumulation of riminophenazines is a key factor in their bioactivity against mycobacteria, which are obligate intracellular pathogens. This intracellular activity is potentiated by phagocyte treatment with IFN-γ or TNF-α (122).
Clofazimine increases superoxide anion production and degranulation by stimulated neutrophils, and TNF-α potentiates this enhancement (189). The prooxidative effect of clofazimine analogs is largely dependent on the nature of the alkylimino group at position 2 on the phenazine nucleus and, to a lesser extent, on halogenation (339). Interestingly, clofazimine also reverses the inhibitory effect of M. tuberculosis-derived factors on the PMN oxidative burst (404). The mechanism underlying this prooxidative effects seems to involve a stimulation of PLA2 activity with subsequent accumulation of arachidonic acid and lysophospholipids, which act as second messengers to activate the oxidase (188). In addition, PLA2 activation and lysophosphotidylcholine accumulation have been held responsible for inhibition of the membrane Na+,K+-ATPase, a key enzyme in various lymphocyte functions (15). Cyclosporine was shown to potentiate the immunosuppressive activity of clofazimine through a PLA2–Na+,K+-ATPase-dependent mechanism (305); other immunosuppressive and anti-inflammatory effects of clofazimine could be related to its ability to scavenge chlorinating oxidants (see above) and to stimulate prostaglandin E2 production by neutrophils (12).
Sulfones and sulfonamides.
Dapsone (4,4′-diaminophenyl sulfone) has been in the armamentarium since 1908, when it was synthesized by Framm and Whitman. Initially developed as an antitubercular drug, it was tested on leprosy in the early 1950s and is still used in combination therapy for this disease. It was later tested in malaria and some inflammatory diseases. Its antibacterial activity is due to inhibition of dihydropteroate synthase. The anti-inflammatory activity of dapsone is less well understood (56). Dapsone inhibits neutrophil functions such as chemotaxis and oxidant production. In addition, it irreversibly inhibits MPO by converting the enzyme into its inactive (ferryl) form (see above). This drug also impairs neutrophil adherence to antibodies bound to the basement membrane (probably by direct interference with antibodies) (376). Dapsone, unlike clofazimine, impairs the production of prostaglandin E2 by neutrophils (11), a possible explanation for dapsone-induced potentiation of cell-mediated immunity. The hematologic toxicity of dapsone is linked to its oxidative metabolism.
The discovery of the antibacterial activity of sulfonamides dates back to the early 1930s, when prontozil entered the anti-infectious armamentarium. Modification of the active derivative (sulfanilamide) has generated hundreds of compounds which all are characterized by the same antibacterial mechanism of action, i.e., inhibition of dihydropteroate synthase. Synergy with dilydrofolate reductase inhibitors has led to the combination of agents from the two classes. The most frequently used antibacterial sulfamide is sulfamethoxazole in combination with TMP (co-trimoxazole). In general, sulfonamides exert an inhibitory effect on phagocyte functions, and many agents in this class have been switched from infections to inflammatory diseases. The mechanisms underlying the effects are unclear. Inhibition of the increase in intracellular Ca2+ concentration after stimulation has been reported with sulfasalazine and sulphapyridine (168). Structure-activity relationships have also been identified for sulfasalazine, which suggests the importance of the azo link between S-aminosalicylic acid and sulfapyridine in the neutrophil-inhibiting effect, rather than release of S-aminosalicylic acid (274). Scavenging of HOCl by sulfapyridine but not sulfamethoxazole has also been reported (289).
Other antibacterial agents.
There are a few studies on the in vitro effect of ethambutol, nitrofurans, and minimally substituted imidazoles (metronidazole and tinidazole) on phagocyte functions, and no significant alterations have been demonstrated.
NONANTIBIOTIC EFFECT OF ANTIBACTERIAL AGENTS: POTENTIAL THERAPEUTIC RELEVANCE?
The therapeutic relevance of the immunomodulatory effects of antibacterial agents is clearly acknowledged for toxic side effects and intracellular bioactivity and does not need further discussion. Other immunomodulating effects of antibiotics which are derived directly from their antibacterial activity are suspected; these include the consequences of antibiotic-induced release of proinflammatory bacterial products (endotoxin in the case of quinolones or β-lactams, depending on the clinical setting [92, 372]). In bacterial meningitis, anti-inflammatory adjunctive therapy has been introduced in clinical practice, but some quinolones have been proposed to reduce or delay the inflammatory response by slowing the release of bacterial cell wall components (273). The rapid bactericidal activity of fluoroquinolones could also be advantageous in treating chronic airway infections by P. aeruginosa, by suppressing excessive immune responses in the lung and thereby preventing the progression of tissue damage (337). Lastly, antibiotic prophylaxis to prevent the development of rheumatic fever and other forms of reactive arthritis is generally accepted for penicillin in streptococcal tonsillitis but is unresolved in other settings (419).
The most intriguing aspect of antibiotic-induced modulation of immune effectors (such as phagocytes) concerns their direct chemical and biochemical interaction with host cell metabolism and functions and whether these effects must be taken into account in the choice of an appropriate antibacterial regimen.
The nonantibiotic effect of antibacterial agents is widely recognized in vitro. These results have led to expectations of additional beneficial effects in infections (and other diseases). With the gradual increase in our knowledge of the ambivalent role of phagocytes and other immune effectors (defense versus destruction), there has been a parallel “modulation” of the physicians' mentalities from what can be called the G. B. Shaw approach (“to stimulate the phagocyte”) to that of Repine and Beehler (to decrease the harmful while retaining the beneficial potential of these cells).
The clinical benefit of the stimulating and restoring effects of antibacterial agents on phagocyte function is, indeed, considered of minimal importance compared to their direct antibacterial activity in the context of infectious diseases. (The growth-stimulating effect of various antibacterial agents given as animal food additives is outside the scope of this review, but I would nonetheless like to stress the potentially disastrous consequences for our bacterial environment.) The example of cefodizime clearly illustrates the surprising lack of interest (after great excitement) in such drugs among manufacturers. On the other hand, new expectations are arising for antibacterials with immunodepressive potential, in the context of inflammatory diseases.
Immunostimulation or Restoration?
Immune response modifiers and immunocompromised patients: the example of cefodizime.
In 1984 the first report on the innovative, nonantibacterial properties of cefodizime, a new 2-amino-5-thiazolyl cephalosporin, appeared in the Journal of Antibiotics (225). This paper summarized in vitro and in vivo data obtained by Hoescht Abteilung Geselschafft in Frankfurt, which suggested that cefodizime (HR-221) displayed original properties unrelated to its antibacterial efficacy. Since this publication, the immunomodulatory activity of cefodizime has been investigated worldwide in vitro, ex vivo, and in vivo in humans and animals (both healthy and immunocompromised), and this has resulted in about 100 publications and conference presentations. Various overviews have summarized the main immunomodulatory properties of cefodizime (24, 195, 196). In the 1990s, cefodizime appeared to be a forerunner of the Holy Grail in anti-infectious therapy, being presented as an IRM antibiotic with both classical antibacterial activity and innovative immunomodulatory potential. The latter included no depression of host cellular defenses (for example, no granulopenia), enhancement (direct or via the release of activating factors) of natural bactericidal systems (microbicidal properties of phagocytes), restoration of deficient antimicrobial systems when required (i.e., in immunocompromised individuals), reduction in bacterial virulence (susceptible and resistant pathogens), and modulation of detrimental host immune factors (excessive cytokine production). Starting with the experimental model of Limbert et al. (225) that demonstrated a prophylactic effect of cefodizime in infections caused by nonsusceptible pathogens (Candida albicans), subsequent results confirmed the potential benefit of cefodizime in the prevention and/or treatment of various infections caused by resistant pathogens such as Plasmodium berghei, C. albicans, and Toxoplasma gondii. Experimental models using immunocompromised animals confirmed the better efficacy of cefodizime than of other cephalosporins. Interestingly, in a model of pulmonary inflammatory responses induced by heat-killed Streptococcus pneumoniae, cefodizime abrogated TNF-α and IL-6 release into the bronchoalveolar lavage fluid and downregulated the strong PMN recruitment otherwise observed (29). In addition, a recent paper reported that cefodizime modulated the pulmonary response to heat-killed Klebsiella pneumoniae by stimulating the early immune response and further reducing late recruitment of neutrophils and IL-1 levels (28). Ex vivo studies have demonstrated strain- and concentration-dependent responsiveness of the immune system to cefodizime with regard to delayed-type hypersensitivity, antibody production, and lymphocyte proliferation. In humans, contrasting results were obtained in healthy subjects and immunocompromised patients: immune parameters in healthy individuals given cefodizime were modestly affected or not modified, whereas those in immunocompromised individuals (with immune systems depressed by cancer, hemodialysis, old age, surgical stress, etc.) were modified after cefodizime administration. In particular, cefodizime administration restored the deficient parameter in the case of depressed phagocytic functions. When placebos or comparator antibiotics were given, the beneficial effect was seen only in the cefodizime-treated group. The chemical structure responsible for the immunomodulatory properties was rapidly identified as the thio-thiazolyl moiety at position 3 of the cephem ring (344), but the cellular mechanism responsible for the multiple immunomodulatory properties remains to be elucidated. It has been shown that in vitro, cefodizime stimulates the proliferative response of lymphocytes, increases the phagocytotic and bactericidal activity of PMNs, and downmodulates the production of proinflammatory cytokines by stimulated monocytes. In contrast to all β-lactams, cefodizime was also reported to significantly increase colony formation by granulocyte-monocyte progenitors (353). Alteration of bacterial virulence in susceptible and resistant bacteria has also been demonstrated with cefodizime.
Despite the abundance of published data, the development of cefodizime as an immunomodulatory antibiotic has been unsuccessful. Ethical (and economic) problems, the good “classical” antibacterial activity of this drug, and the unclarified mechanism of action and cellular targets have made it difficult to establish satisfactory protocols to illustrate the advantages of such an IRM antibiotic. In the study by Wenisch et al. (410), phagocyte function recovered significantly earlier in a group of 15 infected patients receiving cefodizime than in a comparable group treated with ceftriaxone, but the only apparent clinical advantage was earlier defervescence in the cefodizime-treated group. No reports are available on the consequences of prophylactic administration of cefodizime in patients at risk of infections. The loss of interest in IRM antibiotics is clearly illustrated by the drastic fall in publications relevant to this drug. More than half the publications on cefodizime were published in 1984 to 1990, whereas only five papers were referenced in 1996 to 1999 (28, 29, 38, 71, 353).
Fluoroquinolones: a future prospect?
New interest is arising in the potential “immunostimulating” properties of some fluoroquinolones. This may just be because the quinolone class has shown major developments in the last 10 years, whereas interest in β-lactams may be on the decline. Experimental models similar to those used with cefodizime (prophylactic administration in C. albicans infection) have been reported with rufloxacin (64). Other interesting prospects concern the effects of fluoroquinolones on hematopoiesis. Studies using various animal models have shown that in vivo treatment with ciprofloxacin enhances the repopulation of hematopoietic organs in sublethally irradiated mice and in lethally irradiated bone marrow-transplanted mice (182, 183). Accelerated recovery of neutrophils following prophylactic ciprofloxacin treatment of bone marrow transplant recipients has also been observed (155). However, controversial data have been reported in vitro and in vivo (83, 131).
Immunodepression and Anti-Inflammatory Activity of Antibacterial Agents
Two hypotheses have directed the use of antibacterial agents in inflammatory diseases: either the drug displays intrinsic anti-inflammatory activity (empirical observations or demonstrated in vitro or ex vivo effects) or it acts on a latent (unrecognized) pathogen (Chlamydia, Mycoplasma, etc.), causing chronic inflammation. Whatever the putative mechanism (direct or indirect), the modulation of detrimental phagocyte activity is recognized as the basis for antibiotic action.
The use of antibacterial agents as anti-inflammatory drugs falls into four categories: (i) agents which have been in use for a long time (sulfones, sulfonamides, and clofazimine); (ii) drugs which have recently triggered interest, particularly in rheumatoid arthritis (cyclines and ansamycins); (iii) drugs which are effective in specific diseases (for example, macrolides in diffuse panbronchiolitis) and show promise in other inflammatory settings; and (iv) drugs which could be developed in the near future but are at present only being studied in animal models.
Classical use of antibacterial agents in inflammatory diseases.
In addition to its antimycobacterial activity, dapsone exhibits significant anti-inflammatory activity and has been utilized in many neutrophilic dermatoses and other inflammatory diseases such as dermatitis herpetiformis, leukocytoclastic vasculitis, bullous lupus erythematosus, pustular psoriasis, erythema elevatum diutinum, and Crohn's disease (36, 96, 169, 303). Similar indications have been put forward for another antimycobacterial drug, clofazimine, which has proven effective in vitiligo, discoid lupus erythematosus, pyoderma gangrenosum, and pustular psoriasis (53, 233). The effectiveness of these two drugs in mycobacterial diseases has been also ascribed at least partly to their anti-inflammatory activity. As indicated above, both dapsone and clofazimine significantly depress the inflammatory potential of phagocytes; this property clearly seems to underlie their anti-inflammatory efficacy.
Similarly, sulfonamides have proved effective in the treatment of Wegener's granulomatosis (325), and sulfasalazine (and sulfapyridine?) displays antirheumatic activity (308).
Cyclines and ansamycins.
Tetracycline is widely accepted as an effective drug in the treatment of inflammatory acne. One mechanism by which this drug exerts its effect is by inhibiting the proliferation of Propionibacterium acnes. However, the lack of correlation between the drug dose regimen and cutaneous bacterial counts has led to speculation that this drug also interferes with the inflammatory reaction (86).
Similarly, the theory that persistent Mycoplasma infections may cause rheumatoid arthritis has been suggested to explain the benefit of lengthy courses of tetracyclines in this disease (335). Tetracyclines have been also used in reactive arthritis, i.e., nonpurulent inflammation of a joint following urogenital, gastrointestinal, or lower respiratory tract infections (217). However, controversies have arisen, impeding significant conclusions (307, 358). Recently, a multicenter double-blind placebo-controlled trial concluded that minocycline was safe and effective in patients with mild to moderate rheumatoid arthritis (378), supporting the use of this drug (alone or as adjunctive therapy) in rheumatic diseases. A small trial performed in early diffuse scleroderma generated promise, since four of the six patients who completed the trial (minocycline at 50 mg × 2/day for 1 month, increasing to 100 mg × 2/day for the following 11 months) had complete resolution of their skin disease (219). The anti-inflammatory action of tetracyclines seems related to a nonantibacterial mechanism: impairment of phagocyte functions is widely acknowledged, as is the inhibitory effect of these drugs on collagenase and gelatinase activity. These latter effects have also been suggested to play a role in the tetracycline-induced improvement in periodontal disease (120). Further interesting hypotheses include the potential antitumor activity of doxycycline linked to its inhibitory effect on metalloproteases (89). In addition, the anti-inflammatory action of tetracycline has been proposed to be of benefit to prevent endotoxic shock by blockade of LPS-induced TNF-α and IL-1 β secretion (340).
The use of rifampin in the treatment of rheumatoid arthritis was initially linked to anecdotal reports of improvements in rheumatoid arthritis in patients with coexisting tuberculosis who were being treated with rifampin for the infection. Controversial results have been reported on the potential benefit of rifampin in rheumatoid arthritis. Rifamycin, a rifampin derivative, displays antiarthritis activity in ankylosing spondylitis and juvenile pauciarticular or polyarticular rheumatoid arthritis (205).
Macrolides.
The anti-inflammatory activity of macrolides and its potential clinical relevance have been reviewed recently (198, 206, 207, 209). The question whether macrolides can attenuate inflammation was first raised over 20 years ago, when erythromycin and troleandomycin were shown to favorably affect the clinical status of patients with severe asthma. The recent interest in the anti-inflammatory potential of macrolides has been renewed with the Japanese experience of treating diffuse panbronchiolitis (DPB) patients with erythromycin A or its derivatives. DPB is characterized by chronic inflammation of the bronchioles, which progresses insidiously and results in respiratory failure caused by repeated episodes of respiratory infections due mainly to P. aeruginosa. The prognosis of this disease has been radically transformed by the empirical use of long-term low-dose erythromycin A since 1985 (184): the 10-year survival rate of 12.4% among P. aeruginosa-infected DPB patients in 1987 was extended to 94% by 1995. Only erythromycin A and its derivatives (including azithromycin) are effective; 16-member-ring macrolides are not. The nonantibiotic effect of these drugs seems to explain their therapeutic action. In particular, various inflammatory parameters such as neutrophil infiltration and IL-8, LTB4, and elastase levels in bronchoalveolar lavage fluid fall in parallel with the disease improvement during erythromycin A therapy. DPB is similar to cystic fibrosis in its clinical and bacteriological aspects, although it occurs in adults only and does not seem to have a genetic basis. Limited trials have been conducted in cystic fibrosis, but preliminary results with azithromycin argue for more clinical investigations (160). Other clinical developments of macrolides outside their antibacterial effects concern their potential benefit in cancer, an effect demonstrated in animal models (336) and patients with lung cancer (254). The mechanisms underlying the antitumoral and anti-inflammatory activity of macrolides are no doubt multiple: studies done ex vivo and in animal models have demonstrated a modulation of neutrophil functions and proinflammatory cytokine production (200). Structure-activity relationships are in keeping with the results obtained in vitro, since only erythromycin A derivatives display anti-inflammatory activity in vivo.
In addition to the direct anti-inflammatory activity of some macrolides, it has been suggested that these drugs could act indirectly by eliminating persistent pathogens, a possible source of chronic inflammation in the “three A” disease: atherosclerosis, asthma, and arthritis. A large number of studies have shown links between coronary heart disease and Chlamydia pneumoniae, Helicobacter pylori, or Mycoplasma, all of which are macrolide susceptible. This led to trials involving roxithromycin or azithromycin to prevent adverse cardiovascular events. However, in both studies (127, 128), the therapeutic benefit of these drugs could not be strictly ascribed to their antibacterial effect, and anti-inflammatory activity may also have been involved.
The use of macrolides in inflammatory diseases is a stimulating prospect for the future. No in vivo data are yet available for the ketolides, but some animal data suggest that these drugs could also have anti-inflammatory activity (81).
Prospects: fosfomycin and fusidic acid?
Fosfomycin affects various immunologic responses in vitro. In combination with steroids, it improves the clinical symptoms of severe bronchial asthma (266). Recently, the immunomodulatory activity of fosfomycin (and of its enantiomer, which lacks antimicrobial activity) was demonstrated in two animal models. (i) Fosfomycin and its enantiomer significantly increased the survival rate and reduced the levels of TNF-α, IL-1, and IL-6 in serum in a murine model of gut-derived P. aeruginosa sepsis (242). (ii) In addition, in mice injected with LPS, treatment with fosfomycin significantly lowered the peak levels of TNF-α and IL-1β in serum (243).
Fusidic acid downregulates cytokine production and possesses anti-inflammatory activity in vitro. In animal models, this drug protects mice from LPS- and staphylococcal enterotoxin B-induced death and suppresses the release of TNF-α and IFN-γ in vivo (280). Prophylactic administration of fusidin significantly increased survival in neonatal mice challenged with Salmonella enterica serovar Enteritidis LPS and decreased peak levels of TNF-α (110). The potential immunomodulatory effect of fusidic acid has also been demonstrated in a model of concanavalin A (ConA)-induced liver lesions (279). Prophylactic administration of fusidic acid protected mice from ConA-induced hepatitis and was accompanied by markedly diminished levels of IL-2, IFN-γ, and TNF-α, along with increased levels of IL-6, in plasma. Both T cells and macrophages are effectors in ConA-induced hepatic injury, but the precise mechanism underlying the effect of fusidic acid was not clarified. Lastly, an interesting opportunity for future anti-inflammatory strategies concerns the potential in vitro inhibitory activity of various substituted β-lactam derivatives on human leukocyte elastase (93, 130, 374).
DO WE NEED IMMUNOMODULATING ANTIBACTERIAL AGENTS?
The question whether immunomodulatory antibacterial agents have clinical advantages unambiguously implies that the administration during infectious diseases of antibacterial drugs primarily to destroy pathogens independently interferes with host defenses to reinforce their action or downmodulate an exaggerated response. This question also extends to the prophylactic use of antibacterial agents in clinical settings where there is a risk of infections. Theoretically, it does not apply to the use of antibacterial agents for their immunosuppressive potential in noninfectious diseases. Indeed, the potential danger of using antibacterial drugs outside their current indications (for example, in inflammatory diseases) with a theoretically lengthy administration schedule is the induction of microbial resistance. This phenomenon has not been described after more than 10 years of macrolide administration to DPB patients in Japan, but the risk cannot be ignored. The search for antibiotic derivatives devoid of antibacterial activity but retaining immunomodulatory potential is of major interest. This has been proposed with tetracycline derivatives (101) and sulfamide derivatives (274). Similarly, pure immunomodulators have been proposed based on the chemical structure of cefodizime (344). The use of the fosfomycin enantiomer may also be considered in the near future (242).
The theoretical advantages and disadvantages of immunomodulatory antibacterial agents should be considered in two clinical settings: (i) immunocompromised individuals (prophylactic or therapeutic use) and (ii) the risk of septic shock.
Immunocompromised Individuals
The term “immunocompromised” is frequently used to denote patients who have impaired host defenses and who are at risk of infections (87). The range of organisms which cause diseases in these patients extends beyond those which are pathogenic for normal hosts. Pathogen specificity for selected immune deficiencies is also recognized. Compromised patients are predisposed to acquiring resistant bacteria from the hospital environment due to lengthy or frequent hospitalization. The choice of antibiotic regimen will depend on the clinical setting (therapy or prevention, hospital or the community, and severe chronic or transient immunodepression) (181, 270, 276, 401). Early use of an empirical antibiotic regimen with the broadest possible antibacterial spectrum is recommended for prophylaxis in severe immunocompromised individuals. However, no regimen can adequately cover all potential pathogens, and combination therapy may sometimes increase the side effects. Attempts to enhance nonspecific host resistance have been made by using bacterial derivatives, synthetic immune modulators, and cytokines as adjunctive therapies in immune deficiencies (77, 171, 403).
In my opinion, the use of an antibiotic with “immune-enhancing” effects for immunocompromised patients, particularly when a state of transient immunodepression may favor superinfections, is beneficial only if the spectrum and potency are equal to those of comparable antibiotics. This means that in a class of antibacterial agents with comparable activity and pharmacokinetic profiles (the two basic determinants of choice), the drug with immune-enhancing activity should be preferred. (Note that drug costs are not considered in this review.) The advantages would be easier administration and better compliance. Restoration of phagocytic microbicidal function could lead to accelerated clinical improvement or better coverage of potential superinfections. Cefodizime, a broad-spectrum cephalosporin, has been proposed as such a biological response modifier antibiotic (195, 324, 410). It is even possible that quinolones may be active in immunocompromised individuals not only through their bactericidal potency but also through immunoenhancing effects (57, 85).
In conclusion, there do not seem to be theoretical or demonstrated disadvantages in terms of ethical or pharmacological considerations of using “immunostimulating” antibiotics.
Sepsis
Traditionally, sepsis was taken to be the consequence of a bacterial (even occult) infection and was treated empirically with antimicrobial agents. It is now recognized that bacteria trigger pathophysiological events, resulting in an uncontrolled host response. Other events such as burns, pancreatitis, and trauma may also initiate a systemic inflammatory response (284). The discovery of the role of cytokines in the pathophysiology of septic shock has led to major advances in the treatment of sepsis (141). However, other host factors, such as nitric oxide production, complement activation, and hematological disturbances (dependent on or independent of the cytokine cascade) are all involved in the complex pathophysiology of septic shock (reviewed in reference 141).
Despite vast amounts of research on immunomodulatory drugs for the treatment of sepsis and septic shock, no drugs have yet proven useful clinically. Animal models do not seem suitable for assessing new treatments (255), and our poor knowledge of the intricate pathophysiological events in the clinical setting limits therapeutic approaches. In this setting, do we still need immunomodulatory antibacterials? Ritts (324) suggested that such drugs could be important for “the practical and timely treatment of patients … who insidiously develop septic syndrome.” This implies that such drugs would be given early in patients who have not yet developed clinical signs of sepsis, i.e., when bacteria potentially causing septic shock are involved. Such antibiotics would have a downmodulating effect on various proinflammatory cytokines such as TNF-α and IL-1. When a complex host response has developed, it is unlikely that a single drug would be able to act at several levels (bacteria, endotoxin, cytokine production, coagulation cascade, etc.). Such a drug—the Holy Grail of bacterial sepsis management—is hard to develop. An antibiotic might, however, be designed to bind LPS, decrease inflammatory cytokine production, or inhibit the synthesis or various eicosanoids, etc., but the clinical value of such dual-action antibiotics is largely theoretical. Discouraging results have been obtained in clinical trials of host response modulators (reviewed in references 90 and 141).
Unless the specific disturbance from which the pathophysiological cascade of sepsis and septic shock originates is identified, there is no place yet for immunomodulatory antibiotics.
CONCLUSIONS: IMMUNOMODULATING EFFECTS OF ANTIBACTERIAL AGENTS—“NEVER SAY NEVER”
Some philosophical considerations mentioned here have been taken from references 17, 111, and 234. At the dawn of the third millennium, opposing pessimistic and optimistic visions of human evolution are being put forward and so are conflicting view of the promises of immunomodulation. Exploration of new boundaries—outer space and some parts of the Earth and its oceans—will no doubt lead to the discovery of new organisms and potential new pathogens. However, new possibilities for controlling infectious diseases are emerging, as illustrated by the renewal of interest in antibody-based therapies (50), new anti-infectives (43), the potential anti-infective activity of nonantibiotics (191), and the use of probiotics (80). The apocalypse forecast in the early 1990s (16a, 269) will at worst be postponed. Powerful new techniques will help us to unravel the workings of the immune system, together with its interactions with the environment, cell-cell communication, and intracellular language, resulting in the identification of more precise therapeutic targets (32, 62, 253). In the next century, we will probably be able to manipulate our immune system (our genes?) to our liking. One branch of science that has too long been neglected in the field of infectious diseases is chronobiology. Although the first observation of daily rhythms in the plant Kalanchoe blossfeldiana was made by the French astronomer J. J. de Mairan in 1729, our knowledge of the seasonal, circadian, and fertility cycles which contribute to governing the delicate balance between health and disease is still in its infancy. Phagocyte activity is also regulated by an internal clock (249). The benefits of timing have so far been demonstrated for some antitumor agents, but this may well also apply to anti-infective drugs, as suggested for the immunoenhancing effects of cefodizime (66, 414). Is there, then, a place for immunomodulatory antibacterials in the near future? Although I might appear somewhat pessimistic in my discussion of the potential relevance of what we know at present about the interactions between available antibiotics and phagocytes, I would like to end on an optimistic note. As I stressed in the Introduction, our knowledge of antibiotic-host interactions is very limited despite a vast amount of published data. The techniques, models, even fundamental knowledge required to approach this problem remain to be acquired. When these hurdles are overcome, I am confident that what now seem to be miracle drugs endowed with dual antibiotic and immunomodulatory activities will be developed for specific key pathologic processes, patients, and microorganisms.
The explosive development of knowledge in the late nineteenth century was promoted by exchanges among scientists coming from many and varied horizons (chemistry, zoology, and medicine). In the twentieth century, the sciences split into diversified and specialized branches, giving rise to many skillful but narrow-minded scientists. Fundamental (“pure”) research has been considered nobler, more intellectually rewarding, and more challenging than “applied” research. In the coming years, I hope we will see greater interdisciplinary cooperation and closer exchanges between basic and clinical scientists. As put by Pasteur himself, “No category of science exists to which one can give the name of applied Science. Science and the application of science are linked together as a fruit is to the tree that has borne it.”
To conclude, some words from doctors of Salerno (the most famous Western medical school of the Middle Ages) cited in reference 234:
And here I cease to write, but will not cease
To wish you live in health, and die in peace;
And ye our Physicke rules that friendly read,
God grant that Physicke you may never need.
ACKNOWLEDGMENTS
I apologize for not being able to cite all the remarkable publications on this subject, due to space limitation.
The skillful and patient secretarial assistance of C. Azzopardi is greatly appreciated.
REFERENCES
- 1.Abdelghaffar H, Vazifeh D, Labro M T. Comparison of various macrolides for stimulation of human neutrophil degranulation. J Antimicrob Chemother. 1996;38:81–93. doi: 10.1093/jac/38.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Abdelghaffar H, Vazifeh D, Labro M T. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase d-phosphatidate phosphohydrolase transductional pathway. l-Cladinose is involved both in alteration of neutrophil function and modulation of this transductional pathway. J Immunol. 1997;159:3995–4005. [PubMed] [Google Scholar]
- 3.Abdelghaffar H, Mtairag E M, Labro M T. Effects of dirithromycin and erythromycylamine on human neutrophil degranulation. Antimicrob Agents Chemother. 1994;38:1548–1554. doi: 10.1128/aac.38.7.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel G, Szöllösi J, Fachet J. Phagocytosis of fluorescent latex microbeads by peritoneal macrophages in different strains of mice: a flow cytometric study. Eur J Immunogenet. 1991;18:239–245. doi: 10.1111/j.1744-313x.1991.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 5.Adema C M, Harris R A, van Deutekom-Mulder E C. A comparative study of hemocytes from six different snails: morphology and functional aspects. J Invertebr Pathol. 1992;59:24–32. doi: 10.1016/0022-2011(92)90107-f. [DOI] [PubMed] [Google Scholar]
- 6.Ahlstedt S. The antibacterial effects of low concentrations of antibiotics and host defence factors: a review. J Antimicrob Chemother. 1981;18(Suppl. C):59–70. doi: 10.1093/jac/8.suppl_c.59. [DOI] [PubMed] [Google Scholar]
- 7.Aida Y, Pabst M J, Rademacher J M, Hatakayama T, Aono M. Effects of polymyxin B on superoxide anion release and priming in human polymorphonuclear leukocytes. J Leukoc Biol. 1990;47:283–291. doi: 10.1002/jlb.47.3.283. [DOI] [PubMed] [Google Scholar]
- 8.Allen P G, Davidowicz E. Phagocytosis in Acanthamoeba. I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990;145:508–513. doi: 10.1002/jcp.1041450317. [DOI] [PubMed] [Google Scholar]
- 9.Allen P G, Davidowicz E. Phagocytosis in Acanthamoeba. II. Soluble and insoluble mannose-rich ligands stimulate phosphoinositide metabolism. J Cell Physiol. 1990;145:514–521. doi: 10.1002/jcp.1041450318. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Microbiology. Celebrating a century of leadership in microbiology. ASM News. 1999;65:258–380. . American Society for Microbiology, Washington, D.C. [Google Scholar]
- 11.Anderson J M, Adkinson N F. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891–2899. [PubMed] [Google Scholar]
- 12.Anderson R. Enhancement by clofazimine and inhibition by dapsone of production of prostaglandin E2 by human polymorphonuclear leukocytes in vitro. Antimicrob Agents Chemother. 1985;27:257–262. doi: 10.1128/aac.27.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-derived superoxide generation and auto-oxidation. J Infect Dis. 1989;159:966–973. doi: 10.1093/infdis/159.5.966. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R, Theron A J, Feldman C. Membrane-stabilizing, anti-inflammatory interactions of macrolides with human neutrophils. Inflammation. 1996;20:693–705. doi: 10.1007/BF01488805. [DOI] [PubMed] [Google Scholar]
- 15.Anderson R, Smit M J. Clofazimine and B 669 inhibit the proliferative responses and Na+, K+-adenosine triphosphatase activity of human lymphocytes by a lysophospholipid-dependent mechanism. Biochem Pharmacol. 1993;46:2029–2038. doi: 10.1016/0006-2952(93)90645-d. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R A. The effect of antibiotics and of drug associations including antibiotics on the immunodefense system. In: Neumann M, editor. Useful and harmful interactions of antibiotics. Boca Raton, Fla: CRC Press, Inc.; 1985. pp. 185–203. [Google Scholar]
- 16a.Ash C, Perton M, editors. Drug resistance: the new apocalypse. Trends Microbiol Spec Issue. 1994;2:341–425. [Google Scholar]
- 17.Ashoff L. Das reticulo-endotheliale system. Ergeb Inn Med Kinderheilkd. 1924;26:1–119. [Google Scholar]
- 18.Azuma Y, Shinohara M, Murakawa N, Endo M, Ohura K. Possible interaction between new quinolones and immune function in macrophages. Gen Pharmacol. 1999;32:609–614. doi: 10.1016/s0306-3623(98)00243-2. [DOI] [PubMed] [Google Scholar]
- 19.Babior B M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- 20.Babior B M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 21.Baker H J. Effects of penicillin and streptomycin on staphylococci in cultures of mononuclear phagocytes. Ann N Y Acad Sci. 1954;58:1232–1245. doi: 10.1111/j.1749-6632.1954.tb45905.x. [DOI] [PubMed] [Google Scholar]
- 22.Bakker-Woudenberg I A J M. Delivery of antimicrobials to infected tissue macrophages. Adv Drug Delivery Rev. 1995;17:5–20. [Google Scholar]
- 23.Banerjee G, Medda S, Basu M K. A novel peptide-grafted liposomal delivery system targeted to macrophages. Antimicrob Agents Chemother. 1998;42:348–351. doi: 10.1128/aac.42.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barradell L B, Brogden R N. Cefodizime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1992;44:800–834. doi: 10.2165/00003495-199244050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Barrett J F. The immunomodulatory activities of antibacterials. Exp Opin Investig Drugs. 1995;4:551–557. [Google Scholar]
- 26.Barski G. Action de la streptomycine sur l'infection tuberculeuse en culture de tissus. Ann Inst Pasteur. 1948;74:1–11. [Google Scholar]
- 27.Beck G, Habicht G S. Primitive cytokines: harbingers of vertebrate defense. Immunol Today. 1991;12:180–183. doi: 10.1016/0167-5699(91)90049-Y. [DOI] [PubMed] [Google Scholar]
- 28.Bergeron Y, Deslauriers A-M, Ouellet N, Gauthier M-C, Bergeron M. Influence of cefodizime on pulmonary inflammatory response to heat-killed Klebsiella pneumoniae in mice. Antimicrob Agents Chemother. 1999;43:2291–2294. doi: 10.1128/aac.43.9.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Reduction by cefodizime of the pulmonary inflammatory response induced by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:2527–2533. doi: 10.1128/aac.42.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bermudez L E, Young L S. Activity of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988;32:1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermudez L E, Inderlied C, Young L S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991;35:2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard V, Bokoch G M, Diebold B A. Potential drug targets: small GTPases that regulate leukocyte function. Trends Pharmacol Sci. 1999;20:365–370. doi: 10.1016/s0165-6147(99)01367-x. [DOI] [PubMed] [Google Scholar]
- 33.Bjerknes R, Bassoe C F, Sjursen H, Laerum O D, Solberg C O. Flow cytometry for the study of phagocyte functions. Rev Infect Dis. 1989;11:16–33. doi: 10.1093/clinids/11.1.16. [DOI] [PubMed] [Google Scholar]
- 34.Blasi E, Puliti M, Pitzurra L, Barluzzi R, Mazzolla R, Adami C, Cox G W, Bistoni F. Comparative studies on functional and secretory properties of macrophage cell lines derived from different anatomical sites. FEMS Immunol Med Microbiol. 1994;9:207–216. doi: 10.1111/j.1574-695X.1994.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 35.Bokoch G M. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 36.Bor S. Clofazimine (lamprene) in the treatment of vitiligo. South Afr Med J. 1973;47:1451–1454. [PubMed] [Google Scholar]
- 37.Boxer L A, Stossel T P. Qualitative abnormalities of neutrophils. In: Williams W J, Beutler E, Erslev A J, Lichtman M A, editors. Hematology. 3rd ed. New York, N.Y: McGraw-Hill Book Co.; 1983. pp. 802–814. [Google Scholar]
- 38.Braga P C, Dal Sasso M, Mancini L, Sala M T. Influence of sub-inhibitory concentration of cefodizime on the phagocytosis, intracellular killing and oxidative burst of human polymorphonuclear leukocytes. Chemotherapy. 1999;45:166–172. doi: 10.1159/000007179. [DOI] [PubMed] [Google Scholar]
- 39.Braga P C, Dal Sasso M, Maci M, Bondioletti G, Fonti E, Reggio S. Penetration of brodimoprim into human neutrophils and intracellular activity. Antimicrob Agents Chemother. 1996;40:2392–2398. doi: 10.1128/aac.40.10.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briheim G, Dahlgren C. Influence of antibiotics on formyl-methionyl-leucyl-phenylalanine-induced leukocyte chemiluminescence. Antimicrob Agents Chemother. 1987;31:763–767. doi: 10.1128/aac.31.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 42.Brom C, Brom J, König W. Neomycin induces stimulatory and inhibitory effects on leukotriene generation, guanine triphosphatase activity, and actin polymerization within human neutrophils. Immunology. 1992;75:150–156. [PMC free article] [PubMed] [Google Scholar]
- 43.Bryskier A. Novelties in the field of anti-infectives in 1997. Clin Infect Dis. 1998;27:865–883. doi: 10.1086/514929. [DOI] [PubMed] [Google Scholar]
- 44.Burnet F M. The clonal selection theory of acquired immunity. Nashville, Tenn: Vanderbilt University Press; 1959. [Google Scholar]
- 45.Butts J D. Intracellular concentrations of antibacterial agents and related clinical implications. Clin Pharmacokinet. 1994;27:63–84. doi: 10.2165/00003088-199427010-00006. [DOI] [PubMed] [Google Scholar]
- 46.Buxton Hopkins D A. Too rapid destruction of gram-negative organisms. Lancet. 1977;i:603–604. doi: 10.1016/s0140-6736(77)91445-3. [DOI] [PubMed] [Google Scholar]
- 47.Byrani G K, Stokes D C, Fishman M, Shenep J L, Hildner W K, Rufus K, Bradham N, Costlow M E. Binding of polymyxin B to rat alveolar macrophages. J Infect Dis. 1990;162:939–942. doi: 10.1093/infdis/162.4.939. [DOI] [PubMed] [Google Scholar]
- 48.Cao C X, Silverstein S C, Neu H C, Steinberg T H. J774 macrophages secrete antibiotics via organic anion transporters. J Infect Dis. 1992;165:322–328. doi: 10.1093/infdis/165.2.322. [DOI] [PubMed] [Google Scholar]
- 49.Carlone N A, Cuffini A M, Tullio V, Cavallo G. Carbapenems and potential immunomodulating properties. In: Masihi K N, editor. Immunotherapy of infections. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 167–176. [Google Scholar]
- 50.Casadevall A. Antibody-based therapies as anti-infective agents. Exp Opin Investig Drugs. 1998;7:307–321. doi: 10.1517/13543784.7.3.307. [DOI] [PubMed] [Google Scholar]
- 51.Casadevall A, Pirofski L-A. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang H R, Vladoianu I-R, Pechère J-C. Effects of ampicillin, ceftriaxone, chloramphenicol, pefloxacin and trimethoprim-sulphamethoxazole on Salmonella typhi within human monocyte-derived macrophages. J Antimicrob Chemother. 1990;26:689–694. doi: 10.1093/jac/26.5.689. [DOI] [PubMed] [Google Scholar]
- 53.Chuaprapaisilp T, Piamphongsant T. Treatment of pustular psoriasis with clofazimine. Br J Dermatol. 1978;99:303–305. doi: 10.1111/j.1365-2133.1978.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 54.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 55.Cohen G B, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 56.Coleman M D, Tingle M D. Use of a metabolic inhibitor to reduce dapsone-dependent haematological cytotoxicity. Drug Dev Res. 1992;25:1–16. [Google Scholar]
- 57.Connolly M J, Snow M H, Ingham H R. Ciprofloxacin treatment of recurrent Salmonella typhimurium septicaemia in a patient with acquired immune deficiency syndrome. J Antimicrob Chemother. 1986;18:647–648. doi: 10.1093/jac/18.5.647. [DOI] [PubMed] [Google Scholar]
- 58.Couvreur P, Fatal E, Andremont A. Liposomes and nanoparticles in the treatment of intracellular bacterial infections. Pharm Res. 1991;8:1079–1086. doi: 10.1023/a:1015885814417. [DOI] [PubMed] [Google Scholar]
- 59.Coxon P, Summersgill J T, Ramirez J A, Miller R D. Signal transduction during Legionella pneumophila entry into human monocytes. Infect Immun. 1998;66:2905–2913. doi: 10.1128/iai.66.6.2905-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crellin J K. Antibiosis in the 19th century. In: Parascandola J, editor. The history of antibiotics. A symposium. Madison, Wis: American Institute of the History of Pharmacy; 1980. pp. 5–13. [Google Scholar]
- 61.Crockett-Torabi E. Selectins and mechanisms of signal transduction. J Leukoc Biol. 1998;63:1–14. [PubMed] [Google Scholar]
- 62.Cronstein B N, Weissmann G. Targets for antiinflammatory drugs. Annu Rev Pharmacol Toxicol. 1995;35:449–462. doi: 10.1146/annurev.pa.35.040195.002313. [DOI] [PubMed] [Google Scholar]
- 63.Cross W L, Csernok E, Helmchen U. Antineutrophil cytoplasmic antibodies, autoantigens, and systemic vasculitis. APMIS. 1995;103:81–97. doi: 10.1111/j.1699-0463.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 64.Cuffini A M, Tullio V, Alloco A, Paizis G, De Leo C, Carlone N A. Effect of rufloxacin upon non-specific immune defences: in-vitro, ex-vivo, and in-vivo results. J Antimicrob Chemother. 1994;34:545–553. doi: 10.1093/jac/34.4.545. [DOI] [PubMed] [Google Scholar]
- 65.Dallegri F, Dapino P, Arduino N, Bertoletto M, Ottonello L. Cefoperazone prevents the inactivation of a1-antitrypsin by activated neutrophils. Antimicrob Agents Chemother. 1999;43:2301–2303. doi: 10.1128/aac.43.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dammacco F, Halberg E, Carrandente F. Antimicrobial agents and chronoimmunomodulation: an emerging relationship. Chronobiologia. 1988;XV:25–39. [PubMed] [Google Scholar]
- 67.DeLeo F R, Quinn M T. Assembly of the phagocyte NADPH oxidase: molecular interactions of oxidase proteins. J Leukoc Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 68.Descamps-Latscha B, Witko-Sarsat V. Relations polynucléaires neutrophiles et monocytes-macrophages. Rev Fr Allergol. 1999;39:241–247. [Google Scholar]
- 69.Detrick B, Holmes J J. Cytokines in human immunology. In: Leffell M S, Donnenberg A D, Rose N R, editors. Handbook of human immunology. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 234–266. [Google Scholar]
- 70.de Weck A L. Pharmacologic and immunochemical mechanisms of drug hypersensitivity. Immunol Allergy Clin North Am. 1991;11:461–474. [Google Scholar]
- 71.Dhondt A, Vanholder R, Waterloos M-A, Glorieux G, De Smets R, Ringoir S. In vitro effects of cefodizime, imipenem/cilastatin and co-trimoxazole on dexamethasone and cyclosporin A depressed phagocytosis. Infection. 1998;26:120–125. doi: 10.1007/BF02767775. [DOI] [PubMed] [Google Scholar]
- 72.DiPietro L A. Wound healing: the role of the macrophages and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 73.Drabick J J, Bhattacharjee A K, Hoover D L, Siber G E, Morales V E, Young L D, Brown S L, Cross A S. Covalent polymyxin B conjugate with human immunoglobulin as an endotoxin reagent. Antimicrob Agents Chemother. 1998;42:583–588. doi: 10.1128/aac.42.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drevets D A. Dissemination of Listeria monocytogenes by infected phagocytes. Infect Immun. 1999;67:3512–3517. doi: 10.1128/iai.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drevets D A, Canono B P, Leenen P J M, Campbell P A. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drews J, editor. Immunopharmacology. Principles and perspectives. Berlin, Germany: Springer-Verlag KG; 1990. [Google Scholar]
- 77.Drews J. The experimental and clinical use of immune-modulating drug in the prophylaxis and treatment of infections. Infection. 1985;13(Suppl. 2):S241–S250. doi: 10.1007/BF01644438. [DOI] [PubMed] [Google Scholar]
- 78.Drusano G, Labro M T, Cars O, Mendes P, Shah P, Sorgel F, Weber W. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Microbiol Infect. 1998;4(Suppl. 2):27–41. [PubMed] [Google Scholar]
- 79.Duchesne E. Contribution à l'étude de la concurrence vitale chez les microorganismes. M.D. thesis. Lyon, France: Faculté de Lyon; 1897. [Google Scholar]
- 80.Dugas B, Mercenier A, Lenoir-Wijnkoop I, Arnaud C, Dugas N, Postaire E. Immunity and probiotics. Immunol Today. 1999;20:387–390. doi: 10.1016/s0167-5699(99)01448-6. [DOI] [PubMed] [Google Scholar]
- 81.Duong M, Simard M, Bergeron Y, Ouellet N, Côté-Richier M, Bergeron M G. Immunomodulatory effects of HMR 3004 on pulmonary inflammation caused by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:3309–3312. doi: 10.1128/aac.42.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edelman G M. The structure and function of antibodies. Sci Am. 1970;223:34–42. doi: 10.1038/scientificamerican0870-34. [DOI] [PubMed] [Google Scholar]
- 83.Eidelmann G, Shapira Y, Lishner M, Shalit I, Kletter Y, Fabian I. Effect of ciprofloxacin on hematologic parameters in breast cancer patients receiving chemotherapy. Eur J Haematol. 1995;55:202–204. doi: 10.1111/j.1600-0609.1995.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 83b.Elsevier Trends. Drug resistance: the new apocalypse. 1994. Trends Microbial. Spec. Issue. [Google Scholar]
- 84.Elstad M R, McIntyre T M, Prescott S M, Zimmerman G A. The interactions of leukocytes with platelets in blood coagulation. Curr Opin Hematol. 1995;2:47–54. doi: 10.1097/00062752-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 85.Esposito S, Gaeta G B, Galati D, Barber D. Successful treatment with ciprofloxacin of Salmonella typhimurium infection in an immunocompromised host. Infection. 1985;13:288. doi: 10.1007/BF01645442. [DOI] [PubMed] [Google Scholar]
- 86.Esterly N B, Koransky J S, Furey N L, Trevisan M. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308–1313. [PubMed] [Google Scholar]
- 86a.Featherstone C, Elliss C, editors. Phagocytosis. Trends Cell Biol Spec Issue. 1995;5:85–141. [Google Scholar]
- 87.Feld R. The compromised host. Eur J Cancer Clin Oncol. 1989;25(Suppl. 2):S1–S7. [PubMed] [Google Scholar]
- 88.Feldman C, Anderson R, Theron A J, Ramafi G, Cole P J, Wilson R. Roxithromycin, clarithromycin, and azithromycin attenuate the injurious effects of bioactive phospholipids on human respiratory epithelium in vitro. Inflammation. 1997;21:655–665. doi: 10.1023/a:1027342424205. [DOI] [PubMed] [Google Scholar]
- 89.Fife R S, Slede G W., Jr Effects of doxycline on in vitro growth, migration, and gelatinase activity of breast carcinoma cells. J Lab Clin Med. 1995;125:407–411. [PubMed] [Google Scholar]
- 90.Finch R G. Design of clinical trials in sepsis: problems and pitfalls. J Antimicrob Chemother. 1998;41(Suppl. A):95–102. doi: 10.1093/jac/41.suppl_1.95. [DOI] [PubMed] [Google Scholar]
- 91.Finch S C. Neutropenia. In: Williams W J, Beutler E, Erslev A J, Lichtman M A, editors. Hematology. 3rd ed. New York, N.Y: McGraw-Hill Book Co.; 1983. pp. 773–792. [Google Scholar]
- 92.Fink P C, Grunert J H. Endotoxemia in intensive care patients: a longitudinal study with the Limulus amebocyte lysate test. Klin Wochenschr. 1984;62:986–991. doi: 10.1007/BF01728429. [DOI] [PubMed] [Google Scholar]
- 93.Finke P E, Dahlgren M E, Weston H, Maycock A L, Doherty J B. Inhibition of human leukocyte elastase 5. Inhibition by 6-alkyl-substituted penem benzyl esters. Bioorg Med Chem Lett. 1993;3:2277–2282. [Google Scholar]
- 94.Fletcher M P, Seligmann B E. PMN heterogeneity: long-term stability of fluorescent membrane potential responses to the chemoattractant N-formyl-methionyl-leucyl-phenylalanine in healthy adults and correlation with respiratory burst activity. Blood. 1986;68:611–618. [PubMed] [Google Scholar]
- 95.Frank M O, Sullivan G W, Carper H T, Mandell G L. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1992;36:2584–2588. doi: 10.1128/aac.36.12.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fredenberg M, Malkinson F. Sulfone therapy in the treatment of leukocytoclastic vasculitis. J Am Acad Dermatol. 1987;16:772–778. doi: 10.1016/s0190-9622(87)70100-5. [DOI] [PubMed] [Google Scholar]
- 97.Fuji T, Kadota J-I, Morikawa T, Matsubara Y, Kawakami K, Iida K, Shirai R, Taniguchi H, Kaseda M, Kohno S. Inhibitory effect of erythromycin on interleukin-8 production by alpha, 25-dihydroxy vitamin D3-stimulated THP-1 cells. Antimicrob Agents Chemother. 1996;40:1548–1551. doi: 10.1128/aac.40.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabay J E. Microbicidal mechanisms of phagocytes. Curr Opin Immunol. 1988;1:36–40. doi: 10.1016/0952-7915(88)90048-9. [DOI] [PubMed] [Google Scholar]
- 99.Gabler W L. Fluxes and accumulation of tetracyclines by human blood cells. Res Commun Chem Pathol Pharmacol. 1991;72:39–51. [PubMed] [Google Scholar]
- 100.Gabler W L, Creamer H R. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52–58. doi: 10.1111/j.1600-0765.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 101.Gabler W L, Smith J, Tsukuda N. Comparison of doxycycline, and a chemically modified tetracycline inhibition of leukocyte functions. Res Commun Chem Pathol Pharmacol. 1992;78:151–160. [PubMed] [Google Scholar]
- 102.Gallin J I, Fauci A S, editors. Advances in host defence mechanisms. 1. Phagocytic cells. New York, N.Y: Raven Press; 1982. [Google Scholar]
- 103.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 104.Ganz T, Lehrer R I. Antimicrobial peptides of vertebrates. Curr Opin Immunol. 1998;10:41–44. doi: 10.1016/s0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 105.Garaci E, Goldstein A L, editors. Plenum Press, New York, N.Y. 1993. Combination therapies. 2. Biological response modifiers in the treatment of cancer and infectious diseases. [Google Scholar]
- 106.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 107.Geerdes-Fenge H F, Goetschi B, Rau M, Borner K, Koeppe P, Wettich K, Lode H. Comparative pharmacokinetics of dirithromycin and erythromycin in normal volunteers with special regard to accumulation in polymorphonuclear leukocytes and saliva. Eur J Clin Pharmacol. 1997;53:127–133. doi: 10.1007/s002280050350. [DOI] [PubMed] [Google Scholar]
- 108.Gemmell C G. Antibiotics and neutrophil function—potential immunomodulating activities. J Antimicrob Chemother. 1993;31(Suppl. A):23–33. doi: 10.1093/jac/31.suppl_b.23. [DOI] [PubMed] [Google Scholar]
- 109.Gemmell C G. Antibiotics and the expression of staphylococcal virulence. J Antimicrob Chemother. 1995;36:283–291. doi: 10.1093/jac/36.2.283. [DOI] [PubMed] [Google Scholar]
- 110.Genovese F, Mancuso G, Cazzola M, Cusumano V, Nicoletti F, Bendtzen K, Teti G. Improved survival and antagonistic effect of sodium fusidate on tumor necrosis factor alpha in a neonatal mouse model of endotoxin shock. Antimicrob Agents Chemother. 1996;40:1733–1735. doi: 10.1128/aac.40.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Georgiev V S, Yamaguchi H, editors. Immunomodulating drugs. Ann N Y Acad Sci. 1993;685:1–812. [Google Scholar]
- 112.Gilliland B C. Drug-induced autoimmune and hematologic disorders. Immunol Allergy Clin North Am. 1991;11:525–553. [Google Scholar]
- 113.Gillissen G. Side-effects of antibiotics on immune response parameters and their possible implications in antimicrobial chemotherapy. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1988;270:171–199. doi: 10.1016/s0176-6724(88)80154-8. [DOI] [PubMed] [Google Scholar]
- 114.Gladue R P, Bright G M, Isaacson R E, Newborg M F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at site of infection. Antimicrob Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glasser L, Fiederlein R L. Functional differentiation of normal human neutrophils. Blood. 1987;69:937–944. [PubMed] [Google Scholar]
- 116.Golde D W, Hocking W G. Monocyte and macrophage development. In: Gallin J I, Fauci A S, editors. Advances in host defence mechanisms. 1. Phagocytic cells. New York, N.Y: Raven Press; 1982. pp. 13–30. [Google Scholar]
- 117.Gollapudi S, Perumal V, Thadepalli H. Effects of rufloxacin on in-vitro proliferation and differentiation of human mononuclear cells. J Antimicrob Chemother. 1992;29:669–676. doi: 10.1093/jac/29.6.669. [DOI] [PubMed] [Google Scholar]
- 118.Gollapudi S, Thadepalli F, Kim C H, Gupta S. Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related (MRP) gene. Oncol Res. 1995;7:213–225. [PubMed] [Google Scholar]
- 119.Gollapudi S V S, Varjuvegula B, Gupta S, Pok M, Thadepalli H. Aryl-fluoroquinolone derivatives A-56 619 (difloxacin) and A-56 620 inhibit mitogen-induced human mononuclear cell proliferation. Antimicrob Agents Chemother. 1986;30:390–394. doi: 10.1128/aac.30.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Golub L, Ciancio S, Ramamurthy N, Leung M, McNamara T. Low-dose doxycline therapy. J Periodontal Res. 1990;25:321–330. doi: 10.1111/j.1600-0765.1990.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 121.Gomez-Cambronero J, Keire P. Phospholipase D: a novel major player in signal transduction. Cell Signal. 1998;10:387–397. doi: 10.1016/s0898-6568(97)00197-6. [DOI] [PubMed] [Google Scholar]
- 122.Gomez-Flores R, Tucker S D, Kansal R, Tamez-Guerra R, Mehta R T. Enhancement of antibacterial activity of clofazimine against Mycobacterium avium-Mycobacterium intracellulare complex infection induced by IFN-γ is mediated by TNF-α. J Antimicrob Chemother. 1997;39:189–197. doi: 10.1093/jac/39.2.189. [DOI] [PubMed] [Google Scholar]
- 123.Gordon M Y. Origin and development of neutrophils. In: Hellewell P G, Williams T J, editors. Immunopharmacology of neutrophils. London, United Kingdom: Academic Press, Ltd.; 1994. pp. 5–26. [Google Scholar]
- 124.Gordon S, Keshav S, Chung L P. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr Opin Immunol. 1988;1:26–35. doi: 10.1016/0952-7915(88)90047-7. [DOI] [PubMed] [Google Scholar]
- 125.Griem P, Wulferink M, Sachs B, Gonzalez J B, Gleichmann E. Allergic and autoimmune reactions to xenobiotics: how do they arise? Immunol Today. 1998;19:133–141. doi: 10.1016/s0167-5699(97)01219-x. [DOI] [PubMed] [Google Scholar]
- 126.Gunther M R, Mao J, Cohen M S. Oxidant-scavenging activities of ampicillin and sulbactam and their effects on neutrophil functions. Antimicrob Agents Chemother. 1993;37:950–956. doi: 10.1128/aac.37.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta S, Leathan E W, Carrington D, Mendall M A, Kaski J C, Camm A J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events and azithromycin in male survivors of myocardial infections. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 128.Gurfinkel E, Bozowich G, Daroca A, Mautner B. Randomized trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS pilot study. Lancet. 1997;350:404–407. doi: 10.1016/s0140-6736(97)07201-2. [DOI] [PubMed] [Google Scholar]
- 129.Hackstadt T. The diverse habitats of obligate intracellular parasites. Curr Opin Microbiol. 1998;1:82–87. doi: 10.1016/s1369-5274(98)80146-x. [DOI] [PubMed] [Google Scholar]
- 130.Hagmann W, O'Grady L A, Ashe B M, Dahlgren M E, Weston H, Maycock A L, Knight W B, Doherty J B. Inhibition of human leukocyte elastase by C2-substituted cephalosporin sulfones. Eur J Med Chem. 1989;24:599–604. [Google Scholar]
- 131.Hahn T, Barak Y, Liebovich E, Malach L, Dagan O, Rubinstein E. Ciprofloxacin inhibits human hematopoietic cell growth: synergism with tumor necrosis factor and interferon. Exp Hematol. 1991;19:157–160. [PubMed] [Google Scholar]
- 132.Halliwell B, Wasil M. Tetracyclines as antioxidants in rheumatoid arthritis: scavenging of hypochlorous acid. J Rheumatol. 1988;15:530. [PubMed] [Google Scholar]
- 133.Hampton M B, Kettle A J, Winterbourn C C. Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood. 1998;93:1464–1476. [PubMed] [Google Scholar]
- 134.Hand W L, King-Thompson N L. Membrane transport of clindamycin in alveolar macrophages. Antimicrob Agents Chemother. 1982;21:241–247. doi: 10.1128/aac.21.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hand W L, King-Thompson N L. Contrast between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986;29:135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hand W L, King-Thompson N L. The entry of antibiotics into human monocytes. J Antimicrob Chemother. 1989;23:681–689. doi: 10.1093/jac/23.5.681. [DOI] [PubMed] [Google Scholar]
- 137.Hand W L, King-Thompson N L, Johnson J D. Influence of bacterial-antibiotic interactions on subsequent antimicrobial activity of alveolar macrophages. J Infect Dis. 1984;149:271–276. doi: 10.1093/infdis/149.2.271. [DOI] [PubMed] [Google Scholar]
- 138.Hand W L, King-Thompson N L, Holman J W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987;31:1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haslett C, Savill I S, Meagher L. The neutrophil. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 140.Hauser W E, Remington J S. Effect of antibiotics on the immune response. Am J Med. 1982;72:711–716. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- 141.Hawkey P M, Saunders N J, Symonds J M, Wood M J, Daly P J, editors. Sepsis. J Antimicrob Chemother. 1998;41(Suppl. A):1–112. [Google Scholar]
- 142.Heidenreich S. Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sites. J Leukoc Biol. 1999;65:737–743. doi: 10.1002/jlb.65.6.737. [DOI] [PubMed] [Google Scholar]
- 143.Hellewell P G, Williams T J, editors. Immunopharmacology of neutrophils. London, United Kingdom: Academic Press, Ltd.; 1994. [Google Scholar]
- 144.Henneberg G. Die Wirkung der Penicillintherapie auf den gehalt and komplementbindenden Antikörpen bei der Gonorrheae. Arch Dermatol. 1948;187:350–359. [Google Scholar]
- 145.Hirsch F, Kroemer G. The immune system and immune modulation. In: Kresina T F, editor. Immune modulating agents. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 1–19. [Google Scholar]
- 146.Hirsch J G. Host resistance to infectious diseases. A centennial. In: Gallin J I, Fauci A S, editors. Advances in host defence mechanisms. 1. Phagocytic cells. New York, N.Y: Raven Press; 1982. pp. 1–12. [Google Scholar]
- 147.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 148.Hofsli E, Nissen-Meyer J. Reversal of drug resistance by erythromycin: erythromycin increases the accumulation of actinomycin D and doxorubicin in multidrug-resistant cells. Int J Cancer. 1989;44:149–154. doi: 10.1002/ijc.2910440126. [DOI] [PubMed] [Google Scholar]
- 149.Hofstra A H, Li-Muller S M A, Uetrecht J P. Metabolism of isoniazid by activated leukocytes. Possible role in drug-induced lupus. Drug Metab Dispos. 1992;20:205–210. [PubMed] [Google Scholar]
- 150.Hogasen A K M, Abrahamsen T G. Polymyxin B stimulates production of complement components and cytokines in human monocytes. Antimicrob Agents Chemother. 1995;39:529–532. doi: 10.1128/aac.39.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Höger P H, Vosbeck K, Seger R, Hitzig W H. Uptake, intracellular activity and influence of rifampin on normal function of polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1985;28:667–674. doi: 10.1128/aac.28.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holmes B, Quie P G, Windhorst D B, Pollara B, Good R A. Protection of phagocytized bacteria from the killing action of antibiotics. Nature (London) 1966;210:1131–1132. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- 153.Hosker H S R, Kelly C, Corris P A. Assessment of phagocytic function using chemiluminescence. Blood Rev. 1989;3:88–93. doi: 10.1016/0268-960x(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 154.Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumour necrosis factor-alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol Suppl. 1992;157:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 155.Imrie K R, Prince H M, Couture F, Brandwein J M, Keating A. Effect of antimicrobial prophylaxis on hematopoietic recovery following autologous bone marrow transplantation: ciprofloxacin versus cotrimoxazole. Bone Marrow Transplant. 1995;15:267–270. [PubMed] [Google Scholar]
- 156.Ingham E. Modulation of the proliferative response of murine thymocytes stimulated by IL-1 and enhancement of IL-1β secretion from monuclear phagocytes by tetracyclines. J Antimicrob Chemother. 1990;6:61–70. doi: 10.1093/jac/26.1.61. [DOI] [PubMed] [Google Scholar]
- 157.Ireton K, Cossart P. Interaction of invasive bacteria with host signaling pathways. Curr Opin Cell Biol. 1998;10:276–283. doi: 10.1016/s0955-0674(98)80151-8. [DOI] [PubMed] [Google Scholar]
- 158.Itoh K, Okubo K, Utiyama H, Hirano T, Yoshii J, Matsubara K. Expression profile of active genes in granulocytes. Blood. 1998;92:1432–1441. [PubMed] [Google Scholar]
- 159.Jacobs R F, Wilson C B. Activity of antibiotics in chronic granulomatous disease leukocytes. Pediatr Res. 1983;17:916–919. doi: 10.1203/00006450-198311000-00016. [DOI] [PubMed] [Google Scholar]
- 160.Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet. 1998;351:420. doi: 10.1016/S0140-6736(05)78360-4. [DOI] [PubMed] [Google Scholar]
- 161.Jaeschke H, Smith C W. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 162.Jagels M A, Hugli T E. Mechanisms and mediators of neutrophilic leukocytosis. Immunopharmacology. 1994;28:1–18. doi: 10.1016/0162-3109(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 163.Jimenez-Valera M, Sampedro A, Moreno E, Ruiz-Bravo A. Modification of immune response in mice by ciprofloxacin. Antimicrob Agents Chemother. 1995;39:150–154. doi: 10.1128/aac.39.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johansson A, Jesaitis A J, Lundqvist H, Magnusson K E, Sjolin C, Karlsson A, Dahlgren C. Different subcellular localization of cytochrome b and the dormant NADPH-oxidase in neutrophils and macrophages: effect on the production of reactive oxygen species during phagocytosis. Cell Immunol. 1995;161:61–71. doi: 10.1006/cimm.1995.1009. [DOI] [PubMed] [Google Scholar]
- 165.Johnson C M, Rhodes K H, Katzmann J A. Laboratory medicine. Neutrophil function tests. Mayo Clin Proc. 1984;591:431–434. doi: 10.1016/s0025-6196(12)61469-4. [DOI] [PubMed] [Google Scholar]
- 166.Kadota J-I, Iwashita T, Matsubara Y, Ishimatsu Y, Yoshinaga M, Abe K, Kohno S. Inhibitory effect of erythromycin on superoxide anion production by human neutrophils primed with granulocyte-colony stimulating factor. Antimicrob Agents Chemother. 1998;42:1866–1867. doi: 10.1128/aac.42.7.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kadurugamuwa J L, Beveridge T J. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycosides on their release. J Antimicrob Chemother. 1997;40:615–621. doi: 10.1093/jac/40.5.615. [DOI] [PubMed] [Google Scholar]
- 168.Kanerud L, Hafström I, Ringertz B. Effect of sulphasalazine and sulphapyridine on neutrophil superoxide production: role of cytosolic free calcium. Ann Rheum Dis. 1990;49:296–300. doi: 10.1136/ard.49.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Katz S I, Hertz K C, Crawford P S, Gazze L A, Franck M M, Lawley T J. Effect of sulfones on complement deposition in dermatitis herpetiformis and on complement-mediated guinea pig reactions. J Investig Dermatol. 1976;67:688–690. doi: 10.1111/1523-1747.ep12598565. [DOI] [PubMed] [Google Scholar]
- 170.Kaufmann S H E, Reddehase M J. Infection of phagocytic cells. Curr Opin Immunol. 1989;2:43–49. doi: 10.1016/0952-7915(89)90096-4. [DOI] [PubMed] [Google Scholar]
- 171.Kautar A, Oggiano N, Romagnoni G G, Giorgi P L. Effect of oral administration of bacterial extracts on the bactericidal capacity of polymorphonuclear leucocytes in children with recurrent respiratory infections. J Intern Med Res. 1991;19:451–456. doi: 10.1177/030006059101900604. [DOI] [PubMed] [Google Scholar]
- 172.Kawasaki S, Takizawa H, Ohtoshi T, Takeuchi N, Kohyama T, Nakamura H, Kasama T, Kobayashi K, Nakahara K, Morita Y, Yamamoto K. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob Agents Chemother. 1998;42:1499–1502. doi: 10.1128/aac.42.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kenny M T, Balistreri F J, Torney H L. β-Lactam antibiotic modulation of murine neutrophil cytokinesis. Immunopharmacol Immunitoxicol. 1992;14:797–811. doi: 10.3109/08923979209009236. [DOI] [PubMed] [Google Scholar]
- 174.Kerr J F R, Wyllie A H, Currie A H. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kettle A J, Gedye C A, Winterbourn C C. Superoxide is an antagonist of anti-inflammatory drugs that inhibit hypochlorous acid production by myeloperoxidase. Biochem Pharmacol. 1993;45:2003–2010. doi: 10.1016/0006-2952(93)90010-t. [DOI] [PubMed] [Google Scholar]
- 176.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Davies R J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and SiCAM1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 177.Khan A A, Slifer T R, Remington J S. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob Agents Chemother. 1998;42:1713–1717. doi: 10.1128/aac.42.7.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Khan A A, Slifer T R, Araujo F G, Remington J S. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–132. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 179.Kirst H A, Creemer L C, Paschal J W, Preston D A, Alborn W E, Jr, Counter F T, Amos J G, Clemens R L, Sullivan K A, Greene J M. Antimicrobial characterization and interrelationships of dirithromycin and epidirithromycin. Antimicrob Agents Chemother. 1995;39:1436–1441. doi: 10.1128/aac.39.7.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kishi K, Hirai K, Hiramatsu K, Yamasaki T, Nasu M. Clindamycin suppresses endotoxin released by ceftazidime-treated Escherichia coli O55:B5 and subsequent production of tumor necrosis factor alpha and interleukin-1β. Antimicrob Agents Chemother. 1999;43:616–622. doi: 10.1128/aac.43.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klastersky J. Infections in compromised hosts: considerations on preventions. Eur J Cancer Clin Oncol. 1989;25(Suppl. 2):S53–S61. [PubMed] [Google Scholar]
- 182.Kletter Y, Riklis I, Shalit I, Fabian I. Enhanced repopulation of murine hematopoietic organs in sublethally irradiated mice after treatment with ciprofloxacin. Blood. 1991;78:1685–1691. [PubMed] [Google Scholar]
- 183.Kletter Y, Singer A, Nagler A, Slavin S, Fabian I. Ciprofloxacin enhances hematopoiesis and the peritoneal neutrophil functions in lethally irradiated bone marrow-transplanted mice. Exp Hematol. 1994;22:360–365. [PubMed] [Google Scholar]
- 184.Kobayashi H. Airway biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. Am J Med. 1995;99:26S–30S. doi: 10.1016/s0002-9343(99)80282-4. [DOI] [PubMed] [Google Scholar]
- 185.Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987;31:1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 187.Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob Agents Chemother. 1999;43:907–911. doi: 10.1128/aac.43.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Krajewska M M, Anderson R. An in-vitro comparison of the effects of the prooxidative riminophenazines clofazimine and B 669 on neutrophil phospholipase A2 activity and superoxide generation. J Infect Dis. 1992;167:899–904. doi: 10.1093/infdis/167.4.899. [DOI] [PubMed] [Google Scholar]
- 189.Krajewska M M, Anderson R, O'Sullivan J F. Effects of clofazimine analogues and tumor necrosis factor-α individually and in combination on human polymorphonuclear leukocyte functions in vitro. Int J Immunopharmacol. 1993;15:99–111. doi: 10.1016/0192-0561(93)90086-e. [DOI] [PubMed] [Google Scholar]
- 190.Kresina T F, editor. Immune modulating agents. New York, N.Y: Marcel Dekker, Inc.; 1998. [Google Scholar]
- 191.Kristiansen J E, Amaral L. The potential management of resistant infections with non-antibiotics. J Antimicrob Chemother. 1997;40:319–327. doi: 10.1093/jac/40.3.319. [DOI] [PubMed] [Google Scholar]
- 192.Kropec A, Lemmen S W, Grundmann H J, Engels I, Daschner F. Synergy of simultaneous administration of ofloxacin and granulocyte-colony-stimulating factor in killing of Escherichia coli by human neutrophils. Infection. 1995;23:298–300. doi: 10.1007/BF01716290. [DOI] [PubMed] [Google Scholar]
- 193.Kubo S, Matsumoto T, Takahashi K, Haraoka M, Tanaka M, Sakumoto M, Sakamoto Y, Kumazawa J. Enhanced chemiluminescence response of polymorphonuclear leukocytes by new quinolone antimicrobials. Chemotherapy. 1994;40:333–336. doi: 10.1159/000239215. [DOI] [PubMed] [Google Scholar]
- 194.Reference deleted.
- 195.Labro M T. Cefodizime as a biological response modifier: a review of its in-vivo, ex-vivo and in-vitro immunomodulatory properties. J Antimicrob Chemother. 1990;26(Suppl. C):37–47. doi: 10.1093/jac/26.suppl_c.37. [DOI] [PubMed] [Google Scholar]
- 196.Labro M T. Immunological evaluation of cefodizime: a unique molecule among cephalosporins. Infection. 1992;28(Suppl. 1):S45–S47. doi: 10.1007/BF01709951. [DOI] [PubMed] [Google Scholar]
- 197.Labro M T. Effect of antimicrobial agents on polymorphonuclear neutrophil functions. In: Raoult D, editor. Antimicrobial agents and intracellular pathogens. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 87–135. [Google Scholar]
- 198.Labro M T. Effects of macrolides on host natural defenses. In: Bryskier A J, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology, and clinical uses. Paris, France: Arnette-Blackwell; 1993. pp. 389–408. [Google Scholar]
- 199.Labro M T. Immunomodulation by antibacterial agents. Is it clinically relevant? Drugs. 1993;45:319–328. doi: 10.2165/00003495-199345030-00001. [DOI] [PubMed] [Google Scholar]
- 200.Labro M T. Interaction of macrolides and quinolones with the host defence system. Eur Bull Drug Res. 1993;2(Suppl. 1):7–13. [Google Scholar]
- 201.Labro M T. Intraphagocytic penetration of macrolide antibiotics. In: Bryskier A J, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical uses. Paris, France: Arnette-Blackwell; 1993. pp. 379–381. [Google Scholar]
- 202.Labro M T. Interactions between antimicrobial agents and phagocytes: an overview. Int J Antimicrob Agents. 1993;3:73–87. doi: 10.1016/0924-8579(93)90017-y. [DOI] [PubMed] [Google Scholar]
- 203.Labro M T. Interactions entre les agents anti-infectieux et les phagocytes. Presse Med. 1995;24:992–998. [PubMed] [Google Scholar]
- 204.Labro M T. Resistance to and immunomodulation effect of cephalosporin antibiotics. Clin Drug Investig. 1995;9(Suppl. 3):31–44. [Google Scholar]
- 205.Labro M T. Immunomodulatory actions of antibacterial agents. Clin Immunother. 1996;6:454–464. [Google Scholar]
- 206.Labro M T. Effects of macrolides on leukocytes and inflammation. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 101–116. [Google Scholar]
- 207.Labro M T. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother. 1998;41(Suppl. B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 208.Labro M T. Antibacterial agents-phagocytes: new concepts for old in immunomodulation. Int J Antimicrob Agents. 1998;10:11–21. doi: 10.1016/s0924-8579(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 209.Labro M T. Immunological effects of macrolides. Curr Opin Infect Dis. 1998;11:681–688. doi: 10.1097/00001432-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 210.Reference deleted.
- 211.Reference deleted.
- 212.Labro M T, Babin-Chevaye C, Hakim J. Effects of cefotaxime and cefodizime on human granulocyte function in vitro. J Antimicrob Chemother. 1986;18:233–237. doi: 10.1093/jac/18.2.233. [DOI] [PubMed] [Google Scholar]
- 213.Labro M T, El Benna J. Interaction of antibiotics with the phagocyte oxidative burst. In: Faist E, Meakins J L, Schildberg F W, editors. Host defense dysfunction in trauma, shock and sepsis. Berlin, Germany: Springer-Verlag KG; 1993. pp. 953–964. [Google Scholar]
- 214.Labro M T, El Benna J, Abdelghaffar H. Modulation of human polymorphonuclear neutrophil function by macrolides: preliminary data concerning dirithromycin. J Antimicrob Chemother. 1993;31(Suppl. C):51–64. doi: 10.1093/jac/31.suppl_c.51. [DOI] [PubMed] [Google Scholar]
- 215.Labro M T, El Benna J, Charlier N, Hakim J. Cefdinir (CI-983), a new oral amino-2-thiazolyl cephalosporin, inhibits human neutrophil myeloperoxidase in the extracellular medium not the phagolysosome. J Immunol. 1994;152:2447–2455. [PubMed] [Google Scholar]
- 215a.La Penna D, Cellini L, De Gioia S, Mezzetti A, Ciofani G, Festi D, Cuccurullo F. Cephalosporins are scavengers of hypochlorous acid. Biochem Pharmacol. 1995;49:1249–1254. doi: 10.1016/0006-2952(95)00044-z. [DOI] [PubMed] [Google Scholar]
- 216.Laufen H, Wildfeuer A, Lach P. Kinetics of the uptake of antimicrobial agents by polymorphonuclear leucocytes. Arzneim Forsch. 1989;39:233–235. [PubMed] [Google Scholar]
- 217.Lauhio A, Leirisalo-Repo M, Lähdevita J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis with specific reference to Chlamydia arthritis. Arthritis Rheum. 1991;34:6–14. doi: 10.1002/art.1780340103. [DOI] [PubMed] [Google Scholar]
- 218.Reference deleted.
- 219.Le C H, Morales A, Trentham D E. Minocycline in early diffuse scleroderma. Lancet. 1998;352:1755–1756. doi: 10.1016/S0140-6736(05)79828-7. [DOI] [PubMed] [Google Scholar]
- 220.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 221.Lehr H-A, Arfors K-E. Mechanisms of tissue damage by leukocytes. Curr Opin Hematol. 1994;1:92–99. [PubMed] [Google Scholar]
- 222.Levert H, Gressier B, Moutard I, Brunet C, Dine T, Luyckx M, Cazin M, Cazin J C. Azithromycin impact on neutrophil oxidative metabolism depends on exposure time. Inflammation. 1998;22:191–201. doi: 10.1023/a:1022340107017. [DOI] [PubMed] [Google Scholar]
- 223.Lichtman M A. Classification of neutrophil disorders. In: Williams W J, Beutler E, Erslev A J, Lichtman M A, editors. Hematology. 3rd ed. New York, N.Y: McGraw-Hill Book Co.; 1983. pp. 770–772. [Google Scholar]
- 224.Lidbeck A, Nord C E. Reducing the effects of antibiotics on the bowel flora. Eur Bull Drug Res. 1993;2(Suppl. 1):27–32. [Google Scholar]
- 225.Limbert M, Bartlett R R, Dickneite G, Klesel N, Schorlemmer H U, Seibert G, Winkler I, Schrinner E. Cefodizime, an amino-thiazolyl cephalosporin. IV. Influence on the immune system. J Antibiot. 1984;XXXVIII:1719–1726. doi: 10.7164/antibiotics.37.1719. [DOI] [PubMed] [Google Scholar]
- 226.Liu J-P. Protein kinase C and its substrates. Mol Cell Endocrinol. 1996;116:1–29. doi: 10.1016/0303-7207(95)03706-3. [DOI] [PubMed] [Google Scholar]
- 227.Liu W S, Heckmann C A. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 228.Loo K C, Cario A C, Zhong F, Walters J D. Regulation of ciprofloxacin uptake in human promyelocytic leukemia cells and polymorphonuclear leukocytes. J Leukoc Biol. 1997;61:619–623. doi: 10.1002/jlb.61.5.619. [DOI] [PubMed] [Google Scholar]
- 229.Lorian V. Low concentrations of antibiotics. J Antimicrob Chemother. 1985;15(Suppl. A):15–26. doi: 10.1093/jac/15.suppl_a.15. [DOI] [PubMed] [Google Scholar]
- 230.Lowell C A, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukoc Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 231.Lührmann A, Tholke J, Behn I, Schumann J, Tiegs G, Hauschildt S. Immunomodulatory properties of the antibiotic novobiocin in human monocytes. Antimicrob Agents Chemother. 1998;42:1911–1916. doi: 10.1128/aac.42.8.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mackaness G B. The action of drugs on intracellular tubercle bacilli. J Pathol Bacteriol. 1952;64:429–446. doi: 10.1002/path.1700640302. [DOI] [PubMed] [Google Scholar]
- 233.Mackey J P, Barnes J. Clofazimine in the treatment of discoid lupus erythematosus. Br J Dermatol. 1974;91:93–96. doi: 10.1111/j.1365-2133.1974.tb06723.x. [DOI] [PubMed] [Google Scholar]
- 234.Magner L N, editor. A history of medicine. New York, N.Y: Marcel Dekker, Inc.; 1992. [Google Scholar]
- 235.Malech H L, Nauseef W M. Primary inherited defects in neutrophil function: etiology and treatment. Semin Hematol. 1997;34:279–290. [PubMed] [Google Scholar]
- 236.Mandell L A. Effects of antimicrobial and antineoplastic drugs on the phagocytic and microbicidal function of the polymorphonuclear leukocyte. Rev Infect Dis. 1982;4:683–697. doi: 10.1093/clinids/4.3.683. [DOI] [PubMed] [Google Scholar]
- 237.Mandeville J T H, Maxfield F R. Calcium and signal transduction in granulocytes. Curr Opin Hematol. 1996;3:63–70. doi: 10.1097/00062752-199603010-00010. [DOI] [PubMed] [Google Scholar]
- 238.Marcinkiewicz J. Neutrophil chloramines: missing links between innate and acquired immunity. Immunol Today. 1997;18:577–580. doi: 10.1016/s0167-5699(97)01161-4. [DOI] [PubMed] [Google Scholar]
- 239.Martin E, Ganz T, Lehrer R I. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 240.Matera G, Berlinghieri M C, Foca A. Meropenem: effects on human leukocyte function and interleukin release. Int J Antimicrob Agents. 1995;5:129–133. doi: 10.1016/0924-8579(94)00042-s. [DOI] [PubMed] [Google Scholar]
- 241.Mathy-Hartert M, Deby-Dupont G, Deby C, Jadoul L, Vandenberghe A, Lamy M. Cytotoxicity towards human endothelial cells, induced by neutrophil myeloperoxidase: protection by ceftazidime. Mediators Inflamm. 1995;4:437–443. doi: 10.1155/S0962935195000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Immunomodulating effect of fosfomycin on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 1997;41:308–313. doi: 10.1128/aac.41.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Fosfomycin alters lipopolysaccharide-induced inflammatory cytokine production in mice. Antimicrob Agents Chemother. 1999;43:697–698. doi: 10.1128/aac.43.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matsumoto T, Nagafuji T, Takahashi K, Kubo S, Sakumoto M, Mizunoe Y, Kumazawa J. Ofloxacin and fleroxacin enhance superoxide production in human polymorphonuclear leukocytes by increasing phosphorylation in the signal transduction pathway. Int J Antimicrob Agents. 1995;6:85–89. doi: 10.1016/0924-8579(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 245.Matusiewicz R, Rusiecka-Matusiewicz K. The ability of granulocytes from the bone marrow, vascular and tissue pools of healthy subjects to engulf latex particles and reduce nitroblue tetrazolium. Immunol Investig. 1987;16:313–318. doi: 10.3109/08820138709087086. [DOI] [PubMed] [Google Scholar]
- 246.Matzner Y. Acquired neutrophil dysfunction and diseases with an inflammatory component. Semin Hematol. 1997;34:291–302. [PubMed] [Google Scholar]
- 247.Maurin M, Raoult D. Optimum treatment of intracellular infection. Drugs. 1996;52:45–59. doi: 10.2165/00003495-199652010-00004. [DOI] [PubMed] [Google Scholar]
- 248.McDonald P J, Wetherall B L, Pruul H. Post antibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981;3:38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- 249.Melchart D, Martin P, Hallek M, Holzmann M, Jurcic X, Wagner H. Circadian variation of the phagocytic activity of polymorphonuclear leukocytes and of various other parameters in 13 healthy male adults. Chronobiol Int. 1992;9:35–45. doi: 10.3109/07420529209064514. [DOI] [PubMed] [Google Scholar]
- 250.Memin E, Panteix G, Revol A. Carrier-mediated system for pefloxacin uptake in human monocytes. J Antimicrob Chemother. 1997;40:263–268. doi: 10.1093/jac/40.2.263. [DOI] [PubMed] [Google Scholar]
- 251.Metcalf J A, Gallin J I, Nauseef W M, Root R K, editors. Laboratory manual of neutrophil function. New York, N.Y: Raven Press; 1986. [Google Scholar]
- 252.Metchnikoff E. The Nobel Lecture. Reprinted in Scand J Immunol. 1908;30:385–398. , 1989. [Google Scholar]
- 253.Miesel R, Sanocka D, Kurpisz M, Kröger H. Antiinflammatory effects of NADPH oxidase inhibitors. Inflammation. 1995;19:347–362. doi: 10.1007/BF01534392. [DOI] [PubMed] [Google Scholar]
- 254.Mikasa K, Sawaki M, Kita E, Hamada K, Teramoto S, Sakamoto M, Maeda K, Konishi M, Narita N. Significant survival benefit to patients with advanced non-small-cell lung cancer from treatment with clarithromycin. Chemotherapy. 1997;43:288–296. doi: 10.1159/000239580. [DOI] [PubMed] [Google Scholar]
- 255.Mischie H R. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J Antimicrob Chemother. 1998;41(Suppl. A):47–49. doi: 10.1093/jac/41.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 256.Mitsuyama T, Tanaka T, Hidaka K, Abe K, Hara N. Inhibition by erythromycin A of superoxide anion production by human polymorphonuclear leukocytes through the action of cyclic-AMP dependent protein kinase. Respiration. 1995;62:269–273. doi: 10.1159/000196461. [DOI] [PubMed] [Google Scholar]
- 257.Moestrup S K, Cui S, Horum H, Bregengard C, Bjorn S E, Norris K. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Investig. 1995;96:1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moffat J J, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Morikawa K, Oseko F, Morikawa S, Sawada M. Immunosuppressive activity of fosfomycin on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1993;37:2684–2687. doi: 10.1128/aac.37.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Morikawa K, Oseko F, Morikawa S. Immunomodulatory effect of fosfomycin on human B-lymphocyte function. Antimicrob Agents Chemother. 1993;37:270–275. doi: 10.1128/aac.37.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Morikawa K, Watabe H, Araake H, Morikawa S. Modulatory effects of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mosser D M, Karp C L. Receptor mediated subversion of macrophage cytokine production by intracellular pathogens. Curr Opin Immunol. 1999;11:406–411. doi: 10.1016/s0952-7915(99)80068-5. [DOI] [PubMed] [Google Scholar]
- 263.Moulder J W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mtairag E M, Abdelghaffar H, Douhet C, Labro M T. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob Agents Chemother. 1995;39:1574–1579. doi: 10.1128/aac.39.8.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mtairag E M, Abdelghaffar H, Labro M T. Investigation of dirithromycin and erythromycylamine uptake by human neutrophils in vitro. J Antimicrob Chemother. 1994;33:523–536. doi: 10.1093/jac/33.3.523. [DOI] [PubMed] [Google Scholar]
- 266.Mue T, Otsu H, Taniguchi Y, Sato K, Yamaguchi K, Ishihara T, Tamura G, Takishima N. Antibiotic therapy of broncheal asthma—a clinical effect of fosfomycin administration. Prog Med. 1983;3:1582–1588. . (In Japanese.) [Google Scholar]
- 267.Munoz J, Geister R. Inhibition of phagocytosis by aureomycin. Proc Soc Exp Biol Med. 1950;75:367–370. doi: 10.3181/00379727-75-18201. [DOI] [PubMed] [Google Scholar]
- 268.Muranushi N, Horie K, Masuda K, Hirano K. Characteristics of ceftibuten uptake into Caco-2 cells. Pharm Res. 1994;11:2761–2765. doi: 10.1023/a:1018919517839. [DOI] [PubMed] [Google Scholar]
- 269.Murray B E. New aspects of antimicrobial resistance and the resulting therapeutic dilemmas. J Infect Dis. 1991;163:1185–1194. [PubMed] [Google Scholar]
- 270.Murray J A, Warren R E, Wood M J, editors. Infection in the neutropenic patient. J Antimicrob Chemother. 1998;41(Suppl. D):1–105. [Google Scholar]
- 271.Nagafuji T, Matsumoto T, Takahashi K, Kubo S, Haraoka M, Tanaka M, Ogata N, Kumazawa J. Enhancement of superoxide production of polymorphonuclear neutrophils by ofloxacin and the effects of the inhibition of protein kinase C. Chemotherapy. 1993;39:70–76. doi: 10.1159/000238977. [DOI] [PubMed] [Google Scholar]
- 272.Nandan D, Lo R, Reiner N E. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-Fos and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect Immun. 1999;67:4055–4063. doi: 10.1128/iai.67.8.4055-4063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nau R, Zysk G, Schmidt H, Fischer F R, Stringaris A K, Stuertz K, Brück W. Trovafloxacin delays the antibiotic-induced inflammatory response in experimental pneumococcal meningitis. J Antimicrob Chemother. 1997;39:781–788. doi: 10.1093/jac/39.6.781. [DOI] [PubMed] [Google Scholar]
- 274.Neal T M, Winterbourn C C, Vissers M C M. Inhibition of neutrophil degranulation and superoxide production by sulfasalazine. Comparison with 5-aminosalicylic acid, sulfapyridine and olsalazine. Biochem Pharmacol. 1987;36:2765–2768. doi: 10.1016/0006-2952(87)90262-0. [DOI] [PubMed] [Google Scholar]
- 275.Neftel K A, Hauser S P, Muller M R. Inhibition of granulopoiesis in vivo and in vitro by b-lactam antibiotics. J Infect Dis. 1985;152:90–98. doi: 10.1093/infdis/152.1.90. [DOI] [PubMed] [Google Scholar]
- 276.Newland A C, Gaya H. Use of cephalosporins in the immunologically compromised patient. Drugs. 1987;34(Suppl. 2):202–215. doi: 10.2165/00003495-198700342-00015. [DOI] [PubMed] [Google Scholar]
- 277.Nichterlein T, Kretschmar M, Schadt A, Meyer A, Wildfeuer A, Laufen H, Hof H. Reduced intracellular activity of antibiotics against Listeria monocytogenes in multidrug resistant cells. Int J Antimicrob Agents. 1998;10:119–125. doi: 10.1016/s0924-8579(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 278.Nichterlein T, Kretschmar M, Siegsmund M, Hof H. Erythromycin is ineffective against Listeria monocytogenes in multidrug resistant cells. J Chemother. 1995;7:184–188. doi: 10.1179/joc.1995.7.3.184. [DOI] [PubMed] [Google Scholar]
- 279.Nicoletti F, Beltrami B, Raschi E, Di Marco R, Magro G, Grasso S, Bendtzen K, Fiorelli G, Meroni P L. Protection from concavalin A (Con A)-induced T-cell-dependent hepatic lesions and modulation of cytokine release in mice by sodium fusidate. Clin Exp Immunol. 1997;110:479–484. doi: 10.1046/j.1365-2249.1997.4091423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nicoletti F, Zaccone P, Di Marco R, Magno G, Grasso S, Morrone S, Santoni A, Tempera G, Meroni P L, Bendtzen K. Effects of sodium fusidate in animal models of insulin-dependent diabetes mellitus and septic shock. Immunology. 1995;85:645–650. [PMC free article] [PubMed] [Google Scholar]
- 281.Nielsen H. Antibiotics and human monocyte function. I. Chemotaxis. Acta Pathol Microbiol Immunol Scand. 1987;95:293–296. doi: 10.1111/j.1699-0463.1987.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 282.Nielsen H. Antibiotics and human monocyte function. II. Phagocytosis and oxidative metabolism. Acta Pathol Microbiol Immunol Scand. 1989;97:447–451. doi: 10.1111/j.1699-0463.1989.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 283.North R J, Berche P A, Newborg M F. Immunologic consequences of antibiotic-induced abridgement of bacterial infections: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981;127:342–346. [PubMed] [Google Scholar]
- 284.Nyström P O. The systemic inflammatory response syndrome: definition and aetiology. J Antimicrob Chemother. 1998;41(Suppl. A):1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 285.Oleske J M, Philipps I, editors. Clindamycin: bacterial virulence and host defence. J Antimicrob Chemother. 1983;12(Suppl. C):1–124. [Google Scholar]
- 286.Oleske J M, de la Cruz A, Ahdieh H, Sorvino D, La Braico J, Cooper R, Singh R, Lin R, Minnefor A. Effects of antibiotics on polymorphonuclear leukocyte chemiluminescence and chemotaxis. J Antimicrob Chemother. 1983;12(Suppl. C):35–38. doi: 10.1093/jac/12.suppl_c.35. [DOI] [PubMed] [Google Scholar]
- 287.Opdenakker G, Fibbe W E, Van Damme J. The molecular basis of leukocytosis. Immunol Today. 1998;19:182–189. doi: 10.1016/s0167-5699(97)01243-7. [DOI] [PubMed] [Google Scholar]
- 288.Ottonello L, Dallegri F, Dapino P, Pastorino G, Sacchetti C. Cytoprotection against neutrophil-delivered oxidant attack by antibiotics. Biochem Pharmacol. 1991;42:2317–2321. doi: 10.1016/0006-2952(91)90236-x. [DOI] [PubMed] [Google Scholar]
- 289.Ottonello L, Dapino P, Scirocco M C, Balbi A, Bevilacqua M, Dallegri F. Sulphonamides as anti-inflammatory agents: old drugs for new therapeutic strategies in neutrophilic inflammation. Clin Sci. 1995;88:331–336. doi: 10.1042/cs0880331. [DOI] [PubMed] [Google Scholar]
- 290.Ouadrhiri Y, Scorneaux B, Sibille Y, Tulkens P M. Mechanism of intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob Agents Chemother. 1999;43:1242–1251. doi: 10.1128/aac.43.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ozaki M, Komori K, Matsuda M, Yamaguchi R, Honmura T, Tomii Y, Nishimura I, Nishino T. Uptake and intracellular activity of NM-394, a new quinolone, in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1996;40:739–742. doi: 10.1128/aac.40.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pabst M J. Priming of neutrophils. In: Hellewell P G, Williams T J, editors. Immunopharmacology of neutrophils. London, United Kingdom: Academic Press, Ltd.; 1994. pp. 195–221. [Google Scholar]
- 293.Pancholi P, Persing D H. Molecular techniques applied to infectious diseases. In: Leffell M S, Donnenberg A D, Rose N R, editors. Handbook of human immunology. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 153–232. [Google Scholar]
- 294.Parascandola J, editor. The history of antibiotics. A symposium. Madison, Wis: American Institute of the History of Pharmacy; 1980. [Google Scholar]
- 295.Parker R F, Marsh H C. The action of penicillin on staphylococcus. J Bacteriol. 1946;51:181–186. doi: 10.1128/JB.51.2.181-186.1946. [DOI] [PubMed] [Google Scholar]
- 296.Pascual A. Uptake and intracellular activity of antimicrobial agents in phagocytic cells. Rev Med Microbiol. 1995;6:228–235. [Google Scholar]
- 297.Pascual A, Garcia I, Perea E J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leucocytes. Antimicrob Agents Chemother. 1989;33:653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Perry D K, Hand W L, Edmonson D E, Lambeth J D. Role of phospholipase D-derived diradyl glycerol in the activation of the human neutrophil respiratory burst oxidase—inhibition by phosphatidic phosphohydrolase inhibitors. J Immunol. 1992;149:2749–2758. [PubMed] [Google Scholar]
- 299.Perry S, Weinstein I M, Craddock C G, Jr, Laurence J J. The combined use of typhoid vaccine and P32 labeling to assess myelopoiesis. Blood. 1957;12:549–558. [PubMed] [Google Scholar]
- 300.Peters J H, Greseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 301.Philips M R, Pillinger M H, Stand R, Volker C, Rosenfeld M G, Weissmann G, Stock J B. Carboxymethylation of ras-related proteins during signal transduction in neutrophils. Science. 1993;259:977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- 302.Phillips W A, Croatto M, Hamilton J A. The effect of interleukin-4 on the macrophage respiratory burst is species dependent. Biochem Biophys Res Commun. 1992;182:727–732. doi: 10.1016/0006-291x(92)91792-o. [DOI] [PubMed] [Google Scholar]
- 303.Piamphongsant T, Ausawamongkonpan S. Bullous pemphigoid controlled by dapsone. Dermatologica. 1976;152:352–357. doi: 10.1159/000251283. [DOI] [PubMed] [Google Scholar]
- 304.Prins J M, van Deventer S H J, Kuijper E L, Speelman P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Chemother. 1994;38:1211–1218. doi: 10.1128/aac.38.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Prinsloo Y, van Rensburg C E J, van der Walt R, Anderson R. Augmentative inhibition of lymphocyte proliferation by cyclosporin A combined with the riminophenazine compounds clofazimine and B 669. Inflamm Res. 1995;44:379–385. doi: 10.1007/BF01797865. [DOI] [PubMed] [Google Scholar]
- 306.Pruul H, McDonald P J. Damage to bacteria by antibiotics in vitro and its relevance to antimicrobial chemotherapy: a historical perspective. J Antimicrob Chemother. 1982;21:695–698. doi: 10.1093/jac/21.6.695. [DOI] [PubMed] [Google Scholar]
- 307.Pruzanski W, Vadas P V. Should tetracyclines be used in arthritis? J Rheumatol. 1992;19:1495–1497. [PubMed] [Google Scholar]
- 308.Pullar T, Hunter J A, Capell H A. Which component of sulfasalazine is active in rheumatoid arthritis? Br Med J. 1985;290:1535–1538. doi: 10.1136/bmj.290.6481.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pulverer G, Ko H L, Beuth J. Effets immunomodulateurs des antibiotiques influençant les flores digestives. Pathol Biol. 1993;41:753–758. [PubMed] [Google Scholar]
- 310.Pulverer G, Ko H L, Roszkowski W, Beuth J, Yassin A, Jeljaszewicz J. Digestive tract microflora liberates low molecular weight peptides with immunotriggering activity. Zentbl Bakteriol. 1990;272:318–327. doi: 10.1016/s0934-8840(11)80034-4. [DOI] [PubMed] [Google Scholar]
- 311.Raghoebar M, Lindeyer E, Van den Berg W A, Van Ginneken On the mechanisms of association of the macrolide antibiotic erythromycin with isolated human polymorphonuclear leucocytes. Biochem Pharmacol. 1988;37:3221–3227. doi: 10.1016/0006-2952(88)90631-4. [DOI] [PubMed] [Google Scholar]
- 312.Rakita R M, Mychel B R, Rosen H. Inactivation of Escherichia coli penicillin-binding proteins by human neutrophils. Infect Immun. 1994;62:162–165. doi: 10.1128/iai.62.1.162-165.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rammelkamp C H, Keefer C S. Penicillin: its antibacterial effect in whole blood and serum for hemolytic streptococcus and Staphylococcus aureus. J Clin Investig. 1943;22:649–657. doi: 10.1172/JCI101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rappolee D A, Werb Z. Secretory products of phagocytes. Curr Opin Immunol. 1988;1:47–55. doi: 10.1016/0952-7915(88)90050-7. [DOI] [PubMed] [Google Scholar]
- 315.Reato G, Cuffini A M, Tullio V, Palarchio A I, Bonino A, Foa R, Carlone N A. Co-amoxiclav affects cytokine production by human polymorphonuclear cells. J Antimicrob Chemother. 1999;43:715–718. doi: 10.1093/jac/43.5.715. [DOI] [PubMed] [Google Scholar]
- 316.Reddy V M, O'Sullivan J F, Gangadharam P R J. Antimycobacterial activities of riminophenazines. J Antimicrob Chemother. 1999;43:615–623. doi: 10.1093/jac/43.5.615. [DOI] [PubMed] [Google Scholar]
- 317.Reilly J, Compagnon A, Tournier P, Bruit H D. Les accidents du traitement des fièvres typhoïdes par la chloromycétine. Ann Med (Paris) 1950;51:597–629. [PubMed] [Google Scholar]
- 318.Reiner N E. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994;15:374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 319.Repine J E, Beehler C J. Neutrophils and adult respiratory distress syndrome: two interlocking perspectives in 1991. Am Rev Respir Dis. 1991;144:251–252. doi: 10.1164/ajrccm/144.2.251. [DOI] [PubMed] [Google Scholar]
- 320.Ricevuti G, Mazzone A. The neutrophil revisited. Inflammation. 1989;13:475–482. [Google Scholar]
- 321.Riesbeck K, Forsgren A, Henriksson A, Bredberg A. Ciprofloxacin induces an immunomodulatory stress response in human T lymphocytes. Antimicrob Agents Chemother. 1998;42:1923–1930. doi: 10.1128/aac.42.8.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Riesbeck K, Forsgren A. Limited effects of temafloxacin compared with ciprofloxacin on T-lymphocyte functions. Antimicrob Agents Chemother. 1994;38:879–882. doi: 10.1128/aac.38.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rittig M G, Schroppel K, Seack K-H, Sander U, N'Diaye E-M, Maridonneau-Parini I, Solbach W, Bogdan C. Coiling phagocytosis of trypanosomatids and fungal cells. Infect Immun. 1998;66:4331–4339. doi: 10.1128/iai.66.9.4331-4339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ritts R E. Antibiotics as biological response modifiers. J Antimicrob Chemother. 1990;26(Suppl. C):31–36. doi: 10.1093/jac/26.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 325.Roberts D E, Curd J G. Sulfonamides as antiinflammatory agents in the treatment of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1590–1593. doi: 10.1002/art.1780331020. [DOI] [PubMed] [Google Scholar]
- 326.Rodriguez A B, Hernanz A, De la Fuente M. Effect of three β-lactam antibiotics on ascorbate content, phagocytic activity and superoxide anion production in human neutrophils. Cell Physiol Biochem. 1991;1:170–176. [Google Scholar]
- 327.Rodriguez A B, Barriga C, De la Fuente M. Mechanisms of action involved in the chemoattractant activity of three β-lactamic antibiotics upon human neutrophils. Biochem Pharmacol. 1991;41:931–936. doi: 10.1016/0006-2952(91)90198-e. [DOI] [PubMed] [Google Scholar]
- 328.Rodriguez A B, Barriga C, De la Fuente M. Stimulation of phagocytic processes and antibody-dependent cellular cytotoxicity of human neutrophils by cefmetazole. Microbiol Immunol. 1991;35:545–556. doi: 10.1111/j.1348-0421.1991.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 329.Rook G A W, Stanford J L. Give us this day our daily germs. Immunol Today. 1998;19:113–116. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 330.Root R K. Antibacterial properties of polymorphonuclear leukocytes and their interactions with antibiotics. In: Schlesinger D, editor. Microbiology—1981. Washington, D.C.: American Society for Microbiology; 1981. pp. 178–180. [Google Scholar]
- 331.Roszkowski K, Beuth J, Ko H L, Roszkowski W, Jeljaszewicz J, Pulverer G. Influence of antibiotics on anti-tumor activity in Balb/c-mice. Zentbl Bakteriol. 1992;276:280–287. doi: 10.1016/s0934-8840(11)80015-0. [DOI] [PubMed] [Google Scholar]
- 332.Rudin D E, Gao P X, Cao C X, Neu H C, Silverstein S C. Gemfibrozil enhances the listeriacidal effects of fluoroquinolone antibiotics in J774 macrophages. J Exp Med. 1992;176:1439–1447. doi: 10.1084/jem.176.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Saeki T, Ueda K, Tanigawara Y, Hori R, Kamano T. Human P-glycoprotein transports cyclosporin A and FK 506. J Biol Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- 334.Saffer L D, Petri W A., Jr Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. Infect Immun. 1991;59:4681–4683. doi: 10.1128/iai.59.12.4681-4683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sanchez I. Tetracycline treatment in rheumatoid arthritis and other rheumatic diseases. Bras Med. 1968;82:22–31. [Google Scholar]
- 336.Sassa K, Mizushima Y, Fujishita T, Oosaki R, Kobayashi M. Therapeutic effect of clarithromycin on a transplanted tumor in rats. Antimicrob Agents Chemother. 1999;43:67–72. doi: 10.1128/aac.43.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sato A, Kitazawa H, Hajakawa H, Chida K, Iwata M. Effect of 6-fluoro-8-methoxy quinolone (AM-1155) against chronic infection with Pseudomonas aeruginosa in a rat model. J Antimicrob Chemother. 1997;39:217–222. doi: 10.1093/jac/39.2.217. [DOI] [PubMed] [Google Scholar]
- 338.Sato K, Sato N, Shimizu H, Tsutiya T, Takahashi H, Kakizaki S, Takayama H, Takagi H, Mori M. Faropenem enhances superoxide anion production by human neutrophils in vitro. J Antimicrob Chemother. 1999;44:337–341. doi: 10.1093/jac/44.3.337. [DOI] [PubMed] [Google Scholar]
- 339.Savage J E, O'Sullivan J F, Zeis B M, Anderson R. Investigation of the structural properties of dihydrophenazines which contribute to their pro-oxidative interactions with human phagocytes. J Antimicrob Chemother. 1989;23:691–670. doi: 10.1093/jac/23.5.691. [DOI] [PubMed] [Google Scholar]
- 340.Schapira L, Soskolne W A, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxin shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Antimicrob Agents Chemother. 1996;40:825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Scheffer J, Köller J, Cullmann W, König W. Effects of cefaclor, cefetamet and RO 40-6890 on inflammatory response of human granulocytes. J Antimicrob Chemother. 1992;30:57–66. doi: 10.1093/jac/30.1.57. [DOI] [PubMed] [Google Scholar]
- 342.Schentag J J. Tissue-directed pharmacokinetics. Am J Med. 1991;91(Suppl. 3A):5S–11S. doi: 10.1016/0002-9343(91)90394-d. [DOI] [PubMed] [Google Scholar]
- 343.Schlaifer D, Cooper M R, Attal M, Sartor A O, Trepel J B, Laurent G, Myers C E. Myeloperoxidase: an enzyme involved in intrinsic vincristine resistance in human myeloblastic leukemia. Blood. 1993;81:482–489. [PubMed] [Google Scholar]
- 344.Schorlemmer H V, Dickneite G, Blumbach J, Dürckeimer W, Sedlacek H H. Immunomodulation by the new synthetic thiazole derivative tiprotimod. Second communication: immunopharmacological activity. Arzneim Forsch. 1989;39:1085–1089. [PubMed] [Google Scholar]
- 345.Schultz M J, Speelman P, Zaat S, van Deventer S J H, van der Poll T. Erythromycin inhibits tumor necrosis factor alpha and interleukin-6 production by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother. 1998;42:1605–1609. doi: 10.1128/aac.42.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Segal A W. Testing neutrophil function. Clin Immunol Allergy. 1985;5:491–512. [Google Scholar]
- 347.Segel E K, Ellegaard J, Borregaard N. Development of the phagocytic and cidal activity during maturation of myeloid cells: studies on cells from patients with chronic myelogenous leukemia. Br J Haematol. 1987;67:3–10. doi: 10.1111/j.1365-2141.1987.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 348.Selbie L A, Hill S J. G-protein-coupled receptor cross-talk: the fine tuning of multiple receptor-signalling pathways. Trends Pharmacol. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 349.Seligmann B E, Malech H L, Melnick D M, Gallin J I. An antibody binding to human neutrophils demonstrates antigenic heterogeneity detected early in myeloid maturation which correlates with functional heterogeneity of mature neutrophils. J Immunol. 1985;135:2647–2653. [PubMed] [Google Scholar]
- 350.Sewell W A C, Webster A D B. Infection in patients with congenital immune deficiencies. Curr Opin Infect Dis. 1998;11:419–423. doi: 10.1097/00001432-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 351.Shaffer J M, Kucera C J, Spink W W. The protection of intracellular Brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953;97:77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shenep J L, Barton R P, Morgan K A. Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1985;151:1012–1018. doi: 10.1093/infdis/151.6.1012. [DOI] [PubMed] [Google Scholar]
- 353.Shin W S, Min C K, Kim Y R, Hoo J H, Kang M W. In vitro effects of cefodizime on leucocyte functions and colony formation from granulocyte-monocyte progenitors. J Antimicrob Chemother. 1996;37:93–103. doi: 10.1093/jac/37.1.93. [DOI] [PubMed] [Google Scholar]
- 354.Shryock T R, Mortensen J E, Baumholtz M. The effects of macrolides on the expression of bacterial virulence mechanisms. J Antimicrob Chemother. 1998;41:505–512. doi: 10.1093/jac/41.5.505. [DOI] [PubMed] [Google Scholar]
- 355.Silverstein S C, Kabbash C. Penetration, retention, intracellular localization, and antimicrobial activity of antibiotics within phagocytes. Curr Opin Hematol. 1994;1:85–91. [PubMed] [Google Scholar]
- 356.Simchowitz L, Cragoe E J Jr. Na+-Ca2+ exchange in human neutrophils. Am J Physiol. 1988;254:C150–C164. doi: 10.1152/ajpcell.1988.254.1.C150. [DOI] [PubMed] [Google Scholar]
- 357.Simon R. New statistical designs for clinical trials of immunomodulating agents. In: Kresina T F, editor. Immune modulating agents. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 539–550. [Google Scholar]
- 358.Skinner M, Catheart E S, Mills J A, Pinals R S. Tetracyclines in the treatment of rheumatoid arthritis: a double-blind controlled study. Arthritis Rheum. 1971;14:727–732. doi: 10.1002/art.1780140607. [DOI] [PubMed] [Google Scholar]
- 359.Smith J A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 360.Sorensen O, Arnijots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, h-CAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 361.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–433. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 362.Stahl P D. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992;4:49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- 363.Starling E H. Principles of human physiology. 4th ed. Philadelphia, Pa: Lea & Febiger; 1926. [Google Scholar]
- 364.Steele R W. Clinical applications of chemiluminescence of granulocytes. Rev Infect Dis. 1991;13:918–925. doi: 10.1093/clinids/13.5.918. [DOI] [PubMed] [Google Scholar]
- 365.Steele R W, Steele C R, Pilkington N S Jr, Charlton R K. Functional capacity of marginated and bone marrow reserve granulocytes. Infect Immun. 1987;55:2359–2363. doi: 10.1128/iai.55.10.2359-2363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Suhonen J, Hartiala K, Viljanen M K. Tube phagocytosis, a novel way for neutrophils to phagocytize Borrelia burgdorferi. Infect Immun. 1998;66:3433–3435. doi: 10.1128/iai.66.7.3433-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sullivan T J. Allergic reactions to antimicrobial agents: a review of reactions to drugs not in the beta lactam class. J Allergy Clin Immunol. 1984;74:594–599. doi: 10.1016/0091-6749(84)90112-x. [DOI] [PubMed] [Google Scholar]
- 368.Suomalainen K, Sorsa T, Golub L M, Ramamurthy N, Lee H-M, Uitto V-J, Saari H, Konttinen Y T. Specificity of the anticollagenase action of tetracyclines: relevance to their anti-inflammatory potential. Antimicrob Agents Chemother. 1992;36:227–229. doi: 10.1128/aac.36.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Takizawa H, Desaki M, Ohtoshi T, Kikutami T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin-6 expression by human bronchial epithelial cells. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 370.Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–622. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- 371.Tateda K, Ishii Y, Matsumoto T, Furuya N, Nagashima M, Matsunaga T, Ohno A, Miyazaki S, Yamaguchi K. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob Agents Chemother. 1996;40:2271–2275. doi: 10.1128/aac.40.10.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Taüber M G, Schibl A M, Hackbarth C J, Larrick J W, Sande M A. Antibiotic therapy endotoxin concentration in cerebrospinal fluid and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987;156:456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]
- 373.Tentori L, Graziani G, Porcelli S A, Sugita M, Brenner M B, Madaio R, Bonmassar E, Giulani A, Aquino A. Rifampin increases cytokine-induced expression of the CD 1b molecule in human peripheral blood monocytes. Antimicrob Agents Chemother. 1998;42:550–554. doi: 10.1128/aac.42.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Thompson K R, Finke P E, Shah S K, Ashe B M, Dahlgren M E, Dellea P S, Fletcher D S, Hand K M, Maycock A L, Doherty J B. Inhibition of human leukocyte elastase 7. Inhibition by 6-substituted penicillin amides. Bioorganic Med Chem Lett. 1993;3:2289–2294. [Google Scholar]
- 375.Thrasher A J, Keep N H, Wientjes F, Segal A W. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 376.Thuong-Nguyen V, Kadunce D P, Hendrix J D, Gammon W R, Zone J J. Inhibition of neutrophil adherence to antibody by dapsone: a possible therapeutic mechanism of dapsone in the treatment of IgA dermatoses. J Investig Dermatol. 1993;100:349–355. doi: 10.1111/1523-1747.ep12471811. [DOI] [PubMed] [Google Scholar]
- 377.Thys J P, Husson M, Klastersky J. Inactivation of netilmicin and amikacin by intact or disrupted leukocytes. J Antimicrob Chemother. 1984;14:435–438. doi: 10.1093/jac/14.4.435. [DOI] [PubMed] [Google Scholar]
- 378.Tilley B C, Alarcon G S, Heyse S P, Trentham D E, Neuner R, Kaplan D A, Clegg D O, Leisen J C C, Buckley L, Cooper S M, Duncan H, Pillemer S R, Tuttleman M, Fowler S E. Minocycline in rheumatoid arthritis A 48-week, double-blind, placebo-controlled trial. Ann Intern Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- 379.Trautmann M, Zick R, Rukavina T, Cross A S, Marre R. Antibiotic-induced release of endotoxin: in-vitro comparison of meropenem and other antibiotics. J Antimicrob Chemother. 1998;41:163–169. doi: 10.1093/jac/41.2.163. [DOI] [PubMed] [Google Scholar]
- 380.Reference deleted.
- 381.Reference deleted.
- 382.Tufano M A, Cipollaro de l'Ero G, Ianniello R, Baroni A, Galdiero F. Antimicrobial agents induce monocytes to release IL-1α, IL-6, and TNF, and induce lymphocytes to release IL-4 and IFN-γ. Immunopharmacol Immunobiol. 1992;14:769–782. doi: 10.3109/08923979209009234. [DOI] [PubMed] [Google Scholar]
- 383.Uhlinger D J, Burnham D N, Mullins R E, Kalmar S R, Cutler C W, Arnold R R, Lambeth J D, Merrill A H Jr. Functional differences in human neutrophils isolated pre- and post-prandially. FEBS Lett. 1991;286:28–32. doi: 10.1016/0014-5793(91)80933-t. [DOI] [PubMed] [Google Scholar]
- 384.Umeki S. Ampicillin serves as an electron donor. Int J Biochem. 1990;22:1291–1293. doi: 10.1016/0020-711x(90)90311-p. [DOI] [PubMed] [Google Scholar]
- 385.Utili R, Adinolfi L E, Dilillo M, Tripodi M F, Marrone A, Ruggiero G. Activity of aminoglycosides against phagocytosed bacteria. J Antimicrob Chemother. 1991;28:897–904. doi: 10.1093/jac/28.6.897. [DOI] [PubMed] [Google Scholar]
- 386.Van den Broek P J. Antimicrobial drugs, microorganisms, and phagocytes. Rev Infect Dis. 1989;11:213–245. doi: 10.1093/clinids/11.2.213. [DOI] [PubMed] [Google Scholar]
- 387.Van den Broek P J. Activity of antibiotics against microorganisms ingested by mononuclear phagocytes. Eur J Clin Microbiol Infect Dis. 1991;10:114–118. doi: 10.1007/BF01964422. [DOI] [PubMed] [Google Scholar]
- 388.Van der Auwera P, Husson M. Influence of antibiotics on motility and adherence of human neutrophils studied in vitro. Drugs Exp Clin Res. 1989;XV:211–218. [PubMed] [Google Scholar]
- 389.Van der Auwera P, Husson M, Frühling J. Influence of various antibiotics on phagocytosis of Staphylococcus aureus by human polymorphonuclear leucocytes. J Antimicrob Chemother. 1987;20:399–404. doi: 10.1093/jac/20.3.399. [DOI] [PubMed] [Google Scholar]
- 390.Van der Auwera P, Matsumoto T, Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988;22:185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- 391.van Langevelde P, van Dissel J T, Ravensbergen E, Appelmelk B J, Schriver I A, Groenveld P H P. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Van Rensburg C E J, Jooné G, Anderson R. Interactions of the oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, pefloxacin and fleroxacin in the intraphagocytic eradication of Staphylococcus aureus. J Med Microbiol. 1990;32:15–17. doi: 10.1099/00222615-32-1-15. [DOI] [PubMed] [Google Scholar]
- 393.Van Rensburg C E J, Jooné G K, O'Sullivan J F, Anderson R. Antimicrobial activities of clofazimine and B 669 are mediated by lysophospholipids. Antimicrob Agents Chemother. 1992;36:2729–2735. doi: 10.1128/aac.36.12.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Van Zyl J M, Basson K, Kriegler A, Van der Walt B J. Mechanisms by which clofazimine and dapsone inhibit the myeloperoxidase system. Biochem Pharmacol. 1991;42:599–608. doi: 10.1016/0006-2952(91)90323-w. [DOI] [PubMed] [Google Scholar]
- 395.Van Zyl J M, Basson K, Uebel R A, Van der Walt B J. Isoniazid-mediated irreversible inhibition of the myeloperoxidase antimicrobial system of the human neutrophil and the effect of thyronines. Biochem Pharmacol. 1989;38:2363–2373. doi: 10.1016/0006-2952(89)90477-2. [DOI] [PubMed] [Google Scholar]
- 396.Vazifeh D, Bryskier A, Labro M T. Mechanism underlying levofloxacin uptake by human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1999;43:246–252. doi: 10.1128/aac.43.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Vazifeh D, Bryskier A, Labro M T. Effect of proinflammatory cytokines on the interplay between roxithromycin, HMR 3647, or HMR 3004 and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 2000;44:511–521. doi: 10.1128/aac.44.3.511-521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Vazifeh D, Preira A, Bryskier A, Labro M T. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Verbist L. Relevance of antibiotic susceptibility testing for clinical practice. Eur J Clin Microbiol Infect Dis Suppl. 1993;1:2–5. doi: 10.1007/BF02389869. [DOI] [PubMed] [Google Scholar]
- 401.Verhoef J. Transient immunodepression. J Antimicrob Chemother. 1990;26(Suppl. C):23–29. doi: 10.1093/jac/26.suppl_c.23. [DOI] [PubMed] [Google Scholar]
- 402.Vignon-Pennamen M-D, Wallach D. Neutrophilic diseases: a review of extracutaneous neutrophilic manifestations. Eur J Dermatol. 1995;5:449–455. [Google Scholar]
- 403.Vogels M T E, van der Meer J W M. Use of immunomodulators in non specific therapy of bacterial infections. Antimicrob Agents Chemother. 1992;36:1–5. doi: 10.1128/aac.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wadee A A, Anderson R, Rabson A R. Clofazimine reverses the inhibitory effect of Mycobacterium tuberculosis-derived factors on phagocyte intracellular killing mechanisms. J Antimicrob Chemother. 1988;21:65–74. doi: 10.1093/jac/21.1.65. [DOI] [PubMed] [Google Scholar]
- 405.Walker B A M, Seiler A J, Owens C A, Hagenlocker B E, Ward P A. Absence of FMLP receptors on rat macrophages. J Leukoc Biol. 1991;50:600–606. doi: 10.1002/jlb.50.6.600. [DOI] [PubMed] [Google Scholar]
- 406.Walters J D, Zhang F, Nakkula R J. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Weingart C L, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Weiss J W. Leukocyte-derived antimicrobial proteins. Curr Opin Hematol. 1994;1:78–84. [PubMed] [Google Scholar]
- 409.Welte K, Boxer L A. Severe chronic neutropenia: pathophysiology and therapy. Semin Hematol. 1997;34:267–278. [PubMed] [Google Scholar]
- 410.Wenisch C, Parshalk B, Hasenhundl M, Wiesinger E, Graninger W. Effects of cefodizime and ceftriaxone on phagocytic functions in patients with severe infections. Antimicrob Agents Chemother. 1995;39:672–676. doi: 10.1128/AAC.39.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Whiteside T L. Cellular immunology: monitoring of immune therapies. In: Leffell M S, Donnenberg A D, Rose N R, editors. Handbook of human immunology. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 343–380. [Google Scholar]
- 412.Williams M A, Solomkin J S. Integrin-mediated signaling in human neutrophil functioning. J Leukoc Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- 413.Witko-Sarsat V, Descamps-Latscha B. Neutrophil-derived oxidants and proteinases as immunomodulatory mediators in inflammation. Mediators Inflamm. 1994;3:257–273. doi: 10.1155/S0962935194000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wu J, Sanchez de la Pena S, Cornélissen G, Wetterberg I, Halberg E, Lakatera D, Bingham Ch, Harvey J, Bazin H, Zheng T, Leung B, Tran B. Chronosynergistic effect of lighting schedule-shift and cefodizime on plasmocytoma growth and host survival time. Chronobiologia. 1988;XV:105–128. [PubMed] [Google Scholar]
- 415.Yamanouchi T. Recherches expérimentales sur une méthode thérapeutique basée sur la stimulation des phagocytes. Annal Institut Pasteur. 1914;28:420–437. [Google Scholar]
- 416.Yancey R J, Sanchez M S, Ford C W. Activity of antibiotics against Staphylococcus aureus within polymorphonuclear neutrophils. Eur J Clin Microbiol Infect Dis. 1991;10:107–113. doi: 10.1007/BF01964421. [DOI] [PubMed] [Google Scholar]
- 417.Yourtee E L, Root R K. Antibiotic-neutrophil interactions in microbial killing. In: Gallin J I, Fauci A S, editors. Advances in host defence mechanisms. 1. Phagocytic cells. New York, N.Y: Raven Press; 1982. pp. 187–209. [Google Scholar]
- 418.Zak O, Tosch W, Sande M A. Correlation of antibacterial activities of antibiotics in vitro and in animal models of infection. J Antimicrob Chemother. 1985;15(Suppl. A):273–282. doi: 10.1093/jac/15.suppl_a.273. [DOI] [PubMed] [Google Scholar]
- 419.Zhang Y, Gripenberg-Lerche C, Söderström K-O, Towanen A, Toivanen P. Antibiotic prophylaxis and treatment of reactive arthritis. Arthritis Rheum. 1996;39:1238–1243. doi: 10.1002/art.1780390725. [DOI] [PubMed] [Google Scholar]
- 420.Ziegler-Heitbrock H W. The biology of the monocyte system. Eur J Cell Biol. 1989;49:1–12. [PubMed] [Google Scholar]