Key Points
Question
Is the efficacy of HD201, a trastuzumab biosimilar, comparable with referent trastuzumab in a neoadjuvant setting?
Findings
In this randomized clinical trial of 502 patients with ERBB2-positive early breast cancer, the total pathological complete response rates were 45% and 48.7% for HD201 and referent trastuzumab, respectively. The difference between the 2 groups was not significant at −3.8% (95% CI, −12.8% to 5.4%) and fell within the predefined equivalence margins.
Meaning
The results suggest that HD201 demonstrated equivalence to trastuzumab in terms of efficacy for the end point of total pathological complete response rates.
Abstract
Importance
The drug HD201 is a biosimilar candidate for breast cancer treatment as the reference trastuzumab.
Objective
To compare the efficacy of HD201 with referent trastuzumab.
Design, Setting, and Participants
This randomized clinical trial (TROIKA) included 502 women with ERBB2-positive early breast cancer treated with either HD201 or referent trastuzumab. It was conducted across 70 centers in 12 countries, including Western and Eastern Europe and Asian countries. Randomization was stratified by tumor hormone receptor status, clinical stage, and geographic region of recruitment. This analysis was conducted on February 12, 2021, after the completion of the adjuvant phase at a median of 31 months (IQR, 28-33 months) of follow-up.
Interventions
Patients with ERBB2-positive early breast cancer were randomly assigned to receive HD201 or referent trastuzumab in the neoadjuvant setting for 8 cycles, concurrently with 4 cycles of docetaxel, which was followed by 4 cycles of epirubicin and cyclophosphamide. Patients then underwent surgery, which was followed by treatment with 10 cycles of adjuvant HD201 or referent trastuzumab.
Main Outcome and Measures
The primary end point was the total pathological complete response (tpCR) assessed after neoadjuvant treatment. Equivalence was concluded if the 95% CI of the absolute difference in tpCR between arms in the per-protocol set was within the margin of more or less than 15%. Other objectives included the breast pathological complete response, overall response, event-free and overall survival, safety, pharmacokinetics, and immunogenicity.
Results
A total of 502 female patients (mean [range] age, 53 [26-82] years) were randomized to receive either HD201 or referent trastuzumab, and 474 (94.2%) were eligible for inclusion in the per-protocol set. The baseline characteristics were well balanced between the 2 arms; 195 tumors (38.8%) were hormone receptor-negative , and 213 patients (42.4%) had clinical stage III disease. The tpCR rates were 45% and 48.7% for HD201 and referent trastuzumab, respectively. The difference between the 2 groups was not significant at −3.8% (95% CI, −12.8% to 5.4%) and fell within the predefined equivalence margins. The ratio of the tpCR rates between the 2 arms was 0.92 (95% CI, 0.76 to 1.12). A total of 433 patients (86.1%) presented with 2232 treatment-emergent adverse events of special interest for trastuzumab during the entire treatment period, with 220 (88.0%) and 213 (84.5%) patients in the HD201 and referent trastuzumab groups, respectively.
Conclusions and Relevance
The results of this randomized clinical trial found that HD201 demonstrated equivalence to referent trastuzumab in terms of efficacy for the end point of tpCR, with a similar safety profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT03013504
This randomized clinical trial compare the efficacy of HD201 with referent trastuzumab for treating patients with ERBB2-positive breast cancer.
Introduction
The introduction of trastuzumab (Herceptin [Genentech]) into the therapeutic armamentarium for ERBB2-positive breast cancer has substantially changed the natural history of this disease. However, the high cost of referent trastuzumab therapy is a burden for health care systems, and in many countries, patients have only limited access because of the high costs. The need for less expensive alternatives to referent trastuzumab can potentially be met by introducing new biosimilars. In this context, HD201 was developed as a biosimilar to referent trastuzumab by Prestige BioPharma Ltd, Singapore. The development of a biosimilar medicine involves stepwise comparability exercises that are requested by regulatory agencies.1,2,3 Following this guidance, HD201 was extensively characterized and compared with referent trastuzumab, and it was demonstrated that HD201 is similar to referent trastuzumab. During development, to justify the changes in the manufacturing process in addition to the previously reported phase I study in healthy participants, a second phase I study that demonstrated pharmacokinetic equivalence was conducted.4,5 The aim of the present TROIKA study represents the ultimate step and is designed to show similar efficacy and safety between HD201 and referent trastuzumab in patients with ERBB2-positive early breast cancer. The primary objective was to demonstrate the equivalent clinical efficacy in terms of the total pathological complete response (tpCR) rate assessed at the time of surgery.
Methods
Study Design and Participants
TROIKA was a randomized, double-blind, parallel-group, equivalence, multicenter, international phase III clinical trial (NCT03013504) that was reported according to the Enhancing the Quality and Transparency Of Health Research guidelines (Supplement 1 and Supplement 2). It was conducted across 70 centers in 12 countries, including Western and Eastern Europe and Asian countries. The TROIKA trial was conducted according to the ethical principles of good clinical practice and was approved by ethics committees in each involved country. An independent monitoring committee monitored the study. Patients who had histologically confirmed, newly diagnosed, clinical stage II-III, operable ERBB2-positive early breast cancer were eligible to participate. Other inclusion criteria included written informed consent, age of 18 years or older, Eastern Cooperative Oncology Group performance status score of less than 2, left ventricular ejection fraction (LVEF) of 50% or greater by echocardiography or multigated acquisition scan results, and adequate bone marrow, liver, and kidney functions. A chest and abdominal computed tomography scan or magnetic resonance imaging and bone scan were performed before the patient received the study drug to rule out metastasis.
The patients were randomized to receive either HD201 or European Union–sourced referent trastuzumab intravenously every 3 weeks in a neoadjuvant setting for 8 cycles concurrently with 8 cycles of chemotherapy. The study drug was administered at a loading dose of 8 mg/kg and was followed by a maintenance dose of 6 mg/kg for subsequent cycles. Chemotherapy consisted of docetaxel, 75 mg/m2, for 4 cycles, which was followed by 4 cycles of epirubicin, 75 mg/m2, and cyclophosphamide, 500 mg/m2. Treatment-supportive medications, including antiemetics, were administered according to institutional practice. After the completion of neoadjuvant treatment, surgery was performed within 3 to 8 weeks of the last dose of HD201 or referent trastuzumab. Surgery included breast and axillary lymph node dissection. Sentinel node biopsy was allowed as a possible therapeutic option only in the case of clinical node-negative status at baseline. If the sentinel node biopsy results were negative, a full axillary dissection was not required after treatment with neoadjuvant therapy. After surgery, the patients entered the adjuvant period, during which HD201 or referent trastuzumab was administered as intravenous monotherapy with a loading dose of 8 mg/kg followed by a maintenance dose of 6 mg/kg, every 3 weeks, for 10 adjuvant cycles (cycles 9-18) as per the random assignment at baseline. If a patient missed a dose of HD201 or referent trastuzumab by more than 1 week, a reloading dose of HD201 or referent trastuzumab (8 mg/kg) was administered. During the adjuvant period, patients could receive hormonal therapy, radiotherapy, or both as determined by the investigator. All patients underwent an end-of-treatment visit 4 weeks (±2 days) after the last dose of study medication and were followed every 6 months thereafter for an additional 2 years or until death to collect data on cardiac safety and disease status.
Study End Points
The primary objective of this study was to demonstrate the equivalent clinical efficacy of HD201 to referent trastuzumab in terms of the tpCR rate assessed at the time of surgery by study site (local reading) in patients who were treated with HD201 in combination with chemotherapy compared with the tpCR for patients who were treated with referent trastuzumab in combination with chemotherapy. The tpCR rate was defined as the complete absence of cancer cells in the breast and in axillary lymph nodes (ypT0/is, ypN0) assessed in specimens obtained during surgery. The secondary objectives were to compare the breast pathological complete response (bpCR) rates at the time of surgery, overall response rates at the time of surgery, safety, immunogenicity, and trastuzumab concentration trough (Ctrough) values between the 2 arms. Secondary objectives comparing event-free survival and overall survival were planed at 2 years after the completion of treatment, as well as the relationship between pCR status and survival end points.
Study Populations
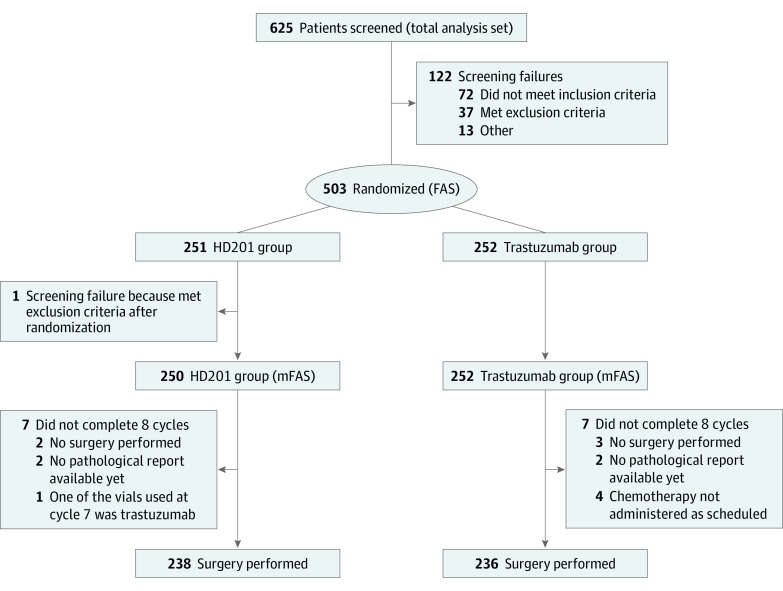
The modified full analysis set (mFAS) included all patients who received at least 1 dose of study medication (Figure). The per-protocol set (PPS) included all patients who received the study treatment (without a major protocol deviation affecting the primary efficacy assessment) and underwent surgery after the completion of neoadjuvant treatment or did not undergo surgery because of lack of efficacy. Protocol deviations were assessed during a preanalysis review meeting held before the database lock. The restricted PPS (rPPS) considered all patients in the PPS, excluding patients who had undergone a sentinel node biopsy procedure and had positive nodes at screening. The safety analysis set (SAF) included all patients in the mFAS, and the adjuvant safety set (aSAF) included all patients who received at least 1 dose of adjuvant study medication.
Figure. CONSORT Flow Diagram Describing the Patient Distribution.
FAS indicates full analysis set; mFAS, modified full analysis set.
The primary data set for the analysis was the PPS. Sensitivity analyses of locally and centrally assessed tpCR and bpCR were also performed based on the mFAS and rPPS. The analyses of the pharmacokinetic (PK) data were primarily performed for the PPS and supportively for the mFAS. The analysis of adverse events was performed for the SAF for the neoadjuvant period and entire treatment period and for the aSAF for the adjuvant treatment period.
Statistical Analysis
All participants who fulfilled the inclusion criteria were randomized using block of 8 in a ratio of 1:1 to either the HD201 arm or the referent trastuzumab arm. Participants were stratified by geographical region, clinical stage (stage II vs III), and estrogen and/or progesterone receptor status (positive vs negative).
The primary hypothesis was that the tpCR rate, based on the result of the local reading, would be equivalent in the 2 treatment groups. Equivalence was concluded if the 95% CI for the difference between the 2 proportions was completely contained within the interval (95% CI, -15.0 to 15.0). As a supportive analysis, the same analysis was performed based on the central reading results.
The acceptance interval was determined by reviewing historical tpCR proportions of chemotherapy and trastuzumab combinations. The difference in the tpCR rates between treatments among the 3 major studies assessing trastuzumab vs control in the neoadjuvant setting was calculated as 36%.6,7,8 The equivalence margin for the difference was derived as 15%, which corresponded to the preservation of 60% of the efficacy seen in these studies as requested by guidance from regulatory agencies.9 For the sample size calculation, the following assumptions were considered: the proportion of responders was 40% in both groups, and the equivalence margin was ±15%. To have 80% power for showing equivalence, data had to be available for 224 patients per treatment group. Considering that approximately 10% of patients could drop out or be nonevaluable, 500 patients were randomized.
For the primary end point, the analysis provided the frequency distribution and exact 95% Clopper-Pearson CI in each treatment group (for all patients and broken down for each stratification factor), difference in the tpCR rate between the 2 treatment groups and exact 95% Santner-Snell CI, and ratio of the tpCR rate in the 2 treatment groups and the 95% CI based on normal approximation. The effect of the stratification factors was evaluated using an adapted logistic model comprising a combination of logistic, binomial, and log-binomial regression (referred to as adapted logistic regression). The adapted logistic regression model compared the 2 treatment groups while adjusting for the geographic region of recruitment, stage, and estrogen receptor/progesterone receptor status. Based on this model, the difference in and ratio of tpCR rates were estimated along with their corresponding 95% CIs. The secondary efficacy variables were analyzed based on 2-sided tests at the 5% level of significance. No adjustments were made to nonprimary end points for multiplicity, and additional sensitivity analyses were performed in the mFAS and rPPS populations. The McNemar test was used in the accordance analysis to evaluate the agreement between central and local readings
The safety analysis was descriptive and performed for the SAF and aSAF. The statistical analysis was performed using SAS, version 9.2 (SAS Institute).
Pharmacokinetics
To demonstrate equivalence based on the Ctrough values at the predose doses of cycles 5 and 8, a 90% CI was determined for the difference between the 2 treatment groups, the limits of which were expressed as a percentage of the mean Ctrough in the reference group. Equivalence was concluded if this CI was completely contained within the predefined equivalence interval of ±20%. In addition, descriptive statistics of the Ctrough at cycles 5 and 8 and the frequency distribution of Ctrough values less than 20 μg/mL are provided.
Results
Patient, Tumor, and Treatment Characteristics
At the time of the report performed on February 12, 2021, at a median follow up of 31 months (IQR, 28-33 months), all patients had completed the treatment. The mFAS comprised 502 randomized women, among whom 250 (49.8%) were in the HD201 treatment group and 252 (50.2%) in the referent trastuzumab treatment group and were included between February 19 and September 21, 2018, across 70 centers in 12 countries. A total of 12 (4.8%) and 16 participants (6.3%) in the HD201 and referent trastuzumab groups, respectively, were excluded from the mFAS to constitute the PPS (Figure). The patient and tumor characteristics were well balanced between the 2 arms (Table 1).
Table 1. Patient, Tumor, and Treatment Characteristics of the mFAS and PPS Populations.
| Characteristic | Patients in the category, No. (%) | |||||
|---|---|---|---|---|---|---|
| mFAS | PPS | |||||
| HD201 | Referent trastuzumab | Total | HD201 | Referent trastuzumab | Total | |
| Patient population, No. | 250 | 252 | 502 | 238 | 236 | 474 |
| Age, median (range), y | 53.7 (26.3-79.4) | 54.2 (28.0-82.1) | 54.1 (26.3-82.1) | 53.7 (26.3-79.4) | 53.6 (28.0-77.9) | 53.6 (26.3-79.4) |
| Region | ||||||
| Asia | 20 (8.0) | 19 (7.5) | 39 (7.8) | 19 (8.0) | 17 (7.2) | 36 (7.6) |
| Western Europea | 14 (5.6) | 14 (5.6) | 28 (5.6) | 10 (4.2) | 8 (3.3) | 18 (3.8) |
| Eastern Europe | 216 (86.4) | 219 (86.9) | 435 (86.7) | 209 (87.8) | 211 (89.4) | 420 (88.6) |
| Weight, median (range), kg | 69 (43-125) | 71 (45-122) | 70 (43-125) | 69 (43-125) | 71 (45-122) | 70 (43-125) |
| ECOG performance status | ||||||
| 0 | 205 (82.0) | 191 (75.8) | 396 (78.9) | 196 (82.4) | 182 (77.1) | 378 (79.7) |
| 1 | 45 (18.0) | 61 (24.2) | 106 (21.1) | 42 (17.6) | 54 (22.9) | 96 (20.3) |
| Left ventricular ejection fraction at baseline, median (range), % | 65.5 (52-77) | 66.0 (51-80) | 66.0 (51-80) | 66.0 (52-77) | 66.0 (52-80) | 66.0 (52-80) |
| Clinical stage | ||||||
| I B | 0 | 1 (0.4) | 1 (0.2) | 0 | 1 (0.4) | 1 (0.2) |
| II A | 64 (25.6) | 70 (27.8) | 134 (26.7) | 61 (25.6) | 65 (27.5) | 126 (26.6) |
| II B | 80 (32.0) | 74 (29.4) | 154 (30.7) | 76 (31.9) | 68 (28.8) | 144 (30.4) |
| III A | 37 (14.8) | 29 (11.5) | 66 (13.1) | 34 (14.3) | 27 (11.4) | 61 (12.9) |
| III B | 50 (20.0) | 53 (21.0) | 103 (20.5) | 48 (20.2) | 51 (21.6) | 99 (20.9) |
| III C | 19 (7.6) | 25 (9.9) | 44 (8.8) | 19 (8.0) | 24 (10.2) | 43 (9.1) |
| Axillary clinical nodal status | ||||||
| N0 | 64 (25.6) | 71(28.2) | 135 (26.9) | 61 (25.6) | 66 (27.9) | 127 (26.8) |
| N1-N2-N3 | 186 (74.4) | 181 (71.8) | 367 (73.1) | 177 (74.4) | 170 (72.1) | 347 (73.2) |
| Hormone receptor status | ||||||
| ER+ or PR+ | 155 (62.0) | 152 (60.3) | 307 (61.2) | 152 (63.9) | 144 (61.0) | 296 (62.4) |
| ER−/PR− | 95 (38.0) | 100 (39.7) | 195 (38.8) | 86 (36.1) | 92 (39.0) | 178 (37.6) |
| ERBB2 status | ||||||
| Positive | 250 (100) | 252 (100) | 502 (100) | 238 (100) | 236 (100.0) | 474 (100) |
| Histological grade | ||||||
| I | 6 (3.3) | 6 (3.1) | 12 (3.2) | 6 (3.6) | 6 (3.4) | 12 (3.4) |
| II | 109 (60.6) | 105 (54.7) | 214 (57.5) | 101 (59.8) | 99 (55.3) | 200 (57.5) |
| III | 65 (36.1) | 81 (42.2) | 146 (39.2) | 62 (36.7) | 74 (41.3) | 136 (39.1) |
| Inflammatory breast | 32 (12.8) | 38 (15.1) | 70 (13.9) | 30 (12.6) | 36 (15.3) | 66 (13.9) |
| Sentinel node biopsy | 9 (3.6) | 7 (2.8) | 16 (3.2) | 8 (3.4) | 4 (1.7) | 12 (2.5) |
| Negative sentinel node | 2 (0.8) | 1 (0.4) | 3 (0.6) | 1 (0.4) | 1 (0.4) | 2 (0.4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; mFAS, modified full analysis set; PPS, per-protocol set; PR, progesterone receptor.
Central Europe was pooled with Western Europe for the analysis.
The relative (SD) dose intensities of the study drugs in the neoadjuvant period were 99.1% (4.9%) and 99.0% (4.8%) in the HD201 and referent trastuzumab treatment arms, respectively. A total of 218 (87.2%) and 218 (86.5%) participants in the HD201 and referent trastuzumab treatment arms, respectively, received 8 administrations of chemotherapy per the package insert. For the adjuvant period, the relative (SD) dose intensity was 100.5% (4.0%) in the HD201 group and 100.3% (5.2%) in the referent trastuzumab group.
Efficacy Results
The locally assessed tpCR rates in the HD201 and referent trastuzumab groups were 45.0% and 48.7%, respectively (Table 2). The difference between the 2 groups was −3.8% (95% CI, −12.8% to 5.4%), which fell within the predefined equivalence margin of −15% to 15%. The unadjusted ratio of the tpCR rate between the 2 groups was 0.92 (95% CI, 0.76 to 1.12). The estimated difference adjusted for stratification factors was −2.1% (95% CI, −10.9% to 6.7%). The estimated ratio of the tpCR rate between the 2 groups (HD201/referent trastuzumab) was 0.90 (95% CI, 0.75 to 1.07). The characteristics of the population assessed by central reading and discordances with local reading are reported in eTable 1 in Supplement 3. The centrally assessed tpCR rates in the HD201 and referent trastuzumab treatment groups in the PPS were 49.8% and 51.9%, respectively. The difference between the 2 groups was −2.1% (95% CI, −11.6% to 7.5%), which fell within the predefined equivalence margins, and the ratio was 0.96 (95% CI, 0.80 to 1.16). A sensitivity analysis of the mFAS and rPPS supported the findings for the difference and ratio criteria (eTable 2 in Supplement 3).
Table 2. Efficacy Results in the PPS Population.
| Group | HD201 | Referent trastuzumab | Difference, % (95% CI)a | Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |||
| PPS, No. | 238 | 236 | NA | NA | NA | NA |
| Locally assessed tpCR | ||||||
| Responders, No. (%) [95% CI] | 107 (45.0) [38.5 to 51.5] | 115 (48.7) [42.2 to 55.3] | −3.8 (−12.8 to 5.4) | −2.1 (−10.9 to 6.7) | 0.92 (0.76 to 1.12) | 0.90 (0.75 to 1.07) |
| Centrally assessed tpCR | ||||||
| Responders, No. (%) [95% CI] | 102 (49.8) [42.7 to 56.8] | 112 (51.9) [45.0 to 58.7] | −2.1 (−11.6 to 7.5) | −2.1 (−11.3 to 7.1) | 0.96 (0.80 to 1.16) | 0.95 (0.80 to 1.14) |
| Locally assessed bpCR | ||||||
| Responders, No. (%) [95% CI] | 126 (52.9) [46.4 to 59.4] | 127 (53.8) [47.2 to 60.3] | −0.9 (−10.0 to 8.2) | −0.4 (−9.2 to 8.5) | 0.98 (0.83 to 1.16) | 0.95 (0.81 to 1.12) |
| Centrally assessed bpCR | ||||||
| Responders, No. (%) [95% CI] | 129 (56.3) [49.6 to 62.9] | 123 (53.9) [47.2 to 60.5] | 2.4 (−7.0 to 11.6) | 2.8 (−6.2 to 11.7) | 1.04 (0.89 to 1.23) | 1.02 (0.87 to 1.19) |
| Overall response at the end of neoadjuvant treatment | ||||||
| Responders, No. (%) | 217 (91.2) | 210 (89.0) | 2.2 (−6.7 to 11.3) | 1.5 (−3.9 to 6.9) | 1.03 (0.97 to 1.09) | 1.02 (0.96 to 1.08) |
Abbreviations: bpCR, breast pathological complete response; NA, not applicable; PPS, per-protocol set; tpCR, total pathological complete response.
Primary end point. The confidence interval was contained between the prespecified margins of equivalence (−15% to 15%). All confidence intervals were contained between the prespecified margins defining equivalence for the primary end point.
Adjusted for stratification factors. The effect of the stratification factors was evaluated using an adapted logistic model comprising a combination of logistic, binomial, and log-binomial regression (referred to as adapted logistic regression).
The results regarding bpCR, overall response, and response based on mammography, ultrasonography, or clinical tumor evaluations supported the comparable efficacy between HD201 and referent trastuzumab (Table 2; eTable 2 in Supplement 3). Survival events were limited at the time of this analysis, and only 8 events counting for event-free survival and 6 for overall survival were reported.
PK Results
The mean (SD) trough levels in the PPS population were 42.7 (20.5) μg/mL and 43.6 (27.7) μg/mL at the onset of cycle 5 and 53.8 (21.5) μg/mL and 53.7 (24.2) μg/mL at the onset of cycle 8 for the HD201 and referent trastuzumab groups, respectively. Expressed as a percentage of the referent trastuzumab group, the mean difference between the treatment groups was −2.0% (95% CI, −10.5% to 6.5%) for cycle 5 and 0.3% (95% CI, −6.2% to 6.8%) for cycle 8. At the onset of cycles 5 and 8, the 90% CI for the mean relative difference was contained within the interval (90% CI, −20% to 20%), implying the bioequivalence of HD201 to referent trastuzumab for Ctrough. The proportions of patients with Ctrough levels of less than 20 μg/mL were 6.4% (15 of 233) and 6.8% (16 of 234) at the onset of cycle 5 and 2.6% (6 of 235) and 2.6% (6 of 230) at the onset of cycle 8 in the HD201 and referent trastuzumab treatment arms, respectively.
Safety Results
Safety was reported for the neoadjuvant, adjuvant, and overall periods (Table 3). For the entire treatment period, treatment-emergent adverse events (TEAEs) were reported for 250 (100%) and 247 (98.0%) participants and serious TEAEs for 24 (9.6%) and 17 (6.7%) participants in the HD201 and referent trastuzumab treatment arms, respectively. Any TEAEs that led to the discontinuation of the study treatment were reported for 16 participants (6.4%) in the HD201 group and 12 (4.8%) in the referent trastuzumab group.
Table 3. Safety Results for the Neoadjuvant, Adjuvant, and Overall Periods.
| Characteristic | Participants with TEAEs, No. (%)a | |||||
|---|---|---|---|---|---|---|
| Neoadjuvant period (SAF) | Adjuvant period (aSAF) | Entire period (SAF) | ||||
| HD201 | Referent trastuzumab | HD201 | Referent trastuzumab | HD201 | Referent trastuzumab | |
| Participants in the analysis set, No. | 250 | 252 | 238 | 242 | 250 | 252 |
| TEAE | ||||||
| Any | 246 (98.4) | 243 (96.4) | 164 (68.9) | 167 (69.0) | 250 (100) | 247 (98.0) |
| Related to study treatment | 85 (34.0) | 90 (35.7) | 74 (31.1) | 72 (29.8) | 127 (50.8) | 136 (54.0) |
| Related to chemotherapy | 243 (97.2) | 238 (94.4) | NA | NA | NA | NA |
| ≥Grade 3 | 76 (30.4) | 65 (25.8) | 15 (6.3) | 8 (3.3) | 86 (34.4) | 70 (27.8) |
| Serious | ||||||
| TEAE | 16 (6.4) | 12 (4.8) | 8 (3.4) | 6 (2.5) | 24 (9.6) | 17 (6.7) |
| Related to study treatment | 0 | 1 (0.4) | 1 (0.4) | 2 (0.8) | 1 (0.4) | 3 (1.2) |
| Related to chemotherapy | 11 (4.4) | 7 (2.8) | NA | NA | NA | NA |
| TEAE leading to discontinuation | 6 (2.4) | 3 (1.2) | 11 (4.6) | 9 (3.7) | 16 (6.4) | 12 (4.8) |
| TEAE of special interest | 204 (81.6) | 197 (78.2) | 117 (49.2) | 110 (45.5) | 220 (88.0) | 213 (84.5) |
| Cardiotoxic effects | 65 (26.0) | 60 (23.8) | 53 (22.3) | 50 (20.7) | 92 (36.8) | 87 (34.5) |
| Infusion site reactions and hypersensitivity | 118 (47.2) | 116 (46.0) | 35 (14.7) | 27 (11.2) | 127 (50.8) | 121 (48.0) |
| Hematotoxic effects | 134 (53.6) | 124 (49.2) | 51 (21.4) | 47 (19.4) | 144 (57.6) | 142 (56.3) |
| Pulmonary disorders | 16 (6.4) | 14 (5.6) | 15 (6.3) | 12 (5.0) | 29 (11.6) | 23 (9.1) |
| Infections | 47 (18.8) | 47 (18.7) | 22 (9.2) | 27 (11.2) | 61 (24.4) | 64 (25.4) |
| TEAEs by PT reported ≥10% | ||||||
| Alopecia | 202 (80.8) | 200 (79.4) | NA | NA | 202 (80.8) | 200 (79.4) |
| Asthenia | 65 (26.0) | 59 (23.4) | 15 (6.3) | 17 (7.0) | 68 (27.2) | 66 (26.2) |
| Fatigue | 59 (23.6) | 60 (23.8) | 11 (4.6) | 10 (4.1) | 62 (24.8) | 67 (26.6) |
| Nausea | 86 (34.4) | 93 (36.9) | 2 (0.8) | 1 (0.4) | 86 (34.4) | 93 (36.9) |
| Diarrhea | 45 (18.0) | 42 (16.7) | 5 (2.1) | 4 (1.7) | 47 (18.8) | 45 (17.9) |
| Neutropenia | 69 (27.6) | 72 (28.6) | 15 (6.3) | 14 (5.8) | 77 (30.8) | 78 (31.0) |
| Anemia | 68 (27.2) | 56 (22.2) | 19 (8.0) | 14 (5.8) | 72 (28.8) | 61 (24.2) |
| Leukopenia | 36 (14.4) | 38 (15.1) | 19 (8.0) | 21 (8.7) | 44 (17.6) | 51 (20.2) |
| Aminotransferase levels increased | ||||||
| Alanine | 28 (11.2) | 34 (13.5) | 12 (5.0) | 13 (5.4) | 35 (14.0) | 41 (16.3) |
| Aspartate | 25 (10.0) | 24 (9.5) | 19 (8.0) | 16 (6.6) | 37 (14.8) | 31 (12.3) |
| Arthralgia | 28 (11.2) | 23 (9.1) | 14 (5.9) | 11 (4.5) | 40 (16.0) | 31 (12.3) |
| Procedural pain | 31 (12.4) | 29 (11.5) | 5 (2.1) | 16 (6.6) | 36 (14.4) | 44 (17.5) |
Abbreviations: aSAF, adjuvant safety set; NA, not applicable; PT, preferred term; SAF, safety set; TEAE, treatment-emergent adverse event.
Percentage calculated based on number for each treatment group.
Four cardiovascular-related deaths occurred in the HD201 group, among which 3 were considered unrelated and 1 was considered unlikely related. One patient was reported to have experienced a myocardial infarction on day 7, 1 patient experienced cardiorespiratory arrest on day 122, 1 sudden death was reported on day 36, and 1 patient experienced a myocardial infarction on day 310. All patients had a reported history of controlled cardiovascular disease. Two deaths related to disease progression were reported during the treatment phase of the study in the HD201 group.
A total of 433 participants presented with 2232 TEAEs of special interest for trastuzumab (cardiotoxic effects, infusion site reactions, hypersensitivity, hematotoxic effects, pulmonary disorders, and infections) during the entire treatment period among 220 (88.0%) and 213 (84.5%) participants in the HD201 and referent trastuzumab groups, respectively. In the HD201 treatment group, 9 participants (3.7%) developed an LVEF value of less than 50% during the entire treatment period as well as 3 participants (1.2%) in the referent trastuzumab treatment group. In the HD201 and referent trastuzumab groups, 7 (2.9%) and 6 (2.4%) patients became positive for antitrastuzumab antibodies after baseline. No neutralizing antibody was identified.
Discussion
The 2017 patent expiration of intravenous trastuzumab across Europe stimulated the development of numerous trastuzumab biosimilar agents. The TROIKA study represents an important step on the path of registration for routine use for HD201.
In such a study, the first parameters to consider are the population studied and the end point criterion used to claim equivalence. In patients with breast cancer who were treated with trastuzumab, assessment in the neoadjuvant setting based on pCR has appeared to be the most robust approach.10 The relationship between pCR status and survival end points has been extensively debated during the past decade, and regulatory agencies answered by registering several compounds on this early criterion of activity. The neoadjuvant setting includes a homogeneous population with fewer uncertainty factors and allows the use of a well-established efficacy assessment criterion related to the survival outcome.11 Definitively, the neoadjuvant setting is considered the new era for development in ERBB2-positive breast cancer.12 Three (CT-P6, SB3, and ABP980) of the 4 available biosimilars of trastuzumab have based their demonstration of equivalent efficacy on such a design.13,14,15 The TROIKA trial included similar inclusion and exclusion criteria and statistical plans as previously reported studies in the same setting.
Another consideration is the robustness of the statistical demonstration for equivalence. The recommended approach by regulatory authorities for deriving equivalence margins relies on the preservation of 50% to 60% of the reference treatment effect estimated in a meta-analysis with major studies.9 The limited variations in the estimated effect of referent trastuzumab led to slightly variable margins of equivalence between all studies assessing trastuzumab biosimilars in this setting. The variation in the criterion of judgment between tpCR and bpCR could be debated and was also a point of variation between these studies.13,14,15,16
In TROIKA, the primary efficacy analysis was aimed at demonstrating equivalence in the tpCR rate between the 2 treatment groups. In the PPS, the difference was −3.8% (95% CI, −12.8% to 5.4%), which was contained within the predefined equivalence margin. The extensive sensitivity analysis supported the robustness of the findings, including the centrally assessed tpCR rates, unadjusted ratio of tpCR, and the difference and ratio of tpCR adjusted for stratification factors, as well as the duplication of these analyses in the rPPS and mFAS populations. In addition, secondary efficacy variables, including the locally and centrally assessed bpCR rates, as well as the overall response rate, were comparable between the 2 arms.
For the entire treatment period, the TEAEs were as expected for this population and class of drug. There were 6 deaths reported during the study, all in the HD201 group, and they were considered unrelated or unlikely related to the study treatment. Overall, the safety profiles seemed comparable between both arms. The decrease of LVEF to less than 50% was contained in the expected ranges for both groups. The decrease of LVEF after the completion of treatment with trastuzumab is rare, and no change is expected with longer follow up. Nevertheless, the assessments within 2 years after the completion of treatment with trastuzumab include a capture of cardiac events and monitoring of the LEVF every 6 months.
The clinical demonstration of PK equivalence in healthy participants between HD201 and referent trastuzumab sourced from the US and European Union was established.5 Additional PK assessment for patients who are receiving iterative cycles of trastuzumab is required and was part of the TROIKA study.3 Assessment of Ctrough at cycles 5 and/or 8 established equivalence, which is relevant because a Ctrough value higher than the threshold of biological activity is the key PK parameter for consideration.17
Limitations
Currently, limitations might be underlined regarding the interpretation of the present trial. To claim a final conclusion in addressing the similarity of HD201 with referent trastuzumab, we have to consider the overall comparative exercise, including preclinical assessment, the PK study in healthy participants, and the present results from TROIKA trial. In this ultimate trial, the confirmation of the relationship between survival outcomes and pCR early criteria of efficacy will be reassuring and should be reported when a longer follow-up will allow reliable estimation.
Conclusions
In this randomized clinical trial, HD201 was comparable with referent trastuzumab in preclinical development and preclinical and clinical pharmacokinetics studies. This phase 3 study demonstrated the equivalence between HD201 and referent trastuzumab in terms of efficacy for the tpCR end point and safety. These results support the application submitted for registration of HD201 as a biosimilar of trastuzumab.
Trial protocol
Statistical analysis plan
eTable 1. Concordance analysis of locally versus centrally assessed breast and total pathological complete response (bpCR and tpCR) in the PPS population
eTable 2. Efficacy results in the mFAS and rPPS populations
Data sharing statement
References
- 1.US European Medecines Agencies . Scientific considerations in demonstrating biosimilarity to a reference product, Guidance for Industry. Accessed April 26, 2021. https://www.fda.gov/downloads/drugs/guidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf
- 2.European Medecines Agency . Guideline on similar biological products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Accessed April 26, 2021. https://www.ema.europa.eu/docs/en_GB/document_library/Scientif_guideline/2015/01/WC500180219.pdf
- 3.European Medecines Agency, Committee for Medicinal Products for Human Use . Guideline on similar biological medicinal products containing monoclonal antibodies—nonclinical and clinical issues. Accessed April 26, 2021. https://www.ema.europa.eu/docs/en_GB/document_library/Scientif_guideline/2012/06/WC500128686.pdf
- 4.Pivot X, Deslypere JP, Park LS, Kim MJ, Lee W, Lee J. A randomized phase I study comparing the pharmacokinetics of HD201, a trastuzumab biosimilar, with European Union-sourced Herceptin. Clin Ther. 2018;40(3):396-405.e4. doi: 10.1016/j.clinthera.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Demarchi M, Coliat P, Shii Hii JC, et al. TROIKA-1: a double-blind, randomized, parallel group study aimed to demonstrate the equivalent pharmacokinetic profile of HD201, a potential biosimilar candidate to trastuzumab, versus EU-Herceptin® and US-Herceptin® in healthy male subjects. Pharmacol Res Perspect. 2021;9(4):839-849. doi: 10.1002/prp2.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377-384. doi: 10.1016/S0140-6736(09)61964-4 [DOI] [PubMed] [Google Scholar]
- 7.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676-3685. doi: 10.1200/JCO.2005.07.032 [DOI] [PubMed] [Google Scholar]
- 8.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024-2031. doi: 10.1200/JCO.2009.23.8451 [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration; US Department of Health and Human Services . Guidance for industry non-inferiority clinical trials. Accessed April 26, 2021. https://www.fda.gov/downloads/Drugs/.../Guidances/UCM201240.pdf
- 10.Pivot X, Petit T. Can we establish a hierarchy among trastuzumab biosimilar candidates? Br J Cancer. 2018;119(3):263-265. doi: 10.1038/s41416-018-0171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 12.Pivot X, Cox DG. A new era for treatment development in HER2-positive breast cancer. Lancet Oncol. 2018;19(2):160-162. doi: 10.1016/S1470-2045(18)30002-0 [DOI] [PubMed] [Google Scholar]
- 13.Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18(7):917-928. doi: 10.1016/S1470-2045(17)30434-5 [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-998. doi: 10.1016/S1470-2045(18)30241-9 [DOI] [PubMed] [Google Scholar]
- 15.Pivot X, Bondarenko I, Nowecki Z, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (Trastuzumab Biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36(10):968-974. doi: 10.1200/JCO.2017.74.0126 [DOI] [PubMed] [Google Scholar]
- 16.Stebbing J, Baranau Y, Manikhas A, et al. Total pathological complete response versus breast pathological complete response in clinical trials of reference and biosimilar trastuzumab in the neoadjuvant treatment of breast cancer. Expert Rev Anticancer Ther. 2018;18(6):531-541. doi: 10.1080/14737140.2018.1457442 [DOI] [PubMed] [Google Scholar]
- 17.Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869-878. doi: 10.1016/S1470-2045(12)70329-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. Concordance analysis of locally versus centrally assessed breast and total pathological complete response (bpCR and tpCR) in the PPS population
eTable 2. Efficacy results in the mFAS and rPPS populations
Data sharing statement