This cohort study develops and validates a prediction score for locoregional failure and distant metastases in oral squamous cell carcinoma that incorporates patient-derived xenograft engraftment and clinicopathological risk factors.
Key Points
Question
What is the prognostic value of patient-derived xenograft (PDX) engraftment in predicting locoregional failure and distant metastases in oral squamous cell carcinoma (OSCC)?
Findings
In this cohort study of 288 patients with OSCC, a prediction model was developed to identify patients at risk for locoregional failure and distant metastases using PDX engraftment data in conjunction with well-established clinical and pathologic predictors. Patients with engraftable tumors, rapid engrafters in particular, were at a higher risk for relapse and poor survival compared with nonengrafters.
Meaning
In this cohort study, PDX engraftment success was a predictor of poor cancer outcomes in OSCC.
Abstract
Importance
Patient-derived xenografts (PDXs) offer the opportunity to identify patients with oral cavity squamous cell carcinoma (OSCC) who are at risk for recurrence and optimize clinical decision-making.
Objective
To develop and validate a prediction score for locoregional failure (LRF) and distant metastases (DM) in OSCC that incorporates PDX engraftment in addition to known clinicopathological risk factors.
Design, Setting, and Participants
In this retrospective cohort study, PDX models were generated from patients with OSCC treated with curative intent at Princess Margaret Cancer Centre (Toronto, Canada) between 2006 and 2018. The cohort included 288 patients (aged ≥18 years) with a new diagnosis of nonmetastatic (M0) OSCC whose tumor samples were available for engraftment under the skin of xenograft mice. Patients were scored as a nonengrafter if PDX formation did not occur within 6 months. Data analysis was performed between August 2006 and May 2018.
Interventions
All patients received up-front curative-intent surgery followed by either observation or postoperative radiation with or without concurrent chemotherapy based on institutional guidelines.
Main Outcomes and Measures
Main outcomes were LRF, DM, and overall survival (OS). Multivariable analysis (MVA) was used to identify predictors of LRF and DM. Factors retained in the final MVA were used to construct a prediction score and classify patients into risk groups.
Results
Overall, 288 patients (mean [SD] age at diagnosis, 63.3 [12.3] years; 112 [39%] women and 176 [61%] men) with OSCC were analyzed. The MVA identified pT3-4, pathologic extranodal extension, and engraftment as predictors of LRF and DM. Patients whose tumors engrafted (n = 198) were more likely to develop LRF (hazard ratio [HR], 1.98; 95% CI, 1.24-3.18) and DM (HR, 2.64; 95% CI, 1.21-5.75) compared with nonengrafters. A prediction score based on the aforementioned variables identified patients at high risk and low risk for LRF (43.5% vs 26.5%), DM (38.2% vs 8.4%), and inferior OS (34% vs 66%) at 5 years. Additionally, rapid engraftment was shown to be similarly prognostic, with rapid engrafters demonstrating higher rates of relapse and poor OS.
Conclusions
In this cohort study, a prediction score using OSCC PDX engraftment, in conjunction with pT3-4 and pathologic extranodal extension, was associated with improved prognostic utility of existing clinical models and predicted patients at risk for LRF, DM, and poor survival.
Introduction
Oral cavity squamous cell carcinoma (OSCC) is a potentially fatal malignant neoplasm of the head and neck with a growing worldwide incidence and a relatively stable 5-year overall survival (OS) rate.1,2 Unfortunately, owing to heterogeneous patterns of locoregional and distant failure, optimal multidisciplinary management remains elusive and is usually based on crude risk stratification methods. Most patients diagnosed with OSCC are treated with primary surgery followed by either observation or postoperative radiation therapy (PORT) plus or minus concurrent chemotherapy.
The decision to offer postsurgical treatments often depends on a clinical estimate of disease recurrence risk. This risk estimate is derived from pathologic risk factors, such as advanced primary tumor or nodal involvement.3,4 However, despite the general utility of these pathologic risk predictors, locoregional failure (LRF) and distant metastases (DM) remain major determinants of mortality and morbidity in OSCC. Therefore, more accurate models of LRF and DM are needed to identify high-risk patients and optimize clinical decision-making in patients diagnosed with OSCC.
Patient-derived xenografts (PDXs) offer the opportunity to observe underlying biological characteristics of OSCC and could potentially identify patients at risk for recurrence. Recently, PDX models of head and neck cancers have been shown to be highly prognostic with the ability to identify patients more likely to experience recurrence and have poor outcomes.5 In this study, we set out to develop a predictive model to estimate the risk of recurrence in patients with OSCC using PDX data in conjunction with well-established clinical and pathologic predictors. We hypothesized that patients with engraftable tumors will have worse clinical outcomes and that including this parameter in prognostic models with known clinicopathological risk factors will improve their effectiveness.
Methods
Study Design and Population
This is a single-institution retrospective study of patients (aged ≥18 years) with a new diagnosis of nonmetastatic (M0) OSCC treated with curative intent at Princess Margaret Cancer Centre between 2006 and 2018. The analysis was restricted to patients whose tumor samples were available for engraftment under the skin of xenograft mice.5 Information on demographic, pathological, and clinical outcomes was extracted from the prospectively collected Head and Neck Anthology of Outcomes6 and/or direct review of patient electronic records. All staging used the 7th edition of the American Joint Committee on Cancer/Union of International Cancer Control guidelines because information on depth of invasion (DOI) for the primary tumor was not available.7 The Research Ethics Board at Princess Margaret Cancer Centre approved this study prior to its initiation, and all animal work complied with the National Institutes of Health guide for the care and use of laboratory animals. Patient informed consent was waived owing to the retrospective nature of the study and use of deidentified patient information.
Patient-Derived Xenografts
Patient-derived xenografts (NOD/SCID/IL2Rγ−/−) were generated from tumor samples of patients with histologically confirmed OSCC as described previously.5 Tumor samples were separated under sterile conditions into small fragments measuring 1 mm3, and 2 fragments were implanted under the flank skin of immunodeficient NOD/SCID/IL2Rγ−/− (NSG) mice. Briefly, mice were anesthetized using an isoflurane chamber, and a small subcutaneous incision pocket was created with scissors midway between the knee and the spine in the right (or left) flank of each mouse. Using forceps, tumor fragments were placed directly into this pocket and kept in place by stapling the wound edge. To account for possible variability in implantation, a total of 5 mice were implanted per patient. Tumor growth was monitored weekly, and patient tumors were classified as engrafters if xenograft formation occurred within 6 months in at least 1 of the 5 mice. All mice were euthanized at either 6 months or if the xenograft tumor grew to a maximum diameter of 1.5 cm. The PDX tumor growth kinetics were collected for a subset of PDX and patients were classified as rapid or slow engrafters based on the optimal cutoff engraftment time.
Standard Treatment
All patients in the study received up-front surgical resection of the primary tumor with or without nodal dissection. Patients with ipsilateral clinical node-positive disease or possibly clinically occult nodal disease (cT2-4, or tumor thickness >4 mm) underwent ipsilateral neck dissection of levels I to III with or without level IV. Patients with contralateral neck disease (cN2c) and/or primary tumor approaching midline were treated with bilateral neck dissection.
PORT decision-making considered the following risk features: pT3-4, pN2-3, level IV to V nodal involvement, pathologic extranodal extension (pENE), depth of primary tumor invasion, involved or close (<5 mm) microscopic resection margins, high histologic grade, lymphovascular invasion, and perineural invasion. Radiation was delivered by intensity-modulated radiation therapy. Patients received 60 Gy in 30 to 33 fractions to the operative bed and dissected nodal regions, whereas undissected at risk nodal regions received 54 to 56 Gy in 30 to 33 fractions. A higher-risk group with involved microscopic margin(s) and/or pENE received an increased dose of radiation (ie, 66 Gy in 33 fractions) and concurrent chemotherapy with cisplatin if fit, as per our institutional guidelines.
Evaluation and Follow-up
Pretreatment imaging (computed tomography scans and/or magnetic resonance imaging of the head and neck) was used for staging. A multidisciplinary team followed patients jointly with comprehensive head and neck examinations every 3 months (year 0-2 posttreatment), then every 4 to 6 months (year 3-5 posttreatment), then annually as needed. Post–radiation therapy imaging was performed 10 to 12 weeks following PORT. Further imaging, including workup of DM and LRF, was based on clinical suspicion and patient symptoms.
Study Outcomes
The outcome of OS was defined from the date of surgery to the date of any cause of death or last follow-up. The LRF and DM outcomes were defined from date of surgery to the date of first locoregional recurrence or DM, respectively, while death without tumor recurrence was considered a competing event.
Statistical Analysis
The nonparametric Kruskal-Wallis tests or Wilcoxon rank sum tests were used to compare continuous variables, while χ2 test was applied to categorical variables. The Kaplan-Meier method with log-rank test was used to analyze OS, while the cumulative incidence method with Fine and Gray test was used to estimate LRF and DM. The Cox proportional-hazards regression, on the other hand, was applied for univariable analysis and multivariable analysis (MVA) to evaluate clinical factors associated with OS.
The MVA with competing risk regression analysis was developed using a backward selection algorithm to identify predictors of LRF and DM. Variables with a P value <.10 in the univariate analysis were considered for inclusion for MVA, and only those achieving a P value <.05 were retained in the final model. To train and validate our MVA models for factors associated with the outcomes of interest, a 10-fold cross-validation approach with 10 replicates was used. The C index is used to assess the discrimination of this model. Risk groups were built based on factors associated with LRF and DM in the final MVA model. The cutoff point for high-risk and low-risk groups was determined using a minimal P value approach for cutoff optimization. Meanwhile, the maximally selected rank statistic method was used to define the optimum cut point for engraftment that can be correlated with LRF and DM. Additional analysis aimed to evaluate the performance of the generated risk group classification using the 8th edition of the American Joint Committee on Cancer/Union of International Cancer Control staging with tumor thickness as a surrogate for DOI.8 All statistical analysis was performed in R, version 3.6.3 (R Foundation for Statistical Computing) using 2-sided statistical tests with a significance (α) level of .05.
Results
Participant and Treatment Characteristics
A total of 288 patients with OSCC were included in the study with a mean (SD) age at diagnosis of 63.3 (12.3) years; 112 (39%) were women and 176 (61%) were men. The median (IQR) follow-up was 3 (1.0-5.3) years. Within this cohort, 171 (59%) patients underwent adjuvant PORT, including 49 (17%) who received concurrent chemotherapy. All patients had tumor engraftment attempted. However, only 198 (69%) patients had tumors that successfully engrafted. Among patients with engrafting tumors, 71 (36%) were classified as rapid engrafters (<8 weeks). The patient and clinical characteristics stratified by engraftment status are summarized in Table 1.
Table 1. Baseline Patient and Tumor Characteristics (n = 288).
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| Whole group (n = 288) | Nonengrafters (n = 90) | Engrafters (n = 198) | ||
| Sex | ||||
| Female | 112 (39) | 32 (36) | 80 (40) | .50 |
| Male | 176 (61) | 58 (64) | 118 (60) | |
| Age at diagnosis, y | ||||
| Mean (SD) | 63.3 (12.3) | 63.8 (10.9) | 63.1 (12.9) | .90 |
| Median (range) | 63.5 (22-94) | 64.0 (33-90) | 63.0 (22-94) | |
| Smoking history | ||||
| No | 96 (33) | 25 (28) | 71 (36) | .40 |
| Yes | 182 (63) | 62 (68) | 120 (61) | |
| Not reported | 10 (3) | 3 (3) | 7 (4) | |
| Subsite | ||||
| Tongue | 152 (53) | 42 (47) | 110 (56) | .20 |
| Floor of mouth | 53 (19) | 24 (27) | 29 (15) | |
| Buccal mucosa | 29 (10) | 12 (13) | 17 (9) | |
| Upper/lower gingiva | 27 (9) | 6 (7) | 21 (11) | |
| Retromolar trigone | 14 (5) | 5 (5) | 9 (4) | |
| Other | 12 (4) | 1 (1) | 11 (5) | |
| pT category | ||||
| T1-2 | 176 (61) | 56 (62) | 120 (61) | .90 |
| T3-4 | 112 (39) | 34 (38) | 78 (39) | |
| pN category | ||||
| N0-1 | 170 (59) | 59 (66) | 111 (56) | .20 |
| N2-3 | 118 (41) | 31 (34) | 87 (44) | |
| Overall stage, 7th edition | ||||
| I | 33 (12) | 13 (14) | 20 (10) | .50 |
| II | 58 (20) | 21 (23) | 37 (19) | |
| III | 52 (18) | 14 (16) | 38 (19) | |
| IVA-B | 145 (50) | 42 (47) | 103 (52) | |
| Histological grade | ||||
| 1-2 | 203 (70) | 67 (74) | 136 (69) | .40 |
| 3 | 85 (30) | 23 (26) | 62 (31) | |
| Max primary tumor size, cm | ||||
| Mean (SD) | 3.3 (1.4) | 3.1 (1.4) | 3.4 (1.4) | .10 |
| Median (range) | 3.0 (0.3-8.6) | 3.0 (1-8.6) | 3.2 (0.3-8.5) | |
| Tumor thickness, mm | ||||
| Mean (SD) | 13.7 (8.8) | 13.0 (8.9) | 14.0 (8.8) | .21 |
| Median (range) | 11 (1-45) | 10 (2-45) | 12 (1-45) | |
| Extranodal extension | ||||
| No | 214 (74) | 80 (89) | 134 (68) | .001 |
| Yes | 74 (26) | 10 (11) | 64 (32) | |
| Total LN positive | ||||
| Mean (SD) | 2.1 (3.3) | 1.3 (1.8) | 2.4 (3.7) | .004 |
| Median (range) | 1 (0-31) | 0 (0-8) | 1 (0-31) | |
| Level IV/V LN involvement | ||||
| No | 246 (85) | 82 (91) | 164 (83) | .10 |
| Yes | 42 (15) | 8 (9) | 34 (17) | |
| PORT | ||||
| No | 117 (41) | 45 (50) | 72 (36) | .04 |
| Yes | 171 (59) | 45 (50) | 126 (64) | |
| Concurrent chemotherapy | ||||
| No | 239 (83) | 82 (91) | 157 (79) | .03 |
| Yes | 49 (17) | 8 (9) | 41 (21) | |
| Early recurrence | ||||
| No | 262 (91) | 89 (99) | 173 (87) | .003 |
| Yes | 26 (9) | 1 (1) | 25 (13) | |
| Overall recurrence | ||||
| No | 167 (58) | 66 (73) | 101 (52) | .001 |
| Yes | 121 (42) | 24 (27) | 97 (48) | |
Abbreviations: LN, lymph node; PORT, postoperative radiation therapy.
P value represents statistical comparison between engrafters and nonengrafters.
Pattern of Failure
During the follow-up period, disease recurrence was detected in 121 (42%) patients, of whom 99 (34%) developed LRF, including 77 (27%) who had local failure and 22 (8%) with regional failure only. In addition, 57 (20%) patients were diagnosed with either synchronous or metachronous DM, including 22 (8%) who developed DM without LRF (eFigure 1 in the Supplement). The most common sites of DMs were lung (42 [74%]) and bone (8 [14%]). A small proportion of patients (26 [8%]) developed early recurrence within 3 months of primary surgical resection.
Outcomes Predictors
In the MVA, patients whose tumors engrafted were more likely to develop LRF (hazard ratio [HR], 1.98; 95% CI, 1.24-3.18) and DM (HR, 2.64; 95% CI, 1.21-5.75) compared with patients with nonengraftable tumors (Table 2). In addition to engraftment, the MVA identified 2 additional features associated with LRF, namely pT3-4 category (HR, 1.66; 95% CI, 1.10-2.48) and pENE (HR, 1.69; 95% CI, 1.09-2.61). Interestingly, these same 2 predictors also were associated with the risk of DM: pT3-4 (HR, 2.23; 95% CI, 1.31-3.83) and pENE (HR, 3.91; 95% CI, 2.28-6.71). The results of the univariable analysis are presented in the eTable in the Supplement.
Table 2. Final Multivariable Analysis Model for Locoregional Failure and Distant Metastasis.
| Variable | HR (95% CI) | |
|---|---|---|
| Locoregional failure | Distant metastasis | |
| pT category | ||
| pT1-2 | 1 [Reference] | 1 [Reference] |
| pT3-4 | 1.66 (1.10-2.48) | 2.23 (1.31-3.83) |
| pENE | ||
| No | 1 [Reference] | 1 [Reference] |
| Yes | 1.69 (1.09-2.61) | 3.91 (2.28-6.71) |
| Engraftment | ||
| No | 1 [Reference] | 1 [Reference] |
| Yes | 1.98 (1.24-3.18) | 2.64 (1.21-5.75) |
Abbreviations: HR, hazard ratio; pENE, pathologic extranodal extension.
Overall Risk Group Classification
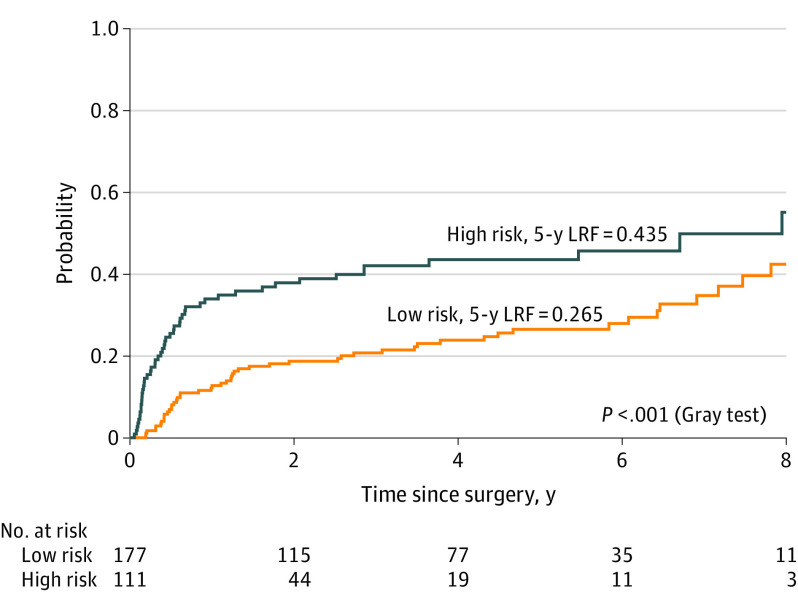
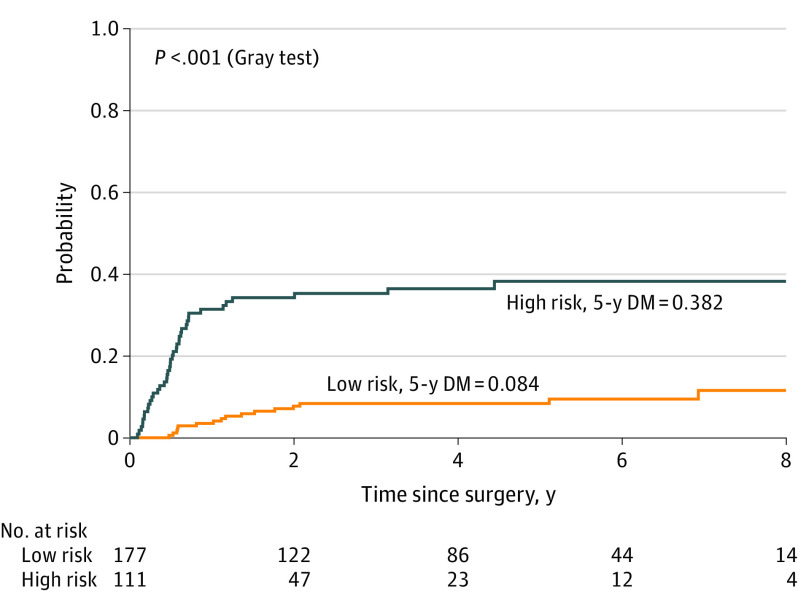
Based on a score of the aforementioned variables (engraftment, pT3-4, pENE), a model was developed to estimate the risk of LRF and DM. Using a minimal P value approach, patients with at least 2 of the 3 high-risk features defined (engraftment, pT3-4, pENE) were classified as high risk, while patients with 1 or no features were classified as low risk for LRF and DM. High-risk patients were shown to have higher rates of LRF (43.5% vs 26.5%; difference, 17%; 95% CI, 5.4%-27.7%) at 5 years and DM (38.2% vs 8.4%; difference, 30%; 95% CI, 19.5%-39.2%) at 5 years, compared with low-risk patients (Figure 1 and Figure 2).
Figure 1. Incidence of Locoregional Failure (LRF) in Oral Cavity Squamous Cell Carcinoma for High-risk (n = 111) vs Low-risk Groups (n = 177).
Figure 2. Incidence of Distant Metastases (DM) in Oral Cavity Squamous Cell Carcinoma for High-risk (n = 111) vs Low-risk Groups (n = 177).
Overall Risk Group Classification Based on 8th Edition Staging
Using tumor thickness as a surrogate for DOI, we sought to evaluate the performance of the risk group classification using 8th-edition TNM staging.8 Consistent with the previous analysis, high-risk patients with 2 of 3 high-risk features (ie, engraftment, pENE and pT3-4 8th edition) were shown to have higher rates of LRF (40% vs 26%; difference, 14%; 95% CI, 7.0%-24.3%) and DM (32% vs 7%; difference, 25%; 95% CI, 19.7%-32.1%) at 5 years compared with low-risk patients (eFigure 2A and 2B in the Supplement). High-risk patients also had inferior OS (38% vs 71%; difference, 33%; 95% CI, 24.2%-41.5%) at 5 years compared with low-risk patients (eFigure 2C in the Supplement).
Clinical Risk Group Classification
The prediction model that included engraftment had the highest C index and the lowest Brier score in the cross-validation analysis (area under the curve [AUC]: 0.68; 95% CI, 0.63-0.73; Brier: 0.18; 95% CI, 0.17-0.23). In contrast, removal of engraftment as a predictor resulted in a lower C index and higher Brier score (AUC: 0.63; 95% CI, 0.57-0.68; Brier: 0.2; 95% CI, 0.17-0.22). In patients classified based on a clinical score only, engraftment remained useful in identifying those with worse outcomes. For example, clinically high-risk patients, based on the presence of pT3-4 and pENE, had higher rates of DM (38% vs 11%; difference, 27%; 95% CI, 18.9%-36.3%) at 5 years if their tumors engrafted compared with nonengrafting patients. Similarly, engraftment was associated with a higher risk of LRF (32% vs 14%; difference, 18%; 95% CI, 8.9%-29.9%) at 5 years in clinically low-risk patients, as defined by the absence of pT3-4 and pENE (eFigure 3 in the Supplement).
Survival Outcomes
The median (range) OS of the cohort was 6.4 (0.1-9.9) years with a 5-year OS of 54% (95% CI, 48%-61%). In the MVA, engraftment was associated with a lower OS (HR, 2.23; 95% CI, 1.21-3.96). The 5-year OS rate for engrafters was lower (46% vs 72%; difference, 26%; 95% CI, 12.1%-39.9%) compared with nonengrafters. The median (range) OS after detection of LRF was 1.5 (0.1-9.4) years, 1.7 (0.1-9.4) years after detection of local-only failure, 0.9 (0.2-7.8) years after detection of regional-only failure, and 1.0 (0.1-7.0) year after detection of DM. Patients classified as high risk in the overall risk group had worse 5-year OS (34% vs 66%; difference, 32%; 95% CI, 19.3%-44.6%) compared with low-risk patients (eFigure 4 in the Supplement). In addition, compared with nonengrafters, engraftment was associated with poor survival in both clinically high-risk (36% vs 65%; difference, 29%; 95% CI, 8.8%-49.2%) and clinically low-risk patients (57% vs 78%; difference, 21%; 95% CI, 2.6%-39.4%) at 5 years (eFigure 5 in the Supplement).
Engraftment Speed
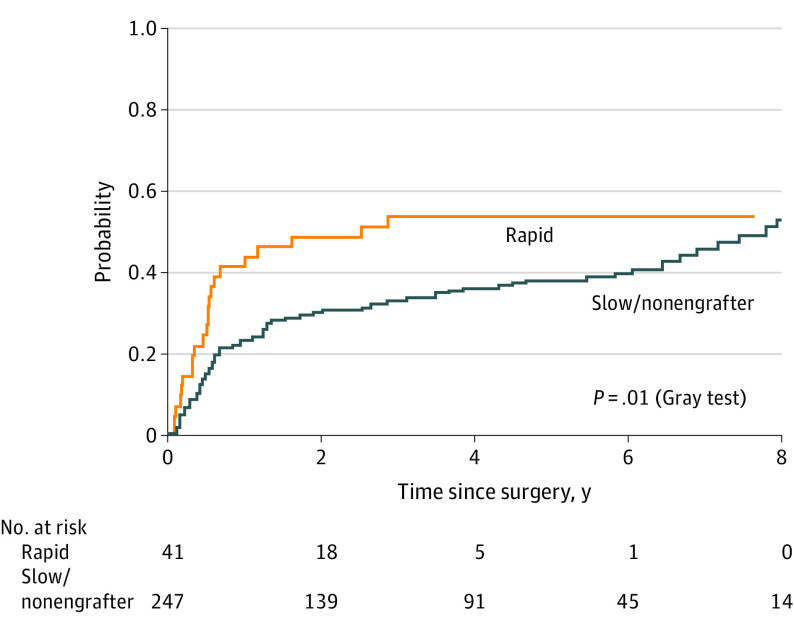
Based on the subset of patients for which engraftment kinetics was available, the median (range) engraftment time was 62 (12-212) days. Given that time to engraftment might represent an important clinical barrier in PDX adoption, we hypothesized that rapid engraftment might be a more timely indicator of aggressiveness and recurrence risk. First, we used rank statistics to estimate the optimal cutoff point for engraftment time as 49 days (ie, 7 weeks). Next, using a more clinically relevant 8-week cutoff during which adjuvant treatment decisions can be made, rapid engraftment (n = 41) was associated with higher rates of LRF and DM (54% vs 38%; difference, 16%; 95% CI, 1.1%-31.9%) at 5 years compared with slow engrafters and nonengrafters (n = 247) (Figure 3). In addition, rapid engrafters had lower rates of OS (48% vs 56%; difference 8%; 95% CI, 5.5%-26.5%) at 5 years. The median (range) OS for rapid engrafters was 3.3 (0.2-7.7) years compared with 6.8 (0.1-9.9) years for slow and nonengrafters (eFigure 6 in the Supplement).
Figure 3. Incidence of Locoregional Failure and Distant Metastases in Rapid Engrafters (n = 41) vs Slow Engrafters or Nonengrafters (n = 247).
PORT Subgroup
In the subgroup analysis of patients receiving PORT (n = 171), the effect of risk stratification on disease recurrence and survival was similar to the overall cohort. Patients treated with PORT only who were classified as high risk had a higher 5-year incidence of LRF (49% vs 33%; difference, 16%; 95% CI, 3.7%-35.7%) and DM (44% vs 10%; difference, 34%; 95% CI, 18.9%-49.1%) and worse 5-year OS (32% vs 65%; difference, 33%; 95% CI, 13.9%-52.1%) compared with low-risk patients (eFigure 7 in the Supplement). Importantly, in the subgroup of patients who did not receive PORT (pT1-2 pN0-1 with negative margins and no pENE), engraftment was associated with a higher incidence of LRF (eFigure 8 in the Supplement).
Discussion
In this study, we present a novel prediction score for the classification of OSCC patients at high and low risk for LRF and DM. In addition to known clinicopathological parameters, this score incorporates PDX as a key predictive variable. First, in the MVA, we show that successful engraftment of OSCC tumors was associated with LRF, DM, and OS. Next, using engraftment, pT category, and pENE status as predictive markers for LRF and DM, we constructed a prediction score that can accurately identify patients at high risk of LRF, DM, and poor OS. We also demonstrate that the addition of engraftment was associated with improved discriminatory capacity of a predictive model based on clinicopathological variables alone. Finally, we show that rapid engraftment was similarly prognostic in terms of predicting patients at risk for recurrence and poor survival.
Previous studies have identified T and N category, histological grade, lymph node ratio, and pENE as prognostic risk factors in OSCC. For example, multiple large retrospective studies of patients with OSCC treated with curative intent have found that pT3-4, pN+, and pENE were associated with the risk of DM.9,10 These same factors were similarly implicated in the risk of early recurrence prior to planned PORT.11 There is also growing evidence that a high positive lymph node ratio is an additional high-risk feature that is associated with poor outcomes, including regional-only failure, distant-only failure, and worse overall survival.4
Interestingly, PDXs have emerged as an important translational tool to develop patient-specific biomarkers in several cancers, including breast and lung cancer.12,13 In a recent study,5 authors demonstrate that PDX engraftment could be used to identify patients with human papillomavirus–negative head and neck cancer who were more likely to relapse and have worse outcomes, suggesting that engraftment is a reliable marker of biological aggressiveness. Thus, while the mainstay of OSCC management remains guided by clinicopathological features, the addition of patient-specific engraftment could stratify patients into treatment risk groups more accurately and result in more personalized treatments.
In recent years, there has been a growing interest in strategies for treatment escalation and de-escalation to improve OSCC cure rates and avoid unnecessary treatment adverse effects. For example, in a phase 2 clinical trial,14 patients with advanced resectable OSCC were found to benefit from treatment escalation in the postoperative setting using concurrent chemoradiation therapy with docetaxel and cetuximab. Conversely, patient populations at low risk for disease recurrence, such as those with pT3N0, pT4aN0, and pT1-2N1 disease without other risk factors, may in fact benefit from treatment de-intensification, as they demonstrated excellent local control and OS rates despite the omission of PORT.15,16
While the 6-month time frame for engraftment makes it impractical for adjuvant decision-making, rapid engraftment (8 weeks or less) offers the potential for more timely prediction of patients at risk for relapse. Interestingly, approximately a third of rapid engrafters in the present cohort (13 of 41) received surgery only without PORT. Given the susceptibility of rapid engrafters for locoregional and distant relapse, this suggests that this patient population may derive the greatest benefit from treatment escalation. For example, patients with local-stage OSCC without any adverse pathological risk factors could be considered for PORT with or without chemotherapy if their tumor rapidly engrafts. Conversely, patients with borderline risk factors but with a nonengrafting or slow-engrafting PDX may theoretically forgo treatment based on their lower overall risk profile.
Limitations
To our knowledge, this is the first study to develop and internally validate a model that incorporates engraftment as a predictive variable in OSCC. However, our study had some limitations. This was a retrospective study based at a single large tertiary cancer center. While the time required to observe engraftment represents a potential barrier toward the integration of PDX in clinical practice, it may be feasible, as our data show, to use rapid engraftment for a more timely characterization of OSCC patient risk. In addition, the PDX models used in this study lack cytotoxic immune cells, which may play a crucial role in immune surveillance and subsequent LRF and DM.
Finally, given that it may be challenging to generate patient-specific xenografts across all treatment centers, it may be important in the future to identify molecular alterations that may act as a potential surrogate for rapid engraftment. For instance, our group previously published a study5 on the association between rapid engraftment and molecular alterations in CCDN1 and CDKN2A in a genetic analysis of 112 samples from engrafting (n = 64) and nonengrafting (n = 48) patients with head and neck cancer, suggesting that deregulation of the G1/S cell cycle checkpoint pathway plays an important role in high-risk patients. Consistent with this proposition, copy number changes identified in the aforementioned tumor samples were found to be retained in their corresponding PDX, while treatment with a CDK4/CDK6 inhibitor (abemaciclib) resulted in growth delay only in PDXs with underlying CCDN1 and CDKN2A alterations.5
Conclusions
In this cohort study, we present a novel prediction score using PDX engraftment to help identify OSCC patient subgroups at high and low risk for disease relapse and poor outcomes. This could pave the way for future prospective studies and randomized clinical trials of modified treatment and surveillance schedules to enhance OSCC cure rates and reduce associated adverse effects.
eTable. Results of Univariable analysis for locoregional failure and distant metastasis
eFigure 1. Venn diagram of patterns of disease failure (N= 121)
eFigure 2. Incidence of locoregional failure (2a), distant metastases (2b) and overall survival (2c) according to high- (n=145) vs low-risk groups (n=143) with 8th edition AJCC staging using Tumor Thickness (TT) as a surrogate for Depth of invasion (DOI)
eFigure 3. Locoregional failure in low-risk clinical group (3a) and distant metastasis in high-risk clinical group (3b) according to engraftment
eFigure 4. Kaplan-Meier curve of overall survival in OSCC high- vs low-risk groups (n=177)
eFigure 5. Kaplan-Meier curve of overall survival in high- (5a) and low-risk (5b) clinical groups according to engraftment
eFigure 6. Kaplan-Meier curve of overall survival in rapid (n=41) vs slow/non-engrafters (n=247)
eFigure 7. Locoregional failure (7a), distant metastasis (7b) and overall survival (7c) in high- vs low- risk groups (PORT cohort)
eFigure 8. Kaplan-Meier curve of LRF in engrafters and non-engrafting patients who did not receive PORT (T1-2 N0-1 with negative margins and no ENE)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386-396. doi: 10.1016/j.mayocp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 3.Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 2006;42(8):789-794. doi: 10.1016/j.oraloncology.2005.11.016 [DOI] [PubMed] [Google Scholar]
- 4.Hosni A, McMullen C, Huang SH, et al. Lymph node ratio relationship to regional failure and distant metastases in oral cavity cancer. Radiother Oncol. 2017;124(2):225-231. doi: 10.1016/j.radonc.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 5.Karamboulas C, Bruce JP, Hope AJ, et al. Patient-derived xenografts for prognostication and personalized treatment for head and neck squamous cell carcinoma. Cell Rep. 2018;25(5):1318-1331.e4. doi: 10.1016/j.celrep.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 6.Wong K, Huang SH, O’Sullivan B, et al. Point-of-care outcome assessment in the cancer clinic: audit of data quality. Radiother Oncol. 2010;95(3):339-343. doi: 10.1016/j.radonc.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti AE. AJCC cancer staging manual. 7th ed. Springer; 2010. [Google Scholar]
- 8.Dirven R, Ebrahimi A, Moeckelmann N, Palme CE, Gupta R, Clark J. Tumor thickness versus depth of invasion—analysis of the 8th edition American Joint Committee on Cancer Staging for oral cancer. Oral Oncol. 2017;74:30-33. doi: 10.1016/j.oraloncology.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto Y, Matsushita Y, Yamada S, et al. Risk factors of distant metastasis in patients with squamous cell carcinoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):474-480. doi: 10.1016/j.oooo.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 10.Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(7):762-766. doi: 10.1001/archotol.132.7.762 [DOI] [PubMed] [Google Scholar]
- 11.Hosni A, Huang SH, Chiu K, et al. Predictors of early recurrence prior to planned postoperative radiation therapy for oral cavity squamous cell carcinoma and outcomes following salvage intensified radiation therapy. Int J Radiat Oncol Biol Phys. 2019;103(2):363-373. doi: 10.1016/j.ijrobp.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 12.John T, Kohler D, Pintilie M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011;17(1):134-141. doi: 10.1158/1078-0432.CCR-10-2224 [DOI] [PubMed] [Google Scholar]
- 13.McAuliffe PF, Evans KW, Akcakanat A, et al. Ability to generate patient-derived breast cancer xenografts is enhanced in chemoresistant disease and predicts poor patient outcomes. PLoS One. 2015;10(9):e0136851. doi: 10.1371/journal.pone.0136851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol. 2014;32(23):2486-2495. doi: 10.1200/JCO.2013.53.9163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell DL, Clinton SK, Old MO. Not so fast: deintensification therapy for locally advanced oral cavity cancer. Int J Radiat Oncol Biol Phys. 2020;106(5):926-927. doi: 10.1016/j.ijrobp.2020.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CY, Fan KH, Lee LY, et al. Precision adjuvant therapy based on detailed pathologic risk factors for resected oral cavity squamous cell carcinoma: long-term outcome comparison of CGMH and NCCN guidelines. Int J Radiat Oncol Biol Phys. 2020;106(5):916-925. doi: 10.1016/j.ijrobp.2019.08.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Results of Univariable analysis for locoregional failure and distant metastasis
eFigure 1. Venn diagram of patterns of disease failure (N= 121)
eFigure 2. Incidence of locoregional failure (2a), distant metastases (2b) and overall survival (2c) according to high- (n=145) vs low-risk groups (n=143) with 8th edition AJCC staging using Tumor Thickness (TT) as a surrogate for Depth of invasion (DOI)
eFigure 3. Locoregional failure in low-risk clinical group (3a) and distant metastasis in high-risk clinical group (3b) according to engraftment
eFigure 4. Kaplan-Meier curve of overall survival in OSCC high- vs low-risk groups (n=177)
eFigure 5. Kaplan-Meier curve of overall survival in high- (5a) and low-risk (5b) clinical groups according to engraftment
eFigure 6. Kaplan-Meier curve of overall survival in rapid (n=41) vs slow/non-engrafters (n=247)
eFigure 7. Locoregional failure (7a), distant metastasis (7b) and overall survival (7c) in high- vs low- risk groups (PORT cohort)
eFigure 8. Kaplan-Meier curve of LRF in engrafters and non-engrafting patients who did not receive PORT (T1-2 N0-1 with negative margins and no ENE)