This randomized clinical trial evaluates the efficacy and safety of pilocarpine hydrochloride, 1.25%, in individuals with presbyopia.
Key Points
Question
Does AGN-190584 (Allergan, an AbbVie company), an investigational pilocarpine formulation, demonstrate efficacy and safety in managing presbyopia?
Findings
In this phase 3 randomized clinical trial, the proportion of participants with improvement of 3 or more lines in mesopic, high-contrast, binocular distance-corrected near visual acuity was statistically significantly higher with AGN-190584 treatment compared with vehicle on day 30, hour 3 (primary end point). AGN-190584 had an acceptable safety profile.
Meaning
AGN-190584 was safe and efficacious through 30 days, improving near and intermediate vision on day 30 for up to 10 hours after administration and supporting its use as a treatment for presbyopia.
Abstract
Importance
AGN-190584 (Allergan, an AbbVie company) is an optimized topical formulation of pilocarpine hydrochloride, 1.25%, designed for managing presbyopia and enhanced with a proprietary vehicle.
Objective
To evaluate the efficacy and safety of pilocarpine hydrochloride, 1.25%, in individuals with presbyopia.
Design, Setting, and Participants
This vehicle-controlled, participant- and investigator-masked, randomized, phase 3 clinical study, GEMINI 1, enrolled individuals with presbyopia, aged 40 to 55 years, at 36 sites in the United States from December 21, 2018, to October 31, 2019. Analysis took place between February 2020 and December 2021.
Interventions
AGN-190584 or the AGN-190584 formulation vehicle was administered bilaterally, once daily for 30 days.
Main Outcomes and Measures
The proportion of participants with improvement of 3 or more lines in mesopic, high-contrast, binocular distance-corrected near visual acuity (DCNVA) at hours 3 and 6 on day 30 were the primary and key secondary efficacy end points, respectively. Safety measures included adverse events.
Results
Of 323 participants who were randomized, 235 (72.8%) were female and 292 (90.4%) were White. The mean (SD) age was 49.6 (3.5) years, and the baseline mean (SD) mesopic DCNVA was 29.2 (6.3) letters. A total of 163 individuals were randomized to AGN-190584 and 160 were randomized to vehicle. GEMINI 1 met its primary and key secondary efficacy end points. On day 30, hour 3, the percentage of participants with improvement of 3 or more lines in mesopic DCNVA was 30.7% (50 of 163) in the AGN-190584 group and 8.1% (13 of 160) in the vehicle group (difference, 22.5% [95% CI, 14.3%-30.8%]; adjusted P < .001). At hour 6, those percentages were 18.4% (30 of 163) and 8.8% (14 of 160), respectively (difference, 9.7% [95% CI, 2.3%-17.0%]; adjusted P = .01). At hour 8, the between-group difference in 3 or more lines of mesopic DCNVA gains was not statistically significant, but clinically relevant prespecified outcome measures demonstrated AGN-190584 superiority to vehicle in least-squares mean (SE) mesopic DCNVA change from baseline at hour 8 (5.4 [0.51] vs 3.6 [0.52] letters; P = .009) and photopic distance-corrected intermediate visual acuity at hour 8 (3.9 [0.44] vs 2.4 [0.45] letters; P = .01) and hour 10 (3.5 [0.46] vs 1.7 [0.47] letters; P = .004). No participants with mesopic DCNVA improvement of 3 or more lines at hour 3 had losses of more than 5 letters in mesopic, high-contrast, binocular-corrected distance visual acuity. The onset of effect was at 15 minutes. AGN-190584 demonstrated an acceptable safety and tolerability profile.
Conclusions and Relevance
AGN-190584 demonstrated superiority over vehicle in mesopic DCNVA on day 30, hours 3 and 6, with an acceptable safety profile. AGN-190584 is a safe and efficacious topical therapy for presbyopia through 30 days.
Trial Registration
ClinicalTrials.gov Identifier: NCT03804268
Introduction
Presbyopia affects approximately 1.8 billion people worldwide1 (typically individuals aged >40 years) and is a characteristic of the age-related, progressive changes of the crystalline lens, or dysfunctional lens syndrome, which includes stage 1 (mild presbyopia and higher-order aberrations), stage 2 (lens opacity with advancing presbyopia and higher-order aberrations), and cataract with severe presbyopia and higher-order aberrations.2,3 Presbyopia progressively reduces the eye’s ability to focus on near objects, likely resulting from gradual lens thickening and loss of lens elasticity and accommodative ability4,5,6,7,8,9 and can impact a person’s daily activities, quality of life, and emotional well-being.10 Advanced and absolute presbyopia also impair intermediate vision as the progressive loss of remaining accommodative ability prevents a wide range of clear vision,11 and individuals with uncorrected presbyopia reported a significant impact on activities requiring near (reading, writing, and using smartphones) and intermediate (computer work or cooking) vision.10 Common presbyopia treatments include corrective glasses/lenses12 and surgery (corneal- or lens-based).13 There is a need for convenient and noninvasive alternatives for managing presbyopia.
Pilocarpine is a cholinergic muscarinic receptor agonist that has been previously investigated for its ability to improve both depth of focus and accommodation.14,15 Pilocarpine acts through the M3 muscarinic receptors on the iris sphincter to constrict the pupil and improve depth of focus.16,17 It also contracts the ciliary muscle to change the lens thickness, stimulating accommodation to allow the eyes to focus on near objects.17 Previous studies of pilocarpine-based glaucoma treatments have reported adverse events (AEs) including brow ache, headaches, vision blur, and discomfort.18,19
Phase 2b, dose-ranging studies (NCT02595528 and NCT02780115) were conducted based on the potential for pilocarpine hydrochloride (0.5%-1.5%) to improve near vision in individuals with presbyopia.20 Results demonstrated robust efficacy for pilocarpine hydrochloride, 1.0%, with a significant percentage of participants maintaining an improvement of 3 lines or more in near vision for up to 8 hours. The studies also showed that concentrations of pilocarpine hydrochloride, 1.5% or less, had acceptable safety and tolerability profiles.20 Moreover, results from in vitro and clinical/phase 1 studies showed that combined with a proprietary vehicle, pilocarpine hydrochloride demonstrated rapid equilibration to the tear film’s physiologic pH, provided greater tolerability, and reduced vision blur compared with a generic formulation.21 Based on these results, AGN-190584 (Allergan, an AbbVie company), an optimized formulation of pilocarpine hydrochloride, 1.25%, in a proprietary vehicle, was developed for managing presbyopia. The objective of the phase 3 GEMINI 1 study was to compare the efficacy and safety of AGN-190584 and vehicle in individuals with presbyopia.
Methods
Study Design
The GEMINI 1 study was a 30-day, multicenter, double-masked, randomized, vehicle-controlled, parallel-group, phase 3 trial conducted at 36 sites in the United States from December 21, 2018, to October 31, 2019, in compliance with the Declaration of Helsinki.22 The study protocol (Supplement 1) was approved by an institutional review board or ethics committee at each site. All participants provided written informed consent and were compensated for their time to complete study visits. This study report follows Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
Participants (aged 40-55 years) were in good general health with objective and subjective evidence of presbyopia. All eligibility criteria are listed in eTable 1 in Supplement 2. Key inclusion criteria included complaints of poor near vision (without near correction) impacting daily activities23; photopic, high-contrast corrected distance visual acuity (CDVA) of 20/25 or better bilaterally; mesopic, high-contrast distance-corrected near visual acuity (DCNVA, measured at 40 cm) of 20/40 to 20/100; photopic, near visual acuity correctable to 20/40 or better bilaterally; and willingness to wear monofocal correction to achieve photopic, binocular CDVA of 20/32 or better during the study. Key exclusion criteria included presence of severe dry eye disease; history of intraocular surgery except photorefractive keratectomy or laser-assisted in situ keratomileusis; history of glaucoma or ocular hypertension; and anisocoria more than 1 mm between pupils under mesopic conditions.
Randomization
Participant randomization (1:1) to AGN-190584 or the AGN-190584 vehicle was stratified by age (≤50 and >50 years), baseline mesopic, high-contrast, binocular DCNVA (20/40 to 20/60 and worse than 20/60), iris color (brown and not brown), and emmetrope status (emmetropes [sphere: −0.50 D to +0.75 D; cylinder: ≤0.75 D] and nonemmetropes). The randomization sequence was computer generated, and an automated interactive electronic response system/method was used to assign participants to study interventions. AGN-190584 and vehicle were provided in identically appearing bottles; both participants and study investigators/staff were masked to the treatment assignment.
Study Intervention and Visits
Participants administered topical AGN-190584 or vehicle bilaterally once daily (in the morning) for 30 days. Study visits were scheduled at screening (1-30 days prebaseline) and days 1 (baseline), 3, 7 (±2), 14 (±2), and 30 (±3)/early exit. On visit days, the study intervention was instilled by designated site personnel at hour 0 (8 am ± 1 hour).
Outcome Measures
The primary efficacy end point was the proportion of participants gaining 3 or more lines in mesopic (10-11 lux at the target), high-contrast, binocular DCNVA on day 30, hour 3. The key secondary efficacy end point was the proportion of participants gaining 3 or more lines in mesopic, high-contrast, binocular DCNVA on day 30, hour 6. Other prespecified efficacy end points included the proportion of participants gaining 3 or more lines in mesopic, high-contrast, binocular DCNVA on day 30, hours 8 and 10; change from baseline in mesopic, high-contrast, binocular DCNVA letters on day 30, hours 0.25 and 0.5; proportion of participants achieving 20/40 or better in photopic (>251 lux at the target), high-contrast, binocular DCNVA on day 30, hours 1 and 3; change from baseline in photopic, high-contrast, binocular distance-corrected intermediate visual acuity (DCIVA; measured at 66 cm) letters on day 30, hour 3; and mean change from baseline on day 30, hour 3 in patient-reported outcomes of mesopic Near Vision Presbyopia Task-based Questionnaire performance and satisfaction scores, and Presbyopia Impact and Coping Questionnaire coping and impact scores. The Near Vision Presbyopia Task-based Questionnaire and Presbyopia Impact and Coping Questionnaire patient-reported outcome instruments were recently developed in accord with US Food and Drug Administration (FDA) standards and validated by the study sponsor.24,25
Preplanned safety outcome measures included AEs; photopic and mesopic, high-contrast, binocular CDVA; biomicroscopy and ophthalmoscopy findings; intraocular pressure; and vital signs. The timing of assessments is provided in the eMethods in Supplement 2.
Statistical Analysis
Sample size calculations were considered for both the primary and key secondary efficacy end points; the calculation requiring the larger sample size, ie, for the key secondary efficacy end point, was used. Assuming proportions of participants gaining 3 or more lines in mesopic DCNVA of 15% with AGN-190584 and 3.6% with vehicle at day 30, hour 6, and 10% dropout, a sample size of approximately 150 participants per group was planned to provide 90% power at a 2-sided significance level of .05.
The intent-to-treat population (all randomized participants) was used for efficacy analyses. The primary and key secondary efficacy end points were tested using the Pearson χ2 test. Point estimates of between-group differences (AGN-190584–vehicle) with a 2-sided 95% CI were provided; missing data were imputed as 3-line gain failures.
Analyses of other secondary efficacy end points used observed data. End points assessing proportions of participants were analyzed with the Pearson χ2 test; end points assessing change from baseline in DCNVA and DCIVA were analyzed using a mixed-effects model for repeated measures including treatment, visit, treatment-by-visit interaction, 4 stratification factors as fixed factors, and baseline value and baseline value-by-visit interactions as covariates under the assumption of missing at random. An unstructured covariance matrix was used for repeated measures. Patient-reported outcomes were analyzed using analysis of covariance including factors of treatment and 4 stratification factors and corresponding baseline score as a covariate. A graphical approach for structured hypotheses (eFigure 1 in Supplement 2) was used to control the overall familywise error rate at α = .05 for the primary and secondary efficacy end points. P values were calculated as adjusted values where indicated.
The safety population included all participants who received 1 or more administrations of study intervention. Analyses using this population were based on the actual study intervention received. Participants self-identified their sex and race and ethnicity on a checklist; these data were required by the regulatory agency and summarized with descriptive statistics. Analysis took place between February 2020 and December 2021.
Results
Disposition, Demographics, and Baseline Characteristics
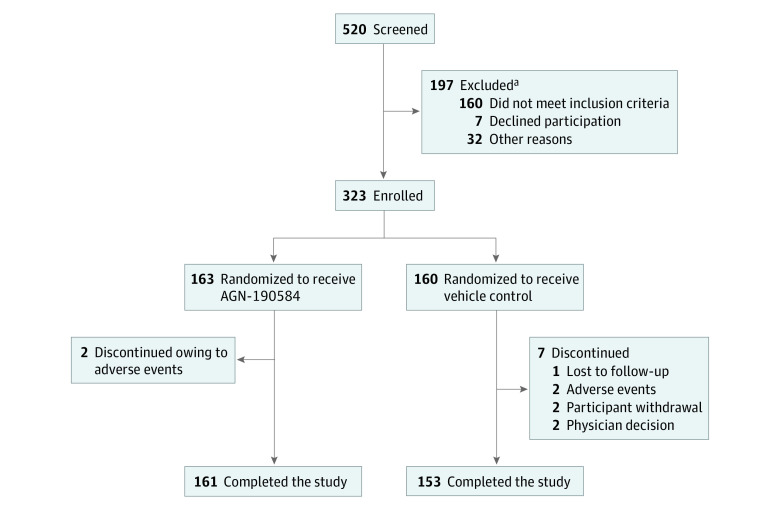
Of 520 participants screened, 323 were randomized to either AGN-190584 (n = 163) or vehicle (n = 160). Of those randomized, 98.8% (161 of 163) and 95.6% (153 of 160) completed the study, respectively (Figure 1).
Figure 1. Participant Disposition.
The adverse events leading to study discontinuation were bradycardia (n = 1) and dyschromatopsia and bilateral visual field defect (n = 1) in the AGN-190584 group and corneal abrasion (n = 1) and headache and migraine (n = 1) in the vehicle group.
aParticipants could fail screening owing to multiple reasons.
The intent-to-treat population included 88 male individuals (27.2%) and 235 female individuals (72.8%); the mean (SD) age was 49.6 (3.5) years, and 185 (57.3%) participants were 50 years or younger. Most participants were White (292 [90.4%]) and not Hispanic or Latino (266 [82.4%]). Demographics and baseline characteristics were balanced between groups (Table 1).
Table 1. Participant Demographics and Characteristics at Baseline (Intent-to-Treat Population).
| Parameter | No. (%) | ||
|---|---|---|---|
| AGN-190584 (n = 163) | Vehicle (n = 160) | Total (N = 323) | |
| Age, y | |||
| Mean (SD) [range] | 49.5 (3.8) [40-55] | 49.7 (3.2) [41-55] | 49.6 (3.5) [40-55] |
| ≤50 | 92 (56.4) | 93 (58.1) | 185 (57.3) |
| >50 | 71 (43.6) | 67 (41.9) | 138 (42.7) |
| Sex | |||
| Male | 50 (30.7) | 38 (23.8) | 88 (27.2) |
| Female | 133 (69.3) | 122 (76.3) | 235 (72.8) |
| Race and ethnicity | |||
| American Indian or Alaska Native | 0 | 2 (1.3) | 2 (0.6) |
| Asian | 2 (1.2) | 1 (0.6) | 3 (0.9) |
| Black or African American | 13 (8.0) | 12 (7.5) | 25 (7.7) |
| Hispanic or Latino | 31 (19.0) | 26 (16.3) | 57 (17.6) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 |
| Not Hispanic or Latino | 132 (81.0) | 134 (83.8) | 266 (82.4) |
| White | 148 (90.8) | 144 (90.0) | 292 (90.4) |
| Multiplea | 0 | 1 (0.6) | 1 (0.3) |
| Iris color | |||
| Brown | 78 (47.9) | 77 (48.1) | 155 (48.0) |
| Not brown | 85 (52.1) | 83 (51.9) | 168 (52.0) |
| Emmetrope status | |||
| Emmetrope | 125 (76.7) | 123 (76.9) | 248 (76.8) |
| Nonemmetrope | 38 (23.3) | 37 (23.1) | 75 (23.2) |
| Mesopic, high-contrast, binocular DCNVA | |||
| Mean (SD) [range], letters | 29.2 (6.6) [18-45] | 29.1 (6.0) [19-49] | 29.2 (6.3) [18-49] |
| 20/40 to 20/60 | 66 (40.5) | 62 (38.8) | 128 (39.6) |
| Worse than 20/60 | 97 (59.5) | 98 (61.3) | 195 (60.4) |
| Photopic, high-contrast, binocular CDVA, mean (SD) [range], lettersb | 60.1 (4.3) [45-70] | 60.0 (3.9) [49-68] | NA |
| Photopic, high-contrast, binocular DCIVA, mean (SD) [range], letters | 53.4 (7.2) [30-73] | 53.4 (6.9) [18-69] | NA |
Abbreviations: CDVA, corrected-distance visual acuity; DCIVA, distance-corrected intermediate visual acuity; DCNVA, distance-corrected near visual acuity; NA, not analyzed.
Participants who reported more than 1 race were only included in the multiple category.
Analyzed for the safety population; AGN-190584: n = 163 and vehicle: n = 159.
Efficacy (Intent-to-Treat Population)
The proportion of participants with an improvement of 3 or more lines of mesopic, high-contrast, binocular DCNVA was higher with AGN-190584 than vehicle on day 30 from hours 0.25 to 6 (P < .05), and comparable in both groups at hours 8 and 10 (Figure 2A). On day 30, hour 3, the percentage of participants with an improvement of 3 lines or more was 30.7% (50 of 163) in the AGN-190584 group and significantly higher compared with 8.1% (13 of 160) in the vehicle group (difference, 22.5% [95% CI, 14.3%-30.8%]; P < .001 after multiplicity adjustment). At hour 6, 18.4% (30 of 163) and 8.8% (14 of 160) of participants had improvement of 3 or more lines with AGN-190584 and vehicle, respectively (difference, 9.7% [95% CI, 2.3%-17.0%]; adjusted P = .01). Preplanned subgroup analyses showed a clinically significant proportion of participants treated with AGN-190584 with gains of 3 or more lines in mesopic DCNVA at day 30, hours 3 and 6, in all evaluated subgroups based on age, baseline binocular DCNVA, iris color, and emmetrope status (eTable 2 in Supplement 2).
Figure 2. High-Contrast, Binocular Visual Outcomes at Day 30.
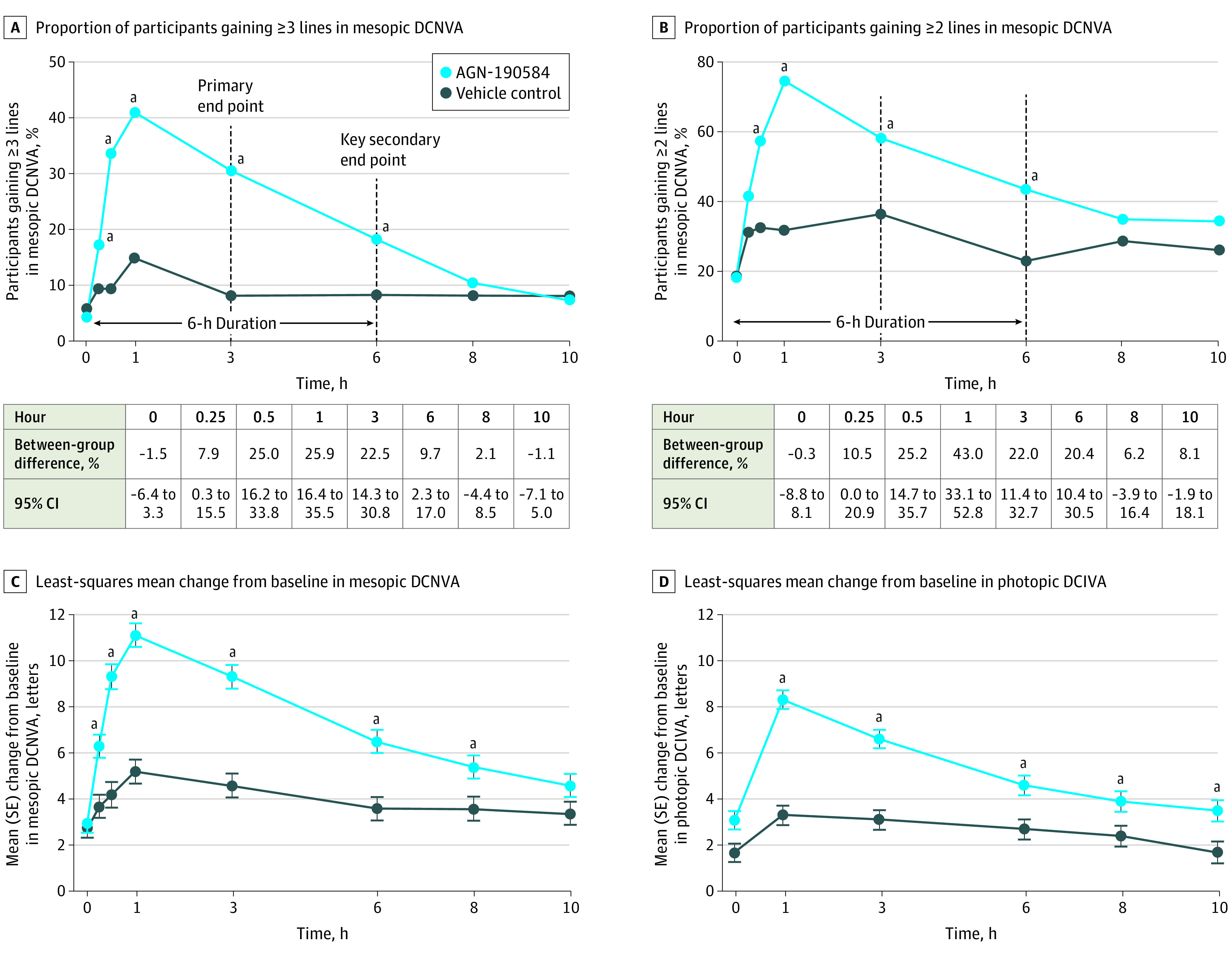
Mesopic and photopic conditions were defined as lighting 10 to 11 lux and 251 lux or more, respectively, measured at the target. DCIVA indicates distance-corrected intermediate visual acuity; DCNVA, distance-corrected near visual acuity.
a P < .05.
Peak efficacy was observed at hour 1, when 41.6% (67 of 161) of participants treated with AGN-190584 had DCNVA improvement of 3 lines or more (Figure 2A). In a post hoc analysis, no participants with DCNVA improvement of 3 lines or more had a loss of more than 5 letters in mesopic, high-contrast, binocular CDVA on day 30, hour 3. The proportion of participants with improvement of 2 lines or more in mesopic, high-contrast, binocular DCNVA was also greater with AGN-190584 than vehicle on day 30 from hours 0.5 to 6 (P < .001; Figure 2B).
Other preplanned analyses demonstrated a longer duration of effect of AGN-190584. Change from baseline in mesopic, high-contrast, binocular DCNVA was greater with AGN-190584 than vehicle on day 30 from 0.25 to 8 hours (P ≤ .01). The least-squares mean (SE) change from baseline with AGN-190584 vs vehicle was 6.3 (0.49) vs 3.7 (0.50) letters at hour 0.25 (P < .001); 9.3 (0.54) vs 4.2 (0.55) letters at hour 0.5 (P < .001); 11.1 (0.51) vs 5.2 (0.52) letters at hour 1 (P < .001); 9.3 (0.51) vs 4.6 (0.52) letters at hour 3 (P < .001); 6.5 (0.50) vs 3.6 (0.51) letters at hour 6 (P < .001); and 5.4 (0.51) vs 3.6 (0.52) letters at hour 8 (P = .009) (Figure 2C). Change from baseline in photopic DCIVA on day 30 was greater with AGN-190584 than vehicle up to 10 hours (P ≤ .01). The least-squares mean (SE) change from baseline in photopic DCIVA with AGN-190584 vs vehicle was 8.3 (0.41) vs 3.3 (0.42) letters at hour 1 (P < .001); 6.6 (0.41) vs 3.1 (0.42) letters at hour 3 (P < .001); 4.6 (0.43) vs 2.7 (0.44) letters at hour 6 (P = .001);3 .9 (0.44) vs 2.4 (0.45) letters at hour 8 (P = .01); and 3.5 (0.46) vs 1.7 (0.47) letters at hour 10 (P = .004) (Figure 2D).
The proportion of participants achieving 20/40 or better photopic, high-contrast, binocular DCNVA was greater with AGN-190584 than vehicle on day 30 from 0.5 to 3 hours (P ≤ .02). The proportion with AGN-190584 vs vehicle was 84.2% (133 of 158) vs 70.6% (108 of 153) at hour 0.5 (P = .004); 92.5% (149 of 161) vs 73.9% (113 of 153) at hour 1 (adjusted P = .01); and 84.5% (136 of 161) vs 71.9% (110 of 153) (adjusted P = .02) at hour 3. The distribution of DCNVA (Snellen equivalent) at hours 0, 3, and 6 is provided in eTable 3 in Supplement 2.
Change from baseline in mesopic Near Vision Presbyopia Task–based Questionnaire performance, Near Vision Presbyopia Task-based Questionnaire satisfaction, Presbyopia Impact and Coping Questionnaire coping, and Presbyopia Impact and Coping Questionnaire impact scores (Table 2) were significant at day 30, hour 3, favoring AGN-190584 (adjusted P = .01). Table 2 summarizes all efficacy end points.
Table 2. Summary of Primary and Secondary Efficacy End Points.
| End point | No./total No. (%) | AGN-190584 vs vehicle, difference (95% CI) | P value | Adjusted P value | |
|---|---|---|---|---|---|
| AGN-190584 | Vehicle | ||||
| P1: proportion of participants gaining 3 lines or more in mesopic, high-contrast, binocular DCNVA at day 30, hour 3a | 50/163 (30.7) | 13/160 (8.1) | 22.5 (14.3 to 30.8) | <.001 | <.001 |
| KS: proportion of participants gaining 3 lines or more in mesopic, high-contrast, binocular DCNVA at day 30, hour 6a | 30/163 (18.4) | 14/160 (8.8) | 9.7 (2.3 to 17.0) | .01 | .01 |
| S1: proportion of participants gaining 3 lines or more in mesopic, high-contrast, binocular DCNVA at day 30, hour 8b | 17/161 (10.6) | 13/153 (8.5) | 2.1 (−4.4 to 8.5) | .53 | >.99 |
| S2: change in mesopic, high-contrast, binocular DCNVA from baseline to day 30, hour 0.5, LS mean (SE), letters | 9.3 (0.54) | 4.2 (0.55) | LS mean difference, 5.1 (3.7 to 6.5) | <.001 | .01 |
| S3: proportion of participants achieving 20/40 or better photopic, high-contrast, binocular DCNVA at day 30, hour 1b | 149/161 (92.5) | 113/153 (73.9) | 18.7 (10.6 to 26.7) | <.001 | .01 |
| S4: change in photopic, high-contrast, binocular DCIVA from baseline to day 30, hour 3, LS mean (SE), letters | 6.6 (0.41) | 3.1 (0.42) | LS mean difference, 3.5 (2.4 to 4.6) | <.001 | .01 |
| S5: change in mesopic NVPTQ performance score from baseline to day 30, hour 3, LS mean (SE) | 1.4 (0.11) | 0.6 (0.11) | LS mean difference, 0.8 (0.6 to 1.1) | <.001 | .01 |
| S6: proportion of participants gaining 3 lines or more in mesopic, high-contrast, binocular DCNVA at day 30, hour 10b | 12/160 (7.5) | 13/152 (8.6) | −1.1 (−7.1 to 5.0) | .73 | >.99 |
| S7: change in mesopic, high-contrast, binocular DCNVA from baseline to day 30, hour 0.25, LS mean (SE), letters | 6.3 (0.49) | 3.7 (0.50) | LS mean difference, 2.6 (1.3 to 3.9) | <.001 | .01 |
| S8: proportion of participants achieving 20/40 or better photopic, high-contrast, binocular DCNVA at day 30, hour 3b | 136/161 (84.5) | 110/153 (71.9) | 12.6 (3.5 to 21.6) | .007 | .02 |
| S9: change in mesopic NVPTQ satisfaction score from baseline to day 30, hour 3, LS mean (SE) | 1.4 (0.1) | 0.6 (0.11) | LS mean difference, 0.8 (0.5 to 1.1) | <.001 | .01 |
| S10: change in PICQ coping score from baseline to day 30, hour 3, LS mean (SE) | −1.0 (0.07) | −0.5 (0.07) | LS mean difference, −0.5 (−0.6 to −0.3) | <.001 | .01 |
| S11: change in PICQ impact score from baseline to day 30, hour 3, LS mean (SE) | −0.7 (0.06) | −0.4 (0.06) | LS mean difference, −0.3 (−0.4 to −0.1) | .001 | .01 |
Abbreviations: DCNVA, distance-corrected near visual acuity; KS, key secondary efficacy end point; LS, least-squares; NVPTQ, Near Vision Presbyopia Task-based Questionnaire; P1, primary efficacy end point; PICQ, Presbyopia Impact and Coping Questionnaire; S, secondary efficacy end point.
Missing data were imputed as failure to achieve gain of 3 or more lines in DCNVA.
Based on observed data for all participants with data at baseline and the time point.
Pupil diameter of the nondominant eye (near vision) decreased with AGN-190584 under both mesopic and photopic conditions. At peak efficacy, the decrease from baseline was 42.3% (–1.5 mm from 3.5 mm at baseline) with AGN-190584 under photopic conditions and 52.1% (–2.4 mm from 4.6 mm at baseline) under mesopic conditions. No obvious change was observed in eyes receiving vehicle under either condition (eFigure 2 in Supplement 2).
Safety
The safety population included 322 participants; 1 participant was excluded from the vehicle group because no treatment doses were administered. Overall, 35.0% (57 of 163) and 23.3% (37 of 159) of participants in the AGN-190584 and vehicle groups, respectively, reported 1 or more treatment-emergent AEs; all reported treatment-emergent AEs are listed in eTable 4 in Supplement 2. Table 3 shows the treatment-emergent AEs reported in 2% or more of participants in either treatment group, with headache being the most common (possibly because participants were prompted/asked to rate temporal/supraorbital headaches per a visual analog scale). In the AGN-190584–treated group, 87% (20 of 23) of headaches were considered mild and none required treatment; no participants discontinued the study owing to headaches. No deaths or serious treatment-emergent AEs occurred during the treatment period.
Table 3. Treatment-Emergent Adverse Events Reported in >2% of Participants in Either Treatment Group.
| System organ class preferred term | No. (%) | |
|---|---|---|
| AGN-190584 (n = 163) | Vehicle (n = 159) | |
| Eye disorders | ||
| Visual impairment | 7 (4.3) | 1 (0.6) |
| Conjunctival hyperemia | 4 (2.5) | 4 (2.5) |
| Vision blur | 4 (2.5) | 2 (1.3) |
| Eye irritation | 4 (2.5) | 1 (0.6) |
| Eye pain | 4 (2.5) | 1 (0.6) |
| Lacrimation increased | 4 (2.5) | 0 |
| Punctate keratitis | 1 (0.6) | 5 (3.1) |
| Nervous system disorders | ||
| Headachea | 23 (14.1) | 15 (9.4) |
| Gastrointestinal disorders | ||
| Nausea | 4 (2.5) | 0 |
Participants were asked to provide a subjective rating of temporal/supraorbital headaches using a visual analog scale, which may have prompted reports of headaches as an adverse event.
Discussion
In this study, AGN-190584 met the primary and key secondary efficacy end points: statistically significant differences in the proportion of participants achieving an improvement of 3 or more lines in mesopic, high-contrast, binocular DCNVA were observed on hours 3 and 6, respectively, on day 30, favoring AGN-190584. Mesopic lighting conditions were used for these outcomes because patients with presbyopia have the most difficulty with reading in dim lighting. Additionally, no participant with an improvement of 3 lines or more in mesopic DCNVA at hour 3 on day 30 lost more than 5 letters in CDVA. The rapid onset of action (15 minutes) and duration of effect (≥6 hours) of AGN-190584 were maintained through day 30. The secondary efficacy end point of functional vision (achieving 20/40 or better photopic, high-contrast, binocular DCNVA), which allows participants to read 6-point fonts from a distance of 14 inches (approximately 35 cm),26 was also met.
Analyses of patient-reported outcome end points demonstrated significant treatment benefits of AGN-190584. Participants receiving AGN-190584 reported greater ability and satisfaction regarding near-vision reading, and a clinically meaningful reduction in use of presbyopia coping mechanisms, compared with participants receiving vehicle. The end point of improvement of 2 lines or more in mesopic, high-contrast, binocular DCNVA was not prespecified but was included because improvement of 2 lines or more was considered clinically meaningful to participants27; improvement of 3 lines or more was used for the prespecified primary and secondary end points, per FDA requirement.28
Presbyopia and vision impairment have been associated with poor quality of life.29,30,31,32,33 Presbyopia can hinder daily living activities, hobbies, and social interactions and cause psychological distress.31 Near vision is used for daily activities such as reading newspapers, smartphones, prescription labels on medications, and menus, while intermediate vision is used for social interactions, computer work, and cooking.34 Commonly used presbyopia treatment options are over-the-counter unifocal reading glasses that primarily correct near vision and multifocal glasses that often have a narrow range for intermediate distance correction. For both options, correction at all viewing distances is limited. AGN-190584 improved near vision and demonstrated improvement in DCIVA through 10 hours on day 30. The mechanism of action of AGN-190584 is through dynamic pupil modulation, in which the iris sphincter is contracted to reduce the pupil size to an optimal range.20 Pupil size reduction increases depth of focus and allows for a greater range of uninterrupted near and intermediate vision that cannot be achieved using eyeglasses.
Treatment with AGN-190584 was well tolerated, with most treatment-related ocular AEs reported as mild in intensity. Participants in both treatment groups had no clinically significant changes in photopic or mesopic, high-contrast, binocular CDVA, biomicroscopy or ophthalmoscopy findings, intraocular pressure, and vital signs. Headache associated with use of pilocarpine eye drops for glaucoma treatment has led to participant discontinuations in clinical studies.35,36,37,38 In GEMINI 1, no discontinuations from the study were due to headache, and the risk of headache (AGN-190584, 14.1%; vehicle, 9.4%) appeared lower than that previously seen with other ocular pilocarpine formulations (>20%),35,37,38 despite being prompted. Furthermore, 87% of headaches that were related to AGN-190584 were mild and transient, requiring no treatment. Overall, AGN-190584 demonstrated an acceptable safety profile and did not impair distance vision; the overall benefit-risk supports the use of AGN-190584 for presbyopia management, and AGN-190584 recently became the first FDA-approved pharmacologic therapy for presbyopia.
Compared with commercially available pilocarpine eye drops used for glaucoma, AGN-190584 is better tolerated, likely owing to its proprietary vehicle that equilibrates to the ocular surface pH within 1 minute21 (rising from approximately 4, needed to ensure drug stability,39,40 to approximately 7) to mitigate AEs (eg, stinging and vision blur). Results from in vitro and clinical/phase 1 studies showed that the proprietary vehicle of AGN-190584 allowed pilocarpine to achieve faster equilibration to the tear film’s physiologic pH, compared with a commercially available generic pilocarpine formulation (pH of approximately 4) that did not reach physiologic pH (approximately 7) in simulated tears, even after 10 minutes.21 Previous studies using a generic pilocarpine formulation have reported that up to 67% of participants had vision blur lasting less than 20 minutes36 and 11% reported burning or stinging.35 In GEMINI 1, vision blur, eye irritation, and eye pain each occurred in only 2.5% of participants treated with AGN-190584. Bioavailability of pilocarpine is increased with the AGN-190584 formulation because of the rapid shift of the drug to a predominantly nonionized form at a pH of approximately 7 that facilitates transcorneal drug penetration. This leads to faster pupillary constriction41 and improved aqueous humor dynamics39 compared with a low-pH pilocarpine formulation.
Limitations
Although the primary end point was assessed under mesopic conditions, the effects of pilocarpine on night vision were not evaluated. A separate clinical trial (NCT04837482) specifically designed to determine the impact of AGN-190584 on night driving performance is underway. Once-daily dosing was evaluated, but more frequent use of AGN-190584 may be needed for adequate reading vision throughout the day; this may be evaluated in future clinical trials. Also, near visual acuity improvements with pilocarpine were not directly compared with those obtained by spectacle correction for near visual acuity.
Conclusions
AGN-190584 applied bilaterally once daily was statistically superior in efficacy compared with vehicle in increasing the proportion of participants with improvement of 3 lines or more in mesopic DCNVA at hours 3 and 6 (but not at hour 8) on day 30, while being safe and well tolerated through 30 days. Achievement of 3-line gains is a rigorous efficacy end point that was required by the FDA. Other efficacy analyses, including change from baseline in mesopic DCNVA and photopic DCIVA letters, demonstrated a duration of AGN-190584 effect out to 8 and 10 hours. The primary and key secondary efficacy end points were met, with AGN-190584 improving functional near and intermediate vision for 30 days without compromising distance vision in individuals with presbyopia.
Trial Protocol
eMethods.
eFigure 1. Graphical Testing Procedure
eFigure 2. Pupil Diameter in the Nondominant Eye on Day 30 (Near Vision)
eTable 1. Participant Eligibility Criteria
eTable 2. Preplanned Subgroup Analyses of Primary and Key Secondary Efficacy Endpoints
eTable 3. Distribution of Mesopic and Photopic High-Contrast, Binocular Distance-Corrected Near Visual Acuity (Snellen Equivalent) on Day 30
eTable 4. All Reported Treatment-Emergent Adverse Events, Number (%) of Participants (Safety Population)
Data Sharing Statement
References
- 1.Fricke TR, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125(10):1492-1499. doi: 10.1016/j.ophtha.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 2.Waring GO, Rocha KM. Characterization of the dysfunctional lens syndrome and a review of the literature. Curr Ophthalmol Rep. 2018;6(4):249-255. doi: 10.1007/s40135-018-0190-3 [DOI] [Google Scholar]
- 3.Mercer RN, Milliken CM, Waring GO IV, Rocha KM. Future trends in presbyopia correction. J Refract Surg. 2021;37(S1):S28-S34. doi: 10.3928/1081597X-20210408-06 [DOI] [PubMed] [Google Scholar]
- 4.Waring GO IV, Chang DH, Rocha KM, Gouvea L, Penatti R. Correlation of intraoperative optical coherence tomography of crystalline lens diameter, thickness, and volume with biometry and age. Am J Ophthalmol. 2021;225:147-156. doi: 10.1016/j.ajo.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 5.Haddad JS, Rocha KM, Yeh K, Waring GO IV. Lens anatomy parameters with intraoperative spectral-domain optical coherence tomography in cataractous eyes. Clin Ophthalmol. 2019;13:253-260. doi: 10.2147/OPTH.S184208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krag S, Andreassen TT. Mechanical properties of the human posterior lens capsule. Invest Ophthalmol Vis Sci. 2003;44(2):691-696. doi: 10.1167/iovs.02-0096 [DOI] [PubMed] [Google Scholar]
- 7.Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Prog Retin Eye Res. 2003;22(6):749-767. doi: 10.1016/S1350-9462(03)00063-6 [DOI] [PubMed] [Google Scholar]
- 8.Schachar RA. Pathophysiology of accommodation and presbyopia: understanding the clinical implications. J Fla Med Assoc. 1994;81(4):268-271. [PubMed] [Google Scholar]
- 9.Smith P. On the growth of the crystalline lens. Trans Ophthalmol Soc UK. 1883;3:79-99. [Google Scholar]
- 10.Kandel H, Khadka J, Goggin M, Pesudovs K. Impact of refractive error on quality of life: a qualitative study. Clin Exp Ophthalmol. 2017;45(7):677-688. doi: 10.1111/ceo.12954 [DOI] [PubMed] [Google Scholar]
- 11.American Optometric Association . Optometric clinical practice guideline: care of the patient with presbyopia. Revised December 2010. Accessed September 8, 2021. https://www.sdeyes.org/docs/CPG-17.pdf
- 12.Lord SR, Dayhew J, Howland A. Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc. 2002;50(11):1760-1766. doi: 10.1046/j.1532-5415.2002.50502.x [DOI] [PubMed] [Google Scholar]
- 13.Wolffsohn JS, Davies LN. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019;68:124-143. doi: 10.1016/j.preteyeres.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 14.Benozzi G, Perez C, Leiro J, Facal S, Orman B. Presbyopia treatment with eye drops: an eight year retrospective study. Transl Vis Sci Technol. 2020;9(7):25. doi: 10.1167/tvst.9.7.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renna A, Vejarano LF, De la Cruz E, Alió JL. Pharmacological treatment of presbyopia by novel binocularly instilled eye drops: a pilot study. Ophthalmol Ther. 2016;5(1):63-73. doi: 10.1007/s40123-016-0050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger-Westphal-stimulated accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2005;46(2):609-617. doi: 10.1167/iovs.04-0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendt M, Glasser A. Topical and intravenous pilocarpine stimulated accommodation in anesthetized rhesus monkeys. Exp Eye Res. 2010;90(5):605-616. doi: 10.1016/j.exer.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron ME. Headaches in relation to the eyes. Med J Aust. 1976;1(10):292-294. doi: 10.5694/j.1326-5377.1976.tb140626.x [DOI] [PubMed] [Google Scholar]
- 19.Urriquia MTB, Marin JDF Jr. Efficacy of topical pilocarpine in the management of primary aqueous tear deficiency: an initial study. Philipp J Ophthalmol. 2014;39(1):6-11. [Google Scholar]
- 20.Price FW, Hom M, Moshirfar M, et al. . Combinations of pilocarpine and oxymetazoline for the pharmacological treatment of presbyopia: two randomized phase 2 studies. Ophthalmol Sci. 2021;1:100065. doi: 10.1016/j.xops.2021.100065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giyanani J, Shabaik Y, Penzner J, Gore A. Novel, fast-equilibrating, ophthalmic vehicle for enhanced patient comfort and tolerability for pilocarpine delivery (poster 895110). Poster presented at: American Association of Pharmaceutical Scientists PharmSci 360 Annual Meeting; October 26-November 5, 2020; virtual. [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.National Eye Institute . National Eye Institute visual functioning questionnaire–25 (VFQ-25) version 2000. Published January 2000. Accessed October 12, 2021. https://www.nei.nih.gov/sites/default/files/2019-06/vfq_ia.pdf
- 24.Johnson N, Shirneshan E, Coon CD, et al. Development of the Presbyopia Impact and Coping Questionnaire. Ophthalmol Ther. 2021;10(4):1057-1075. doi: 10.1007/s40123-021-00391-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirneshan E, Coon CD, Johnson N, et al. Development of the Near Vision Presbyopia Task-based Questionnaire for use in evaluating the impact of presbyopia. J Patient Rep Outcomes. 2021;5(1):125. doi: 10.1186/s41687-021-00378-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwiegerling J. Field guide to visual and ophthalmic optics. SPIE. Published 2004. Accessed January 28, 2022. doi: 10.1117/3.592975 [DOI]
- 27.Evans DG, Coon C, Nichols K, Shirneshan E, Campbell J. Interpreting clinically meaningful near vision improvement in presbyopia with AGN-190584 in the GEMINI 1 phase 3 trial. Presented at: American Academy of Optometry Annual Meeting; November 4, 2021; Boston, MA. [Google Scholar]
- 28.Csaky KG, Richman EA, Ferris FL III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49(2):479-489. doi: 10.1167/iovs.07-1132 [DOI] [PubMed] [Google Scholar]
- 29.Tahhan N, Papas E, Fricke TR, Frick KD, Holden BA. Utility and uncorrected refractive error. Ophthalmology. 2013;120(9):1736-1744. doi: 10.1016/j.ophtha.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 30.Lu Q, Congdon N, He X, Murthy GVS, Yang A, He W. Quality of life and near vision impairment due to functional presbyopia among rural Chinese adults. Invest Ophthalmol Vis Sci. 2011;52(7):4118-4123. doi: 10.1167/iovs.10-6353 [DOI] [PubMed] [Google Scholar]
- 31.Lamoureux EL, Fenwick E, Moore K, Klaic M, Borschmann K, Hill K. Impact of the severity of distance and near-vision impairment on depression and vision-specific quality of life in older people living in residential care. Invest Ophthalmol Vis Sci. 2009;50(9):4103-4109. doi: 10.1167/iovs.08-3294 [DOI] [PubMed] [Google Scholar]
- 32.Patel I, Munoz B, Burke AG, et al. Impact of presbyopia on quality of life in a rural African setting. Ophthalmology. 2006;113(5):728-734. doi: 10.1016/j.ophtha.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 33.McDonnell PJ, Lee P, Spritzer K, Lindblad AS, Hays RD. Associations of presbyopia with vision-targeted health-related quality of life. Arch Ophthalmol. 2003;121(11):1577-1581. doi: 10.1001/archopht.121.11.1577 [DOI] [PubMed] [Google Scholar]
- 34.Institute for Quality and Efficiency in Health Care (IQWiG) . How can presbyopia be corrected? Updated June 4, 2020. Accessed Aug 11, 2021. https://www.ncbi.nlm.nih.gov/books/NBK423827/
- 35.Hartenbaum D, Maloney S, Vaccarelli L, Liss C, Wilson H, Gormley GJ. Comparison of dorzolamide and pilocarpine as adjunctive therapy in patients with open-angle glaucoma and ocular hypertension. Clin Ther. 1999;21(9):1533-1538. doi: 10.1016/S0149-2918(00)80008-9 [DOI] [PubMed] [Google Scholar]
- 36.Kałużny J, Sobecki R, Czechowicz-Janicka K, et al. Efficacy and safety of latanoprost versus pilocarpine/timolol maleate fixed combination in patients with primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2008;86(8):860-865. doi: 10.1111/j.1755-3768.2008.01324.x [DOI] [PubMed] [Google Scholar]
- 37.Laibovitz R, Boyle J, Snyder E, Strohmaier K, Adamsons I. Dorzolamide versus pilocarpine as adjunctive therapies to timolol: a comparison of patient preference and impact on daily life. Clin Ther. 1996;18(5):821-832. doi: 10.1016/S0149-2918(96)80042-7 [DOI] [PubMed] [Google Scholar]
- 38.Nagasubramanian S. A comparison of the ocular hypotensive efficacy, safety and acceptability of brimonidine 0.2% twice daily versus pilocarpine 2.0% thrice daily as adjunct therapy with beta-blockers. In: Glaucoma Update VI. Springer; 2000. doi: 10.1007/978-3-642-57056-8_31 [DOI] [Google Scholar]
- 39.Anderson RA, Cowle JB. Influence of pH on the effect of pilocarpine on aqueous dynamics. Br J Ophthalmol. 1968;52(8):607-611. doi: 10.1136/bjo.52.8.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David R, Goldberg L, Luntz MH. Influence of pH on the efficacy of pilocarpine. Br J Ophthalmol. 1978;62(5):318-319. doi: 10.1136/bjo.62.5.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birmingham AT, Galloway NR, Spencer SA. A comparison of the pupilloconstrictor effect of pilocarpine solution administered to the conjunctival sac as a single drop or as a continuous infusion in normal subjects. Br J Ophthalmol. 1976;60(8):568-572. doi: 10.1136/bjo.60.8.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eFigure 1. Graphical Testing Procedure
eFigure 2. Pupil Diameter in the Nondominant Eye on Day 30 (Near Vision)
eTable 1. Participant Eligibility Criteria
eTable 2. Preplanned Subgroup Analyses of Primary and Key Secondary Efficacy Endpoints
eTable 3. Distribution of Mesopic and Photopic High-Contrast, Binocular Distance-Corrected Near Visual Acuity (Snellen Equivalent) on Day 30
eTable 4. All Reported Treatment-Emergent Adverse Events, Number (%) of Participants (Safety Population)
Data Sharing Statement