Abstract
National guidelines support the use of hydroxyurea (HU) in high-risk patients with polycythemia vera (PV). In this study, we investigated HU treatment patterns in patients with PV. Of patients who received HU for ≥ 3 months, 32.3% had dose adjustments, 23.7% had dose interruptions, and 18.6% discontinued HU. These results emphasize the need for active management of patients with PV.
Background:
Polycythemia vera (PV) is associated with increased blood cell counts, risk of thrombosis, and symptoms including fatigue and pruritus. National guidelines support the use of hydroxyurea (HU) in high-risk patients or those with some other clinical indication for cytoreduction.
Patients and Methods:
REVEAL is a prospective, observational study designed to collect data pertaining to demographics, disease burden, clinical management, patient-reported outcomes, and health care resource utilization of patients with PV in the United States. In this analysis, HU treatment patterns and outcomes were assessed from 6 months prior to enrollment to the time of discontinuation, death, or data cutoff.
Results:
Of the 1381 patients who received HU for ≥3 months, the median HU exposure was 23.6 months (range, 3.1–38.5 months). The most common maximum daily HU doses were 1000 mg (30.6%) and 500 mg (30.1%); only 6.4% received ≥2 g/d HU. Approximately one-third (32.3%) of patients had dose adjustments, 23.8% had dose interruptions, and 257 (18.6%) discontinued HU. The most common reasons for HU discontinuations and interruptions were adverse events/intolerance (37.1% and 54.5%, respectively) and lack of efficacy (35.5% and 22.1%, respectively). Of those who received HU for ≥3 months, 57.1% had hematocrit values > 45% on ≥1 occasion, 33.1% continued to receive phlebotomies, and 27.4% had uncontrolled myeloproliferation.
Conclusion:
The results of this analysis emphasize the need for active management of patients with PV with appropriate HU dose titration to maintain blood count control while monitoring for signs and symptoms of HU intolerance.
Keywords: Cytopenia, Hematocrit, Myeloproliferative neoplasm, Observational study, Prospective
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm associated with erythrocytosis, increased risk of thrombosis, and symptoms including fatigue, early satiety, abdominal discomfort, and pruritus.1,2 The primary goals of risk-adapted therapy for patients with PV include prevention of thromboembolism, reduction of symptom burden, and reduction of bleeding risk.3–5
The European LeukemiaNet (ELN) response criteria for PV include hematocrit (HCT) < 45%.6,7 Hydroxyurea (HU) is the most commonly used first-line cytoreductive medication for patients with high-risk PV. The CYTO-PV study was a prospective, randomized study that demonstrated that an HCT target < 45% was associated with a reduced rate of thrombotic complications.8
An elevated white blood cell (WBC) count has also been implicated in the increased risk of thrombosis in patients with PV. A multivariate, time-dependent sub-analysis of CYTO-PV showed increased risk of thrombosis in patients with WBC count > 11 × 109/L, which has been confirmed by several similar analyses.9–11 However, unlike HCT control, control of leukocytosis has not been shown to reduce thrombosis risk in a prospective study.
The ELN and National Comprehensive Cancer Network (NCCN) Guidelines support the use of HU or interferons in patients with an indication for cytoreductive therapy to achieve and maintain HCT < 45%.4,5 Although in the United States, HU is more commonly used, HU or interferon can lead to HCT control, and both may be supplemented with phlebotomy to achieve HCT < 45%.
A proportion of patients who receive HU fail to achieve HCT and other blood count control.12 Moreover, approximately one-quarter of patients will develop resistance and/or intolerance to HU. The ELN convened a group of experts to develop a consensus definition of clinical resistance and intolerance to HU in patients with PV (Table 1).13 Of note, this consensus definition was developed for use in clinical research and was not entirely based on rigorously designed and controlled trials. A modified version of the definition has been used in clinical trials to identify patients with PV who are resistant/intolerant to HU.14 In addition, the definition has been included in NCCN Guidelines, where it is intended to help health care providers identify high-risk patients who have a potential indication for a change in cytoreductive therapy. Although this definition may not have been universally incorporated into clinical practice, the criteria contained within the definition can provide a useful framework to examine and discuss the treatment patterns of HU in patients with PV.
Table 1.
Definition of Resistance/Intolerance to HU in Patients With PV13
| 1. Need for phlebotomy to keep HCT < 45% after 3 months of ≥ 2 g/d HU, OR |
| 2. Uncontrolled myeloproliferation (ie, PLT count > 400 × 109/L AND WBC count > 10 × 109/L) after 3 months of ≥ 2 g/d HU, OR |
| 3. Failure to reduce massive splenomegalya by > 50% as measured by palpation OR failure to completely relieve symptoms related to splenomegaly after 3 months of ≥ 2 g/d HU, OR |
| 4. ANC < 1.0 × 109/L OR PLT count < 100 × 109/L OR HGB < 10 g/dL at the lowest dose of HU required to achieve a complete or partial clinicohematologic response,b OR |
| 5. Presence of leg ulcers or other unacceptable HU-related nonhematologic toxicities, such as mucocutaneous manifestations, gastrointestinal symptoms, pneumonitis, or fever at any dose of HU |
Abbreviations: ANC = absolute neutrophil count; HCT = hematocrit; HGB = hemoglobin; HU = hydroxyurea; PLT = platelet; PV = polycythemia vera; WBC = white blood cell.
Organ extending > 10 cm below the costal margin.
Complete response defined as HCT < 45% without phlebotomy, PLT count ≤ 400 × 109/L, WBC count ≤ 10 × 109/L, and no disease-related symptoms. Partial response defined as HCT < 45% without phlebotomy or response in ≥ 3 other criteria.
REVEAL (ClinicalTrials.gov, NCT02252159) is a prospective, observational study of patients with PV in the United States, designed to collect data pertaining to the demographics, disease burden, clinical management, patient-reported outcomes, and health care resource utilization of these patients. This analysis of REVEAL characterized HU treatment patterns, including dosing, blood count control, and HU intolerance.
Patients and Methods
This is an exploratory, longitudinal sub-analysis of data from REVEAL. Central (Sterling) and investigative site institutional review board approvals were obtained as applicable. The current analysis includes data collected from July 22, 2014, to May 18, 2017. This study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, and all patients provided written informed consent.
Patients
Patients were aged ≥ 18 years, had a clinical diagnosis of PV established by their treating physician, and were willing and able to complete questionnaires, either alone or with minimal assistance. Patients must have been under physician supervision for management of their PV. Patients who were participating in an active, blinded clinical trial; had a life expectancy of < 6 months; had a diagnosis of myelofibrosis, acute myeloid leukemia, or myelodysplastic syndrome; had a history of, or active plan to proceed to, allogeneic hematopoietic stem cell transplant within 3 months of enrollment; or had a splenectomy were excluded. All decisions regarding patient care were made by the treating physician.
Data Collection
At the time of enrollment, data pertaining to demographics, PV diagnosis, disease course, medical history, and family history were obtained from patient charts and entered into an electronic clinical research form. Retrospective data were collected up to 6 months prior to enrollment, whereas prospective data on PV disease course, phlebotomy procedures, laboratory results, and medications were collected from the time of enrollment.
To ensure maximum data capture for enrolled patients, an index date was defined as 6 months prior to the date of enrollment for each patient. A post-index period was defined as the time from index to patient discontinuation from the study, death, or data cutoff, whichever came first. Patients who had received HU continuously for ≥ 3 months during the post-index period were included in the analyses. All assessments were done after the first 3 months of continuous HU, and while receiving HU during the post index period.
HU Treatment Patterns
During the post-index period, HU treatment patterns, including duration of exposure, maximum daily dose, duration of maximum daily dose, dose changes, dose interruptions (defined as interruption > 14 days), and permanent discontinuations (defined as HU dose discontinued and not restarted during the post index period) were compiled. The reasons for dose changes, interruptions, and discontinuations were also summarized. Physicians were asked to choose from 6 options to describe the reasons for treatment discontinuation: adverse events, disease progression, lack of efficacy, physician decision, patient decision, or other.
Assessments During HU Treatment
During HU treatment, blood counts were examined relative to the ELN definition of response and resistance/intolerance criteria (Table 1) irrespective of HU dose; health care providers were not required to use the ELN definition and may or may not have been aware of the ELN definition when making treatment decisions. The following assessments were made after 3 months of HU exposure during the post-index period: proportion of patients with elevated HCT values (HCT > 45%) with or without phlebotomies; proportion of patients continuing to require phlebotomies; proportion of patients with uncontrolled myeloproliferation (ie, platelet count > 400 × 109/L and WBC count > 10 × 109/L); and presence of splenomegaly as assessed by palpation. The proportion of patients with HCT < 45% and cytopenias, defined as leukopenia (WBC count < 4 × 109/L), thrombocytopenia (platelet count < 100 × 109/L), anemia (hemoglobin < 10 g/dL), and neutropenia (absolute neutrophil count < 1 × 109/L), was evaluated in patients who had a minimum of 2 HCT results. All patients with laboratory values during the post index period while on HU were included in the analyses. Non-hematologic adverse events attributed to HU were also summarized.
All analyses were exploratory in nature and were evaluated with descriptive statistics. Statistical analyses were performed using SAS statistical software (SAS Institute, Cary, NC).
Results
In total, 2510 patients were enrolled from 227 sites; 1432 received HU, of whom 1381 received HU for ≥ 3 months and were evaluable in the post-index period (Table 2). The median age was 69 years (range, 26–94 years), 49.7% were male, and 89.4% were white. The median disease duration was 4.3 years (range, 0–36.5 years). At the time of enrollment, 84.4% of all patients treated with HU for ≥ 3 months had high-risk PV, defined as age ≥ 60 years and/or prior history of thrombosis.
Table 2.
Baseline Demographics and Disease Characteristics at Time of Enrollment
| Variable | All Patients (N = 2510) | Received HU for ≥ 3 Months (n = 1381) |
|---|---|---|
| Median age (range), y | 67.0 (22.0–95.0) | 69.0 (26.0–94.0) |
| Sex, n (%) | ||
| Female | 1150 (45.8) | 695 (50.3) |
| Male | 1360 (54.2) | 686 (49.7) |
| Race, n (%) | ||
| White | 2237 (89.1) | 1235 (89.4) |
| African American | 143 (5.7) | 81 (5.9) |
| Asian | 37 (1.5) | 19 (1.4) |
| Other/no information | 93 (3.7) | 46 (3.3) |
| Median disease duration (range), y | 4.0 (0.0–56.3) | 4.3 (0.0–36.5) |
| Disease duration, y, n (%) | ||
| < 1 | 543 (21.6) | 270 (19.6) |
| 1 to < 3 | 500 (19.9) | 270 (19.6) |
| 3 to < 5 | 391 (15.6) | 230 (16.7) |
| ≥ 5 | 1051 (41.9) | 597 (43.2) |
| Missing | 25 (1.0) | 14 (1.0) |
| Risk category at enrollment, n (%) | ||
| High | 1939 (77.3) | 1165 (84.4) |
| Low | 571 (22.7) | 216 (15.6) |
Abbreviation: HU = hydroxyurea.
HU Treatment Patterns
The median exposure to HU during the post-index period was 23.6 months (range, 3.1–38.5 months). Most (72.1%) patients received between 500 and 1000 mg/d HU; the most common maximum daily HU doses were 1000 mg (30.6%) and 500 mg (30.1%), and the median duration of the continuous maximum daily dose was 19.6 months (range, 0–38.5 months) (Table 3). A small proportion (6.4%) of patients received ≥ 2 g/d HU.
Table 3.
HU Dose Intensity and Exposure
| Received HU for ≥ 3 Months (n = 1381) | |
|---|---|
| Median maximum daily dose (range), mg/d | 1000.0 (71.4–5571.4) |
| Maximum daily dose, mg/d, n (%) | |
| < 400 | 91 (6.6) |
| 500 | 415 (30.1) |
| 750 | 159 (11.5) |
| 1000 | 423 (30.6) |
| 1500 | 204 (14.8) |
| 2000 | 61 (4.4) |
| > 2000 | 28 (2.0) |
| Median duration of maximum daily dose, (range), mos | 19.6 (0.0–38.5) |
| Median HU exposure post-index (range), mos | 23.6 (3.1–38.5) |
Abbreviation: HU = hydroxyurea.
Approximately one-third (32.3%) of patients had HU dose adjustments during the post-index period; 26.1% had dose increases, 19.6% had dose decreases, and 14.1% had both increases and decreases. The most common reasons for dose increases or decreases were lack of efficacy (72.0%) and adverse events (54.5%), respectively (Figure 1). Dose changes were more common among patients who recently started HU (<1 year from enrollment) (24.4% and 17.6%, respectively) within the 6-month post-index period, compared with patients who had the disease for a longer duration (≥5 years; 5.4% and 4.9%, respectively) (Figure 2).
Figure 1.
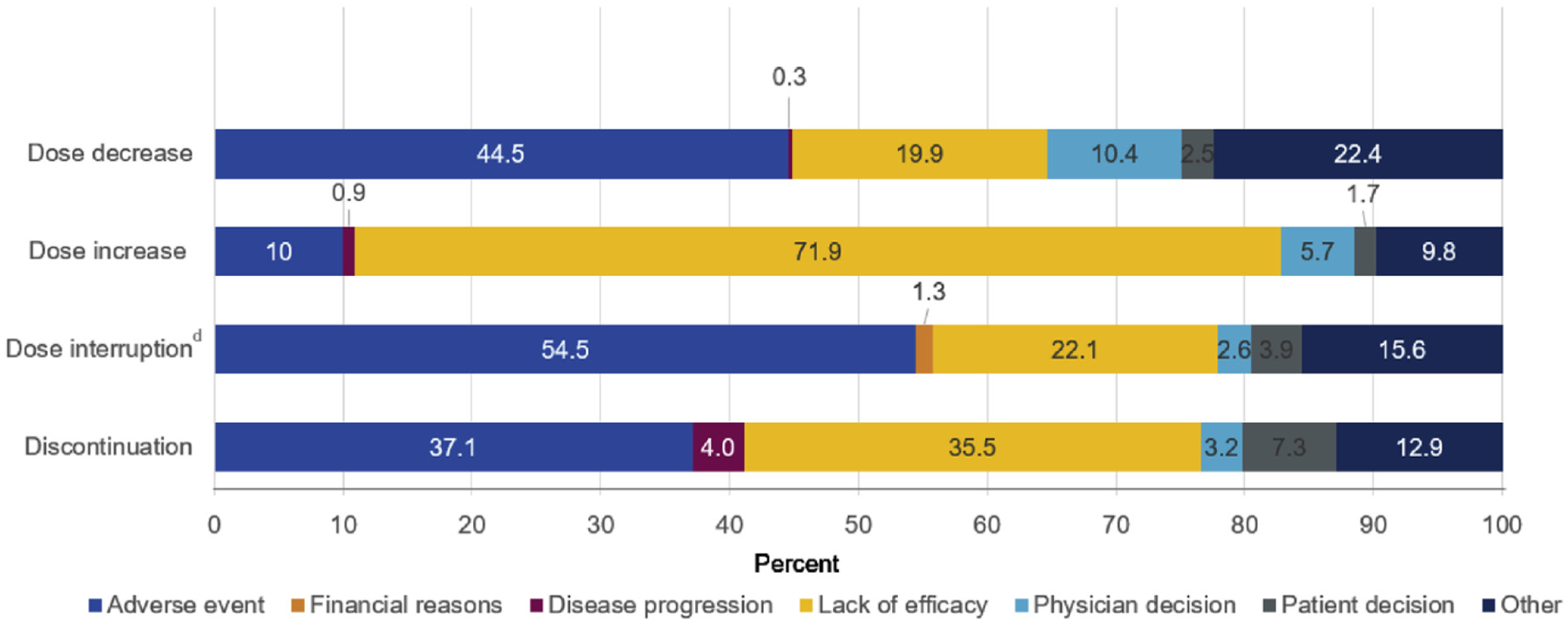
Reasons for HU Dose Adjustment or Discontinuation.a-c aAll Dose Changes, Interruptions, and Discontinuations Were Summarized Across All Patients During Their Complete HU Exposure. bCohorts Were Not Mutually Exclusive. cAny Occurrence of Dose Change was Used as the Denominator; the Same Patient Could Contribute to Multiple Dose Adjustments. dInterrupted for ≥ 14 Days
Abbreviation: HU = hydroxyurea.
Figure 2.
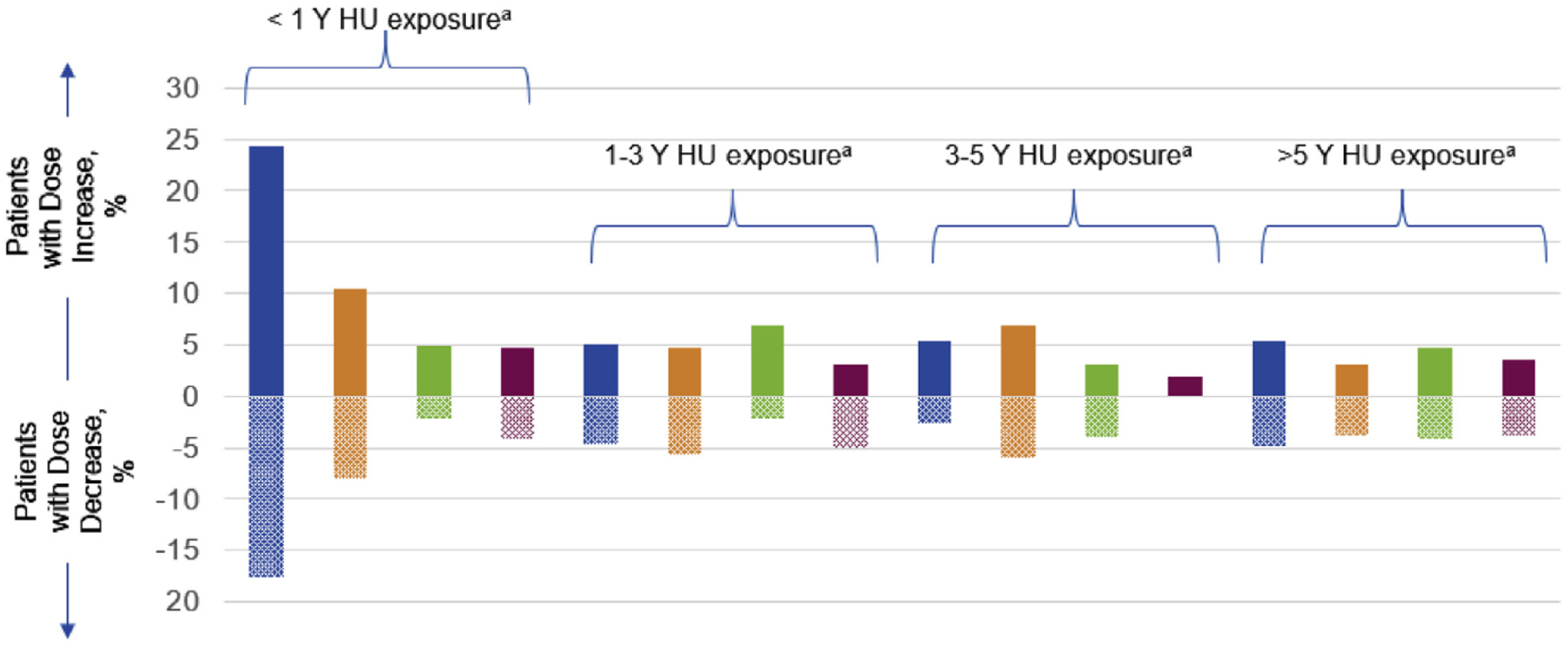
Proportion of HU Dose Adjustments by Time of HU Start and Exposure
Abbreviation: HU = hydroxyurea. aPre-enrollment; bpost-enrollment.
A total of 257 (18.6%) patients discontinued HU treatment by the end of the post-index period. Approximately one-quarter (23.8%; n = 329) of patients had a dose interruption; only 72 (21.9%) of these patients restarted HU during the index period. The most common reasons for HU dose discontinuation were adverse events (37.1%) and lack of efficacy (35.5%); the most common reasons for HU dose interruption were adverse events (54.5%) and lack of efficacy (22.1%). The majority (81.4%; n = 1124) of patients who received HU for at least 3 months continued with HU treatment at the time of data cutoff.
Laboratory Values and Spleen Assessments
Of the 1381 patients, 57.1% of patients had ≥ 1 HCT value > 45% after receiving HU for 3 months (45.2% of patients had ≥ 2 HCT values > 45%, 37.6% of patients had ≥ 3 HCT values > 45%, and 32.1% of patients had ≥ 4 HCT values > 45%). Approximately one-third (33.1%; n = 457) of patients continued to receive phlebotomies; 82.9% of these patients requiring phlebotomies continued to report HCT values > 45%. Over one-quarter (27.4%) of patients had uncontrolled myeloproliferation after being on HU for ≥ 3 months, including 44.9% with ≥ 1 WBC count > 10 × 109/L and 49.2% with ≥ 1 platelet count > 400 × 109/L (Figure 3). Of the 1381 patients who received HU for ≥ 3 months, spleen assessment by physical examination was performed in 973 (70.5%) patients. Of these, 181 (18.6%) had palpable splenomegaly. Spleen length measurements were available for 66 patients, 27.3% of whom had spleen length ≥ 10 cm from the left costal margin to the point of greatest splenic extension.
Figure 3.
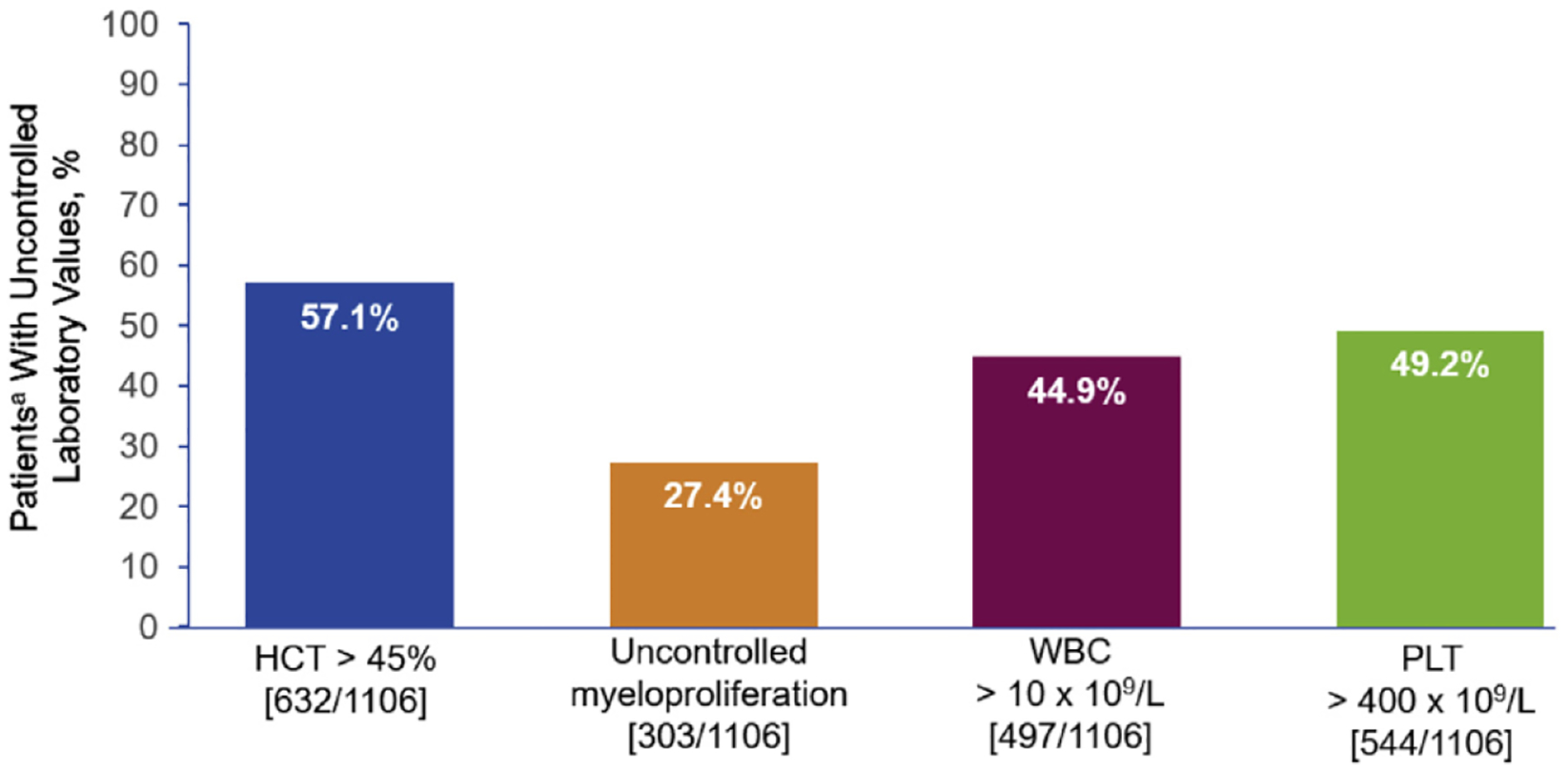
Uncontrolled Laboratory Values in Patients With PV Who Received HU for ≥ 3 Months
Abbreviations: HCT = hematocrit; HU = hydroxyurea; PLT = platelet; PV = polycythemia vera; WBC = white blood cell. aOf patients with > 1 corresponding laboratory value during the post-enrollment period while on HU. Analysis included patients with all 3 laboratory values.
Adverse Events
Of the 1381 patients who received HU for ≥ 3 months during the post-index period, HU-related non-hematologic adverse events occurred in 7.2% of patients after initiating HU (Table 4). Cytopenias were reported for 14.6% of patients while on HU; 7.6% had ≥ 1 hemoglobin value < 10 g/dL, 7.4% had ≥ 1 platelet count < 100 × 109/L, and 1.9% had ≥ 1 absolute neutrophil count < 1 × 109/L.
Table 4.
Summary of Toxicitiesa in Patients Treated With HU for ≥ 3 Months
| Adverse Event | n (%) (n = 1381) |
|---|---|
| Nonhematologic | 100 (7.2) |
| Gastrointestinal disorders | 89 (6.4) |
| Aphthous stomatitis | 2 (0.1) |
| Colitis ulcerative | 1 (0.1) |
| Constipation | 24 (1.7) |
| Diarrhea | 30 (2.2) |
| Duodenal ulcer | 1 (0.1) |
| Intestinal perforation | 1 (0.1) |
| Nausea | 29 (2.1) |
| Stomatitis | 9 (0.7) |
| Vomiting | 13 (0.9) |
| Skin and subcutaneous tissue disorders | 14 (1.0) |
| Decubitus ulcer | 1 (0.1) |
| Skin ulcer | 13 (0.9) |
Abbreviation: HU = hydroxyurea.
These are treatment-emergent adverse events and were not necessarily attributed to HU.
Hematocrit Control and Cytopenias
Of the 1154 evaluable patients with HCT values < 45% while on HU, 14.0% had cytopenias at the same time of HCT < 45%; 6.2% had thrombocytopenia (platelet count < 100 × 109/L), 8.3% had anemia (hemoglobin < 10 g/L), and 1.9% had neutropenia (absolute neutrophil count < 1 × 109/L) (Table 5). A similar number of cytopenias was observed for patients with ≥ 2 HCT values below 45%.
Table 5.
HCT Control and Cytopenias in Patients Treated With HU for ≥ 3 Monthsa
| Variable | Received HU, n/N (%) (n = 1300) |
|---|---|
| HCT evaluable | 1299/1300 (99.9) |
| ≥ 1 HCT value < 45% | 1154/1299 (88.8) |
| Cytopenias at same time of HCT < 45% | 162/1154 (14.0) |
| PLT count < 100 × 109/L | 71/1151 (6.2) |
| HGB < 10 g/L | 96/1153 (8.3) |
| ANC < 1 × 109/L | 20/1042 (1.9) |
Abbreviations: ANC = absolute neutrophil count; HCT = hematocrit; HGB = hemoglobin; HU = hydroxyurea; PLT = platelet.
Denominator includes patients with ≥ 1 corresponding laboratory value during the post-enrollment period while on HU.
Discussion
This analysis of REVEAL provides the largest prospective analysis reported to date of HU treatment patterns in patients with PV. Results of this analysis showed that in the post-index period, patients who received HU for ≥ 3 months were exposed to HU for a median of 23.6 months, and that the majority (72.1%) of patients who received HU received 500 to 1000 mg/d; few (6.4%) patients received the ELN-referenced dose of ≥ 2 g/d.13 Approximately one-third of patients needed dose increases and/or decreases, and one-quarter needed dose interruptions, with only 21.9% of patients restarting after an interruption. Adverse events and lack of efficacy were the most common reasons for permanent discontinuation of HU. Over one-half of patients who received HU for ≥ 3 months continued to have elevated HCT values (>45%), and approximately one-third continued to receive phlebotomies or experience uncontrolled myeloproliferation.
With respect to HU intolerance, when HCT was controlled (<45%) with HU, 14.0% of patients experienced cytopenias, most commonly anemia. In addition, a small proportion (7.2%) of patients had nonhematologic adverse events after starting HU. The adverse events associated with HU in patients enrolled in REVEAL were consistent with those described in previous publications.15–17 An early report of 100 patients with PV who were treated with phlebotomy and HU (mean HU dose, 720 mg/d; mean HU duration, 64.9 months) described 1 patient with skin toxicity (allergic rash); no clinically significant GI toxicity, infection, or cytopenias, were observed.15 A later prospective study randomized patients with PV to treatment with HU (n = 150; initiated at 25 mg/kg/d and maintained at 10–15 mg/kg/d) or pipobroman (n = 142).16 Of the patients who received HU, 133 had > 2 years of follow-up. Stomatitis was reported in 13/133 (10%), leg ulcers in 12/133 (9%), dry skin/acne in 10/133 (7%), gastric pain/diarrhea in 9/133 (7%), and cystitis in 3/133 (2%) of these patients. A more recent retrospective study of 129 patients with PV (n = 54) or essential thrombocythemia (n = 75) treated with a mean dose of 620 mg/d HU described major events necessitating discontinuation in 12 patients, including leg ulcers (n = 4), acute leukemias (n = 3), fever (n = 2), and allergies (n = 2).18 In addition, the drug was withdrawn for symptomatic anemia in 2 patients receiving > 20 mg/kg/d HU.
Similar to findings in this analysis of REVEAL data, doses of HU reported in the above studies were lower than the current ELN-endorsed minimum dose of 2 g/d.13 It is suggested that cumulative exposure to > 1 g/d HU over extended periods (>1 year) may result in HU-related skin toxicity and may require HU discontinuation,18 potentially contributing to the lower doses of HU observed. Indeed, adverse events were the predominant reason for HU dose discontinuation or interruption in REVEAL, although information on the specific AEs leading to discontinuation in REVEAL are not available.
This analysis showed that over one-half of the patients treated with HU failed to achieve HCT values < 45%. Potential reasons for the high proportion of patients with HCT > 45% despite treatment with HU may include development of a more advanced, proliferative disease in a subset of patients, imminent phlebotomy, a lack of appropriate upward titration of HU owing to infrequent follow-up, suboptimal titration of HU, and/or a lack of comfort on the part of the physician or patient with titrating up to 2 g/d. The lack of upward titration may be related to lingering concerns about HU’s role in the risk of transformation into myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).19 Patients with PV are at increased risk of developing acute leukemia, partly as a complication of their disease and partly as a result of the cumulative effects of treatments for PV.20–22 A recent propensity-matched analysis of patients with PV who received phlebotomy (n = 342; median follow-up = 29.9 months) or HU (n = 681; median follow-up 34.7 months) reported 2 patients receiving phlebotomy and 1 patient receiving HU transformed to acute leukemia.12 Similarly, in a nested case-control study of 162 patients with MPNs (110/162 [68%] PV) who developed AML or myelodysplastic syndrome and 242 matched control patients with MPNs (176/242 [73%] PV), 25% of patients with MPNs who developed AML/MDS were not exposed to cytotoxic therapy, which supports a role for nontreatment-related factors. A larger analysis of patients with PV (n = 1545) likewise found no association between HU use and transformation to AML.23 In addition, the risk of AML/MDS development was associated with high exposures of radioactive phosphorus and alkylators but not with HU treatment, even when administered at high doses.24 Despite this evidence, the lingering concern surrounding the leukemogenicity of HU may continue to lead to less aggressive upward titration, even in the context of uncontrolled HTC.
Limitations of this analysis include that it is an exploratory subgroup analysis, with a relatively short observation period, variable durations of disease and/or treatment, variance in the doses of HU used, and the potential confounding effect of age-related adverse events. In addition, reported proportions of patients with increased/decreased laboratory values may be underestimated, as the analyses were performed on patients with ≥ 1 laboratory value, and not all patients had the same number of laboratory value assessments.
Conclusions
In conclusion, this analysis emphasizes the need for active management of patients with PV with appropriate HU dose titration to maintain blood count control while monitoring for signs and symptoms of HU intolerance.
Clinical Practice Points.
National guidelines support the use of HU in high-risk patients with PV.
In this study, we investigated HU treatment patterns in patients with PV.
Of patients who received HU for ≥ 3 months, 32.3% had dose adjustments, 23.7% had dose interruptions, and 18.6% discontinued HU.
These results emphasize the need for active management of patients with PV.
Acknowledgments
The authors wish to thank the patients and their families, the investigators, and the site personnel who participated in this study. This study was sponsored by Incyte Corporation (Wilmington, DE). Medical writing assistance was provided by Michael R. Convente, PhD, of Scientific Pathways, Inc (Hamilton, NJ), and funded by Incyte Corporation.
Disclosure
M.R. Grunwald has provided consultancy to Incyte, AbbVie, Amgen, Cardinal Health, Celgene Corporation, Pfizer, Agios, Merck, and Daiichi Sankyo; and has received research funding from Incyte, Janssen, Forma Therapeutics, and Genentech, Inc/Roche. D.J. Kuter has provided consultancy to Syntimmune, Rigel, Protalex, BMS, Incyte, 3SBIO, Pfizer, Zafgen, Fujifilm, ONO, Merck, Genzyme, Argenx, Shire, Alexion Pharmaceuticals, Inc, Amgen, Novartis, and Dova; has received research funding from Syntimmune, Rigel, Protalex, BMS, Incyte, Shire, and Alexion Pharmaceuticals, Inc; has received royalties from Up-To-Date; and has also served on an advisory committee for Dova. I. Altomare has provided consultancy to Amen, Incyte, Rigel, Bayer, and Novartis; and received research funding from Incyte. J.M. Burke has provided consultancy to Incyte, Celgene Corporation, Bayer, Genentech, Inc, and Gilead. A.T. Gerds has provided consultancy to Incyte, CTI Biopharma, Apexx Oncology, and Celgene Corporation. M.A. Walshauser has no conflicts of interest to disclose. M.R. Savona receives licensing fees from Boehringer Ingelheim; has served as advisory for Astex Therapeutics, Celgene Corporation, Incyte, Karyopharm, and TG Therapeutics, Inc; received research funding from Astex Therapeutics, Incyte Corporation, Sunesis Pharmaceuticals, Inc, Takeda, and TG Therapeutics, Inc; and is a shareholder of Karyopharm. B. Stein has provided consultancy to Incyte and Apexx Oncology. S.T. Oh has provided consultancy to Incyte, Gilead, and Novartis. P. Colucci, S. Parasuraman, and D. Paranagama are employees and shareholders of Incyte Corporation. R. Mesa has provided consultancy to Novartis and received research funding from Incyte, CTI, Genentech, Inc, and Celgene Corporation.
References
- 1.Tefferi A, Barbui T. CME information: polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol 2015; 90:162–73. [DOI] [PubMed] [Google Scholar]
- 2.Harrison C, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol 2017; 96:1653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Lárran A, Pérez-Encinas M, Ferrer-Marín F, et al. Grupo Español de Neoplasias Mieloproliferativas Filadelfia Negativas. Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea. Hematologica 2017; 102:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 2018; 32:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Myeloproliferative neoplasms. Version 2 2018. September 7, 2017. Available at: https://oncolife.com.ua/doc/nccn/Myeloproliferative_Neoplasms.pdf. Accessed: March 6, 2018. [DOI] [PubMed]
- 6.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood 2009; 113:4829–33. [DOI] [PubMed] [Google Scholar]
- 7.Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013; 121:4778–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchioli R, Finazzi G, Specchia G, et al. CYTO-PV Collaborative Group. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013; 368:22–33. [DOI] [PubMed] [Google Scholar]
- 9.Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 2015; 126:560–1. [DOI] [PubMed] [Google Scholar]
- 10.Gangat N, Strand J, Li CY, Wu W, Pardanani A, Tefferi A. Leucocytosis in polycythaemia vera predicts both inferior survival and leukaemic transformation. Br J Haematol 2007; 138:354–8. [DOI] [PubMed] [Google Scholar]
- 11.Landolfi R, Di Gennaro L, Barbui T, et al. European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007; 109:2446–52. [DOI] [PubMed] [Google Scholar]
- 12.Barbui T, Vannucchi AM, Finazzi G, et al. A reappraisal of the benefit-risk profile of hydroxyurea in polycythemia vera: a propensity-matched study. Am J Hematol 2017; 92:1131–6. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Lárran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012; 119: 1363–9. [DOI] [PubMed] [Google Scholar]
- 14.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015; 372: 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West WO. Hydroxyurea in the treatment of polycythemia vera: a prospective study of 100 patients over a 20-year period. South Med J 1987; 80:323–7. [DOI] [PubMed] [Google Scholar]
- 16.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood 1997; 90: 3370–7. [PubMed] [Google Scholar]
- 17.Barosi G, Birgegard G, Finazzi G, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of an European LeukemiaNet (ELN) consensus process. Br J Haematol 2010; 148:961–3. [DOI] [PubMed] [Google Scholar]
- 18.Randi ML, Ruzzon E, Luzzatto G, Tezza F, Girolami A, Fabris F. Safety profile of hydroxyurea in the treatment of patients with Philadelphia-negative chronic myeloproliferative disorders. Haematologica 2005; 90:261–2. [PubMed] [Google Scholar]
- 19.Gotlib J. Hydroxyurea’s leukemogenicity in myeloproliferative neoplasms: a not guilty verdict. Hematologist 2011; 8:1. [Google Scholar]
- 20.Finazzi G, Caruso V, Marchioli R, et al. ECLAP Investigators. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005; 105:2664–70. [DOI] [PubMed] [Google Scholar]
- 21.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004; 117:755–61. [DOI] [PubMed] [Google Scholar]
- 22.Passamonti F, Rumi E, Arcaini L, et al. Leukemic transformation of polycythemia vera: a single center study of 23 patients. Cancer 2005; 104:1032–6. [DOI] [PubMed] [Google Scholar]
- 23.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013; 27: 1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Björkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011; 29:2410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


