Abstract
We aimed to examine whether type 2 diabetes–prevention diet, a dietary pattern previously developed for reducing type 2 diabetes risk, was associated with mortality in a US population. A population-based cohort of 86,633 subjects was identified from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (1993–2015). Dietary information was collected with a food frequency questionnaire. A dietary diabetes risk-reduction score was calculated to reflect adherence to this dietary pattern, with higher scores representing better adherence. Hazard ratios (HRs) and absolute risk differences (ARDs) in mortality rates per 10,000 person-years were calculated. After a mean follow-up of 13.6 years, 17,532 all-cause deaths were observed. Participants with the highest versus the lowest quintiles of dietary diabetes risk-reduction score were observed to have decreased risks of death from all causes (HR = 0.76, 95% CI: 0.72, 0.80; ARD: −81.94, 95% CI: −93.76, −71.12), cardiovascular disease (HR = 0.73, 95% CI: 0.66, 0.81; ARD: −17.82, 95% CI: −24.81, −11.30), and cancer (HR = 0.85, 95% CI: 0.78, 0.94; ARD: −9.92, 95% CI: −15.86, −3.59), which were modified by sex, smoking status, or alcohol consumption in subgroup analyses (P for interaction < 0.05 for all). In conclusion, a type 2 diabetes–prevention diet confers reduced risks of death from all causes, cardiovascular disease, and cancer in this US population.
Keywords: mortality, primary prevention, prospective study, type 2 diabetes–prevention diet
Abbreviation
- CI
confidence interval
- DHQ
diet history questionnaire
- HR
hazard ratio
- PLCO
Prostate, Lung, Colorectal, and Ovarian
Type 2 diabetes is a major public health concern worldwide and is a well-established predisposing factor for cardiovascular disease (1) and cancer (2), which represent 2 leading global causes of death. Dietary behaviors play a critical role in public health; unhealthy diet is ranked as the most common cause of death in the US population (3). Hence, it is essential to investigate the potential associations of dietary behaviors with health outcomes.
A type 2 diabetes–prevention diet was proposed by Rhee et al. in 2015 (4) and features high intakes of cereal fiber, polyunsaturated fatty acids, coffee, and nuts and low intakes of carbohydrates, trans-fatty acids, red and processed meat, and sugar-sweetened beverages (4). Compared with other established dietary patterns (e.g., the Mediterranean diet), the type 2 diabetes–prevention diet captures key dietary elements closely related to the risk of type 2 diabetes and is developed primarily for facilitating the prevention of this disease (4), resulting in inclusion of some components that are not part of other established dietary patterns (e.g., coffee and glycemic index); moreover, adherence to the type 2 diabetes–prevention diet could improve insulin sensitivity and reduce inflammation levels (4–6). Recently, the type 2 diabetes–prevention diet was found to be associated with reduced risks of hepatocellular carcinoma (7), breast cancer (8), and pancreatic cancer (9). However, whether the type 2 diabetes–prevention diet is associated with mortality remains unknown. Some studies have investigated the associations between individual components of the type 2 diabetes–prevention diet with the risk of mortality (10–16), but they fail to consider the potential interactions among dietary components. Therefore, assessment of dietary patterns, which include multiple foods or nutrients simultaneously and thus can capture the potential interactions among them, may provide a more accurate estimate for diet-disease associations.
Hence, in this study, we aimed to examine the hypothesis that adherence to the type 2 diabetes–prevention diet is associated with all-cause and cause-specific mortality in the US population.
METHODS
The results of the present study were reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (17).
Study population
Our study population was identified from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, a large randomized clinical study with 10 enrollment centers (St. Louis, Missouri; Honolulu, Hawaii; Denver, Colorado; Pittsburgh, Pennsylvania: Marshfield, Wisconsin; Birmingham, Alabama; Salt Lake City, Utah; Washington, DC; Minneapolis, Minnesota; and Detroit, Michigan). This trial was designed to investigate the potential beneficial effects of selected screening exams on the risks of death from prostate, lung, colorectal, and ovarian cancers. Study design of the PLCO Cancer Screening Trial has been reported elsewhere (18). Briefly, during November 1993 and September 2001, individuals aged 55–74 years were invited to take part in this trial. A total of 154,887 individuals were qualified for enrollment and individually randomized to the intervention group or the control group in equal proportions, with individuals in the intervention group receiving selected screening exams while those in the control group received usual care. All participants provided written informed consent. The PLCO Cancer Screening Trial was approved by the institutional review boards of the US National Cancer Institute and each enrollment center.
The following participants were further excluded from our study: 1) 4,918 participants failing to return a baseline questionnaire, a baseline risk-factor questionnaire with participant-reported information (e.g., demographic characteristics and medical history); 2) 33,241 participants failing to return a diet history questionnaire (DHQ); 3) 5,221 participants with an invalid DHQ—the valid DHQ refers to having a DHQ completion date, DHQ completion date prior to death date, <8 missing frequency responses, and the absence of extreme energy intake (top 1% and bottom 1%); 4) 9,684 participants with a history of cancer at baseline; 5) 2,046 participants with a history of stroke at baseline; 6) 7,886 participants with a history of heart attack at baseline; and 7) 5,258 participants with a history of diabetes at baseline. Finally, a total of 86,633 participants were included (Figure 1). The reason for excluding participants with a history of cancer, stroke, heart attack, or diabetes at baseline was that they might alter their dietary habits after receiving these diagnoses, which might result in reverse causation.
Figure 1.
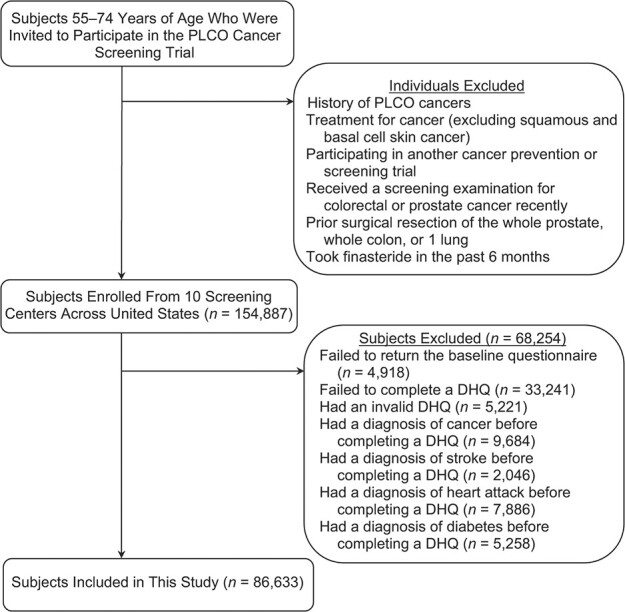
Flow chart identifying subjects included in this study evaluating a type 2 diabetes–prevention diet and multiple causes of mortality, a post hoc analysis of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, United States, 1993–2009. The total number of subjects for an exclusion category box was not available in the PLCO Cancer Screening Trial. DHQ, diet history questionnaire.
Calculation of dietary diabetes risk-reduction score
A dietary diabetes risk-reduction score was calculated to quantify adherence to a type 2 diabetes–prevention diet using the approach described in the literature (4). Briefly, all participants were divided into 5 strata based on quintiles of dietary intake of each component. For favorable components (i.e., cereal fiber, ratio of polyunsaturated to saturated fatty acids, coffee, and nuts), participants in the highest stratum were awarded 5 points and those in the lowest stratum were awarded 1 point; in contrast, for unfavorable components (i.e., glycemic index, trans-fatty acids, red and processed meat, and sugar-sweetened beverages), participants in the highest stratum were awarded 1 point and those in the lowest stratum were awarded 5 points (Web Table 1, available at https://doi.org/10.1093/aje/kwab265). An individual’s dietary diabetes risk-reduction score was calculated as the sum of points for each dietary component, with a range of 8–40 points. Higher scores suggest greater adherence to the diet. Glycemic index was calculated as described previously (19). Notably, in this study, sugar-sweetened beverages referred to soft drinks or fruit drinks, and cereal fiber referred to insoluble fiber. In addition, given that higher consumption of fruits and vegetables has been identified to be associated with a lower risk of type 2 diabetes (20), we calculated a modified dietary diabetes risk-reduction score by regarding these 2 foods as favorable components (Web Table 2).
In the PLCO Cancer Screening Trial, food or nutrient intakes, including those used for the calculation of dietary diabetes risk-reduction score, were evaluated at the study baseline through the DHQ. The DHQ is a 137-item self-administered food frequency questionnaire designed for evaluating food and supplement consumption over the past year; its validity had been confirmed elsewhere (21). Daily food consumption for each participant was estimated by multiplying food frequency by serving size; daily nutrient intake was calculated based on 2 nutrient databases, namely US Department of Agriculture’s 1994–1996 Continuing Survey of Food Intakes by Individuals (22) and Nutrition Data Systems for Research (23).
Outcome assessment
Mortality status of each participant was confirmed predominantly through a mailed annual study update form. Participants failing to return this form were contacted repeatedly by telephone or e-mail. Moreover, mortality status was adjudicated by periodic linkage to the US National Death Index. The ninth revision of International Classification of Diseases was applied to define the underlying causes of death obtained from death certificates: cardiovascular disease (codes 390–459) and cancer (codes 140–209).
Covariate assessment
Age at DHQ completion, alcohol consumption, single or multivitamin supplement use, and food consumption were collected with the above-mentioned DHQ. Of note, dietary intakes of foods and nutrients were adjusted for energy intake from diet with the residual approach (24) before data analysis. Physical activity level was defined as total time of moderate to vigorous activity per week, and was assessed through a self-administered supplemental questionnaire. Healthy Eating Index 2015 and the plant-based diet index were computed as described in the literature (25, 26). Sex, ethnic group, marital status, body weight, height, educational level, smoking status, history of hypertension, family history of cancer, and aspirin use were collected with a self-administered baseline questionnaire. Body mass index was calculated as body weight (kg) divided by height squared (m2).
Statistical analysis
To minimize potential biases and maximize statistical power, multiple imputation with chained equations was applied to impute missing data under the assumption that data were missing at random (the number of imputations = 25) (27); all variables involved in data analysis were applied to yield imputed data sets. Web Table 3 shows the distribution of covariates with missing values before and after multiple imputation. Main data analyses were repeated for participants with complete data to determine the potential influences of data imputation on our results.
To evaluate the associations of the dietary diabetes risk-reduction score with all-cause and cause-specific mortality, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards regression model, with follow-up time as time metric. In our study, follow-up time was calculated as the difference between DHQ completion date and death date, loss to follow-up, or the end of follow-up (December 31, 2015), whichever came first (Figure 2). In regression models, the dietary diabetes risk-reduction score was split into quintiles, with the first quintile as the reference group. For examining linear trends in risk estimates across quintiles of dietary diabetes risk-reduction score, the median of each quintile was assigned to each participant in the quintile at first to yield an ordinal variable, which was then treated as a continuous variable in regression models for testing its significance. No evidence suggesting the violation of the proportional hazards assumption was found, using the Schoenfeld residuals method (all P values for global test >0.05). Covariate selection for multivariable regression was based on the change-in-estimate approach (28) and our knowledge of the existing literature. Specifically, model 1 adjusted for age and sex; model 2 further adjusted for ethnic group, trial arm, educational level, marital status, history of hypertension, family history of cancer (only for all-cause and cancer mortality), aspirin use, single or multivitamin supplement use, smoking status, alcohol consumption, body mass index, physical activity, and energy intake from diet; and model 3 further adjusted for consumption of fruits, vegetables, tea, fish, and dairy. We also performed an analysis treating body mass index as a time-varying covariate (model 4). Moreover, we also calculated absolute risk difference in mortality rate per 10,000 person-years for each HR from the above Cox regression analysis and the below subgroup analysis using the method described in the literature (29).
Figure 2.
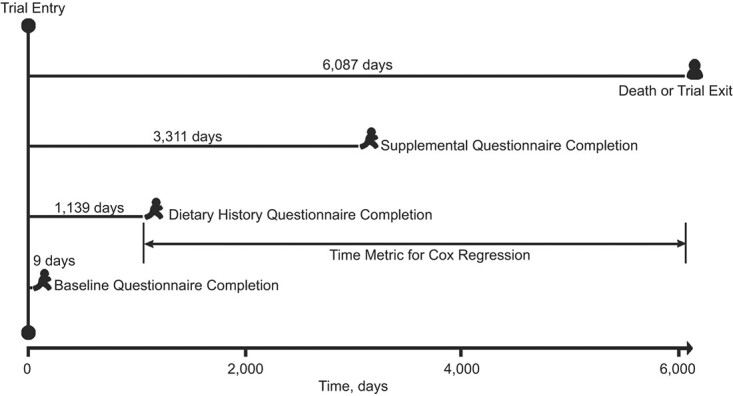
The timeline and follow-up scheme for this study evaluating a type 2 diabetes–prevention diet and multiple causes of mortality, a post hoc analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, United States, 1993–2009. Note that the time span between 2 events represents the average value of all subjects.
Prespecified subgroup analyses were performed after stratifying for age (≥65 vs. <65 years), sex (male vs. female), trial group (intervention group vs. control group), history of hypertension (yes vs. no), body mass index (≥25 vs. <25), smoking status (current or past vs. never), and alcohol consumption (heavy vs. no, light, or moderate). For men, we defined light, moderate, and heavy alcohol consumption as ≤6 g/day, >6 and ≤28 g/day, and >28 g/day, respectively; for women, we defined light, moderate, and heavy alcohol consumption as ≤6 g/day, >6 and ≤14 g/day, and >14 g/day, respectively (30). A P for interaction was estimated by comparing models with and without multiplicative interaction terms prior to performing the above-mentioned subgroup analyses to avert the possible spurious subgroup differences.
Sensitivity analyses were performed to determine the stability of our results: 1) including participants with a history of cancer, stroke, heart attack, or diabetes at baseline; 2) excluding deaths observed within the first 5 years of follow-up to determine the possibility of the observed association resulted from reverse causation; 3) excluding participants with implausible energy intake from diet, defined as <800 or >4,000 kcal/day for men and <500 or >3,500 kcal/day for women (31); 4) repeating analyses with a competing risk regression model (only for cause-specific mortality) to evaluate the potential influences of competing risk bias; 5) adjustment for propensity score on crude model (all covariates included in model 3 were applied to calculate propensity score with logistic regression); 6) additionally adjusting for Healthy Eating Index 2015 or plant-based index in model 3 to test whether the observed associations were mediated by diet quality, and 7) additionally adjusting for intakes of polyunsaturated and saturated fatty acids per reviewer’s suggestion.
To determine the main contributor(s) of the type 2 diabetes–prevention diet, we examined the association between each component of this dietary pattern and the risk of death separately. Statistical analyses were conducted with STATA software (version 12.0; StataCorp LP, College Station, Texas). The statistical significance level was set at P < 0.05 under a 2-tailed test.
RESULTS
Participant characteristics
Participants in the highest versus the lowest quintiles of dietary diabetes risk-reduction score were less likely to be male, be married or living as married, be current smokers, and have a history of hypertension but more likely to be single or multivitamin supplement users, have lower body mass index and energy intake from diet and had higher educational level, alcohol consumption, physical activity level, and Healthy Eating Index 2015 (Table 1). In addition, compared with participants in the lowest quintile of dietary diabetes risk-reduction score, those in the highest quintile had lower glycemic index and lower intakes of trans-fatty acids, sugar-sweetened beverages, red and processed meat, and saturated fatty acids but higher intakes of cereal fiber, nuts, coffee, fruits, and vegetables.
Table 1.
Baseline Characteristics of Study Population According to Quintiles of Dietary Diabetes Risk-Reduction Score, a Post Hoc Analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, United States, 1993–2015
| Quintiles of Dietary Diabetes Risk Reduction Score, Range (Median) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
9–19 (17)
(n = 16,302) |
20–22 (21)
(n = 17,623) |
23–24 (23)
(n = 13,339) |
25–27 (26)
(n = 18,023) |
28–40 (30)
(n = 21,346) |
|||||||||||
| Characteristic | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % |
| Age, years | 64.5 (5.6) | 65.1 (5.7) | 65.3 (5.7) | 65.5 (5.7) | 65.6 (5.7) | ||||||||||
| Male sex | 9,745 | 59.8 | 8,962 | 50.9 | 6,176 | 46.3 | 7,330 | 40.7 | 7,463 | 35.0 | |||||
| Ethnicity | |||||||||||||||
| Non-Hispanic White | 14,982 | 91.9 | 16,316 | 92.6 | 12,263 | 91.9 | 16,470 | 91.4 | 19,241 | 90.1 | |||||
| Non-Hispanic Black | 785 | 4.8 | 552 | 3.1 | 341 | 2.6 | 416 | 2.3 | 454 | 2.1 | |||||
| Hispanic | 239 | 1.5 | 237 | 1.3 | 172 | 1.3 | 246 | 1.4 | 310 | 1.5 | |||||
| Othersa | 296 | 1.8 | 518 | 2.9 | 563 | 4.2 | 891 | 4.9 | 1,341 | 6.3 | |||||
| Married or living as married | 13,082 | 80.2 | 14,052 | 79.7 | 10,611 | 79.5 | 14,191 | 78.7 | 16,197 | 75.9 | |||||
| Body mass indexb | 28.0 (4.9) | 27.5 (4.8) | 27.1 (4.6) | 26.7 (4.5) | 25.8 (4.3) | ||||||||||
| Educational level | |||||||||||||||
| Some college or below | 11,753 | 72.1 | 11,762 | 66.7 | 8,490 | 63.6 | 10,870 | 60.3 | 11,604 | 54.4 | |||||
| College graduate | 2,377 | 14.6 | 2,969 | 16.8 | 2,448 | 18.4 | 3,411 | 18.9 | 4,362 | 20.4 | |||||
| Postgraduate | 2,172 | 13.3 | 2,892 | 16.4 | 2,401 | 18.0 | 3,742 | 20.8 | 5,380 | 25.2 | |||||
| Alcohol consumption, g/day | 7.6 (20.4) | 8.7 (21.6) | 9.7 (24.3) | 10.7 (26.9) | 11.6 (30.1) | ||||||||||
| Smoking status | |||||||||||||||
| Current | 2,114 | 13.0 | 1914 | 10.9 | 1,248 | 9.4 | 1,451 | 8.1 | 1,272 | 6.0 | |||||
| Past | 6,316 | 38.7 | 7,040 | 39.9 | 5,556 | 41.7 | 7,573 | 42.0 | 9,448 | 44.3 | |||||
| Never | 7,872 | 48.3 | 8,669 | 49.2 | 6,535 | 49.0 | 8,999 | 49.9 | 10,626 | 49.8 | |||||
| Physical activity, minutes/weekc | 107.0 (117.2) | 114.1 (120.0) | 120.5 (119.7) | 126.9 (121.9) | 145.8 (129.4) | ||||||||||
| Energy intake from diet, kcal/day | 1937.8 (767.6) | 1790.4 (769.0) | 1697.6 (739.5) | 1646.8 (711.3) | 1639.6 (653.4) | ||||||||||
| HEI-2015 score | 56.5 (7.9) | 62.7 (7.5) | 66.3 (7.2) | 69.5 (7.0) | 75.1 (6.8) | ||||||||||
| Plant-based diet index score | 52.5 (5.9) | 53.4 (6.3) | 53.5 (6.4) | 53.9 (6.5) | 55.0 (6.5) | ||||||||||
| History of hypertension | 5,161 | 31.7 | 5,435 | 30.8 | 3,927 | 29.4 | 5,110 | 28.4 | 5,449 | 25.5 | |||||
| Family history of cancer | 9,082 | 55.7 | 9,906 | 56.2 | 7,465 | 56.0 | 10,310 | 57.2 | 12,088 | 56.6 | |||||
| Aspirin use | |||||||||||||||
| Yes | 6,909 | 42.4 | 7,682 | 43.6 | 5,771 | 43.3 | 7,952 | 44.1 | 9,328 | 43.7 | |||||
| No | 9,393 | 57.6 | 9,941 | 56.4 | 7,568 | 56.7 | 10,071 | 55.9 | 12,018 | 56.3 | |||||
| Single or multivitamin supplement use | |||||||||||||||
| Yes | 11,031 | 67.7 | 13,183 | 74.8 | 10,414 | 78.1 | 14,612 | 81.1 | 18,506 | 86.7 | |||||
| No | 5,271 | 32.3 | 4,440 | 25.2 | 2,925 | 21.9 | 3,411 | 18.9 | 2,840 | 13.3 | |||||
| Energy-adjusted food and nutrient intakes | |||||||||||||||
| Glycemic index | 56.6 (3.6) | 54.8 (3.8) | 53.7 (3.8) | 52.6 (3.8) | 50.7 (3.7) | ||||||||||
| Cereal fiber, g/day | 9.1 (4.8) | 10.4 (5.5) | 11.2 (5.9) | 12.1 (6.2) | 15.1 (7.2) | ||||||||||
| Ratio of polyunsaturated to saturated fatty acids | 0.6 (0.5) | 0.7 (0.2) | 0.7 (0.3) | 0.8 (0.5) | 1.0 (0.4) | ||||||||||
| Trans-fatty acids, g/day | 6.0 (3.4) | 4.9 (3.2) | 4.1 (2.8) | 3.3 (2.5) | 2.1 (1.9) | ||||||||||
| Sugar-sweetened beverages, g/day | 537.9 (705.7) | 283.7 (436.3) | 194.3 (349.0) | 138.5 (263.2) | 78.4 (186.6) | ||||||||||
| Nuts, g/day | 1.9 (4.8) | 3.8 (8.3) | 5.0 (10.4) | 7.1 (13.8) | 13.9 (24.6) | ||||||||||
| Coffee, g/day | 654.1 (769.9) | 827.5 (806.9) | 875.6 (800.0) | 907.6 (788.9) | 950.2 (778.4) | ||||||||||
| Red and processed meat, g/day | 22.0 (20.8) | 15.4 (17.3) | 11.7 (13.9) | 8.9 (11.4) | 4.8 (7.9) | ||||||||||
| Polyunsaturated fatty acids, g/day | 14.8 (7.7) | 14.4 (8.3) | 13.7 (8.2) | 13.5 (8.2) | 14.0 (8.4) | ||||||||||
| Saturated fatty acids, g/day | 26.8 (13.8) | 22.7 (13.1) | 19.8 (11.6) | 17.6 (10.5) | 14.5 (8.4) | ||||||||||
| Fruit, g/day | 199.2 (172.0) | 236.1 (185.8) | 260.1 (200.9) | 285.3 (212.5) | 357.6 (251.5) | ||||||||||
| Vegetable, g/day | 237.8 (146.9) | 258.4 (163.0) | 267.1 (173.0) | 285.2 (179.0) | 344.3 (222.3) | ||||||||||
| Tea, g/day | 277.1 (515.5) | 258.2 (469.8) | 253.5 (453.2) | 254.1 (453.4) | 263.4 (441.7) | ||||||||||
| Fish, g/day | 14.5 (19.1) | 14.3 (17.2) | 14.7 (18.2) | 15.4 (19.1) | 17.4 (21.9) | ||||||||||
| Dairy, servings/day | 1.3 (2.1) | 1.4 (2.2) | 1.4 (2.1) | 1.4 (2.1) | 1.4 (2.0) | ||||||||||
Abbreviations: HEI, Healthy Eating Index; SD, standard deviation.
a “Others” refers to Asian, Pacific Islander, or American Indian.
b Weight (kg)/height (m)2.
c Total time of moderate to vigorous physical activity per week.
Dietary diabetes risk-reduction score and all-cause and cause-specific mortality
During 1,174,401.6 person-years of follow-up, we observed a total of 17,532 all-cause deaths, of which 4,809 (27.4%) were attributable to cardiovascular disease and 5,719 (32.6%) to cancer (Table 2). The mean follow-up was 13.6 (standard deviation, 3.2) years. The crude death rates per 10,000 person-years were 149.28, 40.95, and 48.70 for mortality from all causes, cardiovascular disease, and cancer, respectively, which were obviously lower than those from the National Institutes of Health–AARP study, a contemporary US cohort study involving 521,120 participants (176.99, 53.03, and 62.66 deaths per 10,000 person-years for mortality from all causes, cardiovascular disease, and cancer, respectively) (32). In the fully adjusting model, participants in the highest (5th) vs. the lowest (1st) quintiles of dietary diabetes risk-reduction score were found to be at lower risks of death from all causes (HR = 0.76, 95% CI: 0.72, 0.80; P for trend< 0.001; absolute risk difference = −81.94, 95% CI: −93.76, −71.12), cardiovascular disease (HR = 0.73, 95% CI: 0.66, 0.81; P for trend < 0.001; absolute risk difference = −17.82, 95% CI: −24.81, −11.30), and cancer (HR = 0.85, 95% CI: 0.78, 0.94; P for trend < 0.001; absolute risk difference = −9.92, 95% CI: −15.86, −3.59) (Table 2 and Web Table 4). We obtained similar results when repeating the above-mentioned Cox regression analyses in participants with complete data (Web Table 5) and using the modified dietary diabetes risk-reduction score (Web Table 6).
Table 2.
Hazard Ratios for Associations of Dietary Diabetes Risk-Reduction Score With All-Cause and Cause-Specific Mortality, a Post Hoc Analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, United States, 1993–2015
| Mortality Cause and the Ranges (Medians) of Quintiles of Dietary Diabetes Risk-Reduction Score | No. of Deaths | Death Rate a | Model 1 b | Model 2 c | Model 3 d | Model 4 e | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| All-cause mortality | ||||||||||
| 9–19 (17) | 3,774 | 174.13 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 20–22 (21) | 3,870 | 163.31 | 0.89 | 0.85, 0.93 | 0.93 | 0.89, 0.98 | 0.92 | 0.88, 0.97 | 0.90 | 0.86, 0.93 |
| 23–24 (23) | 2,721 | 150.81 | 0.82 | 0.78, 0.86 | 0.88 | 0.84, 0.93 | 0.87 | 0.82, 0.91 | 0.83 | 0.79, 0.87 |
| 25–27 (26) | 3,451 | 140.11 | 0.75 | 0.71, 0.78 | 0.83 | 0.79, 0.87 | 0.81 | 0.77, 0.85 | 0.82 | 0.78, 0.86 |
| 28–40 (30) | 3,716 | 126.41 | 0.68 | 0.65, 0.71 | 0.79 | 0.76, 0.83 | 0.76 | 0.72, 0.80 | 0.74 | 0.70, 0.78 |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Cardiovascular mortality | ||||||||||
| 9–19 (17) | 1,044 | 48.17 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 20–22 (21) | 1,040 | 43.89 | 0.86 | 0.79, 0.93 | 0.90 | 0.83, 0.99 | 0.89 | 0.81, 0.97 | 0.85 | 0.78, 0.92 |
| 23–24 (23) | 760 | 42.12 | 0.82 | 0.74, 0.90 | 0.89 | 0.81, 0.98 | 0.86 | 0.79, 0.95 | 0.80 | 0.73, 0.88 |
| 25–27 (26) | 968 | 39.3 | 0.75 | 0.68, 0.82 | 0.84 | 0.77, 0.92 | 0.81 | 0.74, 0.89 | 0.82 | 0.75, 0.90 |
| 28–40 (30) | 997 | 33.92 | 0.65 | 0.60, 0.71 | 0.79 | 0.72, 0.87 | 0.73 | 0.66, 0.81 | 0.69 | 0.62, 0.76 |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Cancer mortality | ||||||||||
| 9–19 (17) | 1,219 | 56.24 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 20–22 (21) | 1,285 | 54.23 | 0.95 | 0.87, 1.02 | 1.00 | 0.92, 1.08 | 0.99 | 0.92, 1.08 | 0.97 | 0.90, 1.04 |
| 23–24 (23) | 873 | 48.39 | 0.85 | 0.78, 0.93 | 0.92 | 0.84, 1.00 | 0.91 | 0.83, 1.00 | 0.89 | 0.81, 0.97 |
| 25–27 (26) | 1,120 | 45.47 | 0.80 | 0.73, 0.86 | 0.89 | 0.82, 0.97 | 0.88 | 0.81, 0.96 | 0.90 | 0.83, 0.98 |
| 28–40 (30) | 1,222 | 41.57 | 0.74 | 0.68, 0.80 | 0.87 | 0.80, 0.94 | 0.85 | 0.78, 0.94 | 0.83 | 0.76, 0.92 |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Crude death rate per 10,000 person-years.
b Adjusted for age (years) and sex (male, female).
c Adjustments from model 1 plus ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, others), trial arm (intervention, control), educational level (some college or below, college graduate, postgraduate), marital status (married or living as married, widowed, divorced, separated, never married), history of hypertension (yes, no), family history of cancer (yes, no; only for all-cause and cancer mortality), aspirin use (yes, no), single or multivitamin supplement use (yes, no), smoking status (current, past, never), alcohol consumption (g/day), body mass index, physical activity (minutes/week), and energy intake from diet (kcal/day).
d Adjustments from model 2 plus consumption of fruits (g/day), vegetables (g/day), tea (g/day), fish (g/day), and dairy (servings/day).
e Adjustments from model 2 plus consumption of fruits (g/day), vegetables (g/day), tea (g/day), fish (g/day), and dairy (servings/day), with body mass index treated as a time-varying covariate.
Subgroup analyses
Interestingly, subgroup analyses found that the inverse association with cardiovascular mortality was more pronounced in women than in men (P for interaction = 0.024), whereas the inverse association with cancer mortality was more pronounced among men than women (P for interaction = 0.032) (Table 3 and Web Table 7). Moreover, the inverse associations with all-cause (P for interaction = 0.023) and cancer (P for interaction = 0.023) mortality were more pronounced among participants with heavy alcohol consumption than those with no, light, or moderate alcohol consumption. In addition, the inverse association with cancer mortality was more pronounced among current or past smokers than never smokers (P for interaction = 0.002). No significant interaction effect was found for the remaining stratification factors (all P for interaction > 0.05).
Table 3.
Subgroup Analyses of the Association of Dietary Diabetes Risk-Reduction Score With All-Cause and Cause-Specific Mortality, a Post Hoc Analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, United States, 1993–2015
| Subgroup Variable | All-Cause Mortality | Cardiovascular Mortality | Cancer Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR a | 95% CI | P for Interaction | HR a | 95% CI | P for Interaction | HR a | 95% CI | P for Interaction | |
| Age, years | 0.345 | 0.781 | 0.825 | ||||||
| ≥65 | 0.77 | 0.72, 0.81 | 0.71 | 0.64, 0.80 | 0.86 | 0.77, 0.96 | |||
| <65 | 0.75 | 0.68, 0.83 | 0.81 | 0.65, 1.00 | 0.85 | 0.73, 0.99 | |||
| Sex | 0.280 | 0.024 | 0.032 | ||||||
| Male | 0.77 | 0.72, 0.83 | 0.81 | 0.71, 0.92 | 0.78 | 0.69, 0.89 | |||
| Female | 0.74 | 0.68, 0.80 | 0.61 | 0.52, 0.72 | 0.94 | 0.81, 1.08 | |||
| Trial group | 0.592 | 0.199 | 0.469 | ||||||
| Intervention | 0.76 | 0.71, 0.82 | 0.78 | 0.68, 0.90 | 0.84 | 0.74, 0.95 | |||
| Control | 0.76 | 0.71, 0.82 | 0.68 | 0.59, 0.79 | 0.87 | 0.77, 0.99 | |||
| History of hypertension | 0.997 | 0.595 | 0.504 | ||||||
| Yes | 0.76 | 0.69, 0.82 | 0.73 | 0.62, 0.85 | 0.82 | 0.70, 0.97 | |||
| No | 0.77 | 0.72, 0.82 | 0.74 | 0.65, 0.84 | 0.87 | 0.78, 0.97 | |||
| Body mass indexb | 0.104 | 0.114 | 0.299 | ||||||
| ≥25 | 0.79 | 0.74, 0.84 | 0.78 | 0.69, 0.88 | 0.88 | 0.79, 0.99 | |||
| <25 | 0.72 | 0.66, 0.78 | 0.66 | 0.55, 0.78 | 0.81 | 0.70, 0.95 | |||
| Smoking status | 0.149 | 0.663 | 0.002 | ||||||
| Current or past | 0.70 | 0.66, 0.75 | 0.72 | 0.63, 0.81 | 0.73 | 0.65, 0.82 | |||
| Never | 0.77 | 0.71, 0.84 | 0.69 | 0.59, 0.80 | 1.00 | 0.86, 1.17 | |||
| Alcohol intake, g/dayc | 0.023 | 0.895 | 0.023 | ||||||
| Heavy | 0.67 | 0.59, 0.77 | 0.83 | 0.63, 1.10 | 0.65 | 0.51, 0.82 | |||
| None, light, or moderate | 0.79 | 0.75, 0.84 | 0.74 | 0.66, 0.82 | 0.90 | 0.81, 0.99 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a HRs are for the comparison of quintile 5 to quintile 1 and are adjusted for age (years), sex (male, female), ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, others), trial arm (intervention, control), educational level (some college or below, college graduate, postgraduate), marital status (married or living as married, widowed, divorced, separated, never married), history of hypertension (yes, no), family history of cancer (yes, no; only for all-cause and cancer mortality), aspirin use (yes, no), single or multivitamin supplement use (yes, no), smoking status (current, past, never), alcohol consumption (g/day), body mass index, physical activity (min/week), energy intake from diet (kcal/day), and consumption of fruits (g/day), vegetables (g/day), tea (g/day), fish (g/day), and dairy (servings/day). In subgroup analyses stratified by sex, trial arm, history of hypertension, and smoking status, HRs were not adjusted for the stratification factor.
b Weight (kg)/height (m)2.
c Light, moderate, and heavy alcohol consumption are defined as ≤6 g/day, >6 and <28 g/day for men and >6 and <14 g/day for women, and >28 g/day for men and >14 g/day for women, respectively.
Sensitivity analyses
The initial associations of dietary diabetes risk-reduction score with risks of death from all causes, cardiovascular disease, and cancer did not change materially in a large range of sensitivity analyses (Web Table 8).
Associations by each component of type 2 diabetes–prevention diet
Comparing quintile 5 to quintile 1, higher intake of cereal fiber (HR = 0.79, 95% CI: 0.74, 0.85; P for trend < 0.001), nuts (HR = 0.82, 95% CI: 0.78, 0.86; P for trend < 0.001), or coffee (HR = 0.88, 95% CI: 0.84, 0.93; P for trend< 0.001) was found to be associated with a lower risk of all-cause mortality, whereas higher intake of sugar-sweetened beverages (HR = 1.00, 95% CI: 0.95, 1.05; P for trend = 0.022) was found to be associated with a higher risk of all-cause mortality (Table 4); moreover, an inverse association was found for the ratio of polyunsaturated to saturated fatty acids and all-cause mortality (HR = 0.84, 95% CI: 0.80, 0.89; P for trend< 0.001). A marginally significant positive association was found for red and processed meat consumption and all-cause mortality (HR = 1.01, 95% CI: 0.95, 1.07; P for trend = 0.052). Similar results were obtained for cardiovascular and/or cancer mortality. No significant associations with mortality from all causes, cardiovascular disease, and cancer were found for glycemic index and trans-fatty acid intake.
Table 4.
Association of Each Component of a Type 2 Diabetes–Prevention Diet With All-Cause and Cause-Specific Mortality, a Post Hoc Analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, United States, 1993–2015
| Dietary Component and Quintile Range | All-Cause Mortality | Cardiovascular Mortality | Cancer Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Deaths | HR a | 95% CI | No. of Deaths | HR a | 95% CI | No. of Deaths | HR a | 95% CI | |
| Glycemic index | |||||||||
| <50.09 | 3,453 | 1.00 | Referent | 946 | 1.00 | Referent | 1,111 | 1.00 | Referent |
| 50.09–52.56 | 3,352 | 0.93 | 0.88, 0.97 | 921 | 0.91 | 0.83, 1.00 | 1,069 | 0.95 | 0.87, 1.03 |
| 52.57–54.58 | 3,507 | 0.93 | 0.89, 0.98 | 947 | 0.90 | 0.81, 0.99 | 1,123 | 0.96 | 0.88, 1.05 |
| 54.59–56.93 | 3,418 | 0.89 | 0.84, 0.94 | 938 | 0.87 | 0.79, 0.96 | 1,160 | 0.97 | 0.88, 1.06 |
| ≥56.94 | 3,802 | 0.95 | 0.90, 1.01 | 1,057 | 0.94 | 0.85, 1.05 | 1,256 | 1.00 | 0.91, 1.10 |
| P for trend | 0.061 | 0.225 | 0.817 | ||||||
| Cereal fiber, g/day | |||||||||
| <6.60 | 3,944 | 1.00 | Referent | 1,074 | 1.00 | Referent | 1,283 | 1.00 | Referent |
| 6.60–9.27 | 3,559 | 0.90 | 0.86, 0.95 | 967 | 0.91 | 0.83, 0.99 | 1,177 | 0.94 | 0.86, 1.02 |
| 9.28–12.08 | 3,325 | 0.85 | 0.81, 0.90 | 944 | 0.90 | 0.81, 0.99 | 1,055 | 0.84 | 0.77, 0.92 |
| 12.09–16.16 | 3,330 | 0.82 | 0.78, 0.87 | 882 | 0.80 | 0.72, 0.89 | 1,093 | 0.85 | 0.77, 0.94 |
| ≥16.17 | 3,374 | 0.79 | 0.74, 0.85 | 942 | 0.80 | 0.70, 0.92 | 1,111 | 0.82 | 0.73, 0.94 |
| P for trend | <0.001 | 0.001 | 0.003 | ||||||
| Ratio of polyunsaturated to saturated fatty acids | |||||||||
| <0.52 | 4,268 | 1.00 | Referent | 1,196 | 1.00 | Referent | 1,357 | 1.00 | Referent |
| 0.52–0.65 | 3,634 | 0.91 | 0.87, 0.95 | 1,012 | 0.88 | 0.81, 0.96 | 1,171 | 0.93 | 0.86, 1.01 |
| 0.66–0.78 | 3,392 | 0.88 | 0.84, 0.92 | 945 | 0.84 | 0.77, 0.92 | 1,132 | 0.94 | 0.87, 1.02 |
| 0.79–0.98 | 3,188 | 0.85 | 0.81, 0.90 | 801 | 0.73 | 0.66, 0.80 | 1,058 | 0.91 | 0.84, 1.00 |
| ≥0.99 | 3,050 | 0.84 | 0.80, 0.89 | 855 | 0.77 | 0.70, 0.85 | 1,001 | 0.91 | 0.84, 1.00 |
| P for trend | <0.001 | <0.001 | 0.097 | ||||||
| Trans-fatty acids, g/day | |||||||||
| <1.57 | 3,508 | 1.00 | Referent | 1,017 | 1.00 | Referent | 1,044 | 1.00 | Referent |
| 1.57–2.66 | 3,265 | 0.92 | 0.88, 0.97 | 914 | 0.90 | 0.82, 0.99 | 1,037 | 0.96 | 0.88, 1.05 |
| 2.67–3.95 | 3,428 | 0.95 | 0.90, 1.00 | 940 | 0.91 | 0.83, 1.01 | 1,102 | 0.99 | 0.90, 1.08 |
| 3.96–5.95 | 3,514 | 0.95 | 0.90, 1.00 | 903 | 0.86 | 0.78, 0.96 | 1,240 | 1.06 | 0.96, 1.17 |
| ≥5.96 | 3,817 | 0.97 | 0.91, 1.04 | 1,035 | 0.94 | 0.83, 1.07 | 1,296 | 1.02 | 0.90, 1.15 |
| P for trend | 0.830 | 0.570 | 0.383 | ||||||
| Sugar-sweetened beverages, g/day | |||||||||
| <15.94 | 3,519 | 1.00 | Referent | 973 | 1.00 | Referent | 1,107 | 1.00 | Referent |
| 15.94–48.84 | 3,462 | 0.92 | 0.88, 0.96 | 935 | 0.88 | 0.81, 0.97 | 1,142 | 0.99 | 0.90, 1.09 |
| 48.85–130.34 | 3,479 | 0.93 | 0.89, 0.98 | 970 | 0.92 | 0.84, 1.01 | 1,156 | 1.00 | 0.92, 1.09 |
| 130.35–339.08 | 3,521 | 0.97 | 0.93, 1.02 | 962 | 0.95 | 0.86, 1.04 | 1,128 | 0.99 | 0.91, 1.08 |
| ≥339.09 | 3,551 | 1.00 | 0.95, 1.05 | 969 | 0.99 | 0.90, 1.09 | 1,186 | 1.01 | 0.92, 1.10 |
| P for trend | 0.022 | 0.191 | 0.835 | ||||||
| Nuts, g/day | |||||||||
| <0.08 | 4,231 | 1.00 | Referent | 1,180 | 1.00 | Referent | 1,292 | 1.00 | Referent |
| 0.08–1.09 | 3,651 | 0.93 | 0.89, 0.97 | 1,021 | 0.94 | 0.86, 1.02 | 1,201 | 1.01 | 0.93, 1.09 |
| 1.10–3.41 | 3,284 | 0.84 | 0.81, 0.88 | 913 | 0.85 | 0.78, 0.93 | 1,038 | 0.87 | 0.80, 0.94 |
| 3.42–7.78 | 3,229 | 0.84 | 0.80, 0.88 | 849 | 0.80 | 0.73, 0.87 | 1,097 | 0.93 | 0.86, 1.01 |
| ≥7.79 | 3,137 | 0.82 | 0.78, 0.86 | 846 | 0.80 | 0.73, 0.88 | 1,091 | 0.92 | 0.84, 1.00 |
| P for trend | <0.001 | <0.001 | 0.163 | ||||||
| Coffee, g/day | |||||||||
| <24.17 | 3,307 | 1.00 | Referent | 960 | 1.00 | Referent | 948 | 1.00 | Referent |
| 24.17–441.20 | 3,454 | 0.91 | 0.87, 0.96 | 1,027 | 0.92 | 0.84, 1.00 | 987 | 0.94 | 0.86, 1.02 |
| 441.21–1,050.32 | 3,440 | 0.86 | 0.82, 0.90 | 922 | 0.79 | 0.72, 0.87 | 1,155 | 1.04 | 0.95, 1.13 |
| 1,050.33–1,278.36 | 3,360 | 0.83 | 0.79, 0.87 | 861 | 0.75 | 0.68, 0.83 | 1,200 | 1.03 | 0.94, 1.12 |
| ≥1,278.37 | 3,971 | 0.88 | 0.84, 0.93 | 1,039 | 0.82 | 0.74, 0.90 | 1,429 | 1.07 | 0.98, 1.17 |
| P for trend | <0.001 | <0.001 | 0.010 | ||||||
| Red and processed meat, g/day | |||||||||
| <2.19 | 3,212 | 1.00 | Referent | 946 | 1.00 | Referent | 900 | 1.00 | Referent |
| 2.19–5.01 | 3,246 | 0.96 | 0.91, 1.01 | 897 | 0.90 | 0.82, 0.99 | 1,041 | 1.09 | 1.00, 1.19 |
| 5.02–9.38 | 3,294 | 0.92 | 0.87, 0.97 | 861 | 0.81 | 0.74, 0.89 | 1,094 | 1.07 | 0.98, 1.17 |
| 9.39–18.58 | 3,660 | 0.96 | 0.91, 1.01 | 1,011 | 0.89 | 0.81, 0.98 | 1,266 | 1.16 | 1.06, 1.28 |
| ≥18.59 | 4,120 | 1.01 | 0.95, 1.07 | 1,094 | 0.89 | 0.80, 0.99 | 1,418 | 1.19 | 1.08, 1.31 |
| P for trend | 0.052 | 0.590 | 0.002 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age (years), sex (male, female), ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, others), trial arm (intervention, control), educational level (college below, college graduate, postgraduate), marital status (married or living as married, widowed, divorced, separated, never married), history of hypertension (yes, no), family history of cancer (yes, no; only for all-cause and cancer mortality), aspirin use (yes, no), single or multivitamin supplement use (yes, no), smoking status (current, past, never), alcohol consumption (g/day), body mass index, physical activity (min/week), energy intake from diet (kcal/day), and consumption of fruits (g/day), vegetables (g/day), tea (g/day), fish (g/day), and dairy (servings/day). All individual components were mutually adjusted, with each component treated as a continuous variable in regression models.
DISCUSSION
In this large prospective multicenter study with a mean follow-up of up to 13.6 years, we found that greater adherence to a type 2 diabetes–prevention diet, as indicated by higher dietary diabetes risk-reduction score, was associated with lower risks of death from all causes, cardiovascular disease, and cancer. Subgroup analyses further found that sex, smoking status, and alcohol consumption were effect modifiers of the observed associations between dietary diabetes risk-reduction score and risks of death from all causes, cardiovascular disease, and/or cancer.
Many previous studies in nutritional epidemiology focus on the roles of individual foods or nutrients in health outcomes. However, considering the potential antagonistic or synergistic effects among dietary components and the fact that individuals always consume a variety of foods simultaneously in their daily life, the health effects of a given dietary pattern may be different from the sum of its individual components (31). Therefore, dietary pattern evaluation possibly could provide a better understanding for the roles of diets in health outcomes. In fact, the advantages of analyzing the dietary pattern in the field of public health are increasingly being recognized. For example, the 2015 Dietary Guideline Advisory Committee made its dietary recommendations based on dietary patterns rather than individual foods or nutrients (33). A growing number of studies have shown favorable associations of healthy dietary patterns with mortality risk (34). For example, Patel et al. (35) recently found that adherence to Dietary Approaches to Stop Hypertension, the Alternate Healthy Eating Index, or the Mediterranean diet was associated with a decreased risk of all-cause mortality. In this secondary analysis of the PLCO Cancer Screening Trial, we have assessed, to our knowledge for the first time, the role of the type 2 diabetes–prevention diet in the risk of mortality and we found that adherence to this dietary pattern was associated with reduced risks of all-cause and cause-specific mortality. Our findings are consistent with those from previous studies (34, 35) and extend the favorable associations between healthy dietary pattern and mortality to a type 2 diabetes–prevention diet. Thus, our findings deepen our understanding of the role of dietary exposures in relation to the risk of type 2 diabetes in determining mortality risk. Meanwhile, our findings suggest that increasing intakes of cereal fiber, polyunsaturated fatty acids, coffee, and nuts while decreasing intakes of carbohydrates, trans-fatty acids, red and processed meat, and sugar-sweetened beverages may be helpful for improving longevity, which is particularly significant in that dietary behavior can be modifiable and unhealthy diet is a leading cause of mortality in the US population (3). In addition, our findings highlight the importance of adhering to a healthy dietary pattern in improving health outcomes and provide some supporting evidence for the recommendation of adhering to a healthy eating pattern by the 2015–2020 US dietary guidelines (36).
In this study, we observed inverse associations with all-cause or cause-specific mortality for the ratio of polyunsaturated to saturated fatty acids and intakes of cereal fiber, nuts, and coffee, a positive association for sugar-sweetened beverages, and a null association for glycemic index, which are consistent with the results of previous studies (10–14, 16, 37). However, our study revealed a null association between red and processed meat consumption and cardiovascular mortality, which is inconsistent with a recent prospective cohort study showing a significant positive association (for tertile 3 vs. tertile 1, HR = 1.33, 95% CI: 1.19, 1.49) (38). The inconsistency may be due to the difference in study population; that previous study was conducted among UK adults aged 40–69 years (38). It is also possible that the positive association of red and processed meat consumption with cardiovascular mortality observed in the previous study (37) was due to incomplete adjustment for known confounders (15), such as physical activity. In addition, our study observed a null association of trans-fatty acid intake with all-cause mortality, which is consistent with the results from a prospective study in a British working population (per 1-standard-deviation increase, HR = 1.07, 95% CI: 0.98, 1.18) (39) but is inconsistent with those from several studies showing a positive association (11, 40, 41). The exact reasons for the above phenomenon are unclear and may be attributable to the differences in study population, methodology, and/or the extent of adjustment for potential confounders. Hence, more studies are needed to investigate the associations of intakes of red and processed meat and trans-fatty acid with all-cause and cause-specific mortality.
Interestingly, our study observed that the inverse association with cardiovascular mortality was more pronounced in women than in men, while the inverse association with cancer mortality was more pronounced in men than in women, indicating that sex is an outcome-specific effect modifier in our study setting. The exact reasons for this observation are unclear; it may be related to hormonal differences between the sexes. As almost all women in this study were postmenopausal, estrogen-level difference between men and women is not expected to be a major driver for this observation. Instead, testosterone-level difference between the sexes may be a key inducer. Indeed, observational studies have found that testosterone replacement therapy is associated with an increased risk of cardiovascular events (42) but a decreased risk of aggressive prostate cancer (43); thus, the relatively high testosterone level in men may attenuate the inverse association of the dietary diabetes risk-reduction score with cardiovascular mortality but strengthen the inverse association with cancer mortality. In addition, our subgroup analyses found that the inverse association of dietary diabetes risk-reduction score with cancer mortality was more pronounced in current or past smokers or participants with heavy alcohol consumption, suggesting that diabetes-prevention diet may have interactions with smoking and alcohol drinking in biological pathways. In fact, a prospective cohort study also showed that the inverse association of adherence to Dietary Approaches to Stop Hypertension diet with the risk of all-cause mortality was more pronounced in smokers than in nonsmokers (44). Of note, we cannot rule out a possibility that the above-mentioned interactions are chance findings, although they are biologically possible. Therefore, our findings from subgroup analyses warrant further investigation.
Although the specific mechanisms underlying the inverse associations of the type 2 diabetes–prevention diet with risks of all-cause and cause-specific mortality remain to be explored, intuitively, this dietary pattern possibly exerts its mortality benefits through its individual components. Human and experimental studies have suggested that polyunsaturated fatty acids are capable of improving insulin resistance (45, 46). Moreover, coffee has been found to inhibit inflammatory responses, possibly by reducing the expression of inflammation-related genes (47) and the release of inflammatory mediators (48). In addition, nut consumption has been found to be associated with attenuated oxidative stress (49), which may be through the modulation of nuclear factor-kB and nuclear factor erythroid 2–related factor 2/heme oxygenase-1 pathways (50). Collectively, these facts suggest that the inverse association of the type 2 diabetes–prevention diet with mortality may be explained by improved insulin resistance and decreased levels of inflammation and oxidative stress. Nevertheless, it is also possible that mortality benefits of adhering to this diet are mediated, at least partly, by potential interactions among individual components of the diet.
Our study has several limitations. First, food consumption information used for the construction of dietary diabetes risk-reduction score was evaluated once at baseline in our study. As dietary habits can change over time, food consumption evaluation at 1 time point may result in nondifferential bias. Nonetheless, it has been suggested that the approaches using baseline diet data only in general yield a weaker association than do these using the cumulative averages (51). In addition, in this study, nutrient intake was assessed with the DHQ, a self-administered food frequency questionnaire. However, this questionnaire did not contain the essential information that was required to accurately calculate intakes of some nutrients. For example, some trans fats are artificial and added into processed food products. Thus, the content of trans fats in a food product may depend on the brand of the product. However, the DHQ did not contain this information. Hence, nutrient assessment by the DHQ might be subject to measurement errors. Second, death certificates were employed to obtain the underlying cause of mortality in our study. Of note, the cause of mortality from death certificates may be misclassified in some circumstances (52). Hence, our findings on the association of dietary diabetes risk-reduction score with cause-specific mortality might be susceptible to misclassification bias. Moreover, the validity of mortality assessment in the PLCO Cancer Screening Trial has not been confirmed, raising some concerns on the accuracy of outcome ascertainment. Third, in our study, all participants were US adults between the ages of 55 and 74 years; moreover, 90.9% of participants were non-Hispanic White, 36.6% were college graduates, and 51.0% were current or past smokers. Therefore, our findings may not be generalizable to other populations. Fourth, as shown in Table 4, not all dietary components were associated with all-cause or cause-specific mortality. However, when constructing diabetes risk-reduction score, we assumed that each component contributes equally to the score. Thus, the score used in our study may not precisely reflect the actual role of each dietary component in the real world. Finally, as with any observational study, our results might be influenced by residual confounding due to unmeasured or unrecognized confounders, although a wide range of potential confounders was controlled for. In addition, it should be acknowledged that, based on our findings, the causal association of adhering to a diabetes-prevention diet with mortality risk cannot be established, given the observational design of our study.
In conclusion, the dietary diabetes risk-reduction score is inversely associated with the risks of death from all causes, cardiovascular disease, and cancer in this US population. These findings suggest that adherence to a type 2 diabetes–prevention diet may serve as an attractive strategy for improving longevity. Future studies should clarify the relevant biological mechanisms and validate our findings in other populations.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Infectious Diseases, Institute for Viral Hepatitis, the Key Laboratory of Molecular Biology for Infectious Diseases, Chinese Ministry of Education, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China (Chun-Rui Wang); Department of Cardiology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China (Tian-Yang Hu); Department of Neurosurgery, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao, Shandong, China (Fa-Bao Hao); Department of Anesthesiology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China (Nan Chen); Department of Geriatrics, the Fifth People’s Hospital of Chengdu, Chengdu, China (Yang Peng); Department of Nutrition and Food Hygiene, School of Public Health and Management, Chongqing Medical University, Chongqing, China (Jing-Jing Wu); Department of Nephrology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China (Peng-Fei Yang); and Department of Hepatobiliary Surgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China (Guo-Chao Zhong).
C.-R.W. and T.-Y.H. contributed equally to this work.
Data used in this study are from the PLCO Cancer Screening Trial. Data can be made available upon application and approval.
The authors sincerely thank the National Institutes of Health Prostate, Lung, Colorectal, and Ovarian (PLCO) study group and the National Cancer Institute (NCI) for access to NCI’s data collected by the PLCO Cancer Screening Trial.
The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the National Cancer Institute.
Conflict of interest: none declared.
REFERENCES
- 1. Einarson TR, Acs A, Ludwig C, et al. . Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ling S, Brown K, Miksza JK, et al. . Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43(9):2313–2322. [DOI] [PubMed] [Google Scholar]
- 3. Mokdad AH, Ballestros K, Echko M, et al. . The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhee JJ, Mattei J, Hughes MD, et al. . Dietary diabetes risk reduction score, race and ethnicity, and risk of type 2 diabetes in women. Diabetes Care. 2015;38(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weickert MO, Pfeiffer AFH. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J Nutr. 2018;148(1):7–12. [DOI] [PubMed] [Google Scholar]
- 6. Awika JM, Rose DJ, Simsek S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018;9(3):1389–1409. [DOI] [PubMed] [Google Scholar]
- 7. Luo X, Sui J, Yang W, et al. . Type 2 diabetes prevention diet and hepatocellular carcinoma risk in US men and women. Am J Gastroenterol. 2019;114(12):1870–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang JH, Peng C, Rhee JJ, et al. . Prospective study of a diabetes risk reduction diet and the risk of breast cancer. Am J Clin Nutr. 2020;112(6):1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y, Liu F, Chen AM, et al. . Type 2 diabetes prevention diet and the risk of pancreatic cancer: a large prospective multicenter study. Clin Nutr. 2021;40(11):5595–5604. [DOI] [PubMed] [Google Scholar]
- 10. Huang T, Xu M, Lee A, et al. . Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhuang P, Zhang Y, He W, et al. . Dietary fats in relation to total and cause-specific mortality in a prospective cohort of 521 120 individuals with 16 years of follow-up. Circ Res. 2019;124(5):757–768. [DOI] [PubMed] [Google Scholar]
- 12. Lukic M, Barnung RB, Skeie G, et al. . Coffee consumption and overall and cause-specific mortality: the Norwegian Women and Cancer Study (NOWAC). Eur J Epidemiol. 2020;35(10):913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Souza RJ, Dehghan M, Mente A, et al. . Association of nut intake with risk factors, cardiovascular disease, and mortality in 16 countries from 5 continents: analysis from the Prospective Urban and Rural Epidemiology (PURE) study. Am J Clin Nutr. 2020;112(1):208–219. [DOI] [PubMed] [Google Scholar]
- 14. Ho FK, Gray SR, Welsh P, et al. . Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ. 2020;368:m688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeraatkar D, Han MA, Guyatt GH, et al. . Red and processed meat consumption and risk for all-cause mortality and cardiometabolic outcomes: a systematic review and meta-analysis of cohort studies. Ann Intern Med. 2019;171(10):703–710. [DOI] [PubMed] [Google Scholar]
- 16. Zhang YB, Chen JX, Jiang YW, et al. . Association of sugar-sweetened beverage and artificially sweetened beverage intakes with mortality: an analysis of US National Health and Nutrition Examination Survey. Eur J Nutr. 2021;60(4):1945–1955. [DOI] [PubMed] [Google Scholar]
- 17. Von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prorok PC, Andriole GL, Bresalier RS, et al. . Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 suppl):273S–309S. [DOI] [PubMed] [Google Scholar]
- 19. Augustin LS, Kendall CW, Jenkins DJ, et al. . Glycemic index, glycemic load and glycemic response: an international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. [DOI] [PubMed] [Google Scholar]
- 20. Zheng JS, Sharp SJ, Imamura F, et al. . Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ. 2020;370:m2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subar AF, Thompson FE, Kipnis V, et al. . Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 22. Tippett KS, Cypel YS. Design and Operation: the Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994–96. Washington, DC: US Dept of Agriculture, Agriculture Research Service; (Nationwide Food Surveys Report No 96–1). https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/Design.pdf. Accessed January 14, 2021. [Google Scholar]
- 23. Loth KA. Nutritional Data Systems for Research. Singapore: Springer Singapore. 10.1007/978-981-287-087-2_6-1. Accessed January 14, 2021. [DOI] [Google Scholar]
- 24. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 25. Krebs-Smith SM, Pannucci TE, Subar AF, et al. . Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satija A, Bhupathiraju SN, Spiegelman D, et al. . Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spratt M, Carpenter J, Sterne JA, et al. . Strategies for multiple imputation in longitudinal studies. Am J Epidemiol. 2010;172(4):478–487. [DOI] [PubMed] [Google Scholar]
- 28. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. [DOI] [PubMed] [Google Scholar]
- 29. Di Q, Dai L, Wang Y, et al. . Association of short-term exposure to air pollution with mortality in older adults. JAMA. 2017;318(24):2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xi B, Veeranki SP, Zhao M, et al. . Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70(8):913–922. [DOI] [PubMed] [Google Scholar]
- 31. Willett W. Nutritional Epidemiology. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 32. Zhuang P, Wu F, Mao L, et al. . Egg and cholesterol consumption and mortality from cardiovascular and different causes in the United States: a population-based cohort study. PLoS Med. 2021;18(2):e1003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Millen BE, Abrams S, Adams-Campbell L, et al. . The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr. 2016;7(3):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liese AD, Krebs-Smith SM, Subar AF, et al. . The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel YR, Robbins JM, Gaziano JM, et al. . Mediterranean, DASH, and Alternate Healthy Eating Index dietary patterns and risk of death in the Physicians' Health Study. Nutrients. 2021;13(6):1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. US Department of Health and Human Services . 2015–2020 Dietary Guidelines for Americans. 8th ed.Washington, DC: US Department of Health and Human Services. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf. Published December 2015. Accessed January 14, 2021. [Google Scholar]
- 37. Shahdadian F, Saneei P, Milajerdi A, et al. . Dietary glycemic index, glycemic load, and risk of mortality from all causes and cardiovascular diseases: a systematic review and dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2019;110(4):921–937. [DOI] [PubMed] [Google Scholar]
- 38. Zhang J, Hayden K, Jackson R, et al. . Association of red and processed meat consumption with cardiovascular morbidity and mortality in participants with and without obesity: a prospective cohort study. Clin Nutr. 2021;40(5):3643–3649. [DOI] [PubMed] [Google Scholar]
- 39. Akbaraly TN, Ferrie JE, Berr C, et al. . Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr. 2011;94(1):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiage JN, Merrill PD, Robinson CJ, et al. . Intake of trans fat and all-cause mortality in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) cohort. Am J Clin Nutr. 2013;97(5):1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guasch-Ferré M, Babio N, Martínez-González MA, et al. . Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102(6):1563–1573. [DOI] [PubMed] [Google Scholar]
- 42. Loo SY, Azoulay L, Nie R, et al. . Cardiovascular and cerebrovascular safety of testosterone replacement therapy among aging men with low testosterone levels: a cohort study. Am J Med. 2019;132(9):1069–1077.e4. [DOI] [PubMed] [Google Scholar]
- 43. Loeb S, Folkvaljon Y, Damber JE, et al. . Testosterone replacement therapy and risk of favorable and aggressive prostate cancer. J Clin Oncol. 2017;35(13):1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mokhtari Z, Sharafkhah M, Poustchi H, et al. . Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet and risk of total and cause-specific mortality: results from the Golestan Cohort Study. Int J Epidemiol. 2019;48(6):1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cavaliere G, Trinchese G, Bergamo P, et al. . Polyunsaturated fatty acids attenuate diet induced obesity and insulin resistance, modulating mitochondrial respiratory uncoupling in rat skeletal muscle. PLoS One. 2016;11(2):e0149033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freire TO, Boulhosa RS, Oliveira LP, et al. . n-3 polyunsaturated fatty acid supplementation reduces insulin resistance in hepatitis C virus infected patients: a randomised controlled trial. J Hum Nutr Diet. 2016;29(3):345–353. [DOI] [PubMed] [Google Scholar]
- 47. Kolberg M, Pedersen S, Mitake M, et al. . Coffee inhibits nuclear factor-kappa B in prostate cancer cells and xenografts. J Nutr Biochem. 2016;27:153–163. [DOI] [PubMed] [Google Scholar]
- 48. López-Barrera DM, Vázquez-Sánchez K, Loarca-Piña MG, et al. . Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016;212:282–290. [DOI] [PubMed] [Google Scholar]
- 49. Silveira BKS, Silva A, Hermsdorff HHM, et al. . Effect of chronic consumption of nuts on oxidative stress: a systematic review of clinical trials. [Published online ahead of print October 12, 2020]. Crit Rev Food Sci Nutr. ( 10.1080/10408398.2020.1828262). [DOI] [PubMed] [Google Scholar]
- 50. Fusco R, Cordaro M, Siracusa R, et al. . Consumption of Anacardium Occidentale L. (cashew nuts) inhibits oxidative stress through modulation of the Nrf2/HO-1 and NF-kB pathways. Molecules. 2020;25(19):4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu FB, Stampfer MJ, Rimm E, et al. . Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. [DOI] [PubMed] [Google Scholar]
- 52. Kircher T, Nelson J, Burdo H. The autopsy as a measure of accuracy of the death certificate. N Engl J Med. 1985;313(20):1263–1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


