Abstract
Currently, standard treatment of patients with metastatic colorectal cancer (mCRC) comprises chemotherapy (CT) and/or biological therapy (BT) and/or best supportive care (BSC). The present study performed a meta-analysis on five phase II–III randomized clinical trials, which compared CT/BT/BSC as the control arm with the immune checkpoint inhibitors (ICIs) anti-programmed cell death protein 1 (PD-1) or its ligand (PD-L1) alone or in combination with cytotoxic T lymphocyte antigen 4 or mitogen activated protein kinase kinase inhibitors as the experimental arm, to evaluate whether a standard approach could be overcome using the novel target therapy strategy. Pooled hazard ratio (HR) for progression-free survival was 0.95 in favor of the experimental arm [95% confidence interval (CI), 0.74–1.22; P=0.68]. Heterogeneity was significant: Cochran's Q, 21.0; P=0.0082; I2 index, 76%. Pooled HR for overall survival was 0.88 in favor of the experimental arm (95% CI, 0.75–1.02; P=0.08). Heterogeneity was not significant (Cochran's Q, 6.0; P=0.31; I2 index, 16%). The present meta-analysis demonstrated a trend toward the improvement of survival by PD-1/PD-L1 blockade in mCRC. Further homogeneous studies are necessary to strengthen these results, beyond the known benefits of ICIs in deficient mismatch repair/high microsatellite instability tumors.
Keywords: colorectal cancer, immune checkpoint inhibitor, immunotherapy, meta-analysis, programmed cell death protein 1, programmed death ligand 1
Introduction
Colorectal cancer (CRC) is one of the most frequent causes of disease-associated deaths in industrialized countries and ranks second in terms of cancer-associated mortality worldwide (1). Despite the favorable contribution of screening programs, 25% of patients have advanced disease at diagnosis and 25–50%, who are at an early stage at diagnosis, develop metastatic disease over time (2). The prognosis of patients with metastatic CRC (mCRC) is poor despite the progress of multidisciplinary disease management as well as the current standard systemic treatment that consists of fluoropyrimidine-based chemotherapy (CT) plus oxaliplatin and/or irinotecan combined with biological therapy (BT), such as monoclonal antibodies targeting VEGF or EGFR. Owing to these approaches, average survival has tripled to ~3 years in the last two decades compared with fluorouracil alone (11 months); however, the percentage of patients still alive at 5 years remains only ~10% (3,4).
In this scenario, the challenge of immunotherapy (IT) has emerged with exciting long-term responses. This has been firstly observed in tumors with poor prognosis, such as melanoma and non-small cell lung cancer (5). The up-and-coming efficacy of IT has been reported in other solid tumors, including gastrointestinal tumors, such as programmed death ligand 1 (PD-L1)-positive gastroesophageal junction and hepatocellular carcinoma (6).
Microsatellite instability (MSI) is a hypermutable phenotype caused by the loss of DNA mismatch repair (MMR) activity (7). A total of ~15% of all patients with CRC have deficient MMR (dMMR)/high MSI (MSI-H) tumors, two-thirds of which are categorized as sporadic and one-third as germline, while 3–6% of patients with advanced CRC express a dMMR/MSI-H status (8). The remaining patients are classified as proficient MMR (pMMR) or have microsatellite stable (MSS) tumors or tumors with low MSI, which indicates instability in <30% of the loci and is often regarded as indistinct from MSS (9). In dMMR/MSI-H signature, high tumor mutational burden (TMB; corresponding to ≥10 mutations per 106 DNA bases) and immune cells within the tumor microenvironment, such as tumor infiltrating lymphocytes and macrophages, plus interferon gamma signaling represent the biological background for the role of IT in CRC (10).
Immune checkpoint inhibitors (ICIs) received accelerated Food and Drug Administration (FDA) regulatory approval on May 2017 for patients with dMMR/MSI-H mCRC pretreated with standard therapeutical lines. This was based on the results obtained from the anti-programmed cell death protein 1 (PD-1) humanized IgG4 monoclonal antibody, pembrolizumab, across five uncontrolled, multi-cohort, multicenter, single-arm clinical trials (90 patients affected by CRC) (11). CheckMate-142 was another notable study that obtained FDA approval in August 2017 for the anti-PD-1 fully human IgG4 monoclonal antibody nivolumab. A total of 74 pretreated patients with dMMR/MSI-H mCRC received nivolumab in monotherapy with a dose of 3 mg/kg every 2 weeks. Overall, these patients demonstrated an objective response rate (ORR) of 31%, a progression-free survival (PFS) rate at 12 months of 48.4% and an overall survival (OS) rate at 12 months of 73.8%, regardless of PD-L1 expression level or BRAF/KRAS mutation status, with an acceptable rate of adverse events (12).
Thereafter, in July 2018, nivolumab plus the anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) monoclonal antibody, ipilimumab, obtained regulatory approval owing to the results of further two cohorts developed by CheckMate-142. In the Phase II CheckMate-142/NCT02060188 trial, 119 patients with dMMR/MSI-H mCRC were treated with 3 mg/kg nivolumab plus 1 mg/kg ipilimumab intravenously once every 3 weeks for four times followed by 3 mg/kg nivolumab once every 2 weeks. Overall, these patients demonstrated an ORR of 58%, PFS rates at 12 and 24 months of 71 and 60%, respectively, and OS rates at 12 and 24 months of 85 and 74%, respectively, with treatment-related grade (G)3-4 manageable toxicity for 31% of them (13,14).
In the CheckMate-142 study/LBA18_PR, 45 previously untreated patients with dMMR/MSI-H mCRC received 3 mg/kg nivolumab every 2 weeks and a low dose of 1 mg/kg ipilimumab every 6 weeks until disease progression. These patients demonstrated an ORR of 60% (complete response, 7%), a 12-month PFS rate of 77% and a 12-month OS rate of 83% at a median follow-up of 13.8 months and an exceptionally low rate of G3-4 adverse events (AEs; 16%) (15). More recently, KEYNOTE-177 demonstrated that patients with dMMR/MSI-H mCRC who received pembrolizumab as first line treatment had the probability of living without progression twice on average compared with patients undergoing the conventional approach (16). Based on these data, the present meta-analysis was designed with the aim to clarify and improve interpretation of the results of the heterogeneous studies currently available on ICIs in an advanced CRC setting.
Materials and methods
Systematic review and meta-analysis
The present study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA 2009 checklist) and their extensions (17,18). No study involving human participants and requiring ethics committee approval based on the Declaration of Helsinki and its subsequent revisions was conducted during the present investigation.
Eligibility criteria
Inclusion criteria
The trials, which may be prospective and randomized, concerned the diagnosis of chemo-naive or non-chemo-naive mCRC. Abstracts that contained sufficient information detailing study design, patient characteristics and outcomes were considered. Patients in the experimental arm received treatment with a monoclonal antibody targeting PD-1/PD-L1 alone or in combination (ICI arm). Patients in the control arm received the standard of care with CT and/or BT and/or best supportive care (BSC) (CT/BT/BSC arm).
Exclusion criteria
Non-comparative studies, non-randomized clinical trials and studies that did not involve the target drugs of the present study were excluded. Studies that had no comparable endpoints, cost effective analyses or studies that were written in languages other than English were excluded. Trials with radiotherapy were also excluded due to major heterogeneity.
Data extraction and quality evaluation
The public databases MEDLINE (https://www.nlm.nih.gov/medline/index.html), PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Embase (www.embase.com) and Central Registry of Controlled Trials of the Cochrane Library (www.cochranelibrary.com) were searched for entries from January 01, 1993 until June 15, 2021 and abstracts and relevant full texts were retrieved. A Google academic search (scholar.google.com), including meeting abstracts, was also performed to track relevant references. The search included the following keywords: (‘Colorectal’ OR ‘colorectum’ OR ‘colon’ OR ‘rectum’ OR ‘rectal’) AND (‘adenocarcinoma’ OR ‘carcinoma’ OR ‘tumor’ OR ‘neoplasm’ OR ‘cancer’) AND (‘programmed cell death protein 1’ OR ‘PD-1’ OR ‘programmed death ligand 1’ OR ‘PD-L1’ OR ‘B7-H1’ OR ‘CD274’ OR ‘checkpoint inhibitor’).
Study selection and data collection process
The studies were examined independently by two investigators (MSR, MR) to verify concordance with the eligibility criteria. Variables, such as the number of enrolled patients, year of publication, the treatment program and efficacy endpoints, were extracted and evaluated. All patients were considered for PFS, OS, ORR and safety profile. Any discrepancy was resolved by an arbitrator (MGZ).
Summary measures and statistical analysis
The hazard ratios (HRs) for PFS and OS and the relative ratios (RRs) for ORR and for risk of G≥3 AEs, with their corresponding confidence intervals (CIs), were derived from each included study and were compared in the two groups, ICI vs. CT/BT/BSC arm. The percentage of objective responses (for example, complete or partial response according to the Response Evaluation Criteria in Solid Tumors) and toxicities (G3, 4 and 5 AEs) were collected along with their CIs, separately, for each treatment arm of each study (19,20). The pooled HRs for PFS and OS were calculated using the random-effects model, to generate a more conservative estimate than a fixed-effects model. The pooled RRs for ORR and for G≥3 AEs were also calculated using the random-effects model. HRs, RRs and CIs were translated into logarithm (log) of the HRs, log of the RRs and the corresponding variances. Each study (log)HR and (log)RR were weighted by the inverse of their variance. Weights were considered equal to the inverse of the reported within-study variance plus the between-study variance component τ2. The moment estimator of the between-study variance was used. The Cochran's Q statistics and the associated test were calculated to assess between-study heterogeneity. In addition to Cochran's Q, the I2 statistics, which express the percentage of the total observed variability due to heterogeneity, were also calculated to give an improved measure of the inter-trial consistency. For higher values of the I2 index, heterogeneity is improved (an I2 index of 25, 50 and 75% corresponds to low, medium and high heterogeneity, respectively). Forest plots were reported to display the meta-analysis results. Publication bias was examined in funnel plots using a regression symmetry test. The analyses were conducted using the R package Metafor (Viechtbauer W, 2010; http://doi.org/10.18637/jss.v036.i03) and figures were produced using the R base graphics functions (R Core Team, 2014; http://www.R-project.org/). P<0.05 was considered to indicate a statistically significant difference (21–23).
Results
Study selection
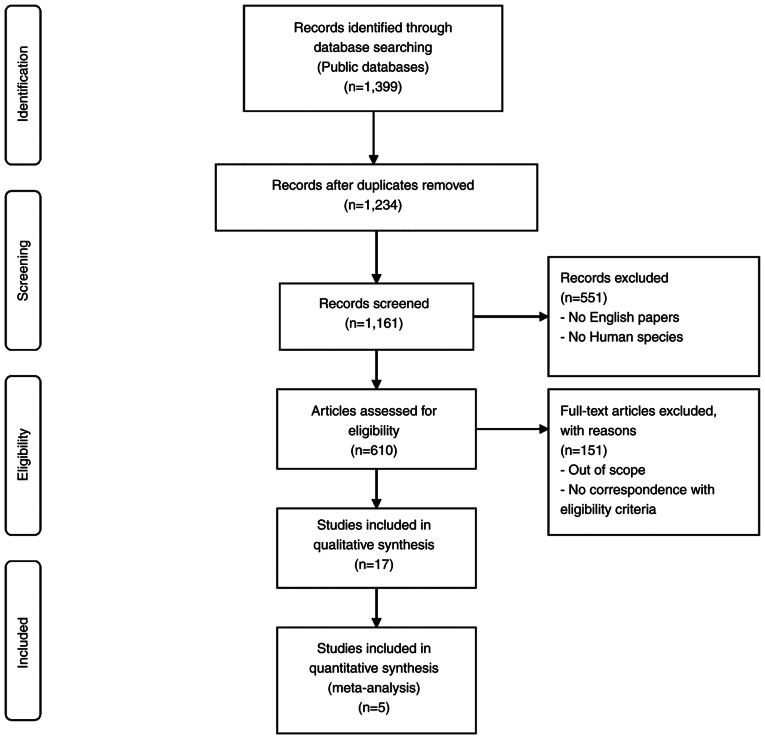
Published within the 1993–2021 timeframe, 1,399 articles were contained in the bibliographic databases. All non-related topic studies, non-comparative studies, non-randomized clinical trials and duplicates were excluded. The remaining 17 articles were further reviewed as potential candidates for the present meta-analysis, but only five articles met the aforementioned inclusion criteria. The searching and selection process is outlined in Fig. 1.
Figure 1.
Flow chart of the literature search used in the present meta-analysis.
Individual study characteristics and results
The included studies were conducted on chemo-naive or non-chemo-naive mCRC. The total number of patients from all trials was 1,423. The characteristics and efficacy results of the selected studies are reported in Table I.
Table I.
Characteristics and efficacy results of the eligible studies.
| First author, year (trial) | Phase | Setting | Target population | Arms | Primary endpoints | SEPs | No. of enrolled patients | PFS (C vs. E) | OS (C vs. E) | ORR (C vs. E) | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mettu et al, 2019 (BACCI) | 2, randomized | Metastatic, refractory | Not selected for MMR status | C: Capecitabine + bevacizumab + placebo; E: Capecitabine + bevacizumab + atezolizumab | PFS | ORR, OS, safety | 128 | 3.3 (95% CI, 2.1-6.2) vs. 4.4 (95% CI, 4.1-6.4) months; HR, 0.725 (95% CI, 0.491-1.07; P=0.051) | 12-month OS: 43% (95% CI, 29–63) vs. 52% (95% CI, 42–65); HR, 0.94 (95% CI, 0.56-1.56; P=0.4) | 4.35 (95% CI, 0.5-14.8) vs. 8.54% (95% CI, 3.5-16.8); P=0.5 | (36) |
| Andre et al, 2021 (KEYNOTE-177) | 3 | Metastatic, 1st line | dMMR/MSI-H | C: SOC CT +/-bevaci zumab/cetuximab; E: Pembrolizumab | PFS, OS | ORR, safety | 307 | 8.2 vs. 16.5 months; HR, 0.59 (95% CI, 0.45-0.79) | 36.7 months vs. median not reached; HR, 0.74 (95% CI, 0.53-1.03; P=0.0359) | 33.1 vs. 45.1% | (16) |
| Chen et al, 2020 (CCTG CO.26) | 2, randomized | Metastatic, refractory | Not selected for MMR status | C: BSC; E: Durvalumab + tremelimumab + BSC | OS | PFS, ORR, AEs | 180 | 1.9 vs. 1.8 months; HR, 1.01 (90% CI, 0.76-1.34; P=0.97) | 4.1 vs. 6.6 months; HR, 0.72 (90% CI, 0.54-0.97; P=0.07) | 0 vs. 0.84% (DCR: 6.6% vs. 22.7%; P=0.006) | (30) |
| Grothey et al, 2018 (MODUL) | 2, randomized, signal-seeking trial | Metastatic, maintenance after 1st line | BRAF wild-type | C: Fluoropyrimidine + bevacizumab; E: Fluoropyrimidine + bevacizumab + atezolizumab | PFS | OS, AEs, ORR, DCR, TTR, DoR | 445 | 7.39 vs. 7.2 months; HR, 0.96 (95% CI, 0.77-1.20; P=0.727) | 21.91 vs. 22.05 months; HR, 0.86 (95% CI, 0.66-1.13; P=0.283) | Ongoing | (35) |
| Eng et al, 2019 (COTEZO IMblaze370) | 3 | Metastatic, at least two prior regimens | Recruitment of patients with MSI-H was capped at 5% | C: Regorafenib; E1: Atezolizumab; E2: Atezolizumab + cobimetinib | OS | PFS, CR, PR, DoR, QoL, AEs, plasmatic concentration of atezolizumab and cobimetinib, % of anti-atezolizumab antibodies | 363 | C vs. E1: 2 (95% CI, 1.87-3.61) vs. 1.94 (95% CI, 1.91-2.1) months; HR, 1.39 (95% CI, 1–1.94; P=0.05); C vs. E2: 2 (95% CI, 1.87-3.61) vs. 1.91 (95% CI, 1.87-1.97) months; HR, 1.25 (95% CI, 0.94-1.65; P=0.13) | C vs. E1: 8.51 (95% CI, 6.41-10.71) vs. 7.10 (95% CI, 6.05-10.05) months; HR, 1.19 (95% CI, 0.83-1.71; P=0.34); C vs. E2: 8.51 (95% CI, 6.41-10.71) vs. 8.87 (95% CI, 7.00-10.61) months; HR, 1.00 (95% CI, 0.73-1.38; P=0.99) | Ongoing | (33) |
AE, adverse event; BSC, best supportive care; C, control arm; CI, confidence interval; CR, complete remission; CT, chemotherapy; DCR, disease control rate; dMMR, deficient mismatch repair; DoR, duration of response; E, experimental arm; HR, hazard ratio; MMR, mismatch repair; MSI-H, high microsatellite instability; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial remission; QoL, quality of life; SEP, secondary endpoint; SOC, standard of care; TTR, time to treatment response.
Pembrolizumab
Besides the phase Ib basket trial KEYNOTE-028, which demonstrated a favorable safety profile in 23 pretreated PD-L1-positive patients with mCRC (24), the efficacy of pembrolizumab, which binds to the PD-1 receptor and blocks its interactions with ligands PD-L1 and PD-L2, was evaluated in the phase 2 study KEYNOTE-016. This trial reported an immune-related ORR of 40% and a 20-week immune-related PFS rate of 78% in a cohort of 41 patients with treatment-refractory dMMR/MSI-H mCRC (25,26). The KEYNOTE-164 study confirmed the antitumor activity of pembrolizumab in 61 patients with treatment-refractory dMMR/MSI-H mCRC, with an ORR of 33%, a PFS of 2.3 months and an OS of 31.4 months at a median follow-up of 31.3 months. In a cohort of 63 patients treated with >1 prior line of therapy, ORR was 33%, PFS was 4.1 months, and OS was not reached at a median follow-up of 24.2 months (27). KEYNOTE-177 is a phase 3, open-label trial, which randomized 307 patients with treatment-naive dMMR/MSI-H mCRC to receive pembrolizumab 200 mg intravenously every 3 weeks for up to 35 cycles or the standard care CT, with or without the biological agents bevacizumab (anti-VEGF) or cetuximab (anti-EGFR). Patients receiving CT could crossover to pembrolizumab after progression. Median follow up was 44.5 months with pembrolizumab and 44.4 months with CT. PFS was markedly increased in the pembrolizumab arm vs. the CT arm (HR, 0.59); however, only a trend toward the improvement of survival was reached with pembrolizumab with respect to CT (HR, 0.74; P=0.0359; for OS significance, the P-value had to meet a prespecified one-sided α=0.0246; Table I) (16). Data reported at the 2021 Gastrointestinal Cancer Symposium and at the 2021 American Society of Clinical Oncology Annual Meeting revealed that the benefit of first line pembrolizumab continued beyond disease progression on the subsequent line of treatment, despite the high crossover to IT (36% of patients of the CT arm plus an additional 37 patients who received an ICI outside of the study, for an effective crossover rate of 60%). The second PFS (PFS2; the time from randomization to disease progression on the next line of therapy or death from any cause) was 24.9 months in the CT arm (62% PFS2 events) and 54.0 months in the ICI arm (44% PFS2 events), with an HR of 0.61 (95% CI, 0.44-0.83) (16,28).
Durvalumab
In a phase I study, 11 patients with mCRC, unselected for MMR status, were treated with durvalumab, a human IgG1 monoclonal antibody directed against the PD-L1 receptor, plus tremelimumab, a monoclonal antibody directed against the CTLA4 protein, reaching one partial response and three stable disease (29). In the phase II trial CCTG CO.26, 180 patients with refractory mCRC, were randomized 2:1 to receive durvalumab (1,500 mg intravenously every 28 days), associated for the first four cycles to tremelimumab (75 mg intravenously), vs. BSC, after failure of all standard regimens. No patients with known dMMR/MSI-H tumors were enrolled. At a median follow-up of 15.2 months, OS was significantly in favor of the experimental arm, where the two-sided P-value was considered statistically significant if <0.10 (HR, 0.72; P=0.07; Table I). In patients confirmed to have pMMR/MSS, the HR for OS was 0.66 in favor of the combined arm (90% CI, 0.49-0.89; P=0.02). No significant difference was reported in median PFS (HR, 1.01; P=0.97). The disease control rate (DCR) was statistically superior for the ICI arm (P=0.006; Table I). Quality of life was preserved, although there was a higher frequency of AEs in the durvalumab plus tremelimumab arm (30).
Atezolizumab
The humanized IgG1 monoclonal antibody atezolizumab dually blocks the PD-L1 and B7.1 receptors, binding to PD-L1 and reactivating the antitumor immune response (25). In preclinical studies, an enhanced immune response was observed by adding mitogen activated protein kinase kinase (MEK)-inhibitors to PD-1/PD-L1 inhibitors (31). In a phase Ib trial with atezolizumab plus the MEK inhibitor cobimetinib, seven responses were observed: A total of six with pMMR/MSS and one with dMMR/MSI-H tumors among 84 patients with mCRC (32). The following multicenter, open-label, phase 3 trial IMblaze 370 randomized 363 patients with mCRC with a 2:1:1 modality. After ≥2 previous CT regimens, patients received atezolizumab 840 mg intravenously every 2 weeks plus cobimetinib 60 mg orally once daily for 21 days every 28 days (183 patients) vs. atezolizumab 1,200 mg intravenously every 3 weeks (90 patients) or the multi-kinase inhibitor regorafenib 160 mg orally once daily for 21 days every 28 days (90 patients). dMMR/MSI-H patients were not to exceed 5%. At a median follow up of 7.3 months, the primary endpoint OS did not improve by the combination treatment with respect to regorafenib (HR, 1.00; P=0.99) or by atezolizumab monotherapy vs. regorafenib (HR, 1.19; P=0.34). G3-4 AEs were prevalent in the combination and regorafenib arms (33).
MODUL is a multicenter phase 2 randomized trial with an adaptable signal-seeking approach based on biomarker-driven maintenance therapy, following the first line standard treatment of mCRC. The study treatment is divided into an induction therapy (FOLFOX regimen plus bevacizumab for 16 weeks) and a maintenance phase for patients without progressive disease, with the assignment in a cohort through tumor tissue biomarkers assessment, and post-treatment follow-up. The cohorts developed to date were ‘BRAF V600E-mutated’ (Cohort 1) and ‘No Biomarker-BRAF wild-type’ (Cohort 2). Patients were randomized (2:1) to: i) Fluoropyrimidine plus cetuximab plus the inhibitor of the mutated BRAF kinase vemurafenib for Cohort 1-experimental; ii) fluoropyrimidine plus bevacizumab plus atezolizumab for Cohort 2-experimental; and iii) fluoropyrimidine plus bevacizumab as the control arm for all cohorts. Among the 824 patients screened, 696 were enrolled to induction therapy. In the primary analysis of Cohort 2, among 445 patients with BRAF wild-type mCRC randomized to maintenance treatment (297 patients in fluoropyrimidine/bevacizumab + atezolizumab; 148 patients in fluoropyrimidine/bevacizumab), no statistically significant difference in the primary endpoint PFS was observed, with an HR of 0.92 (95% CI, 0.72-1.17; P=0.48) at a median follow up of 10.5 months. In the updated analysis, at a median follow up of 18.7 months, the HR for PFS was 0.96 (P=0.727). No advantage in OS was reported either (34,35).
BACCI is a phase II randomized trial conducted in the USA with the aim to co-target the PD-1/PD-L1 and the VEGF axes in 133 patients with refractory mCRC, randomized 2:1 to capecitabine 850-1,000 mg/m2 bidaily, days 1–14, plus bevacizumab 7.5 mg/kg, day 1, plus atezolizumab 1,200 mg, day 1 every 21 days (experimental arm) vs. capecitabine plus bevacizumab plus placebo (control arm). A previous line with bevacizumab, but not with anti-PD-1/PD-L1, was allowed. The primary endpoint was PFS and the secondary endpoints were ORR, OS and safety. At a median follow-up of 12.35 months, with 128 patients included in the analysis, the study reached its prespecified primary endpoint in favor of the atezolizumab arm (110 events required to achieve a PFS HR of 0.65 at one-sided α=0.1 and 80% power), with an HR of 0.725 and a one-sided log-rank P-value of 0.051. In patients with pMMR/MSS (86.7% of the control arm vs. 85.7% of the experimental arm), the HR for PFS was 0.67 (0.44-1.03) in favor of the experimental arm (36).
Meta-analysis results
Efficacy
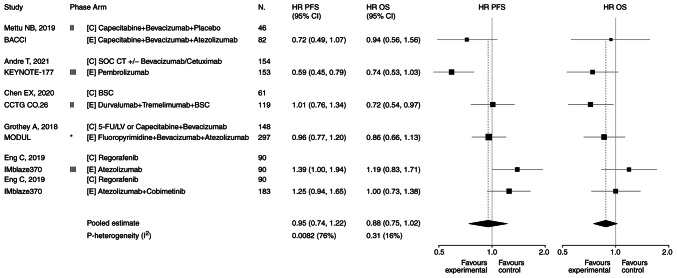
The present study evaluated the PFS and OS to establish the efficacy of the ICI arm vs. the CT/BT/BSC arm. Pooled HR for PFS was 0.95 in favor of the ICI arm (95% CI, 0.74-1.22; P=0.68; Fig. 2). Heterogeneity was significant: Cochran's Q, 21.0; P=0.0082; I2 index, 76%. Pooled HR for OS was 0.88 in favor of the ICI arm (95% CI, 0.75-1.02; P=0.08; Fig. 2). Heterogeneity was not significant (Cochran's Q, 6.0; P=0.31; I2 index, 16%). Forest plots are presented in Fig. 2. Data on ORRs are heterogeneous among trials. Therefore, the pooled ratio of the ORRs in the experimental arm/control arm was calculated, suggesting 1.36 in favor of the ICI arm (95% CI, 1.03-1.80; P=0.027; data not shown).
Figure 2.
Forest plots of PFS and OS. Five trials reported on PFS. Pooled HR for PFS was 0.95 in favor of the experimental arm (95% CI, 0.74-1.22; P=0.68). Heterogeneity was significant: Cochran's Q, 21.0; P=0.0082; I2 index, 76%. Five trials reported on OS. Pooled HR for OS was 0.88 in favor of the experimental arm (95% CI, 0.75-1.02; P=0.08). Heterogeneity was not significant (Cochran's Q, 6.0; P=0.31; I2 index, 16%). *Signal-seeking trial (phase 2, randomized). BSC, best supportive care; C, control arm; CI, confidence interval; CT, chemotherapy; E, experimental arm; 5-FU/LV, 5-fluorouracil/leucovorin; HR, hazard ratio; N, number of patients; OS, overall survival; PFS, progression-free survival; SOC, standard of care.
Safety
Data on toxicity are highly heterogeneous (Table II). The pooled ratio of the risk of G≥3 AEs resulted in 0.87 in favor of the experimental arm (95% CI, 0.40-1.90; P=0.72; data not shown).
Table II.
Data on toxicity reported in the eligible studies.
| Trial | Arms | No. of enrolled patients | Grade ≥3 adverse events (C vs. E) | (Refs.) |
|---|---|---|---|---|
| BACCI | C: Capecitabine + bevacizumab + placebo; E: Capecitabine + bevacizumab + atezolizumab | 128 | Hypertension 7 vs. 9%, Hand-foot syndrome 4 vs. 6%, Diarrhea 2 vs. 7% | (36) |
| KEYNOTE-177 | C: SOC CT +/-bevacizumab/cetuximab; E: Pembrolizumab | 307 | Total 66 vs. 22% | (16) |
| CCTG CO.26 | C: BSC; E: Durvalumab + treme limumab + BSC | 180 | Total 20 vs. 64% (reported version of CTCAE, 4.0); Predominant in E: Abdominal pain, fatigue, white blood cells and eosinophils increase | (30) |
| MODUL | C: Fluoropyrimidine + bevaci zumab; E: Fluoropyrimidine + bevacizumab + atezolizumab | 445 | Ongoing | (35) |
| COTEZO IMblaze370 | C: Regorafenib; E1: Atezolizumab; E2: Atezolizumab + cobimetinib | 363 | Total C 58 vs. E1 31% vs. E2 61%; Predominant in E2: Diarrhea (11%), anemia (6%), increased serum creatine phosphokinase (7%) and fatigue (4%) + two treatment-related deaths (sepsis); In C: One treatment-related death (intestinal perforation). | (33) |
BSC, best supportive care; C, control arm; CT, chemotherapy; CTCAE, common terminology criteria for adverse events; E, experimental arm; SOC, standard of care.
Publication bias
No asymmetry was detected in the funnel plots for PFS and OS, with symmetry P-values of 0.94 and 0.49 with Egger's symmetry test, respectively, providing no statistical evidence of the presence of publication bias (Fig. 3).
Figure 3.
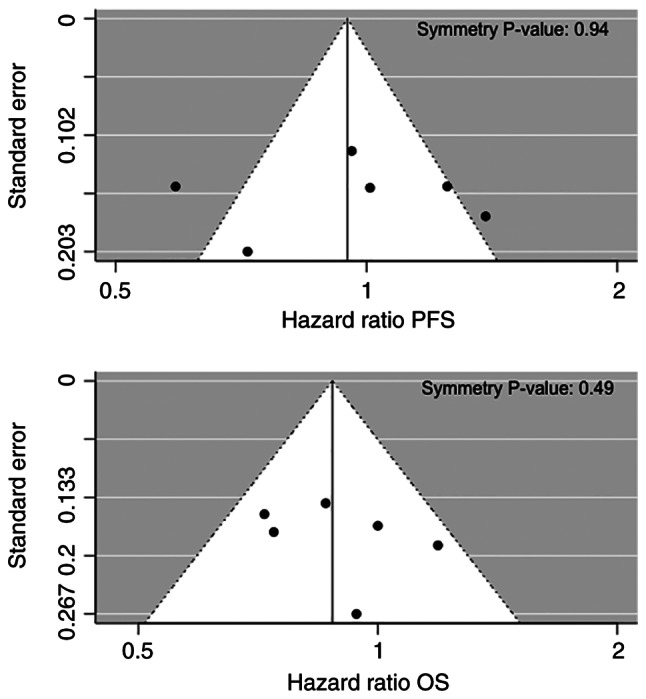
Funnel plots of PFS and OS for publication bias. No asymmetry was detected (symmetry P=0.94 for PFS and P=0.49 for OS, respectively), providing no statistical evidence of the presence of publication bias. OS, overall survival; PFS, progression-free survival.
Discussion
During the last two decades, the ‘CT-only’ approach for mCRC has been evolving through its combination with biological agents targeting EGFR or angiogenesis, involving research of novel specific molecular therapeutic targets, based on emerging biomarkers of tumor cell signaling cascades (for example, HER2, BRAF, MEK, neurotrophic tyrosine receptor kinase and c-Met) and mediators of immune response elicitation. IC pathways, such as the PD-1/PD-L1 and the CD28/CTLA4 systems, serve an important role in the maintenance of self-tolerance and for limiting collateral tissue damage during anti-microbial immune defense. They can be exploited by tumor cells to evade anti-tumor immunity, reducing the cytotoxic activity of T cells, which protects tumor cells from apoptosis (37). In this context, ICIs proved to hold a notable capability to switch on the immune surveillance against cancer (38). Phase I trials reported favorable activity of ICIs in solid tumors (39); however, in CRC, objective responses were observed only for a small subset of patients with dMMR/MSI-H (5% of mCRCs).
Advantages for this population were confirmed in the more advanced phases of clinical studies, highlighting the predictive value of the MMR status and guiding the performance of new studies in this direction (for example, the COMMIT-NCT02997228 study in first line or the ATOMIC-NCT02912559 in adjuvant setting) (40–42). He et al (43) performed a meta-analysis of six early phase studies for a total of 297 patients with mCRC who progressed during or after ≥1 previous line of systemic treatment and received nivolumab (two studies) or pembrolizumab (four studies). The pooled 1-year OS rate, the PFS rate, the ORR and the DCR were 64.2, 38.4, 19.7 and 56.5%, respectively. The outcome was improved in the dMMR/MSI-H subgroup (34% for ORR), with a decrease in the high heterogeneity observed when the studies involving pMMR/MSS cases were excluded (43).
These data are consistent with the results of the present meta-analysis, conducted with the aim to evaluate the role of ICIs when compared with standard treatment for advanced CRC, regardless of the PD-1/PD-L1 expression level, MMR status and line of therapy. Advantages in pooled PFS and OS were obtained in favor of the experimental arm, although without statistical significance potentially due to the different patient characteristics among trials. The favorable results of the CCTG CO.26 trial, where the P-value was considered significant if <0.10, and that of the BACCI trial, which reached its pre-specified primary endpoint (progression-free survival), emerged even if treatment-refractory patients affected by pMMR/MSS mCRC were included. The negative results regarding IMblaze 370, where the recruitment of patients with dMMR/MSI-H was capped at 5%, and MODUL studies weighed against these outcomes. However, the particularity of the MODUL study design, where atezolizumab was evaluated in patients with BRAF wild-type mCRC as a maintenance-therapy after first line therapy, should be considered.
Despite the inclusion in the present meta-analysis of the MODUL trial and other trials that enrolled metastatic refractory patients, the present study observed a counterbalance in the KEYNOTE-177 study due to the success of pembrolizumab as first line therapy specifically for patients with dMMR/MSI-H mCRC. Notably, heterogeneity for OS was not significant. Available data on ORR from the single trials are very heterogeneous, reaching >30% in both experimental and control arms in KEYNOTE-177, while in the remainder of studies the ORR results were <9%. As the ORRs were not reported in all studies, or were based on an exceptionally low number of events, which corresponds to low statistical weight, the present study calculated the pooled ratio of the ORRs. This was driven by the predominant result of KEYNOTE-177 and was statistically significant in favor of the ICI arm (1.36; P=0.027). Regarding toxicity, the G≥3 AEs were also heterogeneous among the single studies and the pooled ratio of the risk of G≥3 AEs (0.87; in favor of the experimental arm) was affected by the >3-times risk of G≥3 AEs of the CCTG CO.26 experimental arm, while inverted results were observed in KEYNOTE-177.
The unmet endpoints of prolonged survival in the described searches, in particular due to the population with unknown MMR status, that may include non-responder cases, reinforced the need for sensitizing the tumor microenvironment to IT. This is more evident for the vast majority of tumors that have lower levels of immune inflammation, such as pMMR/MSS CRC, whose resistance to the innovative IC blockade represents a serious hurdle (44). Therefore, the best use of IC targeting still needs guidance through molecular biomarkers and biological pathways. The underlying mechanism of the emphasized success of IT in the dMMR/MSI-H CRC population remains unclear, although a high TMB was reported in this subgroup and improved outcomes were indicated for tumors, among which CRC, with high TMB with respect to low TMB (even if the cut-off critical value, which is different for cancer types in predicting ICIs efficacy, still needs to be defined), independently by PD-L1 expression (45,46). Tumor-related neoantigens derived from mutations could activate immune cells and increase T-cell tumor infiltration.
In support of this hypothesis, MSI-H CRC has abundant lymphocyte infiltrates and strong expression of IC proteins (47). Strategies to also trigger immune activity in the pMMR/MSS CRC population consist of increasing the mutational load, creating neoantigens or potentiating the immune infiltrate. For this purpose, ICIs are studied in association with radiotherapy or bispecific antibody therapy or cytotoxic agents and/or other agents targeting angiogenesis or other signaling pathway molecules. For example, those involved in the RAS-RAF-MEK-ERK or PI3K-AKT-mTOR cascades. Other regimens, which may potentially elicit an immune response in pMMR/MSS mCRC, combine IT with the DNA-damaging agents poly-ADP-ribose polymerase inhibitors or with cyclo-oxygenase 2 inhibitors or using adoptive cell therapy with chimeric antigen receptor T cells expressing anti-PD-1/PD-L1. Finally, targeting WNT/β-catenin signaling, whose activation is more frequent in pMMR/MSS CRC and is involved in the mechanism of immune exclusion, is under consideration to improve IT efficacy; this is achieved by inducing transcriptional repression of chemokine genes, such as C-C motif chemokine 4, which is important for the intratumoral homing of dendritic cells to the tumor bed. The latest emerging trials that involved combination strategies to overcome resistance are reported in Table III (48–66).
Table III.
Emerging clinical trials focused on combination strategies to overcome resistance.
| A, With radiotherapy | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Trials | Phase | Target population | Treatment strategy | PEPs | Enrollment status | Results | (Refs.) |
| NCT03104439 | 2 | MSS mCRC, MSI-H mCRC, pancreatic cancer (pretreated) | Nivolumab + ipilimumab + palliative RT | DCR | Recruiting (80 pts estimated) | MSS mCRC cohort (40 pts): DCR 17.5%; ORR 7.5%; G≥3 AEs 50%, 1 death for respiratory failure | (48) |
| NSABP FC-9 (NCT03007407) | 2 | MSS mCRC (pretreated) | Durvalumab + tremelimumab following hypofractionated RT | ORR | Recruitment completed (33 pts enrolled) | 14 pts in the first stage: 5 G3 AEs, no G4/5. | (49) |
| ETCTN 10021 (NCT02888743) | 2, randomized | MSS mCRC refractory to 1st line CT, non-small cell lung cancer progressive on PD-1 inhibitor | Durvalumab + tremelimumab +/- repeated low dose fraction ated RT/hypofractionated RT | ORR | Active (180 pts estimated, final data collection date: December 31, 2022) | Prespecified analysis of the CRC cohort (20 pts randomized, 19 treated with IT + RT, 18 evaluable): 1 SD; 16 treatment-related toxicity, 8 G3-5 AEs. | (50) |
|
| |||||||
| B, With bispecific antibody therapy | |||||||
|
| |||||||
| Trials | Phase | Target population | Treatment strategy | PEPs | Enrollment status | Results | (Refs.) |
|
| |||||||
| CO40939 (NCT03866239) | 1b | CEACAM5-high MSS mCRC (pretreated) | Cibisatamab + atezolizumab after pretreatment with obinutuzumaba | %pts with AEs; ORR | Recruiting (46 pts estimated) | Ongoing | (51) |
|
| |||||||
| C, With cytotoxic and/or biological agents | |||||||
|
| |||||||
| Trials | Phase | Target population | Treatment strategy | PEPs | Enrollment status | Results | (Refs.) |
|
| |||||||
| CAVE (EudraCT: 2017-004392-32) | 2 | RAS-BRAF wild-type mCRC, not selected for MMS (pretreated) | Avelumab + cetuximabb | OS | 77 pts enrolled | OS 13.1 months (95% CI, 7.4-18.8 months); PFS 3.6 months (95% CI, 3.3-3.9 months); 1 CR, 3 PR, 32 SD; G3 AEs 22% (13% skin rash, 4% diarrhea). | (52) |
| AVETUX trial (NCT03174405) | 2 | RAS-BRAF wild-type mCRC, not selected for MMS (1st line) | Modified FOLFOX6 + cetux imab + avelumab for up to 18 months | 12-months PFS rate | 43 pts enrolled (39 ITT; 92% MSS, 5% MSI-H, 3% MSI-L) | 12-month PFS rate 40% (PEP of 57% not met); PFS 11.1 months; OS rate 84.6%; ORR 81%; DCR 92% (median follow up 16.2 months). T lymphocytes tumor-infiltration correlates with avelumab reactions (not prognostic). | (53) |
| AVETUXIRI (NCT03608046) | 2 | MSS mCRC (pretreated; allowed previous anti-EGFR if RAS-BRAF wild-type) | Avelumab + cetuximab + irinotecan in RAS-BRAF wild-type pts (cohort A) vs. RAS/BRAF mutated pts (cohort B) | ORR | Recruiting (59 pts estimated) | Interim analysis: 3 PR in cohort A (PEP met, the study continues as 2nd stage in cohort A), no response in cohort B; DCR 60% and 61.5%, PFS 4.2 months and 3.8 months, OS 12.7 months and 14 months in cohort A and B, respectively; G3 21.7% (diarrhea, all related to irinotecan). Encouraging data of cohort B allowed to open the cohort C (PEP: PFS) for RAS/BRAF mutated. | (54) |
| AVETRIC (NCT04513951) | 2 | Initially unresectable RAS wild-type mCRC (1st line) | Modified FOLFOXIRIc + cetuximab + avelumab | PFS | Recruiting (58 pts estimated) | Ongoing | (55) |
| NIVACOR/GOIRC-03-2018 (NCT04072198) | 2 | RAS/BRAF mutated mCRC (1st line) | Nivolumab + FOLFOXIRI + bevaci zumabd for 8 cycles followed by maintenance with bevacizumab + nivolumab | ORR | Recruiting (70 pts estimated) | Preliminary safety analysis (10 pts): G3-4 neutropenia 43%, febrile neutropenia 14%; 1 discontinuation due to serious ileo-urethral fistula not related to nivolumab. | (56) |
| AtezoTRIBE (NCT03721653) | 2, randomized | Initially unresectable mCRC (1st line) | FOLFOXIRI + bevacizumab up to 8 cycles vs. FOLFOXIRI + bevacizumab + atezolizumab up to 8 cycles; Followed by maintenance: 5-FU/LV + bevacizumab vs. 5-FU/LV + bevacizumab + atezolizumab, respectively | PFS | Active (201 pts estimated, final data collection date: April 15, 2021) | Ongoing | (57) |
| REGOMUNE (NCT03475953) | 1/2 | Advanced/metastatic solid tumors-mCRC (pretreated) | Regorafenibe + avelumab for advanced/metastatic solid tumors (ten cohorts), once the RP2D has been determined (phase I trial); low dose of regorafenib (80 mg/day) + avelumab for mCRC pts | For phase 1: RP2D; for phase 2: ORR, PFS | Recruiting (482 pts estimated) | Non-MSI-H mCRC cohort (48 pts, median follow up 7.2 months): 12 TMB reduction, 23 SD; PFS 3.6 months (95% CI, 1.8-5.4); OS 10.8 months (95% CI, 5.9-not reached); G3-4 AEs: Hand Foot Syndrome 29.8%, hypertension 23.4%, diarrhea 12.8%. Low tumor-associated macrophages expression level and low tumor cells to CD8+ T cells distance appear predictive of response to regorafenib + avelumab combination. | (58) |
| ARETHUSA (NCT03519412) | 2 | dMMR mCRC, RAS-extended mutated pMMR mCRC (pretreated) | Pembrolizumab for dMMR mCRC pts; temozolomidef for MGMT IHC-negative, promoter methylation positive RAS-extended mutated pMMR mCRC pts; pts progressing under temo zolomide will proceed to pembro lizumab if TMB is >20 mutations/Mb | ORR | Recruiting (348 pts estimated) | Ongoing | (59) |
| MAYA (NCT03832621) | 2 | MSS, MGMT-silenced mCRC with initial clinical benefit from lead-in treatment with temozolomide (pretreated or not eligible to other conventional treatment) | Nivolumab + ipilimumab + temozolomide | 8-month PFS rate | Active (135 pts estimated, final data collection date: February 2022) | Ongoing | (60) |
| DAPPER (NCT03851614) | 2, randomized, basket | pMMR mCRC, advanced pancreatic adenocarcino ma, advanced leiomyo sarcoma (pretreated or 1st line if no standard therapy exists) | Olaparib (PARP inhibitor) + durvalumab vs. cediranib (VEGFR tyro sine kinases inhibitor) + durvalumab | Changes in genomic and immune biomarkers and in radiomic profiles | Recruiting (90 pts estimated) | Ongoing | (61) |
Carcinoembryonic-T cell bispecific CEA-TCB antibody cibisatamab can cross-link cancer cells and T cells, leading to T cell engagement and activation; obinutuzumab, a glycoengineered CD20 humanized antibody, can abrogate cytokine release of the first TCB administration as for hematologic malignancies (51,66).
Enhancement of antibody-dependent cellular cytotoxicity induced by cetuximab with a consequent anti-PD-L1 activity potentiation is the rationale for a rechallenge strategy after anti-EGFR based first line therapy in RAS-BRAF wild-type mCRC population, independent by MMR status (52).
Immunogenic cell death, induced by CT, or by intensified CT regimens, such as FOLFOXIRI, allows the release of neoantigens that are presented by dendritic cells to cytotoxic T lymphocytes and activate antitumor immune response (55).
Anti-angiogenic agents, such as bevacizumab, can sensitize to the PD-1 checkpoint blockade by the upregulation of the PD-L1 expression via CD8+ T cell secretion of interferon gamma and can normalize tumor vessels promoting intratumoral infiltration of activated T cells (56).
Capability of regorafenib to modulate anti-tumor immunity (also reducing the tumor-associated macrophages) was the basis of its use in synergy with the fully human monoclonal antibody anti-PD-L1 avelumab (58).
Preclinical data demonstrated that, after the induction of somatic mutations in MMR genes, the acquired resistance to temozolomide is accompanied by a high load of neoantigens and translates into an MSI-like phenotype, predictive of response to pembrolizumab (59). AE, adverse event; CEACAM5, carcinoembryonic antigen-related cell adhesion molecule 5; CI, confidence interval; CR, complete remission; CT, chemotherapy; DCR, disease control rate; dMMR, deficient mismatch repair; EudraCT, European Union Drug Regulating Authorities Clinical Trials; FOLFOX, oxaliplatin, folinic acid and 5-fluorouracil; FOLFOXIRI, oxaliplatin, irinotecan, folinic acid and 5-fluorouracil; 5-FU/LV, 5-fluorouracil/leucovorin; G, grade; IHC, immunohistochemistry; IT, immunotherapy; ITT, intention-to-treat; Mb, megabase; mCRC, metastatic colorectal cancer; MGMT, O6-methylguanine-DNA-methyltransferase; MMR, mismatch repair; MSI, microsatellite instability; MSI-H, high microsatellite instability; MSI-L, low microsatellite instability; MSS, microsatellite stable; ORR, objective response rate; OS, overall survival; PARP, Poly ADP-ribose polymerase; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; PEP, primary endpoint; PFS, progression-free survival; pMMR, proficient mismatch repair; PR, partial remission; pt, patient; RP2D, recommended phase II dose; RT, radiotherapy; SD, stable disease; TMB, tumor mutational burden; VEGFR, vascular endothelial growth factor receptor.
Novel prognostic/predictive factors are under evaluation in dMMR/MSI-H mCRC. For example, circulating tumor DNA and microbiota in the phase II randomized trial SAMCO-PRODIGE 54 (comparing the anti-PD-L1 avelumab with standard second line treatment) (67). Predictor variables of the response to IT are a more urgent requirement for patients with pMMR/MSS CRC (68,69). Associations between PD-L1 expression levels and drug efficacy are limited in CRC. However, in the ultramutated phenotype (~1% of pMMR CRCs) of the DNA polymerase ε, which is characterized by the loss of its exonuclease activity, an upregulation of IC genes, such as PD-1/PD-L1 and CTLA4, accompanied by increased immunogenicity, has been associated with clinical advantages similar to dMMR tumors (70,71). Furthermore, a 44-gene signature assay identified 25% of pMMR tumors that possess an innate immune response ability, associated with the upregulation of PD-L1 and indoleamine 2,3-dioxygenase 1, which is similar to 80% of the dMMR population (72). A favorable prognostic role in early CRC, without predictive value for ICIs, has been highlighted for the high immunoscore revealed in the majority of dMMR/MSI-H and in a subgroup of pMMR/MSS CRCs, which was based on CD3+ and CD8+ infiltrating lymphocyte density in the center and invasive margins of the tumor (73).
Forkhead box P3 (FOXP3)-low non-suppressive T cells that are recruited in the presence of tumor colonization by Fusobacterium nucleatum have been observed in an MSI-H tumor microenvironment; while in MSS tumors, FOXP3-high immunosuppressive T-regulatory cells are predominant with associated immune response silencing (74). Identification of the FOXP3 T cell subtype infiltration in tumor tissues and its variation during treatment may be useful in predicting antitumor activity and/or resistance to IT. Despite limitations (for instance, different molecular expression between primary and metastatic sites or between chemo-naive and pretreated patients), the CRC consensus molecular subtype (CMS) classification could also contribute, beyond the MMR status, to identify tumors with an ideal ground for IT. As opposed to the ‘hot’ CMS1 (or ‘immune’; mainly dMMR/MSI-H tumors, exhibiting high TMB and high frequency of BRAF mutations) and CMS4 (or ‘mesenchymal’; tumors with stromal infiltration, angiogenesis activation and involvement of TGF-β), the ‘cold’ CMS2 (or ‘canonical’; with WNT and MYC activation) and CMS3 (or ‘metabolic’; with cancer-cell metabolic dysregulation and KRAS mutation), would require major strategies against their escape mechanisms to ICI activity (75).
The present meta-analysis highlighted favorable results of IT in mCRC, supporting the role of ICIs as a first choice for patients with dMMR/MSI-H, although the small number of trials used may be a limitation of the present study. Efforts are ongoing to evaluate the most effective approach in pMMR/MSS mCRC, where the tumor microenvironment conversion from an immune-silenced to an immune-activated phenotype could be a means to maximize the benefit of ICIs. Further investigations are needed in researching novel combination treatments to overcome resistance and optimize outcomes in the majority of patients with CRC.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MSR and MGZ conceptualized the present study; MSR and VB designed the methodology; VB used software to perform statistical analysis; MSR, VB, MR, MC and MGZ performed validation of the data; MSR and MGZ confirm the authenticity of all the raw data; MSR and VB performed the formal analysis; MSR, VB, MR, MC and MGZ performed the investigation; MSR, VB, MR, MC and MGZ provided the resources; MSR, VB, MR, MC and MGZ curated the data; MSR, VB, MR, MC and MGZ prepared the original draft; MSR, VB, MR, MC and MGZ wrote, reviewed and edited the manuscript; MSR, VB, MR, MC and MGZ checked the data; MSR, MGZ supervised the study; MSR and MGZ performed project administration. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25((Suppl 3)):iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 3.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.6027. [DOI] [PubMed] [Google Scholar]
- 4.Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer: Lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6:39. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein A, Moehler M, Trojan J, Goekkurt E, Vogel A. Immuno-oncology in GI tumours: Clinical evidence and emerging trials of PD-1/PD-L1 antagonists. Crit Rev Oncol Hematol. 2018;130:13–26. doi: 10.1016/j.critrevonc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins MA, Hayashi S, O'Shea AM, Burgart LJ, Smyrk TC, Shimizu D, Waring PM, Ruszkiewicz AR, Pollett AF, Redston M, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: A population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano I, Grassi E, Bertotti A, Trusolino L. Immunogenomics of colorectal tumors: Facts and hypotheses on an evolving Saga. Trends Cancer. 2019;5:779–788. doi: 10.1016/j.trecan.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 12.Overman MJ, Lonardi S, Leone F, McDermott RS, Morse MA, Wong KYM, Neyns B, Leach JL, Garcia Alfonso P, Lee JJ, et al. Nivolumab in patients with DNA mismatch repair deficient/microsatellite instability high metastatic colorectal cancer: Update from CheckMate 142. J Clin Oncol. 2017;35((Suppl 4)):S519. doi: 10.1200/JCO.2017.35.4_suppl.519. [DOI] [Google Scholar]
- 13.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, et al. Durable clinical benefit with Nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2018.36.4_suppl.554. [DOI] [PubMed] [Google Scholar]
- 14.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse M, Van Cutsem E, McDermott RS, Hill AG, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): Long-term follow-up. J Clin Oncol. 2019;37((Suppl 4)):S635. doi: 10.1200/JCO.2019.37.4_suppl.635. [DOI] [Google Scholar]
- 15.Lenz HJJ, Van Cutsem E, Limon ML, Wong KY, Hendlisz A, Aglietta M, Garcia-Alfonso P, Neyns B, Luppi G, Cardin D, et al. Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) Ann Oncol. 2018;29:viii714. doi: 10.1093/annonc/mdy424.019. [DOI] [Google Scholar]
- 16.Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith DM, Garcia-Carbonero R, Alcaide J, Gibbs P, et al. Final overall survival for the phase III KN177 study: Pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) J Clin Oncol. 2021;39((Suppl 15)):S3500. doi: 10.1200/JCO.2021.39.15_suppl.3500. [DOI] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:39. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE-Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 23.R Core Team R, corp-author. A language and environment for statistical computing. http://www.R-project.org/ R Foundation for Statistical Computing, Vienna, Austria. 2014 [Google Scholar]
- 24.O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul Razak AR, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HT, Lee SH, Heo YS. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules. 2019;24:1190. doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiu KK, Andre T, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith DM, Garcia-Carbonero R, Benavides M, Gibbs P, et al. KEYNOTE-177: Phase III randomized study of pembrolizumab versus chemotherapy for microsatellite instability-high advanced colorectal cancer. J Clin Oncol. 2021;39((Suppl 3)):S6. doi: 10.1200/JCO.2021.39.3_suppl.6. [DOI] [Google Scholar]
- 29.Callahan MK, Odunsi K, Sznol M, Nemunaitis JJ, Ott PA, Dillon PM, Park AJ, Schwarzenberger P, Ricciardi T, Macri MJ, et al. Phase 1 study to evaluate the safety and tolerability of MEDI4736 (durvalumab, DUR) + tremelimumab (TRE) in patients with advanced solid tumors. J Clin Oncol. 2017;35((Suppl 15)):S3069. doi: 10.1200/JCO.2017.35.15_suppl.3069. [DOI] [Google Scholar]
- 30.Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al. Effect of combined immune checkpoint inhibition vs. best supportive care alone in patients with advanced colorectal cancer: The Canadian Cancer Trials Group CO.26 study. JAMA Oncol. 2020;6:831–838. doi: 10.1001/jamaoncol.2020.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson C, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: Effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann MD, Kim TW, Lee CB, Goh BC, Miller WH, Jr, Oh DY, Jamal R, Chee CE, Chow LQM, Gainor JF, et al. Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol. 2019;30:1134–1142. doi: 10.1093/annonc/mdz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 34.Schmoll HJ, Arnold D, De Gramont A, Ducreux M, Grothey A, O'Dwyer PJ, Van Cutsem E, Hermann F, Bosanac I, Bendahmane B, et al. MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: An adaptable signal-seeking approach. J Cancer Res Clin Oncol. 2018;144:1197–1204. doi: 10.1007/s00432-018-2632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grothey A, Tabernero J, Arnold D, De Gramont A, Ducreux MP, O'Dwyer PJ, Van Cutsem E, Bosanac I, Srock S, Mancao C, et al. Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs. FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): Findings from Cohort 2 of MODUL-a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann Oncol. 2018;29:VIII714–VIII715. doi: 10.1093/annonc/mdy424.020. [DOI] [Google Scholar]
- 36.Mettu NB, Twohy E, Ou FS, Halfdanarson TR, Lenz HJ, Breakstone R, Boland PM, Crysler O, Wu C, Grothey A, et al. BACCI: A phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): An ACCRU network study. Ann Oncol. 2019;30:v203. doi: 10.1093/annonc/mdz246.011. [DOI] [Google Scholar]
- 37.Messersmith WA. NCCN Guidelines Updates: Management of metastatic colorectal cancer. J Natl Compr Canc Netw. 2019;17:599–601. doi: 10.6004/jnccn.2019.5014. [DOI] [PubMed] [Google Scholar]
- 38.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: Opportunities and challenges. Immunotherapy. 2016;8:821–837. doi: 10.2217/imt-2016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA., Jr Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overman MJ, Yothers G, Jacobs SA, Sanoff HK, Cohen DJ, Guthrie KA, Henry NL, Ganz PA, Kopetz S, Lucas PC, et al. NRG-GI004/SWOG-S1610: Colorectal Cancer Metastatic dMMR Immuno-Therapy (COMMIT) Study-A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC) J Clin Oncol. 2021;39((Suppl 3)):TPS158. doi: 10.1200/JCO.2021.39.15_suppl.TPS3618. [DOI] [Google Scholar]
- 42.Sinicrope FA, Ou FS, Zemla T, Nixon AB, Mody K, Levasseur A, Dueck AC, Dhanarajan AR, Lieu CH, Cohen DJ, et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502) J Clin Oncol. 2019;37((Suppl 15)):e15169. doi: 10.1200/JCO.2019.37.15_suppl.e15169. [DOI] [Google Scholar]
- 43.He S, Hu D, Feng H, Xue Y, Jin J, Wang X. Efficacy of immunotherapy with PD-1 inhibitor in colorectal cancer: A meta-analysis. J Comp Eff Res. 2020;9:1285–1292. doi: 10.2217/cer-2020-0040. [DOI] [PubMed] [Google Scholar]
- 44.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan L, Wang Z, Xue J, Yang H, Zhu Y. Tumor mutation burden predicts response and survival to immune checkpoint inhibitors: A meta-analysis. Transl Cancer Res. 2020;9:5437–5449. doi: 10.21037/tcr-20-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30:1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 47.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh AR, Clark JW, Wo JYL, Yeap BY, Allen JN, Blaszkowsky LS, Ryan DP, Giantonio BJ, Weekes CD, Zhu AX, et al. A phase II study of ipilimumab and nivolumab with radiation in microsatellite stable (MSS) metastatic colorectal adenocarcinoma (mCRC) J Clin Oncol. 2019;37((Suppl 15)):S3514. doi: 10.1200/JCO.2019.37.15_suppl.3514. [DOI] [Google Scholar]
- 49.Lee JJ, Yothers G, George TJ, Fakih M, Basu Mallick A, Maalouf BN, Krauss JC, Heron DE, Allegra CJ, Jacobs SA. NSABP FC-9: Phase II study of dual immune checkpoint blockade (ICB) with durvalumab (D) plus tremelimumab (T) following palliative hypofractionated radiotherapy (SBRT) in patients (pts) with microsatellite-stable (MSS) metastatic colorectal cancer (mCRC) progressing on chemotherapy. J Clin Oncol. 2018;36((Suppl 15)):e15681. doi: 10.1200/JCO.2018.36.15_suppl.e15681. [DOI] [Google Scholar]
- 50.Monjazeb A, Giobbie-Hurder A, Lako A, Tesfaye AA, Stroiney A, Gentzler RD, Jabbour S, Alese OB, Rahma OE, Cleary JM, et al. Analysis of colorectal cancer patients treated on ETCTN 10021: A multicenter randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation. J Clin Oncol. 2019;37((Suppl 8)):S49. doi: 10.1200/JCO.2019.37.8_suppl.49. [DOI] [Google Scholar]
- 51.A Phase Ib study to evaluate the safety, efficacy, pharmacokinetics of cibisatamab in combination with atezolizumab after pretreatment with obinutuzumab in participants with previously treated metastatic colorectal adenocarcinoma. https://clinicaltrials.gov/ct2/show/NCT03866239. [ March 31; 2021 ];ClinicalTrials.gov Identifier. NCT03866239. Other Study ID Numbers: CO40939; EudraCT Number: 2018-003198-93. [Google Scholar]
- 52.Martinelli E, Troiani T, Cardone C, Ciardiello D, Zanaletti N, Borrelli C, Terminiello M, Avallone A, Falcone A, Maiello E, et al. Phase II study of avelumab in combination with cetuximab as a rechallenge strategy in pre-treated RAS wild type metastatic colorectal cancer patients: CAVE (cetuximab-avelumab) colon. Ann Oncol. 2019;30:v251. doi: 10.1093/annonc/mdz246.145. [DOI] [Google Scholar]
- 53.Stein A, Binder M, Goekkurt E, Lorenzen S, Riera-Knorrenschild J, Depenbusch R, Ettrich TJ, Doerfel S, Al-Batran SE, Karthaus M, et al. Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (MCRC): Final results of the phase II AVETUX trial (AIO-KRK-0216) J Clin Oncol. 2020;38((Suppl 4)):S96. doi: 10.1200/JCO.2020.38.4_suppl.96. [DOI] [Google Scholar]
- 54.Van Den Eynde M, Huyghe N, De Cuyper A, Sinapi I, Ferrier M, Gilet M, Van Maanen A, Castella ML, Galon J, Carrasco J. Interim analysis of the AVETUXIRI Trial: Avelumab combined with cetuximab and irinotecan for treatment of refractory microsatellite stable (MSS) metastatic colorectal cancer (mCRC)-A proof of concept, open-label, non-randomized phase IIa study. J Clin Oncol. 2021;39((Suppl 3)):S80. doi: 10.1200/JCO.2021.39.3_suppl.80. [DOI] [Google Scholar]
- 55.Martinelli E, Ciardiello D, Martini G, Troiani T, Cardone C, Vitiello PP, Normanno N, Rachiglio AM, Maiello E, Latiano T, et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann Oncol. 2020;31:30–40. doi: 10.1016/j.annonc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Damato A, Berselli A, Iachetta F, Romagnani A, Larocca M, Garcia Arias A, Antonuzzo L, Nasti G, Bergamo F, Pinto C. Preliminary safety analysis of phase II open-label NIVACOR trial (GOIRC-03-2018) in patients with advanced colorectal cancer RAS or BRAF mutated. J Clin Oncol. 2021;39((Suppl 3)):S37. doi: 10.1200/JCO.2021.39.3_suppl.37. [DOI] [Google Scholar]
- 57.Antoniotti C, Borelli B, Rossini D, Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F, Tamberi S, Corallo S, et al. AtezoTRIBE: A randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer. 2020;20:683. doi: 10.1186/s12885-020-07169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cousin S, Bellera CA, Guégan JP, Gomez-Roca CA, Metges JP, Adenis A, Pernot S, Cantarel C, Kind M, Toulmonde M, et al. REGOMUNE: A phase II study of regorafenib plus avelumab in solid tumors-Results of the non-MSI-H metastatic colorectal cancer (mCRC) cohort. J Clin Oncol. 2020;38((Suppl 15)):S4019. doi: 10.1200/JCO.2020.38.15_suppl.4019. [DOI] [Google Scholar]
- 59.Siena S, Sartore-Bianchi A, Personeni N, Pietrantonio F, Germano G, Amatu A, Bonoldi E, Valtorta E, Barault L, Di Nicolantonio F, et al. Pembrolizumab in MMR-proficient metastatic colorectal cancer pharmacologically primed to trigger dynamic hypermutation status: The ARETHUSA trial. J Clin Oncol. 2019;37((Suppl 15)):TPS2659. doi: 10.1200/JCO.2019.37.15_suppl.TPS2659. [DOI] [Google Scholar]
- 60.Nivolumab Plus Ipilimumab, Temozolomide in Microsatellite Stable, MGMT Silenced Metastatic Colorectal Cancer (MAYA) https://clinicaltrials.gov/ct2/show/NCT03832621. [ March 31; 2021 ];ClinicalTrials.gov Identifier. NCT03832621. Other Study ID Number: INT202-18. [Google Scholar]
- 61.Basket Combination Study of Inhibitors of DNA Damage Response, Angiogenesis and Programmed Death Ligand 1 in Patients with Advanced Solid Tumors (DAPPER) https://clinicaltrials.gov/ct2/show/NCT03851614. [ March 31; 2021 ];ClinicalTrials.gov Identifier. NCT03851614. Other Study ID Number: DAPPER-001. [Google Scholar]
- 62.Tintelnot J, Stein A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges and novel approaches. World J Gastroenterol. 2019;25:3920–3928. doi: 10.3748/wjg.v25.i29.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huyghe N, Baldin P, Van Den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf) 2019;8:11–24. doi: 10.1093/gastro/goz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganesh S, Shui X, Craig KP, Park J, Wang W, Brown BD, Abrams MT. RNAi-Mediated beta-catenin inhibition promotes T cell infiltration and antitumor activity in combination with immune checkpoint blockade. Mol Ther. 2018;26:2567–2579. doi: 10.1016/j.ymthe.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 67.Taïeb J, André T, El Hajbi F, Barbier E, Toullec C, Kim S, Bouche O, Di Fiore F, Chauvenet M, Perrier H, et al. Avelumab versus standard second line treatment chemotherapy in metastatic colorectal cancer patients with microsatellite instability: The SAMCO-PRODIGE 54 randomised phase II trial. Dig Liver Dis. 2020;53:318–323. doi: 10.1016/j.dld.2020.11.031. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Pascual J, Ayuso-Sacido A, Belda-Iniesta C. Drug resistance in cancer immunotherapy: New strategies to improve checkpoint inhibitor therapies. Cancer Drug Resist. 2019;2:980–993. doi: 10.20517/cdr.2019.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghiringhelli F, Fumet JD. Is there a place for immunotherapy for metastatic microsatellite stable colorectal cancer? Front Immunol. 2019;10:1816. doi: 10.3389/fimmu.2019.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shan T, Chen S, Wu T, Yang Y, Li S, Chen X. PD-L1 expression in colon cancer and its relationship with clinical prognosis. Int J Clin Exp Pathol. 2019;12:1764–1769. [PMC free article] [PubMed] [Google Scholar]
- 71.Domingo E, Freeman-Mills L, Rayner E, Glaire M, Briggs S, Vermeulen L, Fessler E, Medema JP, Boot A, Morreau H, et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol. 2016;1:207–216. doi: 10.1016/S2468-1253(16)30014-0. [DOI] [PubMed] [Google Scholar]
- 72.Tsantoulis P, Hill LA, Walker SM, Wirapati P, Graham DM, Wilson RH, Coyle V, Delorenzi M, HarkinD P, Kennedy RD, Tejpar S. Association of a specific innate immune response to DNA damage with DNA repair deficient colorectal cancers. J Clin Oncol. 2016;34:3035. doi: 10.1200/JCO.2016.34.15_suppl.3035. [DOI] [Google Scholar]
- 73.Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of immunoscore in patients with colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2019;8:182–189. doi: 10.1002/cam4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et al. Two FOXP3(+) CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 75.Spallanzani A, Gelsomino F, Caputo F, Santini C, Andrikou K, Orsi G, Rimini M, Pipitone S, Riggi L, Bardasi C, et al. Immunotherapy in the treatment of colorectal cancer: A new kid on the block. J Cancer Metastasis Treat. 2018;4:28. doi: 10.20517/2394-4722.2018.31. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.