Abstract
The number of mesenchymal stromal/stem cell (MSC) therapeutics and types of clinical applications have greatly diversified during the past decade, including rapid growth of poorly regulated “Stem Cell Clinics” offering diverse “Unproven Stem Cell Interventions.” This product diversification necessitates a critical evaluation of the reliance on the 2006 MSC minimal criteria to not only define MSC identity but characterize MSC suitability for intravascular administration. While high-quality MSC therapeutics have been safely administered intravascularly in well-controlled clinical trials, repeated case reports of mild-to-more-severe adverse events have been reported. These are most commonly related to thromboembolic complications upon infusion of highly procoagulant tissue factor (TF/CD142)-expressing MSC products. As TF/CD142 expression varies widely depending on the source and manufacturing process of the MSC product, additional clinical cell product characterization and guidelines are needed to ensure the safe use of MSC products. To minimize risk to patients receiving MSC therapy, we here propose to supplement the minimal criteria used for characterization of MSCs, to include criteria that assess the suitability of MSC products for intravascular use. If cell products are intended for intravascular delivery, which is true for half of all clinical applications involving MSCs, the effects of MSC on coagulation and hemocompatibility should be assessed and expression of TF/CD142 should be included as a phenotypic safety marker. This adjunct criterion will ensure both the identity of the MSCs as well as the safety of the MSCs has been vetted prior to intravascular delivery of MSC products.
Keywords: cellular therapy, mesenchymal stromal/stem cells (MSCs), tissue source, product diversification, safety and efficacy, hemocompatibility, coagulation, coagulopathy, thromboembolism, tissue factor/CD142/Factor III/F3
Graphical Abstract
Graphical Abstract.
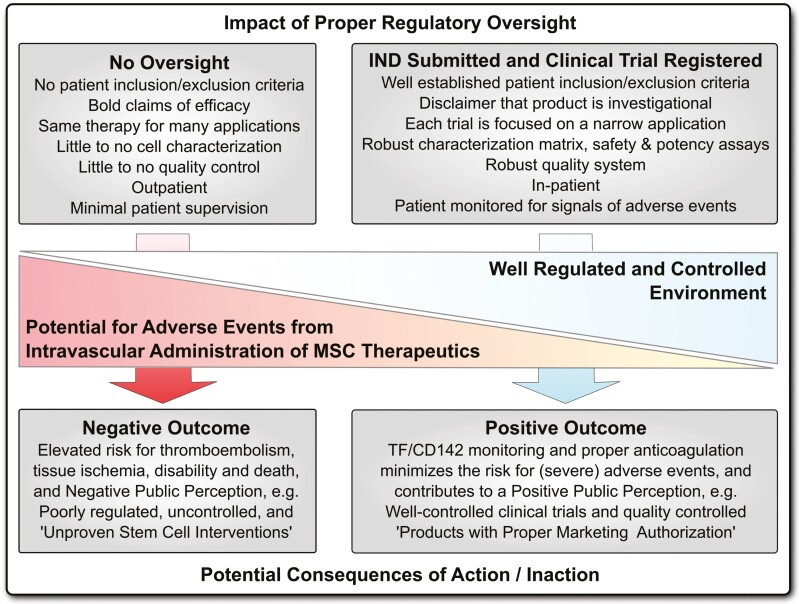
A broad spectrum of oversight impacts on MSC product safety in patients. We here outline the necessary steps toward integration of highly procoagulant tissue factor (TF/CD142) and hemocompatibility assessment of diversified intravascular MSC products as a new safety criterion into the existing MSC minimal criteria. Regulatory authorities and international societies should undertake coordinated efforts to update the already established guidelines.
Significance Statement.
As cell therapies have diversified/grown, so has the frequency of reports on rare but significant adverse thrombotic events. These events are associated with the expression of highly procoagulant tissue factor (TF/CD142) on MSCs triggering coagulation. Current clinical evidence and the supporting research calls for responsible investigators to assess the amount of TF/CD142 and the effects of clinical cell products and to incorporate suitable anticoagulation treatments into their clinical protocols to mitigate the risk of thromboembolic adverse events. Prior to clinical use, MSC expression of TF/CD142 and hemocompatibility should be tested and included as a phenotypic and safety marker.
Introduction
Much ink has been spilled over the definition and name of MSCs (mesenchymal stromal/stem cells).1-7 The first definitions of MSCs relied heavily on the tissue of origin, bone marrow (BM), as a defining characteristic along with the ability of the cells to differentiate down mesodermal lineages, such as cartilage, fat, and bone.2,7-12 In recognition that similar cells can be found in tissues other than BM, in 2006, the International Society for Cell and Gene Therapy (ISCT) proposed a set of MSC “minimal” criteria that defined MSCs based on phenotypic characteristics rather than tissue of origin.13 This coalesced the field around a shared definition and helped advance MSC research and regenerative and immunomodulatory therapies for addressing a great number of unmet medical needs. Today, >50 000 publications on MSCs are listed in PubMed, and >1000 studies using MSCs for diverse indications are registered in clinical trial registries.12,14 While cells meeting the ISCT minimal criteria share much in common, MSCs derived from different tissues and expanded using different processes can have highly divergent properties that impact both their potency and safety profile.2,12,15-20 The rapid progress in the field mandates vigilance for new developments since both MSC products and their treatment indications have greatly diversified during the past decade.2,12,18-30 Recognizing the diversification of MSC products and the increased use of MSCs in humans, guidelines are being established by several regulatory authorities, such as the US Federal Drug Administration (FDA) and European Medicines Agency (EMA),7,20,24,30-36 as well as international societies, such as ISCT and International Society for Stem Cell Research (ISSCR).13,37,38 These guidelines aim to ensure the quality and safety of these novel advanced therapy medicinal products (ATMPs). Critically, these guidelines complement, rather than replace, the definitional criteria to ensure the quality, potency, and safety of MSC products. Continuing in this same spirit of developing safe and effective MSC therapies, we propose the addition of hemocompatibility as a critical characteristic for MSC therapies intended for intravascular use.12,18,19
Clinical Relevance of TF/CD142 Expression for MSC Therapy and Case Reports of Adverse Thrombotic Events
Literature review and meta-analysis have shown that MSC therapeutics used in well-controlled clinical trials exhibit an excellent safety profile (Fig. 1).12,39-42 However, rare but prominent issues with hemocompatibility have become apparent with infused MSC products and need to be incorporated into the existing clinical guidelines to complement the minimal criteria for MSC characterization (Figs. 2, 3).12,18-20,28 The potentially harmful nature of these adverse events mandates great vigilance to avoid any negative outcome for patients, particularly in rather poorly regulated/controlled environments.20,23
Figure 1.
A broad spectrum of oversight impacts on mesenchymal stromal/stem cell (MSC) product safety in patients. While most respected academic centers and professional larger-scale manufacturers and sponsors of well-regulated clinical trials rely on quality systems that minimize the potential of adverse events from intravascular administration of procoagulant tissue factor (TF/CD142) expressing MSC therapeutics (right panel) (eg, submission of an investigational new drug (IND) application to the FDA), there exist hundreds of poorly regulated and poorly controlled “Stem Cell Clinics” internationally, which operate in an environment of lax medical regulations, thus risking potential harm of patients (left panel).20,23 Potential adverse events from intravascular administration include disturbances of hemostatic parameters, which can cause micro-/macro-thrombosis and (pulmonary) thromboembolism, vessel occlusion, and tissue ischemia, that can lead to disability and may potentially end fatal under inappropriate patient supervision.12,43-46
Figure 2.
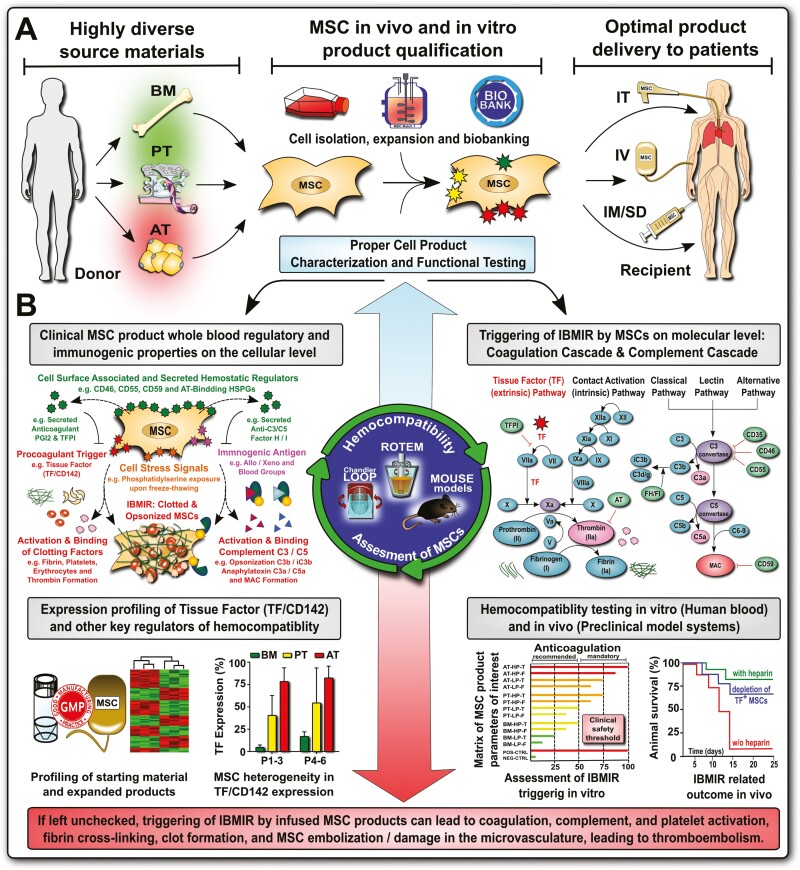
Increasing mesenchymal stromal/stem cell (MSC) product diversification necessitates TF/CD142 screening and hemocompatibility assessment for intravascular MSC applications. (A) MSC products have greatly diversified in the past decade, for example, the tissue source that they are derived from, with bone marrow (BM), perinatal tissue (PT), and adipose tissue (AT) being the most frequent sources, which is decisive for the optimal mode of clinical delivery to patients, for example, intravascular (IV) infusion versus intramuscular or subdermal (IM/SD) injection, or intratracheal (IT) delivery12,18; and (B) Product qualification has shown large differences in expression of procoagulant tissue factor (TF/CD142) between different products (BM lowest, PT intermediate, and AT highest),12 which impacts on the cells’ hemocompatibility properties in vitro and in vivo, and may lead to adverse thrombotic reactions if left unchecked before clinical application. Abbreviations: complement factors C3, C5, C3b, iC3b, C3a, and C5a, and regulatory molecules: membrane cofactor protein (MCP/CD46), decay-accelerating factor (DAF/CD55), protectin (CD59), and factor H and I; coagulation factors I-XII (FI-XII), including their activated intermediates (eg, FXIIa) and regulators antithrombin (AT), tissue factor pathway inhibitor (TFPI) and prostacyclin (PGI2), and instant blood-mediated inflammatory reaction (IBMIR).
Figure 3.
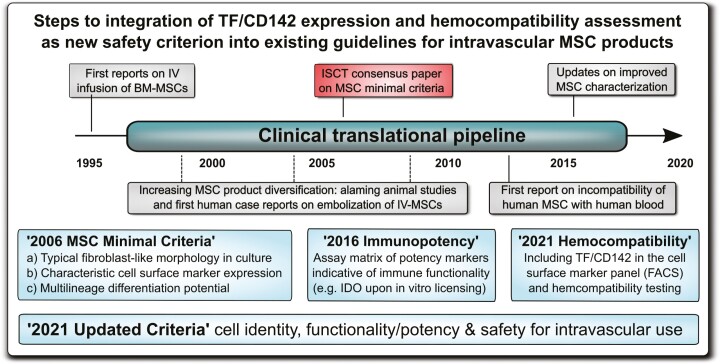
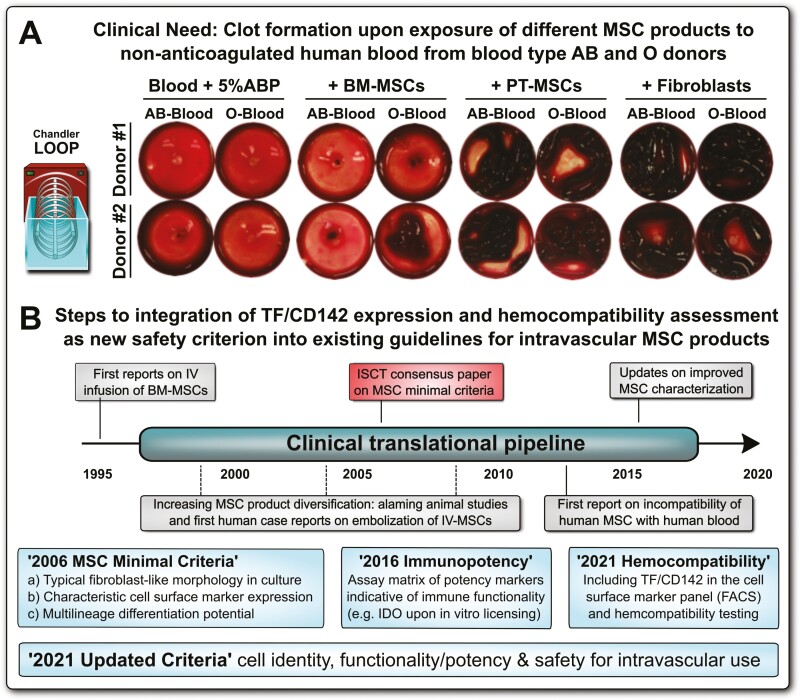
Improved clinical guidelines and minimal criteria for the characterization of diversified mesenchymal stromal/stem cell (MSC) products to include TF/CD142 and hemocompatibility assessment. (A) Clinical need: representative images on blood clot formation upon in vitro exposure of different types of tissue factor (TF/CD142) expressing MSC products to non-anticoagulated human blood from blood type AB and O donors, with comparison of different types of MSC products resuspended in buffer containing 5% human blood type AB plasma (ABP) versus blood supplementation with 5% ABP buffer only, documenting that the blood from O donors containing higher levels of anti-A/B antibodies reacts stronger to therapeutic cells loaded with A/B antigen than the blood from AB donors containing no anti-A/B antibodies, as visualized by stronger clot formation after a 30 minutes cell exposure period to the blood in the Chandler loop model, however, the decisive factor appears to be the tissue source the cells are derived from, with weaker reactions to similar doses (15 000 cells/mL blood) of bone marrow (BM)-derived BM-MSCs than perinatal tissue (PT)-derived PT-MSCs, or human skin fibroblasts used as positive controls.47-50 (B) Historical timeline of integrating hemocompatibility testing of cellular therapies into clinical practice, to mitigate the risk for thromboembolism due to triggering of the instant blood-mediated inflammatory reaction (IBMIR) upon intravascular (IV) delivery of MSC products, with the establishment of first “MSC minimal criteria” in 2006, with suggested updates on “Immunopotency” and “Hemocompatibility” in 2016 and 2021, respectively, with more updated criteria comprising cell identity, activity/potency, and safety in 2021.
As MSCs shifted from being primarily sourced from BM to being more and more frequently sourced from non-hematopoietic tissues like adipose tissue (AT) and perinatal tissue (PT) sources,12,42 the issue of hemocompatibility should have been anticipated. Cells that are not routinely in contact with the blood commonly express variable amounts of highly procoagulant tissue factor (TF/CD142),12,19,51-53 which can cause mild-to-severe thromboembolic adverse events upon intravascular infusion in patients without proper anticoagulation.43,44,51,52,54 If MSCs are considered to be derived from perivascular cells or pericytes,2,55,56 then the expression of TF/CD142 would have the biological function to stop bleeding by initiating thrombosis in response to vascular injuries.57-60
Importantly, if clinicians, patients, and the public are properly informed, the risk for thromboembolic adverse events can be managed with appropriate treatment protocols, for example, the use of suitable anticoagulants such as heparin.61-64 However, under unfortunate circumstances or negligence (eg, in an uninformed and unregulated clinical setting) this can lead to severe injury and even fatal accidents.12,19,20,24,25 At this point, it could be argued that many patient populations undergoing MSC therapy are under tight clinical monitoring, receiving either prophylactic or therapeutic anticoagulation, and that any risk for thromboembolic complications might thus be minimal.18,64,65
However, not all MSC products are developed or administered with the same standard of care. While most culture expanded MSC products developed by companies and academic centers are well regulated, hundreds of stem cell clinics in the US and internationally offer “minimally manipulated” autologous therapies in outpatient settings with variable attention paid to cell identity, quality, or safety (Fig. 1).20,23-25,29 So while it is entirely possible to safely deliver a diverse array of MSC therapies, tragic exceptions prove the rule, and may thus form the baseline for regulatory approaches to prevent such avoidable adverse events in the future.20,25 This has most recently been illustrated by the rapid appearance of many studies administering MSC for COVID-19,19 mainly on the strength of their safety profile rather than discrete therapeutic mechanisms of action (MoA).
Multiple case reports published by independent groups during the past years demonstrate that reports on adverse thrombotic events in response to intravascular MSC therapy are not a singular incident, but that this has occurred repeatedly over the past decade (Table 1), despite the prominent “Nature News Report” by David Cyranoski in 2010, who called for more caution on the matter in his article “Korean deaths spark inquiry.”44 Cyranoski outlined the risks of stem cell tourism, in which patients travel to other countries for unapproved stem cell treatments. Following the death of 2 Koreans who had received stem cell injections, the Korea Food and Drug Administration and health ministry launched an official investigation into the companies offering the treatments for multiple indications. Scrutiny was directed among others against a Seoul-based company, which formulated the autologous AT-MSCs used to treat the 2 Korean patients. One of the stem cell recipients, a 73-year-old man, died in Japan following a pulmonary embolism.44
Table 1.
Key clinical reports on the occurrence of adverse thrombotic reactions upon infusion TF/CD142-bearing cell products and in particular MSCs in patients.
| Ref. | Short description of the article |
|---|---|
| 51,52 | 1999-2002: Bennet and Moberg et al report on the “Incompatibility between human blood and isolated islets of Langerhans” termed triggering of the instant blood-mediated inflammatory reaction (IBMIR) induced by tissue factor (TF/CD142) |
| 44 | 2010: Cyranoski’s “Korean deaths spark inquiry” prominently highlights problems associated with intravascular delivery of MSC therapeutics in a poorly regulated international environment, which may potentially have led to the death of patients |
| 47,49 | 2012: Moll and Le Blanc et al’s “Triggering of IBMIR by bone marrow (BM)-derived MSC products exposed to ABO-compatible human blood in vitro and upon systemic intravascular infusion in patients” mainly attributed to expression of TF/CD142 |
| 61,66,67 | 2012-2019: Stephenne and coworkers “Procoagulant activity of human adult liver derived MSC-like cells and suitable clinical anticoagulation strategies in patients” attributing their procoagulant activity to the expression of TF/CD142 |
| 45 | 2013: Jung et al’s “Familial occurrence of pulmonary thromboembolism after intravenous adipose tissue (AT)-derived stem cell therapy” documents pulmonary emboli in several family members treated in a poorly regulated environment |
| 46,68 | 2013: Acosta and Soria et al document “Occurrence of peripheral microthrombosis in two diabetic patients with critical limb ischemia (CLI) receiving intravascular infusions of autologous AT-derived MSC therapy,” as later also reviewed in Soria-Juan et al68 |
| 48,50,69 | 2014-2016: Moll et al “Impact of cryopreservation and ABO blood group antigens” on the triggering of IBMIR by clinical grade MSC products with increased triggering of IBMIR by freeze-thawed MSCs readily derived from cryopreservation |
| 62,70-73 | 2015-2019: Moll and Ringdén et al report on the “Different procoagulant activity of therapeutic MSCs derived from bone marrow and placental decidua/perinatal tissue” and the need for optimized clinical protocols/anticoagulation for their clinical use |
| 43 | 2017: Wu et al’s “Thromboembolism induced by umbilical cord (UC)-MSC infusion: A report of two cases and literature review” documents prominent macro-thrombi in peripheral veins of 2 patients with kidney disease receiving allogeneic UC-MSCs |
| 18,64,65,74 | 2018-2020: Olson and Cox et al highlight the “Procoagulant nature of various clinical cellular therapeutics” in the healthy and patient blood and mandate more vigilance with their clinical use and optimized anticoagulation protocols in patients |
| 12,15,19 | 2019-2021: Moll and collaborators propose “New clinical guidelines for intravascular MSC therapies” due to the strongly increasing product diversification observed in the past decade and new clinical treatment indications with pronounced coagulopathy |
Note: This table gives a general overview of major clinical developments and does not intend to recapitulate all publications (in particular in vitro studies) on the matter. All adverse thrombotic events and triggering of IBMIR were reported in response to systemic infusion but not in response to local tissue injections.
In 2013, Jung et al reported more detail on the well-documented occurrence of “familial thromboembolism” in several family members undergoing intravenous autologous AT-MSC therapy in a rather poorly regulated environment.45 A 41-year-old man reported with chest pain to a university hospital in Seoul, Korea. Computed tomography (CT) chest scans showed multiple pulmonary artery embolisms and infarcts in the right lung and serum D-dimer was elevated (0.8 µg/mL vs. normal 0-0.5 µg/mL). Full diagnostic workup for hypercoagulability was done for exclusion of common causes and the examining physicians were puzzled about the cause of the emboli and infarcts in his lungs. The patient then admitted to have received multiple intravenous infusions of autologous AT-MSCs in intervals of 1 month, which finished 1 month before the visit to the hospital. The suspicion of AT-MSCs infusions as cause of pulmonary embolisms was solidified when similar pulmonary emboli and elevated D-dimer (1-1.1 µg/mL) were found in the lungs of his parents, who had also received autologous AT-MSC infusions, without any prehistory of hypercoagulability. The index patient was treated with enoxaparin and then overlapped and switched to warfarin. Follow-up chest CT taken 3 months later showed disappearance of pleural effusion in the index patient and pulmonary emboli had resolved in all 3 patients. In 2013, Soria et al reported 2 cases of micro-thrombosis in patients with diabetes with critical limb ischemia (CLI) treated with TF/CD142-expressing patient-derived autologous AT-MSCs within a clinical study.46 In a series of follow-up articles, Soria and collaborators reported that these cells have altered fibrinolytic activity and upregulated TF/CD142 expression compared to AT-MSCs generated from healthy donors, as reviewed in Soria-Juan et al.68
In 2017, Wu et al provided a thorough documentation of macro-thromboembolism upon infusion of the umbilical cord (UC)-derived MSCs in 2 patients with chronic kidney disease (CKD).43 The authors refer at multiple points to their previous publication in JAMA entitled: “Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants,” describing the treatment of >100 CKD patients with autologous BM-derived MSCs.75 Both patients in the case report received UC-MSCs infusions (1 million cells/kg) through the peripheral veins and presented with a swollen and painful forearm post-infusion. Doppler ultrasound showed large venous clots at the proximal end of the puncture site and urokinase and warfarin were used for thrombolytic therapy. The swelling and pain were relieved and cured. The authors concluded that safety concerns are still a major hurdle for stem cell therapy and that thromboembolism as a critical complication should be prevented. These unfortunate case reports, are contrasted by the good safety profile of MSC therapeutics in well-controlled studies with proper cell product characterization, patient inclusion/exclusion criteria, and adjunct safety measures, to identify any harm as early as possible (Fig. 1).19
Among major MSC manufacturers, such as Athersys (BM-MSC-based MultiStem), Mesoblast (BM-MSC-based Remestemcel-L), Pluristem (PT-MSC-based PLX-PAD and PLX-R18), and Tigenix/Takeda (AT-MSC-based Cx611), there has been a general interest in monitoring TF/CD142 and hemocompatibility aspects within their MSC applications.19 For example, Athersys has provided BM-derived multipotent adult progenitor cells (MAPCs) for comparing the effects of different clinically used cell therapies on coagulation and TF/CD142 expression within independent academic studies,65 reviewed independently by Khan and Newsome76 and Caplan et al18 George et al found that MAPCs express only low levels of TF/CD142 in a similar range to that of BM-MSC or BM mononuclear cells, and that they triggered less clotting than AT-, amniotic fluid (AF)-, or UC-derived MSCs, with higher expression levels of TF/CD142.65 Dose-escalation studies by TiGenix/Takeda within their SEPCELL study (AT-MSC-based Cx611 product) and an accompanying preclinical study have shown an increase in the coagulation activation marker thrombin-antithrombin (TAT) complex and D-dimer for infusion of 4 million cells/kg versus placebo controls in healthy volunteer subjects with normal coagulation parameters,77,78 but not for lower cell doses, and attributed this to TF/CD142 expression. Accordingly, MSC products’ dose-limiting toxicity should be carefully monitored particularly in patients with hypercoagulability.19,64 In addition, key aspects of MSC hemocompatibility testing and instant blood-mediated inflammatory reaction (IBMIR) monitoring in patients have also been recently discussed by key opinion leaders and MSC manufacturers within the context of an ISCT workshop (please see the section “Hemocompatibility Testing of Clinical MSC Products and Clinical Monitoring of IBMIR in Patients”, points a-f).
We here postulate that accidental thromboembolic incidences with MSC products can be mitigated through improved problem awareness and dissemination of best practices. This should be accompanied by new guidelines for MSC product characterization and appropriate clinical cell delivery and patient treatment protocols (Figs. 2, 3).12,19 There is no delivery method or patient treatment protocol that is universally applicable.18 Rather, numerous options for cell delivery exist,18 and their applicability for a given clinical indication needs to be evaluated on a case-by-case basis (Fig. 2A), thereby finding a suitable match between individual MSC products and specific patient cohorts regarding optimal safety and efficacy. This is particularly evident for systemic infusion of TF/CD142-bearing MSC products, which may not be fully compatible with the blood. In stark contrast, hematopoietic stem cell (HSC) products are compatible with the blood and are regularly infused in the HSCT (hematopoietic stem cell transplantation) setting, where this is a well-established, safe, and effective method, with evidence of long-term cell engraftment leading to recovery of the hematopoietic system.2
Need for Improved Characterization of MSC Products to Complement the Established “Minimal” Criteria
Modern-day ATMPs rely on stringent phenotypic and functional characterization to guarantee their optimal safety and efficacy in clinical use.79 MSC-based products are among the most popular and commonly used cellular therapeutics tested in clinical trials today,14 which highlights the clinical relevance and need for strong guidelines considering product characterization before clinical use. According to the first comprehensive set of ISCT “minimal” criteria established by Dominici et al in 2006 (Fig. 3),13 MSCs can be characterized by at least 3 phenotypic and functional minimal criteria: (a) their fibroblastic MSC-like morphology in culture, (b) their expression, or lack of expression, of a set of characteristic surface markers (eg, shown by flow cytometry), and (c) their multi-lineage differentiation into several characteristic mesenchymal lineages.11,14
The first “minimal” guidelines were conceived to be just that, minimal criteria for establishing a common nomenclature for a popular cell type being studied around the globe. Today, however, the MSC landscape is more complex and diverse. Indeed, until 2008, clinically administered MSCs were almost entirely BM-derived, while today nearly equal shares of AT-, PT, and BM-derived MSCs are reported in clinical registries,12,42 with an additional large fraction of MSC products of unspecified tissue origin. Particularly PT- and endometrium (EM)-derived MSCs comprise a very large and diverse group of products.80,81 The diversification of MSC products and their clinical uses compels the development of complementary criteria specifically focused on MSC potency and safety. Importantly, MSC product diversification is expanding at a profound rate, thus requiring urgent action to better define the minimal requirements for their safe use under these rapidly changing conditions in addition to requirements for MSC identity and activity/potency (Fig. 3A, 3B).20
The panel of cell surface markers commonly used to identify cells as “MSC” has been initially designed primarily to exclude contaminating cell types (eg, hematopoietic, myeloid cells, and endothelial, using CD34, CD45, CD11b, and CD31, respectively), while using a combination of relatively common cell markers to verify the identity of MSCs (eg, CD105, CD90, CD73, CD44, etc.). It is clear to most researchers and manufacturers working with MSC products today, that this panel was indeed conceived as a “minimal” compromise, to limit the number of surface markers to a feasible amount at the time it was conceived (eg, in 2006 most flow cytometers could only run a handful of colors, thus limiting the number of markers), while providing enough essential information for minimal characterization of the cells to be practical, informative, and useful.
While useful, this panel of surface markers does not address either the therapeutic MoA of MSCs or identify potential risks or even heterogeneity of the product. Recent amendments to the MSC “minimal” criteria include important updates on MSCs’ immune-functional plasticity (Fig. 3B), for example, to upregulate the immunomodulatory molecule indoleamine 2,3-deoxygenase (IDO) upon pro-inflammatory licensing (eg, IFN-γ), to better reflect their functional properties in potency evaluation.38,82 Thus, there exist strong efforts to incorporate functional assays of MSC “potency” that are often associated with immunomodulation and various molecular mechanisms linked to specific indications.38,82-84
In analogy, incorporation of TF/CD142 and hemocompatibility assessment as a new safety marker/criterion would improve both phenotypic and functional characterization in the context of intravascular applications.12,15,19 Thus, we suggest adding TF/CD142 expression and hemocompatibility assessment to these criteria when MSCs are intended for intravascular use (eg, intravenous/intra-arterial infusion). While TF/CD142 expression could be added to the panel of markers assessed by flow cytometry (criteria number 2), the combined assessment of TF/CD142 expression by various methods (eg, see the section “Hemocompatibility Testing of Clinical MSC Products and Clinical Monitoring of IBMIR in Patients”) and concomitant functional hemocompatibility testing could also form a new criterion, in analogy to the functional assessment of multi-lineage differentiation (current criteria number 3), or the recent updates on immune-functional plasticity (Fig. 3B).
Raising Awareness to Mitigate the Risk for Patients and Added Scientific Value of Hemocompatibility Analysis
As outlined earlier, a worrying trend is seen in the multiple recent (2010 onwards) clinical case reports of adverse events and even suspected lethal cases related to thromboembolic complications upon systemic infusion of highly procoagulant TF/CD142 expressing MSC products in patients.12,19 While some well-respected academic centers were among the first to raise awareness to this complication in the literature,20,47,61,64,65,85 the majority of cases typically occurred in poorly or at occasion in completely unregulated clinical environments,20,44,45 such as commercial endeavors and “Stem Cell Clinics” aiming to sell poorly characterized MSC products to vulnerable patients in a fragile/poor state of health, which are especially prone to suffer injury from misguided treatments.23,29,44
A particular concern exists when cells are delivered by systemic infusion, which is still used for half of all registered clinical MSC product applications,12,42 especially when the cells express high levels of TF/CD142. Indeed, TF/CD142-expressing MSC products can only be safely administered to patients with tight clinical monitoring and optimized adjunct clinical treatment protocols, such as appropriate anticoagulation and other safety measures. To date, however, few MSC products are screened for TF/CD142 expression. So it is not clear which products pose a hazard to patients and which are safe. Since MSC products from different tissue sources and manufacturing processes show orders of magnitude differences in TF/CD142 expression,12,65 infusion of under-characterized MSC products can be a potential fatal gamble.44 Thus, practitioners, patients, and public awareness to this problem is essential, to mitigate any risk for patients engaging in MSC therapy. Furthermore, this may also help to mitigate any accidental risk in clinical trials due to a lack of awareness.
Studies on MSC hemocompatibility are not only important for patient safety, but also for understanding the intrinsic biology of MSCs in vivo. The expression of TF/CD142 in multiple perivascular cell types, such as MSCs and vascular smooth muscle cells (VSMCs), has the crucial function to stop bleeding by initiating thrombosis in response to vascular injuries that break the hemocompatible inner lining of blood vessels composed of the highly adapted endothelial cells (ECs).12,57-60 Nonetheless, the idea of blood-resident “circulating” MSCs within the context of systemic healing and repair processes has excited the scientific community over the past decades.12,86 This may be accounted to a presumed analogy of MSCs to HSCs, the latter of which are well known to be recruited from their BM niches and to circulate in circadian fashion, to participate in the bodies repair phases during sleep.87 Indeed, it is well established that HSCs can intrinsically mobilize into and circulate in the blood, while this is still highly disputed for MSCs today.12,86,88 It is not clear if MSCs belong to the prototypic “blood-resident” cell types, or if their detection in the blood and the method of systemic MSC infusion is a mere artifact stemming from a historical misconception.12
By using a methodology developed for HSCT, where this is a well-established, safe, and effective procedure to yield a desired outcome, may not be ideal for the clinical delivery of MSC products at all. Multiple types of circulating mononuclear cells (eg, immune cells) or the highly adapted anti-thrombogenic ECs are found in direct contact with the blood, while decades of research have shown that the TF/CD142-bearing MSCs are typically found in the extravascular compartment, lining the exterior of blood vessels.3,55,56 As perivascular MSCs they maintain the integrity of blood vessels upon injury and prevent bleeding by rapidly promoting a procoagulant state upon de-novo blood contact.57,58,60 Scientific reports of blood-resident circulating MSCs are rare.12,15 In most cases, MSCs have only been found in the blood under rather pathological conditions, such as severe injury or fracture, or other non-physiological insults, such as drug-assisted mobilization, thus raising the questions of being artifacts.86
This does not exclude that small amounts of MSC-like cells can at occasion be found in the blood, which warrants future investigation.86 It may be speculated that circulating MSCs are somehow adapted to circulate in the blood and the mechanisms underlying such adaptation could be of interest to design blood-compatible MSC therapeutics for systemic infusion in the future. It is likely that the blood-resident “circulating” MSCs express only very low amounts of procoagulant TF/CD142, to not trigger instant blood clotting, or that they upregulate molecules that counteract clotting, such as tissue factor pathway inhibitor (TFPI) or prostacyclin (PGI2) (Fig. 2B).12 In addition, MSC may use specific methods to modulate TF/CD142 activity, such as subcellular localization and tissue factor encryption.89 Indeed, our initial report found that TF/CD142 was mainly located in intracellular vesicular deposits in BM MSCs that displayed rather little TF/CD142 on their surface.47
The in vivo phenotype of MSC is rather poorly characterized to date.12,86 It would thus be of great interest to study the amount of TF/CD142 expression of MSCs in vivo (eg, MSCs residing in different tissue compartments, such as AT, BM, and PT or their potentially “circulating” equivalents) and how TF/CD142 changes during in vitro culture and expansion. Furthermore, it is possible that TF/CD142 expression is regulated dynamically in response to changing in vivo stimuli/environments, for example, comorbidities, such as advanced diabetes mellitus (late-stage type 1 or type 2 diabetes), or in response to the uremia/uremic toxins and other detrimental changes resulting from renal failure.16,60,68 The potential influence of hypoxia/normoxia and inflammation on TF/CD142 expression by MSCs should also be anticipated and considered.47,90
In addition, as the interest and use of extracellular vesicles (EVs) as an alternative to live cell therapy grows,91-93 so does the need to develop release criteria that ensure their safe and effective use. Much of the biology of MSC-derived EVs has yet to be defined, although some first preliminary reports on TF/CD142 profiling in MSC-derived EVs exist.94,95 Silachev et al documented similar TF/CD142 expression and procoagulant activity of UC-MSCs and their derived EVs, which could be antagonized by heparin supplementation.94 They also documented an increased positivity of some MSCs and EVs for Annexin V, which may imply the presence of phosphatidylserine on their surface (Fig. 2B). Phosphatidylserine exposure can further promote clot formation, as also identified by others in the triggering of clotting by MSCs.96 Similarly, Adrienne Beth Wright reported the expression of TF/CD142 and initiation of clotting by UC-MSC-derived EVs in her PhD thesis.95 Interestingly, EVs derived from other sources, have also demonstrated the ability to increase coagulation as a possible therapy for patients with hypercoagulable trauma.97
In conclusion, MSC-derived EVs generally contain many of the membrane proteins expressed by their parental MSCs, which include TF/CD142. Further efforts have to be made to study the impact of MSC tissue source, passage number, freeze-thawing, and other crucial manufacturing parameters on EV products.48,95 It remains to be seen, if MSC-EVs entail the same clinical risks concerning coagulation as MSCs and we encourage further investigation in the field.
Hemocompatibility Testing of Clinical MSC Products and Clinical Monitoring of IBMIR in Patients
We have previously given outlines on requirements for clinical hemocompatibility testing of MSC products (Figs. 2, 3),12,18,19,64,65 highlighting the need for robust and simple test systems that can be applied either by trained operators throughout independent laboratories/facilities, or in a more centralized fashion at certified facilities that could take over this task of preclinical quality and safety testing as a service on a commercial or sponsored basis. A key challenge is to precisely determine the effects of each individual MSC product intended for intravascular use on coagulation and other blood-resident hemocompatibility systems (eg, complement and fibrinolytic system) and to find key markers indicative of this effect to standardize these efforts.62 Importantly, in addition to clinical monitoring, principle ex vivo hemocompatibility testing of MSC products intended for intravascular use should already be done before the first use of specific MSC products in patients, to establish their general hemocompatibility profile.12,15,19 There are several ways this could be approached.
MSC products’ TF/CD142 expression has been identified as a key indicator of MSC hemocompatibility and the triggering of the potent TF/CD142 pathway of coagulation (Fig. 2).12 Direct measurements of TF/CD142 protein can be performed directly on the cell surface, or from both cell lysate and supernatant to detect soluble TF/CD142 or detached membrane particle (MP) and EV-associated TF/CD142. Relative amounts of protein can be detected using Western blot, while commercial ELISA kits are available for more accurate quantification. However, we expect that many laboratories will use flow cytometry to assess the amount of TF/CD142 directly on the surface of MSCs to gain a first estimate, as this method is relatively inexpensive, fast, and easy and can be integrated into existing MSC phenotyping panels.12,65,96 A limitation of flow cytometry is the accurate absolute quantification of TF/CD142, although additional cell samples may be used as external reference values for “high” and “low” expression of TF/CD142.98
While several studies have connected TF/CD142 expression on MSC to changes in coagulation assays, there are additional possible mechanisms by which MSCs can affect coagulation that may be tissue or indication-specific. Several research and clinical tools are available to assess the effects of MSCs on coagulation on whole-blood or blood components (Figs. 2, 3),49 including conventional coagulometers, calibrated thrombogram (CAT), thromboelastography (TEG), rotational thromboelastometry (ROTEM), and customized systems, such as the whole-blood Chandler Loop.47,48,50,61,62,64,65,85,94,99-102 Importantly, the establishment of suitable routines and standards considering MSC testing is essential, for example, according to ISO109931/4103 medical devices that contact the blood during clinical routine must be subject to hemocompatibility testing (to show that they are biocompatible with the blood). It should be verified if such principle norms may also be generally extended to intravascularly applied therapeutic cell products in the future.12
Common hemocompatibility test systems include CAT, which is a more controlled, high-throughput research platform, and TEG, which is a relatively common clinical assay that is fairly simple and quick. In addition to being able to quantify the total effects of MSC on coagulation, these systems can be used to select and support an anti-coagulant strategy for clinical administration.61-63,70,104 They can be used as spot test before initiation of anticoagulation, or in a more rigorous repeated blood testing for continuous monitoring, to assess the effects of MSC products on specific patients, as they are being infused.61,64-66 In hypercoagulative or sensitive clinical uses, TEG can be performed with patient blood prior to cell infusion to further mitigate any risk.64 Since most MSC therapeutics are tested in a narrow dose range,42 these test systems could also be of interest to perform cell product dose-escalation/anticoagulation studies on patient blood before clinical use. Importantly, currently, there exists no exact predictive threshold for the tolerable amount of TF/CD142 in a given amount of MSC product that correlates with negative thrombotic outcomes, due to the multifactorial nature of the equation, but first generalizations can be made (Fig. 2B).
Considering optimal clinical use of intravascular MSC therapeutics and adjunct monitoring of IBMIR in patients, physicians, and investigators needs to take into account numerous points that may potentially alter or confound the outcome,12,15,18,19 as follows:
-
(a)
The underlying patient baseline condition (eg, young overall fairly healthy patients vs. elderly very sick patients with multiple comorbidities and pronounced clinical indication/preactivated patient status, may make the occurrence of IBMIR more likely, as seen in CLI studies on patients with diabetes with peripheral vascular disease using autologous AT-MSCs),68
-
(b)
The timing of therapeutic cell administration in relation to disease status (eg, severe vs. critical COVID-19 patients, early treatment with MSCs shortly upon diagnosis and hospitalization might be beneficial and normalize coagulation parameters, while late-stage treatment in critical patients in a procoagulant state may even amplify this condition),19
-
(c)
The type of anticoagulation administered to patients or cell products (eg, no anticoagulation in patients or products vs. prophylactic or therapeutic dose of anticoagulants in patients and/or products, infusion syringe, or cell bag to prevent clotting),12,19
-
(d)
The route and speed of cell administration (eg, IBMIR is expected for intravascular administration, and faster infusion may lead to higher local cell doses in the blood, while slow infusion reduces cell damage and antagonizes TF/CD142 release and clot formation; furthermore, cell aggregation and sedimentation need to be kept in mind),12,105,106
-
(e)
The cell dose administered (eg, typically ranging from 1 to 2 million cells per kg of patient body weight, up to 5, 10, or 20 million cells per kg body weight, please see table S3 in the cited review article,12 relating to a log-fold variation of intravascular TF/CD142 dose, thus affecting the relative and total load of TF/CD142 administered), and finally
-
(f)\t
The timing of analysis (eg, it is of importance to monitor the “MSC infusion period” directly before and after cell administration as contrasted to non-informative time points days after application),12,19,47 as outlined in the next paragraphs.
To monitor the occurrence of IBMIR in clinical cohorts, it is essential to include the correct type of blood samples (eg, choice of the serum vs. anticoagulated plasma) to be able to monitor the respective blood markers that indicate signs of IBMIR (eg, complement, clotting, and fibrinolytic activation).47,61 These include the complement activation marker complement C3 fragment a (C3a), the coagulation activation marker TAT complex, and the hyperfibrinolysis marker D-dimer, respectively (eg, use of EDTA-anticoagulated plasma for C3a and TAT determination). Another key parameter apart from plasma-makers is to monitor the patients for the occurrence of micro-thrombosis and macro-emboli, for example, pulmonary emboli with suitable clinical methods (eg, upon sudden swelling of extremities due to peripheral thrombus formation in arms or legs, or sudden shortness of breath due to pulmonary thromboembolism in association with cell infusion).43,46,68
Considering the timing of analysis, it is important to study the actual “infusion period” of cell application, which has rarely been done so far in studies using MSC infusions. Thus, any potential risk from triggering of IBMIR and concomitant formation of emboli may have been masked.12 Since the term IBMIR relates to an “instant reaction” of the therapeutic cells with patient blood, this may best be done in the 24 hours directly after cell application in acceptable intervals given the actual clinical situation of the patient (eg, for analysis of plasma markers this could be done at infusion, and 3, 6, 12, 24, and 72 hours after infusion for “in-patients”) which should be compared to a “baseline/before sample” taken fairly closely before cell application (eg, directly before cell application, or during prior routine monitoring days before infusion). In our first report on the infusion of weakly TF/CD142 expressing BM-MSC products in patients, we observed a “delayed peak” around 9-12 hours after cell application,47 which may have resulted from the disintegration of therapeutic cells and subsequent release of intracellular TF/CD142. In contrast, MSC products with high surface expression of TF/CD142 may induce a more rapid response 1-3 hours post-infusion, as shown by increased levels of TAT or D-dimer.19,62,77,102
Conclusions and Outlook
As cell therapies have grown, so has the frequency of reports of rare but significant adverse thrombotic events. These events are associated with the expression of varying levels of highly procoagulant TF/CD142 on MSCs, which promotes clotting upon blood exposure (eg, upon intravascular infusion). In this perspective, we have summarized key points that speak for inclusion of TF/CD142 profiling and hemocompatibility testing of clinical MSC products intended for intravascular use, as a new criterion to supplement the already existing “minimal” criteria for MSC characterization. In addition, we have outlined key points of importance considering the added scientific value, and necessary methodology and point-by-point clinical considerations, to practically implement such routines in the near future. In alignment with recent developments,12,15,19,20,23,25,43-45 we feel that the current clinical evidence and the supporting research calls for responsible investigators to assess the amount of TF/CD142 and the adjunct effects of intravascular clinical cell products on human (patient) blood prior to clinical use, and to incorporate suitable anticoagulation strategies into their clinical protocols, to mitigate any risk of thrombotic adverse events in patients. To best reflect and implement this knowledge, regulatory authorities and international societies should undertake coordinated efforts to update the already established clinical guidelines.
Acknowledgments
The content of this perspective has been developed in a collaborative effort by independent research groups located in different countries/continents over the past decade. A tabular overview of major clinical efforts/studies in this field can be found in Table 1. The corresponding author G.M. studied this topic in his PhD thesis and Postdoc studies at Karolinska Institute,12,49 under the guidance of Prof. Olle Ringdén, Prof. Katarina Le Blanc, and Dr. Ida Rasmusson (Postdoc mentor and PhD main and co-supervisors, respectively), who have conceived the project with Prof. Olle Korsgren, Prof. Kristina Nilsson-Ekdahl, and Prof. Bo Nilsson (PhD co-supervisor), located at the Rudbeck Laboratory in Uppsala University, in the context of their islets of Langerhans cell therapy studies to reverse insulin dependence in diabetics. They first conceived the concept of the instant blood-mediated inflammatory reaction (IBMIR) in response to TF/CD142-bearing cellular therapeutics already in the 1990s.51,52 G.M. continued his research on establishing hemocompatibility guidelines for perivascular clinical MSC therapeutics during his Postdoc studies in Berlin.12,15,19,86,98
Funding
G.M.’s contributions were made possible among others, by German Research Foundation (DFG) and German Federal Ministry of Education and Research (BMBF) funding through the BSRT (GSC203) and BCRT and in part by the European Union’s Horizon 2020 research and innovation program under grant agreements No. 733006 (PACE) and No. 779293 (HIPGEN). J.A.A. was supported by the Fraternal Order of Eagles Diabetes Research Center and the Straub Foundation. S.D.O. receives funding support from NINDS R21NS116302, The TIRR Foundation—Mission Connect, the Grace Reynolds Wall Research Fund, and the generous support of the Claire Glassell Stem Cell Gift. In addition, S.D.O. has received research funding through sponsored research agreements between the University of Texas Health Science Center at Houston and Athersys, Biostage, Hope Bio, and Generate Life Sciences. J.A.N. receives funding from the California Institute for Regenerative Medicine (CIRM) and NIH R01NS102486 and R24ODO21606.
Conflict of Interest
Scott D. Olson declared research funding from CBR Systems, Hope Bio, Biostage Inc. and is a technical expert for Bryan Cave Leighton Paisner LLP. All of the other authors declare no potential conflict of interest.
Author Contributions
G.M., J.A.A., S.D.O., and J.A.N. have made substantial contributions to the conception and drafting of the article, given their final approval of the version to be published, and given their agreement to be accountable to all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Caplan AI. What’s in a name? Tissue Eng Part A. 2010;16(8):2415-2417. [DOI] [PubMed] [Google Scholar]
- 2. Bianco P, Cao X, Frenette PS, et al. . The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacchetti B, Funari A, Remoli C, et al. . No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6(6):897-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boregowda SV, Booker CN, Phinney DG.. Mesenchymal stem cells: the moniker fits the science. Stem Cells. 2018;36(1):7-10. [DOI] [PubMed] [Google Scholar]
- 6. Sipp D, Robey PG, Turner L.. Clear up this stem-cell mess. Nature. 2018;561(7724):455-457. [DOI] [PubMed] [Google Scholar]
- 7. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR.. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141-145. [DOI] [PubMed] [Google Scholar]
- 8. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV.. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381-390. [PubMed] [Google Scholar]
- 9. Friedenstein AJ, Chailakhyan RK, Gerasimov UV.. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263-272. [DOI] [PubMed] [Google Scholar]
- 10. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. [DOI] [PubMed] [Google Scholar]
- 11. Pittenger MF, Mackay AM, Beck SC, et al. . Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. [DOI] [PubMed] [Google Scholar]
- 12. Moll G, Ankrum JA, Kamhieh-Milz J, et al. . Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149-163. [DOI] [PubMed] [Google Scholar]
- 13. Dominici M, Le Blanc K, Mueller I, et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [DOI] [PubMed] [Google Scholar]
- 14. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI.. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moll G, Hoogduijn MJ, Ankrum JA.. Editorial: safety, efficacy and mechanisms of action of mesenchymal stem cell therapies. Front Immunol. 2020;11:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrzejewska A, Catar R, Schoon J, et al. . Multi-parameter analysis of biobanked human bone marrow stromal cells shows little influence for donor age and mild comorbidities on phenotypic and functional properties. Front Immunol. 2019;10:2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boland LK, Burand AJ, Boyt DT, et al. . Nature vs. nurture: defining the effects of mesenchymal stromal cell isolation and culture conditions on resiliency to palmitate challenge. Front Immunol. 2019;10:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caplan H, Olson SD, Kumar A, et al. . Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moll G, Drzeniek N, Kamhieh-Milz J, et al. . MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knoepfler P. Harms linked to unapproved stem cell interventions highlight need for greater FDA enforcement. 2021. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/06/harms-linked-to-unapproved-stem-cell-interventions-highlight-need-for-greater-fda-enforcement
- 21. Ankrum J, Karp JM.. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ankrum JA, Ong JF, Karp JM.. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turner L, Knoepfler P.. Selling stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell. 2016;19(2):154-157. [DOI] [PubMed] [Google Scholar]
- 24. Marks PW, Witten CM, Califf RM.. Clarifying stem-cell therapy’s benefits and risks. N Engl J Med. 2017;376(11):1007-1009. [DOI] [PubMed] [Google Scholar]
- 25. Bauer G, Elsallab M, Abou-El-Enein M.. Concise review: a comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Transl Med. 2018;7(9):676-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galipeau J, Sensébé L.. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin I, Galipeau J, Kessler C, et al. . Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019;11. [DOI] [PubMed] [Google Scholar]
- 28. Levy O, Kuai R, Siren EMJ, et al. . Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Master Z, Matthews KRW, Abou-El-Enein M.. Unproven stem cell interventions: a global public health problem requiring global deliberation. Stem Cell Rep. 2021;16(6):1435-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abou-el-Enein M, Angelis A, Appelbaum FR, et al. . Evidence generation and reproducibility in cell and gene therapy research: a call to action. Mol Ther Methods Clin Dev. 2021;22:11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Order from chaos. Nature. 2010;466:7-8. [DOI] [PubMed] [Google Scholar]
- 32. Cyranoski D. FDA challenges stem-cell clinic. Nature. 2010;466(7309):909. [DOI] [PubMed] [Google Scholar]
- 33. Cyranoski D. FDA’s claims over stem cells upheld. Nature. 2012;488(7409):14. [DOI] [PubMed] [Google Scholar]
- 34. Charo RA, Sipp D.. Rejuvenating regenerative medicine regulation. N Engl J Med. 2018;378(6):504-505. [DOI] [PubMed] [Google Scholar]
- 35. European Commission. Evaluation of the EU blood and tissues and cells legislation. 2021. https://ec.europa.eu/health/blood_tissues_organs/policy/evaluation_en
- 36. European Medicines Agency. The European Medicines Agency’s scientific guidelines on cell therapy and tissue engineering. 2021. https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-cell-therapy-tissue-engineering
- 37. Viswanathan S, Shi Y, Galipeau J, et al. . Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019-1024. [DOI] [PubMed] [Google Scholar]
- 38. Nolta JA, Galipeau J, Phinney DG.. Improving mesenchymal stem/stromal cell potency and survival: Proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congrès de Montréal, Organized by the ISCT MSC Scientific Committee. Cytotherapy. 2020;22(3):123-126. [DOI] [PubMed] [Google Scholar]
- 39. Lalu MM, McIntyre L, Pugliese C, et al. , Canadian Critical Care Trials Group . Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lalu MM, Sullivan KJ, Mei SH, et al. . Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta-analyses prior to initiating a first-in-human trial. eLife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson M, Mei SHJ, Wolfe D, et al. . Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kabat M, Bobkov I, Kumar S, Grumet M.. Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Z, Zhang S, Zhou L, et al. . Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. Transplant Proc. 2017;49(7):1656-1658. [DOI] [PubMed] [Google Scholar]
- 44. Cyranoski D. Korean deaths spark inquiry. Nature. 2010;468(7323):485. [DOI] [PubMed] [Google Scholar]
- 45. Jung JW, Kwon M, Choi JC, et al. . Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Acosta L, Hmadcha A, Escacena N, et al. . Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes. 2013;62(12):4266-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moll G, Rasmusson-Duprez I, von Bahr L, et al. . Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30(7):1565-1574. [DOI] [PubMed] [Google Scholar]
- 48. Moll G, Alm JJ, Davies LC, et al. . Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moll G, Le Blanc K.. Engineering more efficient multipotent mesenchymal stromal (stem) cells for systemic delivery as cellular therapy. ISBT Sci Ser. 2015;10:357-365. [Google Scholar]
- 50. Moll G, Hult A, von Bahr L, et al. . Do ABO blood group antigens hamper the therapeutic efficacy of mesenchymal stromal cells? PLoS One. 2014;9(1):e85040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bennet W, Sundberg B, Groth CG, et al. . Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907-1914. [DOI] [PubMed] [Google Scholar]
- 52. Moberg L, Johansson H, Lukinius A, et al. . Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039-2045. [DOI] [PubMed] [Google Scholar]
- 53. Witkowski M, Landmesser U, Rauch U.. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med. 2016;26(4):297-303. [DOI] [PubMed] [Google Scholar]
- 54. Johansson H, Lukinius A, Moberg L, et al. . Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755-1762. [DOI] [PubMed] [Google Scholar]
- 55. Crisan M, Yap S, Casteilla L, et al. . A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313. [DOI] [PubMed] [Google Scholar]
- 56. Bianco P, Robey PG, Simmons PJ.. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drake TA, Morrissey JH, Edgington TS.. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087-1097. [PMC free article] [PubMed] [Google Scholar]
- 58. Morrissey JH. Tissue factor: a key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004;79(2):103-108. [DOI] [PubMed] [Google Scholar]
- 59. Taubman MB, Wang L, Miller C.. The role of smooth muscle derived tissue factor in mediating thrombosis and arterial injury. Thromb Res. 2008;122(Suppl 1):S78-S81. [DOI] [PubMed] [Google Scholar]
- 60. Catar R, Moll G, Hosp I, et al. . Transcriptional regulation of thrombin-induced endothelial VEGF induction and proangiogenic response. Cells. 2021;10:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stephenne X, Nicastro E, Eeckhoudt S, et al. . Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS One. 2012;7(8):e42819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moll G, Ignatowicz L, Catar R, et al. . Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev. 2015;24(19):2269-2279. [DOI] [PubMed] [Google Scholar]
- 63. Liao L, Shi B, Chang H, et al. . Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7(1):106-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. George MJ, Prabhakara K, Toledano-Furman NE, et al. . Procoagulant in vitro effects of clinical cellular therapeutics in a severely injured trauma population. Stem Cells Transl Med. 2020;9(4):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. George MJ, Prabhakara K, Toledano-Furman NE, et al. . Clinical cellular therapeutics accelerate clot formation. Stem Cells Transl Med. 2018;7(10):731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Coppin L, Sokal E, Stéphenne X.. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coppin L, Najimi M, Bodart J, et al. . Clinical protocol to prevent thrombogenic effect of liver-derived mesenchymal cells for cell-based therapies. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Soria-Juan B, Escacena N, Capilla-González V, et al. ; Collaborative Working Group “Noma Project Team” . Cost-effective, safe, and personalized cell therapy for critical limb ischemia in type 2 diabetes mellitus. Front Immunol. 2019;10:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moll G, Geißler S, Catar R, et al. . Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC therapy? Adv Exp Med Biol. 2016;951:77-98. [DOI] [PubMed] [Google Scholar]
- 70. Sadeghi B, Moretti G, Arnberg F, et al. . Preclinical toxicity evaluation of clinical grade placenta-derived decidua stromal cells. Front Immunol. 2019;10:2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Baygan A, Aronsson-Kurttila W, Moretti G, et al. . Safety and side effects of using placenta-derived decidual stromal cells for graft-versus-host disease and hemorrhagic cystitis. Front Immunol. 2017;8:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ringdén O, Baygan A, Remberger M, et al. . Placenta-derived decidua stromal cells for treatment of severe acute graft-versus-host disease. Stem Cells Transl Med. 2018;7(4):325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aronsson-Kurttila W, Baygan A, Moretti G, et al. . Placenta-derived decidua stromal cells for hemorrhagic cystitis after stem cell transplantation. Acta Haematol. 2018;139(2):106-114. [DOI] [PubMed] [Google Scholar]
- 74. Ankrum J. Cell therapies can bring insult to injury. Sci Transl Med. 2020;12:eabb0792. [Google Scholar]
- 75. Tan J, Wu W, Xu X, et al. . Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307(11):1169-1177. [DOI] [PubMed] [Google Scholar]
- 76. Khan RS, Newsome PN.. A comparison of phenotypic and functional properties of mesenchymal stromal cells and multipotent adult progenitor cells. Front Immunol. 2019;10:1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perlee D, de Vos AF, Scicluna BP, et al. . Human adipose-derived mesenchymal stem cells modify lung immunity and improve antibacterial defense in pneumosepsis caused by Klebsiella pneumoniae. Stem Cells Transl Med. 2019;8(8):785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Perlee D, van Vught LA, Scicluna BP, et al. . Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group, placebo controlled trial. Stem Cells. 2018;36(11):1778-1788. [DOI] [PubMed] [Google Scholar]
- 79. Goldsobel G, Von Herrath C, Schlickeiser S, et al. . RESTORE survey on the public perception of advanced therapies and ATMPs in Europe—why the European Union should invest more! Front Immunol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Parolini O, Alviano F, Bagnara GP, et al. . Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300-311. [DOI] [PubMed] [Google Scholar]
- 81. Bozorgmehr M, Gurung S, Darzi S, et al. . Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 2020;8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Galipeau J, Krampera M, Barrett J, et al. . International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chinnadurai R, Rajan D, Qayed M, et al. . Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22(9):2504-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Galipeau J, Krampera M, Leblanc K, et al. . Mesenchymal stromal cell variables influencing clinical potency: the impact of viability, fitness, route of administration and host predisposition. Cytotherapy. 2021;23(5):368-372. [DOI] [PubMed] [Google Scholar]
- 85. Tatsumi K, Ohashi K, Matsubara Y, et al. . Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431(2):203-209. [DOI] [PubMed] [Google Scholar]
- 86. Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Reinke P.. Editorial comment: variables affecting the presence of mesenchymal stromal cells in the peripheral blood and their relationship with apheresis product. Br J Haematol. 2020;189(4):593-596. [DOI] [PubMed] [Google Scholar]
- 87. Méndez-Ferrer S, Chow A, Merad M, Frenette PS.. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16(4):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hoogduijn MJ, Verstegen MM, Engela AU, et al. . No evidence for circulating mesenchymal stem cells in patients with organ injury. Stem Cells Dev. 2014;23(19):2328-2335. [DOI] [PubMed] [Google Scholar]
- 89. Taubman MB, Giesen PL, Schecter AD, Nemerson Y.. Regulation of the procoagulant response to arterial injury. Thromb Haemost. 1999;82(2):801-805. [PubMed] [Google Scholar]
- 90. Rodriguez LA 2nd, Mohammadipoor A, Alvarado L, et al. . Preconditioning in an inflammatory milieu augments the immunotherapeutic function of mesenchymal stromal cells. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lener T, Gimona M, Aigner L, et al. . Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yáñez-Mó M, Siljander PR, Andreu Z, et al. . Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Witwer KW, Van Balkom BWM, Bruno S, et al. . Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Silachev DN, Goryunov KV, Shpilyuk MA, et al. . Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wright AB. The isolation, culture-expansion, cryopreservation, characterization, and properties of umbilical cord-derived mesenchymal stromal cells and their extracellular vesicles [PhD thesis]. Kansas State University; 2021. [Google Scholar]
- 96. O’Rourke B, Nguyen S, Tilles AW, et al. . Mesenchymal stromal cell delivery via an ex vivo bioreactor preclinical test system attenuates clot formation for intravascular application. Stem Cells Transl Med. 2021;10(6):883-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lopez E, Srivastava AK, Pati S, Holcomb JB, Wade CE.. Platelet-derived microvesicles: a potential therapy for trauma-induced coagulopathy. Shock. 2018;49(3):243-248. [DOI] [PubMed] [Google Scholar]
- 98. O’Shea O, Chapman C, Klair H, et al. Collaborative study to assess the suitability of the candidate WHO International Reference Reagent for MSC identity (for flow cytometry) for advanced therapies. WHO/BS/2019.2376—Expert Committee on Biological Standardization. World Health Organization; 2019. [Google Scholar]
- 99. Hoogduijn MJ, de Witte SF, Luk F, et al. . Effects of freeze-thawing and intravenous infusion on mesenchymal stromal cell gene expression. Stem Cells Dev. 2016;25(8):586-597. [DOI] [PubMed] [Google Scholar]
- 100. Christy BA, Herzig MC, Montgomery RK, et al. . Procoagulant activity of human mesenchymal stem cells. J Trauma Acute Care Surg. 2017;83(1 Suppl 1):S164-S169. [DOI] [PubMed] [Google Scholar]
- 101. Christy BA, Herzig MC, Delavan C, et al. . Human primary fibroblasts perform similarly to MSCs in assays used to evaluate MSC safety and potency. Transfusion. 2019;59(S2):1593-1600. [DOI] [PubMed] [Google Scholar]
- 102. Perlee D, de Vos AF, Scicluna BP, et al. . Role of tissue factor in the procoagulant and antibacterial effects of human adipose-derived mesenchymal stem cells during pneumosepsis in mice. Stem Cell Res Ther. 2019;10(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wallin RF. A practical guide to ISO 10993-4: hemocompatibility. 1998. http://www.mddionline.com/practical-guide-iso-10993-4-hemocompatibility
- 104. Gleeson BM, Martin K, Ali MT, et al. . Bone marrow-derived mesenchymal stem cells have innate procoagulant activity and cause microvascular obstruction following intracoronary delivery: amelioration by antithrombin therapy. Stem Cells. 2015;33(9):2726-2737. [DOI] [PubMed] [Google Scholar]
- 105. Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui L, Wagner DC.. The dark side of the force—constraints and complications of cell therapies for stroke. Front Neurol. 2015;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Baker EK, Wallace EM, Davis PG, et al. . A protocol for cell therapy infusion in neonates. Stem Cells Transl Med. 2021;10(5):773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.