Abstract
Background
Mucosal healing (MH) evaluated by endoscopy is a novel target of therapy in UC as it is associated with improved long-term outcomes. It is defined based on the Mayo endoscopic score (MES), but it is still to define whether a value of MES 0 or 1 should be the target. The purpose of this paper is to present the results of a systematic review with meta-analysis which compares long-term outcomes of patients in steroid-free clinical remission with MES 0 with those with MES 1.
Methods
A systematic electronic search of the literature was performed using Medline, Scopus, and CENTRAL through December 2020 (PROSPERO n:CRD42020179333). The studies concerned UC patients, in steroid-free clinical remission, with MES of 0 or 1, and with at least 12-months of follow-up.
Results
Out of 4611 citations, 15 eligible studies were identified. Increases in clinical relapse among patients with MES 1 were observed in all the studies included in this review, suggesting that MES of 1 have a higher risk of relapse than a score of 0. MES 0 patients displayed a lower risk of clinical relapse (OR 0.33; 95% CI 0.26–0.43; I2 13%) irrespective of the follow-up time (12-months or longer). On the other hand, no differences were found comparing MES 0 versus MES 1 about the risk of hospitalization or colectomy.
Conclusions
MES 0 is associated with a lower rate of clinical relapse than is MES 1. For this reason, MES 0, rather than MES 0–1, should be considered the therapeutic target for patients with UC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-022-02157-5.
Keywords: Ulcerative colitis (UC), Mayo endoscopic score (MES), Mucosal healing (MH), Steroid-free clinical remission, Inflammatory bowel disease (IBD)
Background
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disease that generally begins in young adulthood and lasts a lifetime with a chronic relapsing course [1]. The incidence is increasing worldwide, with no definitive cure as yet available. Although the exact etiology of UC remains unknown, the pathogenesis of the colonic inflammatory lesions appears to be due to a dysregulation of the gut mucosa immune system. Medical therapy has been directed to correcting this immunologic imbalance [1–4].
Inflammation of the colonic mucosa is responsible for signs, symptoms, and complications of UC. Both the development of complications and refractoriness to medical therapy may lead to the requirement for colectomy [3–5].
The clinical presentation usually depends on the severity (activity) and the extent of the colonic lesions, although the correlation between symptoms and lesions is not a rule. Patients with UC may lack, or display only minimal, symptoms despite the evidence of active and extensive inflammation at colonoscopy [1, 2]; and symptoms may be present, despite the absence of inflammatory activity at endoscopy [6, 7], which could be related to concomitant irritable bowel syndrome [8, 9].
Resolution of symptoms and maintenance of clinical remission have traditionally been considered as the best targets of therapy in UC [1–4]. Such strategy, however, does not appear to have significantly changed the natural course of the disease [10, 11]. In the last few years, the target of therapy has moved from the resolution of symptoms to the healing of colonic mucosal lesions [3, 4, 12]. Mucosal healing (MH), compared with clinical remission, is associated with a lower risk of recurrence and complications at follow-up [13–16]. Thus, a strategy that aims at MH could allow us to change the natural history of UC.
Current guidelines indicate that endoscopic evaluation represents a necessary tool to define remission and to make clinical decisions [3, 4]. To date, however, no consensus exists regarding the definition of endoscopic remission. Various score systems and cut-off values have been proposed, but the most appropriate one has yet to be established [13–16]. The Mayo endoscopic score (MES) is the most commonly adopted score to measure endoscopic activity in both clinical trials and daily practice [17, 18]. Endoscopic remission is usually defined as either normal mucosa (MES 0) or mild erythema and mild friability (MES 1) appearance [13, 15]. Agrowing number of observations point to the association ofMH with improved long-term clinical outcomes [16, 19].
To identify the best definition of endoscopic remission using the MES, we performed a systematic review and meta-analysis to compare MES 0 versus MES 1 in patients with UC in steroid-free clinical remission. Clinical relapse, hospitalization, and colectomy were considered as outcomes to evaluate the changes in the clinical course.
Methods
Study protocol
Our systematic review and meta-analysis were conducted according to PRISMA guidelines [20]. We used a predetermined protocol (PROSPERO n: CRD42020179333; submitted in April 2020).
A systematic electronic search of the literature was performed using PubMed/MEDLINE, Scopus, and CENTRAL. The search included a combination of Medical Subject headings (MeSH) and keywords (Additional file 1: Table S1). Each of the relevant publication reference sections, Google Scholar, relevant abstracts from United European Gastroenterology Week, and European Crohn’s and Colitis organization conferences were also screened for other applicable publications. The last search was performed in December 2020.
The Authors of the eligible studies were contacted for additional information regarding any inconsistencies in their reported results.
The search was limited to studies conducted on human, adult UC patients in steroid-free clinical remission with MES 0 or MES 1 at endoscopic evaluation. Studies on pediatric populations (0–18 years) were excluded. We considered non-randomized study of intervention (NRSI): prospective and retrospective cohort studies, and case–control studies.
The datasets generated and analysed during the current study are available in the “Mendeley data” repository (https://data.mendeley.com/). The details to access are: Systematic Review and Meta-Analysis: the advantage of Endoscopic Mayo Score 0 over 1 in patients with Ulcerative Colitis. Published: 1 February 2022; Version 1 DOI https://doi.org/10.17632/rkhsvzwr6v.1
Definition of Mayo endoscopic 0 or 1
We identified a dichotomist group of patients with MES of 0 or 1. According to the literature, MES 0 score indicating a normal mucosa; MES 1 score indicating a decreased vascular pattern, erythema, and mild friability [21, 22].
Definition of clinical remission
We included only patients in steroid-free clinical remission. Clinical remission was defined by disease activity scores (e.g., Partial Mayo Score, Truelove and Witts Score, Simple Clinical Colitis Activity Index) below thresholds set in the individual studies [22].
Definition of clinical outcomes
Our primary analysis was the comparison of the proportion of patients with clinical relapse in the MES 0 vs MES 1 group.
Clinical outcomes evaluated in our study included (a) clinical relapse, (b) IBD-related hospitalization rate, and (c) colectomy. Clinical relapse was defined by disease activity scores (e.g., Partial Mayo Score, Truelove and Witts Score, above-set thresholds determined by individual studies) and/or need for medication intensification (Additional file 1: Table S2).
Subgroup analysis and sensitive analysis
A subgroup analysis was performed comparing studies with follow-up periods less than 12 months with those greater than 12 months.Moreover, we performed a subgroup analysis of studies including patients on conventional therapy, biological therapy, or both.
Sensitivity analysis was also conducted excluding the abstracts and the studies with low-moderate quality assessed by the New Castle-Ottawa scale (NOS). Moreover, a sensitivity analysis of studies that included only clinical relapse defined by Partial Mayo Score and among the studies with a prospective design was performed.
Selection of studies
Three authors (MV, AC, and GS) independently reviewed abstracts and manuscripts for eligibility. Conflicts were resolved by consensus with senior authors (AV, GL). Inclusion and exclusion criteria are outlined below.
Inclusion criteria
Human studies comprisingpatients 18 years or older with known UC;
NRSI: prospective and retrospective cohort studies, and case–control studies;
at least 12 months of follow-up;
steroid-free clinical remission;
no prior colectomy;
studies on UC patients evaluating the association between mucosal healing assessed in terms of MES 0 or MES 1 and clinical outcomes (clinical relapse, hospitalization, colectomy).
Exclusion criteria
Non‐Human Studies (cell culture, animal models);
pediatric cohorts (patients less than 18 years of age);
review articles and other systematic reviews or meta-analyses;
absence of steroid-free clinical remission at inclusion;
inability to distinguish between MES 0 and MES 1;
clinical outcomes not reported.
Data extraction and assessment of studies quality
Data extraction was carried out independently by two investigators (MV and GS). Any discrepancies were resolved by consensus in consultation with the senior authors (AV and GL).
Each reviewer extracted the following data: title and reference details (first author, journal, year, country), study population characteristics (number of patients included in study, gender and age, MES, IBD-related medications) outcome data (clinical relapse, hospitalization, colectomy). All data were recorded independently by both literature reviewers in separate databases and were compared at the end of the reviewing process to limit selection bias. The database was then reviewed by a third person (AV). Two authors (MV and GS) independently assessed the quality of included studies using the Newcastle‐Ottawa scale (NOS) for case‐control studies or cohort studies [23]. Significant conflicts between NOS scores were resolved by consensus and consultation of senior authors (AV, GL), otherwise, scores were averaged between the two reviewers. Criteria evaluated for assesment of the quality of the included studies are reported in Additional file 1: Table S4. NOS scores were defined as high (score 7–9), moderate (score 4–6), or low (score 0–3).
Statistical analyses
Adjusted odds ratios (OR) from individual studies were extracted using dichotomous data (MES 0 vs MES 1 groups) for each outcome (clinical relapse, hospitalization, and colectomy). Review Manager v5.4.1 (RevMan 2020, Copenhagen, The Cochrane Collaboration) was used to calculate the pooled odds ratio (95% CI and p values) of clinical relapse, hospitalization, and colectomy among UC patients with MES 0 versus MES 1, generate forest plots, calculate the I2 statistic and to generate a funnel plot. Heterogeneity was assessed using the I2 statistic defined by the Cochrane Handbook for Systematic Reviews [24]. The Odds Ratio for the individual study was combined using a fixed-effect model, with a random-effects model planned in case of substantial heterogeneity (p < 0.10, I2 > 50%). Either Chi2 test p < 0.10 or I2 value > 50% indicated substantial heterogeneity. Publication bias was graphically determined using funnel plots: a symmetric inverted funnel shape arises from a ‘well-behaved’ data set, in which case the publication bias is unlikely [25]. Funnel plot asymmetry was explored using Egger's test [26]. The extracted data were analyzed using the R statistical software (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
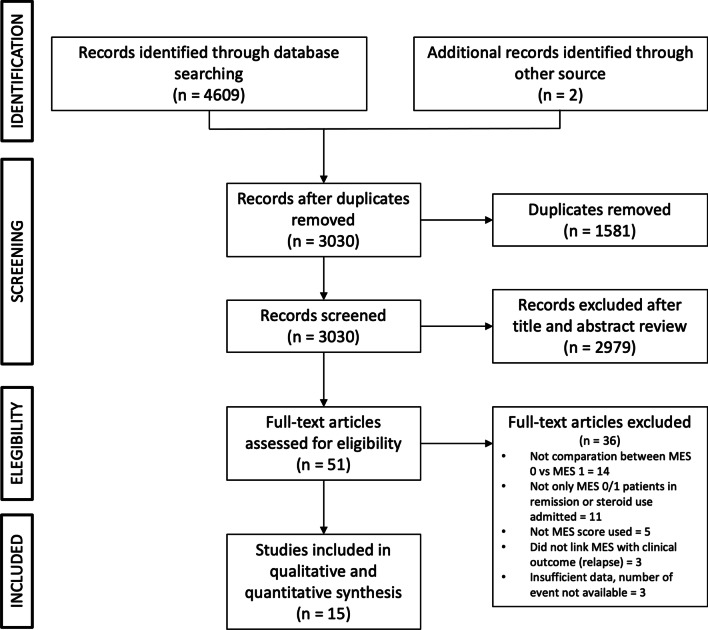
Figure 1 shows the PRISMA flow diagram representing the results of the literature search, as assessed by the three authors (MV, AC, and GS). We found 4611 articles, removing 1581 duplicated records, excluding 2979 records based on their titles and abstracts, and 36 based on their full texts evaluation. We included 15 articles for this review. Characteristics of the 15 selected studies are reported in Additional file 1: Table S3. Additional file 1: Table S2 summarizes the definitions of clinical outcome measures set by individual studies. The quality of the included studies assessed by the NOS is summarized in Additional file 1: Table S4. The mean NOS among the 15 studies included was 6.7 (7 high quality; 8 moderate quality).
Fig. 1.
PRISMA flow diagram
Description of excluded studies
Figure 1 Summarizes the selection process of the studies included in this meta-analysis. The reasons for the exclusion of 36 studies are outlined in Additional file 1: Table S5.
Characteristics of included studies
In assessingclinical relapse, most of the included studies used the clinical Mayo sub-score [27–31] or the need for any treatment escalation [28–30, 32–36]. Of the others, oneused the Truelove and Witts clinical criteria [37], one the Lichtiger Clinical activity Index [38], one the Rachmilewitz Clinical Activity Index [39], and one the Simple Clinical Colitis Activity Index [40]. Finally, two studies evaluated the worsening of stool frequency and/or presence of rectal bleeding [27, 41].
Follow-up lengthsvaried among the studies and details are provided in Table 1.
Table 1.
Mayo endoscopic score and clinical relapse
| Study design | Follow-up (months) | Population | MES 0 or 1 | Clinical relapse | Δ% relapse | |
|---|---|---|---|---|---|---|
| Barreiro-de Acosta et al. [32] (Spain) | Prospective | 12 | 187 |
187 Mayo 0 = 126 (67.3%) Mayo 1 = 61 (32.7%) |
49 (26.2%) Mayo 0 = 24 (19.3%) Mayo 1 = 25 (41%) |
Δ% = 21.7; p < 0.01 |
| Narang et al. [40] (India) | Prospective | 12 | 76 |
46 Mayo 0 = 36 (78.3%) Mayo 1 = 10 (21.7%) |
12 (26.1%) Mayo 0 = 6 (16.7%) Mayo 1 = 6 (60%) |
Δ% = 43.3 |
| Ponte et al. [30] (Portugal) | Retrospective | 46–72 | 82 |
60 Mayo 0 = 32 (53.3%) Mayo 1 = 28 (46.7%) |
19 (31.7%) Mayo 0 = 6 (18.8%) Mayo 1 = 13 (46.4%) |
Δ% = 27.6; p = 0.02 |
| Boal Carvalho et al. [34] (Portugal) | Retrospective | 12 | 138 |
138 Mayo 0 = 61 (44.2%) Mayo 1 = 77 (55.8%) |
28 (20.3%) Mayo 0 = 7 (11.5%) Mayo 1 = 21 (27.3%) |
Δ% = 15.8; p = 0.022 |
| Yokoyama et al. [35] (Japan) | Retrospective | 60 | 38 |
24 Mayo 0 = 9 (37.5%) Mayo 1 = 15 (62.5%) |
11 (45.8%) Mayo 0 = 2 (22%) Mayo 1 = 9 (60%) |
Δ% = 38 |
| Kim et al. [31] (South Korea) | Retrospective | 80 | 215 |
200 Mayo 0 = 113 (56.5%) Mayo 1 = 87 (43.5%) |
51 (25.5%) Mayo 0 = 22 (19.5%) Mayo 1 = 29 (33.3%) |
Δ% = 13.8; p < 0.023 |
| Yoshino et al. [36] (Japan) | Retrospective | 16 | 298 |
88 Mayo 0 = 43 (48.9%) Mayo 1 = 45 (51.1%) |
21 (23.9%) Mayo 0 = 7 (33.3%) Mayo 1 = 14 (66.7%) |
Δ% = 33.4; p = 0.167 |
| López-Palacios et al. [37] (Spain) | Prospective | 27 | 20 |
13 Mayo 0 = 10 (76.9%) Mayo 1 = 3 (23.1%) |
2 (15.4%) Mayo 0 = 1 (10%) Mayo 1 = 1 (33.3%) |
Δ% = 23.3 |
| Yamamoto et al. [27] (Japan) | Prospective | 12 | 164 |
164 Mayo 0 = 84 (51%) Mayo 1 = 80 (49%) |
46 (28%) Mayo 0 = 19 (22.6%) Mayo 1 = 27 (33.8%) |
Δ% = 11.2; p = 0.16 |
| Frieri et al. [33] (Italy) | Prospective | 36 | 52 |
46 Mayo 0 = 29 (63%) Mayo 1 = 17 (37%) |
20 (43.5%) Mayo 0 = 9 (31%) Mayo 1 = 11 (64.7%) |
Δ% = 33.7; p < 0.0001 |
| Lobatón et al. [28] (Belgium, Spain) | Prospective | 12 | 96 |
96 Mayo 0 = 63 (66%) Mayo 1 = 33 (34%) |
22 (23%) Mayo 0 = 13 (21%) Mayo 1 = 9 (27%) |
Δ% = 6; p = 0.438 |
| Osterman et al. [29] (USA) | Prospective | 12 | 100 |
61 Mayo 0 = 5 (8.2%) Mayo 1 = 56 (91.8%) |
8 (13.1%) Mayo 0 = 0 (0%) Mayo 1 = 8 (14.3%) |
Δ% = 14.3 |
| Inoue et al. [41] (Japan) | Retrospective (Abstract) | 39 | 331 |
254 Mayo 0 = 176 (69%) Mayo 1 = 78 (31%) |
53 Mayo 0 = 20 (11.4%) Mayo 1 = 33 (42.3%) |
Δ% = 30.9; p = 0.017 |
| Kanazawa et al. [39] (Japan) | Retrospective | 24 | 166 |
166 Mayo 0 = 91 (54.8%) Mayo 1 = 75 (45.2%) |
9 Mayo 0 = 3 (3.3%) Mayo 1 = 6 (8%) |
Δ% = 4.7 |
| Sakemi et al. [38] (Japan) | Retrospective (Abstract) | 36 | 74 |
74 Mayo 0 = 23 (31%) Mayo 1 = 51 (69%) |
26 Mayo 0 = 2 (9%) Mayo 1 = 23 (46%) |
Δ% = 37 |
Although several studies had 12 months follow-up [27–29, 32, 34, 40], ten studies used a longer period of observation, ranging from 16 to 80 months [30, 31, 33, 35–39, 41]. Seven studies used a prospective design [27–29, 32, 33, 37, 40], while eight studies used a retrospective design [30, 31, 34–36, 38, 39, 41].
Six studies [28, 31–34, 37] assessed the number of patients who underwent colectomy, while three studies [28, 33, 34] reported the hospitalization rate of MES 0 and MES 1 separately (Table 2).
Table 2.
Mayo endoscopic score, colectomy, and hospitalization
| References (Country) | Study design | Follow-up (months) | Population | MES 0 or 1 | Colectomy | Hospitalization |
|---|---|---|---|---|---|---|
| Barreiro-de Acosta et al. [32] (Spain) | Prospective | 12 | 187 |
187 Mayo 0 = 126 (67.3%) Mayo 1 = 61 (32.7%) |
0 | n.a.† |
| Boal Carvalho et al. [34] (Portugal) | Retrospective | 12 | 138 |
138 Mayo 0 = 61 (44.2%) Mayo 1 = 77 (55.8%) |
0 |
3 (2.2%) Mayo 0 = 1 (1.6%) Mayo 1 = 2 (2.6%) |
| Kim et al. [31] (South Korea) | Retrospective | 80 | 215 |
200 Mayo 0 = 113 (56.5%) Mayo 1 = 87 (43.5%) |
0 | n.a.† |
| Frieri et al. [33] (Italy) | Prospective | 36 | 52 |
46 Mayo 0 = 29 (63%) Mayo 1 = 17 (37%) |
0 |
3 (6.5%) Mayo 0 = 0 Mayo 1 = 3 (17.6%) |
| Lobatón et al. [28] (Belgium, Spain) | Prospective | 12 | 96 |
96 Mayo 0 = 63 (66%) Mayo 1 = 33 (34%) |
0 | 0 |
| López-Palacios et al. [37] (Spain) | Prospective | 27 | 20 |
13 Mayo 0 = 10 (76.9%) Mayo 1 = 3 (23.1%) |
0 | n.a.† |
†n.a: not applicable
Mayo endoscopic score and risk of clinical relapse
Fifteen studies assessed the clinical relapse [27–41] and included 1617 patients: 901 with MES of 0, and 716 with MES of 1 (Table 1).
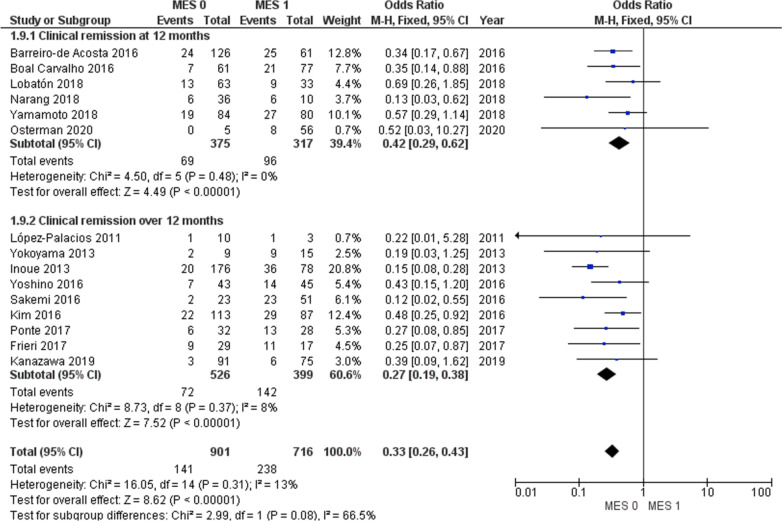
The rate of clinical relapse for MES 1 patients ranged from 8 to 66.7%, and for MES 0 patients from 0 to 33.3%. In MES 0 group the pooled odds ratio (OR) for clinical relapse, irrespective of the time of follow-up, was 0.33 (95% CI 0.26–0.43; I2 = 13%) (Fig. 2). In particular, the OR was 0.42 (95% CI 0.29–0.62; I2 = 0%) in studies with 12 months of follow-up, and 0.27 (95% CI 0.19–0.38, Chi2 = 8.73, I2 = 8%) in studies with follow-ups longer than 12 months. In the latter subgroup analysis, the median follow-up timewas 27 months (range 16–80) (Fig. 2). Therefore, despite the heterogeneity in the length of follow-up (12–80 months), the subgroup analysis showed that the risk of clinical relapse remained significantly higher in patients with MES 1 compared to MES 0 in studies that followed patients up to 12 months and those that followed patients for longer than 12 months.
Fig. 2.
Clinical relapse in MES 0 versus MES 1
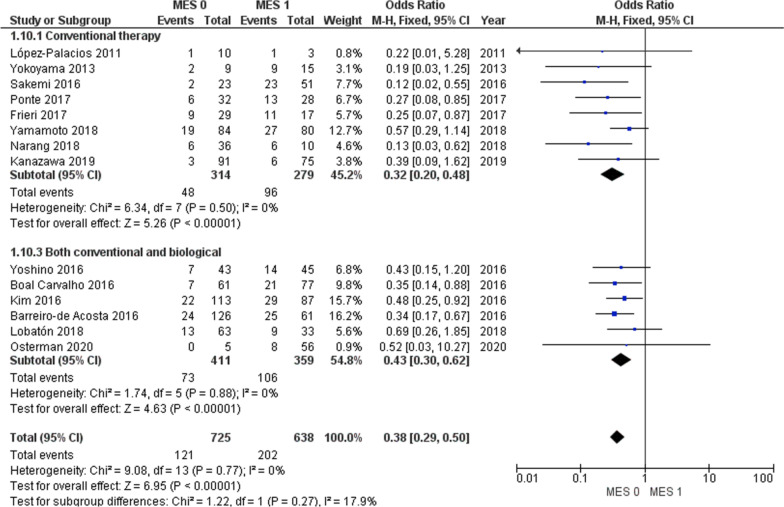
With regard to maintenance therapy, a significantly lower clinical relapse was observed in patients with MES 0 than with those MES 1 in both sub-groups including only patients in conventional therapy and studies including patients on conventional or biological therapy (Fig. 3).
Fig. 3.
Clinical relapse in MES 0 versus MES 1 regarding therapy
Mayo endoscopic score and hospitalization rate
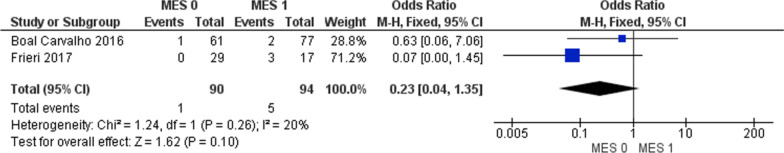
Three studies reported hospitalization rate [28, 33, 34], a zero rate being reportedin one of them [28] (Table 2). Hospitalization rate in the group with a MES of 0 ranged from 0 to 1.6%; on the other hand, it was 0–17.6% in MES 1 group.
Patients achieving MES 0 had a lower risk of hospitalization (OR 0.23; 95% CI 0.04–1.35; I2 = 20%), although the small numbers involved preclude general significance (Fig. 4).
Fig. 4.
Hospitalization in MES 0 versus MES 1
Mayo endoscopic score and risk of colectomy
Six studies [28, 31–34, 37] assessed the number of patients undergoing colectomy (Table 2). Among 680 patients no event was recorded for this outcome.
Sensitivity analysis and publication bias
Sensitivity analysis was conducted excluding the abstracts and the studies with low-moderate quality assessed at NOS. Both analyses showed a lower risk of clinical relapse for the MES 0 group. (OR 0.40; 95% CI 0.30–0.53; I2 0%, and OR 0.34; 95% CI 0.23–0.51; I2 0% respectively) (Additional file 1: Figs. S1 and S2).
After the exclusion of each abstract separately [38, 41], the results were (OR 0.35; 95% CI 0.27–0.45; I2 9%, and OR 0.38; 95% CI 0.29–0.50; I2 0%), respectively (Additional file 1: Figs. S3 and S4).
Furthermore, sensitivity analysis was conducted with studies that included only clinical relapse defined by Partial Mayo Score [28–31]. Among these studies, the MES 0 had a lower risk of clinical relapse compared to MES 1 (OR 0.47; 95% CI 0.29–0.77; I2 = 0%) (Additional file 1: Fig. S5).
A lower rate of clinical relapse was also observed in MES 0 group after the exclusion of studies with a retrospective design [27–29, 32, 33, 37, 40] (RR 0.54; 95% CI 0.41–0.70; I2 = 0) (Additional file 1: Fig. S6).
The asymmetrical shape of the funnel plot and the Egger’s test showed no publication bias among studies analyzing the risk of clinical relapse (p = 0.47) (Additional file 1: Fig. S7).
Discussion
The results of our meta-analysis demonstrate that achieving MES 0 is linked with a lower clinical relapse rate compared to MES 1 in patients with UC in steroid-free clinical remission. Patients with MES 0 had a 67% lower risk of clinical relapse than those with MES 1. A trend toward a lower risk of hospitalization was also observed, but the small number of patients who experienced these outcomes did not allow making firm conclusions. It is nonetheless noteworthy that 88% of the hospitalization occurred in the group of patients with MES 1 at baseline.
MH was firstly reported in 1951 by Kirsner, who observed, using X-ray and proctoscopic examinations, healing of the colonic lesions in a series of patients with severe UC treated with corticotropin. The disappearance of extensive ulcerations of the colon resulted associated with an improved clinical course [42, 43]. More recently, it has been reported that the absence of healing of rectal lesions in patients with severe UC strongly predicts the need for colectomy [44, 45].
In 2015, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) committee defined the treat-to-target approach, based on tailored adjustments of medical therapy, monitoring objective disease activity—i.e., through endoscopy [46].
This strategy has been demonstrated to be feasible in clinical practice and results in high rates of MH long-term prevention of bowel damage and disease complications (hospitalizations, colectomy, dysplasia/cancer) [47].
An unequivocal definition of MH is necessary in order to apply the treat-to-target approach. To date, there is no validated definition of what constitutes endoscopic remission in UC. The most used tool in both routine practice and clinical trials is the MES, being endoscopic remission often defined as both complete (MES 0) and partial (MES 1).
Recently, a prospective multicenter study showed that high definition (HD) electronic chromoendoscopy can detect subtle mucosal and vascular changes that may reflect histologic remission. The Authors develop a new virtual electronic chromoendoscopy score (PICaSSO score) and show this score strongly correlates with histological scores with a better correlation coefficient than the MES and Ulcerative Colitis Endoscopic Index of Severity score in predicting histological remission [48]. These results could represent a valuable tool for a better definition of MH. Unfortunately, only two articles among the included studies in our meta-analysis mentioned technical specifications of the colonoscope in their methods section. These two studies specified the use of virtual chromoendoscopy [28, 35] and neither mentioned the use of HD definition imaging. Therefore, no pooled analysis concerning the risk of clinical relapse with and without HD-defined MES 0 was possible due to inconsistencies in reported outcomes.
Two meta-analyses [49, 50] assessed the impact of MH on long-term outcomes in patients with UC. The authors of both analyses concluded that MH, defined as both complete and partial healing, is a strong predictor of long-term clinical remission. Complete healing predicted higher rates of clinical remission, but it did not result in significantly more favorable than partial healing for predicting surgeries or hospitalizations.These meta-analyses however included studies which used different endoscopic scores (most of them grouping together complete and partial healing) and study populations differing with regard disease activity and therapies.
To overcome these methodologic inadequacies (bias), in our meta-analysis we considered a homogeneous series of studies including only patients with UC in stable clinical remission. The greater strength of our meta-analysis is the rigorous selection of studies including patients in clinical remission without the use of steroids. Moreover, MH was defined based on MES in all studies distinguishing between complete (MES 0) and partial (MES 1) MH. This makes the results of our meta-analysis directly applicable in clinical practice and avoids potential bias in the evaluation of clinical remission.
In all, MES of 0 was associated with greater benefit compared to MES 1 (OR 0.33; 95% CI 0.26–0.43; I2 = 13%). The lower risk of clinical relapse in MES 0 patients has been further demonstrated in a subgroup analysis including studies that followed patients up to 12 months (OR 0.42; 95% CI 0.29–0.62; I2 = 0%) and those that followed patients for longer than 12 months (OR 0.27; 95% CI 0.19–0.38; I2 = 8%). Moreover, in a sub-analysis including only studies with a prospective design (27–29, 32, 33, 37, 40) a lower rate of clinical relapse was observed in the MES 0 group (RR 0.54; 95% CI 0.41–0.70; I2 = 0). Hospitalization rates in the group with the MES 0 ranged from 0 to 1.6%, and from 0 to 17.6% in MES 1 group. Although 5/6 of hospitalization occurred in MES 1 group,the patient numbers involved were too small to permit general conclusions to be drawn from this result. Among the studies that assessed the outcome of colectomy, no event was registered.
However, our meta-analysis has several limitations. First, clinical relapse was defined differently among the included studies, ranging from various clinical indices to the need for steroids or the need for escalation therapy. Second, the quality of studies included in our analysis was acceptable in the NOS, but most studies were lack of comparison of population characteristics between the MES 0 and MES 1 group at baseline.
Conclusions
Our meta-analysis showed a significantly lower risk of clinical relapse in UC patients with an endoscopic finding of MES 0 compared with MES 1, regardless of follow-up length (12 months or longer). MES 0 should therefore be considered the optimal therapeutic goal as it predicts the best clinical course in patients with UC.
Supplementary Information
Additional file 1: Table S1. Research strategy. Table S2 Clinical outcomes and measures. Table S3 Baseline characteristics of the included studies. Table S4 Quality Assessment of Studies by Newcastle-Ottawa Scale. Table S5 Full text excluded. Fig. S1 Sensitivity analysis after abstract exclusion. Fig. S2 Sensitivity analysis after low-moderate quality studies exclusion. Fig. S3 Sensitivity analysis after abstract38 exclusion. Fig. S4 Sensitivity analysis after abstract41 exclusion. Fig. S5 Sensitivity analysis including studies with clinical relapse assessed with Partial Mayo Score evaluation. Fig. S6 Sensitivity analysis after retrospective studies exclusion. Fig. S7 Funnel plot for clinical relapse (N: 15 studies, 1617 patients); Egger’s test: p = 0.47.
Acknowledgements
We thank Keiichi Tominaga and Mimari Kanazawa for answering our queries about their paper and providing us with extra data.
Abbreviations
- MH
Mucosal healing
- UC
Ulcertive colitis
- MES
Mayo endoscopic score
- NRSI
Non-randomized study of intervention
- IBD
Inflammatory bowel disease
- NOS
New castle–ottawa scale
- OR
Odds ratios
Authors' contributions
AV, MV: study concept and design; AV, MV, GS: drafting of the manuscript; MV, AC, GS, CC, EO, AC, FV: acquisition, analysis, and interpretation of data; GL, AV, MV: interpretation of data and critical revision of the manuscript. All authors approved the final draft submitted. Each one of the authors was involved in the writing and revision of the manuscript.
Funding
Not applicable.
Availability of data and material
All data generated or analysed during this study are included in this published article [and its Additional file 1 files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no financial support and no specific conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angelo Viscido and Marco Valvano have contributed equally to this work.
Contributor Information
Angelo Viscido, Email: angelo.viscido@univaq.it.
Marco Valvano, Email: valvano.marco@libero.it.
Gianpiero Stefanelli, Email: giastefanelli@gmail.com.
Annalisa Capannolo, Email: annalisacap@tiscali.it.
Chiara Castellini, Email: chiara.castellini@univaq.it.
Eugenia Onori, Email: eugenia.onori@gmail.com.
Antonio Ciccone, Email: aciccone.md@gmail.com.
Filippo Vernia, Email: filippo.vernia1@gmail.com.
Giovanni Latella, Email: giolatel@tin.it.
References
- 1.Magro F, Gionchetti P, Eliakim R, et al. Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 2.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 5.Pai RK, Jairath V, Vande Casteele N, Rieder F, Parker CE, Lauwers GY. The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2018;88:887–898. doi: 10.1016/j.gie.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Calafat M, Lobatón T, Hernández-Gallego A, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig Liver Dis. 2017;49:1327–1331. doi: 10.1016/j.dld.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Fluxá D, Simian D, Flores L, et al. Clinical, endoscopic and histological correlation and measures of association in ulcerative colitis: correlation in ulcerative colitis. J Dig Dis. 2017;18:634–641. doi: 10.1111/1751-2980.12546. [DOI] [PubMed] [Google Scholar]
- 8.Stanisic V, Quigley EM. The overlap between IBS and IBD—What is it and what does it mean? Expert Rev Gastroenterol Hepatol. 2014;8:139–145. doi: 10.1586/17474124.2014.876361. [DOI] [PubMed] [Google Scholar]
- 9.Aziz I, Simrén M. The overlap between irritable bowel syndrome and organic gastrointestinal diseases. Lancet Gastroenterol Hepatol. 2021;6:139–148. doi: 10.1016/S2468-1253(20)30212-0. [DOI] [PubMed] [Google Scholar]
- 10.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2016;111:1692–1701. doi: 10.1038/ajg.2016.418. [DOI] [PubMed] [Google Scholar]
- 12.Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016 doi: 10.1586/17474124.2016.1174064. [DOI] [PubMed] [Google Scholar]
- 13.Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969–977. doi: 10.1016/j.dld.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Papi C, Aratari A. Mucosal healing as a treatment for IBD? Expert Rev Gastroenterol Hepatol. 2014;8:457–459. doi: 10.1586/17474124.2014.902302. [DOI] [PubMed] [Google Scholar]
- 15.Villanacci V. Histological healing in inflammatory bowel disease: A still unfulfilled promise. World J Gastroenterol. 2013;19:968–978. doi: 10.3748/wjg.v19.i7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battat R, Duijvestein M, Guizzetti L, et al. Histologic healing rates of medical therapies for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Am J Gastroenterol. 2019;114:733–745. doi: 10.14309/ajg.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 17.Viscido A, Latella G, Frieri G. Topical aminosalicylates and histologic healing in ulcerative colitis. Am J Gastroenterol. 2019;114:1922–1923. doi: 10.14309/ajg.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 18.Travis SPL, Higgins PDR, Orchard T, et al. Review article: defining remission in ulcerative colitis: review: remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34:113–124. doi: 10.1111/j.1365-2036.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- 19.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–414. doi: 10.1136/gutjnl-2015-309598. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018;16:1. doi: 10.1002/14651858.CD011450.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24.Higgins JPT, Altman DG, Sterne JAC. Cochrane Handbook for Systematic Reviews of Interventions: version 5.2.0 [updated June 2017]. http://www.training.cochrane.org/handbook.
- 25.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;10:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol. 2018;9:136. doi: 10.1038/s41424-018-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobatón T, Bessissow T, Ruiz-Cerulla A, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: a prospective multicenter study. United European Gastroenterol J. 2018;6:765–772. doi: 10.1177/2050640617752207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osterman MT, Scott FI, Fogt FF, et al. Endoscopic and histological assessment, correlation, and relapse in clinically quiescent ulcerative colitis (MARQUEE) Inflamm Bowel Dis. 2021;27:207–214. doi: 10.1093/ibd/izaa048. [DOI] [PubMed] [Google Scholar]
- 30.Ponte A, Pinho R, Fernandes S, et al. Impact of histological and endoscopic remissions on clinical recurrence and recurrence-free time in ulcerative colitis. Inflamm Bowel Dis. 2017;23:2238–2244. doi: 10.1097/MIB.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Cheon JH, Park Y, et al. Effect of mucosal healing (Mayo 0) on clinical relapse in patients with ulcerative colitis in clinical remission. Scand J Gastroenterol. 2016;51:1069–1074. doi: 10.3109/00365521.2016.1150503. [DOI] [PubMed] [Google Scholar]
- 32.Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis. 2016;10:13–19. doi: 10.1093/ecco-jcc/jjv158. [DOI] [PubMed] [Google Scholar]
- 33.Frieri G, Galletti B, Di Ruscio M, et al. The prognostic value of histology in ulcerative colitis in clinical remission with mesalazine. Therap Adv Gastroenterol. 2017;10:749–759. doi: 10.1177/1756283X17722926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boal Carvalho P, Dias de Castro F, Rosa B, Moreira MJ, Cotter J. Mucosal healing in ulcerative colitis—When zero is better. J Crohns Colitis. 2016;10:20–25. doi: 10.1093/ecco-jcc/jjv180. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013;2013:1–6. doi: 10.1155/2013/192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshino T, Yamakawa K, Nishimura S, Watanabe K, Yazumi S. The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing. Intest Res. 2016;14:37–42. doi: 10.5217/ir.2016.14.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Palacios N, Mendoza JL, Taxonera C, Lana R, López-Jamar JM, Díaz-Rubio M. Mucosal healing for predicting clinical outcome in patients with ulcerative colitis using thiopurines in monotherapy. Eur J Intern Med. 2011;22:621–625. doi: 10.1016/j.ejim.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Sakemi R, Tanaka H, Miyakawa M, Nasuno M, Motoya S. P618. A Mayo endoscopic sub-score of zero predicts long-term remission in patients with ulcerative colitis treated with mesalazine. J Crohns Colitis. 2016;10:14. [Google Scholar]
- 39.Kanazawa M, Takahashi F, Tominaga K, et al. Relationship between endoscopic mucosal healing and histologic inflammation during remission maintenance phase in ulcerative colitis: a retrospective study. Endosc Int Open. 2019;07:E568–575. doi: 10.1055/a-0869-7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narang V, Kaur R, Garg B, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res. 2018;16:55–61. doi: 10.5217/ir.2018.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue N, Takabayashi K, Takayama T, et al. Mucosal healing without endoscopic activity predicts long-term clinical remission in patients with ulcerative colitis. Gastroenterology. 2013;159:s1262–1275. [Google Scholar]
- 42.Kirsner JB, Palmer WL, Klotz AP. Reversibility in ulcerative colitis. Radiology. 1951;57:1–14. doi: 10.1148/57.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Kirsner JB, Palmer WL. Effect of corticotropin (ACTH) in chronic ulcerative colitis; observations in forty patients. J Am Med Assoc. 1951;147:541–549. doi: 10.1001/jama.1951.03670230007003. [DOI] [PubMed] [Google Scholar]
- 44.Carbonnel F, Lavergne A, Lémann M, et al. Colonoscopy of acute colitis: a safe and reliable tool for assessment of severity. Dig Dis Sci. 1994;39:1550–1557. doi: 10.1007/BF02088063. [DOI] [PubMed] [Google Scholar]
- 45.Gustavsson A, Järnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis-3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32:984–989. doi: 10.1111/j.1365-2036.2010.04435.x. [DOI] [PubMed] [Google Scholar]
- 46.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 47.Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014;20:231–239. doi: 10.1097/01.MIB.0000437985.00190.aa. [DOI] [PubMed] [Google Scholar]
- 48.Iacucci M, Smith SCL, Bazarova A, et al. An international multicenter real-life prospective study of electronic chromoendoscopy score PICaSSO in ulcerative colitis. Gastroenterology. 2021;160:1558–1569. doi: 10.1053/j.gastro.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Yoon H, Jangi S, Dulai PS, et al. incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020;159:1262–1275. doi: 10.1053/j.gastro.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SC, Colombel J-F, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245–1255. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Research strategy. Table S2 Clinical outcomes and measures. Table S3 Baseline characteristics of the included studies. Table S4 Quality Assessment of Studies by Newcastle-Ottawa Scale. Table S5 Full text excluded. Fig. S1 Sensitivity analysis after abstract exclusion. Fig. S2 Sensitivity analysis after low-moderate quality studies exclusion. Fig. S3 Sensitivity analysis after abstract38 exclusion. Fig. S4 Sensitivity analysis after abstract41 exclusion. Fig. S5 Sensitivity analysis including studies with clinical relapse assessed with Partial Mayo Score evaluation. Fig. S6 Sensitivity analysis after retrospective studies exclusion. Fig. S7 Funnel plot for clinical relapse (N: 15 studies, 1617 patients); Egger’s test: p = 0.47.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its Additional file 1 files].