Abstract
Background
Coffin-Siris syndrome-8 (CSS8) is a rare autosomal dominant disorder caused by variants in SMARCC2, a core subunit of the chromatin-remodeling complex BRG1-associated factor (BAF). The clinical characteristics of this disorder have not been entirely determined because of the rarity of clinical reports. The BAF complex plays a crucial role in embryogenesis and cardiac development, and pathogenic variants in genes encoding the components of the BAF complex have been associated with congenital heart disease (CHD). However, variants in SMARCC2 have not been reported in patients with CHD.
Case presentation
A 28-year-old primigravida was referred at 24 weeks gestation for prenatal echocardiography. The echocardiographic findings were consistent with a prenatal ultrasound diagnosis of tetralogy of Fallot (TOF). After detailed counseling, the couple decided to terminate the pregnancy and undergo genetic testing. A trio (fetus and the parents) whole-exome sequencing (WES) and copy number variation sequencing (CNV-seq) were performed. CNV-seq identified no chromosomal abnormalities. WES analysis revealed a pathogenic, de novo heterozygous frameshift variant in SMARCC2 (NM_003075.5: c.3561del, p.Leu1188fs). The genetic diagnosis of CSS8 was considered given the identification of the SMARCC2 pathogenic variant.
Conclusions
We report the first prenatal case with the SMARCC2 variant. The presence of CHD further broadens the phenotypic spectrum of SMARCC2-related disease.
Background
Coffin-Siris syndrome-8 (CSS8; OMIM: 618362) is a newly recognized syndromic neurodevelopment disorder characterized by variable degrees of impaired intellectual development and dysmorphic features [1]. It is a rare, autosomal dominant disorder caused by pathogenic variants in SMARCC2 (OMIM: 601734). SMARCC2 is one of the invariable core subunits of the chromatin-remodeling complex BRG1-associated factor (BAF), which plays a crucial role in embryogenesis and cardiac development [2]. To date, only 13 unique SMARCC2 pathogenic variants, identified among 15 variably affected individuals, have been reported, two of which had cardiovascular abnormalities including left coronary distension and mild aortic dilatation [1]. Despite evidence that SMARCC2 is essential for cardiac differentiation and driving stage-specific cardiac gene expression programs [2], none of the individuals with SMARCC2 variants have been associated with congenital heart disease (CHD). At the same time, pathogenic variants in genes encoding other components of the BAF complex have been associated with CHD [3]. In fact, due to the scarcity of clinical reports, the clinical characteristics of CSS8, especially the low penetrant phenotype, such as cardiovascular abnormalities, have not been fully determined. We provide herein the first prenatal case with CHD that may be part of this newly identified SMARCC2-related disorder, which could help define its phenotypic spectrum and diagnostic criteria.
Case presentation
A 28-year-old pregnant woman was referred to our center for prenatal echocardiography at 24 weeks of gestation with fetal cardiac abnormalities identified during routine second-trimester screening. The woman was in good health, with no family history of cardiovascular disease and no medication history during pregnancy. She and her husband are not consanguineous.
Clinical phenotypes
Detailed fetal echocardiographic examination revealed a subaortic ventricular septal defect (3.4 mm) with an enlarged aortic valve (Fig. 1A) and a right ventricular outflow tract and pulmonary stenosis (Fig. 1B). The echocardiographic findings supported the diagnosis of tetralogy of Fallot. After a detailed consultation, the couple decided to terminate the pregnancy and undergo genomic sequencing but declined an autopsy.
Fig. 1.
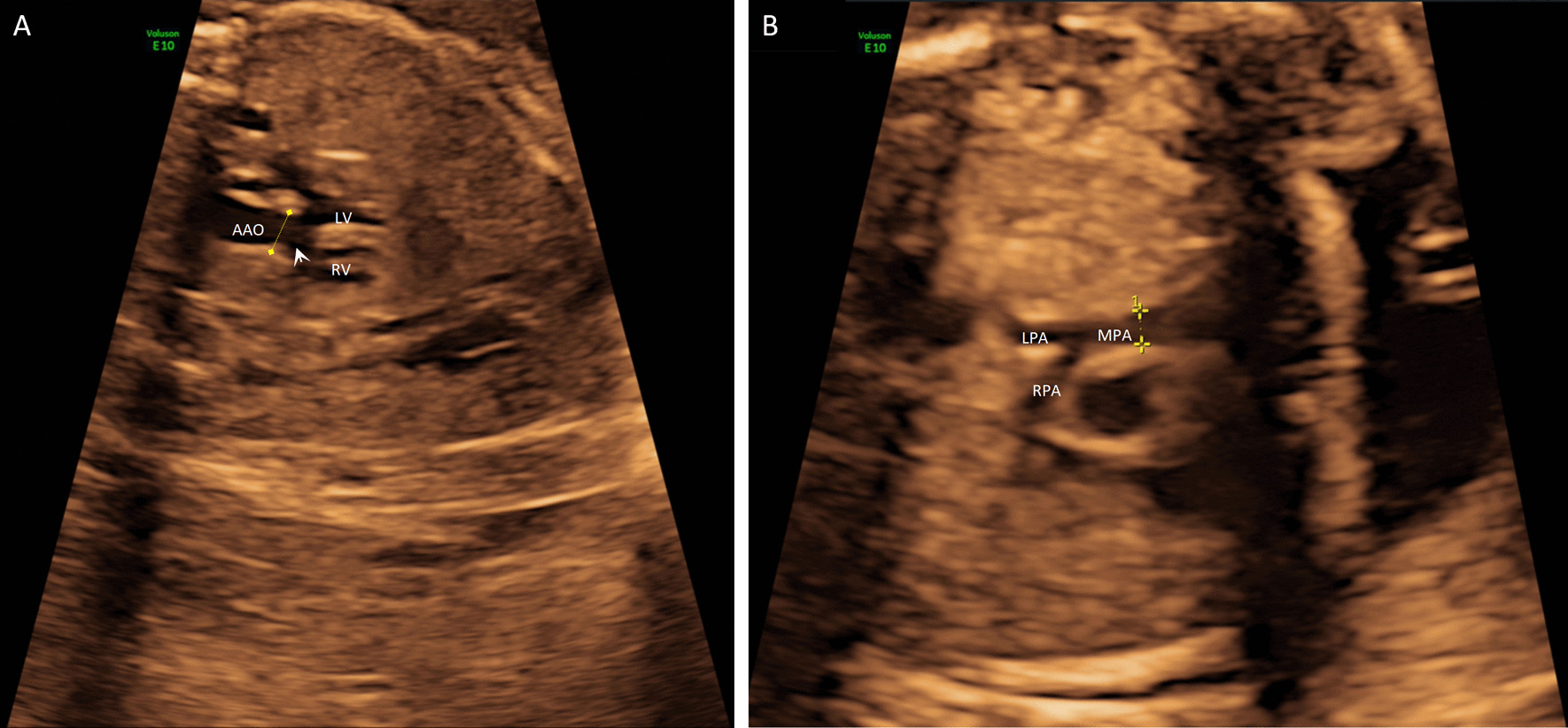
Cardiac defects in the fetus. A The dotted line indicates an enlarged aortic valve; the arrow indicates the ventricular septal defect. B The dotted line refers to a narrowing of the right ventricle outflow tract and pulmonary stenosis. AAO, ascending aorta; L/RPA, left/right pulmonary artery; LV, left ventricle; MPA, main pulmonary artery; RV, right ventricle
Molecular findings
We performed a trio (fetus and the parents) whole-exome sequencing and copy number variation sequencing using methods described previously [4, 5]. We found no chromosomal abnormalities or disease-causing variants in the known CHD genes. However, we identified a de novo heterozygous frameshift variant in SMARCC2 (NM_003075.5: c.3561del, p.Leu1188fs) in the fetus (Fig. 2). This variant has not yet been described as pathogenic or benign and has not been reported in the general population database (gnomAD: https://gnomad.broadinstitute.org). It is predicted to result in either an abnormal truncated protein product or loss of protein from this allele via nonsense-mediated mRNA decay. Furthermore, recent evidence indicates that SMARCC2 is intolerant to loss-of-function variants [1]. In conclusion, we classified this variant as pathogenic according to the American College of Medical Genetics and Genomics guidelines [6].
Fig. 2.
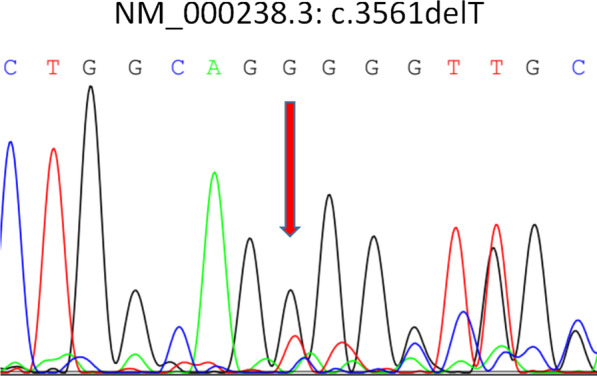
Sanger sequencing shows a frameshift variant in the fetus
Literature review
Since none of the previously reported patients [1] had CHD, the association between SMARCC2 variants and CHD is uncertain. We further conducted literature and public database surveys to determine the association between SMARCC2 variants and CHD. We identified three additional patients with congenital heart disease, two of which carried a contiguous gene deletion containing SMARCC2, and one had a de novo SMARCC2 loss-of-function variant [7, 8]. Detailed genotypes and phenotypes of these patients are summarized in Table 1.
Table 1.
Clinical phenotype and genotype of four unrelated patients with congenital heart disease carrying SMARCC2 deletion or loss-of-function variant
| ID | Sex | Age | Cardiac phenotype | Extra cardiac phenotype | Genetic abnormality | Origin | Reference |
|---|---|---|---|---|---|---|---|
| 401,720 | Female | Infancy | Ventricular septal defect | Broad foot, broad palm, delayed speech and language development, feeding difficulties in infancy, hypertelorism, intellectual disability, muscular hypotonia, open mouth, premature birth, Strabismus, Thick eyebrow, Thick lower lip vermilion, Thick upper lip vermilion,Wide mouth |
[hg19]del(12)(q13.3q14.2p22.3) chr12:g.56554154_63870277del |
De novo | DECIPHER database* |
| F6 | Female | Fetus | Cardiac malposition of the great arteries and multiple ventricular septal defects | Abdominal situs inversus |
SMARCC2 (ENST00000267064: c.1555C > T, p.His519Ter) and NF1(ENST00000456735:c.2747G > A,p.His916Gln) |
De novo | Carss et al. (2014) |
| None | Female | 22 years old | Congenital perimebranous ventricular defect | Neonatal respiratory distress syndrome,neurodevelopmental delay, poor verbal language, dysmorphic facial features, skeletal abnormalities, trigeminal nerve palsy, bilateral mixed hearing loss, rhinolalia, dysarthria and acquired dysphagia for solid foods | 500 Kb-long deletion at 12q13.2-q13.3 that contains SMARCC2 and other 25 genes | De novo | Roberti et al. (2018) |
| None | Female | Fetus | Tetralogy of Fallot | None | SMARCC2 (NM_003075.5: c.3561del, p.Leu1188fs) | De novo | This study |
Discussion and conclusions
We report the first prenatal case of congenital heart disease with a novel variant in SMARCC2, a core subunit of the BAF complex. The discovery of pathogenic variants for pregnancies with structural anomalies during the prenatal period is essential for establishing a precise diagnosis, treatment decision, and correct prognostication and for providing accurate genetic counseling for the perinatal decision-making [9]. Furthermore, our report enlarges the variant spectrum of SMARCC2, and suggests the potential association between CHD and SMARCC2 pathogenic variants.
Growing evidence indicates subunits of the BAF complex are involved in diverse aspects of cardiac development, and the disruption of the BAF complex underlies the pathogenesis of CHD [2]. Actually, CHD is not uncommon in patients with variants in genes encoding BAF complex subunits [1, 3]. For example, in a recent review of patients with variants in genes encoding components of the BAF complex, the incidence of CHD in patients with SMARCB1, SMARCA4, SMARCE1, and ARID1A variants was 45%, 42%, 67%, and 38%, respectively, and the overall incidence was 44% [3]. As for SMARCC2, recent evidence indicates that it is essential for cardiac differentiation through facilitating cardiomyocyte differentiation and controlling temporal steps in cardiac differentiation [2], suggesting a potential general role for the BAF complex in CHD. SMARCC2 variants were recently reported in 15 unrelated patients with impaired intellectual development with speech and behavioral abnormalities, hypotonia, and varying dysmorphism [1]. None of these patients had CHD, although two of them presented coronary distension or aortic dilatation.
To our knowledge, a total of four unrelated CHD patients with SMARCC2 deletion or loss-of-function variants, including the fetus with TOF carrying the novel SMARCC2 pathogenic variant described here, have been reported in the public database or literature [7, 8] (Table 1). The patient 401,720 in DECIPHER database [10] and the patient reported by Roberti et al. [7] carried contiguous gene deletions containing the SMARCC2 gene. They both presented the major features of the CCS8, including intellectual disability, developmental delay with prominent speech delay, behavioral abnormalities, feeding difficulties at the neonatal period, hypotonia, and dysmorphic features, suggesting that the haploinsufficiency of SMARCC2 may contribute significantly to their phenotypes. Interestingly, only the fetus presented herein had no other genetic abnormalities, but the other three patients had either single-gene variants or copy number deletions involving genes other than SMARCC2. It is hard to say whether abnormal SMARCC2 or other genetic abnormalities are the most likely cause of the three patients. The presence of CHD in four unrelated patients with SMARCC2 deletion or loss-of-function variants suggests the possible causal association between SMARCC2 variant and congenital heart disease. Still, it requires further genetic or functional confirmatory studies.
Notably, although we identified no obvious extracardiac malformations by ultrasound, it was still difficult to distinguish whether the congenital heart disease in this fetus was isolated or syndromic. First, subtle dysmorphic features cannot be determined by fetal ultrasound, and some phenotypes, particularly neurodevelopmental disorders, are impossible to identify in the prenatal setting [9]. Furthermore, because of the termination of pregnancy, it is impossible to know if it would have developed other developmental features of CSS8 in the future.
In conclusion, by combining our case and the evidence mentioned above, we conclude that CHD is an intrinsic phenotype of SMARCC2-related disease with reduced penetrance. Further investigations into the pathophysiological mechanism associated with SMARCC2 variants and genotype–phenotype correlations are needed.
Acknowledgements
The authors would like to thank the parents of the fetus.
Abbreviations
- BAF
BRG1-associated factor
- CHD
Congenital heart disease
- CSS8
Coffin-Siris syndrome-8
Authors' contributions
HZ, HY and YH designed the study. XZ, JW and HY collected the study materials or samples. XZ and JW provide representative fetal echocardiograms. HS, JW and SZ completed the sequencing experiments. HS and SZ collected and aggregated the data. XZ, JY and SZ analyzed and interpreted the data. HS and SZ wrote the manuscript. SZ and HS contributed equally to this work. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82170301) to YH and Beijing Lab for Cardiovascular Precision Medicine, Beijing, China (PXM2020_014226_000017_00377132_FCG) to HZ. They both played a role in the collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The data that support the findings of this study are included in our article. The raw data of whole-exome sequencing of the family in this study are not publicly available to protect participant confidentiality. In addition, according to the People's Republic of China regulations on the management of human Genetic Resources, without approval, any unit or individual shall not disclose the original data of genetic resources involving clinical patients. However, the re-analysis of the whole-exome sequencing data are available from the corresponding author on reasonable request. If you want to request access to the data and re-analysis, please contact Prof. Yihua He (Email: heyihuaecho@hotmail.com) at Department of Echocardiography in Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. The parents agreed to participate in this study and provided signed informed consent.
Consent for publication
The parents have provided informed written consent for publication.
Competing interests
The authors declare no competing interests relevant to this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hairui Sun and Siyao Zhang contributed equally to this work
Contributor Information
Hongjia Zhang, Email: zhanghongjia722@ccmu.edu.cn.
Yihua He, Email: heyihuaecho@hotmail.com.
References
- 1.Machol K, Rousseau J, Ehresmann S, Garcia T, Nguyen T, Spillmann RC, Sullivan JA, Shashi V, Jiang YH, Stong N, et al. Expanding the spectrum of BAF-related disorders: de novo variants in SMARCC2 cause a syndrome with intellectual disability and developmental delay. Am J Hum Genet. 2019;104(1):164–178. doi: 10.1016/j.ajhg.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hota SK, Johnson JR, Verschueren E, Thomas R, Blotnick AM, Zhu Y, Sun X, Pennacchio LA, Krogan NJ, Bruneau BG. Dynamic BAF chromatin remodeling complex subunit inclusion promotes temporally distinct gene expression programs in cardiogenesis. Development. 2019;146(19):dev174086. doi: 10.1242/dev.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosho T, Okamoto N. Genotype-phenotype correlation of Coffin–Siris syndrome caused by mutations in SMARCB1, SMARCA4, SMARCE1, and ARID1A. Am J Med Genet C Semin Med Genet. 2014;166C(3):262–275. doi: 10.1002/ajmg.c.31407. [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Hao X, Wang X, Zhou X, Zhang Y, Liu X, Han J, Gu X, Sun L, Zhao Y, et al. Genetics and Clinical Features of Noncompaction Cardiomyopathy in the Fetal Population. Front Cardiovasc Med. 2021;7:617561. doi: 10.3389/fcvm.2020.617561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Yi T, Hao X, Yan H, Wang J, Li Q, Gu X, Zhou X, Wang S, Wang X, et al. Contribution of single-gene defects to congenital cardiac left-sided lesions in the prenatal setting. Ultrasound Obst Gyn. 2020;56(2):225–232. doi: 10.1002/uog.21883. [DOI] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberti D, Conforti R, Giugliano T, Brogna B, Tartaglione I, Casale M, Piluso G, Perrotta S. A novel 12q13.2–q13.3 microdeletion syndrome with combined features of diamond Blackfan Anemia, Pierre Robin sequence and Klippel Feil deformity. Front Genet. 2018;9:549. doi: 10.3389/fgene.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carss KJ, Hillman SC, Parthiban V, McMullan DJ, Maher ER, Kilby MD, Hurles ME. Exome sequencing improves genetic diagnosis of structural fetal abnormalities revealed by ultrasound. Hum Mol Genet. 2014;23(12):3269–3277. doi: 10.1093/hmg/ddu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best S, Wou K, Vora N, Van der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn. 2018;38(1):10–19. doi: 10.1002/pd.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, Vooren SV, Moreau Y, Pettett RM, Carter NP. DECIPHER: Database of chromosomal imbalance and phenotype in humans using ensembl resources. Am J Hum Genet. 2009;84(4):524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included in our article. The raw data of whole-exome sequencing of the family in this study are not publicly available to protect participant confidentiality. In addition, according to the People's Republic of China regulations on the management of human Genetic Resources, without approval, any unit or individual shall not disclose the original data of genetic resources involving clinical patients. However, the re-analysis of the whole-exome sequencing data are available from the corresponding author on reasonable request. If you want to request access to the data and re-analysis, please contact Prof. Yihua He (Email: heyihuaecho@hotmail.com) at Department of Echocardiography in Beijing Anzhen Hospital, Capital Medical University, Beijing, China.


