Abstract
Background
The prevalence of prediabetes is increasing in the global population and its metabolic derangements may expose to a higher risk to develop type 2 diabetes (T2D) and its cardiovascular burden. Lifestyle modifications might have considerable benefits on ameliorating metabolic status. Alternative biomarkers, such as circulating miR-21, has been recently discovered associated with dysglycemia. Here we evaluated, in a longitudinal cohort of dysglycemic population the relation between the circulating miR-21/ROS/HNE levels and the habit-intervention (HI) after 1 year of follow-up.
Methods
1506 subjects from DIAPASON study were screened based on the Findrisc score. Of them, 531 subjects with Findrisc ≥ 9 were selected for dysglycemia (ADA criteria) and tested for circulating miR-21, ROS and HNE levels, as damaging-axis. 207 subjects with dysglycemia were re-evaluated after 1-year of habit intervention (HI). Repeated measures tests were used to evaluate changes from baseline to 1-year of follow-up. The associations between glycemic parameters and miR-21/ROS/HNE were implemented by linear regression and logistic regression models.
Results
After HI, we observed a significant reduction of miR-21/ROS/HNE axis in dysglycemic subjects, concomitantly with ameliorating of metabolic parameters, including insulin resistance, BMI, microalbuminuria, reactive hyperemia index and skin fluorescence. Significant positive interaction was observed between miR-21 axis with glycaemic parameters after HI. Lower miR-21 levels after HI, strongly associated with a reduction of glycemic damaging-axis, in particular, within-subjects with values of 2hPG < 200 mg/dL.
Conclusions
Our findings demonstrated that HI influenced the epigenetic changes related to miR-21 axis, and sustain the concept of reversibility from dysglycemia. These data support the usefulness of novel biological approaches for monitoring glycemia as well as provide a screening tool for preventive programmes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01465-0.
Keywords: Dysglycemia, Prediabetes, microRNA-21, miR-21, ROS, Hydroxynonenal, Habit-intervention, Mediterranean diet, Glucose monitoring
Introduction
Dysglycemia or prediabetes is associated with cardiovascular risk [1] and is mostly described for cardiovascular mortality [2]. Dysglycemia, belonging to the spectrum of glucose abnormalities, arises silently and years before the clinical manifestation [3]; it includes isolated impaired fasting glucose (IFG) and/or isolated impaired glucose tolerance (IGT), exposing people to the risk of type 2 diabetes (T2D) development. Glucose intolerance is detected using a 75-g glucose load test where IGT is defined as a 2-h plasma glucose (2hPG) value of 140–199 mg/dL (7.7–11.1 mmol/L), or IFG of 100–125 mg/dL, or HbA1c of 5.7–6.4% (39–46 mmol/mol) [4, 5]. Recently, the measurements of 1hPG are emerging to define the prediabetic and diabetic status [6, 7] although the consensus on the diagnostic definition of pre-diabetes is still debated [8].
Prediabetes is considered a target for lifestyle intervention in virtue of its peculiarity of “multifactorial and heterogeneous condition” characterized by an asymptomatic transitional state of hyperglycaemia. Therefore, prediabetes—sharing with T2D insulin resistance (IR), over-weight and obesity, which are modifiable risk factors for other cardiometabolic risk factors, such as hypertension [9]—is more liable to a higher risk of the development of cardiovascular disorders [10].
In light of this scenario, preventive programs are focusing on the modification of lifestyle, that notoriously decreases the incidence of T2D, to reduce the risk and delay T2D occurrence and its burden: Diabetes Prevention Program (DPP) recommended healthy habits (diet and physical activity), avoiding smoking, alcohol, and stress as hints to reduce the risk to develop T2D. Intensive lifestyle intervention was able to reduce the incidence of T2D by 58% over 3 years [11, 12].
In the last decade, alternative preventive strategies have focused on genetic approaches, through the investigations on SPNs, to identify loci susceptibility in assessing the risk to develop T2D. However, a strict interaction between unhealthy diet and genetic predisposition was established in cancer [13], but there are inconclusive results about the interactions among common genetic variants, Western diet and lifestyle risk factors on the risk to develop T2D [14]. On the other hand, environmental stimuli, nutritional factors, inflammation, hypoxia, arterial pressure, sex, age, and lifestyle can force changes in epigenetic factors, explaining the heterogeneous phenotypes related to complex and multifactorial diseases, such as dysglycemia. It has been known that dysregulation of epigenetic processes has important consequences for the pathogenesis of T2D and vascular complications [15, 16]. micro-RNAs (miRNAs or miRs), belonging to the epigenetic cluster, may contribute to ascertaining the main mechanisms for dysglycemia and provide a novel tool for a precision preventive strategy. miRNAs, known as highly conserved non-coding RNAs of 25 nucleotides in length, are important forms of biological regulation involving the control of gene expression that may be potentially reversible without altering the DNA sequence [17] because is a process that occurs on the post-transcriptional level. In virtue of their association with the extracellular vesicles (EVs), or carrier proteins, miRNAs may be up-taken by recipient cells influencing their functions [18], and confer high stability in the circulation, making them as biomarkers for T2D, for insulin metabolism, glucose homeostasis [19], and diabetes complications [20, 21]. Current evidence has demonstrated that miRs may predict the progression from pre-diabetic to diabetes state [22]. Many studies reported that glucose, as a nutrient, was able to influence cellular oxidative milieu [23] and miRs expression [24]. Indeed, a particular diet may change the responsiveness of several endogenous miRs in animals [25, 26].
Our previous works demonstrated the ability of the glucose-sensing miR-21 to predict ROS (reactive oxygen species)-mediated damage in prediabetic and T2D subjects, suggesting a role of miR-21 as a novel approach for monitoring glucose, and their relative damage [27, 28].
In this study, we sought to evaluate whether miR-21 damaging-axis would be downregulated by habit intervention (HI, mostly composed by Mediterranean Diet) after one year of follow-up (in high-risk subjects that at baseline showed dysglycemia and a ROS induced damage) delineating with a good approximation a novel mechanism for monitoring damage glycemia-induced.
Research design and methods
Participants and setting
The longitudinal cohort of the DIAPASON (Diabetes Prediction And Screening Observational) Study—a diabetes prevention programme—was used for estimating prediabetes by a procedure based on the Finnish Diabetes Risk Score (FINDRISC) [29]. 1506 participants were selected based on eligible criteria by general practitioners (GP) of Milan and invited for signed informed consent before a laboratory screening. Age of 40–75 years and Findrisc score ≥ 9 were the eligibility criteria adopted [30]. Dysglycemia condition was defined by American Diabetes Association (ADA) as at least one of the glycemic parameters, FPG, 2hPG and HbA1C (respectively, > of 5.6 mmo/L (100 mg/dL), 7.8 mmol/L (140 mg/dL) and 5.7% (39 mmol/mol) [5]). Skin intrinsic fluorescence (SIF) was measured as autofluorescence in human skin with the use of an AGE-Reader (DiagnOptics Technologies BV), that can estimate the accumulation of AGE (advanced glycated end products) in the skin. SIF was determined from the ratio between the emission and reflected excitation light (ratio between the light intensity reflected in the 420–600 nm wavelength range and the light intensity in the 300–420 wavelength) using the AGE-Reader software. EndoPAT 2000 (Itamar Medical, Israel) evaluates acute changes in endothelial function, expressed as RHI (reactive hyperemia index). Subjects were in the supine position for 20 min before measurements, in a quiet, temperature-controlled room with dimmed lights. Each recording consisted of 5 min of baseline/5 min of occlusion/5 min post-occlusion measurement (hyperemic period). Occlusion of the brachial artery was performed on the non-dominant upper arm. The occlusion pressure was at least 60 mmHg above the systolic blood pressure (minimally 200 mmHg, and maximally 300 mmHg).
The DIAPASON protocol was approved by the institutional review boards of the IRCCS MultiMedica [protocol number 24/2012(153)]. Participants were recruited between January 2013 and February 2017. All subjects gave written informed consent.
Selection criteria for habit-intervention (HI) protocol
According to the FINDRISC questionnaire, at baseline, all subjects were at high risk of developing T2D. 207 subjects that at baseline showed at least one glycemic diagnostic (preferentially based on HbA1c) parameter altered was encouraged to correct lifestyle.
A diet was assigned according to the Mediterranean diet (MedDiet) scheme (50–55% carbohydrates, 15–20% proteins, 25–30% lipids) with the caloric quantity chosen for each subject based on the basal metabolic rate calculated with Harris & Benedict formulas [31].
Selection criteria for miR-21 responder group characterization
We selected as Responders group (R) all subjects that, after HI, showed lower miR-21 plasmatic levels than baseline. Subjects (16%) with no changes in miR-21 levels after HI were classified as the Non-Responders group (NR).
Plasma separation and laboratory testing
Peripheral blood sample (5 mL) was extracted in a tube with ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, at room temperature, and centrifuged at 3000 r/min for 10 min. Serum and plasma were separated as routine assessments. Fasting plasma glucose (FPG) was detected by the Slein method using Siemens analyser (Germany); oral glucose tolerance test (OGTT, 75 gr glucose in 300 mL) was used to assess the 2hPG and 1hPG values. Triacylglycerol and total cholesterol were measured using an automated enzymatic colourimetric test (Siemens). HbA1c was detected by a high-performance liquid chromatography automated system (Tosoh, Japan). Insulinemia levels were detected by a Centaur XP analyzer (Siemens). HOMA-IR was calculated by the formula ‘FPG (mg/dL) × fasting insulin (uU/mL)/405’. m-ALB was detected in urine samples previously centrifuged for 10 min at 3000×g to avoid cellular debris using an IMMAGE instrument (Beckman Coulter).
RNA extraction and miRNA determination with real-time PCR analysis
100 μL of plasma was processed for total RNA extraction using an RNA purification kit (NorgenBiotek, Thorold, ON, Canada) following the manufacturer’s instructions. Plasma was centrifuged at 13,000g for 5 min at 4 °C to avoid platelets interferences. Before RNA extraction, 5 μL of cel-miR-39 [(synthetic Caenorhabditis elegans-miR-39), purchased by Applied Biosystems, Life Technologies, Grand Island, NY, USA] was spiked into the plasma to ensure efficiency RNA recovery. The TaqMan MicroRNA Reverse Transcription Kit (Thermo-Fisher) was used to reverse-transcribe miRNAs as recommended by the manufacturer. Real-time qPCR for plasmatic measurements of miR-21 levels was performed with a QuantStudio 6 flex (Applied Biosystems, Foster City, CA, USA) detection system. Data were obtained as Ct values, and the 2 − ΔCt method was applied for analysis of miRNA expression levels, normalizing with external cel-miR-39. In addition, to assess the hemolysis of the samples the ratio between absorbance at 414 nm and 375 nm was calculated [32].
Determination of ROS by electron paramagnetic resonance (EPR)
ROS generation was detected in sera by X band-EPR spectroscopy (E-scan—Bruker BioSpin, GmbH, MA USA) using the EPR method [33, 34]. Sera were incubated with 1 mM CMH (1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrroline) probe prepared in buffer (Krebs-Hepes buffer (KHB) containing 25 μM deferoxamine methane-sulfonate salt (DF) chelating agent and 5 μM sodium diethyldiothio-carbamate trihydrate (DETC) at pH 7.4). An external reference, stable radical CP· (3-Carboxy2,2,5,5-tetramethyl-1-pyrrolidinyloxy) was used to convert ROS determinations in absolute quantitative values (μmol.min−1). All spectra recorded were analysed by standard software (Win EPR 2.11, Bruker).
Determinations of plasmatic 4-hydroxynonenal (HNE) and asymmetrical dimethylarginine (ADMA)
One hundred μL of plasma samples in duplicate were used to analyse HNE-protein adduct concentrations with a commercially available immunoassay kit following the manufacturer’s instructions (Oxiselect, Cell Labs, San Diego, CA, USA). One hundred μL of plasma samples in duplicate were used to analyse asymmetrical dimethylarginine (ADMA) concentration with a commercially available immunoassay kit following the manufacturer’s instructions (Cusabio, Houston, TX, USA).
Statistical analysis
Data were presented as mean ± standard deviation and compared between baseline and after 1 year of follow-up by using the Wilcoxon Signed-Rank test or paired T-test, as appropriate. Comparisons between sex were analysed by T-test or non-parametric Wilcoxon test, as appropriate. The correlation matrix for miR-21/ROS/HNE and other collected variables at follow-up was evaluated. Logistic regression models to evaluate the association between R group and each glycaemic parameter were performed and receiver operating characteristic (ROC) curves were drawn. For each model odds ratio (OR) with 95% CI (confidence interval) and area under the curve (AUC) were calculated. To evaluate the association between ROS/miR-21 values and glycaemic parameters, linear regression models were implemented. All statistical analyses were performed with SAS Software 9.4 and graphs were drawn by GraphPrism v7. P-values < 0.05 were considered statistically significant for all analyses.
Results
Healthy habit intervention (HI) positively influences cardiometabolic traits
Participants at baseline (N = 531) with a moderate/high risk to develop T2D, calculated by Findrisc score ≥ 9, and with at least one of glycemic diagnostic criteria impaired, were encouraged to achieve weight loss and reduce waist circumference, known risk factors for cardiometabolic diseases, recommending a combination of the Mediterranean diet with behavioural support. At 1 year of follow-up, N = 207 subjects were re-evaluated.
Characteristics of subjects
At baseline the cohort had a mean age of 59.7 (± 8.8) years, 57.4% were women. 37.5% of the participants were at slightly elevated risk (FINDRISC 9–11).
The analysis for repeated measures (Table 1) showed significant changes between baseline and after HI 1 year later: overall, BMI was reduced (p = 0.004) and the trend was more evident in women than in men; waist circumference was reduced (p < 0.0001) for both women and men significantly. Also, we notice SIF (p = 0.005) and HbA1C reduction (p < 0.0001), although other glycemic parameters showed a tendency to reduce their values but unreached significance. The increase of HDL is significant, whereas insulinemia, HOMA-IR and micro-albuminuria (m-ALB) were reduced (p < 0.0001, p < 0.0001 and p = 0.04, respectively). Regarding endothelial function measured by EndoPAT, reactive hyperemic index (RHI) showed reduced values (p = 0.015). Augmentation index normalized to 75 bpm (%) as the index of arterial stiffness, showed a tendency to decrease but unreached significance, together with the reduction of heart rate variability (HRV) and asymmetrical dimethylarginine (ADMA), an endogenous competitive inhibitor of nitric oxide (NO) synthesis, used for assessment of endothelial dysfunction (ED) [35].
Table 1.
Characteristics of participants
| Baseline | HI 1-yr | N paired | p-value | |
|---|---|---|---|---|
| Age | 59.7 ± 8.8 | |||
| Women (%) | 57.4 | 55.6 | ||
| DBP (mm Hg) | 76.2 ± 11.5 | 76.5 ± 11.4 | 204 | 0.6627 |
| SBP (mm Hg) | 128.8 ± 15.4 | 129.4 ± 14.2 | 204 | 0.6412 |
| Cardiac frequency (bpm) | 70.3 ± 8.1 | 70.6 ± 8.2 | 201 | 0.9933 |
| BMI (Kg/m2) | 27.5 ± 4.3 | 27.2 ± 4.2 | 203 | 0.0040 |
| Woman | 27.3 ± 4.9 | 26.9 ± 4.9 | 113 | 0.0177 |
| Man | 27.8 ± 3.4 | 27.5 ± 3.2 | 90 | 0.0928 |
| Waist circumference (cm) | 97.6 ± 10.2 | 95.3 ± 10.2 | 195 | < .0001 |
| Woman | 95.0 ± 10.4 | 93.3 ± 10.9 | 108 | 0.0127 |
| Man | 100.9 ± 9.1 | 97.7 ± 8.8 | 87 | < .0001 |
| RHI (a.u.) | 2.41 ± 0.76 | 2.27 ± 0.68 | 189 | 0.0152* |
| AI (75 bpm%) | 22.1 ± 19.9 | 21.7 ± 19.8 | 191 | 0.6636 |
| HRV (a.u.) | 39.3 ± 36.2 | 35.1 ± 17.6 | 188 | 0.5246 |
| SIF (fluor. unit) | 2.26 ± 0.47 | 2.15 ± 0.46 | 196 | 0.0059 |
| FPG (mg/dL) | 95.1 ± 14.0 | 94.5 ± 15.7 | 204 | 0.3941 |
| 1hPG (mg/dL) | 170.9 ± 45.6 | 169.7 ± 61.1 | 196 | 0.7554* |
| 2hPG (mg/dL) | 141.4 ± 49.8 | 137.6 ± 48.9 | 201 | 0.1334* |
| HbA1C (%) | 6.2 ± 0.4 | 6.0 ± 0.4 | 201 | < .0001 |
| HbA1C (mmol) | 44.7 ± 4.6 | 42.1 ± 4.2 | 204 | < .0001 |
| Col (mg/dL) | 207.7 ± 37.4 | 206.5 ± 36.7 | 204 | 0.9169 |
| HDL (mg/dL) | 56.0 ± 14.9 | 58.6 ± 16.2 | 207 | < .0001 |
| TAG (mg/dL) | 120.6 ± 65.0 | 116.7 ± 53 | 207 | 0.8022 |
| LDL (mg/dL) | 127.8 ± 32.5 | 124.8 ± 31.2 | 207 | 0.2645 |
| INS (mIU/L) | 19.5 ± 26.4 | 13.5 ± 11.6 | 200 | < .0001 |
| HOMA-IR | 4.7 ± 6.2 | 3.3 ± 3.4 | 195 | < .0001 |
| m-ALB (mg/dL) | 15.0 ± 34.5 | 11.7 ± 16.2 | 184 | 0.0385 |
| mir-21 (a.u) | 0.035 ± 0.039 | 0.011 ± 0.022 | 116 | < .0001 |
| HNE (ug/mL) | 9.1 ± 9.2 | 6.5 ± 5.8 | 130 | < .0001 |
| ADMA (ng/mL) | 51.7 ± 50.9 | 45.5 ± 24.5 | 85 | 0.3391 |
| ROS (umol/min) | 0.204 ± 0.017 | 0.195 ± 0.020 | 48 | 0.0285* |
N = 207 subjects of the N = 531 enrolled in the DIAPASON study were re-evaluated after 1 year of follow-up under healthy-habit intervention
miR-21: circulating microRNA-21; HI 1-yr: habit intervention of 1 year; DBP: diastolic blood pressure; SBP: systolic blood pressure; BMI: body mass index; RHI: reactive hyperemia index; AI: augmentation index; HRV: heart rate variability; SIF: skin intrinsic fluorescence; FPG: fasting plasma glucose; 1hPG: 1-h plasma glucose; 2hPG: 2-h plasma glucose; HbA1C: glycated hemoglobin; Col: total cholesterol; HDL: high-density lipoprotein; TAG: triacylglycerol; LDL: low-density lipoprotein; INS: insulinemia; HOMA-IR: homeostatic model assessment for insulin resistance; m-ALB: microalbuminuria; HNE: hydroxynonenal; ADMA: asymmetrical dimethylarginine; ROS: reactive oxygen species
Wilcoxon Signed-Rank test; * Paired T-test
Effect of habit-changes on circulating miR-21/HNE/ROS axis 1 year later
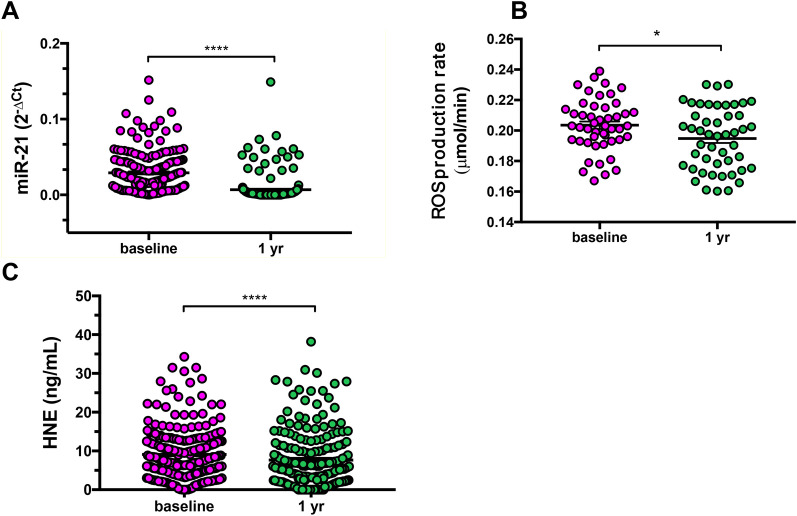
The subjects with higher risk at baseline, determined by Findrisc score, and with at least one of the glycemic criteria impaired, were re-evaluated 1 year after HI, in which subjects become aware of their risk and decided to change their habits ameliorating nutrition and avoid sedentarily. At baseline we found in dysglycemic subjects, increased miR-21/ROS/HNE levels, as reported in our previous work [28], suggesting regulation underlying epigenetic mechanisms. qPCR was used for miR-21 detection in the plasma of subjects (N = 116). Our data showed that the mean value of miR-21 plasma level was decreased after 1 year of HI (p < 0.0001) (Table 1; Fig. 1A), suggesting a strict relationship between miR-21 and glycaemic statuses.
Fig. 1.
miR-21 expression in plasma samples after 1 year of habit-intervention (HI). A Scatter dot plot of miR-21 expression at baseline and 1 year after habit-intervention in which miR-21 was normalised to cel-miR-39 expression using the comparative Ct method in a longitudinal cohort (at baseline, N = 531; at 1 year of HI, n = 207; paired miR21, n = 116). Wilcoxon Signed-Rank test for paired data, p < 0.0001. B Scatter dot plot of ROS extracellular release (measured by EPR instruments) in sera showing significant reduction after habit-intervention (ROS paired n = 48). Paired T-test, p = 0.03. C Scatter dot plot of plasma concentration of HNE (μg/mL) in the dysglycemic cohort (paired HNE n = 130). Wilcoxon Signed-Rank test for paired data, p < 0.0001. ****p < 0.0001; *p < 0.05.
Lifestyle changes reduce both oxidative stress response
Serum was used to detect ROS absolute concentration values by EPR (Fig. 1B), although it clouded the main site of ROS production. After 1-year of HI, the dysglycemic subjects had reduced ROS levels with significant differences compared to baseline (p = 0.03) (Table 1; Fig. 1B). To investigate the free radical-induced lipid damage occurring in plasma and the relative effectiveness of HI power in preventing this damage, we assessed the presence of specific end products of peroxidized lipid as HNE-protein adducts, at baseline and after HI 1 year later. However, the levels were significantly lower when we compared baseline and HI, unveiling a status of improved ROS/HNE-damage in dysglycemia pathogenesis (Table 1; Fig. 1C).
Interestingly, after adjustments of lifestyle, also the correlation analysis (Additional file 1: Table S1) revealed a significant positive association of miR-21 with glycaemic parameters, such as FPG, 1hPG and 2hPG, HbA1C (% and mmol/mol), HNE (rho = 0.2, p = 0.03) and ROS generation (rho = 0.5, p = 0.0002). ADMA showed lower levels after HI, but did not reach significance.
Effect of habit-changes on Responders group
Dysglycemic subjects who after HI significantly reduced miR-21 (classified as Responders, or R, Additional file 1: Table S1) exhibited the greatest metabolic changes (Table 2), compared with those miR-21 unchanged (Non-Responders, or NR) (Table 3). Intriguingly, in R group we found similar miR-21-values in cut-off for discriminating dysglycemic subjects as evaluated in our previous published results [28].
Table 2.
Characterization of the subjects with lower-miR-21 (Responders)
| Baseline | HI 1-yr | N paired | p-value | |
|---|---|---|---|---|
| DBP (mm Hg) | 76.5 ± 12.6 | 77.1 ± 11.7 | 97 | 0.6729 |
| SBP (mm Hg) | 129.8 ± 16.8 | 130.4 ± 14.1 | 97 | 0.7637* |
| Cardiac frequency (bpm) | 70.5 ± 8.0 | 70.8 ± 8.6 | 95 | 0.7702* |
| BMI (Kg/m2) | 27.5 ± 4.4 | 27.0 ± 4.0 | 96 | 0.0036 |
| Waist circumference (cm) | 97.2 ± 10.2 | 95.0 ± 9.4 | 91 | 0.0015* |
| RHI (a.u.) | 2.415 ± 0.756 | 2.197 ± 0.607 | 92 | 0.0275 |
| AI (75 bpm%) | 22.0 ± 19.6 | 20.2 ± 19.2 | 91 | 0.4471 |
| HRV (a.u.) | 43.1 ± 47.3 | 35.2 ± 19.3 | 90 | 0.1595 |
| SIF (fluor. unit) | 2.255 ± 0.393 | 2.206 ± 0.399 | 95 | 0.4586 |
| FPG (mg/dL) | 96.1 ± 12.3 | 94.0 ± 10.4 | 97 | 0.1924 |
| 1hPG (mg/dL) | 178.9 ± 41.8 | 174.7 ± 69.1 | 94 | 0.0080 |
| 2hPG (mg/dL) | 149.4 ± 42.6 | 138.3 ± 38.7 | 96 | 0.0540* |
| HbA1C (%) | 6.2 ± 0.4 | 6.0 ± 0.4 | 95 | < .0001 |
| HbA1C (mmol) | 44.6 ± 4.0 | 42.2 ± 4.1 | 97 | < .0001 |
| Col (mg/dL) | 206.1 ± 34.6 | 204.1 ± 37.1 | 97 | 0.5061* |
| HDL (mg/dL) | 54.0 ± 13.4 | 56.4 ± 15.2 | 97 | 0.0081 |
| TAG (mg/dL) | 123.3 ± 61.3 | 118.9 ± 47.5 | 97 | 0.9806 |
| LDL (mg/dL) | 127.4 ± 30.5 | 123.9 ± 32.3 | 97 | 0.2220* |
| INS (mIU/L) | 18.3 ± 21.1 | 14.1 ± 12.5 | 97 | 0.0007 |
| HOMA-IR | 4.4 ± 4.9 | 3.4 ± 3.3 | 96 | 0.0004 |
| m-ALB (mg/dL) | 14.9 ± 21.1 | 12.1 ± 15.7 | 83 | 0.0230 |
| HNE (ug/mL) | 8.2 ± 7.7 | 5.3 ± 4.1 | 95 | < .0001 |
| ADMA (ng/mL) | 47.5 ± 45.9 | 43.8 ± 22.2 | 62 | 0.1196 |
| ROS (umol/min) | 0.204 ± 0.017 | 0.19 ± 0.018 | 33 | 0.0045* |
| Women | ||||
| DBP (mm Hg) | 72.8 ± 11.4 | 75.4 ± 11.5 | 55 | 0.1999 |
| SBP (mm Hg) | 126.8 ± 17.0 | 130.7 ± 14.6 | 55 | 0.1471* |
| Cardiac frequency (bpm) | 72.4 ± 7.9 | 72.6 ± 9.3 | 53 | 0.9135* |
| BMI (Kg/m2) | 27.3 ± 4.9 | 26.7 ± 4.6 | 55 | 0.0337* |
| Waist circumference (cm) | 94.8 ± 10.1 | 93.1 ± 9.7 | 52 | 0.1170* |
| RHI (a.u.) | 2.555 ± 0.843 | 2.256 ± 0.649 | 52 | 0.0328 |
| AI (75 bpm%) | 27.3 ± 19.3 | 24.2 ± 18.1 | 52 | 0.1943* |
| HRV (a.u.) | 34.4 ± 20.1 | 33.3 ± 19.3 | 51 | 0.3973 |
| SIF (fluor. unit) | 2.296 ± 0.417 | 2.246 ± 0.369 | 54 | 0.9844 |
| FPG (mg/dL) | 93.4 ± 12.3 | 92.0 ± 10.4 | 55 | 0.6405 |
| 1hPG (mg/dL) | 178.0 ± 45.4 | 178.3 ± 88.4 | 52 | 0.0296 |
| 2hPG (mg/dL) | 145.5 ± 47.5 | 138.7 ± 41.8 | 54 | 0.1536* |
| HbA1C (%) | 6.3 ± 0.3 | 6.1 ± 0.4 | 54 | < .0001 |
| HbA1C (mmol) | 45.2 ± 3.6 | 42.7 ± 3.9 | 55 | < .0001 |
| Col (mg/dL) | 211.7 ± 35.7 | 211.1 ± 34.1 | 55 | 0.8807* |
| HDL (mg/dL) | 59.0 ± 13.1 | 62.3 ± 15.0 | 55 | 0.0058 |
| TAG (mg/dL) | 111.9 ± 47.8 | 113.9 ± 43.0 | 55 | 0.2337 |
| LDL (mg/dL) | 130.4 ± 29.9 | 126.0 ± 29.8 | 55 | 0.2092* |
| INS (mIU/L) | 16.8 ± 18.8 | 11.9 ± 8.7 | 55 | 0.0002 |
| HOMA-IR | 3.9 ± 4.0 | 2.7 ± 1.9 | 55 | 0.0002 |
| m-ALB (mg/dL) | 14.3 ± 22.5 | 10.5 ± 11.7 | 46 | 0.0955 |
| HNE (ug/mL) | 8.5 ± 6.6 | 6.0 ± 4.3 | 54 | 0.0001 |
| ADMA (ng/mL) | 45.5 ± 39.9 | 43.1 ± 24.1 | 32 | 0.1642 |
| ROS (umol/min) | 0.203 ± 0.021 | 0.19 ± 0.019 | 15 | 0.1366* |
| Men | ||||
| DBP (mm Hg) | 81.4 ± 12.5 | 79.4 ± 11.6 | 42 | 0.4353* |
| SBP (mm Hg) | 133.7 ± 15.9 | 130.0 ± 13.7 | 42 | 0.1945 |
| Cardiac frequency (bpm) | 68.0 ± 7.4 | 68.5 ± 7.3 | 42 | 0.7313* |
| BMI (Kg/m2) | 27.9 ± 3.6 | 27.5 ± 3.2 | 41 | 0.0691 |
| Waist circumference (cm) | 100.6 ± 9.5 | 97.5 ± 8.5 | 39 | 0.0006* |
| RHI (a.u.) | 2.233 ± 0.586 | 2.121 ± 0.547 | 40 | 0.3240* |
| AI (75 bpm%) | 14.8 ± 17.9 | 14.8 ± 19.4 | 39 | 0.9883* |
| HRV (a.u.) | 54.4 ± 67.0 | 37.8 ± 19.2 | 39 | 0.1983 |
| SIF (fluor. unit) | 2.20 ± 0.356 | 2.15 ± 0.435 | 41 | 0.2293 |
| FPG (mg/dL) | 99.7 ± 11.3 | 96.7 ± 9.8 | 42 | 0.1801 |
| 1hPG (mg/dL) | 180.1 ± 37.4 | 170.2 ± 32.6 | 42 | 0.0713* |
| 2hPG (mg/dL) | 154.5 ± 35.2 | 137.8 ± 34.7 | 42 | 0.0145* |
| HbA1C (%) | 6.2 ± 0.4 | 6.0 ± 0.4 | 41 | < .0001 |
| HbA1C (mmol) | 43.9 ± 4.5 | 41.6 ± 4.2 | 42 | < .0001 |
| Col (mg/dL) | 198.9 ± 32.2 | 194.9 ± 39.3 | 42 | 0.4210* |
| HDL (mg/dL) | 47.6 ± 10.9 | 48.6 ± 11.4 | 42 | 0.3664 |
| TAG (mg/dL) | 138.2 ± 73.4 | 125.4 ± 52.7 | 42 | 0.3812 |
| LDL (mg/dL) | 123.6 ± 31.3 | 121.3 ± 35.4 | 42 | 0.6266* |
| INS (mIU/L) | 20.2 ± 23.8 | 17.1 ± 15.9 | 42 | 0.2601 |
| HOMA-IR | 5.1 ± 5.9 | 4.2 ± 4.4 | 41 | 0.1684 |
| m-ALB (mg/dL) | 15.6 ± 19.5 | 14.0 ± 19.5 | 37 | 0.0991 |
| HNE (ug/mL) | 7.8 ± 9.0 | 4.4 ± 3.5 | 41 | < .0001 |
| ADMA (ng/mL) | 49.6 ± 52.0 | 44.6 ± 20.5 | 30 | 0.3911 |
| ROS (umol/min) | 0.204 ± 0.014 | 0.189 ± 0.018 | 18 | 0.0126* |
Subjects after HI were characterized for lower circulating levels of miR-21
miR-21: circulating microRNA-21; HI 1-yr: habit intervention of 1 year; DBP: diastolic blood pressure; SBP: systolic blood pressure; BMI: body mass index; RHI: reactive hyperemia index; AI: augmentation index; HRV: heart rate variability; SIF: skin intrinsic fluorescence; FPG: fasting plasma glucose; 1hPG: 1-h plasma glucose; 2hPG: 2-h plasma glucose; HbA1C: glycated hemoglobin; Col: total cholesterol; HDL: high-density lipoprotein; TAG: triacylglycerol; LDL: low-density lipoprotein; INS: insulinemia; HOMA-IR: homeostatic model assessment for insulin resistance; m-ALB: microalbuminuria; HNE: hydroxynonenal; ADMA: asymmetrical dimethylarginine; ROS: reactive oxygen species
Wilcoxon Signed-Rank test; * Paired T-test
Table 3.
Characteristics of NR group
| Baseline | HI 1-yr | N paired | p-value | |
|---|---|---|---|---|
| DBP (mm Hg) | 77.1 ± 10.8 | 79.2 ± 9.2 | 19 | 0.4982 |
| SBP (mm Hg) | 127.6 ± 15.7 | 136.8 ± 11.7 | 19 | 0.0429 |
| Cardiac frequency (bpm) | 68.9 ± 8.6 | 69.6 ± 7.1 | 18 | 0.5594 |
| BMI (Kg/m2) | 27.1 ± 3.5 | 27.1 ± 3.9 | 19 | 0.8933 |
| Waist circumference (cm) | 97.7 ± 9.4 | 96.7 ± 12.2 | 17 | 0.4736 |
| Woman | 93.6 ± 8.8 | 90.1 ± 13.3 | 7 | 0.2965 |
| Man | 100.6 ± 9.2 | 100.9 ± 9.8 | 10 | 0.9277 |
| RHI (a.u.) | 2.4 ± 0.7 | 2.4 ± 0.8 | 16 | 0.7715 |
| AI (75 bpm%) | 23.8 ± 20.2 | 20.7 ± 19.1 | 17 | 0.9573 |
| HRV (a.u.) | 37 ± 17.5 | 35.3 ± 16.2 | 17 | 0.7417 |
| SIF (fluor. unit) | 2.4 ± 0.6 | 2.2 ± 0.5 | 18 | 0.0459 |
| FPG (mg/dL) | 105.3 ± 16.7 | 106.1 ± 20.3 | 19 | 0.8142 |
| 1hPG (mg/dL) | 214.3 ± 46.4 | 228.6 ± 58.2 | 19 | 0.0745 |
| 2hPG (mg/dL) | 200.8 ± 77 | 221.4 ± 67.9 | 19 | 0.0916 |
| HbA1C (%) | 6.5 ± 0.6 | 6.3 ± 0.6 | 19 | 0.1114 |
| HbA1C (mmol) | 47.7 ± 6.7 | 45.8 ± 6.3 | 19 | 0.1059 |
| Col (mg/dL) | 202.8 ± 33.1 | 204.6 ± 42.4 | 19 | 0.8262 |
| HDL (mg/dL) | 52.4 ± 15.7 | 54.2 ± 16.6 | 19 | 0.3269 |
| TAG (mg/dL) | 117.6 ± 47.7 | 131.3 ± 71.2 | 19 | 0.2416 |
| LDL (mg/dL) | 126.9 ± 27.1 | 124.2 ± 30.8 | 19 | 0.6712 |
| INS (mIU/L) | 19.6 ± 23.4 | 13.5 ± 7.2 | 18 | 0.3750* |
| HOMA-IR | 5.3 ± 6.8 | 3.7 ± 2.1 | 17 | 0.4586* |
| m-ALB (mg/dL) | 38.9 ± 97.2 | 17.8 ± 32.8 | 17 | 0.0714* |
| HNE (ug/mL) | 10.1 ± 7.7 | 6.8 ± 5.1 | 17 | 0.1634 |
| ADMA (ng/mL) | 67.4 ± 69.9 | 46.1 ± 20.4 | 17 | 0.2435* |
| ROS (umol/min) | 0.20 ± 0.02 | 0.21 ± 0.02 | 14 | 0.8270 |
miR-21: circulating microRNA-21; DBP: diastolic blood pressure; SBP: systolic blood pressure; BMI: body mass index; RHI: reactive hyperemia index; AI: augmentation index; HRV: heart rate variability; SIF: skin intrinsic fluorescence; FPG: fasting plasma glucose; 1hPG: 1-h plasma glucose; 2hPG: 2-h plasma glucose; HbA1C: glycated hemoglobin; Col: total cholesterol; HDL: high-density lipoprotein; TAG: triacylglycerol; LDL: low density lipoprotein; INS: insulinemia; HOMA-IR: homeostatic model assessment for insulin resistance; m-ALB: microalbuminuria; HNE: hydroxynonenal; ROS: reactive oxygen species
T test.; * Wilcoxon test
The R subjects exhibited significantly reduced BMI, waist circumference (WC), RHI, 1hPG, HbA1c, INS, HOMA, m-ALB, HNE and ROS, but increased HDL (Table 2). No changes affected NR group (Table 3). When we detailed in R the changes from baseline to follow-up, accordingly with sex (Table 2), we noticed a reduction of BMI, RHI, insulinemia and HOMA in women, a weight loss more in women than men, which exhibited a reduction of WC, 2hPG and ROS, significantly. HbA1c and HNE levels are reduced for both, significantly; the changes in HNE are carried out more in men than in women.
Dysglycemia predicts miR-21 responsiveness after HI-1 year
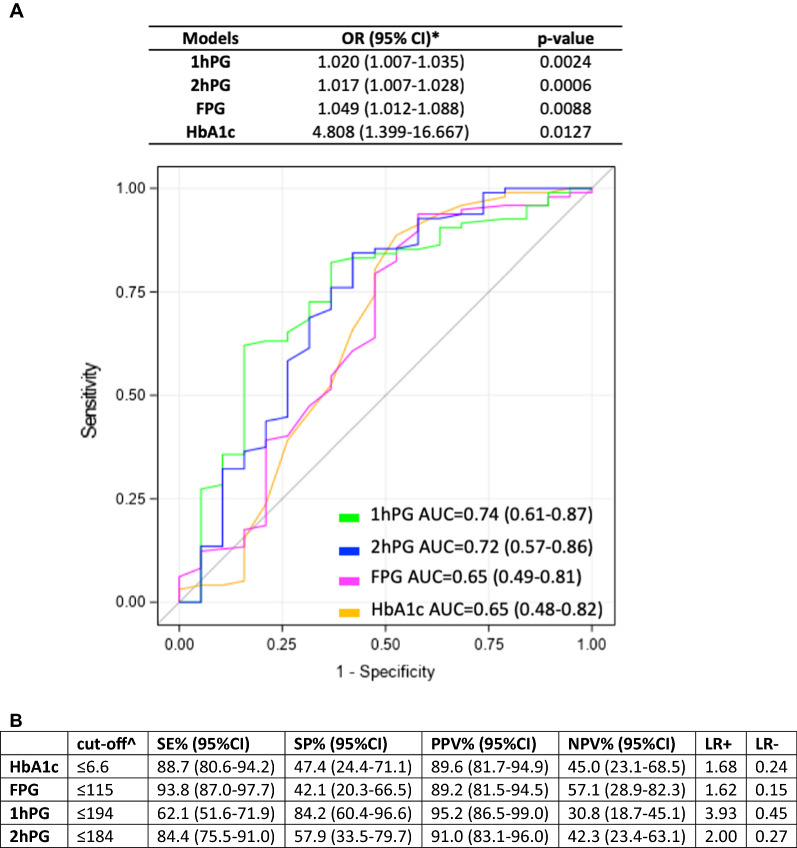
Logistic regression models and multiple ROC curves were drawn for the associations between glycemic parameters and the ability of the diet to reduce miR-21. We observed that for each unit-decrease in 1hPG and 2hPG we expected about 2% increased probability in discriminating Responder group (low-miR-21) after HI. In addition, for 1-unit decrease in HbA1c the probability in the discriminating Responder group is 4.8 times the probability of NR group. Best AUC was observed for 1hPG (0.74) (Fig. 2A), whereas best cut-offs of glycemic parameters in predicting miR-21 reduction and related diagnostic values (Fig. 2B).
Fig. 2.
ROC curves of glycaemic parameters models in detecting miR-21 responders (R). A For each glycaemic parameter, a logistic model was implemented to evaluate the association with R. ROC curves were represented. Yellow-line represents ROC curve for HbA1c model, magenta-line represents ROC curve for FPG model, green-line represents ROC curve for 1hPG model and blue-line represents ROC curve for 2hPG model in discriminating between R and NR. Logistic regression models to evaluate the associations between lower miR-21 levels (at 1-yr) and glycemic parameters (baseline) were performed for predicting the effectiveness of the diet and relatives lower glycemic levels. B Best cut-offs of glycemic parameters in predicting miR-21 reduction were calculated using Youden index. Diagnostic values as sensitivity, specificity, positive and negative predicted values and positive and negative likelihood ratio were also evaluated
In particular, the stratification of glycemic parameters (Table 4), categorized on ADA guidelines for prediabetes and diabetes, highlights that subjects with 2hPG ≥ 200 mg/dL have a minor probability to reduce miR-21 after HI (R group), versus 2hPG < 140 mg/dL or versus 2hPG 140–199 mg/dL. Lower values of FPG lead to a greater probability in discriminating R group if compared with higher values, although not significant (OR = 7.33; p = 0.0604). Moreover, values of HbA1c between 5.7 and 6.4% lead to almost 4-time greater probability in discriminating R group if compared with subjects with HbA1c ≥ 6.5 (p = 0.0128).
Table 4.
Univariable logistic models for the association between Responders and baseline glycemic parameters
| Model | OR (95% CI) | p-value |
|---|---|---|
| HbA1c | ||
| ≥ 6.5 | 1.00 (Ref.) | |
| 5.7–6.4 | 3.89 (1.33–11.32) | 0.0128 |
| < 5.7 | 0.80 (0.13–5.08) | 0.8130 |
| FPG | ||
| ≥ 126 | 1.00 (Ref.) | |
| 100–125 | 3.63 (0.44–29.92) | 0.2316 |
| < 100 | 7.33 (0.92–57.71) | 0.0604 |
| 2hPG | ||
| ≥ 200 | 1.00 (Ref.) | |
| 140–199 | 4.57 (1.38–15.11) | 0.0102 |
| < 140 | 6.00 (1.53–23.53) | 0.0127 |
OR: Odds Ratio; CI: confidence interval; HbA1C: glycated hemoglobin; FPG: fasting plasma glucose; 2hPG: 2-h plasma glucose; Ref: reference category
At linear regression analysis (Additional file 1: Table S3), all glycemic parameters showed a significant association with miR-21 values, with parameter estimates ranging from 0.005 to 1.7. Moreover, ROS was highly associated with 1hPG and 2hPG: for 0.01 unit decrease in ROS, we expected a decrease of 12.7 and 16.3 unit of 1hPG and 2hPG, respectively.
Discussion
Preventive programmes for the global containment of non-communicable diseases (NCD) are centred on the reversibility of dysglycemia, especially for diabetes and its cardiovascular burden, which the World Health Organization (WHO) and the Diabetes Prevention Program (DPP) estimated to be increasing.
In the present work, we sought to demonstrate whether epigenetic changes, based on miR-21 influence, may occur during a habit intervention, in which MedDiet structured by Harris & Benedict could be an eligible method to retain hyperglycemia.
Mediterranean diet (MedDiet)—largely based on plant foods and therefore includes a lot of fruits and vegetables, beans and pulses, nuts and seeds, whole grains and olive oil—notoriously demonstrated beneficial effects on intermediate markers of cardiovascular risk [36]. MedDiet can promote weight loss and improve blood glucose management in people with type 2 diabetes, in metabolic syndrome [37], on endothelial function in T2D [38, 39] and on its capacity for decreasing oxidative stress.
In this work, habit intervention (HI) was able to reduce oxidative stress and the relative axis miR-21/ROS/HNE, interconnected each other as demonstrated by the correlation matrix (Additional file 1: Table S1). In recent work, we demonstrated that the increased glycemic levels are strongly associated with the increased levels of circulating parameters of vascular damage induced by oxidative stress [28]. Furthermore, in our published study about miRs effects on oxidative stress, we showed the protective effects of miR-21 silencing in an experimental model of endothelial cells exposed to glucose variability (GV): functional inhibition of miR-21 lead to upregulation of antioxidant genes, inhibited by GV [27]. The functional over-expression of miR-21 determined the inhibition of ROS-homeostasis target genes, e.g. Krev/Rap1 interaction trapped-1 (KRIT1), Foxo1 and some genes related to antioxidant response, such as Nuclear Erythroid 2 related factor-2 (NRF2) and superoxide-dismutase-2 (SOD2), explaining the causal link between ROS formation and miR-21 [27].
For the first time, in the present work, we noticed that the subjects with a lower-miR-21 (R group) phenotype in HI, besides reducing glycemia significantly, showed lower ROS and HNE levels than baseline. In addition, lower-miR-21 (R group) phenotype in HI exhibits an ameliorating endothelial function expressed as reactive hyperemia index, measured by EndoPAT, reduced BMI and visceral fat, the latter measured as waist circumference. Thus, the HI composed preferentially by MedDiet scheme (50–55% carbohydrates, 15–20% proteins, 25–30% lipids) was able to influence crucial epigenetic mechanisms that in hyperglycemia perpetuate the damage, making the phenomenon reversible. Indeed, it is notorious that hyperglycemia has central trigger factors been recognized as high and prolonged oxidative stress within tissues [40, 41]. The role of miRs in nutritional therapies has been assessed making them novel markers for a successful diet at the molecular level in particular for the prevention of the risk to the development of cardiovascular disease and also for preventing T2D development in subjects with CVD [42].
Gender differences also have been found in our data. The metabolic changes observed in our work during HI concerning baseline can be included in a pathway of genetic influences. In lower-miR-21 (R group) phenotype, women showed a reduction of glycemic parameters, insulinemia and HOMA, and a higher weight loss than in men, which exhibited a reduction in waist circumference (Table 2), mostly attributed to the effects of sex hormones on metabolism [43]. Recent literature evidenced that BMI reflects the major influence of genetic variants than epigenetics: in T2D, polymorphisms on the gene FTO (fat mass and obesity-associated), a gene associated with the common forms of obesity [44], IR in T2D [45], modulating insulin activity and BMI [46], seems to be dependent on the diet [47]. FTO regulates the amount of fat deposition and affects T2D risk through its effect on BMI; indeed, people homozygous for a particular FTO allele weighed about 3 kg more and had a 1.6-fold greater rate of obesity than those who had not inherited this trait.
BMI is less accurate for assessing healthy weight in some groups of people because it does not distinguish between the proportion of weight due to fat or muscle. Waist circumference is a better estimate of visceral fat, become therefore a more accurate predictor of cardiovascular risk, type 2 diabetes in men and metabolic syndrome.
In this study, we revealed putative pathogenic mechanisms exploring an epigenetic approach miR-based to evaluate the effective changes that occurred in lifestyle, measuring plasmatic levels of miR-21 axis, that globally reflects the ROS damage index. Since dysglycemic conditions may remain undetected for many years while silently promoting disease progression and cardiovascular events, a parallel analysis of miRNA values may be helpful for the predictive power over time.
Circulating miR-21 levels are still under-characterized in the diabetic population due to different methods of standardization applied among the populations.
The MIR21 gene, located in TMEM49 gene on chromosome 17q23.2 [48], has been well characterized in many complications diabetes-related, such as diabetic retinopathy [49], kidney fibrosis [50], diabetic nephropathy [19], and insulin resistance (IR) [19]. cardiovascular disease [51], and atherosclerotic plaques [52]. Furthermore, the miR-21 exerts its deleterious effects also on adipose tissues of subjects with diabetes and obesity [53].
We aimed to develop a miRNA-based method as a predictor of worse outcomes in a high-risk population to develop diabetes. Our findings showed the ability of basal glycemic levels to predict miR-21 levels after 1-year of HI, allowing to select of people eligible for habit-intervention efficacy.
All these findings allow us to assert that controlling also plasmatic miR21 levels might ameliorate the deterioration of insulin-resistance that occurs within the pathophysiologic progression towards diabetes. As HbA1c predicts the risk of developing complications, we sought correlations between miR-21 and HbA1c; a positive correlation was found between these factors after HI, suggesting a link to long-term tissue damage. Highlighting these findings, we posit that miR-21 could be an early index of metabolic derangements.
In our results we show that ROS production, quantitatively measured by EPR, the only technique capable of returning ‘intrinsic’ quantitative information of free radical levels [34] that provides absolute quantification of ROS, was reduced after HI. As reported in our previous work, miR-21 could be an important modulator of ROS homeostasis and antioxidant pathways, and defective antioxidant responses are one of the major causes of cellular damage [24]. In diabetes, hyperglycaemia often inhibits the defensive machinery [28, 54] accompanied by increased lipid peroxidation and reduced endogenous antioxidant levels in diabetic patients compared to controls [55, 56].
We showed that miR-21 affects also the index of insulin resistance, which may affect the mitochondrial dysfunction observed in previous studies [27]. During insulin resistance (IR), glucose metabolism converges in mitochondria via insulin signalling causing diabetes progression [57]. In our work, the ameliorating of IR found in our patients after HI might be explained as an improving of mitochondrial biogenesis and functions.
Interestingly, in this work, we noticed reduced levels of lipid peroxidation adducts (HNE) in plasma after HI. The abrogation of the downstream effects of dysglycemia could be attributable to MedDiet because of the reach of antioxidants properties.
This study demonstrated a strong interaction between MedDiet and miR-21 damaging-axis on glycemia after 1-year follow-up. The significant occurrence of reduced glycemia in Responders group (lower-miR-21 phenotype) may be explained by a higher adherence to MedDiet, than the NR group.
Altogether, our data strongly suggest that miR-21 could be considered a predictor of ROS damage before the onset of diabetes, in virtue of its reduction after a habit intervention. These elements strongly argue in favour of using circulating miR-21 as a screening tool in preventive initiatives.
Conclusions
In conclusion, this work demonstrated the ability of the lifestyle changes in ameliorating cardiometabolic traits as well as damaging factors such as miR-21/ROS/HNE axis, clearly demonstrating the huge potentiality to reverse dysglycemia. Accordingly, reduced levels of miR-21 are associated with a reduced abundance of ROS and reduced HNE.
Overall, these findings suggest that an epigenetic approach is a feasible strategy for early detection of metabolic abnormalities and predict worse outcomes using miR-21/ROS/HNE axis as a preventive tool.
Strengths and limitations
The major strength is that the downregulation of miR-21 damage-axis is related to diet, to lower glycemia and weight loss, even if the size of the population is not wide enough.
The limitation of this study may be attributed to lack of adherence to diet (whom data are not annotated), with no predilection in the use of specific components and foods that reach in antioxidants and fibres.
Supplementary Information
Additional file 1: Table S1. Correlation matrix for miR-21, ROS and HNE after 1 year of HI and other variables. Table S2. MiR-21 values between Responders (R) and Non-Responders (NR) at baseline and after 1 year of HI. Table S3. Univariable linear regression models for the association between ROS or miR-21 and each glycemic parameter at 1-year follow-up after HI.
Acknowledgements
This paper is dedicated to the memory of our dear prof. Luigi Rossi Bernardi, who passed away († 16/12/2019) while this paper was being peer-reviewed. Prof. Luigi Rossi Bernardi took part in the design of the DIAPASON study.
Guarantors
Lucia La Sala and Livio Luzi are the guarantors of the work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors' contributions
LLS contributed to the conception and design of the study, analysis and interpretation of data, and wrote the manuscript. LLS and ET performed statistical analyses. SMS contributed to EPR acquisition and interpretation of the data; ACU contributed to clinical data acquisition; PS and IT critically revised the manuscript. ETr and LL contributed to the important intellectual content. All authors contributed to data interpretation and critical revision of the manuscript for important intellectual content and approved the final draft. All authors read and approved the final manuscript.
Funding
DIAPASON study was supported by the Fondazione Romeo ad Enrica Invernizzi – Milano. This research was supported by a research grant award from European Study for the Study of Diabetes (EFSD) as European Research Grant Award (EFSD/Sanofi programme, whose recipient was LLS) and by the Italian Ministry of Health “Ricerca Corrente” grant to IRCCS MultiMedica. The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, and approval of the manuscript, or the decision to submit the manuscript for publication.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Independent Institutional Ethics Committee of IRCCS MultiMedica, Milan (protocol code 24/2012(153)).
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest relevant to this article were reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: 16/12/2019
References
- 1.Emerging Risk Factors C. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117(10):1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja V, Aronen P, Pramodkumar TA, Looker H, Chetrit A, Bloigu AH, Juutilainen A, Bianchi C, La Sala L, Anjana RM, et al. Accuracy of 1-hour plasma glucose during the oral glucose tolerance test in diagnosis of type 2 diabetes in adults: a meta-analysis. Diabetes Care. 2021;44(4):1062–1069. doi: 10.2337/dc20-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Sala L, Tagliabue E, de Candia P, Prattichizzo F, Ceriello A. One-hour plasma glucose combined with skin autofluorescence identifies subjects with pre-diabetes: the DIAPASON study. BMJ Open Diabetes Res Care. 2020;8(1):e001331. doi: 10.1136/bmjdrc-2020-001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman M, Jagannathan R, Buysschaert M, Pareek M, Olsen MH, Nilsson PM, Medina JL, Roth J, Chetrit A, Groop L, et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes Metab Res Rev. 2018;34(5):e2992. doi: 10.1002/dmrr.2992. [DOI] [PubMed] [Google Scholar]
- 9.La Sala L, Tagliabue E, Vieira E, Pontiroli AE, Folli F. High plasma renin activity associates with obesity-related diabetes and arterial hypertension, and predicts persistent hypertension after bariatric surgery. Cardiovasc Diabetol. 2021;20(1):118. doi: 10.1186/s12933-021-01310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403. doi: 10.1016/S2213-8587(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research G: reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, Laakso M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 13.Potter JD. Nutrition and colorectal cancer. Cancer Causes Control. 1996;7(1):127–146. doi: 10.1007/BF00115644. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen R, Gibson R, Aresu M, Heard A, Chan Q, Evangelou E, Gao H, Elliott P, Frost G. Gene-diet quality interactions on haemoglobin A1c and type 2 diabetes risk: the Airwave Health Monitoring Study. Endocrinol Diabetes Metab. 2019;2(4):e00074. doi: 10.1002/edm2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.La Sala L, Crestani M, Garavelli S, de Candia P, Pontiroli AE. Does microRNA perturbation control the mechanisms linking obesity and diabetes? implications for cardiovascular risk. Int J Mol Sci. 2020;22(1):143. doi: 10.3390/ijms22010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato M, Castro NE, Natarajan R. MicroRNAs: potential mediators and biomarkers of diabetic complications. Free Radic Biol Med. 2013;64:85–94. doi: 10.1016/j.freeradbiomed.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien HY, Chen CY, Chiu YH, Lin YC, Li WC. Differential microRNA profiles predict diabetic nephropathy progression in Taiwan. Int J Med Sci. 2016;13(6):457–465. doi: 10.7150/ijms.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spinetti G, Fortunato O, Caporali A, Shantikumar S, Marchetti M, Meloni M, Descamps B, Floris I, Sangalli E, Vono R, et al. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ Res. 2013;112(2):335–346. doi: 10.1161/CIRCRESAHA.111.300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Candia P, Spinetti G, Specchia C, Sangalli E, La Sala L, Uccellatore A, Lupini S, Genovese S, Matarese G, Ceriello A. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS ONE. 2017;12(12):e0188980. doi: 10.1371/journal.pone.0188980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Sala L, Pujadas G, De Nigris V, Canivell S, Novials A, Genovese S, Ceriello A. Oscillating glucose and constant high glucose induce endoglin expression in endothelial cells: the role of oxidative stress. Acta Diabetol. 2015;52(3):505–512. doi: 10.1007/s00592-014-0670-3. [DOI] [PubMed] [Google Scholar]
- 24.La Sala L, Cattaneo M, De Nigris V, Pujadas G, Testa R, Bonfigli AR, Genovese S, Ceriello A. Oscillating glucose induces microRNA-185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc Diabetol. 2016;15:71. doi: 10.1186/s12933-016-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, Abderrahmani A, Regazzi R. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57(10):2728–2736. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrizas M, Brugnara L, Esteban Y, Gonzalez-Franquesa A, Canivell S, Murillo S, Gordillo-Bastidas E, Cusso R, Cadefau JA, Garcia-Roves PM, et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab. 2015;100(3):E407–415. doi: 10.1210/jc.2014-2574. [DOI] [PubMed] [Google Scholar]
- 27.La Sala L, Mrakic-Sposta S, Micheloni S, Prattichizzo F, Ceriello A. Glucose-sensing microRNA-21 disrupts ROS homeostasis and impairs antioxidant responses in cellular glucose variability. Cardiovasc Diabetol. 2018;17(1):105. doi: 10.1186/s12933-018-0748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Sala L, Mrakic-Sposta S, Tagliabue E, Prattichizzo F, Micheloni S, Sangalli E, Specchia C, Uccellatore AC, Lupini S, Spinetti G, et al. Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naive T2D. Cardiovasc Diabetol. 2019;18(1):18. doi: 10.1186/s12933-019-0824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomilehto J, Lindstrom J, Hellmich M, Lehmacher W, Westermeier T, Evers T, Bruckner A, Peltonen M, Qiao Q, Chiasson JL. Development and validation of a risk-score model for subjects with impaired glucose tolerance for the assessment of the risk of type 2 diabetes mellitus-The STOP-NIDDM risk-score. Diabetes Res Clin Pract. 2010;87(2):267–274. doi: 10.1016/j.diabres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Franciosi M, De Berardis G, Rossi MC, Sacco M, Belfiglio M, Pellegrini F, Tognoni G, Valentini M, Nicolucci A. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: the IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care. 2005;28(5):1187–1194. doi: 10.2337/diacare.28.5.1187. [DOI] [PubMed] [Google Scholar]
- 31.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 32.Kirschner MB, Edelman JJ, Kao SC, Vallely MP, van Zandwijk N, Reid G. The impact of hemolysis on cell-free microRNA biomarkers. Front Genet. 2013;4:94. doi: 10.3389/fgene.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mrakic-Sposta S, Gussoni M, Montorsi M, Porcelli S, Vezzoli A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxid Med Cell Longev. 2014;2014:306179. doi: 10.1155/2014/306179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrakic-Sposta S, Gussoni M, Montorsi M, Vezzoli A. Comment on Menzel et al. common and novel markers for measuring inflammation and oxidative stress ex vivo in research and clinical practice-which to use regarding disease outcomes? Antioxidants 2021, 10, 414. Antioxidants. 2021;10(6):836. doi: 10.3390/antiox10060836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98(18):1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 36.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 38.Ryan M, McInerney D, Owens D, Collins P, Johnson A, Tomkin GH. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM. 2000;93(2):85–91. doi: 10.1093/qjmed/93.2.85. [DOI] [PubMed] [Google Scholar]
- 39.Ceriello A, Esposito K, La Sala L, Pujadas G, De Nigris V, Testa R, Bucciarelli L, Rondinelli M, Genovese S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2014;13:140. doi: 10.1186/s12933-014-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120. doi: 10.1186/s12933-017-0604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimenez-Lucena R, Alcala-Diaz JF, Roncero-Ramos I, Lopez-Moreno J, Camargo A, Gomez-Delgado F, Quintana-Navarro GM, Vals-Delgado C, Rodriguez-Cantalejo F, Luque RM, et al. MiRNAs profile as biomarkers of nutritional therapy for the prevention of type 2 diabetes mellitus: from the CORDIOPREV study. Clin Nutr. 2020;40(3):1028–1038. doi: 10.1016/j.clnu.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 43.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 44.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8(9):657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 47.La Sala L, Pontiroli AE. Prevention of diabetes and cardiovascular disease in obesity. Int J Mol Sci. 2020;21(21):8178. doi: 10.3390/ijms21218178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Sala L, Micheloni S, De Nigris V, Prattichizzo F, Ceriello A. Novel insights into the regulation of miRNA transcriptional control: implications for T2D and related complications. Acta Diabetol. 2018;55(10):989–998. doi: 10.1007/s00592-018-1149-4. [DOI] [PubMed] [Google Scholar]
- 49.Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, Ding Y, Liu Q. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34(5):1733–1740. doi: 10.1159/000366374. [DOI] [PubMed] [Google Scholar]
- 50.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4(121):121ra118. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 52.Cao J, Zhang K, Zheng J, Dong R. MicroRNA-146a and -21 cooperate to regulate vascular smooth muscle cell proliferation via modulation of the Notch signaling pathway. Mol Med Rep. 2015;11(4):2889–2895. doi: 10.3892/mmr.2014.3107. [DOI] [PubMed] [Google Scholar]
- 53.Guglielmi V, D'Adamo M, Menghini R, Cardellini M, Gentileschi P, Federici M, Sbraccia P. MicroRNA 21 is up-regulated in adipose tissue of obese diabetic subjects. Nutr Healthy Aging. 2017;4(2):141–145. doi: 10.3233/NHA-160020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 55.Santini SA, Marra G, Giardina B, Cotroneo P, Mordente A, Martorana GE, Manto A, Ghirlanda G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46(11):1853–1858. doi: 10.2337/diab.46.11.1853. [DOI] [PubMed] [Google Scholar]
- 56.Mrakic-Sposta S, Vezzoli A, Maderna L, Gregorini F, Montorsi M, Moretti S, Greco F, Cova E, Gussoni M. R(+)-Thioctic acid effects on oxidative stress and peripheral neuropathy in Type II diabetic patients: preliminary results by electron paramagnetic resonance and electroneurography. Oxid Med Cell Longev. 2018;2018:1767265. doi: 10.1155/2018/1767265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Correlation matrix for miR-21, ROS and HNE after 1 year of HI and other variables. Table S2. MiR-21 values between Responders (R) and Non-Responders (NR) at baseline and after 1 year of HI. Table S3. Univariable linear regression models for the association between ROS or miR-21 and each glycemic parameter at 1-year follow-up after HI.
Data Availability Statement
Not applicable.