Abstract
The unique structure of the human eye as well as exposure of the eye directly to the environment renders it vulnerable to a number of uncommon infectious diseases caused by fungi and parasites. Host defenses directed against these microorganisms, once anatomical barriers are breached, are often insufficient to prevent loss of vision. Therefore, the timely identification and treatment of the involved microorganisms are paramount. The anatomy of the eye and its surrounding structures is presented with an emphasis upon the association of the anatomy with specific infection of fungi and parasites. For example, filamentous fungal infections of the eye are usually due to penetrating trauma by objects contaminated by vegetable matter of the cornea or globe or, by extension, of infection from adjacent paranasal sinuses. Fungal endophthalmitis and chorioretinitis, on the other hand, are usually the result of antecedent fungemia seeding the ocular tissue. Candida spp. are the most common cause of endogenous endophthalmitis, although initial infection with the dimorphic fungi may lead to infection and scarring of the chorioretina. Contact lens wear is associated with keratitis caused by yeasts, filamentous fungi, and Acanthamoebae spp. Most parasitic infections of the eye, however, arise following bloodborne carriage of the microorganism to the eye or adjacent structures.
This is a comprehensive review of the fungal and parasitic diseases of the eye. Numerous fungi and parasites infect the eye either by direct introduction through trauma or surgery, by extension from infected adjacent tissues, or by hematogenous dissemination to the eye. The majority of the clinically important species of fungi and parasites involved in eye infections are reviewed in this article. The fungi are discussed in relation to the anatomical part of the eye involved in disease, whereas parasites are discussed by the diseases they cause. Emphasis has been placed on literature published within this decade, but prior noteworthy reviews and case reports are included. A glossary of the ophthalmologic terms used is provided at the end of the paper (Appendix A). We suggest that the works of Beard and Quickert (26a) and Snell and Lemp (252a) be consulted as references concerning the anatomy of the eye.
ANATOMY OF THE EYE AND ITS RELATIONSHIP TO INFECTIOUS PROCESSES
Orbits, Their Soft Tissue Contents, and Adjacent Structures
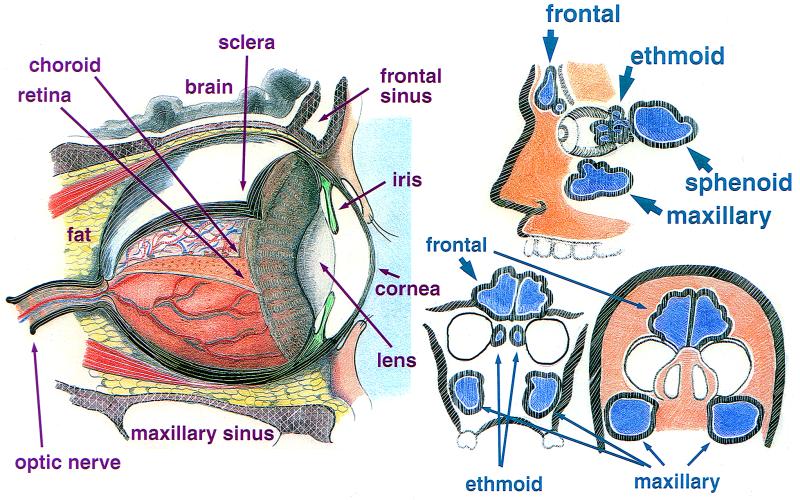
The orbits are pear-shaped bony cavities that contain the globes, extraocular muscles, nerves, fat and blood vessels (Fig. 1, left). The walls of the orbit are comprised of seven bones. The periosteal covering of the orbital bony cavity fuses anteriorly with the orbital septum and posteriorly with the dura mater. Abscesses can localize in the subperiosteal space. The roof, medial wall, and floor of the orbit separate it from adjacent paranasal sinuses, including the maxillary, frontal, ethmoid, and sphenoid sinuses. The paranasal sinuses arise from and drain into the nasal cavity. Thus, an intimate anatomical relationship exists between the orbit and the adjacent paranasal sinuses, and the latter may be the source of an orbital infection (Fig. 1, right).
FIG. 1.
(Left) The human eye in situ with the tunics peeled back, exposing a portion of the vasculature of the retina, lens, and anterior chamber as seen from the side. (Right) The relationship of the paranasal sinuses to the eye. The top figure shows a side view. (Bottom left) Relationship of the sinuses with the orbit as seen by coronal section just posterior to the bridge of the nose. (Bottom right) Relationship of the sinuses with the orbits with the head tilted backwards.
The thinnest bony walls of the orbit are the lamina papyracea, which cover the ethmoid sinuses. They are commonly involved in any fracture of the orbit from force to the periorbital area. As a result of fracture, sinus microbiota has direct ingress to the orbital tissues. Infections of the ethmoid sinus in children commonly extend through the lamina papyracea (without fracture), causing orbital cellulitis. The lateral wall of the sphenoid is also the medial wall of the optic canal. Therefore, infections of the sphenoid sinus may impinge on the optic nerve, resulting in visual loss or visual field abnormalities.
There are several important communications through apertures in the bony orbit to adjacent structures, including the superior and inferior orbital fissures, the lacrimal fossa and nasolacrimal duct, and the optic canal. These apertures may serve as a direct passage for an infectious process between the orbit and surrounding structures.
Blood Supply of the Orbits
The blood supply to the orbit is primarily through the ophthalmic artery and its branches. The majority of orbital venous drainage is via the superior ophthalmic vein, which courses through the superior fissure to the cavernous sinus. The cavernous sinus is a venous plexus located posterior to the apex of the orbit. As the primary venous system receiving orbital drainage, the cavernous sinus is susceptible to venous thrombosis secondary to direct intravascular extension of infection. Veins from the face and many anterior orbital veins anastamose and become tributaries of the superior orbital vein. Thus, facial infections may lead through these communications to infection of the cavernous sinus, which may be a lethal complication.
Eyelids and Lacrimal System
The eyelids possess two protective anatomical barriers preventing the penetration of pathogens beyond the anterior surface of the globe. The first is the orbital septum, a thin multilayer fibrous tissue that divides the orbit from the eyelid into preseptal and postseptal spaces and serves as a physical barrier to prevent infections from spreading posteriorly into orbital fat. The second is the conjunctiva that is reflected back on itself. This prevents material on the anterior surface of the globe from freely moving posteriorly along its surface.
The lacrimal system is comprised of the lacrimal gland, accessory glands, and the excretory system. The lacrimal gland secretes tears that pass down over the cornea and enter the lacrimal excretory system at the puncta. The puncta drains tears into the canalicular system that leads to the lacrimal sac. Tears in the lacrimal sac drain to the nose. The lacrimal system thus forms a direct passage from the anterior ocular adnexa to the nasal cavity. With total nasolacrimal duct obstruction, infected material in the sac may reflux onto the ocular surface.
The Globe, Including the Sclera and Choroid
The adult human eye is approximately 24 mm in anterior-posterior length and is 6 mm3 in volume. The basic structure of the globe consists of three concentric layers or tunics. The outermost tunic is comprised of the cornea and sclera. The middle tunic is the uveal tract. It consists of the choroid, ciliary body, and iris. The innermost tunic is the retina (Fig. 1, left).
The posterior outer layer of the globe is the sclera, which is comprised of collagen and ground substance. The scleral width ranges from 0.3 to 1.0 mm. The sclera is essentially avascular except for superficial episcleral vessels and the intrascleral vascular plexus. The choroid is a vascular tunic that comprises the posterior portion of the uveal tract. The purpose of this highly vascularized tissue is to provide nutritive support to the outer layer of the retina. The blood flow and oxygenation of the choroid are very high compared to the other tissues in the body. Because of these qualities, the choroid may serve as a fertile site for the proliferation of hematogenously spread pathogens.
Anterior Chamber, Aqueous Humor, Cornea, and Iris
The anterior chamber is a space bordered anteriorly by the cornea and posteriorly by the iris diaphragm and pupil and is filled with aqueous humor (Fig. 1, left). The aqueous humor, produced by nonpigmented ciliary epithelium in the posterior chamber, passes through the pupillary aperture into the anterior chamber, where it exits. The cornea is avascular, and its stroma is composed of highly organized collagen fibrils. A tear film comprised of three layers covers the anterior surface of the cornea.
The iris is the anterior extension of the ciliary body that forms a contractile diaphragm in front of the anterior surface of the lens. It separates the anterior and posterior chambers. The central aperture in the iris is the pupil, which constantly changes size in response to light intensity.
Posterior Chamber, Lens, and Vitreous Humor
The posterior chamber is bordered anteriorly by the iris diaphragm and pupil and posteriorly by the lens and zonules (Fig. 1, left). The lens is an avascular biconcave crystalline structure centrally located in the posterior chamber. It continues to grow throughout life, receiving nutrition from the aqueous and vitreous humors.
The vitreous is a gel-like substance occupying the posterior segment of the eye. It consists of a collagen framework interspersed with hyaluronic acid. In its normal state, it is optically clear, whereas during intraocular inflammation it may become hazy.
Retina and Optic Nerve
The retina is the innermost coat of the ocular tunics. It is a thin, transparent, net-like membrane that captures light energy. The retina is comprised of 10 layers, with the layer nearest the interior of the globe containing the photoreceptors called rods and cones. The inner half of the retina receives its blood supply from the central retinal artery, and the outer half receives its blood from the choroid.
The inner cell layer axons in the retina exit the globe to make up the optic nerve (Fig. 1, left). This nerve is surrounded by pia mater, arachnoid, and dura mater meningeal coverings, which are direct extensions from the cranial vault. The optic nerves are vulnerable to infectious processes originating both within the cranial vault and within the orbits.
OCULAR DEFENSE MECHANISMS
Anatomic Defenses
The surface of the eye is armed with mechanical and immunologic functions to defend itself against a hostile environment. The defense mechanisms are native and acquired, both generalized and specific (8). It is manifestly obvious that exposed portions of the eye possess a remarkable defense against microorganisms. To breach this defense, trauma in some form is usually required.
The eyelids provide mechanical protection of the ocular surface. The lashes initiate the blink reflex to protect against airborne particles or trauma. The cornea is exquisitely sensitive, and tactile stimulation of its surface will also initiate the blink reflex. The lids sweep over the anterior surface of the globe directing tears, debris, microbes, and allergens to the lacrimal excretory system. Lipids secreted by the meibomian glands maintain the stability of the tear film.
The nonkeratinized squamous epithelium of the conjunctiva and cornea serves as a protective anatomic barrier against pathogens. The basement membrane and cellular junctional complexes of the cornea contribute to its impermeability (184). Indigenous ocular flora of the lids and mucosal ocular surface serve a protective function by limiting the opportunity for pathogenic organisms to colonize the surface (89).
The vascular supply to the surface of the eye is a major conduit of the immune defenses. The ocular inflammatory response involves vascular dilation and exudation of immunologically active substances and cells, including macrophages, polymorphonuclear leukocytes, and lymphocytes (167).
Defenses of the Tear Film
The tear film is comprised of three layers: oil, aqueous, and mucous. These layers are produced by the meibomian glands, the lacrimal glands, and the goblet cells of the conjunctiva, respectively. The aqueous layer comprises the majority of the 7-μm-thick tear film. It is produced at a rate of ∼1 μl per min. The tear pH, ∼7.14 to ∼7.82, likely contributes to the neutralization of toxic substances (167). Tear flow mechanically bathes the anterior surface of the eye, preventing the adherence of microorganisms, and flushes allergens and foreign particles into the lacrimal excretory system. The mucous layer of the tear film entraps foreign material, which facilitates its removal (1). For example, the mucin contained in tears prevents Candida spp. from adhering to contact lenses, likely by entrapping the microorganisms (34). The tear film contains several immunologically active substances that participate in both general and specific ocular defense (Table 1).
TABLE 1.
Putative host defense components of human tears and their functions
| Tear component(s) | Function |
|---|---|
| Lactoferrin | Protein synthesized by the lacrimal gland; direct bacteriostatic action on bacteria |
| Lysozyme | Protein that targets the cell wall and causes bacteriolysis of gram-positive bacteria |
| β-Lysin | Causes cytolysis of bacteria resistant to lysozyme by targeting the cell membrane |
| Ceruloplasmin | Regulates damage to ocular tissue during inflammation |
| Complement | Promotes lysis of bacteria, chemotaxis, and phagocytosis in tears |
| Immunoglobulins | Human tears possess IgG, IgM, IgE, and IgA; IgA prevents the adherence of bacteria, modulates the normal flora, agglutinates bacteria, and can neutralize toxins |
Conjunctival Defense
Beneath the protective epithelium of the conjunctiva lie a vascular network and lymphoid structures. The conjunctiva-associated lymphoid tissue is subepithelial tissue packed with B and T lymphocytes. B-cell precursors mature when exposed to local antigen, proceed to regional lymph nodes where they transform into plasma cells, and then return via the bloodstream to the conjunctiva, where they produce their specific immunoglobulin A (IgA). Similarly, T-cell precursors are locally sensitized, travel to regional nodes, and then hematogenously return to the conjunctiva to provide cellular defense (46).
Corneal Response
The cornea is avascular and possesses limited immune defenses. The two main components are the Langerhans cells (dendritic cells), which modulate B and T lymphocyte activity in the cornea, and immunoglobulins, which are concentrated in the corneal stroma (167). The corneal surface is covered by a glycocalyx associated with a layer of mucous glycoprotein. A subtype of the IgA cross-links with the mucous glycoprotein to cover and protect the anterior surface of the cornea (184). Upon injury, the corneal epithelium may release a thymocyte-activating factor that incites a local immune response to include polymorphonuclear cells, lymphocytes, and fibroblasts (184).
Cellular Immune Response
Langerhans cells are concentrated in the epithelium of the peripheral cornea and conjunctiva but sparse in the central cornea (184). Like macrophages, they possess receptors for immunoglobulins, complement, and antigen. The Langerhans cell recognizes, phagocytizes, and processes certain antigens for presentation via the epithelial surface and stroma (167). Langerhans cells stimulate helper T and B cells that collaborate with other lymphocytes (killer, suppressor T cells) to enlist a strong cellular immune response. During inflammation Langerhans cells migrate toward the center of the cornea and may participate in the secretion or release of inflammatory mediator substances (105). T cells are mainly present in the conjunctival substantia propria, whereas B cells are more concentrated in the lacrimal gland (167).
Leukocyte Defense
Polymorphonuclear leukocytes possess the ability to ingest and kill microorganisms by two main pathways. The absence of polymorphonuclear leukocytes is associated with fungemia with Candida, Aspergillus, and Fusarium spp. The oxygen-dependent pathway is based on postphagocytic intracellular production of oxygen radicals (oxidants). The oxygen-independent pathway is based mainly on the function of antimicrobial proteins called defensins. Defensins are peptides that possess broad-spectrum antimicrobial activity in vitro, killing a variety of gram-positive and gram-negative bacteria and some fungi (167), including a wide range of ocular pathogens (60).
FUNGAL INFECTIONS OF THE EYE
Epidemiology of Fungal Eye Infections
Ophthalmologists and optometrists, in particular, and clinicians, in general, must be knowledgeable of the pathogenesis of fungal eye infections. Mycotic eye infections are commonplace. For example, the yeast Candida albicans is the most common cause of endogenous endophthalmitis. Filamentous fungi, such as Fusarium solani and Aspergillus flavus, may constitute up to one-third of all cases of traumatic infectious keratitis (157). Furthermore, patients with AIDS may contract many different fungal infections of the eye and adjacent structures (Table 2).
TABLE 2.
Fungal eye disease in patients with AIDS
| Location or nature of lesions | Fungus (reference[s]) |
|---|---|
| Eyelid nodules | Cryptococcus (51) |
| Conjunctivitis, colonization of the conjunctiva | Pneumocystis (220), Candida (112) |
| Cornea | Candida (17) |
| Anterior chamber, limbus | Cryptococcus (17, 47, 182), Histoplasma (88) |
| Choroiditis | Cryptococcus (227), Pneumocystis (90, 239, 241, 284), Histoplasma, Candida, Aspergillus (180a) |
| Retinitis | Cryptococcus, Histoplasma (255) |
| Endogenous endophthalmitis | Aspergillus (203), Fusarium (106), Bipolaris (199) |
| Optic neuropathy | Zygomycetes (154), Histoplasma (297), Cryptococcus (99, 107) |
| Sino-orbital disease | Aspergillus (130) |
In fungal eye disease, the pathogenesis of the infections is inextricably linked to the epidemiology. Therefore, in discussing the epidemiology of fungal eye infections, it is worthwhile at the outset to state several proposed pathogenetic principles of fungal eye disease. (i) It is likely that sustained fungemia with even saprophytic fungi will lead to endophthalmitis. (ii) At the time of initial infection with some of the dimorphic, pathogenic fungi, such as Histoplasma capsulatum and Coccidioides immitis, an unrecognized fungemia occurs and often leads to endophthalmitis. (iii) The paranasal sinuses, because of their direct communication with the ambient air, harbor saprophytic fungi, which may erode the bony walls of the sinus and invade the eye in certain circumstances, e.g., in a patient with neutropenia. (iv) Trauma, either from vegetable matter or surgery, may introduce saprophytic fungi into the cornea and/or adjacent tissue, giving rise to invasive disease.
The epidemiology of endogenous endophthalmitis reflects both the natural habitats of the involved fungi and the habits and health status of the patients (Table 3). Candida endogenous endophthalmitis occurs as a direct result of the success of modern medical practice that sustains patients' lives with broad-spectrum antibiotics, indwelling central venous lines, parenteral nutrition, abdominal surgery, and cytotoxic chemotherapy. The recent origin of this disease is established by the fact that Candida endophthalmitis was first recognized clinically in 1958 (275). Candida and Aspergillus spp. also cause endophthalmitis in intravenous drug users. Virtually any intravascular prosthesis or device may become contaminated by bloodborne opportunistic fungi, and fungemia arising from such infection may lead to endogenous endophthalmitis.
TABLE 3.
Epidemiological characteristics of major fungal eye infections
| Disease | Fungus | Patient characteristic(s) |
|---|---|---|
| Endogenous endophthalmitis | Candida | Intravenous catheters, broad-spectrum antibiotics, neutropenia, intravenous drug abuser |
| Aspergillus | Intravenous drug abuser, corticosteroid use for lung disease, immunocompromised persons | |
| H. capsulatum, B. dermatiditis, C. immitis | Residence in areas of endemicity | |
| Exogenous endophthalmitis | Candida, Paecilomyces | Postoperative infection after lens removal, lens implantation, or corneal transplant |
| Keratitis | Filamentous fungi, Fusarium, Aspergillus | Vegetable matter introduced into the cornea by trauma |
| Candida | Superimposed infection on an abnormal cornea, e.g., chronic corneal ulceration; prolonged use of topical corticosteroids or anesthetics |
Endogenous endophthalmitis occurring as part of disseminated disease with the dimorphic fungi H. capsulatum, Blastomyces dermatitidis, and C. immitis is uncommon. Patients with disease from these fungi have resided in or traveled through the respective areas of endemicity. These are the Ohio and Mississippi river valleys for H. capsulatum; the Lower Sonoran Life Zone for C. immitis, including southern parts of California, Arizona, New Mexico, West Texas, and parts of Mexico and Argentina; and the Southeast and Midwest of the United States for B. dermatitidis. Residence along a waterway may be another important association for exposure to B. dermatitidis (67). H. capsulatum may flourish in bird and bat droppings; therefore, exposure to the fungus may occur through one's occupation, for example, demolition of old bird-infested buildings, or one's hobby, such as camping or spelunking.
Exogenous endophthalmitis, on the other hand, results from trauma to the globe or preceding keratitis. It may also occur as a postoperative complication of lens removal, prosthetic lens implantation, or corneal transplantation. The vast majority of postoperative eye infections are due to coagulase-negative Staphylococcus; however, outbreaks of fungal exogenous endophthalmitis continue to occur episodically. These have been due to perioperative contamination of lens prostheses (204) or contamination of fluids used for irrigation (260) of the perioperative and postoperative eye. Candida species are particularly likely to occur in this setting, and infection may be enhanced by the pre- and postoperative use of topical corticosteroids and antibacterial agents.
Mycotic keratitis is usually caused by filamentous fungi and occurs in conjunction with trauma to the cornea with vegetable matter. In the tropics it is common in male agricultural workers. The fungal genera causing keratitis in the tropics are more diverse and include some, such as Lasiodiplodia theobromae, that do not grow in temperate regions. Eye trauma is the cause of fungal keratitis in temperate areas as well, but the common fungal genera involved are Fusarium, Alternaria, and Aspergillus (71, 293). Keratitis caused by yeasts such as the Candida spp. almost always occur in previously abnormal eyes, e.g., in patients with dry eye, chronic corneal ulceration, or corneal scarring.
Bloodborne Infections: Endogenous Endophthalmitis
Endogenous endophthalmitis is uncommon; however, fungi cause this disease more often than gram-positive or gram-negative bacteria. The term endogenous endophthalmitis implies that bloodborne spread of microorganisms to the eye has occurred. Therefore, infection in the eye is the result of metastatic spread of infection from a distant site, for example, infected heart valves or the urinary tract. In this manner the eye becomes the site of numerous microabscesses. This mechanism of infection is to be contrasted to exogenous endophthalmitis (see below), which arises from the direct introduction of a microorganism(s) into the eye during trauma or surgery. Endogenous endophthalmitis is further distinguished from exogenous endophthalmitis by occurring in a greater number of immunocompromised patients, e.g., patients receiving chemotherapy or total parenteral nutrition, or intravenous drug abusers (Table 4).
TABLE 4.
Risk factors for endogenous and exogenous endophthalmitis
| Disease and fungus | Risk factors or comments |
|---|---|
| Endogenous endophthalmitis | |
| C. albicans | Central venous lines, neutropenia, abdominal surgery, intravenous drug abuse, broad-spectrum antibiotics |
| Candida species | Central venous lines, neutropenia, abdominal surgery, intravenous drug abuse, broad-spectrum antibiotics |
| Aspergillus species | Neutropenia, endocarditis, intravenous drug abuse, pulmonary disease being treated with high-dose steroids, organ transplant |
| Fusarium species | Neutropenia, intravenous drug abuse |
| H. capsulatum, C. immitis, B. dermatitidis, S. schenckii, C. neoformans | May accompany disseminated disease |
| Penicillium species | Intravenous drug abuse |
| Exogenous endophthalmitis | |
| Fusarim species | Posttrauma and postkeratitis |
| Candida species | Postsurgery, contaminated eye irrigants |
| P. lilacinus | Postsurgery, contaminated sterilization solutions |
Endophthalmitis is recognized clinically by the presence of one or more creamy-white, well-circumscribed lesions of the choroid and retina, often accompanied by inflammatory infiltrates in the vitreous. These lesions can be detected using an ophthalmoscope after dilating the pupils. Often, there is inflammation in the anterior chamber manifested by the presence of a hypopyon. Typical lesions of chorioretinitis are shown in Fig. 2, left. Patients complain of eye pain and may have blurred vision or spots in their visual fields. Patients with endogenous endophthalmitis may have positive blood cultures antedating eye symptoms or signs. In the absence of a positive blood culture or characteristic clinical syndrome, aspiration of the vitreous (or biopsy) may be necessary to establish the causative microorganism.
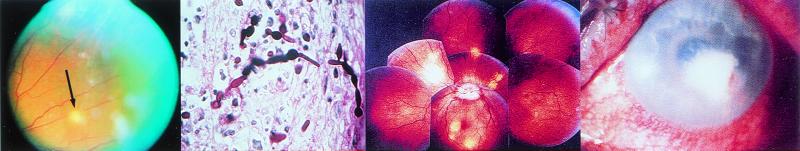
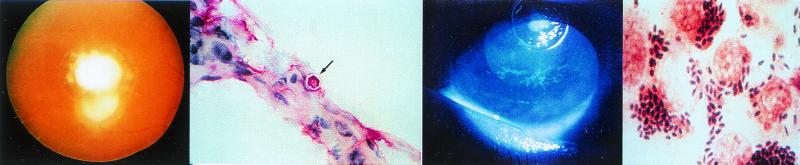
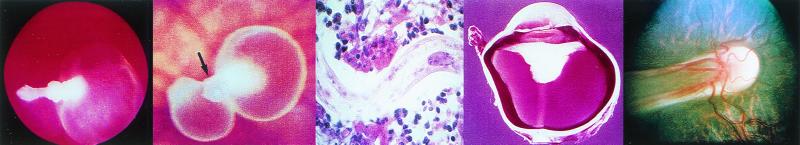
FIG. 2.
(Left) View of the fundus of a patient with C. parapsilosis fungemia complicating total parenteral nutrition for esophageal cancer. A chorioretinal lesion is shown (arrow), and there is accompanying vitreous haze. (Middle left) C. albicans in retinal tissue with both blastoconidia and pseudohyphae present. (Photo courtesy of F. G. LaPiana.) (Middle right) Fundoscopic montage of the eye of a patient with P. carinii. There are edema of the optic nerve head (the circular whitish area in the center from which the blood vessels originate) and scattered multifocal choroidal mass lesions (whitish to yellowish circular lesions). (Reprinted from reference 138 with permission from the publisher.) (Right) C. albicans keratitis in an immunocompromised patient. The dense yellow-white stromal infiltrate resembles bacterial keratitis. It partially covers the pupil. There is a small hypopyon present. (Reprinted from reference 137a with permission from the publisher.)
Why the eye is a common end organ target of fungemia is unknown. However, in a rabbit model of C. albicans endophthalmitis, more fungal elements are found in the eye per gram of tissue than are found in the kidneys of the same animals. Since C. albicans is believed to have a marked tropism for the kidneys and endothelium, the great number of organisms in the eye bespeak a tropism for the eye as well (142, 143). The candidal lesions in the rabbit are identical to those found in humans demonstrating a focal chorioretinitis (Fig. 2, middle left), with a mixture of granulomatous and suppurative host reactions (76). The infection likely begins in the choroid and progresses anteriorly to the retinal layers (226). This may be related mechanistically to the fact that the outer retinal layers, i.e., those considered to be infected first, receive blood from a high-flow system (150 mm/s), whereas the inner layers receive blood from a low-flow system (25 mm/s). It should be noted that drainage from the retinal layers is entirely through the venous system as there is no lymphatic system serving the inside of the globe.
The most common cause of endogenous fungal endophthalmitis is C. albicans (287). Endogenous fungal endophthalmitis by definition follows fungemia; therefore, it is important to note that Candida species are the fourth most common cause of positive nosocomial blood cultures in the United States, exceeding the number of positive cultures of any single gram-negative bacterial genus (21). It is estimated that some 120,000 patients contract disseminated candidiasis (i.e., candidemia) per year in North America (58), and the usual estimates of the incidence of candidal endophthalmitis in patients with candidemia are around 30% (32, 196; J. R. Griffin, R. Y. Foos, and T. H. Pettit, presented at the 22nd Concilium Ophthalmologicum, 1974); thus, the disease is fairly common. If the definition of chorioretinitis is more stringent, i.e., if nonspecific lesions such as cotton wool spots and retinal hemorrhages are eliminated, the incidence is much less, on the order of 9.3% (70, 226).
The pathogenesis of candidemia remains unknown but is likely multifactorial. There are characteristic clinical features of patients with candidemia, with one or another feature being found in each patient. These include the use of broad-spectrum antibiotics that eliminate competing normal microbiota of the host, the presence of central venous catheters, the administration of total parenteral nutrition, prior abdominal surgery, and/or neutropenia (164). One or all of these factors are sufficient to place a patient at risk for candidemia and, hence, for endophthalmitis. Neutropenia, although a risk factor for candidemia, reduces the incidence of candidal endophthalmitis in the rabbit model (122) and perhaps in patients as well (78). This suggests that the chorioretinal lesions are probably a reflection of a vigorous host response rather than just the sheer number of infecting microorganisms.
During the introduction of total parenteral nutrition in the 1970s there was a marked increase in the number of patients with Candida endophthalmitis (77), which is likely related to the prolonged use of central venous catheters. Candida endophthalmitis has also been reported to occur after induced abortion (49), in the postpartum state (43), following treatment of toxic megacolon (123), and as a consequence of intravenous drug abuse. An addict's use of intravenous brown heroin often leads to a characteristic syndrome, at one time common in Europe, that includes pustular cutaneous lesions, endophthalmitis, and osteomyelitis. C. albicans can be isolated from all of these lesions (74). The microorganisms in this syndrome may be acquired from the drug abuser's own skin surface (79). Candida endophthalmitis may also occur after intravenous placement of a foreign device, such as a pacemaker (243), and following repeated intramuscular injections of medications, such as anabolic steroids (285). Species of Candida other than C. albicans are capable of causing endogenous endophthalmitis and may do so in proportion to their ability to cause candidemia (20, 53, 133, 243).
Although Candida species are clearly the most-common causes of endogenous endophthalmitis, other fungi are occasionally encountered. Aspergillus species are the second most-common cause of fungal endophthalmitis (291). Aspergillus spp. may be less capable of causing endophthalmitis than Candida spp.; an example of this is the rabbit endogenous endophthalmitis model, in which larger inocula of Aspergillus spp. are required to cause the disease than with C. albicans (93). Many species of Aspergillus have been reported to cause endophthalmitis, but Aspergillus flavus is probably the most common (219), followed by Aspergillus fumigatus, Aspergillus niger, Aspergillus terreus, Aspergillus glaucus (281), and Aspergillus nidulans (271). Endogenous Aspergillus endophthalmitis may be encountered in neutropenic patients or in patients taking pharmacologic doses of corticosteroids, often for chronic lung disease. Aspergillus endophthalmitis has even been reported to occur following severe periodontitis, although entry of Aspergillus spp. into the bloodstream through the mouth certainly is not common (172). Intravenous drug addicts are at particular risk for disseminated aspergillosis (69). Aspergillus endophthalmitis has been reported in addicts abusing a mixture of intravenous cocaine, pentazocine, and tripelennamine. Three such individuals from Louisville, Ky., were infected with A. flavus in this manner (23). Patients receiving large doses of corticosteroids for lung disease may have negative blood cultures but evidence of severe Aspergillus endogenous endophthalmitis. Endophthalmitis, therefore, is the sole manifestation of disseminated disease and must be established by aspiration of the vitreous (281). Aspergillus endophthalmitis has also arisen in recipients of solid-organ transplants, in which the donated organ was the likely source of the fungus (16, 139). Pathologic specimens of invasive aspergillosis usually demonstrate angioinvasion by the hyphae, and thus Aspergillus species may possess a tropism for vascular tissue (279).
The emerging pathogens of the genus Fusarium have been reported to cause endophthalmitis in neutropenic hosts (160), in an intravenous drug abuser (94), and in a patient with AIDS (106). Penicillium spp. also have caused endogenous endophthalmitis in an intravenous drug abuser (265). As mentioned in connection with C. albicans, endogenous endophthalmitis may occur from fungi seeding the bloodstream from a catheter or endocarditis. Pseudallescheria boydii has caused endophthalmitis from an infected porcine allograft of the aortic valve (259) and even in a patient without risk factors for the disease (193).
The four dimorphic fungi H. capsulatum (165), B. dermatitidis (158), Sporothrix schenckii (2), and C. immitis (96) as well as Cryptococcus neoformans (59) may cause endogenous endophthalmitis as part of disseminated disease. Within the region of H. capsulatum endemicity in North America, roughly the Ohio and Mississippi river valleys, there is a well-described syndrome attributed to infection with H. capsulatum. This entity is known as presumed ocular histoplasmosis (POH), which occurs in immunocompetent individuals and is recognized by the presence of multiple diskiform atrophic chorioretinal scars without vitreous or aqueous humor inflammation. POH is said to affect 2,000 new individuals a year in areas of endemicity and in some cases may lead to visual loss and blindness (165). The lesions are usually burned out, but not all of them are static and some may reactivate (41). The lesions are thought to arise from the hematogenous spread of the fungus following initial infection. The initial infection, acquired by inhalation of microconidia into the lung, spreads throughout the body, including the eye, and is soon controlled by a competent host immune response (175, 249). H. capsulatum is not detectable in the scars of POH. However, there is strong epidemiological evidence, principally deriving from skin test surveys, linking the scars to histoplasmosis (95, 252). A primate model demonstrates pathology identical to that found in humans (250, 251). Similar lesions to those of POH are, however, observed in Europe, where histoplasmosis is rare (264), and therefore, it is likely that similar chorioretinal lesions are the end result of several different infectious agents. Active endophthalmitis in patients with disseminated histoplasmosis secondary to AIDS or immunosuppression occurs and is associated with numerous budding yeast cells in the choroidal tissue and endothelium (41, 88, 231). In some cases the endophthalmitis is accompanied by yeast cells in the anterior chamber angle structures such as the iris, ciliary muscle and canal of Schlemm (41, 88). Two cases of disseminated histoplasmosis, established by elevated H. capsulatum antigen in blood and urine and high complement fixing antibodies, occurred in immunocompetent brothers. Their disease was associated with choroiditis, which appears to progress to typical POH lesions (136). Thus, the link between active histoplasmosis and POH may be made by these and similar cases.
Disseminated blastomycosis is common in dogs and is often accompanied by endophthalmitis (28); for example, 78 eyes in 74 dogs with disseminated disease had endophthalmitis. Canine blastomycosis of the eye always involves the choriocapillaries, and organisms are abundant in the choroid. The disease often progresses to panophthalmitis (38, 39, 246). Endophthalmitis is also seen in humans with disseminated blastomycosis (223), as evidenced by the presence of chorioretinal lesions (108, 223).
Coccidioidomycosis is associated with lesions throughout the eye, including endogenous endophthalmitis (96). Chorioretinal scarring is common in individuals within the region of endemicity with positive skin tests to coccidioidin, a situation reminiscent of histoplasmosis. The chorioretinal lesions presumably occur at the time of initial infection and are usually clinically quiescent and asymptomatic. On the other hand, active chorioretinitis has been described in patients with disseminated disease (96). Anterior chamber disease has been documented in patients with disseminated disease, including iritis and large inflammatory masses in the anterior chamber (61, 96, 180, 298). It is interesting that disseminated coccidioidomycosis in dogs often starts in the posterior chamber and spreads to involve the anterior chamber (15). As previously mentioned, this is believed to be the same route of extension of disease with Candida and B. dermatitidis endophthalmitis.
C. neoformans frequently causes visual symptoms when associated with meningitis. These symptoms are usually due to the swollen brain compressing the optic nerve or edema of the optic nerve itself. However, cryptococcosis may be associated with endophthalmitis manifesting as chorioretinitis, retinal tears, and overlying vitritis (59, 62, 108, 242). Pneumocystis carinii is also an infrequent cause of chorioretinitis in patients with AIDS (Fig. 2, middle right).
Exogenous Endophthalmitis
As the name implies, exogenous endophthalmitis occurs by introduction of microorganisms into the eye from trauma or surgery. It can also be the end result of preexisting scleritis or keratitis (29). Zygomycosis in the surrounding soft tissue and cryptococcal neuroretinitis may also lead to exogenous endophthalmitis. Patients with exogenous endophthalmitis are rarely immunocompromised. Cataract removal followed by placement of a prosthetic lens and corneal transplantation are the surgical procedures most often associated with postoperative fungal exogenous endophthalmitis.
One report describes 19 patients from one hospital with exogenous endophthalmitis, and there was an approximately equal distribution of patients between the categories of postsurgical endophthalmitis, posttrauma endophthalmitis, and endophthalmitis following keratitis (205). Exogenous endophthalmitis may have a period of latency of weeks to months before clinically detectable disease occurs. Even then the infection is often confined to the anterior chamber, pupillary space, or anterior vitreous. Eighty-four percent of patients in one series received topical corticosteroids before diagnosis, and this may have potentiated the disease by reducing local host immunity (205). The most-common causes of postsurgical exogenous endophthalmitis are gram-positive bacteria, including coagulase-negative Staphylococcus, diphtheroids, and Propionibacterium acnes (287). The mycotic causes of exogenous endophthalmitis, such as yeasts (principally Candida species, including Candida glabrata [42] and Candida famata [211]), were found only in the postsurgical group, whereas Fusarium species were found only in the posttraumatic and postkeratitis groups (205). Other Candida spp. have caused exogenous endophthalmitis after lens surgery (211, 294). An epidemic of postsurgical endophthalmitis with Candida parapsilosis has been reported following the placement of anterior and posterior chamber lenses (260). Fifteen patients had ocular surgery over a 3-month period of time. At the time of surgery all eyes were irrigated with a solution from the same lot that was contaminated with C. parapsilosis.
Paecilomyces lilacinus is a ubiquitous soil saprophyte implicated in cases of keratitis and endophthalmitis after trauma (191, 283). However, a large outbreak of P. lilacinus exogenous endophthalmitis followed intraocular lens implantation; the lenses had been contaminated by a bicarbonate solution used to neutralize the sodium hydroxide sterilant added to the lenses. P. lilacinus was cultured from the bicarbonate solution (204). Such fungi as Aspergillus species (29, 64, 194) and Acremonium kiliense (92) have caused infections following lens surgery. These infections, like postoperative P. lilacinus infections, may arise because of fungal contamination of operative and postoperative irrigating solutions (174, 190, 260).
Fungal pathogens in posttraumatic endophthalmitis are legion and similar to those causing fungal keratitis. Recent reports include Fusarium moniliforme (257), Exophiala jeanselmei (114), P. boydii (44), A. niger (129), Scytalidium dimidiatum (9), Helminthosporium spp. (65), S. schenckii (292), Penicillium chrysogenum (82), and L. theobromae (29).
Infections of the Cornea
Fungal infections of the cornea (fungal keratitis or keratomycosis) may constitute 6 to 53% of all cases of ulcerative keratitis, depending upon the country of origin of the study (269). The majority of fungal keratitis occurs after trauma to the cornea in agricultural workers, usually, but not always, with fungus-contaminated plant material (leaves, grain, branches, or wood). The disease may also occur in gardeners and following corneal trauma from indoor plants as well. Occasionally the object striking the cornea is metal. The trauma to the cornea may be so slight as to be forgotten by the patient. Fungal keratitis also occurs with contact lens wear, and this will be discussed later.
Trauma to the cornea with vegetable matter either introduces the fungus directly into a corneal epithelial defect or, alternatively, the defect may become infected following the trauma. The vast majority of cases of fungal keratitis are due to septate, filamentous, saprophytic fungi. Occasionally zygomycetes such as Absidia (168) or Rhizopus (233) spp. may be implicated in keratitis. On the other hand, the abnormal or compromised cornea, e.g., chronic dry eye, is subject to infection with yeasts, usually Candida species. Such uncommon Candida species as Candida lipolytica and Candida humicola have, however, been reported to cause posttraumatic keratitis (187, 188) and Candida guilliermondii after corneal transplant (3). More than 70 species representing 40 genera of fungi have been reported to cause fungal keratitis (269). The most common cause of fungal keratitis is F. solani and other Fusarium species, Aspergillus species, and Curvularia species (269). There may be a hierarchy of fungi capable of producing keratitis, e.g., from most to least capable, Fusarium, Acremonium, and Phialophora spp. This hierarchy is predicated upon their individual ability to invade and destroy the cornea (156).
Fungal keratitis is recognizable by the presence of a coarse granular infiltration of the corneal epithelium and the anterior stroma (Fig. 2, right). The corneal defect usually becomes apparent within 24 to 36 h after the trauma. There is minimal to absent host cellular infiltration. The absence of inflammatory cells is likely a good prognostic finding, since products of polymorphonuclear leukocytes contribute to the destruction of the cornea. The infiltrate is often surrounded by a ring, which may represent the junction of fungal hyphae and host antibodies (156). Descemet's membrane, an interior basement membrane near the aqueous humor, is impermeable to bacteria but can be breached by fungal hyphae, leading to endophthalmitis (212). Even so, endophthalmitis is a rare consequence of fungal keratitis (29). Pathologic specimens of filamentous fungal keratitis demonstrate hyphae following the tissue planes of the cornea, i.e., laying parallel to the corneal collagen lamellae. Examination of multiple scrapings of the cornea establish the agent of fungal keratitis. In some cases a biopsy may need to be performed. Since many of the filamentous fungi grow slowly, the disease often remains unrecognized and untreated for days or weeks until growth is visually detected, and this delay may contribute to a poor response to therapy.
The abnormal cornea in patients with dry eye syndrome, chronic ulceration, erythema multiforme, and perhaps human immunodeficiency virus (HIV) infection (particularly those with AIDS) is subject to fungal infection, most commonly with Candida species. Candida keratitis usually appears as a small demarcated ulcer with an underlying opacity of the cornea resembling bacterial keratitis. C. albicans was found to be the most common cause of microbial keratitis in a series of 13 AIDS patients (121). Candida keratitis has occurred as well in patients who chronically abused corneal anesthetics (49).
The wearing of hard and soft extended-wear contact lenses is associated with infectious keratitis usually caused by Pseudomonas aeruginosa. Both P. aeruginosa (36) and C. albicans (34) adhere to contact lenses, and the adherence of the former to lens surfaces is greatly enhanced in the presence of tear deposits (35), some of which could conceivably serve as carbohydrate receptors for the microorganisms (147). Adherent microorganisms secrete an extensive exopolymer that is virtually impenetrable to antibiotics and difficult to remove. Contact lenses coated with such biofilms likely increase the risk of infectious keratitis (80). The wearing of contact lenses leads to a relative hypoxia of the corneal epithelium that may lead to measurable changes in the cell surface glycoproteins (145). Perhaps microscopic defects are introduced by lens wear that enhance microorganism adherence to the otherwise nonadherent corneal epithelium (144). Fungal keratitis in association with contact lens wear is almost always due to Candida spp., although Cryptococcus laurentii (217) has been reported. Filamentous fungal keratitis occurs less often with lens wear (200, 261, 296), but the filamentous fungi can actually penetrate the lens matrix (141, 200, 245, 261, 289, 296). Fungi and the bacteria adherent to contact lenses arise from patient handling, including the cleaning and storage of the lenses. These adherent microorganisms also derive from the normal flora of the conjunctiva (181).
Infections of Adjacent Structures
Infections of the eyelids, conjunctiva, and lacrimal system.
The eyelids may be the site of inoculation of S. schenckii, resulting in a chronic suppurating ulcer (2). It has been contended that eyelid lesions are common in patients with disseminated blastomycosis; however, in a recent survey of 79 patients, only one had an eyelid lesion (24). Blastomycosis, however, may present solely as a conjunctival lesion apart from any external eyelid involvement (248). Paracoccidioides brasiliensis often causes disease of the eyelids and conjunctiva resulting in disfiguring lesions. This systemic mycosis, acquired by inhalation of conidia into the lungs, is endemic to Mexico and Central and South America and has a predilection for mucocutaneous surfaces, particularly the mouth and nares (215). Occasionally the cornea may become infected and lead to blindness (244).
Eyelashes may become infected, and the individual cilia become matted from the host inflammatory response. This is usually attributed to coagulase-negative staphylococci, although blepharitis may rarely occur with dermatophytes such as Microsporum canis (57). Dermatophyte infection, however, is limited to keratinized tissue, and therefore, when the eyelid is involved, it is usually an extension of disease from the face. The dermatophytes may affect the eyebrows as well (262). Yeasts such as Candida species have been associated with cases of ulcerative blepharitis in patients with skin atopy (127). The lipophilic fungus Malassezia furfur, likely normal microbiota of the adult pilosebaceous unit, can be cultured from normal as well as seborrheic dermatitis lesions of the eyelid (197). M. furfur has been implicated as a cause of blepharitis (J. Toth, M. Bausz, and L. Imre, Letter, Br. J. Ophthalmol. 80:488, 1996). The treatment of facial and scalp seborrheic dermatitis with azoles restores normal to near-normal skin in patients infected with HIV, thus giving impetus to the notion that M. furfur is integral to the skin disease. Malassezia pachydermatis, a congener that does not require exogenous lipids for growth like M. furfur, has been isolated from a conjunctival discharge in a neonate with a sepsis syndrome with the same microorganism (152).
The eyelid and surrounding soft tissue of the eye may be swollen or impinged upon by adjacent tissue involved in mycotic infections. For example, proptosis of the eye is commonly seen with rhino-orbital-cerebral mucormycosis or zygomycosis, which may arise from the buccal or nasal cavity with extension to the eye and optic nerve (10, 134, 220, 236, 256). Furthermore, fungi may cause sinusitis with extension into the orbit, and when this occurs, proptosis of the eye is common (120). (This will be discussed in detail below.) Chronic rhinofacial zygomycosis caused by Conidiobolus coronatus (214), which involves the eye and orbit secondary to chronic facial destruction, requires biopsy of involved tissue for diagnosis and isolation of the fungus. Blunt trauma to the orbital region can also introduce fungi such as Bipolaris spp., leading to an orbital cellulitis and proptosis (128).
The normal microbiota of the conjunctival mucosal epithelium consists of micrococci and aerobic diptheroids (73). Isolation of fungi from the normal conjunctival sac occurs in ∼6 to 25% of normal patients, although it is uncommon to repeatedly isolate the same fungus from the eye. There may be a seasonal increase in conjunctival fungal isolation, possibly related to the airborne carriage of conidia (14). Genera of filamentous fungi predominated in three large studies of conjunctival cultures involving over 1,000 patients (14, 234, 235). In one study of the conjunctiva and eyelid margins, C. parapsilosis was found in ∼25% of the eyes (290). The incidence of fungi in human conjunctival cultures is much greater than has been found in wild animals. For example, fungi were cultured from the eye in only 2 of 65 birds of prey (75).
Perhaps the high incidence of fungal isolation from the conjunctiva of humans is related to the frequency with which fungi can be isolated from cosmetics. Shared-use cosmetics, including eye products, yielded fungi in 10.4% of the products, representing 69 different species of fungi (178). Chronic use of topical ophthalmic antibiotics predisposes humans to fungal carriage in the conjunctiva (185), likely by removing the normal microbiota. This change in flora may be important if followed by trauma or contact lens wear, thus allowing saprophytic fungi direct ingress to the cornea.
P. carinii has been documented to cause conjunctivitis apart from retinal or choroidal lesions. One case of palpebral conjunctivitis was reported in a patient with AIDS receiving aerosolized pentamidine (220). Pentamidine administered in this manner does not have systemic distribution and predisposes the patient to extrapulmonary pneumocytosis. C. immitis has been isolated in a case of granulomatous conjunctivitis in an immunocompetent individual (166).
Infection and obstruction of the lacrimal duct system or dacryocystitis may be due to fungal infection. Where investigated, yeasts such as Candida (30, 120, 208) or Rhodotorula (183) spp. have been implicated. Fungi can be isolated from ∼30% of eyes with congenital dacryocystitis, and C. albicans is most often cultured. Bacteria are commonly isolated simultaneously with fungi from affected eyes in children (101). An unidentified species of Paecilomyces has been reported as a cause of dacryocystitis (124).
Infections arising from the paranasal sinuses and other sites within or near the orbit.
Invasive Aspergillus and zygomycete infections have a marked predilection for the orbit and surrounding tissues, including the paranasal sinuses. Many different presentations of eye disease by Aspergillus occur even in the healthy host. However, patients who are immunocompromised by the use of chemotherapy, corticosteroids, and principally, neutropenia are at particular risk for invasive disease arising in or near the orbit. The conidia of Aspergillus species are common airborne particles that are often found in the paranasal sinuses of healthy hosts that may on occasion be associated with sinusitis or a paranasal sinus fungus ball. These infections are not invasive, and drainage or excision may lead to clinical resolution. Invasive disease in the compromised host may begin as dacryocystitis, masquerade as an optic nerve tumor, or present as an entirely retrobulbar process (155). Orbital disease with Aspergillus in the immunocompromised host may also begin as sphenoid and/or ethmoid sinusitis with erosion of the bony orbit, leading to invasion of the orbital space and proptosis. Proptosis may be the initial sign of fungal sinusitis even in immunocompetent individuals. For example, four otherwise healthy hosts were described with proptosis and sinusitis with Drechslera, Aspergillus, and Curvularia (120) species; the proptosis was present for >5 months in three of the patients.
Invasive zygomycosis occurs in immunocompromised patients, not unlike those at risk for aspergillosis. Rhino-orbito-cerebral zygomycosis is a devastating complication of diabetic ketoacidosis and the use of immunosuppressive drugs following organ transplant. It may follow deferoxamine treatment for hemochromatosis as well (10), whereas pulmonary zygomycosis usually occurs only in neutropenic patients. Infarcted and necrotic tissue is often visible to the naked eye on the hard and soft palate and in the nose and can extend into the orbit. In the typical patient with diabetic ketoacidosis, rhino-orbito-cerebral zygomycosis is a fulminant disease requiring emergent surgical removal of dead tissue and the institution of appropriate antifungal therapy. The common fungi involved in this disease are Rhizopus, Mucor, and Absidia spp. A more indolent form of this disease has been described in some diabetic patients (116) where proptosis, drooping eyelid, or an eye palsy was present for weeks to months. Blindness in one eye may be the presenting symptom of invasive zygomycosis. Blindness can be secondary to central retinal artery occlusion (85), optic nerve infarction (72), or infarction of the optic chiasm (154).
Infections of the empty eye socket.
Removal of the globe leads to colonization of the exposed tissue by microorganisms. Although conjunctivae of the anophthalmic socket may support more bacteria than the normal eye, there is no difference in the kinds of bacteria that occur (66, 276). Apparently, patients who manipulate their prosthetic eyes frequently have a significantly greater percentage of gram-negative bacteria (276). Fungi do not appear to pose a problem in patients with anophthalmus.
Establishing Diagnosis of Fungal Infections of the Eye and Adjacent Structures
The most critical pieces of information regarding infections of the eye are the clinical history, clinical examination, and accurate identification of the causative microorganism(s). A good history and eye examination may provide sufficient information to suggest the pathogenesis of the disease and likely microorganisms. For example, diminishing vision and pain in the eye of a patient wearing contact lenses in the presence of a corneal ulcer strongly suggest an infectious keratitis caused by bacteria, saprophytic fungi, or amoebae. Appropriate material obtained from the cornea may establish the precise pathogen. Similarly, the complaint of dark spots in the visual field of a patient recently discharged following abdominal surgery who had blood cultures which yielded C. parapsilosis is highly suggestive of endophthalmitis and can be confirmed by careful observation of the fundus of the affected eye(s). The history and eye examination are often characteristic of fungal eye infections and thus provide clues to the pathogenesis of disease and likely pathogens.
There is no pathognomonic lesion of mycotic infection of the eye; therefore, the clinician must also consider viral, bacterial, and parasitic causes. The diagnosis of fungal infections requires the clinician to (i) establish the presence of ophthalmic pathology (which may require special instruments, such as a scanning slit confocal microscope [87]); (ii) obtain tissue in which the fungus is visualized; and (iii) isolate the responsible fungus. Fungal isolation by culture is particularly important since tissue strains frequently do not allow one to determine the identity of filamentous fungi or yeasts with any degree of certainty. Isolation allows one to perform both authoritative identification and antifungal testing when necessary.
Exceptions to the rule requiring isolation of the fungus from eye tissue would include such entities as endogenous endophthalmitis in which fungi known to cause this disease have been isolated from blood culture and the clinical presentation is compatible with vascular dissemination of the microorganism. Similarly, histoplasmosis and coccidioidomycosis are commonly associated with characteristic chorioretinal lesions, and isolation of the fungus from another anatomical site or measurement of antibody to the fungus is sufficient to establish one of these microorganisms as the cause of the eye disease. Lastly, C. neoformans is capable of causing a number of different ophthalmic presentations, usually in conjunction with meningoencephalitis, and its isolation from blood and/or cerebrospinal fluid is usually sufficient explanation for the associated eye findings. In most other circumstances the clinician will be obligated to establish the diagnosis by isolating the causative microorganism directly from the eye or adnexal tissue.
Cilia and eyelid.
Cilia can be removed with forceps and observed directly under the microscope as well as placed on suitable culture media. When entertaining the possibility of fungal infection of the cilia, lids, and conjunctiva, the use of an olive oil overlay to solid medium should be considered in order to isolate M. furfur, which has been invoked as a cause of blepharitis (263, 272). Tissue should be obtained whenever possible, since convincing evidence that an isolate obtained from a swab is a pathogen may be difficult to collect. The presence of a chronic ulcer on or near the eyelid should suggest sporotrichosis or perhaps blastomycosis, and culture of biopsied tissue in the former case will establish the disease, whereas in the latter case, the fungus is usually readily visible in scrapings from the lesion.
Conjunctiva and lacrimal duct and gland.
Aspergillus, Fusarium spp. (14, 235), and occasionally C. albicans (232) are cultured from the conjunctiva of healthy and diseased eyes; therefore, it is difficult to establish saprophytic fungal isolates as pathogens of the conjunctiva unless a biopsy is performed, and this is rarely necessary. P. carinii may cause conjunctivitis, but this organism cannot be cultured (220). Material may be expressed from an infected lacrimal duct, or if required, an incision can be made and tissue can be obtained for culture and appropriate stains.
Paranasal sinuses and adnexa.
Not infrequently, invasive orbital disease arises from the paranasal sinuses, usually the ethmoid and sphenoid sinuses. A computerized tomography (CT) scan of the orbit and paranasal sinuses will establish the extent of disease, and biopsy, curettage, and drainage of the infected sinus can obtain adequate material. Aspergillus spp., zygomycetes, and other filamentous fungi are the usual pathogens. Rhinocerebral zygomycosis involving the orbit can often be diagnosed by biopsy of necrotic tissue from the hard palate or nose.
Cornea.
The use of an exceedingly thin, round-ended platinum spatula or, alternatively, a scalpel blade or small needle allows for scrapings to be obtained from the corneal surface for stains and cultures (288). Ample tissue is needed, so multiple corneal scrapings are usually performed. Biopsy of the cornea or keratoplasty may be required to provide sufficient diagnostic material. Saprophytic filamentous fungi more often than not cause posttraumatic keratomycosis. Rarely are yeasts involved in posttrauma keratitis (269). The detection of fungal elements in tissue or smears may be enhanced and thus detected with significantly greater sensitivity using acridine orange (135), Calcofluor white (45), or lactophenol cotton blue (40, 270) stains. The first two stains have the added advantage of demonstrating other pathogens, such as bacteria, amoebic exocysts, and microsporidial spores. This may be important, because traumatic injuries to the cornea may involve more than one pathogen. Similarly, the use of a battery of fluorescein-conjugated lectins has been shown to be useful in detection of ocular mycoses (218). The use of such tests as a chitin assay (151) or PCR (192) may prove useful, but these assays currently suffer from a cumbersome technique in the former and lack of detection across fungal genera in the latter.
Remainder of the globe.
Establishing the identity of the fungus in some cases of endogenous and exogenous endophthalmitis, scleritis, and panophthalmitis may require biopsy of tissue (sclera) or aspiration of vitreous fluid. Aspiration of aqueous fluid is rarely done to establish the presence of infection. The yield from vitreous fluid is greater than aqueous fluid in cases of endophthalmitis (25). In a recent study of 420 patients with endophthalmitis, it was concluded that the performance of vitrectomy did not enhance the yield of pathogens over aspiration alone. Thus, the authors specifically advise against the use of vitrectomy for diagnostic reasons (25). The surgical implantation of intraocular lenses may lead to pseudophakic endophthalmitis, and the removal of the lens may be required. This material should be sent to the laboratory and divided among appropriate bacterial and fungal stains and growth media.
Contact lenses, prostheses, and ophthalmic drugs and paraphernalia.
Contact lens wear is often complicated by bacterial keratitis and occasionally fungal keratitis. The inner surface of the involved lens should be swabbed, and the lens should be sectioned to obtain appropriate stains and cultures (288). Corneal transplants may be a source of infection, and thus, it is recommended that prior to transplantation a small piece of tissue be removed from the corneal rim along with an aliquot of the storage medium, and both should be submitted for culture (288). However, the storage media for corneal tissue do not serve as growth media in and of themselves (224). Ophthalmic drops and contact lens solutions may be the source of infection of the conjunctiva and cornea and of exogenous endophthalmitis. Samples of suspected contaminated solutions can be inoculated directly into liquid media.
Culture media.
For almost all specimens, the use of blood agar and Sabouraud dextrose agar containing gentamicin is sufficient for isolation of fungi. Agars containing cycloheximide should be avoided since some saprophytic fungi and C. neoformans are inhibited by this additive. The use of brain heart infusion broth for material from swabs may be helpful in isolation (288).
Brief Discussion of the Therapy of Fungal Eye Infections
A combination of surgery and antifungal drugs is the usual therapeutic approach to most fungal eye infections. We will discuss briefly the therapeutic approach to the fungal eye infections discussed previously.
The presence of endogenous endophthalmitis without a positive blood culture yielding the causative microorganism requires aspiration of the vitreous to establish the cause. Removal of a nidus of fungemia, if such exists—for example, an infected catheter, heart valve, or suppurative thrombophlebitis—is usually necessary for cure. Candida endogenous endophthalmitis can be treated with intravenous amphotericin B alone (26). The concomitant use of oral 5-flucytosine has been tried with success. Intravitreal injections of 5 to 10 μg of amphotericin B may be used adjunctively, but the intravenous administration of amphotericin B or an oral azole is required, as the endophthalmitis is just one of the many manifestations of disseminated candidiasis. Oral fluconazole, 100 to 200 mg/day for several months, has successfully treated endophthalmitis as well as disseminated disease (5, 163), although in the rabbit endophthalmitis model intravenous amphotericin B was superior to oral fluconazole (86). In one series of endogenous endophthalmitis with Candida species, 17 eyes were treated with a combination of vitrectomy, intravitreal amphotericin B, and systemic therapy for those patients with marked vitreous infiltrates. All demonstrated a satisfactory visual outcome (83). A combination of vitrectomy and oral fluconazole for 3 weeks was effective in six patients (50). Current regimens with this azole for disseminated candidiasis use 400 to 800 mg/day. These same regimens would be suitable for the treatment of Candida spp. and other yeasts causing exogenous endophthalmitis as well. In one study involving 15 patients with postoperative exogenous endophthalmitis due to C. parapsilosis, the patients were treated with success with a combination of intravenous and intravitreal amphotericin B without removing the lens (260). Success without removing the lens prostheses has been reported in another series of cases of postoperative C. parapsilosis endophthalmitis (137). The efficacy of lipid-associated forms of amphotericin B in Candida endophthalmitis is yet to be determined (186).
Filamentous fungi involved in endogenous and exogenous endophthalmitis may require repeated vitrectomy and injection of amphotericin B (282) or miconazole. Aspergillus spp. and many other filamentous fungi can be treated with amphotericin B, although cure is not predictable. Itraconazole has good activity against Aspergillus spp. and may be more suitable than amphotericin B for dematiaecious fungi, many of which were formerly treated with oral ketoconazole (132). Itraconazole may be the most useful agent for the treatment of Paecilomyces (221) and orbital infections with Aspergillus (171).
Keratitis is usually treated with a topical antifungal, sometimes in conjunction with subconjunctival injections of the same drug and/or oral antifungals. Natamycin applied on an hourly basis is the preferred polyene for topical administration as the deoxycholate salt in amphotericin B is toxic to the cornea. However, lipid-associated amphotericin B may offer an effective but safer alternative and should be further studied (206). Collagen shields impregnated with antimicrobials appear to be a promising approach, and such shields soaked in amphotericin B and replaced daily have been used with success with Aspergillus keratitis (176). The topical use of the antiseptic agent chlorhexidine gluconate at a 0.2% concentration showed efficacy in vitro (169) and in patients with fungal keratitis (209).
Failure to respond to initial therapy may require subconjunctival miconazole and or oral fluconazole or itraconazole. Both azoles have been used to treat Candida keratitis as well (146). Surgical intervention with biopsy, partial keratectomy or keratoplasty may be required. Frequent debridement of the affected corneal surface may be beneficial in some cases.
Treatment of eye infections associated with disseminated disease by the dimorphic fungi is generally that of treating the disseminated infection, although intravitreal and subconjunctival injections of antifungals may be helpful (96).
PARASITIC INFECTIONS OF THE EYE
While relatively uncommon in the United States, various parasitic infections are important causes of ophthalmic disease worldwide. For example, toxoplasmosis and onchocerciasis affect millions of persons, with a significant proportion manifesting ocular involvement. As with fungal infections, the epidemiology of parasitic ocular disease also reflects the habitats of causative parasites as well as the habits and health status of the patient. Additionally, congenital infections may pose diagnostic and treatment challenges. The classes of infection are varied and include protozoa, nematodes, cestodes, trematodes, and ectoparasites. Like fungal endophthalmitis, the geographic location is an important determining factor in the development of parasitic infections. Additional considerations must include local sanitation and the presence of a vector for transmission as well as the more-complicated life cycles of the parasites and definitive hosts. Because of this somewhat more complex scenario, as well as the tendency for the parasites to cause a wider variety of pathologic lesions, the various parasitic etiologies of ocular disease will be addressed individually, including epidemiology, pathogenesis, diagnosis, and treatment.
Ocular Disease Caused by Protozoans
Toxoplasmosis.
Infection by Toxoplasma gondii is thought to affect approximately one-third of the adult human population (91). It is common in warm moist climates such as the Caribbean and Central America (84). However, infection in Europe is common, with prevalence rates as high as 90% in France (173). Two forms of T. gondii are infectious: the tissue cyst which is found in raw or undercooked meat and the oocyst which is present in the feces of the domestic cat, which is considered to be the definitive host. Rarely, individuals may be exposed through the consumption of contaminated drinking water.
While most individuals who are infected with T. gondii will not develop ocular disease, two specific populations are at particularly high risk: the immunocompromised patient (specifically, those infected with HIV) and neonates who have been exposed transplacentally by the mother's acute infection (84).
The noncongenital pathogenesis of toxoplasma endophthalmitis involves the ingestion of tissue cysts or oocysts. After digestion by enteric enzymes, sporozooites are released to become trophozooites, leading to invasion of the lymphatic system and thereby gaining access to various internal organs, including the eye (138). Congenital infection occurs when the mother acquires infection during pregnancy and trophozoites are passed to the fetus via the placenta. Cyst formation occurs once the trophozoites reach the end organs of infection.
Acute infection in newborns and patients infected with HIV may lead to an intense necrotizing chorioretinitis. More commonly, however, chorioretinitis is the result of necrotizing inflammation following the rupture of an older, slowly growing cyst, thus releasing bradyzoites (Fig. 3, left) (138). Congenital disease frequently involves both eyes, while acquired disease is usually unilateral (6, 56).
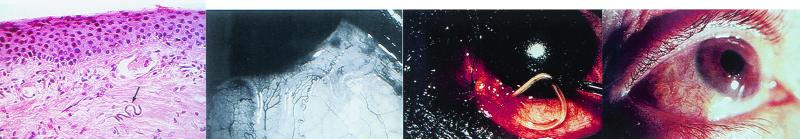
FIG. 3.
(Left) Recurrent toxoplasmosis around the periphery of an old scar, which is the white central disc. (photo courtesy of F. G. LaPiana.) (Middle left) Corneal scraping stained with Masson trichrome stain demonstrating a polygonal cyst of Acanthamoeba (arrow). (Middle right) Dendritiform epithelial lesions of the lower part of the cornea demonstrated by fluorescein staining in a patient with Acanthamoeba keratitis. (Right) Gram stain of a conjunctival scraping from a patient with microsporidial keratoconjunctivitis demonstrating large gram-positive ovoid microorganisms within conjunctival epithelial cells. (The last three panels reprinted from reference 137a with permission from the publisher.)
Ocular symptoms in the patient with congenital ocular toxoplasmosis may include strabismus, nystagmus, and blindness (201). Acute, acquired disease is associated with scotoma, photophobia, and loss of central vision due to macular involvement. Oculomotor nerve involvement may result in ptosis.
The diagnosis of chorioretinitis due to T. gondii is based on the combination of slit lamp examination and serologic confirmation. Slit lamp findings are that of a focal, necrotizing retinitis and are typically yellow-white, cottony lesions with indistinct borders, often occurring in clusters (138). The healing process, which usually takes several months, results in a sharper border, often accompanied by peripheral hyperpigmentation. Acquired disease may resemble lesions of Mycobacterium tuberculosis, syphilis, leprosy, and presumed ocular histoplasmosis (189). Lesions of congenital ocular toxoplasmosis must be distinguished from lesions of herpes simplex virus, cytomegalovirus, and rubella virus as well as syphilis (189).
Serologic testing for toxoplasmosis is available utilizing a number of methods detecting IgG or IgM, including enzyme-linked immunosorbent assay, direct agglutination assay, indirect immunofluorescence assay, immunosorbent agglutination assay, and immunocapture and immunoblot assays (98). As would be expected, antibody detection in the neonate is complicated by passive transmission of maternal IgG, making serologic diagnosis more challenging. The use of gamma interferon, PCR, and antigen detection hold promise in the determination of both acquired and congenital infections.
First-line therapy of T. gondii-related chorioretinitis includes the use of pyrimethamine with sulfadiazine (81). Folinic acid is added to prevent bone marrow toxicity. Therapy should continue for 1 to 2 weeks beyond the resolution of symptoms. Pregnant women should receive spiramycin, available from the U.S. Food and Drug Administration, for therapy. Alternative regimens include the use of pyrimethamine with clindamycin, clarithromycin, or azithromycin. Atovaquone has been used alone with success as well (230). Patients infected with HIV and who show signs of previous infection as demonstrated by serologic testing and show evidence of CD4 depletion (CD4 count of <100 cells/mm3) should receive primary prophylaxis with trimethoprim-sulfamethoxazole (27). In patients with AIDS who have demonstrated toxoplasmic chorioretinitis, chronic suppressive therapy with sulfadiazine, pyrimethamine, and folinic acid should continue indefinitely following initial therapy.
Chagas' disease.
Chagas' disease, or American trypanosomiasis, results from infection by Trypanosoma cruzi. This infection is endemic in Central and South America and may be responsible for up to 10% of all deaths in some areas (98). Infection occurs when an infected reduvid bug (also called kissing bug) bites a human. At the time of the bite, the insect excretes trypomastigotes of T. cruzi. As the saliva of the insect is irritating, the human will commonly scratch or rub the bite site, thereby introducing the trypomastigotes into the lesion. Once introduced, the trypomastigotes circulate throughout the body with a preference for invading muscle cells, neural tissue, and the reticuloendothelial system. Once intracellular, the trypomastigote divides to become an amastigote which, in turn, continues to divide, ultimately leading to the destruction of the cell with release of amastigotes and trypomastigotes. The T. cruzi life cycle is completed with the ingestion of trypomastigotes during a blood meal by the reduvid bug. Additionally, Chagas' disease may be acquired through blood transfusion, organ transplantation, placental transfer, and accidental (laboratory) ingestion (267).
Once introduced to the body, T. cruzi may cause an intense, nodular inflammatory response termed a chagoma. If the initial bite is near the orbit, the patient may experience significant palpebral and periorbital edema (Romana's sign), with the chagoma lasting several months. This is a hallmark clue to the identification of Chagas' disease. The edema is painless and is frequently followed by constitutional symptoms of fever, malaise, and anorexia. While there are no long-term sequelae related to Romana's sign, there are a number of potentially severe complications of chronic Chagas' disease, including cardiomyopathy, megaesophagus, and megacolon.
The diagnosis of acute Chagas' disease is made by the detection of trypomastigotes in the bloodstream by direct examination of uncoagulated blood or buffy coat preparation. Likewise, trypomastigotes and amastigotes may be demonstrated on aspiration near the site of initial bite or chagoma. Direct culturing of blood on NNN or other suitable media may result in positive cultures in 7 to 10 days (7). The technique of xenodiagnosis may be used for diagnosis, if available. With this technique, laboratory-bred trypanosome-free reduvid bugs are allowed to feast on a sample of the patient's blood. The feces are then examined for the presence of trypomastigotes 10 to 20 days later. Serologic testing is of little value in the diagnosis of acute Chagas' disease as antibodies do not usually appear for 2 to 40 days following the onset of symptoms. Additionally, serologic studies may falsely detect the cross-reactivity of antibodies to nonpathogenic Trypanosoma rangeli (98). Alternative diagnostic techniques, including PCR, appear to be of increasing utility.
Therapy of Chagas' disease with antitrypanosome therapy is most successful in the acute stage. Two medications are available: nifurtimox and benznidazole. Therapy is usually extended for a period of months, and parasitologic cure rates are somewhat disappointing. Both medications carry a long list of significant side effects.
Malaria.
Affecting over 3 million humans, malaria is one of the most important infections of humans. Malaria is the result of human infection with one of four distinct parasites: Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium falciparum. These species are transmitted to humans through the passage of Plasmodium sporozoites through the Anopheles mosquito. Infection is widely distributed throughout the world, including Africa, Central America, South America, Oceania, and Asia, with specific species showing varied geographic and resistance patterns. Transfusion-related malaria is rare, as is disease associated with shared needles (33). Immigrants and travelers to foreign countries represent the vast majority of cases seen in the United States.
Plasmodium spp. lead a complicated life cycle in which humans are the intermediate hosts. At the time of a bite by the anopheles mosquito, sporozoites are injected into the bloodstream and then migrate to the liver. With acute infection, the sporozoites invade hepatocytes to form schizonts, leading to merozoites. Merozoites are then released into the vascular system to infect red corpuscles, producing additional merozoites. The cycle is completed when some of the erythrocytic parasites develop into a sexual stage, the gametocyte, which is the form responsible for infection of the mosquito.
The pathogenesis of disease with respect to malaria can generally be traced to problems related to the physical effects of the parasite (microvascular obstruction and hemolysis) and the direct metabolic effects of the parasite (tissue hypoxia and hypoglycemia). Here, ocular disease certainly follows this pattern, as the most-common ocular finding in malaria is retinal hemorrhage and is thought to be a consequence of anemia (149). Other eye-related findings include amaurosis fugax, optic neuritis, glaucoma, panuveitis, oculomotor paralysis, and cortical blindness. It is held that retinal hemorrhage is a poor prognostic indicator in cerebral malaria.
Retinal hemorrhage may be seen in a number of infectious entities and is not pathognomic of ocular malaria. However, when coupled with the observation of Plasmodium spp. on blood smear, with the trophozoite, merozoite, or gametocyte form, the diagnosis becomes apparent. The specific intricacies of species identification by visual inspection of the blood smear are beyond the scope of this text. However, it should be noted that the most-crucial point lies in the differentiation of infection due to P. falciparum from that of other Plasmodium spp. due to the potential severity of disease and drug resistance.
Ocular malaria is not an isolated symptom of systemic disease, and therapy should proceed according to current Centers for Disease Control and Prevention recommendations. As an aside, it is important to note that antimalarials, specifically chloroquine and its derivatives, have been associated with significant retinal disease seen either at the time of therapy or perhaps years later. Symptoms may include blurred vision, scotoma, and visual-field defects. Retinal pigmentation may be disturbed with this therapy, and an abnormal threshold to red light may be seen on testing.
Leishmaniasis.
Leishmania spp. are obligate intracellular protozoans which infect an estimated 12 million persons. There are numerous species within the genus, and disease manifestation is, in part, species specific. Leishmaniasis occurs worldwide, with concentrations in Asia, the Middle East, and Africa.
Two life forms of Leishmania spp. are important in understanding the epidemiology and parasitology: the amastigote, which develops in the human reticuloendothelial system, and the promastigote, the form which develops in the gut of the sandfly vector. Once injected into humans during the sandfly blood meal, the promastigote develops into an amastigote after being engulfed by tissue macrophages. Within these cells, the amastigotes replicate and may spread either systemically or cutaneously. Accordingly, human disease is termed visceral leishmaniasis, cutaneous leishmaniasis, or mucocutaneous leishmaniasis. Not surprisingly, these forms exhibit distinctly different ocular findings.
Visceral leishmaniasis, or that which represents systemic disease, is known as kala-azar. Symptoms and signs are usually seen 2 to 4 months after exposure and are characterized by lymphadenopathy, fever, hepatosplenomegaly, cachexia, and pancytopenia. The original site of infection (or bite) may remain inflamed for long periods of time. The ocular manifestations of kala-azar are relatively uncommon and include chorioretinitis, central retinal vein thrombosis, iritis, papillitis, and keratitis (138). Additionally, flame-shaped retinal hemorrhages have been described. Glaucoma has been reported to develop after the successful treatment of kala-azar. Generally, ocular symptoms and findings are not the initial manifestation of disease.
Ocular findings in cutaneous leishmaniasis represent a local phenomenon resulting from the initial site of infection near the eye with occasional spread to the lacrimal duct. Ptosis may be a presenting complaint (48, 138, 225). Periorbital structures, including the eyelid, may show intense inflammation and necrosis (253). If the initial bite occurs on the conjunctival mucosa, the disease is termed mucocutaneous leishmaniasis. This state may lead to severe ulceration and possible loss of the eye.
The diagnosis of leishmaniasis is made by direct demonstration of organisms on tissue smears or biopsy. Amastigotes are usually demonstrated fairly easily in the case of cutaneous or mucocutaneous ocular disease. However, amastigotes have not been directly identified in cases of ocular disease associated with kala azar. When present, Leishmania spp. may be cultured on Novy, MacNeal, Nicolle's medium as well as Schneider's Drosophila medium supplemented with 30% fetal bovine serum (98). While available, serologic testing is not particularly useful for diagnosing cutaneous and mucocutaneous disease due to cross-reactivity with T. cruzi and Mycobacterium leprosum.
Acanthamoeba infection.
Discovered recently, ocular infection by Acanthamoeba spp. is associated with the use of contact lenses. Acanthamoeba spp. are small, free-living protozoans which exist in both fresh and marine environments and are resistant to chlorination. Cases of acanthamoebic keratitis have been recognized mainly in the United States (138). Acanthamoeba exhibits two life forms: trophozoites and cysts. With respect to ocular disease, the infectious form is the trophozoite, which measures 15 to 45 μm in diameter. Trophozoites revert to cyst form with adverse changes in pH, oxygen and food supply, and crowding (Fig. 3, middle left).
The pathogenesis of ocular disease due to Acanthamoeba spp. involves direct contact between cornea and trophozoites. Corneal injury is a predisposing factor and may be due to contact lenses or corneal surgery (115). Contact lens solutions made from tap water have been found to harbor the microorganisms (258). Symptoms of acanthamoebic keratitis are generally quite protracted and include severe pain, conjunctival edema, and loss of vision (98). The clinical scenario may be confused with herpes simplex virus keratitis, resulting in delay of diagnosis. Examination findings include a ring infiltrate around the cornea, with possible corneal penetration (Fig. 3, middle right) (118). Hypopyon, hyphema, and uveitis may be present as well (119). Histologic examination demonstrates the presence of both trophozoites and cysts in the cornea (113, 119, 131, 162, 295). It has been postulated that the intense inflammation associated with Acanthamoeba keratitis occurs only after the death of the parasite (138).
Demonstration of trophozoites and cysts in stained preparations of corneal scrapings is the traditional method of diagnosis. Additionally, Acanthamoeba may be cultured on nonnutrient agar which has been flooded with Page's saline solution and overlaid with Escherichia coli (277). The usefulness of serologic testing has not been proven. An important point in diagnosing Acanthamoeba infection is that all patients with unresponsive keratitis, whether contact lens wearers or not, must be evaluated for possible Acanthamoeba infection.
Treatment of Acanthamoeba infection is quite difficult. Current recommendations include using a solution of 0.1% propamadine along with a combination antibacterial preparation. Another option is the use of polyhexamethylene biguanide (0.02% solution) or chlorhexidine (0.02% solution) (104). Unfortunately, medical therapy usually fails, leading to surgical therapy, including penetrating keratoplasty. Preventive measures include counseling patients to avoid homemade contact lens solutions, as tap water may contain Acanthamoeba spp.
Microsporidiosis.
Microsporidiosis is a term used to identify infection due to microorganisms of the phylum Microspora, of which two genera appear to be important in the pathogenesis of ocular disease: Encephalitozoon and Nosema. Another genus, Microsporidium, is classified as Nosema-like. Recently, Septata spp. have been implicated in keratoconjunctivitis (161). It should be noted that knowledge of microsporidiosis seems to be rapidly expanding, given its important role as an opportunistic infection in patients with AIDS. These microorganisms are a diverse group of obligate intracellular organisms and are common pathogens in nonhuman hosts. The life cycle is somewhat complex, involving three general stages: infection, merogony, and sporogony. The infectious spore is quite small (1 to 2 μm in diameter). Therefore, electron microscopy is usually needed to establish diagnosis.
Ocular infection is presumed to occur either by direct inoculation into eye structures or by dissemination systemically, with the latter proposed to be the pathogenesis in patients with AIDS. Ocular findings are generally limited to the conjunctiva and cornea. Conjunctival hyperemia, a distinctive punctate epithelial keratitis, hyphema, necrotizing keratitis, and corneal ulcer are typical findings. Symptoms of infection include photophobia, foreign body sensation, and decreased visual acuity. With respect to diagnosis, spores have been demonstrated in most cases in which corneal scrapings or biopsy specimens are examined by light or electron microscopy (Fig. 3, right). Rarely, these microorganisms have been isolated in vitro (240). Where available, serologic testing may assist in the diagnosis of microsporidiosis (68). Current recommendations for treatment include the use of albendazole, which has shown some promise in the treatment of corneal disease. Historically, severe, progressive cases of ocular microsporidiosis have resulted in enucleation.
Giardiasis.
Giardiasis is an infection due to the protozoan Giardia lamblia. Infection generally manifests as intestinal illness, but ocular findings are being recognized with increasing frequency. G. lamblia is found worldwide and is relatively common in the United States. It is found in contaminated water sources as well as food which has been contaminated by a human carrier. Person-to-person transmission may occur, and giardiasis has been shown to cause outbreaks of diarrhea among children attending day care centers.
Infection with G. lamblia occurs after the consumption of cysts. Excystation then occurs in the duodenum, resulting in the formation of the trophozoite, which then attaches to the mucosa, at which time gastrointestinal illness begins. Interestingly, ocular disease has not been shown to be related to the presence of microorganisms within eye structures. Rather, it appears that ocular disease is allergic in nature (13, 148). The pathogenesis of this is poorly understood.
The ocular manifestations associated with giardiasis include chorioretinitis, iridocyclitis, retinal hemorrhage, retinal arteritis, vitreous hemorrhage, and uveitis. Retinal changes have been described as “salt and pepper” changes (55). Diagnosing ocular giardiasis is a challenge and is generally made by exclusion. Retrospective diagnosis may be made by demonstration of improvement in ocular findings following a course of treatment with antigiardial agents (138). Diagnosing intestinal disease by the identification Giardia antigen may help support the diagnosis of ocular giardiasis.
Recommended treatment of giardiasis includes a 5-day course of either metronidazole or albendazole, with respective cure rates of 92 and 84%. Pregnant women should receive paromomycin as first-line therapy.
Rhinosporidiosis.
Rhinosporidiosis, caused by Rhinosporidium seeberi, is a mucocutaneous disease that involves the palpebral conjunctiva in ∼15% of all cases of rhinosporidiosis (216). It is an infrequent cause of disease in India and tropical South America. Reproduction of R. seeberi in tissue produces polypoid or papillary growths that arise from mucous epithelium. Early in this century it was proposed that R. seeberi had taxonomic features of an aquatic fungus (19). In that light, it is interesting that in a recent outbreak of conjunctival rhinosporidiosis in Serbia there was a high correlation of disease with patients swimming in a stagnant lake (278). However, in India, conjunctival rhinosporidiosis occurs mainly in desert regions. Autochthonous cases have been reported from the United States, usually Texas, but a recent case occurred in a child from New York state (213). Recent investigations of RNA genes from this microorganism disclose that it may be more closely related to fish parasites than to fungi (125), and it is, therefore, included in protozoan diseases of the eye.
Ocular Disease Caused by Nematodes
Onchocerciasis.
Onchocerciasis is an important cause of blindness. It is estimated that 40 million humans have been infected with Onchocercus volvulus and 5% of those are blind due to the infection (river blindness) (54). Onchocerciasis is endemic in tropical Africa, South America, and the Arabian peninsula. It appears that humans are the main reservoir, with infection occurring from the bite of an infected female blackfly, Simulium spp. After biting an infected person and ingesting microfilariae, the microfilariae mature to the larval stage as they migrate to the proboscis of the fly. There, the larvae may be injected into a human with the next bite, where there is further maturation in the subcutaneous tissues for approximately 1 year, resulting in the formation of an adult worm capable of producing microfilariae. These microfilariae migrate throughout skin and connective tissue, where they die after several years. Adult worms (the male and female measure 30 by 0.2 mm and 400 by 0.3 mm, respectively) may live in the subcutaneous tissue for years, with a female producing one-half to one million microfilariae yearly. The site of the adult worm is usually found over a bony prominence and may develop into a firm, nontender nodule, or onchocercoma.
It is the migration of microfilariae through skin and connective tissue which is responsible for the majority of clinical findings in onchocerciasis. Migration may lead to pruritis manifesting as dermatitis. The skin may become thickened, edematous, wrinkled, and depigmented (12, 37, 54).
As with generalized cutaneous disease, ocular onchocerciasis is due to the presence and/or migration of microfilariae in and through ocular structures as well as the host's response to the migration (12, 37, 54, 103, 198). There are five predominant ocular findings that correlate with the location of microfilariae: punctate keratitis, sclerosing keratitis, iridocyclitis, chorioretinitis, and optic atrophy (138). Punctate keratitis is characterized by a number of small fluffy infiltrates in the cornea (11, 12, 198). These infiltrates often involve the peripheral margins of the cornea and individual microfilariae are usually identified in the lesions (Fig. 4, left). Symptoms of punctate keratitis include photophobia and lacrimation. Sclerosing keratitis is a more advanced lesion due to the prolonged presence of microfilariae (11, 12, 198). It is seen as a generalized white haziness across the cornea. Visual impairment occurs when the pupil becomes obscured. Iridocyclitis is present when the microfilariae make their way into the anterior chamber and may lead to the development of glaucoma or cataract formation (11, 12, 198). Other findings may include distortion of the pupil, which may also be covered with exudate. Chorioretinitis, which may involve the optic disk, is due to the presence of microfilariae in these layers of the eye and is accompanied by the host response (11, 12, 198). The lesions typically appear as focal areas of depigmentation with atrophy of the retinal pigment epithelium. Chorioretinitis usually involves both eyes. Optic atrophy is the predominant cause of blindness due to onchocerciasis and may accompany chorioretinitis (11). Optic atrophy is the end result of optic neuritis and it is thought to be due to the presence of microfilariae in the optic nerve.
FIG. 4.
(Left) Microfilaria of O. volvulus (arrow) in the superficial corneal stroma (hematoxylin and eosin stain). (Middle left) L. loa beneath the conjunctiva. (Left and middle left panels reprinted from reference 137a with permission from the publisher.) (Middle right) Adult L. loa being extracted from the subconjunctival space. (Right) A serpentine Dirofilaria migrating beneath the lateral aspect of the bulbar conjunctiva. (Middle right and right panels reprinted from reference 53a with permission from the publisher.)
The diagnosis of onchocerciasis is accomplished by a combination of clinical symptoms and signs with histopathologic examination of specimens. The skin manifestations must be differentiated from findings of insect bites, leprosy, dermatomycosis, and syphilis. Slit lamp examination may confirm the presence of microfilariae in the anterior chamber. A sclerocorneal punch biopsy may aid in the diagnosis as well (138). Additionally, multiple skin snips may lead to the diagnosis. Rarely, microfilariae are demonstrated in blood and/or urine samples. PCR may aid in the diagnosis of disease associated with a low burden of microfilariae. Xenodiagnosis, using laboratory-bred blackflies, may provide a clue as well. Finally, a trial of diethylcarbamazine (DEC), 50 mg orally, may result in marked worsening of pruritis and rash, suggesting the diagnosis of onchocerciasis.
Therapy includes both manual removal of adult worms and pharmacologic methods of killing microfilariae. Traditional therapy has centered around the use of DEC, but this is active only against microfilariae, allowing adult worms to repopulate the microfilariae in several months. Ivermectin is a promising agent which leads to a rapid decline in the microfilaria burden. Suramin is toxic to both microfilariae and adult worms but has significant toxicity.
Loiasis.
Loiasis, caused by the so-called African eyeworm, represents another microfilarial infection, differing from onchocerciasis in that the adult worms migrate through subcutaneous and deep connective tissues, producing microfilaria loiasis which may be easily detected in appropriately collected blood smears. Affecting an estimated 3 million persons in Central and West Africa, loiasis is an important cause of parasitic ocular disease. The agent of loiasis is Loa loa. Infection is acquired by humans through the bite of the tabinid flies, including the mango fly and horsefly of the genus Chrysops. When humans are bitten, larvae pass from the fly to the human, where they develop over 1 year into mature adult worms (100). These adults, which live up to 15 years, migrate through cutaneous and deep connective tissue, producing microfilariae. The migration of adult worms is generally painless but may be noticed as a tingling sensation when occurring of the bridge at the nose, the bulbar conjunctiva, and the eyelid (102, 153). Additionally, an intense atopic reaction may occur, leading to localized angioedema termed calabar swelling (98).
Ocular disease may be due to both the presence of microfilaria and the presence of the adult worm. The presence of adult worms in the conjunctiva is rather dramatic and is responsible for the name African eyeworm (Fig. 4, middle left). The presence of the worm is associated with conjunctival injection and pain with movement of the eye and may affect vision transiently. Additionally, adult worms have been found in the vitreous, eyelid, and anterior chamber. Microfilariae may travel via the bloodstream to involve the retina and choroidal vessels, leading to retinal hemorrhages resulting from aneurysmal dilatation of retinal vessels (98, 207, 273). Perivascular inflammation may be seen as well. These changes may be seen on slit lamp examination.
The diagnosis of loiasis is generally made by the detection of circulating microfilariae. Given the periodicity of microfilariae in the blood, specimens should be drawn at midday, and a direct, unstained smear may demonstrate microorganisms. Giemsa-stained slides may aid in the identification of microfilariae, and biopsy of an infected area may demonstrate the presence of an adult worm. In cases of conjunctival involvement, extraction of an adult worm confirms the diagnosis (138).
Therapy of loiasis involves the manual removal of adult worms present in the conjunctiva (Fig. 4, middle right) in addition to the use of DEC in escalating doses over a period of 3 weeks. Severe hypersensitivity responses may occur due to the killing of both microfilariae and adult worms (138).
Dirofilariasis.
Dirofilaria immitis, commonly known as dog heartworm, has been increasingly recognized as a cause of human disease in the form of pulmonary, cardiovascular, subcutaneous, and ocular infection. Human dirofilariasis is found throughout the world, and in some places, up to one-half of the canine population may be infected (109). Infection is transmitted to human skin by infected mosquitoes in the form of larvae. Following infection, the larvae migrate through subcutaneous tissue to the right heart or other organs, where there is maturation to the adult worm. Microfilariae have not been demonstrated in human infection. Ocular disease occurs with the migration of larvae through periorbital or palpebral tissue (Fig. 4, right). Rarely, larvae may migrate to intraocular structures.
Ocular symptoms of dirofilariasis depend on the location of affected structures. Involvement of the eyelid leads to pruritis, pain, edema, and congestion of the conjunctiva, while ocular involvement leads to photophobia, diplopia, foreign body sensation, and floaters (138). Invasion of the vitreous may be detected by ophthalmoscopy. Diagnosis is generally accomplished by histologic demonstration of the adult worm. Occasionally, the adult worm may be extracted from the conjunctiva (18, 97). Unfortunately, serologic studies lack sensitivity and specificity. However, disease may be associated with mild to moderate eosinophilia. Surgical excision of the adult worm is the recommended treatment, although DEC has been used with success in some cases.
Gnathostomiasis.
Gnathostomiasis is a significant cause of ocular disease in East Asia, with sporadic cases reported worldwide. It is the second most-common ocular parasite in Thailand (268). Ocular disease is due to the migration and metabolites of the Gnathostoma spp., with Gnathostoma spinigerum being the most-common species in humans. Infection begins with ingestion of contaminated fish, pork, chicken, frog, or snake. Rarely, transplacental transmission may occur (63). Contaminated meat contains third-stage larvae, and upon ingestion, the larvae penetrate the viscera and travel to internal organs and subcutaneous tissue. This migration may lead to significant symptoms due to the presence of by-products of the larvae. Orbital symptoms include erythema, pruritis, and localized warmth (63). Eosinophilia may be quite pronounced during this migratory phase.
Gnathostomiasis may manifest as cutaneous, visceral, or cerebral disease, depending on the route of the larvae. Both intraocular and corneal or conjunctival invasion may occur, leading to corneal ulceration, orbital cellulitis, uveitis, cataract formation, glaucoma, central retinal vein occlusion, retinitis, and vitreous hemorrhage (138). Fibrinous scarring along the migratory path may have severe complications, including retinal detachment (274).
Diagnosis of ocular gnathostomiasis is difficult and must be considered in patients with marked eosinophilia and elevated IgE. A history of painless subcutaneous edema may assist in the diagnosis, but it also appears that a high degree of suspicion may be of greatest help in areas of nonendemicity (138). An enzyme-linked immunosorbent assay is available and may assist in the diagnosis short of histopathologic examination of biopsy material. Surgical treatment remains the only treatment.
Ocular Disease Caused by Cestodes
Cysticercosis.
Infection with members of the Taenia tapeworm genus represents an important etiology of parasitic ocular disease. Taenia solium (along with its larval form, cysticercus cellulosae) is the most-common species causing cysticercosis in humans. Infection is seen worldwide, including China, Eastern Europe, India, Indonesia, Pakistan, and Central America. Risk factors include the consumption of undercooked pork and contaminated food and water.
Taxonomically, taeniasis (infection by the adult worm) must be differentiated from cysticercosis (infection by the larvae). However, patients may harbor both taeniasis and cysticercosis. Taeniasis is an intestinal infection caused by consumption of the adult worm through undercooked pork and is not associated with ocular disease. In cysticercosis, the human is as an intermediate host following the consumption of eggs in contaminated food or water. After ingestion, the eggs hatch and mature to larvae which are carried by mesenteric vessels to various parts of the body, where they are filtered through subcutaneous and intramuscular tissues, with preference for the brain and eyes. Ocular disease is reported to occur in a significant number of all cases of cysticercosis (98). Affected structures include the eyelid, conjunctiva, vitreous, anterior chamber, and subretina. Symptoms may include periorbital pain, diplopia, ptosis, blurring or loss of vision, distortion of images, and the sensation of light flashes (31, 111, 177, 202, 237, 247).
Diagnosis of ocular cysticercosis is usually accomplished by direct ophthalmoscopic demonstration of the larval worm. The living form of cysticercosis has the features of an undulating, expanding and contracting “pearl” with intermittent evagination and invagination of the protoscolex (Fig. 5, left and middle left) (98). This may result in an inflammatory chorioretinitis at the sites of previous “bites” by the protoscolex. Ocular ultrasonography may assist in the diagnosis of a subretinal cyst (280). Subcutaneous disease involving the periorbital structures may mimic neurofibromatosis, presenting as a painless, firm nodule up to 2 cm in diameter which may be caseated or calcified with significant edema. Other testing which may improve the diagnosis includes CT and assays for eosinophilia in anterior chamber fluid. Histologic examination helps to differentiate cysticercosis from hydatid disease and coenurosis. While useful for the treatment of neurocysticercosis, praziquantil is ineffective in the treatment of ocular disease. Metrifonate has shown some success in treatment (138). Interestingly, spontaneous extrusion of cystercerci from the eye may occur (22, 210). Vitrectomy along with photocoagulation has shown some success in removing cystercerci from the vitreous cavity (238).
FIG. 5.
(Left) Cysticercosis of the eye, fundoscopic view. While being observed by the ophthalmologist, the protoscolex inverted and everted rhythmically. Here it is everted and pointed to the left. (Reprinted from reference 53a with permission from the publisher.) (Middle left) Two cystercerci in the vitreous. One shows an evaginated protoscolex (arrow) (Reprinted from reference 138 with permission from the publisher.) (Middle) Toxocara endophthalmitis. Serial section of the eye shows an intact Toxocara larva. (Middle right) Enucleated eye from a patient with toxocaral endophthalmitis. Note the large retrolental mass (white) associated with a funnel-shaped retinal detachment. (Middle and middle right panels reprinted from reference 53a with permission from the publisher.) (Right) The optic disk of the eye “dragged” by a T. canis larva. (Photo courtesy of F. G. LaPiana).
Toxocariasis.
Toxocariasis is the result of infection by Toxocara spp., with Toxocara canis being responsible for the majority of human disease. It is a common pathogen found in dogs, and seroepidemiologic studies have demonstrated significant exposure of human populations in the United States. Infection is established after the ingestion of eggs found in soil contaminated by canine feces. After ingestion, the eggs (which may remain viable for months) hatch in the small intestine, with the resulting larvae penetrating the mucosa and migrating to the liver, lung, and trachea. This is termed visceral larva migrans (VLM). More rarely, and usually without signs of other organ involvement, the larvae may travel to the eye leading to ocular larva migrans (OLM).
OLM is thought to caused by larvae which have escaped the usual pattern of migration from the intestinal tract to the liver or pulmonary structures (299). The host response appears to be weaker in OLM than VLM. OLM may be seen as a broad spectrum of ophthalmic disease, including retinal detachment, posterior pole granuloma, uveitis, vitreous abscess, optic neuritis, keratitis, iritis, and hypopyon (Fig. 5, middle, middle right, and right) (150, 179, 254, 299). Strabismus, decreased or loss of vision, esotropia, and leukocoria are typical symptoms (179, 228, 229, 299).
The diagnosis of OLM is based on the findings of previously listed conditions combined with eosinophilia as well as serologic testing. An enzyme immunoassay using larval antigen is highly specific. Measurement of antibody in vitreous humor increases the specificity, and this is of particular importance in differentiating OLM from retinoblastoma (159, 179). Of interest, a recent study from Egypt suggests that concomitant infection with Toxocara spp. and T. gondii may occur in approximately 8% of patients with serologic evidence of toxocariasis (222). The most-common finding on examination is the presence of a granuloma on the retina which appears as a gray or yellow semispherical lesion.
While therapy with DEC, albendazole, and mebendazole have shown promise in the treatment of VLM, medical therapy has not shown benefit in treating ocular disease. Oral steroids have been suggested as therapy along with photocoagulation.
Echinococcosis.
Ocular echinococcosis, also called hydatid disease, is the result of infection by the larval stage of Echinococcus spp. Echinococcus granulosus is the species responsible for the majority of cases in humans. Disease is found in Australia, East Africa, India, the Mediterranean, the Middle East, South America, and Russia, with the highest incidence reported in Africa. Risk factors include raising swine in the presence of dogs (195). In this disease, humans act as intermediate hosts, and approximately 1% of infections manifest ocular findings.
Hydatid disease is contracted by the consumption of food or water which has been contaminated by egg-containing feces, usually that of the dog. These eggs, or oncospheres, hatch in the human duodenum and penetrate the intestinal wall, gaining access to the portal venous system. The oncospheres may lodge in the liver or travel to other organs. Over time, the oncosphere develops into a cyst, termed a hydatid cyst, which harbors protoscolex-containing brood capsules.
Proptosis, resulting from an extraorbital space-occupying hydatid cyst, is the most common ocular finding. Proptosis may lead to exposure keratitis and conjunctival congestion as well as ulceration of the cornea. Severe cases may lead to blindness (138). Other complications include erosion of the orbital wall into the cranium, optic atrophy, and optic neuritis. One case of subretinal hydatid disease has been reported.
When hydatid disease is suspected, CT may detect the low-density cyst with high specificity. Ultrasonography and magnetic resonance imaging are gaining in popularity in the diagnosis of ocular hydatid disease as well. Serologic testing may be used to confirm the diagnosis with an initial screening by indirect hemagglutination assay and the highly specific arc-5 antigen detection. However, only 50 to 70% of patients with hydatid disease test positive by the arc-5 antigen detection.
Therapy for hydatid disease is surgical removal of the cyst, which may be performed percutaneously (4). Medical therapy with mebendazole or albendazole has been found to be useful in the prevention of recurrence when cyst contents contaminate the surgical site.
Ocular Disease Caused by Ectoparasites
Myiasis.
Ocular myiasis is the result of invasion of the eye by larvae of flies. Genera important to human myiasis include Dermatobia, Gasterophilus, Oestra, Cordylobia, Chrysomia, Wohlfahrtia, Cochliomyia, and Hypoderma. Geographic location and local practices (e.g., shepherding) as well as sanitation are important determining factors of infection. Ophthalmomyiasis may be categorized into three categories: ophthalmomyiasis externa, ophthalmomyiasis interna, and orbital myiasis (138).
Ophthalmomyiasis externa is usually seen in areas of shepherding and is typically due to larvae of the sheep nasal botfly, Oestra ovis (117, 126). Infection involves the superficial tissues, including the eyelid, conjunctiva, lacrimal sac, and nasolacrimal ducts (52, 286). Conjunctivitis is most commonly found and is marked by the sensation of a foreign body with watery or mucopurulent drainage. Superficial follicular conjunctivitis and punctate keratitis may be seen on examination. A crawling or wriggling sensation accompanied by swelling and cellulitis may be seen in palpebral myiasis. Diagnosis of ophthalmomyiasis externa is made by demonstration of maggots, and histologic examination may show granuloma formation. As a small number of larvae may be present, a high index of suspicion is necessary to make the diagnosis. Treatment involves the manual removal of larvae. Anticholinesterase ointment may help kill or paralyze the larvae. Steroids and antibiotics may be necessary to control inflammation and secondary bacterial infection.
Ophthalmomyiasis interna is most commonly caused by a single larva of the Hypoderma spp. Infection is due to invasion of the tissues, leading to uveitis. More serious complications may include lens dislocation and retinal detachment (170, 266). Diagnosis is usually confirmed by the observation of migratory tracks across the subretina. In general, the larva dies and vision is not affected. However, if significant inflammation exists, steroid therapy may be necessary, with surgical extraction being reserved for the most severe cases.
Orbital myiasis may be due to a number of fly species and is generally seen in patients who are unable to care for themselves (140). Foul odor is the most likely attraction of the flies, and disease is due to the local destruction of tissue by the maggots. Orbital myiasis is easy to diagnose with significant numbers of larvae present. An entomologist is usually needed to determine the exact species. Therapy is directed at removal of all maggots and control of secondary infection.
Lice.
Lice belong to the order Anoplura, of which two genera are parasitic for humans: Pediculus and Phthirus. Of these, medically important species include Pediculus humanus var. corporis, the human body louse; Pediculus humanus var. capitis, the human head louse; and Phthirus pubis, the crab louse. Members of the two genera are easily distinguished from each other by microscopic examination. Depending on the species, eggs, or nits, are laid and glued to body hairs or clothing fibers. Following this, nymphs emerge to feed on the host, giving rise to symptoms of pruritis. Of the species mentioned, P. pubis is most likely to involve the eyebrows and eye lashes. In addition to pruritis, small erythematous papules with evidence of excoriation may be present. Involvement of the eyelash may cause crusting of the lid margins. In this case, diagnosis is relatively simple as nits are easily seen at the base of the eyelash (Fig. 6). Noneyelid involvement by P. pubis may be treated with lindane, permethin, pyrethin, or malathion. Eyelid disease is treated with a thick layer of petrolatum along the lid, twice a day for 8 days, or the application of 1% yellow oxide of mercury four times a day for 2 weeks.
FIG. 6.
Scanning electron micrograph of P. pubis adult and nit attached to an eyelash. (Reprinted from reference 137a with permission from the publisher.)
Ticks.
Ticks are arthropods belonging to the class Arachnida. There are a number of different species of ticks which may cause disease in humans and animals. Ticks are important vectors for the transmission of infection such as Rocky Mountain spotted fever, ehrlichiosis, Lyme disease, tularemia, and Colorado tick fever. Ticks exist in three life stages—larva, nymph, and adult—all of which require blood meals. Most tick bites are uncomplicated, and prompt removal of the tick is all that is necessary. Ticks have been reported to attach to ocular structures and may be appear to be a meibomian gland mass. In one such case, the nymph was associated with a stinging sensation. Following removal of the tick, a firm nodule, representing a tick bite granuloma, may remain for several weeks. This granuloma likely represents retained tick material and generally resolves spontaneously.
Glossary
amaurosis fugax Temporary blindness caused by reduced blood flow. anterior chamber An aqueous filled space bordered anteriorly by the cornea and posteriorly by the iris diaphragm and pupil. aqueous humor Watery clear fluid that fills the anterior and posterior chamber of the eye. It is formed continuously and drains behind the iris. blepharitis Inflammation of the eyelids. May be due to dermatophytes or M. furfur. canaliculitis Inflammation of the lacrimal excretory system, usually caused by Candida or Rhodotorula when caused by fungi. cellulitis Preseptal cellulitis is inflammation in front of the orbital septum. Postseptal cellulitis is inflammation of the connective tissue behind the orbital septum. Aspergillus and the zygomycetes may cause this disease. chorioretinitis Inflammation of the choroid and retinal layers of the eye. May be focal, multifocal, or diffuse. It is a manifestation of disseminated, bloodborne infections, such as candidiasis, histoplasmosis, and toxoplasmosis. conjunctivitis Inflammation of the conjunctiva, rarely caused by fungi. Often associated with redness, edema, and watery or purulent discharge. Gram stain of the exudate can establish the usual bacterial causes of conjunctivitis. dacryocystitis Inflammation of the lacrimal sac usually occurring after duct obstruction. endophthalmitis Intraocular inflammation involving the anterior chamber and the vitreous cavity of the eye. Endogenous endophthalmitis is bloodborne, whereas exogenous endophthalmitis occurs after trauma, surgery, or keratitis. hypopyon Pus in the anterior chamber. hyphema Hemorrhage into the anterior space of the eye. keratitis Inflammation of the epithelial surface of the cornea is known as epithelial keratitis. Stromal keratitis is inflammation of the deep tissue of the cornea. Fungal elements often course in parallel with the collagen strands. panophthalmitis Inflammation involving the whole interior of the eye. proptosis Forward displacement of the eye, usually from an infectious or neoplastic process in the retrobulbar space. A prominent feature of rhino-orbito-cerebral zygomycosis and aspergillosis in the neutropenic patient. ptosis Drooping of the upper eyelid. Often seen with acute Chagas' disease, when it is known as Romana's sign. retinitis Inflammation of the retinal layer of the eye. A prominent feature of endogenous fungal endophthalmitis such as that caused by Candida species. scleritis Inflammation of the scleral tunic of the eye. slit lamp A device through which an intense beam of light is projected to allow microscopic visualization of the eye. vitrectomy Microsurgical operation that removes the vitreous humor of the eye. vitreous humor Transparent, jelly-like substance that occupies the posterior segment of the eye. vitreitis Inflammation of the vitreous humor, which may lead to loss of visual acuity. It appears as a haze in the vitreous on ophthalmoscopy.
REFERENCES
- 1.Adams A D. The morphology of human conjunctival mucus. Arch Ophthalmol. 1979;97:730–734. doi: 10.1001/archopht.1979.01020010382023. [DOI] [PubMed] [Google Scholar]
- 2.Agger W A. Sporotrichosis. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 79–80. [Google Scholar]
- 3.Ainbinder D, Parmley V C, Mader T H, Nelson M L. Infectious crystalline keratopathy caused by Candida guilliermondii. Am J Ophthalmol. 1998;125:723–725. doi: 10.1016/s0002-9394(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 4.Akham O, Bilgi S, Akata D, Kiratli H, Ozmen M. Percutaneous treatment of an orbital hydatic cyst: a new therapeutic approach. Am J Ophthalmol. 1998;125:827–829. doi: 10.1016/s0002-9394(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 5.Akler M, Vellend H, McNeely D M, Walmsley S L, Gold W L. Use of fluconazole in the treatment of candidal endophthalmitis. Clin Infect Dis. 1995;20:657–664. doi: 10.1093/clinids/20.3.657. [DOI] [PubMed] [Google Scholar]
- 6.Akstein R, Wilson L, Teutsch S. Acquired toxoplasmosis. Ophthalmology. 1982;89:1299–1302. doi: 10.1016/s0161-6420(82)34629-1. [DOI] [PubMed] [Google Scholar]
- 7.Albesa I, Eraso A. Primary isolation of Trypanosoma cruzi by hemoculture: method of glucose concentration. Am J Trop Med Hyg. 1983;32:963–967. doi: 10.4269/ajtmh.1983.32.963. [DOI] [PubMed] [Google Scholar]
- 8.Allansmith M R. The eye and immunology. St. Louis, Mo: Mosby; 1982. [Google Scholar]
- 9.al-Rajhi A, Awad A H, al-Hedaithy S S, Forster R K, Caldwell K C. Scytalidium dimidiatum fungal endophthalmitis. Br J Ophthalmol. 1993;77:388–390. doi: 10.1136/bjo.77.6.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammon A, Rumpf K W, Hommerich C P, Behrens-Baumann W, Ruchel R. Rhinocerebral mucormycosis during deferoxamine therapy. Dtsch Med Wochenschr. 1992;117:1434–1438. doi: 10.1055/s-2008-1062461. [DOI] [PubMed] [Google Scholar]
- 11.Anderson J, Front R. Ocular onchocerciasis. In: Binford C, Connor D, editors. Pathology of tropical and extraordinary diseases. Washington, D.C.: Armed Forces Institute of Pathology; 1976. pp. 373–381. [Google Scholar]
- 12.Anderson J, Fuglasang H, Al-Zubaidy A. Onchocerciasis in Yemen with special reference to Sowda. Trans R Soc Trop Med Hyg. 1973;67:30–31. doi: 10.1016/0035-9203(73)90300-3. [DOI] [PubMed] [Google Scholar]
- 13.Anderson M, Griffith D. Intestinal giardiasis associated with ocular inflammation. J Clin Gastroenterol. 1985;7:169. doi: 10.1097/00004836-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Ando N, Takatori K. Fungal flora of the conjunctival sac. Am J Ophthalmol. 1982;94:67–74. doi: 10.1016/0002-9394(82)90193-3. [DOI] [PubMed] [Google Scholar]
- 15.Angell J, Merideth R E, Shively J N, Sigler R L. Ocular lesions associated with coccidioidomycosis in dogs: 35 cases (1980–1985) J Am Vet Med Assoc. 1987;190:1319–1322. [PubMed] [Google Scholar]
- 16.Anteby I, Kramer M, Rahav G, Benezra D. Necrotizing choroiditis-retinitis as presenting symptom of disseminated aspergillosis after lung transplantation. Eur J Ophthalmol. 1997;7:294–296. doi: 10.1177/112067219700700316. [DOI] [PubMed] [Google Scholar]
- 17.Aristimuno B, Nirankari V S, Hemady R K, Rodrigues M M. Spontaneous ulcerative keratitis in immunocompromised patients. Am J Ophthalmol. 1993;115:202–208. doi: 10.1016/s0002-9394(14)73924-8. [DOI] [PubMed] [Google Scholar]
- 18.Arvantis P, Vakalis N, Damanakis A, Theodossiadis G. Ophthalmic dirofilariasis. Am J Ophthalmol. 1997;123:689–691. doi: 10.1016/s0002-9394(14)71083-9. [DOI] [PubMed] [Google Scholar]
- 19.Ashworth J W. On Rhinosporidium seeberi (Wernicke 1903) with special reference to its sporulation and affinities. Trans R Soc Edinburgh. 1923;53:301–342. [Google Scholar]
- 20.Baley J, Kliegman R M, Annable W L, Dahms B B, Fanaroff A A. Torulopsis glabrata sepsis appearing as necrotizing enterocolitis and endophthalmitis. Am J Dis Child. 1984;138:965–966. doi: 10.1001/archpedi.1984.02140480067020. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S N, et al. Secular trends in nosocomial primary bloodstream infections in the United States. Am J Med. 1991;91:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 22.Bansal R, Gupta A, Grewal S, Mohan K. Spontaneous extrusion of cysticercosis: a report of three cases. Indian J Ophthalmol. 1992;40:59–60. [PubMed] [Google Scholar]
- 23.Barr C C, Walsh A, Wainscott B, Finger R. Aspergillus endophthalmitis in intravenous-drug users—Kentucky. Morb Mortal Wkly Rep. 1990;39:48–49. [PubMed] [Google Scholar]
- 24.Bartley G. Blastomycosis of the eyelid. Ophthalmology. 1995;102:2020–2023. doi: 10.1016/s0161-6420(95)30760-9. [DOI] [PubMed] [Google Scholar]
- 25.Barza M, Pavan P, Doft B, Wisniewski S, Wilson L, Han D, Kelsey S. Evaluation of microbiological diagnostic techniques in postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 1997;115:1142–1150. doi: 10.1001/archopht.1997.01100160312008. [DOI] [PubMed] [Google Scholar]
- 26.Baum J, Barza M. Infections of the eye. In: Gorbach S L, Bartlett J G, Blacklow N R, editors. Infectious diseases. W. B. Philadelphia, Pa: Saunders Company; 1998. pp. 1355–1373. [Google Scholar]
- 26a.Beard C, Quickert M H. Anatomy of the orbit: a dissection manual. Birmingham, Ala: Aesculapius Publishing Co.; 1988. [Google Scholar]
- 27.Behbahani R, Moshfeghi M, Baxter J. Therapeutic approaches for AIDS-related toxoplasmosis. Ann Pharmacother. 1995;29:760–768. doi: 10.1177/106002809502907-819. [DOI] [PubMed] [Google Scholar]
- 28.Bloom J, Hamor R E, Gerding P A., Jr Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985–1993) J Am Vet Med. 1996;209:1271–1274. [PubMed] [Google Scholar]
- 29.Borderie V, Bourcier T M, Poirot J L, Baudrimont M, Prudhomme de Saint-Maur P, Laroche L. Endophthalmitis after Lasiodiplodia theobromae corneal abscess. Graefes Arch Clin Exp Ophthalmol. 1997;235:259–261. doi: 10.1007/BF00941769. [DOI] [PubMed] [Google Scholar]
- 30.Brook I, Frazier E H. Aerobic and anaerobic microbiology of dacryocystitis. Am J Ophthalmol. 1998;125:552–554. doi: 10.1016/s0002-9394(99)80198-6. [DOI] [PubMed] [Google Scholar]
- 31.Brooks A, Essex W, West R. Cysticercosis of the superior oblique muscle. Aust J Ophthalmol. 1983;11:119–122. [PubMed] [Google Scholar]
- 32.Brooks R G. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Int Med. 1989;149:2226–2228. [PubMed] [Google Scholar]
- 33.Bruce-Chwatt L. Transfusion malaria revisited. Trop Dis Bull. 1982;79:827–840. [PubMed] [Google Scholar]
- 34.Butrus S I, Klotz S A. Blocking Candida adherence to contact lenses. Curr Eye Res. 1986;5:745–750. doi: 10.3109/02713688609000015. [DOI] [PubMed] [Google Scholar]
- 35.Butrus S I, Klotz S A. Contact lens surface deposits increase the adhesion of Pseudomonas aeruginosa. Curr Eye Res. 1990;9:717–724. doi: 10.3109/02713689008999566. [DOI] [PubMed] [Google Scholar]
- 36.Butrus S I, Klotz S A, Misra R P. The adherence of Pseudomonas aeruginosa to soft contact lenses. Ophthalmology. 1987;94:1310–1314. doi: 10.1016/s0161-6420(87)80017-9. [DOI] [PubMed] [Google Scholar]
- 37.Buttner D, Laer G, Mannveiller E, Buttner M. Clinical, parasitological, and serological studies on onchocerciasis in the Yemen Arab Republic. Troponmed Parasitol. 1982;33:201–212. [PubMed] [Google Scholar]
- 38.Buyukmihci N. Ocular lesions of blastomycosis in the dog. J Am Vet Med Assoc. 1982;180:426–431. [PubMed] [Google Scholar]
- 39.Buyukmihci N C, Moore P F. Microscopic lesions of spontaneous ocular blastomycosis in dogs. J Comp Pathol. 1987;97:321–328. doi: 10.1016/0021-9975(87)90096-x. [DOI] [PubMed] [Google Scholar]
- 40.Byrne K A, Burd E M, Tabbara K F, Hyndiuk R A. Diagnostic microbiology and cytology of the eye. Boston, Mass: Butterworth-Heinemann; 1995. [Google Scholar]
- 41.Callanan D, Fish G E, Anand R. Reactivation of inflammatory lesions in ocular histoplasmosis. Arch Ophthalmol. 1998;116:470–474. doi: 10.1001/archopht.116.4.470. [DOI] [PubMed] [Google Scholar]
- 42.Cameron J, Badr I A, Miguel Risco J, Abboud E, Gaonnah el S. Endophthalmitis cluster from contaminated donor corneas following penetrating keratoplasty. Can J Ophthalmol. 1998;33:8–13. [PubMed] [Google Scholar]
- 43.Cantrill H, Rodman W P, Ramsay R C, Knobloch W H. Postpartum Candida endophthalmitis. JAMA. 1980;243:1163–1165. [PubMed] [Google Scholar]
- 44.Carney M D, Tabassian A, Guerry R K. Pseudo-Allescheria boydii endophthalmitis. Retina. 1996;16:263–264. [PubMed] [Google Scholar]
- 45.Chander J, Chakrabarti A, Sharma A, Saini J S, Panigarhi D. Evaluation of Calcofluor staining in the diagnosis of fungal corneal ulcer. Mycoses. 1993;36:243–245. doi: 10.1111/j.1439-0507.1993.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 46.Chandler J W, Gillette T E. Immunologic defense mechanism of the ocular surface. Ophthalmology. 1983;90:583–591. doi: 10.1016/s0161-6420(83)34510-3. [DOI] [PubMed] [Google Scholar]
- 47.Charles N C, Boxrud C A, Small E A. Cryptococcosis of the anterior segment in acquired immune deficiency syndrome. Ophthalmology. 1992;99:813–816. doi: 10.1016/s0161-6420(92)31895-0. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhry I, Hylton C, DesMarchais B. Bilateral ptosis and lower eyelid ectropion secondary to cutaneous leishmaniasis. Arch Ophthalmol. 1998;116:1244–1245. [PubMed] [Google Scholar]
- 49.Chen S, Chung Y M, Liu J H. Endogenous Candida endophthalmitis after induced abortion. Am J Ophthalmol. 1998;125:873–875. doi: 10.1016/s0002-9394(98)00052-x. [DOI] [PubMed] [Google Scholar]
- 50.Christmas N, Smiddy W E. Vitrectomy and systemic fluconazole for treatment of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1996;27:1012–1018. [PubMed] [Google Scholar]
- 51.Coccia L, Calista D, Boschini A. Eyelid nodule: a sentinel lesion of disseminated cryptococcosis in a patient with acquired immunodeficiency syndrome. Arch Ophthalmol. 1999;117:271–272. [PubMed] [Google Scholar]
- 52.Cogen M, Hays S, Dixon J. Cutaneous myiasis of the eyelid due to Cuterebra larva. JAMA. 1987;258:1795–1796. [PubMed] [Google Scholar]
- 53.Cohen M, Montgomerie J Z. Hematogenous endophthalmitis due to Candida tropicalis: report of two cases and review. Clin Infect Dis. 1993;17:270–272. doi: 10.1093/clinids/17.2.270. [DOI] [PubMed] [Google Scholar]
- 53a.Connor D H, Chandler F W. Pathology of infectious diseases. Stamford, Conn: Appleton and Lange; 1997. [Google Scholar]
- 54.Connor D, Neafie R. Onchocerciasis. In: Binford C, Connor D, editors. Pathology of tropical and extraordinary diseases. Washington, D.C.: Armed Forces Institute of Pathology; 1976. pp. 360–372. [Google Scholar]
- 55.Corsi A, Nucci C, Knafelz D, Bulgarini D, Dilorio L, Polito A, DeRisi F, Ardenti Morini F, Paone F. Ocular changes associated with Giardia lamblia infection in children. Br J Ophthalmol. 1998;82:59–62. doi: 10.1136/bjo.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couvreur J, Thulliez P. Acquired toxoplasmosis of ocular or neurologic site: 49 cases. Presse Med. 1996;25:438–442. [PubMed] [Google Scholar]
- 57.Creach P, Auffret N, Buot G, Binet O. Microsporum canis tinea ciliaris and blepharitis. Ann Dermatol Venereol. 1995;122:773–774. [PubMed] [Google Scholar]
- 58.Crislip M A, Edwards J E. Candidiasis. Infect Dis Clin N Am. 1989;3:103–133. [PubMed] [Google Scholar]
- 59.Crump J R, Elner S G, Elner V M, Kaufmann C A. Cryptococcal endophthalmitis: case report and review. Clin Infect Dis. 1992;14:1069–1073. doi: 10.1093/clinids/14.5.1069. [DOI] [PubMed] [Google Scholar]
- 60.Cullor J, Mannis M J, Murphy C J, Smith W L, Selsted M E, Reid T W. In vitro antimicrobial activity of defensins against ocular pathogens. Arch Ophthalmol. 1990;108:861–864. doi: 10.1001/archopht.1990.01070080105044. [DOI] [PubMed] [Google Scholar]
- 61.Cunningham E, Jr, Seiff S R, Berger T G, Lizotte P E, Howes E L, Jr, Horton J C. Intraocular coccidioidomycosis diagnosed by skin biopsy. Arch Ophthalmol. 1998;116:674–677. doi: 10.1001/archopht.116.5.674. [DOI] [PubMed] [Google Scholar]
- 62.Custis P, Haller J A, de Juan E., Jr An unusual case of cryptococcal endophthalmitis. Retina. 1995;15:300–304. doi: 10.1097/00006982-199515040-00006. [DOI] [PubMed] [Google Scholar]
- 63.Daengrang S. Gnathostomiasis in Southeast Asia. Southeast Asian J Trop Med Public Health. 1981;12:319–332. [PubMed] [Google Scholar]
- 64.Das T, Vyas P, Sharma S. Aspergillus terreus postoperative endophthalmitis. Br J Ophthalmol. 1993;77:386–387. doi: 10.1136/bjo.77.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das T, Gopinathan U, Sharma S. Exogenous Helminthosporium endophthalmitis. Br J Ophthalmol. 1994;78:492–493. doi: 10.1136/bjo.78.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dayal Y, Rao S S, Mahajan V M. Comparative study of bacterial and fungal floras of contracted sockets and fellow eyes. Ann Ophthalmol. 1984;16:154. , 156, 158. [PubMed] [Google Scholar]
- 67.Deepe G S J, Klein B S. Blastomyces. In: Gorbach S L, Bartlett J G, Blacklow N R, editors. Infectious diseases. W. B. Philadelphia, Pa: Saunders Company; 1998. pp. 2365–2369. [Google Scholar]
- 68.Didier E, Shadduck J, Didier P, Millichanp N, Vossbrinck C. Studies on ocular microsporidia. J Protozool. 1991;38:635–638. [PubMed] [Google Scholar]
- 69.Doft B H, Clarkson J G, Rebell G, Forster R K. Endogenous Aspergillus endophthalmitis in drug users. Arch Ophthalmol. 1980;98:859–862. doi: 10.1001/archopht.1980.01020030853010. [DOI] [PubMed] [Google Scholar]
- 70.Donahue S P, Greven C M, Zuravleff J J, et al. Intraocular candidiasis in patients with candidemia: clinical implications derived from a prospective multicenter study. Ophthalmology. 1994;101:1302–1309. doi: 10.1016/s0161-6420(94)31175-4. [DOI] [PubMed] [Google Scholar]
- 71.Doughman D, Leavenworth N M, Campbell R C, Lindstrom R L. Fungal keratitis at the University of Minnesota: 1971–1981. Trans Am Ophthalmol Soc. 1982;80:235–247. [PMC free article] [PubMed] [Google Scholar]
- 72.Downie J A, Francis I C, Arnold J J, Bott L M, Kos S. Sudden blindness and total ophthalmoplegia in mucormycosis: a clinicopathologic correlation. J Clin Neuroophthalmol. 1993;13:27–34. [PubMed] [Google Scholar]
- 73.Duke-Elder S. System of ophthalmology. 8, part 1. St. Louis, Mo: Mosby; 1965. [Google Scholar]
- 74.Dupont B, Drouhet E. Cutaneous, ocular, and osteoarticular candidiasis in heroin addicts: new clinical and therapeutic aspects in 38 patients. J Infect Dis. 1985;152:577–591. doi: 10.1093/infdis/152.3.577. [DOI] [PubMed] [Google Scholar]
- 75.Dupont C, Carrier M, Higgins R. Bacterial and fungal flora in healthy eyes of birds of prey. Can Vet J. 1994;35:699–701. [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards J, Montgomerie J, Foos R, Shaw V, Guze L. Experimental hematogenous endophthalmitis caused by Candida albicans. J Infect Dis. 1975;131:649–657. doi: 10.1093/infdis/131.6.649. [DOI] [PubMed] [Google Scholar]
- 77.Edwards J E J. Candida endophthalmitis. In: Bodey G P, Fainstein V, editors. Candidiasis. New York, N.Y: Raven Press; 1985. pp. 211–225. [Google Scholar]
- 78.Edwards J E J. Candida species. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2289–2306. [Google Scholar]
- 79.Elbaze P, Lacour J P, Cottalorda J, Le Fichoux Y, Ortonne J P. The skin as the possible reservoir for Candida albicans in the oculocutaneous candidiasis of heroin addicts. Acta Dermatol Venereol. 1992;72:180–181. [PubMed] [Google Scholar]
- 80.Elder M, Stapleton F, Evans E, Dart J K. Biofilm-related infections in ophthamology. Eye. 1995;9:102–109. doi: 10.1038/eye.1995.16. [DOI] [PubMed] [Google Scholar]
- 81.Engstrom R J, Holland G, Nussenblatt R, Jabs D. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 1991;111:601–610. doi: 10.1016/s0002-9394(14)73706-7. [DOI] [PubMed] [Google Scholar]
- 82.Eschete M, King J W, West B C, Oberle A. Penicillium chrysogenum endophthalmitis. Mycopathologia. 1981;74:125–127. doi: 10.1007/BF01259468. [DOI] [PubMed] [Google Scholar]
- 83.Essman T, Flynn H W, Jr, Smiddy W E, Brod R D, Murray T G, Davis J L, Rubsamen P E. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28:185–194. [PubMed] [Google Scholar]
- 84.Feldman H. Epidemiology of toxoplasma infections. Epidemiol Rev. 1982;4:204–213. doi: 10.1093/oxfordjournals.epirev.a036247. [DOI] [PubMed] [Google Scholar]
- 85.Ferry A P, Abedi S. Diagnosis and management of rhino-orbitocerebral mucormycosis (phycomycosis): a report of 16 personally observed cases. Ophthalmology. 1983;90:1096–1104. doi: 10.1016/s0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]
- 86.Filler S, Crislip M A, Mayer C L, Edwards J E., Jr Comparison of fluconazole and amphotericin B for treatment of disseminated candidiasis and endophthalmitis in rabbits. Antimicrob Agents Chemother. 1991;35:288–292. doi: 10.1128/aac.35.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Florakis G, Moazami G, Schubert H, Koester C J, Auran J D. Scanning slit confocal microscopy of fungal keratitis. Arch Ophthalmol. 1997;115:1461–1463. doi: 10.1001/archopht.1997.01100160631019. [DOI] [PubMed] [Google Scholar]
- 88.Font R, Parsons M A, Keener M J, Shaver R P, Foos R Y. Involvement of anterior chamber angle structures in disseminated histoplasmosis: report of three cases. Geriatr J Ophthalmol. 1995;4:107–115. [PubMed] [Google Scholar]
- 89.Fredrickson A B. Behavior of mixed culture of microorganisms. Annu Rev Microbiol. 1969;31:63. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- 90.Freeman W R, Gross J G, Labelle J, Oteken K, Katz B, Wiley C A. Pneumocystis carinii choroidopathy. A new clinical entity. Arch Ophthalmol. 1989;107:863–867. doi: 10.1001/archopht.1989.01070010885036. [DOI] [PubMed] [Google Scholar]
- 91.Frenkel J. Toxoplasmosis. In: Marcial-Rojas R, editor. Pathology of protozoal and helminthic diseases. Baltimore, Md: Williams and Wilkins; 1971. pp. 254–290. [Google Scholar]
- 92.Fridkin S, Kremer F B, Bland L A, Padhye A, McNeil M M, Jarvis W R. Acremonium kiliense endophthalmitis that occurred after cataract extraction in an ambulatory surgical center and was traced to an environmental reservoir. Clin Infect Dis. 1996;22:222–227. doi: 10.1093/clinids/22.2.222. [DOI] [PubMed] [Google Scholar]
- 93.Fujita N K, Henderson D K, Hockey L J, et al. Comparative ocular pathogenicity of Cryptococcus neoformans, Candida glabrata, and Aspergillus fumigatus in the rabbit. Investig Ophthalmol Vis Sci. 1982;22:410. [PubMed] [Google Scholar]
- 94.Gabriele P, Hutchins R K. Fusarium endophthalmitis in an intravenous drug abuser. Am J Ophthalmol. 1996;122:119–121. doi: 10.1016/s0002-9394(14)71976-2. [DOI] [PubMed] [Google Scholar]
- 95.Ganley J P. Epidemiologic characteristics of presumed ocular histoplasmosis. Acta Ophthalmol. 1973;51:1–63. [PubMed] [Google Scholar]
- 96.Ganley J P, Klotz S A. Coccidioidomycosis. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 70–73. [Google Scholar]
- 97.Garaffini T, Ducasse A, Jaussaud R, Strady A, Pinon J. Human periorbital dirofilariasis. J Fr Ophthalmol. 1996;19:55–57. [PubMed] [Google Scholar]
- 98.Garcia L, Bruckner D. Diagnostic medical parasitology. 3rd ed. Washington, D.C.: ASM Press; 1997. [Google Scholar]
- 99.Garrity J, Herman D C, Imes R, Fries P, Hughes C F, Campbell R J. Optic nerve sheath decompression for visual loss in patients with acquired immunodeficiency syndrome and cryptococcal meningitis with papilledema. Am J Ophthalmol. 1993;116:472–478. doi: 10.1016/s0002-9394(14)71407-2. [DOI] [PubMed] [Google Scholar]
- 100.Gendelman D, Blumberg R, Sadun A. Ocular Loa loa with cryoprobe extraction of subconjunctival worm extraction. Ophthalmology. 1984;91:300–303. doi: 10.1016/s0161-6420(84)34302-0. [DOI] [PubMed] [Google Scholar]
- 101.Ghose S, Mahajan V M. Fungal flora in congenital dacryocystitis. Indian J Ophthalmol. 1990;38:189–190. [PubMed] [Google Scholar]
- 102.Gibbs R. Loiasis: report of three cases and literature review. J Nat Med Assoc. 1979;71:853–84. [PMC free article] [PubMed] [Google Scholar]
- 103.Gibson D, Heggie C, Connor D. Clinical and pathologic aspects of onchocerciasis. Pathol Annu. 1980;15:195–240. [PubMed] [Google Scholar]
- 104.Gilbert D, Moellering R, Sande M. The Sanford guide to antimicrobial therapy. 28th ed. Vienna, Va: Antimicrobial Therapy Inc.; 1998. [Google Scholar]
- 105.Gillette T E, Chandler J W, Greiner J V. Langerhans cells of the ocular surface. Ophthalmology. 1982;89:700–711. doi: 10.1016/s0161-6420(82)34737-5. [DOI] [PubMed] [Google Scholar]
- 106.Glasgow B, Engstrom R E, Jr, Holland G N, Kreiger A E, Wool M G. Bilateral endogenous Fusarium endophthalmitis associated with acquired immunodeficiency syndrome. Arch Ophthalmol. 1996;114:873–877. doi: 10.1001/archopht.1996.01100140087017. [DOI] [PubMed] [Google Scholar]
- 107.Golnik K C, Newman S A, Wispelway B. Cryptococcal optic neuropathy in the acquired immune deficiency syndrome. J Clin Neuroophthalmol. 1991;11:96–103. [PubMed] [Google Scholar]
- 108.Gottlieb J, McAllister I L, Guttman F A, Vine A K. Choroidal blastomycosis. A report of two cases. Retina. 1995;15:248–252. [PubMed] [Google Scholar]
- 109.Grieve R, Lok J, Glickman L. Epidemiology of canine heartworm infection. Epidemiol Rev. 1983;5:220–246. doi: 10.1093/oxfordjournals.epirev.a036260. [DOI] [PubMed] [Google Scholar]
- 110.Reference deleted.
- 111.Guillory S, Zinn K. Intravitreal Cysticercus cellulosae: ultrasonographic and fluoresceinangiographic features. Bull N Y Acad Med. 1980;56:655–661. [PMC free article] [PubMed] [Google Scholar]
- 112.Gumbel H, Ohrloff C, Shah P M. The conjunctival flora of HIV-positive patients in an advanced stage. Fortschr Ophthalmol. 1990;87:382–383. [PubMed] [Google Scholar]
- 113.Hamburg A, De Jonckheere J. Amoebic keratitis. Ophthalmologica. 1980;181:74–80. doi: 10.1159/000309030. [DOI] [PubMed] [Google Scholar]
- 114.Hammer M, Harding S, Wynn P. Post-traumatic fungal endophthalmitis caused by Exophiala jeanselmei. Ann Ophthalmol. 1983;15:853–855. [PubMed] [Google Scholar]
- 115.Hanssens M, de Jonck Heere J, de Meunynck C. Acanthamoebic keratitis. Int Ophthalmol. 1985;7:203–213. doi: 10.1007/BF00128367. [DOI] [PubMed] [Google Scholar]
- 116.Harrill W C, Stewart M G, Lee A G, Cernoch P. Chronic rhinocerebral mucormycosis. Laryngoscope. 1996;106:1292–1297. doi: 10.1097/00005537-199610000-00024. [DOI] [PubMed] [Google Scholar]
- 117.Harvey J. Sheep botfly: ophthalmomyiasis externa. Can J Ophthalmol. 1986;21:92–95. [PubMed] [Google Scholar]
- 118.Harwood C, Rich G, McAleer R, Cherian G. Isolation of Acanthamoeba from a cerebral abscess. Med J Austr. 1988;148:47–49. doi: 10.5694/j.1326-5377.1988.tb104486.x. [DOI] [PubMed] [Google Scholar]
- 119.Heffler K, Echhardt T, Reboli A, Stieritz D. Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am J Ophthalmol. 1996;122:584–586. doi: 10.1016/s0002-9394(14)72126-9. [DOI] [PubMed] [Google Scholar]
- 120.Heier J, Gardner T A, Hawes M J, McGuire K A, Walton W T, Stock J. Proptosis as the initial presentation of fungal sinusitis in immunocompetent patients. Ophthalmology. 1995;102:713–717. doi: 10.1016/s0161-6420(95)30964-5. [DOI] [PubMed] [Google Scholar]
- 121.Hemady R. Microbial keratitis in patients infected with the human immunodeficiency virus. Ophthalmology. 1995;102:1026–1030. doi: 10.1016/s0161-6420(95)30917-7. [DOI] [PubMed] [Google Scholar]
- 122.Henderson D K, Hockey L B, Vukalcic L J, et al. Effect of immunosuppression on the development of experimental hematogenous Candida endophthalmitis. Infect Immun. 1980;27:628–631. doi: 10.1128/iai.27.2.628-631.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Henderson T, Irfan S. Bilateral endogenous Candida endophthalmitis and chorioretinitis following toxic megacolon. Eye. 1996;10:755–757. doi: 10.1038/eye.1996.179. [DOI] [PubMed] [Google Scholar]
- 124.Henig F, Lehrer N, Gabbay A, Kurz O. Paecilomycosis of the lacrimal sac. Mykosen. 1973;16:25–28. doi: 10.1111/j.1439-0507.1973.tb04047.x. [DOI] [PubMed] [Google Scholar]
- 125.Herr R A, Ajello L, Taylor J W, Arseculeratne S N, Mendoza L. Phylogenetic analysis of Rhinosporidium seeberi's 18S small-unit ribosomal DNA groups this pathogen among members of the protoctistan Mesomycetozoa clade. J Clin Microbiol. 1999;37:2750–2754. doi: 10.1128/jcm.37.9.2750-2754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heyde R, Seiff S, Mucia J. Ophthalmomyiasis externa in California. W J Med. 1986;144:80–81. [PMC free article] [PubMed] [Google Scholar]
- 127.Huber-Spitzy V, Bohler-Sommeregger K, Arocker-Mettinger E, Grabner G. Ulcerative blepharitis in atopic patients—is Candida species the causative agent? Br J Ophthalmol. 1992;76:272–274. doi: 10.1136/bjo.76.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacobson M, Galetta S L, Atlas S W, Curtis M T, Wule A W. Bipolaris-induced orbital cellulitis. J Clin Neuroophthalmol. 1992;12:250–256. [PubMed] [Google Scholar]
- 129.Jager M, Chodosh J, Huang A J, Alfonso E C, Culbertson W W, Forster R K. Aspergillus niger as an unusual cause of scleritis and endophthalmitis. Br J Ophthalmol. 1994;78:584–586. doi: 10.1136/bjo.78.7.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johnson T E, Casiano R R, Kronish J W, Tse D T, Meldrum M, Chang W. Sino-orbital aspergillosis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1999;117:57–64. doi: 10.1001/archopht.117.1.57. [DOI] [PubMed] [Google Scholar]
- 131.Jones D, Visvesvara G, Robinson N. Acanthamoeba polyphagia keratitis and acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol. 1975;95:221–232. [PubMed] [Google Scholar]
- 132.Jones D B. Chemotherapy of fungal infections. In: Srinevasan B D, editor. Ocular therapeutics. New York, N.Y: Masson; 1980. pp. 35–50. [Google Scholar]
- 133.Joshi N, Hamory B H. Endophthalmitis caused by non-albicans species of Candida. Rev Infect Dis. 1991;13:281–287. doi: 10.1093/clinids/13.2.281. [DOI] [PubMed] [Google Scholar]
- 134.Kameh D, Gonzalez O R, Pearl G S, Walsh A F, Gambon T, Kropp T M. Fatal rhino-orbital-cerebral zygomycosis. South Med J. 1997;90:1133–1135. doi: 10.1097/00007611-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 135.Kanungo R, Srinivasan R, Rao R S. Acridine orange staining in early diagnosis of mycotic keratitis. Acta Ophthalmol. 1991;69:750–753. doi: 10.1111/j.1755-3768.1991.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 136.Katz B J, Scott W E, Folk J C. Acute histoplasmosis choroiditis in 2 immunocompetent brothers. Arch Ophthalmol. 1997;115:1470–1472. doi: 10.1001/archopht.1997.01100160640023. [DOI] [PubMed] [Google Scholar]
- 137.Kauffman C A, Bradley S F, Vine A K. Candida endophthalmitis associated with intraocular lens implantation: efficacy of fluconazole therapy. Mycoses. 1993;36:13–17. doi: 10.1111/j.1439-0507.1993.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 137a.Kaufman H E, Barron B A, McDonald M B, editors. The cornea. Woburn, Mass: Butterworth-Heinemann; 1998. [Google Scholar]
- 138.Kean B, Sun T, Ellsworth R. Color atlas/text of ophthalmologic parasitology. 1st ed. New York, N.Y: Igaku-Shoin; 1991. [Google Scholar]
- 139.Keating M, Guerrero M A, Daly R C, Walker R C, Davies S F. Transmission of invasive aspergillosis from a subclinically infected donor to three different organ transplant recipients. Chest. 1996;109:1119–1124. doi: 10.1378/chest.109.4.1119. [DOI] [PubMed] [Google Scholar]
- 140.Kersten R, Showkrey N, Tabbara K. Orbital myiasis. Ophthalmology. 1986;93:1228–1232. doi: 10.1016/s0161-6420(86)33592-9. [DOI] [PubMed] [Google Scholar]
- 141.Kirsch L, Brownstein S. Fungal invasion of seven hydrophilic contact lenses. Am J Opthalmol. 1993;115:460–465. doi: 10.1016/s0002-9394(14)74447-2. [DOI] [PubMed] [Google Scholar]
- 142.Klotz S A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992;14:340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- 143.Klotz S A. Plasma and extracellular matrix proteins mediate in the fate of Candida albicans in the human host. Med Hypotheses. 1994;42:328–334. doi: 10.1016/0306-9877(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 144.Klotz S A, Au Y-K, Misra R P. A partial-thickness epithelial defect increases the adherence of Pseudomonas aeruginosa to the cornea. Investig Ophthalmol Vis Sci. 1989;30:1069–1074. [PubMed] [Google Scholar]
- 145.Klotz S A, Misra R P, Butrus S I. Contact lens wear enhances adherence of Pseudomonas aeruginosa and binding of lectins to the cornea. Cornea. 1990;9:266–270. [PubMed] [Google Scholar]
- 146.Klotz S A, Zahid M A, Bartholomew W, Revera P M, Butrus S I. Candida albicans keratitis treated successfully with itraconazole. Cornea. 1996;15:102–104. [PubMed] [Google Scholar]
- 147.Klotz S I, Misra R P, Butrus S I. Carbohydrate deposits on the surfaces of worn extended-wear soft contact lenses. Arch Ophthalmol. 1987;105:974–977. doi: 10.1001/archopht.1987.01060070118039. [DOI] [PubMed] [Google Scholar]
- 148.Knox D, King J., Jr Retinal arteritis, iridocyclitis and giardiasis. Ophthalmology. 1982;89:1303–1308. doi: 10.1016/s0161-6420(82)34636-9. [DOI] [PubMed] [Google Scholar]
- 149.Kochar D, Shubhakaran, Kumawat B, Thanvi I, Joshi A, Vyas S. Ophthalmoscopic abnormalities in adults with falciparum malaria. Q J Med. 1998;91:845–852. doi: 10.1093/qjmed/91.12.845. [DOI] [PubMed] [Google Scholar]
- 150.Krukar-Baster K, Zygulska-Mach H, Sajak-Hydzik K, Kubicka-Trzaska A, Dymon M. Long term observations of ocular toxocariasis in children and youth. Klin Oczna. 1996;98:445–448. [PubMed] [Google Scholar]
- 151.Lamps C, Oeltmann T N, Collins M J, Jr, Robinson R D, Logan R A, Head W S, O'Day D M. Development of a chitin assay for the quantification of fungus. Curr Eye Res. 1995;14:637–641. doi: 10.3109/02713689508998490. [DOI] [PubMed] [Google Scholar]
- 152.Larocco M, Dorenbaum A, Robinson A, Pickering L K. Recovery of Malassezia pachydermatis from eight infants in a neonatal intensive care nursery: clinical and laboratory features. J Pediatr Infect Dis. 1988;7:398–401. doi: 10.1097/00006454-198806000-00006. [DOI] [PubMed] [Google Scholar]
- 153.Lee B, McMillian R. Ocular filariasis in an African student in Missouri. Ann Ophthalmol. 1984;16:456–458. [PubMed] [Google Scholar]
- 154.Lee B L, Holland G N, Glasgow B J. Chiasmal infarction and sudden blindness caused by mucormycosis in AIDS and diabetes mellitus. Am J Ophthalmol. 1996;122:895–896. doi: 10.1016/s0002-9394(14)70392-7. [DOI] [PubMed] [Google Scholar]
- 155.Levin L A, Avery R, Shore J W, Woog J J, Baker A S. The spectrum of orbital aspergillosis: a clinicopathological review. Survey Ophthalmol. 1996;41:142–154. doi: 10.1016/s0039-6257(96)80004-x. [DOI] [PubMed] [Google Scholar]
- 156.Liesegang T J. Fungal keratitis. In: Kaufman H E, Barron B A, McDonald M B, editors. The cornea. Boston, Mass: Butterworth-Heinemann; 1998. pp. 219–245. [Google Scholar]
- 157.Liesegang T J, Forster R F. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:80. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 158.Logan R A, O'Day D M. Blastomycosis. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 66–67. [Google Scholar]
- 159.López-Vílez R, Súarez de Figueroa M, Gimeno L, Garcia-Camacho A, Fenoy S, Guillén J. Ocular toxocariasis or retinoblastoma? Enferm Infect Microbiol Clin. 1995;13:242–245. [PubMed] [Google Scholar]
- 160.Louie T, el Baba F, Shulman M, Jimenez-Lucho V. Endogenous endophthalmitis due to Fusarium: case report and review. Clin Infect Dis. 1994;18:585–588. doi: 10.1093/clinids/18.4.585. [DOI] [PubMed] [Google Scholar]
- 161.Lowder C, McMahon J, Meilser D, Dodds E, Calabrese L, Didier E, Cali A. Microsporidial keratoconjunctivitis caused by Septata intestinalis in a patient with acquired immunodeficiency syndrome. Am J Ophthalmol. 1996;121:715–717. doi: 10.1016/s0002-9394(14)70642-7. [DOI] [PubMed] [Google Scholar]
- 162.Lund O, Stefani F, Dechant W. Amoebic keratitis: a clinicopathologic case report. Arch Ophthalmol. 1980;98:475–479. [Google Scholar]
- 163.Luttrull J, Wan W L, Kubak B M, Smith M D, Oster H A. Treatment of ocular fungal infections with oral fluconazole. Am J Ophthalmol. 1995;119:477–481. doi: 10.1016/s0002-9394(14)71234-6. [DOI] [PubMed] [Google Scholar]
- 164.Maenza J R, Merz W G. Candida albicans and related species. In: Gorbach S L, Bartlett J G, Blacklow N R, editors. Infectious diseases. W. B. Philadelphia, Pa: Saunders Company; 1998. pp. 2313–2322. [Google Scholar]
- 165.Magie S K. Ocular histoplasmosis. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 75–77. [Google Scholar]
- 166.Maguire L, Campbell R J, Edson R S. Coccidioidomycosis with necrotizing granulomatous conjunctivitis. Cornea. 1994;13:539–542. [PubMed] [Google Scholar]
- 167.Mannis M J, Smolin G. Natural defense mechanism of the ocular surface. Ocular infection and immunity. St. Louis, Mo: Mosby; 1996. [Google Scholar]
- 168.Marshall D, Browstein S, Jackson W B, Mintsioulis G, Gilberg S M, al-Zerah B F. Post-traumatic corneal mucormycosis caused by Absidia corymbifera. Ophthalmology. 1997;104:1107–1111. doi: 10.1016/s0161-6420(97)30177-8. [DOI] [PubMed] [Google Scholar]
- 169.Martin M, Rahman M R, Johnson G J, Srinivasan M, Clayton Y M. –1996. Mycotic keratitis: susceptibility to antiseptic agents. Int Ophthalmol. 1995;19:299–302. doi: 10.1007/BF00130925. [DOI] [PubMed] [Google Scholar]
- 170.Mason G. Bilateral ophthalmomyiasis interna. Am J Ophthalmol. 1981;91:65–70. doi: 10.1016/0002-9394(81)90350-0. [DOI] [PubMed] [Google Scholar]
- 171.Massry G G, Hornblass A, Harrison W. Itraconazole in the treatment of orbital aspergillosis. Ophthalmology. 1996;103:1467–1470. doi: 10.1016/s0161-6420(96)30482-x. [DOI] [PubMed] [Google Scholar]
- 172.Matsuo T, Nakagawa H, Matsuo N. Endogenous Aspergillus endophthalmitis associated with periodonitis. Ophthalmologica. 1995;209:109–111. doi: 10.1159/000310592. [DOI] [PubMed] [Google Scholar]
- 173.McCabe R, Remmington J. Toxoplasmosis: the time has come. N Engl J Med. 1988;318:313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- 174.McCray E, Rampell N, Solomon S L, et al. Outbreak of Candida parapsilosis endophthalmitis after cataract extraction and intraocular lens implantation. J Clin Microbiol. 1986;24:625–628. doi: 10.1128/jcm.24.4.625-628.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McMillan T A, Lashkari K. Ocular histoplasmosis. Int Ophthalmol Clin. 1996;36:179–186. doi: 10.1097/00004397-199603630-00016. [DOI] [PubMed] [Google Scholar]
- 176.Mendicute J, Ondarra A, Eder F, Ostolaza J I, Salaberria M, Lamsfus J M. The use of collagen shields impregnated with amphotericin B to treat Aspergillus keratomycosis. CLAO J. 1995;21:252–255. [PubMed] [Google Scholar]
- 177.Messner K, Kaminerer M. Intraocular cysticercosis. Arch Ophthalmol. 1979;97:1103–1105. doi: 10.1001/archopht.1979.01020010557010. [DOI] [PubMed] [Google Scholar]
- 178.Mislivec P, Bandler R, Allen G. Incidence of fungi in shared-use cosmetics available to the public. J AOAC Int. 1993;76:430–436. [PubMed] [Google Scholar]
- 179.Molk R. Ocular toxocariasis: a review of the literature. Ann Ophthalmol. 1983;15:216–231. [PubMed] [Google Scholar]
- 180.Moorthy R, Rao N A, Sidikaro Y, Foos R Y. Coccidioidomycosis iridocyclitis. Ophthalmology. 1994;101:1923–1928. doi: 10.1016/s0161-6420(94)31082-7. [DOI] [PubMed] [Google Scholar]
- 180a.Morinelli E N, Dugel P U, Riffenburgh R, Rao N A. Infectious multifocal choroiditis in patients with acquired immune deficiency syndrome. Ophthalmology. 1993;100:1014–1021. doi: 10.1016/s0161-6420(93)31543-5. [DOI] [PubMed] [Google Scholar]
- 181.Mowrey-McKee M, Sampson H J, Proskin H M. Microbial contamination of hydrophilic contact lenses. Part II. Quantitation of microbes after patient handling and after septic removal from the eye. CLAO J. 1992;18:240–244. [PubMed] [Google Scholar]
- 182.Muccioli C, Belfort Junior R, Neves R, Rao N. Limbal and choroidal Cryptococcus infection in the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;120:539–540. doi: 10.1016/s0002-9394(14)72677-7. [DOI] [PubMed] [Google Scholar]
- 183.Muralidhar S, Sulthana C M. Rhodotorula causing chronic dacryocystitis: a case report. Indian J Ophthalmol. 1995;43:196–198. [PubMed] [Google Scholar]
- 184.Nassif K F. Ocular surface defense mechanisms. Infections of the eye. New York, N.Y: Little, Brown and Company; 1996. [Google Scholar]
- 185.Nema H, Ahuja O P, Bal A, Mohapatra L N. Effects of topical corticosteroids and antibiotics on mycotic flora of conjunctiva. Am J Ophthalmol. 1968;65:747–750. doi: 10.1016/0002-9394(68)94393-6. [DOI] [PubMed] [Google Scholar]
- 186.Neppert B, Guthoff R, Heidemann H T. Endogenous Candida endophthalmitis in a drug dependent patient: intravenous therapy with liposome encapsulated amphotericin B. Klin Monatsbl Augenheilkd. 1992;201:122–124. doi: 10.1055/s-2008-1045881. [DOI] [PubMed] [Google Scholar]
- 187.Nitzulescu V, Niculescu M. Three cases of ocular candidiasis caused by Candida lipolytica. Arch Roum Pathol Exp Microbiol. 1976;35:269–272. [PubMed] [Google Scholar]
- 188.Nitzulescu V, Niculescu M. Ophthalmopathy determined by Candida humicola. Arch Roum Pathol Exp Microbiol. 1975;34:357–361. [PubMed] [Google Scholar]
- 189.O'Connor G. Manifestations and management of ocular toxoplasmosis. Bull N Y Acad Med. 1974;50:192–210. [PMC free article] [PubMed] [Google Scholar]
- 190.O'Day D M, Head W S, Robinson R D. An outbreak of Candida parapsilosis endophthalmitis analysis of strains by enzyme profile and antifungal susceptibility. Br J Ophthalmol. 1987;71:126–129. doi: 10.1136/bjo.71.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Okhravi N, Dart J K, Towler H M, Lightman S. Paecilomyces lilacinus endophthalmitis with secondary keratitis: a case report and literature review. Arch Ophthalmol. 1997;115:1320–1324. doi: 10.1001/archopht.1997.01100160490021. [DOI] [PubMed] [Google Scholar]
- 192.Okhravi N, Adamson P, Mant R, Matheson M, Midgley G, Towler H, Lightman S. Polymerase chain reaction and restriction fragment length polymorphism mediated detection and speciation of Candida spp. causing intraocular infection. Investig Ophthalmol Vis Sci. 1998;39:859–866. [PubMed] [Google Scholar]
- 193.Orr P, Safneck J R, Napier L B. Monosporium apiospermum endophthalmitis in a patient without risk factors for infection. Can J Ophthalmol. 1993;28:187–190. [PubMed] [Google Scholar]
- 194.Oxford K, Abbott R L, Fung W E, Ellis D S. Aspergillus endophthalmitis after sutureless cataract surgery. Am J Ophthalmol. 1995;120:534–535. doi: 10.1016/s0002-9394(14)72673-x. [DOI] [PubMed] [Google Scholar]
- 195.Pappaioanou M, Schwabe C, Sand D. An evolving pattern of human hydatid disease transmission in the United States. Am J Trop Med Hyg. 1977;26:732–742. doi: 10.4269/ajtmh.1977.26.732. [DOI] [PubMed] [Google Scholar]
- 196.Parke D W, Jones D B, Gentry L O. Endogenous endophthalmitis among patients with candidemia. Ophthalmology. 1982;89:789–796. doi: 10.1016/s0161-6420(82)34722-3. [DOI] [PubMed] [Google Scholar]
- 197.Parunovic A, Halde C. Pityrosporum orbiculare: its possible role in seborrheic blepharitis. Am J Ophthalmol. 1967;63:815. [PubMed] [Google Scholar]
- 198.Paul E, Zimmerman L. Some observations on the ocular pathology of onchocerciasis. Hum Pathol. 1970;1:581–594. doi: 10.1016/s0046-8177(70)80058-2. [DOI] [PubMed] [Google Scholar]
- 199.Pavan P R, Margo C E. Endogenous endophthalmitis caused by Bipolaris hawaiiensis in a patient with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:644–645. doi: 10.1016/s0002-9394(14)73211-8. [DOI] [PubMed] [Google Scholar]
- 200.Perlman E, Binns L. Intense photophobia caused by Arthrographis kalrae in a contact lens-wearing patient. Am J Ophthalmol. 1997;123:547–549. doi: 10.1016/s0002-9394(14)70182-5. [DOI] [PubMed] [Google Scholar]
- 201.Perry D, Merritt J. Congenital ocular toxoplasmosis. J Natl Med Assoc. 1983;75:169–174. [PMC free article] [PubMed] [Google Scholar]
- 202.Perry H, Font R. Cysticercosis of the eyelid. Arch Ophthalmol. 1978;96:1255–7. doi: 10.1001/archopht.1978.03910060081017. [DOI] [PubMed] [Google Scholar]
- 203.Petersen M, Althaus C, Santen R, Gerhaz C D. Endogenous Aspergillus endophthalmitis in AIDS. Klin Monatsbl Augenheilkd. 1997;211:400–402. doi: 10.1055/s-2008-1035156. [DOI] [PubMed] [Google Scholar]
- 204.Pettit T H, Olson R J, Foos R Y, Martin W J. Fungal endophthalmitis following intraocular lens implantation. A surgical epidemic. Arch Ophthalmol. 1980;98:1025–1039. doi: 10.1001/archopht.1980.01020031015002. [DOI] [PubMed] [Google Scholar]
- 205.Pflugfelder S C, Flynn H W, Zwickey T A, Forster R K, Tsiligianni A, Culbertson W W, Mandelbaum S. Endogenous fungal endophthalmitis. Ophthalmology. 1988;95:19–30. doi: 10.1016/s0161-6420(88)33229-x. [DOI] [PubMed] [Google Scholar]
- 206.Pleyer U, Grammer J, Pleyer J H, Kosmidis P, Fries D, Schmidt K H, Thiel H J. Amphotericin B—bioavailability in the cornea. Studies with local administration of liposome incorporated amphotericin B. Ophthalmologenetics. 1995;92:469–475. [PubMed] [Google Scholar]
- 207.Price D, Hopps H. Loiasis (the eye worm, loa worm) In: Marcel-Rojas R, editor. Pathology of protozoal and helminthic diseases. Baltimore, Md: Williams and Wilkins; 1971. pp. 917–926. [Google Scholar]
- 208.Purgason P, Hornblass A, Loeffler M. Atypical presentation of fungal dacryocystitis. A report of two cases. Ophthalmology. 1992;99:1430–1432. doi: 10.1016/s0161-6420(92)31788-9. [DOI] [PubMed] [Google Scholar]
- 209.Rahman M, Minassian D C, Srinivasan M, Martin M J, Johnson G J. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiol. 1997;4:141–149. doi: 10.3109/09286589709115721. [DOI] [PubMed] [Google Scholar]
- 210.Raina U, Taneja S, Lamba P, Bansal R. Spontaneous extrusion of extraocular cysticercus cysts. Am J Ophthalmol. 1996;121:438–441. doi: 10.1016/s0002-9394(14)70442-8. [DOI] [PubMed] [Google Scholar]
- 211.Rao N, Nerenberg A V, Forster D J. Torulopsis candida (Candida famata) endophthalmitis simulating Propionibacterium syndrome. Arch Ophthalmol. 1991;109:1718–1721. doi: 10.1001/archopht.1991.01080120102037. [DOI] [PubMed] [Google Scholar]
- 212.Rebell G, Forster R K. Lasiodiplodia theobromae as a cause of keratomycoses. Sabouraudia. 1976;14:155–170. doi: 10.1080/00362177685190231. [DOI] [PubMed] [Google Scholar]
- 213.Reidy J, Sudesh S, Klafter A B, Olivia C. Infection of the conjunctiva by Rhinosporidium seeberi. Surv Ophthalmol. 1997;41:409–413. doi: 10.1016/s0039-6257(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 214.Rippon J W. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes. Philadelphia, Pa: W. B. Saunders Company; 1988. [Google Scholar]
- 215.Rippon J W. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes. Philadelphia, Pa: W. B. Saunders Company; 1988. pp. 506–531. [Google Scholar]
- 216.Rippon J W. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes. Philadelphia, Pa: W. B. Saunders Company; 1988. pp. 362–372. [Google Scholar]
- 217.Ritterband D, Seedor J A, Shah M K, Waheed S, Schoor I. A unique case of Cryptococcus laurentii keratitis spread by a rigid gas permeable contact lens in a patient with onychomycosis. Cornea. 1998;17:115–118. doi: 10.1097/00003226-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 218.Robin J, Arffa R C, Avni I, Rao N A. Rapid visualization of three common fungi using fluorescein-conjugated lectins. Investig Ophthalmol Vis Sci. 1986;27:500–506. [PubMed] [Google Scholar]
- 219.Roney P, Barr C C, Chun C H, et al. Endogenous Aspergillus endophthalmitis. Rev Infect Dis. 1986;8:955–958. doi: 10.1093/clinids/8.6.955. [DOI] [PubMed] [Google Scholar]
- 220.Ruggli G, Weber R, Messmer E P, Font R L, Moll C, Bernauer W. Pneumocystis carinii infection of the conjunctiva in a patient with acquired immune deficiency syndrome. Ophthalmology. 1997;104:1853–1856. doi: 10.1016/s0161-6420(97)30017-7. [DOI] [PubMed] [Google Scholar]
- 221.Saberhagen C, Klotz S A, Bartholomew W, Drews D, Dixon A. Infection due to Paecilomyces lilacinus: a challenging clinical identification. Clin Infect Dis. 1997;25:1411–1413. doi: 10.1086/516136. [DOI] [PubMed] [Google Scholar]
- 222.Safar E H, Abd-el Ghaffar F M, Saffar S A, Makled K M, Habib K S, el Abiad R, Shabrawy E. Incidence of Toxoplasma and Toxocara antibodies among outpatients in the Ophthalmic Research Institute, Egypt. J Egypt Soc Parasitol. 1995;25:839–852. [PubMed] [Google Scholar]
- 223.Safneck J, Hogg G R, Napier L B. Endophthalmitis due to Blastomyces dermatitidis. Case report and review of the literature. Ophthalmology. 1990;97:212–216. doi: 10.1016/s0161-6420(90)32604-0. [DOI] [PubMed] [Google Scholar]
- 224.Saggau D, Bourne W M, Sinkeldam I R, Roberts G D. Replication of fungi in K-Sol corneal preservation medium at 4 degrees C. Arch Ophthalmol. 1986;104:1362–1363. doi: 10.1001/archopht.1986.01050210116036. [DOI] [PubMed] [Google Scholar]
- 225.Saltici A, Gurler B, Gurel M. Mechanical ptosis and lagophthalmos in cutaneous leishmaniasis. Br J Ophthalmol. 1998;82:975. doi: 10.1136/bjo.82.8.974a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Samiy N, D'Amico D J. Endogenous fungal endophthalmitis. Int Opthalmol Clin. 1996;36:147–162. doi: 10.1097/00004397-199603630-00014. [DOI] [PubMed] [Google Scholar]
- 227.Saran B R, Pomilla P V. Retinal vascular nonperfusion and retinal neovascularization as a consequence of cytomegalovirus retinitis and cryptococcal choroiditis. Retina. 1996;16:510–512. doi: 10.1097/00006982-199616060-00007. [DOI] [PubMed] [Google Scholar]
- 228.Schantz D, Meyer D, Glickman L. Clinical serologic, and epidemiologic characteristics of ocular toxocariasis. Am J Trop Med Hyg. 1979;28:24–28. doi: 10.4269/ajtmh.1979.28.24. [DOI] [PubMed] [Google Scholar]
- 229.Schantz P, Glickman L. Toxocaral larva migrans. N Engl J Med. 1978;298:426–429. doi: 10.1056/NEJM197802232980806. [DOI] [PubMed] [Google Scholar]
- 230.Schimkat M, Althaus C, Armbrecht C, Jablonowski H, Sundmacher R. Treatment of toxoplasmosis retinochoroiditis with atovaquone in an AIDS patient. Klin Monatsbl Augenheilkd. 1995;206:173–177. doi: 10.1055/s-2008-1035425. [DOI] [PubMed] [Google Scholar]
- 231.Scholz R, Green W R, Kutys R, Sutherland J, Richards R D. Histoplasma capsulatum in the eye. Ophthalmology. 1984;91:1100–1104. doi: 10.1016/s0161-6420(84)34190-2. [DOI] [PubMed] [Google Scholar]
- 232.Schwab I R, Dawson C R. Conjunctiva. In: Vaughan D G, Asbury T, Riordan-Eva P, editors. General ophthalmology. Norwalk, Conn: Appleton and Lange; 1995. pp. 95–122. [Google Scholar]
- 233.Schwartz L, Loignon L M, Webster R G., Jr Posttraumatic phycomycosis of the anterior segment. Arch Ophthalmol. 1978;96:860–863. doi: 10.1001/archopht.1978.03910050462014. [DOI] [PubMed] [Google Scholar]
- 234.Segal E, Romano A, Eylan E, Stein R. Fungal flora of the normal conjuctival sac. Mykosen. 1977;20:9–14. doi: 10.1111/j.1439-0507.1977.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 235.Sehgal S, Dhawan S, Chhiber S, Sharma M, Talwar P. Frequency and significance of fungal isolations from conjunctival sac and their role in ocular infections. Mycopathologia. 1981;73:17–19. doi: 10.1007/BF00443007. [DOI] [PubMed] [Google Scholar]
- 236.Selcen D, Secmeer G, Aysun S, Kanra G, Onerci M, Gokoz A, Ecevit Z, Ceyhan M, Anlar Y. Mucormycosis in a diabetic child and its treatment with fluconazole: a case report. Turkish J Pediatr. 1995;37:165–168. [PubMed] [Google Scholar]
- 237.Sen D. Cysticercus cellulosae in the lacrimal gland, orbit and eyelid. Acta Ophthalmol. 1980;58:144–147. doi: 10.1111/j.1755-3768.1980.tb04578.x. [DOI] [PubMed] [Google Scholar]
- 238.Seo M, Woo J, Park Y. Intravitreal cysticercosis. Korean J Ophthalmol. 1996;10:55–59. doi: 10.3341/kjo.1996.10.1.55. [DOI] [PubMed] [Google Scholar]
- 239.Sha B, Benson C A, Deutsch T, Noskin G A, Murphy R L, Pottage J C, Jr, Finn W G, Roth S I, Kessler H A. Pneumocystis carinii choroiditis in patients with AIDS: clinical features, response to therapy, and outcome. J Acquir Immune Defic Syndr. 1992;5:1051–1058. [PubMed] [Google Scholar]
- 240.Shadduck J, Meccoli R, Davis R, Font R. Isolation of a microsporidian from a human patient. J Infect Dis. 1990;162:773–776. doi: 10.1093/infdis/162.3.773. [DOI] [PubMed] [Google Scholar]
- 241.Shami M J, Freeman W, Friedberg D, Siderides E, Listhaus A, Ai E. A multicenter study of Pneumocystis choroidopathy. Am J Ophthalmol. 1991;112:15–22. doi: 10.1016/s0002-9394(14)76206-3. [DOI] [PubMed] [Google Scholar]
- 242.Sheu S, Chen Y C, Kuo N W, Wang J H, Chen C J. Endogenous cryptococcal endophthalmitis. Ophthalmology. 1998;105:377–381. doi: 10.1016/s0161-6420(98)93679-x. [DOI] [PubMed] [Google Scholar]
- 243.Shmuely H, Kremer I, Sagie A, Pitlik S. Candida tropicalis multifocal endophthalmitis as the only initial manifestation of pacemaker endocarditis. Am J Ophthalmol. 1997;123:559–560. doi: 10.1016/s0002-9394(14)70189-8. [DOI] [PubMed] [Google Scholar]
- 244.Silva M, Mendes R P, Lastoria J C, Barraviera B, Marques S A, Kamegasawa A. Paracoccidioidomycosis: study of six cases of ocular involvement. Mycopatholgia. 1988;102:87–96. doi: 10.1007/BF00437445. [DOI] [PubMed] [Google Scholar]
- 245.Simmons R B, Buffington J R, Ward M, Wilson L A, Ahearn D G. Morphology and ultrastructure of fungi in extended-wear soft contact lenses. J Clin Microbiol. 1986;24:21–25. doi: 10.1128/jcm.24.1.21-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Simon J, Helper L C. Ocular disease associated with blastomycosis in dogs. J Am Vet Med Assoc. 1970;157:922–925. [PubMed] [Google Scholar]
- 247.Singh G, Kaur J. Cysticercosis of the eyelid. Ann Ophthalmol. 1982;14:947–950. [PubMed] [Google Scholar]
- 248.Slack J, Hyndiuk R A, Harris G J, Simons K B. Blastomycosis of the eyelid and conjunctiva. Ophthalmol Plastic Reconstructive Surg. 1992;8:143–149. doi: 10.1097/00002341-199206000-00011. [DOI] [PubMed] [Google Scholar]
- 249.Smith R E. Commentary on histoplasmosis. Ocular Immunol Inflamm. 1997;5:69–70. doi: 10.3109/09273949709085053. [DOI] [PubMed] [Google Scholar]
- 250.Smith R E, Dunn S, Jester J W. Natural history of experimental histoplasmic choroiditis in the primate. I. Histopathologic features. Investig Ophthalmol Vis Sci. 1984;25:801–809. [PubMed] [Google Scholar]
- 251.Smith R E, Dunn S, Jester J W. Natural history of experimental histoplasmic choroiditis in the primate. II. Clinical features. Investig Ophthalmol Vis Sci. 1984;25:810–819. [PubMed] [Google Scholar]
- 252.Smith R E, Ganley J P. An epidemiological study of presumed ocular histoplasmosis. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:672–693. [PubMed] [Google Scholar]
- 252a.Snell R S, Lemp M A. Clinical anatomy of the eye. Boston, Mass: Blackwell Scientific Publications; 1989. [Google Scholar]
- 253.Sodafy M, Aminlari A, Resaei H. Ophthalmic leishmaniasis. Clin Exp Dermatol. 1981;6:485–488. doi: 10.1111/j.1365-2230.1981.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 254.Sorr E. Meandering ocular toxocariasis. Retina. 1984;4:90–96. doi: 10.1097/00006982-198400420-00003. [DOI] [PubMed] [Google Scholar]
- 255.Specht C, Mitchell K T, Bauman A E, Gupta M. Ocular histoplasmosis with retinitis in a patient with acquired immune deficiency syndrome. Ophthalmology. 1991;98:1356–1359. doi: 10.1016/s0161-6420(91)32126-2. [DOI] [PubMed] [Google Scholar]
- 256.Sponsler T, Sassani J W, Johnson L N, Towfighi J. Ocular invasion in mucormycosis. Surv Ophthalmol. 1992;36:345–350. doi: 10.1016/0039-6257(92)90111-6. [DOI] [PubMed] [Google Scholar]
- 257.Srdic N, Radulovic S, Nonkovic Z, Velimirovic S, Cvetkovic L, Vico I. Two cases of exogenous endophthalmitis due to Fusarium moniliforme and Pseudomonas species as associated aetiological agents. Mycoses. 1993;36:441–444. [PubMed] [Google Scholar]
- 258.Stehr-Grean J, Bailey T, Visvesvara G. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 259.Stern R M, Zakov Z N, Meisler D M, Hall G S, Martin A. Endogenous Pseudoallesheria boydii endophthalmitis. Cleveland Clin Q. 1986;53:197–203. doi: 10.3949/ccjm.53.2.197. [DOI] [PubMed] [Google Scholar]
- 260.Stern W H, Tamura E, Jacobs R A, et al. Epidemic postsurgical Candida parapsilosis endophthalmitis. Ophthalmology. 1985;92:1701–1709. doi: 10.1016/s0161-6420(85)34095-2. [DOI] [PubMed] [Google Scholar]
- 261.Strelow S A, Kent H D, Eagle R C, Cohen E J. A case of contact lens related Fusarium solani keratitis. CLAO J. 1992;18:125–127. [PubMed] [Google Scholar]
- 262.Sugar A. Dermatophytosis. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 73–74. [Google Scholar]
- 263.Sullivan J H. Lids and lacrimal apparatus. In: Vaughan D G, Asbury T, Riordan-Eva P, editors. General ophthalmology. Norwalk, Conn: Appleton and Lange; 1995. pp. 78–94. [Google Scholar]
- 264.Suttorp-Schulten M S A. The etiology of the presumed ocular histoplasmosis syndrome. Ocular Immunol Inflamm. 1997;5:71–72. doi: 10.3109/09273949709085054. [DOI] [PubMed] [Google Scholar]
- 265.Swan S, Wagner R A, Myers J P, Cinelli A B. Mycotic endophthalmitis caused by Penicillium sp. after parenteral drug abuse. Am J Ophthalmol. 1985;100:408–410. doi: 10.1016/0002-9394(85)90502-1. [DOI] [PubMed] [Google Scholar]
- 266.Syrdalen P, Nitter T, Mehl R. Ophthalmomyiasis interna posterior. Report of case caused by the reindeer warble fly larva and review of previous reported cases. Br J Ophthalmol. 1982;66:589–593. doi: 10.1136/bjo.66.9.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tanowitz H, Kirchoff L, Simon D, Monsis S, Weiss M, Wilber M. Chagas' disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Teckhasaenee C, Ritch R, Kanchanaranya C. Ocular parasitic infections in Thailand. Rev Infect Dis. 1986;8:350–356. doi: 10.1093/clinids/8.3.350. [DOI] [PubMed] [Google Scholar]
- 269.Thomas P. Mycotic keratitis—an underestimated mycosis. J Med Vet Mycol. 1994;32:235–256. doi: 10.1080/02681219480000321. [DOI] [PubMed] [Google Scholar]
- 270.Thomas P, Kuriakose T, Kirupashanker M P, Maharajan V S. Use of lactophenol cotton blue mounts of corneal scrapings as an aid to the diagnosis of mycotic keratitis. Diagn Microbiol Infect Dis. 1991;14:219–224. doi: 10.1016/0732-8893(91)90035-e. [DOI] [PubMed] [Google Scholar]
- 271.Tong Q, Chai W X, Wang Z F, Kou J F, Qi Z T, Wang D L. A case of cerebral aspergillosis caused by Aspergillus nidulans. Clinical, pathologic and mycologic identifications. Chinese Med J. 1990;103:518–522. [PubMed] [Google Scholar]
- 272.Reference deleted.
- 273.Toussaint D, Danis P. Retinopathy in generalized Loa loa filariasis. A clinicopathological study. Arch Ophthalmol. 1965;74:470–476. doi: 10.1001/archopht.1965.00970040472007. [DOI] [PubMed] [Google Scholar]
- 274.Tudor R, Blair E. G. spinigerum: an unusual cause of ocular nematodiasis in the Western Hemisphere. Am J Ophthalmol. 1971;72:185–190. doi: 10.1016/0002-9394(71)91612-6. [DOI] [PubMed] [Google Scholar]
- 275.Van Buren J M. Septic retinitis due to Candida albicans. Arch Pathol. 1958;65:137. [PubMed] [Google Scholar]
- 276.Vasquez R, Linberg J V. The anophthalmic socket and the prosthetic eye. A clinical and bacteriologic study. Opthalmic Plastic Reconstruct Surg. 1989;5:277–280. doi: 10.1097/00002341-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 277.Visvesvara G. Pathogenic and opportunistic free-living amoebae. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 278.Vukovic Z, Bobic-Radovanovic A, Latkovic Z, Radovanovic Z. An epidemiological investigation of the first outbreak of rhinosporidiosis in Europe. J Trop Med Hyg. 1995;98:333–337. [PubMed] [Google Scholar]
- 279.Watts J C, Chandler F W. Aspergillosis. In: Connor D H, editor. Pathology of infectious diseases. Vol. 2. Stamford, Conn: Appleton-Lange; 1997. pp. 933–941. [Google Scholar]
- 280.Wei J. Ultrasonic diagnosis of intraocular cysticercosis. Chung-Hua Yen Ko Tsa Chih. 1990;26:230–231. [Google Scholar]
- 281.Weishaar P, Flynn H W, Jr, Murray T G, Davis J L, Barr C C, Gross J G, Mein C E, McLean W C, Jr, Killian J H. Endogenous Aspergillus endophthalmitis. Clinical features and treatment outcomes. Ophthalmology. 1998;105:57–65. doi: 10.1016/s0161-6420(98)71225-3. [DOI] [PubMed] [Google Scholar]
- 282.Weissgold D, Maguire A M, Brucker A J. Management of postoperative Acremonium endophthalmitis. Ophthalmology. 1996;103:749–756. doi: 10.1016/s0161-6420(96)30620-9. [DOI] [PubMed] [Google Scholar]
- 283.Westenfeld F, Alston W K, Winn W C. Complicated soft tissue infection with prepatellar bursitis caused by Paecilomyces lilacinus in an immunocompetent host: case report and review. J Clin Microbiol. 1996;34:1559–1562. doi: 10.1128/jcm.34.6.1559-1562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Whitcup S M, Fenton R M, Pluda J M, De Smet M D, Nussenblatt R B, Chan C C. Pneumocystis carinii and Mycobacterium avium-intracellulare infection of the choroid. Retina. 1992;12:331–335. doi: 10.1097/00006982-199212040-00006. [DOI] [PubMed] [Google Scholar]
- 285.Widder R A, Bartz-Schmidt K U, Geyer H, Brunner R, Kirchhof B, Donike M, Heinmann K. Candida albicans endophthalmitis after anabolic steroid abuse. Lancet. 1995;345:330–331. doi: 10.1016/s0140-6736(95)90325-9. [DOI] [PubMed] [Google Scholar]
- 286.Wilhelmus K. Myiasis palpebrarum. Am J Ophthalmol. 1986;101:496–498. doi: 10.1016/0002-9394(86)90661-6. [DOI] [PubMed] [Google Scholar]
- 287.Wilhelmus K. The pathogenesis of endophthalmitis. Int Ophthalmol Clin. 1987;27:74–81. doi: 10.1097/00004397-198702720-00003. [DOI] [PubMed] [Google Scholar]
- 288.Wilhelmus K R, Liesegang T J, Osato M S, Jones D B. Laboratory diagnosis of ocular infections. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 289.Wilson L, Ahearn D G. Association of fungi with extended-wear soft contact lenses. Am J Ophthalmol. 1986;101:434–436. doi: 10.1016/0002-9394(86)90642-2. [DOI] [PubMed] [Google Scholar]
- 290.Wilson L, Ahern D G, Jones D B, Sexton R R. Fungi from the normal outer eye. Am J Ophthalmol. 1969;67:52–56. doi: 10.1016/0002-9394(69)90007-5. [DOI] [PubMed] [Google Scholar]
- 291.Wilson L A, Ajello L. Agents of oculomycosis: fungal infections of the eye. In: Collier L, Balows A, Sussman M, editors. Topley & Wilson's microbiology and microbial infections. 4. Medical mycology. London, United Kingdom: Arnold; 1998. pp. 525–567. [Google Scholar]
- 292.Witherspoon C, Kuhn F, Owens S D, White M F, Kimble J A. Endophthalmitis due to Sporothrix schenckii after penetrating ocular injury. Ann Ophthalmol. 1990;22:385–388. [PubMed] [Google Scholar]
- 293.Wong T, Fong K S, Tan D T. Clinical and microbial spectrum of fungal keratitis in Singapore: a 5-year retrospective study. Int Ophthalmol. 1997;21:127–130. doi: 10.1023/a:1026462631716. [DOI] [PubMed] [Google Scholar]
- 294.Wong V K W, Tasman W, Eagle R C J, Rodriguez A. Bilateral Candida parapsilosis endophthalmitis. Arch Ophthalmol. 1997;115:670–672. doi: 10.1001/archopht.1997.01100150672022. [DOI] [PubMed] [Google Scholar]
- 295.Wright P, Warhurst D, Jones B. Acanthamoeba keratitis successfully treated medically. Br J Ophthalmol. 1985;69:778–82. doi: 10.1136/bjo.69.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yamamoto G, Pavan-Langston D, Stowe III G C, Albert D M. Fungal invasion of a therapeutic soft contact lens and cornea. Ann Ophthalmol. 1978;11:1731–1735. [PubMed] [Google Scholar]
- 297.Yau T, Rivera-Velazquez P M, Mark A S, Cytryn A S, Levy C S, Shmookler B M, Kolsky M P. Unilateral optic neuritis caused by Histoplasma capsulatum in a patient with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1996;121:324–326. doi: 10.1016/s0002-9394(14)70285-5. [DOI] [PubMed] [Google Scholar]
- 298.Zakka K, Foos R Y, Brown W J. Intraocular coccidioidomycosis. Surv Ophthalmol. 1978;22:313–321. doi: 10.1016/0039-6257(78)90176-5. [DOI] [PubMed] [Google Scholar]
- 299.Zinkham W. Visceral larva migrans. Am J Dis Child. 1978;32:627–633. doi: 10.1001/archpedi.1978.02120310091020. [DOI] [PubMed] [Google Scholar]