Abstract
Decades after its discovery in East Africa, Zika virus (ZIKV) emerged in Brazil in 2013 and infected millions of people during intense urban transmission. Whether vertebrates other than humans are involved in ZIKV transmission cycles remained unclear. Here, we investigate the role of different animals as ZIKV reservoirs by testing 1723 sera of pets, peri-domestic animals and African non-human primates (NHP) sampled during 2013–2018 in Brazil and 2006–2016 in Côte d'Ivoire. Exhaustive neutralization testing substantiated co-circulation of multiple flaviviruses and failed to confirm ZIKV infection in pets or peri-domestic animals in Côte d'Ivoire (n=259) and Brazil (n=1416). In contrast, ZIKV seroprevalence was 22.2% (2/9, 95% CI, 2.8–60.1) in West African chimpanzees (Pan troglodytes verus) and 11.1% (1/9, 95% CI, 0.3–48.3) in king colobus (Colobus polycomos). Our results indicate that while NHP may represent ZIKV reservoirs in Africa, pets or peri-domestic animals likely do not play a role in ZIKV transmission cycles.
Keywords: Zika virus, flavivirus, serology, animal reservoir, zoonoses, antibody
The Zika virus (ZIKV) was first isolated from a sentinel rhesus monkey (Macaca mulatta) in Uganda during the 1950s and later reported in native non-human primate (NHP) species and in forest-dwelling Aedes mosquitos in Africa and therefore transmission in parts of Africa is thought to be maintained through interepidemic sylvatic cycles [1, 2]. ZIKV was not considered a major threat to human health [3, 4] until its emergence in Latin America, which was associated with severe congenital malformations including microcephaly [5]. According to evolutionary reconstructions, ZIKV was likely introduced into Brazil during 2013 [5] and infected approximately 8.5 million people during 2015 and 2016 [6]. Community protective immunity in urban centers likely contributed to cease of the epidemic [7].
Sylvatic transmission cycles may permit ZIKV maintenance until the pool of susceptible humans is replenished by birth and migration, potentially contributing to a resurge of intense urban ZIKV transmission cycles. Whether animal reservoirs play a role in ZIKV transmission in the Americas remains unknown. The available data on ZIKV infection of NHP in the Americas are inconclusive. Some South American NHP species, such as marmosets (Callithrix jacchus) and tamarins (Saguinus labiatus) were susceptible to ZIKV in vivo [8]. Low seroprevalence and low levels of neutralizing antibodies have been reported in capuchin monkeys in Northeastern and Central Brazil [9, 10]. Finally, ZIKV has been detected in NHP carcasses in the Southeastern region, but since contamination of the remains could not be excluded, the relevance of those findings still needs to be confirmed [11].
Several flaviviruses can infect pets and peri-domestic animals. For instance, experimental infection and serological evidence indicate circulation of West Nile virus (WNV) and Japanese Encephalitis virus (JEV) in cats and dogs [12–15] and of Wesselsbron virus (WSLV) in cattle, goat and sheep [15–21]. Virus detection by molecular methods, virus isolation and serological evidence substantiate that horses can be infected with WNV, Saint Louis Encephalitis virus (SLEV), JEV and Usutu virus (USV) [22, 23]. Livestock species, such as sheep and goats, were susceptible to ZIKV in vivo [24, 25], and although ZIKV-specific neutralizing antibodies were reported in sero-epidemiological studies in cattle and sheep from Brazil and in horses from French Pacific Islands [26, 27], only few flaviviruses beyond ZIKV were used in those studies to rule out potentially unspecific test results elicited by cross-reactive flavivirus antibodies. Exposure of vertebrates to ZIKV depends on the feeding preferences of the mosquito vectors. In Latin America, ZIKV vectors include predominantly Aedes aegypti and, to a lesser extent, Ae. albopictus [2, 28, 29]. In Africa, the invertebrate host range of ZIKV is not entirely known. However, major vectors likely include Aedes aegypti formosus, Ae. africanus, Ae. albopictus, Ae. apicoargenteus, Ae. furcifer and Ae. vitattus [28]. Whereas Ae. aegypti and, to a lesser extent, Ae. albopictus are highly anthropophilic, they are also known to opportunistically feed on other vertebrates, including rodents, cattle, sheep and dogs [29–34]. Those animals are reared in high numbers and in close proximity to humans and may thus be important components of (peri-)urban ZIKV transmission cycles. Therefore, it is currently unclear whether pets and peri-domestic animals play a role in the maintenance of ZIKV in Africa or the Americas.
Here we investigated ZIKV spread in pets (i.e. dogs and cats) and peri-domestic animals (i.e. equids, cattle, sheep and goats) sampled in northeastern Brazil, the 2015–2016 outbreak’s epicentre, and in wild NHP, pets and peri-domestic animals sampled in Côte d’Ivoire, where ZIKV has been documented in forest-dwelling mosquitos [4, 35].
In Brazil, equid, cattle, sheep, goat, dog and cat samples were collected in the northeastern state of Bahia during 2013–2018 within state routine veterinary surveillance activities or from dogs and cats presenting clinical signs, and in 2015 from dogs in the neighbouring state of Pernambuco for a Leishmania spp. serosurvey [36]. In Côte d'Ivoire, peri-domestic animals were sampled in 2012 and 2014. Unhabituated wild NHP such as king colobus (Colobus polycomos) and western red colobus (Piliocolobus badius) were sampled in the Taï National Park during 2006 and 2016 [37, 38]. Samples were also collected from a habituated group of sooty mangabeys (Cercocebus atys) that has been under observation since 2012 [37, 38]. Additionally, nine blood samples from deceased West African chimpanzees (Pan troglodytes verus) were included; this population of chimpanzees has been followed daily since 1979 and necropsy samples have been systematically performed on all dead individuals recovered, since the inception of the veterinary programme in 2002 [37, 39].
A total of 1416 sera of pets and peri-domestic animals from Brazil and 298 sera of dogs, peri-domestic animals and NHP from Côte d'Ivoire were screened for ZIKV antibodies using the NS1-based Electrochemiluminescence immunoassay (ECLIA) Elecsys Zika IgG (Roche, Penzberg, Germany). This double-antigen test enables multispecies testing because it does not require species-specific secondary antibodies, but it was only validated for human sera [40]. ECLIA-positive sera were further tested by a plaque reduction neutralization test (PRNT90) to identify anti-ZIKV neutralizing antibodies (Asian ZIKV lineage, strain H/PF/2013; used for samples from Brazil because of limited volume of available sera and African ZIKV lineage, strain MR766; used for samples from Côte d’Ivoire because of limited volume of available sera). Because cross-reacting antibodies against co-circulating flaviviruses can yield false-positive ZIKV test results [41, 42], we compared ZIKV PRNT90 endpoint titres with endpoint titres against co-circulating flaviviruses representing diverse serocomplexes, including Rocio virus (ROCV; strain UVE/ROCV/1975/BR/5P H34 675), Saint Louis Encephalitis virus (SLEV; strain MSI-7), Bussuquara virus (BSQV; strain BeAn 4073), Wesselsbron virus (WSLV; strain UVE/WESSV/UNK/ZA/SAH-177), Spondweni virus (SPOV; strain UVE/SPOV/UNK/ZA/SM-6 V-1s), Dengue virus 2 (DENV-2; strain Thailand/16681/84), Yellow Fever virus (YFV; strain 17D), and West Nile virus (WNV; strain NY-99). We used antigenic cartography to discern neutralizing antibody reactivity patterns [43]. Due to the lack of sufficient volume of serum samples, chimpanzee sera were only tested by PRNT90 and not by ECLIA.
PRNT90 was conducted in 12-well plates seeded 1 day before the infection with 1.6×105 Vero FM cells for ZIKV, SPOV, YFV, WSLV, WNV and BSQV, 1.6×105 Vero B4 cells for DENV-2 and ROCV, and 1.2×105 BHK-21 cells for SLEV. Forty plaque-forming units were incubated with respective serum dilutions of 1 : 40, 1:80, 1:160, 1:320, 1:640 and 1:1280 for 1 h, added onto the cell monolayer, and incubated for 1 h before adding an overlayer containing DMEM with 2% FCS and 1.25% carboxymethyl cellulose. Cells were incubated for 3 days for WNV and SLEV, 4 days for ZIKV, 5 days for SPOV, DENV-2 and BSQV, 6 days for ROCV and WSLV and 7 days for YFV. The overlayer medium was removed, and cells were fixated with 6% paraformaldehyde and stained with crystal violet. PRNT90 endpoint reciprocal titres were calculated using a logistic regression function in GraphPad Prism 6 (GraphPad Software, www.graphpad.com).
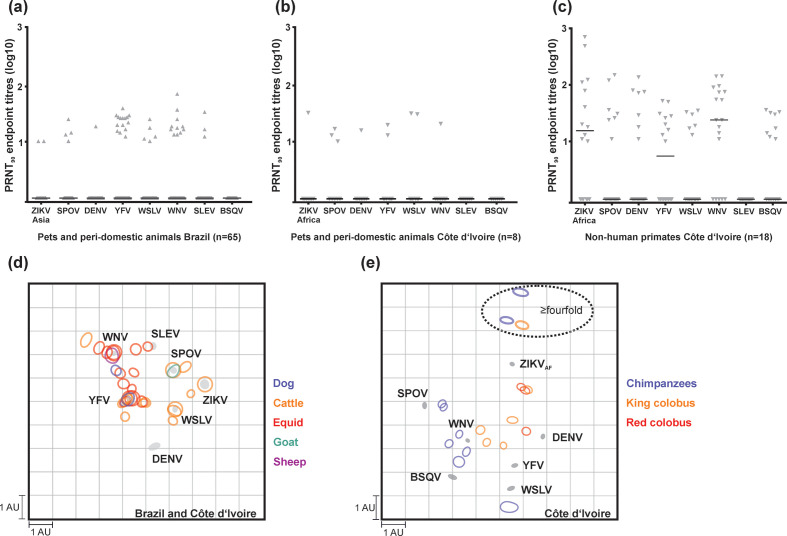
The initial ECLIA-based ZIKV reactivity rate among pets and peri-domestic animals was 4.6% (65/1416, 95% CI, 3.5–5.8) in Brazil and 3.6% (8/220, 95% CI, 1.6–7.0) in Côte d’Ivoire (Fig. 1, Table 1). However, based on PRNT90 fourfold titre differences, commonly considered decisive in flavivirus serology [42], ZIKV-specific antibodies could neither be confirmed in pets and peri-domestic animals from Brazil, nor from Côte d'Ivoire (Fig. 2a; Fig. 2b). Instead, among the Brazilian ECLIA-reactive sera, reciprocal PRNT90 endpoint titres were fourfold or higher for WNV in five (7.7%), YFV in three (4.6%), SPOV in one (1.5%) and WSLV in one serum (1.5%). A total of 31 ECLIA-positive sera (47.7% of all ZIKV ECLIA-reactive sera from Brazil) were negative for all flaviviruses tested by PRNT90, likely due to differential sensitivity of those tests. Among the eight ECLIA-reactive sera from pets and peri-domestic animals from Côte d'Ivoire, we found a monotypic reaction to YFV in one serum and to SPOV in another serum (Table S1, available in the online version of this article).
Fig. 1.
Sampling sites and endemic flaviviruses. Sampling sites in Brazil and in Côte d’Ivoire (a). Maximum-likelihood phylogeny of flaviviruses relevant for this study inferred using a dataset containing translated polyprotein genes and a Whelan and Goldman amino acid substitution model. Black circles at nodes represent support of grouping of ≥0.75 from 1000 bootstrap replicates. Viruses that were used in this study are highlighted in bold (b).
Table 1.
Sampling table and Zika virus antibody test reactivity rate
|
Country sampled |
Group |
Animal species |
Total |
ECLIA % (95% CI) |
PRNT90 % (95% CI) |
Year |
|---|---|---|---|---|---|---|
|
Peri-domestic |
Donkey |
55 |
9.1 (3.0–19.9) |
0 |
2013–2018 |
|
|
Peri-domestic |
Mule |
95 |
4.2 (1.2–10.4) |
0 |
2014–2018 |
|
|
Peri-domestic |
Horse |
620 |
4.0 (2.6–5.9) |
0 |
2015–2018 |
|
|
Brazil |
Peri-domestic |
Cow |
278 |
6.5 (3.9–10.0) |
0 |
2016–2018 |
|
Peri-domestic |
Sheep |
64 |
4.7 (1.0–13.1) |
0 |
2017, 2018 |
|
|
Peri-domestic |
Goat |
231 |
2.6 (1.0–5.6) |
0 |
2018 |
|
|
Pet |
Cat |
5 |
20.0 (0.5–71.6) |
0 |
2015, 2018 |
|
|
Pet |
Dog |
68 |
4.4 (0.9–12.4) |
0 |
2015, 2018 |
|
|
Peri-domestic |
Cow |
16 |
12.5 (1.6–38.4) |
6.3 (0.2–30.2) |
2012–2014 |
|
|
Peri-domestic |
Goat |
97 |
3.1 (0.6–8.8) |
1.0 (0.1–5.6) |
2012–2014 |
|
|
Peri-domestic |
Pig |
18 |
0.0 |
0 |
2012–2014 |
|
|
Peri-domestic |
Sheep |
89 |
2.2 (0.3–7.8) |
0 |
2012–2014 |
|
|
CIV |
NHP |
King colobus (Colobus polycomos) |
9 |
66.7 (29.9–92.5) |
11.1 (0.3–48.3) |
2006–2016 |
|
NHP |
Western red colobus (Piliocolobus badius) |
13 |
23.1 (5.0–53.8) |
0 |
2006–2016 |
|
|
NHP |
Sooty mangabey (Cercocebus atys) |
17 |
0.0 |
0 |
2006–2016 |
|
|
NHP |
Chimpanzees (Pan troglodytes verus) |
9 |
100.0 (66.4–100.0) |
22.2(2.8–60.1) |
2006–2016 |
|
|
Pet |
Dog |
39 |
2.6 (0.1–13.5) |
0 |
2006–2016 |
|
|
Total |
1723 |
5.3 (4.1–6.1) |
|
Detailed information is available in supplementary table S1.
CI, confidence intervals; ECLIA, electrochemiluminescence immunoassay; NHP, non-human primates; PRNT, plaque reduction neutralization test.
Fig. 2.
Flavivirus antibody reactivity patterns. Reciprocal PRNT90-specific endpoint titres for the African ZIKV lineage strain (ZIKVAF), the Asian ZIKV lineage strain (ZIKVAS), SPOV, DENV-2, YFV, WSLV, WNV, SLEV, ROCV and BSQV of ZIKV among ECLIA-positive sera in pets and peri-domestic animals in Brazil (a), in Côte d’Ivoire (b) and in NHP from Côte d’Ivoire (c). 2D antigenic cartography showing neutralizing activity against related flaviviruses among domestic animals in Brazil (d), Côte d’Ivoire (d) and in NHP in Côte d’Ivoire (e). Each unit of antigenic distance (length of one square grid side, measured in any direction) is equivalent to a fourfold dilution in the PRNT90. Each circle corresponds to one tested serum sample showing titres (sera with a negative PRNT90 or an endpoint titre <10 are not shown) and circle size suggests intra-sample differences. Grey circles indicate the antigens or antisera (tested viruses). In (d), titers obtained with the African ZIKV lineage strain were used for animals from Côte d’Ivoire and with the Asian ZIKV lineage strain for animals from Brazil. Horizontal lines plotted in (a), (b) and (c) show median
Antigenic cartography did not provide a robust separation of flavivirus serocomplexes in pets and peri-domestic animals from either Côte d`Ivoire or Brazil (Fig. 2d). The detection of sera with monotypic reaction or titres ≥fourfold for SPOV, and WSLV in Brazilian animals must be carefully interpreted. WSLV has not been reported in the Americas, and although SPOV has been isolated in Culex quinquefasciatus in Haiti [44] it is unlikely that either SPOV or WSLV would be widely dispersed among pets or peri-domestic animals from Brazil because there are no robust data on isolation or molecular detection of those viruses in vertebrates from Latin America. A more plausible explanation for those PRNT results are cross-reactive flavivirus antibodies, which complicate the interpretation of flavivirus serological assays in hyperendemic settings, even when considering only ≥fourfold PRNT90 titres as decisive serological support [42, 45]. This interpretation is supported by the overall low titres against all tested flaviviruses and by previous evidence showing the difficulties to confirm human ZIKV infection in DENV-endemic areas [42, 46].
In NHP, ZIKV ECLIA reactivity was observed in 23.1% (3/13, 95% CI, 11.1–39.3) of red colobus, 66.7% (6/9, 95% CI, 29.9–92.5) of king colobus and in none of the 17 sooty mangabeys, while 88.9% (8/9, 95% CI, 51.8–99.7) of the chimpanzees tested positive for ZIKVAF using PRNT90. Fourfold or higher ZIKV PRNT90 titres compared to other flaviviruses were found in 11.1% (1/9, 95% CI, 0.3–48.3) of king colobus and in 22.2% (2/9, 95% CI, 2.8–60.1) of chimpanzees (Table 1, Fig. 2c, e) and in none of the previously ECLIA-reactive red colobus. Moreover, one chimpanzee (1/9, 95% CI, 0.3–48.3) presented titres ≥fourfold for WNV compared to all other flaviviruses. The difference in NHP ZIKV seroprevalence might be related to differential host susceptibility or different ecological niches occupied by those NHP since the mosquito species from which ZIKV was isolated in Côte d’Ivoire (i.e. Aedes vitattus, Ae. furcifer and Ae. aegypti formosus) are predominantly found in the tree canopy [28, 35, 47]. Notably, ZIKV was linked to NHP already in the initial viral isolation from a sentinel Rhesus macaque caged in the canopy [48]. Our data suggest that some NHP species like chimpanzees and king colobus, which are at least partly arboreal, might play a role as ZIKV reservoirs in Côte d’Ivoire. Our interpretation is supported by previous studies indicating ZIKV exposure of native African NHP by viral isolation from Patas (Erythrocebus patas) and Vervet (Chlorocebus pygerythrus) monkeys in Senegal [49] and by the detection of ZIKV-neutralizing antibodies in different NHP species such as in red-tailed monkey (Cercopithecus ascanius), grey-cheeked mangabey (Lophocebus albigena), mantled guereza (Colobus guereza) in Uganda [50], and tantalus (Chlorocebus tantalus) and Mona (Cercopithecus mona) monkey in Nigeria [51]. On the other hand, our data do not provide conclusive evidence on whether those NHP species may serve as amplification or reservoir hosts, because of our small sample size and because our methods were not targeted towards detecting active infections. Future longitudinal studies including younger NHP, as performed for CHIKV reservoir studies in Senegal [52], could clarify the role of those NHP species for ZIKV transmission in Africa.
Our study was limited by the lack of longitudinal sampling from the different animal species in both locations in Brazil and Côte d’Ivoire, since long-term titre comparison would be more effective for understanding antibody responses after multiple flavivirus infections. We also did not test other synanthropic animals such as marsupials, known to be relevant arbovirus hosts, e.g. for the alphavirus Ross River virus [53] and which may be exposed to ZIKV vectors [54]. However, while most of the previous studies on ZIKV animal reservoirs either used tests with lower specificity only (e.g. ELISA, complement fixation and haemagglutination) or assessed flaviviral cross-reactivity using fewer viruses in PRNT [27, 50, 51], we provided data from a large number of sera of pets and peri-domestic animals from two continents and performed extensive neutralization testing, considered the gold-standard technique.
In brief, our data confirm that co-circulating flaviviruses challenge unambiguous ZIKV antibody test results in hyper-endemic areas [41, 42]. Despite those technical limitations, our data imply that while some NHP species in Côte d’Ivoire may serve as ZIKV reservoir hosts, pets and peri-domestic animals are neither involved in ZIKV transmission cycles in Brazil, nor in Côte d’Ivoire.
Supplementary Data
Funding information
J.F.G. was supported by the Deutsche Forschungsgemeinschaft (DFG) Research Group ‘Sociality and Health in Primates’ (FOR2136; CA 1108/3–1). This work was supported by the European Union’s Horizon 2020 research and innovation programme through the ZIKAlliance project (grant agreement no. 734548) to J.F.D. and X.d.L. and by Roche Diagnostics through the investigator-initiated study ZikaStock to J.F.D.
Acknowledgements
We thank Sandra Junglen and Anne Kopp for providing the SLEV strain. We thank the authorities in Côte d’Ivoire for their support of this research, in particular the Ministry of the Environment and Forests and the Ministry of Research, the Ivorian Office of National Parks (Office Ivorian des Parcs et Reserves), the directorship of the Taï National Park and the Centre Suisse de Recherches Scientifiques en Côte d'Ivoire in Abidjan. We are grateful to the field assistants of Taï National Park for their commitment and efforts that enabled this study.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
In Brazil, sampling and analyses were approved by the Federal University of Bahia’s animal ethics committee under authorization no. 55/2017, by the Animal Ethics Committee (CEUA) of the Aggeu Magalhães Institute (Fiocruz) for dog sampling under authorization no. 56/2013. In Côte d'Ivoire, sampling of peri-domestic animals was permitted through the ethic commission, ‘Comité national d’éthique et de la recherche (CNER)’ of the ‘Ministère de la santé et de l’hygiène publique –République de Côte d`Ivoire’ (permit number 101–10/MSHP/CENR/P); sampling followed Directive 86/609/EEC on the Protection of Animals Used for Experimental and Other Scientific Purposes and permission was issued by LANADA/LCPA, Laboratoire national d`appui au développement agricole/Laboratoire Nationale de la Pathologie Animale, Bingerville, CI. Sampling of NHP in Côte d'Ivoire was performed with the permission of the research ministries of Côte d’Ivoire and ethical approval of the Ivorian Office of National Parks, with approval by the Centre Suisse de Recherche Scientifique en Côte d’Ivoire and the Laboratoire National de la Pathologie Animale, Bingerville, Côte d’Ivoire.
Footnotes
Abbreviations: AU, antigenic unit; CI, confidence intervals; ECLIA, electrochemiluminescence immunoassay; NHP, non-human primates; PRNT, plaque reduction neutralization test.
A supplementary table is available with the online version of this article.
References
- 1.Petersen LR, Jamieson DJ, Honein MA. Zika Virus. N Engl J Med. 2016;375:294–295. doi: 10.1056/NEJMc1606769. [DOI] [PubMed] [Google Scholar]
- 2.Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faye O, Freire CCM, Iamarino A, Faye O, de Oliveira JVC, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentine MJ, Murdock CC, Kelly PJ. Sylvatic cycles of arboviruses in non-human primates. Parasit Vectors. 2019;12:463. doi: 10.1186/s13071-019-3732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faria NR, Azevedo R do S da S, Kraemer MUG, Souza R, Cunha MS, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady OJ, Osgood-Zimmerman A, Kassebaum NJ, Ray SE, de Araújo VEM, et al. The association between Zika virus infection and microcephaly in Brazil 2015-2017: An observational analysis of over 4 million births. PLoS Med. 2019;16:e1002755. doi: 10.1371/journal.pmed.1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netto EM, Moreira-Soto A, Pedroso C, Höser C, Funk S, et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. mBio. 2017;8:e01390-17. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry N, Ferguson D, Ham C, Hall J, Jenkins A, et al. High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci Rep. 2019;9:14495. doi: 10.1038/s41598-019-50918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira-Filho EF, Oliveira RAS, Ferreira DRA, Laroque PO, Pena LJ, et al. Seroprevalence of selected flaviviruses in free-living and captive capuchin monkeys in the state of Pernambuco, Brazil. Transbound Emerg Dis. 2018;65:1094–1097. doi: 10.1111/tbed.12829. [DOI] [PubMed] [Google Scholar]
- 10.Moreira-Soto A, Carneiro I de O, Fischer C, Feldmann M, Kümmerer BM, et al. Limited evidence for infection of urban and peri-urban nonhuman primates with zika and chikungunya viruses in Brazil. mSphere. 2018;3 doi: 10.1128/mSphere.00523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terzian ACB, Zini N, Sacchetto L, Rocha RF, Parra MCP, et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci Rep. 2018;8:16034. doi: 10.1038/s41598-018-34423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ, et al. Experimental infection of cats and dogs with West Nile virus. Emerg Infect Dis. 2004;10:82–86. doi: 10.3201/eid1001.020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoda H, Tamaru S, Morimoto M, Hayashi T, Shimojima M, et al. Experimental infection of Japanese encephalitis virus in dogs. J Vet Med Sci. 2011;73:1241–1242. doi: 10.1292/jvms.11-0142. [DOI] [PubMed] [Google Scholar]
- 14.Davoust B, Leparc-Goffart I, Demoncheaux JP, Tine R, Diarra M, et al. Serologic surveillance for West Nile virus in dogs, Africa. Emerg Infect Dis. 2014;20:1415–1417. doi: 10.3201/eid2008.130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar K, Arshad SS, Selvarajah GT, Abu J, Toung OP, et al. Prevalence and risk factors of Japanese encephalitis virus (JEV) in livestock and companion animal in high-risk areas in Malaysia. Trop Anim Health Prod. 2018;50:741–752. doi: 10.1007/s11250-017-1490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durand B, Haskouri H, Lowenski S, Vachiery N, Beck C, et al. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: dog as an alternative WNV sentinel species? Epidemiol Infect. 2016;144:1857–1864. doi: 10.1017/S095026881600011X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coetzer JA, Theodoridis A. Clinical and pathological studies in adult sheep and goats experimentally infected with Wesselsbron disease virus. Onderstepoort J Vet Res. 1982;49:19–22. [PubMed] [Google Scholar]
- 18.Coetzer JA, Barnard BJ. Hydrops amnii in sheep associated with hydranencephaly and arthrogryposis with wesselsbron disease and rift valley fever viruses as aetiological agents. Onderstepoort J Vet Res. 1977;44:119–126. [PubMed] [Google Scholar]
- 19.Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, et al. Epidemiology of usutu virus: The European Scenario. Pathogens. 2020;9:E699. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladreyt H, Auerswald H, Tum S, Ken S, Heng L, et al. Comparison of Japanese encephalitis force of infection in pigs, poultry and dogs in cambodian villages. Pathogens. 2020;9:E719. doi: 10.3390/pathogens9090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Bocanegra I, Jurado-Tarifa E, Cano-Terriza D, Martínez R, Pérez-Marín JE, et al. Exposure to West Nile virus and tick-borne encephalitis virus in dogs in Spain. Transbound Emerg Dis. 2018;65:765–772. doi: 10.1111/tbed.12801. [DOI] [PubMed] [Google Scholar]
- 22.Lecollinet S, Pronost S, Coulpier M, Beck C, Gonzalez G, et al. Viral equine encephalitis, a growing threat to the horse population in Europe? Viruses. 2019;12:E23. doi: 10.3390/v12010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira-Filho EF, Fischer C, Berneck BS, Carneiro IO, Kühne A, et al. Ecologic determinants of west nile virus seroprevalence among equids, Brazil. Emerg Infect Dis. 2021;27:2466–2470. doi: 10.3201/eid2709.204706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz ER, Pozor MA, Pu R, Barr KL, Beachboard SE, et al. Experimental infection of pregnant female sheep with zika virus during early gestation. Viruses. 2019;11:E795. doi: 10.3390/v11090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragan IK, Blizzard EL, Gordy P, Bowen RA. Investigating the potential role of North American Animals as hosts for zika virus. Vector Borne Zoonotic Dis. 2017;17:161–164. doi: 10.1089/vbz.2016.2099. [DOI] [PubMed] [Google Scholar]
- 26.Pauvolid-Corrêa A, Gonçalves Dias H, Marina Siqueira Maia L, Porfírio G, Oliveira Morgado T, et al. Zika Virus Surveillance at the Human-Animal Interface in West-Central Brazil, 2017-2018. Viruses. 2019;11:E1164. doi: 10.3390/v11121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck C, Leparc-Goffart I, Desoutter D, Debergé E, Bichet H, et al. Serological evidence of infection with dengue and Zika viruses in horses on French Pacific Islands. PLoS Negl Trop Dis. 2019;13:e0007162. doi: 10.1371/journal.pntd.0007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilakis N, Weaver SC. Flavivirus transmission focusing on Zika. Curr Opin Virol. 2017;22:30–35. doi: 10.1016/j.coviro.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira Dos Santos T, Roiz D, Santos de Abreu FV, Luz SLB, Santalucia M, et al. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg Microbes Infect. 2018;7:191. doi: 10.1038/s41426-018-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delatte H, Desvars A, Bouétard A, Bord S, Gimonneau G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector Borne Zoonotic Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 31.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- 32.Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors. 2012;5:57. doi: 10.1186/1756-3305-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omondi D, Masiga DK, Ajamma YU, Fielding BC, Njoroge L, et al. Unraveling host-vector-arbovirus interactions by two-gene high resolution melting mosquito bloodmeal analysis in a kenyan wildlife-livestock interface. PLoS One. 2015;10:e0134375. doi: 10.1371/journal.pone.0134375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick DM, Hattaway LM, Hsueh AN, Ramos-Niño ME, Cheetham SM. PCR-Based Bloodmeal Analysis of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) in St. George Parish, Grenada. J Med Entomol. 2019;56:1170–1175. doi: 10.1093/jme/tjz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akoua-Koffi C, Diarrassouba S, Bénié VB, Ngbichi JM, Bozoua T, et al. Investigation surrounding a fatal case of yellow fever in Côte d’Ivoire in 1999. Bull Soc Pathol Exot. 2001;94:227–230. [PubMed] [Google Scholar]
- 36.Sales K da S, de Oliveira Miranda DE, Costa PL, da Silva FJ, Figueredo LA, et al. Home sweet home: sand flies find a refuge in remote indigenous villages in north-eastern Brazil, where leishmaniasis is endemic. Parasit Vectors. 2019;12:118. doi: 10.1186/s13071-019-3383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann F, Köhler SM, Nowak K, Dupke S, Barduhn A, et al. Low antibody prevalence against Bacillus cereus biovar anthracis in Taï National Park, Côte d’Ivoire, indicates high rate of lethal infections in wildlife. PLoS Negl Trop Dis. 2017;11:e0005960. doi: 10.1371/journal.pntd.0005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gogarten JF, Davies TJ, Benjamino J, Gogarten JP, Graf J, et al. Factors influencing bacterial microbiome composition in a wild non-human primate community in Taï National Park, Côte d’Ivoire. ISME J. 2018;12:2559–2574. doi: 10.1038/s41396-018-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gogarten JF, Akoua-Koffi C, Calvignac-Spencer S, Leendertz SAJ, Weiss S, et al. The ecology of primate retroviruses - an assessment of 12 years of retroviral studies in the Taï national park area, Côte d׳Ivoire. Virology. 2014;460–461:147–153. doi: 10.1016/j.virol.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laengin T, Augenstein S, Stadlbauer E, Girgnhuber H, Gloeck M, et al. Performance of an Automated Zika IgG Immunoassay in the Detection of Zika IgG Specific Antibodies-A Validation Approach in Samples from Prevalence Areas and Non-Endemic Countries. Trop Med Infect Dis. 2020;5:E97. doi: 10.3390/tropicalmed5020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer C, de Oliveira-Filho EF, Drexler JF. Viral emergence and immune interplay in flavivirus vaccines. Lancet Infect Dis. 2020;20:15–17. doi: 10.1016/S1473-3099(19)30697-8. [DOI] [PubMed] [Google Scholar]
- 42.Fischer C, Jo WK, Haage V, Moreira-Soto A, de Oliveira Filho EF, et al. Challenges towards serologic diagnostics of emerging arboviruses. Clin Microbiol Infect. 2021;27:1221–1229. doi: 10.1016/j.cmi.2021.05.047. [DOI] [PubMed] [Google Scholar]
- 43.Katzelnick LC, Fonville JM, Gromowski GD, Bustos Arriaga J, Green A, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science. 2015;349:1338–1343. doi: 10.1126/science.aac5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White SK, Lednicky JA, Okech BA, Dunford JC. Spondweni Virus in Field-Caught Culex quinquefasciatus Mosquitoes, Haiti, 2016. Emerg Infect Dis. 2018;24:1765–1767. doi: 10.3201/eid2409.171957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inouye S, Matsuno S, Tsurukubo Y. “Original antigenic sin” phenomenon in experimental flavivirus infections of guinea pigs: studies by enzyme-linked immunosorbent assay. Microbiol Immunol. 1984;28:569–574. doi: 10.1111/j.1348-0421.1984.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 46.Lindsey NP, Staples JE, Powell K, Rabe IB, Fischer M, et al. Ability To Serologically Confirm Recent Zika Virus Infection in Areas with Varying Past Incidence of Dengue Virus Infection in the United States and U.S. Territories in 2016. J Clin Microbiol. 2018;56:e01115-17. doi: 10.1128/JCM.01115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diallo D, Sall AA, Diagne CT, Faye O, Hanley KA, et al. Patterns of a sylvatic yellow fever virus amplification in southeastern Senegal, 2010. Am J Trop Med Hyg. 2014;90:1003–1013. doi: 10.4269/ajtmh.13-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 49.Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, et al. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. Am J Trop Med Hyg. 2015;92:88–97. doi: 10.4269/ajtmh.13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76:552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 51.Monath TP, Kemp GE. Importance of nonhuman primates in yellow fever epidemiology in Nigeria. Trop Geogr Med. 1973;25:28–38. [PubMed] [Google Scholar]
- 52.Althouse BM, Guerbois M, Cummings DAT, Diop OM, Faye O, et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat Commun. 2018;9:1046. doi: 10.1038/s41467-018-03332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephenson EB, Peel AJ, Reid SA, Jansen CC, McCallum H. The non-human reservoirs of Ross River virus: a systematic review of the evidence. Parasit Vectors. 2018;11:188. doi: 10.1186/s13071-018-2733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bueno MG, Martinez N, Abdalla L, Duarte Dos Santos CN, Chame M. Animals in the Zika Virus Life Cycle: What to Expect from Megadiverse Latin American Countries. PLoS Negl Trop Dis. 2016;10:12. doi: 10.1371/journal.pntd.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.