Abstract
Background
Hypertension is highly prevalent and associated with the elevated risks of cardiovascular diseases, dementia, and physical disabilities among adults. Although the correlation between bilirubin and hypertension has been reported, the observation in quinquagenarian population is scarce. We aimed to examine bilirubin-hypertension association in Guankou Ageing Cohort Study.
Methods
Participants ≥ 55 years were recruited and their questionnaires and physical examination data were collected. Kaplan–Meier survival analysis and Cox proportional hazards regression were implemented to assess the hypertension risk. The non-liner dose–response relationships of bilirubin-hypertension were determined by restricted cubic spline (RCS) models. Receiver operating characteristic (ROC) curves and multiple factors analysis (MFA) were performed to evaluate the predictive abilities.
Results
1881 eligible participants (male 43.75%, female 56.25%) with the median age of 61.00 (59.00–66.00) were included. The hazard ratio (HR, 95% CI) of serum total bilirubin (STB) and unconjugated bilirubin (UCB) were 1.03 (1.01–1.05) and 1.05 (1.03–1.07), while conjugated bilirubin (CB) showed a weak protective effect with the HR of 0.96 (0.92–0.99), and the associations remained significant in all models. RCS analyses further indicated the similar bidirectional effects of STB and UCB with the cut-off of 12.17 μmol/L and 8.59 μmol/L, while CB exhibited inverse bidirectional dose–response relationship with a cut-off of 3.47 μmol/L. ROC curves and MFA showed baseline STB combined with age, BMI, and waist circumference could well discriminate the low and high of hypertension risk.
Conclusions
Our findings suggested the higher levels of total and unconjugated bilirubin were hazardous factors of hypertension, while an inverse effect presented when more bilirubin was conjugated.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-022-03309-7.
Keywords: Ageing, Bilirubin, Cohort study, Hypertension
Background
Ageing is defined as a gradual decline in the ability to maintain whole-body homeostasis, causing the onset of ageing-related diseases (ARDs) and eventually death [1]. As the ageing tendency of the population accelerates, the elderlies are becoming an increasingly important subpopulation that merits special attention regarding health and social issues [2]. Hypertension, a representative ageing syndrome which is common in the middle old-ages (quinquagenarian), is characterized by persistently elevated systemic arterial blood pressure (BP) and may be accompanied by functional or organic damage to the heart, brain, and kidney, which is ranked third as a cause of disability-adjusted life-years and affected over 1/5 adults (26.4%) worldwide [3–5]. Although the age-standardized prevalence of hypertension decreased in high-income countries over the past decade, it has been increasing in low- and middle-income countries [5]. In China, along with the urbanization, economic growth, and the population ageing, the prevalence of hypertension has been elevated markedly. Some surveys have revealed that 26.6–33.6% of the total population was diagnosed with hypertension [6, 7], which estimated to cause 23 million deaths per year (increased 89% compared with 1990) [8]. Besides the high prevalence, the rates of awareness, treatment, and control were also inadequate, which exerts heavy burdens on the public health system in China, even in the entire world [9]. In view of the severe situations, discovering novel hypertension-related biomarkers and distinguishing high-/low-risk population through biomarker-based models would better facilitate the prevention and control of hypertension [10].
Bilirubin (C33H36N4O6), conventionally considered as the ultimate product of heme metabolism, is produced starting from the breakdown of red blood cells to generate carbon monoxide, iron, and biliverdin. Biliverdin is an unstable intermediate and rapidly converts into bilirubin by biliverdin reductases [11]. In hepatocytes, bilirubin is conjugated with glucuronic acid by uridine-diphosphate glucuronosyltransferase 1A1 (UGT1A1), known as conjugated bilirubin (CB) [12]. Comparing with unconjugated bilirubin (UCB), CB is soluble, which can be filtered and excreted via kidney but does not cross the brain barrier [13]. Animal experiments showed bilirubin mediated cytoprotection effects, including anti-oxidation and anti-inflammatory in vivo [14]. Several epidemiological studies have also shown the inverse correlation between bilirubin and the risk of cardiovascular diseases [15, 16]. However, bilirubin presents the potential cytotoxic effects either. Toxicological studies manifested that high serum concentration of bilirubin would bound to and deposited on various tissues, and further pleaded to deleterious events such as jaundice, mental disorders, cerebral palsy, brain damage, and even death [17]. Beyond that, hyperbilirubinemia was associated with a worse world health organization functional class, higher right atrial pressure, higher brain natriuretic peptide, and a larger Doppler right ventricular index [18]. Hence, the subtle role of this substance in human metabolism keeps unclear.
Until now, some epidemiological studies have aimed to uncover the relationship between bilirubin and BP among children and middle-age adults. However, very limited studies have been conducted among the ageing population, especially in China [19–21]. Given the potential indication of bilirubin towards hypertension, we aimed to examine the association between the baseline levels of bilirubin and the incident risk of hypertension with the adjustments for key covariates on the dataset of the Guankou Ageing Cohort Study (GACS).
Material and methods
Study population
The research protocol of the GACS has been approved by the Medical Ethics Committee of School of Medicine, Xiamen University. All procedures were conducted in accordance with the Declaration of Helsinki, and all patients were required to provide written informed consent prior to participation. No adverse events were reported during or after completion of the study. The GACS was a prospective dynamic cohort study nested in the public health service system in Xiamen. Through tracing the disease process of ARDs (hypertension, diabetes mellitus, and dementia), this cohort study aimed at recognizing risk factors in the ageing process and providing clues to possible pathogenesis. We recruited the ageing inhabitants (≥ 55 years) from a rural area of Jimei District in Xiamen, China. The participants have relatively stable sociological features and can be followed over a long period. Subjects underwent annual comprehensive health check-ups in the Xiamen Guankou hospital from July 1st, 2013, until onset of hypertension, death, or the end of observation (December 31st, 2019). The participants’ lifestyle questionnaires, demographic information, and comprehensive medical check-up data were collected along with the biological samples. The data obtained at the initial medical check-up were served as the baseline information.
Data collection
Before recruitment, all investigators and staff members accepted specific trainings to be familiar with the aims of the study as well as the application methods of equipment. The standardized questionnaire developed by the School of Public Health, Xiamen University, was used to determine the information regarding of marriage status, lifestyle factors (e.g., smoking, drinking and physical exercise). All questionnaires were collected through computer-assisted face-to-face interviews during clinic visits under the guidance of investigators. After at least 10-min relaxation, three consecutive BP readings were obtained with a five-minute interval and the average was used as BP value. Subjects with BP variation beyond 10 mm Hg among the measurements were required to take a repeated measurement in three days later. Suspected hypertensive patients were asked to take further consultation until confirmation. Waist circumference (WC), which is the horizontal circumference of the mid-point line between the lowest rib and the upper edge of the iliac crest (about 1 cm to the upper edge of navel), was measured with errors of 0.5 cm. Body height and weight were measured when the subjects were taken in light clothing and without shoes (with errors 0.5 cm and 0.1 kg, respectively). Body mass index (BMI) was computed as weight in kg divided by height in m2. A 5-mL of venous blood was drawn from each subject was drawn into sodium citrate anticoagulant tube after overnight fasting (about 12 h) and then sent immediately to biochemistry laboratory in an ice cooler for the further processing and analyses.
Definitions and participant classifications
Hypertension was defined as sustainably systolic blood pressure (SBP) ≥ 140 mm Hg and/or diastolic blood pressure (DBP) ≥ 90 mm Hg taken in clinic or with a history of taking antihypertensive medications. Cases of incident hypertension were defined as those who had no baseline hypertension and were diagnosed during follow-up. Prehypertension was defined as SBP 120 to 139 mm Hg and DBP 80 to 89 mm Hg without antihypertensive medication. Stage 1 hypertension was defined as SBP 140 to 159 mm Hg and DBP 90 to 99 mm Hg, Stage 2 as 160 to 179 mm Hg and 100 to 109 mm Hg, and Stage 3 as ≥ 180 mm Hg and ≥ 110 mm Hg [22]. Four subsets of participants in GACS were initially categorized based on the STB, CB, and UCB quartiles at baseline to examine the overall relationship between hypertension and bilirubin. Type 2 diabetes mellitus (T2DM) was defined as fasting plasma glucose at least 7.0 mmol/L or if the subjects being on antidiabetic agents currently [23]. And hyperuricemia was diagnosed as serum uric acid ≥ 420 μmol/L (7.0 mg/dL) in males and serum uric acid ≥ 360 μmol/L (6.0 mg/dL) in females [24].
Statistical analysis
Multiple imputation by chained equations (MICE) was implemented to fill out the missing covariate values prior to statistical analysis [25]. Kolmogorov–Smirnov test and Levene’s test were performed to assess the normality and homogeneity of variance, respectively. All data are displayed as mean standard deviation (± SD), median (lower and upper quartiles), and frequencies (percentages) depending on the type of data. Student’s t-test or one-way analysis of variance (ANOVA), and Manne-Whitney U test or Kruskal–Wallis test, and Pearson’s Chi-square test were performed for comparing normally distributed continuous variables, uneven distributed variables, and categorical variables, respectively. The incidence density of hypertension was calculated as the total event number divided by the sum of follow-ups (per 100 person-years).
The Kaplan–Meier log-rank analyses and Cox proportional hazards regression models were applied after adjusting for potential key confounders to calculate the hazard rations (HR) and 95% confidence intervals (CI) for studying the relationship between hypertensive incidence and bilirubin levels. As the levels of fasting plasma glucose (FPG), STB, CB, and serum creatinine (SCr) in the population did not meet normal distribution, different types of data transformation methods were performed before the above-mentioned analyses. These data were converted by base 10 logarithmic, Box-Cox [26], and square root (SQRT) transformation to obtain normal distributions. Among them, the calculation formula of Box-Cox transformation is shown as follows:
where y represents the novel variable obtained after Box-Cox transformation, w is the original continuous dependent variable, and λ is the transformation parameter to be identified [27].
A multivariable Cox model with restricted cubic spine (RCS) with 4 knots were further constructed to check the bilirubin-hypertension dose–response associations to avoid the inappropriate linearity assumptions [28], as the RCS model is a smoothly joined sum of polynomial functions that do not assume linearity of the relationship. The threshold was determined as the identification of the risk function inflexion point. The 95% CI was derived by bootstrap resampling. The calculation formula of multivariable Cox proportional hazards regression is described below [29]:
The above formula gave the underlying hazard at time t for subject i with covariates (explanatory variables) Xi.
To evaluate if the model of bilirubin combined with other statistically significant risk factors could improve sensitivity to distinguish the high and low risk of hypertensive group, we calculated the area under the ROC curve (AUC) for each model by using pROC package [30]. The goodness of model fits was evaluated by the ANOVA and Akaike information criterion (AIC). And the distinguishing ability of hypertensive or non-hypertensive population was further determined by subsequent unsupervised clustering analysis based on multiple factor analysis (MFA) algorithm [31]. Two-tailed probability values < 0.05 were considered being statistically significant at 0.05 level. We performed all analyses using R software version 4.0.5 (R foundation, Vienna, Austria) for Windows and SPSS software version 26.0 (IBM SPSS Inc., Chicago, IL, USA).
Patient and public involvement
Neither patient was involved in setting the research question or the outcome measures, nor of them have involved in developing the plans for recruitment, design, or implementation of the study. No patient was asked of advice on interpreting or writing up of the results.
Results
Baseline characteristics of participants
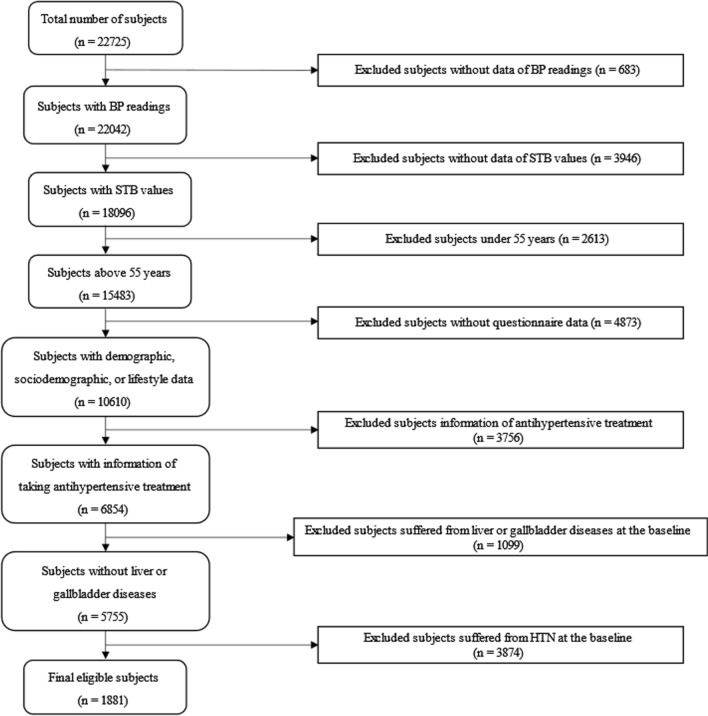
At the beginning of this study, 22,725 subjects were included in the GACS, who took part in at least 2 times of the follow-up visits and medical tests. As shown in Fig. 1, we excluded 20,844 subjects according to the following criteria: (1) lack of BP readings (n = 683); (2) lack of STB (n = 3946); (3) under the age of 55 years (n = 2613); (4) lack of demographic, sociodemographic, or lifestyle data (n = 4873); (5) lack of information on whether taking antihypertensive treatment (n = 3756); (6) suffered from liver diseases (hepatitis, alcoholic liver, cirrhosis) or gallbladder diseases (cholecystitis, gallstones, choledocholithiasis) which resulted in the elevation of bilirubin levels (n = 1099) during the follow-up process. As displayed in Additional file 1: Table S1, the hypertensive prevalence at the baseline was 67.32% (41.79% in males; 58.21% in females) and there was no gender composition difference between the 2 subgroups (χ2 = 2.00, P = 0.158). We further excluded 3874 subjects suffered from hypertension at the baseline, thus, 1881 subjects were enrolled in the next stage statistical analyses.
Fig. 1.
Flow diagram of participant inclusion and exclusion criteria in the GACS. BP, blood pressure; HTN, hypertension; STB, serum total bilirubin
Missing values of BMI (n = 12), WC (n = 43), leukocyte (n = 64), platelet (n = 63), hemoglobin (n = 60), FPG (n = 12), alanine transaminase (ALT) (n = 3), aspartate aminotransferase (AST) (n = 3), and alcohol consumption status (n = 10) were interpolated using MICE method. The median age of subjects at recruitment was 61.00 years, with 823 (43.75%; 62.00 years) males. Here, smoking status was classified as “current smoker” (smoked at least one cigarette per day for over 12 months), “former smoker” (former daily smoker who quit smoking > 6 months), and “non-smoker” (including occasional smoking and former occasional smoking); status of alcohol consumption was categorized as “current drinker” (individuals who drunk at least one cup (approximately 125 mL) of alcoholic beverages (beer, wine, and liquors) per day for over 12 months) and “never drinker” (including occasional drinking and former occasional drinking). The frequency of exercise was roughly defined as how many times they took various forms of physical exercise per week (≥ once monthly as the low, ≥ once weekly as the medium, and daily as the high). The study population included 491 current smokers (26.10%), 311 current drinkers (16.62%), and 779 participants (41.41%) with the medium or high frequency of exercise. Table 1 showed the specific baseline information of the subjects in the GACS, which revealed that male individuals had higher age, DBP, leukocyte, hemoglobin, ALT, STB, CB, and SCr. Female participants exhibited larger percentages of widowhood, non-smoker, non-drinker, as well as low and medium exercise frequency compared with male participants. The baseline SBP of male (125.00 (117.00–132.00)) and female participants (124.00 (116.75–131.00)) were not statistically different (P = 0.289), while the DBP of male subjects (76.00 (71.00–81.00)) were slightly higher (P < 0.001) than females (75.00 (70.00–80.00)). In addition, the prevalence of T2DM and hyperuricemia were not significantly different between genders (PT2DM = 0.750; Phyperuricemia = 0.629).
Table 1.
Characteristics of the 1881eligible participants at recruitment
| Variables | Overall | Male | Female | P value |
|---|---|---|---|---|
| N = 1881 | n = 823 (43.75%) | n = 1058 (56.25%) | ||
| Age (years) | 61.00 (59.00–66.00) | 62.00 (60.00–67.00) | 60.00 (59.00–65.00) | < 0.001 |
| WC (cm) | 81.00 (75.00–88.00) | 81.00 (75.00–87.00) | 81.00 (75.00–87.00) | 0.482 |
| BMI (kg/m2) | 24.13 ± 60.92 | 21.97 (20.06–23.99) | 22.52 (20.78–24.92) | < 0.001 |
| SBP (mm Hg) | 124.00 (117.00–131.00) | 125.00 (117.00–132.00) | 124.00 (116.75–131.00) | 0.289 |
| DBP (mm Hg) | 76.00 (70.00–80.00) | 76.00 (71.00–81.00) | 75.00 (70.00–80.00) | < 0.001 |
| Leukocyte (× 109/L) | 5.90 (5.10–7.01) | 6.40 (5.46–7.50) | 5.60 (4.80–6.52) | < 0.001 |
| Platelet (× 109/L) | 217.00 (190.00–252.00) | 213.00 (186.00–246.00) | 220.00 (192.75–255.00) | 0.001 |
| Hemoglobin (g/L) | 135.00 (126.00–144.00) | 143.00 (135.00–151.00) | 130.00 (123.00–136.00) | < 0.001 |
| FPG (mmol/L) | 4.99 (4.50–5.60) | 4.85 (4.40–5.47) | 5.10 (4.50–5.70) | < 0.001 |
| ALT (U/L) | 20.90 (16.30–27.50) | 21.90 (17.00–28.60) | 20.40 (15.70–26.33) | < 0.001 |
| AST (U/L) | 21.00 (18.00–24.10) | 21.00 (18.00–24.10) | 21.00 (18.00–24.20) | 0.650 |
| Albumin (g/L) | 44.50 (42.90–46.00) | 44.50 (42.90–46.00) | 44.60 (42.88–46.10) | 0.386 |
| STB (μmol/L) | 11.90 (9.25–15.20) | 12.50 (9.50–16.00) | 11.50 (9.00–14.50) | < 0.001 |
| CB (μmol/L) | 3.20 (2.20–4.50) | 3.60 (2.50–5.00) | 2.90 (1.90–3.90) | < 0.001 |
| UCB (μmol/L) | 8.30 (5.90–11.50) | 8.40 (5.90–11.60) | 8.20 (5.90–11.10) | 0.316 |
| SCr (μmol/L) | 62.80 (51.70–75.80) | 72.50 (64.00–83.80) | 54.70 (47.30–64.43) | < 0.001 |
| TC (mmol/L) | 5.00 (4.20–6.00) | 4.84 (4.19–5.55) | 5.16 (4.53–5.82) | < 0.001 |
| TG (mmol/L) | 5.03 (4.40–5.71) | 0.99 (0.74–1.43) | 1.08 (0.79–1.52) | 0.001 |
| T2DM (n, %) | (176) 9.36 | 79 (9.60) | 97 (9.17) | 0.750 |
| Hyperuricemia (n, %) | 468 (24.88) | 200 (24.30) | 268 (25.35) | 0.629 |
| Marriage Status (n, %) | < 0.001 | |||
| Married | 1638 (87.10) | 767 (93.20) | 871 (82.33) | |
| Widowed | 229 (12.20) | 49 (5.95) | 180 (17.01) | |
| Unspecified | 14 (0.70) | 7 (0.85) | 7 (0.66) | |
| Smoking Status (n, %) | < 0.001 | |||
| Non-smoker | 1337 (71.08) | 294 (35.72) | 1043 (98.58) | |
| Former Smoker | 53 (2.82) | 52 (6.32) | 1 (0.09) | |
| Current Smoker | 491 (26.10) | 477 (57.96) | 14 (1.32) | |
| Drink Status (n, %) | < 0.001 | |||
| Non-drinker | 1560 (83.38) | 553 (67.69) | 1007 (95.54) | |
| Current Drinker | 311 (16.62) | 264 (32.31) | 47 (4.46) | |
| Exercise Frequency | < 0.001 | |||
| Low | 1102 (58.59) | 488 (52.30) | 614 (58.03) | |
| Medium | 322 (17.12) | 124 (15.07) | 198 (18.71) | |
| High | 457 (24.29) | 211 (25.63) | 246 (23.26) | |
Normally distributed variables with even variance were presented as mean ± SD, skewed variables as median (lower quartile to upper quartile), and categorical variables as n (%). Continuous variables were compared using Student’s t-test or Mann–Whitney U test depending on distribution. Pearson’s χ2 tests were used to compare categorical values
HTN, hypertension; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasm blood; ALT, alanine aminotransferase; AST, aspartate aminotransferase; STB, serum total bilirubin; CB, conjugated bilirubin; UCB, unconjugated bilirubin; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus
Kaplan–Meier analysis
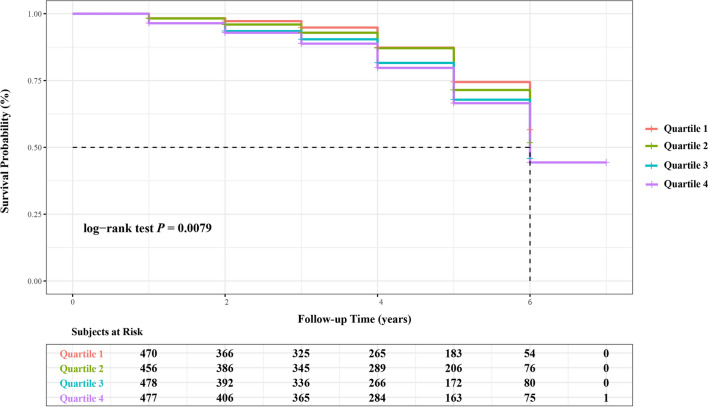
These 1881 participants were further divided into 4 subgroups on the basis of the STB quartiles, and the total and the average length of follow-up was 6916 and 3.68 ± 1.69 person-years, respectively. Their detail information and the hypertensive incidence were summarized in Table 2, which revealed the individuals who had the higher STB level were likely to have the higher values of hemoglobin, ALT, AST, CB, and UCB. When the STB level increased, the incident density of hypertension also presented an elevating trend (P = 0.018). During the seven-year of follow-up, 435 cases were diagnosed with hypertension and the overall accumulative incident rate was 6.29 per 100 person-years (5.97 and 6.51 per 100 person-years in men and women, respectively) in the GACS. The cumulative hazards of hypertensive incidence were generated by Kaplan–Meier log-rank analysis was shown in Fig. 2, and the results of pairwise comparisons between quartiles were shown in Additional file 1: Table S4. As expected, the cumulative hazards increased with follow-up time. And the positive correlation seemed to exist between STB levels and the hypertensive incidence in the GACS, as the highest STB quartile exhibited the highest onset risk for hypertension among the four subgroups. Moreover, the hypertension risk in quartile 1 was significantly lower than quartile 3 (p = 0.013) and quartile 4 (p = 0.003), while the similar pattern could also be observed between quartile 2 and quartile 4 (p = 0.028).
Table 2.
Baseline characteristics of initial non-HTN participants according to baseline bilirubin quartiles
| Variable | Overall | STB quartiles | ||||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 | 4 | P value | ||
| < 9.3 (μmol/L) | 9.3 ~ 11.8 (μmol/L) | 11.9 ~ 15.2 (μmol/L) | > 15.2 (μmol/L) | |||
| N = 1881 | n = 470 | n = 456 | n = 478 | n = 477 | ||
| Age (years) | 61.00 (59.00–66.00) | 62.00 (59.00–67.00) | 61.00 (59.25–66.00) | 61.00 (59.00–66.00) | 61.00 (59.00–65.00) | 0.107 |
| Length of follow-up (Person-years) | 3.68 ± 1.69 | 3.54 ± 1.73 | 3.86 ± 1.67 | 3.61 ± 1.72 | 3.71 ± 1.61 | 0.025 |
| Male (n, %) | 823 (43.75) | 189 (40.21) | 174 (38.16) | 212 (44.35) | 248 (51.99) | < 0.001 |
| WC (cm) | 81.00 (75.00–88.00) | 81.00 (74.00–87.00) | 81.00 (76.00–88.00) | 81.00 (75.00–87.00) | 80.00 (74.00–87.00) | 0.211 |
| BMI (kg/m2) | 22.26 (20.45–24.46) | 22.15 (20.44–24.34) | 22.66 (20.95–24.94) | 22.21 (20.44–24.44) | 22.04 (20.03–24.25) | 0.007 |
| SBP (mm Hg) | 127.00 (117.00–138.00) | 127.00 (116.75–138.00) | 129.00 (118.00–139.00) | 125.50 (116.00–137.00) | 127.00 (117.50–137.50) | 0.094 |
| DBP (mm Hg) | 78.00 (72.00–84.00) | 77.00 (71.00–84.00) | 78.00 (72.00–85.00) | 78.00 (72.00–84.00) | 78.00 (72.00–85.00) | 0.389 |
| Leukocyte (× 109/L) | 5.90 (5.10–7.01) | 5.90 (5.29–7.10) | 5.80 (5.00–7.10) | 6.00 (5.20–7.10) | 5.96 (4.90–7.90) | 0.169 |
| Platelet (× 109/L) | 217.00 (190.00–252.00) | 225.00 (201.00–261.25) | 217.00 (190.25–251.00) | 215.00 (185.00–250.25) | 212.00 (184.00–242.00) | < 0.001 |
| Hemoglobin (g/L) | 135.00 (126.00–144.00) | 132.00 (124.75–142.00) | 134.00 (126.00–142.00) | 136.00 (126.75–145.00) | 138.00 (129.50–147.00) | < 0.001 |
| FPG (mmol/L) | 4.99 (4.50–5.60) | 4.94 (4.50–5.60) | 4.90 (4.40–5.50) | 4.99 (4.67–5.60) | 5.10 (4.50–5.70) | 0.278 |
| ALT (U/L) | 20.90 (16.30–27.50) | 19.20 (14.68–25.63) | 20.70 (16.40–27.10) | 21.90 (17.00–29.25) | 21.90 (17.65–27.50) | < 0.001 |
| AST (U/L) | 21.00 (18.00–24.10) | 19.95 (17.30–24.00) | 20.00 (17.93–23.30) | 21.00 (18.20–25.00) | 21.00 (19.00–25.00) | < 0.001 |
| Albumin (g/L) | 44.50 (42.90–46.00) | 44.45 (42.50–45.93) | 44.20 (42.60–46.00) | 44.60 (42.90–46.10) | 44.80 (43.30–46.10) | 0.024 |
| CB (μmol/L) | 3.20 (2.20–4.50) | 2.40 (1.60–3.10) | 3.10 (2.30–3.70) | 3.50 (2.48–4.50) | 4.50 (2.85–5.80) | < 0.001 |
| UCB (μmol/L) | 8.50 (5.90–11.50) | 5.00 (3.70–6.00) | 7.40 (6.50–8.38) | 9.90 (8.60–11.13) | 14.00 (11.60–16.75) | < 0.001 |
| SCr (μmol/L) | 62.80 (51.70–75.80) | 63.70 (51.48–77.33) | 60.05 (48.10–73.75) | 62.55 (52.43–75.43) | 64.40 (54.00–77.00) | 0.001 |
| TC (mmol/L) | 5.03 (4.40–5.71) | 4.94 (4.20–5.65) | 5.07 (4.35–5.70) | 5.07 (4.43–5.72) | 5.04 (4.46–5.76) | 0.135 |
| TG (mmol/L) | 1.04 (0.77–1.48) | 1.08 (0.81–1.62) | 1.07 (0.78–1.46) | 1.03 (0.77–1.49) | 0.99 (0.72–1.39) | 0.007 |
| T2DM (n, %) | 178 (9.46) | 52 (11.06) | 42 (9.21) | 43 (8.81) | 41 (8.78) | 0.510 |
| Hyperuricemia (n, %) | 468 (24.88) | 82 (17.44) | 107 (23.46) | 142 (29.71) | 137 (28.78) | < 0.001 |
| Marriage (n, %) | 0.976 | |||||
| Married | 1638 (87.08) | 405 (86.17) | 395 (86.62) | 421 (88.08) | 417 (87.42) | |
| Widowed | 229 (12.17) | 61 (12.98) | 58 (12.72) | 53 (11.09) | 57 (11.95) | |
| Unspecified | 14 (0.74) | 4 (0.85) | 3 (0.66) | 4 (0.84) | 3 (0.63) | |
| Smoking Status (n, %) | 0.069 | |||||
| Non-smoker | 1331 (70.76) | 335 (71.28) | 335 (73.46) | 340 (71.13) | 321 (67.30) | |
| Former Smoker | 53 (2.82) | 9 (1.91) | 10 (2.19) | 11 (2.30) | 23 (4.82) | |
| Current Smoker | 491 (26.10) | 125 (26.60) | 110 (24.12) | 124 (25.94) | 132 (27.67) | |
| Drinking Status (n, %) | 0.019 | |||||
| Non-drinker | 1560 (82.93) | 396 (84.26) | 388 (85.09) | 402 (84.10) | 374 (78.41) | |
| Current Drinker | 311 (16.53) | 70 (14.89) | 67 (14.69) | 73 (15.27) | 101 (21.74) | |
| Exercise Frequency (n, %) | 0.013 | |||||
| Low | 1102 (58.59) | 307 (65.32) | 257 (56.36) | 269 (56.28) | 269 (56.39) | |
| Medium | 322 (17.12) | 55 (11.70) | 87 (19.08) | 89 (18.62) | 91 (19.08) | |
| High | 457 (24.30) | 108 (22.98) | 112 (24.56) | 120 (25.10) | 117 (24.53) | |
| Incident HTN (n, %) | 435 (6.29) | 81 (4.87) | 104 (5.92) | 121 (7.01) | 129 (7.28) | 0.018 |
Normally distributed variables are presented as mean ± SD, skewed variables as median (interquartile range), and categorical variables as n (%). Incidence rate was calculated as the number of hypertension incident cases divided by 100 person-years of follow-up. Continuous variables were compared using one-way ANOVA or Kruskal–Wallis test depending on distribution. Pearson’s χ2 tests were used to compare categorical values
HTN, hypertension; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasm blood; ALT, alanine aminotransferase; AST, aspartate aminotransferase; STB, serum total bilirubin; CB, conjugated bilirubin; UCB, unconjugated bilirubin; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus
Fig. 2.
Cumulative risk curves for hypertension incidence by Kaplan–Meier analysis. Groups of STB concentration were defined as: Quartile 1, < 9.3 (μmol/L); Quartile 2, 9.3 ~ 11.8 (μmol/L); Quartile 3, 11.9 ~ 15.2 (μmol/L); Quartile 4, > 15.3 (μmol/L)
Cox proportion hazards regression model
Cox proportional hazards regression model was further performed to elaborate the quantitative profile between bilirubin and the hypertension risks. Multicollinearity diagnostics was conducted to detect the multicollinearity between key variables. The values of Tolerance (TOL) and Variance Inflation Factor (VIF) greater than 0.1 and 10 indicates the multicollinearity is acceptable [32–34]. The variable of hyperuricemia was not included in the model construction due to its values of TOL and VIF exceeded thresholds. As summarized in Table 3, variables of BMI, WC, FPG, AST, STB, CB, UCB, SCr, and current smoking were significantly associated with the incident risk of hypertension, and current smoking (1.99 (1.60–2.46)) and DM (1.67 (1.28–2.19)) were the two most significant risk factors. HR (95% CI) for STB was 1.03 (1.01–1.05), while for BMI, WC, FPG, UCB, STB, AST, and SCr, these ratios were 1.23 (1.00–1.26), 1.08 (1.07–1.09), 1.07 (1.05–1.09), 1.05 (1.03–1.07), 1.03 (1.01–1.05), 1.02 (1.01–1.03), and 1.01 (1.00–1.01), respectively. However, CB showed the weak inverse relationship with incident hypertension with the HR of 0.96 (0.92–0.99).
Table 3.
Hazard ratios of serum total bilirubin levels for hypertension in the GACS
| Variables | HR (95% CI) | P value |
|---|---|---|
| Female | 1.08 (0.89–1.31) | 0.430 |
| Age | 1.01 (0.99–1.03) | 0.244 |
| BMI | 1.23 (1.20–1.26) | < 0.001 |
| WC | 1.08 (1.07–1.09) | < 0.001 |
| SBP | 1.00 (0.99–1.00) | 0.962 |
| DBP | 1.00 (0.99–1.01) | 0.885 |
| FPG | 1.07 (1.05–1.09) | < 0.001 |
| AST | 1.02 (1.01–1.03) | 0.003 |
| STB | 1.03 (1.01–1.05) | 0.002 |
| CB | 0.96 (0.92–0.99) | 0.022 |
| UCB | 1.05 (1.03–1.07) | < 0.001 |
| SCr | 1.01 (1.00–1.01) | 0.005 |
| T2DM | 1.67 (1.28–2.19) | < 0.001 |
| Current Smoker | 1.99 (1.60–2.46) | < 0.001 |
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasm glucose; AST, aspartate aminotransferase; STB, serum total bilirubin; CB, conjugated bilirubin; UCB, unconjugated bilirubin; SCr, serum creatinine; T2DM, type 2 diabetes mellitus
*P < 0.05 was considered statistically significant
Table 4 showed the HRs calculated by multivariable adjusted Cox proportional hazards regressions. Raw data of FPG, STB, and SCr were transformed by base 10 logarithmic, Box-Cox (λ = − 0.418), and SQRT to meet the requirement of normal and homoscedastic distribution prior to analysis. Our findings suggested that the hypertensive risk for subjects in the highest STB quartile was approximately 1.5 times as that of the lowest quartile in the crude model (HR 1.49, 95% CI 1.13–1.97), and the correlation remained significant in all 4 models after the adjustment of multivariable. And after adjusting for age, gender, BMI, WC, baseline BP level (SBP and DBP), DM, FPG, AST, and SCr (Model 3), the HR of the highest quartile was 1.76 (95% CI 1.32–2.34). The similar positive relationship could also be observed between hypertensive risk and UCB levels; individuals in the highest UCB quartile group exhibited 2.26 times higher incident risk compared with those in the lowest level group in model 1 (Additional file 1: Table S3). However, as it was displayed in Additional file 1: Table S2, a weak negative association could be observed between the hypertension incident risks and serum CB levels with the gradual declined tendency of HRs, which hinted the risk of hypertension decreased with the increasement of CB levels.
Table 4.
Prospective analysis of associations between STB levels and hypertension incidence
| STB quartiles | |||||
|---|---|---|---|---|---|
| 1 < 9.3 μmol/L |
2 9.3 ~ 11.8 μmol/L |
3 11.9 ~ 15.2 μmol/L |
4 > 15.3 μmol/L |
P for trend | |
| Crude Model | Reference | 1.14 (0.85–1.52) | 1.40 (1.05–1.85) | 1.49 (1.13–1.97) | 0.017 |
| Model 1 | Reference | 1.10 (0.82–1.47) | 1.49 (1.12–1.97) | 1.68 (1.27–2.22) | < 0.001 |
| Model 2 | Reference | 1.11 (0.83–1.48) | 1.50 (1.13–1.99) | 1.68 (1.27–2.23) | < 0.001 |
| Model 3 | Reference | 1.21 (0.90–1.63) | 1.60 (1.20–2.12) | 1.76 (1.32–2.34) | < 0.001 |
| Model 4 | Reference | 1.19 (0.89–1.60) | 1.46 (1.10–1.94) | 1.68 (1.26–2.23) | 0.002 |
Multivariable-adjusted Cox regression models were used to assess the hypertension incidence risk by STB quartiles. Crude model: Only included STB level at the baseline. Multivariable model 1: Included variables of age, gender, BMI, and WC on the basis of the Crude model. Multivariable model 2: Further included variables of SBP, DBP, and DM at baseline on the basis of the Multivariable model 1. Multivariable model 3: Further included variables of FPGlog10, AST, and SCrSQRT on the basis of the Multivariable model 2. Multivariable model 4: Further included the variable of smoking status on the basis of the Multivariable model 3
STB, serum total bilirubin; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; T2DM, type 2 diabetes mellitus; AST, aspartate aminotransferase; FPG, fasting plasma glucose; SCr, serum creatinine
Restricted cubic spline analyses
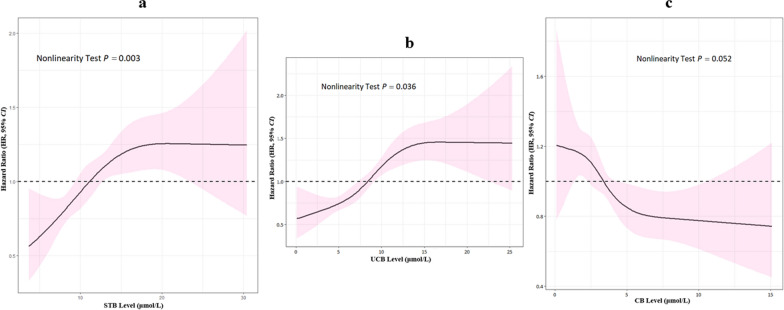
The RCS analyses have been performed to avoid the potential effects of inappropriate linearity and to check the relationship of the precise dose–response associations between predictor and response variables. As shown in Fig. 3, the P value of nonlinearity test (P = 0.003) confirmed the non-linear association between STB and hypertension. Beyond this, STB exhibited bidirectional physiological effects in different vivo concentration range with the cut-off of 12.17 μmol/L. STB levels below the cut-off point of 12.17 μmol/L were considered to show protective effects, while the opposite effects presented beyond the cut-off. The similar situation arose between hypertension incident risks and UCB in indicated the bidirectional effects of UCB with the cut-off of 8.59 μmol/L. Nevertheless, serum CB concentrations presented the unstable correlation with the hypertensive hazards, which was in contrast to STB and UCB, as 3.47 μmol/L was the cut-off point (Additional file 1: Table S2).
Fig. 3.
The non-linear dose–response relationship between bilirubin and hypertension. Restricted cubic spine with 4 knots was performed to determine the bilirubin (STB, UCB, and CB)-hypertension dose–response relationship. The solid blue line represents the dose–response curve with multivariable Cox model, and the pink shading indicates the 95% confidence interval. STB, UCB, and CB exhibited bidirectional effects to hypertensive risk with the threshold concentration of 12.17 μmol/L, 8.59 μmol/L, and 3.47 μmol/L, respectively
The association between bilirubin and hypertension stage
In addition, participants were divided into 4 hypertension stages at the end of follow-up according to the classification requirements, including normal group of 485 subjects (25.78%), pre-hypertension group of 938 subjects (49.87%), Stage 1 hypertension group of 385 subjects (20.47%), Stage 2 of 63 subjects (3.35%), and Stage 3 of 10 subject (0.53%). Due to the number of cases greater than or equal to Stage 1 hypertension was too small, we combined these cases into one ≥ Stage 1 hypertension group. Table 5 showed that variables of age (P < 0.001), WC (P < 0.001), BMI (P = 0.006), ALT (P < 0.001), AST (P = 0.006), ALT (P = 0.006), albumin (P = 0.017), and TG (P = 0.050) exhibited different distribution and increased as the condition of hypertension got worse. Interestingly, the consistently decreasing trends in STB and UCB appeared to exist along with the deterioration of hypertension (PSTB = 0.723; PUCB = 0.782), but an increasing trend seemed to be observed in CB (PCB = 0.053).
Table 5.
Participants’ characteristics of the GKASC grouped by HTN stage
| Variables | Overall | Normal | Pre-HTN | ≥ Stage 1 HTN | P value |
|---|---|---|---|---|---|
| N = 1881 | n = 485 (25.78%) | n = 938 (49.87%) | n = 458 (24.35%) | ||
| Age (years) | 65.00 (62.00–70.00) | 65.00 (62.00–69.00) | 65.00 (62.00–69.00) | 66.00 (63.00–72.00) | < 0.001 |
| Male (n, %) | 823 (43.75) | 200 (41.24) | 423 (45.10) | 200 (43.67) | 0.380 |
| WC (cm) | 81.00 (75.00–88.00) | 80.00 (74.00–87.00) | 80.00 (75.00–87.00) | 82.00 (76.00–89.00) | < 0.001 |
| BMI (kg/m2) | 22.23 (20.45–24.46) | 22.06 (20.17–24.12) | 22.23 (20.36–24.46) | 22.54 (20.85–24.77) | 0.006 |
| Leukocyte (× 109/L) | 5.90 (5.10–7.00) | 5.80 (4.80–6.92) | 5.90 (5.10–7.10) | 6.00 (5.30–7.00) | 0.095 |
| Platelet (× 109/L) | 217.00 (190.00–252.00) | 217.00 (188.00–251.50) | 217.00 (189.00–250.00) | 220.00 (193.00–254.00) | 0.409 |
| Hemoglobin (g/L) | 135.00 (126.00–144.00) | 134.00 (126.00–143.00) | 135.00 (127.00–144.00) | 135.00 (126.00–145.00) | 0.324 |
| FPG (mmol/L) | 4.99 (4.50–5.60) | 5.00 (4.40–5.55) | 4.93 (4.50–5.60) | 4.98 (4.50–5.60) | 0.617 |
| ALT (U/L) | 20.95 (16.33–27.50) | 20.40 (15.70–25.45) | 20.70 (16.50–27.50) | 22.80 (17.10–30.20) | < 0.001 |
| AST (U/L) | 21.00 (18.00–24.20) | 20.00 (18.00–23.90) | 21.00 (18.00–24.00) | 21.00 (18.00–26.00) | 0.006 |
| Albumin (g/L) | 44.60 (42.90–46.00) | 44.20 (42.75–45.50) | 44.60 (42.80–46.10) | 44.75 (43.20–46.30) | 0.017 |
| STB (μmol/L) | 11.90 (9.25–15.20) | 12.00 (9.10–15.10) | 11.90 (9.28 -15.23) | 11.85 (9.40–15.40) | 0.723 |
| CB (μmol/L) | 3.20 (2.20–4.40) | 3.00 (2.10–4.10) | 3.10 (2.10–4.50) | 3.30 (2.38–4.60) | 0.053 |
| UCB (μmol/L) | 8.30 (5.90–11.45) | 8.60 (5.95–11.60) | 8.30 (5.70–11.50) | 8.10 (6.10–11.13) | 0.782 |
| SCr (μmol/L) | 62.80 (51.70–75.80) | 62.70 (50.70–76.35) | 63.10 (51.98–75.50) | 62.60 (52.25–76.13) | 0.853 |
| TC (mmol/L) | 5.03 (4.40–5.71) | 5.06 (4.42–5.69) | 5.05 (4.42–5.79) | 5.00 (4.32–5.64) | 0.107 |
| TG (mmol/L) | 1.04 (0.77–1.48) | 1.00 (0.74–1.38) | 1.05 (0.77–1.52) | 1.07 (0.81–1.55) | 0.050 |
| T2DM (n, %) | 176 (9.36) | 39 (8.04) | 93 (9.91) | 44 (9.61) | |
| Hyperuricemia (n, %) | 468 (24.88) | 124 (25.57) | 216 (23.05) | 128 (27.95) | |
| Marriage Status (n, %) | 0.486 | ||||
| Married | 1649 (87.67) | 432 (89.07) | 818 (87.21) | 399 (87.12) | |
| Widowed | 220 (11.70) | 50 (10.31) | 116 (12.37) | 54 (11.79) | |
| Unspecified | 12 (0.64) | 3 (0.62) | 4 (0.43) | 5 (0.11) | |
| Smoking Habits | 0.958 | ||||
| Non-smoker | 1395 (74.16) | 365 (75.26) | 689 (73.45) | 341 (74.45) | |
| Former Smoker | 59 (3.14) | 14 (0.29) | 30 (3.20) | 15 (3.28) | |
| Current Smoker | 427 (22.70) | 106 (21.86) | 219 (23.35) | 102 (22.27) | |
| Drinking Habits | 0.375 | ||||
| Non-drinker | 831 (87.81) | 425 (87.81) | 800 (85.29) | 390 (85.15) | |
| Current Drinker | 121 (12.70) | 59 (12.19) | 138 (14.71) | 68 (14.85) | |
| Exercise frequency | 0.880 | ||||
| Low | 1109 (58.96) | 294 (60.62) | 552 (58.85) | 263 (57.42) | |
| Medium | 171 (9.09) | 41 (8.45) | 88 (9.38) | 42 (9.17) | |
| High | 601 (31.95) | 150 (30.93) | 298 (31.77) | 153 (33.41) | |
Normally distributed variables with even variance were presented as mean ± SD, skewed variables as median (interquartile range), and categorical variables as n (%)
Continuous variables were compared using Student’s t-test or Mann–Whitney U test depending on distribution. Pearson’s χ2 tests were used to compare categorical values
HTN, hypertension; WC, waist circumference; BMI, body mass index; FPG, fasting plasm blood; ALT, alanine aminotransferase; AST, aspartate aminotransferase; STB, serum total bilirubin; CB, conjugated bilirubin; UCB, unconjugated bilirubin; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus
Model predictive abilities
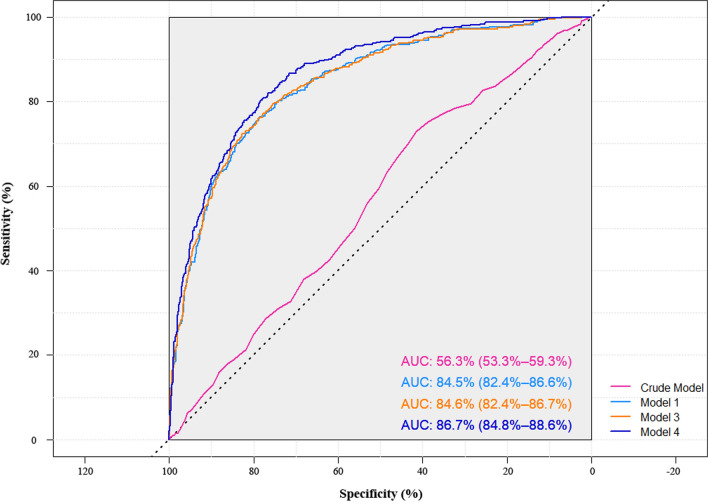
Due to STB represented the co-regulating effects of CB and UCB, we constructed hypertension predicting models based on STB and evaluated their predictive powers among the ageing population by generating ROC curves and calculating AUC (Fig. 4). The discriminative performance showed a significant improvement compared with the crude model when only baseline STB was included (AUCCrude 0.56, 95% CI 0.53–0.59; AUCModel1 0.85, 95% CI 0.82–0.87; AUCModel2 0.85, 95% CI 0.83–0.88; AUCModel3 0.85, 95% CI 0.82–0.87; AUCModel4 0.87, 95% CI 0.85–0.89). Both ANOVA and AIC were used to access the goodness of the above fits, which suggested the addition of baseline SBP and DBP of model 2 had little enhancement of predictive power compared with model 1 (PANOVA = 0.228). Regarding of the balance of cost and predicative performance, STB at baseline combined with age, BMI, and WC showed a good practical potential to discriminate the high-risk of hypertension.
Fig. 4.
ROC curves and AUC of models. Crude model: Used STB as the single variable and unadjusted other baseline variables; Multivariable model 1: Adjusted for age, gender, BMI, and WC on the basis on crude model; Multivariable model 2: Further adjusted for SBP, DBP, and DM at baseline on the basis on model 1; Multivariable model 3: Further adjusted for FPGlog10, AST, and SCrSQRT on the basis on model 2; Multivariable model 4: Further adjusted for smoking status on the basis on model 3. The ROC curve of Model 2 had little enhancement of predictive power with the supplement of baseline SBP, DBP, and DM of on the basis of model 1. Thus, the ROC curve of Model 2 is not shown in the figure. ROC, receiver operating characteristic curve; AUC, area under curve; CB, conjugated bilirubin; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; AST, aspartate aminotransferase; FPG, fasting plasma glucose; SCr, serum creatinine
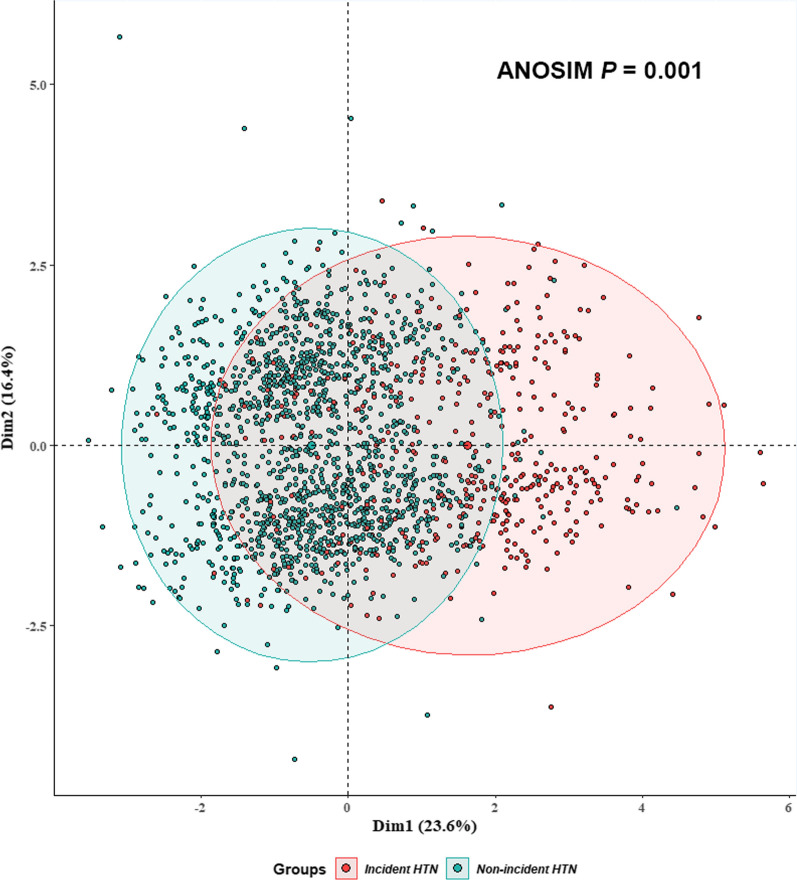
We conducted clustering analysis based on MFA algorithm to further examine discernibility power of Model 1, and the results supported the conclusions generated by ROC curves. Specifically, by applying MFA, we identified the first and second principal component (Dim1; Dim2) that explained most of the variance in our data (23.6% and 16.4%) as shown in Fig. 5. Research subjects with high- and low-risk hypertension could be well clustered into 2 distinct clusters with STB at baseline combined with age, BMI, and WC.
Fig. 5.
Clustering analysis based on MFA algorithm. Red dots represent participants diagnosed with hypertension at the end of follow-up, while green dots are non-incident ones. Participants with high- and low-risk hypertension were clustered into two distinct clusters with STB at baseline combined with age, BMI, and WC. Ellipses represent the 95% CI. BMI, body mass index; WC, waist circumference; MFA, multiple factor analysis
Discussion
Hypertension represents a primary risk factor of cardiovascular and cerebrovascular diseases linked with endothelial dysfunction, which is the leading cause of mortality in the world. The population ageing, rapid urbanization, as well as the changes of environment and lifestyle count great significance for hypertension preventing and controlling [35–37]. In addition, more attention should be paid to the molecular risk factors, which may indicate the pathology and strengthen prevention of hypertension. Bilirubin could be served as one of the molecular factors that has been associated with hypertension in few studies, however, the previous conclusions are still controversial.
In our present work, we analyzed the relationship between bilirubin concentration and hypertension risk according to the data obtained from the GACS. The bidirectional effects of the different bilirubin species were observed along their concentration changes. The lower levels of STB and UCB showed the protective effects towards hypertension, while the opposite effects were observed at the higher levels. The inverse associations have been observed between serum CB and hypertension. The validity of our research is bolstered by previous research that has reached the similar conclusions. Yasuko Takeda et al. selected 37 patients with pulmonary arterial hypertension (PAH) as the research objects and manifested that elevated serum bilirubin is a risk factor for death in patients with PAH [18]. Beyond that, according to the research of 37,544 newborns (31,819 term and 5725 preterm births) from the U.S. Collaborative Perinatal Project conducted from 1959 to 1965, Huan Yu et al. proposed that neonatal serum bilirubin levels at 48 h after birth were positively associated with childhood blood pressure/hypertension in the preterm infants at 7 years [19]. However, other experts offered diverse perspectives that high serum bilirubin may decrease hypertensive risk. For example, Lina Wang et al. analyzed data from the National Health and Nutrition Examination Surveys (NHANES) 1999–2012 (N = 31,069) and demonstrated that SBP decreased progressively up to − 2.5 mmHg (P < 0.001) and the prevalence of hypertension was up to 25% lower (P < 0.001) in those with bilirubin ≥ 1.0 mg/dL-the highest two deciles-compared with those with 0.1–0.4 mg/dL-the lowest decile. The author supposed that the fundamental mechanism was high serum bilirubin level might inactivate and inhibit the synthesis of reactive oxygen species in vascular cells to decrease the risk of hypertension [20]. Ho Jun Chin et al. also proposed that bilirubin concentration was negatively correlated with hypertension incident risk among normotensive Korean adults [21]. Such protective effects were mainly attributed to the fact that bilirubin has significant antioxidant properties, such as preventing vitamin A and polyunsaturated fatty acids from oxidation [17]. Meanwhile, bilirubin was supposed to be a potent substance of scavenging hydrogen peroxide radicals and therefore fulfills the anti-oxidative function. However, when exploring the effects of bilirubin in some ageing-related diseases, such as cardiovascular and metabolic disorders, some large-scale cross-sectional and cohort studies have suggested the potential protective effects of bilirubin [38, 39]. We would like to offer a perspective view that STB and UCB mainly exerted both harmful and preventive influence to hypertension, which have the patterns similar to hormesis effect [40]. We also observed that CB was weakly and negatively correlated with hypertension, which was partially in agreement with those of previous studies [41]. The metabolism cross-talk of STB, UCB and CB may well illustrate the role of bilirubin, which suggested that different bilirubin metabolites should be treated dialectically when assessing their risk of hypertension.
The pathological mechanisms of hypertension remain extremely complicated. The partial characteristic of hypertension is mild symptoms of inflammation, and the main mechanisms to pathogenesis involving in the up-regulation of the sympathetic nervous system and the increased renin–angiotensin–aldosterone system (RAAS)-activity [42]. CB and UCB are the two major species of bilirubin, which display unique chemical properties, and UCB constitutes larger proportion than CB. As a lipophilic molecule, UCB is able to bind to human neurons, which is enriched in phospholipids. While the excitability of sympathetic nervous system exhibited a positive relationship with the bilirubin deposited in the central nervous system, which matters in the hypertensive pathogenesis [19, 43]. In contrast, CB presented the inverse effect as UCB, which can be plausibly interpreted by its chemical properties of water-soluble and urinary ready excretion. Therefore, phase-II conjugation is important to balance serum CB and avoid UCB accumulation. The breakthrough increasing of UCB may add hypertension risk. Anyway, how the adverse action of bilirubin initiating and/or progressing hypertension remains unclear.
Because of the bidirectional effect, bilirubin appears not to be suitable as an ideal hypertension biomarker as the AUC of crude model is relatively low (AUCBase 0.56, 95% CI 0.53–0.59), but this result hinted at the significance of subsequent researches. Regarding of the balance of cost and predicative performance, Model 1 showed a good practical potential to discriminate the high-hypertension risks. In addition, the 7-year hypertension incident rate in the GACS was 6.29 per 100 person-years (5.97 and 6.51 per 100 person-years in men and women, respectively), which was relatively lower in the similar researches conducted in German [44] and Korea [45]. The possible reasons might be as follows: (1) The participants in the GACS were relatively younger than those in other researches. The CARLA study, conducted among Germany general population, was comprised of 1779 subjects with a mean age of 64.9 (SD = 10.2) years for men and 63.8 (SD = 9.9) years for women at baseline. The positive association between increasing age and incidence of hypertension was in agreement with many literatures, which could be attributed to lower-level physical activity, differences in dietary intake, the age-dependent hardening of the vascular system and worsening of kidney function [46, 47]; (2) The participants in the GACS were shown to be more “slim” than other cohorts. The male and female participants showed the mean BMI values of 22.13 (SD = 0.10) and 22.88 (SD = 0.10) in the GACS, which were relatively lower than Germany and Korean population involved. BMI is a well-recognized risk factor for hypertension [48], and in a meta-analysis, the mean SBP and DBP reductions associated with an average weight loss of 5.1 kg were 4.4 and 3.6 mmHg, respectively [49]; (3) Another possible reason might be attributed to the annual routine health examination of the subjects, which may be the relatively healthy part of the population have been involved.
In our study, the prevalence of T2DM and hyperuricemia were not significantly different between genders. He et al. used real-world data to estimate the changing tendencies in the prevalence of T2DM in Xiamen City from 2014 to 2019. Interestingly, the overall prevalence among the male and female adults were 4.18% and 5.52% in Xiamen, and T2DM prevalence exhibited an increasing trend with advancing age regardless of gender [50]. In our work, female participants were younger than males, which could be one possible reason to explain the prevalence of T2DM were not significantly different between genders. The discrepancy of gender-specific hyperuricemia prevalence is also existed, which might be attributed to the lifestyles, dietary habits, regional economic level, and individual living standards [51, 52]. Besides, women in post-menopausal could lead to estrogen deficiency. Estrogens may promote renal clearance of serum urate and its deficiency can result in changes in the endocrine system and increase of metabolic diseases [53]. However, the underlying mechanisms that lead to the gender-specific prevalence of hyperuricemia requires further exploration.
In our risk discrimination analysis, the predictive performance of model exhibited a stepwise increase with the processive inclusion of significant associated factors and relevant biological variables, especially in Model 4. A large body of previous studies proposed smoking was a risk factor for hypertension [54–56], thus, the ability of risk discrimination in Model 4 improved drastically. However, there were very few differences of the predictive performance between Model 1 and Model 3, which hinted variables of age, gender, BMI, and WC could be considered as the main parameters in the risk discrimination model. Moreover, as shown in Crude Model, the AUC was relatively low (AUCBase 0.56, 95% CI 0.53–0.59), which suggested STB appears not to be suitable as an ideal hypertension biomarker because of the bidirectional effect. Regarding of the balance of cost and predicative performance, Model 1 showed a good practical potential to screen the subjects with high-hypertension risks.
The current analyses had several strength and limitations. The first strength was that our research was based on a large prospective study, the GACS with high-quality design, and the tracing of diseases made it possible to recognize potential protective and harmful factors as well as their modes of action on ARDs. The data of physiology, lifestyle factors, socioeconomics, and biological samples during the long-term follow-up increased our confidence to infer the underlying mechanisms of certain questions raised from the observation. And the treatment regimens based on bilirubin in clinical practice may be available when it is verified by the future studies. As for limitations, one of the limitations was hypertensive state is unstable, which can be affected by environmental and psychological causes, and the case might inevitably include the false positive confirmation. The standard definition of hypertension clinical diagnosis was based on the three BP measurements in at least two different occasions [57]. The BP readings of our subjects were measured only in the same hospital. It is also difficult to require subjects to use 24 h blood pressure monitoring equipment during the follow-up. The second limitation was hypertension subtypes (primary and secondary hypertension) were not well discriminated because of technical and cost limits during follow-up, which might introduce bias into further analysis. At last, the state of hyperuricemia in our research was relied on anamnesis and medical records of participants, rather than dynamic monitoring data, which might pose a potential risk of bias towards our conclusions.
All in all, this study provides the valuable clues of the associations between bilirubin and hypertension, even though the underlying mechanisms remain ambiguous to date. Advancing theories and methodologies is urgently called for to uncover the bilirubin-hypertension association from the more refined perspective [58].
Conclusions
Our findings suggested STB and UCB exhibited consistently hazardous effects to the incident risk of hypertension higher levels after multivariable adjustments, while an inverse effect could be observed in conjugated bilirubin. And current findings did not identify an association between bilirubin level and hypertension severity.
Supplementary Information
Additional file 1. The supplementary files of this article
Acknowledgements
The authors are sincerely appreciated to all the participants in GACS for participating in questionnaire survey, and undergoing the annual medical examinations.
Abbreviations
- ALT
Alanine transaminase
- ANOVA
Analysis of variance
- ARDs
Ageing-related diseases
- AST
Aspartate aminotransferase
- BMI
Body mass index
- BP
Blood pressure
- CB
Conjugated bilirubin
- CI
Confidence intervals
- DBP
Diastolic blood pressure
- T2DM
Type 2 diabetes mellitus
- FPG
Fasting plasma glucose
- GACS
Guankou Ageing Cohort Study
- MFA
Multiple factor analysis
- MICE
Multiple imputation by chained equations
- HR
Hazard rations
- SBP
Systolic blood pressure
- SCr
Serum creatinine
- SQRT
Square root
- STB
Serum total bilirubin
- UCB
Unconjugated bilirubin
- WC
Waist circumference
Authors' contributions
CT and H–X J contributed to design the study, research data and wrote the manuscript. BZ contributed to data interpretation and the discussion of the results. YL and S–N L contributed to methodology. T-M C and Y-H S contributed to design the study and critically reviewed the manuscript. Y-Q Z, L-N Z, L-M L, and J-C L contributed to questionnaire survey and reach data. YW, Z-Y Z contributed to the collection and deposition of biological samples. L-J L contributed to critically review the manuscript and English editing. H-Q S and JZ conceptualized and designed the protocol and critically reviewed the manuscript. Y-X W financial supported the cohort study and provided suggestions to the content of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 22076157), the Fundamental Research Funds for the Central Universities (No. 20720190074), and the Planning Guidance Project of Fujian Provincial Department of Science and Technology (2019D015).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The research protocol of the GACS has been approved by the Medical Ethics Committee of School of Medicine, Xiamen University. All patients were required to provide written informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Tang, Hanxiang Jiang and Bin Zhao contributed equally to this work
Contributor Information
Yuxin Wang, Email: 3146786287@qq.com.
Jie Zhang, Email: jie.zhang@xmu.edu.cn.
Heqing Shen, Email: hqshen@xmu.edu.cn.
References
- 1.Timmers PRHJ, Wilson JF, Joshi PK, Deelen J. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat Commun. 2020;11(1):3570. doi: 10.1038/s41467-020-17312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard JR, Officer A, de Carvalho IA, Sadana R, Margriet A, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 4.M Ezzati, AD Lopez, A Rodgers, Hoorn S Vander, CJ Murray. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360. [DOI] [PubMed]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhang L, Wang F, Liu L, Wang H; China National Survey of Chronic Kidney Disease Working Group. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens 2014; 27:1355–1361. [DOI] [PMC free article] [PubMed]
- 7.Gao Y, Chen G, Tian H, Lin L, Lu J, Weng J, et al. Prevalence of hypertension in China: a cross-sectional study. PLoS ONE. 2013;8:e65938. doi: 10.1371/journal.pone.0065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390(10112):2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 10.Ke C, Zhu X, Zhang Y, Shen Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics. 2018;14(9):117. doi: 10.1007/s11306-018-1408-y. [DOI] [PubMed] [Google Scholar]
- 11.Hamoud AR, Weaver L, Stec DE, Hinds TD., Jr Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol Metab. 2018;29(3):140–150. doi: 10.1016/j.tem.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15(25):2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- 13.Franchini M, Targher G, Lippi G. Serum bilirubin levels and cardiovascular disease risk: a Janus Bifrons? Adv Clin Chem. 2010;50:47–63. doi: 10.1016/s0065-2423(10)50003-9. [DOI] [PubMed] [Google Scholar]
- 14.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A. 2009;106(13):5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novotný L, Vítek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228(5):568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 16.Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999–2004. Am J Med. 2008;121(9):781–788. doi: 10.1016/j.amjmed.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooda V, Gahlaut A, Gothwal A, Hooda V. Bilirubin enzyme biosensor: potentiality and recent advances towards clinical bioanalysis. Biotechnol Lett. 2017;39(10):1453–1462. doi: 10.1007/s10529-017-2396-0. [DOI] [PubMed] [Google Scholar]
- 18.Takeda Y, Takeda Y, Tomimoto S, Tani T, Narita H, Kimura G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med. 2010;10:22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Zou L, He Y, Luo L, Dong W, Zhang Y, et al. Associations between neonatal serum bilirubin and childhood hypertension. PLoS ONE. 2019;14(7):e0219942. doi: 10.1371/journal.pone.0219942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Bautista LE. Serum bilirubin and the risk of hypertension. Int J Epidemiol. 2015;44(1):142–152. doi: 10.1093/ije/dyu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin HJ, Song YR, Kim HS, Park M, Yoon HJ, Na KY, et al. The bilirubin level is negatively correlated with the incidence of hypertension in normotensive Korean population. J Korean Med Sci. 2009;24(1):S50–S56. doi: 10.3346/jkms.2009.24.S1.S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015. Circulation. 2018;137(22):2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Zhang Y, Wei F, Song J, Cao Z, Chen C, et al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J Transl Med. 2019;17(1):403. doi: 10.1186/s12967-019-02156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang M, Yu X, Wei F, Chen C, Zhang K, et al. Association of hypertension and hypertriglyceridemia on incident hyperuricemia: an 8-year prospective cohort study. J Transl Med. 2020;18(1):409. doi: 10.1186/s12967-020-02590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Buuren S, Groothuis-Oudshoorn KM. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 26.Yang R, Yi N, Xu S. Box-Cox transformation for QTL mapping. Genetica. 2006;128(1–3):133–143. doi: 10.1007/s10709-005-5577-z. [DOI] [PubMed] [Google Scholar]
- 27.Xiong S, Lu S, Shang F, Li X, Yan J, Cen K. Online predicting PCDD/F emission by formation pathway identification clustering and Box-Cox Transformation. Chemosphere. 2021;274:12978.0. doi: 10.1016/j.chemosphere.2021.129780. [DOI] [PubMed] [Google Scholar]
- 28.Chen YJ, Liu C, Huang LL, Ai SH, Sun L, Huang Z, et al. First-trimester blood concentrations of drinking water trihalomethanes and neonatal neurobehavioral development in a Chinese birth cohort. J Hazard Mater. 2019;362:451–457. doi: 10.1016/j.jhazmat.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 29.Katz MH, Hauck WW. Proportional hazards (Cox) regression. J Gen Intern Med. 1993;8(12):702–711. doi: 10.1007/BF02598295. [DOI] [PubMed] [Google Scholar]
- 30.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husson F, Josse J, Le S, J Mazet. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining with R. 2014.
- 32.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 33.Whynes DK, Baines DL. Explaining variations in the frequency of night visits in general practice. Fam Pract. 1996;13(2):174–178. doi: 10.1093/fampra/13.2.174. [DOI] [PubMed] [Google Scholar]
- 34.Smith AC, Koper N, Francis CM, Fahrig L. Confronting collinearity: comparing methods for disentangling the effects of habitat loss and fragmentation. Landsc Ecol. 2009;24(10):1271. [Google Scholar]
- 35.Sheppard JP, Stevens S, Stevens R, Martin U, Mant J, Gobbs FDR, et al. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Intern Med. 2018;178(12):1626–1634. doi: 10.1001/jamainternmed.2018.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 38.Khoei NS, Grindel A, Wallner M, Mölzer C, Doberer D, Marculescu R, et al. Mild hyperbilirubinaemia as an endogenous mitigator of overweight and obesity: Implications for improved metabolic health. Atherosclerosis. 2018;269:306–311. doi: 10.1016/j.atherosclerosis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Li M, Xu M, Bi Y, Li X, Chen Y, et al. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J Diabetes. 2011;3(3):217–224. doi: 10.1111/j.1753-0407.2011.00138.x. [DOI] [PubMed] [Google Scholar]
- 40.Vaiserman AM. Hormesis and epigenetics: is there a link? Ageing Res Rev. 2011;10(4):413–421. doi: 10.1016/j.arr.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Hwang HJ, Kim SH. Inverse relationship between fasting direct bilirubin and metabolic syndrome in Korean adults. Clin Chim Acta. 2010;411(19–20):1496–1501. doi: 10.1016/j.cca.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Cabandugama PK, Gardner MJ, Sowers JR. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101(1):129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morioka I, Nakamura H, Koda T, Sakai H, Kurokawa D, Yonetani M, et al. Serum unbound bilirubin as a predictor for clinical kernicterus in extremely low birth weight infants at a late age in the neonatal intensive care unit. Brain Dev. 2015;37(8):753–757. doi: 10.1016/j.braindev.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Lacruz ME, Kluttig A, Hartwig S, Löer M, Tiller D, Greiser KH, et al. Prevalence and Incidence of Hypertension in the General Adult Population: Results of the CARLA-Cohort Study. Medicine (Baltimore) 2015;94(22):e952. doi: 10.1097/MD.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JH, Yang DH, Park HS, Cho Y, Jun JE, Park WH, et al. Incidence of hypertension in Korea: 5-year follow-up study. J Korean Med Sci. 2011;26(10):1286–1292. doi: 10.3346/jkms.2011.26.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian Z, Liang M. Renal metabolism and hypertension. Nat Commun. 2021;12(1):963. doi: 10.1038/s41467-021-21301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 49.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 50.He W, Xu Q, Han L, Wu T, Shi X, Ye L, et al. Using real-world data to estimate the changing trends in the prevalence and incidence of type 2 diabetes mellitus in Xiamen of China from 2014 to 2019. BMC Endocr Disord. 2021;21(1):92. doi: 10.1186/s12902-021-00759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X, Zhang H, Wang F, et al. Epidemiology and prevalence of hyperuricemia among men and women in Chinese rural population: The Henan Rural Cohort Study. Mod Rheumatol. 2020;30(5):910–920. doi: 10.1080/14397595.2019.1660048. [DOI] [PubMed] [Google Scholar]
- 52.Qi D, Liu J, Wang C, Wang L, Zhang X, Lin Q, et al. Sex-specific differences in the prevalence of and risk factors for hyperuricemia among a low-income population in China: a cross-sectional study. Postgrad Med. 2020;132(6):559–567. doi: 10.1080/00325481.2020.1761133. [DOI] [PubMed] [Google Scholar]
- 53.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women-the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10(5):R116. doi: 10.1186/ar2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Yang S, He Y, et al. Association between passive smoking and hypertension in Chinese non-smoking elderly women. Hypertens Res. 2017;40(4):399–404. doi: 10.1038/hr.2016.162. [DOI] [PubMed] [Google Scholar]
- 55.Omboni S. Smoking and hypertension: what is behind the mask? J Hypertens. 2020;38(6):1029–1030. doi: 10.1097/HJH.0000000000002423. [DOI] [PubMed] [Google Scholar]
- 56.Liu SH, Liu B, Sanders AP, Saland J, Wilson KM. Secondhand smoke exposure and higher blood pressure in children and adolescents participating in NHANES. Prev Med 2020; 134: 106052. [DOI] [PMC free article] [PubMed]
- 57.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 58.Huang Q, Hu D, Wang X, Chen Y, Wu Y, Pan L, et al. The modification of indoor PM25 exposure to chronic obstructive pulmonary disease in Chinese elderly people: A meet-in-metabolite analysis. Environ Int. 2018;121(2):1243–1252. doi: 10.1016/j.envint.2018.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The supplementary files of this article
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.