Abstract
Purpose
To determine the incidence and clinical predictors of intrathoracic complications in COVID-19 patients, and the association with outcomes.
Methods
In this retrospective cross-sectional study, we included 976 patients (age 61 ± 17 years, 62% male) who tested positive for SARS-CoV-2 between March 3–April 4, 2020 and underwent chest imaging. 3836 radiographs from 976 patients and 105 CTs from 88 patients were reviewed for intrathoracic complications, including pneumothorax, pneumomediastinum, pneumopericardium, lobar collapse, pleural effusion, and pneumatocele formation.
Results
There was a high rate of intrathoracic complications (197/976, 20%). Pleural effusion was the most common complication (168/976, 17%). Pneumothorax (30/976, 3%) and pneumatoceles (9/88, 10%) were also frequent. History of hypertension and high initial CXR severity score were independent risk factors for complications. Patients with any intrathoracic complication during admission had an over 11-fold risk of ICU admission (adjusted odds ratio [aOR] 11.2, p < 0.0001) and intubation (aOR 12.4, p < 0.0001), over 50% reduction in successful extubation (aOR 0.49, p = 0.02) and longer length of stay (median 13 versus 5 days, p < 0.0001). There was no difference in overall survival between patients with and without any complication (log-rank p = 0.94).
Conclusion
In COVID-19 patients who underwent chest imaging, 1 in 5 patients have an intrathoracic complication, which are associated with higher level of care and prolonged hospital stay. Hypertension history and high CXR severity score confer an increased risk of complication.
Summary
Intrathoracic complications in COVID-19 are common and are predictive of ICU admission, need for intubation, less successful extubation, and longer length of stay but are not predictive of mortality.
Abbreviations: SARS-CoV, severe acute respiratory syndrome; MERS-CoV, Middle East respiratory syndrome (MERS-CoV); RT-PCR, reverse-transcriptase polymerase chain reaction; CT, computed tomography; ICU, intensive care unit; LOS, length of stay
Keywords: SARS-CoV-2, COVID-19, Pneumothorax, Length of stay, Intubation
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered in December 2019 and resulted in a devasting pandemic, declared by the World Health Organization in March 2020. SARS-CoV-2 causes a respiratory illness termed COVID-19, which ranges from a mild, self-limiting respiratory tract illness to a severe pneumonia that can progress to multiorgan failure and death.1
Clinical features of SARS-CoV-2 are similar to prior coronavirus-related pulmonary syndromes, specifically those seen in the 2003 outbreak of severe acute respiratory syndrome (SARS-CoV) and the 2012 outbreak of Middle East respiratory syndrome (MERS-CoV).2 Additionally, SARS-CoV-2 and SARS-CoV appear to share the same human host cell receptor, angiotensin-converting enzyme 2 (ACE2), indicating similar mechanisms of infection.3
The most common complication of COVID-19 pneumonia is acute respiratory distress syndrome (ARDS).4 However, there are additional intrathoracic complications may occur in conjunction with or separate from ARDS including pneumothorax, pneumomediastinum, subcutaneous emphysema, lobar collapse, pleural effusion and pneumatocele formation. These are important as they may affect management and can potentially increase morbidity and mortality.5., 6. Such complications were also reported with prior coronavirus-related syndromes. For example, SARS-CoV had a high incidence of pneumothorax and pneumomediastinum.7., 8., 9., 10., 11., 12. Pneumothorax and pleural effusion were associated with poor prognosis in MERS-CoV.13
Early small retrospective studies and case reports suggest overall low rates of these ancillary intrathoracic complications in COVID-19.14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27. One large scale study found a high incidence of barotrauma in patients with COVID-19 on mechanical ventilation compared to patients with ARDS without COVID-19.28 Spontaneous pneumothorax, pneumomediastinum, and pneumatoceles in patients not on mechanical ventilation have also been reported.29., 30., 31.
There is lack of large-scale studies examining the overall rate of intrathoracic complications in COVID-19, risk factors for developing complications, or their associations with outcomes. In this study, we evaluated patients who tested positive for SARS-CoV-2 and underwent chest CT or chest radiograph (CXR). The goal was to establish the rate of intrathoracic complications in COVID-19 patients, determine clinical predictors of complications and assess the association of complications with outcomes.
2. Materials and methods
2.1. Study design and patients
The institutional review board approved this cross-sectional observational study and a waiver of consent was granted (IRB 20-04021777, approved April 10, 2020). Between March 3, 2020 and April 4, 2020, we used the COVID-19 Institutional Data Repository (IDR) to identify a total of 4110 patients who underwent nasopharyngeal or oropharyngeal reverse-transcriptase polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 at NewYork-Presbyterian (NYP) per clinical routine (Fig. 1 ). NYP is a large healthcare system comprised of both academic and community hospitals in Manhattan, Brooklyn, and Queens. The COVID-19 IDR is a registry of suspected COVID-19 patients that were tested by RT-PCR at NYP. The authors have access to the complete medical records and radiology images for NYP-Weill Cornell (NYP-WC) and NYP-Lower Manhattan Hospital (NYP-LMH).
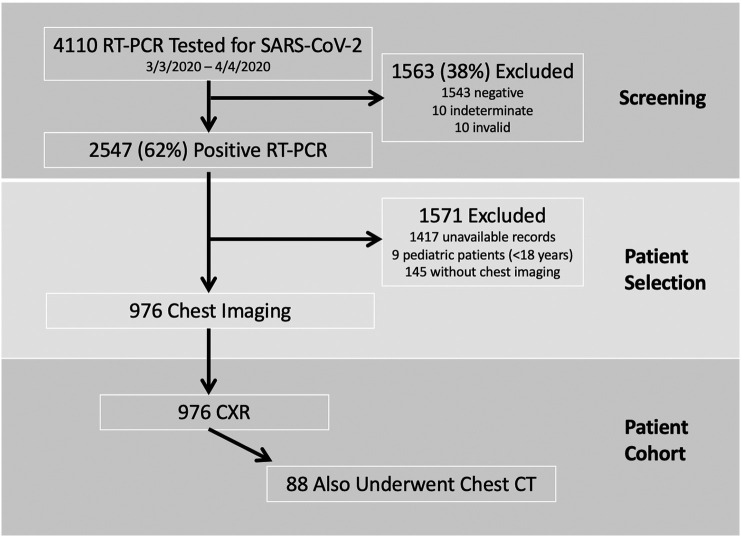
Fig. 1.
Consort flow diagram showing the screening and selection of the 976 RT-PCR (+) patients with chest imaging included in the data analysis. RT-PCR, reverse-transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CXR, chest radiograph; CT, computed tomography.
The details of the study population and design were previously reported.32 In brief, as shown in Fig. 1, we excluded patients with RT-PCR test results that were negative, indeterminate or invalid (n = 1563), those whose medical records were unavailable for review (n = 1417; 1392 from other NYP Hospitals to which EMR access was inaccessible, 25 restricted healthcare workers), and pediatric cases (n = 9). For this analysis, patients were excluded if they did not have at least one CXR or one chest CT (n = 145).
A total of 976 patients who tested positive on RT-PCR for COVID-19 and underwent chest imaging were included in the final cohort. All 976 patients had at least one CXR and 88 of those 976 patients also underwent at least one chest CT.
Manual chart review from the electronic medical records was performed for each patient from the time period of April 24, 2020 to May 7, 2020 for the clinical history and outcomes. For the survival analyses, we used the date of the RT-PCR specimen collection of the first positive COVID-19 test to death date or to the last documented note in the electronic medical record when the patient was noted to be alive.
2.2. Imaging protocol
A total of 3836 CXRs from 976 patients were reviewed, of which 3812 were AP portable radiographs (99%) and 24 were PA/lateral radiographs. A total of 105 chest CT scans were performed on 88 patients on at least 32-slice multidetector CT scanners (General Electric Medical Systems, Milwaukee, WI or Siemens, Erlangen, Germany). The CT scans were performed with (n = 67) or without (n = 34) intravenous contrast media. All studies were reconstructed with slice thickness ≤ 2.5 mm using soft tissue and lung reconstruction kernels.
2.3. Image analysis
The [Blinded] Core Laboratory, which consisted of 6 board-certified radiologists (5 of which were fellowship-trained cardiothoracic radiologists and 1 was a fellowship-trained emergency/musculoskeletal radiologist), interpreted all CXRs. All chest CT scans were interpreted by board-certified cardiothoracic radiologists. All imaging interpretations were performed as group consensus reads between one junior (1–2 years of post-fellowship experience) and one senior radiologist (5, 12, or 14 years of post-fellowship experience), with disagreement resolved by a third senior cardiothoracic radiologist. The readers were aware of the positive RT-PCR but otherwise blinded to the clinical history and outcomes.
Readers reviewed all initial CXRs performed on each patient at presentation and recorded the presence of a complication. Readers then reviewed all additional available CXRs and CTs performed during each patient's emergency department visit or hospitalization related to their positive RT-PCR to determine if complications were present on at least one imaging study.
CXRs were reviewed for the presence or absence of the following findings: (1) pneumothorax with or without loculation, (2) chest tube, (3) pneumomediastinum, (4) pneumopericardium, (5) subcutaneous emphysema, (6) lobar collapse and (7) pleural effusion. Pleural effusion size was not graded. Initial CXRs were also given a severity grading score ranging from 0 to 6, whereby the frontal chest radiograph is divided into 6 zones (right upper, middle, lower, and left upper, middle, and lower), and each zone was scored with either a 0 (absence of lung abnormality) or 1 (presence of an abnormality which could be interpretated as having COVID-19 involvement). This scoring system was validated by Toussie et al.33
CT images were reviewed for the presence or absence of the following findings: (1) pneumothorax, (2) chest tube, (3) pneumomediastinum, (4) pneumopericardium, (5) subcutaneous emphysema, (6) lobar collapse and associated airway findings, (7) pleural effusion and (8) pneumatocele within the lung parenchyma. Presence or absence of pleural thickening or enhancement associated with pleural effusion was also documented. Pneumatoceles were defined as abnormal air-filled spaces in the lungs and characterized as air-filled only or containing an air-fluid level. Pneumatoceles were documented to allow assessment of their potential relationship to pneumothorax. Additional lung parenchymal findings were not evaluated as they have been well documented in the literature.
2.4. Outcomes data
The outcome end points included intensive care unit (ICU) admission, intubation rate, successful extubation rate, hospitalization length of stay (LOS) and all-cause mortality.
2.5. Statistical analysis
Descriptive statistics were used to summarize continuous variables, with mean ± standard deviation or median with interquartile range [IQR], and comparisons were made using t-test or Kruskal-Wallis test. Frequencies and percentages were used to summarize categorical variables and comparisons were made using Fisher's exact test, Chi-square test, or Chi-square trend test. Univariate and multivariable logistic regression analyses were employed to determine which clinical predictors were independently associated with having any complications. Clinical predictors for the multivariable analysis were selected based on patient characteristics and presenting vital signs with p < 0.1 in Table 1 (age, smoking, coronary artery disease [CAD], heart failure [HF], diastolic blood pressure [DBP], respiratory rate [RR]), initial CXR severity score, and also included BMI and sex. To determine the association between any complication and the outcomes for ICU stay, intubation, and successful extubation, multivariable logistic regression models were adjusted for potential confounders of baseline clinical non-imaging covariates with a p < 0.1 in Table 1 (age, smoking, CAD, HF, presenting DBP,RR, and initial CXR severity score). Comparison of mortality between patients with and without complications were estimated using the product limit (Kaplan-Meier) method and log-rank test. Cox-proportional hazard regression analysis was performed for any initial CXR complications, and adjusted for age, sex, RR, BMI, DBP, initial CXR severity score, and history of smoking, hypertension, CAD, and HF. A 2-tailed p-value < 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used to perform all statistical analyses.
Table 1.
Demographics and characteristics of patients testing positive for SARS-CoV-2 who underwent chest imaging.
| All patients (n = 976) |
Complications (n = 197) |
No complications (n = 779) |
P-value | |
|---|---|---|---|---|
| Age, mean ± SD | 61.2 ± 17.1 | 66.1 ± 14.7 | 59.9 ± 17.5 | <0.0001 |
| Male, n (%) | 606 (62%) | 131 (67%) | 475 (61%) | 0.16 |
| BMI, kg/m2, mean ± SD | 28.4 ± 6.8 n = 923 |
29.5 ± 6.2 n = 727 |
28.4 ± 7.0 n = 196 |
0.76 |
| Prior medical historya | ||||
| Smoking history, n (%) | 239 (24%) | 62 (31%) | 177 (23%) | 0.01 |
| Asthma, n (%) | 93 (10%) | 19 (10%) | 74 (9%) | 1.00 |
| COPD, n (%) | 44 (5%) | 13 (7%) | 31 (4%) | 0.12 |
| ILD, n (%) | 5 (0.5%) | 2 (1%) | 3 (0.4%) | 0.27 |
| Hypertension, n (%) | 528 (55%) n = 968 |
134 (69%) | 394 (51%) | <0.0001 |
| CAD, n (%) | 143 (15%) n = 967 |
45 (23%) n = 194 |
98 (13%) n = 773 |
0.0004 |
| Heart failure, n (%) | 65 (7%) n = 966 |
25 (13%) n = 192 |
40 (5%) n = 774 |
0.0003 |
| Presenting vital signs | ||||
| Temperature (°C), mean ± SD | 37.4 ± 0.9 n = 959 |
37.5 ± 0.9 n = 196 |
37.4 ± 0.9 n = 767 |
0.49 |
| Heart rate (bpm), mean ± SD | 95.4 ± 18.5 n = 965 |
96.5 ± 19.2 n = 192 |
95.2 ± 18.3 n = 770 |
0.37 |
| Systolic BP, mean ± SD | 129.7 ± 21.7 n = 964 |
130.8 ± 23.1 n = 195 |
129.5 ± 21.4 n = 769 |
0.43 |
| Diastolic BP, mean ± SD | 77.3 ± 12.9 n = 964 |
75.8 ± 13.5 n = 195 |
77.7 ± 12.7 n = 769 |
0.08 |
| Respiratory rate, mean ± SD | 20.4 ± 5.3 n = 958 |
21.9 ± 6.3 n = 194 |
20.0 ± 5.0 n = 764 |
0.0001 |
| No. of chest imaging | ||||
| All chest radiograph, n (%) | 976 (100%) | 197 (20%) | 779 (80%) | – |
| Chest radiograph portable, n (%) | 968 (99%) | 197 (100%) | 771 (99%) | 0.37 |
| Chest radiograph PA/lateral, n (%) | 24 (2%) | 6 (3%) | 18 (2%) | 0.61 |
| All cardiothoracic CT, n (%) | 88 (9%) | 45 (23%) | 43 (6%) | <0.0001 |
| Chest CT without contrast, n (%) | 32 (3%) | 21 (11%) | 11 (1%) | <0.0001 |
| Chest CT with contrast, n (%) | 34 (3%) | 16 (8%) | 18 (2%) | 0.0003 |
| Chest CT PE, n (%) | 33 (3%) | 18 (9%) | 15 (2%) | <0.0001 |
| Chest CT angiography, n (%) | 2 (0.22%) | 2 (1%) | 0 (0%) | 0.04 |
| Initial CXR severity score | <0.0001 | |||
| 0 | 191 (20%) | 18 (9%) | 173 (22%) | |
| 1 | 85 (9%) | 12 (6%) | 73 (9%) | |
| 2 | 132 (14%) | 25 (13%) | 107 (14%) | |
| 3 | 109 (11%) | 21 (11%) | 88 (11%) | |
| 4 | 259 (27%) | 58 (29%) | 201 (26%) | |
| 5 | 86 (9%) | 24 (12%) | 62 (8%) | |
| 6 | 114 (12%) | 39 (20%) | 75 (10%) |
Abbreviations: BMI, Body Mass Index; CT, Computed Tomography; bpm, Beats Per Minute; BP, Blood Pressure; COPD, Chronic Obstructive Pulmonary Disease; ILD, Interstitial Lung Disease; CAD, Coronary Artery Disease; CXR, chest x-ray.
Medical history was determined based on clinical notes in the electronic medical records.
3. Results
3.1. Demographics and clinical predictors of complications
There were 976 patients testing positive for SARS-CoV-2 who underwent chest imaging. Demographics and clinical characteristics are detailed in Table 1.
Patients with complications were older than patients without complications (p-value < 0.0001). Patients with complications were more likely to have a history of smoking, hypertension, CAD or heart failure (all p ≤ 0.01) and presented with a higher respiratory rate (p = 0.0001). Patients with complications were more likely to have a CXR severity score of 4 or higher (p ≤ 0.0004). In multivariable analysis, history of heart failure or hypertension, elevated presenting respiratory rate, and CXR severity score of 5 or 6 remained independent risk factors for complication (p ≤ 0.01, Table 2 ). Given that pleural effusions may be secondary to heart failure, repeat multivariable analysis was performed excluding pleural effusions. 76 (76/976, 8%) patients had a non-pleural effusion complication. Only hypertension and CXR severity score of 5 or 6 remained independent risk factors for complication (both p ≤ 0.04, Table 2). There was a 2-fold increase risk for a complication (not including pleural effusion) if the patient had a history of hypertension (adjusted OR 2.09, 95% CI 1.15–3.75, p = 0.01).
Table 2.
Univariate and multivariable analysis of clinical predictors for any complications including and excluding pleural effusions. Clinical predictors for the multivariable analysis were selected based on patient characteristics and presenting vital signs with p < 0.1 in Table 1, and also included BMI and sex.
| Univariate predictors (Any complication) |
Multivariable analysis (Any complication) |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-Value | Odds ratio (95% CI) | p-Value | |
| Age | 1.02 (1.01–1.03) | <0.0001 | 1.00 (0.99–1.02) | 0.49 |
| BMI | 1.01 (0.98–1.03) | 0.50 | 1.01 (0.98–1.04) | 0.59 |
| Male sex | 1.27 (0.91–1.77) | 0.15 | 1.29 (0.89–1.88) | 0.18 |
| Smoking | 0.83 (0.63–1.08) | 0.17 | 0.97 (0.70–1.35) | 0.86 |
| Hypertension | 2.20 (1.57–3.08) | <0.0001 | 1.66 (1.11–2.47) | 0.01 |
| CAD | 1.91 (1.33–2.74) | 0.0004 | 1.36 (0.86–2.17) | 0.19 |
| HF | 2.27 (1.47–3.51) | 0.0002 | 2.20 (1.22–3.96) | 0.01 |
| DBP | 0.99 (0.98–1.00) | 0.08 | 0.999(0.99–1.01) | 0.83 |
| RR | 1.06 (1.03–1.09) | <0.0001 | 1.04 (1.01–1.07) | 0.002 |
| Initial CXR score | ||||
| 1 vs 0 | 1.58 (0.072–3.45) | 0.25 | 1.12 (0.48–2.60) | 0.80 |
| 2 vs 0 | 2.25 (1.17–4.31) | 0.02 | 1.66 (0.84–3.30) | 0.15 |
| 3 vs 0 | 2.29 (1.16–4.53) | 0.02 | 1.86 (0.91–3.81) | 0.09 |
| 4 vs 0 | 2.77 (1.57–4.89) | 0.0004 | 1.78 (0.97–3.26) | 0.06 |
| 5 vs 0 | 3.72 (1.89–7.32) | 0.0001 | 2.78 (1.35–5.73) | 0.006 |
| 6 vs 0 | 5.00 (2.69–9.30) | <0.0001 | 2.94 (1.51–5.74) | 0.002 |
| Univariate predictors (Pleural effusions excluded) |
Multivariable analysis (Pleural effusions excluded) |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-Value | Odds ratio (95% CI) | p-Value | |
| Age | 1.01 (0.99–1.02) | 0.45 | 0.99 (0.97–1.01) | 0.14 |
| BMI | 1.01 (0.97–1.04) | 0.67 | 1.00 (0.96–1.04) | 0.82 |
| Male sex | 1.45 (0.87–2.40) | 0.15 | 1.21 (0.70–2.08) | 0.49 |
| Smoking | 0.75 (0.51–1.09) | 0.13 | 0.73 (0.47–1.13) | 0.15 |
| Hypertension | 1.89 (1.15–3.13) | 0.01 | 2.09(1.16–3.75) | 0.01 |
| CAD | 1.38 (0.80–2.37) | 0.25 | 1.34 (0.70–2.58) | 0.38 |
| HF | 0.95 (0.43–2.09) | 0.90 | 0.998 (0.98–1.02) | 0.60 |
| DBP | 1.00 (0.97–1.02) | 0.82 | 1.00 (0.98–1.02) | 0.74 |
| RR | 1.05 (1.01–1.09) | 0.007 | 1.04 (0.997–1.08) | 0.07 |
| Initial CXR score | ||||
| 1 vs 0 | 1.64 (0.51–5.33) | 0.41 | 1.37 (0.41–4.51) | 0.61 |
| 2 vs 0 | 1.04 (0.32–3.33) | 0.96 | 0.82 (0.25–2.68) | 0.41 |
| 3 vs 0 | 2,66 (0.98–7.19) | 0.05 | 2.25 (0.81–6.21) | 0.12 |
| 4 vs 0 | 1.85 (0.75–4.55) | 0.18 | 1.42 (0.56–3.58) | 0.46 |
| 5 vs 0 | 6.96 (2.78–17.4) | <0.0001 | 6.12 (2.35–15.9) | 0.0002 |
| 6 vs 0 | 3.68 (1.44–9.42) | 0.007 | 2.47 (0.93–6.59) | 0.04 |
Abbreviations: BMI, Body Mass Index; CAD, Coronary Artery Disease; DBP, diastolic blood pressure; RR, respiratory rate; CXR, chest x-ray.
Table 1 summarizes the number of chest imaging studies performed and CXR severity scores for all patients as well as stratified by those with and without complications. All 976 patients underwent at least one CXR (AP CXR, PA/lateral, or both) with a total number of performed studies as follows: total CXRs (n = 3836), AP portable CXRs (n = 3812), PA/lateral CXRs (n = 24). 88 patients also underwent chest CT (n = 108). Nearly all patients received at least one AP CXR (99%). There was no difference in the proportion of patients who underwent CXRs, either portable or PA/lateral, between patients with complications and those without (both p ≥ 0.37). Patients with complications were more likely to undergo chest CT of any type (p-value < 0.0001).
3.2. Incidence of intrathoracic complications
Intrathoracic complication rates are detailed in Table 3 and two illustrative cases are presented in Fig. 2, Fig. 3 . A complication was deemed present if identified on either any CXR or CT obtained during the patient's ED visit or hospitalization (n = 976) with the exception of pleural enhancement, pleural thickening and pneumatoceles, which were only assessed in patients with CTs (n = 88). Subgroup rates are also presented for initial CXR alone, any CXR during admission, and any chest CT during admission.
Table 3.
Intrathoracic complications found on CT and CXR in patients testing positive for SARS-CoV-2
| Complication n (%) |
Initial CXR (n = 976) |
Any CXR (n = 976) |
Chest CT (n = 88) |
Chest CT or CXRb (n = 976) |
|---|---|---|---|---|
| Any complication | – | – | – | 197 (20%) |
| Pneumothorax | 3 (0.3%) | 28 (3%) | 7 (8%) | 30 (3%) |
| Loculated | 2 (0.2%) | 9 (32%) | 3 (43%) | 11 (1%) |
| Bilateral | – | – | 2 (29%) | – |
| Chest tube | 0 | 21 (2%) | 5 (6%) | 22 (2%) |
| Pneumomediastinum | 2 (0.2%) | 15 (2%) | 3 (3%) | 16 (2%) |
| Pneumopericardium | 0 | 3 (0.3%) | 0 (0%) | 3 (0.3%) |
| Subcutaneous emphysema | 0 | 14 (1%) | 2 (2%) | 15 (2%) |
| Lobar collapse | 2 (0.2%) | 26 (3%) | 2 (2%) | 27 (3%) |
| + mucoid impaction? | – | – | 1 (50%) | – |
| + tracheal thickening? | – | – | 0 (0%) | – |
| + bronchial thickening? | – | – | 0 (0%) | – |
| Pleural effusion | 51 (5%) | 149 (15%) | 31 (35%) | 168 (17%) |
| + loculated | – | – | 8 (9%) | – |
| + pleural thickening or enhancement | – | – | 2 (2%)a | – |
| Cavity | – | – | 9 (10%) | – |
| Air only | – | – | 6 (67%) | – |
| Fluid level | – | – | 3 (33%) | – |
Pleural enhancement could not be assessed in the 23 patients with non-contrast CT scans.
Complication was deemed present if identified on either any CXR or CT obtained during the patient's ED visit or hospitalization.
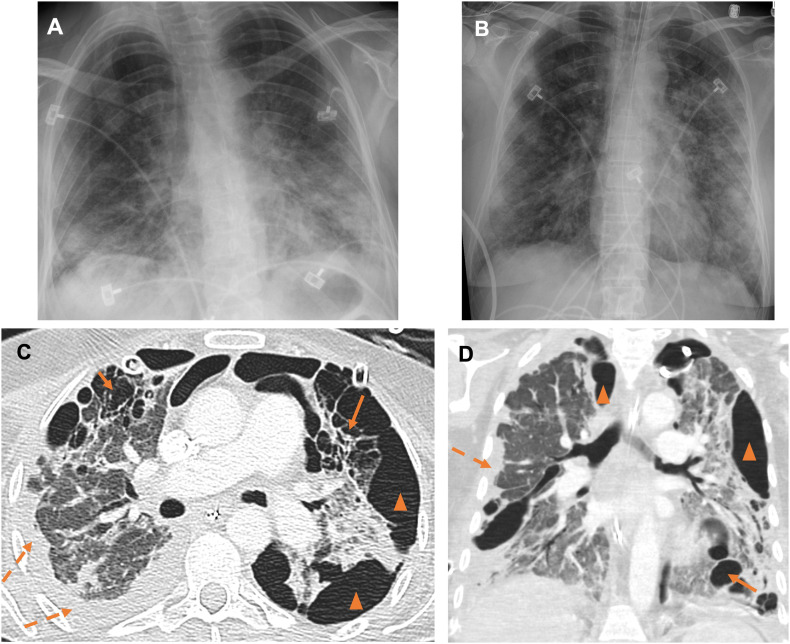
Fig. 2.
53 year-old female triathlon runner with severe COVID-19 infection and multiple complications. On the day of presentation and PCR testing, AP portable radiograph (A) shows ill-defined airspace opacities in the lower lungs and small pleural effusions. The patient was intubated on hospital day (HD) 3 with repeat AP portable radiograph (B) showing worsening multifocal airspace opacities. As seen on axial (C) and coronal (D) images from a contrast-enhanced CT chest performed on HD 84, course was complicated by multiple pneumatoceles (arrows), loculated pneumothoraces (arrowheads), loculated pleural effusions (dashed arrow). Bilateral chest tubes are noted. Barotrauma and infection likely resulted in pneumatoceles with parenchymal-pleural and bronchopleural fistualization and pneumothoraces.
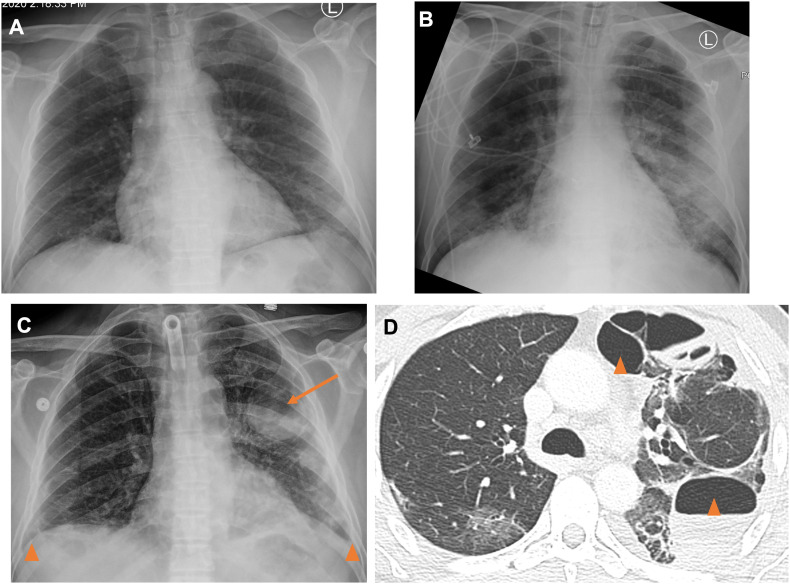
Fig. 3.
47 year old male with history of hypertension admitted for COVID-19 pneumonia complicated by acute respiratory distress syndrome requiring intubation/tracheostomy. On the day of presentation and PCR testing, AP portable radiograph (A) shows ill-defined left lower lobe opacities. The patient was intubated on HD 2 and repeat AP radiograph (B) on HD 7 shows worsening now bilateral airspace opacities. On HD 26, AP portable radiograph (C) showed improved airspace opacities, but a new pneumotocele containing an air-fluid level in the left lung (arrow) and small pleural effusions (arrowheads). Axial (D) images from a same day contrast-enhanced CT chest show multi-septated spaces containing air and fluid, felt to represent a combination of pneumatoceles and loculated hydropneumothorax (arrowheads).
197 patients (197/976, 20%) had at least one complication on either CXR or CT. Pleural effusion was the most common complication (168/976, 17%) followed by pneumothorax (30/976, 3%) and lobar collapse (27/976, 3%). Chest tube placement, subcutaneous emphysema, pneumomediastinum and pneumopericardium were rare. On initial CXR alone, pleural effusion was the most common complication (51/976, 5%). Other complications were rare including spontaneous pneumothorax (3/976, 0.3%) and pneumomediastinum (2/976, 0.2%).
In patients who underwent CT, pleural effusion was again the most common complication, present in more than one-third of patients (31/88, 35%) and complex by imaging in a minority of patients (<10%). Pneumotocele formation was the second most common complication (9/88, 10%) with two-thirds being air-only and one-third demonstrating an air-fluid level. Pneumothorax was the third most common complication (7/88, 8%). Pneumothoraces were bilateral in approximately one-third of patients (2/7, 29%) and loculated in nearly half of patients (3/7, 43%). Of the 9 patients who had a pneumothorax on either radiograph or CT and underwent CT during their admission, 2 (2/9, 22%) had either a pneumatocele with air-fluid level or an air-filled pneumatocele. Chest tube placement was required in only 6% (5/88) of patients. Pneumomediastinum, subcutaneous emphysema and lobar collapse were rare findings.
3.3. Association of complications with outcomes
A total of 277 patients (277/976, 28%) were admitted to the ICU. 252 (252/976, 26%) patients were intubated and 78 (78/252, 31%) had been successfully extubated by the time of study termination.
The differences in the proportion of patients with and without ICU admission, intubation, and successful extubation by complication types are detailed in Supplemental Table 1 (based on any CXR or CT during admission) and Supplemental Table 2 (based on initial CXR alone).
3.3.1. Complication on any chest imaging
Any complication (combined analysis), pneumothorax, chest tube, subcutaneous emphysema, pneumomediastinum, pneumopericardium, lobar collapse and pleural effusion were more prevalent in those with ICU admission and intubation than those without. Any complication, pneumothorax, chest tube and pleural effusion were less frequent in patients who were successfully extubated.
Of the 197 patients with any complication, 69% (135/197) were intubated and 31% (62/197) were not. Of the 62 patients who were not intubated and had a complication, 7% (4/62) had pneumothorax, 5% (3/62) had pneumomediastinum and 3% (2/62) had subcutaneous emphysema. Of the 135 that were intubated and had a complication, 19% (26/135) had pneumothorax, 10% (13/135) had pneumomediastinum, 2% (3/135) had pneumopericardium and 9.6% (13/135) had subcutaneous emphysema.
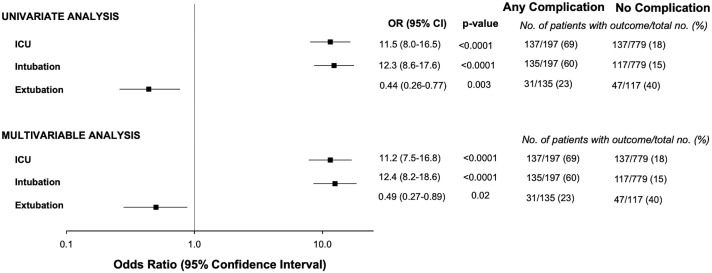
Patients with any complication had higher rates of ICU admission (adjusted odds ratio [aOR] 11.2, p < 0.0001), intubation requirement (aOR 12.4, p < 0.0001) and were over 50% less likely to have a successful extubation (aOR 0.49, p = 0.02) (Fig. 4 ) when compared to those without complications.
Fig. 4.
Univariate and multivariable analysis of any complications for ICU stay, intubation, and successful extubation. Multivariable models were adjusted for baseline clinical non-imaging covariates with a p < 0.1 in Table 1 (age, smoking, hypertension, coronary artery disease, heart failure, presenting diastolic blood pressure and respiratory rate).
The differences in LOS between those with and without complications are provided in Table 4 . Patients with any complication, pneumothorax, subcutaneous emphysema, pneumomediastinum, lobar collapse, and pleural effusion had longer LOS (all p ≤ 0.02) compared to those who did not have those complications.
Table 4.
Differences in length of hospital stay in patients with and without complications.
| Predictor variable (n) | Length of stay days, median [IQR] | p-Value |
|---|---|---|
| Any complication | <0.0001 | |
| Present (122) | 13.0 [6.0–22.0] | |
| Absent (624) | 5.0 [2.0–9.0] | |
| Pneumothorax | 0.001 | |
| Present (12) | 15.5 [7.5–27.0] | |
| Absent (734) | 5.0 [3.0–10.0] | |
| Chest tube | 0.12 | |
| Present (8) | 9.5 [5.5–23.5] | |
| Absent (738) | 6.0 [3.0–10.0] | |
| Pneumomediastinum | 0.003 | |
| Present (8) | 16.5 [8.5–23.0] | |
| Absent (738) | 5.0 [3.0–10.0] | |
| Pneumopericardium | 0.17 | |
| Present (2) | 18.0 [7.0–29.0] | |
| Absent (744) | 6.0 [3.0–11.0] | |
| Subcutaneous emphysema | 0.02 | |
| Present | 18.0 [7.0–23.0] | |
| Absent | 6.0 [3.0–10.0] | |
| Lobar collapse | <0.0001 | |
| Present (16) | 20.5 [11.0–27.5] | |
| Absent (730) | 5.0 [3.0–10.0] | |
| Pleural effusion | <0.0001 | |
| Present (105) | 12.0 [6.0–21.0] | |
| Absent (641) | 5.0 [2.0–9.0] | |
| Air-filled cavity | 0.06 | |
| Present (6) | 23.5 [12.0–35.0] | |
| Absent (52) | 6.5 [2.0–15.5] | |
| Air-fluid Cavity | 0.39 | |
| Present (1) | 18.0 [18.0–18.0] | |
| Absent (57) | 8.0 [2.0–17.0] | |
| Pleural thickening or enhancement | 0.73 | |
| Present | 10.5 [6.0–15.0] | |
| Absent | 8.0 [2.0–19.5] |
Abbreviations: IQR = interquartile range.
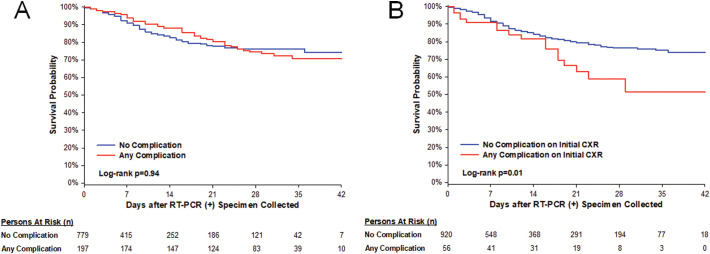
There were 143 deaths (143/976, 15%). There was no difference in overall survival between patients with and without any complication (Fig. 5 , log-rank p = 0.94).
Fig. 5.
Kaplan-Meier survival curve. Patients with and without any complication are represented by the red and blue curves, respectively. Patients with a complication on any CXR or CT during admission had no difference in overall survival (A) (log-rank p = 0.94). Patients with a complication on initial CXR had worse survival (B) (log-rank = 0.01), but there was not survival difference after adjusting for age, sex, respiratory rate, body mass index, diastolic blood pressure, and history of smoking, hypertension, coronary artery disease, or heart failure (adjusted HR 1.11 [0.62–1.97], p = 0.74). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.2. Complication on initial CXR
Median days from positive RT-PCR to initial CXR was 0 [interquartile range 0–0]. Pleural effusion was significantly more prevalent in those with ICU admission than those without (p = 0.02). There was no difference in complication rates on initial CXR between patients that were ultimately intubated or in whom extubation was unsuccessful. Patients with any initial CXR complication had worse survival (unadjusted HR [95% CI]: 1.87 [1.13–3.10], p = 0.02) when compared to those without any initial CXR complications. However, there was no survival difference after adjusting for age, sex, respiratory rate, body mass index, diastolic blood pressure, CXR severity score, and history of smoking, hypertension, coronary artery disease, or heart failure (adjusted HR 101[0.56–181], p = 0.98).
4. Discussion
In this study we observed an intrathoracic complications rate of 20% in a large cohort of patients with COVID-19 who underwent chest imaging. We provide a detailed assessment of the rates of intrathoracic complications in COVID-19 and their association with co-morbidities, presenting symptoms and outcomes. This adds to our understanding of the pathophysiology of COVID-19 and has several implications for patient care.
Many of the complications we evaluated (namely, pneumothorax, pneumomediastinum, pneumopericardium, subcutaneous emphysema) can occur due to barotrauma or spontaneously. In prior coronavirus-related pulmonary syndromes (SARS-CoV and MERS-CoV), mechanically ventilated patients demonstrated high rates of barotrauma (12–34%).7., 8., 9. McGuinness et al. observed a similar high incidence of barotrauma in patients with COVID-19 related acute respiratory syndrome (ARDS) (24%), greater than non-COVID-19 related ARDS.28 Our study had similarly high rates of complications in intubated patients (19% with pneumothorax and 10% with pneumomediastinum) compared to never intubated patients (6.5% and 5%, respectively) and at presentation (0.3% and 0.2%, respectively), supporting the notion that coronavirus uniquely increases barotrauma risk. It is known that patients with ARDS are more likely to develop pneumatoceles and pneumothorax related to barotrauma31, but the high rates of barotrauma in COVID-19 compared to typical pneumonia induced ARDS raises concern for a distinct lung pathophysiology underlying COVID-19.28., 34. The exact mechanism of spontaneous pneumothorax and pneumomediastinum in COVID-19 is likely multi-factorial centering around alveolar damage and rupture related to infection and increased intrathoracic pressure from coughing leading to interstitial emphysema and pneumatocele formation.29., 35.
Of note, our rates of pneumothorax (0.3%) and pleural effusion (5%) based on initial CXR in COVID-19 were similar to those previously reported (pneumothorax rates of 1–2%14., 15., 21. and effusion rates of 1–10%.22., 24., 26., 27.). Our rates of pneumothorax (3%) and pleural effusion (17%) on any chest imaging during admission were higher than previously reported. This difference is likely related to our larger sample size, inclusion of portable CXRs, and inclusion of a breadth of illness severity ranging from those discharged from the ED to those requiring protracted ICU stays. Additionally, pleural effusion rate could be overestimated on CXR if extensive basilar consolidation was present. Moreover, some studies have reported as association between pneumothorax, subcutaneous emphysema, and pneumomediastinum and worse prognosis. This discrepancy is likely related to a focus on only hospitalized/ICU patients or small sample size.5., 36.
The ability to predict who may need a higher level of care may aid in clinical decision-making such as degree of patient monitoring (i.e. remote home monitoring versus admission). We observed that history of hypertension and higher respiratory rate on presentation were independent risk factors for developing an intrathoracic complication. Presence of any intrathoracic complication was a predictor for ICU admission, intubation, failure to extubate and longer LOS without difference in overall survival. This is in keeping with a recent study of mechanically ventilated patients with COVID-19 showing no difference in the survival for patients with and without barotrauma.28
Furthermore, prognostic information gleaned from imaging can inform conversations between physicians, patients and their families, potentially alleviating patient and family anxiety and facilitating goals of care discussions. For example, pneumothorax, chest tube placement, pneumomediastinum, and pleural effusions were predictors of failure to extubate while other complications such as pneumomediastinum and lobar collapse were not. It may provide comfort that certain otherwise disconcerting findings do not necessarily correlate with mortality, although they may predict significant morbidity (e.g. tracheostomy).
Many institutions have successfully instituted a multidisciplinary approach to the care of patients with COVID-19; however, the radiologist has been notably absent from the table.37., 38. The association between imaging findings and outcomes highlights the vital role of the radiologist and supports the need for radiologists to be actively involved with interdisciplinary care teams to provide imaging interpretation and insight into the implication of complications.
This study has important limitations. Our cohort was derived from two large tertiary academic hospitals and ambulatory outpatient practices in Manhattan at the earliest stages of the pandemic and may therefore not be generalizable to dissimilar hospitals or practices, such as community or rural hospitals and practices, or those that are underfunded or under-resourced. Patients hospitalized with COVID-19 today may face a lower incidence of thoracic complications and outcomes may be improved given advances in therapy and management. Our cohort is composed of symptomatic RT-PCR (+) patients identified during the first month of the pandemic when the testing availability was limited. We hope our inclusion of a broad range of illness severities makes our findings more widely applicable and adds to the literature a more comprehensive assessment not solely focusing on severe presentations. Timing of complications relative to each other or to other events was not recorded. Specifically, timing of complications relative to intubation or chest tube placement (before or after) was not determined and; therefore, it was not determined whether these complications were secondary to barotrauma, chest tube placement or spontaneous. Additionally, the timing or purpose (drainage of effusion or treatment of pneumothorax) of chest tube placement was not known. Our aim was to determine the overall rate rather than the specific etiologies of these complications as mechanically ventilated patients have been independently studied. Finally, severity of pneumonia was not documented as it is now well known to be associated with poor prognosis in COVID-19 yet this may be a confounding factor.
5. Conclusion
Patients with COVID-19 infection who undergo chest imaging have intrathoracic complications associated with higher level of care and prolonged hospital stay. Clinical teams should be aware of comorbidities and presenting symptoms that place the patient at increased risk for such complications. Radiologists' recognition of these complications can aid in prognostication, mitigation of complications, and improve allocation of resources for patients with COVID-19.
The following are the supplementary data related to this article.
Differences in proportions of patients with or without ICU stay, intubation, and successful extubation by complication types.
Differences in proportions of patients with or without ICU stay, intubation, and successful extubation by complication types seen on initial CXR.
Declaration of competing interest/funding
This study received support from NewYork-Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO). Dr. Mahmood was supported by the Glorney-Raisbeck Award from the New York Academy of Medicine. The other authors have no conflict to disclose relative to this manuscript. Authors were involved in acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, technical, and material support of this study. The content of this manuscript has not been previously published or presented.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 02 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol. 05 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 3.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. Jun 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozsoy I.E., Tezcan M.A., Guzeldag S., Ozdemir A.T. Is spontaneous pneumomediastinum a poor prognostic factor in Covid-19? J Coll Physicians Surg Pak. 2021 Feb;31(2):132–137. doi: 10.29271/jcpsp.2021.02.132. [DOI] [PubMed] [Google Scholar]
- 6.Zhan N., Guo Y., Tian S., et al. Clinical characteristics of COVID-19 complicated with pleural effusion. BMC Infect Dis. 2021 Feb 15;21(1):176. doi: 10.1186/s12879-021-05856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao H.K., Wang J.H., Sung C.S., Huang Y.C., Lien T.C. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Crit Care. Aug 2005;9(4):R440–R445. doi: 10.1186/cc3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler R.A., Lapinsky S.E., Hallett D., et al. Critically ill patients with severe acute respiratory syndrome. JAMA. Jul 2003;290(3):367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 9.Lew T.W., Kwek T.K., Tai D., et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. Jul 2003;290(3):374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 10.Peiris J.S., Chu C.M., Cheng V.C., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. May 2003;361(9371):1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sihoe A.D., Wong R.H., Lee A.T., et al. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. Jun 2004;125(6):2345–2351. doi: 10.1378/chest.125.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan M.S., Chan I.Y., Fung K.H., Poon E., Yam L.Y., Lau K.Y. High-resolution CT findings in patients with severe acute respiratory syndrome: a pattern-based approach. AJR Am J Roentgenol. Jan 2004;182(1):49–56. doi: 10.2214/ajr.182.1.1820049. [DOI] [PubMed] [Google Scholar]
- 13.Das K.M., Lee E.Y., Al Jawder S.E., et al. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. AJR Am J Roentgenol. Sep 2015;205(3):W267–W274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 02 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 05 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K., Zeng Y., Xie P., et al. COVID-19 with cystic features on computed tomography: a case report. Medicine (Baltimore) May 2020;99(18) doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiolfi A., Biraghi T., Montisci A. Management of persistent pneumothorax with thoracoscopy and bleb resection in COVID-19 patients. Ann Thorac Surg. 11 2020;110(5) doi: 10.1016/j.athoracsur.2020.04.011. e413-e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Gao R., Zheng Y., Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. Aug 2020;27(5) doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Su X., Zhang T., Zheng C. Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 05 2020;21(5):627–628. doi: 10.3348/kjr.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 05 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. Apr 2020 doi: 10.1002/jmv.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 06 2020;295(3) doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 06 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 24.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 04 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 07 2020;215(1):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 26.Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 04 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K.C., Xu P., Lv W.F., et al. CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol. May 2020;126 doi: 10.1016/j.ejrad.2020.108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhan C., Rosenberg N., McGuinness G. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 11 2020;297(2) doi: 10.1148/radiol.2020202352. E252-E262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López Vega J.M., Parra Gordo M.L., Diez Tascón A., Ossaba Vélez S. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg Radiol. Jun 2020 doi: 10.1007/s10140-020-01806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen J., Kamps M.J.A., Joosten T.M.B., Barten D.G. Spontaneous pneumomediastinum in a male adult with COVID-19 pneumonia. Am J Emerg Med. Jul 2020 doi: 10.1016/j.ajem.2020.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. May 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 32.Toy D., Mahmood S.S., Rotman J., et al. Imaging utilization and outcomes in vulnerable populations during COVID-19 in New York City. Radiol Cardiothorac Imaging. 2020 Dec 17;2(6) doi: 10.1148/ryct.2020200464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toussie D., Voutsinas N., Finkelstein M., et al. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19. Radiology. 2020 Oct;297(1):E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kangas-Dick A., Gazivoda V., Ibrahim M., et al. Clinical characteristics and outcome of pneumomediastinum in patients with COVID-19 pneumonia. J Laparoendosc Adv Surg Tech A. 2021 Mar;31(3):273–278. doi: 10.1089/lap.2020.0692. [DOI] [PubMed] [Google Scholar]
- 35.González-Pacheco H., Gopar-Nieto R., Jiménez-Rodríguez G.M., Manzur-Sandoval D., Sandoval J., Arias-Mendoza A. Bilateral spontaneous pneumothorax in SARS-CoV-2 infection: a very rare, life-threatening complication. Am J Emerg Med. Jul 2020 doi: 10.1016/j.ajem.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alijehani Y., Othman S.A., Almubarak Y., et al. Crit care res pract. Thoracic surgery consultations in COVID-19 critically ill patients. Beyond Conserv Approach. 2021 Mar;27(2021):6626150. doi: 10.1155/2021/6626150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aliberti S., Amati F., Pappalettera M., et al. COVID-19 multidisciplinary high dependency unit: the Milan model. Respir Res. Oct 2020;21(1):260. doi: 10.1186/s12931-020-01516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin K.M., Karas M.G., Ivascu N.S., Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 06 2020;201(11):1337–1344. doi: 10.1164/rccm.202004-1037CP. E252-E262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in proportions of patients with or without ICU stay, intubation, and successful extubation by complication types.
Differences in proportions of patients with or without ICU stay, intubation, and successful extubation by complication types seen on initial CXR.