Abstract
BACKGROUND
The COVID-19 pandemic presents unique social, economic, and psychological challenges for individuals globally. Thus, women who are pregnant face unprecedented mental health challenges.
OBJECTIVE
We sought to determine the impact of the pandemic on perinatal depression and anxiety in a longitudinal pregnancy cohort. We hypothesized increased depression and anxiety scores in women during pregnancy and after birth in the pandemic at all time points.
STUDY DESIGN
Participants were enrolled in the Ontario Birth Study, a pregnancy cohort embedded in clinical care at Mount Sinai Hospital, Toronto, Canada. Perinatal depression and anxiety were assessed using the 2-Item Patient Health Questionnaire and 2-Item Generalized Anxiety Disorder Questionnaire in early pregnancy, whereas the Edinburgh Postnatal Depression Scale and 2-Item Generalized Anxiety Disorder Questionnaire were used in late pregnancy and after birth. Logistic regression models were created to examine the association of the pandemic with clinically elevated mental health scores in the prepandemic group vs pandemic group while adjusting for covariates.
RESULTS
A total of 1159 survey responses from 649 participants between March 1, 2019, and February 28, 2021, were used to conduct this study. Participants were assessed in early pregnancy (n=416), in late pregnancy (n=373), and after birth (n=370). Responses received on or before February 29, 2020, were considered the “prepandemic” responses, whereas responses after the aforementioned date were considered the “pandemic” responses. Mean rank scores of depression and anxiety were significantly higher in the pandemic group (P=.02 and P=.003, respectively) in the postpartum period. There was no significant association between pandemic time and antenatal scores. However, postnatally, mothers were 2.6 times more likely to score ≥13 on the Edinburgh Postnatal Depression Scale during the pandemic than before the pandemic (95% confidence interval, 1.2–5.7; P=.02). Adjustment for ethnicity and income strengthened this association as the odds ratio increased to 3.3 (95% confidence interval, 1.4–8.0; P=.007).
CONCLUSION
Pandemic-associated increases in depression and anxiety scores were confined to the postpartum period, highlighting a need for increased screening and interventions for perinatal mood and anxiety disorders postnatally as this pandemic continues.
Keywords: anxiety, COVID-19 pandemic, depression, developmental programming, Edinburgh Postnatal Depression Scale, Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, perinatal mental health, perinatal mood and anxiety disorders, pregnancy
AJOG MFM at a Glance.
Why was this study conducted?
The COVID-19 pandemic presents additional social, economic, and psychological challenges that are especially concerning for pregnant women. Perinatal mood and anxiety disorders (PMADs) during gestation can lead to negative health outcomes for both the parent and neonate. As this pandemic continues, it is necessary to evaluate perinatal mental health at multiple points during pregnancy to identify when an individual is most at risk of PMADs and to optimize the distribution of healthcare resources.
Key findings
In this longitudinal pregnancy cohort, significant increases in depression and anxiety scores because of the COVID-19 pandemic were limited to the postpartum period.
What does this add to what is known?
Our findings suggested that prevention and treatment interventions that target postpartum PMADs are a high priority during pandemic times.
Introduction
COVID-19 remains a rapidly evolving situation that has dramatically changed the global healthcare landscape. Beyond the immediate health consequences of a major viral outbreak, there have been profound psychological, economic, and social consequences as people are forced to leave their jobs and to isolate themselves from their families, friends, and communities. The US Centers for Disease Control and Prevention reported that 40% of adults experienced mental health problems during this pandemic and up to 31% experienced depression and anxiety.1 Symptoms of depression, anxiety, and psychological distress are reported more frequently by women than men during large-scale disease outbreaks,2 such as during the 2003 severe acute respiratory syndrome (SARS) outbreak,3 and are currently being observed during the COVID-19 pandemic.4 This is especially concerning in the pregnant population as perinatal psychological distress not only can lead to negative health outcomes for the birthing parent and child5 but also can cause spousal or partner stress,6 induce household instability for siblings,7 and contribute to economic burden on a societal level.8
During pregnancy, an individual undergoes substantial physiological and psychological changes, which put them at a higher risk of experiencing distressing feelings, referred to as perinatal mood and anxiety disorders (PMADs).9 An estimated 13% to 21% of pregnant individuals experience PMADs, which most commonly presents as depression and anxiety but includes obsessive-compulsive disorder and posttraumatic stress disorder.10 The current research will focus on depression and anxiety when referring to PMADs. There has been a push for routine clinical PMAD screening for all those receiving prenatal care with more consistent follow-up11 because as many as half of those with PMADs are not identified.12 The symptoms of perinatal depression overlap heavily with major depressive disorder, but they differ in their onset as PMADs are triggered by pregnancy.13 Furthermore, anxiety disorders are still highly comorbid with depression even during the perinatal period.14 Some of the risk factors for developing PMADs are low socioeconomic status, exposure to interpersonal or domestic violence, and lack of social support.15 These risk factors can be exacerbated by the stressors associated with the COVID-19 pandemic, which has the potential to increase the incidence of PMADs in an already high-risk group.
There is strong evidence for links between PMADs and adverse birth outcomes, such as preterm birth, intrauterine growth restriction,16 , 17 decreased mother-infant bonding, and delayed infant cognitive and emotional development.18 , 19 Consequently, the effects of in utero exposure to PMADs can be observed in infancy and into adulthood.20, 21, 22 It has been postulated that the dysregulation of cortisol during pregnancy, while depressive symptoms are occurring, is one of the mechanisms contributing to the increased behavioral problems seen in children. Cortisol can cross the placenta to influence the developing fetal hypothalamic-pituitary-adrenal axis and limbic system, subsequently affecting emotional reactivity in children.23 Similarly, anxiety during pregnancy has been shown to have a negative impact on executive function in children. Higher state anxiety during pregnancy was associated with lower visuospatial working memory performance at 6 to 9 years of age.24 Postpartum depression has a prevalence of 10% to 15%,25 and this condition hinders mother-infant bonding, which creates an adverse environment for optimal child neurodevelopment. It has been strongly associated with poor offspring cognitive functioning, behavioral inhibition, emotional maladjustment, and psychiatric disorder risk in later life.14, 15, 16 Although research has focused predominantly on postpartum depression, postpartum anxiety has also been related to negative offspring psychological outcomes, such as increased behavioral and emotional problems.22 , 26
Pregnant individuals may be at higher risk of PMADs during pandemic conditions because of the lifestyle restrictions, financial uncertainty, and stress from decreased social support and childcare. Racine et al27 reviewed 18 unique studies investigating PMADs and infant health during this pandemic, concluding that depression has increased 2-fold and anxiety 3-fold compared with prepandemic levels. Tomfohr-Madsen et al28 evaluated 46 studies of PMADs during COVID-19 conducted between December 2019 and February 2021 and have found that the prevalence of both depression and anxiety were higher in studies conducted later in the pandemic. To the best of our knowledge, most studies that have investigated PMADs during the COVID-19 pandemic have been cross-sectional and thus based on a particular phase of pregnancy or time in the perinatal period. As healthcare resources are highly strained during pandemic conditions, it would be helpful to evaluate mental health at multiple points during the perinatal period to identify when individuals are most at risk of PMADs. These additional data could help to optimize the distribution and timing of limited healthcare resources. This study aimed to examine the effect of COVID-19 on all phases of pregnancy, starting with the first trimester of pregnancy to the postpartum period. We hypothesized that the pandemic would be associated with an increase in depression and anxiety scores at all the time points evaluated. We presented results from the Ontario Birth Study (OBS), an ongoing longitudinal pregnancy cohort based at Mount Sinai Hospital, Toronto, Ontario, Canada.
Materials and Methods
This study was approved by the Mount Sinai Hospital Research Ethics Board, Toronto, Ontario. Through the OBS, all participants provided written informed consent for their participation in both study components and access to their clinical data for research.
Study participants
The OBS is an open longitudinal pregnancy cohort developed by the Lunenfeld-Tanenbaum Research Institute and Mount Sinai Hospital, Toronto, Ontario. There are approximately 7000 deliveries per year at Mount Sinai Hospital. Women at <17 weeks of gestation, English speaking, and at least 18 years old are eligible to participate. Data from lifestyle questionnaires (LSQs), diet questionnaires, clinical records, and biospecimens are collected throughout pregnancy, using methodology as described previously.29
The LSQs are administered in early pregnancy (12–16 weeks of gestation), late pregnancy (24–28 weeks of gestation), and postnatally (6–10 weeks after birth). The LSQs collect data on previous pregnancies, mental health, demographics, lifestyle, and environmental exposures. To investigate the potential effects of the pandemic on mental health, we analyzed LSQ responses received in the year before and during the COVID-19 pandemic between March 1, 2019, and February 28, 2021. Responses received on or before February 29, 2020, were considered the “prepandemic” responses, and responses from March 1, 2021, onward were considered the “pandemic” responses. Questionnaire response rates were 92.2% before the pandemic and 95.1% during the pandemic in early pregnancy, 81.2% before the pandemic and 75.9% during the pandemic in late pregnancy, and 65.4% before the pandemic and 53.1% during the pandemic in the postpartum period.
Assessment of depression and anxiety
All depression and anxiety measures were embedded into each LSQ. Anxiety was measured with the 2-Item Generalized Anxiety Disorder Questionnaire (GAD-2) at all time points. The GAD-2 was derived from the 7-item GAD. A GAD-2 score of ≥3 indicates clinically elevated anxiety with 83% sensitivity and 90% specificity.30 Depression in early pregnancy was assessed by the 2-Item Patient Health Questionnaire (PHQ-2), derived from the 9-item PHQ and Primary Care Evaluation of Mental Disorders. A PHQ-2 score of ≥3 can be used to screen for major depression with 83% sensitivity and 92% specificity.31 The late pregnancy and postnatal questionnaires included the Edinburgh Postnatal Depression Scale (EPDS), a 10-item instrument commonly used in prenatal care for depression screening. The EPDS is the most used depression screening tool in postnatal care. It has a scale of 0 to 30, and individuals who score ≥13 are considered likely to be suffering from perinatal depression. This screening tool with a cutoff value of ≥13 has 66% sensitivity and 95% specificity.32 Clinical literature has shown that cutoff values of 11 and 13 are both valid; however, we chose to use the higher symptom levels of perinatal depression with greater specificity and somewhat lower sensitivity.33 Moreover, a 3-question anxiety-focused derivative of the EPDS (EPDS-3A) was used to examine anxiety at the late pregnancy and postpartum time points. A cutoff of ≥5 was used to identify persons experiencing clinically elevated anxiety, which has a sensitivity of 70.9% and specificity of 92.2%.34
Statistical analyses
Initial analyses involved the comparisons of depression and anxiety scores for each demographic variable. The primary demographic characteristics evaluated were age, body mass index (BMI), education, ethnicity, income, and parity. The Kendall tau-b correlation coefficient was used to assess the association of continuous demographic variables with maternal mental health scores. Maternal mental health scores were compared for categorical demographic variables using the Mann-Whitney U test or the Kruskal-Wallis H test. Maternal depression and anxiety scores before the pandemic and during the pandemic were directly compared using Mann-Whitney U tests. Chi-square tests were used to test the association between pandemic time and depression and anxiety scores above the clinical cutoff: PHQ-2 and GAD-2 scores of ≥3, EPDS-3A scores of ≥5, and EPDS scores of ≥13, regardless of whether the participant endorsed suicidal ideation. Furthermore, logistic regression models were created to examine the associations of pandemic timing (before or during the pandemic) with clinically relevant maternal depression and anxiety scores, adjusting for covariates. Any demographic characteristic significantly associated with depression and/or anxiety scores consistently at multiple time points during pregnancy and postnatally were included in all the final multivariable logistic regression models. Thus, only income and ethnicity were included as covariates. Individuals missing either of these were excluded from the analyses. Statistical analysis was conducted using IBM SPSS (version 27; Armonk, NY) and R (version 4.1.0; Vienna, Austria). Figures were generated using GraphPad (San Diego, CA). Of note, 2-sided P values of <.05 were considered statistically significant.
Results
Descriptive analysis
A total of 416 participants (280 in the prepandemic group and 136 in the pandemic group) were assessed in early pregnancy, 373 (263 in the prepandemic group and 110 in the pandemic group) were assessed in late pregnancy, and 370 (285 in the prepandemic group and 85 in the pandemic group) were assessed after delivery. A total of 8 participants were missing mental health variables for both groups in early pregnancy, 2 in late pregnancy, and 4 in the postpartum period, all of whom were excluded from analyses. The distributions of demographics at each time point before and during the pandemic are summarized in Table 1 .
Table 1.
Demographic variables in perinatal women before and during the COVID-19 pandemic
| Variable | Early pregnancy |
Late pregnancy |
Postpartum period |
||||
|---|---|---|---|---|---|---|---|
| Group | Prepandemic,n (%) | Pandemic,n (%) | Prepandemic,n (%) | Pandemic, n (%) | Prepandemic,n (%) | Pandemic,n (%) | |
| Group total | 280 | 136 | 263 | 110 | 285 | 85 | |
| Total | 416 | 373 | 370 | ||||
| Prepregnancy BMI | Normal | 187 (66.5) | 95 (69.9) | 183 (69.6) | 76 (69.1) | 205 (71.9) | 61 (71.8) |
| Overweight | 60 (21.8) | 29 (21.3) | 46 (17.5) | 23 (20.9) | 51 (17.9) | 16 (18.8) | |
| Obese | 31 (11) | 12 (8.8) | 31 (11.8) | 10 (9.1) | 28 (9.8) | 8 (9.4) | |
| Missing Mean±SD |
2 (0.7) 23.9±4.7 |
0 (0) 23.8±5.0 |
3 (1.1) 23.8±4.7 |
1 (0.9) 23.8±4.6 |
1 (0.4) 23.7±4.2 |
0 (0) 23.9±5.0 |
|
| Ethnicity | White | 162 (58) | 71 (52.2) | 153 (58.2) | 57 (51.8) | 166 (58.2) | 49 (57.6) |
| Non-White | 118 (42) | 65 (47.8) | 106 (40.3) | 50 (45.5) | 113 (39.6) | 33 (38.8) | |
| Missing | 0 (0) | 0 (0) | 4 (1.5) | 3 (2.7) | 6 (2.1) | 3 (3.5) | |
| Income | <$100,000 | 103 (37.0) | 40 (29.4) | 85 (32.3) | 35 (31.8) | 107 (37.5) | 34 (40.0) |
| $100,000–$149,999 | 52 (18.5) | 36 (26.5) | 65 (24.7) | 28 (25.5) | 63 (22.1) | 13 (15.3) | |
| ≥$150,000 | 107 (38.1) | 56 (41.2) | 96 (36.5) | 41 (37.2) | 90 (31.6) | 32 (37.6) | |
| Missing | 18 (6.4) | 4 (2.9) | 17 (6.5) | 6 (5.5) | 25 (8.8) | 6 (7.1) | |
| Parity | Primiparous | 162 (58.0) | 93 (68.4) | 146 (55.5) | 75 (68.2) | 165 (57.9) | 65 (76.5) |
| Multiparous | 117 (41.6) | 43 (31.6) | 116 (44.1) | 35 (31.8) | 120 (42.1) | 19 (22.4) | |
| Missing | 1 (0.4) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 1 (1.1) | |
| Education | Bachelor's degree | 114 (40.9) | 63 (46.3) | 109 (41.4) | 52 (47.3) | 108 (37.9) | 42 (49.4) |
| Graduate degree | 122 (43.4) | 58 (42.6) | 118 (44.9) | 42 (38.2) | 140 (49.1) | 29 (34.1) | |
| Other | 42 (15.0) | 13 (9.6) | 32 (12.2) | 11 (10.0) | 31 (10.9) | 11 (13.0) | |
| Missing | 2 (0.7) | 2 (1.5) | 4 (1.5) | 5 (4.5) | 6 (2.1) | 3 (3.5) | |
| Age (y) | Mean age±SEM | 34.5±0.24 | 33.6±0.35 | 34.6±0.24 | 34.1±0.35 | 33.9±0.22 | 34.6±0.45 |
| Maximum–minimum | 45–22 | 44–25 | 45–24 | 44–26 | 45–21 | 44–25 | |
| Missing | 3 | 1 | 5 | 4 | 4 | 3 | |
BMI, body mass index; SEM, standard error of the mean; SD, standard deviation.
Zhang. Impact of COVID-19 on perinatal maternal depression and anxiety. Am J Obstet Gynecol MFM 2022.
Most participants were White and primiparous with a mean prepregnancy BMI of 23.7 to 23.9 kg/m2 across all groups. The study population was generally highly educated with most participants having a bachelor's degree or higher. Depression scores in early pregnancy (P<.001) and depression and anxiety scores postnatally (P=.02 and P=.006, respectively) differed significantly by ethnicity with higher scores in non-Whites than Whites. Depression and anxiety scores were significantly different between income levels (<$100,000, $100,000–$149,999, and ≥$150,000) at all time points: in early pregnancy (P<.001 and P=.003, respectively), in late pregnancy (P<.001 and P=.05, respectively), and in the postpartum period (P=0.001 and P=0.01, respectively). The mean ranks of depression scores were the highest in the lowest income category (<$100,000) compared with both upper-income categories ($100,000–$150,000 and >$150,000) in early pregnancy (P=.008 and P<.001, respectively), late pregnancy (P=.002 and P<.001, respectively), and the postpartum period (P=.008 and P=.001, respectively). Moreover, participant anxiety scores were highest in the lowest income category compared with $100,000 to 150,000 and >$150,000 in early pregnancy (P=.04 and P=.001, respectively) and the postpartum period (P=.02 and P=.01, respectively). Primiparous mothers reported higher depression scores (P=.002) and anxiety scores (P=.03) postnatally and higher depression scores in late pregnancy (P=.04) than multiparous mothers. Participant age was inversely associated with anxiety scores only in the postpartum period (r=−0.13; P=.01) but was not related to depression scores at any time point. Depression and anxiety scores did not differ between BMI categories.
The means, medians, and mean ranks of maternal depression and anxiety scores before and during the pandemic are shown in Table 2 . Both mean and median scores were well below clinical cutoff values in all groups. Direct pandemic time comparisons of maternal depression and anxiety scores revealed higher mean ranks of depression scores during the pandemic than before the pandemic (208.7 and 178.6, respectively) in the postpartum period (P=.02). Similarly, mean ranks of maternal anxiety scores were significantly higher during the pandemic than before the pandemic (203.8 and 177.4, respectively) in the postpartum period (P=.03). There was no significant difference between either maternal depression or maternal anxiety scores before and during the pandemic in early or late pregnancy. The distribution of maternal depression and anxiety scores at all time points is shown in the Figure .
Table 2.
Mean ranks of perinatal maternal depression and anxiety scores before and during the COVID-19 pandemic
| Variable | Early pregnancy (12–16 wk of gestation) | Late pregnancy (24–28 wk of gestation) | Postpartum period (6–10 wk of gestation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal depressiona | ||||||||||||
| Mean (range) | Median | Mean rank | P value | Mean (range) | Median | Mean rank | P value | Mean (range) | Median | Mean rank | P value | |
| Pre-pandemic | 0.7 (0–5) | 0 | 206.4 | .7 | 5.4 (0–25) | 1.0 | 185.1 | .6 | 5.4 (0–24) | 5.0 | 178.6 | .02 |
| Pandemic | 0.8 (0–6) | 0 | 211.2 | 5.8 (0–27) | 1.0 | 191.4 | 6.8 (0–20) | 6.0 | 208.7 | |||
| Maternal anxietyb | ||||||||||||
| Mean (range) | Median | Mean rank | P value | Mean (range) | Median | Mean rank | P value | Mean (range) | Median | Mean rank | P value | |
| Pre-pandemic | 1.0 (0–6) | 1.0 | 204.1 | .6 | 1.0 (0–6) | 1.0 | 182.5 | .3 | 0.9 (0–6) | 1.0 | 177.4 | .03 |
| Pandemic | 1.0 (0–6) | 1.0 | 210.0 | 1.1 (0–6) | 1.0 | 194.3 | 1.2 (0–6) | 1.0 | 203.8 | |||
Depression scores were calculated using the 2-item Patient Health Questionnaire in early pregnancy and the Edinburgh Postnatal Depression Scale in late pregnancy and the postpartum period
Anxiety scores were calculated using the 2-item General Anxiety Disorder Questionnaire.
Zhang. Impact of COVID-19 on perinatal maternal depression and anxiety. Am J Obstet Gynecol MFM 2022.
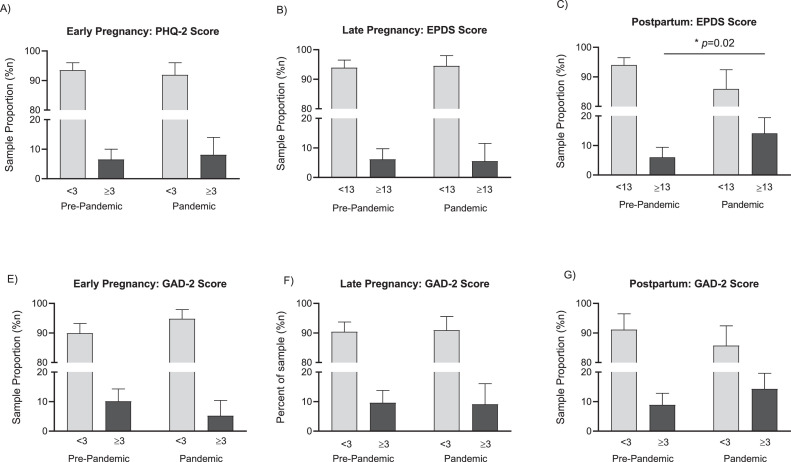
Figure.
Prevalence of perinatal depression and anxiety before and during COVID-19
A–C,Prevalence of clinically elevated depression scores (PHQ-2, ≥3; EPDS, ≥13). D–F, Prevalence of clinically elevated anxiety scores (GAD-2, ≥3). There was a significant association between clinically elevated maternal depression scores and pandemic time only in the postpartum period (P=.02). Data are sample proportions with 95% confidence intervals.
Zhang. Impact of COVID-19 on perinatal maternal depression and anxiety. Am J Obstet Gynecol MFM 2022.
Association of the COVID-19 pandemic with clinically relevant depression and anxiety scores
Table 3 provides the unadjusted and adjusted results of the logistic regression models, where ethnicity and income are also included in the adjusted model. In early and late pregnancy, both the unadjusted and adjusted models showed no significant association between pandemic time and maternal depression or anxiety scores. However, in the postpartum unadjusted model, mothers were 2.6 times more likely to report clinically elevated depression scores during the pandemic (95% CI, 1.2–5.7; P=.02). This association strengthened after adjusting for ethnicity and income as the odds ratio increased to 3.3 (95% CI, 1.4–8.0; P=.007). Similarly, study participant anxiety as measured by the EPDS-3A was higher during the pandemic only in the postpartum period. In the unadjusted model, participants were 1.8 times more likely to experience clinically elevated anxiety scores (95% CI, 1.1–3.1; P=.02). This association was stronger after adjusting for covariates with an increased odds ratio of 2.1 (95% CI, 1.2–3.7; P=.009).
Table 3.
Unadjusted and adjusted logistic regression analyses of the association between maternal depression and anxiety and the COVID-19 pandemic
| Period | Score cutoffs | Prepandemica n (%) | Pandemic, n (%) | Unadjusted OR (95% CI) | Adjusted ORb (95% CI) | P value |
|---|---|---|---|---|---|---|
| Early pregnancy | Depression PHQ-2<3 PHQ-2≥3 Anxiety GAD-2<3 GAD-2≥3 |
261 (92.8) 18 (6.4) 248 (88.3) 28 (9.9) |
151 (91.9) 11 (8.1) 128 (94.1) 7 (5.1) |
Reference 1.3 (0.6–2.8) Reference 0.5 (0.2–1.1) |
Reference (0.5–2.5) Reference 0.4 (0.2–1.1) |
.8 .06 |
| Late pregnancy | Depression EPDS<13 EPDS≥13 Anxiety GAD-2<3 GAD-2≥3 EPDS-3A<5 EPDS-3A≥5 |
247 (93.9) 16 (6.1) 236 (89.7) 25 (9.5) 200 (76.0) 62 (23.6) |
104 (94.5) 6 (5.5) 100 (90.9) 10 (9.1) 79 (71.8) 31 (28.2) |
Reference 0.9 (0.3–2.4) Reference 0.9 (0.4–2.0) Reference 1.3 (0.8–2.1) |
Reference 0.9 (0.3–2.4) Reference 0.9 (0.4–2.0) Reference 1.3 (0.8–2.3) |
.9 .9 .3 |
| Postpartum period | Depression EPDS<13 EPDS≥13 Anxiety GAD-2<3 GAD-2≥3 EPDS-3A<5 EPDS-3A≥5 |
268 (94.0) 17 (5.90) 257 (90.2) 25 (8.8) 220 (77.2) 63 (22.1) |
73 (85.9) 12 (14.11) 72 (84.7) 12 (14.1) 55 (64.7) 29 (34.1) |
Reference 2.6 (1.2–5.7) Reference 1.7 (0.8–3.6) Reference 1.8 (1.1–3.1) |
Reference 3.3 (1.4–8.0) Reference 1.9 (0.9–4.1) Reference 2.1 (1.2–3.7) |
.007 .1 .009 |
CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale; EPDS-3A, 3-question anxiety-focused derivative of the EPDS; GAD-2, 2-Item Generalized Anxiety Disorder Questionnaire; OR, odds ratio; PHQ-2, 2-Item Patient Health Questionnaire.
Pandemic time (reference=prepandemic period)
Adjusted for ethnicity and income.
Zhang. Impact of COVID-19 on perinatal maternal depression and anxiety. Am J Obstet Gynecol MFM 2022.
Comment
Principal findings
The current study advances our understanding of how the COVID-19 pandemic has contributed to PMADs. Unexpectedly, in this longitudinal pregnancy cohort, the effect of pandemic conditions on increasing depression scores was limited to the postpartum period. This suggested that prevention and treatment interventions that target postpartum depression and anxiety are a high priority during pandemic times.
Results in the context of what is known
Like other forms of depression, postpartum depression is multifactorial with genetics, psychosocial factors, and circadian rhythm disruption all playing a significant role.35 Fewer studies have examined postpartum anxiety, but most attribute its incidence to the same mechanisms as depression because of their comorbidity.36 Although we cannot determine the specific mechanisms to account for the current findings, it is of note that diminished social support and sleep have been linked to pandemic-related depression in recent work.37 , 38 It is highly plausible that postpartum depression may be particularly sensitive to these effects.
In contrast to previous studies,28 but similar to our findings, Zilver et al39 did not observe an effect of COVID-19 on PMADs during pregnancy. Although related, the risk factor profiles differ between antenatal and postnatal depression whereby the largest predictor of antenatal depression is low self-esteem and anxiety, whereas the largest predictor for postnatal depression is parenting stress.40 An international collaboration (including 64 countries) found that the most commonly reported worries for birthing parents during the COVID-19 pandemic were related to family and social support after delivery and inadequate childcare and the risk of their child contracting COVID-19 as this pandemic continues.30 These results were in line with our findings as we observed an association of the pandemic with PMADs that was confined to the postnatal period. In other studies, birthing parents reported that the perception of decreased social and informal support from family and friends because of pandemic restrictions was a negative psychosocial outcome of the COVID-19 pandemic.31 Several other recent studies have evaluated the role of perceived social support during the pandemic. Moreover, those who reported higher levels of perceived social support reported lower levels of depression and anxiety alongside improved sleep quality.38 Kent de Grey et al32 proposed improved sleep quality as 1 pathway through which social support could promote health. They argued that the positive aspects of social support, such as the feeling or perception of belonging or being valued, can elevate mood and decrease anxiety to improve sleep quality. Of all the physiological changes experienced by mothers in the perinatal period, increased sleep disturbance is often disregarded. Mothers who experience increased insomnia or sleep disturbance are more likely to experience depressive symptomatology or postpartum depression.41 , 42
Clinical implications and future directions
According to Elsenbruch et al,43 pregnant women with low social support lack effective psychosocial resources as they have less social participation and insufficient emotional and instrumental support. In addition, the postpartum period represents an important and stressful life change for mothers. These conditions can lead to hypersecretion of cortisol, which has been considered a biologic risk factor of depression.44 Although both primiparous and multiparous mothers are affected by PMADs, primiparous mothers in the early postnatal period may be more vulnerable to psychosocial challenges. In addition to experiencing physiological changes, they must navigate their new role as a mother with their partner, extended family, and healthcare professionals.31
It has been suggested that the COVID-19 pandemic has magnified social and economic inequalities for mothers with mental health problems.45 As many of the OBS participants are highly educated people in the upper-income bracket, it is plausible that they had more job and income stability during the pandemic and, therefore, would not have been as susceptible to this risk factor during pregnancy as lower-income women. However, social support is a major buffer for postpartum depression and would have been a significant factor for all new mothers during the pandemic. Epidemiologic studies have shown that the most important and relevant predictors of PMADs are job concerns, coping mechanisms, and resilience and adaptability scores measured by scales, such as the Connor-Davidson Resilience Scale.46 Future work could make use of this scale in the pregnant population to assist clinicians in the identification of individuals at the highest risk of PMAD in routine clinical care. Recent studies have shown a lack of social support for new mothers is associated with increased psychological distress.47 Furthermore, Kinser et al48 evaluated maternal attitudes postpartum regarding resources during the COVID-19 pandemic. Mothers reported feeling “cheated” out of their postpartum resources, such as doulas and early child-rearing classes after receiving prenatal care during pregnancy. When we accounted for income in the analysis, the relationship between pandemic timing and both depression and anxiety scores in the postpartum period became even stronger.
Research implications
Women participating in the OBS have the option to enroll their child into OBS Kids, which collects early child development information and biospecimens from birth to 4 years. Future studies will assess whether maternal mental health scores relate to child developmental outcomes.
Strengths and limitations
A major strength of the current research was the opportunity to examine a study population recruited from the same hospital both before and during the pandemic. Additional value was provided by the availability of data at multiple perinatal time points. The OBS continued recruitment through most of the pandemic, allowing the generation of a substantial prenatal sample size for analysis. A limitation was the relatively small size of the postpartum group during the pandemic. In general, questionnaire response rates are lower in the postpartum period as new parents are dealing with other challenges. In addition, although we could continue following those already recruited and to restart recruitment, there was an unavoidable approximate 3-month gap in recruitment. Another limitation is that maternal mental health was self-reported via questionnaires. Ideally, these measures would be verified with a thorough clinical assessment. However, all the assessments used were established, validated tools.
Conclusions
The perinatal period is a sensitive time during which intrauterine exposures or impaired parental caregiving can alter the development of key infant organ systems, leading to long-term effects on neurologic and cardiometabolic functions. In addition, PMADs can result in stress for spouses or partners, affect other children in the household, and become a financial burden because of impacting an individual's ability to work. Our study evaluated the association of the COVID-19 pandemic with PMAD, specifically perinatal anxiety and depression at multiple time points perinatally. Furthermore, our study identified a significant increase in depression and anxiety scores in the postpartum period only. This observation highlights the need for increased screening and treatment for PMAD in the postpartum period as this pandemic continues.
Acknowledgments
We are extremely grateful to all the women who participated in the Ontario Birth Study (OBS). In addition, the authors acknowledge the contribution and support of the OBS team members.
Footnotes
C.X.W.Z. and J.C.O. contributed equally to this work.
The authors report no conflict of interest.
The datasets generated from the Ontario Birth Study cohort are available from the corresponding author on reasonable request.
This research was supported by the Canadian Institutes for Health Research (S.G.M.; grant number FDN-148368). The Ontario Birth Study is funded by Mount Sinai Hospital, Mount Sinai Hospital Foundation, the Mount Sinai Hospital/University Health Network Department of Obstetrics and Gynaecology, and the Lunenfeld-Tanenbaum Research Institute.
Cite this article as: Zhang CXW, Okeke JC, Levitan RD, et al. Evaluating depression and anxiety throughout pregnancy and postpartum: impact of the COVID-19 pandemic. Am J Obstet Gynecol MFM 2022;XX:x.ex–x.ex.
References
- 1.Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54:302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vehmeijer FOL, Guxens M, Duijts L, El Marroun H. Maternal psychological distress during pregnancy and childhood health outcomes: a narrative review. J Dev Orig Health Dis. 2019;10:274–285. doi: 10.1017/S2040174418000557. [DOI] [PubMed] [Google Scholar]
- 6.Hanington L, Heron J, Stein A, Ramchandani P. Parental depression and child outcomes–is marital conflict the missing link? Child Care Health Dev. 2012;38:520–529. doi: 10.1111/j.1365-2214.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201–215. doi: 10.1016/j.ehb.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health. 2020;20:173. doi: 10.1186/s12889-020-8293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meltzer-Brody S, Rubinow D. In: Women's mood disorders. Cox E, editor. Springer Nature; New York, NY: 2021. An overview of perinatal mood and anxiety disorders: epidemiology and etiology; pp. 5–16. [Google Scholar]
- 10.Kendig S, Keats JP, Hoffman MC, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. Obstet Gynecol. 2017;129:422–430. doi: 10.1097/AOG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accortt EE, Wong MS. It is time for routine screening for perinatal mood and anxiety disorders in obstetrics and gynecology settings. Obstet Gynecol Surv. 2017;72:553–568. doi: 10.1097/OGX.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 12.Gjerdingen DK, Yawn BP. Postpartum depression screening: importance, methods, barriers, and recommendations for practice. J Am Board Fam Med. 2007;20:280–288. doi: 10.3122/jabfm.2007.03.060171. [DOI] [PubMed] [Google Scholar]
- 13.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 14.Falah-Hassani K, Shiri R, Dennis CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47:2041–2053. doi: 10.1017/S0033291717000617. [DOI] [PubMed] [Google Scholar]
- 15.Byrnes L. Perinatal mood and anxiety disorders. J Nurse Pract. 2018;14:507–513. [Google Scholar]
- 16.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heun-Johnson H, Seabury SA, Menchine M, Claudius I, Axeen S, Lakshmanan A. Association between maternal serious mental illness and adverse birth outcomes. J Perinatol. 2019;39:737–745. doi: 10.1038/s41372-019-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehnig F, Nagl M, Stepan H, Wagner B, Kersting A. Associations of postpartum mother-infant bonding with maternal childhood maltreatment and postpartum mental health: a cross-sectional study. BMC Pregnancy Childbirth. 2019;19:278. doi: 10.1186/s12884-019-2426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. 2009;52:425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70:1312–1319. doi: 10.1001/jamapsychiatry.2013.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 23.Swales DA, Winiarski DA, Smith AK, Stowe ZN, Newport DJ, Brennan PA. Maternal depression and cortisol in pregnancy predict offspring emotional reactivity in the preschool period. Dev Psychobiol. 2018;60:557–566. doi: 10.1002/dev.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress. 2011;14:665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anokye R, Acheampong E, Budu-Ainooson A, Obeng EI, Akwasi AG. Prevalence of postpartum depression and interventions utilized for its management. Ann Gen Psychiatry. 2018;17:18. doi: 10.1186/s12991-018-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor TG, Heron J, Golding J, Glover V, Study Team ALSPAC. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 27.Racine N, Eirich R, Cooke J, et al. When the bough breaks: a systematic review and meta-analysis of mental health symptoms in mothers of young children during the COVID-19 pandemic. Infant Ment Health J. 2022;43:36–54. doi: 10.1002/imhj.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomfohr-Madsen LM, Racine N, Giesbrecht GF, Lebel C, Madigan S. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021;300 doi: 10.1016/j.psychres.2021.113912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson LN, Knight JA, Hung RJ, et al. The Ontario Birth Study: a prospective pregnancy cohort study integrating perinatal research into clinical care. Paediatr Perinat Epidemiol. 2018;32:290–301. doi: 10.1111/ppe.12473. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Kim HH, Basaldua R, et al. A cross-national study of factors associated with women's perinatal mental health and wellbeing during the COVID-19 pandemic. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallon V, Davies SM, Silverio SA, Jackson L, De Pascalis L, Harrold JA. Psychosocial experiences of postnatal women during the COVID-19 pandemic. A UK-wide study of prevalence rates and risk factors for clinically relevant depression and anxiety. J Psychiatr Res. 2021;136:157–166. doi: 10.1016/j.jpsychires.2021.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent de Grey RG, Uchino BN, Trettevik R, Cronan S, Hogan JN. Social support and sleep: a meta-analysis. Health Psychol. 2018;37:787–798. doi: 10.1037/hea0000628. [DOI] [PubMed] [Google Scholar]
- 33.Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD. DEPRESsion Screening Data (DEPRESSD) EPDS Group. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371:m4022. doi: 10.1136/bmj.m4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith-Nielsen J, Egmose I, Wendelboe KI, Steinmejer P, Lange T, Vaever MS. Can the Edinburgh Postnatal Depression Scale-3A be used to screen for anxiety? BMC Psychol. 2021;9:118. doi: 10.1186/s40359-021-00623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guintivano J, Sullivan PF, Stuebe AM, et al. Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol Med. 2018;48:1190–1200. doi: 10.1017/S0033291717002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali E. Women's experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237–249. doi: 10.2147/IJWH.S158621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terada M, Ohtsu H, Saito S, et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grey I, Arora T, Thomas J, Saneh A, Tohme P, Abi-Habib R. The role of perceived social support on depression and sleep during the COVID-19 pandemic. Psychiatry Res. 2020;293 doi: 10.1016/j.psychres.2020.113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zilver SJM, Broekman BFP, Hendrix YMGA, et al. Stress, anxiety and depression in 1466 pregnant women during and before the COVID-19 pandemic: a Dutch cohort study. J Psychosom Obstet Gynaecol. 2021;42:108–114. doi: 10.1080/0167482X.2021.1907338. [DOI] [PubMed] [Google Scholar]
- 40.Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008;8:24. doi: 10.1186/1471-244X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iranpour S, Kheirabadi GR, Esmaillzadeh A, Heidari-Beni M, Maracy MR. Association between sleep quality and postpartum depression. J Res Med Sci. 2016;21:110. doi: 10.4103/1735-1995.193500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okun ML. Sleep and postpartum depression. Curr Opin Psychiatry. 2015;28:490–496. doi: 10.1097/YCO.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 43.Elsenbruch S, Benson S, Rücke M, et al. Social support during pregnancy: effects on maternal depressive symptoms, smoking and pregnancy outcome. Hum Reprod. 2007;22:869–877. doi: 10.1093/humrep/del432. [DOI] [PubMed] [Google Scholar]
- 44.Qin DD, Rizak J, Feng XL, et al. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci Rep. 2016;6:30187. doi: 10.1038/srep30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibaut F, van Wijngaarden-Cremers PJM. Women's mental health in the time of Covid-19 pandemic. Front Glob Womens Health. 2020;1 doi: 10.3389/fgwh.2020.588372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma X, Wang Y, Hu H, Tao XG, Zhang Y, Shi H. The impact of resilience on prenatal anxiety and depression among pregnant women in Shanghai. J Affect Disord. 2019;250:57–64. doi: 10.1016/j.jad.2019.02.058. [DOI] [PubMed] [Google Scholar]
- 47.Arnold M, Kalibatseva Z. Are “superwomen” without social support at risk for postpartum depression and anxiety? Women Health. 2021;61:148–159. doi: 10.1080/03630242.2020.1844360. [DOI] [PubMed] [Google Scholar]
- 48.Kinser PA, Jallo N, Amstadter AB, et al. Depression, anxiety, resilience, and coping: the experience of pregnant and new Mothers During the first few months of the COVID-19 pandemic. J Womens Health (Larchmt) 2021;30:654–664. doi: 10.1089/jwh.2020.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]