Abstract
Background
The role of skeletal muscle index (SMI) and systemic inflammation index (SII) for patients with lymph node-positive breast cancer remain controversial. This retrospective study aims to evaluate the individual and synergistic value of SMI and SII in outcomes prediction in this population.
Methods
Lymph node-positive breast cancer patients who received mastectomy between January 2011 and February 2013 were included in this retrospective study. We used abdominal computed tomography (CT) to measure skeletal muscle mass at the third lumbar (L3) level. The optimal cut-off values of SMI and SII were determined through maximizing the Youden index on the receiver operating characteristic (ROC) curves. Kaplan–Meier method was used to assess the correlation between SMI, SII, and overall survival (OS). The prognostic value of SMI and SII were analyzed with the multivariable Cox proportional hazards model.
Results
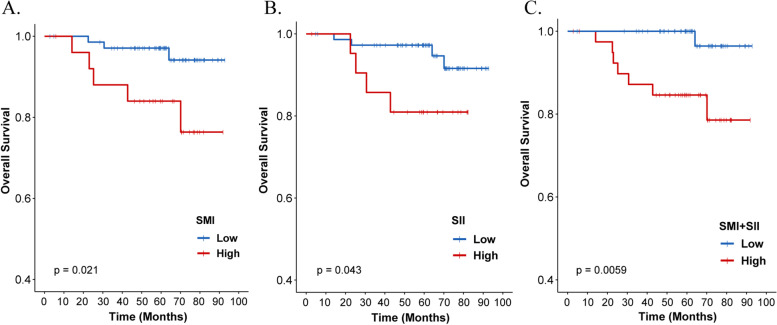
Of 97 patients included in our study (mean age: 46 [range: 27–73] years; median follow-up: 62.5 months), 71 had low SMI (sarcopenia), 59 had low SII, and 56 had low SMI + SII. Kaplan–Meier survival curves showed that both high SMI (P = 0.021, 5-year OS: 84.0% vs. 94.1%) and high SII (P = 0.043, 5-year OS: 81.0% vs. 97.3%) were associated with worse OS. Additionally, patients with either low SMI or low SII had significantly better OS (P = 0.0059, 5-year OS: 100.0% vs. 84.6%) than those with high SMI + SII. Multivariable analysis confirmed the predictive values of high SMI (P = 0.024, hazard ratio [HR]: 9.87) and high SII (P = 0.048, HR: 6.87) for poor OS. Moreover, high SMI + SII was significantly associated with poor survival (P = 0.016, HR: 16.36).
Conclusions
In this retrospective analysis, both SMI and SII independently predicted the prognosis of patients with lymph node-positive breast cancer. SMI + SII might be a stronger prognostic factor than either alone based on our findings, but should be further verified in a larger study.
Keywords: Skeletal muscle index (SMI), Systemic inflammation index (SII), Breast cancer, Overall survival
Background
Skeletal muscle index (SMI) is an important parameter to measure the body composition. Low SMI indicates the presence of sarcopenia, a condition generally induced by multiple causes including lack of exercise, endocrine dysfunction, chronic diseases, systemic inflammation, and malnutrition [1]. A previous study found that sarcopenia correlates with the nutrition indicator such as prognostic nutritional index (PNI) [2]. In patients with cancer, sarcopenia is mostly observed during disease progression [3], and it is also associated with higher toxicities from chemotherapy, higher rates of treatment complications [4], and worse outcomes [5].
Chronic systemic inflammation is involved in tumor occurrence, development, and metastasis [6, 7]. Previous studies have shown that long-term inflammation may predispose to muscle loss and sarcopenia [8], which may aggravate systemic inflammation and lead to higher risk of mortality [9]. Therefore, biomarkers of systemic inflammation have unique values in predicting outcomes in patients with cancer.
Breast cancer is one of the most common cancers worldwide and the leading cause of malignant disease among women. With early detection and systemic therapies, patients with breast cancer can generally have a satisfactory outcome [10]. To achieve individualized treatment for breast cancer, it is vital to identify patients with potential adverse prognosis. Previous studies have noted an inverse relationship between SMI and chemotherapy toxicity in patients with either early-stage [11] or metastatic [12] breast cancer. Among patients with breast cancer who received postoperative adjuvant radiotherapy, SMI is also a promising prognostic indicator for predicting outcomes [13]. Indeed, low SMI indicates sarcopenia, which is associated with an increased risk of overall mortality in breast cancer survivors [5, 14–16]. Additionally, biomarkers of systemic inflammation such as systemic immune-inflammation index (SII), have shown their prognostic value in various malignant tumors [17–20] and are recommended as useful prognostic indicators for patients with breast cancer [21, 22]. However, the association of SMI and SII with survival in patients with lymph node-positive breast cancer remains to be determined.
In this study, we measured the skeletal muscle area at the level of the third lumbar vertebrae (L3) by computed tomography (CT) to calculate the value of SMI. We analyzed the prognostic values of SMI and SII in the survival of patients with lymph node-positive breast cancer. Our findings may offer guidance for developing individualized management and treatment programs for patients with lymph node-positive breast cancer.
Methods
Patient selection
In our retrospective study, we included 97 patients with lymph node-positive breast cancer who received surgical treatment between January 2011 to February 2013 at Sun Yat-sen University Cancer Center (SYSUCC). The follow-up data of each patient was collected through clinic visits or phone interviews. All data are complete at the time of analysis. The following inclusion criteria were applied: lymph-node metastasis; pathological diagnosis of stage II and III breast cancer; female sex; available abdominal CT images; and complete clinical data on age, body mass index (BMI), TNM stage, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (Her2), Ki67, complete blood count, and liver function tests. The exclusion criteria were as follows: distant metastasis; breast cancer recurrence; prior diagnosis of other cancers; other comorbidities that may affect survival such as cardiopathy and systemic immune disease; and prior usage of any immunosuppressants. The tumor staging was based on the International Union Against Cancer TNM classification system for breast cancer (7th edition) [23]. The classification of molecular subtypes was based on the guidelines established at the 13th St. Gallen International Breast Cancer Conference [18]. The Research Ethics Committee of SYSUCC approved this study. All patients provided written consent.
The assessment of SMI and patient groupings
Abdominal CT images at the L3 level obtained from Monaco TPS version 5.1 (Elekta CMS, Maryland 72 Heights, MO, USA) before the surgical operation and chemotherapy was used to measure skeletal muscle area and fat tissue (Fig. 1). The measurement was based on their specific Hounsfield unit (HU) ranges. We calculated SMI as follows: SMI = skeletal muscle area (cm2)/height (m2).
Fig. 1.
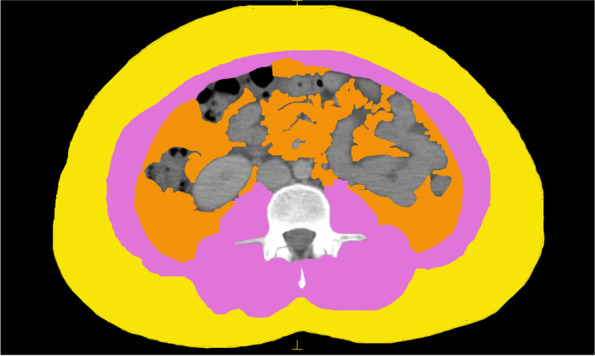
Body composition at the third lumbar vertebra level. Yellow: visceral adipose tissue (VAT); Pink: skeletal muscle mass (SMM); Orange: subcutaneous adipose tissue (SAT)
Currently, there is no gold standard to define sarcopenia in cancer patients, and many studies generally seek to an approach that best separates patients with sarcopenia and those did not have sarcopenia with respect to the outcome of interest (i.e., mortality in this study). To this end, we defined patients with sarcopenia with the use of receiver operating characteristic curve (ROC) analysis, with the Youden index (sensitivity + specificity -1) being maximized to determine the optimal cut-off point of SMI. Because of the time-dependent nature of survival outcome data, we also used survival-ROC to determine the cut-off values and the results were similar. Patients with SMI value lower than the cut-point were categorized having sarcopenia. Similar categorization procedure was performed for SII, and prognostic nutritional index (PNI). In addition, we combined SMI with SII to obtain a new variable—SMI + SII. The low SMI + SII group includes patients with both low SMI and low SII values. The rest of the patients were included in the high SMI + SII group.
The PNI value was calculated using the following formula: PNI = serum albumin (g/L) + 5*peripheral blood lymphocyte count (*109/L). The classification of BMI was based on the criteria specific for Chinese population: BMI < 18.5 kg/m2 (underweight), 18.5–23.9 kg/m2 (normal), 24–27.9 kg/m2 (overweight), and ≥ 28 kg/m2 (obese) [24, 25].
Statistical analysis
We used Mann–Whitney U test to compare age, BMI, T stage, N stage, clinical stage, PNI, and SII between the high SMI and low SMI groups. The Pearson chi-squared test was used to compare ER, PR, Her2, Ki67 stage, and subtype. Overall survival (OS) was defined as the interval from the date of surgery to the date of death or latest follow-up. Survival curves were constructed with the Kaplan–Meier method, and the log-rank test was used for between-group comparisons. Univariable analysis was used to identify clinical variables associated with survival. Multivariable Cox proportional hazards regression models were used to adjust for clinically relevant variables from univariable analysis. All statistical analysis were performed in SPSS version 26.0 (IBM Corp., Armonk, NY) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value < 0.05 was considered to indicate statistical significance.
Results
Clinical baseline data
The median age of the 97 included patients was 46 (range, 27–73) years. After a median follow-up of 62.5 months, a total of 8 deaths occurred. The baseline characteristics are shown in Table 1. The median BMI was 22.72 kg/m2 (range: 18.83–32.05 kg/m2). We measured the skeletal muscle area and calculated the SMI value (Fig. 1). The area under the ROC curve (AUC) determines the optimal cut-off points for SMI (44.91 cm2/m2, median: 41.62 cm2/m2, range: 21.1–55.53 cm2/m2); SII (610.79 × 109/L, median: 445.22, range: 118.67–2434.89); and PNI (55.53 g/L, median: 52.2 g/L, range: 44–63 g/L) with an AUC value of 0.61, 0.56, and 0.65, respectively. Low SMI (sarcopenia) was observed in 71 (73.2%) patients and was associated with lower BMI (P < 0.001). A total of 59 (60.8%) and 56 (57.7%) patients were categorized into the low SII and low SMI + SII groups, respectively.
Table 1.
Baseline characteristics of study patients
| Variable | N (%) | Low SMI | High SMI | P |
|---|---|---|---|---|
| Age, years [mean: 45.69] | 0.970 | |||
| < 60 | 86 | 63 | 23 | |
| ≥ 60 | 11 | 8 | 3 | |
| BMI | 0.001 | |||
| Normal (18.5–23.9) | 66 | 48 | 18 | |
| Overweight (24.0–27.9) | 24 | 18 | 6 | |
| Obese (≥ 28.0) | 7 | 5 | 2 | |
| T stage | 0.087 | |||
| 1 | 24 | 15 | 9 | |
| 2 | 53 | 39 | 14 | |
| 3 | 10 | 8 | 2 | |
| 4 | 10 | 9 | 1 | |
| N stage | 0.772 | |||
| 1 | 30 | 22 | 8 | |
| 2 | 37 | 28 | 9 | |
| 3 | 30 | 21 | 9 | |
| Clinical stage | 0.987 | |||
| II | 26 | 19 | 7 | |
| III | 71 | 52 | 19 | |
| ER | 0.434 | |||
| Negative | 36 | 28 | 8 | |
| Positive | 61 | 43 | 18 | |
| PR | 0.715 | |||
| Negative | 44 | 33 | 11 | |
| Positive | 53 | 38 | 15 | |
| Her2 | 0.423 | |||
| Negative | 57 | 40 | 17 | |
| Positive | 40 | 31 | 9 | |
| Ki67 | 0.113 | |||
| Negative | 22 | 19 | 3 | |
| Positive | 75 | 52 | 23 | |
| Subtype | 0.764 | |||
| Luminal A | 11 | 6 | 5 | |
| Luminal B | 52 | 41 | 11 | |
| Her2 + | 21 | 15 | 6 | |
| TNBC | 13 | 9 | 4 | |
| PNI | 0.769 | |||
| Low | 68 | 49 | 19 | |
| High | 27 | 21 | 6 | |
| Missing | 2 | 1 | 1 | |
| SII | 0.548 | |||
| Low | 59 | 56 | 3 | |
| High | 38 | 15 | 23 | |
SMI skeletal muscle index, ER estrogen receptor, PR progesterone receptor, Her2 human epidermal growth factor receptor-2, TNBC triple negative breast cancer, PNI prognostic nutritional index, SII systemic inflammation index
Prognostic value and survival analysis
Kaplan–Meier curves demonstrated that patients with low SMI had more favorable OS than those with high SMI (P = 0.021, 5-year OS: 94.1% vs. 84.0%) (Fig. 2a). In terms of SII, patients with low SII had better OS than patients with high SII (P = 0.043, 5-year OS: 97.3% vs. 81.0%) (Fig. 2b). In addition, patients with both low SMI and low SII (low SMI + SII group) were associated with significantly better OS (P = 0.0059, 5-year OS: 100.0% vs. 84.6%) (Fig. 2c).
Fig. 2.
Kaplan–Meier curves indicating the overall survival (OS) rates of SMI (a), SII (b), and SMI + SII (c), respectively. SMI, skeletal muscle index; SII, systemic inflammation index
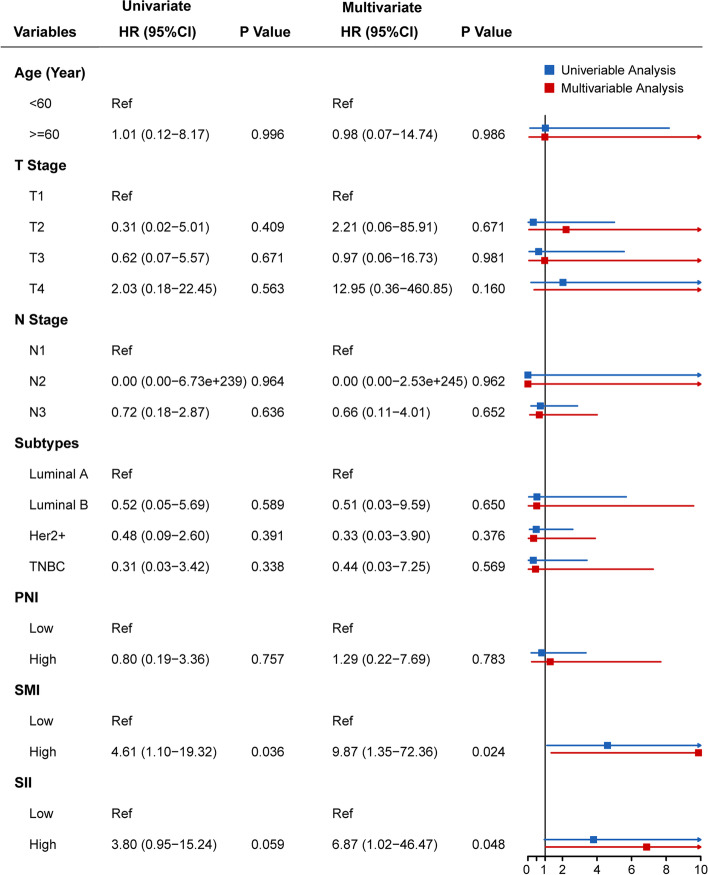
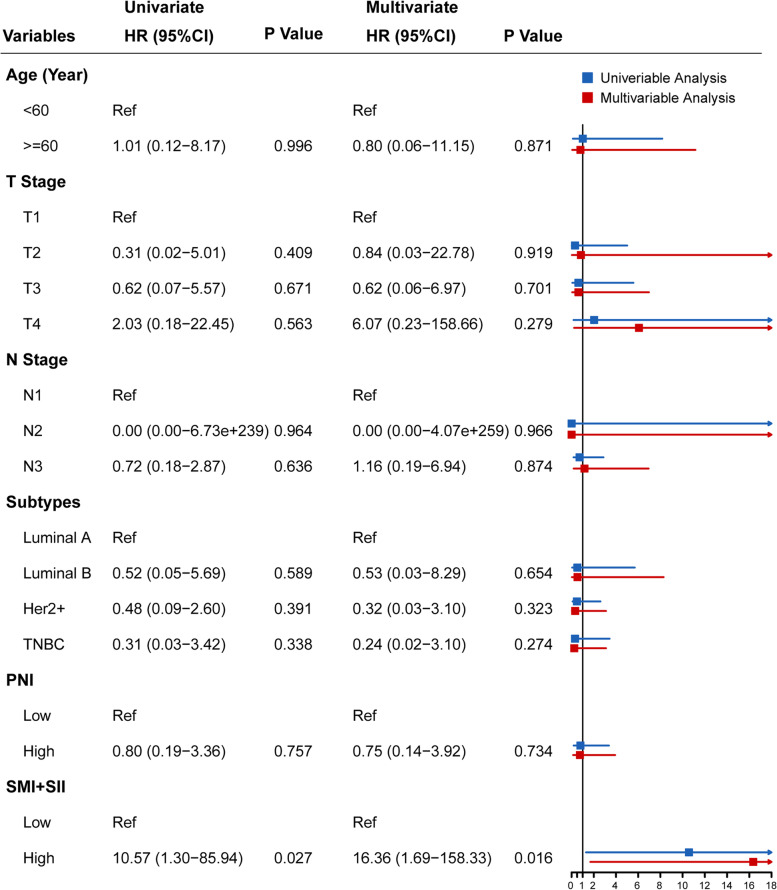
Univariable Cox proportional hazards regression analysis (Figs. 3 and 4) showed that high SMI (P = 0.036; hazard ratio [HR]: 4.613, 95% confidence interval [CI], 1.101–19.322) and high SMI + SII (P = 0.027, HR: 10.571, 95% CI, 1.300–85.936) were significantly associated with worse survival. In the multivariable Cox proportional hazards regression models adjusted for clinical variables, high SMI (P = 0.024), high SII (P = 0.048), and high SMI + SII (P = 0.016) were independently associated with worse survival. Additionally, the high SMI + SII groups (HR [95% CI]: 16.361 [1.691–158.325]) had significantly higher risk of mortality than the high SMI (HR [95% CI]: 9.867 [1.346–72.355]) or high SII (HR [95% CI]: 6.870 [1.015–46.472]) groups.
Fig. 3.
Cox proportional hazards models showing the correlation between clinical variables and overall survival (n = 97): Model A. PNI, prognostic nutritional index; SMI, skeletal muscle index; SII, systemic inflammation index
Fig. 4.
Cox proportional hazards models showing the correlation between clinical variables and overall survival (n = 97): Model B. PNI, prognostic nutritional index
Discussion
In this study, we examined the SMI and SII prognostic values in a retrospective cohort of patients with lymph node-positive breast cancer without distant metastasis. The results indicate that lower SMI and SII values were both independently associated with more favorable OS in these patients, and the combination of SMI and SII might better predict the survival outcomes.
Our study cohort had several notable factors that may contribute to fewer deaths. First, most patients had a pathological diagnosis of certain molecular subtypes (type luminal A and B) that are shown to be associated with satisfactory prognosis in previous studies [26]. Second, the patients were relatively young, and all of them had received standardized treatment modalities in our hospital. Nonetheless, SMI and SII still emerged as an important prognostic factor in both univariable and multivariable analysis, indicating their unique value for this patient population.
Our finding that low SMI was probably associated with better survival was consistent with a previous report [27], but appeared different from other studies. Caan et al. [5] and Deluche et al. [28] reported that sarcopenia was associated with a higher mortality among patients with nonmetastatic breast cancer. For those with metastatic breast cancer, Franzoi et al. [29] observed that sarcopenia is an indicator for poor prognosis. Both patients with early and advanced stage breast cancer struggle with serious chemotherapy-related toxicities. We speculated that the association of SMI with cancer survival may differ among patients of different age groups who may suffer from different chemotherapy-related toxicities resulted from diverse skeletal muscle index. In our study, the median age of breast cancer patients was 45 years and 75% were aged < 55 years, which was relatively young compared with previous studies [5, 28, 29]. Of note, the onset of menopause in women is generally associated with decreased skeletal muscle mass and increased adipose tissue [28]. Older patients generally have more adipose tissue than younger patients, which can be associated with more toxicity from chemotherapies [5]. For older patients receiving chemotherapy, a higher SMI value indicates better nutritional status and is predictive of better survival. However, for younger patients, a higher SMI value may be associated with a higher level of substance exchange within capillaries in the muscle tissue [4, 11], which could predispose to the development of chemotherapy-induced toxicities. In this regard, the increased risk of toxicities from chemotherapy may outweigh the benefits associated with a high SMI. Therefore, low SMI could be beneficial in younger patients and confer better treatment response and tolerance of chemotherapy instead of higher toxicities.
Moreover, the European Working Group on sarcopenia recommends to evaluate the presence of both low muscle mass and low muscle function (strength or performance) to determine sarcopenia [30]. Versteeg et al. [31] demonstrated that higher muscle strength could be beneficial for the survival of old patients with advanced cancer. Therefore, clinicians should not only focus on the value of SMI, but also incorporate patients’ nutritional status, muscle strength, extent of weight loss, and the daily lifestyle into the comprehensive assessment of prognosis.
Inflammation is closely connected with cancer, and previous studies have explored the prognostic value of a combination of SMI and common inflammatory markers. Demirelli et al. [32] showed that several inflammatory markers are independent prognostic factors in patients with metastatic gastric cancer. Cho et al. [33] found that sarcopenia accompanied by higher neutrophil/lymphocyte ratio is associated with inferior OS and progression-free survival in patients with head and neck cancer. The SII is a systemic inflammatory marker that takes into account the role of lymphocytes, neutrophils, and platelets. Lymphocytes plays a significant role in the inhibition of tumor proliferation and migration [34]. Lymphocytes can not only prevent cytotoxic cell death [35], but also secrete IFN-γ and TNF-α to control tumor growth [36]. Lack of lymphocytes may lead to insufficient immunoreaction, and thus promote disease progression [37]. In contrast, neutrophils can secrete a series of inflammatory mediators [38], which could positively affect tumor growth and metastasis. Neutrophils can also impair the cytolytic ability of activated T cells and NK cells [39]. In addition, platelets activated by tumor-related inflammation accelerate tumor cell migration and protect tumor cells from immune attack [40]. In essence, the development and progression of tumors are dependent on the balance between tumor-promoting factors (neutrophils and platelets) and tumor-inhibiting factors (lymphocytes). The SII integrates these relative factors and is considered a better inflammatory marker than other inflammatory prognostic factors that reflect this balance, such as neutrophil-to-lymphocyte ratio (NLR) or monocyte-to-lymphocyte ratio (MLR). In this study, we found that the OS of patients with low SMI and SII were significantly better than those with both high SMI and high SII. Therefore, our finding adds to the growing body of evidence that shows the important value of combining SMI with SII to predict prognosis in patients with solid tumors.
The improved predictive value of SMI and systemic inflammatory markers may be based on the mutual effects between skeletal muscle and inflammation. Skeletal muscle can secrete cytokines such as IL-6, IL-8, and IL-15 in an autocrine, paracrine, or endocrine manner, which promotes systemic inflammation and subsequently tumor growth [41]. Meanwhile, during chronic inflammation, the elevated production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) can damage proteins through carbonylation that can lead to irreversible protein modification [7]. In fact, the primary cause for skeletal muscle loss in breast cancer patients receiving chemotherapies is tumor-related cytokines and chronic inflammation rather than the chemotherapy itself [11]. In the present study, patients with a higher SII value may have had an increased level of proinflammatory cytokines, which promotes the development of chronic inflammation and worse survival.
This study has some limitations. Firstly, the retrospective nature of this analysis indicates that certain confounding factors may not have been accounted for owing to incomplete clinical information. Some important demographic factors such as socioeconomic status may affect patient survival. Secondly, the relatively small sample size and fewer deaths could have prevented us from identifying significant relationships for TNM stage and pathological and molecular subtype with patient prognosis. Finally, our study sample consists of a relatively young Chinese population and thus, our conclusions may not be directly applicable to patients with different demographics.
Conclusions
In summary, this retrospective analysis suggested that high SMI and high SII could be associated with poor OS in a relatively young group of patients with lymph node-positive breast cancer without distant metastasis. In clinical practice, a combination of SMI and SII may provide better prognostic values in predicting the prognosis for these patients to some extent. This warrants further confirmation in studies with a larger patient cohort in the future.
Acknowledgements
The authors appreciate all the patients who participated in this study. This study was supported by the National Natural Science Foundation of China (No. 81872459 to ZY He), the National Natural Science Foundation of China (No. 81803050 to WW Zhang) and the Natural Science Foundation of Guangdong Province (No. 2018A030313666 to ZYH).
Abbreviations
- SMI
Skeletal muscle index
- SII
Systemic inflammation index
- CT
Computed tomography
- L3
The third lumbar
- ROC
The receiver operating characteristic
- OS
Overall survival
- PNI
Prognostic nutritional index
- BMI
Body mass index
- ER
Estrogen receptor
- PR
Progesterone receptor
- Her2
Human epidermal growth factor receptor-2
- TNBC
Triple negative breast cancer
- HU
Hounsfield unit
- AUC
The area under the ROC curve
- HR
Hazard ratio
- CI
Confidence interval
- NLR
Neutrophil-to-lymphocyte ratio
- MLR
Monocyte-to-lymphocyte ratio
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
Authors’ contributions
Z.Y.H., S.G.W., R.T., J.P.D. and L.Z. contributed to the study conception and design. Z.Y.H., S.G.W. and W.W.Z. provided the administrative support. Z.Y.H., W.W.Z., L.Z., J.Y.S. and J.Z. prepared the study materials and patients. R.T., J.P.D., L.Z. and F.C. collected the data. R.T., J.P.D. and L.Z. finished the data analysis and interpretation. The first draft of the manuscript was written by R.T. and all authors modified it based on their opinions. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81803050 to WW Zhang), the National Natural Science Foundation of China (No. 81872459 to ZY He) and the Natural Science Foundation of Guangdong Province (No. 2018A030313666 to ZYH). The first fund is mainly used to recruit volunteers for data collection. The other two funds help us with study design, data analysis and manuscript writing.
Availability of data and materials
According to our institution’s regulation, the original data will not be shared.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of SYSUCC and was conducted in agreement with the Helsinki Declaration, ethical guidelines for medical research on humans, and ethics guidelines for human genome/gene analysis research. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ru Tang, Jia-Peng Deng and Lei Zhang contributed equally.
Contributor Information
San-Gang Wu, Email: unowu12345@hotmail.com.
Zhen-Yu He, Email: hezhy@sysucc.org.cn.
References
- 1.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, KeimeGuibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Directors Ass. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, Seto Y. Poor nutritional status and sarcopenia influences survival outcomes in gastric carcinoma patients undergoing radical surgery. Eur J Surg Oncol. 2020;46(10 Pt A):1963–1970. doi: 10.1016/j.ejso.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/s1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 4.Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28(9):2107–2118. doi: 10.1093/annonc/mdx271. [DOI] [PubMed] [Google Scholar]
- 5.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA, Chen WY. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018;4(6):798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, Kawanishi S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev. 2013;2013:387014. doi: 10.1155/2013/387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age-Related Sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer Nat Rev Dis Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 11.Mazzuca F, Onesti CE, Roberto M, Di Girolamo M, Botticelli A, Begini P, Strigari L, Marchetti P, Muscaritoli M. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget. 2018;9(39):25714–25722. doi: 10.18632/oncotarget.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, Benbow JM, Muss HB. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(3):658–665. doi: 10.1158/1078-0432.Ccr-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua X, Deng JP, Long ZQ, Zhang WW, Huang X, Wen W, Guo L, He ZY, Lin HX. Prognostic significance of the skeletal muscle index and an inflammation biomarker in patients with breast cancer who underwent postoperative adjuvant radiotherapy. Curr Probl Cancer. 2020;44(2):100513. doi: 10.1016/j.currproblcancer.2019.100513. [DOI] [PubMed] [Google Scholar]
- 14.Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consul N, Guo X, Coker C, Lopez-Pintado S, Hibshoosh H, Zhao B, Kalinsky K, Acharyya S. Monitoring Metastasis and Cachexia in a Patient with Breast Cancer: A Case Study. Clin Med Insights Oncol. 2016;10:83–94. doi: 10.4137/cmo.S40479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng JP, Hua X, Long ZQ, Zhang WW, Lin HX, He ZY. Prognostic value of skeletal muscle index and monocyte-to-lymphocyte ratio for lymph node-positive breast cancer patients after mastectomy. Annals of translational medicine. 2019;7(23):775. doi: 10.21037/atm.2019.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil-Bernabé AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, Pollard JW, Ruf W, Muschel RJ. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–3175. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 18.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Placke T, Salih HR, Kopp HG. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol. 2012;189(1):154–160. doi: 10.4049/jimmunol.1103194. [DOI] [PubMed] [Google Scholar]
- 20.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Shi Z, Bai Y, Liu L, Cheng K. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer management and research. 2019;11:4471–4480. doi: 10.2147/cmar.S197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020;24(5):2993–3021. doi: 10.1111/jcmm.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun KH, Lee JS, Kim JH, Kim JJ, Chin HM, Park SM. The rationality of N3 classification in the 7th edition of the International Union Against Cancer TNM staging system for gastric adenocarcinomas: a case-control study. Int j sur. (London, England) 2014;12(9):893–896. doi: 10.1016/j.ijsu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 25.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 26.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 27.Del Fabbro E, Parsons H, Warneke CL, Pulivarthi K, Litton JK, Dev R, Palla SL, Brewster A, Bruera E. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17(10):1240–1245. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 2018;26(3):861–868. doi: 10.1007/s00520-017-3902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzoi MA, Vandeputte C, Eiger D, Caparica R, Brandão M, De Angelis C, Hendlisz A, Awada A, Piccart M, de Azambuja E. Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast Cancer Res Treat. 2020;181(1):199–209. doi: 10.1007/s10549-020-05617-2. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versteeg KS, Blauwhoff-Buskermolen S, Buffart LM, de van der Schueren MAE, Langius JAE, Verheul HMW, Maier AB, Konings IR, Higher Muscle Strength Is Associated with Prolonged Survival in Older Patients with Advanced Cancer. Oncologist. 2018;23(5):580–585. doi: 10.1634/theoncologist.2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demirelli B, Babacan NA, Ercelep Ö, Öztürk MA, Kaya S, Tanrıkulu E, Khalil S, Hasanov R, Alan Ö, Telli TA, Koca S, Arıbal ME, Kuzan B, Dane F, Yumuk PF (2020) Modified Glasgow Prognostic Score, Prognostic Nutritional Index and ECOG Performance Score Predicts Survival Better than Sarcopenia, Cachexia and Some Inflammatory Indices in Metastatic Gastric Cancer. Nutr Cancer:1–9. 10.1080/01635581.2020.1749290 [DOI] [PubMed]
- 33.Cho Y, Kim JW, Keum KC, Lee CG, Jeung HC, Lee IJ. Prognostic Significance of Sarcopenia With Inflammation in Patients With Head and Neck Cancer Who Underwent Definitive Chemoradiotherapy. Front Oncol. 2018;8:457. doi: 10.3389/fonc.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto W, Kashiwagi S, Asano Y, Takada K, Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Hirakawa K, Ohira M. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. 2018;18(1):1137. doi: 10.1186/s12885-018-5051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marra P, Mathew S, Grigoriadis A, Wu Y, Kyle-Cezar F, Watkins J, Rashid M, De Rinaldis E, Hessey S, Gazinska P, Hayday A, Tutt A. IL15RA drives antagonistic mechanisms of cancer development and immune control in lymphocyte-enriched triple-negative breast cancers. Can Res. 2014;74(17):4908–4921. doi: 10.1158/0008-5472.Can-14-0637. [DOI] [PubMed] [Google Scholar]
- 36.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(26):4045–4051. doi: 10.1200/jco.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Giorgi U, Mego M, Scarpi E, Giuliano M, Giordano A, Reuben JM, Valero V, Ueno NT, Hortobagyi GN, Cristofanilli M. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer. 2012;12(4):264–269. doi: 10.1016/j.clbc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils Dependence on the myeloperoxidase system. J Immunol. 1987;139(7):2406–2413. [PubMed] [Google Scholar]
- 40.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratesi A, Tarantini F, Di Bari M. Skeletal muscle: an endocrine organ. Clin Cases Miner Bone Metab. 2013;10(1):11–14. doi: 10.11138/ccmbm/2013.10.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
According to our institution’s regulation, the original data will not be shared.