Abstract
Melanins represent virulence factors for several pathogenic fungi; the number of examples is growing. Thus, albino mutants of several genera (in one case, mutated precisely in the melanizing enzyme) exhibit decreased virulence in mice. We consider the phenomenon in relation to known chemical properties of melanin, beginning with biosynthesis from ortho-hydroquinone precursors which, when oxidized enzymatically to quinones, polymerize spontaneously to melanin. It follows that melanizing intermediates are cross-linking reagents; melanization stabilizes the external cell wall against hydrolysis and is thought to determine semipermeability in the osmotic ram (the appressorium) of certain plant pathogens. Polymeric melanins undergo reversible oxidation-reduction reactions between cell wall-penetrating quinone and hydroquinone oxidation states and thus represent polymeric redox buffers; using strong oxidants, it is possible to titrate the melanin on living cells and thereby demonstrate protection conferred by melanin in several species. The amount of buffering per cell approximately neutralizes the amount of oxidant generated by a single macrophage. Moreover, the intermediate oxidation state, the semiquinone, is a very stable free radical and is thought to trap unpaired electrons. We have suggested that the oxidation state of external melanin may be regulated by external Fe(II). An independent hypothesis holds that in Cryptococcus neoformans, an important function of the melanizing enzyme (apart from melanization) is the oxidation of Fe(II) to Fe(III), thereby forestalling generation of the harmful hydroxyl radical from H2O2. Thus, problems in fungal pathogenesis have led to evolving hypotheses regarding melanin functioning.
In an era of exuberant development of informational biological macromolecules, it seems anachronistic to discuss polymers as primitively and randomly formed as the melanins. Yet, the properties of melanins provide explanations of defensive and aggressive functions in microbes. Moreover, melanins, rather than being only simple sunshades, have the properties of semiconductors (20) and are essential for hearing and sight in higher animals. In this review I will emphasize the chemical and physicochemical roles played by melanin in the pathogenesis of various fungal infections. The reader is referred to prior reviews for discussions of the biosynthesis and distribution of fungal melanins (4, 90), the role of melanin in plant pathogens (18a), the physicochemical properties of melanins (61, 62, 76) and a recent, excellent general overview of the synthesis and functions of fungal melanins (6).
DEFINITION OF MELANIN
Melanins are pigments of high molecular weight formed by oxidative polymerization of phenolic compounds and usually are dark brown or black. They are widely distributed in the living world. In general, they are conjugated polymers of ortho-dihydroxyphenols. The individual residues of polymeric melanin likewise contain two ortho oxygens. Melanins are among the most stable, insoluble, and resistant of biochemical materials; a typical melanin isolation involves extraction of lipids with various solvents and digestion of all other biopolymers by refluxing in strong acid (56). Most fungal melanins are derived from the precursor molecule 1,8-dihydroxynaphthalene (DHN) and are known as DHN-melanins; the biosynthetic pathway which furnishes DHN has been termed the polyketide pathway and resides primarily in ascomycetes and related deuteromycetes (4). Recognized human pathogens which form melanin precursors by the polyketide pathway include Aspergillus nidulans, A. niger, Alternaria alternata, Cladosporium carionii, Exophiala jeanselmei, Fonsecaea compacta, F. pedrosoi, Hendersonula toruloidii, Phaeoannellomyces wernickii, Phialophora richardsiae, P. verrucosa, Wangiella dermatitidis, and Xylohypha bantiana (81, 89, 90). The tyrosine ring represents a second source of precursor molecules, either as such or as dihydroxyphenylalanine (DOPA), and because the oxidized product of DOPA, dopaquinone, is able to cyclize to form the 5,6-dihydroxyindole ring, tyrosine or DOPA melanin typically contains indole rings (54). Additional monomeric precursors of melanin include γ-glutaminyl-4-hydroxybenzene and catechol (90); these precursors are characteristic of basidiomycetes (4). In the actual synthesis of melanin, the precursor diphenols are destabilized by enzymatic oxidation to quinones, whereupon the oxidation products polymerize spontaneously to make melanin. The enzymes that effect the one-step oxidation of dihydroxyphenols to quinones and thereby catalyze their polymerization into melanin are called polyphenoloxidases or laccases; enzymes that effect synthesis by a two-step oxidation of tyrosine are called tyrosinases. Because the polymerization does not follow a precise pattern, as does the synthesis of most other biopolymers, a given sample of melanin contains molecules with various structures, and any diagramed structure represents an oversimplification (4). Moreover, the usual drastic conditions of melanin isolation precludes the recovery of more labile polymers which might have been associated with the melanin in vivo.
Biosynthesis of DHN-melanin via the polyketide pathway has been intensively studied in W. dermatitidis; the late stages of the pathway, in which 1,3,6,8-tetrahydroxynaphthalene is successively converted to scytalone, vermelone, and DHN, are well understood. Tricyclazole, an inhibitor of the reductive enzymes which form scytalone and vermelone, prevented the darkening of cultures. Pigment-defective mutants Mel1 and Mel2 secreted intermediates that were able to reconstitute melanization in Mel3; therefore these mutants must be blocked after the block in Mel3. Mel1, which secretes the intermediate scytalone, was shown to lack the dehydratase enzyme which converts scytalone to vermelone, the next stable intermediate in the pathway. Mel3, with the earlier metabolic block, must contain all the enzymes required for the conversion of scytalone to DHN-melanin. Mel2, which secretes DHN and a variety of prior metabolites, was shown to lack the final, DHN oxidase which polymerizes melanin (16a). The mutants could be divided into complementation groups by protoplast fusion studies. Interestingly, the fusion studies appeared to resolve certain early metabolic mutants which had not been defined biochemically (12). Recently a polyketide synthase gene, termed WdPKS1, was described in W. dermatitidis. Using primers based on conserved regions of polyketide synthase 1 from Colletotrichum lagenarium, a PCR product was obtained from genomic DNA, and transformation was carried out with a vector containing a hygromycin B resistance gene. Albino transformants were tested for site-specific integration, were rescued, and were sequenced. The deduced protein showed 60% identity to the C. lagenarium polyketide synthase 1 (B. Feng and P. J. Szaniszlo, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. F-66, p. 264, 1998).
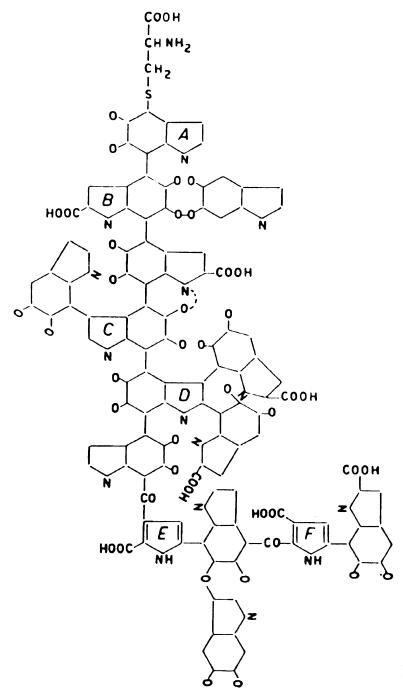
Figure 1 represents a simplified structure for squid melanin (46, 56). The structure was deduced through chromatographic and electrophoretic analysis of reductive and oxidative degradation products (47). Because of the great difficulty of such work, few melanins have been structurally analyzed, and great reliance is placed on analogies to model melanins. Thus, early criteria for provisional identification of a brown or black pigment as melanin were based on resistance to solvents and concentrated acids, solubilization and degradation by alkali, oxidation by ammoniacal silver salts, and bleaching by oxidizing agents (47, 61). To these should be added the more modern techniques of electron paramagnetic resonance (7, 74, 86) and mass spectrography (22). Cryptococcus neoformans melanins have been analyzed by oxidative and reductive degradation, and the degradation products expected for DOPA melanin and dopamine melanin have been reported (92). This was satisfying, since substrates for cryptococcal melanin are entirely exogenous and since the respective precursor had been supplied to each melanizing culture.
FIG. 1.
Structure of squid melanin proposed by Nicolaus et al. (47). Rings are aromatic. Each pair of o-oxygen substutituents can exist as either quinone, hydroquinone, or semiquinone groups.
The localization of melanin in the fungal cell wall is supported by three types of evidence. First, removal of the cell wall removes most of the dark color (12). Second, cell walls of albino mutants of W. dermatitidis (16a) and C. neoformans (38) appear hyaline in electron micrographs, whereas those of the parental wild types have an electron-dense outer layer. Feeding of scytalone to the albino culture of W. dermatitidis allowed the mutant to become melanized and reestablished the electron-dense layer. Finally, melanized cells of W. dermatitidis are more resistant to enzymatic hydrolysis than are the same strains whitened by tricyclazole or Mel− strains having metabolic blocks in melanin synthesis, indicating participation by melanin in the structure of the cell wall (12).
TANNINS AND CATECHOLS AS BIOACTIVE PRECURSORS
Tannins as Cross-Linkers
Insight into melanin function is gained by consideration of the properties and functions of plant tannins. Tannins have the chemical structure of monomeric polyhydroxyphenols (79). They are good reducing agents and oxy-radical scavengers (8, 18, 94); in this way they are thought to protect plant epithelium from the results of UV irradiation. In the sclerotization reactions of plants, polyphenol oxidases are liberated from vesicles in response to injury and, in the presence of oxygen, catalyze the two-electron oxidation of tannins to produce very reactive quinones. The quinones add to and cross-link surface proteins, forming a cuticle which tends to resist further injury (69). Cross-linking by quinones occurs typically through the mechanism of the Michael addition reaction, in which the electrophilic α, β-unsaturated quinone ring (see Fig. 3) is replaced at the β position by a nucleophilic group in a protein, such as a thiol or amine (55), to give what is called a melanoprotein. The result is blackening and hardening at a site of injury on a plant, and the reaction is familiar as the browning reaction of cut fruits. Another example of quinonic addition reactions is provided by byssal threads, the attachment organ of mussels, which produce DOPA-containing biological glues. These are oxidized to quinones to facilitate the attachment of the mollusk to a substrate (84). It follows that oxidized polyphenols are alkylating agents and potentially toxic and that production of melanin must be kept away from the cytoplasm of the cell. Thus, in fungi, melanization takes place outside the plasma membrane (90), while in vertebrates, it occurs in specialized vesicles, the melanosomes (77).
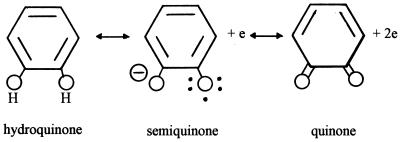
FIG. 3.
Oxidation states of the quinonic residues of melanin: the hydroquinone, the free radical semiquinone, and the quinone.
Melanin Biosynthetic Intermediates as Phytotoxins
Given the reactivity and potential toxicity of the quinonic oxidative products of melanin precursors, it might be expected that certain melanotic fungi might secrete such products in order to injure their hosts. Indeed, numerous phytotoxins derived from DHN have been described (93). One of these, alteichin, is a toxic quinone which is secreted by the fungus Alternaria eichhorniae (68).
MELANIN AS A CROSS-LINKER OF THE FUNGAL CELL WALL
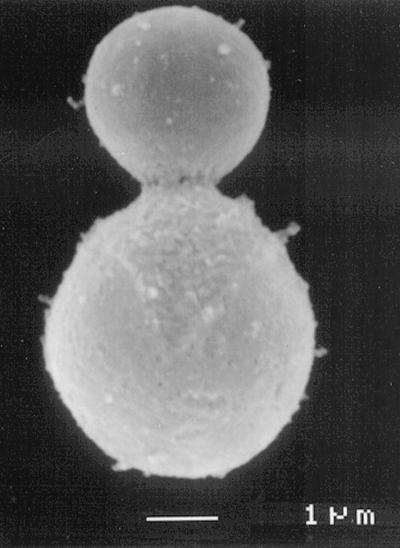
Because the extracellular precursors of melanin are cross-linking reagents, one might expect melanized cell walls to exhibit evidence of cross-linking. A striking confirmation is provided by what has been termed the “melanin ghost” phenomenon, in which melanized cells contain a visible, hydrolysis-resistant cell wall skeleton which remains after drastic hydrolysis of everything else (Fig. 2) (86). Such structures are not seen after treatment of nonmelanized cells. Supporting evidence for cross-linking is provided by the widespread finding that melanized cells are more resistant to enzymatic lysis (5, 6, 12, 36, 40, 52, 63, 90), although this phenomenon may reflect steric hindrance in addition to or instead of cross-linking.
FIG. 2.
Electron micrograph of a “melanin ghost” of C. neoformans. Organs from a mouse infected with Mel+ C. neoformans were extracted with solvents, digested in hot HCl, and centrifuged. These structures were not seen when a Mel− infection was so treated. Courtesy of Rosas et al. (For a related study, see reference 71).
RELATION OF FUNGAL MELANIN TO VIRULENCE
Correlation Studies
While not all pathogenic fungi are melanotic, there is a large class of potentially invasive fungi which have in common the production of melanin. These have been termed dematiaceous or phaeohyphomycetous fungi (66). In a study of numerous melanizing and nonmelanizing strains of Basidiobolus species, the melanizing cultures were associated with human disease (9). In a similar vein, it has been noted that the melanizing species of Cryptococcus, namely C. neoformans, is the most common cryptococcal pathogen of humans and animals; this observation led to a genetic study implicating melanin in the pathogenesis of cryptococcosis (see below) (38).
Studies of Mel− Fungal Mutants
UV-induced or spontaneous Mel− point mutants of C. neoformans have exhibited reduced virulence on several occasions. These mutants were selected on Guizotia abyssinica, l-DOPA, dopamine, or l-norepinephrine agar. A particular strain selected for study, 92t-1 (38), also exhibited an inability to grow at 37°C (it is not clear why the project was complicated by simultaneous study of the seemingly trivial temperature trait). On electron microscopy, the mutant was noted to lack an electron-dense layer in the cell wall. When this strain was sexually crossed to the wild type, 1:1 segregation of each of the parental melanization, temperature tolerance, and mating compatibility traits were observed. Three temperature-tolerant Mel− progeny were selected for virulence testing in mice and, under the conditions used, did not kill any mice, while the Mel+ progeny killed all mice inoculated. The mutant strains all exhibited defects in the uptake of l-DOPA and in phenoloxidase activity. A similar set of experiments was based on a mutant with a spontaneous Mel− mutation (65). This mutant was passed through a sexual cross, and Mel− progeny were reisolated. When these were inoculated into mice, the Mel− reisolates multiplied less well in mouse brains and exhibited less virulence than the wild type did, but they appeared still capable of killing mice; most, but not all, of the dead mice contained organisms which had reverted to Mel+ (termed MelR), but some mice died with unreverted mutants in their brains. MelR strains were considered to be isogenic to Mel− strains; when MelR and Mel− pairs were inoculated into mice, the MelR strain was consistently more virulent.
Genes governing melanization in C. neoformans were surveyed and classified by meiotic genetic analysis after the construction of inbred parents homogeneous for pigmentation. A total of 20 Mel− mutants were induced with ethyl methanesulfonate. The mutations in these mutants mapped to no less than seven loci. Four of these classes exhibited suppression (melanization) on supplementation with copper sulfate, consistent with a requirement for copper in the laccase (82a).
An attempt was made to compare the relative contributions to virulence of the melanizing and capsule-forming systems in C. neoformans, using a temperature-conditional melanin-forming trait, discovered in acapsular mutants, as though it were a Mel− mutation (39). Later evidence that melanization is decreased in many wild-type strains of C. neoformans at 37°C (28) has made it difficult to interpret the former study.
The use of random point mutations has its drawbacks. First, such mutants have a characteristic rate of reversion which can confound experiments involving large numbers of cells, such as animal inoculations and transformations. Second is the possibility of multiple mutations. This problem is not unique to random point mutation studies; however, the availability of sexual analysis allows the purification of mutant strains in the C. neoformans serotype D system. Third is the combination of nonspecific mutation and indirect causation: the physiologic basis of a random mutation often is different or more complex than envisioned.
Purification and characterization of the cryptococcal phenoloxidase (25, 91) and cloning of its gene in C. neoformans (91) made possible molecular genetic studies of melanization in that pathogen. The enzyme was identified as a typical, multicopper laccase of 65 kDa. Since the loci of the UV-induced Mel− point mutations had not been characterized in earlier mouse experiments, virulence experiments were repeated with an albino mutant containing a known disruption of the structural gene for the laccase. The mutation was passed through several crosses to the wild type in order to remove unknown secondary mutations, after which it still caused albinism and decreased virulence. The CNLAC1 transcript could be detected in a mutant with a point mutation in this genetic locus, but no detectable melanin was made by the point mutant. Transformation of the disruption mutant with the wild-type but not the point-mutated gene led to complementation. Cloning of the laccase gene also allowed detection of the laccase gene RNA transcript in yeast isolated from infected rabbit cerebrospinal fluid, indicating that the laccase gene was expressed in the rabbit infection (72).
Mel− mutants of W. dermatitidis were also found to have reduced virulence for mice compared with the melanized wild type. Under typical conditions, the wild-type strain killed mice in 3 days while a spontaneous Mel3 mutant killed no mice during the standard observation period of 21 days. When the observation period was extended to 60 days, a number of deaths by mutant strains were recorded; just as in C. neoformans, virulence was decreased but not totally abolished by the defects in melanization (11). The UV-induced Mel1 and Mel2 mutants also exhibited reductions in virulence. Phenotype seemed as important as genotype: Me13 mutants darkened by prior feeding with the pentaketide pathway intermediate, scytalone, were more virulent, while the wild type lightened by prior treatment with tricyclazole (an inhibitor of DHN-melanin synthesis) was less virulent (60a). Thus, the outcome of the infection appeared to be influenced strongly by the initial degree of melanization of the fungal cell. UV-induced albino mutants of Aspergillus fumigatus also exhibited decreased virulence (33; see below).
Anti-Melanin Drug Treatment Study
The drug glyphosate was found to inhibit melanization in C. neoformans; when fed to mice, it improved survival in experimental cryptococcosis (44; J. D. Nosanchuk and A. Casadevall, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. F-49, p. 305, 1999). However, the significance of this therapeutic effect is not clear, since, on the one hand, the drug is thought to inhibit de novo synthesis of aromatic compounds (including precursors of melanin in organisms which synthesize them [93]) while, on the other hand, C. neoformans makes melanin only from exogenous catecholamines (34). For the sake of consistency, it would make sense if the anticryptococcal effect resulted from inhibition not of melanization but of biosynthesis of essential aromatic compounds, as is the case in plants.
Role of Melanin in Penetration of Cells
Penetration of the plant cell wall by certain plant-pathogenic fungi is accomplished by a specialized cellular apparatus, the appressorium, which develops from newly deposited spores. This structure exerts a large physical pressure on a small area of the plant cell wall, thereby punching a hole through the wall; penetration of Mylar coverslips has been cited as evidence against participation by specific hydrolytic enzymes. The pressure is generated osmotically by up to 3.22 M glycerol concentrated within the appressorial cell; appressoria of the ascomycete rice blast fungus, Magnaporthe grisea, generate up to 80 atm of pressure (5.8 MPa) when exposed to distilled water (or morning dew), while incubation of appressoria in comparably concentrated external solutions collapsed them (10). The osmotically generated pressure has been estimated indirectly by measuring the freezing-point depression of appressorial cellular fluid by observing intracellular ice crystals on a refrigerated microscope stage (24) and directly by an optical method (3). DHN-melanin is a necessary component of the functioning appressorium in M. grisea (but not in Alternaria alternata, whose appressoria are normally nonpigmented [33a]): appressoria of albino Magnaporthe mutants are abnormally permeable and morphologically abnormal and are unable to generate high pressures (23). Melanin reduces the porosity of the appressorial wall, enabling the fungal cell to retain glycerol (23, 24). Compounds which inhibit the biosynthesis of DHN-melanin, such as tricyclazole, fthalide, and pyroquilon, are used as “antipenetrant” agricultural antifungals; they do not kill plant pathogens, but they cause the development of defective appressoria lacking melanin; these appressoria are unable to penetrate the plant epithelium and establish infection. One of these chemicals, tricyclazole, inhibits melanin synthesis in a variety of phaeohyphomycetes which cause human disease (90). Development of an appressorium has not been reported to play a role in animal infections, but it represents an interesting model of melanin functioning.
Does melanin play a role in fungal penetration of animal tissues? W. dermatitidis grows through agar at a rate determined by the hardness of the gel. The melanized wild type grows faster than Mel− mutants. However, the DHN-melanin inhibitor tricyclazole slowed the wild type, while repair of the mutant block in a Mel3 strain by feeding the intermediate scytalone restored melanization and boosted the growth rate (5a). Thus, melanin appears important in hyphal tip protrusion; by analogy to the appressorium, one wonders about a possible osmotic mechanism.
MELANIN AS A PHYSIOLOGIC REDOX BUFFER
Electronic Properties of Melanin
A survey of the paramagnetic properties of biological materials identified the strong electron spin resonance signal given by melanized tissues (especially frog eggs) as evidence of stabilized free radicals in biological systems; it was suggested that melanin represented a trap for unpaired electrons (7). This phenomenon was interpreted as evidence for three oxidation states of the quinonic residues of melanin: a hydroquinone (the fully reduced state), a semiquinone (the paramagnetic free radical, oxidation product), and a quinone (the two-electron oxidation product) (Fig. 3). Semiquinones are known to be stable free radicals, and since polymeric melanin contains a widely conjugated aromatic structure, it was suggested that the energy of the free-radical semiquinone of melanin was further reduced by the possibility of resonance (45). Thus, melanin might function as a sink for potentially harmful unpaired electrons.
Melanin as a Redox Buffer
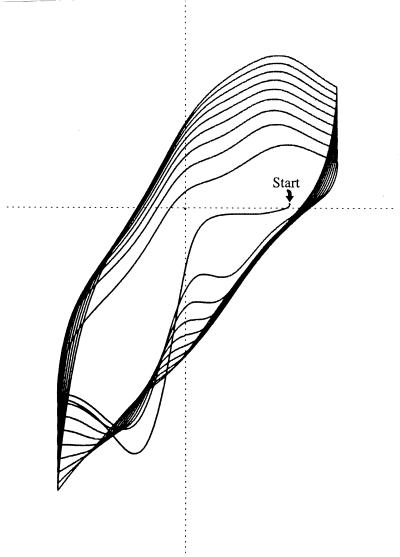
The multiple oxidation states predicted for melanin were confirmed by demonstrating electrochemical reduction of melanin with Ti(III) and oxidation with Fe(III) or oxygen (21). Melanin was shown to catalyze the reduction of Fe(III) by NADH, thereby showing that melanin could act as a mediator of electron exchanges (16). The participation of the free radical semiquinone as an intermediate stage in oxidation-reduction reactions was shown by monitoring the electrochemical reactions of Aspergillus niger melanin by electron spin resonance. When the sample was progressively reduced, a species giving an electron spin resonance signal increased and then decreased; when the sample was reoxidized, the same phenomenon occurred (43). Horak and Weeks (22) deposited melanin onto a carbon electrode by electrochemically oxidizing a solution of 5,6-dihydroxyindole (a precursor of dopamine-melanin); they monitored the electrochemical properties of the resulting synthetic melanin film by cyclic voltammetry. The voltammetric tracing (Fig. 4) demonstrated incremental increases in redox buffering capacity resulting from incremental increases in the thickness of the melanin film and can be considered to represent redox buffering by melanin “in real time.”
FIG. 4.
Redox buffering by melanin. The electrochemical potential of an inert electrode was rhythmically cycled between positive and negative (indicated on horizontal axis) in the presence of the dissolved melanin precursor, 5,6-dihydroxyindole. The precursor was incrementally oxidized and melanin was incrementally precipitated with each positive sweep. Current to and from the electrode was recorded on the vertical axis. Because voltage varied linearly with time, the horizontal “voltage axis” is also a time axis. Since charge equals current times time and is proportional to area, the increasing area circumscribed indicates that buffer capacity increases with melanin film thickness (22). Reprinted from reference 29.
Demonstration of Physiologic Redox Buffering
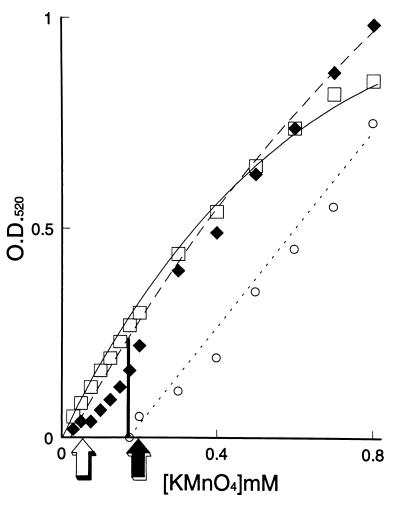
Jacobson and Emery noted albinism, decreased polyphenol oxidase activity, and decreased catechol uptake in mutants of C. neoformans selected for sensitivity to hyperbaric oxygen (27). They took the results as support for biological redox buffering by catechols, melanin, or both. However, only polymeric melanin, but not various tannins or catechols, protected C. neoformans against hypochlorite: the minimal fungicidal concentration for melanized cells was five- to sixfold higher than that for nonmelanized cells (32). Melanin on washed, melanized cells was titrated with potassium permanganate in vivo. Nonmelanized cells gave a plot identical to that of buffer (Fig. 5). Melanized cells gave a plot shifted to the right, indicating neutralization of permanganate by melanin. Mel− mutants grown in dopamine gave plots identical to those of wild-type cells lacking dopamine, but the addition of purified melanin to such suspensions restored the ability to neutralize permanganate. The equivalence points in these titrations were also the minimal fungicidal concentrations of permanganate, which were severalfold higher for melanized cells. Protection by melanin was a quantitative phenomenon; reconstitution of nonmelanized cells required 25 to 50 μg of purified melanin per ml of suspension. Biosynthesis of this amount of melanin required 5 days of culture of the wild type in 1 mM dopamine hydrochloride, resulting in a liquid culture that appeared almost black. The maximum reactivity of melanin with strong oxidants on a cellular basis was approximately 10 fmol per cell, roughly equal to the cellular production of hydrogen peroxide by macrophages (15). Interestingly, melanin did not protect yeast cells against hydrogen peroxide. Several explanations for this exception were offered: (i) hydrogen peroxide is only 1/100 as fungicidal as hypochlorite; (ii) while melanin is extracellular, hydrogen peroxide is neutral and diffuses readily into the cell; and (iii) hydrogen peroxide reacts with melanin only under alkaline conditions.
FIG. 5.
Permanganate titration of washed cells of C. neoformans. Absorbance at 520 nm (O.D. 520) was read after 3 h of incubation of cells with the oxidant. Squares indicate permanganate in buffer; diamonds indicate nonmelanized cells; circles indicate melanized cells. The minimal fungicidal concentrations (arrows) corresponded to the equivalence points of the redox titrations.
Melanin as Protection against Free Radicals
The electron paramagnetic resonance signal of a melanin sample was shown to increase in the presence of superoxide ions, consistent with transfer of single electrons from superoxide and their stabilization on melanin (35). Wang and Casadevall (88) studied protection against oxygen free radicals and nitrogen free radicals by melanin on live C. neoformans. The oxygen free radical was generated by a metal-catalyzed Fenton reaction in which Fe(III), H2O2, and epinephrine produced hydroxyl radical; the nitrogen free radical was generated by treating NaNO3 with acid to produce nitric oxide. Melanized cells survived approximately 10-fold better than did nonmelanized cells, and the system was proposed to model a role for melanin in virulence as protection for the pathogen against immunologically generated free radicals.
The free radical property of cryptococcal melanin was itself investigated (85). Melanized but not nonmelanized C. neoformans cells gave an electron spin resonance spectrum and transmission electron micrograph characteristic of melanin. The electron spin resonance signal was somewhat increased when melanized cells were incubated in a solution containing nitric oxide and (very slightly) increased when melanized cells were incubated in the Fenton reagent. These results were thought to demonstrate the transfer of unpaired electrons to the melanin of melanized cells. The study was extended by selection and study of a Mel− mutant with 1.5% of the wild-type phenoloxidase activity. Growth of the mutant in the melanin precursor l-DOPA increased resistance to nitric oxide but did not increase resistance to hydroxyl radical, suggesting that a very small amount of melanin might suffice to protect cells against nitric oxide but that a larger amount was required to protect against the oxygen radical-generating system.
Polacheck et al. had earlier studied fungal sensitivity to oxidants generated in very similar metal-catalyzed Fenton reagents (59). Although they had found poorly melanizing mutants of C. neoformans to be relatively sensitive and had attributed the result to inadequate melanization, the lack of exogenous catecholamine melanin precursors during the growth of cultures prior to the experiment seems to have precluded melanin synthesis, even in the wild type, since C. neoformans requires exogenous catechols for melanization (34). Indeed, the use of exponential-phase cells grown in high glucose concentration for the experiment also argues against participation of the laccase, since that enzyme is made by stationary-phase cells lacking glucose (58). Since the genetic loci of the mutations in the spontaneous albino mutants employed were not proven to be phenoloxidase genes, the experimental result may have resulted from a mutational effect only indirectly related to melanization. For instance, oxidation of Fe(II) by the laccase itself might account for the protection (41) (see “Discrepancy between melanization in vitro and in vivo” below).
Heat Resistance of Melanized Cells
Melanization of fungi has been noted to confer resistance to heating in some instances (64) but not in others (95). Exogenous l-DOPA supplied to a wild-type culture of C. neoformans allowed melanization and slightly increased resistance to heating, while the same chemical supplied to a Mel− mutant did not increase its resistance (70). The basis for the protection is not understood. It may be that the melanin traps toxic reactive oxygen free radicals which are generated from the cell membrane as a result of heating. An alternative explanation for the protective effect might relate to cross-linking of the cell wall by melanin.
Antioxidant Pigment Mutants in A. fumigatus
A rough, melanin-like, green, antioxidant surface pigment appears to participate in the pathogenesis of aspergillosis. White, pigmentless mutants induced by UV were shown to have an abnormally smooth external surface, as though the pigment were also responsible for the surface texture. The white mutants were 10- to 12-fold more sensitive to exogenous oxidants than was the wild type. Upon incubation with phagocytes, eightfold more reactive oxygen was detected in incubations with mutant conidia than in incubations with wild-type conidia, consistent with quenching of reactive oxygen compounds by pigment in the wild type. Mutant conidia were shown by electron microscopy to have been damaged more extensively than wild-type ones had. Moreover, mutant conidia were less lethal when inoculated into mice. Finally, pigment revertants were found simultaneously to regain normally rough surface texture, resistance to oxidants, suppression (or quenching) of oxidant production by phagocytes, and virulence (33).
Effect of Fungal Melanin on Oxidative Killing of Black Fungi
Melanin-lacking mutants of several species are sensitive to damage from oxidants. In Pyricularia oryzae (synonymous with Magnaportha grisea), Mel− mutants are nonpathogenic and sensitive to damage from oxygen radicals secreted by infected plant tissues (2). In the zoopathogens Wangiella dermatitidis and Alternaria alternata, the availability of albino mutants allowed an estimation of the contribution of melanin to the resistance to oxidants in fungi which normally express DHN-melanin. The studies were performed by titrating melanized and nonmelanized cells with hypochlorite or permanganate. In each case, the melanized strain neutralized more oxidant and was severalfold more resistant than was the corresponding albino strain (30). In agreement with those data, melanized strains of W. dermatitidis were found to resist oxidative killing by human neutrophils better than nonmelanized strains were. Moreover, normal strains whose melanization was blocked by growth in acidic medium were made as sensitive to oxidative killing as albino mutants, while an albino mutant melanized by feeding with the DHN pathway intermediate, scytalone, was made resistant (75). Thus, the DHN-melanin of dematiaceous fungi functions as an antioxidant.
Reciprocal Relationship between Melanin and Superoxide Dismutase
Because melanin undergoes single electronic oxidations and reductions among three stable and interconvertible-oxidation states, it might be expected to mediate single-electron exchanges between various molecules, and it has been shown to facilitate the dismutation of superoxide in vitro (17, 35, 78). Reciprocal relationships between the amount of cellular melanin and the superoxide dismutase activity have been described in the frog (17) and in C. neoformans (31), possibly suggesting that increased melanin is able to substitute and compensate for decreased superoxide dismutase by itself dismutating superoxide, but the physiologic significance of the phenomenon is not known.
INTERACTION OF MELANIN WITH METALS
Chelation versus Oxidation-Reduction
Melanin binds many transition metals (80). The utility of such binding has been variously interpreted as decreasing the concentrations of free metals, in the case of toxic metals, and creating a depot adjacent to the cell, in the case of essential metals. In its role of electron exchanger, melanin can either oxidize or reduce metals and can either facilitate or inhibit single-electron transfers, leading to free radical formation, depending on whether it binds the metal effectively. For instance, Fe(II) in the presence of the weak chelator ADP is bound by melanin, which inhibits hydroxyl radical formation from Fe(II) and H2O2. In the presence of the strong chelator, EDTA, melanin does not bind Fe(II) and does not inhibit hydroxyl radical formation. If Fe(III) is chelated by EDTA, melanin reduces but does not bind Fe(III); the resulting Fe(II) reacts with H2O2 to produce hydroxyl radicals (57). Thus, in the presence of the stronger chelator, EDTA, melanin does not bind Fe(III) effectively but does serve as a reducing agent for Fe(III). It has been proposed that melanin produced by the bacterium Azotobacter salinestris serves to sequester iron and prevent damage resulting from Fenton reactions (53). However, a Mel− mutant of the fungus Gaeumannomyces graminis did not exhibit sensitivity to cadmium or copper (15a), suggesting that melanin does not protect against transition metals in all fungi.
Physiologic Control of the Oxidation State of Melanin
If melanin functions as a fungal extracellular redox buffer, then one might expect the fungal cell to exert some active control over its oxidation state, since if the buffer system were completely passive, the melanin might be permanently oxidized in the cell's first encounter with oxidants. After noting that the process of reductive iron uptake involves extracellular reduction of Fe(III) to Fe(II) and pooling of Fe(II) in extracellular fluid, we wondered whether the pool of Fe(II) might serve to reduce or rereduce melanin (29). By performing electrochemical experiments employing a melanin electrode (see above), we determined that in the reaction Fe(II) + melaninox ⇌ Fe(III) + melaninred, the equilibrium lies to the right, indicating that the hypothesis is thermodynamically feasible. The conclusion was based on two types of observations. First, exposure of melanin to Fe(II) made the electrochemical potential of the melanin film more negative; second, exposure of melanin to Fe(II) diminished the ability of the melanin film to absorb additional electrons from the voltammograph. Both of these observations were consistent with reduction of melanin by Fe(II). Moreover, the reaction between Fe(II) and melanin was not inhibited or reversed by Fe(III). As evidence that the same reaction can occur in intact, melanized C. neoformans, we noted that melanized cell suspensions absorbed less light in the presence of Fe(II) (reduced melanin absorbs less light than oxidized melanin [73]). We therefore proposed that the oxidation state of extracellular melanin may be regulated indirectly through the physiologic reduction of extracellular Fe(III) to Fe(II), which reduces melanin in turn (29, 51).
INTERACTION OF MELANIN WITH DRUGS
It is possible to find examples of indifference, apparent protection, and apparent potentiation when melanized and nonmelanized cells are exposed to various drugs. Melanin did not protect W. dermatitidis against antifungal drugs, since Mel− mutants were no more susceptible to a variety of antifungals than was the Mel+ wild type (60). However, the opposite conclusion was drawn with C. neoformans. When catechols were withheld, thereby preventing melanization, cryptococcal cultures survived a 1-h treatment with amphotericin B less well (87). The authors offered two explanations for the result: that melanin decreased cell wall permeability to amphotericin B or that melanin quenched free radicals released by cell membrane damaged by the drug. Melanized cells of C. neoformans were found to be slightly more susceptible to the calmodulin antagonist drug trifluoperazine, which is known to bind strongly to melanin (88a). The reason for the differential susceptibility is not known, but trifluperazine has been reported to be effective in the treatment of experimental murine cryptococcosis (14).
INTERACTION OF MELANIN WITH EXTRACELLULAR ENZYMES
The melanin of the apple scab pathogen, Venturia inequalis, has been noted to bind the organism's extracellular hydrolytic enzymes and to release them very slowly. This property has been proposed to be a mechanism for localizing the pathogenic factors to the site of the infection (19). Cryptococcal melanin binds many (cryptococcal) cellular proteins and gives the yeast some protection from leukocyte microbicidal proteins, presumably by binding them before they can reach the plasma membrane of the fungus (13). Melanized fungi are also more resistant to hydrolytic enzymes, possibly because of sequestration of enzymes on melanin, possibly because of cross-linking of cell wall polysaccharides by melanin (5, 6, 36, 40, 52, 63), or possibly because of steric hindrance by melanin attached to the cell wall polysaccharides.
DISCREPANCY BETWEEN MELANIZATION IN VITRO AND IN VIVO
Visible melanization has not been noted in pathologic specimens with cryptococcosis, even though silver staining for melanin gives a positive result (37, 67). Because the silver stain is not totally specific for melanin, the presence of polymeric melanin has been questioned. This discrepancy has been approached in several ways. Nosanchuk et al. (49) selected melanin-binding variants from a phage display library and demonstrated binding to cryptococcal cells recovered from infected murine brain tissue; they also detected antimelanin antibodies in sera of infected mice and inferred that epitopes of melanin were indeed present on C. neoformans in infectious sites. Next, they showed cross-linking of the cell wall by melanin in vivo. They found that dark melanin ghosts could be recovered from infected mouse tissues after drastic solvent extraction and acid digestion if the mouse had been infected with a Mel+ strain (Fig. 2). If the mouse had been infected with a Mel− strain, melanin ghosts could not be demonstrated (71). At a minimum, it appears that the cell wall of infecting cells is cross-linked and studded by oxidized catecholamine derivatives.
The remaining weakness in the redox model of cryptococcal pathogenesis is the apparently small amount of melanin made by C. neoformans. We have shown that in several species, protection against oxidants is a quantitative phenomenon which depends on the amount of melanin in the culture (30, 32). Thus, to prove participation by melanin in pathogenesis requires more than simple detection. In light microscopy of histopathologic sections, chromomycetes contain much more visible melanin than does C. neoformans. It is possible, then, that melanin itself participates in the pathogenesis of chromomycosis more than of cryptococcosis. Admittedly, it has been argued that melanin may function as a catalytic electron exchanger rather than as an exhaustable substrate (16, 29). Nevertheless, the quantitative problem prompted the formulation of a new hypothesis.
This new hypothesis addressed the role of the laccase. Liu et al. (42) recovered cryptococcal cells from infected murine brains, noted visually that the cells lacked dark pigment, subjected them to alkaline hydrogen peroxide, and chromatographically demonstrated pyrrole-2,3,5-tricarboxylic and pyrrole-2,3-dicarboxylic acids, derivatives of oxidized 3,4-dihydroxyphenylalanine and dopamine. Although they inferred the oxidation of catecholamines, they concluded somewhat circularly that polymeric melanin was not present. However, the presence of catechol oxidative products and laccase mRNA in infected sites and the decreased virulence of laccase-negative mutants seemed clearly to implicate the laccase in pathogenesis. Liu et al. (41) considered alternative functions for the laccase, a multicopper oxidase. They noted that the homologues, ceruloplasmin (in vertebrates) and pFET3 (in Saccharomyces cerevisiae), functioned as ferrous oxidases and suggested that the cryptococcal laccase might have the same pathogenetic function [oxidizing Fe(II) to Fe(III)]. They reasoned that since Fe(II) can donate an electron to immunologically generated H2O2 to produce the very toxic hydroxyl radical, interception and oxidation of Fe(II) ions by the fungal laccase might enable the pathogen to escape killing by phagocytes. In support of their hypothesis, they demonstrated that recombinant cryptococcal laccase catalyzes air oxidation of Fe(II). They confirmed that killing of C. neoformans by mouse alveolar macrophages in vitro is increased by the addition of superoxide dismutase or Fe(II) and decreased by the addition of mannitol [as would be predicted from a mechanism of killing based on generation of hydroxyl radicals from immunologically generated H2O2 and Fe(II)]. Finally, they noted that the laccase-containing wild type was always somewhat more resistant to mouse alveolar macrophages than was a laccase-deleted strain, even though the cultures were not allowed to make melanin (41). The hypothesis is interesting and sophisticated for several reasons. It suggests an independent pathogenic function, not dependent on melanin synthesis, for the laccases of pathogenic fungi; it hypothesizes an oxidative defense against strong oxidants as an alternative or a supplement to the more obvious notion of reductive neutralization; and it proposes a counterforce to the active membrane-bound ferric reductase of C. neoformans (51). Moreover, the hypothesis is consistent with current notions of Fenton reaction chemistry. While the cell membrane ferric reductase, by making a pool of extracellular Fe(II) (29), might be imagined to make the fungus more susceptible to H2O2, the laccase might decrease the susceptibility to H2O2 by oxidizing Fe(II) to Fe(III). Inasmuch as the reductase is made during exponential growth (51) whereas the laccase is made during stationary phase (32, 58), a futile cycle is not necessarily implied. Perhaps the ferroxidative laccase hypothesis (41) may be considered somewhat parallel to the ferroxidative melanin hypothesis, in which Fe(II) is hypothesized to react with melanin to give Fe(III) and reduced melanin (29). It must be admitted that the data on laccase functioning are not yet compelling, since the experimental effects have been relatively small. Additional confirmatory data would be desirable.
UV PROTECTION BY MELANIN
Although UV resistance would seem to have no direct relation to the host-parasite relationship, melanized strains of fungi are more resistant than nonmelanized strains to UV irradiation (6a, 12, 13a, 84a). It follows that, when aerosolized in sunlight, melanized spores of any species might be more infectious.
REGULATION OF MELANIN PRODUCTION IN C. NEOFORMANS
Given that melanin functions as an antioxidant, it is curious that the melanizing enzyme, the laccase, of C. neoformans is not induced by hyperbaric oxygen or H2O2 (our unpublished data); rather, it is made in response to glucose starvation (50, 58). Interestingly, a cryptococcal GPA1 gene cloned to study cryptococcal pheromone receptors was found to respond more strongly to starvation conditions (82); Alspaugh et al. (1) imagined it to be an environmental sensory receptor related to nutrition. They then disrupted the gene and examined mating and melanin synthesis. The deletion mutant was deficient in both. Moreover, it was also deficient in capsular synthesis (which depends on low Fe concentrations [83]) and less virulent in the rabbit meningitis model of virulence. When putative intracellular messenger for GPA1, cyclicAMP (cAMP) was supplied, the deficiencies in mating, melanization, and capsular production were corrected. The authors suggested that in response to nutrient deprivation, the cryptococcal Gpa1 protein turns on the mating response and the two virulence factors through the mediation of cAMP.
CONCLUSION
Melanin clearly promotes infectivity in a number of species of zoopathogenic fungi. Mel− mutants of C. neoformans, W. dermatitidis, and A. fumigatus all exhibit reduced virulence in mice, which is to say that both catechol-melanins (in the first) and DHN-melanins (in the last two) participate in invasive disease. Known properties of melanin which have been proposed to explain its pathogenic effect include cross-linking or shielding of cell wall constituents against hydrolytic enzymes, sequestration of host defensive proteins, redox buffering, trapping of single electrons, dismutation of superoxide ion, a role in osmotic penetration of the (plant) cell wall by the appressorium, and a possible role in other penetrations. Given the importance of host-derived oxidants in defense against infection, a growing body of literature supports redox buffering (reductive neutralization of immunologically generated oxidants) by melanin as a pathogenic mechanism. Interestingly, a potentially antioxidant ferroxidase function, quite distinct from melanization, has been proposed for the cryptococcal laccase in pathogenesis. According to this hypothesis, the laccase itself inhibits the immunologic generation of hydroxyl radicals by oxidizing Fe(II) to Fe(III) before the reactive electron of the Fe(II) can reduce H2O2 to hydroxyl radicals and hydroxide. There is a little bit of evidence to support the trapping of single electrons by melanin in pathogenesis. Protection against hydrolytic enzymes by precursors of melanin cross-linking the cell wall (or, alternatively, by polymerized melanin covering cell wall linkages) is an additional pathogenetic mechanism for which there are considerable data. The many properties of melanin support a wide variety of functional models, and new and imaginative pathogenic hypotheses are needed.
ADDENDUM IN PROOF
The pathogen Sporothrix schenkii makes DHN melanin. UV-induced albino mutants are susceptible to oxidants, phagocytosis, and UV, but a certain mutant could be repaired by supplying the melanin precursor scytolone (R. Romero-Martinez, M. Wheeler, A. Guerrero-Plata, R. Guadelupe, and H. Torres-Guerrero, Infect. Immun. 68:3696–3703, 2000).
REFERENCES
- 1.Alspaugh J A, Perfect J R, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aver'yanov A A, Lapikova V P, Petelina G G, Dzhavakhiya V G. Increased sensitivity of pigment mutants of Pyricularia oryzae to toxic excretions of rice leaves. Fiziol Rast (Moscow) 1989;36:1088–1095. [Google Scholar]
- 3.Bechinger C, Giebel K-F, Schnell M, Leiderer P, Deising H B, Bastmeyer M. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science. 1999;285:1896–1899. doi: 10.1126/science.285.5435.1896. [DOI] [PubMed] [Google Scholar]
- 4.Bell A A, Wheeler M H. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24:411–451. [Google Scholar]
- 5.Bloomfield B J, Alexander M. Melanins and resistance to fungi to lysis. J Bacteriol. 1967;93:1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Brush L, Money N P. Invasive hyphal growth in Wangiella dermatitidis is induced by stab inoculation and shows dependence upon melanin biosynthesis. Fungal Genet Biol. 1999;28:190–200. doi: 10.1006/fgbi.1999.1176. [DOI] [PubMed] [Google Scholar]
- 6.Butler M J, Day A W. Fungal melanins: a review. Can J Microbiol. 1998;44:1115–1136. [Google Scholar]
- 6a.Butler M J, Lazarovits G, Higgins B G, Lachance M-A. Identification of a black yeast isolated from oak bark as belonging to genus Phaeococcomyces sp. Analysis of melanin produced by the yeast. Can J Microbiol. 1989;35:728–734. [Google Scholar]
- 7.Commoner B, Townsend J, Pike G E. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 8.Cotelle N, Bernier J L, Henichart J P, Catteau J P, Gaydou E, Wallet J C. Scavenger and antioxidant properties of ten synthetic flavones. Free Radical Biol Med. 1992;13:211–219. doi: 10.1016/0891-5849(92)90017-b. [DOI] [PubMed] [Google Scholar]
- 9.Cutler J E, Swatek F E. Pigment production by Basidiobolus in the presence of tyrosine. Mycologia. 1969;61:130–135. [PubMed] [Google Scholar]
- 10.De Jong J C, McCormack B J, Smirnoff N, Talbot N J. Glycerol generates turgor in rice blast. Nature. 1997;389:244–245. [Google Scholar]
- 11.Dixon D M, Polak A, Szaniszlo P J. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J Med Vet Mycol. 1987;25:97–106. doi: 10.1080/02681218780000141. [DOI] [PubMed] [Google Scholar]
- 12.Dixon D M, Szaniszlo P J, Polak A. Dihydroxynaphthalene (DHN) melanin and its relationship with virulence in the early stages of phaeohyphomycosis. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 297–318. [Google Scholar]
- 13.Doering T L, Nosanchuk J D, Roberts W K, Casadevall A. Melanin as a potential cryptococcal defense against microbicidal proteins. Med Mycol. 1999;37:175–181. [PubMed] [Google Scholar]
- 13a.Durrell L W. The composition and structure of walls of dark fungus spores. Mycopathol Mycol Appl. 1964;23:339–345. doi: 10.1007/BF02049005. [DOI] [PubMed] [Google Scholar]
- 14.Eilam Y, Polacheck I, Ben-Gigi G, Chernichovsky D. Activity of phenothiazines against medically important yeasts. Antimicrob Agents Chemother. 1987;31:834–836. doi: 10.1128/aac.31.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fels A O, Nathan C F, Cohn Z A. Hydrogen peroxide release by alveolar macrophages from sarcoid patients and by alveolar macrophages from normals after exposure to recombinant interferons αA, β and γ and 1,25-dihydroxyvitamin D3. J Clin Investig. 1987;80:381–386. doi: 10.1172/JCI113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Frederick B A, Caesar Tonthat T-C, Wheeler M H, Sheehan K B, Edens W A, Henson J M. Isolation and characterization of Gaeumannomyces graminis var. graminis melanin mutants. Mycol Res. 1999;103:99–110. [Google Scholar]
- 16.Gan E V, Haberman H F, Menon I A. Electron-transfer properties of melanin. Arch Biochem Biophys. 1976;173:666–672. doi: 10.1016/0003-9861(76)90304-0. [DOI] [PubMed] [Google Scholar]
- 16a.Geis P A, Wheeler M H, Szaniszlo P J. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol. 1984;137:324–328. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- 17.Geremia E, Corsaro C, Bonomo R, Giardinelli R, Pappalardo P, Vanella A, Sichel G. Eumelanins as free radicals trap and superoxide dismutase activities in amphhibia. Comp Biochem Physiol Ser B. 1984;79:67–69. [Google Scholar]
- 18.Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:2016–2021. [Google Scholar]
- 18a.Henson J M, Butler M J, Day A W. The dark side of the mycelium: melanins of pathogenic fungi. Annu Rev Phytopathol. 1999;37:447–471. doi: 10.1146/annurev.phyto.37.1.447. [DOI] [PubMed] [Google Scholar]
- 19.Hignett R C, Kirkham D S. The role of extracellular melanoproteins of Venturia inequalis in host susceptibility. J Gen Microbiol. 1967;48:269–275. doi: 10.1099/00221287-48-2-269. [DOI] [PubMed] [Google Scholar]
- 20.Hill Z H. The function of melanin or 6 people examine an elephant. Bioessays. 1992;14:49–56. doi: 10.1002/bies.950140111. [DOI] [PubMed] [Google Scholar]
- 21.Horak V, Gillette J R. A study of the oxidation-reduction state of synthetic 3,4-dihydroxy-dl-phenylalanine melanin. Mol Pharmacol. 1971;7:429–433. [PubMed] [Google Scholar]
- 22.Horak V, Weeks G. Poly(5,6-dihydroxyindole) melanin film electrode. Bioorg Chem. 1993;21:24–33. [Google Scholar]
- 23.Howard R J, Ferrari M A. Role of melanin in appressorium function. Exp Mycol. 1989;13:403–418. [Google Scholar]
- 24.Howard R J, Ferrari M A, Roach D H, Money N P. Penetration of hard substances by a fungus employing enormous turgor pressures. Proc Natl Acad Sci USA. 1991;88:11281–11284. doi: 10.1073/pnas.88.24.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda R, Shinoda T, Morita T, Jacobson E S. Characterization of a phenoloxidase from Cryptococcus neoformans var. neoformans. Microbiol Immunol. 1993;37:759–764. doi: 10.1111/j.1348-0421.1993.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 26.Ito S, Wakamatsu K. Chemical degradation of melanins: applications to identification of dopamine melanin. Pigment Cell Res. 1998;11:120–126. doi: 10.1111/j.1600-0749.1998.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson E S, Emery H S. Catecholamine uptake, melanization and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson E S, Emery H S. Temperature regulation of the cryptococcal phenoloxidase. J Med Vet Mycol. 1991;29:121–124. doi: 10.1080/02681219180000201. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson E S, Hong J D. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J Bacteriol. 1997;179:5340–5346. doi: 10.1128/jb.179.17.5340-5346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson E S, Hove E, Emery H S. Antioxidant function of melanin in black fungi. Infect Immun. 1995;63:4944–4945. doi: 10.1128/iai.63.12.4944-4945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson E S, Jenkins N D, Todd J M. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994;62:4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson E S, Tinnell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage A A. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun. 1997;65:5110–5117. doi: 10.1128/iai.65.12.5110-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Kimura N, Tsuge T. Gene cluster involved in melanin biosynthesis of the filamentous fungus Alternaria alternata. J Bacteriol. 1993;175:4427–4435. doi: 10.1128/jb.175.14.4427-4435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korth H, Pulverer G. Pigment formation for differentiating Cryptococcus neoformans from Candida albicans. Appl Microbiol. 1971;21:541–542. doi: 10.1128/am.21.3.541-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korytowsski W, Kalyanaraman B, Menon I A, Sarna T, Sealy R C. Reaction of superoxide anions with melanins: electron spin resonance and spin trapping studies. Biochim Biophys Acta. 1986;882:145–153. doi: 10.1016/0304-4165(86)90149-2. [DOI] [PubMed] [Google Scholar]
- 36.Kuo M-J, Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967;94:624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon-Chung K J, Hill W B, Bennett J E. New special stain for histopathologic diagnosis of cryptococcosis. J Clin Microbiol. 1981;13:383–387. doi: 10.1128/jcm.13.2.383-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon-Chung KJ, Polacheck I. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindermann R G, Toussoun T A. Behaviour of albino chlamydospores of Thielaviopsis basicola. Phytopathology. 1966;56:887. [PubMed] [Google Scholar]
- 41.Liu L, Tewari R P, Williamson P R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun. 1999;67:6034–6039. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Wakamatsu K, Ito S, Williamson P R. Catecholamine oxidative products, but not melanin, are produced by Cryptococcus neoformans during neuropathogenesis in mice. Infect Immun. 1999;67:108–112. doi: 10.1128/iai.67.1.108-112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukiewicz S, Reszka K, Matusak Z. Simultaneous electrochemical-electron spin resonance (SEESR) studies on natural and synthetic melanins. Bioelectrochem Bioenerg. 1980;7:153–165. [Google Scholar]
- 44.Malik J, Barry G, Kishore G. The herbicide glyphosate. Biofactors. 1989;2:17–25. [PubMed] [Google Scholar]
- 45.Mason H S, Ingram D J E, Allen B. The free radical property of melanins. Arch Biochem Biophys. 1960;86:225–230. doi: 10.1016/0003-9861(60)90409-4. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaus R A. Melanins. Paris, France: Hermann; 1968. [Google Scholar]
- 47.Nicolaus R A, Piatelli M, Fattoruuso E. On some natural melanins. Tetrahedron. 1964;20:1163–1172. doi: 10.1016/s0040-4020(01)98983-5. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted
- 49.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nurudeen T A, Ahearn D G. Regulation of melanin production by Cryptococcus neoformans. J Clin Microbiol. 1979;10:724–729. doi: 10.1128/jcm.10.5.724-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyhus K J, Wilborn A T, Jacobson E S. Ferric iron reduction by Cryptococcus neoformans. Infect Immun. 1997;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Old K M, Robertson W M. Effects of lytic enzymes and natural soil on the fine structure of conidia of Cochliobolus sativus. Trans Br Mycol Soc. 1970;54:343–350. [Google Scholar]
- 53.Page W J, Shivprasad S. Iron binding to Azotobacter salinestris melanin, iron mobilization and uptake mediated by siderophores. BioMetals. 1995;8:59–64. doi: 10.1007/BF00156159. [DOI] [PubMed] [Google Scholar]
- 54.Pawelek J M, Korner M M. The biosynthesis of mammalian melanin. Am Sci. 1982;70:136–145. [PubMed] [Google Scholar]
- 55.Peter M G. Chemical modifications of biopolymers by quinones and quinone methides. Angew Chem Int Ed Engl. 1989;28:555–570. [Google Scholar]
- 56.Piattelli M, Fattorusso E, Nicolaus R A, Magno S. The structure of melanins and melanogenesis. V. Ustilago melanin. Tetrahedron. 1965;21:3229–3236. doi: 10.1016/0040-4020(63)85021-8. [DOI] [PubMed] [Google Scholar]
- 57.Pilas B, Sarna T, Kalyanaraman B, Swartz H M. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radical Biol Med. 1988;4:285–293. doi: 10.1016/0891-5849(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 58.Polacheck I, Hearing V J, Kwon-Chung K J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982;150:1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polacheck I, Platt Y, Aronovitch J. Catecholamines and virulence of Cryptococcus neoformans. Infect Immun. 1990;58:2919–2922. doi: 10.1128/iai.58.9.2919-2922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polak A, Dixon D M. Loss of melanin in Wangiella dermatitidis does not result in greater susceptibility to antifungal agents. Antimicrob Agents Chemother. 1989;33:1639–1640. doi: 10.1128/aac.33.9.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60a.Polak A, Dixon D M. Pathogenicity of Wangiella dermatitidis. In: Vanden Bossche H, Kerridge D, Odds F, editors. Dimorphic fungi in biology and medicine. London, United Kingdom: Plenum Press; 1993. pp. 307–312. [Google Scholar]
- 61.Prota G. Melanins and melanogenesis. San Diego, Calif: Academic Press, Inc.; 1992. [Google Scholar]
- 62.Prota G, D'Ischia M, Napolitano A. The chemistry of melanins and related metabolites. In: Nordlund J J, Boissy R E, Hearing V J, King R A, Ortonne J-P, editors. The pigmentary system. Oxford, United Kingdom: Oxford University Press; 1998. pp. 307–332. [Google Scholar]
- 63.Ray R M, Desai J D. Effect of melanin on enzymatic hydrolysis of cellulosic waste. Biotechnol Bioeng. 1984;24:699–701. doi: 10.1002/bit.260260711. [DOI] [PubMed] [Google Scholar]
- 64.Rehnstrom A L, Free S J. The isolation and characterization of melanin-deficient mutants of Monilinia frùcticola. Physiol Mol Plant Pathol. 1997;49:321–330. [Google Scholar]
- 65.Rhodes J C, Polacheck I, Kwon-Chung K J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rippon J W. Medical mycology. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1982. [Google Scholar]
- 67.Ro J Y, Lee S S, Ayala A G. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53–57. [PubMed] [Google Scholar]
- 68.Robeson D, Strobel G, Matsumoto G K, Fisher E L, Chen M H, Clardy J. Alteichin: an unusual phytotoxin from Alternaria eichorniae, a fungal pathogen of water hyacinth. Experientia. 1984;40:1248–1250. doi: 10.1007/BF01946657. [DOI] [PubMed] [Google Scholar]
- 69.Rohringer R, Samborski D J. Aromatic compounds in the host-parasite interaction. Annu Rev Phytopathol. 1967;5:77–86. [Google Scholar]
- 70.Rosas A L, Casadevall A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett. 1997;153:265–272. doi: 10.1111/j.1574-6968.1997.tb12584.x. [DOI] [PubMed] [Google Scholar]
- 71.Rosas A L, Nosanchuk J D, Feldmesser M, Cox G M, McDade H C, Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun. 2000;68:2845–2853. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarna T, Pilas B, Land E J, Truscott T G. Interaction of radicals from water radiolysis with melanin. Biochem Biophys Acta. 1986;883:162–167. doi: 10.1016/0304-4165(86)90147-9. [DOI] [PubMed] [Google Scholar]
- 74.Sarna T, Swartz H M. Identification and characterization of melanin in tissues and body fluids. Folia Histochem Cytochem. 1978;16:275–286. [PubMed] [Google Scholar]
- 75.Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zuendorf J, Lluetticken R, Haase G. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect Immun. 1999;67:94–101. doi: 10.1128/iai.67.1.94-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sealy R C, Felix C C, Hyde J S, Swartz H M. Structure and reactivity of melanins: influence of free radicals and metal ions. In: Prior W A, editor. Free radicals in biology. IV. New York, N.Y: Academic Press, Inc.; 1980. pp. 209–259. [Google Scholar]
- 77.Seiji M, Fitzpatrick T B, Birbeck M S C. The melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J Investig Dermatol. 1961;36:243–252. doi: 10.1038/jid.1961.42. [DOI] [PubMed] [Google Scholar]
- 78.Sichel G, Corsaro C, Scalia M, Di Bilio A J, Bonomo R P. In vitro scavenger activity of some flavonoids and melanins against O2−·. Free Radical Biol Med. 1991;10:1–8. doi: 10.1016/0891-5849(91)90181-2. [DOI] [PubMed] [Google Scholar]
- 79.Swain T. Secondary compounds as protective agents. Annu Rev Plant Physiol. 1977;28:479–501. [Google Scholar]
- 80.Swartz H M, et al. Modulation by neuromelanin of the availability and reactivity of metal ions. Ann Neurol. 1992;32:S69–S75. doi: 10.1002/ana.410320712. [DOI] [PubMed] [Google Scholar]
- 81.Taylor B E, Wheeler M H, Szaniszlo P J. Evidence for pentaketide melanin biosynthesis in dematiaceous human pathogenic fungi. Mycologia. 1987;79:320–322. [Google Scholar]
- 82.Tolkacheva T, McNamara P, Piekarz E, Courchesne W. Cloning of a Cryptococcus neoformans gene, GPA1, encoding a G-protein a-subunit homolog. Infect Immun. 1994;62:2849–2856. doi: 10.1128/iai.62.7.2849-2856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82a.Torres-Guererro H, Edman J C. Melanin-deficient mutants of Cryptococcus neoformans. J Med Vet Mycol. 1994;32:303–313. [PubMed] [Google Scholar]
- 83.Vartivarian S E, Anaissie E J, Cowart R E, Sprigg H A, Tingler M J, Jacobson E S. Regulation of cryptococcal polysaccharide by iron. J Infect Dis. 1993;167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- 84.Waite JH, Hansen DC, Little KT. The glue protein of ribbed mussels (Geukensia demissa): a natural adhesive with some features of collagen. J Comp Physiol Ser B. 1989;159:517–525. doi: 10.1007/BF00694376. [DOI] [PubMed] [Google Scholar]
- 84a.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol. 1995;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Aisen P, Casadevall A. Melanin, melanin “ghosts” and melanin composition in Cryptococcus neoformans. Infect Immun. 1996;64:2420–2424. doi: 10.1128/iai.64.7.2420-2424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Casadevall A. Growth of Cryptococcus neoformans in presence of l-DOPA decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother. 1994;38:2648–2650. doi: 10.1128/aac.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88a.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob Agents Chemother. 1996;40:541–545. doi: 10.1128/aac.40.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wheeler M H. Comparisons of fungal melanin biosynthesis in ascomycetous, imperfect and basidiomycetous fungi. Trans Br Mycol Soc. 1983;81:29–36. [Google Scholar]
- 90.Wheeler M H, Bell A A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]
- 91.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williamson P R, Wakamatsu K, Ito S. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998;180:1570–1572. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoder O C, Turgeon B G. Molecular-genetic evaluation of fungal molecules for roles in pathogenesis to plants. J Genet. 1996;75:425–440. [Google Scholar]
- 94.Yoshida T, et al. Studies on inhibition mechanism of autoxidation by tannins and flavononids. V. Radical-scavenging effects of tannins and related polyphenols on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:1919–1921. [Google Scholar]
- 95.Zhdanova N N, Melezhik A V, Vasilevskaya A I. Thermostability of some melanin containing fungi. Biol Bull Acad Sci USSR. 1980;7:305–310. [PubMed] [Google Scholar]