Purpose:
The purpose of this study was to evaluate the efficacy of topical ivermectin 1% cream application on the eyelashes in combination with eyelid hygiene in the treatment of Demodex blepharitis.
Methods:
One hundred two eyes of 102 patients with symptomatic Demodex blepharitis were divided into 2 groups according to the use of topical ivermectin in this retrospective case–control study. The ivermectin group (n = 51) applied topical ivermectin 1% cream on the eyelashes for 15 minutes once weekly, but the control group (n = 51) did not. In both groups, eyelid hygiene was performed once daily. The Standard Patient Evaluation of Eye Dryness (SPEED) symptom questionnaire score, Oxford staining score, eyelid debris, eyelid redness/swelling, and telangiectasia were assessed during the follow-up visits.
Results:
The mean follow-up periods of the ivermectin and control groups were 15.1 ± 9.7 weeks and 14.8 ± 8.6 weeks, respectively. The SPEED score and eyelid debris grade were significantly improved in both groups during the follow-up, although the SPEED score and eyelid debris grade showed greater changes in the ivermectin group than in the control group. The Oxford staining score, eyelid redness/swelling grade, and telangiectasia grade were significantly improved only in the ivermectin group but not in the control group.
Conclusions:
In patients with Demodex blepharitis, the use of topical ivermectin 1% cream for 15 minutes once weekly in addition to eyelid hygiene had more significantly improved symptoms, ocular surface staining, eyelid debris, redness/swelling, and telangiectasia as compared with eyelid hygiene alone. These findings support the efficacy of topical ivermectin 1% cream application in the treatment of Demodex blepharitis.
Key Words: Demodex, demodicosis, ivermectin, blepharitis, eyelid hygiene
Demodex species are the most common ectoparasite living in or near hair follicles in humans and feed on skin cells and the sebum that accumulates in the hair follicles. 1–4 Demodex is one of the causes of chronic skin diseases such as rosacea, and in ophthalmic areas, it causes chronic blepharitis, conjunctivitis, corneal inflammation, and meibomian gland dysfunction. 5–7 For the treatment of Demodex blepharitis, tea tree oil (TTO) can be applied to the eyelashes or eyelid hygiene can be performed. 8,9 However, eyelid scrubs containing TTO often cause irritation and allergic reactions, and patient compliance is poor because of the inconvenience of use, 8 although it reduces the number of Demodex organisms and improves the ocular surface disease index in patients with Demodex blepharitis. 9
Ivermectin is a broad-spectrum antiparasite drug first derived from Streptomyces avermitilis by Satoshi Omura and William C. Campbell in the 1970s. 10 Oral ivermectin is well known for its effect in reducing the number of Demodex organisms and improving tear film stability. 11–13 A previous study reported that topical application of ivermectin–metronidazole gel ointment to the eyelashes at 15-day intervals in Demodex blepharitis effectively reduced the number of Demodex and severity of eyelid inflammation without side effects. 14 We hypothesized that applying commercially available topical ivermectin cream alone to the eyelashes could effectively treat Demodex blepharitis. To verify this hypothesis, this study investigated the efficacy of topical ivermectin cream in Demodex blepharitis by comparing the application of topical ivermectin cream to the eyelashes once weekly for 15 minutes in combination with daily eyelid hygiene with daily eyelid hygiene alone.
PATIENTS AND METHODS
This retrospective case–control study was conducted adhering to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB No. 2020AS0289). The Ethics Committee approved a waiver of informed consent because of the retrospective collection of existing data for this study, and the data used in this study were deidentified for the sake of participants' privacy.
Study Population
This study included 102 eyes from 102 patients diagnosed with symptomatic Demodex blepharitis who participated in the follow-up after treatment for at least 4 weeks at Korea University Ansan Hospital, Gyeonggi-do, Republic of Korea between November 1, 2019, and August 30, 2020. Through a retrospective review of medical records, 51 patients met the inclusion/exclusion criteria and were treated with topical ivermectin 1% cream (Soolantra; Galderma, Lausanne, Switzerland) and were suggested to maintain eyelid hygiene using an eyelid cleansing product that contains TTO (Oust Demodex cleanser; OCuSOFT, Rosenberg, TX) during the study period (ivermectin group). They were matched according to age (±4 yrs) and sex at a ratio of 1:1 to 51 patients with Demodex blepharitis who were not treated with topical ivermectin 1% cream but instead with eyelid hygiene (control group). Demodex blepharitis was diagnosed when all 4 of the following conditions were met 15,16 : 1) when cylindrical dandruff was observed at the eyelashes; 2) when ocular discomfort related to blepharitis, such as itching and irritation, was present 17 ; 3) when all 3 anterior blepharitis signs of eyelid debris, eyelid redness/swelling, and eyelid telangiectasia had a grade of mild or higher in both eyes 18–21 ; and 4) when 4 or more mites were observed on 1 eyelash during the observation of a total of 8 eyelashes under a microscope by pulling out 2 eyelashes from each eyelid (Fig. 1). 9,15,22 The inclusion criteria were as follows: 1) patients who were previously treated with eye drops for more than 1 month to relieve ocular discomfort but whose symptoms persisted and 2) patients who had no other diseases, such as aqueous-deficient dry eye and cicatricial conjunctivitis, that can cause ocular surface disease and eyelid inflammation. Exclusion criteria included 1) history of active ocular infection other than demodicosis; 2) presence of uncontrolled systemic disease except for hypertension and diabetes; 3) use of contact lenses within 1 month of baseline; 4) history of ocular surgery or ocular trauma within 3 months of baseline; 5) systemic use of a corticosteroid, cyclosporine, tetracycline derivatives, antihistamine, or isotretinoin; 6) presence of eyelid disease or structural abnormality other than Demodex blepharitis; and 7) age younger than 20 years. The study eye selection criteria were as follows: 1) if both eyes met the diagnostic criteria for Demodex blepharitis, the eye with the higher eyelid debris grade (cylindrical dandruff) was selected or 2) if both eyes had the same eyelid debris grade, the right eye was selected.
FIGURE 1.
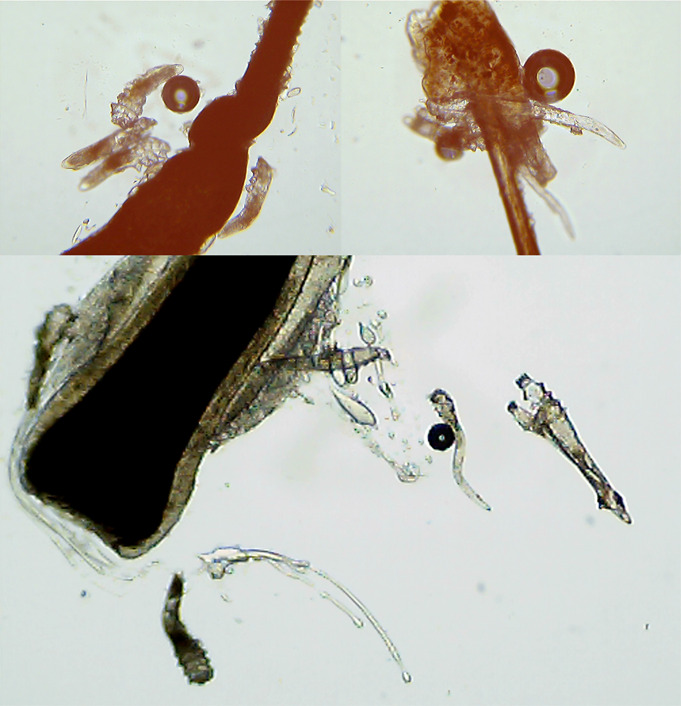
Confirmation of the presence of Demodex. Demodex was observed under a microscope by pulling eyelashes from eyelids.
Study Protocol
The ivermectin group applied topical ivermectin 1% cream (Soolantra) to the upper and lower eyelashes once weekly using a cotton swab and then carefully wiped it with an eyelid cleanser 15 minutes later. The participants were instructed to be careful not to let the cream enter the eye or make contact with the conjunctival mucosa when applied to the eyelashes. Eyelid hygiene was performed once daily using the eyelid cleansing product that contains TTO (Oust Demodex) by both the ivermectin and control groups. Both groups were prescribed hyaluronic acid and/or diquafosol eye drops depending on their symptoms of eye discomfort.
Patient Examinations
All study participants completed a medical and medication history questionnaire. Subjective symptoms, ocular surface, and eyelids were evaluated before and after the treatment for Demodex blepharitis in all patients. The Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire (0–28 points) was performed to assess the frequency and severity of ocular discomfort (0 point = best score and 28 points = worst score). The corneal and conjunctival staining grade was evaluated according to the Oxford scheme (0–5 points) after staining the ocular surface with a fluorescein strip (0 point = best score and 5 points = worst score). The eyelid debris grade for evaluating cylindrical dandruff in the eyelashes and the eyelid redness/swelling grade were all evaluated as absent, mild, moderate, severe, or very severe (0–4 points). 18–20 Eyelid telangiectasia was graded as no findings, mild, moderate, or severe (0–3 points). 21 In the ivermectin group, the occurrence of side effects from the use of topical ivermectin 1% cream on the eyelashes was evaluated.
Statistical Analyses
Statistical analyses were performed with the IBM SPSS Statistics Standard version 20 software program (IBM Corp, Armonk, NY). Age, sex, laterality, and follow-up period among the study participants were compared between the ivermectin and control groups with the Student t test and the χ2 test. Subjective symptoms (SPEED score) and objective signs (Oxford staining score, eyelid debris, eyelid redness/swelling, and telangiectasia grade) at baseline and follow-up visits were compared between the ivermectin and control groups with repeated-measures analysis of variance with Tukey's honestly significant difference post hoc test. A P value of less than 0.05 was considered to be statistically significant. A post hoc power analysis using the difference between 2 independent means (2 groups) option of the G*power version 3.1.9.6 software program (Franz Paul, Kiel, Germany) was conducted to determine study power.
RESULTS
The mean ages (±SD) of the ivermectin and control groups were 63.6 ± 12.8 years (range: 31–86 years) and 63.6 ± 12.7 years (range: 31–84 years), respectively. Each of the 2 groups included 30 women (58.8%). There were 40 right eyes (78.4%) in the ivermectin group and 34 right eyes (66.7%) in the control group. The mean follow-up periods of the ivermectin and control groups were 15.1 ± 9.7 weeks (range: 4–40 weeks) and 14.8 ± 8.6 weeks (range: 4–32 weeks), respectively. There were no significant differences in age, sex distribution, laterality, or follow-up period between the 2 groups (Table 1).
TABLE 1.
Comparison of Patient Characteristics Between the Ivermectin and Control Groups
| Ivermectin (n = 51) | Control (n = 51) | P * | |
| Age, yr, mean ± SD | 63.6 ± 12.8 | 63.6 ± 12.7 | >0.999 |
| Sex, n (%) | |||
| Male:female | 21 (41.2):30 (58.8) | 21 (41.2):30 (58.8) | >0.999† |
| Laterality, n (%) | |||
| Right eye:left eye | 40 (78.4):11 (21.6) | 34 (66.7):17 (33.3) | 0.267† |
| Follow-up period, wk, mean ± SD | 15.1 ± 9.7 | 14.8 ± 8.6 | 0.872 |
Student t test.
χ2 test.
The mean SPEED score of the ivermectin group (12.0 ± 6.4 points) was significantly greater than that of the control group (9.6 ± 4.8 points) at baseline (P = 0.037). In comparison with baseline, both the ivermectin and control groups showed significantly improved SPEED scores at their respective follow-up visits (7.4 ± 5.0 and 8.0 ± 5.1 points; P < 0.001 and P = 0.009, respectively). As such, there was no significant difference found in SPEED scores between the 2 groups at the follow-up visit (P = 0.515) (Fig. 2).
FIGURE 2.
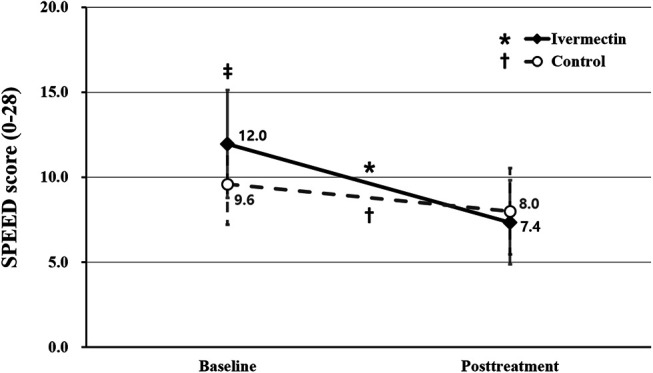
Comparison of subjective symptom scores between the ivermectin and control groups. The SPEED questionnaire was used to assess the symptom score (0–28 points). The asterisk indicates statistically significant changes in the SPEED score in the ivermectin group. The dagger indicates statistically significant changes in the SPEED score in the control group. The double dagger indicates statistically significant differences between the ivermectin and control groups. The asterisk, dagger, and double dagger indicate P < 0.05 per repeated-measures ANOVA with Tukey's HSD post hoc test. ANOVA, analysis of variance; HSD, honestly significant difference.
The mean Oxford staining scores of the ivermectin and control groups were 0.6 ± 0.8 and 0.6 ± 0.7 points, respectively, at baseline (P = 0.678). During the follow-up, the mean Oxford staining scores of the ivermectin group (0.3 ± 0.6 points) showed significant improvement relative to that recorded at baseline (P = 0.005), but the control group (0.4 ± 0.6 points) did not (P = 0.065) (Fig. 3).
FIGURE 3.
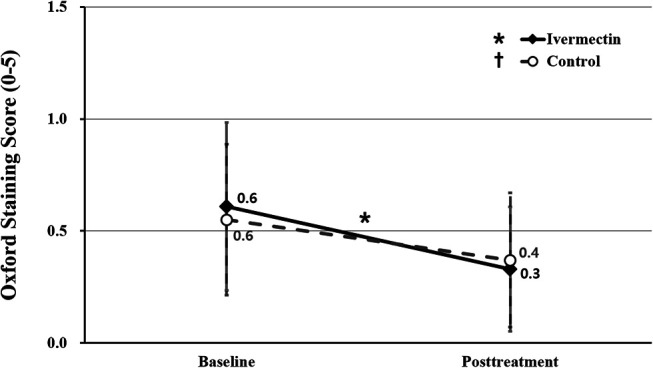
Comparison of Oxford staining scores between ivermectin and control groups. The asterisk indicates statistically significant changes in the Oxford staining score in the ivermectin group. The asterisk indicates P < 0.05 per repeated-measures ANOVA with Tukey's HSD post hoc test. ANOVA, analysis of variance; HSD, honestly significant difference.
The ivermectin group had a greater mean eyelid debris grade (2.2 ± 0.7) than the control group (2.0 ± 0.6) at baseline (P = 0.029). Both the ivermectin and control groups showed a significantly decreased eyelid debris grade at the follow-up visit compared with baseline (1.1 ± 0.7 and 1.6 ± 0.5; P < 0.001 and P = 0.001, respectively). The mean eyelid debris grade of the ivermectin group was significantly less than that of the control group during the follow-up (P < 0.001) (Figs. 4, 5).
FIGURE 4.
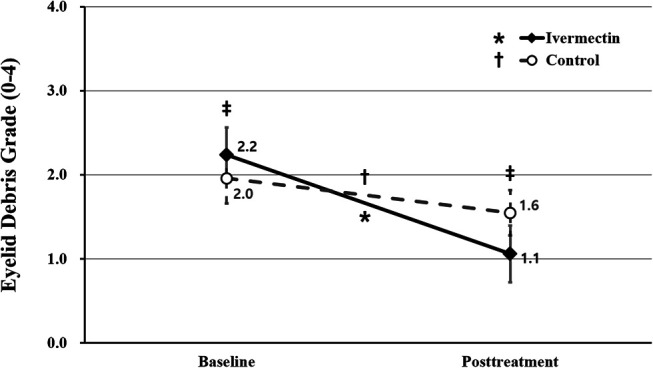
Comparison of eyelid debris grade between the ivermectin and control groups. The asterisk indicates statistically significant changes in the eyelid debris grade in the ivermectin group. The dagger indicates statistically significant changes in the eyelid debris grade in the control group. The double dagger indicates statistically significant differences between the ivermectin and control groups. The asterisk, dagger, and double dagger indicate P < 0.05 per repeated-measures ANOVA with Tukey's HSD post hoc test. ANOVA, analysis of variance; HSD, honestly significant difference.
FIGURE 5.
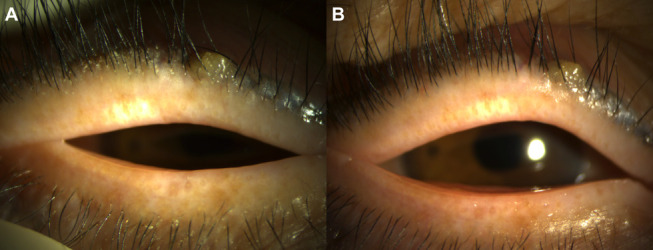
Anterior-segment photographs of the right eye of a 78-year-old man treated with a combination of topical ivermectin 1% cream and eyelid hygiene. A, Baseline and (B) post-treatment (+6 wk) photographs show improvement in eyelid debris grade.
The mean eyelid redness/swelling grade of the ivermectin group (2.2 ± 0.8 points) was greater than that of the control group (1.8 ± 0.8 points) at baseline (P = 0.014) yet became significantly decreased during the follow-up (1.6 ± 0.7 points; P < 0.001) compared with baseline, whereas there were no significant changes in the mean eyelid redness/swelling grade between baseline and follow-up (1.7 ± 0.8; P = 0.180) in the control group. As a result, there was no significant difference in eyelid redness/swelling grade between the 2 groups during the follow-up (P = 0.515) (Figs. 6, 7).
FIGURE 6.
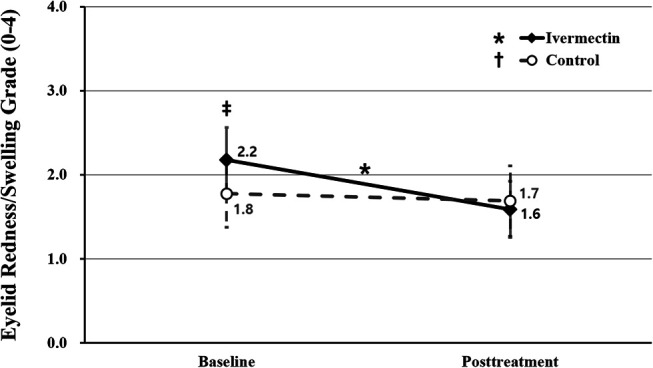
Comparison of eyelid redness/swelling grade between the ivermectin and control groups. The asterisk indicates statistically significant changes in the eyelid redness/swelling grade in the ivermectin group. The double dagger indicates statistically significant differences between the ivermectin and control groups. The asterisk and double dagger indicate P < 0.05 per repeated-measures ANOVA with Tukey's HSD post hoc test. ANOVA, analysis of variance; HSD, honestly significant difference.
FIGURE 7.
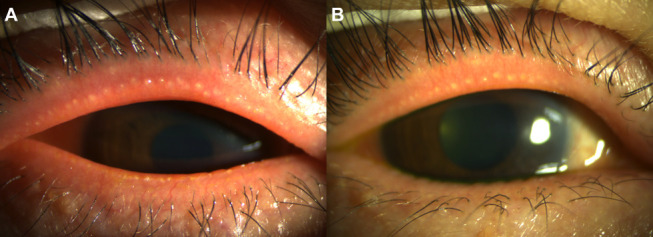
Anterior-segment photographs of the right eye of a 54-year-old woman who treated with a combination of topical ivermectin 1% cream and eyelid hygiene. A, Baseline and (B) post-treatment (+5 wk) photographs show improvement in eyelid redness/swelling and telangiectasia grade.
The mean eyelid telangiectasia grade showed a decrease from 2.4 ± 0.7 points at baseline to 2.2 ± 0.7 points during the follow-up in the ivermectin group, constituting a statistically significant change (P = 0.006). Conversely, the control group experienced no significant change in the mean eyelid telangiectasia grade (2.0 ± 0.8 points at baseline vs. 1.9 ± 0.8 points during the follow-up) (P = 0.420). The mean eyelid telangiectasia grade of the ivermectin group was greater than that of the control group at both baseline and follow-up (P = 0.006 and P = 0.035, respectively) (Fig. 8).
FIGURE 8.
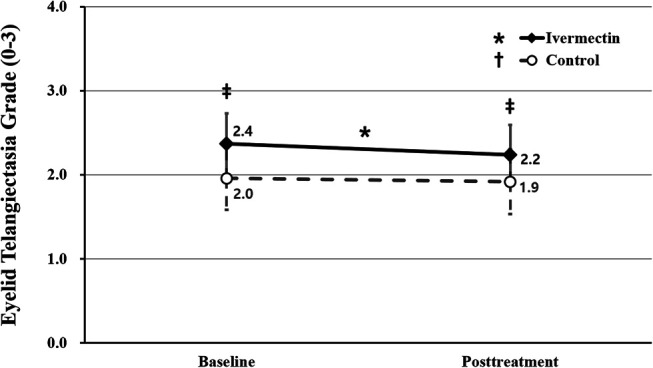
Comparison of eyelid telangiectasia grade between the ivermectin and control groups. The asterisk indicates statistically significant changes in the eyelid telangiectasia grade in the ivermectin group. The double dagger indicates statistically significant differences between the ivermectin and control groups. Both the asterisk and double daggers indicate P < 0.05 per repeated-measures ANOVA with Tukey's HSD post hoc test. ANOVA, analysis of variance; HSD, honestly significant difference.
There were no reports of side effects except mild irritation in 5 participants from the use of topical ivermectin 1% cream on the eyelashes in the ivermectin group. The eyelid debris grade variation in the ivermectin and control groups was 1.18 ± 0.93 and 0.41 ± 0.70 points, respectively. From these values, the effect size was calculated as 0.9355 by a post hoc power analysis. This effect size of 0.9355 and a 2-tailed alpha value of 0.05 in the subgroup analysis, with 51 patients included in each group, led to a power of 0.99.
DISCUSSION
This study compared the subjective symptoms and objective signs between patients receiving topical ivermectin 1% cream and performing eyelid hygiene and those performing eyelid hygiene only to investigate the therapeutic effect of topical ivermectin 1% cream in the treatment of Demodex blepharitis. The results of this study reveal that the application of ivermectin 1% cream on the eyelashes for 15 minutes once weekly in addition to eyelid hygiene once daily led to a superior therapeutic effect both in subjective symptoms and objective signs compared with eyelid hygiene alone. Evidence-based methods that are effective in treating Demodex blepharitis include oral ivermectin and TTO lid scrub. 13 In a previous study, 6 mg of ivermectin taken orally twice daily at 2-week intervals reduced the average number of Demodex mites in chronic Demodex blepharitis. 11 Meanwhile, Holzchuh et al 12 reported that 200 μg/kg of ivermectin taken orally once daily at 1-week intervals significantly reduced the average number of Demodex mites and improved the tear break-up time and Schirmer I test values in patients with chronic Demodex blepharitis who did not respond to conventional treatment. Oral ivermectin treatment may result in moderate-to-severe adverse reactions, although most adverse reactions are transient and mild. 23–25 On the other hand, the side effects of topical ivermectin are mostly mild, including irritation, allergic dermatitis, and redness. 25,26 In this study, topical application of ivermectin 1% cream improved clinical signs and symptoms without moderate-to-severe side effects from the medication in Demodex blepharitis.
Antiparasitic and anti-inflammatory activities are the 2 main mechanisms that are believed to be effective against Demodex infestations. The mechanism of ivermectin's antiparasitic effect is believed to be due to the selective and high-affinity binding of ivermectin with glutamate-gated or γ-aminobutyric acid–gated chloride ion channels in the peripheral synapses of neurons. This binding leads to an increase in the permeability of chloride ion channels, resulting in the inhibition of nerve or muscle cells, which can cause paralysis and death of the offending parasite. 25,27–29 Meanwhile, the anti-inflammatory effects of ivermectin are likely due to the inhibition of the production of lipopolysaccharide-induced inflammatory cytokines, such as tumor necrosis factor-α and interleukin-8, and the upregulation of anti-inflammatory cytokines, such as interleukin-10, by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathways. 30 Demodex blepharitis is a chronic condition that results from inflammation of the eyelashes, eyelids, and ocular surface by Demodex infestation. Thus, ivermectin can have a useful therapeutic effect on Demodex blepharitis because of its antiparasitic and anti-inflammatory effects, which can eliminate both the cause of Demodex infestations and the inflammation caused by the progression of the infection.
Oral ivermectin–metronidazole combined therapy showed superior efficacy for reducing the number of Demodex-infected eyelashes compared with oral ivermectin therapy alone. 15 Similarly, topical application of ivermectin 0.1%–metronidazole 1% gel achieved complete eradication of Demodex in 96.6% of patients with Demodex blepharitis. 14 Notably, the complete remission rate attained by topical ivermectin–metronidazole gel was greater than that achieved by systemic ivermectin treatment. 14 Although it is not known whether oral ivermectin or topical ivermectin have a better therapeutic effect in Demodex blepharitis patients, it is known that the addition of metronidazole for both oral and/or topical ivermectin can provide additional therapeutic effects. Metronidazole has acaricidal, anti-inflammatory, inhibition of reactive oxygen species, and antibacterial activity through its active metabolites. 15,31–33 Therefore, it is believed that adding these several activities of metronidazole to the antiparasitic and anti-inflammatory activities of ivermectin could further reduce the number of Demodex-infected eyelashes and improve clinical signs and symptoms.
TTO 5% to 50% lid scrub may be recommended as the first-line treatment method in Demodex blepharitis. 34 Previous studies have shown that topical TTO lid scrub reduced the average number of Demodex mites and improved symptoms and signs of Demodex blepharitis. 9,35 In this study, no changes in eyelid redness/swelling or eyelid telangiectasia in the control group after using the eyelid cleansing product that contains TTO were noted, although symptoms and eyelid debris grade were improved during the follow-up. In particular, the symptom score and eyelid debris grade were significantly milder in the control group than in the ivermectin group at baseline, whereas, at the follow-up visit, those of the control group were similar or worsened relative to the ivermectin group. In a previous study comparing the anti-Demodectic effects of commercially available eyelid cleaners through in vitro experiments, it was reported that eyelid cleaners containing less terpinen-4-ol or linalool, which exhibit anti-Demodectic effects, have relatively limited antiparasitic effects. 36 Although the eyelid cleansing product used for eyelid hygiene in this study contains 2% TTO, it is believed that it had a lesser effect on Demodex eradication than ivermectin because of its relatively low concentration of TTO. 36 However, TTO concentration of above 25% is known to be cytotoxic. 37 Thus, patients were routinely prescribed to use a safe, commercially available eyelid cleansing product with a low concentration of terpinen-4-ol when used at home. In addition, a previous meta-analysis showed that usual lid hygiene significantly improves the subjective symptoms of Demodex blepharitis. 34
There are several limitations to this study. First, the number of participants enrolled in the study was small because of the nature of retrospective research. Second, only the patient statements that their eyelid hygiene and/or topical ivermectin was used consistently during the follow-up period could be evaluated at the follow-up, whereas the actual rate of patient compliance with treatment was not confirmed. Third, the presence of Demodex was directly confirmed for definitive diagnosis of Demodex blepharitis at baseline. However, no follow-up microscopic examination of eyelashes was performed to confirm the absence of Demodex post-treatment. Instead, the efficacy of the treatment of Demodex blepharitis was evaluated according to the degree of improvement in cylindrical dandruff, which is the key pathognomonic finding of Demodex. 38 In fact, as cylindrical dandruff was improved in most patients treated with topical ivermectin in this study, it was believed to be unnecessary to pull out eyelashes for confirmation. In a previous study, the use of topical ivermectin–metronidazole gel showed a complete remission rate of 96.6% of Demodex blepharitis. 14 Finally, because of the nature of a retrospective study, there is a bias that patients with severe signs and symptoms were more likely to have been prescribed weekly treatment with topical ivermectin cream in this study. Thus, a large-scale randomized controlled trial is necessary to confirm the effect of topical ivermectin in the treatment of Demodex blepharitis and to confirm the possibility that definitive eradication of blepharitis could be achieved by a combination of oral and topical treatments.
In conclusion, this study showed that the application of topical ivermectin 1% cream on the eyelashes for 15 minutes once weekly together with a daily eyelid hygiene regimen was more effective in improving the subjective symptoms and objective signs of Demodex blepharitis without adverse effects as compared with a control group performing daily eyelid hygiene only. Based on these results, it is believed that a combination of topical ivermectin 1% cream and eyelid hygiene can be prescribed as an effective treatment for Demodex blepharitis.
Footnotes
Supported by the following: a TRC Research Grant of the Korea University Medicine and Korea Institute of Science and Technology; a Korea University Ansan Hospital Grant; a Korea University Grant (K1625491, K1722121, K1811051, K1913161, and K2010921); the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 9991007583, KMDF_PR_20200901_0296); and Korea Environment Industry & Technology Institute (KEITI) through the Technology Development Project for Safety Management of Household Chemical Products, funded by Korea Ministry of Environment (MOE) (2020002960007, NTIS-1485017184). The funding organization had no role in the design or conduct of this research.
The authors have no conflicts of interest to disclose.
Contributor Information
Young Choi, Email: skflto@naver.com.
Eun Gyu Yoon, Email: caitlin.yoon@gmail.com.
Jong Suk Song, Email: crisim@korea.ac.kr.
Il-Hwan Kim, Email: kumcihk@korea.ac.kr.
Hyo Myung Kim, Email: hyomkim@daum.net.
REFERENCES
- 1. Basta-Juzbasić A, Subić JS, Ljubojević S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin Dermatol. 2002;20:135–140. [DOI] [PubMed] [Google Scholar]
- 2. English FP, Nutting WB. Demodicosis of ophthalmic concern. Am J Ophthalmol. 1981;91:362–372. [DOI] [PubMed] [Google Scholar]
- 3. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophthalmol Soc. 1967;65:361–392. [PMC free article] [PubMed] [Google Scholar]
- 4. Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618–626. [DOI] [PubMed] [Google Scholar]
- 5. Kamoun B, Fourati M, Feki J, et al. Blepharitis due to Demodex: myth or reality? [in French]. J Fr Ophtalmol. 1999;22:525–527. [PubMed] [Google Scholar]
- 6. Humiczewska M. Demodex folliculorum and Demodex brevis (Acarida) as the factors of chronic marginal blepharitis [in Polish]. Wiad Parazytol. 1991;37:127–130. [PubMed] [Google Scholar]
- 7. Heacock CE. Clinical manifestations of demodicosis. J Am Optom Assoc. 1986;57:914–919. [PubMed] [Google Scholar]
- 8. Gao YY, Di Pascuale MA, Elizondo A, et al. Clinical treatment of ocular demodicosis by lid scrub with tea tree oil. Cornea. 2007;26:136–143. [DOI] [PubMed] [Google Scholar]
- 9. Koo H, Kim TH, Kim KW, et al. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. J Korean Med Sci. 2012;27:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen IS, Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol. 2018;596:1833–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filho PA, Hazarbassanov RM, Grisolia AB, et al. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. Br J Ophthalmol. 2011;95:893–895. [DOI] [PubMed] [Google Scholar]
- 12. Holzchuh FG, Hida RY, Moscovici BK, et al. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am J Ophthalmol. 2011;151:1030–1034.e1. [DOI] [PubMed] [Google Scholar]
- 13. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. [DOI] [PubMed] [Google Scholar]
- 14. Ávila MY, Martínez-Pulgarín DF, Rizo Madrid C. Topical ivermectin-metronidazole gel therapy in the treatment of blepharitis caused by Demodex spp.: a randomized clinical trial. Cont Lens Anterior Eye. 2021;44:101326. [DOI] [PubMed] [Google Scholar]
- 15. Salem DA, El-Shazly A, Nabih N, et al. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin-metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int J Infect Dis. 2013;17:e343–e347. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25:858–870. [DOI] [PubMed] [Google Scholar]
- 19. Fadlallah A, Rami HE, Fahd D, et al. Azithromycin 1.5% ophthalmic solution: efficacy and treatment modalities in chronic blepharitis. Arq Bras Oftalmol. 2012;75:178–182. [DOI] [PubMed] [Google Scholar]
- 20. Efron N. Efron Grading Scales for Contact Lens Complications (Millennium Edition). Oxford, UK: Butterworth-Heinemann; 2000. Available at: https://eprints.qut.edu.au/11857/1/11857a.pdf. Accessed December 22, 2020. [Google Scholar]
- 21. Arita R, Minoura I, Morishige N, et al. Development of definitive and reliable grading scales for meibomian gland dysfunction. Am J Ophthalmol. 2016;169:125–137. [DOI] [PubMed] [Google Scholar]
- 22. Fromstein SR, Harthan JS, Patel J, et al. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pacqué M, Muñoz B, Greene BM, et al. Safety of and compliance with community-based ivermectin therapy. Lancet. 1990;335:1377–1380. [DOI] [PubMed] [Google Scholar]
- 24. Gardon J, Gardon-Wendel N, Demanga-Ngangue N, et al. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. [DOI] [PubMed] [Google Scholar]
- 25. Lam NSK, Long XX, Li X, et al. Comparison of the efficacy of tea tree (Melaleuca alternifolia) oil with other current pharmacological management in human demodicosis: a systematic review. Parasitology. 2020;147:1587–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0·75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103–1110. [DOI] [PubMed] [Google Scholar]
- 27. Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981–988. [DOI] [PubMed] [Google Scholar]
- 28. Kobylinski KC, Foy BD, Richardson JH. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malar J. 2012;11:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cully DF, Paress PS, Liu KK, et al. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J Biol Chem. 1996;271:20187–20191. [DOI] [PubMed] [Google Scholar]
- 30. Ci X, Li H, Yu Q, et al. Avermectin exerts anti‐inflammatory effect by downregulating the nuclear transcription factor kappa‐B and mitogen‐activated protein kinase activation pathway. Fundam Clin Pharmacol. 2009;23:449–455. [DOI] [PubMed] [Google Scholar]
- 31. Metronidazole in the treatment of rosacea. Arch Dermatol. 1985;121:307–308. [PubMed] [Google Scholar]
- 32. Shelley WB, Shelley ED, Burmeister V. Unilateral demodectic rosacea. J Am Acad Dermatol. 1989;20(5 pt 2):915–917. [DOI] [PubMed] [Google Scholar]
- 33. Schmadel LK, McEvoy GK. Topical metronidazole: a new therapy for rosacea. Clin Pharm. 1990;9:94–101. [PubMed] [Google Scholar]
- 34. Navel V, Mulliez A, Benoist d'Azy C, et al. Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. Ocul Surf. 2019;17:655–669. [DOI] [PubMed] [Google Scholar]
- 35. Karakurt Y, Zeytun E. Evaluation of the efficacy of tea tree oil on the density of Demodex mites (Acari: Demodicidae) and ocular symptoms in patients with demodectic blepharitis. J Parasitol. 2018;104:473–478. [DOI] [PubMed] [Google Scholar]
- 36. Cheung IMY, Xue AL, Kim A, et al. In vitro anti-demodectic effects and terpinen-4-ol content of commercial eyelid cleansers. Cont Lens Anterior Eye. 2018;41:513–517. [DOI] [PubMed] [Google Scholar]
- 37. Homeyer DC, Sanchez CJ, Mende K, et al. In vitro activity of Melaleuca alternifolia (tea tree) oil on filamentous fungi and toxicity to human cells. Med Mycol.. 2015;53:285–294. [DOI] [PubMed] [Google Scholar]
- 38. Cheng AM, Sheha H, Tseng SC. Recent advances on ocular Demodex infestation. Curr Opin Ophthalmol. 2015;26:295–300. [DOI] [PubMed] [Google Scholar]


