Supplemental Digital Content is Available in the Text.
Key Words: atrial fibrillation, sodium-glucose cotransporter 2 inhibitors, diabetes mellitus, meta-analysis
Abstract:
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have well-documented effects on reducing hospitalization for heart failure and cardiovascular mortality, although the effect on atrial fibrillation (AF) has not been comprehensively investigated. Therefore, we performed a meta-analysis to assess the association between SGLT2 inhibitors and AF risk by systematically searching PubMed, Embase, and ClinicalTrials.gov. Two investigators independently identified randomized controlled trials, which compared SGLT2 inhibitors with control in patients with type 2 diabetes, heart failure, or chronic kidney disease. Primary outcomes were incident AF and stroke. We included 20 randomized trials involving 63,604 patients. The SGLT2 inhibitors used were dapagliflozin (7 studies, 28,834 patients), canagliflozin (7 studies, 17,440 patients), empagliflozin (5 studies, 9082 patients), and ertugliflozin (1 study, 8246 patients). Follow-up ranged from 24 weeks to 202 weeks. SGLT2 inhibitors treatment was associated with a significant attenuation in the risk of incident AF (odds ratio = 0.82; 95% confidence interval, 0.72–0.93; P = 0.002) compared with control. No significant difference in stroke between SGLT2 inhibitors and control groups was found (odds ratio = 0.99; 95% confidence interval, 0.85–1.15; P = 0.908). This present meta-analysis indicates that SGLT2 inhibitors are associated with a lower risk of incident AF and do not significantly affect stroke risk for patients with and without type 2 diabetes.
INTRODUCTION
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, is a leading cause of mortality worldwide. As is reported, the estimated prevalence of AF is 1%–4% in western countries and China, 1 and its prevalence is expected to surge in the next 3 decades owing to factors such as an aging population, economic growth, and increased prevalence of risk factors for AF. 2 AF increases the risk of heart failure and stroke that causes a huge burden on human health and social economy. Diabetes mellitus has been well established as a critical risk factor for AF. 3 Accumulating evidence demonstrates that diabetes is closely associated with increased risk of AF promotion. 4–6 As far back as the Framingham Heart Study, it was suggested that the risk of developing AF was significantly increased in patients with diabetes during a long-term follow-up. 5 A meta-analysis of 29 studies, which included 8,037,756 individuals, also found similar results that patients with diabetes had a 49% increased risk in the development of AF. 7 In addition, higher glycemic levels were shown to be correlated with increased risk of AF. 8 Generally, the link between diabetes mellitus and AF is clear and close.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a novel class of oral antihyperglycemic agents for treating type 2 diabetes by selectively inhibiting renal reabsorption of glucose and increasing urinary glucose excretion, 9,10 have been fully demonstrated to attenuate the risk of hospitalization for worsening heart failure and the risk of cardiovascular death or serious renal outcomes, in recent, large, multicenter, randomized controlled trials (RCTs). 11–14 Recently, an animal study suggested that canagliflozin had an effect on preventing developing AF and suppressing the promotion of atrial remodeling. 15 In addition, Zelniker et al 16 reported that dapagliflozin was associated with a decreased risk of AF in patients with type 2 diabetes regardless of the history of AF or other cardiovascular diseases in a post hoc analysis of the DECLARE-TIMI58. SGLT2 inhibitors seem to possess therapeutic potential for AF. However, the association has not been comprehensively established, and evidence is inconclusive. Thus, we carried out this present meta-analysis, pooling data from all available RCTs, which compared SGLT2 inhibitors with placebo and reported AF as an adverse event, to further evaluate the effect of SGLT2 inhibitors on AF risk, including sensitivity and subgroup analyses.
METHODS
This present meta-analysis was performed in accord with the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. 17
Search Strategy
A comprehensive literature search of PubMed, Embase, and ClinicalTrials. gov was carried out for all relevant publications on SGLT2 inhibitors and the risk of AF from inception to May 2021 without language restriction. Keywords or mesh terms used in the searches included “AF” combined with “SGLT2 inhibitor”, “empagliflozin”, “canagliflozin”, “dapagliflozin”, “ertugliflozin”, “sotagliflozin”, “ipragliflozin”, “remogliflozin”, “sergliflozin”, or “tofogliflozin”. The detailed search strategies are shown in Supplemental Digital Content 1 (see Supplementary file, http://links.lww.com/JCVP/A733. In addition, a manual search of retrieving the references listed in identified studies and previous reviews to identify any additional studies was conducted.
Selection Criteria
Studies considered eligible for inclusion were RCTs that met the following criteria: (1) included patients with SGLT2 inhibitors exposure or nonexposure and (2) reported the risks of AF using hazard ratio, odds ratio (OR), or risk ratio (RR) estimates with 95% confidence intervals (CI) or sufficient data to calculate them. Letters to the editor, review articles, and case reports were excluded. Two reviewers independently identified all records by title and abstract and subsequently retrieved and evaluated the full text of any potential articles. When there were multiple RCTs from the same patient cohorts, only the trial with a larger sample size and more informative data was included. Disagreements were resolved by consensus.
Data Extraction and Outcome Assessment
Data were extracted onto a standardized form manually by 2 independent authors. Data extracted from the eligible studies were as follows: information regarding the quality of study, first author, year of publication, study design, participants characteristics, study period, interventions (dose and agent type of SGLT2 inhibitors), and effect estimates with 95% CIs for each outcome. We attempted to contact the corresponding authors to obtain additional data when complete information was unavailable. Any discrepancies were resolved by referring back to the original report.
The primary analyses were focused on evaluating the risk of overall AF among SGLT2 inhibitors users and nonusers. Besides, we also checked stroke as the primary outcome. Further subgroup analyses were performed to investigate the performance of SGLT2 inhibitor treatment between different agent types and length of follow-up.
Quality Assessment
Two authors independently assessed the methodological quality of the RCTs using the Cochrane risk-of-bias tool. 18 Any disagreement on the risk-of-bias assessments was resolved by discussion of the 2 authors.
Statistical Analysis
All meta-analyses were performed with the random-effect model using STATA SE software (version 16.0, StataCorp, TX). The Mantel–Haenszel test with fixed-effects model, which predicted a low heterogeneity for the outcomes of interest, with pooled OR and corresponding 95% CIs was applied to summarized dichotomous data. Statistical heterogeneity was assessed using the Cochrane Q test and quantified by the I2 statistics. A P value less than 0.1 was considered statistically significant for the Q test, and I2 of ≥50% indicated a substantial level of heterogeneity. When significant heterogeneity was identified, random-effects model was performed. Several subgroup analyses were conducted to evaluate the robustness of results. Publication bias was investigated graphically with a funnel plot and quantitatively with Egger's test if numbers of included studies were more than 10 in accordance with Cochrane Handbook.
RESULTS
Study Selection and Characteristics
The search strategy is outlined in Figure 1. A total of 1284 records were initially identified. No records were identified through other sources. After the removal of duplicates, 384 records were further excluded with respect to titles and abstracts, leaving 138 records for full-text review. After careful assessment, 20 studies, comprising a total of 63,604 participants, matched our prespecified criteria and were included for the final meta-analysis. All of the included studies were RCTs. Table 1 summarized the characteristics of the included studies. These studies were published between 2012 and 2020 and had sample sizes ranging from 269 to 17,160 patients. The proportion of female subjects ranged from 28.5% to 51.6%. The study population was type 2 diabetes for all studies except for 2 trials, DAPA-HF, 12 and DAPA-CKD. 19 DAPA-HF included patients with symptomatic heart failure and an ejection fraction of 40% or less, and it had 42% of participants with type 2 diabetes. DAPA-CKD included patients with chronic kidney disease and an estimated glomerular filtration rate of 25–75 mL per minute per 1.73 m2, and eit had 67.5% of patients with type 2 diabetes. Of all the included studies, 7 RCTs 12,19–24 assessed the effect of dapagliflozin, 7 RCTs 11,25–29 assessed the effect of canagliflozin, 5 RCTs 30–34 assessed the effect of empagliflozin, and 1 RCT 35 assessed the effect of ertugliflozin. Across all 20 studies, 14 studies evaluated the effects of different pharmacologic dosages.
FIGURE 1.
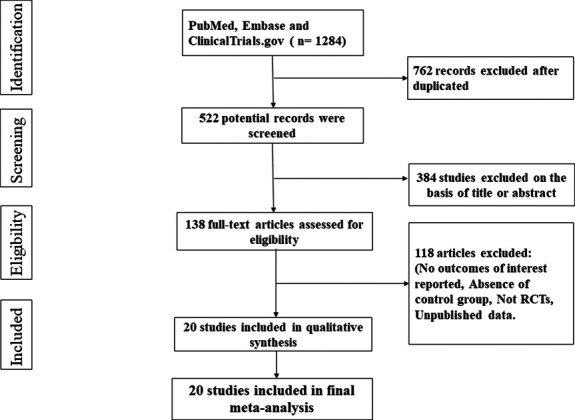
Flow diagram of study selection.
TABLE 1.
Baseline Characteristics of Included Trials and Quality Assessment
| Study (Trial Name) | Study Design (NCT Number) | Mean Age (SD) | Study Population | No. of patients | Female (%) | Interventions | Background Hypoglycaemic Therapy | Mean Follow-up (wk) | Quality Assessment | ||
| Treatment | Control | Treatment (Female/Male) | Control (Female/Male) | ||||||||
| Wilding 2012 | RCT (NCT00673231) | 59.5 ± 8.1 | 58.8 ± 8.6 | Type 2 diabetes | 607 (320/287) | 193 (98/95) | 52.2 | Dapagliflozin (2.5/5/10 mg once daily) matching placebo | Insulin | 48 | A |
| Bailey 2013 | RCT (NCT00528879) | 53.7 (NA) | 54.0 (NA) | Type 2 diabetes | 409 (194/215) | 137 (62/75) | 46.9 | Dapagliflozin (2.5/5/10 mg once daily) matching placebo | Metformin | 102 | A |
| Leiter 2014 | RCT (NCT01042977) | 63.9 ± 7.6 | 63.6 ± 7.0 | Type 2 diabetes, cardiovascular disease | 480 (159/321) | 482 (159/323) | 33.1 | Dapagliflozin (10 mg once daily) matching placebo | Insulin | 24 | B |
| Mathieu 2015 | RCT (NCT01606320) | 55.2 ±8.6 | 55.0 ±9.6 | Type 2 diabetes | 160 (90/70) | 160 (84/76) | 54.4 | Dapagliflozin (10 mg once daily) matching placebo | Metformin saxagliptin | 24 | A |
| DARELARE-TIMI58 | RCT (NCT01730534) | 63.9 ± 6.8 | 64.0 ± 6.8 | Type 2 diabetes, cardiovascular disease | 8582 (3171/5411) | 8578 (3251/5327) | 37.4 | Dapagliflozin (10 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i GLP-1 receptor agonist | 102 | A |
| DAPA-HF 2019 | RCT (NCT03036124) | 66.2 ± 11.0 | 66.5 ± 10.8 | Type 2 diabetes, heart failure with reduced ejection fraction | 2373(564/1809) | 2371(545/1826) | 23.4 | Dapagliflozin (10 mg once daily) matching placebo | Metformin insulin sulfonylurea | 72 | A |
| DAPA-CKD 2020 | RCT (NCT03036150) | 61.8 ± 12.1 | 61.9 ± 12.1 | Type 2 diabetes, chronic kidney disease | 2152 (709/1443) | 2152(716/1436) | 33.1 | Dapagliflozin (10 mg once daily) matching placebo | Metformin insulin sulfonylurea | 125 | A |
| CANTATA-MSU 2013 | RCT (NCT01106625) | 56.8 ± 9.7 | 56.7 ± 8.3 | Type 2 diabetes | 313 (150/163) | 156 (80/76) | 49.0 | Canagliflozin (100/300 mg once daily) matching placebo | Metformin suiphonylurea | 52 | A |
| Yale 2014 | RCT (NCT01064414) | 68.7 ± 8.2 | 68.2 ± 8.4 | Type 2 diabetes, chronic kidney disease | 179 (73/106) | 90 (33/57) | 36.1 | Canagliflozin (100/300 mg once daily) matching placebo | Insulin sulphonylurea | 52 | A |
| Bode 2014 | RCT (NCT01106651) | 63.9 ± 6.2 | 63.2 ± 6.2 | Type 2 diabetes | 477 (224/253) | 237 (94/143) | 44.5 | Canagliflozin (100/300 mg once daily) matching placebo | Insulin sulphonylurea | 104 | A |
| CANVAS-R 2017 | RCT (NCT01989754) | 63.9 ± 8.42 | 64 ± 8.28 | Type 2 diabetes, chronic kidney disease | 2904 (1111/1794) | 2903 (1053/1854) | 37.3 | Canagliflozin (100/300 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i GLP-1 receptor agonist | 187 | A |
| CANVAS 2017 | RCT (NCT01032629) | 62.5 ± 8.1 | 62.3 ± 7.9 | Type 2 diabetes, cardiovascular disease | 2888(983/1905) | 1442(486/956) | 33.9 | Canagliflozin (100/300 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i GLP-1 receptor agonist | 202 | A |
| CREDENCE 2019 | RCT (NCT02065791) | 62.9 ± 9.2 | 63.2 ± 9.2 | Type 2 diabetes, chronic kidney disease | 2202(762/1440) | 2199(732/1467) | 33.9 | Canagliflozin (100 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i GLP-1 receptor agonist | 125 | A |
| CANTATA-SU 2013 | RCT (NCT00968812) | 56.2 ± 9.2 | 56.3 ± 9.0 | Type 2 diabetes | 968/(475/493) | 482/(219/263) | 47.9 | Canagliflozin (100/300 mg once daily) matching glimepiride | Metformin | 52 | A |
| Kovacs 2014 | RCT (NCT01210001) | 54.5 ± 9.4 | 54.6 ± 10.5 | Type 2 diabetes | 333 (165/168) | 165(92/73) | 51.6 | Empagliflozin (10/25 mg once daily) matching placebo | Metformin pioglitazone | 24 | A |
| Barnett 2014 | RCT (NCT01164501) | 63.7 ± 8.9 | 64.1 ± 8.7 | Type 2 diabetes, chronic kidney disease | 419 (170/249) | 319 (138/181) | 41.7 | Empagliflozin (10/25 mg once daily) matching placebo | Metformin pioglitazone insulin | 52 | A |
| Rosenstock 2016 | RCT (NCT01011868) | 59.2 (NA) | 58.1(NA) | Type 2 diabetes | 324 (138/186) | 170 (80/90) | 42.4 | Empagliflozin (10/25 mg once daily) matching placebo | Insulin | 78 | A |
| Softeland 2017 | RCT (NCT01734785) | 54.9 ± 9.7 | 55.9 ± 9.6 | Type 2 diabetes | 222 (85/137) | 110 (49/61) | 40.4 | Empagliflozin (10/25 mg once daily) matching placebo | Metformin | 24 | A |
| EMPA-REG OUTCOME 2015 | RCT (NCT01131676) | 63.1 ± 8.6 | 63.2 ± 8.8 | Type 2 diabetes | 4687 (1351/3336) | 2333 (653/1680) | 28.5 | Empagliflozin (10/25 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i | 161 | A |
| VERTIS CV | RCT (NCT01986881) | 64.4 ± 8.1 | 64.4 ± 8.1 | Type 2 diabetes | 5499 (1633/3866) | 2747 (844/1903) | 30.0 | Ertugliflozin (5/15 mg once daily) matching placebo | Metformin insulin sulfonylurea DPP-4i GLP-1 receptor agonist | 182 | A |
Quality Assessment
Randomized sequence generation and allocation concealment were conducted adequately in most studies. The risk of bias for most studies was assessed as low, and all data were derived from randomized trials (Table 1).
Effects of Sodium-Glucose Cotransporter 2 Inhibitors on AF
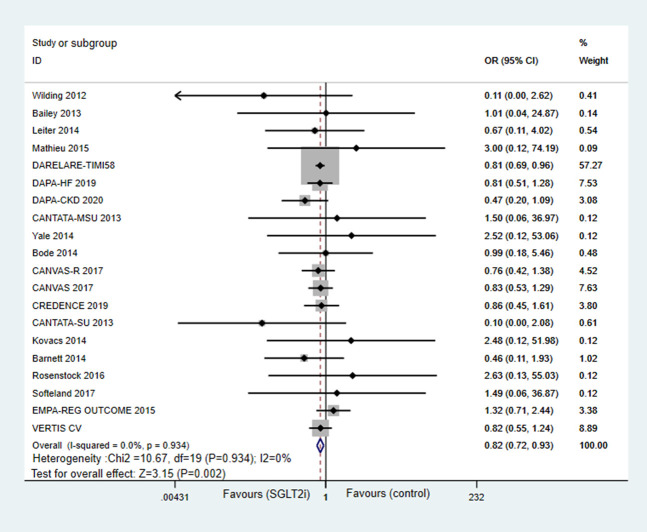
AF was reported in all 20 studies. SGLT2 inhibitors therapy was associated with a significant 18% reduction in the odds of incident AF compared with control (OR = 0.82; 95% CI, 0.72–0.93; P = 0.002; Fig. 2). In subgroup analysis based on the types of SGLT2 inhibitors used (see Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCVP/A733), only dapagliflozin was associated with significant attenuation on the risk of AF (OR = 0.80; 95%CI, 0.68–0.93; P = 0.003). Canagliflozin showed a similar trend but not a statistically significant risk reduction of AF (OR = 0.82; 95% CI, 0.60–1.10; P = 0.187). As for empagliflozin, there was no difference in the risk of AF (OR = 1.19; 95% CI, 0.69–2.04; P = 0.537). In the subgroup analysis regarding the length of follow-up (see Figure 2, Supplemental Digital Content 1, http://links.lww.com/JCVP/A733), SGLT2 inhibitors significantly attenuated the risk of incident AF in trials with less than 2 years of follow-up (OR = 0.81; 95% CI, 0.70–0.94; P = 0.008). A trend for lower risk of incident AF in the SGLT2 inhibitors group was detected in studies with more than 2 years of follow-up when compared with controls (OR = 0.82; 95%CI, 0.55–1.24; P = 0.117). Notably, the subgroup analyses were more susceptible to type II errors because of less power than the overall pooled analysis, which includes all studies regardless of the length of follow-up.
FIGURE 2.
Forest plot for meta-analyses comparing the effects of SGLT2 inhibitors with control in AF risk.
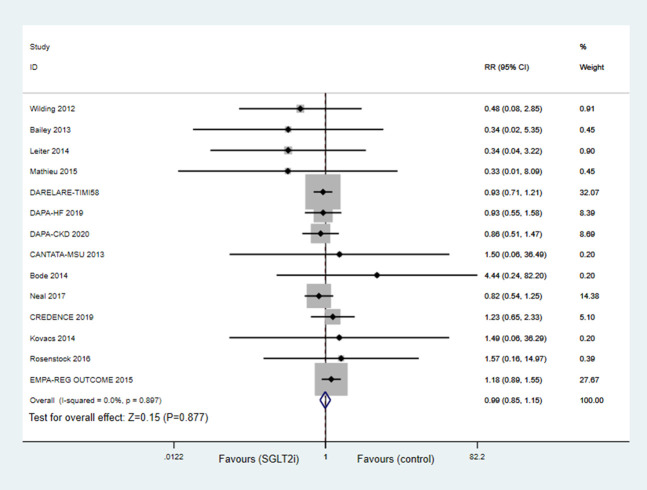
Effects of SGLT2 Inhibitor on Stroke
Apart from 5 studies, 26,29,31,33,35 15 studies reported stroke. Compared with controls, SGLT2 inhibitors treatment did not increase the risk of stroke (OR = 0.99; 95%CI, 0.85–1.15; P = 0.908; Fig. 3). In subgroup analysis, there were 7 studies in the dapagliflozin group, 4 studies in the canagliflozin group, and 3 studies in the ertugliflozin group, and all suggested no significant differences between SGLT2 inhibitors treatment and control groups (see Figure 3, Supplemental Digital Content 1, http://links.lww.com/JCVP/A733).
FIGURE 3.
Forest plot for meta-analyses comparing the effects of SGLT2 inhibitors with control in stroke.
Publication Bias
No obvious asymmetry was revealed on the funnel plot (see Figure 4, Supplemental Digital Content 1, http://links.lww.com/JCVP/A733). Additionally, no evidence of publication bias was indicated by Egger's test (P = 0.858).
DISCUSSION
This large meta-analysis of 20 RCTs involving 63,604 patients suggests that SGLT2 inhibitors treatment significantly attenuated the risk of incident AF for patients with type 2 diabetes, heart failure, or chronic kidney disease. In addition, we also found that the use of SGLT2 inhibitors did not affect the risk of stroke in individuals with and without type 2 diabetes. According to previous animal experiments and clinical studies, SGLT2 inhibitors have been hypothesized a sympathetic inhibitory effect, which may be involved in the antiarrhythmic effect. 36 However, up to now, the molecular mechanisms underlying the antiarrhythmic effect of SGLT2 inhibitors remain unclear. Thus, more studies should be conducted to further explain how SGLT2 inhibitors exert antiarrhythmic effects.
Except for the well-established effect on rehospitalization for heart failure and cardiovascular mortality, SGLT2 inhibitors appear to attenuate the incidence of AF. Experimental and clinical data have provided several possible explanations for the protective heart benefits of SGLT2 inhibitors. SGLT2 inhibitors are thought to play a key role in reducing glucose, body weight, and blood pressure. By suppressing SGLT2 in the proximal renal tube, SGLT2 inhibitors induce glucosuria and natriuresis. 37,38 Increased glucose and sodium excretion, causing osmotic diuresis and contraction of plasma volume, thereby reducing atrial blood pressure and atrial dilation. 39 Owing to the natriuretic and diuretic effects, a 7% reduction in the plasma volume in patients with type 2 diabetes using dapagliflozin was found at week 12. 40 In diabetic patients, the sodium–hydrogen exchanger 1 upregulates, leading to a significant increase in the intracellular sodium content, which results in a higher activity of the Na+/Ca2+ exchanger and subsequently an increase in the calcium levels in the sarcoplasmic reticulum. However, this mechanism is burdened by a high risk of arrhythmia because of the mitochondrial activation of the Na+/Ca2+ exchanger. 41,42 SGLT2 inhibitors suppress sodium–hydrogen exchange, promote natriuresis, and lower cardiac intracellular Na+ and Ca2+, which have been connected to the reduction of myocardial hypertrophy, 43,44 adverse remodeling and fibrosis, and hence decrease the risk of arrhythmia. Epicardial fat could lead to localized inflammation and is thought to increase the incidence of AF. Besides, some studies have shown that SGLT2 inhibitors are associated with a reduction in epicardial fat, contributing to the antiarrhythmic effect. 45 Recently, a population-based propensity score-matched cohort study involving 79,150 diabetic patients on SGLT2 inhibitors compared with 79,150 matched diabetic patients on DDP-4 inhibitors 46 showed that there was a 17% reduction of new-onset AF. This is the first real-world study focusing on the risk of AF among type 2 diabetes patients treated with SGLT2 inhibitors. More studies should be conducted to further understand the relationship between SGLT2 inhibitors and arrhythmia.
AF as a known risk factor for incident stroke has been well demonstrated in previous studies. However, it is still unclear whether a reduced risk of incident AF associated with SGLT2 inhibitors affects the incidence of stroke. In EMPA-REG OUTCOME, there was a higher incidence of AF in the empagliflozin-treated group, as well as fatal or nonfatal stroke. Similarly, CANVAS-R and CANVAS trials suggested that a lower risk of incident AF in the canagliflozin group was associated with a decreased risk of stroke compared with control. In contrast, similar incidence of ischemic stroke was found in the dapagliflozin and placebo groups, whereas the DECLARE-TIMI 58 trial reported the lowest incidence of AF with dapagliflozin. In this present meta-analysis, we found that SGLT2 inhibitors do not significantly affect the risk of stroke (95% CI, 0.85–1.15; P = 0.908). Taken all together, the association between SGLT2 inhibitors and the risk of stroke ought to be interpreted cautiously due to currently insufficient data.
There are several limitations to this meta-analysis. AF in the included studies was reported as a serious adverse event, not as an outcome. Thus, no clear predefinitions and no standard method for assessing AF events were described, which may cause reporting bias. Because AF was not the outcome of interest in the included studies, risk factors for AF may not balance in SGLT2 inhibitors and control groups. Although a significantly decreased incidence of AF with SGLT2 inhibitors was detected in this present meta-analysis, more studies are needed to conduct and identify whether it is clinically important.
CONCLUSIONS
Overall, SGLT2 inhibitors are associated with a lower risk of incident AF and do not significantly affect stroke risk in patients with and without type 2 diabetes. Based on the above limitations, these findings should be interpreted with caution until more specifically designed trials are available.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
Bachelor of Science: Ru-Jie Zheng, Yue Wang, Jie-Ying Duan and Ming-Yue Yuan are in masters, Jun-Nan Tang and Jin-Ying Zhang are PhDs.
REFERENCES
- 1. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 2. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 3. Lau DH, Nattel S, Kalman JM, et al. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. [DOI] [PubMed] [Google Scholar]
- 4. Echouffo-Tcheugui JB, Shrader P, Thomas L, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF registry. J Am Coll Cardiol. 2017;70:1325–1335. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6. Wang A, Green JB, Halperin JL, et al. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1107–1115. [DOI] [PubMed] [Google Scholar]
- 7. Huxley RR, Filion KB, Konety S, et al. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dublin S, Glazer NL, Smith NL, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. [DOI] [PubMed] [Google Scholar]
- 10. Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53:875–883. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Mahaffey KW; CANVAS Program Collaborative Group, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJV, Solomon SD, Inzucchi SE, et al. DAPA-HF trial committees and investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 13. Packer M, Anker SD, Butler J, et al. EMPEROR-reduced trial investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 14. Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishinarita R, Niwano S, Niwano H, et al. Canagliflozin suppresses atrial remodeling in a canine atrial fibrillation model. J Am Heart Assoc. 2021;10:e017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. [DOI] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Sterne AC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. London, United Kingdom: The Cochrane Collaboration; 2011. [Google Scholar]
- 19. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. DAPA-CKD trial committees and investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 20. Wilding JP, Woo V, Soler NG, et al. Study GroupLong-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 0062;156:405–415. [DOI] [PubMed] [Google Scholar]
- 21. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leiter LA, Cefalu WT, de Bruin TW, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62:1252–1262. [DOI] [PubMed] [Google Scholar]
- 23. Wiviott SD, Raz I, Bonaca MP, et al. DECLARE–TIMI 58 investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 24. Mathieu C, Ranetti AE, Li D, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–2017. [DOI] [PubMed] [Google Scholar]
- 25. Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yale JF, Bakris G, Cariou B, et al. ; DIA3004 Study Group. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. [DOI] [PubMed] [Google Scholar]
- 27. Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. [DOI] [PubMed] [Google Scholar]
- 28. Perkovic V, Jardine MJ, Neal B, et al. CREDENCE trial investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 29. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52-week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 30. Kovacs CS, Seshiah V, Swallow R, et al. EMPA-REG PIO™ trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. [DOI] [PubMed] [Google Scholar]
- 31. Barnett AH, Mithal A, Manassie J, et al. EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. [DOI] [PubMed] [Google Scholar]
- 32. Rosenstock J, Jelaska A, Zeller C, et al. EMPA-REG BASALTM trial investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Søfteland E, Meier JJ, Vangen B, et al. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40:201–209. [DOI] [PubMed] [Google Scholar]
- 34. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 35. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. [DOI] [PubMed] [Google Scholar]
- 36. Palmiero G, Cesaro A, Vetrano E, et al. Impact of SGLT2 inhibitors on heart failure: from pathophysiology to clinical effects. Int J Mol Sci. 2021;22:5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka H, Takano K, Iijima H, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. [DOI] [PubMed] [Google Scholar]
- 39. Ni L, Yuan C, Chen G, et al. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lambert R, Srodulski S, Peng X, et al. Intracellular Na+ concentration ([Na+]i) is elevated in diabetic hearts due to enhanced Na+-Glucose cotransport. J Am Heart Assoc. 2015;4:n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 44. Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res. 2014;102:205–213. [DOI] [PubMed] [Google Scholar]
- 46. Chen HY, Huang JY, Siao WZ, et al. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:73. [DOI] [PMC free article] [PubMed] [Google Scholar]