Objective:
There is increasing evidence supporting the efficacy of sacubitril/valsartan for treating left heart failure, but few studies have investigated its effects on right ventricular (RV) dysfunction. This study aimed to explore the effects of sacubitril/valsartan on RV dysfunction among patients with heart failure with reduced ejection fraction (HFrEF).
Methods:
A total of 93 patients with HFrEF with RV dysfunction who were hospitalized from January 2018 through June 2019 were included in this retrospective observational study. All patients received their first sacubitril/valsartan treatment as in patients during the study period. We excluded 11 patients who were lost to follow-up or had incomplete heart echocardiography data. After 6 months of follow-up, we re-evaluated New York Heart Association Functional Classification and performed echocardiography to identify changes in relevant variables after treatment.
Results:
At baseline, 24% of the patients had an initial sacubitril/valsartan regimen of 12/13 mg twice daily and 76% of the patients had an initial dose of 24/26 mg twice daily. During follow-up, 27% of patients increased their dosage to 49/50 mg twice daily, 68% of patients were taking 24/26 mg twice daily, and 5% of the patients were still taking 12/13 mg twice daily. We found that sacubitril/valsartan treatment was associated with significant improvements in the following RV function indicators: tricuspid annular plane systolic excursion, tricuspid annular s′ peak velocity (S′), RV fractional area change, and pulmonary artery systolic pressure (PASP). Crude linear regression analysis revealed that a tricuspid annular plane systolic excursion improvement was positively correlated with a change in left ventricular ejection fraction (LVEF) and negatively correlated with a change in left ventricular end-systolic volume (LVESV). However, these correlations were nonexistent after adjusting for multiple echocardiographic variables.
Conclusions:
In patients with RV dysfunction and HFrEF, sacubitril/valsartan may improve RV remodeling. This influence may be independent of left cardiac remodeling.
Key Words: sacubitril/valsartan, heart failure, right ventricular dysfunction
INTRODUCTION
Sacubitril/valsartan, an angiotensin receptor–neprilysin inhibitor, has attracted considerable attention in heart failure (HF) therapeutics. Aside from inhibiting the renin–angiotensin–aldosterone system, it can also increase the concentration of neprilysin to exert positive effects on human metabolism, diuresis, natriuresis, and vasodilation. 1 Many large multicenter randomized studies have demonstrated the superiority and substitutability of this drug in the treatment of HF with reduced ejection fraction (HFrEF). For patients with HFrEF, compared with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), sacubitril/valsartan can clearly decrease the risk of cardiovascular death and all-cause death, reduce rehospitalization rates, reverse cardiac remodeling, and improve quality of life. 2–6
Right ventricular (RV) dysfunction frequently occurs secondary to decompensation of left cardiac function. Reduced RV ejection has been associated with decreased survival among patients with chronic HF and an increased risk of HF rehospitalization. 7,8 Unfortunately, there remains a lack of clear evidence of the benefits of a specific treatment for RV dysfunction.
Despite a wealth of high-quality evidence regarding the advantages of sacubitril/valsartan for left heart remodeling, data on the effects of sacubitril/valsartan on RV function are still lacking. Thus, we aimed to explore the feasibility and effects of sacubitril/valsartan for treating right heart insufficiency in patients with HFrEF.
METHODS
Population and Procedures
In this single-center, retrospective observational study, the data of all consecutive patients with HFrEF who were prescribed sacubitril/valsartan between January 2018 and June 2019 were retrieved from a computerized clinical management database. The specific inclusion criteria were as follows: (1) age >18 years, (2) clinical symptoms consistent with New York Heart Association (NYHA) class II–IV, (3) sacubitril/valsartan was first used after a diagnosis of cardiac insufficiency, and (4) complete standard Doppler echocardiographic evaluation data, with a left ventricular ejection fraction (LVEF) ≤40% and feature of RV dysfunction with tricuspid annular plane systolic excursion (TAPSE) ≤17 mm. We excluded 8 patients without available quantitative right ventricular assessment data during follow-up after sacubitril/valsartan initiation, 10 patients who were not followed up in the outpatient clinic within 6 months from discharge, and 1 patient who died before sufficient follow-up data were collected. Finally, 82 patients were included in the analysis. This study was approved by the study hospital's Ethical Review Board (Sir Run Run Shaw Hospital, Zhejiang University, China).
Drug Administration
Considering that all patients with HF had been depending on ACEI or ARB therapy if with no contraindications and that there were various medication and dosage options, the initial sacubitril/valsartan dosages were determined by attending physicians based on each patient's previous medication regimen, clinical symptoms, and blood pressure at baseline. To reduce the risk of angioedema, patients were required to stop taking ACEIs or ARBs at least 36 hours before initiating sacubitril/valsartan therapy. During follow-up, we assessed blood pressure, weight, kidney function, and clinical complaints every 2–4 weeks. If a patient tolerated the initial dosage, the dosage was titrated up in a stepwise fashion until reaching the maximum tolerated dose of sacubitril/valsartan. If there was increased blood potassium concentration (>5.5 mmol/L), abnormal renal function [creatinine >221 μmol/L or estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2], symptomatic hypotension, or symptoms of dizziness during treatment, the dosage was reduced and the patient kept under close observation. Severe adverse effects were grounds for discontinuing sacubitril/valsartan administration.
Data Collection
Standard transthoracic 2-dimensional echocardiography and Doppler examinations were used for capturing cardiac function indicators. The conventional indicators to assess the RV systolic function include TAPSE, right ventricular fractional area change (RVFAC), and tricuspid annular s′ peak velocity (S′). TAPSE was measured as the peak excursion at the lateral aspect of the tricuspid annulus from the apical 4-chamber view, based on achieving proper orientation for M-mode echocardiography. RVFAC was calculated as the fractional change between RV end diastole and end systole from the apical 4-chamber view. S′ was calculated using pulsed tissue Doppler. According to the consensus document of the international ultrasonographic standard, patients were regarded as having right heart dysfunction if they presented with one or more of the following criteria 9 : (1) RVFAC ≤35%, (2) TAPSE ≤17 mm, and (3) S′ ≤9.5 cm/s. Pulmonary artery systolic pressure (PASP) was determined using an estimated right atrial pressure (estimated by assessing the diameter and collapsibility of the inferior vena cava) and combining this value with the systolic pressure gradient between the right atrium and ventricle, reflecting the maximum velocity of tricuspid regurgitation. Tricuspid regurgitation velocity was determined by continuous wave Doppler through the tricuspid valve. Left cardiac function indicators were measured using M-mode. All left echocardiograms were completed as the standard of care. All echocardiographic variables were measured twice, and trained technicians documented the mean value of each variable before a patient started taking sacubitril/valsartan and after 6 months treatment of sacubitril/valsartan.
For all patients, collected and analyzed data consisted of sociodemographic characteristics (age and sex), clinical and anthropometric characteristics (systolic blood pressure, diastolic blood pressure, heart rate, and body mass index), disease history, medical history, NYHA classification, and biochemical indicators. Baseline N-terminal pro–B-type natriuretic peptide and eGFR were measured to assess cardiac and kidney function.
Statistical Analysis
Continuous variables are presented as mean ± SD or median and interquartile range, according to the normality of the distribution. Categorical variables are summarized as frequencies and percentages. Considering the potential bias that exists in the measurement of indicators, we used Bonferroni's correction, a repeated measures analysis of variance to reduce the differences between mean data over time.
In the subgroup analysis, paired-samples t tests or Wilcoxon rank tests were performed to compare values over time. “Δ” represents the difference between the pretreatment value and post-treatment value for a continuous variable. Multiple linear regression was performed to evaluate the correlation between ΔTAPSE and investigated variables. Two-tailed P values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY).
RESULTS
Baseline Characteristics
Table 1 summarizes the baseline characteristics of the patients who met the study's inclusion criteria. At baseline, the mean age of the 82 included patients was 59.4 ± 11.9 years. 74.1% of study patients were men. 21.9%, 51.3%, and 26.8% of the patients were classified as NYHA class IV, III, and II, respectively. In our cohort, hypertension was noted in 25.6%, diabetes in 17%, coronary artery disease in 20.7%, myocardial infarction (MI) in 9.7%, and atrial fibrillation in 35.3% of patients. All patients had taken ACEIs, ARB, or beta blockers selected to the study.
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Variable | N = 82 |
| Age, yrs | 59.4 ± 11.9 |
| Male (%) | 61 (74.3) |
| Systolic blood pressure, mm Hg | 114.7 ± 17.4 |
| Diastolic blood pressure, mm Hg | 70.1 ± 16.1 |
| Heart rate, b·min−1 | 85.0 ± 21.5 |
| BMI, kg/m2 | 23.5 ± 3.6 |
| NYHA class | |
| II | 22 (26.8) |
| III | 41 (51.3) |
| IV | 19 (21.9) |
| Diseases history, n (%) | |
| Hypertension | 21 (25.6) |
| Diabetes | 14 (17.0) |
| Coronary artery disease | 17 (20.7) |
| MI | 8 (9.7) |
| Atrial fibrillation | 29 (35.3) |
| Therapeutic measure, n (%) | |
| β-blocker | 82 (100) |
| Diuretic | 75 (91.4) |
| Antiplatelet therapy | 17 (20.7) |
| Anticoagulants | 26 (31.7) |
| CRTP/CRTD | 24 (29.2) |
| Baseline laboratory results | |
| eGFR, mL·min−1·1.73 m2 | 76.1 ± 23.5 |
| NT-proBNP, pg/mL | 4181.5 ± 3217.8 |
Values are presented as mean ± SD, number (%), or median (interquartile range).
BMI, body mass index; CRT-D, cardiac resynchronization therapy–defibrillator; CRTP, cardiac resynchronization therapy pacemaker; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Dose Titration of Sacubitril/Valsartan
Figure 1 shows the longitudinal changes of sacubitril/valsartan dosage from baseline and through follow-up. Initially, 20 patients (24%) were taking 12/13 mg twice daily, and 62 patients (76%) were taking 24/26 mg twice daily. The titration dose of sacubitril/valsartan was gradually increased during follow-up. After 6 months, 22 patients (27%) were taking 49/51 mg twice daily, 56 patients (68%) were taking 24/26 mg twice daily, whereas 4 patients (5%) continued with 12/13 mg twice daily. Some patients maintained on baseline doses because they developed symptoms, such as hypotension or dizziness, after dose titration. None of the patients developed drug-induced hyperkalemia or deterioration of renal function during follow-up.
FIGURE 1.
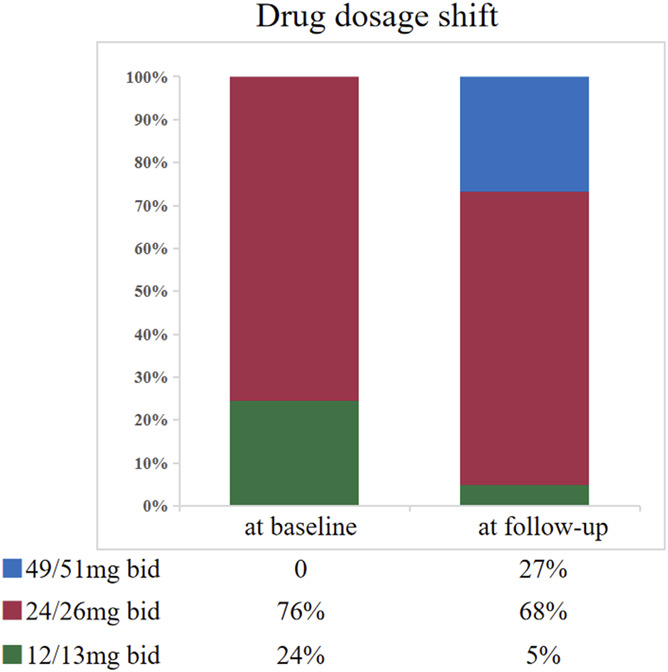
Longitudinal changes of sacubitril/valsartan dosage at baseline and during follow-up.
Echocardiographic Changes
Table 2 summarizes the echocardiographic changes from baseline through follow-up after sacubitril/valsartan initiation. After 6 months drug treatment, we found significant improvements in indicators of left cardiac function, including LVEF (30.2 ± 5.5 vs. 37.0 ± 10.0, P < 0.001), left ventricular end-systolic volume (LVESV; 164.1 ± 54.4 vs. 145.4 ± 61.4, P = 0.017), left ventricular end-diastolic volume (247.4 ± 64.1 vs. 236.4 ± 80.9, P = 0.018), and left atrial volume index (LAVI) (36.2 ± 3.9 vs. 33.7 ± 3.1, P = 0.003). Simultaneously, the measurements of RV function also significantly improved, specifically TAPSE (13.7 ± 2.3 vs. 16.0 ± 4.3, P < 0.001), RVFAC (30.3 ± 11.7 vs. 42.7 ± 15.2, P < 0.001), S′ (9.0 ± 2.0 vs. 9.8 ± 2.5, P < 0.001), and PASP (49.9 ± 19.3 vs. 42.7 ± 15.2, P = 0.002).
TABLE 2.
Changes in the Echocardiographic Parameters From Baseline Through Follow-up
| Variable | Baseline | 6 mo | P |
| NYHA class | 3.0 ± 0.7 | 2.4 ± 0.6 | <0.001 |
| LVEF, % | 30.2 ± 5.5 | 37.0 ± 10.0 | <0.001 |
| LVESV, mL | 164.1 ± 54.4 | 145.4 ± 61.4 | 0.017 |
| LVEDV, mL | 247.4 ± 64.1 | 236.4 ± 80.9 | 0.018 |
| E/E′ ratio | 15.5 ± 6.9 | 15.3 ± 6.4 | 0.725 |
| LAVI, mL/m2 | 36.2 ± 3.9 | 33.7 ± 3.1 | 0.003 |
| TAPSE, mm | 13.7 ± 2.3 | 16.0 ± 4.3 | <0.001 |
| RVFAC, % | 30.3 ± 11.7 | 42.7 ± 15.2 | <0.001 |
| S′, m/s | 9.0 ± 2.0 | 9.8 ± 2.5 | 0.001 |
| TRV, m/s | 3.1 ± 0.7 | 2.9 ± 0.6 | 0.005 |
| PASP, mm Hg | 49.9 ± 19.3 | 42.7 ± 15.2 | 0.002 |
| TAPSE/PASP | 0.31 ± 0.13 | 0.42 ± 0.21 | <0.001 |
Values are presented as mean ± SD. P values <0.05 were considered statistically significant.
E/E′ ratio, transmitral to mitral annular early diastolic velocity ratio; TRV, tricuspid regurgitation velocity.
Table 3 summarizes the changes in the TAPSE between baseline and follow-up in different subgroups to further elaborate the changes in RV function under different conditions. The subgroup analyses revealed that long-term administration of sacubitril/valsartan can significantly ameliorate previously abnormal TAPSE, irrespective of final titration dose, NYHA class, sex, and status of hypertension, diabetes, MI history, severe pulmonary arterial hypertension, or cardiac resynchronization implantation.
TABLE 3.
Change in the TAPSE Between Baseline and 6 mo Follow-up
| Variable | Total | Baseline | 6 mo | P |
| Titration dose | ||||
| 49/51 mg | N = 22 | 13.9 ± 2.6 | 16.8 ± 4.1 | <0.001 |
| 24/26 mg | N = 56 | 13.4 ± 3.3 | 15.8 ± 4.7 | 0.013 |
| 12/13 mg | N = 4 | 15.3 ± 2.6 | 17.4 ± 1.6 | 0.046 |
| NYHA class | ||||
| II | N = 22 | 13.2 ± 2.6 | 16.1 ± 2.8 | 0.002 |
| III | N = 41 | 14.3 ± 2.1 | 16.6 ± 4.8 | 0.026 |
| IV | N = 19 | 13.0 ± 2.1 | 16.7 ± 4.5 | 0.005 |
| Gender | ||||
| Male | N = 61 | 13.5 ± 2.3 | 15.8 ± 4.5 | 0.001 |
| Female | N = 21 | 14.2 ± 2.3 | 16.6 ± 3.7 | 0.035 |
| Hypertension | ||||
| Yes | N = 21 | 14.5 ± 2.3 | 17.1 ± 4.3 | 0.043 |
| No | N = 61 | 13.4 ± 2.2 | 15.6 ± 4.2 | 0.001 |
| Diabetes | ||||
| Yes | N = 14 | 14.4 ± 1.8 | 18.0 ± 3.9 | 0.016 |
| No | N = 68 | 13.5 ± 2.4 | 15.5 ± 4.2 | 0.001 |
| MI | ||||
| Yes | N = 8 | 13.7 ± 1.9 | 17.5 ± 4.9 | 0.036 |
| No | N = 74 | 13.7 ± 2.3 | 15.8 ± 4.2 | <0.001 |
| PASP >60 mm Hg | ||||
| Yes | N = 22 | 13.5 ± 2.4 | 15.9 ± 3.3 | 0.012 |
| No | N = 60 | 13.7 ± 2.3 | 16.0 ± 4.5 | 0.001 |
| CRTP/CRTD | ||||
| Yes | N = 24 | 14.5 ± 2.6 | 16.4 ± 3.6 | 0.046 |
| No | N = 58 | 14.2 ± 3.1 | 16.3 ± 4.6 | 0.001 |
Values are presented as mean ± SD. P values <0.05 were considered statistically significant.
CRT-D, cardiac resynchronization therapy–defibrillator; CRTP, cardiac resynchronization therapy pacemaker.
Linear Regression Analysis of ΔTAPSE
Table 4 shows the multiple linear models testing the correlation of ΔTAPSE between baseline and follow-up with echocardiographic and demographic variables. In model 1 (adjusted for age), ΔTAPSE correlated positively with ΔLVEF and negatively with ΔLVESV. After adjustments for multiple variables—including sex, hypertension, diabetes, MI, and a series of echocardiographic indexes—these associations were not significant.
TABLE 4.
Multiple Linear Regression Analysis on the ΔTAPSE
| Model 1 | Model 2 | |||
| r | P | r | P | |
| ΔLVEF | 0.430 | <0.001 | 0.567 | 0.092 |
| ΔLVESV | −0.674 | <0.001 | −0.391 | 0.136 |
| ΔLVEDV | −0.304 | 0.138 | 0.088 | 0.677 |
| ΔPASP | −0.182 | 0.112 | −0.119 | 0.522 |
| ΔE/E′ ratio | −0.075 | 0.670 | −0.483 | 0.704 |
| ΔLAVI | −0.045 | 0.705 | −0.643 | 0.094 |
| Male | 0.041 | 0.718 | 0.216 | 0.335 |
| Hypertension | −0.005 | 0.968 | −0.043 | 0.802 |
| Diabetes | 0.173 | 0.131 | 0.101 | 0.610 |
| MI | 0.136 | 0.226 | 0.146 | 0.419 |
Δ presented difference value between baseline and follow-up. P values <0.05 were considered statistically significant. Model 1, adjusted for age. Model 2, adjusted for ΔLVEF, ΔLVESV, ΔLVEDV, ΔPASP, ΔE/E′ ratio, ΔLAVI, male, hypertension, diabetes, AF, and MI.
E/E′ ratio, transmitral to mitral annular early diastolic velocity ratio; LVEDV, left ventricle end-diastolic volume.
DISCUSSION
Our study was the first to suggest the striking effect of sacubitril/valsartan therapy in a Chinese population with RV dysfunction combined with HFrEF. The clinical outcome improvements and good drug tolerance without drug withdrawal highlight the effectiveness and safety of sacubitril/valsartan for RV dysfunction treatment and add convincing evidence to the real-world clinical data in a Chinese context.
The doses of sacubitril/valsartan in this study were somewhat different from those reported in previous clinical trials. All patients in the PARADIGM-HF trial underwent an adaptation period to ensure that drug side effects were tolerable at the target dose (97/103 mg twice daily), and this was finally achieved in 74.76% of patients. 2,10 Owing to the influence of blood pressure, impaired renal function, fluctuations in blood potassium levels, and irregular follow-up, the proportions of patients receiving the target dose varied remarkably among different subgroups of patients with HF during the up-titration period. 11,12 In a real-world study in Germany, 64% of patients were initially prescribed 24/26 mg of sacubitril/valsartan twice daily, 32% of patients were prescribed 49/51 mg twice daily, and 4% of patients were prescribed 97/103 mg twice daily. Almost two-thirds (62%) of all patients were still taking the initial dose after 6 months from the first prescription. 13 In a similar cohort in China, only 31% of patients prescribed sacubitril/valsartan at a maximum dose of 49/51 mg twice daily after 1-year assessment. 14 In our study, 27% of patients ultimately achieved the dose of 49/51 mg twice daily after 6 months. A recent research preliminarily observed that the optimally effective and tolerated dose among Chinese patients with HFrEF was possibly lower than that among Western patients. 14 This might be attributable to differences in genetic and clinical characteristics and medical treatment of patients with HFrEF between China and other areas of the world. 15 Therefore, more studies are required to further investigate and optimize dose management of sacubitril/valsartan for Chinese patients with HF.
TAPSE is one of the major indicators reflecting RV systolic function. 16 Reduced TAPSE was associated with a poor prognosis. 17,18 Dini et al enrolled 706 patients with chronic HFrEF to explore the association between reversible abnormal TAPSE and survival. They found that the participants whose abnormal baseline TAPSE values were successfully corrected during the treatment period had better short-term outcomes and long-term improvement in prognosis than participants with worsened or persistently abnormal TAPSE. 19 In this study, we observed significant TAPSE improvements during follow-up, irrespective of NYHA classification, sex, hypertension status, diabetes status, MI history, and cardiac resynchronization implantation status. In addition, we found RVFAC, S′, and PASP were substantially improved. These results indicated important therapeutic value of sacubitril/valsartan for the patients with both HFrEF and RV systolic dysfunction.
Some real-world studies have reported similar results regarding the benefits of sacubitril/valsartan on RV function. Masarone et al 20 demonstrated substantial TAPSE improvements after long-term sacubitril/valsartan treatment in patients with HFrEF during 1-year follow-up, and these improvements persisted after 2 years. In a previous observational cohort in Italy, the mean TAPSE significantly increased from 7.8 ± 3.9 mm at baseline to 16.5 ± 4.0 mm after 1-year treatment (P < 0.001). 21 Our study further found that the RV benefit of using sacubitril/valsartan was persistent at different titration doses.
Reverse remodeling is a critical goal of HF management. Notably, the value of sacubitril/valsartan treatment for reversing LV remodeling in patients with HFrEF has been confirmed in recent years. 3,22,23 In our study, we found that the degree of TAPSE improvement was closely associated with improvements in LVEF and LVESV after adjusting for age. This association between RV function and LV remodeling was revealed to be an independent one after adjusting for echocardiographic variables. Our results that sacubitril/valsartan independently improved RV function seem to be consistent with those of other small-scale studies. 20,21 Our findings suggest that the inhibitory effects of sacubitril/valsartan on the neprilysin and renin–angiotensin–aldosterone system activity might directly improve RV function, not just by compensatory effects through improvements in the LV pulmonary circulation. Clements et al 24 observed that RV function indicators significantly improved in a rat model of pulmonary hypertension after 6 weeks of sacubitril/valsartan treatment. Kia et al found similar effects of sacubitril/valsartan on the inhibition of maladaptive RV remodeling through reductions in RV pressure and hypertrophy. 25 These animal experiments implied that sacubitril/valsartan might be appropriate for treating RV dysfunction because of other causes except for LV dysfunction. However, more fundamental and clinical research is still required to further confirm and analyze the mechanisms underlying the effects of sacubitril/valsartan on RV function.
This study has enriched the evidence supporting sacubitril/valsartan as a treatment option for RV dysfunction, promoting a further understanding of the therapeutic indications of this drug for Chinese patients with HF. Our study had some limitations. First, as a single-center, small-scale, retrospective observational study, selection bias and residual confounding are inherent and hinder the generalizability of our results. Second, our study lacked a control group of patients taking ACEI or ARB drugs, which may affect RV remodeling. Thus, studies with larger randomized cohorts and longer follow-up periods are required to validate our results. Third, the affordability and medical insurance–related aspects of sacubitril/valsartan prescriptions, which might influence the titration of the drug, were not assessed in our study.
CONCLUSIONS
In patients with RV dysfunction and HFrEF, sacubitril/valsartan may improve RV remodeling and dysfunction in patients with HFrEF with RV dysfunction. This influence may be independent of the left cardiac dysfunction.
Footnotes
Supported by Medical Health Science and Technology program of Zhejiang Province (2018KY492 and 2020KY165), Clinical research fund project of Zhejiang Medical Association (2018ZYC-A13), and Natural Science Foundation of Zhejiang Province (LY21H020006).
The authors report no conflicts of interest.
Y. Yang and C. Shen authors contributed to the work equally and should be regarded as cofirst authors. Y. Yang, C. Shen, J. Lu, and C. Xiong designed the study and drafted the manuscript. Y. Yang and G. Fu participated in revising the manuscript. All authors approved the final manuscript.
Contributor Information
Chao Shen, Email: shencdjym@163.com.
Jiangting Lu, Email: lujiangting@zju.edu.cn.
Guosheng Fu, Email: fugs@zju.edu.cn.
Cui Xiong, Email: xiongcui921@163.com.
REFERENCES
- 1. Feng L, Karpinski PH, Sutton P, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53:275–276. [Google Scholar]
- 2. Mcmurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3. Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA . 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA . 2019;322:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–361. [DOI] [PubMed] [Google Scholar]
- 7. de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. [DOI] [PubMed] [Google Scholar]
- 8. Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation . 2010;121:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging . 2017;18:1301–1310. [DOI] [PubMed] [Google Scholar]
- 10. Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du AX, Westerhout CM, McAlister FA, et al. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. J Cardiovasc Pharmacol. 2019;73:149–154. [DOI] [PubMed] [Google Scholar]
- 12. Martens P, Verluyten L, Van de Broek H, et al. Determinants of maximal dose titration of sacubitril/valsartan in clinical practice. Acta Cardiol . 2021;76:20–29. [DOI] [PubMed] [Google Scholar]
- 13. Wachter R, Fonseca AF, Balas B, et al. Real-world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen W, Liu Y, Li Y, et al. Sacubitril/valsartan improves cardiac function in Chinese patients with heart failure: a real-world study. ESC Heart Fail. 2021;8:3783–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dewan P, Jhund PS, Shen L, et al. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail. 2019;21:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aloia E, Cameli M, D'Ascenzi F, et al. TAPSE: an old but useful tool in different diseases. Int J Cardiol. 2016;225:177–183. [DOI] [PubMed] [Google Scholar]
- 17. Damy T, Kallvikbacka-Bennett A, Goode K, et al. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. [DOI] [PubMed] [Google Scholar]
- 18. Kjaergaard J, Akkan D, Iversen KK, et al. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. [DOI] [PubMed] [Google Scholar]
- 19. Dini FL, Carluccio E, Simioniuc A, et al. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2016;18:1462–1471. [DOI] [PubMed] [Google Scholar]
- 20. Masarone D, Errigo V, Melillo E, et al. Effects of sacubitril/valsartan on the right ventricular arterial coupling in patients with heart failure with reduced ejection fraction. J Clin Med. 2020;9:3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Correale M, Mallardi A, Mazzeo P, et al. Sacubitril/valsartan improves right ventricular function in a real-life population of patients with chronic heart failure: the Daunia Heart Failure Registry. Int J Cardiol Heart Vasc. 2020;27:100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy SP, Prescott MF, Camacho A, et al. Atrial natriuretic peptide and treatment with sacubitril/valsartan in heart failure with reduced ejection fraction. JACC Heart Failure . 2021;9:127–136. [DOI] [PubMed] [Google Scholar]
- 23. Ibrahim NE, Piña IL, Camacho A, et al. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur J Heart Fail. 2020;22:2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clements RT, Vang A, Fernandez-Nicolas A, et al. Treatment of pulmonary hypertension with angiotensin II receptor blocker and neprilysin inhibitor sacubitril/valsartan. Circ Heart Fail. 2019;12:e005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharifi Kia D, Benza E, Bachman TN, et al. Angiotensin receptor-neprilysin inhibition attenuates right ventricular remodeling in pulmonary hypertension. J Am Heart Assoc. 2020;9:e015708. [DOI] [PMC free article] [PubMed] [Google Scholar]


