Abstract
Introduction
WHO has launched updated cervical screening guidelines, including provisions for primary HPV screen-and-treat. Papua New Guinea (PNG) has a high burden of cervical cancer, but no national cervical screening programme. We recently completed the first field trials of a screen-and-treat algorithm using point-of-care self-collected HPV and same-day treatment (hereafter self-collected HPV S&T) and showed this had superior clinical performance and acceptability to visual inspection of the cervix with acetic acid (VIA). We, therefore, evaluated the effectiveness, cost-effectiveness and resource implications of a national cervical screening programme using self-collected HPV S&T compared with VIA in PNG.
Methods
An extensively validated platform (‘Policy1-Cervix’) was calibrated to PNG. A total of 38 strategies were selected for investigation, and these incorporated variations in age ranges and screening frequencies and allowed for the identification of the optimal strategy across a wide range of possibilities. A selection of strategies that were identified as being the most effective and cost-effective were then selected for further investigation for longer-term outcomes and budget impact estimation. In the base case, we assumed primary HPV testing has a sensitivity to cervical intraepithelial neoplasia 2 (CIN2+) + of 91.8% and primary VIA of 51.5% based on our earlier field evaluation combined with evidence from the literature. We conservatively assumed HPV sampling and testing would cost US$18. Costs were estimated from a service provider perspective based on data from local field trials and local consultation.
Results
Self-collected HPV S&T was more effective and more cost-effective than VIA. Either twice or thrice lifetime self-collected HPV S&T would be cost-effective at 0.5× gross domestic product (GDP) per capita (incremental cost-effectiveness ratio: US$460–US$656/life-years saved; 1GDPper-capita: US$2829 or PGK9446 (year 2019)) and could prevent 33 000–42 000 cases and 23 000–29 000 deaths in PNG over the next 50 years, if scale-up reached 70% coverage from 2023.
Conclusion
Self-collected HPV S&T was effective and cost-effective in the high-burden, low-resource setting of PNG, and, if scaled-up rapidly, could prevent over 20 000 deaths over the next 50 years. VIA screening was not effective or cost-effective. These findings support, at a country level, WHO updated cervical screening guidelines and indicate that similar approaches could be appropriate for other low-resource settings.
Keywords: screening, cancer, mathematical modelling, health economics
Key questions.
What is already known?
Papua New Guinea (PNG) is a lower-middle-income country with a high burden of cervical cancer.
In 2020, WHO launched a strategy to eliminate cervical cancer as a public health problem globally through wide-scale uptake of HPV vaccination, cervical cancer screening, and improving access to cancer treatment and care, and in July 2021, WHO launched updated cervical screening guidelines recommending primary HPV testing in a screen-and-treat approach or screen-triage-and-treat for ages 30–50 years. To make progress towards cervical cancer elimination, it is essential for PNG to identify the screening strategies which are locally appropriate.
The first field trials of point-of-care (PoC) HPV testing using self-collected vaginal specimens followed by same-day thermal ablation (or gynaecological referral if a cervical lesion seen on examination) have been conducted in PNG. This screening modality was found to be acceptable to women and effective for detection of high-grade precancerous lesions. However, optimal screening ages and frequencies, and the cost-effectiveness of a national roll-out of such an approach has not been determined.
Key questions.
What are the new findings?
Evaluation of HPV self-collection is increasingly being performed in the context of LMICs. Different models have been assessed for delivery of self-collection and modalities of ‘test and treat’ or ‘test, triage and treat’. Here, we aimed to combine all the elements for delivery of a pragmatic approach in a high burden population never reached by screening, including HPV testing with point-of-care and self-collection modalities and a screen-and-treat approach involving thermal ablation (self-collected HPVS&T).
This is the first modelled analysis which captures all elements of point-of-care HPV self-collected screen and treat (self-collected HPV S&T), an approach designed for a high-burden and low-resource setting like PNG.
Either twice (at 30 and 40 years or at 35 and 40 years) or thrice per lifetime (at 30, 35 and 40 years) self-collected HPV S&T could reduce cervical cancer incidence by 35%–48% and mortality by 39%–49% over the long term.
What do the new findings imply?
The study findings support the WHO cervical cancer elimination strategy and their recently updated cervical screening guidelines.
The results in this paper will also support major new implementation effort in the Western Pacific to scale up HPV vaccination, screen-and-treat, and cancer treatment services towards achieving cervical cancer elimination in PNG and more broadly in the region (Eliminate Cervical Cancer in the Western Pacific (ECCWP) initiative). This practically realisable HPV-based screening modality is likely to be applicable for other LMICs where resources for cervical screening are limited.
Introduction
Cervical cancer is the second most common cancer among women in low- and lower-middle income countries with a population-weighted average age-standardised incidence rate (ASR) of 17.8 new cases per 100 000 women in 2018.1 Papua New Guinea (PNG) has a high estimated burden of cervical cancer with an ASR incidence rate of 29.1/100 000 women (all ages) and mortality rate of 19.1/100 000 in 2018,1 which is 1.6 times higher the average incidence rate for all low- and lower-middle income countries.2 Although the burden of disease is high, cervical screening or human papillomavirus (HPV) vaccination programmes are not currently available in PNG. A pilot cervical cytology-based screening project was conducted in 30 health facilities in 16 provinces by an Australian non-government organisation3 and screened around 45 000 women (~4% of target population aged 30–59 years) in the period 2001–2011.3 As Pap test specimens were sent to Australia for testing, over 60% of those with high-grade disease needing further investigation or treatment were lost to follow-up due to the time between testing and recall. In 2009, a Ministerial Task Force on Cervical Cancer recommended the discontinuation of Pap test based screening in PNG, and the evaluation of the ‘screen and treat’ approach using visual inspection of the cervix with acetic acid (VIA), followed by cervical cryotherapy if lesions were identified, which was based on the 2014 WHO cervical cancer screening guidelines.4 5 A subsequent evaluation found the sensitivity of VIA to detect high-grade precancerous lesions in PNG was 51.5% and specificity 81.4%.6 We recently conducted the first field trials to evaluate a new screening model comprising point-of-care (PoC) HPV testing (GeneXpert; Cepheid, Sunnyvale, California, USA) using self-collected vaginal specimens followed by same-day curative treatment using a new, battery-operated, portable, thermal ablation device (WISAP Medical Technology).6–11 In our first trial among 1005 women in PNG (2013–2015), we showed that (1) HPV testing of self-collected vaginal specimens had comparable sensitivity (91.7%) and specificity (87.0%) to clinician-collected cervical specimens for the detection of cervical pre-cancer using the GeneXpert platform6 7 12; (2) VIA alone, or VIA in combination with HPV testing had poor performance for the detection of underlying High-Grade Squamous Intraepithelial Lesions (HSIL) or worse (sensitivity 51.5% and 45.5%, respectively) compared with HPV testing alone (sensitivity 91.7%)6; and (3) with suitable training and support, PoC HPV self-collected screen and treat (thereafter self-collected HPV S&T) can be provided routinely in primary care facilities.7 The poor performance of VIA in this trial is similar to the findings reported in India and Rwanda.13 14 In a second field trial among 4000 women in PNG (2017–2020), we confirmed the clinical performance of our self-collected HPV S&T modality for the detection and treatment of cervical pre-cancer, and its high acceptability among women and health providers.15
In May 2018, the Director-General of the WHO announced a global call to action towards achieving the elimination of cervical cancer as a public health problem. In November 2020, WHO launched the global elimination strategy16 that included the ‘90-70-90’ triple intervention targets to be met by 2030: (1) 90% of girls fully vaccinated with the HPV vaccine by age 15; (2) 70% of women screened with a high-precision HPV test by age 35 and 45 years of age; and (3) 90% of women with cervical precancer treated, and 90% of women with invasive cancer managed and treated appropriately. Achieving the triple-intervention targets in the next decade would put countries on the path to achieving elimination in the next century, reducing cervical cancer mortality by 99% and saving more than 62 million women’s lives over the next century.2 To support elimination effort, WHO has recently released updated cervical screening guidelines which recommend primary HPV screen-and-treat or primary HPV screen-triage-and-treat for women aged 30–49 years.17
To make progress towards elimination, it will be essential for countries to implement locally appropriate, context-specific intervention strategies. In this paper, we evaluated new WHO screen-and-treat approach informed by local data from a field trial of self-collected HPV S&T modality to identify the optimal cervical screening strategy for PNG. Here, we reported on the estimated effectiveness and cost-effectiveness of national roll-out of self-collected HPV S&T in PNG, the long-term impact on cervical cancer incidence and mortality, and the resource implications of scaling up such an algorithm.
Methods
Model platform
A validated dynamic individual-based microsimulation model (‘Policy1-Cervix’) of HPV transmission, type-specific natural history of cervical intraepithelial neoplasia (CIN) and invasive cancer staging, and cervical screening, diagnosis, and treatment was used. This model platform has been used to evaluate the effectiveness and cost-effectiveness of cervical cancer screening for both vaccinated and unvaccinated cohorts across different settings, including the renewal of the cervical screening programme in Australia,18 19 the impact of HPV testing using self-collected samples in Australia,20 the impact of primary HPV testing in New Zealand,21 England22 and China23–25 and vaccine evaluations in Japan.26 Most recently, this model was also used to evaluate the timeline to cervical cancer elimination for 78-low-income and lower-middle income countries (LMICs), in Australia, USA and globally2 27–30 and is also being used to inform development of updated WHO screening guidelines.17 (online supplemental figure A1. More details of the model platform present in the online supplemental appendix, p1-2 and via Policy1-Cervix website https://www.policy1.org/models/cervix/documentation).
bmjgh-2021-007380supp001.pdf (2.1MB, pdf)
Model calibration
We calibrated the model to the cervical cancer incidence rate in PNG using age-specific GLOBOCAN 2018 data1 (ASR=28.4/100 000 women (0–84 years)) as shown in online supplemental figure A2 (A)). Additionally, the model was also calibrated to the age-specific high-risk HPV prevalence among women aged 18–54 years, based on data from a local HPV prevalence survey31 (online supplemental figures A2 (C) and A3).
Background hysterectomy rates
Although there is some hysterectomy being done on benign conditions, this information is not well documented or there is paucity of information, we assumed there was no background hysterectomy for benign conditions.
Screening and management pathways
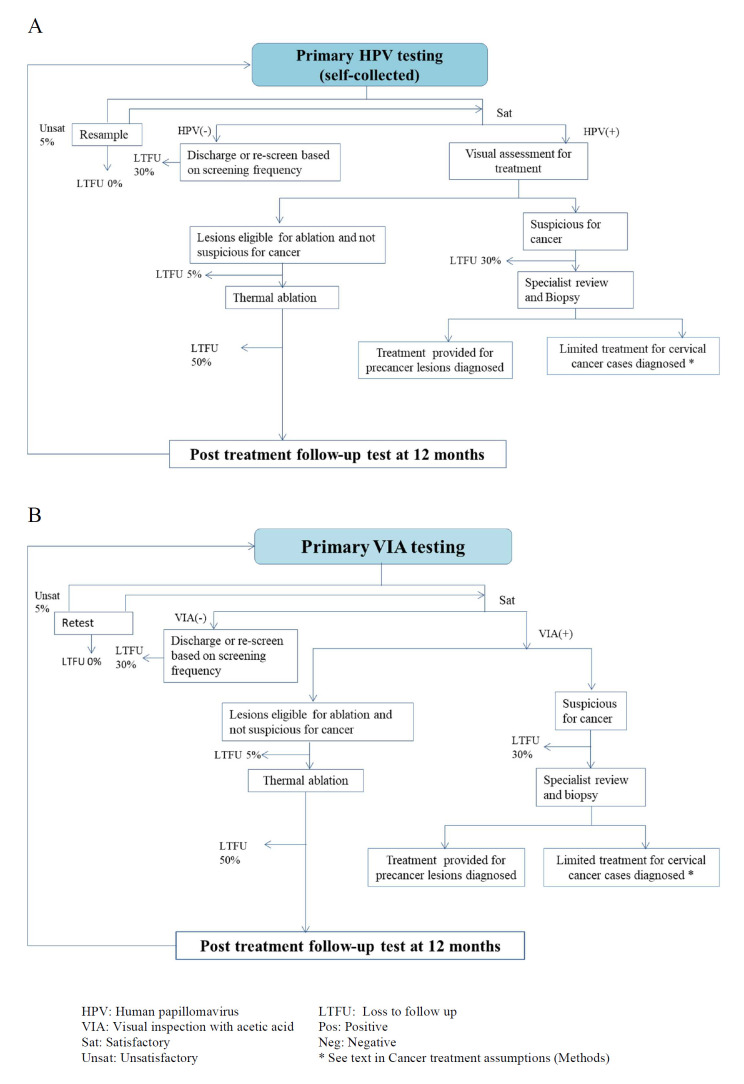
We considered two screening and management pathways. The first is the same pathway as in the field project, utilising self-collected HPV S&T (figure 1A). In this pathway, women who are positive at primary HPV testing are treated with thermocoagulation of the transformation zone of the cervix. For the second pathway, we considered screening with VIA (figure 1B). In both screening pathways, women who have a cervical lesion at visual assessment, but are not suspected of harbouring a cancer, are immediately treated with ablation and that women whose lesions are large or suspicious for cancer are referred to a specialist for further assessment. For both pathways, we assumed that women who were referred for diagnosis with suspicion of cancer, but found to have CIN3, would be treated with hysterectomy or conisation depending on patient individual clinical circumstances (based on local expert opinion). We assumed that women who are negative may return for screening at a set time, depending on the screening frequency, and that women who received treatment for precancer for a ‘test of cure’ using the same test as the primary test.
Figure 1.
Screening management pathway. (A) Point-of-care (PoC) HPV self-collected screen and treat (self-collected HPV S&T), (B) VIA screening.
Screening ages and frequencies
Although initial field trials focused on women aged 30–59 years, in the modelled analysis we considered various screening frequencies (once, twice, thrice lifetime and 5 yearly) at different initiating ages (30 years, 35 years and 40 years).
For this analysis, we considered two overall steps. In the first step (step 1), a total of 38 screening strategies were assessed to identify the optimal screening strategies for PNG, considering both ‘self-collected HPV S&T’ and VIA approaches and varying screening ages and frequencies. Single cohort modelling approaches were used for this step to identify the lifetime impacts of each screening strategy and cost-effective screening strategies. In step 2, strategies that appeared on the cost-effectiveness frontier from step 1 were selected to assess the long-term impact of scaling-up screening on ASR cancer incidence and mortality, cases and deaths, resource utilisation and projected financial costs nationwide (table 1). In addition to the scenarios that appeared at the cost-effectiveness frontier, we also included the WHO elimination strategy (twice per lifetime at ages 35, 45)17 in step 2.
Table 1.
Screening strategies
| Strategy no | Step 1: Strategies included, varying ages and frequencies for both primary HPV and VIA approaches | Step 2: Strategies assessed to predict impact on health outcomes, resource utilisation and budget impact |
| 38 strategies were assessed using the screening pathways as in figure 1A (self-collected HPV S&T) and 1B (VIA) | Strategies that appeared on the cost-effectiveness frontier from step 1 were assessed, as well as the WHO elimination strategy (twice lifetime at ages 35, 45) | |
| 0 | No intervention | No intervention |
| 1 | 1X-lifetime screening | |
| 1.1 | Once lifetime at age 30 (1X) | |
| 1.2 | Once lifetime at age 35 (1X) | Once lifetime at age 35 (1X) |
| 1.3 | Once lifetime at age 40 (1X) | |
| 1.4 | Once lifetime at age 45 (1X) | |
| 1.5 | Once lifetime at age 50 (1X) | |
| 2 | 2X-lifetime screening | |
| 2.1 | Twice lifetime at age 30, 35 (2X) | |
| 2.2 | Twice lifetime at age 35, 40 (2X) | Twice lifetime at age 35, 40 (2X) |
| 2.3 | Twice lifetime at age 40, 45 (2X) | |
| 2.4 | Twice lifetime at age 45, 50 (2X) | |
| 2.5 | Twice lifetime at age 30, 40 (2X) | Twice lifetime at age 30, 40 (2X) |
| 2.6 | Twice lifetime at age 35, 45 (2X)—WHO global elimination strategy and 2021 WHO guideline’s recommended strategy | Twice lifetime at age 35, 45 (2X)-WHO global elimination strategy and 2021 WHO guideline’s recommended strategy |
| 2.7 | Twice lifetime at age 40, 50 (2X) | |
| 2.8 | Twice lifetime at age 45, 55 (2X) | |
| 3 | 3X-lifetime screening | |
| 3.1 | Thrice lifetime at age 30, 35, 40 (3X) | Thrice lifetime at age 30, 35, 40 (3X) |
| 3.2 | Thrice lifetime at age 35, 40, 45 (3X) | |
| 3.3 | Thrice lifetime at age 40, 45, 50 (3X) | |
| 3.4 | Thrice lifetime at age 30, 40, 50 (3X)-2021 WHO guideline’s recommended strategy | |
| 3.5 | Thrice lifetime at age 35, 45, 55 (3X) | |
| 4 | 5-yearly screening 30–55 | |
| 4.1 | 5-yearly at age 30–55 (6X) | 5-yearly at age 30–55 (6X) |
| Total: 38 screening strategies | Total: 5 screening strategies plus WHO elimination strategy |
S&T, screen and treat; VIA, visual inspection with acetic acid.
Screening compliance assumptions
We assumed 10% of women never attend screening in their lifetime for all scenarios. We assumed that 70% of women will attend routine screening at each invitation, selected from 90% of the population of ever-screeners. Given that treatment with ablation is performed on the same day, we assumed 5% lost to follow-up for ablation as this is consistent with experiences on-the-ground in PNG. 17
These assumptions on compliance with routine attendance were similar to the assumptions used in the modelling to support WHO’s updated cervical screening guidelines. However, for women referred to diagnostic services and for women who received treatment and need to attend post-treatment follow-up at 12 months, we assumed 50% lost to follow-up, based on local experience. For women who were referred for diagnostic evaluation for suspicion of cancer, we assumed a 30% lost to follow-up, due to limited facilities and difficulties in travel, particularly for rural women (see figure 1A, B).
Screening test characteristics
The test characteristics for primary self-collected HPV testing were obtained from an international systematic review on the sensitivity and specificity of PCR-based HPV testing using self-collected samples as well as from the local trial of PoC HPV self-collected testing in PNG.32 The international systematic review has found that PCR self-collected HPV testing was as sensitive as clinician-collected HPV testing to detect CIN2+ (pooled relative ratio 0.99 (95% CI 0.97 to 1.02).32 Based on a local trial in PNG, Toliman et al found that self-collected HPV testing had sensitivity of 91.7% to detect high-grade lesions.6 Therefore, in this study, we took a conservative approach and assumed that HPV had sensitivity to CIN2 +of 91.7% and specificity of 89.8% in the base case, which is slightly lower than performance reported for clinician-collected HPV samples. We also considered 89.1% (lower bound) and 95.3% (upper bound) sensitivity to CIN2 +in sensitivity analysis. VIA test characteristics estimated from a screening trial in PNG was used in the base case analysis.6 The VIA screening trial in PNG involved ~1000 women and identified 51.5% sensitivity and 81.4% specificity to detect CIN2 +and this test performance was assumed in the model.6 This performance assumption for VIA is consistent with studies from India13 and Rwanda14 and consistent with the outcomes of large population-based experiences of VIA in India, in which VIA testing of 70 000 women over 12 years did not reduce the incident cancer, and only reduced mortality rates through stage-shifting.33 A more favourable sensitivity (70% sensitivity to CIN2+) of VIA testing inferred from international systematic review was also used in sensitivity analysis34 35 (table 2).
Table 2.
Summary of model parameters for screening, diagnosis, and treatment procedures, and ranges for sensitivity analysis
| Parameters | Baseline value | Range for sensitivity analysis (lower bound and upper bound) | Sources |
| Preintervention burden of disease | |||
| Incidence | ASR-W (0–84)=28.4/100 000 | N/A | GLOBOCAN, 20181 |
| Mortality | ASR-W (0–74)=18.6/100 000 | N/A | GLOBOCAN, 20181 |
| Screening participation and compliance | |||
| Screening participation rate | 90% of women ever screen; 70% return for their next scheduled routine visit (selected from the 90% ever-screeners) |
50%–90% | Based on WHO elimination targets by 203016 |
| Rate of lost to follow-up for same-day treatment with thermal ablation | 5% | NA | Assumption based on the trial outcomes |
| Rate of loss to follow-up (LTFU) of referral for treatment of larger lesions or suspicious cancer investigation | 30% for self-collected HPV S&T 30% for VIA |
Based on limited health facilities that can offer cancer diagnosis and treatment, as well as the limited access for rural women. (Personal communication with local experts) | |
| LTFU of women after ablative treatment for precancer at 12 m follow-up visit | 50% for self-collected HPV S&T 50% for VIA |
Lower bound: 10% for self-collected HPV S&T and 10% for VIA screening |
Base case: based on limited health facilities and as well as the limited access for rural women. Lower bound: based on the experience of the field trial in PNG. |
| Screening test characteristics | |||
| self-collected HPV | Sensitivity of 91.7% and specificity of 89.8% to detect CIN2+.* | Sensitivity: 89.1%–95.3% and Specificity: 88.6%–90.6% to detect CIN2+ | Arbyn et al48
32; Toliman et al6 |
| Primary VIA | Sensitivity of 51.5% and specificity of 81.4% for CIN2+.* | Upper bound: Sensitivity of 70% and specificity of 78% for CIN2+. |
Base case: Toliman et al6 Upper bound: Based on the upper 95% CI of VIA test performance Toliman et al6 and a systematic review on VIA test performance categorised for high quality studies.35 |
| Ablation treatment success rate | 84.3%–92.4% for CIN1-3; 0% for cancer | Randall et al49 | |
| Cancer treatment | |||
| % Cancer treatment uptake for symptomatically detected cancers | 20% treatment access rate overall. (Detailed assumptions in the Methods section and online supplemental appendix) |
Lower bound: 8%. Upper bound: 90% treatment access overall |
Base case: Only a few health facilities can offer cancer diagnosis and treatment, which is limited to radical hysterectomy (Based on personal communication with local experts). Lower bound: Based on access rate to radiotherapy estimated by Datta et al.50 Upper bound: Based on WHO cancer treatment target for cervical cancer elimination.16 |
| 5- year survival by FIGO stage (%) | FIGO I: 0.64; FIGO II: 0.52; FIGO III: 0.12; FIGO IV: 0.01 (online supplemental table A1) |
Lower bound: FIGO I: 0.625; FIGO II: 0.48; FIGO III: 0.101; FIGO IV: 0.011 Upper bound: FIGO I: 0.869; FIGO II: 0.774; FIGO III: 0.599; FIGO IV: 0.117 (online supplemental table A1) |
Base case: Informed by stage-specific survival rates for countries with ~20% cancer treatment access to radiotherapy,2 assuming the survival benefits are mostly targeted to Stage 1 and 2, and further adjusted to fit to GLOBOCAN2018 mortality rates. Lower bound: We assumed the same survival rates estimated for PNG as reported in Canfell et al.2 Upper bound: We assumed the same survival rates estimated for countries with 90% treatment access rate across all stages as reported in Canfell et al.2 |
| Costs † (US$) and other health economic parameters | |||
| self-collected HPV test cost ‡ | US$18 | US$8 |
Base case costs were estimated based on the current screening trial in PNG. Lower cost of HPV test in sensitivity analysis was assumed based on discussions with experts regarding future reduction in HPV test costs. |
| VIA test cost ‡ | US6 | NA | |
| Biopsy | US$59 | NA | |
| Ablation | US$15 | NA | |
| Cancer treatment costs | |||
| FIGO I | US$1614 (applied to 80% of FIGO I diagnosed cases) | Upper bound: US$1937 |
Base case: costs were estimated based on personal communication with local experts. Upper bound: Cancer treatment cost for FIGO I and II was assumed 20% higher than base case. In a sensitivity analysis considering a 90% cancer treatment access, we assumed some treatment options for advanced cancer stages would be available in PNG. We assumed cancer treatment costs for FIGO III were 40% higher than treatment cost for FIGO I and cost for FIGO IV was equal to cost for FIGO I treatment. These factors were derived from previous study.51 (see online supplemental tables A2 and A3) |
| FIGO II | US$1614 (applied to 20% of FIGO II diagnosed cases) | Upper bound: US$1937 | |
| FIGO III | 0 | Upper bound: 0 | |
| FIGO IV | 0 | Upper bound: 0 | |
| Threshold for willingness-to-pay | 0.5 X PNG GDP per capita, US$1415 (PGK4723) | 1 X PNG GDP per capita, US$ 2829 (PGK9446) | PNG GDP per capita was based on World Bank, 2019.52 |
| Discount rates | |||
| Effects | 3% | 0% | WHO-CHOICE cost-effectiveness analysis guideline. |
| Costs | 3% | 3% | WHO-CHOICE cost-effectiveness analysis guideline. |
We assumed the test performance for HSIL are equivalent for CIN2+.
Costs were collected in PGK currency and converted to US$, using exchange rate of PGK1=US$0.3, 17 October 2019, Commonwealth Bank, Australia)
*The Toliman et al study reported test performance of PoC HPV self-collected testing and VIA testing for HSIL.
†Costs were estimated from service provider’s perspective, considering direct medical costs that associated with each screening, diagnostic tests or treatment procedures.
‡Including costs of test and test delivery.
ASR, age-standardised rate; CIN, cervical intraepithelial neoplasia; FIGO, International Federation of Gynaecology and Obstetrics; GDP, gross domestic product; HSIL, high-grade squamous intraepithelial lesions; PNG, Papua New Guinea; PoC, point-of-care; S&T, screen and treat; VIA, visual inspection with acetic acid.
Vaccination assumptions
Although the PNG government is committed to HPV vaccine introduction and pilot HPV vaccine projects were completed in some provinces for schoolgirls some years ago,36 at this point a national HPV vaccination programme has not been recommended in PNG. In the first couple of decades after vaccination, most women over 30 years, and thus eligible for screening, will not be vaccinated. Therefore, in this analysis, we assumed that cervical screening strategies were conducted in women who have not received the HPV vaccine.
Cancer treatment assumptions
The current infrastructure for cancer treatment in PNG is very limited, based on consultation with local experts. Only one radiotherapy treatment unit has been established, which was reported to be non-functional since 201537. We therefore assumed that radiotherapy access is limited and unreliable. We assumed that only radical hysterectomy (available in a few hospitals) was used for women with early-stage cancers. Given this situation, at base case analysis we assumed that 80% of cervical cancer diagnosed at International Federation of Gynaecology and Obstetrics (FIGO) stage I, 20% of those diagnosed at FIGO II would be treated with radical hysterectomy, those diagnosed at FIGO III and IV were not treated, and that these treatment rates did not vary for screen-detected or symptomatically detected cancers. The modelled distribution of cervical cancer stage in PNG was 14%, 55%, 27% and 3% for FIGO I, II, III and IV, respectively. Assuming 80% of FIGO I and 20% of FIGO II cancers receive treatment resulted in 80×14%+20×55%+0×27%+0×3%=20% of any diagnosed cancer would be treated in base case analysis, and therefore costs of cancer for these stages were adjusted accordingly. Our survival inputs produced similar mortality rates to those reported in GLOBOCAN2018 for PNG1 (online supplemental figure A2 (B)). We also considered lower (8%) and higher (90%) cancer treatment access rates in sensitivity analysis. (more details in online supplemental appendix 1, part 3. Cancer treatment access rate and survival assumptions)
Costs
Costs were estimated from a service provider perspective. Direct medical costs were considered only. Costs were originally assessed in the field in PGK (PNG currency) and were converted to US$, using the 2019 exchange rate (PGK1=US$0.3, 17 October 2019, Commonwealth Bank, Australia).38 Costs associated with cervical cancer screening and thermal ablation were estimated from our screening trials in PNG. Based on financial expenditures on personnel, equipment, consumables, and the number of women screened during the trial period, we estimated unit costs of HPV testing, VIA testing, visual assessment for ablation and thermal ablative treatment (table 2). The number of screened women was based on the actual number of women was screened and treated each day in the ongoing trials in PNG. Using this method, the cost of HPV testing (including costs associated with test and test delivery) was US$18 per test as currently estimated. In the context of expected mass HPV testing in the next decade, we conservatively assumed a unit test cost for HPV testing of US$18 in the base case. However, it is possible the HPV test cost would be decreased in the context of a national screening programme as higher volumes could result in more competitive test prices. Therefore, we considered a lower HPV test cost (US$8) in sensitivity analysis. The cost of VIA as a primary or as an assessment for same-day treatment was estimated to be US$6 per test. The unit cost of thermal ablation was estimated to be US$15. Other unit costs, including costs of biopsy and treatment for lesions ineligible for ablation and for early-stage cancers with full course of treatment with hysterectomy (that are currently available in PNG) were estimated based on consultation with local experts. In consultation with local experts, the costs of biopsy and cancer treatment for FIGO I and FIGO II were estimated to be US$59 and US$1614, respectively. At present, treatments for stage III and stage IV cancers and for palliative care are not available in PNG, therefore no treatment cost was assumed to be incurred for cancer at these stages in base case analysis. However, in sensitivity analysis considering a 90% treatment access rate, we assumed that treatment for advanced-stage cancers would be available. (Details in online supplemental appendix, part 4. Cost estimates) Based on the analysis of the cost profile by different cost components, which show substantial proportions of screening costs and cancer treatment costs (which mainly were treatment costs for early-stage cancers), we checked for the robustness of the results by considering variations in these costs in sensitivity analysis.
Outcomes assessed
For step 1, we report on outcomes over the lifetime of unvaccinated women who would turn 30 in 2023, the first cohort to be fully impacted by scale-up of cervical screening in PNG. Outcomes assessed include ASR of cervical cancer incidence and mortality, and cost-effectiveness. For step 2, we selected the strategies that appeared on the cost-effectiveness frontier from step 1 as well as the WHO elimination strategy, and further assessed the longer-term outcomes of scaling-up screening to reach 70% coverage from 2023 onwards and reported on the ASR of cervical cancer incidence and mortality out to 2072, and total cancer cases and deaths predicted. We also estimated the total undiscounted financial budget impact of a national screening programme over a 5-year (2023–2027) and 10-year (2023–2032) horizon. We did not account for inflation in this budget impact analysis. This financial cost projection was estimated at service provider perspective, including direct medical costs associated with cervical cancer screening, precancer treatment, cancer diagnosis and treatment for PNG; however, they do not include overhead costs of the programme, including administrative costs and capital costs of existing buildings, equipment, vehicles, that allocated to these services. These costs also do not include start-up costs associated with the establishment of a national screening programme, including costs of screening registry system, training for health staff and community mobilisation that usually occur at a configuration stage of a national cervical screening programme. For step 2, we also estimated the annual number of HPV tests, annual number of women diagnosed with precancerous lesions and eligible for ablation, annual number of women diagnosed with precancerous lesions but ineligible for ablation, and the number with symptomatic cancer and screen-detected cancer. The annual resource utilisation numbers were calculated as an average over the first 5 years of a potential national cervical screening programme in PNG.
The cost-effectiveness analysis (CEA) was conducted from the service provider perspective for cervical cancer prevention. A single cohort CEA over the lifetime of the cohort was performed across 38 screening strategies assuming a 3% discount rate for effects and costs (starting at age 12), based on WHO recommendations.39 A 0% discount for effects and 3% discount for costs was considered in sensitivity analysis. In CEA, the incremental cost-effectiveness ratios (ICERs) were used to compare to willingness-to-pay (WTP) threshold. Half of the gross domestic product (GDP) per capita for PNG (0.5GPDpc (US$1415 or PGK4723, World Bank 2019)) was used as the indicative WTP threshold for the evaluation.40 We also considered a WTP threshold of 1GPDpc (1GDPpc=US$2829 or PGK9446).
Sensitivity analysis
Univariate sensitivity analysis was performed on key model assumptions to evaluate the robustness of the results. Key parameters were considered, including PoC HPV self-collected test accuracy (best sensitivity (95.3%) and worst sensitivity (89.1%) vs 91.7% at base case) of HPV and VIA screening, lower costs of HPV tests (US$8/test vs US$18/test at base case), higher cancer treatment costs (20% higher vs costs at base case), variation in screening coverage (50% and 90% vs 70% at base case), lower loss to follow-up rates at post-treatment follow-up (10% vs 50%), lower and upper cancer treatment access rate (8% and 90% vs 20% at base case), and lower discount rates for effects (0% vs 3% at base case) (table 2).
Patient involvement
This modelling study has been done as part of a programme ‘Prospective cohort study to evaluate PoC HPV-DNA self-collected testing for the early detection and treatment of cervical pre-cancer in high-burden, low resource settings’ funded by the National Health and Medical Research Council (NHMRC) Australia Project Grant 1104938. The modelling assumptions have been informed by data/information regarding screening management pathways, PoC HPV self-collected test performance, VIA test performance, costing data from past and ongoing field trials in PNG in collaboration with local investigators, who directly working with patients and understand the local context.
As many other field trials, patient involvement concept has been integrated through different study stages. The previous VIA and ongoing self-collected HPV S&T field trials were designed in partnership with national and provincial health authorities, expert groups (PNG OBGYN Society, Technical working group on cervical cancer)—whose works are focused on improving population health in general and women health in particular, and civil society groups (PNG Cancer Foundation)—where including patient representatives/cancer survivors. The design of the field trials had been informed by findings of a qualitative study, in which we engaged women and their families at community level to explore understandings around cervical cancer causation, stigma, women’s health priorities.41 As a result, the self-collected HPV S&T modality was designed to create the most convenient environment for women to get screened and reduced waiting time.
At recruitment and implementation stage of screening trials, women were provided general information about the screening trials, including key study objectives and procedures; eligibility and inclusion/exclusion criteria; and the benefits and potential risks of study participation. Women can choose to participate or not in the study based on formal informed consent procedures.
Six-monthly newsletters have been prepared for study participants, local stakeholders and clinical staff at study sites documenting progress with each stage of the trial, emerging issues, upcoming events and new staffing. These newsletters have been disseminated to study sites and key local and national stakeholders including community leaders, national and provincial health departments, and other relevant organisations. Cancer survivors who participated in the trials have been invited to talk about her experience of being early diagnosed and treated cervical cancer.
Results
Effectiveness outcomes
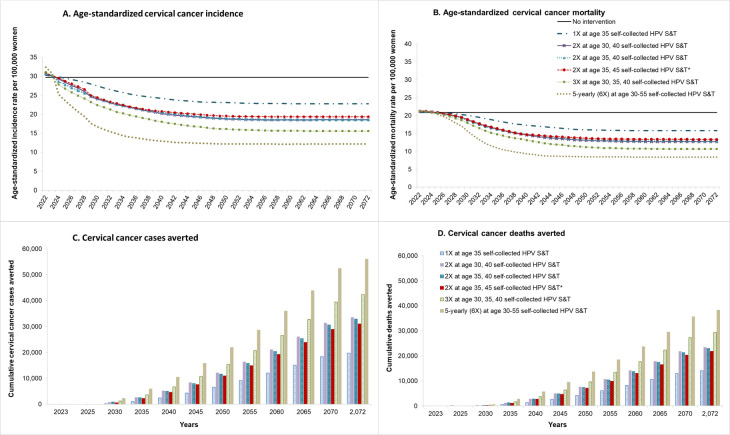
Assuming no further intervention in PNG (‘no intervention’), the ASR of cervical cancer incidence was predicted to remain at 29.7 per 100 000 women (0–84 years) and mortality at 20.8 per 100 000 women (0–84 years) for the next 50 years, and generate 1085 new cases and 737 deaths in the year 2020 alone (figure 2 and online supplemental figure A4). Screening either two or three times in a lifetime for women aged 30, 40 years and 30, 35, and 40 years, respectively, using self-collected HPV S&T reduced the ASR of cervical cancer incidence to 15.6–18.6 per 100 000 (35%–48% reduction) and reduced ASR mortality to 10.6–12.7 per 100 000 women (39%–49% reduction) over the longer term. In contrast, either two or three times screening with VIA in a lifetime reduced the ASR of cervical cancer incidence by 17%–24%, and the ASR of mortality by 18%–25% over a lifetime. With a favourable sensitivity of VIA for CIN2/3 (70% sensitivity), two or three times in a lifetime reduced the reduced the ASR of cervical cancer incidence by 19%–30% and reduced mortality by 20%–32%, and therefore, remained less effective than self-collected HPV S&T even in this favourable scenario (online supplemental figure A5).
Figure 2.
Age-standardised incidence and mortality and cumulative cervical cancer cases and deaths averted over time of the strategies which were the most cost-effective as identified in step 1. *WHO recommendation for cervical screening for cervical cancer elimination. S&T, screen and treat.
Cost-effectiveness outcomes (step 1)
Once lifetime (age 35 years, 1X), twice lifetime (30&40 years and 35, 40 years, 2X) and thrice lifetime (age 30, 35 and &40 years, 3X) screening strategies with self-collected HPV S&T were on the cost-effectiveness frontier (figure 3). The ICERs of these strategies were US$311/life-years saved (LYS), US$460/LYS-US$568/LYS, US$656/LYS, respectively, and therefore, these strategies were considered as cost-effective at the 0.5GDP per capita threshold (US$1415). At 1GDPpc (US$2829), once, twice and thrice lifetime screening, and even 5-yearly screening were cost-effective. When assuming 70% VIA sensitivity for CIN2/3, once, twice and thrice lifetime self-collected HPV S&T remained cost-effective at either 0.5GDP or 1GDP per capita thresholds, and therefore primary VIA screening was not cost-effective even under these favourable assumptions around test performance (online supplemental figure A6).
Figure 3.
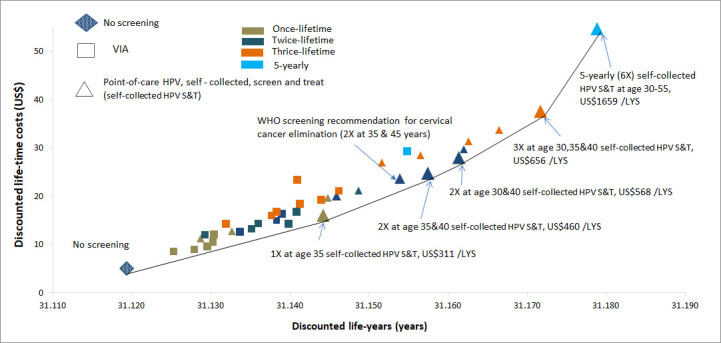
Cost-effectiveness analysis. The performance of VIA screening test (51% sensitivity) was derived from VIA screening trial in PNG reported in Toliman et al. The cost-effectiveness analysis included current situation (no screening) and 38 self-collected HPV S&T and VIA screening scenarios. The gross domestic product (GDP) per capita for PNG (0.5GDPpc (US$1415 or PGK4723, world bank 2019) was used as the indicative willingness-to-pay (WTP) threshold for the evaluation. We also secondarily considered a WTP threshold of 1GDPpc (1GDPpc=US$2829 or PGK9446). LYS, life-years saved; PNG, Papua New Guinea; S&T, screen and treat; VIA, visual inspection with acetic acid.
Long-term impact of PoC self-collected HPV screening strategies that appeared on the cost-effectiveness frontier as identified in step 1
Figure 2 shows the ASR and mortality rates and cumulative cases and deaths averted over the next 50 years (2023–2072) for the five self-collected HPV S&T strategies that appeared on the cost-effectiveness frontier plus the WHO elimination strategy, and table 3 shows the cumulative number of cases and deaths in PNG, assuming screening was scaled up in PNG from 2023. Screening women three times per lifetime with self-collected HPV S&T was predicted to prevent 42 352 cases and 29 353 deaths over the 50-year period 2023–2072, compared with no screening. Screening every 5 years at ages 30–55 years could avert 56 047 cervical cancer cases and 38 244 deaths over the next 50 years (figure 2 and table 3).
Table 3.
Cumulative cases and deaths over 50 years (2023–2072) of the strategies which were the most cost-effective as identified in step 1 in PNG
| Scenarios | No intervention | 1X at age 35 self-collected HPV S&T | 2X at age 30, 40 self-collected HPV S&T | 2X at age 35, 40 self-collected HPV S&T | 2X at age 35, 45 self-collected HPV S&T* | 3X at age 30, 35, 40 self-collected HPV S&T | 5-yearly (6X) 30–55 self-collected HPV S&T |
| Incidence | |||||||
| Cumulative cases over 2023–2072 | 108 204 | 88 509 | 74 623 | 75 323 | 77 183 | 65 852 | 52 158 |
| Cases averted (2023–2072) | – | 19 695 | 33 581 | 32 881 | 31 021 | 42 352 | 56 047 |
| Mortality | |||||||
| Cumulative deaths over 2023–2072 | 75 731 | 61 716 | 52 247 | 52 789 | 53 899 | 46 378 | 37 487 |
| Deaths averted (2023–2072) | – | 14 015 | 23 484 | 22 942 | 21 833 | 29 353 | 38 244 |
*WHO recommendation of cervical screening for cervical cancer elimination was added.
PNG, Papua New Guinea; S&T, screen and treat.
Resource utilisation outcomes of the strategies which were the most cost-effective as identified in step 1
Table 4A presents average annual resources of optimal screening strategies required (averaged over the first 5 years of implementation, 2023–2027) assuming the programme is scaled-up nationally in PNG to reach 70% coverage from 2023 onwards. We found that once lifetime self-collected HPV S&T would require 42 500 HPV tests per year. As expected, more frequent screening strategies would require more HPV tests (71 400–83 800 for twice lifetime, depending on screening ages, up to 124 900 for thrice lifetime and 197 400 for 5-yearly screening). Similarly, more frequent screening strategies would require a higher number of ablative treatments, ranging from an annual number of 5892 ablative treatment with once lifetime screening to 20 484 ablative treatments with 5-yearly screening (table 4A). Without screening, on average 1085 women would be diagnosed with cervical cancer through symptomatic presentation annually over the next 5-years. With 70% scale up of the screening programme using self-collected HPV S&T in 2023, on average annually over the next 5-years 1028 women would be diagnosed with cervical cancer (of which 53 cases are screen detected) for once lifetime screening, or 919 cases for 5-yearly screening (of which 193 cases are screen detected). The number of women with lesions ineligible for ablation (and therefore referred for more advanced treatment) was predicted to range from 58 cases in once lifetime screening to 182 cases in 5-yearly screening (table 4A).
Table 4.
Estimated resource utilisation and budget required for a national screening programme of the most cost-effective strategies in PNG. (A) Average annual resources§ required for a national screening program in PNG estimated over the first 5 years of implementation (2023–2027). (B) Estimated total budget* (US$) over the first 5-year and 10-year periods †
| (A) Annual resource utilisation§ | No screening | 1X at age 35 self-collected HPV S&T | 2X at age 35 and 40 self-collected HPV S&T | 2X at age 30 and 40 self-collected HPV S&T | 2X at age 35 and 45 self-collected HPV S&T‡ | 3X at age 30, 35 and 40 self-collected HPV S&T | 5-yearly 30–55 self-collected HPV S&T (6X) |
| No of HPV tests | 0 | 42 500 | 78 200 | 83 800 | 71 400 | 124 900 | 197 400 |
| No of women diagnosed with precancerous lesions and eligible for ablation | 0 | 5892 | 8802 | 12 391 | 8186 | 16 147 | 20 484 |
| No of women diagnosed with cervical cancer through symptomatic presentation | 1085 | 974 | 900 | 907 | 913 | 854 | 726 |
| No of women diagnosed with cervical cancer through screening | 0 | 53 | 89 | 88 | 108 | 108 | 193 |
| No of women diagnosed with precancerous lesions but ineligible for ablation | 0 | 58 | 83 | 110 | 84 | 130 | 182 |
| (B) Budget * | No screening | 1X at age 35 self-collected HPV S&T | 2X at age 35 and 40 self-collected HPV S&T | 2X at age 30 and 40 self-collected HPV S&T | 2X at age 35 and 45 self-collected HPV S&T‡ | 3X at age 30, 35 and 40 self-collected HPV S&T |
5-yearly 30–55 self-collected HPV S&T (6X) |
| 5-year budget (2023–2027) | 1.9M | 6.9M | 10.9M | 11.9M | 10.2M | 16.5M | 24.8M |
| 10-year budget (2023–2032) | 3.8M | 13.9M | 21.3M | 23.4M | 20.3M | 31.7M | 46.9M |
*5-year and 10-year budgets were calculated as the financial costs (US$, 2019) associated with cervical cancer screening, diagnosis, and treatment over the first 5 years (2023-2027) and 10 years (2023-2032) of implementation. This budget is a broad estimate of that required for a future national cervical cancer screening programme in PNG (inflation was not considered).
†We used UN population structure estimated for PNG (year 2020) and assumed this population structure remained over 2023–2032 to estimate budget and resources.
‡WHO recommendation for cervical screening for cervical cancer elimination.
§ Annual resources for a national cervical screening program in PNG were estimated as an average of the resources required over the first 5 years of implementation (2023-2027)
PNG, Papua New Guinea; S&T, screen and treat.
Budget impact and profile of financial costs associated with screening, diagnosis and treatment using self-collected HPV S&T modality
Without a cervical screening programme, it was estimated that total 5-year and 10-year undiscounted financial costs of ~US1.9 million and US$3.8 million, respectively would be spent on diagnosis and treatment of cervical cancer, due to the limited amount of treatment available for cervical cancer (table 4B). If a national cervical screening programme were to be implemented, there would be additional costs incurred for HPV testing, visual assessment for ablation, and precancer treatment and cancer treatment. We estimated that the total undiscounted 5-year financial costs (2023–2027) for cervical cancer screening, diagnosis and treatment would range from ~US$6.9 million (once lifetime screening) to US$24.8 million (5-yearly (6X lifetime) screening) (table 4B). For the WHO’s ‘elimination strategy’ (twice lifetime screening at age 35 and 45 years), the total 5-year cost would be about US$10.2 million, which averages to US$2.1 million per annum. The cost of the HPV test alone contributed the largest amount to the total 5-year cost, ranging from ~US$3.7 million (53% of total cost, once lifetime) to ~US$17.4 million (70% of total cost, 5-yearly (6X) screening) (figure 4A, B). The costs of treatment for lesions ineligible for ablation and early-stage cancer contributed the second largest cost category, which ranged from US$2.7 million (38% of the total cost, once lifetime) to US$4.9 million (20% of the total cost, 5-yearly (6X) screening). The 10-year budget was approximately double that of the 5-year budget, noting slight differences generated because of the impact of screening as it is introduced over time. On average, estimated undiscounted financial costs over a lifetime per women screened ranged from US$25 per woman (5-yearly screening) to US$40/woman (once lifetime) (data not shown).
Figure 4.
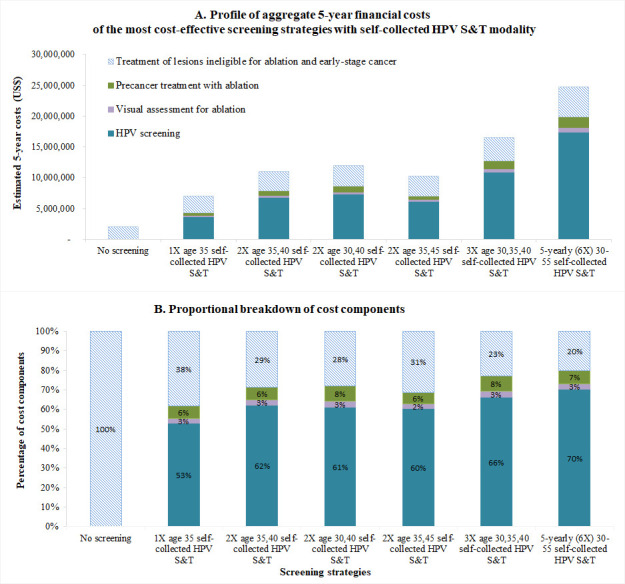
Budget impact and cost profile associated with cervical cancer screening, diagnosis and treatment in PNGNote: Budget was calculated as the financial costs (US$, 2019) of cost-effective screening strategies. This budget is a broad estimate of that required for a future national cervical cancer screening programme in PNG (inflation was not considered). The United Nations population structure estimated for PNG (year 2020) was used and assumed this population structure remained over 2023–2032 to estimate budget. PNG, Papua New Guinea; S&T, screen and treat.
Sensitivity analysis
Assumptions around screening coverage and loss to follow-up rates after treatment are the most influential factors on the ASR incidence rates (online supplemental figure A7). In terms of CEA, 0% discount rate for effect, lower self-collected HPV S&T test cost (US$8/test), lower screening coverage, and lower (8%) cancer treatment access rate generally result in reduced ICERs compared with the base case. In contrast, higher screening coverage, higher cancer treatment costs, and a higher assumed cancer treatment access rate (90%) resulted in higher ICERs in almost all strategies (online supplemental table A4). It should be noted that under the (currently counter factual) 90% cancer treatment access assumption, 5-yearly (6X) self-collected HPV S&T screening would not be considered cost-effective at either 1GDPpc or 0.5GDPpc in PNG, however, thrice lifetime screening remained cost-effective at both thresholds in this high cancer treatment scenario.
Discussion
We evaluated the long-term impact of national scale-up of self-collected HPV S&T modality using local field data and found that self-collected HPV S&T was highly effective and cost-effective in PNG when screening up to thrice in a lifetime from age 30 years. Rapid scale-up twice or thrice per lifetime self-collected HPV S&T strategies were cost-effective and could prevent tens of thousands of deaths over the next 50 years. In contrast, primary VIA screening was substantially less effective and was not cost-effective, even when assuming favourable assumptions about test performance for VIA. Our findings were consistent with our modelled evaluations that informed WHO’s updated cervical screening guidelines, which found that primary HPV testing was cost-effective at an average across 78 LMICs.17 These findings were also consistent with previous published literature on the effectiveness and cost-effectiveness of PoC HPV screen and treat modality in a modelling study on HPV screening in LMICs: Based on data from demonstration projects in Nicaragua, India and Uganda, a modelling study using the Harvard model42 found that PoC-HPV screen and treat modality would be value-for-money in settings with high lost to follow-up rates and the same-day-treatment availability.42 Our findings about the efficacy of HPV screening in this setting are consistent with results from other modelled evaluations considering HPV self-collection, based on data from ASPIRE and START-UP trials in Uganda.43 44 In our study, we found that twice lifetime screening at age 35 and 45 (the WHO screening age and frequency recommendation for cervical cancer elimination) with self-collected HPV S&T can prevent over 20 000 deaths after 50 years of achieving 70% screening coverage in PNG. In comparison, previous work has shown that twice lifetime screening at age 35 and 45 in addition to 90% HPV vaccination coverage in adolescent females and 90% cancer treatment access (the triple-intervention strategy recommended by WHO) would avert twice as many deaths (42 000) after 50 years in PNG, mostly due to the additional benefits of scaled-up access to cervical cancer treatment.2
We showed that once a national screening programme was established in PNG, a population of under 10 million people, its average annual costs of HPV screening (for thrice lifetime screening) over the first 5 years would be US$3.3 million per annum, including the costs of HPV screening and precancer and cancer treatment. In terms of preparedness for the national HPV screening programme, PNG needs to secure more than 100 000 HPV tests (for thrice lifetime screening) annually over the first 5 years of scaling-up to 70% coverage across the whole population of age-eligible women. In preparation for the full-scale programme roll-out, the health system also needs to improve capacity to provide adequate colposcopy, biopsy and cancer treatment services for more than 1000 women annually who would be diagnosed with cervical cancer and would therefore require cancer treatment and care. Additionally, PNG also needs to provide up to 16000 ablative treatment (for thrice lifetime screening) annually for women who would be detected with eligible cervical precancerous lesions. These estimates will support major new implementation effort in the Western Pacific to scale up HPV vaccination, screen- and-treat, and cancer treatment services towards achieving cervical cancer elimination in PNG and more broadly in the region (ECCWP), a collaboration between C4 and the Minderoo foundation.45
In this study, we found that cost of HPV testing accounted for over 50% of the total costs associated with screening, diagnosis and treatment. The current cost of HPV screening used in this model were based on a field trial of self-collected HPV S&T in PNG, and we made the assumption that this cost would be the same (US$18/test) under national roll-out. However, this cost may reduce for a national screening programme, due to HPV test market shaping and pricing negotiations and lower programme costs when the screening is integrated in the existing health system, which would improve cost-effectiveness as shown in our sensitivity analysis. Given the limited facilities in PNG for cancer diagnosis and treatment, lost to follow-up rates at diagnosis were high and we assumed that late-stage cancers were not treated in the base case. If cancer treatment services for women can be scaled-up in line with the WHO targets for increasing cancer treatment and care, for instance, by increasing radiotherapy services, costs associated with cancer treatment will increase, and deaths associated with cervical cancer would decline. In this case, our sensitivity analysis showed that self-collected HPV S&T remained cost-effective.
There are several limitations to this study. Data sources on the burden of disease for PNG is limited. Cervical cancer incidence and mortality assumptions were based on GLOBOCAN2018 estimates. Because a population-based cancer registry has not been established in PNG, the GLOBOCAN’s estimate utilised data from neighbouring countries in the region.46 For cancer treatment access rates, we incorporated local expert information on the availability of hysterectomy for early-stage cancers (table 2) and mortality rates were compared well against GLOBOCAN2018 estimates (online supplemental figure A2 (B)). We also assumed that screening could be scaled up rapidly to reach 70% coverage nationally. There will be many challenges to scale-up screening rapidly in lower-resource settings, particularly challenges for scaling-up screening in hard-to-reach rural areas.
This study has many strengths. First, the Policy1-Cervix model has been extensively validated across a range of settings and used to evaluate various cervical screening strategies for many countries. It has been explicitly used to evaluate policy questions for some high-income countries19 21 and was the sole model to be used to evaluate the benefits, harms and cost-effectiveness of cervical screening algorithms to inform WHO updated 2021 cervical screening guidelines.17 The model was one of three models used by the CCEMC to assess the impacts of cervical cancer elimination strategies on cervical cancer incidence and mortality to inform the WHO global strategy towards cervical cancer elimination.2 29 This model incorporated data on cervical cancer incidence and mortality from GLOBOCAN 2018 and local data on age-specific and type-specific HPV prevalence was used. Second, key model inputs, including loss-to follow-up rates and costing data were derived from the self-collected HPV S&T trial that are currently being conducted in PNG. Third, the ‘screen and treat’ management pathway was consistent with new 2021 WHO cervical screening recommendations and we evaluated it in context of data from local field screening experience and consultation with local experts.
Findings of our study support the WHO strategy for cervical cancer elimination that investing in interventions to meet the 90-70-90 targets offers immense economic and societal benefits. These findings will support major new implementation effort in the Western Pacific to scale up HPV vaccination, screen- and-treat, and cancer treatment services towards achieving cervical cancer elimination in PNG and more broadly in the region (ECCWP).45 However, given the limitations on human resource and infrastructure of the existing health system in PNG, particularly for cervical cancer screening, diagnosis and treatment, in order to scale up cervical screening nationwide, the country would need to develop a cervical screening programme and integrate this screening with the existing primary healthcare services. More importantly, investment in infrastructure and human resource for radiotherapy is needed, which would improve survival across all cancers and not just cervical cancer. A range of practical steps to implement cervical cancer screening and precancer treatment have been developed by WHO.47 The understanding of social, cultural, and religious barriers are crucial to establish referral systems that connect all screening, diagnosis and treatment services, as recommended by the WHO.16
In the 2014 WHO cervical cancer screening guidelines, primary HPV testing was recommended and VIA testing was recommended as an alternative for low-resource settings.5 The recently updated 2021 WHO guidelines now recommend all countries consider primary HPV testing.17 The local experience of self-collected HPV S&T modality has shown that primary HPV testing is feasible and acceptable in PNG, and here we demonstrated that it is also more effective and cost-effective than primary VIA screening; together these findings support the updated 2021 WHO cervical screening guidelines. Our findings are highly relevant for other low-income countries considering screen-and-treat modalities for primary HPV screening.
Footnotes
Handling editor: Seye Abimbola
Twitter: @DiepN99
Contributors: DTNN: reviewed literature and field data for model inputs; calibrated the model; performed model simulations and data analyses; drafted and revised the manuscripts. KTS: reviewed data for model inputs; guided for modelling outcomes; reviewed and commented for model outcomes; reviewed and revised manuscripts. AK: provided technical support for the model and reviewed the manuscripts. GM: provided insights on local context; provided local information that has been used for model assumptions, reviewed and commented on the manuscripts. JWB: provided insights on local context; provided local information that has been used for model assumptions, reviewed and commented on the manuscripts. JK: provided insights on local context; provided local information that has been used for model assumptions, reviewed and commented on the manuscripts. PJT: provided local information that has been used for model assumptions, reviewed and commented on the manuscripts. SGB: provided support for field screening trials in PNG, reviewed and comments on the manuscripts. MS: provided support for field screening trials in PNG, reviewed and comments on the manuscripts. JK: provided support for field screening trials in PNG, reviewed and comments on the manuscripts. AV: conceptualised the field screening trials in PNG, worked closely with local collaborators and gathered very important data from field screening trials in PNG and provided insights on the local context that have been used for model inputs, reviewed and commented on the manuscripts. KC: responsible for the overall content of the manuscript and controlled the decision to publish; conceptualised the modelling study as part of the grant; oversaw the modelling steps and outcomes; reviewed and commented for the data analysis; reviewed and commented on the final version of the manuscript.
Funding: This study was part-funded by the National Health and Medical Research Council (NHMRC), Australia (GNT1104938). KTS receives salary support from Cancer Institute (CDF1004). KC receives salary support from the National Health and Medical Research Council (NHMRC; APP1194679).
Competing interests: MS is Executive Director at VCS Foundation which has developed the canSCREEN digital health platform used in Project ROSE, which is a partnership between the University of Malaya and VCS Foundation. VCS Foundation is a not-for-profit organisation that offers services to implement, support, monitor and manage population health programs. KC and MS are co-PIs of an investigator-initiated trial of cytology and primary HPV screening (Compass; ACTRN12613001207707 and NCT02328872), which is conducted and funded by the VCS Foundation (VCS), a government-funded health promotion charity. KC is also an investigator of Compass New Zealand (ACTRN12614000714684), which was conducted and funded by Diagnostic Medlab (DML), now Auckland District Health Board. The VCS Foundation received equipment and a funding contribution from Roche Molecular Systems and Ventana USA and DML received equipment and a funding contribution for Compass from Roche Molecular Systems. However, neither KC nor her institution on her behalf (Daffodil Centre) receives direct funding from industry for this trial or any other project.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing is not applicable.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global cancer Observatory: cancer today, 2018. Available: https://gco.iarc.fr/today [Accessed 10 Oct 2020].
- 2.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving who cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:591–603. 10.1016/S0140-6736(20)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyen J. Cervical screening in Papua New Guinea: 10 years exprience of the MeriPath program. Proceedings of the 47th Annual Symposium of the Medical Society of Papua New Guinea, Kimbe, West New Britain, 2011. [Google Scholar]
- 4.Mola G, Posanai E, Augerea L, et al. Final report of the Ministerial Task force on prevention and treatment of cancer of the cervix in PNG. Port Moresby: Government of Papua New Guinea, 2009. [Google Scholar]
- 5.World Health Organization . Comprehensive cervical cancer control: a guide to essential practice -2nd ed World Health organization 2014. [PubMed]
- 6.Toliman PJ, Kaldor JM, Badman SG, et al. Performance of clinical screening algorithms comprising point-of-care HPV-DNA testing using self-collected vaginal specimens, and visual inspection of the cervix with acetic acid, for the detection of underlying high-grade squamous intraepithelial lesions in Papua New Guinea. Papillomavirus Res 2018;6:70–6. 10.1016/j.pvr.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toliman P, Badman SG, Gabuzzi J, et al. Field evaluation of Xpert HPV point-of-care test for detection of human papillomavirus infection by use of self-collected vaginal and clinician-collected cervical specimens. J Clin Microbiol 2016;54:1734–7. 10.1128/JCM.00529-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toliman PJ, Kaldor JM, Tabrizi SN, et al. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric 2018;21:235–8. 10.1080/13697137.2018.1439917 [DOI] [PubMed] [Google Scholar]
- 9.Ekeroma A, Dyer R, Palafox N, et al. Cancer management in the Pacific region: a report on innovation and good practice. Lancet Oncol 2019;20:e493–502. 10.1016/S1470-2045(19)30414-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saweri OP, Batura N, Adawiyah RA, et al. Cost and cost-effectiveness of point-of-care testing and treatment for sexually transmitted and genital infections in pregnancy in low-income and middle-income countries: a systematic review protocol. BMJ Open 2019;9:e029945. 10.1136/bmjopen-2019-029945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallely A. Field trial of a novel point-of-care HPV ‘self-collect, test and treat’ cervical screening strategy for women in low- and middle-income countries: the HPV-STAT study, Papua New Guinea, 2018. Available: https://www.isrctn.com/ISRCTN13476702 [Accessed 23 Aug 2021].
- 12.Toliman PJ, Kaldor JM, Badman SG, et al. Evaluation of self-collected vaginal specimens for the detection of high-risk human papillomavirus infection and the prediction of high-grade cervical intraepithelial lesions in a high-burden, low-resource setting. Clin Microbiol Infect 2019;25:496-503. 10.1016/j.cmi.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 13.Basu P, Mittal S, Banerjee D, et al. Diagnostic accuracy of via and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer 2015;137:859–67. 10.1002/ijc.29458 [DOI] [PubMed] [Google Scholar]
- 14.Umulisa MC, Franceschi S, Baussano I, et al. Evaluation of human-papillomavirus testing and visual inspection for cervical cancer screening in Rwanda. BMC Womens Health 2018;18:59. 10.1186/s12905-018-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallely AJ. Point of care testing and treatment to improve birth outcomes and to prevent cervical cancer in high-burden settings: Update from two ongoing field trials in PNG. PNG Obstetrics & Gynaecology Society Annual Meeting; 2019 Sept; Port Moresby, PNG 2019.
- 16.World Health Organization . Global strategy to accelerate the elimination of cervical cancer as a public health problem, 2020. Available: https://www.who.int/publications/i/item/9789240014107 [Accessed 2 Dec 2020].
- 17.World Health Organization . Who guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. Geneva: World Health Organization, 2021: 115. [PubMed] [Google Scholar]
- 18.Simms KT, Hall M, Smith MA, et al. Optimal management strategies for primary HPV testing for cervical screening: cost-effectiveness evaluation for the National cervical screening program in Australia. PLoS One 2017;12:e0163509. 10.1371/journal.pone.0163509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew J-B, Simms KT, Smith MA, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National cervical screening program. Lancet Public Health 2017;2:e96–107. 10.1016/S2468-2667(17)30007-5 [DOI] [PubMed] [Google Scholar]
- 20.Smith MA, Hall MT, Saville M, et al. Could HPV testing on self-collected samples be routinely used in an organized cervical screening program? A modeled analysis. Cancer Epidemiol Biomarkers Prev 2021;30:268–77. 10.1158/1055-9965.EPI-20-0998 [DOI] [PubMed] [Google Scholar]
- 21.Lew J-B, Simms K, Smith M, et al. Effectiveness modelling and economic evaluation of primary HPV screening for cervical cancer prevention in New Zealand. PLoS One 2016;11:e0151619. 10.1371/journal.pone.0151619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C Kitchener H, Canfell K, Gilham C, et al. The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014;18:1–196. 10.3310/hta18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canfell K, Shi J-F, Lew J-B, et al. Prevention of cervical cancer in rural China: evaluation of HPV vaccination and primary HPV screening strategies. Vaccine 2011;29:2487–94. 10.1016/j.vaccine.2010.12.085 [DOI] [PubMed] [Google Scholar]
- 24.Shi J-F, Canfell K, Lew J-B, et al. Evaluation of primary HPV-DNA testing in relation to visual inspection methods for cervical cancer screening in rural China: an epidemiologic and cost-effectiveness modelling study. BMC Cancer 2011;11:239. 10.1186/1471-2407-11-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane A, Shi J-F, Simms KT, et al. Health economic evaluation of primary human papillomavirus screening in urban populations in China. Cancer Epidemiol 2021;70:101861. 10.1016/j.canep.2020.101861 [DOI] [PubMed] [Google Scholar]
- 26.Simms KT, Hanley SJB, Smith MA, et al. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health 2020;5:e223–34. 10.1016/S2468-2667(20)30010-4 [DOI] [PubMed] [Google Scholar]
- 27.Hall MT, Simms KT, Lew J-B, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health 2019;4:e19-e27. 10.1016/S2468-2667(18)30183-X [DOI] [PubMed] [Google Scholar]
- 28.Simms KT, Steinberg J, Caruana M, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. Lancet Oncol 2019;20:394–407. 10.1016/S1470-2045(18)30836-2 [DOI] [PubMed] [Google Scholar]
- 29.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:575–90. 10.1016/S0140-6736(20)30068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger EA, Smith MA, Killen J, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health 2020;5:e213–22. 10.1016/S2468-2667(20)30006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toliman P. Innovative approaches for cervical cancer screening in Papua New Guinea: evaluation of novel point-of-care test and treat algorithms in a high-burden setting. New South Wales, Australia: UNSW Sydney, 2020. [Google Scholar]
- 32.Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. 10.1136/bmj.k4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shastri SS, Mittra I, Mishra GA, et al. Effect of via screening by primary health workers: randomized controlled study in Mumbai, India. J Natl Cancer Inst 2014;106:dju009. 10.1093/jnci/dju009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustafa RA, Santesso N, Khatib R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet 2016;132:259–65. 10.1016/j.ijgo.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 35.Qiao L, Li B, Long M, et al. Accuracy of visual inspection with acetic acid and with Lugol's iodine for cervical cancer screening: meta-analysis. J Obstet Gynaecol Res 2015;41:1313–25. 10.1111/jog.12732 [DOI] [PubMed] [Google Scholar]
- 36.Kelly-Hanku A, Newland J, Aggleton P, et al. Hpv vaccination in Papua New Guinea to prevent cervical cancer in women: gender, sexual morality, outsiders and the de-feminization of the HPV vaccine. Papillomavirus Res 2019;8:100171. 10.1016/j.pvr.2019.100171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiting Natalie . Papua New Guinea’s only radiation machine won't work, and the consequences can be fatal, 2018. Available: https://www.abc.net.au/news/2019-01-21/papua-new-guinea-cancer-patients-miss-out-on-radiation-therapy/10729160 [Accessed 16 Oct 2019].
- 38.Commonwealth Bank . Foreign exchange rates, 2019. Available: https://www.commbank.com.au/personal/international/foreign-exchange-rates.html [Accessed 18 Oct 2019].
- 39.WHO Commission on Macroeconomics and Health . Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and health. Geneva: World Health Organization, 2001. [Google Scholar]
- 40.Tan-Torres ET, Baltussen R, Adam T. Making choices in health: who guide to cost-effectiveness analysis. Geneva: World Health Organization, 2003. [Google Scholar]
- 41.Kelly-Hanku A, Ase S, Fiya V, et al. Ambiguous bodies, uncertain diseases: knowledge of cervical cancer in Papua New Guinea. Ethn Health 2018;23:659–81. 10.1080/13557858.2017.1283393 [DOI] [PubMed] [Google Scholar]
- 42.Campos NG, Tsu V, Jeronimo J, et al. Estimating the value of point-of-care HPV testing in three low- and middle-income countries: a modeling study. BMC Cancer 2017;17:791. 10.1186/s12885-017-3786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mezei AK, Pedersen HN, Sy S, et al. Community-Based HPV self-collection versus visual inspection with acetic acid in Uganda: a cost-effectiveness analysis of the ASPIRE trial. BMJ Open 2018;8:e020484. 10.1136/bmjopen-2017-020484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campos NG, Tsu V, Jeronimo J, et al. Cost-Effectiveness of an HPV self-collection campaign in Uganda: comparing models for delivery of cervical cancer screening in a low-income setting. Health Policy Plan 2017;32:956–68. 10.1093/heapol/czw182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The Daffodil Centre . Collaborative cervical cancer program established in the Western Pacific through landmark investment, 2021. Available: https://daffodilcentre.org/news/collaborative-cervical-cancer-program-established-in-the-western-pacific-through-landmark-investment/ [Accessed 13 Jul 2021].
- 46.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization . Who guidelines for the use of thermal ablation for cervical pre-cancer lesions, 2019. Available: https://apps.who.int/iris/handle/10665/329299 [Accessed 19 Nov 2020]. [PubMed]
- 48.Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol 2014;15:172–83. 10.1016/S1470-2045(13)70570-9 [DOI] [PubMed] [Google Scholar]
- 49.Randall TC, Sauvaget C, Muwonge R, et al. Worthy of further consideration: an updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Prev Med 2019;118:81–91. 10.1016/j.ypmed.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 50.Datta NR, Samiei M, Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int J Radiat Oncol Biol Phys 2014;89:448–57. 10.1016/j.ijrobp.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 51.Campos NG, Sharma M, Clark A, et al. The health and economic impact of scaling cervical cancer prevention in 50 low- and lower-middle-income countries. Int J Gynaecol Obstet 2017;138 Suppl 1:47–56. 10.1002/ijgo.12184 [DOI] [PubMed] [Google Scholar]
- 52.World Bank . GDP per capita (current US$) - Papau New Guinea, 2019. Available: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=PG [Accessed 21 Oct 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007380supp001.pdf (2.1MB, pdf)
Data Availability Statement
Data sharing is not applicable.