Abstract
Growth directly influences production rate and therefore is one of the most important and well-studied traits in animal breeding. However, understanding the genetic basis of growth has been hindered by its typically complex polygenic architecture. Here, we performed quantitative trait locus mapping and genome-wide association studies for 10 growth traits that were observed over 2 years in 1,100 F1 captive-bred trevally (Pseudocaranx georgianus). We constructed the first high-density linkage map for trevally, which included 19,861 single nucleotide polymorphism markers, and discovered 8 quantitative trait loci for height, length, and weight on linkage groups 3, 14, and 18. Using genome-wide association studies, we further identified 113 single nucleotide polymorphism-trait associations, uncovering 10 genetic hot spots involved in growth. Two of the markers found in the genome-wide association studies colocated with the quantitative trait loci previously mentioned, demonstrating that combining quantitative trait locus mapping and genome-wide association studies represents a powerful approach for the identification and validation of loci controlling complex traits. This is the first study of its kind for trevally. Our findings provide important insights into the genetic architecture of growth in this species and supply a basis for fine mapping quantitative trait loci, genomic selection, and further detailed functional analysis of the genes underlying growth in trevally.
Keywords: linkage map, QTL, genome-wide association studies, selective breeding, genomic prediction, GenPred, shared data resource
Introduction
Growth facilitates essential functions such as reproduction or the ability to adapt to environments. Although there are exceptions, increase in body size is usually positively correlated with numerous fitness traits such as higher mating success and fecundity, increased offspring quality and lengthened longevity (Gebhardt-Henrich and Richner 1998; Dmitriew 2011). Most vertebrates exhibit a finite amount of growth. In fish, however, the process of growing is indeterminate, or indefinite, and continues throughout their life (Weatherley 1972), although the rate tends to decline as body size increases (Pedersen and Jobling 1989). This highly complex process is the result of interactions between environmental effects and genetic differences. Factors including sex (e.g. Imsland and Jonassen 2003), age, or food availability (e.g. Jones 1986) influence growth rate, as well as abiotic factors changing seasonally such as temperature (e.g. Karås and Klingsheim 1997; Imsland et al. 2007), photoperiod (e.g. Imsland and Jonassen 2003), or oxygen levels (e.g. Brett and Groves 1979). The genetic basis of growth traits is typically highly polygenic (Wellenreuther and Hansson 2016). Functional relationships between genetic variations and physiological parameters of growth have been described in commercially relevant species such as cod (Gadus morhua) [see review by Imsland and Jónsdóttir (2002)], Atlantic salmon (Salmo salar) (Tsai et al. 2015), or tilapia (Tilapia mossambica) (Liu et al. 2014), but are still poorly understood in many nonmodel species.
With the development of next-generation sequencing technologies the cost of high-density genotyping has drastically decreased, therefore enabling the more widespread application of quantitative trait locus (QTL) mapping experiments. Many variants associated with complex phenotypes have since been found in a variety of nonmodel species. However, QTL mapping approaches only allow the identification of genetic regions that are polymorphic between 2 parents, and relevant in a particular environment, therefore, potentially missing some common variants associated with the traits (Mackay 2001). For this reason, the location and effects of detected QTLs can vary between mapping populations. For example, in the Atlantic salmon, QTLs involved in growth have been found on different linkage groups (LGs) according to different studies (Baranski et al. 2010; Gutierrez et al. 2012; Tsai et al. 2015; Besnier et al. 2020).
Recently, genome-wide association studies (GWAS) have been widely employed to detect QTLs both in captive (Palaiokostas et al. 2018) and natural populations (Santure and Garant 2018). This technique identifies associations between markers and phenotypes based on linkage disequilibrium (LD) (Meuwissen and Goddard 2010). Compared with QTL mapping, GWAS can be conducted on genetically diverse, unrelated individuals and is particularly advantageous when controlled crossing and generation of large segregating populations is difficult. GWAS has proved useful for the identification of loci associated with numerous growth traits in fish (e.g. Yang et al. 2020). However, the power of GWAS relies on the number of markers in relation to the extent of LD in the population (Newell et al. 2011). When applied to natural populations or collections of outcrossing individuals, characterized by a rapid LD decay, tens of thousands of markers are often required to obtain an adequate level of resolution. Because of the intrinsic limitations of statistical methods comparing thousands of tests, GWAS has a higher frequency of false discovery of loci than QTL mapping (Hayes et al. 2013). A final disadvantage of GWAS is its low power for detecting rare allelic variants. However, their frequency can be increased in controlled crosses and therefore captured with QTL mapping. Hence, combining GWAS and QTL mapping often gives more complete and reliable results than using one of the methods alone (Fraslin et al. 2020).
Aquaculture is the fastest growing food-production sector in Aotearoa New Zealand. Currently, the local industry relies almost exclusively on the farming of 3 species: Greenshell mussels (Perna canaliculus), Pacific oysters (Crassostrea gigas), and only 1 finfish, king/chinook salmon (Oncorhynchus tshawytscha), which is an introduced species (Camara and Symonds 2014; Symonds et al. 2019). Hence, there is a strong interest in diversifying the range of species available for aquaculture. The native finfish silver trevally (Pseudocaranx georgianus, Cuvier 1833) has been identified as a suitable candidate for New Zealand aquaculture. In Aotearoa, its Māori name is araara. Indigenous Māori people have a strong cultural connection to trevally, where it is considered as taonga (i.e. has value, or is treasured). Trevally is a shoaling pelagic species found throughout the coastal waters of southern Australia and around New Zealand (Smith-Vaniz and Jelks 2006). It is most common at depths of approximately 80 m, although its range is thought to be 10–238 m and can reach over 40 years of age (Ministry for Primary Industries 2014; Bray 2020). In many regions of New Zealand, trevally is a major component of recreational and commercial fisheries (Ministry for Primary Industries 2014). For it to be suitable for commercial aquaculture, however, trevally’s growth rate must be improved, which can be done using selective breeding (Valenza-Troubat et al. 2021b).
In 2016, the Institute of Plant and Food Research (PFR) started a selective breeding program on trevally, and has induced a wild broodstock to create F1 offspring for this purpose. The primary goal of the breeding program is to improve the growth rate, which has been demonstrated to show both high heritability and transcriptional plasticity in response to temperature (Valenza-Troubat et al. 2021a, 2021b). Individuals attain sexual maturity between years 3 and 4, which is also around the time when this species reaches harvest size (Valenza-Troubat et al. 2021b). The long-term breeding goal is to reduce the time to harvest to around 2 years. In this study, we investigated the genetic architecture of 10 growth traits in the F1 new population of trevally. More specifically, we genotyped and phenotyped 1,100 F1 trevally to (1) generate a high-density linkage map; (2) detect rare QTLs associated with growth using linkage mapping in a subfamily comprising 89 individuals; (3) identify common SNPs associated with growth using GWAS on the whole population; and (4) overlap the results and annotate supported regions.
Methods
Study population
The trevally population used in this study was generated as part of a breeding program started at the PFR finfish research facility in Nelson, New Zealand. The full description of holding conditions and the pedigree are described in Valenza-Troubat et al. (2021a, 2021b). Briefly, the population comprised of 13 wild caught F0 broodstock and 1,100 F1 captive-bred offspring. In 2015, induced mass spawning of the F0 generation in a single tank was used to produce the offspring F1 generation. This resulted in a complex pedigree, including a combination of unrelated, full-, and half-siblings in the F1 generation. F1 offspring were held in a single tank from hatch receiving the same feeding regime, light, water flow, and aeration until the end of this experiment. The seawater tanks in the facility are located on the seaward side of Port Nelson and receive ambient seawater from an underground bore that is filtered using mesh filters and UV treatment.
Phenotyping and trait estimation
Ten growth traits were used in the current study, namely peduncle length (PL), height at 25 (H25), 50 (H50), and 75% (H75) of the PL, estimated weight (EW), and related net gain traits (ΔPL, ΔH25, ΔH50, ΔH75, and ΔEW, respectively) (Valenza-Troubat et al. 2021b). These measurements were recorded on 3 occasions throughout the experiment, at the beginning (November 2017), in the middle (October 2018), and at the end (November 2019), when the fish were a little over 2, 3, and 4 years old, respectively. Using the Morphometric Software (https://www.plantandfood.co.nz/page/morphometric-software-home/), the outline of each fish was extracted from images and morphometric were located, and then used to make measurements. PL was measured by assessing the distance between the upper lip and narrowest cross-section of the tail. Height was measured at 3 positions along the fish: 25%, 50%, and 75% of the PL. The weight estimations (EW) were done following a Bayesian hierarchical approach as described in Froese et al. (2014). The net gain for each trait was calculated as the difference from the initial measurement (November 2017) with subsequent ones. The normality of the data was assessed visually using quantile–quantile (QQ) plots generated in the R statistical environment version 3.2.3 (R Development Core Team 2016).
Genotyping and variant calling
Samples of fin tissue from 13 tagged F0 and 1,100 F1 individuals were collected and stored as described in Valenza-Troubat et al. (2021a, 2021b). Total DNA was extracted as described in Ashton et al. (2019) with minor modifications, and then quantified and quality-checked by fluorescence, spectrophotometry and agarose gel electrophoresis. The 13 F0 individuals were whole genome sequenced (paired-end, 125 bp reads) over 3 lanes of the HiSeq 2500 platform at the Australian Genome Research Facility (AGRF, Melbourne, Australia). The F1 were genotyped using a modified GBS approach (Elshire et al. 2011), as described in Valenza-Troubat et al. (2021a, 2021b). A total of 12 pools of 96 samples each were prepared and sent to AGRF for sequencing on a HiSeq 2500 platform (single-end, 100 bp reads). Sequencing data quality for both F0 and F1 generations were checked using FastQC v0.11.7 (Andrews 2010). Raw reads from the F0 were trimmed using trimmomatic v0.36 (Bolger et al. 2014) (using the parameters HEADCROP: 9; TRAILING: 10; SLIDINGWINDOW: 5:20; MINLEN: 75). The F1 samples were demultiplexed from the 12 sequencing libraries using the process_radtags module available in the STACKs version 2.1 pipeline (Catchen et al. 2013) and the reads were trimmed using Fastq-mcf in ea-utils v1.1.2-806 (minimum sequence length = 50, quality threshold causing base removal = 33) (Aronesty 2013). Read groups were added to all sequences and bam files were sorted and indexed using Picard toolkit (Broad_Institute 2015). Reads were then mapped to the reference genome (Catanach et al. 2021) using the Burrows–Wheeler Aligner v0.7.17 (Li and Durbin 2010) and variants were called jointly with the parallel module of freebayes v1.3.1 (Garrison and Marth 2012), with minimum of 5 observations and minimum mapping quality of 10. The SNPs with single-sample sequence coverage (sequencing depth) < 3 were removed to reduce the number of putatively erroneous genetic variants, and missing data and minor allele frequency (MAF) were set to <20% and >0.05, respectively.
Linkage map construction and QTL identification
The parents of each F1 individual in the dataset were identified with Sequoia v2.0.7 (Huisman 2017) as reported in Valenza-Troubat et al. (2021a, 2021b). The full SNP dataset (i.e. before the stringent filtering performed for the parentage analysis) was filtered for Mendelian errors (>5%), and checked for distorted segregation using a chi-square test with α = 0.05. The linkage map was constructed in Lep-MAP v3.0 (Rastas et al. 2013), using the largest family. Markers were separated into linkage groups (LGs) with the SeparateChromosomes module [logarithm of odds (LOD) limit = 14, minimum markers per LG = 50]. The order of the markers was computed with the OrderMarkers module. Single markers at the end of each LG were removed if they were more than 3 cM apart from the next closest marker. MapChart v2.32 (Voorrips 2002) was used to visualize the genetic map. As the F0 were assumed to be outbred, the linkage map and the genotypes of the mapping family were input as a 4-way cross in the R package R/qtl version 1.47-9 (Broman et al. 2003) for interval mapping. Standard interval mapping was performed and a genome-wide permutation test (Doerge and Churchill 1996) with 1,000 permutations was used to determine the LOD significance thresholds (P-value = 0.05).
Genome-wide association study
GWAS was carried out on the entire genotyped F1 population (n = 1,100). SNPs were removed if the call rate was smaller than 0.8, MAF < 0.01, if Mendel error rate >5% (based on trios identified with the parentage analysis carried out above), and they were LD-pruned using an r2 > 0.80 in a 50-kb sliding window with 5 variants. Association analysis were performed using the Fixed and random model Circulating Probability Unification (FarmCPU) method (Liu et al. 2016) implemented in GAPIT3 v3.1.0 (Wang and Zhang 2021). A Bayesian information criterion (BIC)-based model selection was used to find the optimal number of principal components (PCs) for each time measure, to account for family and population structure. The cutoff for significant association was a false discovery rate (FDR)-adjusted P-value = 0.05 (Benjamini and Hochberg 1995), to control for multiple testing. To assess how well the model used in GWAS accounted for population structure and family relatedness, results of the GWAS were visualized with QQ plots implemented in GAPIT3, which depicted the distribution of the actual P-values compared with the theoretical ones. Manhattan plots were used to visualize the SNPs associated with the different phenotypes, using the physical position of the markers on the reference genome.
Ethics statement
All research carried out in this study was reviewed and approved by the animal ethics committee of Victoria University of Wellington in New Zealand (application number 25976).
Results
Phenotypic values of growth traits
All offspring were phenotyped at the first measurement (November 2017), while numbers decreased at the 2 subsequent time points because of natural mortality that occurred during the study. All traits showed a normal distribution and exhibited large levels of phenotypic variation, as discussed in Valenza-Troubat et al. (2021a, 2021b). A summary of the mean values and standard deviations of the 10 growth phenotypes is shown in Table 1. In both the family used for QTL mapping and in the entire population used for GWAS, a normal distribution of the residuals was observed for the 10 traits investigated (Fig. 1). Transgressive lines (i.e. offspring that have more extreme phenotypes than the parents) were observed in the population.
Table 1.
Summary of the phenotypes height at 25%, 50%, and 75% of the body (H25, H50, and H75, respectively), PL, EW, and net gains traits associated (ΔH25, ΔH50, ΔH74, ΔPL, and ΔEW, respectively) across the whole F1 population and for the largest family.
| November 2017 |
October 2018 |
November 2019 |
||||
|---|---|---|---|---|---|---|
| All |
n = 1,094 |
n = 719 |
n = 694 |
|||
| Mean | SD | Mean | SD | Mean | SD | |
| H25 | 49.71 | 9.02 | 66.94 | 13.51 | 90.10 | 15.70 |
| H50 | 59.45 | 11.02 | 79.99 | 16.39 | 105.53 | 18.65 |
| H75 | 48.58 | 9.91 | 61.26 | 12.69 | 85.63 | 15.80 |
| PL | 156.79 | 23.92 | 200.64 | 35.36 | 264.09 | 40.44 |
| EW | 89.90 | 41.85 | 191.93 | 99.89 | 426.34 | 177.93 |
| ΔH25 | 15.85 | 7.80 | 39.08 | 11.21 | ||
| ΔH50 | 18.75 | 8.42 | 44.34 | 12.36 | ||
| ΔH75 | 11.08 | 6.77 | 35.54 | 10.19 | ||
| ΔPL | 40.03 | 18.00 | 103.59 | 27.31 | ||
| ΔEW | 95.83 | 64.45 | 330.78 | 147.12 | ||
|
| ||||||
| Family |
n = 87 |
n = 68 |
n = 67 |
|||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| H25 | 52.21 | 7.95 | 73.71 | 11.32 | 96.52 | 12.64 |
| H50 | 63.10 | 9.29 | 86.87 | 13.41 | 111.90 | 14.77 |
| H75 | 52.85 | 8.99 | 66.72 | 9.75 | 92.36 | 12.39 |
| PL | 167.59 | 21.32 | 218.19 | 30.04 | 280.78 | 33.07 |
| EW | 107.43 | 37.85 | 238.01 | 89.61 | 498.52 | 159.82 |
| ΔH25 | 20.43 | 6.53 | 42.78 | 9.21 | ||
| ΔH50 | 22.46 | 6.92 | 46.84 | 9.89 | ||
| ΔH75 | 12.88 | 5.54 | 37.79 | 8.33 | ||
| ΔPL | 47.91 | 15.91 | 109.31 | 23.15 | ||
| ΔEW | 125.99 | 60.54 | 384.63 | 135.00 | ||
Included are the number offspring phenotyped (n), mean, and SD.
Fig. 1.
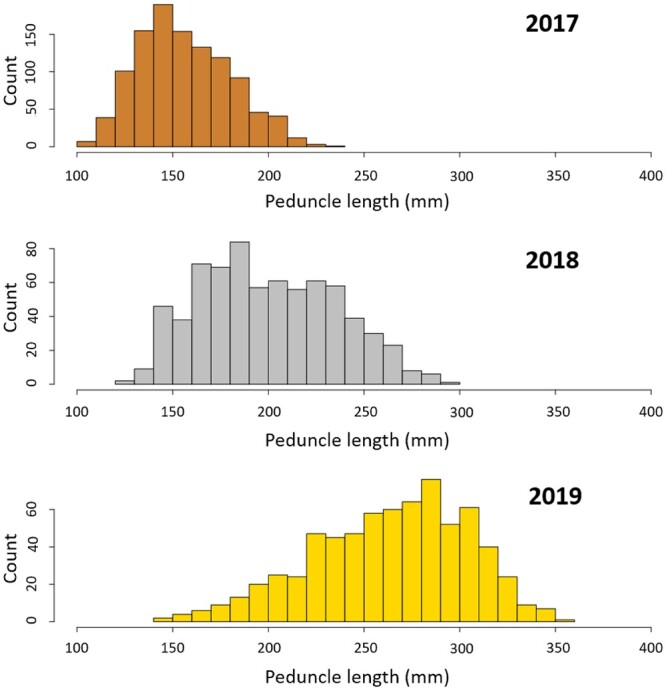
Distribution of the PL measurements in November 2017 (n = 1,094), October 2018 (n = 719), and November 2019 (n = 694).
High-density linkage map
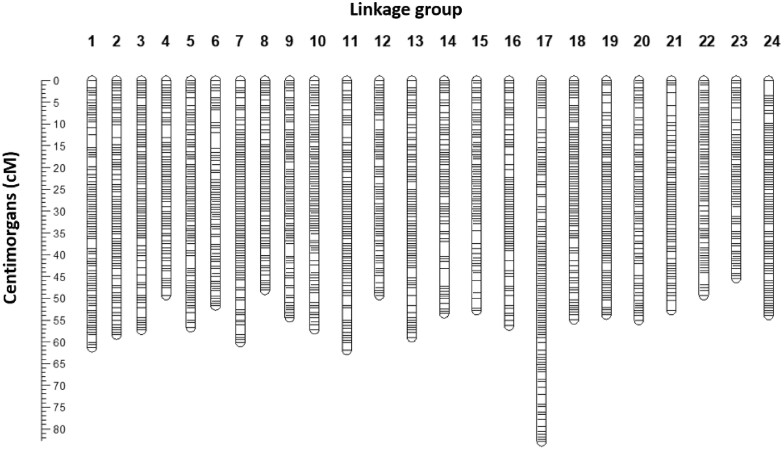
Sequencing of the 13 parental fishes generated 1.23 billion reads, corresponding to an average genome coverage of 13× per individual. Of the 1,100 offspring, 1,094 F1 were successfully genotyped via GBS, resulting in 0.42× genome coverage per individual fish. Variant calling and basic quality filtering generated a dataset of 171,923 SNPs. Overall, 21 families from 10 out of the 13 sequenced F0 fishes were identified by Valenza-Troubat et al. (2021a, 2021b) when reconstructing the pedigree of the population. The largest family included 87 offspring and was used to assemble the sex-averaged linkage map. A total of 21,665 SNPs were polymorphic in this family and passed the chi-square test, and 19,861 were successfully mapped to 24 LGs, amounting to 1,830 when not accounting for the comapping loci (Fig. 2). Inspection of SNPs showed that there was good congruence between the physical location of SNPs on the map vs the genome. LG numbers were randomly assigned, as no previous reference had been published. The genetic map spanned 1,335.46 cM, with an average marker distance of 0.73 cM. The largest gap was on LG14 and was 4.10 cM long. The longest and shorter LGs were 17 (82.89 cM) and 8 (48.14 cM), respectively (Table 2).
Fig. 2.
Visualization of the sex-averaged map built for the largest segregating family (n = 87). The 24 LGs represent the expected 24 Pseudocaranx georgianus chromosomes.
Table 2.
Marker statistics of the linkage map constructed from the largest trevally family.
| LG | Number of markersa | Length (cM) | Average distance between markers (cM) |
|---|---|---|---|
| 1 | 85 | 61.27 | 0.72 |
| 2 | 80 | 58.41 | 0.73 |
| 3 | 81 | 57.26 | 0.71 |
| 4 | 68 | 49.34 | 0.73 |
| 5 | 81 | 56.69 | 0.70 |
| 6 | 65 | 51.72 | 0.80 |
| 7 | 87 | 60.09 | 0.69 |
| 8 | 70 | 48.14 | 0.69 |
| 9 | 78 | 54.43 | 0.70 |
| 10 | 84 | 57.22 | 0.68 |
| 11 | 82 | 61.88 | 0.75 |
| 12 | 71 | 49.30 | 0.69 |
| 13 | 79 | 59.00 | 0.75 |
| 14 | 64 | 53.51 | 0.84 |
| 15 | 67 | 52.84 | 0.79 |
| 16 | 67 | 56.27 | 0.84 |
| 17 | 108 | 82.89 | 0.77 |
| 18 | 82 | 54.95 | 0.67 |
| 19 | 80 | 53.82 | 0.67 |
| 20 | 71 | 55.04 | 0.78 |
| 21 | 65 | 52.83 | 0.81 |
| 22 | 73 | 49.29 | 0.68 |
| 23 | 63 | 45.39 | 0.72 |
| 24 | 79 | 53.88 | 0.68 |
| Total | 1,830 | 1.335.46 | 0.73 |
Count after removal of comapping.
QTL mapping revealed rare variants associated with growth
The genome-wide significant thresholds for QTL mapping were established at LOD values between 4.60 and 4.81 for the 10 phenotypic traits across 3 years (Table 3). In total, 8 significant QTLs were identified on 4 locations in 3 LGs (Fig. 3). Six of these QTLs were for traits recorded during the first measurement (November 2017), and 2 for the last measurement (November 2019). In particular, 3 QTLs detected for H25, PL, and EW measured at the first time point comapped on LG3 (between 23.2 and 24.9 cM). A second QTL was mapped to LG3 at 52.1 cM for H75 from the first measurement. Additionally, the traits PL and EW from 2017 were associated to a second locus, at the top of LG18. The percentage of phenotypic variance explained (PVE) by the 2017 QTLs ranged from 22.1% to 25.3%. In the last measurement, 2 QTLs for ΔH75 and ΔPL were found to comap to LG14 (between 17 and 17.1 cM), with PVE of 39.7 and 31.1, respectively (Table 3). No QTLs were found for the middle measurement (October 2018) and for all other traits in 2017 and 2019.
Table 3.
List of significant QTL detected for height at 25% and 75% of the peduncle length (H25 and H75, respectively), PL, EW, and net gain for H75 (ΔH75) and PL (ΔPL) in November 2017 (Nov17) and November 2019 (Nov19), using a high-density linkage map.
| Time | Trait | LG | Peak position (cM) | SNP at peak | LOD threshold | LOD at peak | PVE (%) |
|---|---|---|---|---|---|---|---|
| Nov17 | H25 | 3 | 23.2 | trevally000114_4454829 | 4.71 | 4.73 | 22.1 |
| Nov17 | PL | 3 | 24.9 | trevally000114_4498123 | 4.71 | 4.83 | 22.6 |
| Nov17 | EW | 3 | 24.9 | trevally000114_4498123 | 4.60 | 5.39 | 24.8 |
| Nov17 | H75 | 3 | 52.1 | trevally000114_23355470 | 4.64 | 5.50 | 25.3 |
| Nov17 | PL | 18 | 0.0 | trevally001200_2242455 | 4.71 | 4.88 | 22.8 |
| Nov17 | EW | 18 | 0.0 | trevally001200_2242455 | 4.60 | 4.82 | 22.5 |
| Nov19 | ΔPL | 14 | 17.0 | trevally001025_25205374 | 4.81 | 5.41 | 31.0 |
| Nov19 | ΔH75 | 14 | 17.6 | trevally001025_25180484 | 4.69 | 7.36 | 39.7 |
For each QTL the position on the LG, the significance threshold, the LOD at the peak, the SNP name, and the PVE are shown.
Fig. 3.
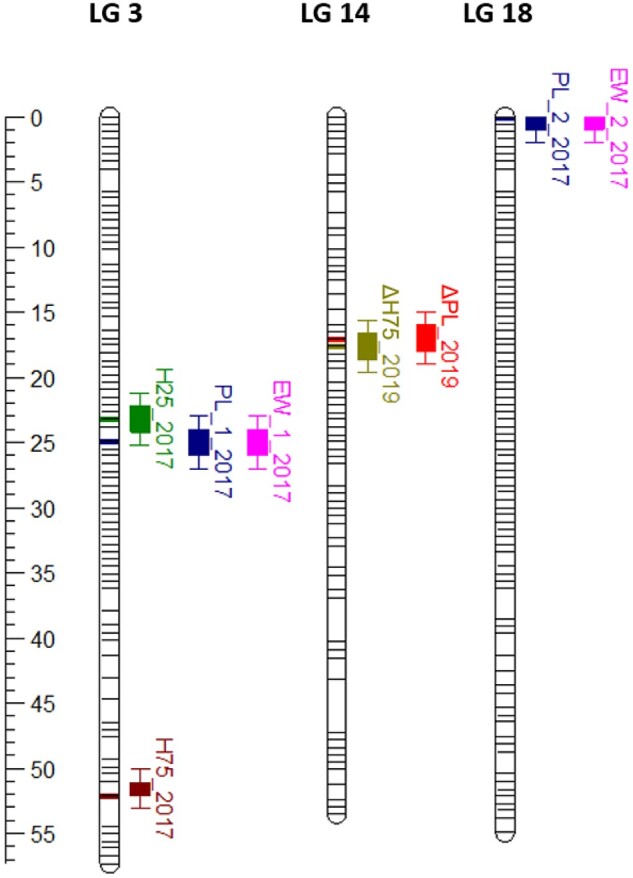
Eight significant QTL associated with growth traits were found in trevally, across 3 LGs (LG3, LG14, and LG18). In November 2017, 2 QTLs were found associated with peduncle length (PL_1_2017 and PL_2_2017, on LG3 and LG18, respectively), which were also associated with EW (EW_1_2017 and EW_2_2017). In the same year, 2 more QTLs were found associated with height at 25% (H75_2017) and 75% (H75_2017) of the peduncle length, both on LG3. Finally, 2 QTLs were found in November 2019, 1 for net gain in peduncle length (PL_2019) and one for net gain in height at 75% of the peduncle length (H75_2019), both on LG14.
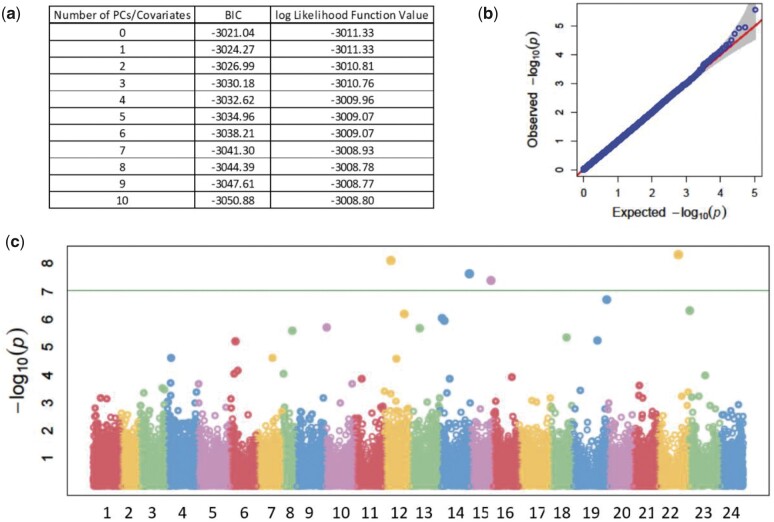
Genome-wide association found strongly associated SNPs
A total of 1,024 F1 individuals had both genotypic and phenotypic data for all measurements. After filtering based on Mendelian errors, MAF, and LD pruning, 107,067 SNPs were left and used in the GWAS. Model selection in GAPIT resulted in no PCs to be used as covariates in any of the traits (Fig. 4a). QQ plots showed that FarmCPU adequately accounted for the confounding effects of family and population structure (Fig. 4b). A total of 93 SNPs were significantly associated [−log 10(ρ) > 7.03] with at least 1 of the 10 traits measured at each year of phenotyping (Fig. 4c;Supplementary Table 1). Only ΔH75 in October 2018 and November 2019 had no significant association. Of the 93 SNPs, 15 were associated with more than 1 trait. These included 4, 7, and 4 SNPs associated with measurements at the first, second, and third time point, respectively. No common SNPs were identified among different years. Significant associations were in some cases found for SNPs <0.5 Mb apart, highlighting hot spots on chromosomic regions. A total of 10 hot spots were identified: 2 on LG1, 1 on LG2, 2 on LG3, 2 on LG5, 1 on LG6, 1 on LG10, and 1 on LG18. Six of these included associations for traits measured in different years (Supplementary Table 1).
Fig. 4.
GWAS results for the net gain in PL (ΔPL) in November 2019. a) Summary of BIC for the optimal number of PCs to use in the model. b) QQ-plot of P-values. On the Y-axis is the observed negative base 10 logarithm of the P-values, and on the X-axis the expected observed negative base 10 logarithm of the P-values under the assumption that the P-values follow a uniform [0 to 1] distribution. The gray area shows the 95% confidence interval for the QQ-plot under the null hypothesis of no association between the SNP and the trait. c) Manhattan plot of the results of the GWAS. On the X-axis is the physical position of the SNPs on the genome divided by chromosomes, and on the Y-axis is the negative log base 10 of the P-values. The horizontal green line represents the significance threshold.
Discussion
We constructed the first genetic map for trevally, and we were able to use this map to determine genomic regions associated with phenotypic growth traits. Our map included 19,861 SNPs across 24 LGs and confirmed the 24 mega-scaffolds of the trevally reference genome (Catanach et al. 2021). A linkage map built for yellowtail kingfish (Seriola lalandi), the closest species to trevally for which a map has been constructed, was also resolved into 24 LGs (Nguyen et al. 2018), consistent with our findings. The map built here for trevally was 1,335.46 cM in length, which is within the range of map length expected for many teleost fish. For example, the map assembled here is longer when compared with the yellowtail kingfish map, similar in length to the map of the Australasian snapper (Chrysophrys auratus) (Ashton et al. 2019) and shorter when compared with the European sea bass (Dicentrarchus labrax) (Griot et al. 2021).
Eight significant QTLs related to growth were mapped to 3 of the trevally LGs. Other studies in teleost have found multiple QTLs affecting growth such as in Atlantic salmon (Tsai et al. 2015; Besnier et al. 2020), Australasian snapper (Ashton et al. 2019), spotted sea bass (Lateolabrax maculatus) (Liu et al. 2020), or yellowtail kingfish (Nguyen et al. 2018), supporting the hypothesis of a polygenic regulation. No significant QTLs were identified with QTL mapping for the second time point, October 2018. In the segregating family used for QTL mapping, the number of offspring decreased from 87 in 2017 to 68 in 2018 and 67 in 2019 as the result of natural mortality. Although some statistically significant QTLs were found in the measurements made in the latter period of the experiments, the smaller sample sizes in the 2 last measurements reduced the power of the study, which could explain the absence of QTLs detected in October 2018. Indeed, sample size is known to influence the power of a study to detect QTLs (Hong and Park 2012) and is regularly discussed as one of the most important concerns when designing a mapping experiment (Ashton et al. 2017). For the same reason, QTL mapping was not performed on the smaller families present in this breeding population. Interestingly, 2 traits (PL and EW) shared the same QTLs on 2 LGs in the first measurement period (November 2017). This is consistent with the level of high genetic and phenotypic correlations reported for body length and body weight (Valenza-Troubat et al. 2021b). These findings suggest that selection applied on easily measurable traits such as length will result in the concomitant improvement of more difficult to assess, yet valuable, growth traits (such as weight) in the breeding of trevally.
GWAS identified 113 associations with growth, corresponding to 93 different SNPs spanning 22 LGs, further supporting the hypothesis of a polygenic basis of growth-related traits in trevally. Growth is considered as a complex trait and has been found to be polygenic across the tree of life, in very diverse taxa from plants, like in the model species Arabidopsis thaliana (Wieters et al. 2021), to vertebrates like humans (Sinnott-Armstrong et al. 2021) and other fish species (e.g. Liu et al. 2014; Yang et al. 2020; Debes et al. 2021). In our study, a lower number of loci were found with the QTL mapping experiment than with the GWAS, which can be explained by the smaller amount of genetic variation represented in the single F1 family compared with the overall breeding population, which was derived from 13 parental individuals. Four QTLs and 15 SNPs were found to have a significant association with more than 1 trait. This was expected, as the different phenotypes were all deduced from the PL and they all then measured the same process underlying growth.
For each trait, the markers found in association were different from a year to another, both in QTL mapping and in GWAS. These differences could be due either to significant loci changing over time, by switching on or off, or to environmental variation, as it was observed in other QTL mapping studies that investigated traits highly affected by the environment (Sun et al. 2017; Ashton et al. 2019). However, there were some hot spot regions of 0.5 Mb that contained SNPs associated with different traits and different years. Noteworthy is the chromosomic region at the top of LG5, where 2 hot spots were found, 1 between 0 and 0.5 Mb (for ΔEW and PL in 2018, and H75 and ES in 2019) and one between 1.4 and 1.8 Mb (for ΔEW, H25, H50, and PL in 2019). Future studies should investigate these further. In addition to these hot spots, we also identified SNP regions that were significant in both QTL and GWAS analyses. Specifically, by comparing the relative physical positions of the SNPs, 2 regions found with QTL mapping appeared to be in close proximity with 2 significant SNPs identified by GWAS. In particular, the QTLs for H25, PL, and EW measured in 2017 spanned the 4.45- to 4.50-Mb region on chromosome 3 (corresponding to 23.20–24.90 cM on LG3) and SNP trevally000114_4956408, located at 4.96 Mb on chromosome 3, was found to be significant for PL in 2019 with GWAS; and the QTLs for ΔH75 and ΔPL in 2019, encompassing the 25.18–25.21 Mb region on chromosome 14 (17.02–17.59 on LG14), are close to SNP trevally001025_25939455, located at 25.94 Mb on chromosome 14 and significant for ΔH50 and ΔPL in 2019 (Fig. 5 and Table 3; Supplementary Table 1). Being identified with 2 different statistical analyses and for multiple traits across 2 years, these 2 regions are then of particular interest for the understanding of the genetic determinism of growth in trevally. A BLAST search of the 100-kb regions flanking those markers against the NCBI nucleotide database did not return any sequence similarities, indicating that they are located in noncoding or in nonannotated regions. Intergenic regions can still have a functional role in gene expression and regulation (Wyrick and Young 2002). For example, these SNPs could be located in an intron that acts as a regulatory region (promoter, enhancer, silencer, or insulator).
Fig. 5.
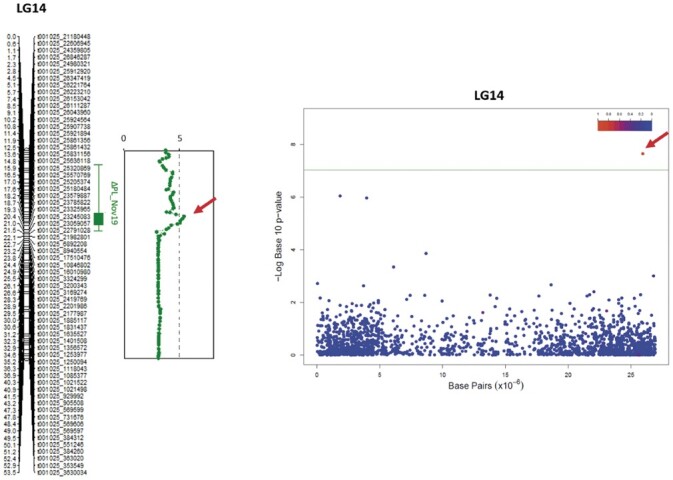
Comparison of QTL (left) and SNP associated (right) with the net gain in PL (ΔPL) in November 2019.
Compared with the heritability estimates found in Valenza-Troubat et al. (2021a, 2021b), the present analysis still seems underpowered. The range of heritability estimates was moderate to high (0.67 ± 0.05 to 0.76 ± 0.06) for the measured traits (H25, H50, H75, PL, and EW) and moderate (ranging from 0.28 ± 0.07 to 0.68 ± 0.07) for the net gain traits (ΔH25, ΔH50, ΔH75, ΔPL, and ΔEW), and it remained consistent throughout the experiment. In the present study, PVEs ranged from 22.1% to 47.36% for the QTL mapping study and from 4.74% to 46.81% for GWAS, indicating that neither techniques were able to capture all of the genetic components of growth traits (Table 4). Additional genetic interactions, other than additive effects, should be considered. While heritability is a key feature of a trait indicating its potential for improvement via selection, polygenic traits are often influenced by nonadditive genetic effects such as dominance or epistasis (Glover et al. 2017). Understanding the genetic mechanisms that underlie a trait is an important part of explaining phenotypic diversity. This is particularly relevant when looking at traits related to fitness (e.g. growth, shyness, foraging, or predator awareness) in populations that are undergoing domestication but still occasionally interbreed with wild conspecifics (e.g. when new broodstock is caught and added).
Table 4.
Comparison of heritability estimates found in Valenza-Troubat et al. (2021a, 2021b) and PVE by QTL found in interval mapping and markers from the GWAS.
| Time | Trait | Heritability | PVE QTL (%) | PVE GWAS (%) |
|---|---|---|---|---|
| Nov17 | H25 | 0.67 ± 0.05 | 22.1 | 18.4 |
| Nov17 | H50 | 0.75 ± 0.05 | — | 24.2 |
| Nov17 | H75 | 0.73 ± 0.05 | 25.3 | 38.5 |
| Nov17 | PL | 0.74 ± 0.05 | 45.3 | 28.8 |
| Nov17 | EW | 0.75 ± 0.05 | 47.4 | 42.3 |
| Oct18 | H25 | 0.74 ± 0.05 | — | 38.6 |
| Oct18 | H50 | 0.75 ± 0.05 | — | 24.2 |
| Oct18 | H75 | 0.68 ± 0.05 | — | 4.7 |
| Oct18 | PL | 0.70 ± 0.05 | — | 40.6 |
| Oct18 | EW | 0.70 ± 0.05 | — | 5.3 |
| Oct18 | ΔH25 | 0.46 ± 0.05 | — | 10.9 |
| Oct18 | ΔH50 | 0.52 ± 0.05 | — | 43.5 |
| Oct18 | ΔH75 | 0.28 ± 0.05 | — | — |
| Oct18 | ΔPL | 0.47 ± 0.05 | — | 46.8 |
| Oct18 | ΔEW | 0.63 ± 0.05 | — | 18.0 |
| Nov19 | H25 | 0.73 ± 0.05 | — | 20.3 |
| Nov19 | H50 | 0.72 ± 0.05 | — | 41.6 |
| Nov19 | H75 | 0.68 ± 0.05 | — | 31.2 |
| Nov19 | PL | 0.69 ± 0.05 | — | 26.4 |
| Nov19 | EW | 0.72 ± 0.05 | — | 21.4 |
| Nov19 | ΔH25 | 0.56 ± 0.05 | — | 16.1 |
| Nov19 | ΔH50 | 0.54 ± 0.05 | — | 15.2 |
| Nov19 | ΔH75 | 0.43 ± 0.05 | 39.7 | — |
| Nov19 | ΔPL | 0.51 ± 0.05 | 31.0 | 21.7 |
| Nov19 | ΔEW | 0.68 ± 0.05 | — | 11.9 |
Data are shown for phenotypes height at 25%, 50%, and 75% of the body (H25, H50, and H75, respectively), PL, EW, and net gains traits associated (ΔH25, ΔH50, ΔH74, ΔPL, and ΔEW, respectively) in November 2017 (Nov17), October 2018 (Oct18), and November 2019 (Nov19).
Future directions
In this study, the combination of QTL mapping and GWAS enabled the identification of genomic regions that control growth in a large captive trevally breeding population. From a farming perspective, parameters such as stocking density (Irwin et al. 1999) or feed availability and quality (Holm et al. 1990) can be carefully managed to accelerate fish growth in a land-based aquaculture facility. However, when aiming to develop new species for aquaculture, it is fundamental to understand the genetic architecture of commercially important traits that can potentially be enhanced through selective breeding. The findings of this study provide a useful framework for determining the genetic basis for growth traits in trevally. The identification of multiple QTLs through QTL mapping and genetic markers commonly involved in growth-related traits via GWAS represents an essential step for the implementation of a molecular breeding program for trevally. With a highly polygenic trait such as growth, genomic selection might be the most effective strategy to improve economic traits in this species. With this objective in mind, we are in the process of developing a medium-density SNP array to be used for the routine screening of the trevally breeding population (though note that such an SNP chip would also be useful for screening wild populations). Additionally, fine mapping, confirmation, and annotation of relevant regions identified in this study will bring a deeper understanding of the genetic architecture of growth in this species, and possibly also closely related teleost species.
Data availability
Supplementary Table 1 contains a list of SNP-trait associations detected with GWAS for height at 25%, 50%, and 75% of the PL (H25, H50, and H75 respectively), PL, EW, and net gain traits (ΔH25, ΔH50, ΔH75, ΔPL, ΔEW) in November 2017 (Nov17), October 2018 (Oct18), and November 2019 (Nov19). For each SNP, the table shows the physical position on the LG, the P-value, the MAF, the FDR-adjusted P-value, and the percentage of variance explained (PVE).
Trevally (araara) are a taonga (treasured) species to Māori, the Indigenous people of Aotearoa New Zealand. All genomic data obtained from taonga species have whakapapa (genealogy that includes people, plants and animals, mountains, rivers, and winds) and are therefore taonga in their own right. These data are tapu (sacred) and tikanga (customary practices, protocols, and ethics) determine how people interact with these data. Thus, all the genomic data have been deposited in a managed repository that controls access. Raw and analyzed data are available through the Genomics Aotearoa data repository at https://repo.data.nesi.org.nz/. This was done to recognize Māori as important partners in science and innovation and as intergenerational guardians of significant natural resources and indigenous knowledge.
Supplemental material is available at G3 online.
Supplementary Material
Acknowledgments
We would like to acknowledge the PFR staff that assisted with the breeding and husbandry operation for the trevally populations; in particular Warren Fantham who oversees the larvae rearing of finfish and Therese Wells who manages the post-juvenile husbandry.
Funding
This research was funded through the MBIE Endeavour Programme “Accelerated breeding for enhanced seafood production” (No. C11X1603) to MW.
Conflicts of interest
None declared.
Literature cited
- Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data; 2010.
- Aronesty E. Comparison of sequencing utility programs. Open Bioinform J. 2013;7(1):1–8. [Google Scholar]
- Ashton DT, Hilario E, Jaksons P, Ritchie PA, Wellenreuther M.. Genetic diversity and heritability of economically important traits in the Australasian snapper (Chrysophrys auratus). Aquaculture. 2019;505:190–198. [Google Scholar]
- Ashton DT, Ritchie PA, Wellenreuther M.. 15 years of QTL studies in fish: challenges and future directions. Mol Ecol. 2017;26(6):1465–1476. [DOI] [PubMed] [Google Scholar]
- Ashton DT, Ritchie PA, Wellenreuther M.. High-density linkage map and QTLs for growth in snapper (Chrysophrys auratus). G3 (Bethesda). 2019;9(4):1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranski M, Moen T, Våge DI.. Mapping of quantitative trait loci for flesh colour and growth traits in Atlantic salmon (Salmo salar). Genet Sel Evol. 2010;42(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B (Methodol). 1995;57(1):289–300. [Google Scholar]
- Besnier F, Solberg MF, Harvey AC, Carvalho GR, Bekkevold D, Taylor MI, Creer S, Nielsen EE, Skaala Ø, Ayllon F, et al. Epistatic regulation of growth in Atlantic salmon revealed: a QTL study performed on the domesticated-wild interface. BMC Genet. 2020;21(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray DJ. Silver Trevally, Pseudocaranx georgianus (Cuvier 1833). In: Fishes of Australia; 2020. [accessed 2022 Jan 24] https://fishesofaustralia.net.au/home/species/4279.
- Brett J, Groves T.. Physiological energetics. Fish Physiol. 1979;8(6):280–352. [Google Scholar]
- Broad_Institute. Picard-tools; 2015. https://broadinstitute.github.io/picard/.
- Broman KW, Wu H, Sen Ś, Churchill GA.. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19(7):889–890. [DOI] [PubMed] [Google Scholar]
- Camara MD, Symonds JE.. Genetic improvement of New Zealand aquaculture species: programmes, progress and prospects. N Z J Mar Fresh. 2014;48:466–491. [Google Scholar]
- Catanach A, Ruigrok M, Chagné D, Bowatte D, Davy M, Storey R, Valenza-Troubat N, López-Girona E, Hilario E, Wylie MJ, et al. Genomic basis of sex determination in the marine teleost Pseudocaranx georgianus. BMC Genomics. 2021. 22(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA.. Stacks: an analysis tool set for population genomics. Mol Ecol. 2013;22(11):3124–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes PV, Piavchenko N, Ruokolainen A, Ovaskainen O, Moustakas-Verho JE, Parre N, Aykanat T, Erkinaro J, Primmer CR.. Polygenic and major-locus contributions to sexual maturation timing in Atlantic salmon. Mol Ecol. 2021;30(18):4505–4519. [DOI] [PubMed] [Google Scholar]
- Dmitriew CM. The evolution of growth trajectories: what limits growth rate? Biol Rev. 2011;86(1):97–116. [DOI] [PubMed] [Google Scholar]
- Doerge RW, Churchill GA.. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142(1):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE.. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One. 2011;6(5):e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraslin C, Quillet E, Rochat T, Dechamp N, Bernardet J-F, Collet B, Lallias D, Boudinot P.. Combining multiple approaches and models to dissect the genetic architecture of resistance to infections in fish. Front Genet. 2020;11(677):677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese R, Thorson JT, Reyes R Jr. A Bayesian approach for estimating length-weight relationships in fishes. J Appl Ichthyol. 2014;30(1):78–85. [Google Scholar]
- Garrison E, Marth G.. Haplotype-based variant detection from short-read sequencing. arXiv:1207.3907 [q-bio]; 2012.
- Gebhardt-Henrich S, Richner H.. Causes of growth variation and its consequences for fitness. Oxf Ornithol Ser. 1998;8:324–339. [Google Scholar]
- Glover KA, Solberg MF, McGinnity P, Hindar K, Verspoor E, Coulson MW, Hansen MM, Araki H, Skaala Ø, Svåsand T.. Half a century of genetic interaction between farmed and wild Atlantic salmon: status of knowledge and unanswered questions. Fish. 2017;18(5):890–927. [Google Scholar]
- Griot R, Allal F, Phocas F, Brard-Fudulea S, Morvezen R, Bestin A, Haffray P, François Y, Morin T, Poncet C, et al. Genome-wide association studies for resistance to viral nervous necrosis in three populations of European sea bass (Dicentrarchus labrax) using a novel 57k SNP array DlabChip. Aquaculture. 2021;530:735930. [Google Scholar]
- Gutierrez AP, Lubieniecki KP, Davidson EA, Lien S, Kent MP, Fukui S, Withler RE, Swift B, Davidson WS.. Genetic mapping of quantitative trait loci (QTL) for body-weight in Atlantic salmon (Salmo salar) using a 6.5 K SNP array. Aquaculture. 2012;358:61–70. [Google Scholar]
- Hayes BJ, Lewin HA, Goddard ME.. The future of livestock breeding: genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends Genet. 2013;29(4):206–214. [DOI] [PubMed] [Google Scholar]
- Holm JC, Refstie T, Bø S.. The effect of fish density and feeding regimes on individual growth rate and mortality in rainbow trout (Oncorhynchus mykiss). Aquaculture. 1990;89(3–4):225–232. [Google Scholar]
- Hong EP, Park JW.. Sample size and statistical power calculation in genetic association studies. Genomics Inform. 2012;10(2):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman J. Pedigree reconstruction from SNP data: parentage assignment, sibship clustering and beyond. Mol Ecol Resour. 2017;17(5):1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsland AK, Jonassen TM.. Growth and age at first maturity in turbot and halibut reared under different photoperiods. Aquacult Int. 2003;11(5):463–475. [Google Scholar]
- Imsland AK, Jónsdóttir ÓDB.. Is there a genetic basis to growth in Atlantic cod? Fish Fisheries. 2002;3(1):36–52. [Google Scholar]
- Imsland AK, Schram E, Roth B, Schelvis-Smit R, Kloet K.. Improving growth in juvenile turbot (Scophthalmus maximus Rafinesque) by rearing fish in switched temperature regimes. Aquacult Int. 2007;15(5):403–407. [Google Scholar]
- Irwin S, O'halloran J, FitzGerald R.. Stocking density, growth and growth variation in juvenile turbot, Scophthalmus maximus (Rafinesque). Aquaculture. 1999;178(1–2):77–88. [Google Scholar]
- Jones G. Food availability affects growth in a coral reef fish. Oecologia. 1986;70(1):136–139. [DOI] [PubMed] [Google Scholar]
- Karås P, Klingsheim V.. Effects of temperature and salinity on embryonic development of turbot (Scophthalmus maximus L.) from the North Sea, and comparisons with Baltic populations. Helgoländer Meeresunters. 1997;51(2):241–247. [Google Scholar]
- Li H, , Durbin R.. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Sun F, Xia JH, Li J, Fu GH, Lin G, Tu RJ, Wan ZY, Quek D, Yue GH.. A genome scan revealed significant associations of growth traits with a major QTL and GHR2 in tilapia. Sci Rep. 2014;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang M, Fan B, Buckler ES, Zhang Z.. Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet. 2016;12(2):e1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang H, Wen H, Shi Y, Zhang M, Qi X, Zhang K, Gong Q, Li J, He F, et al. First high-density linkage map and QTL fine mapping for growth-related traits of spotted sea bass (Lateolabrax maculatus). Mar Biotechnol. 2020;22(4):526–538. [DOI] [PubMed] [Google Scholar]
- Mackay TF. The genetic architecture of quantitative traits. Annu Rev Genet. 2001;35(1):303–339. [DOI] [PubMed] [Google Scholar]
- Meuwissen T, Goddard M.. Accurate prediction of genetic values for complex traits by whole-genome resequencing. Genetics. 2010;185(2):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry for Primary Industries. Trevally (TRE). Fisheries Assessment Plenary. Wellington (New Zealand: ): Ministry for Primary Industries; 2014. [Google Scholar]
- Newell M, Cook D, Tinker N, Jannink J-L.. Population structure and linkage disequilibrium in oat (Avena sativa L.): implications for genome-wide association studies. Theor Appl Genet. 2011;122(3):623–632. [DOI] [PubMed] [Google Scholar]
- Nguyen NH, Rastas P, Premachandra H, Knibb W.. First high-density linkage map and single nucleotide polymorphisms significantly associated with traits of economic importance in Yellowtail Kingfish Seriola lalandi. Front Genet. 2018;9(127). doi: 10.3389/fgene.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaiokostas C, Kocour M, Prchal M, Houston RD.. Accuracy of genomic evaluations of juvenile growth rate in common carp (Cyprinus carpio) using genotyping by sequencing. Front Genet. 2018;9(82). https://doi.org/10.3389/fgene.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T, Jobling M.. Growth rates of large, sexually mature cod Gadus morhua, in relation to condition and temperature during an annual cycle. Aquaculture. 1989;81(2):161–168. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Vienna: R Foundation for Statistical Computing. 2016. [accessed 2016 Dec 12]. http://www.R-project.org/.
- Rastas P, Paulin L, Hanski I, Lehtonen R, Auvinen P.. Lep-MAP: fast and accurate linkage map construction for large SNP datasets. Bioinformatics. 2013;29(24):3128–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santure AW, Garant D.. Wild GWAS—association mapping in natural populations. Mol Ecol Resour. 2018;18(4):729–738. [DOI] [PubMed] [Google Scholar]
- Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, Venkataraman GR, Wainberg M, Ollila HM, Kiiskinen T, et al. ; FinnGen. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. 2021;53(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vaniz WF, Jelks HL.. Australian trevallies of the genus Pseudocaranx (Teleostei: carangidae), with description of a new species from Western Australia. Mem Mus Vic. 2006;63(1):97–106. [Google Scholar]
- Sun C, Niu Y, Ye X, Dong J, Hu W, Zeng Q, Chen Z, Tian Y, Zhang J, Lu M.. Construction of a high-density linkage map and mapping of sex determination and growth-related loci in the mandarin fish (Siniperca chuatsi). BMC Genomics. 2017;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds JE, Clarke SM, King N, Walker SP, Blanchard B, Sutherland D, Roberts R, Preece MA, Tate M, Buxton P.. Developing successful breeding programs for New Zealand aquaculture: a perspective on progress and future genomic opportunities. Front Genet. 2019;10(27):doi: 10.3389/fgene.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-Y, Hamilton A, Tinch AE, Guy DR, Gharbi K, Stear MJ, Matika O, Bishop SC, Houston RD.. Genome wide association and genomic prediction for growth traits in juvenile farmed Atlantic salmon using a high density SNP array. BMC Genomics. 2015;16(1):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza-Troubat N, Davy M, Storey R, Wylie MJ, Ritchie PA, Wellenreuther M.. Differential expression analyses reveal extensive transcriptional plasticity induced by temperature in New Zealand silver trevally (Pseudocaranx georgianus). Evol Appl. 2021. a;https://doi.org/10.1111/ eva.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza-Troubat N, Hilario E, Montanari S, Morrison‐Whittle P, Ashton D, Ritchie P, Wellenreuther M.. Evaluating new species for aquaculture: a genomic dissection of growth in the New Zealand silver trevally (Pseudocaranx georgianus). Evol Appl. 2021. b; https://doi.org/10.1111/eva.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorrips R. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93(1):77–78. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Z.. GAPIT Version 3: boosting power and accuracy for genomic association and prediction. Genomics Proteomics Bioinformatics. 2021; https://doi.org/10.1016/j.gpb.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherley AH. Growth and Ecology of Fish Populations. New York (NY: ): Academic Press; 1972. [Google Scholar]
- Wellenreuther M, Hansson B.. Detecting polygenic evolution: problems, pitfalls, and promises. Trends Genet. 2016;32(3):155–164. [DOI] [PubMed] [Google Scholar]
- Wieters B, Steige KA, He F, Koch EM, Ramos-Onsins SE, Gu H, Guo Y-L, Sunyaev S, de Meaux J.. Polygenic adaptation of rosette growth in Arabidopsis thaliana. PLos Genet. 2021;17(1):e1008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick JJ, Young RA.. Deciphering gene expression regulatory networks. Curr Opin Genet Dev. 2002;12(2):130–136. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu L, Wu X, Li B, Huang W, Weng Z, Lin Z, Song L, Guo Y, Meng Z, et al. Identification of candidate growth-related SNPs and genes using GWAS in brown-marbled grouper (Epinephelus fuscoguttatus). Mar Biotechnol. 2020;22(2):153–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary Table 1 contains a list of SNP-trait associations detected with GWAS for height at 25%, 50%, and 75% of the PL (H25, H50, and H75 respectively), PL, EW, and net gain traits (ΔH25, ΔH50, ΔH75, ΔPL, ΔEW) in November 2017 (Nov17), October 2018 (Oct18), and November 2019 (Nov19). For each SNP, the table shows the physical position on the LG, the P-value, the MAF, the FDR-adjusted P-value, and the percentage of variance explained (PVE).
Trevally (araara) are a taonga (treasured) species to Māori, the Indigenous people of Aotearoa New Zealand. All genomic data obtained from taonga species have whakapapa (genealogy that includes people, plants and animals, mountains, rivers, and winds) and are therefore taonga in their own right. These data are tapu (sacred) and tikanga (customary practices, protocols, and ethics) determine how people interact with these data. Thus, all the genomic data have been deposited in a managed repository that controls access. Raw and analyzed data are available through the Genomics Aotearoa data repository at https://repo.data.nesi.org.nz/. This was done to recognize Māori as important partners in science and innovation and as intergenerational guardians of significant natural resources and indigenous knowledge.
Supplemental material is available at G3 online.