Abstract
Objective
Changes in thyroid function will be accompanied by changes in urinary N-acetyl-β-D-glucosaminidase (uNAG) levels. Therefore, whether thyroid hormones interfere the ability of uNAG in detecting acute kidney injury (AKI) has raised concern in patients with critical illness.
Design
A prospectively recruited, observational study was performed.
Setting
Adults admitted to the intensive care unit of a grade A tertiary hospital in China.
Participants
A total of 1919 critically ill patients were enrolled in the study.
Main outcome measures
To investigate the variations of the ability of uNAG to detect AKI in patients with critical illness under different thyroid hormones levels (differences in area under the curve (AUC) for uNAG diagnosis and prediction of AKI with different thyroid hormones levels).
Results
The bivariate correlation analysis revealed that FT3 and TT3 levels were independently associated with uNAG levels (p<0.001). FT3 and uNAG also showed correlation in multivariable linear regression analysis (p<0.001). After stratification according to the levels of FT3 or TT3, significant variation was observed in the uNAG levels with different quartiles (p<0.05). However, in patients with varying FT3 and TT3 levels, no significant difference was found in the AUCs of uNAG to detect AKI (p>0.05).
Conclusions
Even if uNAG levels varied with FT3 and TT3 levels, these hormones did not interfere with uNAG’s ability to detect AKI in patients with critical illness.
Keywords: acute renal failure, adult intensive & critical care, diabetes & endocrinology
Strengths and limitations of this study.
This is the first study to investigate the influences of thyroid hormones for the performance of urinary urinary N-acetyl-β-D-glucosaminidase (uNAG) in detecting acute kidney injury (AKI).
This prospectively recruited, observational study was of a long duration and had an adequate sample size.
Intensive care unit patients were divided into four groups according to quartiles of thyroid hormones levels to represent the population with different thyroid function status.
We evaluated the ability of uNAG to diagnose and predict AKI primarily by calculating area under the curve.
A limitation of this study was that the relationship between uNAG and thyroid hormones could not be dynamically assessed because we only measured uNAG and thyroid hormones levels at the time of admission.
Introduction
Acute kidney injury (AKI) is a growing burden in critically ill patients.1 2 Early effective treatments can alleviate burden due to high morbidity and mortality of AKI, especially in the intensive care unit (ICU).3–6 However, individual differences in serum creatinine (SCr) levels and clinical inconveniences in recording hourly urine output usually lead to delayed initiation of treatments.7–9 Therefore, confronting the challenges of AKI management demands a biomarker that can predict AKI onset as early as possible.10
Urinary N-acetyl-β-D-glucosaminidase (uNAG) is a lysosomal enzyme that is secreted predominantly by the proximal renal tubules.11–13 Its macromolecular characteristics prevent it from being filtered by the glomerulus, so high levels of uNAG are likely derived from the kidney.14–16 As acute tubular necrosis is one of the histopathological types in AKI,17 18 uNAG is considered to be a sensitive tubular biomarker for AKI.11 12 uNAG is valuable in the early detection of AKI, as demonstrated in previous studies.19–23 Besides, recent studies have indicated that a combination of functional markers and renal tubular damage markers could improve AKI detection in patients with critical illness,24 25 once again proving the diagnostic value of uNAG.
Thyroid disease was thought to be associated with renal disorders and significant changes in renal markers such as creatinine and cystatin C.26 27 Many studies have suggested that uNAG levels would increase in patients with hyperthyroidism.28–31 High levels of uNAG in patients with hyperthyroidism might be caused by glomerular hyperfiltration, hyperactive tubular secretory function, and proteinuria.28 32 33 Abnormal thyroid metabolism is usually present in patients with critical illness.34 35 Thus, the question about the effect of fluctuations in thyroid hormones levels on uNAG in patients with critical illness is worth investigating. However, most previous studies have analysed patients with thyroid disease, and the influence of thyroid hormones levels on the performance of uNAG for detecting AKI was not explored.
Therefore, this prospectively recruited, observational study was performed in a large cohort of patients with critical illness to explore which types of thyroid hormones are associated to uNAG levels, and elucidate the accuracy of uNAG in the identification of AKI under different levels of correlated thyroid hormones.
Materials and methods
Study design and participants
This prospectively recruited, observational study was undertaken in the ICU of Guangdong Provincial People’s Hospital. All adult patients (≥18 years) admitted between October 2014 and December 2017 were assessed for inclusion. Patients were excluded if they were (1) suffering from hyperthyroidism or hypothyroidism, (2) had preexisting end-stage renal disease or undergoing renal replacement therapy before ICU admission, (3) had a history of renal transplantation or nephrectomy and (4) had missing clinical data or refused consent.
Samples and data collection
Blood samples used to determine thyroid hormones, thyroid-stimulating hormone (TSH), serum albumin, and urine samples used to measure uNAG levels were obtained once from patients within 1 hour after ICU admission. SCr was measured at least once a day as part of routine clinical care to monitor AKI onset. Then, we recorded the following data of patients during their hospital stay: occurrence of AKI within 1 week after ICU admission, AKI grade, length of ICU stay, length of hospital stay, ICU mortality and in-hospital mortality. Comprehensive baseline clinical data were collected prospectively, including age, sex, body mass index, preexisting clinical conditions, admission type, baseline SCr, baseline-estimated glomerular filtration rate (eGFR), SCr at admission, serum albumin, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, free triiodothyronine (FT3), free thyroxine (FT4), total triiodothyronine (TT3), total thyroxine (TT4), TSH and uNAG. We calculated the eGFR based on the Chronic Kidney Disease (CKD) Epidemiology Collaboratio creatinine equation.36
Definitions
In the light of the Kidney Disease Improving Global Outcomes (KDIGO) criteria, AKI was diagnosed if there was an increase in SCr level by 0.3 mg/dL (26.5 mmol/L) within 48 hours, increase in SCr to 1.5 times of the baseline level within 1 week, or urine output <0.5 mL/kg/h for 6 hours.37 Because recording hourly urine output was inconvenient in clinical practice and insensitive after diuretic administration, AKI was diagnosed by detecting changes in SCr.38 A patient’s baseline SCr was determined in the following order of preference39: (1) the most recent pre-ICU value between 30 and 365 days before ICU admission, (2) a stable pre-ICU value >365 days for patients aged <40 years (stable defined as within 15% of the lowest ICU measurement) before ICU admission, (3) pre-ICU value >365 days before ICU admission and lower than the initial SCr value at ICU admission, (4) a pre-ICU value (between 3 and 39 days before ICU admission) less than or equal to the initial on-admission SCr value to ICU and not distinctly during AKI, and (5) the lowest SCr on initial admission to the ICU, last ICU value, or minimum value at follow-up to 365 days. AKI diagnosed at ICU admission or within 1 week after ICU admission was defined as established AKI or later-onset AKI, respectively. Mild AKI was defined as KDIGO stage 1 and severe AKI was defined as KDIGO stage 2 or 3.37 40 The primary outcome was the presence of AKI, and secondary outcomes included length of ICU and hospital stay, as well as ICU and hospital mortality.
Patient groups
Based on the results of the bivariate correlation analysis between uNAG and thyroid hormones, patients were stratified into four groups according to the quartiles of relevant thyroid hormones levels. Baseline characteristics, outcome and performance of uNAG in determining AKI were compared among these groups. Besides, to shut off the influences of renal function, we also divided patients into two subgroups, namely, AKI group and non-AKI group, and these subgroups were also split into four quartiles according to relevant thyroid hormones.
Laboratory methods
All specimen assays were accomplished by the central laboratory of Guangdong Provincial People’s Hospital within 24 hours after collection. SCr, uNAG and serum albumin were analysed using the UniCel DxC 800 Synchron System (Beckman Coulter, Brea, California, USA). Values of uNAG were normalised to that of the urinary creatinine concentrations. The coefficients of inter-assay and intra-assay variations for uNAG were both ≤10%. Thyroid hormones were measured by chemiluminescent immunoassay using Unicel DxI800 Synchron System (Beckman Coulter). The normal value ranges of TT3, TT4, FT3, and FT4 are 1.34–2.73 nmol/L, 78.40–158.40 nmol/L, 3.80–6.00 pmol/L and 7.50–21.10 pmol/L, respectively.
Patient and public involvement
Patients were not involved in the study.
Statistical analysis
A two-tailed p<0.05 was regarded as significant. Continuous variables were summarised as median (IQR, IQR) depending on their abnormal distribution and were compared using the non-parametric tests. Categorical variables were reported as frequency (percentage) and compared using the χ2 test or Fisher’s exact test. Bivariate correlation analysis was used to examine the association between two variables to confirm representative indicators of thyroid function for further analysis. Multivariable linear regression analysis with a stepwise variable selection was also used to assess the relationship between uNAG and other variables. Receiver operating characteristic curve analysis and area under the curve (AUC), as assessed using the Hanley-McNeil method, were used to evaluate the ability of uNAG to detecting AKI. The optimal cut-off value for AKI detection was determined with Youden’s index. All statistical analyses were performed using SPSS V.23.0 (SPSS) and MedCalc V.18.2.1 (MedCalc Software, Ostend, Belgium) software programs.
Results
Participants
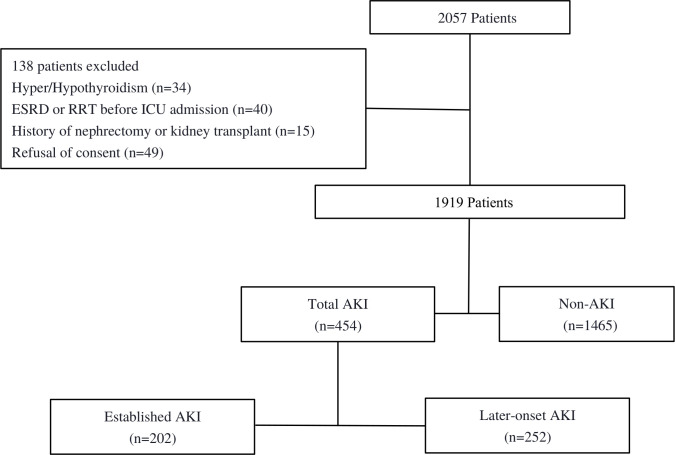
In strict accordance with the selection criteria, 138 patients were excluded from among 2057 patients; finally, 1919 patients with critical illness were included in the analysis (figure 1). The total number of patients with AKI was 454, of which 202 had established AKI and 252 had later-onset AKI. The incidence of total AKI was approximately 23.7%.
Figure 1.
Recruitment of patients into the study. Established AKI indicated the diagnosis of AKI at ICU admission. Later-onset AKI was defined as no AKI diagnosis at ICU admission but reaching the KDIGO criteria within 1 week after admission. AKI, acute kidney injury; ESRD, end-stage renal disease; ICU, Intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; RRT, renal replacement therapy.
Factors related to uNAG
The bivariate correlation between TT3, TT4, FT3 and FT4 and uNAG were determined to identify indicators for thyroid function that influenced uNAG (online supplemental table 1). The results revealed that both FT3 and TT3 were significantly related to uNAG (p<0.001). FT3 and TT3 showed consistent negative correlation with uNAG, so we could conclude that uNAG levels decreased at higher FT3 and TT3 levels. However, FT4, TT4 and TSH levels were not related to uNAG (p>0.05). According to the results of the bivariate correlation analysis, the factors that are correlated with uNAG were included in the multivariable linear regression analysis. In multivariable linear regression analysis (online supplemental table 2), we found uNAG was negatively correlated with FT3, even though the correlation was weak (standardised β=−2.893, p=0.016). Based on the above results, we divided the patients into four quartile groups according to FT3 or TT3 levels.
bmjopen-2021-055787supp001.pdf (87.3KB, pdf)
bmjopen-2021-055787supp002.pdf (87.7KB, pdf)
Patient’s characteristics and outcomes under different FT3 or TT3 levels
Table 1 shows the characteristics and outcomes of patients under different FT3 levels. From the table, patients with higher FT3 levels were younger, had fewer complications, were less likely to require emergency surgery, had a lower APACHE II score, had a higher THS levels and had a better basic renal function. Essentially, high thyroid hormones levels help the body respond better to critical situations. Patient outcomes were also in line with this explanation. Patients with high FT3 levels had a lower incidence of AKI. Even if AKI occurs, it has a milder form. A downward trend was shown in uNAG levels with the increase of FT3 and TT3 levels. Table 2 indicates that patients with different TT3 levels demonstrate comparable characteristics and outcomes.
Table 1.
Characteristics and outcomes of patients according to quartiles of FT3
| Variables | Quartile I | Quartile II | Quartile III | Quartile IV | P value |
| FT3 | 2.98 (2.63–3.21) | 3.66 (3.52–3.80) | 4.13 (4.00–4.25) | 4.76 (4.54–5.07) | <0.001 |
| No | 486 | 482 | 473 | 478 | |
| Baseline characteristics | |||||
| Age, years | 59 (47–70) | 54 (43–65) | 53 (40–63) | 49 (36–59) | <0.001 |
| Male sex, n (%) | 262 (53.9) | 253 (52.5) | 244 (51.6) | 251 (52.5) | 0.912 |
| BMI, kg/m2 | 22.20 (20.82–24.20) | 22.20 (20.52–24.00) | 22.46 (21.05–24.76) | 22.36 (20.57–24.89) | 0.085 |
| Preexisting clinical conditions | |||||
| Hypertension, n (%) | 101 (20.8) | 91 (18.9) | 89 (18.8) | 72 (15.1) | 0.138 |
| DM, n (%) | 52 (10.7) | 40 (8.3) | 35 (7.4) | 26 (5.4) | 0.025 |
| CKD, n (%) | 43 (8.8) | 13 (2.7) | 6 (1.3) | 4 (0.8) | <0.001 |
| CHD, n (%) | 23 (4.7) | 13 (2.7) | 11 (2.3) | 10 (2.1) | 0.062 |
| Stroke, n (%) | 90 (18.5) | 68 (14.1) | 52 (11.0) | 41 (8.6) | <0.001 |
| CHF, n (%) | 24 (4.9) | 11 (2.3) | 5 (1.1) | 2 (0.4) | <0.001 |
| Malignancy, n (%) | 88 (18.1) | 59 (12.2) | 51 (10.8) | 41 (8.6) | <0.001 |
| COPD, n (%) | 23 (4.7) | 5 (1.0) | 3 (0.6) | 7 (1.5) | <0.001 |
| Chronic liver disease, n (%) | 11 (2.3) | 3 (0.6) | 3 (0.6) | 0 (0.0) | 0.001 |
| Sepsis, n (%) | 145 (29.8) | 51 (10.6) | 21 (4.4) | 18 (3.8) | <0.001 |
| Admission type | <0.001 | ||||
| Elective surgical, n (%) | 304 (62.6) | 407 (84.4) | 415 (87.7) | 418 (87.4) | |
| Emergency surgical, n (%) | 79 (16.3) | 28 (5.8) | 20 (4.2) | 13 (2.7) | |
| Medical, n (%) | 103 (21.2) | 47 (9.8) | 38 (8.0) | 47 (9.8) | |
| uNAG, U/g Cr | 34.09 (20.78–58.62) | 27.01 (15.39–41.56) | 23.61 (15.30–36.25) | 21.04 (13.97–31.92) | <0.001 |
| Baseline eGFR, ml/min/1.73 m2 | 98.35 (79.56–110.61) | 103.31 (99.89–113.39) | 103.10 (90.37–115.23) | 104.67 (92.29–117.55) | <0.001 |
| Baseline SCr, umol/L | 64.34 (52.20–80.75) | 61.85 (51.28–75.33) | 64.00 (53.54–75.30) | 65.00 (54.88–77.43) | 0.091 |
| Scr at ICU admission umol/L | 78.02 (61.88–105.06) | 70.00 (58.95–85.68) | 71.96 (59.90–87.00) | 71.80 (59.86–88.50) | <0.001 |
| Serum albumin, g/L | 28.70 (25.00–32.45) | 30.90 (27.49–34.20) | 32.40 (29.20–34.90) | 34.55 (31.90–37.80) | <0.001 |
| TSH uIU/ml | 1.00 (0.52–2.05) | 1.22 (0.64–2.10) | 1.33 (0.78–2.48) | 1.38 (0.74–2.42) | <0.001 |
| APACHE Ⅱ score | 12 (8–20) | 9 (6–12) | 8 (5–12) | 6 (4–10) | <0.001 |
| Primary outcomes | |||||
| Total AKI, n (%) | 214 (44.0) | 99 (20.5) | 81 (17.1) | 60 (12.6) | <0.001 |
| Established AKI, n (%) | 123 (25.3) | 41 (8.5) | 23 (4.9) | 15 (3.1) | <0.001 |
| Later-onset AKI, n (%) | 91 (18.7) | 58 (12.0) | 58 (12.3) | 45 (9.4) | <0.001 |
| Grade of AKI | <0.001 | ||||
| Non-AKI, n (%) | 272 (56.0) | 383 (79.5) | 392 (82.9) | 418 (87.4) | |
| Mild AKI, n (%) | 132 (27.2) | 75 (15.6) | 62 (13.1) | 53 (11.1) | |
| Severe AKI, n (%) | 82 (16.9) | 24 (5.0) | 19 (4.0) | 7 (1.5) | |
The non-normally distributed continuous variables are expressed as median (25–75th percentile (IQR)). Categorical variables are expressed as n (%).
Established AKI, defined as diagnosis of AKI at ICU admission; later-onset AKI, indicated no AKI diagnosis at ICU admission but reaching the KDIGO criteria within 1 week after admission; mild-AKI: defined as reaching KDIGO stage 1 diagnostic criteria of AKI; severe-AKI, defined as reaching KDIGO stage 2 or stage 3 diagnostic criteria of AKI.
P value for global comparisons among groups by rank sum test and χ2 test for continuous and categorical variables, respectively.
AKI, acute kidney injury; APACHE Ⅱ score, Acute Physiology and Chronic Health Evaluation Ⅱ score; BMI, body mass index; CHD, coronary heart disease; CHF, chronic heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FT3, free triiodothyronine; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; n, sample size; SCr, serum creatinine; TSH, thyroid-stimulating hormone; uNAG, urinary N-acetyl-β-D-glucosaminidase.
Table 2.
Characteristics and outcomes of patients according to quartiles of TT3
| Variables | Quartile I | Quartile II | Quartile III | Quartile IV | P value |
| TT3 | 0.63 (0.51–0.72) | 0.90 (0.85–0.95) | 1.09 (1.04–1.15) | 1.38 (1.29–1.51) | <0.001 |
| No | 484 | 482 | 486 | 467 | |
| Baseline characteristics | |||||
| Age, years | 59 (46–69) | 54 (42–65) | 53 (41–63) | 50 (37–60) | <0.001 |
| Male sex, n (%) | 285 (58.9) | 238 (49.4) | 236 (48.6) | 251 (53.7) | 0.004 |
| BMI, kg/m2 | 22.19 (20.56–24.34) | 22.39 (20.83–24.57) | 22.41 (20.81–24.60) | 22.32 (20.50–24.18) | 0.206 |
| Preexisting clinical conditions | |||||
| Hypertension, n (%) | 114 (23.6) | 86 (17.8) | 73 (15.0) | 80 (17.1) | 0.005 |
| DM, n (%) | 57 (11.8) | 45 (9.3) | 27 (5.6) | 24 (5.1) | <0.001 |
| CKD, n (%) | 35 (7.2) | 17 (3.5) | 8 (1.6) | 6 (1.3) | <0.001 |
| CHD, n (%) | 25 (5.2) | 11 (2.3) | 9 (1.9) | 12 (2.6) | 0.010 |
| Stroke, n (%) | 94 (19.4) | 59 (12.2) | 61 (12.6) | 37 (7.9) | <0.001 |
| CHF, n (%) | 24 (5.0) | 5 (1.0) | 10 (2.1) | 3 (0.6) | <0.001 |
| Malignancy, n (%) | 84 (17.4) | 65 (13.5) | 54 (11.1) | 36 (7.7) | <0.001 |
| COPD, n (%) | 20 (4.1) | 8 (1.7) | 6 (1.2) | 4 (0.9) | 0.001 |
| Chronic liver disease, n (%) | 9 (1.9) | 6 (1.2) | 2 (0.4) | 0 (0.0) | 0.002 |
| Sepsis, n (%) | 151 (31.2) | 40 (8.3) | 27 (5.6) | 17 (3.6) | <0.001 |
| Admission type | <0.001 | ||||
| Elective surgical, n (%) | 279 (57.6) | 419 (86.9) | 426 (87.7) | 420 (89.9) | |
| Emergency surgical, n (%) | 86 (17.8) | 21 (4.4) | 16 (3.3) | 17 (3.6) | |
| Medical, n (%) | 119 (24.6) | 42 (8.7) | 44 (9.1) | 30 (6.4) | |
| uNAG, U/g Cr | 34.75 (20.69–57.78) | 25.86 (16.80–42.18) | 23.48 (14.94–35.99) | 21.13 (13.88–33.73) | <0.001 |
| Baseline eGFR, ml/min/1.73 m2 | 99.05 (82.74–113.07) | 100.03 (89.21–112.97) | 103.72 (91.64–114.05) | 105.94 (92.32–117.21) | <0.001 |
| Baseline SCr, umol/L | 64.00 (52.25–79.90) | 63.50 (53.98–78.00) | 63.40 (51.95–75.20) | 64.00 (54.00–76.70) | 0.447 |
| SCr at ICU admission umol/L | 78.05 (62.93–102.00) | 71.00 (59.33–89.47) | 71.00 (58.68–86.47) | 71.50 (59.50–87.00) | <0.001 |
| Serum albumin, g/L | 28.45 (24.93–32.25) | 30.80 (27.50–34.20) | 32.65 (29.50–35.24) | 34.60 (31.60–37.10) | <0.001 |
| TSH uIU/ml | 0.91 (0.49–1.81) | 1.21 (0.64–2.12) | 1.30 (0.78–2.27) | 1.50 (0.79–2.91) | <0.001 |
| APACHE Ⅱ score | 13 (8–20) | 9 (6–12) | 8 (5–12) | 7 (4–10) | <0.001 |
| Primary outcomes | |||||
| Total AKI, n (%) | 221 (45.7) | 101 (21.0) | 69 (14.2) | 63 (13.5) | <0.001 |
| Established AKI, n (%) | 119 (24.6) | 41 (8.5) | 27 (5.6) | 15 (3.2) | <0.001 |
| Later-onset AKI, n (%) | 102 (21.1) | 60 (12.4) | 42 (8.6) | 48 (10.3) | <0.001 |
| Grade of AKI, | <0.001 | ||||
| Non-AKI, n (%) | 263 (54.3) | 381 (79.0) | 417 (85.8) | 404 (86.5) | |
| Mild AKI, n (%) | 140 (28.9) | 75 (15.6) | 52 (10.7) | 55 (11.8) | |
| Severe AKI, n (%) | 81 (16.7) | 26 (5.4) | 17 (3.5) | 8 (1.7) | |
The non-normally distributed continuous variables are expressed as median (25–75th percentile (IQR)). Categorical variables are expressed as n (%).
Established AKI, defined as diagnosis of AKI at ICU admission; later-onset AKI, indicated no AKI diagnosis at ICU admission but reaching the KDIGO criteria within 1 week after admission; mild-AKI: defined as reaching KDIGO stage 1 diagnostic criteria of AKI; severe-AKI, defined as reaching KDIGO stage 2 or stage 3 diagnostic criteria of AKI.
P value for global comparisons among groups by rank sum test and χ2 test for continuous and categorical variables, respectively.
AKI, acute kidney injury; APACHE Ⅱ score, Acute Physiology and Chronic Health Evaluation Ⅱ score; BMI, body mass index; CHD, coronary heart disease; CHF, chronic heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; n, sample size; SCr, serum creatinine; TSH, thyroid-stimulating hormone; TT3, total triiodothyronine; uNAG, urinary N-acetyl-β-D-glucosaminidase.
Variations of uNAG levels under different FT3 or TT3 levels
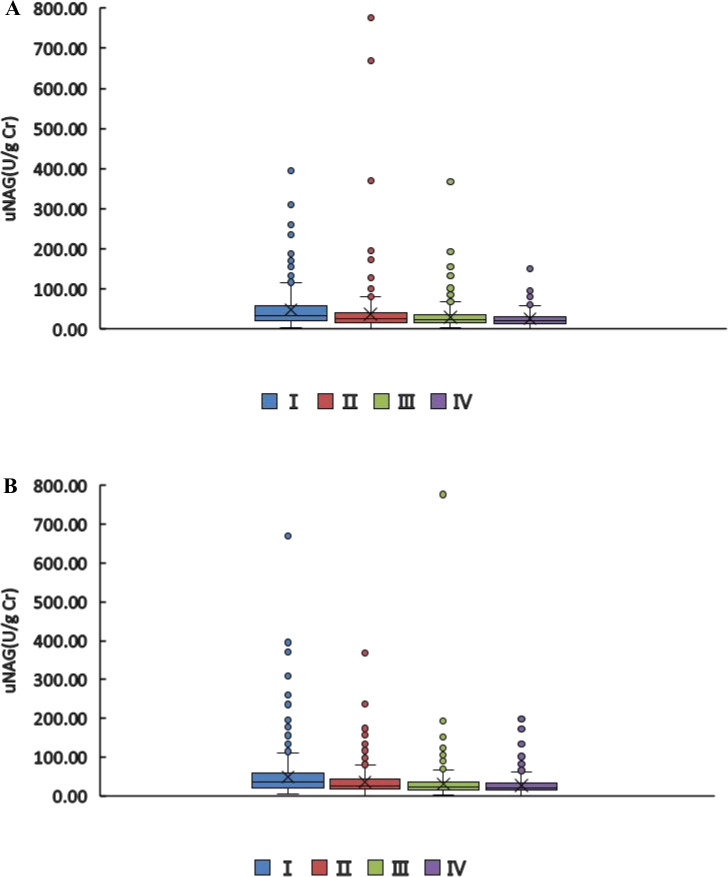
Although the variations of uNAG levels were moderate, a significant difference could be demonstrated among different FT3 levels (figure 2A). Under different TT3 levels, uNAG levels also showed significant differences, but changes in uNAG levels in quartiles III and IV were not statistically significant (figure 2B). In this study, as the level of FT3 or TT3 increases, the level of uNAG gradually decreases. We additionally analysed the changes in the levels of uNAG in the quartiles of FT4, TT4 and TSH. It was found that the level of uNAG had no obvious regularity in different levels of FT4, TT4 and TSH (online supplemental figure 1).
Figure 2.
The median of uNAG levels in patients with different FT3 or TT3 levels. (A) FT3: Ⅰ vs Ⅱ p<0.05, Ⅰ vs Ⅲ p<0.05, Ⅰ vs IV p<0.05; Ⅱ vs Ⅲ p<0.05, Ⅱ vs Ⅳ p<0.05; Ⅲ vs Ⅳ p<0.05; B) TT3: Ⅰ vs Ⅱ p<0.05, Ⅰ vs Ⅲ p<0.05, Ⅰ vs IV p<0.05; Ⅱ vs Ⅲ p<0.05, Ⅱ vs Ⅳ p<0.05; Ⅲ vs Ⅳ p>0.05. FT3, free triiodothyronine; TT3, total triiodothyronine; uNAG, urinary N-acetyl-β-D-glucosaminidase.
bmjopen-2021-055787supp003.pdf (980.9KB, pdf)
Variations of uNAG levels in the non-AKI group and AKI group under different FT3 or TT3 levels
To eliminate influences of changes in renal function, the patients were also divided into non-AKI and AKI subgroups and then categorised into four quartiles according to the levels of FT3 or TT3 (online supplemental figure 2). The trend of uNAG in the non-AKI and AKI groups was consistent, which decreased with the increase in FT3 or TT3 levels. This showed that thyroid hormones independently affect the levels of uNAG.
bmjopen-2021-055787supp004.pdf (1.1MB, pdf)
Performance of uNAG in detecting AKI in patients with different FT3 or TT3 levels at ICU admission
We quantified the performance of uNAG to detect AKI based on AUC values (table 3). In the entire cohort, uNAG detected total AKI with an AUC of 0.682, diagnosed established AKI with an AUC of 0.700, and predicted later-onset AKI with an AUC of 0.668.
Table 3.
Detection of AKI using uNAG by different quartiles
| Total AKI | Established AKI | Later-onset AKI | ||||||||||
| AUC-ROC | 95% CI | Cut-off | P value | AUC-ROC | 95% CI | Cut-off | P value | AUC-ROC | 95% CI | Cut-off | P value | |
| Total | 0.682±0.015 | 0.661 to 0.703 | 41.11 | <0.001 | 0.700±0.021 | 0.677 to 0.722 | 41.11 | <0.001 | 0.668±0.019 | 0.645 to 0.690 | 22.02 | <0.001 |
| FT3 | ||||||||||||
| Quartiles Ⅰ | 0.650±0.025 | 0.606 to 0.692 | 32.27 | <0.001 | 0.663±0.031 | 0.614 to 0.709 | 32.27 | <0.001 | 0.632±0.033 | 0.580 to 0.682 | 23.17 | <0.001 |
| Quartiles Ⅱ | 0.637±0.032 | 0.592 to 0.680 | 53.23 | <0.001 | 0.651±0.050 | 0.604 to 0.697 | 76.22 | 0.003 | 0.627±0.039 | 0.580 to 0.672 | 21.93 | 0.001 |
| Quartiles Ⅲ | 0.660±0.035 | 0.615 to 0.703 | 48.36 | <0.001 | 0.649±0.059 | 0.601 to 0.695 | 20.34 | 0.012 | 0.664±0.042 | 0.618 to 0.708 | 48.36 | <0.001 |
| Quartiles Ⅳ | 0.668±0.038 | 0.624 to 0.711 | 40.23 | <0.001 | 0.652±0.051 | 0.606 to 0.697 | 20.34 | 0.003 | 0.674±0.047 | 0.629 to 0.716 | 33.67 | <0.001 |
| TT3 | ||||||||||||
| Quartiles Ⅰ | 0.636±0.025 | 0.592 to 0.679 | 65.78 | <0.001 | 0.663±0.032 | 0.613 to 0.710 | 65.78 | <0.001 | 0.605±0.033 | 0.553 to 0.656 | 65.78 | 0.002 |
| Quartiles Ⅱ | 0.654±0.031 | 0.610 to 0.697 | 21.22 | <0.001 | 0.668±0.048 | 0.620 to 0.712 | 58.53 | <0.001 | 0.645±0.037 | 0.599 to 0.690 | 21.93 | <0.001 |
| Quartiles Ⅲ | 0.656±0.038 | 0.612 to 0.698 | 40.23 | <0.001 | 0.664±0.054 | 0.618±0.708 | 24.14 | 0.002 | 0.651±0.050 | 0.605 to 0.694 | 40.23 | 0.003 |
| Quartiles Ⅳ | 0.693±0.036 | 0.649 to 0.735 | 45.00 | <0.001 | 0.651±0.061 | 0.603 to 0.697 | 20.34 | 0.014 | 0.706±0.043 | 0.662 to 0.748 | 45.00 | <0.001 |
Established AKI, defined as diagnosis of AKI at ICU admission; later-onset AKI, indicated no AKI diagnosis at ICU admission but reaching the KDIGO criteria within 1 week after admission.
FT3: total AKI: Ⅰ vs Ⅱ: Z=0.320, p=0.749; Ⅰ vs Ⅲ: Z=−0.232, p=0.816; Ⅰ vs Ⅳ: Z=−0.396, p=0.692;Ⅱ vs Ⅲ: Z=0.485, p=0.628; Ⅱ vs Ⅳ: 0.624, p=0.533; Ⅲ vs Ⅳ: Z=0.155, p=0.877; established AKI: Ⅰ vs Ⅱ: Z=0.204, p=0.838; Ⅰ vs Ⅲ: Z=0.210, p=0.834; Ⅰ vs Ⅳ: Z=0.184, p=0.854; Ⅱ vs Ⅲ: Z=0.026, p=0.979; Ⅱ vs Ⅳ: −0.014, p=0.989; Ⅲ vs Ⅳ: Z=−0.039, p=0.969; later-onset AKI: Ⅰ vs Ⅱ: Z=0.098, p=0.922; Ⅰ vs Ⅲ: Z=−0.599, p=0.549; Ⅰ vs Ⅳ: Z=−0.731, p=0.465; Ⅱ vs Ⅲ: Z=−0.646, p=0.519; Ⅱ vs Ⅳ: −0.770, p=0.442; Ⅲ vs Ⅳ: Z=−0.159, p=0.874.
TT3: total AKI: Ⅰ vs Ⅱ: Z=−0.452, p=0.651; Ⅰ vs Ⅲ: Z=−0.440, p=0.660; Ⅰ vs Ⅳ: Z=−1.301, p=0.193; Ⅱ vs Ⅲ: Z=−0.041, p=0.968; Ⅱ vs Ⅳ: −0.821, p=0.412; Ⅲ vs Ⅳ: Z=−0.707, p=0.478; established AKI: Ⅰ vs Ⅱ: Z=−0.087, p=0.931; Ⅰ vs Ⅲ: Z=−0.016, p=0.987; Ⅰ vs Ⅳ: Z=0.174, p=0.862; Ⅱ vs Ⅲ: Z=0.055, p=0.956; Ⅱ vs Ⅳ: 0.219, p=0.827; Ⅲ vs Ⅳ: Z=0.160, p=0.873; later-onset AKI: Ⅰ vs Ⅱ: Z=−0.807, p=0.294; Ⅰ vs Ⅲ: Z=−0.768, p=0.443; Ⅰ vs Ⅳ: Z=−1.863, p=0.062; Ⅱ vs Ⅲ: Z=−0.097, p=0.923; Ⅱ vs Ⅳ: −1.075, p=0.253; Ⅲ vs Ⅳ: Z=−0.834, p=0.4.
AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; FT3, free triiodothyronine; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; TT3, total triiodothyronine; uNAG, urinary N-acetyl-β-D-glucosaminidase.
To explore the effects of thyroid hormones on the efficacy of uNAG to detect AKI, the patients were divided into four quartiles according to FT3 levels and TT3 levels at ICU admission, and the AUC values of uNAG were calculated and compared with detect AKI between different groups (table 3). From the results in table 3, the performance of uNAG to detect total AKI, diagnose established AKI, and predict later-onset AKI was not distinct under different thyroid hormones levels. No significant difference in the AUC was observed between any two groups, whether divided into four quartiles by FT3 levels or by TT3 levels. On the contrary, the optimal cut-off value of uNAG for detecting total AKI, diagnosing established AKI, and predicting later-onset AKI showed fair discrimination in different quartiles based on FT3 or TT3 levels. However, changes in the cut-off values of uNAG were not as simple as uNAG increasing with higher FT3 or TT3 level.
Discussion
Recently, combining functional and renal tubular injury biomarkers as a new strategy to detect AKI has attracted research attention.24 41 uNAG was considered an important biomarker of renal tubular damage in combination with AKI biomarkers.24 This prospectively recruited cohort study investigated the variations of the ability of uNAG to detect AKI in patients with critical illness under different thyroid hormones levels. Patients were divided into four quartile groups according to FT3 and TT3 levels based on the results of the bivariate correlation analysis and multivariate linear regression analysis between uNAG and thyroid hormones, and the AUC values of uNAG in diagnosing and predicting AKI were calculated for each group. In this study, significant differences were found uNAG levels of patients between different quartiles, but the performance of uNAG in detecting AKI was not different among patients under different quartiles.
As a sensitive marker of renal tubular damage,11 12 elevated uNAG levels were considered an early sign of AKI.19–22 However, uNAG levels were also influenced by non-renal factors as reported previously.23 29–31 33 42–45 The animal experiment results of Lapointe et al showed that the uNAG levels of hyperthyroid cats without CKD and hyperthyroid cats with CKD were higher than those of healthy cats and the uNAG level of hyperthyroid cats without CKD decreased after treatment with methimazole.33 Tominaga et al also found similar situation in patients with hyperthyroidism.31 Their results suggested that patients with hyperthyroidism had higher uNAG levels than normal people and patients with diabetes without kidney damage. Another study further proved that elevated uNAG level not only occurred in Graves’ disease but also in subacute thyroiditis and silent thyroiditis.30 Undoubtedly, the aforementioned studies indicated that excessive thyroid hormones level could cause an increase in uNAG levels.
uNAG is a lysosomal hydrolase that is derived from the lysosome of the proximal tubule cells of the kidney.11–13 Thyroid hormones could increase uNAG levels by changing the lysosomal function or affecting kidney function. Lysosomal enzyme activity is dependent on the state of thyroid function.46 47 The possible hypothetical mechanism was that thyroid hormones stimulated the biosynthesis of lysosomal enzymes,48 whereas excessive thyroid hormones could lead to lysosomal enzyme leak because changes in lysosomal membrane permeability resulted from the increase in the production of reactive oxygen species.49–51 Thyroid hormones might cause changes in uNAG levels by affecting kidney function and damaging kidney structure. In case of hyperthyroidism, hyperfiltration, proteinuria, hypertrophy and hyperplasia altogether damage the glomerular basement membrane and renal tubules.28 32 33 Animal experiments have found that the increased activity of angiotensin II will lead to interstitial fibrosis and matrix protein accumulation by inducing an increase in the production of transforming growth factor β1 and platelet derived growth factor and ultimately resulting in tubulointerstitial nephritis.52–54 An increase in angiotensin II levels should also have the same influence in patients with hyperthyroidism.55
However, compared with previous studies, this study presented opposite results in exploring the relationship between thyroid hormones and uNAG. Our results indicated that FT3 and TT3 levels were negatively correlated with uNAG in the bivariate correlation analysis. In the multivariate linear regression analysis, similar conclusions were drawn in the correlation between FT3 and uNAG. This finding could be explained by the fact that triiodothyronine was the main physiologically active substance. Triiodothyronine has been shown more biologically active than tetraiodothyronine in terms of pathophysiological effects.56 Even in critically ill patients without thyroid disease, thyroid hormones levels might be abnormal, and the incidence in AKI patients was as high as 80%.57 Among them, the reduction of triiodothyronine was the most common.58 Low thyroid hormones levels have been shown to be a risk factor for poor prognosis in critically ill patients,58 which is consistent with this study finding that critically ill patients with low thyroid hormones levels had higher APACHE Ⅱ score. uNAG levels decreased with the increase in FT3 and TT3 levels, and significant differences were noted in uNAG levels between different FT3 and TT3 levels. Even if the study patients were divided into AKI and non-AKI groups for analysis, we still obtained the same results. The most important reason for the contrasting results between our study and previous studies might be the difference in research objects. Previous studies have mainly explored the effect of excessive thyroid hormones on uNAG levels caused by thyroid diseases. However, the study excluded patients with thyroid disease to explore changes in uNAG levels in patients with critical illness under different thyroid function states. Our analysis found that patients whose thyroid function remained intact despite having a severe disease had lower APACHE II score and incidence of AKI. This might explain our study finding that patients with higher thyroid hormones levels had lower uNAG levels.
This study did not display a significant variable difference in the AUC values of uNAG in discovering AKI among patients with different FT3 or TT3 levels. In the absence of thyroid disease, the results suggested that uNAG levels in patients with critical illness were negatively correlated with FT3 and TT3 levels, and the ability of uNAG to diagnose and predict AKI was not interfered by thyroid hormones.
This study still has some limitations. First, we used thyroid hormones levels at admission rather than continuous thyroid hormones variability to test our hypotheses. Second, our study has not verified the influences of thyroid hormones on the ability of uNAG to detect AKI in patients with thyroid diseases. We thought a large sample size of patients with thyroid diseases was needed to reveal this influence.
Conclusion
Although FT3 and TT3 affected the level of uNAG, these hormones did not affect the performance of uNAG to recognise AKI in patients with critical illness.
Supplementary Material
Acknowledgments
The authors thank all the doctors, nurses, technicians and patients at the Guangdong Provincial People’s Hospital for their dedication in the study.
Footnotes
Contributors: CBC is the guarantor of this work. CBC, SLL, DDL and LHH equally contributed to the design of the research. SLL and LHH equally contributed to analyze and interpret the data. CBC and DDL contributed to the conception of the research and critically revised the manuscript. SLL, DDL, LHH, MXF, JXL, JD, HF, HDZ, LLH, JX and YFL performed the research and collected data. All authors contributed to the acquisition and analysis of the data, drafted the manuscript, and agreed to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Funding: The study was supported by Chunbo Chen’s research grants from the Science and Technology Planning Project of Guangdong Province (Key Programme) (2014B020212023), the National Natural Science Foundation of China (82172162), the Guangzhou Livelihood Science and Technology Programme (201803010058) and the Major Program of Summit Project, Guangdong Province High-level Hospital Construction Project of Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences (DFJH2020028). Author Linhui Hu is currently receiving a grant (zx2020017) from the High-level Hospital Construction Research Project of Maoming People's Hospital.
Competing interests: The authors have no conflicts of interest to disclose.
Patient and public involvement statement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets generated and/or analysed during this study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The current study received the approval of ethics committee of the Guangdong Provincial People’s Hospital (No.GDREC2015396H(R1)). The ethics committee of the Guangdong Provincial People’s Hospital supervised the study, including the study design, protocol, ethical issue and data and sample collection. Written informed consent was obtained from each patient or from the appropriate guardian. Participants gave informed consent to participate in the study before taking part.
References
- 1.Arora T, Martin M, Grimshaw A, et al. Prediction of outcomes after acute kidney injury in hospitalised patients: protocol for a systematic review. BMJ Open 2020;10:e042035. 10.1136/bmjopen-2020-042035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sako K, Furuichi K, Yamamura Y, et al. Association between the recurrence period of acute kidney injury and mortality: a single-centre retrospective observational study in Japan. BMJ Open 2019;9:e023259. 10.1136/bmjopen-2018-023259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans administration study. Crit Care Med 2009;37:2552–8. 10.1097/CCM.0b013e3181a5906f [DOI] [PubMed] [Google Scholar]
- 4.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190–9. 10.1001/jama.2016.5828 [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015;10:1510–8. 10.2215/CJN.02140215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou FF, Yang X. Advances in the management of acute cardiorenal syndrome in China: biomarkers for predicting development and outcomes. Kidney Dis 2017;2:145–50. 10.1159/000449026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol 2007;2:356–65. 10.2215/CJN.03280906 [DOI] [PubMed] [Google Scholar]
- 8.Martins CB, De Bels D, Honore PM, et al. Early prediction of acute kidney injury by machine learning: should we add the urine output criterion to improve this new tool? J Transl Int Med 2020;8:201–2. 10.2478/jtim-2020-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Wang L, Xiong M, et al. New criterion to evaluate acute-on-chronic kidney injury based on the creatinine reference change. Am J Nephrol 2020;51:453–62. 10.1159/000506664 [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Ge B, Liu Y, et al. The efficacy of biomarkers in the diagnosis of acute kidney injury secondary to liver cirrhosis. Medicine 2021;100:e25411. 10.1097/MD.0000000000025411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 2014;29:1301–11. 10.1093/ndt/gft510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care 2016;20:299. 10.1186/s13054-016-1478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 2008;1:200–8. 10.1111/j.1752-8062.2008.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprenkle P, Russo P. Molecular markers for ischemia, do we have something better then creatinine and glomerular filtration rate? Arch Esp Urol 2013;66:99–114. [PubMed] [Google Scholar]
- 15.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 2008;48:463–93. 10.1146/annurev.pharmtox.48.113006.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo J-J, Kwon JH, Kim YS, et al. The role of urinary N-acetyl-β-D-glucosaminidase in cirrhotic patients with acute kidney injury: multicenter, prospective cohort study. J Clin Med 2021;10:4328. 10.3390/jcm10194328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the rifle and akin criteria. Nat Rev Nephrol 2011;7:201–8. 10.1038/nrneph.2011.14 [DOI] [PubMed] [Google Scholar]
- 18.Wen Y, Yang C, Menez SP, et al. A systematic review of clinical characteristics and histologic descriptions of acute tubular injury. Kidney Int Rep 2020;5:1993–2001. 10.1016/j.ekir.2020.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng B, Jin Y, Liu G, et al. Urinary N-acetyl-beta-D-glucosaminidase as an early marker for acute kidney injury in full-term newborns with neonatal hyperbilirubinemia. Dis Markers 2014;2014:1–6. 10.1155/2014/315843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi K, Negishi K, Ishizu T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011;39:2464–9. 10.1097/CCM.0b013e318225761a [DOI] [PubMed] [Google Scholar]
- 21.Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 2007;18:904–12. 10.1681/ASN.2006030221 [DOI] [PubMed] [Google Scholar]
- 22.Peng Z-Y. The biomarkers for acute kidney injury: a clear road ahead? J Transl Int Med 2016;4:95–8. 10.1515/jtim-2016-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Deng Y, Zhai Y, et al. Impact of blood glucose levels on the accuracy of urinary N-acety-β-D-glucosaminidase for acute kidney injury detection in critically ill adults: a multicenter, prospective, observational study. BMC Nephrol 2019;20:186. 10.1186/s12882-019-1381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y, Chi R, Chen S, et al. Evaluation of clinically available renal biomarkers in critically ill adults: a prospective multicenter observational study. Crit Care 2017;21:46. 10.1186/s13054-017-1626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Y, Ma J, Hou Y, et al. Combining serum cystatin C and urinary N-acetyl-beta-D-glucosaminidase improves the precision for acute kidney injury diagnosis after resection of intracranial space-occupying lesions. Kidney Blood Press Res 2020;45:142–56. 10.1159/000504599 [DOI] [PubMed] [Google Scholar]
- 26.Simeoni M, Cerantonio A, Pastore I, et al. The correct renal function evaluation in patients with thyroid dysfunction. J Endocrinol Invest 2016;39:495–507. 10.1007/s40618-015-0402-8 [DOI] [PubMed] [Google Scholar]
- 27.Greco M, Foti DP, Aversa A, et al. Cystatin C, a controversial biomarker in hypothyroid patients under levothyroxine therapy: THYRenal, a pilot cohort observational study. J Clin Med 2020;9. 10.3390/jcm9092958. [Epub ahead of print: 13 09 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 2012;16:204–13. 10.4103/2230-8210.93737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komosińska-Vassev K, Olczyk K, Koźma EM, et al. Graves' disease-associated changes in the serum lysosomal glycosidases activity and the glycosaminoglycan content. Clin Chim Acta 2003;331:97–102. 10.1016/S0009-8981(03)00090-1 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S, Ishiyama M, Kosaka J, et al. Urinary N-acetyl-beta-D-glucosaminidase (Nag) activity in patients with Graves' disease, subacute thyroiditis, and silent thyroiditis: a longitudinal study. Endocrinol Jpn 1991;38:303–8. 10.1507/endocrj1954.38.303 [DOI] [PubMed] [Google Scholar]
- 31.Tominaga M, Fujiyama K, Hoshino T, et al. Urinary N-acetyl-beta-D-glucosaminidase in the patients with hyperthyroidism. Horm Metab Res 1989;21:438–40. 10.1055/s-2007-1009256 [DOI] [PubMed] [Google Scholar]
- 32.Iglesias P, Bajo MA, Selgas R, et al. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord 2017;18:131–44. 10.1007/s11154-016-9395-7 [DOI] [PubMed] [Google Scholar]
- 33.Lapointe C, Bélanger M-C, Dunn M, et al. N-Acetyl-Beta-D-Glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2008;22:1103–10. 10.1111/j.1939-1676.2008.0168.x [DOI] [PubMed] [Google Scholar]
- 34.Economidou F, Douka E, Tzanela M, et al. Thyroid function during critical illness. Hormones 2011;10:117–24. 10.14310/horm.2002.1301 [DOI] [PubMed] [Google Scholar]
- 35.Zaloga GP, Chernow B, Smallridge RC, et al. A longitudinal evaluation of thyroid function in critically ill surgical patients. Ann Surg 1985;201:456–64. 10.1097/00000658-198504000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 38.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: Workgroup statements from the tenth acute dialysis quality initiative consensus conference. Contrib Nephrol 2013;182:13–29. 10.1159/000349963 [DOI] [PubMed] [Google Scholar]
- 39.Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 2010;77:1020–30. 10.1038/ki.2010.25 [DOI] [PubMed] [Google Scholar]
- 40.Basu RK, Wang Y, Wong HR, et al. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 2014;9:654–62. 10.2215/CJN.09720913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014;64:2753–62. 10.1016/j.jacc.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alderman MH, Melcher L, Drayer DE, et al. Increased excretion of urinary N-acetyl-beta-glucosaminidase in essential hypertension and its decline with antihypertensive therapy. N Engl J Med 1983;309:1213–7. 10.1056/NEJM198311173092004 [DOI] [PubMed] [Google Scholar]
- 43.Kohno M, Kanayama Y, Yasunari K, et al. Significance of the measurement of urinary alanine aminopeptidase and N-acetyl-beta-D-glucosaminidase activity in evaluating patients with essential hypertension. Clin Exp Hypertens A 1985;7:1347–60. 10.3109/10641968509073596 [DOI] [PubMed] [Google Scholar]
- 44.Ouchi M, Suzuki T, Hashimoto M, et al. Urinary N-acetyl-β-D-glucosaminidase levels are positively correlated with 2-hr plasma glucose levels during oral glucose tolerance testing in prediabetes. J Clin Lab Anal 2012;26:473–80. 10.1002/jcla.21549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agirbasli M, Radhakrishnamurthy B, Jiang X, et al. Urinary N-acetyl-beta-D-glucosaminidase changes in relation to age, sex, race, and diastolic and systolic blood pressure in a young adult biracial population. The Bogalusa heart study. Am J Hypertens 1996;9:157–61. 10.1016/0895-7061(95)00336-3 [DOI] [PubMed] [Google Scholar]
- 46.Guillou H, David V, Lorcy Y, et al. Serum lysosomal acid hydrolase activities in Graves' disease. Clin Chim Acta 1982;120:219–24. 10.1016/0009-8981(82)90158-9 [DOI] [PubMed] [Google Scholar]
- 47.Wład H, Suszka B, Lacka K, et al. Enzymes depolymerizing glycosaminoglycans in blood serum of patients with HyPer and hypothyroidism. Endokrynol Pol 1985;36:215–20. [PubMed] [Google Scholar]
- 48.Ullrich K, Gieselmann V, Mersmann G, et al. Endocytosis of lysosomal enzymes by non-parenchymal rat liver cells. Comparative study of lysosomal-enzyme uptake by hepatocytes and non-parenchymal liver cells. Biochem J 1979;182:329–35. 10.1042/bj1820329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mano T, Sinohara R, Sawai Y, et al. Effects of thyroid hormone on coenzyme Q and other free radical scavengers in rat heart muscle. J Endocrinol 1995;145:131–6. 10.1677/joe.0.1450131 [DOI] [PubMed] [Google Scholar]
- 50.Pehowich DJ. Thyroid hormone status and membrane n-3 fatty acid content influence mitochondrial proton leak. Biochim Biophys Acta 1999;1411:192–200. 10.1016/S0005-2728(99)00041-9 [DOI] [PubMed] [Google Scholar]
- 51.Venditti P, De Rosa R, Di Meo S. Effect of thyroid state on susceptibility to oxidants and swelling of mitochondria from rat tissues. Free Radic Biol Med 2003;35:485–94. 10.1016/S0891-5849(03)00331-9 [DOI] [PubMed] [Google Scholar]
- 52.Gilbert RE, Wu LL, Kelly DJ, et al. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol 1999;155:429–40. 10.1016/S0002-9440(10)65139-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int 2003;63:809–25. 10.1046/j.1523-1755.2003.00840.x [DOI] [PubMed] [Google Scholar]
- 54.Tryggvason K, Pettersson E. Causes and consequences of proteinuria: the kidney filtration barrier and progressive renal failure. J Intern Med 2003;254:216–24. 10.1046/j.1365-2796.2003.01207.x [DOI] [PubMed] [Google Scholar]
- 55.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–9. 10.1056/NEJM200102153440707 [DOI] [PubMed] [Google Scholar]
- 56.Surks MI, Oppenheimer JH. Concentration of L-thyroxine and L-triiodothyronine specifically bound to nuclear receptors in rat liver and kidney. Quantitative evidence favoring a major role of T3 in thyroid hormone action. J Clin Invest 1977;60:555–62. 10.1172/JCI108807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iglesias P, Olea T, Vega-Cabrera C, et al. Thyroid function tests in acute kidney injury. J Nephrol 2013;26:164–72. 10.5301/jn.5000106 [DOI] [PubMed] [Google Scholar]
- 58.Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism 2007;56:239–44. 10.1016/j.metabol.2006.09.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055787supp001.pdf (87.3KB, pdf)
bmjopen-2021-055787supp002.pdf (87.7KB, pdf)
bmjopen-2021-055787supp003.pdf (980.9KB, pdf)
bmjopen-2021-055787supp004.pdf (1.1MB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets generated and/or analysed during this study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.