Abstract
Introduction
Reducing unplanned hospital readmissions is an important priority for all hospitals and health systems. Hospital discharge can be complicated by discrepancies in the medication reconciliation and/or prescribing processes. Clinical pharmacist involvement in the medication reconciliation process at discharge can help prevent these discrepancies and possibly reduce unplanned hospital readmissions.
Methods
We report the results of our quality improvement intervention at Duke University Hospital, in which pharmacists were involved in the discharge medication reconciliation process on select high-risk general medicine patients over 2 years (2018–2020). Pharmacists performed traditional discharge medication reconciliation which included a review of medications for clinical appropriateness and affordability. A total of 1569 patients were identified as high risk for hospital readmission using the Epic readmission risk model and had a clinical pharmacist review the discharge medication reconciliation.
Results
This intervention was associated with a significantly lower 7-day readmission rate in patients who scored high risk for readmission and received pharmacist support in discharge medication reconciliation versus those patients who did not receive pharmacist support (5.8% vs 7.6%). There was no effect on readmission rates of 14 or 30 days. The clinical pharmacists had at least one intervention on 67% of patients reviewed and averaged 1.75 interventions per patient.
Conclusion
This quality improvement study showed that having clinical pharmacists intervene in the discharge medication reconciliation process in patients identified as high risk for readmission is associated with lower unplanned readmission rates at 7 days. The interventions by pharmacists were significant and well received by ordering providers. This study highlights the important role of a clinical pharmacist in the discharge medication reconciliation process.
Keywords: medication reconciliation, hospital medicine, transitions in care, pharmacists
Introduction
Unplanned hospital readmissions are a persistent healthcare value concern and opportunity that are associated with higher costs,1 decreased patient satisfaction,2 increased length of stay, and increased mortality.3 Because of these negative consequences, hospital readmissions have been identified as a priority for multiple healthcare agencies including the Center for Medicare and Medicaid Services,4 Institute for Healthcare Improvement,5 Joint Commission for Hospital Accreditation,6 and others. In addition, there are significant financial penalties for hospitals and health systems that perform below target in readmission performance.4
Hospital discharge is viewed as a critical transition in the healthcare trajectory of patients and represents a time when errors can occur. At the time of discharge, medications are initiated, changed, or discontinued. Errors during discharge medication reconciliation can result in complications, including, but not limited to, adverse drug events that can worsen patients’ health status leading to hospital readmissions.7 Many studies have shown that significant errors occur with medication management at the time of admission to and discharge from the hospital,8–18 with some studies reporting medication errors as high as 97% in hospital admissions and 80% in hospital discharges.12 Therefore, management of medications at transitions in and out of the hospital is critical to maintaining good health status and preventing readmissions.
Medication reconciliation is the process of comparing a patient’s active medication orders with those on their home medication list at the time of admission, level of care transfers and at discharge.6 The practice ensures that the home medication list is accurate by taking a best possible medication history (BPMH), which involves completing a thorough patient interview and confirming the information with another objective source of information, including medication fill history from a home pharmacy or nursing facility records.19–21 A medication reconciliation determines which medications are continued or discontinued in the next phase of care. During the process of medication reconciliation, multiple inaccuracies can occur, including errors of omission (not continuing a medication), commission (inappropriately continuing or starting a medication) or prescribing errors (such as frequency or dose errors).21 Multiple studies have indicated all of these errors are possible, but errors of omission are considered to be the most common.8 11 12 16 18 Medication reconciliation is more easily and accurately performed with electronic health record support.17
With the risk of medication reconciliation errors propagating through discharge potentially leading to adverse drug events or readmission, research has been focused on interventions that can reduce medication errors at discharge. Many interventions have focused on involving pharmacists or pharmacy personnel, such as pharmacy technicians or students, in the medication history and/or reconciliation process. The benefit of pharmacist participation in this process has been shown in multiple studies, particularly in reducing adverse drug events or potential adverse drug events. Unfortunately, multiple systematic reviews have not shown consistent evidence that pharmacist involvement in medication reconciliation results in a decrease in hospital readmissions or use.12 13 16 22–30
Duke University Hospital (DUH) is a tertiary, academic medical centre in Durham, North Carolina. We use the Epic electronic medical record for all clinical documentation and ordering processes including medication reconciliation. At DUH, we have acknowledged opportunities and implemented strategies to further reduce readmission rates. In 2016, we completed an internal quality improvement initiative of clinical pharmacist involvement in the discharge medication reconciliation process for two adult general medicine teams and discovered that we had significant numbers of medication-related interventions that could be identified and addressed by clinical pharmacists. At that time, we already had pharmacy technicians obtaining BPMH at hospital admission. We then developed and instituted a programme at DUH for clinical pharmacists to participate in the discharge medication reconciliation process as a quality improvement project. We also recognised that there was little evidence reporting the benefit of using the Epic readmission risk score model to guide pharmacists to which patients to intervene on at discharge. Our goals were therefore to use the Epic readmission risk model to identify high-risk patients for the clinical pharmacists to intervene on to reduce medication errors at discharge from DUH for general medicine patients and achieve reductions in unplanned readmissions. We started the pharmacist medication reconciliation process in October 2018, and in this article we report on the findings of this quality improvement study.
Methods
Study design and setting
This quality improvement interventional study focused on clinical pharmacists supporting the discharge medication reconciliation process with patients discharged from adult general medicine services at DUH between 1 October 2018 and 29 February 2020. We chose 29 February 2020 as our stop date because the COVID-19 pandemic disrupted our usual patient population, census and workflows.
Using all patients identified as ‘high risk’ based on the Epic readmission risk score model, we created three comparative cohorts. Group 1 (intervention group) consisted of high-risk patients who had their discharge medication reconciliation reviewed by a clinical pharmacist. Group 2 (historic controls) were high-risk patients discharged between 1 April 2018 and 1 October 2018. In that group, all discharge medication reconciliation was performed by the general medicine provider only, without clinical pharmacist review. Finally, group 3 (concurrent controls) were high-risk patients discharged during the intervention time period, 1 October 2018–29 February 2020, but who did not have a clinical pharmacist medication reconciliation review concurrently performed because the patient was discharged outside of the service hours (7 days a week from 07:00 to 14:30) when a pharmacist was unavailable to review the discharge medication reconciliation. We report this quality improvement project using the Standards for Quality Improvement Reporting Excellence V.2.0 guidelines.31
Intervention
The DUH electronic medical record, Epic, was used for all medication reconciliation processes. We used the Epic Unplanned Readmission Model V.132 to identify patients in the highest-risk quartile to be included in our intervention group. The Epic unplanned readmission risk model has been investigated on our Duke patient population previously and reported out separately.33 In this previous work, the area under curve (C-statistic) for Duke Hospital general medicine for this model in predicting unplanned readmissions was 0.694. The Epic readmission risk model V.1 calculates a score for readmission risk every 4 hours for inpatients. The score is a continuous variable from 0 to 100, which increases with readmission risk but does not assign a specific probability. The model variables include patient age, clinical diagnoses variables, laboratory variables, medication numbers and classes, order types and healthcare use variables. The model shows a patient’s risk score visually as a coloured icon on the provider patient lists. High-risk score icons are coloured red; medium risk icons are yellow; and low risk icons are green. We set the high-risk threshold to identify the top 25% at risk of readmission. The next 25% would score medium risk, and the lowest 50% would score low risk. Therefore, we set the model threshold based on capacity to provide the intervention rather than metrics of sensitivity or positive predictive value. We reviewed the performance of the risk score model quarterly to determine if thresholds were still set appropriately and the threshold for high risk remained a score of 27 (out of 100 possible total) for the duration of this study.
The process of discharge medication reconciliation review by the clinical pharmacists was initiated on 1 October 2018, with services available 7 days a week from 07:00 to 14:30. The process for our intervention on high-risk patients was such that the general medicine provider would complete the preliminary discharge medication reconciliation on the discharge day by 14:30 and notify the clinical pharmacist to review the completed discharge medication reconciliation before the patient’s discharge order was signed. The clinical pharmacist discharge medication reconciliation review process included focused reviews of the following areas:
Accuracy and completeness of the clinical provider completed discharge medication reconciliation by comparing home medications with inpatient orders and new orders at discharge.
Medication dosing and frequency as well as renal and hepatic adjustments.
Duration of therapy and days of supply for antimicrobials and short course medications such as opiates.
Therapeutic duplications.
Drug–drug interactions.
Cost or financial barriers of certain medications.
After visit summary accuracy and clarity.
The clinical pharmacist would then communicate findings and suggestions for changes to the provider. The provider would make any changes to the discharge medication orders at their discretion before discharge. Also, an admission medication history throughout the study period was attempted by a pharmacy technician through previously established hospital programmes. While no standardised evaluation tool was used, all reviewing pharmacists were trained and familiar with these items to review. Reviewing pharmacists participating in this programme were also trained in technical aspects of discharge medication reconciliation within Epic.
Clinical pharmacy leadership and DUH Medication Reconciliation Committee reviewed the workflows of this study and made one substantive iterative change in several quality improvement cycles over the 2 years of the study. The process of notifying the pharmacist that the provider had completed the discharge medication reconciliation on a high-risk patient and was ready for review changed from a manual process to an automated process in September 2019. When finished with the discharge medication reconciliation process for a high-risk patient, the system now generates an automated consult to the pharmacy, notifying the pharmacist by page to start their workflow.
Outcome variables
Readmissions definition
We measured readmission rates of 7, 14 and 30 days. We report the raw readmissions rates for each group. Our definitions for readmissions were as follows:
Index hospitalisation and associated clinical pharmacist medication reconciliation review were at DUH and readmissions were same-site (DUH).
Patients included were inpatient adults ages 18 and older. We excluded patients whose index admissions were based on psychiatric diagnoses, sickle cell disease, rehabilitation care and non-surgical cancer Medicare Severity Diagnosis Related Groups (DRG), or admitted for inpatient hospice. Patients who were transferred to other acute facilities, died during index hospitalisation or left against medical advice were also excluded.
We excluded patients whose readmission was based on psychiatric diagnoses, sickle cell disease and rehabilitation care, or who had planned readmissions (based on the Center for Medicare and Medicaid Services (CMS) algorithm).4 We excluded sickle cell patients because they have such a unique transitions of care requirement and care system within DUH that would not fit in this specific pharmacy programme design. In order to approximate the CMS Hospital-Wide Readmission Measure exclusions, we excluded from index cases (denominator) patients who had a primary cancer DRG.34 Patients with a secondary cancer DRG were included in our cohorts.
Pharmacist interventions
During the first 6 months of the programme (1 October 2018–31 March 2019), more detailed data were abstracted regarding clinical pharmacist interventions when reviewing the discharge medication reconciliation. Data collection included the number of interventions made by the clinical pharmacist; if the intervention involved a high-risk medication (defined as interventions related to antithrombotics, antiepileptics, antihypertensives, antimicrobials, diuretics, insulin, immunosuppressants and opioids); and if the recommendations were accepted by the medical provider. The aforementioned medications were deemed high risk by the opinion of the involved pharmacists and providers due to the high propensity for errors to occur at transitions of care, which could place a patient at high risk of adverse event or readmission.
Data collection
All high-risk patient encounters, as defined previously, that had a clinical pharmacist review of their discharge medication reconciliation were maintained on an intervention list by pharmacy leadership. Comparison groups were obtained by querying Epic’s Clarity database. The inpatient readmission risk score flowsheet data for intervention and comparison groups were also queried from Epic’s Clarity database. These data were then joined to an internally maintained unplanned readmission database which closely mimics the CMS methodology (using aforementioned readmissions definition), allowing comparison of the intervention and comparison groups.
Statistical analysis
Patient demographics were summarised and compared using standardised mean differences (SMDs). SMDs are an effect metric that measure the degree of imbalance across groups. While not a test of statistical significance, an SMD greater than 0.10 indicates that there are meaningful differences in averages across groups, and therefore potential unequal allocation of the interventions. We performed a multivariable logistic regression, regressing readmission onto the intervention group. We fit separate models for outcomes of 7, 14 and 30 days. Since the readmission score accounts for many clinical factors, we used a minimally adjusted model, adjusting for the abstracted demographic factors as well as baseline risk score. We reported ORs and 95% CIs. Kaplan-Meier curves were plotted for the time to readmission for intervention and comparison groups.
Results
Baseline analysis
A total of 1569 patients identified as high risk by the Epic readmission risk model were in intervention group 1, receiving clinical pharmacist support for discharge medication reconciliation in the period from 1 October 2018 to 29 February 2020. A total of 873 patients were in comparison group 2 (preintervention) and 940 patients were in comparison group 3 (concurrent to intervention). Baseline characteristics of intervention group 1 and comparison groups 2 and 3 are shown in table 1 and were similar (no variable with SMD exceeding 10%) with two exceptions: the intervention group had a higher readmission risk score and a higher percentage of black patients.
Table 1.
Patient baseline characteristics by intervention and comparison groups
| Characteristics | Group 1 (pharmacist intervention) | Group 2 (preintervention comparison group) | Group 3 (concurrent comparison group) | Total all groups | SMD |
| n=1569 | n=873 | n=940 | N=3382 | ||
| Age | 0.067 | ||||
| Median (IQR) | 63 (51–73) | 61 (50–72) | 63 (53–73) | 62 (51–73) | |
| Race/ethnicity, n (%) | 0.117 | ||||
| Hispanic | 58 (3.7) | 19 (2.2) | 42 (4.5) | 119 (3.5) | |
| Non-Hispanic black | 793 (50.5) | 428 (49.0) | 440 (46.8) | 1661 (49.1) | |
| Non-Hispanic white | 664 (42.3) | 400 (45.8) | 416 (44.3) | 1480 (43.8) | |
| Other/unknown | 54 (3.4) | 26 (3.0) | 42 (4.5) | 122 (3.6) | |
| Sex, n (%) | 0.049 | ||||
| Female | 810 (51.6) | 463 (53.0) | 464 (49.4) | 1737 (51.4) | |
| Male | 759 (48.4) | 410 (47.0) | 476 (50.6) | 1645 (48.6) | |
| Length of stay | 0.044 | ||||
| Median (IQR) | 6.6 (3.6–13.2) | 6.1 (3.4–11.1) | 5.9 (3.2–11.5) | 6.2 (3.5–12) | |
| Discharge disposition, n (%) | 0.077 | ||||
| Facility | 594 (37.9) | 290 (33.2) | 304 (32.3) | 1188 (35.1) | |
| Home | 975 (62.1) | 583 (66.8) | 636 (67.7) | 2194 (64.9) | |
| Insurance status, n (%) | |||||
| Medicaid | 279 (17.8) | 154 (17.6) | 136 (14.5) | 569 (16.8) | 0.085 |
| Medicare | 1085 (69.2) | 618 (70.8) | 681 (72.4) | 2384 (70.5) | |
| Private | 145 (9.2) | 71 (8.1) | 94 (10.0) | 310 (9.2) | |
| Other/unknown | 60 (3.8) | 30 (3.4) | 29 (3.1) | 119 (3.5) | |
| Average risk score at discharge | 38.8 | 36.5 | 34.8 | 37.1 | 0.212 |
SMD, standardised mean difference.
Main analysis
Intervention group 1 had a 7-day readmission rate of 5.8%, which was significantly less than comparison groups 2 and 3, which had a 7-day readmission rate of 7.6% (table 2). The multivariable model showed those patients who did not receive the pharmacist participation in medication reconciliation at discharge had a significantly higher odds of readmission at 7 days (table 2).
Table 2.
Readmission rate, ORs and 95% CIs
| Readmission in 7 days | Readmission in 14 days | Readmission in 30 days | ||
| Analysis group 1 (pharmacist intervention) |
Readmission rate | 5.8% | 13.7% | 25.8% |
| OR | – | – | – | |
| Analysis group 2 (preintervention comparison group) | Readmission rate | 7.6% | 14.2% | 26.0% |
| OR | 1.41 (1.01 to 1.97) | 1.10 (0.86 to 1.40) | 1.06 (0.87 to 1.29) | |
| Analysis group 3 (concurrent comparison group) | Readmission rate | 7.6% | 13.8% | 24.6% |
| OR | 1.49 (1.07 to 2.07) | 1.12 (0.8 to 1.43) | 1.06 (0.87 to 1.28) |
Adjusted covariables: readmission risk score, discharge disposition, age, sex, race/ethnicity and length of stay.
Kaplan-Meier event curve of time to readmission shows the difference in readmission event timing between the intervention group and the comparison groups diverges early after discharge with a maximum difference present at 7 days with the curves subsequently converging again at approximately 10 days (figure 1). There was no significant difference in unplanned readmission rates at 14 or 30 days.
Figure 1.
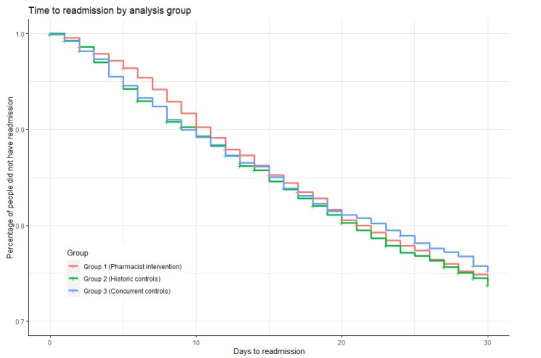
Kaplan-Meier curve—time to readmission.
Secondary analysis of pharmacist interventions
During the first 6 months of the programme, we performed a targeted review of the number and kind of interventions performed by the clinical pharmacists to better understand opportunities. In this review, 780 patients received a clinical pharmacist review of their discharge medication reconciliation, resulting in a significant number of interventions per patient with multiple interventions involving high-risk medications (table 3). Most of the intervention suggestions were accepted by the medical team.
Table 3.
Targeted review of interventions performed by clinical pharmacists during first 6 months of the programme
| Total number of patients reviewed by a pharmacist | 780 |
| Total number of interventions | 1366 |
| Total number of patients with at least one intervention by a pharmacist | 523 |
| Average number of interventions/patient | 1.75 |
| Patients with at least one intervention (%) | 67% |
| Interventions that involved high-risk medication* (%) | 71% |
| Interventions accepted by the primary medical team (%) | 89% |
*High-risk medications: antithrombotics, antiepileptics, antihypertensives, antimicrobials, diuretics, insulin, immunosuppressants and opioids.
Discussion
In this quality improvement project, we show that patients at high risk of an unplanned readmission who receive a clinical pharmacist review of discharge medication reconciliation had a significantly lower rate of unplanned hospital readmissions at 7 days with no impact on readmission rates of 14 or 30 days. Additionally, our analysis of the first 6 months of this project showed that clinical pharmacists made a significant number of recommendations per patient, and many of these recommendations involved high-risk medications. In addition, the majority of the pharmacist recommendations were agreed to by the medical providers. One of the strengths of this project is the high number of patients involved and the use of the Epic electronic record readmission risk model to identify which high-risk patients to include in the intervention group.
Although we were unable to show a difference in readmission rates at 14 and 30 days, we feel that the significant reduction in readmissions at 7 days is an important finding. Despite our quality improvement project focusing on a single pharmacy intervention before discharge, we were still able to show a significant reduction in 7-day readmission rates. We hypothesise that 7-day readmission rates may be more representative of factors that can be controlled by the discharging hospital and teams. Chin et al found that a hospital’s variability in discharge factors dramatically reduced at 7 days, supporting the concept that hospitals may be more able to affect early readmissions within 7 days of discharge rather than late readmissions after 7 days.35 Graham et al found early readmissions (before 7 days) were more likely to be assessed as preventable by the hospital or its provider teams, and those late readmissions (after 7 days) were more likely to be influenced by the clinic practices or various home interventions.36 We feel that our results are consistent with this concept and that the structure of our intervention would impact early postdischarge readmissions.
In the first 6 months of our study, the findings of our detailed pharmacy intervention data were similar to what has been reported in major systematic reviews of this topic in the literature12 13 16 22–26 28–30: a significant number of interventions per patient, a significant number of interventions involving high-risk medications and overall high acceptance of recommendations by pharmacists by the ordering provider.
We believe this is the first study to examine using the Epic readmission risk model to guide which patients could be a focus for a pharmacy-supported medication reconciliation process. This risk model has been previously evaluated by our institution and confirmed to have a reasonable discriminatory function to identify patients at higher risk of readmission.33 Our understanding of the Epic readmission model is there is some possibility of customisation of the model’s variables and weightings, but our institution has not pursued that, so this project represents the implementation of the standard risk model. Using this risk model, we can more efficiently focus our interventions on those patients at the highest risk of unplanned readmission. Based on the results we have shown, our hospital has committed to sustaining the pharmacy staffing necessary to perform this medication reconciliation work.
Our work has limitations to acknowledge. First, this is a quality improvement project meant to improve our local institution’s performance on unplanned readmission rates using accepted best practices of clinical pharmacist support during the discharge medication reconciliation process. As it is a quality improvement project, these specific results may not generalise to other institutions. Further limitations of our study include that providing discharge patient counselling or postdischarge follow-up was not included in our clinical pharmacist interventions. Previous studies have shown benefits in pharmacist postdischarge follow-up.22 During the study time frame, there were other projects and work within our institution focused on reducing readmissions from a variety of different groups. Due to the inability to pinpoint these other studies in terms of time and patient population, we are unable to develop a system to control for the effect of these other projects on readmission rates. However, these other potentially confounding projects would have had the same effect on all groups, so despite this, we were still able to show a significant difference in 7-day readmission rates compared with both control groups. This project was a single site readmission project (DUH), so we were not capturing readmissions at other hospitals, which could have resulted in an imbalance of readmissions in our data for those who received clinical pharmacist review versus those who did not. A review of 2019 data shows that 87% of all readmissions within our health system for DUH general medicine discharges are ‘same-site’ returns to DUH with 13% being readmitted at the other two Duke affiliated hospitals.
In reviewing these findings and how they may guide future studies, we feel that the use of an electronic health record readmission risk model or scoring system can be very useful to identify patients for readmission reduction interventions. We feel that it is important to examine the most successful practices after discharge, which could involve a clinical pharmacist to help impact readmissions.
Conclusion
At our local institution, this quality improvement project showed that involving clinical pharmacists in the discharge medication reconciliation process is associated with a significant reduction in 7-day unplanned readmissions. The Epic readmission risk score model allowed our pharmacists to focus their efforts on the highest-risk patients rather than randomly decide which patients to intervene on. The work of our pharmacists involved making a significant number of recommendations per patient, many of which were adopted by the medical providers, with many suggestive changes involving high-risk medications.
Footnotes
Twitter: @DGallagherMD
Contributors: We followed the ICMJE authorship guidelines in that all authors contributed substantially to the design of the work (including data acquisition, analysis and interpretation); helped draft the manuscript in all phases; had final approval of the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Specific author main responsibilities include concept and design: DG, MG, LB and BG; acquisition of data: DL, LS, MG, LB, CS and KK; analysis and interpretation of data: DG, BG, CZ, LB, CS and KK; drafting of the manuscript: DG, DL, LS, MG, CS, KK, CZ, BG and LB; critical revision of paper for important intellectual content: DG, DL, LS, MG, CS, KK, CZ, BG and LB; statistical analysis: CZ and BG; provision of study materials or patients: N/A; obtainment of funding: DG; administrative, technical or logistic support: DG, BG and LB; supervision: DG, BG and LB; other (implementation of pharmacist medication reconciliation): MG, DL, LS and LB. DG functioned as guarantor and accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon request. Deidentified Data are available upon reasonable request and after approval of request by Duke Health research leadership.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study (Protocol ID: Pro00107559) was approved by the Institutional Review Board of Duke University as exempt without the need for informed consent as it is a quality improvement project to apply best practices to reduce unplanned readmissions.
References
- 1.Jencks SF. Defragmenting care. Ann Intern Med 2010;153:757–8. 10.7326/0003-4819-153-11-201012070-00010 [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui Z, Berry S, Bertram A, et al. Does patient experience predict 30-day readmission? A patient-level analysis of HCAHPS data. J Hosp Med 2018;13:681–7. 10.12788/jhm.3037 [DOI] [PubMed] [Google Scholar]
- 3.Burke RE, Jones CD, Hosokawa P, et al. Influence of Nonindex Hospital readmission on length of stay and mortality. Med Care 2018;56:85–90. 10.1097/MLR.0000000000000829 [DOI] [PubMed] [Google Scholar]
- 4.Center for Medicare and Medicaid services (CMS) . Hospital readmissions reduction program (HRRP), 2020. Available: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program [Accessed 16 Jun 2020].
- 5.Institute for Healthcare Improvement . Medication reconciliation to prevent adverse drug events. Available: http://www.ihi.org/explore/adesmedicationreconciliation [Accessed 01 Dec 2021].
- 6.The Joint Commision . National patient safety goals effective January 2021 for the hospital program national patient safety goals NPSG.03.06.01. Available: https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2021/hap_npsg_jan2021.pdf [Accessed 01 Dec 2021].
- 7.Howard RL, Avery AJ, Howard PD, et al. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care 2003;12:280–5. 10.1136/qhc.12.4.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008;42:1373–9. 10.1345/aph.1L190 [DOI] [PubMed] [Google Scholar]
- 9.Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012;157:1–10. 10.7326/0003-4819-157-1-201207030-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71. 10.1001/archinte.166.5.565 [DOI] [PubMed] [Google Scholar]
- 11.Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med 2008;23:1414–22. 10.1007/s11606-008-0687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehnbom EC, Stewart MJ, Manias E, et al. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother 2014;48:1298–312. 10.1177/1060028014543485 [DOI] [PubMed] [Google Scholar]
- 13.Michaelsen MH, McCague P, Bradley CP, et al. Medication reconciliation at discharge from Hospital: a systematic review of the quantitative literature. Pharmacy 2015;3:53–71. 10.3390/pharmacy3020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman EA, Smith JD, Raha D, et al. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005;165:1842–7. 10.1001/archinte.165.16.1842 [DOI] [PubMed] [Google Scholar]
- 15.Cornu P, Steurbaut S, Leysen T, et al. Effect of medication reconciliation at hospital admission on medication discrepancies during hospitalization and at discharge for geriatric patients. Ann Pharmacother 2012;46:484–94. 10.1345/aph.1Q594 [DOI] [PubMed] [Google Scholar]
- 16.Tam VC, Knowles SR, Cornish PL, et al. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005;173:510–5. 10.1503/cmaj.045311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med 2009;169:771–80. 10.1001/archinternmed.2009.51 [DOI] [PubMed] [Google Scholar]
- 18.Gleason KM, McDaniel MR, Feinglass J, et al. Results of the medications at transitions and clinical handoffs (match) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med 2010;25:441–7. 10.1007/s11606-010-1256-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadasivaiah S, Smith DE, Goldman S, et al. Improving best possible medication history with vulnerable patients at an urban safety net academic Hospital using pharmacy technicians. BMJ Open Qual 2017;6:e000102. 10.1136/bmjoq-2017-000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnipper JL, Mixon A, Stein J, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the Marquis study. BMJ Qual Saf 2018;27:954–64. 10.1136/bmjqs-2018-008233 [DOI] [PubMed] [Google Scholar]
- 21.Olavo F, Kaveh GS. Medication reconciliation in the hospital: what, why, where, when, who and how? Healthc Q 2012;15 Spec No:42–9. 10.12927/hcq.2012.22842 [DOI] [PubMed] [Google Scholar]
- 22.Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-Based medication reconciliation practices: a systematic review. Arch Intern Med 2012;172:1057–69. 10.1001/archinternmed.2012.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geurts MME, Talsma J, Brouwers JRBJ, et al. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol 2012;74:16–33. 10.1111/j.1365-2125.2012.04178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med 2012;157:417–28. 10.7326/0003-4819-157-6-201209180-00006 [DOI] [PubMed] [Google Scholar]
- 25.Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 2013;158:397–403. 10.7326/0003-4819-158-5-201303051-00006 [DOI] [PubMed] [Google Scholar]
- 26.Holland R, Desborough J, Goodyer L, et al. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol 2008;65:303–16. 10.1111/j.1365-2125.2007.03071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensing HT, Stuijt CCM, van den Bemt BJF, et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm 2015;21:614–36. 10.18553/jmcp.2015.21.8.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNab D, Bowie P, Ross A, et al. Systematic review and meta-analysis of the effectiveness of pharmacist-led medication reconciliation in the community after hospital discharge. BMJ Qual Saf 2018;27:308–20. 10.1136/bmjqs-2017-007087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mekonnen AB, McLachlan AJ, Brien J-AE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6:e010003. 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bethishou L, Herzik K, Fang N, et al. The impact of the pharmacist on continuity of care during transitions of care: a systematic review. J Am Pharm Assoc 2020;60:163–77. 10.1016/j.japh.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 31.Ogrinc G, Davies L, Goodman D, et al. Squire 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016;25:986–92. 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EPIC . Cognitive computing model brief: risk of unplanned readmission (version 1), 2016. Available: www.epic.com [Accessed 16 Jun 2020].
- 33.Gallagher D, Zhao C, Brucker A, et al. Implementation and continuous monitoring of an electronic health record embedded readmissions clinical decision support tool. J Pers Med 2020;10. 10.3390/jpm10030103. [Epub ahead of print: 26 08 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Center for Medicare and Medicaid Services (CMS) . Hospital-Wide readmission measure updates and specifications report – version 10.0, 2021. Available: https://qualitynet.cms.gov/files/6094393d2be51c001edf6163?filename=2021_HWR_AUS_Report.pdf [Accessed 22 Jul 2021].
- 35.Chin DL, Bang H, Manickam RN, et al. Rethinking Thirty-Day Hospital readmissions: shorter intervals might be better indicators of quality of care. Health Aff 2016;35:1867–75. 10.1377/hlthaff.2016.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham KL, Auerbach AD, Schnipper JL, et al. Preventability of early versus late Hospital readmissions in a national cohort of general medicine patients. Ann Intern Med 2018;168:766–74. 10.7326/M17-1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request. Deidentified Data are available upon reasonable request and after approval of request by Duke Health research leadership.


