Abstract
The hepatitis A virus (HAV), a picornavirus, is a common cause of hepatitis worldwide. Spread of infection is generally person to person or by oral intake after fecal contamination of skin or mucous membranes; less commonly, there is fecal contamination of food or water. Hepatitis A is endemic in developing countries, and most residents are exposed in childhood. In contrast, the adult population in developed countries demonstrates falling rates of exposure with improvements in hygiene and sanitation. The export of food that cannot be sterilized, from countries of high endemicity to areas with low rates of infection, is a potentially important source of infection. After ingestion and uptake from the gastrointestinal tract, the virus replicates in the liver and is excreted into the bile. Cellular immune responses to the virus lead to destruction of infected hepatocytes with consequent development of symptoms and signs of disease. Humoral immune responses are the basis for diagnostic serologic assays. Acute HAV infection is clinically indistinguishable from other causes of acute viral hepatitis. In young children the disease is often asymptomatic, whereas in older children and adults there may be a range of clinical manifestations from mild, anicteric infection to fulminant hepatic failure. Clinical variants include prolonged, relapsing, and cholestatic forms. Management of the acute illness is supportive, and complete recovery without sequelae is the usual outcome. Research efforts during World War II led to the development of passive immunoprophylaxis. Pooled immune serum globulin is efficacious in the prevention and attenuation of disease in exposed individuals. More recently, active immunoprophylaxis by vaccination has been accomplished. Future eradication of this disease can now be contemplated.
INTRODUCTION
“Viruses”
Before the precise recognition of what we now define as viruses, bacteria, fungi, and protozoa, all infectious agents were referred to as viruses, from the Latin for poison. The work of scientists such as Pasteur, Lister, and Koch in the 1800s led to the isolation of pure cultures of bacteria and the demonstration of their causal role in infectious diseases. By the turn of the century, experimental evidence was accumulating that culture-sterile, filtered preparations could transmit infection 151, 185. For example, Walter Reed published his observations on the transmission of yellow fever by inoculation of human volunteers with filtered serum isolated from patients with clinical disease in 1902. He commented “Yellow fever, like the foot and mouth disease of cattle, is caused by a micro-organism so minute in size that it might be designated as ultra-microscopic” 206. Transmission of foot-and-mouth disease observed in cattle followed earlier reports that tobacco mosaic disease could be transmitted with a filtered inoculum 151, 185. In contrast, the viral etiology of epidemic jaundice was not widely accepted by physicians until the mid-20th century. Indeed, an editorial in the Journal of the American Medical Association used the term “catarrhal jaundice” as late as 1943 15. The basic science of viruses and the clinical science of their associated diseases have made enormous progress this century. The Nobel laureate Peter Medawar succinctly put it when he said that “No virus is known to do good; it has been well said that a virus is ‘a piece of bad news wrapped up in protein’ ” 169.
Historical Epidemic Jaundice
According to Cockayne, catarrhal jaundice was recognized in ancient Greece and Rome 46. The ancient Chinese were also apparently aware of its existence. Cockayne accepts the first reference to epidemic jaundice as that occurring in Minorca in 1745, recorded by Cleghorn in Epidemic Diseases of Minorca 1744 to 1749, and he reports numerous other instances in the 1700s and 1800s. Clearly, by the time of his review in 1912, there was ample evidence of its occurrence.
Infectious Agent of Epidemic Jaundice
McDonald is credited 115, 272 as the first person to implicate a virus as the etiologic agent of what we now call hepatitis A 166. However, in his report of acute yellow atrophy of the liver being “produced when some special virus acts on a previously damaged liver,” he may have used the term in the sense of any infectious agent, not the filterable agent of Reed and other investigators. Similarly, Cockayne in his treatise on the relationship between epidemic and catarrhal (sporadic) jaundice writes “due to virus remaining active” and “a virulent condition” 46. Cockayne considered that many of the features of epidemic and sporadic cases of jaundice were like those of mumps, another disease of uncertain etiology at that time; he concluded that both epidemic and sporadic jaundice was caused by a specific organism of unknown nature.
Viral Etiology of Epidemic Hepatitis
In 1931, Findlay, Dunlop, and Brown presented a paper entitled “Observations on Epidemic Catarrhal Jaundice” at the Royal Society of Tropical Medicine and Hygiene 81. After reviewing the history of epidemic jaundice, current knowledge, and a contemporary outbreak in Surrey, they concluded that it was likely due to an “ultra-microscopic virus which is pathogenic only to man,” similar to varicella, herpes zoster, rubella, and dengue. Deliberate experimental transmission to human volunteers was first reported from Germany in 1942 255 and the Middle East in 1943 42, more than 25 years before successful transmission in an animal model 116. H. C. Brown, one of the authors of the 1931 paper ascribing the etiology of epidemic jaundice to a virus, developed an illness with the characteristics of a sporadic case of epidemic jaundice. He became ill <5 weeks after handling sera from the epidemic jaundice outbreak in Yorkshire described by Pickles 196. The timing of Brown's symptoms was consistent with what we now know is the incubation period of hepatitis A and perhaps the first documentation of viremic serum transmitting epidemic hepatitis 81.
Virologic Classification
All viruses are classified by their virion properties (morphology, physicochemical and physical properties, genome, proteins, lipids, carbohydrates, genome organization, and replication), antigenic properties, and biologic properties (Table 1). The viral genome is either RNA or DNA and double stranded or single stranded. In addition, the viral particle may be enveloped (host-derived lipid envelope) or nonenveloped. Each of the five major hepatitis viruses (Table 1)—hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), delta hepatitis virus (HDV), and hepatitis E virus (HEV)—belongs to a separate family (the taxonomy of viruses includes family, genus, and species).
TABLE 1.
The major hepatitis viruses
| Virus | Classification | Genome | Envelope | Spread |
|---|---|---|---|---|
| HAV | Picornaviridae, genus Hepatovirus | RNA | Nonenveloped | Fecal-oral |
| HBV | Hepadnaviridae | DNA | Lipid enveloped | Parenteral |
| HCV | Flaviviridae, genus Hepacivirus | RNA | Lipid enveloped | Parenteral |
| HDV | Unclassified | RNA | Lipid enveloped (from HBV) | Parenteral |
| HEV | Caliciviridae, genus proposed | RNA | Nonenveloped | Fecal-oral |
HEPATITIS A VIRUS
The Picornaviridae are small, nonenveloped, single-stranded RNA viruses. Human pathogens include species in the genera Rhinovirus (human rhinoviruses) and Enterovirus (poliovirus, coxsackieviruses, echoviruses, and human enteroviruses). Although HAV shares some major characteristics with other genera of the picornavirus family, it is sufficiently different that it is classified as the only species in the genus Hepatovirus. There are naturally occurring strains that infect nonhuman primates (three genotypes) as well as four genotypes that comprise the human-infectious viruses 148. The strains belonging to each genotype have ≥85% nucleotide identity. Most human strains belong to either genotype I or III. The prototypic laboratory strains HM175, originally isolated in Melbourne, Australia, and CR326, from Costa Rica, are closely related genotype I strains 148. HAV, unlike other members of the Picornaviridae family, is stable at pH 1 and resistant to heat (56°C for 30 min) and shows no cross-hybridization with enteroviruses, rhinoviruses, or other picornaviruses. Details of these characteristics are amply referenced in standard texts 115.
Genome Organization
The organization of the HAV genome is similar to that of the other picornaviruses 115. The positive-sense (i.e., translatable), single-stranded RNA is 7.5 kb in length and consists of a 5′ noncoding region (NCR) of 734 to 740 nucleotides, a coding region of 2,225 to 2,227 nucleotides, and a 3′ noncoding region of 40 to 80 nucleotides 115. The secondary structure of the 5′ NCR is important in translation initiation. The Picornaviridae RNA genomes lack the cap assembly found at the 5′ end of mRNA species that normally guides the ribosomal complex to the translation start site. An internal ribosome entry site formed by the 5′ NCR functions to initiate translation in the picornaviruses, including HAV. The 5′ NCR of HCV, another single-stranded, positive-sense, uncapped RNA genome, also includes an internal ribosome entry site.
Proteins
Although HAV was first successfully adapted to cell culture 20 years ago 201, its protein constituents have not been completely defined 115. Infected cells contain only low titers of virus, and consequently protein chemistry has been limited. The P1 region encodes the three major proteins of the viral capsid, VP1, VP2, and VP3. A fourth viral capsid protein (VP4), essential for virion formation 199, is not detected in mature viral particles. Each of the capsid proteins is cleaved from the precursor polyprotein by the viral protease 3C, encoded in the P3 region. The native conformation of the capsid proteins VP1 and VP3 forms a single, dominant, serologic epitope on the viral capsid and elicits a neutralizing antibody response. Nonstructural proteins encoded in the P2 and P3 regions are predicted to function in RNA synthesis and virion formation. VPg (virion protein, genome linked), also encoded in the P3 region, is covalently linked to the 5′ genome terminus and involved in initiation of RNA synthesis.
Life Cycle
Available data as to the exact fate of virions immediately after oral intake are sketchy (see Fig. 1). In experimental infection of owl monkeys with human HAV, viral antigen was detectable by immunofluorescence in the stomach, small intestine, and large intestine not only after the initial oral inoculation but also later in the course of the disease 20. The ability to detect viral antigens in intestinal crypt cells using immunofluorescence suggests that viral replication can occur in the intestine 20. Virions presumably reach the liver in the portal blood (or after systemic circulation) and are taken up by hepatocytes. An attachment receptor for HAV in nonliver primate cells has been characterized 21, 77, 131; however, the relationship of this mucin-like class I integral membrane glycoprotein to hepatocyte uptake of virus is not clear. Once HAV has replicated in the liver and been released into bile (see below), the enterohepatic cycle of gastrointestinal uptake and transfer to the liver could continue until neutralizing or other antibodies interrupted the cycle.
FIG. 1.
Possible “enterohepatic” cycling of HAV.
Replication
Current evidence indicates that HAV replication is probably exclusive to hepatocytes and gastrointestinal epithelial cells in vivo, although cell culture infection and replication in nonhepatocyte cell lines are well documented 115. Virus-encoded proteins replicate the RNA genome via a negative-strand intermediate and are themselves synthesized from the genomic positive strand. Intact virions contain the RNA genome, the covalently linked VPg protein, and a capsid of the coat proteins VP1, VP2, and VP3 with icosahedral symmetry. Virus particles appear in bile and blood, presumably being released across the apical hepatocyte membrane into the biliary canaliculus and across the basolateral membrane into the bloodstream. The mechanism of viral release and secretion is unknown but clearly is not dependent on cell destruction, since high viral titers are present in stool before there is any evidence of hepatocyte necrosis 51, 139, 245.
Detection
HAV was first visualized after aggregation of fecal material with serum containing specific homologous antibodies 78. The fecal material was collected from Joliet prison volunteers 34 inoculated with the MS-1 strain of hepatitis virus characterized by Krugman and colleagues 138. The technique of immune electron microscopy of stool was then used to assay for specific anti-HAV antibodies in convalescent-phase sera after episodes of naturally occurring hepatitis and to investigate the transmission of virus 67, 70. HAV can now be detected by a variety of immunologic and molecular techniques, including radioimmunoassay (RIA), DNA-RNA hybridization 245, and reverse transcriptase PCR (RT-PCR) amplification. RT-PCR amplification was used to identify specific viral strains implicated in parenteral transmission of virus 7, 159.
TRANSMISSION
Physicians in the early 1900s recognized that hepatitis A was spread by person-to-person contact 46, food, and possibly water 262. Cockayne extensively reviewed previous literature, generally selecting statements and observations that we now know to be correct. For example, he reports “One man already infected travelled to Flintshire and there passed on the disease to three others.” Whereas person-to-person contact was evident, an alimentary mode of spread was not generally accepted. Although most physicians considered that a respiratory-type droplet infection was more likely 33, 54, 81, 84, 92, 154, gastrointestinal transmission was predicted by some authors in Europe 2 and the United States 181.
Oral Transmission
Experimental transmission of infective hepatitis by feeding duodenal juice was first reported by Voegt 255; cited in reference 82). Of note, although published in Germany (in Muenchener medizinische Wochenschrift [Munich Medical Weekly]) in 1942, the findings were referenced by British investigators in 1943 82 and Americans in 1944 104 despite World War II. An underground network apparently procured scientific publications through neutral countries (H. Mayo, personal communication), permitting efficient dissemination of knowledge.
In the United States, Havens and colleagues successfully transmitted jaundice by feeding either serum or a filtrate of stool extract to 12 conscientious objectors who volunteered for studies at Yale University 104. The incubation period was 20 to 30 days in the four persons who became icteric, consistent with transmission of hepatitis A. Stool samples obtained during convalescence were not infectious, whereas hepatitis was transmitted with stool samples collected 5 days after the onset of symptoms 105. In parallel studies, three of five volunteers became jaundiced after intracutaneous inoculation of serum. The incubation period was longer, suggesting hepatitis B 104. In later publications from this group, a distinction between infectious hepatitis (short incubation) and serum jaundice (long incubation) was made 189, 190. Infectious hepatitis (hepatitis A) was transmitted to four of five volunteers by ingestion of preicteric sera and to two of three volunteers after ingestion of stool 190. Hepatitis was apparent less than 40 days after exposure in all volunteers. In contrast, serum jaundice (hepatitis B) was transmitted to 10 of 23 volunteers inoculated with serum but not to three volunteers ingesting serum 190. All in this group developed hepatitis more than 50 days after exposure.
Parenteral Transmission
Voegt injected preicteric serum and produced jaundice in studies performed at the same time as those investigating oral transmission (255; cited in reference 190). Working with British troops in Palestine in 1941 to 1942 and unaware of Voegt's findings (as judged by the references cited and the geographic separation), Cameron injected whole blood or serum from jaundiced patients into seven volunteers 42. One recipient developed jaundice a month after injection, consistent with the incubation period of hepatitis A (10 to 50 days). The serum for this recipient was collected 2 days after the onset of jaundice in the donor. Jaundice eventually developed in five volunteers after various periods of time on active duty. Cameron abandoned further human experiments when he became aware of case fatalities (not from his own series).
Havens and colleagues at Yale also transmitted infectious hepatitis (hepatitis A) by parenteral means to 6 of 11 recipients. Using preicteric serum obtained from volunteers who developed short-incubation hepatitis following ingestion of infected material 190, they observed a short incubation period similar to that noted after ingestion of infected material. Serum obtained 11 days before and 31 days after the onset of symptoms did not transmit hepatitis to any of six volunteers (three in each group), whereas serum collected 4 days after the onset was infectious in three of six recipients 105. Three volunteers were infected sequentially with hepatitis B and then hepatitis A, demonstrating a lack of cross-reactive immunity. The authors interpreted their findings conservatively, as not indicating a fundamental difference between the two diseases, since the clinical features were indistinguishable with the exception of the incubation phase 190.
Infectious Hepatitis versus Serum Jaundice
By the late 1940s, the differentiation between infectious hepatitis and serum jaundice was distinct enough, and the nomenclature was confusing enough, that MacCallum proposed using the terms hepatitis A and hepatitis B in 1947 16. Whether the differences were explained by two distinct viruses or different strains of the same organism remained uncertain. The term catarrhal jaundice was finally abandoned following the general acceptance that a virus(es) was the etiologic agent(s), because the transmission experiments used filtered material 106, 189.
Willowbrook State School MS-1 and MS-2
Definitive evidence of two different types of hepatitis virus was provided by a series of experiments carried out at the Willowbrook State School on Staten Island. The goal of the original studies was to control hepatitis, which was endemic in this residential school for the mentally disabled. The early work demonstrated the period of infectivity of serum and fecal material 139, 140, 257 and also the usefulness of measurements of serum glutamic oxaloacetic transaminase (SGOT) in the diagnosis of anicteric and asymptomatic infections 139.
Second attacks of hepatitis were observed in some residents 138. To investigate this observation more thoroughly, pools of serum obtained during the two separate episodes in one resident (Mir) were inoculated into newly admitted residents kept in isolation. One pool, designated MS-1 (Mir serum-1), caused hepatitis in seven of eight children after a relatively short incubation (hepatitis A), whereas the second pool, MS-2, resulted in long-incubation hepatitis (hepatitis B) in seven of nine children 138. The MS-1 pool was used in the first successful transmission of hepatitis A to animals (marmosets) 116 and to transmit hepatitis to volunteers (in Joliet prison) whose clinical samples were the sources of material for the first detection of virus particles 78. Marmoset livers became the source of infectious material not only for the development of serologic assays but also for cell culture experiments.
MODERN-ERA TRANSMISSION
Hepatitis A is the most common cause of acute hepatitis in the United States, as reported to the Centers for Disease Control and Prevention (CDC) and shown for 1998 in Fig. 2 and 3 10. The numbers of hepatitis A cases reported in 1996 and 1997 were substantially higher (31,032 and 30,021, respectively); there are no data for 1999 at this time. The latest data from the Viral Hepatitis Surveillance Program (1993) indicate that contact with a person infected with hepatitis A is the most common identifiable source of infection (22%), with day care centers the possible source in 17%, international travel in 6%, homosexual activity in 5%, injection drug use in 2%, and a food- or waterborne outbreak suspected in 2% (www.cdc.gov/nciod/diseases/hepatitis/h96surve.htm). The largest percentage of infected persons, however, have no identifiable source (47%).
FIG. 2.
Age group distribution of reported cases of HAV and HBV infection in 1998. (CDC data.)
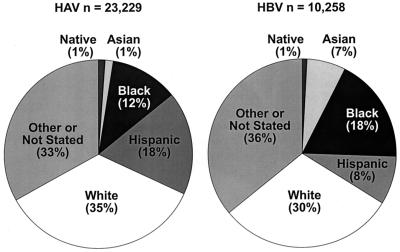
FIG. 3.
Race and ethnic distribution of reported cases of HAV and HBV infection in 1998. (CDC data.) Native, American Indian or Alaskan native; Asian, Asian or Pacific islander.
Personal Contact
Besides accounting for 22% of attributed sources (website cited above), personal contact with an unidentified source shedding HAV is likely to explain many or all of the cases with no identifiable risk factor. In Salt Lake County, Utah, 98 of 390 (25%) household contacts of 167 persons without identified risk factors demonstrated serologic evidence of recent hepatitis A infection (C. Staes, T. Schlenker, I. Risk, L. Bogdanow, K. Cannon, R. Winn, H. Harris, C. Shapiro, A. Pavia, and B. Bell, Clin. Infect. Dis. 25:411, 1997, abstract). The highest rate of retrospectively diagnosed recent hepatitis A was in children who were <7 years of age (38 of 81 [47%] household contacts in this age group). Young children are often asymptomatic when infected with hepatitis A 99, 226 and, with their less scrupulous hygiene, may serve as a source of infection.
The virus is hardy, surviving on human hands and inanimate objects (fomites) 163. Studies indicate that viral particles are excreted fecally during clinical illness 223 and that fecal excretion can be prolonged, as determined by detection of viral nucleic acids (by RT-PCR amplification) for 3 to 11 months 126, 269. In a neonatal intensive care unit outbreak of nosocomial hepatitis A, there was prolonged excretion of virus by neonates, as evidenced by detection of viral protein and nucleic acid for 4 to 5 months after initial identification of infection 213. Furthermore, in a large-scale field trial of the efficacy of a formalin-inactivated hepatitis A vaccine, HAV RNA was detected in stool collected 61 to 90 days after onset of illness in 16 of 19 cases of infection in the control group 125. The infectivity of fecal material was not demonstrated in any of these cases. However, taken together, these observations reinforce the need for rigorous personal hygiene in the prevention of transmission. The prolonged excretion of infectious virus plus the hardiness of the virus may well explain the continued occurrence of sporadic cases of hepatitis A in developed countries as well as the endemicity in underdeveloped countries.
Food-Borne Hepatitis A
One of the earliest documented outbreaks of hepatitis A associated with consumption of contaminated material was the demonstration of a rising titer of specific antibody in members of a family who contracted acute hepatitis after eating mussels 68. The largest known modern epidemic of hepatitis A was also from consumption of contaminated seafood. In Shanghai, China, 292,301 cases of acute hepatitis were attributed to eating raw clams 100. Oysters 49, 64 and cockles 183 have also been implicated. HAV may survive for extended periods of time in seawater. Viral nucleic acids were detectable 232 days after being seeded in artificial seawater, whereas they were only detectable for 35 days in cell culture 18. Consequently, the filtering of seawater by bivalves, with the potential for retaining infectious HAV particles resulting from fecal contamination, can lead to the transmission of infection to those who consume the seafood without adequate cooking. Spread of hepatitis A has been reported in the United States and Europe following consumption of contaminated lettuce 212, ice slush beverages 27, frozen strawberries 11, 121, 179, and salad food items 155, 192. The global movement of food items that cannot be heated for viral inactivation may be a major cause of outbreaks in developed countries in the future. The recent multistate outbreak of hepatitis A 121 following the illegal use of non-U.S. produce in school lunches 109 illustrates the problem.
Waterborne Outbreaks
In the United States, waterborne transmission of hepatitis A accounts for only a fraction of cases. In one small outbreak, HAV contaminated well water 32. A swimming pool with potential sewage contamination was implicated as the common source in another 157. Hepatitis A is considered an occupational hazard for sewage workers in some countries 65 but not in Israel or the United States 150, 247. HAV was detected in the final effluent from wastewater treatment plants in the Mediterranean 71, demonstrating a potential source for seafood contamination. Waterborne transmission is less important in the spread of hepatitis A than person-to-person contact. In contrast, hepatitis E, particularly in epidemic form, appears to be transmitted by waterborne outbreaks.
Nosocomial Hepatitis A
Transmission of hepatitis A from hospitalized patients with unsuspected disease to staff is well recognized 93. For example, an adult patient with diarrhea after an elective cholecystectomy 94, premature infants 135 with prolonged viral excretion 213, burn patients incubating HAV in hospital 72, and a patient who was immunodeficient and negative for HAV antibodies 41 have all been sources of nosocomial infection. One example of nosocomial spread emphasizes the natural life cycle of the virus. A patient with an overdose and trauma from a motor vehicle accident had a T-tube draining bile during the incubation phase of hepatitis A. The bile was the only apparent source of infection in five cases of nosocomial hepatitis A 101. Vertical transmission is rare 74, 146 but was the apparent source of hepatitis A in a nursery outbreak 258.
Parenteral Hepatitis A Transmission
Once considered rare outside experimental studies 218, parenteral transmission of hepatitis A complicating transfusion of blood and blood products has now been reported many times. Blood from a single donor who became ill 1 week after donation transmitted disease to 11 recipient neonates and thence secondarily to an additional 44 persons 180. Transmission associated with platelet and plasma donation processing 170 and anticancer immunotherapy reagents 259 has also been documented. More recently, identical HAV sequences were detected in clotting factor concentrates and hemophiliac recipients in Italy 159. The solvent-detergent method of viral inactivation was considered inadequate for nonenveloped viruses such as HAV. In late 1995, a similar outbreak occurred in the United States 7. Vapor heating of clotting factor concentrates is experimentally effective in eradicating infective HAV 25.
The other group at risk for HAV infection by parenteral transmission is the injection drug-using population 4, 97. Hepatitis A can also be potentially spread within this group by contamination from rectally carried drugs 237 as well as by unsanitary living conditions, crowding, and lack of the necessary personal hygiene to prevent infection. Approximately 40 to 50% of injection drug users in northern Europe are anti-HAV positive 136, and in Scandinavia and France the seropositive rate is significantly higher than in a matched control population 117, 216, 256, 261. At Johns Hopkins Hospital, seropositive rates were more than twice as high in injection drug users (66%) as in homosexual men (27%) and correlated with poverty 254. Targeting this group for vaccination to prevent HAV infection may decrease their infection rates in the future.
Homosexual Transmission
Although early seroprevalence studies of hepatitis A did not demonstrate increased positivity in homosexual men 238, two prospective studies of seroconversion clearly document a high rate of ongoing infection 50, 53. More recently, hepatitis A outbreaks among homosexual men were reported in the United States and abroad 5, 110, 236. Higher seroprevalence rates of hepatitis A infection are associated with oral-anal contact regardless of sexual orientation 23, not with homosexuality per se 254.
PATHOLOGY
Before the late 1930s, pathologic examination of the liver required either an open-liver biopsy at the time of surgery or death of the patient and an autopsy. Consequently, little information was available regarding the pathologic changes in uncomplicated infectious hepatitis. The findings in acute yellow atrophy were well documented 166, and the relationship between acute yellow atrophy and nonfatal hepatitis was increasingly recognized 46, 80, 88, although the heterogeneity of etiologic factors contributing to cases of acute yellow atrophy was probably underappreciated.
Percutaneous Liver Biopsy
Iversen and Roholm revolutionized liver biopsies by developing a percutaneous technique that is remarkably similar to that in use today—a transthoracic approach with a suction needle 128. In their paper describing the histopathology of acute hepatitis, “sporadic acute benign jaundice of the catarrhal type,” they reported on the findings in 38 aspiration biopsy specimens 128. Their descriptions are as apt today as they were 60 years ago. They observed hepatocellular necrosis with ballooning and eosinophilic degeneration, an inflammatory infiltrate of mostly mononuclear cells, and a variable amount of collagen. In follow-up biopsies on 12 patients 25 to 35 days later, there was less inflammation and the connective tissue was unchanged. One patient was biopsied 16 h before succumbing to fulminant hepatitis. The biopsy showed findings similar to those following a benign course but of greater severity, with destruction of the parenchyma and an inflammatory infiltrate.
The technique of percutaneous liver biopsy was rapidly adopted by others. A British study from 1943 reported findings in 14 patients with epidemic hepatitis (mostly HAV), 7 with serum jaundice (HBV), and 35 with jaundice in relation to arsenotherapy (mostly HBV) 66. No histopathological differences were perceived between the various supposed etiologies. The researchers observed necrosis that was most marked pericentrally and inflammation that was maximal in the portal areas. The extent of involvement was increased with more severe disease. Similar findings were observed in large series reported over the ensuing 30 years 127, 158, 195, 225. All agreed that the cardinal features of acute hepatitis were the presence of hepatocellular degeneration, characterized as either ballooning or acidophilic (apoptotic) change, together with various degrees of portal and lobular inflammation and hepatocyte regeneration.
Modern-Era Liver Biopsy
Although the number of biopsy samples examined was extensive, the older studies of the pathology of acute hepatitis lacked the ability to compare hepatitis A with hepatitis B and hepatitis C in well-documented serologically defined cases. This distinction is of only modest importance since the overwhelming bulk of the findings, like the clinical manifestations, are qualitatively identical 141, 184, 242. As part of the Copenhagen Hepatitis Acuta Programme that monitored over 100,000 patients, liver biopsies were performed routinely until 1980. With the advent of serodiagnosis for acute hepatitis A as a research tool in the late 1970s, a comparison study was undertaken 141. The parenchymal changes (focal necrosis, ballooning, and acidophilic degeneration) were less marked in the biopsy specimens from 86 patients with hepatitis A than in specimens from 78 patients with hepatitis B, whereas the degree of portal inflammation was similar. Follow-up liver biopsy samples (two to four per patient) were obtained for 36 patients at intervals of 1 to 24 months after the acute illness. The biopsy samples were classified as normal (17 of 54 biopsies) and nonspecific reactive changes (24 of 54); continuing acute hepatitis was observed for 1 to 5 months after initial presentation for 13 141. Fibrin ring granulomas, more often associated with diseases such as Q fever, were described in acute hepatitis A biopsy samples by some investigators 197, 268.
HAV Detection
The technique of in situ hybridization was recently used to localize HAV sequences in human liver biopsies 241. Viral RNA was detected in hepatocytes, sinusoidal cells, and inflammatory cells. Replicative intermediates were not detected. Using a macrophage-specific marker, the investigators confirmed the presence of viral nucleic acids in the cytoplasm of phagocytic cells. Clearance of virus by uptake of antigen-antibody complexes between virions and anti-HAV into these cells seems a plausible explanation for this observation, rather than phagocytosis of virus directly.
IMMUNOLOGY
The immunology of hepatitis A is important for two reasons. First, specific diagnostic tests for the confirmation of HAV as the etiologic agent are dependent on the production of antibody by the humoral immune response (see below). The humoral immune response also leads to the development of circulating immune complexes 160, 248 with associated symptoms and signs in some patients 122, 123. Second, clearance of viral infection and the disease manifestations associated with this process is almost certainly produced by the cellular immune response.
Humoral Immune Response
Immunoglobulin M (IgM), IgG, and IgA antibodies directed against conformational epitopes on the HAV particle are induced and can usually be detected by the onset of clinical illness 228. In addition, total IgM levels are often elevated in acute hepatitis A infection (28 of 33 cases [85%]) but not in acute hepatitis B infection (3 of 24 cases [13%]) 175. The hepatitis A-specific IgM response is limited to the initial infection except in rare instances and thus becomes a useful marker of acute disease. IgA is also produced for a limited period of time. Its role in immunity is uncertain. Theoretically, if antibodies such as secretory IgA were transported into the intestinal tract, then enterohepatic circulation of viral particles could be interrupted by neutralizing the virus. In experimental and naturally acquired hepatitis A, however, neutralizing antibodies are uncommonly found in fecal extracts 229. In contrast, another picornavirus, the poliovirus, elicits effective intestinal and salivary neutralizing antibody 229. The IgG response to HAV is delayed compared with IgM and IgA responses but is long-lived and accounts for resistance to reinfection. In an isolated Amerindian tribe, anti-HAV antibody was present in everyone over the age of 50 years but in no one younger 31. This observation suggests that the tribe members had not been exposed to HAV for 50 years and that anti-HAV IgG persisted for that length of time without need for additional exposure. Loss of detectable antibody following immunosuppression for organ transplantation may occur 19. Whether this represents risk for repeat infection has not been documented.
The antibodies are usually directed against surface proteins. The capsid proteins VP1 and VP3 and the precursor protein VP0 may be recognized 256. Almost all patients expressed both IgG and IgM antibodies to VP1. The IgG response to VP3 was detectable for years after disease resolution 256. Antibodies to nonstructural proteins are also induced. Although they are less abundant and lack neutralizing activity, they are produced in most individuals early in the infection 210. Detection of antibodies recognizing P2 permits differentiation between infection (antibody present) and vaccination (no antibody) as sources of antigenic determinants 210.
Cellular Immune Response
The pathologic changes described above were initially considered to be secondary to viral infection alone. However, large quantities of infectious virus are produced in the liver and excreted in stool before the onset of any recognizable hepatic disease 51, 139, 245. Furthermore, HAV is not directly cytopathic in cell culture but rather is associated with persistent infection without cell injury 115, 201. Taken together, these observations led to the recognition of immune-mediated injury as the most plausible explanation for hepatic inflammation. Consistent with this hypothesis is the observation that cytotoxic lymphocytes isolated from patients with acute hepatitis A infection lyse autologous, HAV-infected target cells 83, 249. Other cytotoxic cells, such as natural killer cells, may also be involved 83, 142. Their role may be limited, since they lack antigen specificity. Overall, therefore, hepatitis A and hepatitis B are similar not only in their clinical manifestations but also in the mechanism underlying their production, that of cytotoxic T-cell recognition and destruction of virus-infected cells.
SEROLOGY
The specific detection of hepatitis A infection was first accomplished in 1973 using immune electron microscopy of fecal extracts to visualize virus-like particles 78. Antibody in convalescent-phase serum samples from persons with experimentally or naturally acquired infection aggregated the virus and permitted its visualization by electron microscopy. When serum collected before infection or early in the disease was used, few or no virus particles were identified. This research technique was successfully used to investigate the period of infectivity but could not be employed for routine diagnosis of large numbers of clinical samples. The next step was the development of complement fixation 200 and immune adherence 172 tests for detection of serum antibody to HAV antigens in 1974. This required a source of HAV antigen, supplied by liver extracts from marmosets infected with a Costa Rican strain of HAV, CR326.
Despite being cumbersome, the immune adherence test quickly provided a wealth of important data about hepatitis A infection. With the original description of the test came demonstration of simultaneous infection with both HAV and HBV; evidence that HAV antibodies were acquired early in life in areas of high prevalence; association of low socioeconomic status with seropositivity in areas of low incidence; persistence of antibody for at least 7 years; an antigenically related or identical virus infection in chimpanzees, grivets, and rhesus monkeys not experimentally infected; and detection of various quantities of antibody in lots of immune serum globulin 172.
Both the complement fixation test and the immune adherence test were used to examine seroconversion following experimental infection with the MS-1 strain of hepatitis 137. The complement fixation test was not as specific or as sensitive as the immune adherence test. Using the immune adherence test, seroconversion was demonstrated in 20 of 20 infected persons. Antibody was detectable soon after the onset of clinical hepatitis but was present within the first week in only 45%; in 20% detectable seroconversion was delayed for at least 2 weeks after disease onset. Nevertheless, a test that could be used for diagnosis of acute hepatitis A infection was now available. Hemagglutination assays for HBV surface antigen and antibody to HBV core and surface proteins were developed in 1970. Consequently, by 1975, acute viral hepatitis could be ascribed to either HAV or HBV, permitting the recognition of viral non-A, non-B hepatitis (hepatitis C and hepatitis E).
In 1975, solid-phase RIAs developed for the detection of HAV antigen were modified to measure antibody 203. A comparison between immune adherence, immune electron microscopy, and RIA demonstrated that each test was able to detect seroconversion following inoculation with MS-1 virus 69. Antigen partially purified from stool was equivalent to marmoset liver-derived viral antigen in these assays. RIAs were also modified to use minimal quantities of viral antigen and to assay anti-HAV IgM antibody 38.
A competitive binding assay (HAVAB; Abbott Laboratories, North Chicago, Ill.) was developed by 1978 to improve sensitivity 37. In this assay, antibody in patient serum competes with radiolabeled antibody for HAV. The assay was also adapted to measure anti-HAV IgM, which was detected in acute-phase sera but not in convalescent-phase sera 37. However, the absorption of IgG from samples to measure IgM reactivity was difficult to perform, and the resultant assay lacked reliable specificity. In 1980, an alternative technique was developed (HAVAB-M; Abbott Laboratories) in which IgM antibody was directly selected and anti-HAV activity was then measured 63. With these improvements, anti-HAV IgM antibody was detected at the time of onset of symptoms in most patients. IgM titers decreased in the weeks after onset and then became undetectable. This assay could thus be used to diagnose acute hepatitis A at the time of clinical symptoms.
Diagnostic Accuracy
The sensitivity, specificity, and positive predictive value of quantitating IgM-specific anti-HAV was determined in a cluster of cases that occurred in 1979 using normal blood donors as the control population 234. The sensitivity of IgM anti-HAV measurement for acute hepatitis was 100%, the specificity was 99%, and the positive predictive value was 88%. Since its introduction and widespread use, diagnostic difficulties have been uncommon. Occasionally, the test is negative at the time of clinical presentation, but repeat testing 1 to 2 weeks later usually demonstrates positivity 112. One possible explanation for this observation is that dilution of serum before assay, in order to prevent false-negative results, could result in loss of reactivity in sera with low titers. In two episodes of mild acute infection in vaccinees, the appearance of IgM anti-HAV positivity was delayed until convalescence 125, an observation that has not been explained. False-positive Epstein-Barr virus serologies 79 are less of a diagnostic problem than is prolonged positivity in the absence of hepatitis 130, 220.
The length of time that the IgM anti-HAV test remains positive varies 130. In 37 patients followed until the disappearance of antibody, the majority, 32 of 37 (86%), were IgM anti-HAV negative by 7 months after onset, defined as jaundice in all but two anicteric cases, for which the onset of symptoms was used. Twenty-six of 37 (70%) were negative for IgM anti-HAV by 4 months. All cases demonstrated a decrease in titer (positive test value closer to the negative cut-off) before becoming negative in the assay. In contrast, IgM anti-HAV positivity was prolonged beyond 7 months in five individuals whose last positive test was recorded between 9 and 12 months after onset. Eventually, they each had negative IgM anti-HAV test results. In most patients (47 of 50), the biochemical evidence of hepatitis had resolved either prior to or by the time of disappearance of IgM anti-HAV. Of the remaining three patients, two eventually normalized biochemical hepatitis and the third was lost to follow-up. In a second study, two of six patients were IgM anti-HAV positive (low titer) 30 to 32 months after the onset of hepatitis A 220. A diagnostic dilemma may arise if a patient has unrecognized chronic hepatitis before contracting HAV. The persistence of IgM anti-HAV positivity for more than 12 months together with an unrelated and unidentified cause of hepatitis could potentially lead to an incorrect diagnosis of chronic hepatitis A.
Future Assays
In vaccine trials, the detection of antibody often requires a more sensitive assay because vaccine-induced antibody titers are generally lower than those induced by natural infection. Noninvasive tests, i.e., not requiring blood samples, are also useful for screening large populations. The development of a highly sensitive assay that is specific for IgG anti-HAV and can measure antibody in saliva after vaccination is promising 182. The assay was validated using paired saliva samples from travelers undergoing vaccination. In practice, antibody titers are not measured routinely after vaccination since the response rate to the vaccine and its effectiveness are so high (see below), and a highly sensitive assay therefore has little utility. In contrast, a cheap and robust assay for screening prior to vaccination would be extremely useful in many clinical scenarios (see below). Most assays have used HAV produced in tissue culture as a source of antigen. In the future, recombinant HAV antigen may provide a less costly alternative 143.
CLINICAL DISEASE
The clinical features of viral hepatitis, once symptoms commence, are similar regardless of the specific hepatotrophic alphabet virus involved. Extrahepatic manifestations and complications may differ quantitatively, but qualitatively they also are common. There are unique aspects of clinical hepatitis A, however, because of the different patient populations in which the disease is observed. Thus, hepatitis A can be sporadic, endemic, or epidemic.
Sporadic Hepatitis A
Cockayne was the first observer to recognize that the sporadic form of the disease (at that time referred to as catarrhal jaundice) was identical to the epidemic form of the disease 46. He cites Rolleston 211 as thinking that the febrile cases of sporadic jaundice were the same as the epidemic cases, although few in number. In contrast, Cockayne pointed out that the geographic range, age, seasonal incidence, symptoms, physical signs, variable prevalence (“a peculiarity of all infectious diseases”), occurrence of prolonged jaundice, and relapses were comparable in the sporadic and epidemic cases. He concluded that “Sporadic and epidemic catarrhal jaundice are found somewhat in the same way as sporadic and epidemic poliomyelitis, except that jaundice is more common in the sporadic form and met with more often and over a wider area in the epidemic form.”
Endemic Hepatitis A
The endemic form of the disease is more difficult to recognize because of the high incidence of asymptomatic and anicteric cases when the disease is acquired in early childhood. Passive transmission of maternal antibody protects the neonate, but protection wanes during infancy, and young children are ideal fecal-oral transmitters of infection. In the developing world, where sanitation is limited or absent, infection remains almost universal. In Egyptian children 1 to 3 years old, the seroprevalence rate was 100% 58, remaining at this level until age 67. Similarly, 2- to 4-year-old Nicaraguan children have a seroprevalence rate of 73% 194, and in Pune, India, virtually 100% of children are infected by late childhood 17. Immigrants and travelers from areas of low disease rates are therefore at high risk of infection when residing in countries where infection is endemic.
Cameron, who reported on epidemic hepatitis among British troops in Palestine during World War II, recognized the existence of endemic infection in the region 42. He wrote that “a large number of [indigent] children acquire the disease in a mild form and are immune for life, thus reducing the incidence in the adult native population. With each new immigration of settlers [from Germany, for example], a new non-immune child population is added, and this accounts for epidemics. … The arrival of British troops represents another immigration.”
The immunity of Indian and Maori troops to epidemic hepatitis during World War II and the relative incidence (10:1) in white and nonwhite American troops 266 can be explained by differences in childhood exposure rates. Similarly, the observation that officers in the British Army and flying personnel in the Royal Air Force had a fourfold-higher rate of infection than ordinary ranks and ground staff 266, a difference not seen in U.S. forces, is also understandable by differences in childhood exposure rates secondary to socioeconomic disparities.
Epidemic Hepatitis A
Long before the advent of serologic testing for hepatitis A and before the development of quantitative tests of hepatocellular necrosis, large series of epidemic jaundice cases were carefully observed and the manifestations were reported. Epidemics of jaundice from HAV (infectious hepatitis) usually commenced early in the fall and peaked in winter, waning then until the next yearly cycle started. This seasonal pattern is unexplained. The clinical picture was and is remarkably similar in all epidemics, with little change despite major differences in age and geography. The cases manifesting specific symptoms and signs in three series are shown in Tables 2 and 3. The cases in these epidemics varied in age as well as place of infection. Of 194 cases reported in Detroit between November 1937 and March 1938, only 27 involved persons more than 14 years old 173. In the military epidemics of World War II, the cases reflected the ages of the troops, with the majority ranging from 19 to 40 years old 102, 113.
TABLE 2.
Findings in epidemic jaundice in the 1930s and 1940sa
| Symptom | % with symptom
|
||
|---|---|---|---|
| Detroit civilians (n = 194) | Mediterranean military (n = 200) | New York military (n = 200) | |
| Anorexia | 93 | 82 | 92 |
| Nausea, vomiting | 84 | 75 | 79 |
| Malaise | —b | 82 | 69 |
| Fever | 61 | 53 | 42 |
| Headache | 57 | 35 | 27 |
| Abdominal pain | 50 | — | 57 |
TABLE 3.
Physical examination findings in epidemic jaundice during World War IIa
| Sign | % with symptom
|
|
|---|---|---|
| Mediterranean (n = 200) | New York (n = 200) | |
| Hepatomegaly | 59 | 51 |
| Hepatic tenderness | 54 | 38 |
| Splenomegaly | 11 | 14 |
| Bradycardia | 9 | 25 |
Epidemic Hepatitis and War
Epidemics of jaundice are common in military medical history. Blumer reported that the first known epidemic in the United States was in conjunction with the War of 1812 33. In “The Medical and Surgical History of the War of the Rebellion” (the Civil War), Smart recorded 71,691 cases of jaundice in Federal troops 224. Details in the individual histories are consistent with infectious hepatitis (hepatitis A). The peak incidence occurred in the fall and winter of 1863, also suggestive of the seasonal occurrence of hepatitis A. Cockayne quoted different statistics: 22,569 cases of epidemic jaundice with 161 deaths among 2,218,599 Federal troops during the war between the North and the South (10% infection rate, 0.7% case fatality rate) 46. I have not found an original source for these latter statistics, but based on my assessment of Cockayne (from his published analysis of jaundice, which included 142 references), I consider that they are likely to be more accurate.
In World War I, British, French, and other Allied forces reported epidemics of jaundice starting in 1915 and continuing intermittently 14, 161, 262. The Mesopotamian epidemic is curious in that Indian and British troops were equally affected, and Willcox reported that there was little evidence of person-to-person contact 263. One possible explanation is that hepatitis E accounted for some or all of the cases. Hepatitis A is endemic in India, whereas hepatitis E is more often associated with large epidemics. Furthermore, in hepatitis E, there is less person-to-person spread. Purcell likewise suggests that at least some of the epidemics of hepatitis in Europe in past centuries may have been due to hepatitis E infection in that they predominantly affected young adults and fulminant hepatitis occurred in pregnant women 202. In two recent studies, there was cooccurrence of HAV and HEV as causes of acute hepatitis. Interestingly, in both reports, the hepatitis E cases occurred in the indigent or native population (Nepalese in one instance, Djibouti natives in the other), whereas hepatitis A was found in nonnatives (tourists in Nepal, French troops in Djibouti) 44, 52. The United States escaped most of the epidemics in World War I by late entry into the conflict. Paul and Gardner consider this one of the reasons for U.S. military unpreparedness for the impact of hepatitis epidemics on troops during World War II, since plans for dealing with infectious disease were developed based on experience in the previous war 188.
Campaign jaundice was of major military importance in World War II. Epidemics occurred in British troops in Palestine in 1940 to 1941 42 and in Allied forces in North Africa in 1942 to 1943 102, 113, 188, 266; every theater of military operations was affected by the end of hostilities 188. The large numbers of active servicemen involved are illustrated in the El Alamein campaign. At the peak of the epidemic in November 1942, the number of men hospitalized with jaundice (1,861 for the month) was only exceeded by those hospitalized with battle casualties (3,602). Overall, there were ∼200,000 recognized cases of hepatitis in the U.S. Army alone 188, with a total in the millions likely in the combined Allied forces. In Germany, the situation was identical or even worse, with 190,000 cases in September 1941, 5 to 6 million cases in their armed forces over a 3-year period, and more than 10 million estimated military and civilian cases during the war 98.
The scientific thinking about hepatitis was further complicated in this era by the recognition of sporadic and epidemic forms of a long-incubation hepatitis (see above). Appreciating the military importance of the disease, the U.S. Army sponsored research that investigated experimental transmission of hepatitis, characterized the features of short-incubation and long-incubation disease, and examined prevention strategies 188. The success of the latter initiative is reported below, and some of the transmission and clinical studies are specifically referenced herein.
Common Clinical Features
After an incubation phase of 15 to 50 days (mean, 30 days), most infected persons developed nonspecific constitutional symptoms followed by gastrointestinal symptoms (Table 2). This preicteric or prodromal period averaged 5 to 7 days but varied in length from 1 day to more than 2 weeks 102, 113, 173. In approximately 15% of cases, however, there was no obvious prodrome before the appearance of jaundice. The findings resemble other viral prodromes and are indistinguishable from them. Less common manifestations than those tabulated included chills, myalgias and arthralgias, cough and upper respiratory symptoms, constipation, diarrhea, pruritus, and urticaria. The nonspecificity of symptoms is such that the diagnosis of an anicteric case of infectious hepatitis cannot be made with certainty unless modern testing is used. This is illustrated in a retrospective analysis of an outbreak of hepatitis affecting the Holy Cross football team in 1969. The epidemic was considered on clinical grounds to have involved almost all members of the team. However, when anti-HAV antibody was measured in stored sera, the attack rate was only 33% 85. Only the icteric cases were truly infected; all the supposedly anicteric cases were not. The Holy Cross outbreak is unusual in this regard. In other outbreaks of hepatitis A among adults, the percentage with jaundice varies between 40 and 70% 144, 214. One potential explanation for the finding of jaundice in 100% of true hepatitis A cases in the Holy Cross outbreak is that there are strain-related differences in disease severity; there is no supportive evidence for this explanation. Alternatively, another systemic infection may have occurred simultaneously with the hepatitis A. This explanation could account for not only the gastrointestinal symptoms in the non-HAV cases but also the increase in jaundiced cases if the additional infection were associated with an increase in bilirubin load (hemolysis) or another mechanism of interference with bilirubin transport or excretion.
The onset of the icteric phase is heralded by dark urine (conjugated bilirubinuria) before jaundice becomes apparent. The nonspecific and gastrointestinal symptoms often subside but may persist. The duration of jaundice is quite variable. In the Detroit series, the mean length was 7 days, with a range of 4 to >22 days 173. In contrast, the modal length reported by Havens was 20 to 29 days 102. Actual quantitation of bilirubin (in milligrams per deciliter rather than the earlier “icterus index”) was not performed routinely until the 1950s, and consequently precise levels from the older epidemics are sparse. The maximum bilirubin in 60 patients from Havens' series was 10.8 mg/dl 102. In the series from the Rockefeller Institute Hospital in New York 113, the average was 6.7 mg/dl. Since all patients in these case series were icteric, they form a more homogeneous group than later series, in which infections could be identified by biochemical, serologic, or virologic means. Abnormal physical examination findings apart from jaundice occurred in approximately half the patients or fewer (Table 3).
Disease duration, not unexpectedly, varied with the duration of jaundice. In Detroit, the mean length was 15 days. This relatively short duration may reflect their younger age. In Havens' cases, hospitalization length averaged 30 days, ranging from 7 to 87 days. Patients recovered uneventfully; relapse and other complications were uncommon in most series. Three relapses among 200 patients were observed by Havens 102 although Hoagland and Shank reported retention of sulfobromophthalein, an organic anion transported like bilirubin, in 18.5% of cases after initial normalization 113, indicating a decrease in hepatic function during relapse. The military burden, however, was quite considerable because of the large numbers of men involved and the length of time before return to full duty.
Anicteric and Asymptomatic Hepatitis
The accurate diagnosis of anicteric hepatitis and recognition of the existence of asymptomatic hepatitis required the development of an objective measurement of hepatic injury as indirect evidence of acute hepatitis. In 1955, Wróblewski and LaDue reported their work on the release of GOT with liver injury 267. They measured SGOT activity in 10 patients with jaundice from parenterally transmitted hepatitis and in 5 patients with jaundice without any recognized parenteral risk factors. SGOT levels were elevated in all patients and returned to normal with recovery from the acute illness. Using measurement of SGOT, Krugman and colleagues demonstrated the existence of asymptomatic infection with HAV following ingestion of infectious material 140. Serum collected at the time of modest elevation of SGOT transmitted infection to additional recipients, demonstrating a temporal relationship between the elevated SGOT levels and infectivity. In addition, measurement of SGOT levels permitted the unequivocal detection of anicteric cases of both hepatitis A and hepatitis B 138.
Careful analysis of a food-borne outbreak of hepatitis A at a naval facility in San Diego demonstrated that 14% of patients were asymptomatic and that 30% were not jaundiced 214. This study used aminotransferase levels in case finding and also confirmed etiology by demonstrating rising titers of anti-HAV antibodies. Common symptoms and signs in this outbreak occurred less frequently than in the epidemics from the late 1930s and early 1940s but were qualitatively similar, with few exceptions (Tables 4 and 5). Arthralgias were noted in 10% and a rash in 14%, symptoms that are more often associated with acute hepatitis B. A recent review of 59 patients hospitalized in Pasadena, Calif., for sporadic hepatitis A between 1985 and 1994 reveals virtually identical findings (Table 6) 246. Arthralgias (19%) and rash (7%) were also observed in this cohort. Findings on physical examination were qualitatively similar for both epidemic and sporadic hepatitis A to those reported in earlier outbreaks.
TABLE 4.
Findings in epidemic and sporadic hepatitis Aa
TABLE 5.
Physical examination findings in epidemic and sporadic hepatitis Aa
TABLE 6.
Laboratory investigations among hospitalized patients with sporadic hepatitis Aa
| Test | Pasadena, 1985–1994 | Dallas, 1997–1998 |
|---|---|---|
| Bilirubin (mg/dl) | 7 | 13.3b (4.9–46.4) |
| Alkaline phosphatase (U/liter) | 319 | 335 (117–1,104) |
| AST (mIU/liter) | 1,754 | 3,664 (428–10,420) |
| ALT (mIU/liter) | 1,952 | 3,628 (1,029–9,220) |
| Albumin (g/dl) | —c | 2.6 (1.5–3.1) |
| Globulin (g/dl) | — | 4.0 (3.2–5.6) |
| Prothrombin time(s) | — | 15.1 (11.7–26.4) |
Data are mean peaks (ranges). Data are from reference 246 and unpublished sources.
Mean peak of 10 mg/dl, excluding data for a patient with hemolysis and hepatitis A (bilirubin, 46.4 mg/dl).
—, not reported.
The ratio of anicteric to icteric cases (1:3.5) in the San Diego epidemic likely reflects the ages of the individuals. In young children, the fraction of inapparent infections can be much higher. In an outbreak of hepatitis A in a religious community, where all diagnosed patients were under 20 years old, a limited household serosurvey detected IgM anti-HAV in 15 individuals, only 2 of whom developed jaundice, a ratio of 7.5:1 191. Clinically obvious disease, however, can occur even in infancy. In a series of six infants aged 2 weeks to 8 months reported by Linder et al., bilirubin levels were 5 to 12 mg/dl and the alkaline phosphatase was strikingly elevated (mean, 5.9-fold; range, 1.2 to 12.1-fold) 153. This may represent a cholestatic form of hepatitis A in infancy.
The attack rate in members of households exposed to infection is consistent with asymptomatic disease in young children 84. Thus, Ford observed that the rate of clinically apparent disease was much lower in children under 5 years of age (2 of 73 [3%] compared with 20 of 72 [28%] for 5- to 10-year-old children and 18 of 66 [27%] for 10- to 15-year-old children) despite apparently identical risks of infection. The low attack rate in adults (8 of 438 [2%]) almost certainly reflects immunity rather than inapparent infection 84. In 1937, Hugh Barber correctly predicted the natural history of hepatitis A infections based on his own observations and a review of the literature. He wrote, “If infective hepatic jaundice is due to a virus, which sets up acute hepatitis; if it is highly infectious in children, but well resisted by them; if most adults have acquired immunity, but those who become infected have a liver less capable of regeneration than the child, the natural history of epidemic and sporadic cases may be explained” 24.
Laboratory Investigations of Acute Hepatitis A
As with the clinical symptoms and signs, there are no pathognomonic findings in the laboratory investigations that distinguish HAV from other hepatotrophic viruses. The maximum elevation of alanine aminotransferase and aspartate aminotransferase can be substantially higher than that observed in acute hepatitis B, but there is a wide range. In general the degree of aminotransferase elevation roughly correlates with the severity of the acute hepatitis A in that asymptomatic cases have lower aminotransferase levels. The overall severity of the infection, however, is demonstrated by the bilirubin level as well as the prothrombin time. Most cases of hepatitis A have a bilirubin of ≤10 mg/dl in the absence of hemolysis, an indication that hepatitis A is usually not severe.
Relapsing, Prolonged, and Cholestatic Hepatitis A
Relapses in the course of hepatitis A occur in some patients 45, 46, 91, 102, 129, 240, 246. For example, Cockayne wrote that “Relapse may occur after it has completely disappeared …” 46. However, the cases that he documents are difficult to distinguish from second infections with a different etiologic agent without the availability of specific diagnostic tests. Similar criticisms can be applied to most reports of relapse prior to the isolation of HAV and development of specific assays for the virus. Demonstration of HAV in stool during relapse 223 provides the best evidence of causality. The techniques of immune electron microscopy, RIA, and molecular hybridization were used, indicating that both protein and nucleic acid components of the virus were present and thus suggesting continued infectivity.
The rate of hepatitis A relapse varies: 3 of 200 (1.5%) in Havens' case series, 17 of 256 (6.6%) in Argentina, and 7 of 59 (11.9%) patients hospitalized in Pasadena, Calif., in the 10 years between 1985 and 1994 102, 223, 246. The severity of symptoms and biochemical abnormalities during the second phase are essentially the same as observed during the initial illness except for a tendency to greater cholestasis 91, 240. A relapse necessarily lengthens the course, and the overall duration of disease is similar to those with a prolonged (but not biphasic) illness 240.
In some individuals, the course of hepatitis A is unusually prolonged. Havens observed jaundice for up to 120 days (17 weeks) in his series, for example 102. Complete follow-up of almost all the cases in the San Diego naval outbreak revealed a prolonged course (abnormal aminotransferase levels after 14 weeks) in 11 of 130 cases (8.5%). Liver biopsies performed at that time demonstrated portal inflammation with piecemeal necrosis, periportal fibrosis, and lobular hepatitis. All biochemical abnormalities eventually resolved by 5 months. Since prolonged excretion of virus (i.e., viral nucleic acid detected by RT-PCR) may occur in patients with persistent elevation of alanine aminotransferase 269, any patient with either relapse or a prolonged course should be regarded as potentially infectious. Fatigue may persist after resolution of biochemical abnormalities in some patients. When patients were questioned up to 30 months postinfection, fatigue was more frequent in those hospitalized for hepatitis A or B than in those hospitalized with other infections 28. Most patients, however, recover completely in 6 months or less 246.
The occurrence of “cholangiolytic” or cholestatic variants of acute hepatitis A was described in 1984 95 after the advent of specific diagnostic testing that permitted identification of the etiologic agent. Previous accounts of this variant (reviewed in reference 95) did not have the benefit of such tests. Severe pruritus, diarrhea, weight loss, and malabsorption may accompany the cholestasis. Although resolution occurred in all patients, symptomatic relief was obtained with corticosteroids in some patients without untoward sequelae 95. However, the report of persistent aminotransferase elevation and viral excretion with progressive hepatic fibrosis in one patient treated with corticosteroids 166 is cautionary when considering treating what is otherwise a relatively benign variant.
Fulminant Hepatitis A
Like hepatitis B, delta hepatitis, and hepatitis E, hepatitis A can cause acute hepatic failure. Fulminant hepatitis A was diagnosed in 20 of 295 patients in a recent retrospective study of acute hepatic failure in the United States, less frequent than acetaminophen toxicity (60 of 295) and hepatitis B (30 of 295) 217. The fatality rate for hepatitis A is generally low, quoted as <1.5% of all hospitalized icteric cases 115. Between 1983 and 1987, 381 deaths due to hepatitis A were reported to the CDC 147. With ∼30,000 reported cases yearly, this gives an estimated fatality rate of 1.3%, likely a maximum rate because of relative underreporting of nonfatal disease. Fulminant disease occurs more frequently in adults than children 264 but can occur in childhood 62. The spontaneous recovery rate for patients with fulminant acute hepatitis A in the recent retrospective U.S. study, which included all age groups, was 35%, whereas it was 39% in a French pediatric population 62. Other patients may survive following liver transplantation 62, 217. Occasionally, hepatitis A infection recurs following transplantation 75, 86.
In the largest recent epidemic, in Shanghai, where 292,301 cases were reported between January and March 1988, there were 32 deaths 100, a minuscule fatality rate of 0.01%. In contrast, five deaths were associated with a large urban epidemic in Tennessee in 1994 and 1995. Of 256 patients hospitalized in Tennessee for severe disease, 3 developed classic fulminant hepatic failure, of whom 2 died. One patient with underlying chronic liver disease also died. Two cases of prolonged illness were classified as autoimmune hepatitis on the basis of positive antinuclear antibody (titer, >1:640) and liver biopsy samples consistent with that diagnosis. These patients also died. Factors that contributed to mortality in those with severe disease included age of >40 years (deaths of 3 of 53 hospitalized patients who were older than 40 years) and other comorbid conditions (e.g., chronic hepatitis C).
Chronic Liver Disease and Acute Hepatitis A
The risk of fulminant hepatitis is increased in patients with underlying chronic liver disease who develop acute viral hepatitis, regardless of etiology 133, and patients without prior exposure should be vaccinated 13. However, the report from Italy of an unexpectedly high rate of fulminant hepatitis A in patients with underlying chronic hepatitis C (7 of 17 [41%]) but not chronic hepatitis B (0 of 10) 253 has not been confirmed by other investigators 22, 108.
The classic teaching for many years has been that hepatitis A infection does not cause chronic liver disease and that there is no chronic carrier state. With the advent of highly sensitive assays for HAV detection, it has become clear that in rare patients, viral nucleic acids can be detected in stool for many weeks after the onset of infection, even when hepatic enzymes have returned to normal 269. Does this represent chronic infection or one end of the normal spectrum? One patient had HAV RNA in stool (by RT-PCR) 11 months after onset of illness, at which time he also had persistent aminotransferase elevation and detectable anti-HAV of the IgM class 126. A liver biopsy at that time showed portal inflammation and interface hepatitis. The patient developed esophageal varices at 25 months, and aminotransferase elevations and IgM anti-HAV were still present after 31 months. Although reported as chronic hepatitis A, it may represent two separate diseases, prolonged hepatitis A and a second, unidentified cause of chronic liver disease.
Similarly, a case report of chronic hepatitis A with persistent IgM class anti-HAV antibody and progressive liver disease 165 may represent observations that are true but unrelated. In the absence of chronic liver disease, low-level IgM anti-HAV can be detected up to 32 months after acute infection 220. Furthermore, the titer of IgM anti-HAV is normally such that early in the course of infection samples are diluted 1:4,000 before assaying to avoid a false-negative prozone effect. IgM class antibodies may therefore be detectable, albeit at a lower level, for many months as the titer gradually declines. Thus, it would seem that persistence of detectable IgM class anti-HAV antibody does not prove persistent infection.
Chronic liver disease can appear to follow acute hepatitis A but lack a direct etiologic relationship 120, 204, 252. The triggering of autoimmune hepatitis by HAV infection in two subjects was reported in 1991 in a prospective study of relatives of patients with autoimmune chronic active hepatitis 252. With the overwhelming advantage of a prospective evaluation and the observation of autoimmune hepatitis occurring in two study subjects, these data are difficult to refute. Similarly, when aminotransferase levels are normal before HAV infection and the illness is characterized as steroid-responsive liver disease that recurs on steroid withdrawal 204, the assumption that HAV infection is triggering or unmasking autoimmune hepatitis seems reasonable. However, when the diagnosis of autoimmune hepatitis is made in persons with concurrent HAV infection, it is quite problematic, since viral hepatitis is associated with antinuclear antibody positivity and the features on liver biopsy are sufficiently similar to preclude absolute diagnoses.
Extrahepatic Manifestations
A variety of extrahepatic manifestations can be observed in patients with acute hepatitis A. In order of frequency, as seen in 256 patients hospitalized in Tennessee in 1994 to 1995, these include hemolysis (10 patients), acalculous cholecystitis (10 patients), acute renal failure (3 patients), and pleural or pericardial effusion, acute reactive arthritis, and pancreatitis (1 patient each) 264. Neurologic manifestations, although not reported in this particular series, may also be seen.
Hemolysis is precipitated by viral hepatitis, including hepatitis A, in patients with glucose-6-phosphate dehydrogenase deficiency 119, 219. In addition, red cell survival in the absence of an underlying red cell abnormality can be shortened by acute infectious hepatitis (presumptive hepatitis A) 132. Hemolysis may be autoimmune in nature, associated with antibodies to triosephosphate isomerase 208, 209, and can be severe 156, 244. Other hematologic abnormalities include aplastic anemia 73, autoimmune thrombocytopenic purpura 47, and pure red cell aplasia 221.
Acute cholecystitis and acute pancreatitis may complicate hepatitis A. The exact pathogenesis of acute cholecystitis is uncertain. In one patient, HAV antigen was detected in bile duct epithelium and the gallbladder wall, suggesting a direct effect of viral infection rather than a secondary phenomenon 176. Most cases of acute pancreatitis complicating viral hepatitis occur in fulminant hepatitis (reviewed in reference 61). Occasionally, however, pancreatitis may be encountered in nonfulminant disease 61, 264.
Occasionally, patients with HAV infection manifest symptoms consistent with circulating immune complex formation. These include cutaneous vasculitis, arthritis, and cryoglobulinemia 57, 122, 123. Either IgM or IgG anti-HAV is detected in the cryoglobulins 122, 123. The symptoms resolve spontaneously with resolution of the hepatitis A.
Interstitial nephritis 90, renal failure with proteinuria and hypocomplementemia suggesting immune complex disease 43, immune complex mesangial proliferative glomerulonephritis 164, 271, and acute tubular necrosis 76, 152 occur in the absence of fulminant hepatitis. The lack of severe liver disease precludes a missed diagnosis of hepatorenal syndrome. The exact mechanism(s) involved has not been defined. Immune complex formation may be an important etiologic factor.
Mononeuritis 193, mononeuritis multiplex 215, Guillain-Barré syndrome 239, postviral encephalitis 60, 243, and transverse myelitis 39 have been described in patients with acute hepatitis A. The etiology of these findings is uncertain; they may be caused by vasculitis.
MANAGEMENT
No specific management is necessary for most patients with uncomplicated HAV infection. Common sense prescribes appropriate rest (when necessary) and diet (avoiding foods that may cause digestive discomfort, such as fatty food). In the past, however, strict bed rest until complete resolution of all symptoms was common. Hoagland and Shank analyzed the relationship between the length of time from onset of symptoms until hospitalization and the average duration of illness 113. They found that when hospitalization was delayed for 30 or more days, the illness lasted an average of 81 days. In contrast, when the patient was hospitalized within the first 14 days, the illness lasted for an average of 46 days. Their conclusions were that prompt hospitalization and freedom from activity were important. An alternative explanation is that a slower, more subfulminant course was associated with a longer period until complete resolution and that the degree of bed rest (or need for hospitalization) was unproven. In 1969, a randomized study that compared “early and vigorous exercise” with traditional rest was published 207. No difference in the duration of illness was observed with the institution of a deliberate exercise program. Early, however, was defined as when symptoms (anorexia and malaise) and signs (liver tenderness) were graded as slight or 2+ (on a 1+ to 4+ scale). Nevertheless, this study led to the abandonment of strict bed rest in the management of acute hepatitis.
In hepatitis complicated by fulminant hepatic failure, management is determined by the complications that develop and the availability of transplantation. Similarly, extrahepatic manifestations such as renal failure and pancreatitis are managed in a routine manner. The expectation for all patients is for complete recovery without sequelae, which occurs in the vast majority.
PREVENTION AND RISK OF INFECTION
Gamma Globulin (Passive Immunoprophylaxis)
Almost 25 years before the successful transmission of hepatitis A to animals and nearly 30 years before visualization of the virus and development of assays for detection, prevention of clinical hepatitis A was achieved 233 as the result of a series of events. First, the recognition that human serum could attenuate or prevent clinical measles in susceptible individuals was reported in 1937 167. Convalescent-phase serum (enriched in specific antibody) was superior to pooled adult serum, and placental extract (containing passively transferred maternal antibody only) was ineffective. Second, methods were developed in the early 1940s to separate serum into component fractions 48. An average 25-fold concentration in antibody was achieved in fraction III, containing the immune globulins. Large-scale plasma fractionation programs were then undertaken by the Red Cross during World War II to provide plasma expanders. Gamma globulin was another product of the separation procedure. Finally, the demonstration of the effectiveness of the gamma globulin fraction in attenuation and prevention of measles in susceptible individuals exposed by household contact was made in 1944 187, 232.
During the summer of 1944, an outbreak of hepatitis occurred at a children's camp near Philadelphia 177. Joseph Stokes, Jr., a pediatrician on the faculty of the University of Pennsylvania School of Medicine, who knew that gamma globulin prevented or attenuated measles in susceptible, exposed individuals 232, was consulted regarding the epidemic. With knowledge of the effectiveness of gamma globulin in measles epidemics, Stokes took the next step, using gamma globulin to prevent the further transmission of epidemic hepatitis (hepatitis A) 233.
In the children's camp, icteric hepatitis developed in 125 of 278 (45%) putatively exposed individuals who did not receive gamma globulin and in 3 of 53 (6%) randomly selected individuals who were given gamma globulin (0.15 ml/lb of body weight). At this dose, they used all the available gamma globulin, hence the difference in numbers in the two groups 233. Confirmation of similar efficacy quickly followed in both military 89 and civilian 103 populations. Immune serum (gamma) globulin (ISG) was protective for up to 9 months, and doses as small as 0.01 ml/lb were effective 231, 257.
More recently, immune globulin was used to arrest an ongoing outbreak of hepatitis A in a religious community in 1989 191. Preliminary data indicated that persons under age 20 were uniformly susceptible, whereas the majority of older individuals were predicted to be immune (16 of 18 [89%] tested were anti-HAV positive and IgM anti-HAV negative). After administration of gamma globulin (0.02 ml/kg) to 2,287 individuals (total cost for vaccine and syringes, $3,620), there were only seven further cases of hepatitis A diagnosed between 2 weeks and 7 months after injection. The effectiveness of gamma globulin was calculated at 89% 191. Low levels of neutralizing antibody can be detected in recipients after ISG administration, although commercial tests for antibody are negative 265.
The current question is whether ISG preparations will continue to provide protection. The decrease in anti-HAV seropositivity in the general population may result in failure of protection from standard doses. The report of clinical hepatitis A in two recipients of standard doses of ISG in the United Kingdom is of great concern 26. In one individual who developed acute hepatitis A, transmission of infection was calculated to have occurred 2 months after the individual received 2.5 ml of ISG. In a second individual in whom fatal hepatitis A developed, symptoms commenced 10.5 weeks after inoculation. The titers of anti-HAV in the immune globulin preparations were 103 and 120 IU/ml 26, lower than those previously reported for similar preparations. The minimum protective level in recipients of ISG is unknown 265, but estimates of 10 mIU/ml are used. Simple mathematics indicate that 2.5 ml would administer 300 IU (120 IU/ml) initially. If the volume of distribution was 3 liters, then the level immediately after injection would be 100 mIU/ml. In one study, anti-HAV titers were measured 5 days after injection of ISG and again at 1, 2, 5, and 6 months 270. The half-life for the first interval (day 5 to 1 month) was 35 days, and for the remaining two intervals it was 21 days. Using these half-lives, an initial level of 100 mIU/ml would reach 6.25 mIU/ml in 98 days (14 weeks). With a larger volume of distribution, such as 4 liters, then the level falls below 10 mIU/ml before 11 weeks. These calculations are consistent with failure of protection for more than 2 to 2.5 months, as observed 26.
Since there may be a continued need for ISG unless universal vaccination is carried out, one solution may be to prepare ISG from donors with a history of jaundice, as suggested by Hopkins (118). He demonstrated a 10-fold-higher titer of anti-HAV activity in immunoglobulin preparations from HBV surface antigen-negative donors with a history of jaundice 118. Persons with a history of jaundice are usually dissuaded from donating blood because of the risk of transmitting disease. Despite sensitive assays for antigens and antibodies to detect evidence of various hepatitis viruses, there will be a small risk of transmission unless an assay for viral nucleic acids is introduced. Inevitably, some donated blood will be obtained during the “window” period, when the blood may be negative for antigen and antibody and yet contain virus.
Vaccination (Active Immunoprophylaxis)
The advent of an efficacious vaccine to prevent hepatitis A solves some (but not all) of the problems, from waning titers of anti-HAV antibody to increasing numbers of susceptible persons in the population. In 1991, a preliminary study of a vaccine manufactured at the Biologics Research Department, Walter Reed Army Institute of Research (appropriate, since Reed was the first person to transmit a viral disease, yellow fever, in human experiments), was published 222. The authors demonstrated acquisition of neutralizing anti-HAV antibody in recipients of a formalin-inactivated viral vaccine preparation. A live attenuated HAV vaccine was also capable of eliciting neutralizing antibody 171. Unlike those administered the inactivated vaccine, recipients developed IgM anti-HAV positivity without clinical hepatitis or evidence of significant liver dysfunction, although 40% reported gastrointestinal side effects.
By 1992, the clinical efficacy of the formalin-inactivated hepatitis A vaccines HAVRIX (Smith-Kline Beecham) 3 and VAQTA (Merck, Sharpe and Dohme) was apparent 260. Both use laboratory-attenuated strains of hepatitis A (HM175 for HAVRIX and CR326F for VAQTA) for production of a formalin-inactivated vaccine. A large-scale, double-blind, randomized, controlled field trial in elementary school children in Thailand demonstrated the efficacy of HAVRIX 124, 125. Of 40 cases of clinical hepatitis A that occurred among 40,119 children in the year following a single dose of vaccine, only 2 occurred in vaccinated individuals 125. The adverse reactions reported for the vaccine were minimal, and seroconversion after two doses was 99.8% in healthy individuals 3.
In a double-blind, placebo-controlled trial, the VAQTA vaccine was administered to 519 seronegative children aged 2 to 16 years living in a religious community in New York State, where hepatitis A was highly endemic. From day 21 after a single-dose injection, there were no cases of hepatitis A in the vaccine group, whereas 34 cases occurred in the placebo group. The seven cases in the vaccine group that occurred before day 21 were almost certainly due to transmission before vaccination. Similarly, hepatitis A outbreaks in two villages in Slovakia 198 and in rural communities in Alaska 168 were controlled with vaccination programs. Combined passive-active immunoprophylaxis is also effective 3, 96, 270, although the titers achieved are generally lower than with vaccine only 96, 270.
The Food and Drug Administration (FDA) licensed HAVRIX in February 1995 for administration to children who were ≥2 years old and adults. The CDC recommendations included use for travelers to areas other than western Europe, Scandinavia, Canada, Japan, Australia, and New Zealand 6. They indicated that screening for existing antibody should be considered for potential recipients aged >40 years and those who had resided in areas of high endemicity (see below). VAQTA has also been licensed in the United States by the FDA. In addition to travelers, the American College of Physicians recommends that other high-risk groups be vaccinated 87. These include homosexual men, injection drug users, persons with chronic liver disease, and workers with an occupational risk of infection. When HBV vaccination is also recommended, combined HAV and HBV vaccination can be undertaken 1, 3. The Advisory Committee on Immunization Practices issued guidelines for hepatitis A prevention in late 1996 9. They recommended routine vaccination of travelers to countries with high or intermediate endemicity of infection and of children who were >2 years of age and living in U.S. communities that have high rates of hepatitis A infection (700 to 1,000 cases per 100,000 population annually). Other populations at increased risk of HAV infection or at increased risk of adverse outcomes, i.e., the high-risk groups identified by the American College of Physicians, were also recommended for vaccination.
Trials of efficacy, safety, and reactogenicity are carried out in healthy persons. However, if those with chronic liver disease are to be vaccinated, it is important to determine the response to vaccination in this group. The anti-HAV titer achieved in those with chronic liver disease was lower in two separate studies 134, 145, although most patients (94% in reference 134) did achieve detectable antibody levels. Similarly, titers were lower in homosexual men with human immunodeficiency virus (HIV) infection than in those without HIV infection 111, 178, and overall, seroconversion rates were somewhat lower (88% in reference 178) compared with 100% in non-HIV-infected individuals. The vaccines were well tolerated in all studies. Vaccine-induced antibody is predicted to be long-lived and persist for >20 years 250.
The most recent recommendations for prevention of hepatitis A from the Advisory Committee on Immunization Practice 13 broaden the target groups for vaccination. Routine vaccination is now recommended for children in states, counties, and communities with rates of hepatitis A infection that are twice the 1987 to 1997 national average of 10 cases per 100,000 population (Table 7) 13. In order of incidence, the states are Arizona (48 cases per 100,000 population), Alaska (45 cases), Oregon (40 cases), New Mexico (40 cases), Utah (33 cases), Washington (30 cases), Oklahoma (24 cases), South Dakota (24 cases), Idaho (21 cases), Nevada (21 cases), and California (20 cases). In addition, states, counties, and communities with rates exceeding the national average should consider routine childhood vaccination. These states include Missouri (19 cases per 100,000 population), Texas (16 cases), Colorado (16 cases), Arkansas (14 cases), Montana (11 cases), and Wyoming (11 cases). The recommendations for protection of travelers, men who have sex with men, users of illegal drugs, persons who are at occupational risk for infection, persons who have clotting factor disorders, and persons with chronic liver disease were unchanged.
TABLE 7.
Reported cases of acute hepatitis A in the United Statesa
| Site | No. of cases/100,000 population
|
Total cases
|
||
|---|---|---|---|---|
| 1985–1994 | 1987–1997 | 1996 | 1997 | |
| Total U.S. | 31,032 | 30,021 | ||
| California | 21.9 | 20 | 6,653 | 6,422 |
| Texas | 15.0 | 16 | 3,460 | 4,511 |
| Arizona | 48.1 | 48 | 1,767 | 2,330 |
| Oklahoma | 12.5 | 24 | 2,586 | 1,445 |
| Michigan | NS | NS | 506 | 1,372 |
| New York | 3.6 | NS | 1,047 | 1,302 |
| Missouri | 14.4 | 19 | 1,414 | 1,151 |
| Washington | 5.6 | 30 | 1,001 | 1,015 |
| Utah | 27.2 | 33 | 1,073 | 550 |
CDC data (10, 13; www.cdc.gov/nciod/diseases/hepatitis/h96surve.htm). NS, not stated.
Screening Prior to Vaccination
Considerable differences exist in recommendations for serologic screening before vaccination 40, 230, 251. The aim is to reduce the cost of vaccination by eliminating those with previous natural infection. The economics are determined by the rate of seropositivity, the cost of screening, and the cost of vaccination. If two doses of vaccine are administered, then cost neutrality occurs when the fractional rate of seropositivity is equal to the screening cost divided by the vaccination cost. For example, anti-HAV seropositivity was 33% (or 1 in 3) in the National Health and Nutrition Examination Survey III (NHANES III), and therefore, the costs of screening before vaccination and of vaccinating all persons are identical if the screening cost (total anti-HAV testing) is one third the vaccination cost, e.g., $20 for screening tests and $60 for two vaccinations. If screening is cheaper, e.g., $10, or vaccination is more expensive, e.g., $80, then screening first generates cost savings on a population basis. Similarly, if the seropositive rate is higher in a given population, screening will be cost beneficial. Alternatively, if vaccination is cheaper, e.g., $40, then it is economically better to vaccinate without screening.
There are some disadvantages to screening before vaccination of high-risk groups other than the potential cost and the need to obtain samples for testing. Because the test results are not available immediately, screened persons must return for vaccinations. Consequently, there is a possible loss of vaccinees who do not follow up after screening. Another difficulty in deciding between screening first and universal vaccination is the lack of valid seroprevalence data for many populations. We have analyzed seropositivity in the Parkland Memorial Hospital Liver Clinic population with chronic HCV infection (J. A. Cuthbert, G. Vinson, J. S. Reisch, and M. Q. Ansari, submitted for publication). There were ethnic differences in seroprevalence. In Hispanics, 42 of 47 (89%) were anti-HAV positive, whereas 51 of 74 (69%) African-Americans and 49 of 102 (48%) non-Hispanic Caucasians were anti-HAV positive. There was no difference with age of <50 years in any ethnic group. The estimated screening cost is $17.50, and the vaccine cost (without supplies or labor charges) is currently $16.17 per dose (government contract rate). For the Dallas County Hospital District, screening will be cost-beneficial in Hispanics and African-Americans, but vaccination without screening appears to be rational for non-Hispanic Caucasians. Similarly, in Ireland, universal vaccination without screening is considered the best strategy if the seroprevalence rate is <45% 205.
Why Prevent Hepatitis A?
Different populations have different goals. Governments want to save money, armies want to have healthy troops, and travelers want to enjoy their vacations. For prospective travelers, an estimate of the risk of hepatitis A infection can be obtained by examining seroprevalence data. The rates are lowest in Scandinavia. Thus, the anti-HAV positive rate was 2% in Swedes born after 1950 35. In heterogeneous populations, particularly those with immigrants, the overall prevalence of anti-HAV is predictably higher and differs with birthplace. For example, 45% of U.K.-born Londoners are anti-HAV positive but 70% of those born in a foreign country are positive 30. Intermediate rates of seropositivity are found in the Mediterranean. In Spain, the effect of socioeconomic level is shown by the 63% anti-HAV positive rate among gypsy children aged 1 to 14 years, compared with 46% for children in an orphanage and 23% for nongypsy families 174. In Italy, seropositive rates are higher in the southern part of the country, e.g., 27% anti-HAV positivity in persons aged 3 to 19 years, compared with 5% in northern Italy 235. Among U.S. military personnel, deployment in the Carribean was associated with an increased seroprevalence rate 107. Consistent with endemicity in the Carribean, 73% of children 2 to 4 years of age in Nicaragua were seropositive 194. At the far end of the spectrum, 97% of elementary school children in Sierra Leone were anti-HAV positive 114, as were 100% of Australian aboriginals 11 to 15 years of age living at the “top end” of the Northern Territory 36; 100% of Egyptian village children aged 1 to 3 years were seropositive 58; and 100% of Indian children in Pune were anti-HAV positive by late childhood 17. From these data, it is apparent that there is a considerable risk of hepatitis A infection with travel to Africa, the Indian subcontinent, and the Carribean, lower risk in the Mediterranean area, and negligible risk in Scandinavia.
From the economic perspective, hepatitis A is a costly burden to society that could be mitigated by universal childhood vaccination. The cost of HAV infection was recently calculated to be $332 million to $580 million annually in the United States 29. A food-borne outbreak of hepatitis A in Denver, for example, was calculated to cost $689,314 for disease control, including $450,397 for 16,293 ISG injections 56. The medical costs for hepatitis A cases requiring hospitalization are estimated to be $1,070 to $2,460 each 56, 149. One economic analysis indicated that either universal vaccination or screening and vaccination of 2-year-old children should be considered cost-effective in developed countries 59. In another analysis, neither universal vaccination nor screening and vaccination of adults who were >50 years old were cost-effective 183. These data support the most recent recommendation of universal vaccination of children in U.S. areas of high endemicity as a method of disease control.
CONCLUDING REMARKS
Hepatitis A is common worldwide. Although it remains endemic in developing countries, there are falling seroprevalence rates in residents of developed countries. In young children, acute HAV infection is often asymptomatic. In contrast, older children and adults demonstrate a range of clinical manifestations from mild, anicteric infection to fulminant hepatic failure, with substantial morbidity and economic consequences. Passive immunoprophylaxis, using pooled ISG, prevents or attenuates disease in exposed individuals but does not provide long-term protection. Vaccination (active immunoprophylaxis) with predicted long-term protection against and future eradication of this disease is now possible.
REFERENCES
- 1.Ambrosch F, Wiedermann G, André F E, Delem A, Grepor H, Hofmann H, D'Hondt E, Kundi M, Wynen J, Kunz C. Clinical and immunological investigation of a new combined hepatitis A and hepatitis B vaccine. J Med Virol. 1994;44:452–456. doi: 10.1002/jmv.1890440426. [DOI] [PubMed] [Google Scholar]
- 2.Andersen T T. The etiology of hepatitis epidemica. Acta Med Scand. 1937;93:209–227. [Google Scholar]
- 3.Andre F E, D'Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10(Suppl. 1):S160–S168. doi: 10.1016/0264-410x(92)90576-6. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Hepatitis A among drug abusers. Morb Mortal Wkly Rep. 1988;37:297–300. , 305. [PubMed] [Google Scholar]
- 5.Anonymous. Hepatitis A among homosexual men—United States, Canada, and Australia. Morb Mortal Wkly Rep. 1992;41:155. , 161–164. [PubMed] [Google Scholar]
- 6.Anonymous. Licensure of inactivated hepatitis A vaccine and recommendations for use among international travelers. Morb Mortal Wkly Rep. 1995;44:559–560. [PubMed] [Google Scholar]
- 7.Anonymous. Hepatitis A among persons with hemophilia who received clotting factor concentrate—United States, September–December 1995. Morb Mortal Wkly Rep. 1996;45:29–32. [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Anonymous. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1996;45(RR-15):1–30. [PubMed] [Google Scholar]
- 10.Anonymous. Summary of notifiable diseases, United States 1997. Morb Mortal Wkly Rep. 1997;46:3. , 10–13. [PubMed] [Google Scholar]
- 11.Anonymous. Hepatitis A associated with consumption of frozen strawberries—Michigan, March 1997. Morb Mortal Wkly Rep. 1997;46:288. , 295. [PubMed] [Google Scholar]
- 12.Anonymous. Summary of notifiable diseases, United States 1998. Morb Mortal Wkly Rep. 1998;47:7. , 12–15. [PubMed] [Google Scholar]
- 13.Anonymous. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1999;48(RR-12):1–37. [PubMed] [Google Scholar]
- 14.Anonymous. Jaundice at Alexandria. Br Med J. 1916;1:320–321. [Google Scholar]
- 15.Anonymous. Epidemic hepatitis, or catarrhal jaundice. JAMA. 1943;123:636–637. [Google Scholar]
- 16.Anonymous. Homologous serum hepatitis. Lancet. 1947;ii:691–692. [Google Scholar]
- 17.Arankalle V A, Tsarev S A, Chadha M S, Alling D W, Emerson S U, Banerjee K, Purcell R H. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;17:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 18.Arnal C, Crance J M, Gantzer C, Schwartzbrod L, Deloince R, Billaudel S. Persistence of infectious hepatitis A virus and its genome in artificial seawater. Zentbl Hyg Umweltmed. 1998;201:279–284. [PubMed] [Google Scholar]
- 19.Arslan M, Wiesner R H, Poterucha J J, Gross J B, Zein N N. Hepatitis A (HAV) antibodies in liver transplant (OLT) recipients: evidence for loss of immunity posttransplantation. Hepatology. 1998;28:235A. doi: 10.1002/lt.500060216. [DOI] [PubMed] [Google Scholar]
- 20.Asher L V S, Binn L N, Mensing T L, Marchwicki R H, Vassell R A, Young G D. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus) J Med Virol. 1995;47:260–268. doi: 10.1002/jmv.1890470312. [DOI] [PubMed] [Google Scholar]
- 21.Ashida M, Hamada C. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J Gen Virol. 1997;78:1565–1569. doi: 10.1099/0022-1317-78-7-1565. [DOI] [PubMed] [Google Scholar]
- 22.Asselah T, Bernuau J, Marcellin P. Prevalence of hepatitis C virus infection in patients hospitalized for hepatitis A. Ann Intern Med. 1999;130:451. doi: 10.7326/0003-4819-130-5-199903020-00010. [DOI] [PubMed] [Google Scholar]
- 23.Ballesteros J, Dal-Re R, Gonzalez A, del Romero J. Are homosexual males a risk group for hepatitis A infection in intermediate endemicity areas? Epidemiol Infect. 1996;117:145–148. doi: 10.1017/s0950268800001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber H. Infective hepatic jaundice. Br Med J. 1937;1:67–68. doi: 10.1136/bmj.1.3966.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett P N, Meyer H, Wachtel I, Eibl J, Dorner F. Inactivation of hepatitis A virus in plasma products by vapor heating. Transfusion. 1997;37:215–220. doi: 10.1046/j.1537-2995.1997.37297203527.x. [DOI] [PubMed] [Google Scholar]
- 26.Behrens R H, Doherty J F. Severe hepatitis A despite passive immunisation. Lancet. 1993;341:972. doi: 10.1016/0140-6736(93)91274-p. [DOI] [PubMed] [Google Scholar]
- 27.Beller M. Hepatitis A outbreak in Anchorage, Alaska, traced to ice slush beverages. West J Med. 1992;156:624–627. [PMC free article] [PubMed] [Google Scholar]
- 28.Berelowitz G J, Burgess A P, Thanabalasingham T, Murray-Lyon I M, Wright D J. Post-hepatitis syndrome revisited. J Viral Hepatol. 1995;2:133–138. doi: 10.1111/j.1365-2893.1995.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 29.Berge J J, Drennan D P, Jacobs R J, Jakins A, Meyerhoff A S, Stubblefield W, Weinberg M. The cost of hepatitis A infections in American adolescents and adults in 1997. Hepatology. 2000;31:469–473. doi: 10.1002/hep.510310229. [DOI] [PubMed] [Google Scholar]
- 30.Bernal W, Smith H M, Williams R. A community prevalence study of antibodies to hepatitis A and E in inner-city London. J Med Virol. 1996;49:230–234. doi: 10.1002/(SICI)1096-9071(199607)49:3<230::AID-JMV12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Black F L, Jacobson D L. Hepatitis A antibody in an isolated Amerindian tribe fifty years after exposure. J Med Virol. 1986;19:19–21. doi: 10.1002/jmv.1890190104. [DOI] [PubMed] [Google Scholar]
- 32.Bloch A B, Stramer S L, Smith J D, Margolis H S, Fields H A, McKinley T W, Gerba C P, Maynard J E, Sikes R K. Recovery of hepatitis A virus from a water supply responsible for a common source outbreak of hepatitis A. Am J Public Health. 1990;80:428–430. doi: 10.2105/ajph.80.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumer G. Infectious jaundice in the United States. JAMA. 1923;81:353–358. [Google Scholar]
- 34.Boggs J D, Melnick J L, Conrad M E, Felsher B F. Viral hepatitis: clinical and tissue culture studies. JAMA. 1970;214:1041–1046. doi: 10.1001/jama.214.6.1041. [DOI] [PubMed] [Google Scholar]
- 35.Bottiger M, Christenson B, Grillner L. Hepatitis A immunity in the Swedish population: a study of the prevalence of markers in the Swedish population. Scand J Infect Dis. 1997;29:99–102. doi: 10.3109/00365549709035867. [DOI] [PubMed] [Google Scholar]
- 36.Bowden F J, Currie B J, Miller N C, Locarnini S A, Krause V L. Should aboriginals in the “top end” of the Northern Territory be vaccinated against hepatitis A? Med J Aust. 1994;161:372–373. doi: 10.5694/j.1326-5377.1994.tb127490.x. [DOI] [PubMed] [Google Scholar]
- 37.Bradley D W, Fields H A, McCaustland K A, Maynard J E, Decker R H, Whittington R, Overby L R. Serodiagnosis of viral hepatitis A by a modified competitive binding radioimmunoassay for immunoglobulin M anti-hepatitis A virus. J Clin Microbiol. 1979;9:120–127. doi: 10.1128/jcm.9.1.120-127.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley D W, Maynard J E, Hindman S H, Hornbeck C L, Fields H A, McCaustland K A, Cook E H., Jr Serodiagnosis of viral hepatitis A: detection of acute-phase immunoglobulin M anti-hepatitis A virus by radioimmunoassay. J Clin Microbiol. 1977;5:521–530. doi: 10.1128/jcm.5.5.521-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breningstall G N, Belani K K. Acute transverse myelitis and brainstem encephalitis associated with hepatitis A infection. Pediatr Neurol. 1995;12:169–171. doi: 10.1016/0887-8994(94)00153-s. [DOI] [PubMed] [Google Scholar]
- 40.Bryan J P, Nelson M. Testing for antibody to hepatitis A to decrease the cost of hepatitis A prophylaxis with immune globulin or hepatitis A vaccines. Arch Intern Med. 1994;154:663–668. [PubMed] [Google Scholar]
- 41.Burkholder B T, Coronado V G, Brown J, Hutto J H, Shapiro C N, Robertson B, Woodruff B A. Nosocomial transmission of hepatitis A in a pediatric hospital traced to an anti-hepatitis A virus-negative patient with immunodeficiency. Pediatr Infect Dis J. 1995;14:261–266. doi: 10.1097/00006454-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Cameron J D S. Infective hepatitis. Q J Med. 1943;12:139–155. [Google Scholar]
- 43.Chio F, Jr, Bakir A A. Acute renal failure in hepatitis A. Int J Artif Organs. 1992;15:413–416. [PubMed] [Google Scholar]
- 44.Clayson E T, Innis B L, Myint K S, Snitbhan R, Vaughan D W, Shrestha M P. Short report: relative risk of hepatitis A and E among foreigners in Nepal. Am J Trop Med Hyg. 1995;52:506–507. doi: 10.4269/ajtmh.1995.52.506. [DOI] [PubMed] [Google Scholar]
- 45.Cobden I, James O F. A biphasic illness associated with acute hepatitis A virus infection. J Hepatol. 1986;2:19–23. doi: 10.1016/s0168-8278(86)80004-6. [DOI] [PubMed] [Google Scholar]
- 46.Cockayne E A. Catarrhal jaundice, sporadic and epidemic, and its relation to acute yellow atrophy of the liver. Q J Med. 1912;6:1–29. [Google Scholar]
- 47.Cohen O, Mevorach D, Ackerman Z, Oren R. Thrombocytopenic purpura as a manifestation of acute hepatitis A. J Clin Gastroenterol. 1993;17:166–167. doi: 10.1097/00004836-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Cohn E J, Oncley J L, Strong L E, Hughes W L, Jr, Armstrong S H. Chemical, clinical, and immunological studies on the products of human plasma fractionation. I. The characterization of the protein fractions of human plasma. J Clin Investig. 1944;23:417–432. doi: 10.1172/JCI101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conaty S, Bird P, Bell G, Kraa E, Grohmann G, McAnulty J M. Hepatitis A in New South Wales, Australia, from consumption of oysters: the first reported outbreak. Epidemiol Infect. 2000;124:121–130. doi: 10.1017/s0950268899003386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corey L, Holmes K K. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. N Engl J Med. 1980;302:435–438. doi: 10.1056/NEJM198002213020804. [DOI] [PubMed] [Google Scholar]
- 51.Coulepis A G, Locarnini S A, Lehmann N I, Gust I D. Detection of hepatitis A virus in the feces of patients with naturally acquired infection. J Infect Dis. 1980;141:151–156. doi: 10.1093/infdis/141.2.151. [DOI] [PubMed] [Google Scholar]
- 52.Coursaget P, Buisson Y, Enogat N, Bercion R, Baudet J M, Delmaire P, Prigent D, Desrame J. Outbreak of enterically transmitted hepatitis due to hepatitis A and hepatitis E viruses. J Hepatol. 1998;28:745–750. doi: 10.1016/s0168-8278(98)80222-5. [DOI] [PubMed] [Google Scholar]
- 53.Coutinho R, Lent A, Albrecht-Van P, Rijsdijk T, Lelie N, Nagelkerke N, Kuipers H. Prevalence and incidence of hepatitis A among male homosexuals. Br Med J. 1983;287:1743–1750. doi: 10.1136/bmj.287.6407.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cullinan E R. The epidemiology of jaundice. Proc R Soc Med. 1939;32:933–950. [PMC free article] [PubMed] [Google Scholar]
- 55.Reference deleted.
- 56.Dalton C B, Haddix A, Hoffman R E, Mast E E. The cost of a food-borne outbreak of hepatitis A in Denver, Colo. Arch Intern Med. 1996;156:1013–1016. [PubMed] [Google Scholar]
- 57.Dan M, Yaniv R. Cholestatic hepatitis, cutaneous vasculitis, and vascular deposits of immunoglobulin M and complement associated with hepatitis A virus infection. Am J Med. 1990;89:103–104. doi: 10.1016/0002-9343(90)90107-o. [DOI] [PubMed] [Google Scholar]
- 58.Darwich M A, Faris R, Clemens J D, Rao M R, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in the Nile Delta: a pilot study. Am J Trop Med Hyg. 1996;54:554–558. doi: 10.4269/ajtmh.1996.54.554. [DOI] [PubMed] [Google Scholar]
- 59.Das A. An economic analysis of different strategies of immunization against hepatitis A virus in developed countries. Hepatology. 1999;29:548–552. doi: 10.1002/hep.510290225. [DOI] [PubMed] [Google Scholar]
- 60.Davis L E, Brown J E, Robertson B H, Khanna B, Polish L B. Hepatitis A post-viral encephalitis. Acta Neurol Scand. 1993;87:67–69. doi: 10.1111/j.1600-0404.1993.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 61.Davis T V, Keeffe E B. Acute pancreatitis associated with acute hepatitis A. Am J Gastroenterol. 1992;87:1648–1650. [PubMed] [Google Scholar]
- 62.Debray D, Cullufi P, Devictor D, Fabre M, Bernard O. Liver failure in children with hepatitis A. Hepatology. 1997;26:1018–1022. doi: 10.1053/jhep.1997.v26.pm0009328329. [DOI] [PubMed] [Google Scholar]
- 63.Decker R H, Kosakowski S M, Vanderbilt A S, Ling C M, Chairez R, Overby L R. Diagnosis of acute hepatitis A by HAVAB-M, a direct radioimmunoassay for IgM anti-HAV. Am J Clin Pathol. 1981;76:140–147. doi: 10.1093/ajcp/76.2.140. [DOI] [PubMed] [Google Scholar]
- 64.Desenclos J C, Klontz K C, Wilder M H, Nainan O V, Margolis H S, Gunn R A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991;81:1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Serres G, Laliberte D. Hepatitis A among workers from a waste water treatment plant during a small community outbreak. Occup Environ Med. 1997;54:60–62. doi: 10.1136/oem.54.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dible J H, McMichael J, Sherlock S P V. Pathology of acute hepatitis: aspiration biopsy studies of epidemic, arsenotherapy and serum jaundice. Lancet. 1943;ii:402–408. [Google Scholar]
- 67.Dienstag J L, Feinstone S M, Kapikian A Z, Purcell R H, Boggs J D, Conrad M E. Faecal shedding of hepatitis-A antigen. Lancet. 1975;ii:765–767. doi: 10.1016/s0140-6736(75)92434-4. [DOI] [PubMed] [Google Scholar]
- 68.Dienstag J L, Gust I D, Lucas C R, Wong D C, Purcell R H. Mussel-associated viral hepatitis, type A: serological confirmation. Lancet. 1976;i:561–564. doi: 10.1016/s0140-6736(76)90358-5. [DOI] [PubMed] [Google Scholar]
- 69.Dienstag J L, Krugman S, Wong D C, Purcell R H. Comparison of serological tests for antibody to hepatitis A antigen, using coded specimens from individuals infected with the MS-1 strain of hepatitis A virus. Infect Immun. 1976;14:1000–1003. doi: 10.1128/iai.14.4.1000-1003.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dienstag J L, Routenberg J A, Purcell R H, Hooper R R, Harrison W O. Foodhandler-associated outbreak of hepatitis type A. An immune electron microscopy study. Ann Intern Med. 1975;83:647–650. doi: 10.7326/0003-4819-83-5-647. [DOI] [PubMed] [Google Scholar]
- 71.Divizia M, Ruscio V, Degener A M, Pana A. Hepatitis A virus detection in wastewater by PCR and hybridization. New Microbiol. 1998;21:161–167. [PubMed] [Google Scholar]
- 72.Doebbeling B N, Li N, Wenzel R P. An outbreak of hepatitis A among health care workers: risk factors for transmission. Am J Public Health. 1993;83:1679–1684. doi: 10.2105/ajph.83.12.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domenech P, Palomeque A, Martinez-Gutierrez A, Vinolas N, Vela E, Jimenez R. Severe aplastic anaemia following hepatitis A. Acta Haematol. 1986;76:227–229. doi: 10.1159/000206061. [DOI] [PubMed] [Google Scholar]
- 74.Erkan T, Kutlu T, Cullu F, Tumay G T. A case of vertical transmission of hepatitis A virus infection. Acta Paediatr. 1998;87:1008–1009. doi: 10.1080/080352598750031725. [DOI] [PubMed] [Google Scholar]
- 75.Fagan E, Yousef G, Brahm J, Garelick H, Mann G, Wolstenholme A, Portmann B, Harrison T, Mowbray J F, Mowat A, Zuckerman A, Williams R. Persistence of hepatitis A virus in fulminant hepatitis and after liver transplantation. J Med Virol. 1990;30:131–136. doi: 10.1002/jmv.1890300210. [DOI] [PubMed] [Google Scholar]
- 76.Faust R L, Pimstone N. Acute renal failure associated with nonfulminant hepatitis A viral infection. Am J Gastroenterol. 1996;91:369–372. [PubMed] [Google Scholar]
- 77.Feigelstock D, Thompson P, Mattoo P, Kaplan G G. Polymorphisms of the hepatitis A virus cellular receptor 1 in African green monkey kidney cells result in antigenic variants that do not react with protective monoclonal antibody 190/4. J Virol. 1998;72:6218–6222. doi: 10.1128/jvi.72.7.6218-6222.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feinstone S M, Kapikian A Z, Purcell R H. Hepatitis A: detection by immune electron microscopy of a virus-like antigen associated with acute illness. Science. 1973;182:1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- 79.Fikar C R, McKee C. False positivity of IgM antibody to Epstein-Barr viral capsid antigen during acute hepatitis A infection. Pediatr Infect Dis. 1994;13:413–414. doi: 10.1097/00006454-199405000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Findlay G M, Dunlop J L. A fatal case of acute necrosis of the liver associated with epidemic catarrhal jaundice. Br Med J. 1932;1:652–656. doi: 10.1136/bmj.1.3718.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Findlay G M, Dunlop J L, Brown H C. Observations on epidemic catarrhal jaundice. Trans R Soc Trop Med Hyg. 1931;25:7–24. [Google Scholar]
- 82.Findlay G M, Martin N H. Jaundice following yellow-fever immunisation. Lancet. 1943;i:678–680. [Google Scholar]
- 83.Fleischer B, Fleischer S, Maier K, Wiedmann K H, Sacher M, Thaler H, Vallbracht A. Clonal analysis of infiltrating T lymphocytes in liver tissue in viral hepatitis A. Immunology. 1990;69:14–19. [PMC free article] [PubMed] [Google Scholar]
- 84.Ford J C. Infective hepatitis: 300 cases in an outer London borough. Lancet. 1943;i:675–678. [Google Scholar]
- 85.Friedman L S, O'Brien T F, Morse L J, Chang L W, Wacker W E, Ryan D M, Dienstag J L. Revisiting the Holy Cross football team hepatitis outbreak (1969) by serological analysis. JAMA. 1985;254:774–776. [PubMed] [Google Scholar]
- 86.Gane E, Sallie R, Saleh M, Portmann B, Williams R. Clinical recurrence of hepatitis A following liver transplantation for acute liver failure. J Med Virol. 1995;45:35–39. doi: 10.1002/jmv.1890450107. [DOI] [PubMed] [Google Scholar]
- 87.Gardner P, Eickhoff T, Poland G A, Gross P, Griffin M, LaForce F M, Schaffner W, Strikas R. Adult immunizations. Ann Intern Med. 1996;124:35–40. doi: 10.7326/0003-4819-124-1_part_1-199601010-00007. [DOI] [PubMed] [Google Scholar]
- 88.Gaskell J F. The changes in the liver in a fatal case of epidemic “catarrhal” jaundice. J Pathol Bacteriol. 1933;36:257–262. [Google Scholar]
- 89.Gellis S S, Stokes J, Jr, Brother G M, Hall W M, Gilmore H R, Beyer E, Morrissey R A. The use of human immune serum globulin (gamma globulin) in infectious (epidemic) hepatitis in the Mediterranean theater of operations. I. Studies on prophylaxis in two epidemics of infectious hepatitis. JAMA. 1945;128:1062–1063. [Google Scholar]
- 90.Geltner D, Naot Y, Zimhoni O, Gorbach S, Bar-Khayim Y. Acute oliguric renal failure complicating type A nonfulminant viral hepatitis: a case presentation and review of the literature. J Clin Gastroenterol. 1992;14:160–162. doi: 10.1097/00004836-199203000-00019. [DOI] [PubMed] [Google Scholar]
- 91.Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A: review of 14 cases and literature survey. Medicine (Baltimore) 1992;71:14–23. doi: 10.1097/00005792-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 92.Glover J A, Wilson J. An extensive epidemic of catarrhal jaundice. Lancet. 1931;i:722–725. [Google Scholar]
- 93.Goodman R A. Nosocomial hepatitis A. Ann Intern Med. 1985;103:452–454. doi: 10.7326/0003-4819-103-3-452. [DOI] [PubMed] [Google Scholar]
- 94.Goodman R A, Carder C C, Allen J R, Orenstein W A, Finton R J. Nosocomial hepatitis A transmission by an adult patient with diarrhea. Am J Med. 1982;73:220–226. doi: 10.1016/0002-9343(82)90182-6. [DOI] [PubMed] [Google Scholar]
- 95.Gordon S C, Reddy K R, Schiff L, Schiff E R. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101:635–637. doi: 10.7326/0003-4819-101-5-635. [DOI] [PubMed] [Google Scholar]
- 96.Green M S, Cohen D, Lerman Y, Sjogren M, Binn L N, Zur S, Slepon R, Robin G, Hoke C, Bancroft W, Safary A, Danon Y, Wiener M. Depression of the immune response to an inactivated hepatitis A vaccine administered concomitantly with immune globulin. J Infect Dis. 1993;168:740–743. doi: 10.1093/infdis/168.3.740. [DOI] [PubMed] [Google Scholar]
- 97.Grinde B, Stene-Johansen K, Sharma B, Hoel T, Jensenius M, Skaug K. Characterisation of an epidemic of hepatitis A virus involving intravenous drug abusers—infection by needle sharing? J Med Virol. 1997;53:69–75. doi: 10.1002/(sici)1096-9071(199709)53:1<69::aid-jmv12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 98.Gutzeit K. Die hepatitis epidemica. Muench Med Wochenschr. 1950;92:1295–1301. [PubMed] [Google Scholar]
- 99.Hadler S C, Webster H M, Erben J J, Swanson J E, Maynard J E. Hepatitis A in day-care centers: a community-wide assessment. N Engl J Med. 1980;302:1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- 100.Halliday M L, Kang L Y, Zhou T K, Hu M D, Pan Q C, Fu T Y, Huang Y S, Hu S L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J Infect Dis. 1991;164:852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- 101.Hanna J N, Loewenthal M R, Negel P, Wenck D J. An outbreak of hepatitis A in an intensive care unit. Anaesth Intensive Care. 1996;24:440–444. doi: 10.1177/0310057X9602400405. [DOI] [PubMed] [Google Scholar]
- 102.Havens W P., Jr Infectious hepatitis in the Middle East: a clinical review of 200 cases seen in a military hospital. JAMA. 1944;126:17–23. [Google Scholar]
- 103.Havens W P, Jr, Paul J R. Prevention of infectious hepatitis with gamma globulin. JAMA. 1945;129:270–272. [Google Scholar]
- 104.Havens W P, Jr, Ward R, Drill V A, Paul J R. Experimental production of hepatitis by feeding icterogenic materials. Proc Soc Exp Biol Med. 1944;57:206–208. [Google Scholar]
- 105.Havens W P. Period of infectivity of patients with experimentally induced infectious hepatitis. J Exp Med. 1946;83:215–258. [PubMed] [Google Scholar]
- 106.Havens W P. The etiology of infectious hepatitis. JAMA. 1947;134:653–655. doi: 10.1001/jama.1947.02880250001001. [DOI] [PubMed] [Google Scholar]
- 107.Hawkins R E, Malone J D, Cloninger L A, Rozmajzl P J, Lewis D, Butler J, Cross E, Gray S, Hyams K C. Risk of viral hepatitis among military personnel assigned to US Navy ships. J Infect Dis. 1992;165:716–719. doi: 10.1093/infdis/165.4.716. [DOI] [PubMed] [Google Scholar]
- 108.Helbling B, Renner E L, Kammerlander R. Acute hepatitis A in patients with chronic hepatitis C. Ann Intern Med. 1999;131:314. doi: 10.7326/0003-4819-131-4-199908170-00025. [DOI] [PubMed] [Google Scholar]
- 109.Henkel J. Food firm gets huge fine for tainted strawberry harvest. FDA Consum. 1999;33:37–38. [PubMed] [Google Scholar]
- 110.Henning K J, Bell E, Braun J, Barker N D. A community-wide outbreak of hepatitis A: risk factors for infection among homosexual and bisexual men. Am J Med. 1995;99:132–136. doi: 10.1016/s0002-9343(99)80132-6. [DOI] [PubMed] [Google Scholar]
- 111.Hess G, Clemens R, Bienzle U, Schonfeld C, Schunck B, Bock H L. Immunogenicity and safety of an inactivated hepatitis A vaccine in anti-HIV positive and negative homosexual men. J Med Virol. 1995;46:40–42. doi: 10.1002/jmv.1890460109. [DOI] [PubMed] [Google Scholar]
- 112.Hirata R, Hoshino Y, Sakai H, Marumo F, Sato C. Patients with hepatitis A with negative IgM-HA antibody at early stages. Am J Gastroenterol. 1995;90:1168–1169. [PubMed] [Google Scholar]
- 113.Hoagland C L, Shank R E. Infectious hepatitis: a review of 200 cases. JAMA. 1946;130:615–621. doi: 10.1001/jama.1946.02870100001001. [DOI] [PubMed] [Google Scholar]
- 114.Hodges M, Sanders E, Aitken C. Seroprevalence of hepatitis markers; HAV, HBV, HCV and HEV amongst primary school children in Freetown, Sierra Leone. West Afr J Med. 1998;17:36–37. [PubMed] [Google Scholar]
- 115.Hollinger F B, Ticehurst J. Hepatitis A virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 735–782. [Google Scholar]
- 116.Holmes A W, Wolfe L, Rosenblate H, Deinhardt F. Hepatitis in marmosets: induction of disease with coded specimens from a human volunteer study. Science. 1969;165:816–817. doi: 10.1126/science.165.3895.816. [DOI] [PubMed] [Google Scholar]
- 117.Holter E, Siebke J C. Hepatitis A in young Norwegian drug addicts and prison inmates. Infection. 1988;16:91–94. doi: 10.1007/BF01644310. [DOI] [PubMed] [Google Scholar]
- 118.Hopkins R. HBsAg-negative blood donors with a history of jaundice as a source of plasma for preparation of hyperimmune hepatitis type A globulin. J Infect. 1981;3:166–171. doi: 10.1016/s0163-4453(81)91361-x. [DOI] [PubMed] [Google Scholar]
- 119.Huo T I, Wu J C, Chiu C F, Lee S D. Severe hyperbilirubinemia due to acute hepatitis A superimposed on a chronic hepatitis B carrier with glucose-6-phosphate dehydrogenase deficiency. Am J Gastroenterol. 1996;91:158–159. [PubMed] [Google Scholar]
- 120.Huppertz H I, Treichel U, Gassel A M, Jeschke R, Meyer zum Buschenfelde K H. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23:204–208. doi: 10.1016/0168-8278(95)80336-x. [DOI] [PubMed] [Google Scholar]
- 121.Hutin Y J, Pool V, Cramer E H, Nainan O V, Weth J, Williams I T, Goldstein S T, Gensheimer K F, Bell B P, Shapiro C N, Alter M J, Margolis H S. A multistate, foodborne outbreak of hepatitis A. National Hepatitis A Investigation Team. N Engl J Med. 1999;340:595–602. doi: 10.1056/NEJM199902253400802. [DOI] [PubMed] [Google Scholar]
- 122.Ilan Y, Hillman M, Oren R, Zlotogorski A, Shouval D. Vasculitis and cryoglobulinemia associated with persisting cholestatic hepatitis A virus infection. Am J Gastroenterol. 1990;85:586–587. [PubMed] [Google Scholar]
- 123.Inman R D, Hodge M, Johnston M E, Wright J, Heathcote J. Arthritis, vasculitis, and cryoglobulinemia associated with relapsing hepatitis A virus infection. Ann Intern Med. 1986;105:700–703. doi: 10.7326/0003-4819-105-5-700. [DOI] [PubMed] [Google Scholar]
- 124.Innis B L, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik C A, Suntayakorn S, Suknuntapong T, Safary A, Boslego J W. A field efficacy trial of inactivated hepatitis A vaccine among children in Thailand. Vaccine. 1992;10:S159. [Google Scholar]
- 125.Innis B L, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik C A, Suntayakorn S, Suknuntapong T, Safary A, Tang D B, Boslego J W. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994;271:1328–1334. [PubMed] [Google Scholar]
- 126.Inoue K, Yoshiba M, Yotsuyanagi H, Otsuka T, Sekiyama K, Fujita R. Chronic hepatitis A with persistent viral replication. J Med Virol. 1996;50:322–324. doi: 10.1002/(SICI)1096-9071(199612)50:4<322::AID-JMV7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 127.Ishak K G. Light microscopic morphology of viral hepatitis. Am J Clin Pathol. 1976;65:787–827. [PubMed] [Google Scholar]
- 128.Iversen P, Roholm K. On aspiration biopsy of the liver, with remarks on its diagnostic significance. Acta Med Scand. 1939;102:1–16. [Google Scholar]
- 129.Jacobson I M, Nath B J, Dienstag J L. Relapsing viral hepatitis type A. J Med Virol. 1985;16:163–169. doi: 10.1002/jmv.1890160208. [DOI] [PubMed] [Google Scholar]
- 130.Kao H W, Ashcavai M, Redeker A G. The persistence of hepatitis A IgM antibody after acute clinical hepatitis A. Hepatology. 1984;4:933–936. doi: 10.1002/hep.1840040525. [DOI] [PubMed] [Google Scholar]
- 131.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone S M. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 132.Katz R, Velasco M, Guzman C, Alessandri H. Red cell survival estimated by radioactive chromium in hepatobiliary disease. Gastroenterology. 1964;46:399–404. [PubMed] [Google Scholar]
- 133.Keeffe E B. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 134.Keeffe E B, Iwarson S, McMahon B J, Lindsay K L, Koff R S, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A, Clemens R, Krause D S. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881–886. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 135.Klein B S, Michaels J A, Rytel M W, Berg K G, Davis J P. Nosocomial hepatitis A. A multinursery outbreak in Wisconsin. JAMA. 1984;252:2716–2721. doi: 10.1001/jama.252.19.2716. [DOI] [PubMed] [Google Scholar]
- 136.Krook A, Albert J, Andersson S, Biberfeld G, Blomberg J, Eklund I, Engstrom A, Julander I, Kall K, Martin C, Stendahl P, Struve J, Sonnerborg A. Prevalence and risk factors for HTLV-II infection in 913 injecting drug users in Stockholm, 1994. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:381–386. doi: 10.1097/00042560-199708150-00009. [DOI] [PubMed] [Google Scholar]
- 137.Krugman S, Friedman H, Lattimer C. Identification by specific complement fixation and immune adherence tests. N Engl J Med. 1975;292:1141–1143. doi: 10.1056/NEJM197505292922201. [DOI] [PubMed] [Google Scholar]
- 138.Krugman S, Giles J P, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 139.Krugman S, Ward R, Giles J P. The natural history of infectious hepatitis. Am J Med. 1962;32:717–728. doi: 10.1016/0002-9343(62)90161-4. [DOI] [PubMed] [Google Scholar]
- 140.Krugman S, Ward R, Giles J P, Bodansky D, Jacobs A M. Infectious hepatitis: detection of virus during the incubation period and in clinically inapparent infection. N Engl J Med. 1959;261:729–734. doi: 10.1056/NEJM195910082611501. [DOI] [PubMed] [Google Scholar]
- 141.Kryger P, Christoffersen P. Liver histopathology of the hepatitis A virus infection: a comparison with hepatitis type B and non-A, non-B. J Clin Pathol. 1983;36:650–654. doi: 10.1136/jcp.36.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kurane I, Binn L N, Bancroft W H, Ennis F A. Human lymphocyte responses to hepatitis A virus-infected cells: interferon production and lysis of infected cells. J Immunol. 1985;135:2140–2144. [PubMed] [Google Scholar]
- 143.LaBrecque F D, LaBrecque D R, Klinzman D, Perlman S, Cederna J B, Winokur P L, Han J Q, Stapleton J T. Recombinant hepatitis A virus antigen: improved production and utility in diagnostic immunoassays. J Clin Microbiol. 1998;36:2014–2018. doi: 10.1128/jcm.36.7.2014-2018.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lednar W M, Lemon S M, Kirkpatrick J W, Redfield R R, Fields M L, Kelley P W. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122:226–233. doi: 10.1093/oxfordjournals.aje.a114093. [DOI] [PubMed] [Google Scholar]
- 145.Lee S D, Chan C Y, Yu M I, Wang Y J, Chang F Y, Lo K J, Safary A. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol. 1997;52:215–218. doi: 10.1002/(sici)1096-9071(199706)52:2<215::aid-jmv16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 146.Leikin E, Lysikiewicz A, Garry D, Tejani N. Intrauterine transmission of hepatitis A virus. Obstet Gynecol. 1996;88:690–691. doi: 10.1016/0029-7844(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 147.Lemon S M. Inactivated hepatitis A vaccines. JAMA. 1994;271:1363–1364. [PubMed] [Google Scholar]
- 148.Lemon S M, Jansen R W, Brown E A. Genetic, antigenic and biological differences between strains of hepatitis A virus. Vaccine. 1992;10(Suppl. 1):S40–S44. doi: 10.1016/0264-410x(92)90540-z. [DOI] [PubMed] [Google Scholar]
- 149.Lemon S M, Shapiro C N. The value of immunization against hepatitis A. Infect Agents Dis. 1994;3:38–49. [PubMed] [Google Scholar]
- 150.Lerman Y, Chodik G, Aloni H, Ribak J, Ashkenazi S. Occupations at increased risk of hepatitis A: a 2-year nationwide historical prospective study. Am J Epidemiol. 1999;150:312–320. doi: 10.1093/oxfordjournals.aje.a010004. [DOI] [PubMed] [Google Scholar]
- 151.Levine A J. The origins of virology. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1–14. [Google Scholar]
- 152.Lin C C, Chang C H, Lee S H, Chiang S S, Yang A H. Acute renal failure in non-fulminant hepatitis A. Nephrol Dial Transplant. 1996;11:2061–2066. doi: 10.1093/oxfordjournals.ndt.a027098. [DOI] [PubMed] [Google Scholar]
- 153.Linder C R, Karetnyi Y V, Kuint J, Mendelson E, Dagan R. Symptomatic hepatitis A virus infection during the first year of life. Pediatr Infect Dis. 1995;14:628–629. doi: 10.1097/00006454-199507000-00017. [DOI] [PubMed] [Google Scholar]
- 154.Lisney A A. Epidemic catarrhal jaundice in school children. Br Med J. 1937;1:703–706. doi: 10.1136/bmj.1.3978.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lowry P W, Levine R, Stroup D F, Gunn R A, Wilder M H, Konigsberg C., Jr Hepatitis A outbreak on a floating restaurant in Florida, 1986. Am J Epidemiol. 1989;129:155–164. doi: 10.1093/oxfordjournals.aje.a115104. [DOI] [PubMed] [Google Scholar]
- 156.Lyons D J, Gilvarry J M, Fielding J F. Severe haemolysis associated with hepatitis A and normal glucose-6-phosphate dehydrogenase status. Gut. 1990;31:838–839. doi: 10.1136/gut.31.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahoney F J, Farley T A, Kelso K Y, Wilson S A, Horan J M, McFarland L M. An outbreak of hepatitis A associated with swimming in a public pool. J Infect Dis. 1992;165:613–618. doi: 10.1093/infdis/165.4.613. [DOI] [PubMed] [Google Scholar]
- 158.Mallory T B. The pathology of epidemic hepatitis. JAMA. 1947;134:655–662. doi: 10.1001/jama.1947.02880250003002. [DOI] [PubMed] [Google Scholar]
- 159.Mannucci P M, Gdovin S, Gringeri A, Colombo M, Mele A, Schinaia N, Ciavarella N, Emerson S U, Purcell R H. Transmission of hepatitis A to patients with hemophilia by factor VIII concentrates treated with organic solvent and detergent to inactivate viruses. The Italian Collaborative Group. Ann Intern Med. 1994;120:1–7. doi: 10.7326/0003-4819-120-1-199401010-00001. [DOI] [PubMed] [Google Scholar]
- 160.Margolis H S, Nainan O V. Identification of virus components in circulating immune complexes isolated during hepatitis A virus infection. Hepatology. 1990;11:31–37. doi: 10.1002/hep.1840110107. [DOI] [PubMed] [Google Scholar]
- 161.Martin C J. Concerning the pathology and etiology of the infectious jaundice common at the Dardenelles, 1915. Br Med J. 1917;1:445. doi: 10.1136/bmj.1.2936.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reference deleted.
- 163.Mbithi J N, Springthorpe V S, Boulet J R, Sattar S A. Survival of hepatitis A virus on human hands and its transfer on contact with animate and inanimate surfaces. J Clin Microbiol. 1992;30:757–763. doi: 10.1128/jcm.30.4.757-763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McCann U G, 2nd, Rabito F, Shah M, Nolan C R, 3rd, Lee M. Acute renal failure complicating nonfulminant hepatitis A. West J Med. 1996;165:308–310. [PMC free article] [PubMed] [Google Scholar]
- 165.McDonald G S, Courtney M G, Shattock A G, Weir D G. Prolonged IgM antibodies and histopathological evidence of chronicity in hepatitis A. Liver. 1989;9:223–228. doi: 10.1111/j.1600-0676.1989.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 166.McDonald S. Acute yellow atrophy of the liver. Edin Med J. 1908;1:83–88. [Google Scholar]
- 167.McKhann C F. The prevention and modification of measles. JAMA. 1937;109:234. [Google Scholar]
- 168.McMahon B J, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med. 1996;150:733–739. doi: 10.1001/archpedi.1996.02170320079014. [DOI] [PubMed] [Google Scholar]
- 169.Medawar P B, Medawar J S. Viruses. In: Medawar P B, Medawar J S, editors. Aristotle to zoos: a philosophical dictionary of biology. Cambridge, Mass: Harvard University Press; 1983. p. 275. [Google Scholar]
- 170.Meyers J D, Huff J C, Holmes K K, Thomas E D, Bryan J A. Parenterally transmitted hepatitis A associated with platelet transfusions: epidemiologic study of an outbreak in a marrow transplantation center. Ann Intern Med. 1974;81:145–151. doi: 10.7326/0003-4819-81-2-145. [DOI] [PubMed] [Google Scholar]
- 171.Midthun K, Ellerbeck E, Gershman K, Calandra G, Krah D, McCaughtry M, Nalin D, Provost P. Safety and immunogenicity of a live attenuated hepatitis A virus vaccine in seronegative volunteers. J Infect Dis. 1991;163:735–739. doi: 10.1093/infdis/163.4.735. [DOI] [PubMed] [Google Scholar]
- 172.Miller W J, Provost P J, McAleer W J, Ittensohn O L, Villarejos V M, Hillmean M R. Specific immune adherence assay for human hepatitis A antibody. Application to diagnostic and epidemiologic investigations. Proc Soc Exp Biol Med. 1975;149:254–261. doi: 10.3181/00379727-149-38783. [DOI] [PubMed] [Google Scholar]
- 173.Molner J G, Meyer M F. Jaundice in Detroit. Am J Public Health. 1940;30:509–515. doi: 10.2105/ajph.30.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morales J L, Huber L, Gallego S, Alvarez G, Diez-Delgado J, Gonzalez A, Aguilar L, Dal-Re R. A seroepidemiologic study of hepatitis A in Spanish children: relationship to age and socio-environmental factors. Infection. 1992;20:194–196. doi: 10.1007/BF02033057. [DOI] [PubMed] [Google Scholar]
- 175.Mosley J W, Visoná K A, Villarejos V M. Immunoglobulin M level in the diagnosis of type A hepatitis. Am J Clin Pathol. 1981;75:86–87. doi: 10.1093/ajcp/75.1.86. [DOI] [PubMed] [Google Scholar]
- 176.Mourani S, Dobbs S M, Genta R M, Tandon A K, Yoffe B. Hepatitis A virus-associated cholecystitis. Ann Intern Med. 1994;120:398–400. doi: 10.7326/0003-4819-120-5-199403010-00008. [DOI] [PubMed] [Google Scholar]
- 177.Neefe J R, Stokes J., Jr An epidemic of infectious hepatitis apparently due to a water-borne agent. JAMA. 1945;128:1063–1075. [Google Scholar]
- 178.Neilsen G A, Bodsworth N J, Watts N. Response to hepatitis A vaccination in human immunodeficiency virus-infected and -uninfected homosexual men. J Infect Dis. 1997;176:1064–1067. doi: 10.1086/516512. [DOI] [PubMed] [Google Scholar]
- 179.Niu M T, Polish L B, Robertson B H, Khanna B K, Woodruff B A, Shapiro C N, Miller M A, Smith J D, Gedrose J K, Alter M J, Margolis H S the National Hepatitis A Investigation Team. Multistate outbreak of hepatitis A associated with frozen strawberries. J Infect Dis. 1992;166:518–524. doi: 10.1093/infdis/166.3.518. [DOI] [PubMed] [Google Scholar]
- 180.Noble R C, Kane M A, Reeves S A, Roeckel I. Posttransfusion hepatitis A in a neonatal intensive care unit. JAMA. 1984;252:2711–2715. [PubMed] [Google Scholar]
- 181.Norton J A. Acute infectious jaundice. JAMA. 1939;113:916–917. [Google Scholar]
- 182.Ochnio J J, Scheifele D W, Ho M, Mitchell L A. New, ultrasensitive enzyme immunoassay for detecting vaccine- and disease-induced hepatitis A virus-specific immunoglobulin G in saliva. J Clin Microbiol. 1997;35:98–101. doi: 10.1128/jcm.35.1.98-101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O'Connor J B, Imperiale T F, Singer M E. Cost-effectiveness analysis of hepatitis A vaccination strategies for adults. Hepatology. 1999;30:1077–1081. doi: 10.1002/hep.510300422. [DOI] [PubMed] [Google Scholar]
- 184.Okuno T, Sano A, Deguchi T, Katsuma Y, Ogasawara T, Okanoue T, Takino T. Pathology of acute hepatitis A in humans. Comparison with acute hepatitis B. Am J Clin Pathol. 1984;81:162–169. doi: 10.1093/ajcp/81.2.162. [DOI] [PubMed] [Google Scholar]
- 185.Oldstone M B A, editor. Viruses, plagues, and history. New York, N.Y: Oxford University Press; 1998. [Google Scholar]
- 186.O'Mahony M C, Gooch C D, Smyth D A, Thrussell A J, Bartlett C L R, Noah N D. Epidemic hepatitis A from cockles. Lancet. 1983;i:518–520. doi: 10.1016/s0140-6736(83)92203-1. [DOI] [PubMed] [Google Scholar]
- 187.Ordman C W, Jennings C G, Jr, Janeway C A. Chemical, clinical, and immunological studies on the products of human plasma fractionation. XII. Use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prevention and attenuation of measles. J Clin Investig. 1944;23:541–549. doi: 10.1172/JCI101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paul J R, Gardner H T. Viral hepatitis: communicable diseases transmitted through contact or by unknown means. In: Hoff E C, Coates J B Jr, editors. Preventive medicine in World War II. V. Washington, D.C.: Office of the Surgeon General, Department of the Army; 1960. pp. 411–462. [Google Scholar]
- 189.Paul J R, Havens W P., Jr Recent advances in the study of infectious hepatitis and serum jaundice. Trans Assoc Am Phys. 1946;59:133–141. [Google Scholar]
- 190.Paul J R, Havens W P, Sabin A B, Philip C B. Transmission experiments in serum jaundice and infectious hepatitis. JAMA. 1945;128:911–915. [Google Scholar]
- 191.Pavia A T, Nielsen L, Armington L, Thurman D J, Tierney E, Nichols C R. A community-wide outbreak of hepatitis A in a religious community: impact of mass administration of immune globulin. Am J Epidemiol. 1990;131:1085–1093. doi: 10.1093/oxfordjournals.aje.a115601. [DOI] [PubMed] [Google Scholar]
- 192.Pebody R G, Leino T, Ruutu P, Kinnunen L, Davidkin I, Nohynek H, Leinikki P. Foodborne outbreaks of hepatitis A in a low endemic country: an emerging problem? Epidemiol Infect. 1998;120:55–59. doi: 10.1017/s0950268897008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pelletier G, Elghozi D, Trepo C, Laverdant C, Benhamou J P. Mononeuritis in acute viral hepatitis. Digestion. 1985;32:53–56. doi: 10.1159/000199217. [DOI] [PubMed] [Google Scholar]
- 194.Perez O M, Morales W, Paniagua M, Strannegard O. Prevalence of antibodies to hepatitis A, B, C, and E viruses in a healthy population in Leon, Nicaragua. Am J Trop Med Hyg. 1996;55:17–21. [PubMed] [Google Scholar]
- 195.Petersen P, Christoffersen P, Elling P, Juhl E, Dietrichson O, Faber V, Iversen K, Nielsen J O, Poulsen H. Clinical, biochemical, immunological, and morphological features at time of diagnosis. Scand J Gastroenterol. 1974;9:607–613. [PubMed] [Google Scholar]
- 196.Pickles W N. Epidemic catarrhal jaundice: an outbreak in Yorkshire. Br Med J. 1930;1:944–946. doi: 10.1136/bmj.1.3620.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ponz E, Garcia-Pagan J C, Bruguera M, Bruix J, Rodes J. Hepatic fibrin-ring granulomas in a patient with hepatitis A. Gastroenterology. 1991;100:268–270. doi: 10.1016/0016-5085(91)90612-o. [DOI] [PubMed] [Google Scholar]
- 198.Príkazsky V, Oleár V, Cernoch A, Safary A, Andre F E. Interruption of an outbreak of hepatitis A in two villages by vaccination. J Med Virol. 1994;44:457–459. doi: 10.1002/jmv.1890440427. [DOI] [PubMed] [Google Scholar]
- 199.Probst C, Jecht M, Gauss-Muller V. Intrinsic signals for the assembly of hepatitis A virus particles: role of structural proteins VP4 and 2A. J Biol Chem. 1999;274:4527–4531. doi: 10.1074/jbc.274.8.4527. [DOI] [PubMed] [Google Scholar]
- 200.Provost J J, Ittensohn O L, Villarejos V M, Hilleman M R. A specific complement-fixation test for human hepatitis A employing CR 326 virus antigen. Diagnosis and epidemiology. Proc Soc Exp Biol Med. 1975;148:962–968. doi: 10.3181/00379727-148-38669. [DOI] [PubMed] [Google Scholar]
- 201.Provost P J, Hilleman M R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979;160:213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- 202.Purcell R H. Hepatitis viruses: changing patterns of human disease. Proc Natl Acad Sci USA. 1994;91:2401–2406. doi: 10.1073/pnas.91.7.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Purcell R H, Wong D C, Moritsugu Y, Dienstag J L, Routenberg J A, Boggs J D. A microtiter solid phase radioimmunoassay for hepatitis A antigen and antibody. J Immunol. 1976;116:349–356. [PubMed] [Google Scholar]
- 204.Rahaman S M, Chira P, Koff R S. Idiopathic autoimmune chronic hepatitis triggered by hepatitis A. Am J Gastroenterol. 1994;89:106–108. [PubMed] [Google Scholar]
- 205.Rajan E, Shattock A G, Fielding J F. Cost-effective analysis of hepatitis A prevention in Ireland. Am J Gastroenterol. 2000;95:223–226. doi: 10.1111/j.1572-0241.2000.01689.x. [DOI] [PubMed] [Google Scholar]
- 206.Reed W. Recent researches concerning the etiology, propagation, and prevention of yellow fever by the United States Army Commission. J Hyg. 1902;2:101–119. doi: 10.1017/s0022172400001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Repsher L H, Freebern R K. Effects of early and vigorous exercise on recovery from infectious hepatitis. N Engl J Med. 1969;281:1393–1396. doi: 10.1056/NEJM196912182812504. [DOI] [PubMed] [Google Scholar]
- 208.Ritter K, Uy A, Ritter S, Thomssen R. Hemolysis and autoantibodies to triosephosphate isomerase in a patient with acute hepatitis A virus infection. Scand J Infect Dis. 1994;26:379–382. doi: 10.3109/00365549409008608. [DOI] [PubMed] [Google Scholar]
- 209.Ritter S, Schroder S, Uy A, Ritter K. Haemolysis in hepatitis A virus infections coinciding with the occurrence of autoantibodies against triosephosphate isomerase and the reactivation of latent persistent Epstein-Barr virus infection. J Med Virol. 1996;50:272–275. doi: 10.1002/(SICI)1096-9071(199611)50:3<272::AID-JMV10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 210.Robertson B H, Jia X Y, Tian H, Margolis H S, Summers D F, Ehrenfeld E. Antibody response to nonstructural proteins of hepatitis A virus following infection. J Med Virol. 1993;40:76–82. doi: 10.1002/jmv.1890400115. [DOI] [PubMed] [Google Scholar]
- 211.Rolleston R D. Encyclopedia medica. 1905. Diseases of the liver. [Google Scholar]
- 212.Rosenblum L S, Mirkin I R, Allen D T, Safford S, Hadler S C. A multifocal outbreak of hepatitis A traced to commercially distributed lettuce. Am J Public Health. 1990;80:1075–1079. doi: 10.2105/ajph.80.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rosenblum L S, Villarino M E, Nainan O V, Melish M E, Hadler S C, Pinsky P P, Jarvis W R, Ott C E, Margolis H S. Hepatitis A outbreak in a neonatal intensive care unit: risk factors for transmission and evidence of prolonged viral excretion among preterm infants. J Infect Dis. 1991;164:476–482. doi: 10.1093/infdis/164.3.476. [DOI] [PubMed] [Google Scholar]
- 214.Routenberg J A, Dienstag J L, Harrison W O, Kilpatrick M E, Hooper R R, Chisari F V, Purcell R H, Fornes M F. Foodborne outbreak of hepatitis A: clinical and laboratory features of acute and protracted illness. Am J Med Sci. 1979;278:123–137. [PubMed] [Google Scholar]
- 215.Safadi R, Ben-Hur T, Shouval D. Mononeuritis multiplex: a rare complication of acute hepatitis A. Liver. 1996;16:288–289. doi: 10.1111/j.1600-0676.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 216.Scheutz F, Skinhrj P, Mark I. Viral hepatitis among parenteral drug addicts attending a Danish addiction clinic. Scand J Infect Dis. 1983;15:139–143. doi: 10.3109/inf.1983.15.issue-2.02. [DOI] [PubMed] [Google Scholar]
- 217.Schiodt F V, Atillasoy E, Obaid Shakil A, Schiff E R, Caldwell C, Kowdley K V, Stribling R, Crippen J S, Flamm S, Somberg K A, Rosen H, McCashland T M, Hay J E, Lee W M the Acute Liver Failure Study Group. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 218.Sherertz R J, Russell B A, Reuman P D. Transmission of hepatitis A by transfusion of blood products. Arch Intern Med. 1984;144:1579–1580. [PubMed] [Google Scholar]
- 219.Siddiqui T, Khan A H. Hepatitis A and cytomegalovirus infection precipitating acute hemolysis in glucose-6-phosphate dehydrogenase deficiency. Mil Med. 1998;163:434–435. [PubMed] [Google Scholar]
- 220.Sikuler E, Keynan A, Hanuka N, Zagron-Bachir G, Sarov I. Persistence of a positive test for IgM antibodies to hepatitis A virus in late convalescent sera. Isr J Med Sci. 1987;23:193–195. [PubMed] [Google Scholar]
- 221.Simmons J, Stein L, Kaufman A. Pure red cell aplasia and hepatitis A. South Med J. 1993;86:1274–1276. doi: 10.1097/00007611-199311000-00021. [DOI] [PubMed] [Google Scholar]
- 222.Sjogren M H, Hoke C H, Binn L N, Eckels K H, Dubois D R, Lyde L, Tsuchida A, Oaks S, Jr, Marchwicki R, Lednar W, Chloupek R, Ticehurst J, Bancroft W H. Immunogenicity of an inactivated hepatitis A vaccine. Ann Intern Med. 1991;114:470–471. doi: 10.7326/0003-4819-114-6-470. [DOI] [PubMed] [Google Scholar]
- 223.Sjogren M H, Tanno H, Fay O, Sileoni S, Cohen B D, Burke D S, Feighny R J. Hepatitis A virus in stool during clinical relapse. Ann Intern Med. 1987;106:221–226. doi: 10.7326/0003-4819-106-2-221. [DOI] [PubMed] [Google Scholar]
- 224.Smart C. The medical and surgical history of the war of the rebellion. I. Washington, D.C.: Government Printing Office; 1888. Medical history, part III, being the third medical volume; pp. 874–879. [Google Scholar]
- 225.Smetana H F. The histologic diagnosis of viral heptatitis by needle biopsy. Gastroenterology. 1954;26:612–625. [PubMed] [Google Scholar]
- 226.Smith P F, Grabau J C, Werzberger A, Gunn R A, Rolka H R, Kondracki S F, Gallo R J, Morse D L. The role of young children in a community-wide outbreak of hepatitis A. Epidemiol Infect. 1997;118:243–252. doi: 10.1017/s0950268897007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reference deleted.
- 228.Stapleton J T. Host immune response to hepatitis A virus. J Infect Dis. 1995;171:S9–S14. doi: 10.1093/infdis/171.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 229.Stapleton J T, Lange D K, LeDuc J W, Binn L N, Jansen R W, Lemon S M. The role of secretory immunity in hepatitis A virus infection. J Infect Dis. 1991;163:7–11. doi: 10.1093/infdis/163.1.7. [DOI] [PubMed] [Google Scholar]
- 230.Steffen R, Kane M A, Shapiro C N, Billo N, Schoellhorn K J, van Damme P. Epidemiology and prevention of hepatitis A in travelers. JAMA. 1994;272:885–889. [PubMed] [Google Scholar]
- 231.Stokes J, Jr, Farquhar J A, Drake M E, et al. Infectious hepatitis: length of protection by immune serum globulin (gamma globulin) during epidemics. JAMA. 1951;147:714–719. doi: 10.1001/jama.1951.03670250006003. [DOI] [PubMed] [Google Scholar]
- 232.Stokes J, Jr, Maris E P, Gelliss S S. Chemical, clinical, and immunological studies on the products of human plasma fractionation. XI. Use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prophylaxis and treatment of measles. J Clin Investig. 1944;23:531–540. doi: 10.1172/JCI101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stokes J, Jr, Neefe J R. The prevention and attenuation of infectious hepatitis with gamma globulin (preliminary note) JAMA. 1945;127:144–145. [Google Scholar]
- 234.Storch G A, Bodicky C, Parker M, Blecka L J, Aach R D. Use of conventional and IgM-specific radioimmunoassays for anti-hepatitis A antibody in an outbreak of hepatitis A. Am J Med. 1982;73:663–668. doi: 10.1016/0002-9343(82)90408-9. [DOI] [PubMed] [Google Scholar]
- 235.Stroffolini T, Chiaramonte M, Franco E, Rapicetta M, De Mattia D, Mura I, Trivello R, Giammeco A, Rigo G, Scarpa B. Baseline seroepidemiology of hepatitis A virus infection among children and teenagers in Italy. Infection. 1991;19:97–100. doi: 10.1007/BF01645576. [DOI] [PubMed] [Google Scholar]
- 236.Sundkvist T, Aitken C, Duckworth G, Jeffries D. Outbreak of acute hepatitis A among homosexual men in East London. Scand J Infect Dis. 1997;29:211–212. doi: 10.3109/00365549709019028. [DOI] [PubMed] [Google Scholar]
- 237.Sundkvist T, Johansson B, Widell A. Rectum carried drugs may spread hepatitis A among drug addicts. Scand J Infect Dis. 1985;17:1–4. doi: 10.3109/00365548509070411. [DOI] [PubMed] [Google Scholar]
- 238.Szmuness W, Dienstag J L, Purcell R H, Harley E J, Stevens C E, Wong D C. Distribution of antibody to hepatitis A antigen in urban adult populations. N Engl J Med. 1976;295:755–759. doi: 10.1056/NEJM197609302951404. [DOI] [PubMed] [Google Scholar]
- 239.Tabor E. Guillain-Barré syndrome and other neurologic syndromes in hepatitis A, B, and non-A, non-B. J Med Virol. 1987;21:207–216. doi: 10.1002/jmv.1890210303. [DOI] [PubMed] [Google Scholar]
- 240.Tanno H, Fay O H, Rojman J A, Palazzi J. Biphasic form of hepatitis A virus infection: a frequent variant in Argentina. Liver. 1988;8:53–57. doi: 10.1111/j.1600-0676.1988.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 241.Taylor M, Goldin R D, Ladva S, Scheuer P J, Thomas H C. In situ hybridization studies of hepatitis A viral RNA in patients with acute hepatitis A. J Hepatol. 1994;20:380–387. doi: 10.1016/s0168-8278(94)80012-x. [DOI] [PubMed] [Google Scholar]
- 242.Teixeira M R, Jr, Weller I V, Murray A, Bamber M, Thomas H C, Sherlock S, Scheuer P J. The pathology of hepatitis A in man. Liver. 1982;2:53–60. doi: 10.1111/j.1600-0676.1982.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 243.Thomas W J, Bruno P, Holtzmuller K. Hepatitis A virus anicteric encephalitis coexistent with hepatitis C virus infection. Am J Gastroenterol. 1993;88:279–281. [PubMed] [Google Scholar]
- 244.Tibble J A, Ireland A, Duncan J R. Acute auto immune haemolytic anaemia secondary to hepatitis A infection. Clin Lab Haematol. 1997;19:73–75. doi: 10.1046/j.1365-2257.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 245.Ticehurst J R, Feinstone S M, Chestnut T, Tassopoulos N C, Popper H, Purcell R H. Detection of hepatitis A virus by extraction of viral RNA and molecular hybridization. J Clin Microbiol. 1987;25:1822–1829. doi: 10.1128/jcm.25.10.1822-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tong M J, el-Farra N S, Grew M I. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(Suppl. 1):S15–S18. doi: 10.1093/infdis/171.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 247.Trout D, Mueller C, Venczel L, Krake A. Evaluation of occupational transmission of hepatitis A virus among wastewater workers. J Occup Environ Med. 2000;42:83–87. doi: 10.1097/00043764-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 248.Tsai J F, Margolis H S, Jeng J E, Ho M S, Chang W Y, Hsieh M Y, Lin Z Y, Tsai J H. Increased IgM class circulating immune complexes in acute hepatitis A virus infection. Clin Immunol Immunopathol. 1996;78:291–295. doi: 10.1006/clin.1996.0041. [DOI] [PubMed] [Google Scholar]
- 249.Vallbracht A, Gabriel P, Maier K, Hartmann F, Steinhardt H J, Muller C, Wolf A, Manncke K H, Flehmig B. Cell-mediated cytotoxicity in hepatitis A virus infection. Hepatology. 1986;6:1308–1314. doi: 10.1002/hep.1840060614. [DOI] [PubMed] [Google Scholar]
- 250.Van Damme P, Thoelen S, Cramm M, De Groote K, Safary A, Meheus A. Inactivated hepatitis A vaccine: reactogenicity, immunogenicity, and long-term antibody persistence. J Med Virol. 1994;44:446–451. doi: 10.1002/jmv.1890440425. [DOI] [PubMed] [Google Scholar]
- 251.Van Doorslaer E, Tormans G, Van Damme P. Cost-effectiveness analysis of vaccination against hepatitis A in travellers. J Med Virol. 1994;44:463–469. doi: 10.1002/jmv.1890440429. [DOI] [PubMed] [Google Scholar]
- 252.Vento S, Garofano T, Di Perri G, Dolci L, Concia E, Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337:1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 253.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 254.Villano S A, Nelson K E, Vlahov D, Purcell R H, Saah A J, Thomas D L. Hepatitis A among homosexual men and injection drug users: more evidence for vaccination. Clin Infect Dis. 1997;25:726–728. doi: 10.1086/513757. [DOI] [PubMed] [Google Scholar]
- 255.Voegt H. Zur aetiologie der hepatitis epidemica. Muench Med Wochenschr. 1942;89:76–79. [Google Scholar]
- 256.Wang C H, Tschen S Y, Heinricy U, Weber M, Flehmig B. Immune response to hepatitis A virus capsid proteins after infection. J Clin Microbiol. 1996;34:707–713. doi: 10.1128/jcm.34.3.707-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ward R, Krugman S, Giles J P, Jacobs A M, Bodansky O. Infectious hepatitis: studies of its natural history and prevention of viral hepatitis. N Engl J Med. 1958;258:407–416. doi: 10.1056/NEJM195802272580901. [DOI] [PubMed] [Google Scholar]
- 258.Watson J C, Fleming D W, Borella A J, Olcott E S, Conrad R E, Baron R C. Vertical transmission of hepatitis A resulting in an outbreak in a neonatal intensive care unit. I Infect Dis. 1993;167:567–571. doi: 10.1093/infdis/167.3.567. [DOI] [PubMed] [Google Scholar]
- 259.Weisfuse I B, Graham D J, Will M, Parkinson D, Snydman D R, Atkins M, Karron R A, Feinstone S, Rayner A A, Fisher R I, Mills B J, Dutcher J P, Weiss G R, Glover A, Kuritsky J N, Hadler S C. An outbreak of hepatitis A among cancer patients treated with interleukin-2 and lymphokine-activated killer cells. J Infect Dis. 1990;161:647–652. doi: 10.1093/infdis/161.4.647. [DOI] [PubMed] [Google Scholar]
- 260.Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G, Ryan J, Provost P, Nalin D. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992;327:453–457. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- 261.Widell A, Hansson B G, Moestrup T, Nordenfeldt E. Increased occurrence of hepatitis A with cyclic outbreaks among drug addicts in a Swedish community. Infection. 1983;11:198–200. doi: 10.1007/BF01641196. [DOI] [PubMed] [Google Scholar]
- 262.Willcox W H. The epidemic jaundice of campaigns. Br Med J. 1916;1:297–300. doi: 10.1136/bmj.1.2878.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Willcox W H. Jaundice: with special reference to types occurring during the war. Lecture III, part I: epidemic catarrhal jaundice. Br Med J. 1919;1:671–675. doi: 10.1136/bmj.1.3048.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Willner I R, Uhl M D, Howard S C, Williams E Q, Riely C A, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128:111–114. doi: 10.7326/0003-4819-128-2-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 265.Winokur P L, Stapleton J T. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992;14:580–586. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]
- 266.Witts L J. Some problems of infective hepatitis. Br Med J. 1944;1:739–743. doi: 10.1136/bmj.1.4352.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wróblewski F, LaDue J S. Serum glutamic oxaloacetic transaminase activity as an index of liver cell injury: a preliminary report. Ann Intern Med. 1955;43:345–360. doi: 10.7326/0003-4819-43-2-345. [DOI] [PubMed] [Google Scholar]
- 268.Yamamoto T, Ishii M, Nagura H, Miyazaki Y, Miura M, Igarashi T, Toyota T. Transient hepatic fibrin-ring granulomas in a patient with acute hepatitis A. Liver. 1995;15:276–279. doi: 10.1111/j.1600-0676.1995.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 269.Yotsuyanagi H, Koike K, Yasuda K, Moriya K, Shintani Y, Fujie H, Kurokawa K, Iino S. Prolonged fecal excretion of hepatitis A virus in adult patients with hepatitis A as determined by polymerase chain reaction. Hepatology. 1996;24:10–13. doi: 10.1053/jhep.1996.v24.pm0008707246. [DOI] [PubMed] [Google Scholar]
- 270.Zaaijer H L, Leentvaar-Kuijpers A, Rotman H, Lelie P N. Hepatitis A antibody titres after infection and immunization: implications for passive and active immunization. J Med Virol. 1993;40:22–27. doi: 10.1002/jmv.1890400106. [DOI] [PubMed] [Google Scholar]
- 271.Zikos D, Grewal K S, Craig K, Cheng J C, Peterson D R, Fisher K A. Nephrotic syndrome and acute renal failure associated with hepatitis A virus infection. Am J Gastroenterol. 1995;90:295–298. [PubMed] [Google Scholar]
- 272.Zuckerman A J. Viral hepatitis and the Australia-SH antigen. Nature. 1969;223:569–572. doi: 10.1038/223569a0. [DOI] [PubMed] [Google Scholar]