Abstract
Background:
The goal of root canal therapy depends on chemomechanical debridement and three-dimensional filling of the root canal system.[1]
Aim:
The aim of this study is to assess the effect of NaOCl + Ethylenediaminetetraacetic acid (EDTA) and Twin Kleen as a final irrigating solution on the depth of penetration of AH Plus and Perma Evolution sealers into the dentinal tubules.
Materials and Methods:
Forty mandibular premolars were decoronated and instrumented up to size 30. Moreover, randomly assigned into two groups based on final rinse Group A (n = 20): 5.25% NaOCl + 17% EDTA. Group B (n = 20): Twin Kleen solution. Resin sealers were labeled with few grains of fluorescent rhodamine B dye and subdivided into two subgroups, Subgroup A1and B1 (n1= 10): AH Plus; Subgroup A2 and B2 (n2= 10): Perma Evolution and obturated. Confocal laser scanning microscopy was used to examine the sections taken 2, 5, and 8 mm from the apex. Images were exported to Image J software to determine the sealer penetration depth.
Statistical Analysis:
Independent t-test and one-way analysis of variance followed by Tukey's HSD post hoc test.
Results:
Maximum depth of sealer penetration was seen in Twin Kleen in all sections. Perma Evolution showed highest sealer penetration at the middle and apical third region of root canal for both groups.
Conclusions:
Final irrigation with Twin Kleen produced highest sealer penetration than with EDTA.
Keywords: AH plus, confocal laser scanning microscope, ethylenediaminetetraacetic acid
INTRODUCTION
A successful endodontic treatment outcome requires complete three-dimensional cleaning, shaping, and filling of the root canal system with an impermeable, biocompatible, and dimensionally stable filling material.[1] Sodium hypochlorite is perhaps the most widely used broad-spectrum antimicrobial agent. It has the tissue-dissolving capacity, ability to remove the organic part of the smear layer, and can act as a lubricant for endodontic instrumentation as well. Therefore, sodium hypochlorite pertains to be the primary endodontic irrigating solution worldwide.[2]
Root canal instrumentation produces a layer of organic and inorganic material called the smear layer that may also contain bacteria and their by-products. It can prevent the penetration of intracanal medicaments into dentinal tubules and influence the adaptation of filling materials to canal walls. The inorganic portion of the smear layer is unaffected by sodium hypochlorite (NaOCl) solution. Therefore, chelating agents can be used for its removal.[3]
Crumpton et al. demonstrated effective smear layer removal with a final 1 min rinse of 1 mL of 17% ethylenediaminetetraacetic acid (EDTA) followed by 3 mL of 5.25% NaOCl.[4] The EDTA can be used as a final flush or in combination with NaOCl during the instrumentation of the root canal.[4] However, sodium hypochlorite and EDTA can interact, resulting in the loss of free available chlorine for NaOCl,[5] which decreases the tissue dissolution capacity, antimicrobial activity, and dentin structural integrity. A rinse with saline or distilled water between two endodontic irrigating solutions has been suggested to reduce their interaction.[6]
A newly suggested chelating agent, 1-hydroxyethylidene 1,1-bisphosphonate (HEDP), has been recommended as a chelating agent in the root canal treatment because of its biocompatibility and combined availability with NaOCl.[7] The combination of HEDP and NaOCl can effectively remove the smear layer while having a minimal impact on the root dentin wall; furthermore, HEDP has no effect on the proteolytic and analytic processes of NaOCl.[8]
HEDP, unlike EDTA, is a weak chelating agent and therefore cannot be used as a bare final rinse; instead, it is recommended that HEDP to be combined with NaOCl and used as a full root canal irrigation solution.[9] Recently, Twin Kleen (MaarcDental Innovationsendo, India) which consists of 9% HEDP has to be freshly mixed with 3%NaOCl is used as a one-step final rinse. The manufacturer claims that it is safe to use with NaOCl and has been suggested for the continuous soft chelation of the root canals.
A meticulous root canal obturation can be accomplished not only by eliminating root canal debris and contaminants but also by ensuring that the filling materials are highly adaptable. Sealer is a radiopaque luting agent that is used in conjunction with the solid core material during obturation to fill voids and seal root canals. An ideal sealer should be able to destroy bacteria on the dentinal walls of root canals as well as diffuse within the dentinal tubules. This is only possible if the sealer possesses both antimicrobial and flow properties.[10]
In endodontic therapy, variety of root canal sealing agents have been used. AH Plus (Dentsply DeTrey, Konstanz, Germany) is an epoxy resin-based sealer with long-term sealing properties, good handling characteristics, and good physical properties. Perma Evolution (Becht, Germany) is a recently released epoxy resin-based sealer in the market, which combines epoxide chemistry with innovative microcapsule technology.[11]
However, the literature does not provide adequate data about the new epoxy resin-based sealer “Perma Evolution.” The amount of sealer that penetrates into the dentinal tubules helps to determine which sealers are best for obturating root canals with the minimum gaps and voids.[12]
As a result, the focus of this investigation was to see how 17% EDTA and Twin Kleen, used as a final irrigating solution, affected the penetration depth of AH Plus and Perma Evolution root canal sealers into dentinal tubules at the coronal, middle, and apical levels using Confocal laser Scanning Microscope.
MATERIALS AND METHODS
The research protocol was accepted by the ethical committee (KIMS/IEC/D23/2018).
Preparation of specimens
Forty human single-rooted mandibular premolars with matured apex and those free from cracks, fractures and extracted for periodontal reasons were selected for the study. Teeth with curvatures (0°–10°) were selected by following Schneider's method[13] and single canal was confirmed by examining buccolingual and mesiodistal directions with digital radiograph (RVG6100; Carestream Dental LLC, Atlanta, GA, USA). Teeth with calcified canals, internal resorption, and root canal without apical patency were omitted. Teeth were then cleaned off of soft-tissue debris and calculus and disinfected in 0.5% chloramine T solution and were decoronated at CEJ level. The canal working length measurement was taken by placing a #15 K file into the canal till it reaches the apical foramen. The working length was assessed by reducing 1 mm from this length. Canals were prepared using ProTaper Gold rotary system up to an apical preparation of #F3 (tip size 30 with a taper of 0.09). Canals were irrigated using 5 ml of 5.25% NaOCl irrigating solution over 2 min at every change in file, using a 5 ml syringe and 30 G side-vented needle.
Then samples were randomly assigned into two groups based on the final irrigation sequence.
Group A (n = 20): 3 ml 5.25% sodium hypochlorite followed by 3 ml 17% EDTA
Group B (n = 20): 10 ml Twin Kleen solution.
According to the manufacturer recommendations, 10 ml 0f 3%NaOCl is to be used with two capsules of HEDP (each capsule consists of 0.42 g HEDP). Then each canal was flooded with 5 ml of distilled water after final irrigation and dried with sterile paper points. Two coats of nail varnish were applied to the roots, and the apical foramen was sealed with Type II glass ionomer cement.
OBTURATION OF THE ROOT CANALS
Based on the root canal sealer chosen, each group was further divided into two subgroups.
Subgroup A1 (n1= 10): (NaOCl + EDTA) + AH plus
Subgroup B1 (n1= 10): (NaOCl + EDTA) + Perma evolution
Subgroup A2 (n2= 10): Twin Kleen + AH plus
Subgroup B2 (n2= 10): Twin Kleen + Perma evolution.
All teeth were obturated with a matching tapered gutta-percha cone of the ProTaper gold instruments (ProTaper Gold Gutta-Percha Points F3) and resin sealers. The sealer was labeled with 0.1% fluorescent rhodamine B. A standard volume of 0.05 ml of sealer was delivered into the root canal and size #30 lentulo spiral was used with a slow-speed hand piece at a speed of 300 rpm with an up-and-down motion within the canal six times. With the use of a hot instrument, excess gutta-percha in the access cavity was removed and temporary filling material was placed. All procedures were performed by the same operator.
The specimens were then sealed at the coronal end with intermediate restorative material, and the specimens were stored for 7 days at 37°C and 100% humidity to enable the sealer to fully set. Transverse sections of 0.5–1 mm thickness were cut with slow speed water cooling hard tissue microtome saw at distances of 8, 5, and 2 mm from apex, representing the coronal, middle, and apical regions, respectively.
Penetration depth measurement
The slices were photographed at × 10 magnification using a confocal laser scanning microscope and a dry lens (numeric aperture 0.3). The images were acquired and analyzed using the Leica Application Suite Advanced Fluorescence 3.3 programme (Leica Microsystems). For each specimen, the photographs were assessed using software Image J, and the greatest sealer penetration depth was measured in micrometers buccal, lingual, mesial, and distal areas circumferentially surrounding the root canal [Figure 1].
Figure 1.
Measurement of sealer penetration at (a) buccal, (b) mesial, (c) distal and (d) lingual locations in the same specimen
Assessment of penetration depth
The average sealer penetration was determined from the four measurements and then used to depict sealer penetration. Finally, for visualization, images of the same specimen were stitched together using Image J's stitching plugin [Figure 2].
Figure 2.
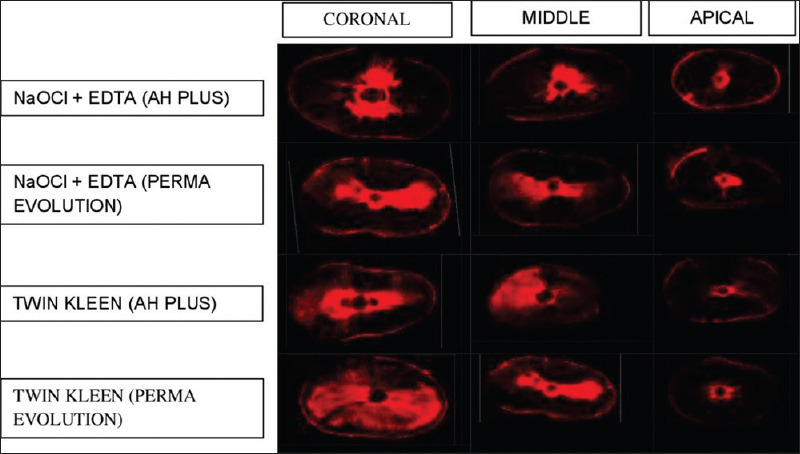
A representative confocal laser scanning microscopy image of a sample from each group at 8 mm, 5 mm, and 2 mm levels as coronal, middle, and apical third regions of root canal, respectively
Statistical analysis
The data were statistically analyzed using the SPSS software 22.0 (SPSS Inc., Chicago, IL, USA), and the significance level was set at P < 0.05. The normality assumption was validated using the Shapiro–Wilk test. The nonparametric independent t-test and one-way ANOVA tests were performed to determine sealer penetration in each category, followed by Tukey's Post hoc analysis. In each group, the same method was used to assess sealer penetration at the 2 mm, 5 mm, and 8 mm levels.
RESULTS
A characteristic confocal laser scanning microscopic images at 8 mm, 5 mm, and 2 mm levels from the apex is shown in Figure 2. Table 1 shows the extent of sealer penetration values (mean standard deviation; median [interquartile range]) at all the three levels. [Graph 1] represents mean sealer penetration depth. Independent t-test analysis indicated a significant difference among Groups 1 and 2 (P < 0.05). Group 2 Twin Kleen irrigation protocol exhibited a higher mean depth than Group 1 NaOCl + EDTA at all three levels. At the coronal third level, there was no statistically significant difference in sealer subgroups between the two groups, whereas, Perma Evolution showed higher tubular penetration depth in the middle and apical third regions of the root canal in both the groups. In comparison to the middle and apical, the coronal thirds of all groups showed the highest penetration of sealer, regardless of the sealer and final irrigant used.
Table 1.
The depth of sealer penetration values (mean ± standard deviation; median [interqu artile range]) among all groups at the 8 mm, 5 mm, and 2 mm sections
| CORONAL mean ±sd | MIDDLE mean ±sd | APICAL mean ±sd | P value | POST HOC TEST | |||
|---|---|---|---|---|---|---|---|
| NAOCL+EDTA | DEPTH OF PENETRATION | AH PLUS | 406.29±3.59 | 183.08±2.19 | 23.31±1.58 | 0.0001* | C vs M=S* |
| C vs A=S* | |||||||
| M vs A=S* | |||||||
| PERMA EV | 411.10±6.68 | 191.84±2.13 | 33.79±6.48 | 0.0001* | C vs M=S* | ||
| C vs A=S* | |||||||
| M vs A=S* | |||||||
| P VALUE | T TEST | 0.06 | 0.0001* | 0.0001* | |||
| TWINKLEEN | DEPTH OF PENETRATION | AH PLUS | 596.96±6.54 | 237.44±3.80 | 55.91±1.48 | 0.0001* | C vs M=S* |
| C vs A=S* | |||||||
| M vs A=S* | |||||||
| PERMA EV | 600.69±5.63 | 248.91±12.89 | 61.89±2.43 | 0.0001* | C vs M=S* | ||
| C vs A=S* | |||||||
| M vs A=S* | |||||||
| P VALUE | T TEST | 0.11 | 0.0001* | 0.0001* |
Graph 1.
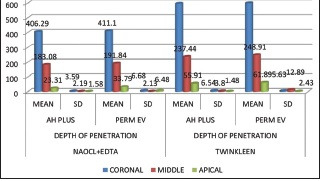
Shows the depth of sealer penetration values (mean ± SD; median [IQR]) among all groups at the 8 mm, 5 mm, and 2 mm sections
DISCUSSION
The findings revealed that at all three levels using a new final rinse Twin Kleen group resulted in greater depth of sealer penetration than using EDTA for both sealer subgroups. This could be due to the improved smear layer removal with more patent dentinal tubules.[14,15] The findings were consistent with previous research conducted by Zehnder et al. 2018, who found that the persistent presence of HEDP inhibits the formation of smear layer along the walls.[16] As a result, sodium hypochlorite can act directly on dentinal tubules and lateral canals in the apical third, resulting in improved smear layer removal. According to Paqué et al., the deposition of hard tissue debris was substantially lower when a mixture of NaOCl and HEDP was used to irrigate the root canals rather than 2.5% NaOCl alone.[8] As a whole, using HEDP as an irrigant enhances the elimination of smear layer thereby retaining the activity of NaOCl.
Tubular sealer penetration is important for optimal root canal therapy. Removal of smear layer and penetration of sealer into dentinal tubules can effectively prevent re-contamination of the root canals by depriving residual germs of nutrition.[17] By modifying the permeability of the tubules, some studies advise keeping the smear layer in place to trap bacteria and bacterial byproducts and prevent leakage.[18]
AH Plus and Perma Evolution showed deeper tubular penetration, due to acceptable physical features such as flow, surface tension, solubility, viscosity, working, and setting time. The flow qualities of the sealers dictate how well they can penetrate the dentinal tubules, lateral canals, and accessory canals inside the root canal.[19] Perma Evolution has antimicrobial efficacy and optimum flow similar to AH Plus root canal sealer as per ADA specification no. 57.[20]
The coronal aspect of the root canal had the greatest depth of tubular sealer penetration in both the irrigation groups. Regardless of the type of sealer utilized, the coverage and tubular sealer penetration depth were substantially greater (P < 0.05) at the 8 mm rather than at the 5-mm and 2-mm levels. It could be because the greater smear layer removal in coronal third region as well the number and diameter of tubules decreases as the root canal descends apically. Primary dentinal tubules, which often exist in irregular directions and densities, with specific parts tubule-free, can be found in the apical section of roots. Due to the presence of cementum-like tissue, tubules might become obstructed. As the irrigant gets closer to the apex, its ability to dissolve the smear layer declines, limiting the flow of sealer into the dentinal tubules. Due to more patent tubules, the buccal and lingual parts of the root canal have deeper sealer penetration than the mesial and distal sections.[21]
AH Plus and Perma Evolution sealers showed similar penetration depth in the coronal third area of the root canal, whereas, in the middle and apical third regions, Perma evolution sealer penetrated into dentinal tubules better than AH plus sealer. It could be because epoxide-based sealer (Perma Evolution) is a permanent root filling substance based on proven epoxide technology with good dentin adherence, owing to trusted epoxide chemistry and the added benefit of microcapsule innovation. The product's microcapsules, which comprise a novel reactive adhesive, improve the durability and tightness of the two component materials, allowing the sealer to more effectively infiltrate into the dentinal tubules. The sealer's hydrophilic composition may also play a role in the sealer's high dentin adherence.
Therefore, Group 2 showed greater penetration depth of both the sealers AH Plus and Perma Evolution when compared to Group 1 at all the three levels. Whereas no difference was seen between both the sealers in coronal third, Perma Evolution showed higher penetration in middle and apical thirds of root canal.
This research's drawback is that, as it is an in vitro study, root cross-sections are less important in the context of simulating microbiological clinical conditions. The effect of fluorescent dye concentration on dentin sealer penetration has not really been studied in the dental literature. In some experiments, the Rhodamine B dye was used at a concentration of 0.1% by weight, whereas in other studies, it is mentioned as the use of traces or “few grains.” As a result, proposed research should concentrate on the use of fluorescent dyes in endodontics to develop a consistent concentration that works best with endodontic sealers. There is currently a scarcity of information on the usage of the HEDP and the latest epoxy resin-based sealer Perma evolution. Future research should concentrate on the benefits of the continuous soft-chelating irrigation (HEDP) protocol.
CONCLUSIONS
Within the confines of this investigation, it can be claimed that Twin Kleen group showed greater and statistically significant sealer penetration depth when compared to NaOCl + EDTA group at all the three levels. There was no substantial difference between the two sealers AH Plus and Perma Evolution in the coronal third of the root canal, although in the middle and apical thirds of root canals, Perma Evolution had a greater penetration depth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chadha R, Taneja S, Kumar M, Gupta S. An in vitro comparative evaluation of depth of tubular penetration of three resin-based root canal sealers. J Conserv Dent. 2012;15:18–21. doi: 10.4103/0972-0707.92600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal A. Antimicrobial irrigants in the endodontic therapy. Int J Health Sci (Qassim) 2012;6:186–92. [PMC free article] [PubMed] [Google Scholar]
- 3.Violich DR, Chandler NP. The smear layer in endodontics – A review. Int Endod J. 2010;43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 4.Crumpton BJ, Goodell GG, McClanahan SB. Effects on smear layer and debris removal with varying volumes of 17% REDTA after rotary instrumentation. J Endod. 2005;31:536–8. doi: 10.1097/01.don.0000148871.72896.1d. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson RM, Podlich HM, Moule AJ. Influence of ethylenediaminetetraacetic acid on the active chlorine content of sodium hypochlorite solutions when mixed in various proportions. J Endod. 2011;37:538–43. doi: 10.1016/j.joen.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Arias-Moliz MT, Ordinola-Zapata R, Baca P, Ruiz-Linares M, Ferrer-Luque CM. Antimicrobial activity of a sodium hypochlorite/etidronic acid irrigant solution. J Endod. 2014;40:1999–2002. doi: 10.1016/j.joen.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Girard S, Paqué F, Badertscher M, Sener B, Zehnder M. Assessment of a gel-type chelating preparation containing 1-hydroxyethylidene-1, 1-bisphosphonate. Int Endod J. 2005;38:810–6. doi: 10.1111/j.1365-2591.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 8.Paqué F, Rechenberg DK, Zehnder M. Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. J Endod. 2012;38:692–5. doi: 10.1016/j.joen.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42:335–43. doi: 10.1111/j.1365-2591.2008.01514.x. [DOI] [PubMed] [Google Scholar]
- 10.Berman LH, Hargreaves KM. Cohen's pathways of the pulp expert consult. Elsevier sci. 2015:290–7. [Google Scholar]
- 11.Moogi P, Sayyad A, Rathore V, Ghosh S, Ambalia S, Amin A, et al. Comparative evaluation of marginal adaptation of two resin-based sealers: A scanning electron microscopic study. Endodontology. 2020;32:137. [Google Scholar]
- 12.Weis MV, Parashos P, Messer HH. Effect of obturation technique on sealer cement thickness and dentinal tubule penetration. Int Endod J. 2004;37:653–63. doi: 10.1111/j.1365-2591.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 13.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32:271–5. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 14.De-Deus G, Zehnder M, Reis C, Fidel S, Fidel RA, Galan J, Jr, et al. Longitudinal co-site optical microscopy study on the chelating ability of etidronate and EDTA using a comparative single-tooth model. J Endod. 2008;34:71–5. doi: 10.1016/j.joen.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Tartari T, de Almeida Rodrigues Silva E Souza P, Vila Nova de Almeida B, Carrera Silva Júnior JO, Facíola Pessoa O, Silva E Souza Junior MH. A new weak chelator in endodontics: Effects of different irrigation regimens with etidronate on root dentin microhardness. Int J Dent. 2013;2013:743018. doi: 10.1155/2013/743018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehnder M, Schmidlin P, Sener B, Waltimo T. Chelation in root canal therapy reconsidered. J Endod. 2005;31:817–20. doi: 10.1097/01.don.0000158233.59316.fe. [DOI] [PubMed] [Google Scholar]
- 17.Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, et al. Critical review on methacrylate resin-based root canal sealers. J Endod. 2010;36:383–99. doi: 10.1016/j.joen.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Assouline LS, Fuss Z, Mazor Y, Weiss EI. Bacterial penetration and proliferation in root canal dentinal tubules after applying dentin adhesives in vitro. J Endod. 2001;27:398–400. doi: 10.1097/00004770-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Kwak SW, Ha JH, Lee W, Kim HC. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg Chem Appl. 2017;2017:2582849. doi: 10.1155/2017/2582849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiwari S, Murthy CS, Usha HL, Shivekshith AK, Kumar NN, Vijayalakshmi L. A comparative evaluation of antimicrobial efficacy and flow characteristics of two epoxy resin-based sealers-AH plus and Perma Evolution: An in vitro study. J Conserv Dent. 2018;21:676–80. doi: 10.4103/JCD.JCD_305_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parirokh M, Eghbal MJ, Asgary S, Ghoddusi J, Stowe S, Forghani F, et al. Effect of 808nm diode laser irradiation on root canal walls after smear layer removal: A scanning electron microscope study. Iran Endod J. 2007;2:37–42. [PMC free article] [PubMed] [Google Scholar]


