Abstract
Obesity-linked diabetes is associated with accumulation of proinflammatory macrophages into adipose tissue leading to inflammasome activation and pyroptotic secretion of interleukin (IL)-1β and IL-18. Targeting fatty acid binding protein 4 (FABP4) uncouples obesity from inflammation, attenuates characteristics of type 2 diabetes and is mechanistically linked to the cellular accumulation of monounsaturated fatty acids in macrophages. Herein we show that pharmacologic inhibition or genetic deletion of FABP4 activates silent mating type information regulation 2 homolog 1 (SIRT1) and deacetylates its downstream targets p53 and signal transducer and activator of transcription 3 (STAT3). Pharmacologic inhibition of fatty acid synthase or stearoyl-coenzyme A desaturase inhibits, whereas exogenous addition of C16:1 or C18:1 but not their saturated acyl chain counterparts, activates SIRT1 and p53/STAT3 signaling and IL-1β/IL-18 release. Expression of the p53 target gene ASC [apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (CARD)] required for assembly of the NLR family pyrin domain containing 3 (NLRP3) inflammasome is downregulated in FABP4 null mice and macrophage cell lines leading to loss of procaspase 1 activation and pyroptosis. Concomitant with loss of ASC expression in FABP4−/− macrophages, inflammasome activation, gasdermin D processing, and functional activation of pyroptosis are all diminished in FABP4 null macrophages but can be rescued by silencing SIRT1 or exogenous expression of ASC. Taken together, these results reveal a novel lipid-regulated pathway linking to SIRT1-p53-ASC signaling and activation of inflammasome action and pyroptosis.
Keywords: fatty acid binding protein 4, SIRT1, ASC, p53, inflammasome, pyroptosis
Obesity-linked metabolic disorders, such as diabetes mellitus, atherosclerosis, and insulin resistance are often associated with chronic systematic inflammation and accumulation of proinflammatory macrophages in visceral adipose depots (1-3). Adipose-resident macrophages produce proinflammatory cytokines [eg, tumor necrosis factor α, interleukin (IL)-1β, IL-18, monocyte chemoattractant protein-1] that contribute to recruitment of other immune cells (B cells, dendritic cells) and the development of whole-body metabolic dysfunction (4-6). Fatty acid binding protein 4 (FABP4), also known as adipocyte FABP or aP2, is mainly expressed in adipocytes and macrophages and has been shown to play an important role in regulating lipid metabolism and insulin resistance (7-9). Studies have shown that whole body FABP4 null mice are protected from hyperinsulinemia and insulin resistance associated with dietary or genetic obesity (10), and FABP4 null macrophages exhibit increased level of intracellular monounsaturated fatty acids (MUFA) such as oleic acid (18:1n9), palmitoleic acid (cis 16:1n7), and palmitelaidic acid (trans 16:1n7) (11). Loss of FABP4 and increased intracellular MUFA leads to upregulated expression of mitochondrial UCP2, reduced reactive oxygen species production, reduced endoplasmic reticulum (ER) stress, and attenuated nuclear factor kappa B (NF-κB) activation and cytokine release (11, 12).
SIRT1 belongs to the sirtuin family of nicotinamide adenosine dinucleotide–dependent deacetylase/deacylases and is a critical modulator of physiologic processes such as cancer, apoptosis, senescence, and inflammation (13-15). SIRT1 deacetylation targets such as p53, forkhead box O1, forkhead box O3, hypoxia-inducible factor-1α, proliferator activated receptor γ coactivator 1α (PGC-1α), and signal transducer and activator of transcription 3 (STAT3) play a variety of roles (16-20), and Najt et al recently reported that in hepatocytes, MUFAs can allosterically activate SIRT1 leading to the deacetylation of downstream targets such as forkhead box O3 and PGC-1α (21). Moreover, perilipin 5 (PLIN5), a member of the perilipin family, likely serves as a conduit for lipid signaling by mediating MUFA transfer from the cytoplasm to the nucleus where it donates a bound lipid to SIRT1 thereby promoting PGC-1α/peroxisome proliferator activated receptor α activation (21, 22).
Apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC; also named PYCARD) is an adaptor protein that contains a N-terminal PYRIN-PAAD-DAPIN domain (PYD) and a C-terminal caspase-recruitment domain (CARD) and plays a key role in mediating inflammation (23, 24). ASC interacts with NLR family pyrin domain containing 3 (NLRP3) through PYD-PYD domain assembly and then recruits procaspase-1 through CARD-CARD interaction to cleave procaspase1 into its mature activated form (24, 25). Activated caspase-1 mediates the proteolytic maturation of several key proteins including pro-IL-1β, pro-IL-18, and gasdermin D (GSDMD) (26, 27). Recent work showed that cleaved GSDMD N-terminal domain undergoes polymerization and lipidation with phosphatidyl ethanolamine that inserts into the plasma membrane to form an annulus producing an active pyroptotic assembly (28). The processed forms of IL-1β and IL-18 are secreted via GSDMD-mediated pyroptosis producing the proinflammatory state of classically activated macrophages (29).
Previous studies showed that the lack of FABP4 in macrophages suppressed inflammatory signaling by attenuating the NF-κB pathway and diminishing NLRP3 activation (12, 30). However, the molecular mechanism(s) by which FABP4 uncouples obesity from inflammation is complex but involves monounsaturated fatty acid mediated signaling. Herein, we provide a mechanism whereby lipid regulated SIRT1 controls p53 acetylation leading to modulation of the NLRP3 inflammasome and pyroptosis. Moreover, we identified that ASC not only plays a vital role in inflammasome activation but is also a key target of the lipid-regulated SIRT1-p53 axis.
Materials and Methods
Reagents and Chemicals
The fatty acid synthase (FAS) inhibitors cerulenin (17397-89-6) and C75 (191282-48-1) and the stearoyl coenzyme A desaturase (SCD) inhibitor CAY10566 (944808-88-2), were purchased from Santa Cruz biotechnology. EX527 (E7034), lipopolysaccharide (LPS; 297-473-0), adenosine 5′-triphosphate (ATP) disodium salt hydrate (34369-07-8), fatty acid free bovine serum albumin (A6003), and the FABP inhibitor HTS01037 (SML2374) were purchased from Sigma-Aldrich. C16:1, C16:0, C18:0 and C18:1 fatty acid were purchased from Nu-Check. All other reagents were highest grade commercially available.
Cell Culture and Cell Lines
Wild-type (WT) and FABP4 null macrophages were immortalized from bone marrow-derived macrophages of WT and FABP4 null C57BL/6J mice as previously reported (31). WT and FABP4 null macrophages were maintained in RPMI 1640 medium (11875119, Invitrogen) with 10% fetal bovine serum (FBS). THP-1 cells (TIB-202, ATCC) were maintained in Dulbecco’s Modified Eagle Medium (11965092, Invitrogen) with 10% FBS. Primary bone marrow-derived macrophages (described as BMDM in this article) were isolated directly from C57BL/6J mice, plated, and maintained in RPMI plus 10% FBS, and 10 ng/mL macrophage colony-stimulating factor for 7 days prior to treatment (32).
Animals
Mice were fed either a standard chow diet or a diet containing saturated-fat (lard; F3282; BioServe, Flemington) for 12 weeks after weaning to achieve obesity (33). Male C57BL/6J WT and FABP4 null mice were sacrificed between 15 and 16 weeks age as described previously (11, 12). All experimental procedures using animals were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Immunoblotting
Cells were washed twice in cold phosphate-buffered saline (PBS; 11666789001, Millipore Sigma) and lysed in radioimmunoprecipitation assay buffer (R0278, Millipore Sigma) supplemented with protease inhibitors (539134, Millipore), phosphatase inhibitors (P0044, P5726, Sigma), and deacetylase inhibitors (HY-K0030, MedChem Express). Equal amounts of protein were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (926-31090, Li-Cor Biosciences). After blocking in blocking buffer (926-32099, Li-Cor Biosciences) for 1 hour at room temperature, the membrane was incubated in primary antibody overnight, washed and incubated with secondary antibody, and imaged using an Odyssey infrared imaging system (Li-Cor Biosciences). The antibodies used were anti-SIRT1(Cell Signaling Technology Cat# 9475, RRID:AB_2617130; http://antibodyregistry.org/AB_2617130), anti-acetyl-p53 (Millipore Cat# 06-758, RRID:AB_310240; http://antibodyregistry.org/AB_310240), anti-p53 (Cell Signaling Technology Cat# 2524, RRID:AB_331743; http://antibodyregistry.org/AB_331743), anti-acetyl-STAT3 (Cell Signaling Technology Cat# 2523, RRID:AB_561524; http://antibodyregistry.org/AB_561524), anti-STAT3 (CellCell Signaling Technology Cat# 9132, RRID:AB_331588; http://antibodyregistry.org/AB_331588), anti-actin (Sigma-Aldrich Cat# A5441, RRID:AB_476744; http://antibodyregistry.org/AB_476744), anti-ASC (AdipoGen Cat# AG-25B-0006PF, RRID:AB_2490441; http://antibodyregistry.org/AB_2490441), anti-GSDMD (Abcam Cat# ab219800, RRID:AB_2888940; http://antibodyregistry.org/AB_2888940), anti-caspase1(Abcam Cat# ab179515, RRID:AB_2884954; http://antibodyregistry.org/AB_2884954), anti-NLRP3 (AdipoGen Cat# AG-20B-0014, RRID:AB_2490202; http://antibodyregistry.org/AB_2490202), and anti-PLIN2 (Proteintech Cat# 15294-1-AP, RRID:AB_2878122; http://antibodyregistry.org/AB_2878122).
Quantitative Reverse Transcription-Polymerase Chain Reaction
Cells were washed twice using cold PBS and total RNA isolated using TRIzol reagent (15596018, Invitrogen). For complimentary DNA synthesis, 1 μg total RNA was used for reverse transcription using iScript (1708840, Bio-Rad) following the manufacturer’s instructions. SYBR Green supermix (1725271, Bio-Rad) were used to detect amplified target sequences using a Bio-Rad CFX 96 real-time system. Transcription factor II E (TFIIE) was used as an internal control to normalize expression. The following primers were used: TFIIE forward 5’- CAAGGCTTTAGGGGACCAGATAC-3’, TFIIE reverse 5’-CATCCATTGACTCCACAGTGACAC-3’, ASC forward 5’- CCAGTGTCCCTGCTCAGAGT-3’, ASC reverse 5’-TCATCTTGTCTTGGCTGGTG-3’, PLIN2: forward: GTCAGCTCTCCTGTTAGGCGT, and PLIN2 reverse: AGGTTGGCCACTCTCATCAC.
Intracellular Fatty Acid Analysis and Lipidomics
Fatty acids extraction and analysis was performed as previously reported (11). Briefly, cells from 10-cm plates were washed twice using cold PBS and harvested in 100 mM sodium acetate (pH 3.9). Total lipids were extracted in hexane-isopropanol-H2O (3:2:2) and centrifuged at 3000 rpm for 10 min. The organic layer was dried under nitrogen and resuspend in 1 mL of chloroform. Samples were loaded onto a pre-equilibrated HF Bond Elut NH2 column (Agilent Technology) to remove neutral lipids and the fatty acid fraction eluted from the column followed by quantitation using the nonessential fatty acid kit (434-91795,Wako). For fatty acid compositional determination, analysis was carried out at the Metabolomics Resources Core of the Mayo Clinic (Rochester, MN, USA). Fatty acids were identified and quantitated using a triple quadrupole mass spectrometer coupled with an ultrapressure liquid chromatography system as previously described (34). Data acquisition was performed under negative electrospray ionization condition in selective reaction monitoring mode.
Short Hairpin RNA Gene Silencing
WT and FABP4 knockout macrophages were transduced with a short hairpin RNA lentivirus targeting either SIRT1 or PLIN2 using recombinant lentivirus packaged from HEK293FT cells using the RNA interference vector pLKO1 as described previously (35). Briefly, recombinant lentiviruses were packaged in HEK-293T cells and harvested 48 hours after transfection. 3T3-L1 preadipocytes were transduced with lentiviruses for 16 hours in the presence of 6 μg/mL polybrene. Cells were allowed to recover for 24 hours after transduction before the addition of selection media containing 2 μg/mL puromycin. Green fluorescent protein (GFP), SIRT1 and PLIN2 targeting plasmids were obtained from Open Biosystems. The following target sequences were used: PLIN2 (GenBank accession number NM_007408) targeting sequence, 5′-CCGGCCTCCGCTTATGTCAGTACAACTCGAGTTGTACTGACATAAGCGGAGGTTTTTG -3′ and SIRT1 full hairpin sequence 5’-CCGGCGCGGATAGGTCCATATACTTCTCGAGAAGTATATGGACCTATCCGCGTTTTTG-3’.
Transient Transfection
WT and FABP4−/− macrophages were transfected with plasmid cloning DNA (pcDNA)-ASC1 plasmids (+ASC) or pcDNA-GFP plasmids (Control) using Lipofectamine™ 3000 (L3000015, Invitrogen) according to the manufacturer’s instructions. Cells were transfected with 5 μg plasmid per 1 × 105 cells for 24 hours and allowed to recover for 24 hours before analysis. Plasmids were obtained from Addgene.
RNAseq Analysis
Total RNA was purified by using TRIzol as described, resuspended in RNase-free water, and repurified using the RNeasy Mini Kit (74104, Qiagen). RNA library preparation and sequencing was carried out by the University of Minnesota Genomic Center. Briefly, total RNA samples were converted to Illumina sequencing libraries using Illumina’s (San Diego, CA, USA) TruSeq RNA Sample Preparation Kit (catalog no. RS-122-2001 or RS-122-2002) or single-stranded messenger RNA (mRNA) Sample Preparation Kit (catalog no. RS-122-2101). The libraries were then loaded onto the HiSeq2500 paired end flow cell and loaded on the instrument. Base call (.bcl) files for each cycle of sequencing were generated by Illumina Real-Time Analysis software and were saved at the Minnesota Supercomputing Institute. RNAseq analysis was performed by University of Minnesota Genomic Center as well. Briefly, quality of data in fastq files is assessed using FastQC. Low-quality bases and adapter sequences are removed using Trimmomatic Reads are aligned using Hisat2. Fragments per kilobase of transcript per million mapped reads expression values are generated using Cuffquant and Cufnorm from the Cufflinks package, and raw read counts are generated using featureCounts from the Subread R package. Differential gene expression testing was performed via edgeR. Absolute fold change > 2 and false discovery rate < 0.05 were considered significantly changes between samples. Ingenuity Pathway Analysis (IPA) software was used to identify canonical pathways.
Isolation of Stromal Vascular Cells
Stromal vascular fraction cells were purified as previously described (11). Adipose tissues were dissected from WT and FABP4 null C57BL/6J mice. Tissues were minced and digested with collagenase in Krebs-Ringers- N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid buffer at 37°C for 1 hour. The mixture was then filtered with 100-μm cell strainer, and the stromal vascular fraction (SVF) was collected by centrifugation at 500× g for 10 minutes. Then the SVF was washed 5 times by Krebs-Ringers-N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid buffer and used for further experiments (36).
Enzyme-linked Immunosorbent Assay Analysis
Cell culture media obtained from cells was analyzed via enzyme-linked immunosorbent assay (ELISA) using the IL-1β/IL-1F2 Quantikine ELISA Kit (R and D Systems, Cat# DY7625-05, RRID:AB_2895548; http://antibodyregistry.org/AB_2895548) or the IL-18/IL-1F4 ELISA kit (R and D Systems, Cat# MLB00C, RRID:AB_2895547; http://antibodyregistry.org/AB_2895547) according to the manufacturer’s instructions. Optical imaging was carried out using a SpectraMax M2 plate reader (Molecular Devices) calibrated against a standard curve obtained with dilutions of purified IL-1β or IL-18.
Chromatin Immunoprecipitation
Cells were washed in cold PBS and chromatin isolated using the ChIP-IT High Sensitivity Kit (53040, Active Motif). Briefly, protein-DNA complexes were cross-linked with formaldehyde and anti-p53 or control antibody used to immunoprecipitate sheared DNA fragments. The resultant fragments were digested with proteinase K and target sequences amplified as described (37).
Immunofluorescence Assay
THP-1 cells were resuspended at 2 × 106 cells/mL in RPMI followed by addition of LPS to a final concentration of 1 μg/mL for 4 hours. As indicated, HTS01037 was added at a final concentration of 30 μM at various times prior to stimulation with 20 μM nigericin for 90 minutes. Formaldehyde was added to the cells at a final concentration of 2%, and samples were incubated for 20 minutes at room temperature. Cells were pelleted by centrifugation at 500 × g for 5 minutes followed by 2 washes with PBS and spread onto polylysine-coated slides and allowed to dry. Slides were submerged in PBS + 0.1% Triton-X 100 for permeabilization and blocked in 2% bovine serum albumin + 300 mM glycine followed by staining with mouse anti-ASC (1:50, B3, Santa Cruz Biotechnology) followed by Alexa 488-conjugated goat anti-mouse secondary antibody (1:500, Life Technologies). DNA was labeled by Hoechst 33342 (1 μg/mL, Immunochemistry), and samples were mounted in 90% glycerol in 20 mM Tris pH 8.5. Fields were imaged on an Olympus FluoView FV1000 confocal microscope using a 60× Plan Apo water immersion objective (NA = 1.2). ImageJ [National Institutes of Health (NIH)] was used for image processing and Cell Profiler (NIH) was used for unbiased scoring of cells with ASC specks.
p53 Activity Assay
Equal amounts of protein were loaded to the p53 Transcription Factor Assay Kit (600020, Cayman) following the manufacturer’s instructions. Plates were scanned for absorbance at 450 nm, and results were normalized to controls for each individual experiment.
Flow Cytometry Analysis
Cells were counted and fixed in 1% paraformaldehyde (47608, Sigma) for 15 minutes at room temperature, washed with PBS, and then incubated with 0.5% bovine serum albumin + 0.1%Triton in PBS for 20 min. Cells were incubated with the indicated antibodies at 4°C in the dark for 30 minutes, washed, and resuspended in 0.5 mL PBS for follow cytometry. Flow cytometry was performed on a BD LSRII H1160 analyzer and quantitated using FlowJo software. The antibody used were APC Rat Anti-CD11b (553312), BV421 Rat Anti-mouse F4/80 (565411) purchased from BD Biosciences. Goat anti-rabbit immunoglobulin G Alexa Fluor 555 (ab15007) was purchased from Abcam. Gating strategy was following macrophages population were gated by CD11b + F4/80+ and then mean fluorescence intensity of Acetyl-p53 channel (Alexa Fluor 555) was quantified in CD11b + F4/80 + populations and showed in the figures.
Statistical Analysis
All data are expressed as means ± SE of the mean. Statistical significance was determined using an unpaired, 2-tailed Student t test. Individual experiments were repeated a minimum of 3 times.
Results
Pharmacologic Inhibition or Genetic Deletion of FABP4 Activates SIRT1-p53/STAT3 Signaling in Macrophages
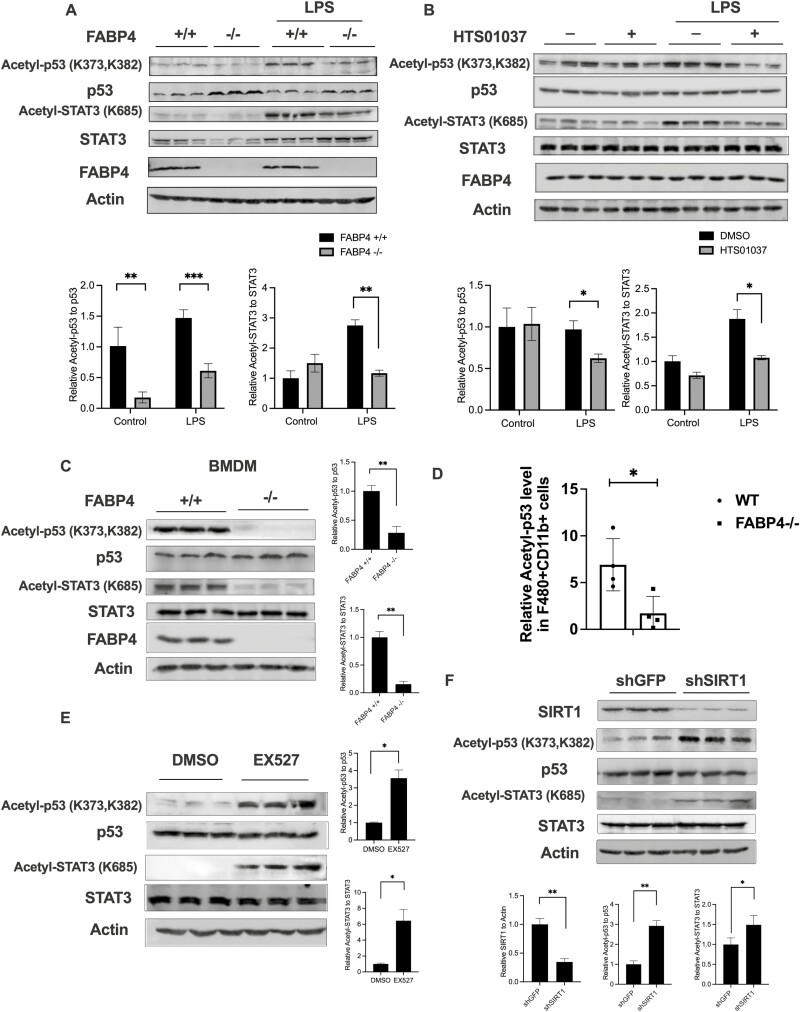
Since Najt et al reported that monounsaturated fatty acids are allosteric activators of SIRT1 (21) and Xu et al reported that C16:1 and C18:1 levels are selectively elevated in FABP4 null macrophages (11), we hypothesized that absence of FABP4 in macrophages would increase SIRT1 activity and its downstream target signaling. To test this, we evaluated the acetylation level of known SIRT1 targets such as p53 and STAT3 (17, 19, 20) in response to an LPS challenge in immortalized macrophage cell lines derived from WT or FABP4 C57Bl/6J null mice (31). The acetylation level of both p53 (K373, K382) and STAT3 (K685) were decreased under stimulated conditions in response to genetic deletion of FABP4 (Fig. 1A). To pharmacologically mimic FABP4 deficiency in macrophages, the FABP4 chemical inhibitor HTS01037 (38) was used. Similarly, both the acetylation level of p53 and STAT3 were decreased in response to inhibition of FABP4 by HTS01037 in Wt macrophages (Fig. 1B) when challenged with LPS. Further, in bone marrow–derived macrophages purified from WT and FABP4 null mice, the acetylation level of both p53 and STAT3 were similarly decreased in response to FABP4 deletion (Fig. 1C). The same pattern was also revealed using human THP-1 cells where the acetylation level of p53 and STAT3 are decreased in response to FABP4 inhibitor HTS01037 treatment (39). To further confirm FABP4-SIRT1 connection in mice, stromal vascular fraction cells (SVF) were purified from 16-week high-fat diet WT and FABP4 null mice and CD11b+ F4/8+ macrophage populations (40) were gated by flow cytometry. Acetylated p53 level in CD11b+ F4/80+ SVF cells was also significantly decreased in FABP4 null mice compared to WT animals (Fig. 1D). To further test whether this change of acetylation/deacetylation level of p53 and STAT3 is due to SIRT1, a SIRT1 inhibitor EX527 (41) was used to attenuate SIRT1 activity in macrophages. The acetylation level of both p53 and STAT3 were markedly increased in response to pharmacologic inhibition of SIRT1 by EX527 (Fig. 1E). Moreover, when Sirt1 (but not Sirt3) were silenced in FABP4 null macrophages, the acetylation level of p53 and STAT3 were also upregulated (Fig. 1F) (39), suggesting that the acetylation level of p53 and STAT3 in macrophages is regulated by SIRT1. Taken together, pharmacologic inhibition or genetic deletion of FABP4 modulates the acetylation level of p53 and STAT3 in a SIRT1-dependent manner in macrophages.
Figure 1.
Pharmacologic inhibition or genetic deletion of fatty acid binding protein 4 (FABP4) activates silent mating type information regulation 2 homolog 1 (SIRT1)-p53/signal transducer and activator of transcription 3 (STAT3) signaling in macrophages. (A) Wild-type (WT) and FABP4−/− macrophages were stimulated with lipopolysaccharide (LPS) (100 ng/mL) for 24 hours. Acetyl-p53, total p53, acetyl-STAT3, total STAT3, and FABP4 protein levels were measured by immunoblotting. (B) WT macrophages were treated with 30 μM HTS01037 and 100 ng/ml LPS for 24 hours and the indicated antigen measured by immunoblotting. (C) Bone marrow–derived macrophages were isolated and cultured from WT and FABP4−/− mice and the acetyl-p53, total p53, acetyl-STAT3, total STAT3, and FABP4 protein levels were measured by immunoblotting. (D) Stromal vascular fraction cells were purified from WT and FABP4 null high-fat diet mice visceral adipose tissue. The relative acetyl-p53 level in CD11b+ F4/80+ stromal vascular fraction cells was measured by flow cytometry. (E) FABP4−/− macrophages were treated with either dimethylsulfoxide or 50 μM EX527 for 4 hours and acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein levels were measured by immunoblotting. (G) SIRT1 knockdown (shSIRT1) and control GFP (shGFP) made in FABP4−/− macrophages were evaluated for SIRT1, acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein levels by immunoblotting. Quantification results represent the mean ± SE of the mean. *P < 0.05, **P < 0.01.
De Novo Lipogenesis–derived MUFA Regulate p53 and STAT3 in Macrophages
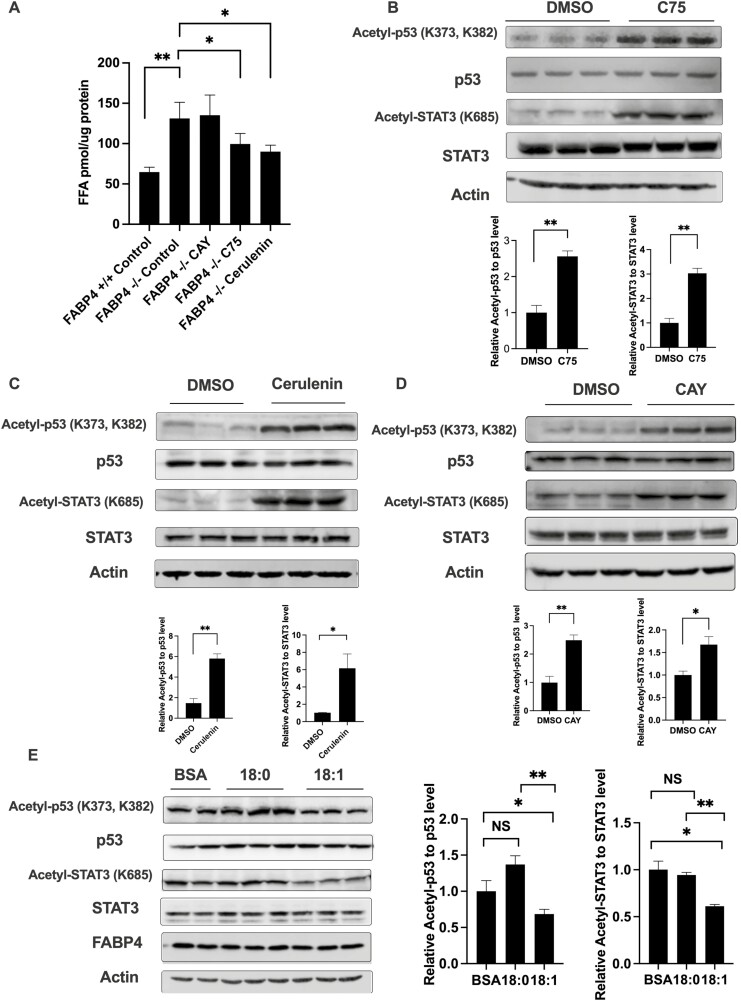
Since SIRT1 activity is increased in response to absence of FABP4 and monounsaturated fatty acids are allosteric activators of SIRT1, we further want to explore whether this increased SIRT1 activity in FABP4 null macrophages is due to increased intracellular monounsaturated fatty acid level. Previous work by Xu et al demonstrated that genetic or pharmacologic loss of FABP4 leads to elevated levels of C16:1 and C18:1 fatty acid (11). This increased MUFA is associated with an upregulation of SCD1, a key enzyme that converts saturated fatty acids to unsaturated form by inserting the double bonds into acyl chains (42). Since de novo lipogenesis is 1 of the major monounsaturated fatty acids sources in macrophages, FAS inhibitors C75 and cerulenin were used to block both saturated and unsaturated fatty acids generation while SCD1 inhibitor CAY10566 was used to specifically block MUFA generation process without changing saturated fatty acids level. To confirm the efficiency of the inhibitors, total intracellular fatty acids levels were measured, and both C75 and cerulenin decreased the total pool of fatty acids in macrophages while SCD1 inhibitor CAY10566 did not (Fig. 2A). Lipidomic analysis showed that both C75 and cerulenin treatment reduced the absolute levels of both saturated (such as palmitic acids) and unsaturated fatty acids (such as palmitoleic acids and oleic acids), while SCD1 inhibitor CAY10566 only reduced MUFA levels (such as palmitoleic acids and oleic acids) (39). Concomitant with reduced levels of C16:1 and C18:1, the acetylation of p53 and STAT3 were both increased with FAS inhibition (Fig. 2B and 2C), which suggested that inhibition of de novo lipogenesis deactivated SIRT1 activity. Further, SCD1 inhibitor also increased levels of p53 and STAT3 acetylation in macrophages (Fig. 2D) concomitant with reduced monounsaturated fatty acid levels although total fatty acid abundance was not statistically changed (Fig. 2A) (39). To contrast the effect of reduced MUFA pools on p53 and STAT3 acetylation, we exogenously supplemented WT macrophages with monounsaturated oleic acid or saturated stearic acid and profiled acetylation of SIRT1 target proteins p53 and STAT3. As shown in Figure 2E, addition of C18:1 attenuated p53 and STAT3 acetylation while saturated C18:0 had no effect. These results suggest that modulation of cellular MUFA pools, either via disruption of FABP4, inhibition of de novo lipogenesis or fatty acid supplementation, modulates SIRT1 activity and the acetylation levels of p53 and STAT3.
Figure 2.
De novo lipogenesis–derived monounsaturated fatty acid activates silent mating type information regulation 2 homolog 1 (SIRT1)-p53/signal transducer and activator of transcription 3 (STAT3) signaling in macrophages. (A) Wild-type (WT) and fatty acid binding protein 4 (FABP4−/−) macrophages were treated with the stearoyl-coenzyme A desaturase 1 (SCD1) inhibitor CAY10566 (30 nM), fatty acid synthesis inhibitor C75 (50 μM), or Cerulenin (25 μM) for 24 hours and intracellular free fatty acids were measured. (B) FABP4−/− macrophages were treated with the fatty acid synthesis inhibitor C75 (50 μM) for 24 hours, and the levels of acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein were measured by immunoblotting. (C) FABP4−/− macrophages were treated with the fatty acid synthesis inhibitor Cerulenin (25 μM) for 24 hours and acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein levels were measured by immunoblotting. (D) FABP4−/− macrophages were treated with the SCD1 inhibitor CAY10566 (30 nM) for 24 hours and acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein levels were measured by immunoblotting. (E) WT macrophages were treated with 50 μM fatty acid free bovine serum albumin only or plus 200 μM C18:0 and C18:1 fatty acids for 48 hr and acetyl-p53, total p53, acetyl-STAT3, and total STAT3 protein levels were measured by immunoblotting. Quantification results represent the mean ± SE of the mean. *P < 0.05, **P < 0.01.
Recent work from Mashek et al using cultured hepatocytes has demonstrated that PLIN5 mediates the delivery of cytoplasmic monounsaturated fatty acids from the cytoplasm to the nucleus SIRT1 (21, 22). PLIN5 belongs to the perilipin family has been positively correlated with lipolysis and lipid metabolism (43, 44). Since PLIN2 is the major perilipin in macrophages (39, 45), we wanted to explore whether PLIN2 plays a role in p53 or STAT3 acetylation. To test this, PLIN2 was silenced in WT macrophages, and SIRT1 activity was evaluated by assessing the acetylation status of p53 and STAT3. Silencing PLIN2 in WT macrophages led to a modest but not significant increase in acetylation of p53 and STAT3 compared to GFP-silenced control cells (39). In contrast, silencing of Plin2 in FABP4 null macrophages, where SIRT1 activity is high, robustly increased acetylation levels of both p53 and STAT3 (39), suggesting that PLIN2 may play a role in mediating the FABP4-MUFA-SIRT1 activation process. Lastly, whereas monounsaturated fatty acids reduced p53 DNA binding activity due to deacetylation, loss of PLIN2 negates such regulation and renders monounsaturated C18:1 unable to affect DNA binding activity (39). These data suggested that de novo lipogenesis-derived MUFA activates SIRT1 in a PLIN2-dependent manner leading to decreased p53 and STAT3 acetylation.
Loss of FABP4 Reduced p53 Activity and Expression of Its Downstream Target ASC
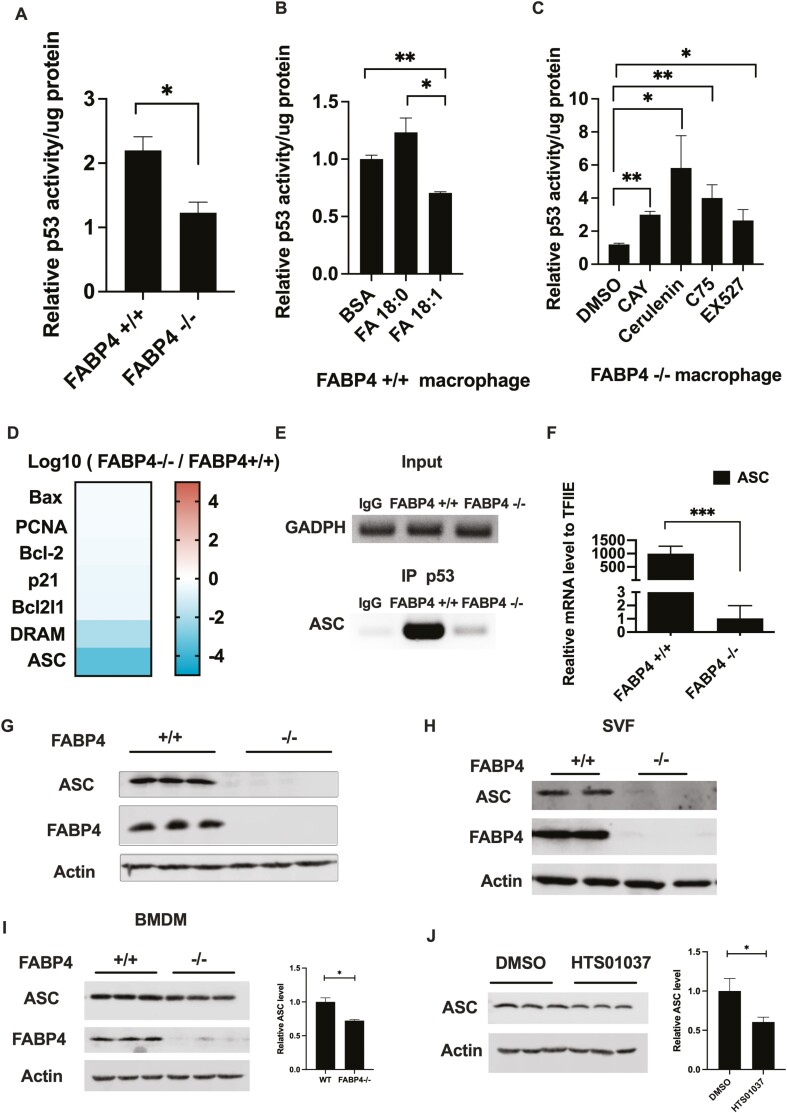
p53 regulates the expression of genes affecting proliferation, apoptosis, autophagy, and metabolism (46, 47) and is controlled by posttranslational modifications such as ubiquitylation, phosphorylation, and acetylation (48-50). Previous studies have shown that acetylation of p53 affects its DNA binding ability, protein-protein interaction, and protein stability (51-53). To parallel the acetylation status of p53 with its transcriptional activity, we profiled the DNA binding activity of p53 in response to lipid modulation. Consistent with reduced acetylated p53 in response to loss of FABP4, p53 activity was also reduced in FABP4 null macrophage cells compared to WT macrophages (Fig. 3A). As shown in Figure 3B, addition of stearate resulted in a trend toward increased p53 DNA binding activity whereas monounsaturated C18:1 reduced p53 activity. Consistent with increased p53 acetylation level, p53 DNA binding activity was increased in response to inhibition of de novo lipogenesis by C75 and cerulenin (Fig. 3C). Since both acetylation and DNA binding ability of p53 were regulated in response to lipid modulation, we further evaluated whether the downstream targets and signaling pathway of p53 was coordinately affected.
Figure 3.
Loss of fatty acid binding protein 4 (FABP4) reduced p53 activity and expression of its downstream target apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC). (A) Wild-type (WT) and FABP4−/− macrophages were treated with 100 ng/mL lipopolysaccharide for 24 hours, and p53 activity was measured. (B) WT macrophages were treated with 50 μM fatty acid free bovine serum albumin only or plus 200 μM C18:0 and C18:1 fatty acid for 48 hours, and the p53 activity was measured. (C) FABP4−/− macrophages were treated with either the stearoyl-coenzyme A desaturase 1 inhibitor CAY10566 (30 nM), the fatty acid synthesis inhibitor C75 (50 μM), Cerulenin (25 μM) for 24 hours, or 50 μM EX527 for 4 hours and p53 activity were measured. (D) Messenger RNA (mRNA) level of p53 downstream targets in FABP4−/− macrophages compared to WT from RNAseq database. (E) Chromatin immunoprecipitation assay showed that p53 DNA-binding ability on ASC promoter in WT and FABP4−/− macrophage. (F) ASC mRNA level were measured in WT and FABP4−/− macrophages by reverse transcription-polymerase chain reaction. (G) ASC protein expression in WT and FABP4−/− macrophages were measured by immunoblotting. (H) The stromal vascular fraction cells were purified from WT and FABP4−/− mice, and ASC and FABP4 protein expression were measured by immunoblotting. (I) Bone marrow derived macrophage were purified and cultured from WT and FABP4−/− high-fat diet mice, and ASC and FABP4 protein expression measured by immunoblotting. (J) WT macrophages were treated with 30 μM HTS01037 for 48 hours, and ASC protein expression was measured by immunoblotting. All p53 activity assays were normalized to intracellular lysates protein level for each condition. All results represent the mean ± SE of the mean. *P < 0.05, **P < 0.01.
A RNAseq analysis of WT and FABP4 null macrophages revealed a variety of p53 regulated genes and pathways were downregulated including changes in inflammatory response, cell signaling, and cell death survival pathways. Several p53 regulated genes were identified (Fig. 3D) by IPA and of those genes most robustly downregulated, apoptosis-associated, speck-like protein containing a CARD domain (ASC, also known as PyCard) (37, 54) was notable for its mechanistic connectivity to NLRP3 inflammasome assembly and pyroptosis (23, 28, 55, 56). Consistent with reduced ASC expression, IPA analysis revealed that inflammatory response pathways are significantly down in FABP4 null cells compared to WT macrophages (39). Since previous publications identified ASC as a p53-responsive gene (37, 54), we carried out chromatin immunoprecipitation of genomic DNA from both WT and FABP4 null macrophages. As shown in Figure 3E, p53 was preferentially bound to the ASC promoter in WT but not in FABP4 null macrophages, this might be due to the reduced p53 DNA binding ability in response to loss of FABP4. Consistent with the chromatin immunoprecipitation results, real-time polymerase chain reaction results further confirmed that ASC mRNA level is decreased over 1000-fold in FABP4 null macrophages compared to WT cells and that ASC protein is undetectable in cells lacking FABP4 (Figs. 3F and 3G). Extending this analysis to stromal vascular fraction of WT and FABP4 null mice, ASC expression was also abolished in response to genetic deletion of FABP4 (Fig. 3H). Taken together, these data suggest a lipid-regulated p53-ASC signaling system exists in macrophages and that the presence or absence of fatty acid binding protein 4 (FABP4) modulates this equilibrium.
Pharmacologic and Genetic Ablation of FABP4 Blocks GSDMD Processing, Pyroptosis, and IL-1β and IL-18 Secretion
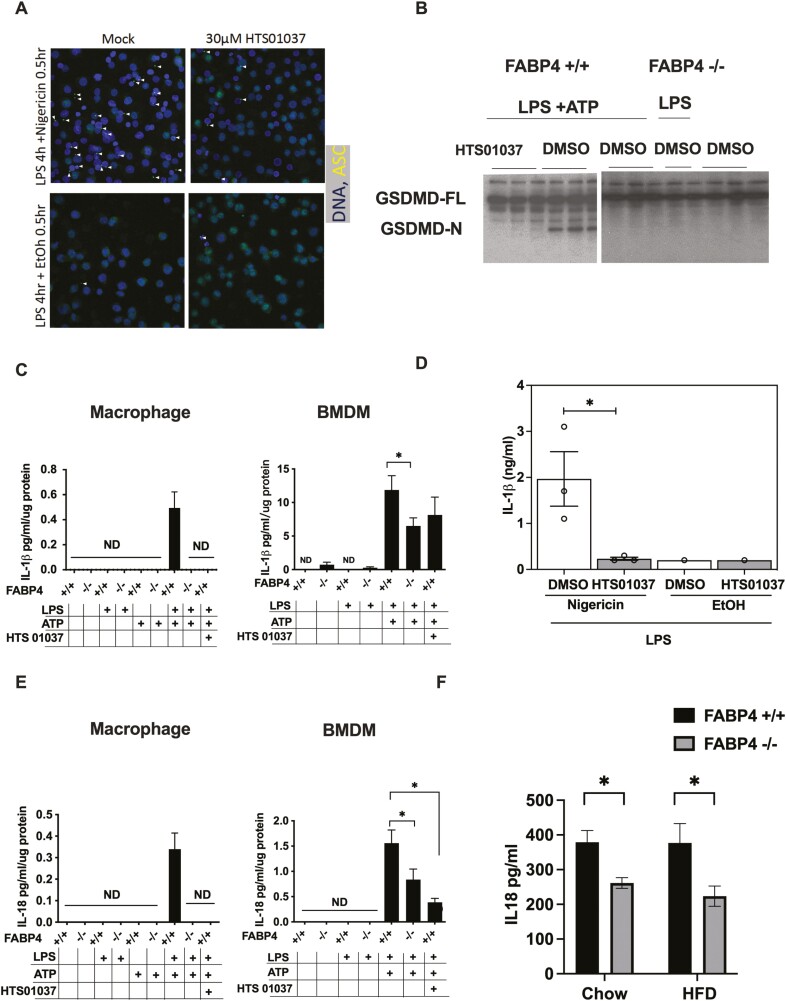
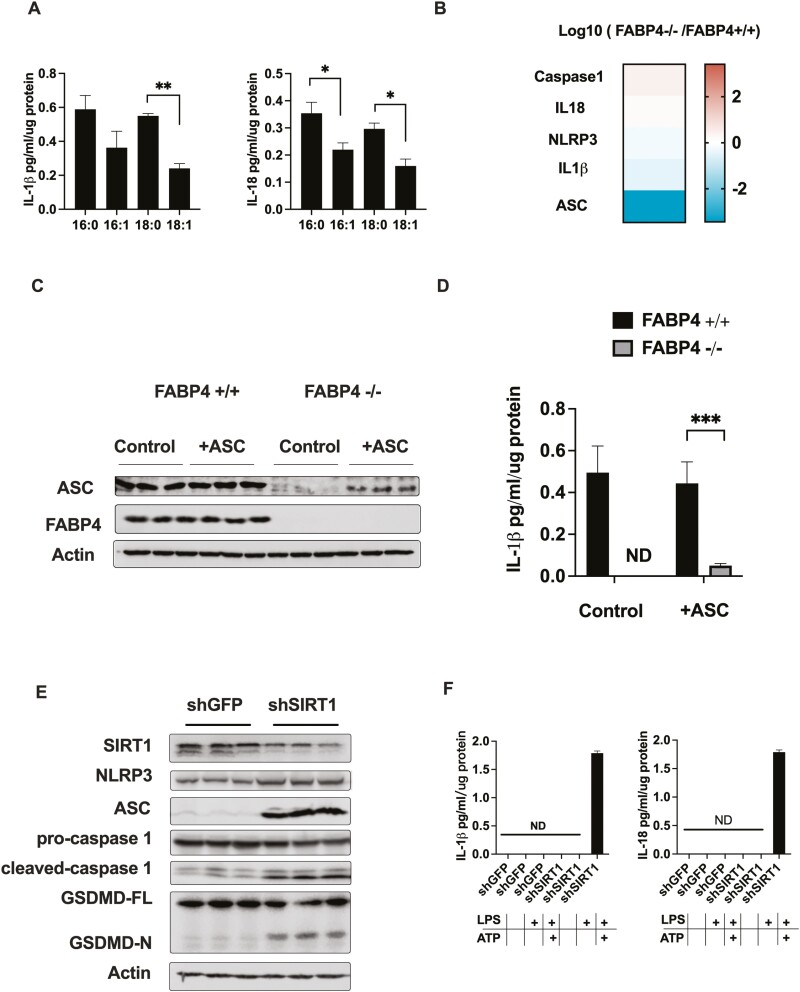
Since ASC expression is diminished in response to loss of FABP4 and ASC has a vital role in inflammasome assembly and activation, we want to examine whether loss of FABP4 could inhibit functional inflammasome formation. To test this, we visualized inflammasome localization using ASC as a marker, which forms a single centrosome-associated puncta (specks) upon inflammasome activation. As shown in Figure 4A, HTS01037 pretreatment significantly reduced ASC speck formation. Interestingly, HTS01037 strongly inhibited ASC speck formation even when administered simultaneously with nigericin stimulation suggesting a rapid and dramatic reduction in inflammasome signaling competency (Fig. 4A). To further confirm inflammasome inactivation in FABP4 null macrophages, the processing of GSDMD was evaluated. GSDMD has been shown to mediate host defense against infection and following cleavage by the activated inflammasome, the processed GSDMD N-terminal domain polymerizes, binds phosphatidyl choline and inserts into the plasma membrane, forming an annulus from which cleaved IL-1β and IL-18 are secreted (29). Herein, we showed that in response to LPS + ATP activation, GSDMD is processed in WT macrophages, but not in FABP4 null macrophages (Fig. 4B). Paralleling this result, pharmacological inhibition of FABP4 by HTS01037 in WT macrophages also blocked GSDMD processing. To confirm the inflammasome inactivation and functionality of cleaved GSDMD, ELISA analysis revealed that IL-1β and IL-18 secretion were dramatically reduced in pharmacologic inhibition or genetic deletion of FABP4 macrophages in both cell culture (Figs. 4C-4E) and mouse models of FABP4 deletion (Fig. 4F). Taken together, these results suggest that by reducing ASC expression, inflammasome activation, GSDMD processing, and IL-1β and IL-18 secretion are all diminished in FABP4 null macrophages.
Figure 4.
Pharmacologic and genetic ablation of fatty acid binding protein 4 (FABP4) blocks gasdermin D (GSDMD) processing, pyroptosis and interleukin (IL)-1β and IL-18 secretion. (A) Immunofluorescence of apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) on THP-1 cells treated with 30 μM HTS01037. Immunofluorescence micrographs of THP-1 cells stained for ASC (green) and nucleus (blue). White arrowheads marked characteristic punctate single speck. (B) Wild-type (WT) and FABP4−/− macrophages were treated with 30 μM HTS01037 and 500 ng/mL lipopolysaccharide (LPS) for 4 hours plus 2 mM adenosine 5′-triphosphate (ATP) for the last hour. Full length and N-terminal GSDMD were evaluated by immunoblotting. (C) WT and FABP4−/− macrophages were treated with 30 μM HTS01037 and 500 ng/mL LPS for 4 hours plus 2 mM ATP for the last hour, and IL-1β secretion into the media was measured by enzyme-linked immunosorbent assay (ELISA). Bone marrow derived macrophage were purified and cultured from WT and FABP4−/− mice, treated with 30 μM HTS01037 and 500 ng/mL LPS for 4 hours plus 2 mM ATP for the last hour, and IL-1β secretion levels in the media were measured by ELISA. (D) Quantification of IL-1β in media taken from THP-1 cells treated with LPS + Nigericin with (white bars) or without (grey bars) pretreatment with 30 μM HTS01037 prior to Nigericin addition. (E) WT and FABP4−/− macrophages were treated with 30 μM HTS01037 and 500 ng/ml LPS for 4 hours plus 2 mM ATP for the last hour, and IL-18 secretion measured by ELISA. Bone marrow derived macrophage were purified and cultured from WT and FABP4−/− mice, treated with 30 μM HTS01037 and 500 ng/mL LPS for 4 hours plus 2 mM ATP for the last hour, and IL-18 secretion in the media was measured by ELISA. (F) IL-18 levels were measured in serum from WT and FABP4−/− mice fed with chow or high-fat diet for 16 weeks. ELISA results were normalized to intracellular lysates protein level for each condition. All results represent the mean ± SE of the mean. *P < 0.05, **P < 0.01.
Proinflammatory Cytokine Secretion by FABP4−/− Macrophages Is Rescued by Reintroducing ASC or Silencing SIRT1
To further confirm lipid-dependent inflammasome signaling, we evaluated IL-1β and IL-18 secretion in response to saturated and monounsaturated fatty acid treatment. Secretion of both IL-1β and IL-18 were decreased following MUFA treatment compared to saturated fatty acid treated macrophages (Fig. 5A). The mRNA level of IL-1β and IL-18 and other components of inflammasome, such as NLRP3 and caspase-1, were not changed significantly in cells lacking FABP4 while, while ASC was downregulated 1000-fold (Fig. 5B). To evaluate both the necessity and sufficiency of ASC down regulation as the key determinant in inflammasome activation, recombinant ASC was re-expressed into FABP4 null macrophages and IL-1β secretion evaluated. As shown in Figure 5D, ectopic introduction of ASC into FABP4 null macrophages partially rescued IL-1β release. These results imply a key regulatory node in inflammasome activation and pyroptosis is ASC expression.
Figure 5.
Proinflammatory cytokine secretion by fatty acid binding protein 4 (FABP4−/−) macrophages is rescued by reintroducing apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) or silencing silent mating type information regulation 2 homolog 1 (SIRT1). (A) Wild-type (WT) macrophages were treated with 200 μM saturated or unsaturated fatty acid plus 50 μM fatty acid free bovine serum albumin for 16 hours. Interleukin (IL)-1β and IL-18 secretion levels in the media were evaluated by enzyme-linked immunosorbent assay (ELISA). (B) Messenger RNA level of major components of NLR family pyrin domain containing 3 (NLRP3) inflammasome and IL-1 family cytokines were evaluated in RNAseq database. (C) WT and FABP4−/− macrophages were transfected with plasmid cloning DNA (pcDNA), apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain 1 (ASC1) plasmids (+ASC), or pcDNA-green fluorescent protein (GFP) plasmids (Control), and ASC protein expression were measured by immunoblotting. (D) WT and FABP4−/− macrophages were transfected with pcDNA-ASC1 plasmids or pcDNA-GFP plasmids and IL-1β secretion level in the media was evaluated by ELISA. (E) SIRT1 (shSIRT1) and control GFP (shGFP) were silenced in FABP4−/− macrophages and treated with 500 ng/mL lipopolysaccharide for 4 hours plus 2 mM adenosine 5′-triphosphate for the last hour. SIRT1, NLRP3, ASC, pro- and cleaved caspase1, and full length and N-terminal gasdermin D were evaluated by immunoblotting. (F) IL-1β and IL-18 secretion levels in the media were measured by ELISA in SIRT1 knock down (shSIRT1) and control GFP (shGFP) FABP4−/− macrophages. ELISA results were normalized to intracellular lysates protein level for each condition. All results represent the mean ± SE of the mean. *P < 0.05, **P < 0.01, ***P < 0.001.
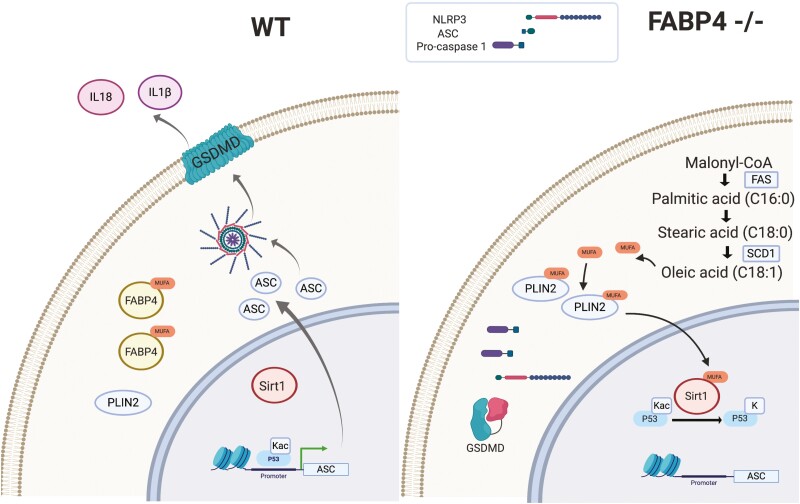
As an alternate strategy toward demonstrating the SIRT1-p53-ASC axis, Sirt1 was gene silenced in FABP4 null macrophages. As shown in Figure 5E and 5F, loss of Sirt1 expression in FABP4−/− macrophages fully rescued ASC expression and caspase1 activation leading to GSDMD processing and IL-1β and IL-18 secretion. Taken together, these results suggest SIRT1 functions as a lipid-controlled master regulator of inflammation in macrophage cells (Fig. 6).
Figure 6.
Schematic representation of lipid signaling and inflammasome activation in wild-type (WT) and fatty acid binding protein 4 (FABP4) null macrophage cells. Left: In WT macrophage cells monounsaturated fatty acids are bound to FABP4 leading to reduced activation of silent mating type information regulation 2 homolog 1 (SIRT1) and acetylation of p53. Acetylated p53, in turn, activates apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) expression and promotes activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome and proinflammatory cytokines secretion, such as interleukin (IL)-1β and IL-18. Right: In FABP4 null macrophages, loss of FABP4 leads to allosteric activation of SIRT1 by de novo lipogenesis derived monounsaturated fatty acids. p53 is deacetylated and deactivated by SIRT1 leading to loss of expression of ASC. Lack of ASC prevents NLRP3 inflammasome activation and assembly, gasdermin D processing, and secretion of proinflammatory IL-1β and IL-18. Created with BioRender.com.
Discussion
FABP4 null mice have been shown to be protected from chronic inflammation and metabolic dysfunction in response to dietary and genetic models of obesity (57). FABP4 is basally expressed primarily in adipocytes, peripheral macrophages, and microglial cells and a variety of studies have suggested that protection from metabolic dysfunction is linked to FABP4 function in both adipocytes and peripheral macrophages (58, 59). Of the analyses carried out in macrophages, reduced inflammation as measured by attenuated inflammatory cytokine secretion and NF-κB activation are the primary determinants of metabolic improvement. The molecular mechanisms that link loss of FABP4 to inflammasome dysfunction have been obscure, and the results shown herein reveal an unappreciated regulatory axis that links monounsaturated fatty acids to regulation of SIRT1. Moreover, exogenous or endogenous lipid activation of SIRT1 in macrophages results in loss of p53 acetylation that, in turn, controls expression of ASC, inflammasome assembly and cytokine secretion.
Interestingly, in murine and human macrophages, FABP4 is a minor FABP isoform and the major FABP is FABP5. In vitro ligand binding analysis of FABP4 and FABP5 reveal similar binding specificities and affinities although FABP4 exhibits the highest affinity for monounsaturated fatty acids. Pharmacologic or molecular loss of FABP4 eliminates a protein-lipid equilibrium and makes FABP4-bound fatty acids available for other interactions. Paradoxically, genetic loss of FABP5 does not result in robust regulation of macrophages as do FABP4 null mice suggesting that in vivo, the lipid pools may not partition between all lipid binding proteins equivalently. A third FABP in macrophages is perilipin 2, a member of the multigene family of perilipins implicated in lipid droplet biology and fatty acid trafficking (60, 61). Recent studies showed that PLIN5 can also act as a FABP and deliver MUFA to SIRT1 in the nucleus. This implies that within the cellular environment, fatty acids may be bound to multiple lipid binding proteins and the relative affinity, specificity, and abundance of each governs fatty acid distribution. Silencing of PLIN2 in control macrophages (39) leads to potentiated p53 and STAT3 acetylation where silencing of PLIN2 in FABP4 null macrophages (39) potentiates p53/STAT3 acetylation, suggesting that PLIN2 might play similar roles as PLIN5 to deliver fatty acid to SIRT1 in the nucleus. Consistent with this model, pharmacologic inhibition of FABP4 using HTS01037 that liberates bound fatty acids similarly increased p53/STAT3 acetylation. Whereas de novo lipogenesis produces fatty acids that activate SIRT1, exogenously added C18:1 regulates p53 activity only in control cells expressing PLIN2. In contrast, when PLIN2 is silenced, exogenous C18:1 no longer mediates p53 regulation implying that 2 lipid pools, 1 from the de novo pathway and 1 from fatty acid uptake, have the potential to regulate p53, and both pathways are mediated by PLIN2.
SIRT1 plays a key role in the anti-inflammatory response, and while the detailed mechanism is likely multifactorial, results presented in Figures 3 and 4 strongly suggest p53 acetylation regulates expression of ASC in macrophages. Inflammasome activation, GSDMD processing, and functional activation of pyroptosis are all diminished in FABP4 null macrophages but can be rescued by silencing SIRT1 or exogenous expression of ASC (Fig. 5). IL-1β and IL-18 secretion are abolished in FABP4 null macrophage due to the lack of ASC expression that leads to failure to process GSDMD. These results suggest an underappreciated role for ASC not only as a protein scaffold that links NLRP3 to caspase1 activation but also a key transcriptional target for lipid activated SIRT1. Whereas previous studies in other systems have linked SIRT1 to NLRP3 inflammasome (62-64) and p53 to ASC (37, 54), the work presented herein describes the SIRT1-p53-ASC axis in macrophages and its regulation by monounsaturated fatty acids. This concept is diagrammatically presented in Figure 6. Although previous studies showed that saturated fatty acids induce or increase NLRP3 expression and IL-1β and IL-18 secretion in macrophages (65-67), our studies link monounsaturated fatty acids to ASC through SIRT1, implying a bimodal regulation of NLRP3 inflammasome by fatty acids. Moreover, the loss of FABP4, either by molecular or pharmacologic means, likely liberates monounsaturated fatty acids that activate SIRT1 in a PLIN2-dependent manner. Such regulation negatively regulates inflammasome assembly and markedly diminishes pyroptosis, leading to the generalized anti-inflammatory properties of the FABP4 null mouse. Similarly, patients with a polymorphism in the FABP4 promoter that diminishes expression also exhibit diminished inflammation (68, 69), and the results suggest that loss of FABP4 in humans may similarly regulate pyroptosis and cytokine secretion.
Acknowledgments
We would like to thank the University of Minnesota Genomic Center for RNAseq and Juan E. Abrahante Lloréns for analyzing the RNAseq database. Lipids analyses were supported by the Mayo Metabolomics Core.
Glossary
Abbreviations
- ASC
apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain
- ATP
adenosine 5′-triphosphate
- CARD
C-terminal caspase recruitment domain
- ELISA
enzyme-linked immunosorbent assay
- FABP
fatty acid binding protein
- FAS
fatty acid synthase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GSDMD
gasdermin D
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- LPS
lipopolysaccharide
- mRNA
messenger RNA
- MUFA
monounsaturated fatty acids
- NIH
National Institutes of Health
- NLRP3
NLR family pyrin domain containing 3
- PBS
phosphate-buffered saline
- pcDNA
plasmid cloning DNA
- PGC-1α
proliferator activated receptor γ coactivator 1α
- PLIN
perilipin
- PYD
PYRIN-PAAD-DAPIN domain
- SCD
stearoyl-coenzyme A desaturase
- SIRT1
silent mating type information regulation 2 homolog 1
- STAT3
signal transducer and activator of transcription 3
- SVF
stromal vascular fraction
- TFIIE
transcription factor II E
- WT
wild-type
Financial Support
Funding was provided by NIH R01 DK053189 and NIH R01 AG069819 to D.A.B.
Author Contributions
Y.H. carried out experiments, data analysis, and manuscript preparation. P.Y. performed experiments leading to Figure 4B. S.M.V. and H.W. carried out experiments linked to Figure 4A and 4D and edited the manuscript. D.D. made the short hairpin PLIN2 and short hairpin GFP in WT and FABP4−/− macrophages, evaluated PLIN3 expression in macrophages, and contributed to manuscript preparation. D.A.B. developed the conceptual framework for the study and contributed to manuscript preparation and editing. D.A.B. is the guarantor of all information in the manuscript.
Disclosures
Each of the authors declares that no conflict of interest exists in the preparation, analysis, or development of the manuscript or its conclusions.
Data Availability
All animals and cell lines described in this manuscript are available via request to the corresponding author. All other materials and reagents, including antibodies, are commercially available without license. Suppliers and catalog numbers (Research Resource Identifiers) for specific reagents are indicated in the text. Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.
References
- 1. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118(11):1786-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu J, Zhao J, Meng H, Zhang X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front Immunol. 2019;10:101173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285(43):32679-32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs-mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015;11(10):592-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2019;26(3):216-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuhashi M, Tuncman G, Goerguen CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu HL, Hertzel AV, Steen KA, Wang Q, Suttles J, Bernlohr DA. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol Cell Biol. 2015;35(6):1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steen KA, Xu HL, Bernlohr DA. FABP4/aP2 regulates macrophage redox signaling and inflammasome activation via control of UCP2. Mol Cell Biol. 2017;37(2):UNSP e00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filipe Costa-Machado L, Fernandez-Marcos PJ. The sirtuin family in cancer. Cell Cycle. 2019;18(18):2164-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vachharajani VT, Liu TF, Wang XF, Hoth JJ, Yoza BK, McCall CE. Sirtuins link inflammation and metabolism. J Immunol Res. 2016:2016:20168167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu TF, McCall CE. Deacetylation by SIRT1 reprograms inflammation and cancer. Genes Cancer. 2013;4(3-4):135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551-563. [DOI] [PubMed] [Google Scholar]
- 17. Vaziri H, Dessain SK, Eagon EN, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149-159. [DOI] [PubMed] [Google Scholar]
- 18. Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011-2015. [DOI] [PubMed] [Google Scholar]
- 19. Bernier M, Paul RK, Martin-Montalvo A, et al. Negative Regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286(22):19270-19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie YZ, Erion DM, Yuan ZL, et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11(4):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Najt CP, Khan SA, Heden TD, et al. Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1. Mol Cell. 2020;77(4):810-824.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallardo-Montejano VI, Saxena G, Kusminski CM, et al. Nuclear perilipin 5 integrates lipid droplet lipolysis with PGC-1 alpha/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat Commun. 2016;7:12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009;182(5):3173-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171(11):6154-6163. [DOI] [PubMed] [Google Scholar]
- 25. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821-832. [DOI] [PubMed] [Google Scholar]
- 26. Fernandes-Alnemri T, Wu J, Yu JW, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Zhang ZB, Ruan JB, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He WT, Wan HQ, Hu LC, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1 beta secretion. Cell Res. 2015;25(12):1285-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GKS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and I kappa B kinase activities. J Biol Chem. 2005;280(13):12888-12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ying W, Cheruku PS, Bazer FW, Safe SH, Zhou BY. Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp. 2013;(76):e50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baar RA, Dingfelder CS, Smith LA, Bernlohr DA, Parks EJ. Investigation of in vivo fatty acid metabolism in AFABP/aP2−/− mice. FASEB J. 2004;18(4):A6-A6. [DOI] [PubMed] [Google Scholar]
- 34. Persson XMT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51(9):2761-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-Transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hertzel AV, Smith LA, Berg AH, et al. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am J Physiol Endocrinol Metab. 2006;290(5):E814-E823. [DOI] [PubMed] [Google Scholar]
- 37. Ohtsuka T, Ryu H, Minamishima YA, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6(2):121-128. [DOI] [PubMed] [Google Scholar]
- 38. Hertzel AV, Hellberg K, Reynolds JM, et al. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J Med Chem. 2009;52(19):6024-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernlohr D. Supplemental data for: Huang et al., Regulation of inflammasome activation and pyroptosis via a lipid-regulated SIRT1-p53-ASC axis in macrophages. Dryad. Deposited October 8, 2021. 10.5061/dryad.wpzgmsbnt [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orr JS, Kennedy AJ, Hasty AH. Isolation of adipose tissue immune cells. J Vis Exp. 2013;75:e50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gertz M, Fischer F, Nguyen GTT, et al. Ex-527 inhibits Sirtuins by exploiting their unique NAD(+)-dependent deacetylation mechanism. Proc Natl Acad Sci U S A. 2013;110(30):E2772-E2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Enoch HG, Catala A, Strittmatter P. Mechanism of rat-liver microsomal stearyl-CoA desaturase—studies of substrate-specificity, enzyme-substrate interactions, and function of lipid. J Biol Chem. 1976;251(16):5095-5103. [PubMed] [Google Scholar]
- 43. Dalen KT, Dahl T, Holter E, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta Mol Cell Biol Lipids. 2007;1771(2):210-227. [DOI] [PubMed] [Google Scholar]
- 44. Wang H, Sreenevasan U, Hu H, et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52(12):2159-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saliba-gustafsson P, Pedrelli M, Werngren O, Parini P, Ehrenborg E. The lipid-droplet associated protein perilipin 2 (PLIN2) plays a central role in lipid accumulation and cholesterol efflux via effects on lxr signaling in human macrophages. Atherosclerosis. 2018;275:e32E32-e32E32. [Google Scholar]
- 46. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413-431. [DOI] [PubMed] [Google Scholar]
- 47. Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harbor Perspect Biol. 2009;1(6):a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu YQ, Tavana O, Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11(7):564-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bode AM, Dong ZG. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4(10):793-805. [DOI] [PubMed] [Google Scholar]
- 51. Reed SM, Quelle DE. p53 acetylation: regulation and consequences. Cancers. 2015;7(1):30-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595-606. [DOI] [PubMed] [Google Scholar]
- 53. Luo JY, Li MY, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A. 2004;101(8):2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ohtsuka T, Liu XF, Koga Y, et al. Methylation-induced silencing of ASC and the effect of expressed ASC on p53-mediated chemosensitivity in colorectal cancer. Oncogene. 2006;25(12):1807-1811. [DOI] [PubMed] [Google Scholar]
- 55. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377-1379. [DOI] [PubMed] [Google Scholar]
- 58. Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Investig. 2008;118(7):2640-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty Acid-Binding Protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2014;8(suppl 3):23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McIntosh AL, Storey SM, Atshaves BP. Intracellular lipid droplets contain dynamic pools of sphingomyelin: ADRP binds phospholipids with high affinity. Lipids. 2010;45(6):465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McIntosh AL, Senthivinayagam S, Moon KC, et al. Direct interaction of Plin2 with lipids on the surface of lipid droplets: a live cell FRET analysis. Am J Physiol Cell Physiol. 2012;303(7):C728-C742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li YX, Yang XF, He YH, et al. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology. 2017;222(3):552-561. [DOI] [PubMed] [Google Scholar]
- 63. Li YX, Wang P, Yang XF, et al. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol. 2016;77:148-156. [DOI] [PubMed] [Google Scholar]
- 64. Han Y, Sun WJ, Ren D, et al. SIRT1 agonism modulates cardiac NLRP3 inflammasome through pyruvate dehydrogenase during ischemia and reperfusion. Redox Biol. 2020;34:101538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gianfrancesco MA, Dehairs J, L’Homme L, et al. Saturated fatty acids induce NLRP3 activation in human macrophages through K+ efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(7):1017-1030. [DOI] [PubMed] [Google Scholar]
- 66. Moon JS, Lee S, Park MA, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Investig. 2015;125(2):665-680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Wood LG, Li Q, Scott HA, et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J Allergy Clin Immunol. 2019;143(1):305-315. [DOI] [PubMed] [Google Scholar]
- 68. Shafey HI, Mahrous KF, Hassan AAM, Rushdi HE, Ibrahim MAM. Single-nucleotide polymorphisms in FABP4 gene associated with growth traits in Egyptian sheep. Vet World. 2020;13(6):1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ordovas JM. Identification of a functional polymorphism at the adipose fatty acid binding protein gene (FABP4) and demonstration of its association with cardiovascular disease: a path to follow. Nutr Rev. 2007;65(3):130-134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All animals and cell lines described in this manuscript are available via request to the corresponding author. All other materials and reagents, including antibodies, are commercially available without license. Suppliers and catalog numbers (Research Resource Identifiers) for specific reagents are indicated in the text. Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.