Abstract
Purpose
GLP-1 based therapy represents a new treatment option for inflammatory bowel disease. Ban-Lan-Gen (BLG) granule, a known anti-viral TCM formulation, exhibits potential anti-inflammatory activities in treating various kinds of inflammation. However, its anti-inflammatory effect on colitis and the underlying mechanisms remain unknown.
Methods
Dextran sulfate sodium (DSS)-induced chronic relapsing colitis in mice was established. The disease activity index, histological sign of damage, and levels of proinflammatory cytokines were performed to assess the protective effects of BLG. Serum GLP-1 level and colonic Gcg, GPR41 and GRP43 expression, the community compositions of gut microbiota, the levels of SCFAs in the feces and GLP-1 release from primary murine colon epithelial cells were performed to characterize the effects of BLG on gut microbiota and gut SCFA derived-GLP-1 production.
Results
BLG treatment significantly alleviated body weight loss, DAI, colon shortening, colon tissue damage, and pro-inflammatory cytokine levels of TNF-α, IL-1β and IL-6 in the colon tissues. Moreover, BLG treatment could observably restore colonic Gcg, GPR41 and GRP43 expression and serum GLP-1 level of colitic mice, as well as correct the alteration of gut microbiota in colitic mice by increasing the abundances of SCFA-producing bacteria, eg, Akkermansia and Prevotellaceae_UCG-001, and decreasing the abundances of bacteria, eg, Eubacterium_xylanophilum_group, Ruminococcaceae_UCG-014, Intestinimonas, and Oscillibacter. Furthermore, BLG treatment could markedly increase the levels of SCFAs in feces of colitic mice. In parallel, ex vivo assay also showed that and the extract of feces from BLG-treatment mice could greatly stimulate the secretion of GLP-1 from primary murine colon epithelial cells.
Conclusion
These findings suggest that the anti-colitis effects of BLG are achieved at least partly by regulating gut microbiota and restoring gut SCFA derived-GLP-1 production, and BLG has the potential to be developed as a promising agent for the treatment of chronic relapsing colitis.
Keywords: colitis, Ban-Lan-Gen granule, gut microbiota, short-chain fatty acids, GLP-1
Introduction
Ulcerative colitis (UC) is a long-term inflammatory condition in the colon and rectum characterized by recurrent diarrhea, abdominal pain, weight loss, and mucopurulent bloody stool.1 Recently, with the increasing spread of the Western lifestyle, the prevalence of UC has been increasing in previously low-incidence countries, including China.2 This increase imposes significant problems in public health and has a serious impact on the working ability and quality of life of the sufferers. Notably, the pathogenesis of UC is still largely unclear, but it is generally accepted that genetics, environmental factors, gut microbiota, and immune systems all contribute to the development of UC.3 Even now, UC cannot be cured, and the goal of treatment in clinics is to control clinical symptoms, induce and maintain remission, promote mucosal healing, and reduce recurrence. Classical therapeutic drugs consist of aminosalicylates, corticosteroids, immunosuppressants, and biologic agents. However, these drugs cannot achieve ideal results due to their various side effects.4 Recently, many case studies have reported that traditional Chinese medicine (TCM) exhibits great potential to help induce remission of UC with low toxicity, suggesting that developing new remedies from TCM is a promising therapeutic strategy for UC.5–7
The Ban-Lan-Gen (BLG) granule is a TCM formulation produced from the aqueous extract of Radix Isatidis, which has been recorded in the Chinese Pharmacopoeia and recognized as an important TCM for the prevention and treatment of cold and malignant infectious diseases, especially SARS coronavirus and H1N1 influenza virus.8 Except for its anti-viral efficacy, BLG also exhibits potential anti-inflammatory activities in treating various kinds of inflammation.9,10 Additionally, several soluble compounds including glucosinolates (R,S-goitrin, progoitrin, epiprogoitrin and gluconapin) and nucleosides (hypoxanthine, adenosine, uridine and guanosine) and indigoid alkaloids (eg, indigo and indirubin) have been isolated and identified from the aqueous extract of Radix Isatidis.11,12 Previous studies have well documented that the compounds adenosine, uridine, and indirubin exhibit potent anti-colitis effects in different animal colitis models.13–17 However, no evidence-based study has been conducted to assess the efficacy of BLG against colitis. In the present study, we investigated the protective effect of BLG on dextran sulfate sodium (DSS)-induced chronic relapsing colitis in C57BL/6 mice and found that oral administration of BLG significantly alleviated DSS-induced chronic relapsing colitis in mice, and its regulatory mechanism was associated with regulating gut microbiota and restoring gut derived-glucagon-like peptide-1 (GLP-1) production.
Materials and Methods
Materials
BLG granule (without sugar, National medicine approval Z11020357; Beijing Tongrentang Science and Technology Development Co., Ltd, Beijing, China; batch number: 20110966) was purchased from the drugstore. DSS (molecular weight: 36,000–50,000 Daltons) was obtained from MP Biologicals (Santa Ana, USA). Sulfasalazine (SASP) (purity ≥ 98%), hematoxylin, and eosin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse TNF-α, IL-1β, and IL-6 luminex Elisa assay kit was purchased from R&D systems (Minneapolis, MN, USA). Acetic acid, propionic acid, and butyric acid were purchased from Aladdin Industrial Corporation (Shanghai, China). 2-ethylbutyric acid was bought from Merck KGaA (Darmstadt, Germany).
Animals and Experiment
Male C57BL/6 mice aged 6–8 weeks (weighing 18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed in a temperature of 22 ± 2°C with a 12 h light/dark cycle. The mice were fed a standard rodent diet with free access to drinking water to adapt to the new environment for one week. The mice were then randomly divided into four groups: control group, DSS model group, SASP treated group (200 mg/kg, po.), and the BLG-treated group (1 g/kg, po.). As shown in Figure 1A, experimental chronic relapsing colitis was induced by three cycles of 1.8% DSS for five days and then distilled water for seven days to mice, according to our previous study.18 The mice in the SASP- and BLG-treated groups were treated daily with SASP and BLG from day 0, respectively. The dosage of BLG was set as 1 g/kg according to the preliminary experiment. In parallel, the dosage of SASP was set to 200 mg/Kg according to the literature.4 The control group and DSS model group received the same volume of water throughout the entire experimental period.
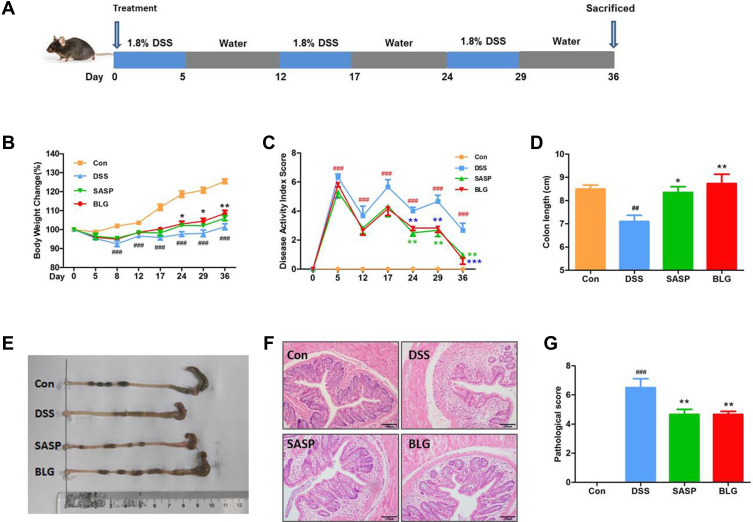
Figure 1.
BLG ameliorated DSS-induced chronic relapsing colitis in mice. (A) experimental design for chronic relapsing colitis and treatment, (B) body weight change, (C) disease activity index (DAI) score, (D) colon length, (E) representative images of the colon, (F) H&E staining of the colon (magnification, ×100), and (G) histological score. Data are expressed as the mean ± SEM (n = 6). ##p < 0.01 or ###p < 0.001 vs control (Con) group; *p < 0.05 or **p < 0.01 or ***p < 0.001 vs DSS group.
Evaluation of Disease Activity Index (DAI)
Body weight, stool consistency, and rectal bleeding were recorded daily. The disease activity index (DAI) was determined by combining the scores of body weight, stool consistency, and rectal bleeding, as previously described.19 At the end of the experiments, all mice were euthanized, and blood, feces, and colon were harvested for further experiments.
Histological Examination
Colon tissues were fixed in formalin and embedded in paraffin. Five micrometers of sections were made and stained with hematoxylin-eosin (H&E), and then evaluated and scored in a blind manner as previously described.19
RNA Extraction and RT-PCR Analysis
Total RNA of colon tissues was extracted by Trizol reagent (Invitrogen, Carlsbad, CA) and followed by reverse transcriptase (TaKaRa, Kusatsu, Shiga, Japan) for cDNA. Quantitative PCR was performed using a real-time PCR system with SYBR Green Master (Roche, Basel, Switzerland). Target-gene transcriptions were normalized to β-actin, and the data were analyzed using the 2 −ΔΔCT method. The primer sequences of genes were listed in Table 1.
Table 1.
Primer Sequences of Genes
| Name | Sequence |
|---|---|
| Mouse β-actin | Forward 5′- GGC TGT ATT CCC CTC CAT CG −3′ |
| Reverse 5′- CCA GTT GGT AAC AAT GCC ATG T - 3′ | |
| Mouse TNF-α | Forward 5′- CAG GCG GTG CCT ATG TCT C-3′ |
| Reverse 5′- CGA TCA CCC CGA AGT TCA GTA G-3′ | |
| Mouse IL-6 | Reverse 5′- CTG CAA GAG ACT TCC ATC CAG-3′ |
| Reverse 5′- AGT GGT ATA GAC AGG TCT GTT GG-3′ | |
| Mouse IL-1β | Reverse 5′- GAA ATG CCA CCT TTT GAC AGT G-3′ |
| Reverse 5′- TGG ATG CTC TCA TCA GGA CAG-3′ | |
| Mouse Gcg | Reverse 5′- CTA CAC CTG TTC GCA GCT CA-3′ |
| Reverse 5′- CTG GGG TTC TCC TCT GTG TC-3′ | |
| Mouse GPR41 | Reverse 5′- TCT ACC TAG GTC CCG TGT GG-3′ |
| Reverse 5′- GGT GTA GAG GCA GGA GAG GA-3′ | |
| Mouse GPR43 | Reverse 5′- ATC CTC ACG GCC TAC ATC CT-3′ |
| Reverse 5′- CAG CAG CAA CAA CAG CAA GT-3′ |
Primary Murine Colon Epithelial Cell Isolation and Culture
Primary murine colon epithelial cell isolation and culture were performed as described previously.20 In brief, the colon of mice aged 6–8 weeks was firstly excised after execution by cervical dislocation, and then opened longitudinally, rinsed in Hanks’ balanced salt solution (HBSS, calcium- and magnesium-free) and cut into 0.5–1 mm pieces. Subsequently, the tissues were digested with 0.4 mg/mL collagenase XI (Sigma, Poole, UK) in free DMEM medium and centrifuged at 300×g for 5 min at room temperature. The pellets were resuspended in DMEM medium (supplemented with 10% fetal bovine serum, 100 Units/mL penicillin and 100 µg/mL streptomycin) at 37 ° C and passed through a nylon mesh (pore size ~250μm). Aliquots of colon epithelial cells were plated in glass-bottom culture dishes and then incubated with acetic acid, propionic acid, butyric acid and fecal extracts of mice for 2h at 37°C, 5% CO2.
ELISA Analysis
Colon tissues were homogenized with PBS, and levels of cytokines IL-6, TNF-α and IL-1β in colon tissues were detected using a luminex ELISA assay kit (R&D systems, Minneapolis, MN, USA). As well, GLP-1 levels in the serum and culture medium of primary murine colon epithelial cells were assayed ELISA kit (Bioswamp, Wuhan, China) according to the manufacturer’s instruction.
Fecal 16S rRNA Analysis
The total DNA of the feces was extracted using a DNA extraction kit (TIANGEN, China). The quality and quantity of DNA were measured with ratios of 260 nm/280 nm and 260 nm/230 nm, respectively. Subsequently, each extracted DNA was used as a template, and their V3-V4 region of 16S rRNA genes of distinct regions were amplified with specific primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). The PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Germany), quantified by real-time PCR, and sequenced using the IlluminaMiseq PE300 sequencing platform (Illumina Inc., CA, USA). For the bioinformatics analysis, the data processing was followed as per the previously reported protocol.21,22 Briefly, the raw fast files were filtered using Cutadapt (V1.9.1). The OTUs were clustered with a cut-off value of 97% similarity using UPARSE (version 7.0.1001), and chimeric sequences were removed using UCHIME. The community composition analysis was performed and classified using the RDP classifier (http://rdp.cme.msu.edu/) based on the SILVA ribosomal RNA gene database.
Short-Chain Fatty Acids (SCFA) Analysis
The levels of SCFAs (acetic acid, propionic acid, and butyric acid) were measured as described previously by Tao et al, with some modifications.23 In short, 100 mg of feces were first suspended in 0.4 mL deionized water, and 0.1 mL 50% sulfuric acid and 0.5 mL of 2-ethylbutyric acid (internal standard) were added and then homogenized and centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was extracted with 0.5 mL ethyl ether and then injected into the GC for analysis. For the gas chromatography (GC) analysis, a GC-2010 Plus gas chromatograph (Shimadzu, Inc.) equipped with a flame ionization detector (FID) was used to analyze the samples. Separation was achieved using a ZKAT-624 column, 30 m × 0.53 mm × 0.3 μm (Lanzhou Zhongke Antai Analytical Technology Co. Ltd, China). The data were acquired using GC Solution software (Shimadzu, Inc.). The split ratio was 10:1, and the carrier gas was nitrogen at a flow rate of 6 mL/min. The injection volume was 1 μL. The injector and detector temperatures were 300°C. The oven temperature was held at 140°C for 13.5 min and then increased to 250°C at a rate of 120°C/min; the temperature was held for 5 min.
Statistical Analysis
The data are expressed as mean ± standard error of the mean (SEM). The significances for data were evaluated by one-way ANOVA, followed by Duncan’s multiple range tests. GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA) was used for all calculations, and p < 0.05 was considered statistically significant.
Results
BLG Ameliorated DSS-Induced Chronic Relapsing Colitis in Mice
It is well known that UC is a chronic relapsing colonic inflammatory condition with severe abdominal pain, diarrhea, and bleeding. Accordingly, DSS-induced chronic relapsing colitis in mice was established to evaluate the anti-colitis efficacy of BLG (Figure 1A). Compared to the control group, the mice in the DSS model group had significant body weight loss and a high DAI, while these changes were dramatically reversed after BLG treatment for 24 days (Figures 1B and C). Colon shortening is an important characteristic marker of UC. As shown in Figure 1D and E, the colon length of the mice receiving DSS administration was significantly shortened, but this was relieved by the treatment of BLG. Subsequently, histopathological analysis was performed to evaluate the colonic inflammation. The H&E staining images and pathological score revealed that DSS administration significantly disrupted the colonic architecture and caused crypt destruction, whereas BLG treatment could greatly reduce the crypt destruction and pathological score (Figures 1F and G). Notably, the protective effect of BLG at a dosage of 1g/Kg was comparable to SASP at a dosage of 200 mg/Kg. Collectively, these findings indicate that BLG was effective in reducing the severity of DSS-induced chronic relapsing colitis in mice.
BLG Suppressed Gene Expression and Production of Proinflammatory Cytokines TNF-α, IL-1β, and IL-6 in the Colon of DSS-Treated Mice
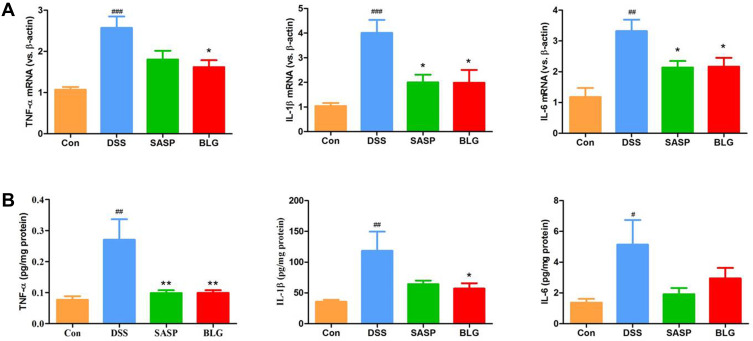
TNF-α, IL-1β, and IL-6 are important inflammatory markers of colonic inflammation. As shown in Figure 2A, DSS induced a significant increase in gene expression of TNF-α, IL-1β, and IL-6 in the colon compared with the control group. The administration of BLG could markedly reverse these DSS-mediated changes. Next, we used ELISA to determine the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in the colon tissues. The results also demonstrated that colonic levels of TNF-α, IL-1β, and IL-6 in mice treated with DSS were substantially increased, and these increases were relieved by the treatment of BLG (Figure 2B).
Figure 2.
BLG suppressed gene expression and production of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in the colon of DSS-treated mice. (A) colonic gene expressions of TNF-α, IL-1β, and IL-6; (B) colonic protein levels of TNF-α, IL-1β, and IL-6. Data are expressed as the mean ± SEM (n = 4–6). #p < 0.05 or ##p < 0.01 or ###p < 0.001 vs control (Con) group; *p < 0.05 or **p < 0.01 vs DSS group.
BLG Changed Gut Microbiota Diversity of DSS-Induced Colitis Mice
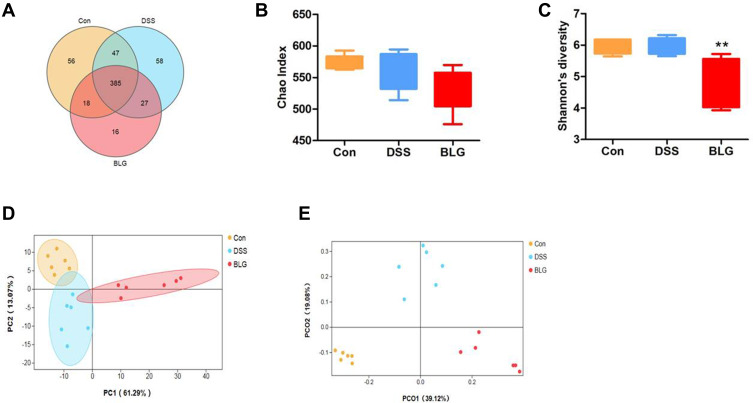
Dysbiosis of gut microbiota is crucial in the pathogenesis of UC.24 To investigate whether BLG regulated the gut microbiota of DSS-treated mice, 16S rRNA sequencing was performed to analyze the bacterial community of intestinal contents. The Venn diagram showed that 385 OTUs were shared by three groups. Meanwhile, each group had unique OTUs (Figure 3A). Also, the Chao1 index and the Shannon index presented in Figures 3B and C showed that the community diversity of the gut microbiota in BLG-treated mice was reduced, since the Shannon index in BLG-treated group was significantly declined. Principal components analysis (PCA) and Principal coordinate analysis (PCoA) were used to determine clustering patterns among three groups and showed that the community structure of DSS-treated mice was clearly separated after BLG treatment (Figures 3D and E). These data indicated that BLG treatment significantly affected the community structure of DSS-induced colitis mice.
Figure 3.
BLG changed gut microbiota diversity of DSS-induced colitis mice. (A) Venn diagram for OTUs, (B) Chao1 index, (C) Shannon’s richness index, (D) score plot for principal component analysis (PCA) of OTUs, (E) score plot for principal coordinate analysis (PCoA) of OTUs. Data are expressed as the mean ± SEM (n = 6). **p < 0.01 vs DSS group.
BLG Altered Gut Microbiota Abundance of DSS-Induced Colitis Mice
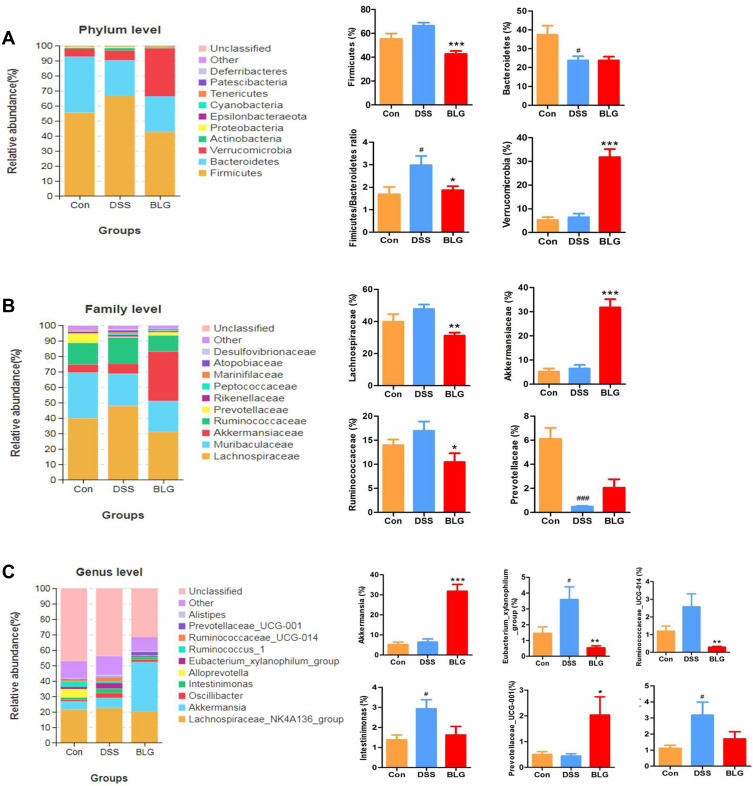
To assess specific changes in the fecal microbiota, we analyzed the composition of gut microbiota at all taxonomic levels. As shown in Figure 4A, the predominant phyla among all groups were Firmicutes and Bacteroidetes, followed by Verrucomicrobia. Compared to the mice in the control group, the fecal microbiota community from the DSS-treated mice showed a dramatic increase in the relative abundance of the Firmicutes and Firmicutes/Bacteroidetes ratio, and these changes were significantly reversed after BLG treatment. In particular, BLG treatment sharply increased the relative abundance of Verrucomicrobia in the feces of DSS-induced colitis mice. At the family level, the fecal microbiota community was occupied by Lachnospiraceae, Muribaculaceae, Akkermansiaceae, Ruminococcaceae, and Prevotellaceae (Figure 4B). Compared to the DSS group, the consumption of BLG increased the abundance of Akkermansiaceae but decreased the abundance of Lachnospiraceae and Ruminococcaceae. Of note, at the genus level, the fecal microbiota was occupied by the Lachnospiraceae_NK4A136_group, Akkermansia, and Prevotellaceae_UCG-001 (Figure 4C). This finding also showed that BLG treatment effectively reversed the flora imbalance in response to DSS challenge, characterized by decreased Eubacterium_xylanophilum_group, Ruminococcaceae_UCG-014, Intestinimonas and Oscillibacter, and increased Akkermansia and Prevotellaceae_UCG-001.
Figure 4.
BLG altered gut microbiota abundance of DSS-induced colitis mice. (A) the abundance of gut microbiota at the phylum level; (B) the abundance of gut microbiota at the family level; (C) the abundance of gut microbiota at the genus level. Data are expressed as the mean ± SEM (n = 6). #p < 0.05 or ###p < 0.001 vs control (Con) group; *p < 0.05 or **p < 0.01 or ***p < 0.001 vs DSS group.
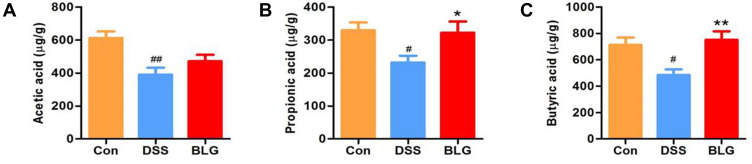
BLG Increased the Levels of SCFAs in the Feces of DSS-Induced Colitis Mice
Considering that short-chain fatty acids (SCFAs) are the main metabolites of Akkermansia and Prevotellaceae_UCG-001, and acetic acid, propionic acid, and butyric acid are the most abundant SCFAs in the intestinal lumen,25–27 we also measured these SCFAs in our study. As shown in Figure 5, the fecal acetic acid, propionic acid, and butyric acid concentrations were markedly decreased in the DSS-treated group, whereas BLG treatment could greatly repress this reduction.
Figure 5.
BLG increased the levels of SCFAs in the feces of DSS-induced colitis mice. (A) acetic acid content in feces; (B) propionic acid content in feces; (C) butyric acid content in feces. Data are expressed as the mean ± SEM (n = 6). #p < 0.05 or ##p < 0.01 vs control (Con) group; *p < 0.05 or **p < 0.01 vs DSS group.
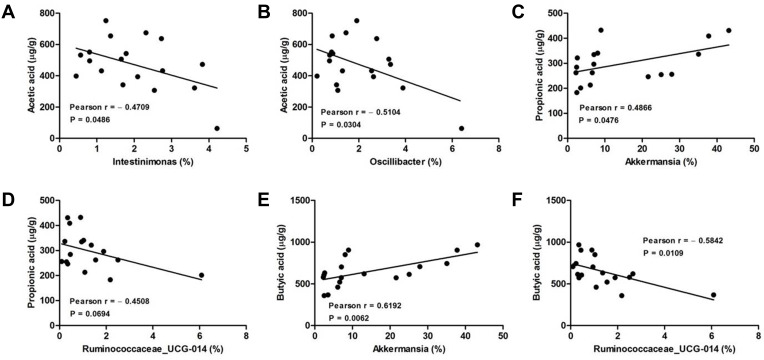
Analysis of the Correlation Between Gut Microbiota and SCFAs in the Feces of DSS-Induced Colitis Mice
We further calculated the Pearson correlation coefficient between differential SCFAs and fecal microbiota at the genus level. As shown in Figure 6, Akkermansia was positively correlated propionic acid (Pearson = 0.4866) and butyric acid (Pearson = 0.6192) production. Oppositely, both Intestinimonas and Oscillibacter were negatively correlated to acetic acid production, and their Pearson coefficients were 0.4709 and 0.5104, respectively. As well, Ruminococcaceae_UCG-014 was negatively correlated to both propionic acid (Pearson = 0.4508) and butyric acid (Pearson = 0.5842) production, respectively.
Figure 6.
Analysis of Pearson correlations between differential SCFAs and colonic microbial genera. (A) Intestinimonas vs acetic acid; (B) Oscillibacter vs acetic acid; (C) Akkermansia vs propionic acid; (D) Ruminococcaceae_UCG-014 vs propionic acid; (E) Akkermansia vs butyric acid; (F) Ruminococcaceae_UCG-014 vs butyric acid.
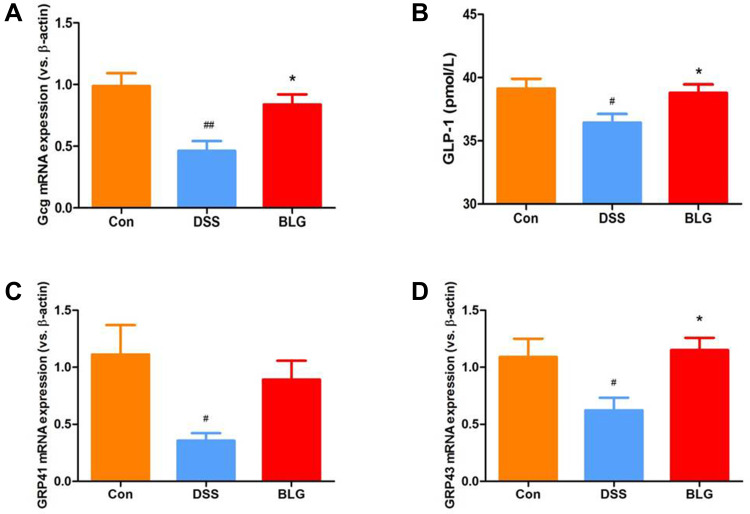
BLG Increase Serum GLP-1 Level and Colonic Gcg, GPR41 and GRP43 mRNA Expression of DSS-Treated Mice
Glucagon-like peptide-1 (GLP-1) is a cell type-specific post-translational product of proglucagon (Gcg) with anti-inflammatory property.28 As shown in Figure 7, DSS induced a significant decrease of Gcg mRNA expression in the colon compared with the control group, while BLG treatment could markedly reverse DSS-induced Gcg reduction (Figure 7A). In parallel, the level of GLP-1 in the serum was markedly decreased in the DSS-treated group, whereas BLG treatment could greatly prevent this decrease (Figure 7B). As the secretion of GLP-1 can be stimulated by SCFAs via G protein-coupled receptor 43 (GRP43) and G protein-coupled receptor 41 (GRP41) activation,29 we also detected the expression of GPR41 and GRP43 in the colon of colitic mice, and found that the colonic mRNA expression of GRP43 and GPR41 were dramatically decreased after DSS challenged, while these declines can be effectively rescued by BLG treatment (Figure 7C and D).
Figure 7.
BLG increased serum GLP-1 level and colonic Gcg, GPR41 and GRP43 mRNA expression of DSS-treated mice. (A) Gcg mRNA expression in the colon tissue; (B) GLP-1 level in the serum; (C) GPR41 mRNA expression in the colon tissue; (D) GPR43 mRNA expression in the colon tissue. Data are expressed as the mean ± SEM (n = 5–6). #p < 0.05 or ##p < 0.01 vs control (Con) group; *p < 0.05 vs DSS group.
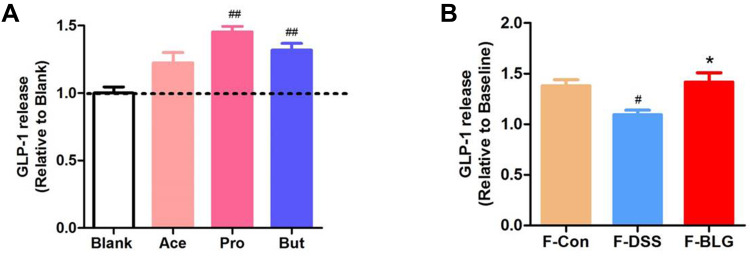
BLG-Derived SCFA Stimulate the Release of GLP-1 from Primary Murine Colon Epithelial Cells
As BLG treatment could increase serum GLP-1 level, colonic Gcg mRNA expression and the fecal SCFA level of DSS-treated mice, we further detected the effect of acetic acid, propionic acid, and butyric acid, as well as the extract of feces from Control (F-Con), DSS colitis (F-DSS) and BLG-treated colitis (F-BLG) mice on the release of GLP-1 from primary murine colon epithelial cells. As shown in Figure 8A, primary murine colon epithelial cells treated with 2 mM acetic acid, propionic acid, and butyric acid respectively, all could greatly stimulate GLP-1 release, which were consistent with previous studies.29,30 As well, all F-Con, F-DSS and F-BLG (equivalent to 0.25 g feces) could also greatly stimulate GLP-1 release from primary murine colon epithelial cells. Of note, the amount of GLP-1 release from F-DSS-treated primary murine colon epithelial cells was much less than the amount of GLP-1 release from both F-Con and F-BLG-treated primary murine colon epithelial cells, respectively (Figure 8B). These data indicated that BLG treatment significantly restore gut SCFA derived-GLP-1 production.
Figure 8.
BLG-derived SCFAs stimulate the release of GLP-1 from primary murine colon epithelial cells. (A) the amounts of GLP-1 release from primary murine colon epithelial cells were stimulated by acetic acid, propionic acid, butyric acid; (B) the amounts of GLP-1 release from primary murine colon epithelial cells were stimulated by fecal extracts F-Con, F-DSS, and F-BLG. Aliquots of colon epithelial cells were plated in glass-bottom culture dishes and then incubated with 2 mM acetic acid, propionic acid, butyric acid respectively, as well as fecal extracts F-Con, F-DSS, and F-BLG (equivalent to 0.25 g feces) respectively for 2h at 37°C, 5% CO2. The amount of GLP-1 release from primary murine colon epithelial cells was detected by ELISA. Data are expressed as the mean ± SEM (n =3). #p < 0.05 or ##p < 0.01 vs blank or F-Con group; *p < 0.05 vs F-DSS group.
Abbreviations: Ace, acetic acid; Pro, propionic acid; But, butyric acid; F-Con, fecal extract from control mice; F-DSS, fecal extract from colitis mice; F-BLG, fecal extract from BLG-treated colitis mice.
Discussion
UC is listed as a refractory disease by the World Health Organization and is becoming a globally dangerous disease; however, effective methods for predicting, preventing, and treating the disease remain limited. There is consequently an urgent need to explore and develop new safe and effective strategies for the treatment of UC. TCM formulations are the promising choice since many TCM formulations have been proven to effectively treat UC in the Chinese population for centuries, and they are all biological organics and natural materials mostly harmless to humans and animals.31,32 The present study aimed to seek a safe and effective TCM formulation for the treatment of UC and explore its action mechanism. BLG is a well-known TCM formulation prescribed for influenza treatment.8,33 Work from our laboratory and others has demonstrated that indigo naturalis, a TCM processed product with the same raw material as BLG, exhibited significant efficacy in treating UC in humans and animals.4,34 However, the anti-colitis effect of BLG and its action mechanism are unclear. In the current study, our results demonstrated that BLG potently alleviated DSS-induced colonic inflammation, which was associated with regulating gut microbiota and restoring gut derived-GLP-1 production.
It is well known that UC is characterized by periods of relapse with typical clinical features such as loss of body weight, diarrhea, rectal bleeding, and extensive colonic mucosal damage.35 Accordingly, chronic relapsing colitis was established by giving three cycles of 1.8% DSS for five days followed by seven days of drinking water. As shown in Figure 1B, the fluctuating body weight lost and DAI score demonstrated that chronic relapsing colitis was successfully induced. Mice in groups treated with BLG showed an upshift recovery from day 8 and a significant difference from day 24. The same change was also observed in the DAI score, which indicated that a clinical improvement in colitis was achieved. In terms of colonic injury and inflammatory conditions, the observations in colon length, colon tissue damage, and gene expression and production of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in the colon tissues also showed great improvement after BLG treatment. Collectively, these results clearly indicate that BLG is effective in treating chronic relapsing colitis in mice.
How does BLG exert its pharmacologic action? Numerous previous studies have shown that the gut microbiota plays a key role in the pathogenesis of UC, and microbiome-based and microbiome-targeted therapies have been emerging as a very attractive strategy to treat UC. In the present study, we demonstrate that BLG treatment results in significant alterations in the composition of gut microbiota, suggesting that the protective effect of BLG against DSS-induced colitis was associated with regulating gut microbiota. This observation was consistent with the notion that reprogramming the homeostasis of gut microbiota is an important way to understand the efficacy of TCM formulations.36,37 Notably, Akkermansia are gram-negative and strictly anaerobe bacteria living in the mucus layer of the intestine, which functions to degrade mucin, produce propionic acid, stimulate goblet-cell differentiation, and maintain the integrity of the mucosal barrier.26 Multiple clinical and animal data have shown that Akkermansia is highly associated with healthy mucosa,38 and oral administration of bacteria in the Akkermansia genus could significantly ameliorate mucosal inflammation.39 Our present data showed that the relative abundance of Akkermansia was significantly increased after BLG treatment. Further, Prevotellaceae_UCG-001 is an SCFA producing bacteria.27 More than one study demonstrated that a lower relative abundance of Prevotellaceae_UCG-001 was found in the feces of animal colitis.40,41 Our present data also showed that BLG treatment could sharply elevate the relative abundance of Prevotellaceae_UCG-001 in the colon of DSS-treated mice. On the contrary, Oscillibacter is a mesophilic, strictly anaerobic bacterium.42 It has been reported that the relative abundance of Oscillibacter was significantly increased in UC mice and significantly positively correlated with IL-6 and IL-1β levels and pathological score.43,44 Notably, BLG treatment significantly reduced the relative abundance of Oscillibacter in the feces of DSS-treated mice. Of note, these BLG-altered bacteria are the most SCFA-producing bacteria. Numerous previous studies have documented that SCFAs exert potential beneficial effects against colonic inflammation and protection of intestinal epithelial integrity.45,46 Our present data also observed that the concentrations of SCFA acetic acid, propionic acid, and butyric acid in the feces of DSS-treated mice were greatly increased after BLG treatment. Taken together, these findings clearly indicate that BLG treatment is effective to enhance SCFA-producing bacteria of DSS-induced chronic relapsing colitic mice.
GLP-1 is an incretin mainly produced in the ileum and the colon and plays an important role in delaying gastric emptying and lowers postprandial blood glucose.47 Recently, it found that GLP-1 based therapy represents a new treatment option for inflammatory bowel disease, evidenced by that dipeptidyl peptidase (DPP)-4, GLP-1 receptor agonists, and GLP-1 nanomedicine could effective alleviate gut inflammation in mice.48–51 As previous studies have reported that high SCFA concentrations have been associated with elevated plasma levels of GLP-1 in humans and mice.52 Our present data showed that the serum GLP-1 level and Gcg mRNA expression of DSS-treated mice was significantly elevated after BLG treatment. As well, compared to the stimulation of the extract of feces from DSS-treated colitis mice, the secretion of GLP-1 from the colonic culture was significantly increased after the stimulation of the extract of feces from BLG-treated colitis mice. How SCFAs influence the release of GLP-1? Gwen Tolhurst et al reported that SCFAs could stimulate GLP-1 secretion via GRP43 and GPR41.29 Our present data also indicated that BLG treatment significantly increased the mRNA expression of GRP43 and GPR41 in the colon of DSS-treated mice. These data indicated that BLG treatment could restore SCFA promoted-GLP-1 production via activation of GRP43 and GPR41.
Conclusions and Prospect
BLG is a long-standing over-the-counter (OTC) medicine in China, and no acute toxicity was observed at the maximum tolerance dose of 80g/Kg of BLG used in Kunming breed mice.53 At present, the recommended dosage of BLG (without sugar) in humans was 9–15g/day (for 3 times per day). Our study demonstrated that BLG at 1g/Kg could ameliorate DSS-induced chronic relapsing colitis in mice. This dosage was close to that of BLG used in clinics. Our study also found its action mechanism was at least partly mediated by alteration of gut microbiota, especially SCFA-producing bacteria, such as Akkermansia and Prevotellaceae_UCG-001, to restore gut derived-GLP-1 production. These findings suggest that BLG deserves further consideration as a potential therapeutic drug for clinical colitis treatment. However, its exact mechanisms in regulating gut microbiota are still needed to be confirmed using microbiota-deleted mice and fecal bacteria transplantation.
Funding Statement
This work was kindly funded by the National Natural Science Foundation of China (81560676 and 81660479), SZU Top Ranking Project (86000000210) and Foundations of Shenzhen Science and Technology Innovation Committee (JCYJ20210324093810026), Medical Science and Technology Research Foundation of Guangdong province, China (A2020157 and A2020272), and fund from Guizhou Provincial Key Laboratory of Pharmaceutics, Guizhou Medical University (YWZJ2020-01) and Peking University Shenzhen Hospital (JCYJ2018009).
Abbreviations
Ace, Acetic acid; But, Butyric acid; BLG, Ban-Lan-Gen; DSS, Dextran sulfate sodium; DAI, Disease activity index; DPP, Dipeptidyl peptidase; FID, Flame ionization detector; F-Con, The extract of feces from Control mice; F-DSS, The extract of feces from DSS colitis mice; F-BLG, The extract of feces from BLG-treated colitis mice; GLP-1, Glucagon-like peptide-1; Gcg, Proglucagon; GC, Gas chromatography; GRP43, G protein-coupled receptor 43; GRP41, G protein-coupled receptor 41; H&E, Hematoxylin-eosin; HBSS, Hanks’ balanced salt solution; OTC, Over-the-counter; PCA, Principal components analysis; PCoA, Principal coordinate analysis; Pro, Propionic acid; SASP, Sulfasalazine; SCFA, short-chain fatty acid; TCM, traditional Chinese medicine; UC, Ulcerative colitis.
Data Sharing Statement
All data generated during study has been included in the manuscript.
Ethics Approval
All experimental protocols were approved by the Animal Ethics Committees of Shenzhen Peking University-Hong Kong University of Science and Technology Medical Center (Shenzhen, China) in accordance with the “Institutional Guidelines and Animal Ordinance” (Ethics No. A2020157).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tang B, Zhu J, Zhang B, et al. Therapeutic potential of triptolide as an anti-inflammatory agent in dextran sulfate sodium-induced murine experimental colitis. Front Immunol. 2020;11:592084. doi: 10.3389/fimmu.2020.592084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 3.Peng J, Zheng TT, Li X, et al. Plant-Derived Alkaloids: the Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front Pharmacol. 2019;10:351. doi: 10.3389/fphar.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao HT, Peng J, Wen B, et al. Indigo Naturalis Suppresses Colonic Oxidative Stress and Th1/Th17 Responses of DSS-Induced Colitis in Mice. Oxid Med Cell Longev. 2019;2019:9480945. doi: 10.1155/2019/9480945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Ding Y, Tong Z. Efficacy and Safety of Sophora flavescens (Kushen) Based Traditional Chinese Medicine in the Treatment of Ulcerative Colitis: clinical Evidence and Potential Mechanisms. Front Pharmacol. 2020;11:603476. doi: 10.3389/fphar.2020.603476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao F, Liu J, Sha BX, Pan HF. Natural Products: experimental Efficient Agents for Inflammatory Bowel Disease Therapy. Curr Pharm Des. 2019;25:4893–4913. doi: 10.2174/1381612825666191216154224 [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol. 2013;44:274–283. doi: 10.1007/s12016-012-8328-9 [DOI] [PubMed] [Google Scholar]
- 8.Li ZT, Li L, Chen TT, et al. Efficacy and safety of Ban-Lan-Gen granules in the treatment of seasonal influenza: study protocol for a randomized controlled trial. Trials. 2015;16:126. doi: 10.1186/s13063-015-0645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Li L, Zhou H, et al. Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules. 2017;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan D, Liu W, Shi Y, et al. Protective Effects of Aqueous Extract of Radix Isatidis on Lipopolysaccharide-Induced Sepsis in C57BL/6J Mice. J Med Food. 2020;23:79–89. doi: 10.1089/jmf.2019.4476 [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Xie Y, Hu X, et al. Qualitative and quantitative analysis of glucosinolates and nucleosides in Radix Isatidis by HPLC and liquid chromatography tandem mass spectrometry. Acta Pharm Sin B. 2013;3:337–344. doi: 10.1016/j.apsb.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Zheng C, Li J, Yang L, Wang Z, Wang R. Separation and Quantification of Four Main Chiral Glucosinolates in Radix Isatidis and Its Granules Using High-Performance Liquid Chromatography/Diode Array Detector Coupled with Circular Dichroism Detection. Molecules. 2018;23:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–861. doi: 10.1007/s00535-016-1220-2 [DOI] [PubMed] [Google Scholar]
- 14.Tokuyasu N, Shomori K, Amano K, et al. Indirubin, a Constituent of the Chinese Herbal Medicine Qing-Dai, Attenuates Dextran Sulfate Sodium-induced Murine Colitis. Yonago Acta Med. 2018;61:128–136. doi: 10.33160/yam.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao W, Guo Y, Wang C, et al. Indirubin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice through the inhibition of inflammation and the induction of Foxp3-expressing regulatory T cells. Acta Histochem. 2016;118:606–614. doi: 10.1016/j.acthis.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Ercan G, Yigitturk G, Erbas O. Therapeutic effect of adenosine on experimentally induced acute ulcerative colitis model in rats. Acta Cir Bras. 2020;34:e201901204. doi: 10.1590/s0102-865020190120000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeengar MK, Thummuri D, Magnusson M, Naidu VGM, Uppugunduri S. Uridine Ameliorates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Sci Rep. 2017;7:3924. doi: 10.1038/s41598-017-04041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YH, Xiao HT, Hu DD, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227–239. doi: 10.1016/j.phrs.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 19.Xiao HT, Lin CY, Ho DH, et al. Inhibitory effect of the gallotannin corilagin on dextran sulfate sodium-induced murine ulcerative colitis. J Nat Prod. 2013;76:2120–2125. doi: 10.1021/np4006772 [DOI] [PubMed] [Google Scholar]
- 20.Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39:424–429. doi: 10.1038/ijo.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, He Y, Pan D, Cao J, Sun Y, Zeng X. Associations of Gut Microbiota With Heat Stress-Induced Changes of Growth, Fat Deposition, Intestinal Morphology, and Antioxidant Capacity in Ducks. Front Microbiol. 2019;10:903. doi: 10.3389/fmicb.2019.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Wang S, Sun Y, et al. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr Polym. 2020;230:115726. doi: 10.1016/j.carbpol.2019.115726 [DOI] [PubMed] [Google Scholar]
- 23.Tao JH, Duan JA, Jiang S, Guo JM, Qian YY, Qian DW. Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat by gas chromatography with flame ionization detector. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1029-1030:88–94. doi: 10.1016/j.jchromb.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 24.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Xu LZ, Zhao S, Shen ZF, Shen H, Zhan LB. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol. 2020;104:5449–5460. doi: 10.1007/s00253-020-10527-w [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Wu X, Cao S, et al. Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice. Nutrients. 2017;9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5 [DOI] [PubMed] [Google Scholar]
- 28.Foreman RE, George AL, Reimann F, Gribble FM, Kay RG. Peptidomics: a Review of Clinical Applications and Methodologies. J Proteome Res. 2021;20:3782–3797. doi: 10.1021/acs.jproteome.1c00295 [DOI] [PubMed] [Google Scholar]
- 29.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ducastel S, Touche V, Trabelsi MS, et al. The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids. Sci Rep. 2020;10:174. doi: 10.1038/s41598-019-56743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kou FS, Shi L, Li JX, et al. Clinical evaluation of traditional Chinese medicine on mild active ulcerative colitis: a multi-center, randomized, double-blind, controlled trial. Medicine. 2020;99:e21903. doi: 10.1097/MD.0000000000021903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao HT, Zhong L, Tsang SW, Lin ZS, Bian ZX. Traditional Chinese medicine formulas for irritable bowel syndrome: from ancient wisdoms to scientific understandings. Am J Chin Med. 2015;43:1–23. doi: 10.1142/S0192415X15500019 [DOI] [PubMed] [Google Scholar]
- 33.Liang X, Huang Y, Pan X, et al. Erucic acid from Isatis indigotica Fort. suppresses influenza A virus replication and inflammation in vitro and in vivo through modulation of NF-kappaB and p38 MAPK pathway. J Pharm Anal. 2020;10:130–146. doi: 10.1016/j.jpha.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naganuma M, Sugimoto S, Fukuda T, et al. Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: a post hoc analysis from the INDIGO study. J Gastroenterol. 2020;55:169–180. doi: 10.1007/s00535-019-01625-2 [DOI] [PubMed] [Google Scholar]
- 35.Chiba M, Nakane K, Tsuji T, et al. Relapse Prevention by Plant-Based Diet Incorporated into Induction Therapy for Ulcerative Colitis: a Single-Group Trial. Perm J. 2019;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue SJ, Wang WX, Yu JG, et al. Gut microbiota modulation with traditional Chinese medicine: a system biology-driven approach. Pharmacol Res. 2019;148:104453. doi: 10.1016/j.phrs.2019.104453 [DOI] [PubMed] [Google Scholar]
- 37.Feng W, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 38.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian X, Wu W, Yang L, et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front Microbiol. 2019;10:2259. doi: 10.3389/fmicb.2019.02259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu L, Jin L, Xia D, et al. Nitrate ameliorates dextran sodium sulfate-induced colitis by regulating the homeostasis of the intestinal microbiota. Free Radic Biol Med. 2020;152:609–621. doi: 10.1016/j.freeradbiomed.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 41.Zou J, Shen Y, Chen M, et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104:5999–6012. doi: 10.1007/s00253-020-10665-1 [DOI] [PubMed] [Google Scholar]
- 42.Katano Y, Fujinami S, Kawakoshi A, et al. Complete genome sequence of Oscillibacter valericigenes Sjm18-20(T) (=NBRC 101213(T)). Stand Genomic Sci. 2012;6:406–414. doi: 10.4056/sigs.2826118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu M, Li P, An Y, et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150:104489. doi: 10.1016/j.phrs.2019.104489 [DOI] [PubMed] [Google Scholar]
- 44.Wang CS, Li WB, Wang HY, et al. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J Gastroenterol. 2018;24:4254–4262. doi: 10.3748/wjg.v24.i37.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009 [DOI] [PubMed] [Google Scholar]
- 47.Zatorski H, Salaga M, Fichna J. Role of glucagon-like peptides in inflammatory bowel diseases-current knowledge and future perspectives. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1321–1330. doi: 10.1007/s00210-019-01698-z [DOI] [PubMed] [Google Scholar]
- 48.Anbazhagan AN, Thaqi M, Priyamvada S, et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine. 2017;13:659–665. doi: 10.1016/j.nano.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villumsen M, Schelde AB, Jimenez-Solem E, Jess T, Allin KH. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine. 2021;37:100979. doi: 10.1016/j.eclinm.2021.100979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning MM, Yang WJ, Guan WB, Gu YP, Feng Y, Leng Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protected against dextran sulfate sodium-induced experimental colitis by potentiating the action of GLP-2. Acta Pharmacol Sin. 2020;41:1446–1456. doi: 10.1038/s41401-020-0413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato S, Sato T, Fujita H, Kawatani M, Yamada Y. Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms. Sci Rep. 2021;11:9167. doi: 10.1038/s41598-021-88612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larraufie P, Martin-Gallausiaux C, Lapaque N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q. Study on acute toxicity of the compound Radix Spray. Sci Edu Article Coll. 2013;3:92–93. [Google Scholar]