SUMMARY
The human gut microbiota resides within a diverse chemical environment challenging our ability to understand the forces shaping this ecosystem. Here, we reveal that fitness of the Bacteroidales, the dominant order of bacteria in the human gut, is an emergent property of glycans and one specific metabolite, butyrate. Distinct sugars serve as strain-variable fitness switches activating context-dependent inhibitory functions of butyrate. Differential fitness effects of butyrate within the Bacteroides are mediated by species-level variation in Acyl-CoA thioesterase activity and nucleotide polymorphisms regulating an Acyl-CoA transferase. Using in vivo multi-omic profiles, we demonstrate Bacteroides fitness in the human gut is associated together, but not independently, with Acyl-CoA transferase expression and butyrate. Our data reveal that each strain of the Bacteroides exists within a unique fitness landscape based on the interaction of chemical components unpredictable by the effect of each part alone mediated by flexibility in the core genome.
Graphical Abstract

In brief
Each member of the human gut microbiome Bacteroides has a unique fitness landscape whereby depending on which glycan a strain uses, the metabolite butyrate is differentially inhibitory, and defense to butyrate is mediated by genetic variation among Bacteroides in Acyl-CoA metabolism.
INTRODUCTION
The gut microbiome is composed of an enormous magnitude and diversity of microorganisms residing in a chemically and molecularly complex ecosystem. Increasing evidence has highlighted the critical role of individual species’ and strains’ roles of the microbiome in human health, such as in protection from infection (Leshem et al., 2020; Pamer, 2016), immunity (Belkaid and Harrison, 2017; Honda and Littman, 2016; Macpherson et al., 2017), efficacy of vaccines (Hagan et al., 2019), immunotherapy (Zitvogel et al., 2018), drug metabolism (Koppel et al., 2017; Zimmermann et al., 2019), nutrition (Kolodziejczyk et al., 2019), and a multitude of human diseases (Barcik et al., 2020; Elinav et al., 2019; Plichta et al., 2019; Sherwin et al., 2019). Given the widespread impact of the microbiome on human health, there is an imperative to understand the forces that shape the microbiome, in particular at the community and strain level (Ferreiro et al., 2018; Foster et al., 2017; Schmidt et al., 2018).
Carbohydrates are a major factor shaping the composition of the gut microbiome and mediate many of the effects of the microbiome on human health (Koropatkin et al., 2012; Porter and Martens, 2017; Sonnenburg and Bäckhed, 2016). Sugars such as dietary glycans are critical determinants of the fitness of the Bacteroidales, the predominant order of bacteria in the gut (Arumugam et al., 2011; Eckburg et al., 2005). The Bacteroidales are central to host health and disease, with specific Bacteroidales species and strains playing protective and pathogenic roles in human physiology and disease (Mazmanian et al., 2008; Wexler and Goodman, 2017). Survival of the Bacteroidales in the mammalian gut is dependent on the ability of each individual member to utilize and compete for the diverse array of glycans in the intestine (Patnode et al., 2019; Rakoff-Nahoum et al., 2014; Sonnenburg et al., 2010).
In addition to the wide range of carbohydrates, the gut ecosystem is rich in chemical and metabolic diversity (Donia and Fischbach, 2015; Integrative HMP (iHMP) Research Network Consortium, 2019). The chemical and metabolic composition of the gut, derived from components of diet as well as microbial and host metabolism, has important roles in human health (Sonnenburg and Bäckhed, 2016). One class of small molecules, the short-chain fatty acids (SCFAs), primary metabolites of host and microbial metabolism, is both abundant and dynamic across health and disease. SCFAs are critical to host activities from colonic epithelial metabolism and homeostasis, immunoregulation, cancer, and the gut-brain axis (Koh et al., 2016; Rooks and Garrett, 2016). The impact of the chemical and metabolite diversity of the intestinal ecosystem on the gut microbiome is largely unknown.
Here, we reveal that a microbial-derived small molecule, butyrate, is a context-dependent inhibitor of the human gut Bacteroidales. We find that fitness of Bacteroidales is a function of sugars and butyrate, whereby butyrate acts as a fitness-switch dependent on which specific sugar is utilized. Individual strains have idiosyncratic butyrate fitness switches across diverse sugars. The mechanism of species- and sugar-dependent inhibitory effects of butyrate is genetic variation in the function and regulation of Acyl-CoA metabolism within the Bacteroides. Our work demonstrates that strain-level fitness of the dominant order of bacteria in the human gut is an emergent property of specific dietary glycans and one microbial-derived small molecule butyrate, mediated by enzymatic and transcriptional regulatory variation in the core genome.
RESULTS
Butyrate displays strain-dependent inhibitory activity against the Bacteroidales
As abundant gut ecological commodities, we asked if the primary metabolite SCFAs affected the fitness of the Bacteroidales (Figure 1). We assayed a panel of SCFAs prevalent in the human gut (Koh et al., 2016) composed of a range of length (1–4 carbons), isomerization, and number of carboxylic acids groups (1 or 2) at physiological concentrations for effects on growth of a panel of 7 species of the Bacteroidales. Most SCFAs had either no or minimal effects on the Bacteroidales. However, the C4-monocarboxylated SCFA butyrate, exclusively produced by microbes (Lavelle and Sokol, 2020), was found to be highly growth inhibitory to five of the seven Bacteroidales species tested (B. eggerthi, Be; B. ovatus, Bo; B. uniformis, Bu; B. vulgatus, Bv; Parabacteroides merdae, Pm), without notable effects on two (B. fragilis, Bf; B. thetaiotaomicron, Bt). Growth inhibition was specific to butyrate as compared to minimal fitness effects of the C3-monocarboxylated SCFA, propionate, or the C4-dicarboxylated SCFA, succinate.
Figure 1. The effect of microbial metabolites on growth of Bacteriodales.
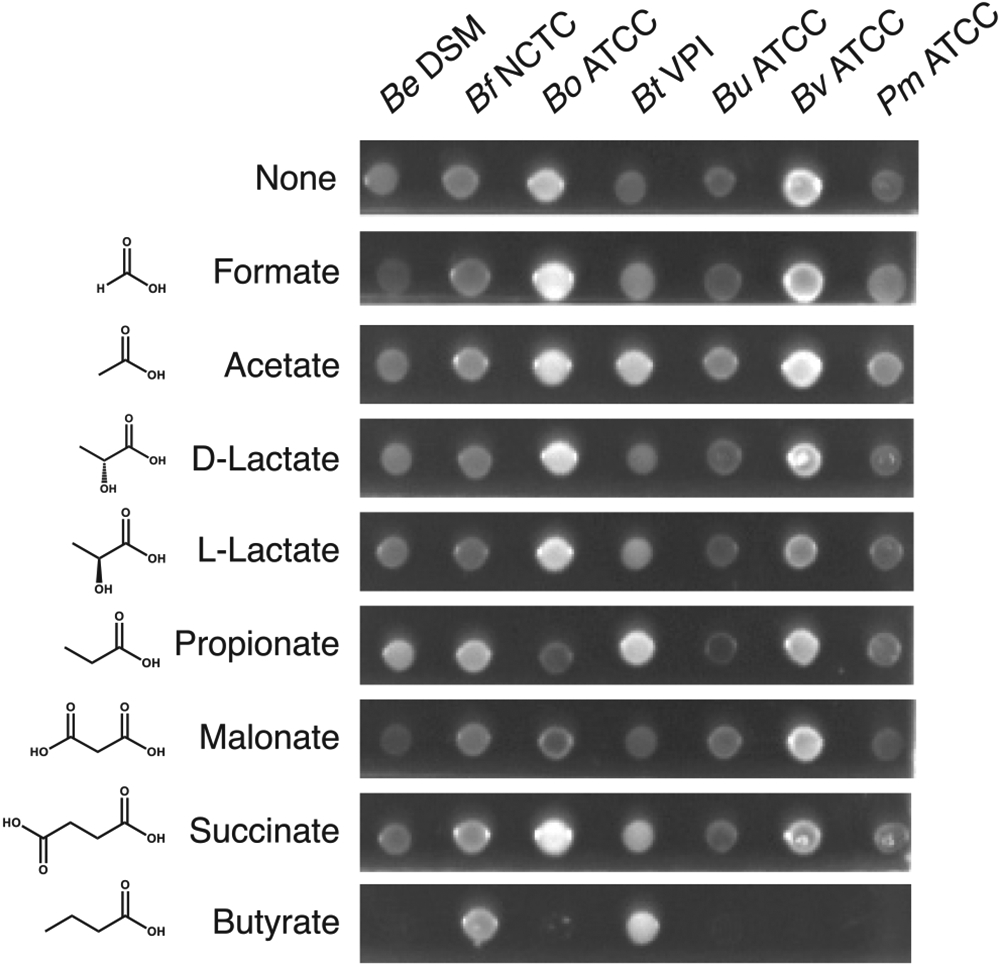
Type strains of Bacteriodales were grown in the presence or absence of SCFAs with Glc as the carbon source. Be DSM: B. eggerthii DSM 20697, Bf NCTC: B. fragilis NCTC 9343, Bo ATCC: B. ovatus ATCC 8483, Bt VPI: B. thetaiotaomicron VPI-5482, Bu ATCC: B. uniformis ATCC 8492, Bv ATCC: B. vulgatus ATCC 8482, Pm ATCC: P. merdae ATCC 43184. Shown is a representative of three biological replicates.
We next examined the fitness effects of butyrate on an expanded collection of 53 human gut Bacteroidales isolates, encompassing multiple strains within a species (Figure 2A). Within the species Bo and Bv, for example, Bo CL03 and Bv CL09 strains were resistant to butyrate, while Bo ATCC 8483 and Bv ATCC 8482 strains were sensitive (Figure 2A). Together, these results identified the specific microbial metabolite butyrate as inhibitory to the Bacteroidales at the species and strain level.
Figure 2. Strain-level fitness in the Bacteroides is dependent on the interactions of sugars and butyrate.
(A) Heatmap of sensitivity to butyrate as a function of monosaccharide utilization (See STAR Methods for details on inhibition scoring). Row presents the phylogenic tree of the Bacteroides and Parabacteroides.
(B–E) Strains as indicated were grown in monosaccharides (B), human milk oligosaccharides (C), glycans (D), and monosaccharides and cognate homodimers disaccharides (E) with and without butyrate. See also Figures S1D and S1E.
(F) B. nordii CL02, B. ovatus ATCC, B. salyersiae DSM, and B. caccae ATCC grown in inulin or pectin −/+ butyrate.
(G) Schematic of gnotobiotic experiments (top). Log fold change of relative abundance (post/pre butyrate in drinking water for given dietary glycan) of Bn CL02, Bo ATCC, Bs DSM, and Bc ATCC (bottom). p value by two-tailed Student’s t test as indicated (n = 5/group). See STAR Methods for details.
Shown is a representative of two biological replicates in (A–F).
Bacteroidales fitness is dependent on interactions of sugars and butyrate
The Bacteroidales use a diverse range of monosaccharides for energy to compete within the mammalian intestine (Koropatkin et al., 2012). We next assayed whether Bacteroidales susceptibility to butyrate was robust to a range of monosaccharides. These experiments yielded the striking finding that the inhibitory activity of butyrate was conditional on the monosaccharide utilized (Figure 2). For example, strains highly resistant to butyrate when grown in glucose (Glc) as the sole carbon source were rendered severely sensitive when utilizing N-Acetylgalactosamine (GalNAc) (Figures 2A and 2B). This inhibitory property was specific to butyrate, as growth in GalNAc did not render Bacteroidales strains sensitive to other SCFAs (Figure S1A). The differential inhibitory effects of butyrate across strains and sugars were not associated with growth rate (Figure S1B). Inhibition by butyrate was observed at concentrations as low as 2 mM (Figure S1C) within the physiological range of butyrate in the mammalian gut (Cummings et al., 1987; Koh et al., 2016). We reveal a hierarchy of strength of monosaccharides to tune fitness effects of butyrate: Glc, fructose (Fru) and mannose (Man) render most Bacteroidales resistant to butyrate, N-Acetylglucosamine (GlcNAc), galactose (Gal) and xylose (Xyl) mediate intermediate sensitivity, and rhamnose, ribose, and GalNAc render strains highly sensitive to butyrate (e.g., Bt VPI-5482) (Figures 2A and 2B). Importantly, we observed variation in the hierarchical strength of individual monosaccharides to tune butyrate-mediated Bacteroides fitness. For example, B. fragilis 9343 is highly resistant to butyrate in GlcNAc but highly sensitive in Xyl, whereas B. ovatus CL03 is substantially more resistant to butyrate in Xyl than in GlcNAc (Figures 2A and 2B). Together, these data reveal that butyrate interacts with individual monosaccharides on the fitness of the Bacteroidales. These interactions occur as a function of context, whereby the strength of distinct sugars differentially tunes butyrate-mediated fitness (sugar-strength), and as a function of intrinsic properties of the microbe in the same sugar, whereby specific strains are differentially resistant to butyrate (strain-strength).
While monosaccharides are the monomeric terminal products entering central metabolism, dietary oligomeric and polymeric glycans (which arrive undigested to the colon as microbiota-accessible carbohydrates; MACs) (Sonnenburg and Bäckhed, 2016) are critical forms of glycan utilized by the Bacteroides and are major drivers of Bacteroides composition (Patnode et al., 2019; Sonnenburg et al., 2010). Across a panel of 7 distinct dietary glycans there was a range of strain-level variation in susceptibility to butyrate (Figure S1D). Among B. fragilis species, utilizers of human milk oligosaccharides (HMOs) critical for colonizing the infant gut (Charbonneau et al., 2016; Marcobal et al., 2011), we observed a within-species, strain-level hierarchical strength of HMOs to tune butyrate-mediated fitness with strength increasing from 3-SL to LnNT to 2-FL (Figure 2C). As exemplified by Bo CL03, distinct plant- and animal-derived glycans can control the fitness effects of butyrate depending on which MAC is being utilized (Figure 2D). Cumulatively, these results demonstrate that depending on which MAC among the glycan diversity of the human diet composed of milk, plant, and animal glycans a given Bacteroides strain utilizes, there is a dynamic strain-specific range of fitness effects of butyrate.
Unpredictable interactions of glycan components with butyrate on strain-level fitness
Comparing the fitness effects of Bacteroides strains for monosaccharides and MACs revealed a striking discordance of the inhibitory effects of butyrate for specific polymers and the monomers of which the glycan is composed (cognate polymer-monomer pairs). For some MACs, there was concordance between cognate polymer-monomer pairs, such as Bacteroides members resistant to butyrate in the glucan pullulan and the fructan levan, which also demonstrated resistance to butyrate in Glc or Fru monomers (Figures 2A, 2B, 2D, and S1B). However, for certain polymers, we observed phenotypic discordance in the effects of butyrate for polymer-monomer pairs. For example, Bo CL03 was highly resistant to butyrate in the monomers arabinose, Gal, Glc, and Man but highly sensitive to butyrate in cognate heteropolymers, arabinogalactan, orglucomannan (Figures 2A, 2B, and 2D). Sensitivity to butyrate in these polymers was not a property of the individual monomers of these heteropolymers in combination (Figure S1E).
We next analyzed the inhibitory function of butyrate for Bacteroides species in cognate pairs of monomer and homo- and hetero-dimers. Bo CL03, resistant to butyrate in Glc and Fru, was also resistant in the Glc homodimer maltose (α1,4) and the Glc-Fru heterodimer sucrose (α1,β2) (Figure 2E). However, this strain was highly sensitive to butyrate in the Glc homodimers cellobiose (β1,4) and the Glc-Fru heterodimer palatinose (α1,6) (Figure 2E). Furthermore, we find species-level variation in discordance of butyrate-mediated fitness between monomers and dimers. B. xylanisolvens (Bx) CL03 was resistant to butyrate in Glc but sensitive in all Glc homodimers including cellobiose (Figure 2E). In contrast, Bu CL03 was resistant to butyrate in maltose and cellobiose (Figure 2E). Together, these results demonstrate species-level properties of glycan components independent of their overall combination in mediating the fitness effects of butyrate on the Bacteroides.
Butyrate affects microbiome community composition in a glycan-dependent manner
The composition of Bacteroides communities is driven by competition based on utilization of dietary glycans (Patnode et al., 2019; Sonnenburg et al., 2010). Our findings suggested that the composition of Bacteroides communities could be a property of species- and glycan-specific fitness as a function of both the specific glycan and butyrate. To determine if butyrate impacts the relative abundance of Bacteroides species in vivo, we colonized germ-free mice that were fed diets with either one or both of two prebiotic and health-relevant MACs, inulin and pectin (Koh et al., 2016), as the sole dietary glycan with a community of four Bacteroides species demonstrating distinct fitness effects of butyrate as a function of inulin and pectin in vitro (Figure 2F). Administration of butyrate led to significant impact on the fitness of each of the four species of Bacteroides in mice fed both dietary glycans (Figure 2G). With the exception of Bc, the fitness effects of butyrate on each Bacteroides species were glycan specific, with significant differences of the impact of butyrate on each Bacteroides species fitness depending on the specific dietary glycan (Figure 2G) correlating with the glycan-specific fitness effects of butyrate on each species in vitro. Together, these experiments demonstrate both that butyrate can impact the relative fitness of Bacteroides in vivo and impact species-level fitness in a glycan-dependent manner.
Butyrate induces sugar- and species-dependent stress responses
We next sought to determine the mechanistic basis of the interaction between butyrate and sugars on Bacteroides species-level fitness. To begin, we investigated the global transcriptional responses via RNA sequencing (RNA-seq) of the butyrate-resistant B. thetaiotaomicron VPI 5482 (herein Bt) and the butyrate-sensitive B. vulgatus ATCC 8482 (herein Bv) in Glc after a short pulse with different SCFAs (Table S2). Few genes were specifically regulated by butyrate in either strain, with the notable exception of upregulation in Bt of genes involved in biosynthesis of branched-chain amino acids (BCAAs) (ilv and leu operons) (Figures 3A and S2A). In both species, all SCFAs downregulated NADH-dehydrogenase genes (Figure 3A), which have been shown to be upregulated in response to intracellular acidification in bacteria (Kannan et al., 2008), suggesting that species-specific resistance was not a result of differential intracellular acidification by butyrate. Exposure to SCFAs did not result in intracellular acidification in the butyrate-sensitive Bv, nor did we observe differences in intracellular pH between inhibitory (butyrate) or non-inhibitory SCFAs (acetate or succinate) (Figure S2B). To determine the transcriptional response to butyrate as a function of sugar strength, we compared transcriptional responses of Bv to butyrate when grown in Glc, GlcNAc, Gal, or Xyl, which have increasing tuning of butyrate sensitivity (Table S2). Across sugars there was greater induction of stress-response genes such as Hsp family members and GroES with increasing sugar strength (Figure 3B). This suggests that butyrate caused a different degree of cellular stress depending on which sugar was being utilized. Indeed, we found differential cell membrane damage by butyrate dependent on sugar, with minimal membrane damage and bacteriostatic effect of butyrate in Glc in Bv, but not Bt other SCFAs, and severe membrane damage and subsequent cell death after treatment with butyrate in Gal (Figures 3C, 3D, and S2C). Together, these results demonstrate that butyrate induces species-specific cellular stress responses, membrane damage, and cell death in the Bacteroides and that the strength of cellular stress and bacteriostatic versus bactericidal function is sugar dependent.
Figure 3. Butyrate induces sugar-dependent stress responses and membrane damage.
(A) RNA-seq of Bt VPI (left) and Bv ATCC (right) after 1 h pulse with and without specific SCFAs in Glc. Heatmap illustrates expression of genes regulated by butyrate (∣log2(fold change)∣> 1, padj < 0.05) and the differential expression of these genes in acetate and/or succinate. See also Figure S2A and Table S2.
(B) RNA-seq of Bv ATCC after 1 h pulse −/+ butyrate in various sugars. Volcano plots showing the significance and fold change of genes in each sugar condition (right). Significantly differentially expressed genes are shown in red (∣log2(fold change)∣> 1, padj < 0.05). Heatmap illustrates gradient of differential expression of significant genes across sugars in response to butyrate (left). See also Table S2 and STAR Methods.
(C) Scatterplot of flow cytometry analysis of low and high propidium iodide (PI) staining of Bt VPI and Bv ATCC with and without specific SCFAs in Glc or Gal. See also Figure S2C and STAR Methods for gating strategy.
(D) Colony forming units of Bv ATCC after pulse −/+ butyrate in Glc or Gal.
In (A and B), fold change and padj were calculated for two biological replicates. In (C and D), error bars represent mean ± SEM of three biological replicates. Nonsignificant p > 0.05, *p < 0.05, ***p < 0.001, ****p < 0.0001; paired two-tailed t test.
An Acyl-Coenzyme A metabolic defense system in the Bacteroides
To dissect the mechanism by which butyrate is differentially inhibitory to the Bacteroides, we constructed a genome-wide transposon (Tn) mutagenesis library of Bt to identify the genetic determinants of butyrate resistance in Bt which demonstrates high level resistance to butyrate. Tn insertion in the BT_3942 gene conferred the greatest fitness disadvantage in butyrate among 14 genes with significant loss of fitness (Figure 4A). BT_3942 is an Acyl-coenzyme A (Acyl-CoA) thioesterase, predicted to hydrolyze Acyl-CoAs into free fatty acyl groups and coenzyme A (CoA) (Figure 4B). Targeted non-polar deletion of BT_3942 demonstrated sensitivity to butyrate at concentrations as low as 2 mM (Figure S3A) and in complex media with variable sugar concentration (Figure S3B). Complementation of Bt Δ3942 confirmed that this Acyl-CoA thioesterase gene confers resistance to butyrate (Figure 4C). Resistance was specific to butyrate as Bt Δ3942 did not become sensitive to other SCFAs (Figure S3C). Together, these data demonstrate that resistance to butyrate in Bt is mediated by an Acyl-CoA thioesterase.
Figure 4. An Acyl-CoA metabolic defense system in the Bacteroides.
(A) Tn-seq of Bt VPI grown in Glc −/+ butyrate. Specific genes are highlighted in green (BT_3942) and blue (ilv and leu operons). See also Table S3.
(B) Genomic locus of BT_3942 encoding Acyl-CoA thioesterase and predicted enzymatic reaction.
(C) Bt WT, Bt Δ3942 and the complemented strain grown in Glc −/+ butyrate.
(D) Tn-seq of Bv ATCC grown in Glc −/+ butyrate. Specific genes are highlighted in green (BVU_1164/ 1163 operon). See also Table S3.
(E) Genomic locus of the BVU_1164/1163 operon encoding a hypothetical protein and Acyl-CoA transferase and predicted enzymatic reaction.
(F) Bv WT, Bv Δ1163, and the complemented strain (two left), and Bt WT complemented with BVU_1163 (third from left). Bv Δ1163 complemented with BT_3942 (right) grown in Glc −/+ butyrate.
(G) Relative abundance of 13C butyryl-CoA in Bt WT, Bv WT, and their isogenic mutant strains 30 min post pulse at mid log phase in Glc with 13C butyrate. See also Figure S3E.
(H) Relative abundance of Acyl-CoAs in Bt WT, Bv WT, and their isogenic strains, as indicated, in Glc with butyrate or water control. See also Figure S3G.
In (A and D), plots are the combined fold change and p value from two independent replicates (See STAR Methods). Shown is a representative of three biological replicates in (C and F). Error bars represent mean ± SEM of two (G) and three (H) biological replicates. Nonsignificant p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; unpaired two-tailed t test in (G), two-way ANOVA with Tukey’s multiple comparisons test in (H).
To identify the genetic determinants that confer butyrate sensitivity in Bv, which demonstrates high sensitivity to butyrate, we performed transposon sequencing (Tn-seq) in Bv (Table S3). Tn insertion in the genes BVU_1163 and BVU_1164, which compose an operon, generated the greatest gain of resistance to butyrate among 91 genes with significant gain of resistance phenotype (Figures 4D and 4E). BVU_1163 is annotated as a putative Acyl-CoA transferase predicted to reversibly transfer CoAs from Acyl-CoAs to acetate (Figure 4E). Enzymatic assays with recombinant protein BVU_1163 revealed transferase activity for propionyl, butyryl, and succinyl-CoA substrates, but not the medium-chain hexanoyl-CoA (Figure S3D). Deletion of BVU_1163 (Bv Δ1163) rendered Bv partially resistant to butyrate in Glc, which was phenotypically complemented by expression of BVU_1163 (Figure 4F). These findings demonstrate that sensitivity to butyrate in Bv is mediated by an Acyl-CoA transferase. Expression of Acyl-CoA thioesterase BT_3942 conferred resistance to the naturally sensitive Bv, while expression of the Acyl-CoA transferase BVU_1163 conferred sensitivity in the naturally resistant Bt, demonstrating a common, opposing pathway of Acyl-CoA metabolism susceptibility in these two strains (Figure 4F). Together, our data demonstrate that Acyl-CoA metabolism acts in metabolic defense to the inhibitory effects of butyrate in the Bacteroides.
Butyrate uptake and metabolism by the Bacteroides
To determine if extracellular butyrate is taken up and converted metabolically by the Bacteroides, we pulsed naturally resistant Bt and naturally sensitive Bv with 13C butyrate and measured 13C labeling of intracellular Acyl-CoA metabolites in bacterial lysates after 30 min. 13C labels were present in the forms of butyryl-CoA and to a lesser extent, acetyl-CoA, but not propionyl CoA in both Bt and Bv with notably a greater extent of 13C-incorporation into butyryl-CoA in Bv (Figures 4G and S3E).
We next measured Acyl-CoA and free CoA pools after butyrate pulse yet before growth inhibition (Figure S3F) in Bt wild type (WT) and the butyrate-sensitive isogenic mutant Bt Δ3942 as well as in Bv WT and the partially butyrate-resistant isogenic mutant Bv Δ1163. In Bt WT, there was no significant change after butyrate pulse in acetyl-CoA, propionyl-CoA, or free CoA but moderate yet significant accumulation of butyryl-CoA (2-fold) (Figures 4H and S3G). In Bv WT, butyrate pulse led to a greater increase in butyryl-CoA levels compared to Bt and depletion of acetyl-CoA, propionyl CoA, and free CoA (Figures 4H and S3E). There was significant accumulation of butyryl-CoA in the butyrate-sensitive Bt Δ3942 compared to Bt WT and depletion of acetyl-CoA and CoA, but not of propionyl-CoA (Figures 4H and S3G). Accumulation of butyryl-CoA as a result of BT_3942 deletion was confirmed by 13C butyrate tracing (Figure 4G). Propionyl-CoA levels were unchanged between Bv WT and Bv Δ1163 (Figure 4H). Bv Δ1163, despite being partially sensitive to butyrate, had higher levels of acetyl-CoA after butyrate pulse then that of healthy Bv WT in the absence of butyrate (Figure 4H). Bv Δ1163 demonstrated significantly less accumulation of butyryl-CoA after butyrate pulse compared to Bv WT (Figures 4H and S3G). Decreased accumulation of butyryl-CoA as a result of BVU_1163 deletion was confirmed by 13C butyrate tracing (Figure 4G). Together, these data demonstrate that extracellular butyrate is taken up by Bacteroides and metabolized predominantly into butyryl-CoA, differentially impacts Acyl-CoA pools among Bacteroides species and as a function of Acyl-CoA thioesterase and Acyl-CoA transferase activity, and that butyryl-CoA accumulation correlates with the toxic effects of butyrate.
Sugar tuning of butyrate-mediated fitness via synergy in Acyl-CoA metabolism
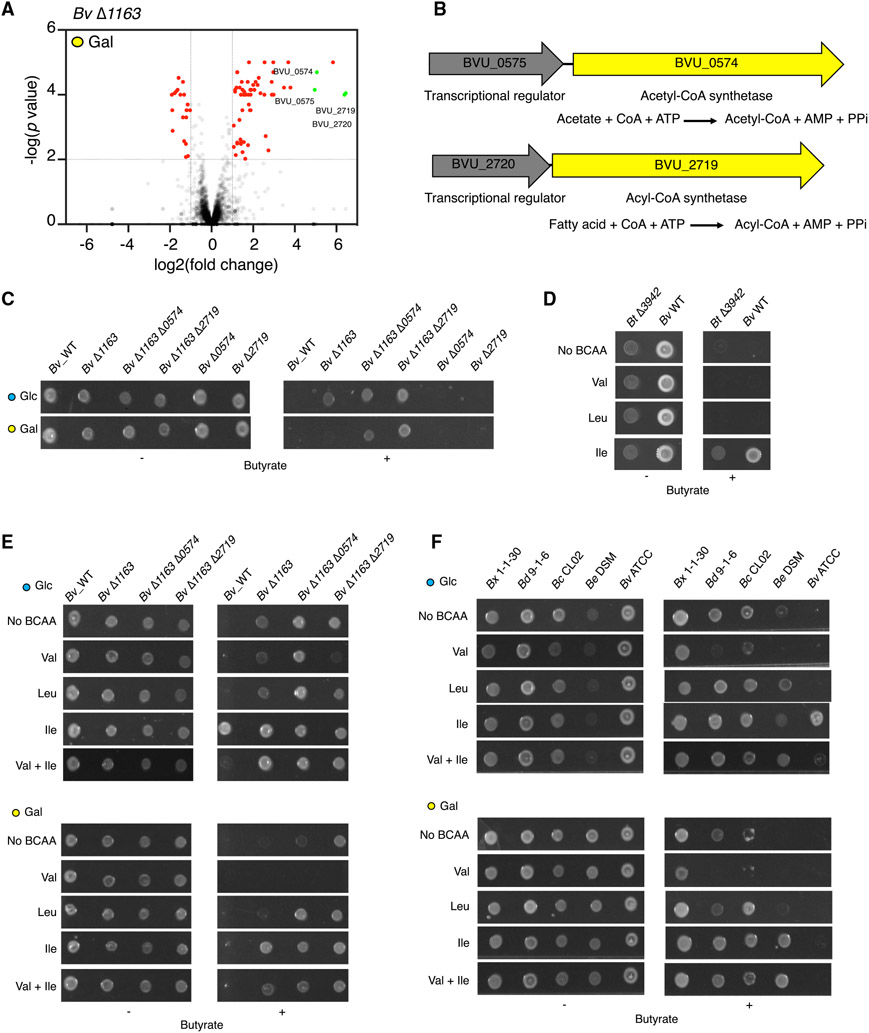
In the absence of the Acyl-CoA transferase, Bv Δ1163 is partially resistant to butyrate in Glc but remains sensitive in Gal (Figure S4A), suggesting the existence of mechanisms in addition to the Acyl-CoA transferase BVU_1163 that mediate sugar-specific sensitivity to butyrate. To gain insight into the mechanism by which different sugars tune Bacteroides fitness to butyrate, we performed a synthetic fitness Tn-seq in Bv Δ1163 in Gal (Table S3). Four of the top five genes in which insertions leading to the greatest gain of fitness to butyrate in Gal occurred in two operons, BVU_2720/2719 and BVU_0575/0574 (Figure 5A). Remarkably, both BVU_0574 and BVU_2719 are predicted Acyl-CoA synthetases (Figure 5B). Notably, BVU_2719 (and the Bt homolog, BT_2782) was induced to a greater extent in Gal compared to Glc in both Bv and Bt and is repressed by butyrate in Bv in Gal (Figure S4B). Deletion of the BVU_0574 or BVU_2719 resulted in full resistance to butyrate in Glc and Gal in the Bv Δ1163 background but not in Bv WT background (Figure 5C). The Acyl-CoA synthetases did not have the same synergistic strength, as Bv Δ1163 Δ2719 showed greater resistance to butyrate in Gal compared with Bv Δ1163 Δ0574 (Figure 5C). Importantly, butyrate pulse in the Bv Δ1163 Δ0574 strain did not result in accumulation of butyryl-CoA, consistent with full resistance to butyrate (Figure S4C).
Figure 5. Synergy in Acyl-CoA metabolism interacts with BCAAs to tune sugar-dependent inhibitory effects of butyrate.
(A) Tn-seq of Bv Δ1163 grown in Gal −/+ butyrate. Two operons, BVU_0575/0574 and BVU_2720/2719, are highlighted in green. Fold change and p value were calculated by combining two biological replicates. See also Table S3.
(B) Genomic locus of BVU_0575/0574 encoding transcriptional regulator and Acetyl-CoA synthetase, and BVU_2720/2719 encoding transcriptional regulator and Acyl-CoA synthetase and predicted enzymatic reactions.
(C) Bv WT and isogenic strains with deletions in Acyl-CoA transferase (BVU_1163) and Acyl-CoA synthetases (BVU_2719, BVU_0574) grown in Glc or Gal −/+ butyrate.
(D) Bt Δ3942 and Bv WT grown in Glc supplemented with Val, Leu, or Ile −/+ butyrate.
(E and F) Bv WT and isogenic strains with deletions in Acyl-CoA transferase (BVU_1163) and Acyl-CoA synthetases (BVU_2719, BVU_0574) (E) and natural human gut Bacteroides strains (F) grown in Glc or Gal supplemented with Val, Leu, or Ile −/+ butyrate.
Shown is a representative of three biological replicates in (C–F).
To address genetic mechanisms mediating the fitness effects of butyrate at the strain-level, we performed Tn-seq in Bv CL09 which is resistant to butyrate in Glc but sensitive in Gal and Xyl (Figures 2A and 2B) after selection in Gal in the presence of butyrate (Table S3; Figure S4D). This revealed gain-of-resistance insertions in AGXZ_01713 (Acyl-CoA transferase BVU_1163) and AGXZ_02243 and AGXZ_00341 (each of the Acyl-CoA synthetases, BVU_0574 and BVU_2719), each of which is 100% identical by amino acid (AA) between Bv ATCC and Bv CL09 (Table S3; Figure S4D). In Bv CL09, deletion of BVU_1163 or BVU_2719 conferred resistance to butyrate in Gal but not Xyl (Figure S4E). Deletion of both BVU_1163 and BVU_2719 resulted in resistance to butyrate in Xyl (Figure S4E). Together, these data demonstrate that synergy in Acyl-CoA metabolic genes mediates the hierarchical strength of sugars on fitness effects of butyrate in the Bacteroides at the species and strain level.
Coenzyme A metabolic defense is tuned by a hierarchy of branched-chain AAs
Tn-seq in butyrate-resistant Bt revealed that 7 of the 14 genes with significant loss of resistance to butyrate belonged to the ilv (4 of 5 genes) and leu (3 of 5 genes) operon (Figures 4A and S4F), many of which were upregulated in Glc compared to Gal (Table S4). We reasoned that the BCAAs, end products of biosynthesis mediated by ilv and leu operons, might control resistance to butyrate in Bt. Remarkably, isoleucine (Ile), but not leucine (Leu) or valine (Val), conferred resistance to butyrate in Bt Δ3942 and Bv in Glc (Figure 5D) but not in Gal (Figure 5E). However, in Bv Δ1163, Ile conferred resistance to butyrate in Gal (Figure 5E). In Bv Δ1163 Δ0574, but not Bv Δ1163, Leu conferred resistance to butyrate in Gal (Figure 5E). Unexpectedly, in Bv Δ1163 Δ2719, which is resistant to butyrate in Glc and Gal, we observed that the addition of Val resulted in reversal of resistance, rendering this mutant sensitive to butyrate via a pathway counteracted by Ile (Figure 5E). We found examples of sugar-specific BCAA tuning in natural strains (Figure 5F) analogous to each Bv mutant of Acyl-CoA metabolism (Figure 5E). For example, Ile, but not Leu, conferred resistance in Gal in Be DSM (analogous to Bv Δ1163 in Gal), while Leu, but not Val, was able to confer resistance to butyrate in Glc (analogous to Bv Δ1163 Δ0574 in Gal). Addition of Val resulted in reversal of resistance in B. dorei 9-1-6 (analogous to Bv Δ1163 Δ2719) but not in Bx 1-1-30 (Figure 5F). These results suggest that Bacteroides species have a spectrum of Acyl-CoA metabolic states tunable by specific sugars and distinct BCAAs.
Species-level variation in Acyl-CoA thioesterase function among the Bacteroides
We next sought to determine the mechanism of species-level variation of the fitness effects of butyrate among the Bacteroides. Comparison of AA sequences revealed that the predicted Acyl-CoA thioesterase BT_3942 was poorly conserved among the Bacteroides (Figure S5A), sharing only 51% AA identity with a homolog BVU_0767 from butyrate-sensitive Bv (Figures 6A and S5B). Expression of BT_3942, but not BVU_0767, conferred resistance to both Bt Δ3942 and Bv WT (Figure 6B). Recombinant protein BT_3942 and BVU_0767 demonstrated different substrate specificity for hydrolysis of short chain lengths of Acyl-CoAs and propionyl- and butyryl-CoA but not medium or long chain Acyl-CoAs, with BT_3942 demonstrating substrate preference for butyryl-CoA (Figures 6C and S5C). Together, these results demonstrate that within Bacteroides, differences in the gene function of an Acyl-CoA thioesterase can mediate the variable fitness effects of butyrate.
Figure 6. Species-level genetic variation in Acyl-CoA metabolic defense among the Bacteroides.
(A) Protein identity of Acyl-CoA thioesterases, BT_3942 (Bt VPI), and BVU_0767 (Bv ATCC). See also Figures S5A and S5B.
(B) Bt Δ3942 and Bv WT complemented with BT_3942 or BVU_0767 grown in Glc −/+ butyrate.
(C) Acyl-CoA thioesterase activity of the recombinant proteins, BT_3942, and BVU_0767 with different chain lengths of acyl-CoAs as substrate. See also Figure S5C.
(D) Protein identity of Acyl-CoA transferases, BVU_1163 (Bv ATCC) and BT_3193 (Bt VPI). See also Figures S5D and S5E.
(E) Bv Δ1163 and Bt WT complemented with BVU_1163 or BT_3193 grown in Glc −/+ butyrate.
(F) Relative mRNA expression of Acyl-CoA transferase in Bt VPI (BT_3193) and Bv ATCC (BVU_1163) grown in Glc across growth phases. See also Figure S5F.
(G) mRNA levels of Acyl-CoA transferase genes (left) and inhibitory effect of butyrate (right) in Bv Δ1163 strains harboring plasmids expressing BVU_1163 or BT_3193 under their native or swapped promoters.
(H) Schematic of RNA polymerase binding sites showing nucleotide polymorphisms within the promoter regions of BVU_1163 and BT_3193.
(I) Schematic of the nucleotide polymorphism constructs on pBv and pBt promoter backbones (left), mRNA levels of BVU_1163 (middle), and inhibitory effect of butyrate (right) in Bv Δ1163 strains harboring plasmids driving BVU_1163 under the promoter with each strain-specific nucleotide polymorphism.
Error bars represent mean ± SEM of two biological replicates in (C and F) and three biological replicates in (G and I). Nonsignificant p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001; unpaired two-tailed t test in (C, F, G, and I), One-Way ANOVA with Dunnett multiple comparisons test in (C, red asterisks). Shown is a representative of three biological replicates in (B, E, G, and I).
Promoter polymorphisms mediate species-level variation in inhibitory effects of butyrate
As opposed to poor conservation of the Acyl-CoA thioesterase between Bt and Bv, the Acyl-CoA transferase BVU_1163 was highly conserved among the Bacteroides (Figure S5D), showing 83% AA identity with BT_3193 (Figures 6D and S5E). BT_3193 rendered both Bv Δ1163 and Bt sensitive to butyrate, comparable to that of BVU_1163 (Figure 6E). This suggests that the difference in butyrate sensitivity of Bt and Bv may be due to differential regulation of BT_3193 versus BVU_1163. Remarkably, BT_3193 was downregulated in Bt, while BVU_1163 was upregulated in Bv (Figure 6F) with corresponding differences in protein levels between these two species (Figure S5F). At the strain level, similar to the species level, there was greater expression of BVU_1163 in Bv ATCC (sensitive to butyrate in Glc) compared to Bv CL09 (resistant to butyrate in Glc) (Figure S5G). Thus, we observe an association between gene expression of this conserved Acyl-CoA metabolic gene and species- and strain-level variation in the fitness effect of butyrate.
To determine if differential expression of the Acyl-CoA transferase genes in Bt versus Bv is due to intrinsic properties of a specific promoter among Bacteroides, we cloned the promoter regions of BT_3193 (pBt) and BVU_1163 (pBv) upstream of either the BT_3193 or BVU_1163 operon, respectively, generating a panel of species-specific promoter-gene hybrid constructs (Figures 6G). The Bv promoter drove significantly greater expression of either gene compared to the Bt promoter, suggesting that differential gene regulation is due to species-specific intrinsic promoter strength (Figure 6G, left). To determine if high or low gene expression driven by the Bv or Bt specific promoters was sufficient to confer sensitivity to butyrate, the engineered strains harboring plasmids expressing each of the six hybrid pairs (no promoter, pBv, or pBt with BT_3193 or BVU_1163) were grown with or without butyrate. Remarkably, the strong, high-expression Bv promoter, but not the weak, low-expression Bt promoter (Figure 6G, middle), was sufficient to confer butyrate sensitivity to Bv Δ1163 (Figure 6G, right) and across a panel of naturally resistant Bacteroides species (Figure S5F) and strains (Figure S5I). Controlled inducible expression of BVU_1163 induced dose-dependent sensitivity in Bv as a function of sugar strength and Bv Δ1163 and Bv Δ1163 Δ0574 background (Figure S5J). Together, these data demonstrate that species-specific expression of a conserved Acyl-CoA transferase gene is regulated by species-specific promoter region to confer Bacteroides differential sensitivity to butyrate.
Species-level variation in butyrate sensitivity was encoded within 200 base pairs of the strain-specific promoter region of the Acyl-CoA transferase BVU_1163/BT_3193 (Figure 6G). Transcription initiation occurs by binding of the bacterial RNA polymerase holoenzyme complex to specific sequences of the promoter. Comparison of the Bacteroides −7 and −33 regions (Bayley et al., 2000; Lim et al., 2017; Mimee et al., 2016) revealed conservation of −7 region sequence TANNTTTG (Figure 6H). We observed a double nucleotide polymorphism (TTAG for pBt and TTTA for pBv) and a four-nucleotide difference in location of the −33 region between the Bt and Bv Acyl-CoA transferase promoters (Figure 6H). To determine the role of these species-specific promoter sequence variations in regulation of Acyl-CoA transferase gene expression, we made a series of constructs in −33 region nucleotide and location polymorphisms on the Bv high expression endogenous promoter backbone and the Bt low expression endogenous promoter backbone (Figure 6I, left). Remarkably, replacement of the Bv −33 region with the Bt −33 region nucleotide polymorphism (TTTA to TTAG) at its own or the Bt −33 region location (four-nucleotide spacer deletion) converted the Bv promoter into a low-expression promoter (Figure 6I, middle) and conferred resistance to butyrate (Figure 6I, right). Conversely, replacement of the Bt −33 region with the Bv −33 region nucleotide polymorphism (TTAG to TTTA) at its own or the Bv −33 region location (4 nucleotide spacer addition) converted the Bt promoter into a high-expression promoter (Figure 6I, middle) and conferred sensitivity to butyrate (Figure 6I, right). Together these results demonstrate that species-specific nucleotide and location polymorphisms encoded in cis at the −33 promoter region of a conserved Acyl-CoA transferase gene mediate variation in sensitivity of the Bacteroides to butyrate.
Lower expression of BVU_1163 in Bv CL09, compared to Bv ATCC, correlated with strain-level differences in sensitivity to butyrate (Figure S5G). However, the promoter sequences of BVU_1163 are 100% identical within species between Bv ATCC and Bv CL09 (Figure S5K), suggesting that while −33 promoter cis-acting factors are responsible for regulation of Acyl-CoA transferase, c/s-independent factors may be responsible for differential mRNA levels of Acyl-CoA transferase at the strain level. To identify candidate strain-specific regulators of Acyl-CoA transferase expression, we performed RNA-seq in Bv CL09 after pulse of butyrate in Glc (Table S2) and compared global transcriptional responses to butyrate between Bv CL09 and Bv ATCC. We identified 87 genes demonstrating strain-specific regulation in response to butyrate (Table S2; Figure S5L). Of these, 74 were induced by butyrate specifically in Bv CL09, including 7 transcriptional regulators, two of which were anti-sigma factors AGXZ_01245 and AGXZ_00606 (Table S2) serving as candidates of trans-acting factors mediating cis-independent regulation of Acyl-CoA transferase gene expression at the strain level.
Acyl-CoA transferase expression and butyrate levels correlate with Bacteroides fitness in vivo
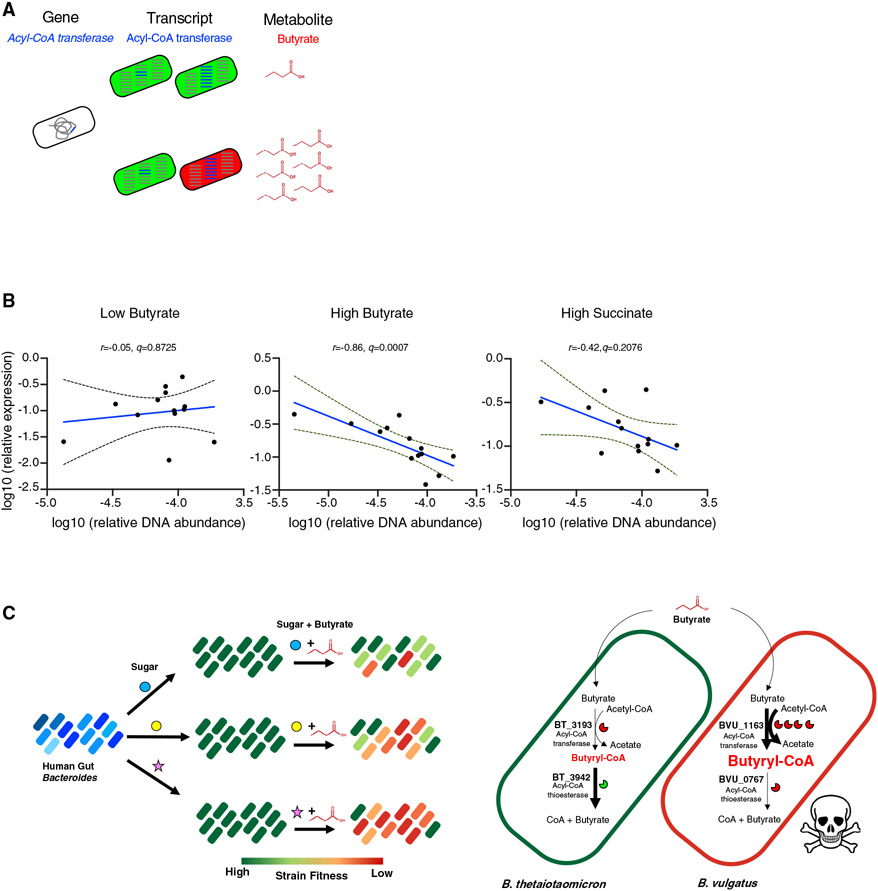
Our in vitro approach revealed that Bacteroides fitness is conditional on both butyrate and the expression level of the Acyl-CoA transferase, BT_3193/BVU_1163, whereby butyrate is inhibitory if the Bacteroides member expresses high levels of BT_3193/BVU_1163 (Figure 7A). We next sought evidence of whether Bacteroides fitness in the human gut is a function of butyrate and expression level of this conserved Acyl-CoA transferase. This required a multi-omics in vivo dataset simultaneously capturing metagenomes (MGX; BT_3193/BVU_1163 DNA abundance as a proxy for Bacteroides fitness), metatranscriptomes (MTX; relative expression of BT_3193/BVU_1163 RNA), and metabolomes (MBX; concentration of SCFAs, including butyrate), all from the same human fecal sample. Using the Inflammatory Bowel Disease Multi’omics Database (Lloyd-Price et al., 2019), we selected 88 longitudinal samples from 26 healthy subjects with matched MGX, MTX, and MBX data. We tested for associations between Bacteroides abundance and expression level of BT_3193/BVU_1163 under different gut metabolite levels. This analysis, independent of choice of metabolomics normalization method, revealed a significant negative correlation between Bacteroides abundance and the relative expression of BT_3193/BVU_1163 in high butyrate, and no correlation in either low butyrate or high succinate (Figures 7B and S6A) or between butyrate and BCAA (Figure S6B). Together, these data suggest that Bacteroides fitness in vivo is a function of both butyrate and expression of a conserved Acyl-CoA transferase in the human gut.
Figure 7. Bacteroides fitness is conditional on Acyl-CoA transferase expression and butyrate in vivo.
(A) Schematic demonstrating Bacteroides fitness based on interactions of BT_3193/BVU_1163 gene function, expression, and presence of a specific metabolite butyrate. Fitness represented in green (high) and red (low).
(B) Correlation between relative DNA abundance and relative expression of the Acyl-CoA transferase BT_3193/BVU_1163 of fecal samples from 26 human subjects in low versus high levels of fecal butyrate or high succinate (Benjamini-Hochberg false discovery rate (FDR) approach with a conservative FDR q-value < 0.05). See also Figure S6A.
(C) (left) Schematic of butyrate acting as a sugar- and strain-specific fitness switch in the Bacteroides. For three representative sugars, for which each Bacteroides species/strains (indicated by shades of blue) utilize and grow (high fitness indicated in green), butyrate results in differential impact on fitness at the species and strain level. (right) Model of the genetic and molecular mechanisms mediating variation in the inhibitory effect of butyrate among the Bacteroides. Butyrate enters the cell and is converted to butyryl-CoA through the activity of an Acyl-CoA transferase. An Acyl-CoA thioesterase then hydrolyzes butyryl-CoA decreasing cellular accumulation of this candidate toxic product. In the sensitive Bv, BVU_1163 (Acyl-CoA transferase) is highly expressed (4 enzyme symbols) leading to greater production of butyryl-CoA, and the poorly conserved Acyl-CoA thioesterase homolog in Bv (BVU_0767) results in butyryl-CoA accumulation.
DISCUSSION
For the Bacteroides, the predominant Gram-negative bacteria of the human intestine, survival in the gut is dependent on the ability of each strain to utilize dietary and host sugars. We have revealed a context-dependent inhibitory function of the specific metabolite butyrate where one microbe-derived small molecule acts as a fitness switch depending on the specific sugar utilized and in which individual strains have distinct switches across sugars (Figure 7C, left). Our work demonstrates strain-level fitness of members of the gut microbiome as an emergent property (West-Eberhard, 2019) based on the interaction of components across molecular class unpredictable by the effect of each part alone. Furthermore, our findings uncover that each strain of the Bacteroides has a unique norm of reaction (Woltereck, 1909) for each sugar it can utilize. The mechanism of these non-linear reaction norms is within Bacteroides genetic variation in Coenzyme A metabolism which integrates these environmental chemical factors into dynamic fitness phenotypes. Elucidation of the emergent properties of the fitness of members of the gut is instrumental to our understanding of the context-dependent rules that govern the structure of the gut microbiome, its hierarchical organization, and attempts to engineer this ecosystem in health and disease.
Our study reveals a role of the specific SCFA butyrate in gut microbiota ecology. SCFAs play important roles in mammalian health. In microbe-microbe interactions in the gut, these primary metabolites have long been appreciated to be important in positive fitness interactions in microbial ecosystems based on syntrophy, in which bacteria utilize these substrates as energy sources via assimilation or as electron acceptors (Fischbach and Sonnenburg, 2011). Recent work has shown that SCFAs can inhibit Enterobacteriaciae, such as Klebsiella pneumonia and Salmonella enterica, through acidification of the intracellular compartment (Jacobson et al., 2018; Sorbara et al., 2019). Of the SCFAs, butyrate is distinctive in a numbers of ways: (1) it is uniquely made by specific bacteria, notably the Lachnospiraceae (as opposed to other SCFAs that may be made by a wide range of microbes and also the host) (Lavelle and Sokol, 2020), (2) with rare exceptions (Ziels et al., 2019), it is not used as an energy source by bacteria, and (3) it plays unique roles in host biology and fitness from roles in gut epithelial homeostasis and immunoregulation as an energy source, signaling molecule, and epigenetic modifier (Koh et al., 2016). Thus, it is tempting to speculate that the emergent properties of distinct sugars and butyrate reflects eco-evolutionary interactions playing out between butyrate-producing organisms and individual Bacteroides strains across glycan niches balanced over evolutionary time by host fitness.
We found that butyrate, but not other SCFAs, caused rapid cellular stress, membrane damage, and cell death, conditional on which sugar is being utilized and varying among the Bacteroides. Species- and sugar-dependent inhibitory function of butyrate is due to variation within the Bacteroides in both the function and regulation of Acyl-Coenzyme A metabolism. By identifying a specific inhibitory function of butyrate and elucidating a species-specific and sugar-dependent Coenzyme A-based metabolic defense system, our work presents a connection between growth inhibition and metabolism distinct from known links with growth rate, nutrient limitation, the stringent response, and oxidative stress (Stokes et al., 2019). It will be of interest to determine whether these Acyl-CoA metabolic enzymes evolved primarily in defense to butyrate or as a secondary function and whether the role of these enzymes is a general feature of bacteria across environments or specific to the metabolic versatility of Bacteroides and the gut ecosystem.
Our work suggests that several non-mutually exclusive mechanisms may mediate species- and sugar-level variation in the Bacteroides butyrate fitness switch. We discovered that the mechanism governing resistance to butyrate was variation in short Acyl-CoA thioesterase and transferase activity among the Bacteroides. 13C tracing experiments and metabolite analysis suggest the conversion and accumulation of butyryl-CoA as a mechanism mediating the toxic effects of butyrate on the Bacteroides (Figure 7C, right) as an antimetabolite and/or metabolic poison. We found that specific BCAAs tuned the strain- and sugar-dependent toxicity of butyrate. This may occur through modulating specific Acyl-CoA and/or free acyl intracellular metabolites, such as acetyl-CoA, by Ile and Leu or propionyl-CoA by Val via BCAA catabolism by the BCKDH enzyme complex. Alternatively, our demonstration of a mechanistic role of a putative medium/long chain Acyl-CoA synthetase suggests that the protective (Ile, Leu) and detrimental (Val) role of specific BCAAs may be due to their role as precursors in the synthesis of functionally distinct and strain- and sugar-variable odd- (Ile, Leu) or even- (Val) length outer-membrane branched lipids (Kaiser and Heinrichs, 2018). We found phenotypic discordance between monomeric, dimeric, and polymeric sugars, suggesting that sensing of specific sugars, such as through two components systems (Schwalm et al., 2017), may play a regulatory role in modulating the effects of butyrate in the Bacteroides.
We found that variation in the enzymatic function of an Acyl-CoA thioesterase and regulation of an Acyl-CoA transferase by nucleotide polymorphisms in the RNA polymerase binding site at the −33 promoter region mediated differential resistance to butyrate among the Bacteroides. Strain-level variation in accessory genomes (Greenblum et al., 2015; Pasolli et al., 2019; Sberro et al., 2019; Zeevi et al., 2019) is well appreciated in adaptive functions in microbe-microbe, environmental, and host interactions both among the Bacteroides and in the gut microbiota at large, such as by encoding type-VI secretion systems, biosynthetic gene clusters, and polysaccharide utilization. By demonstrating a role for variation in Acyl-CoA metabolic genes, our work suggests that in addition to the accessory genome, the core genome may be under selection in members of the gut microbiota with strain-specific trade-offs between the role of central metabolism in bacterial physiology and ecological roles in diet- and microbe-microbe metabolic defense. Altogether, our work demonstrates a proof-of-principle of the power of “bottom-up” approaches to reveal the forces determining the fitness of each member of the microbiome as a function of the interaction of parts unpredictable by studying each part alone nor reducible from the sum.
Limitations of the study
There are certain limitations of our work that will be the focus of future research. The first focus will be to understand the significance of glycan-specific impacts on the strain-level fitness of butyrate on the Bacteroides within a complex community ecological context such in competition for glycans with butyrate producing bacteria in vivo. Secondly, while we find a negative correlation between Bacteroides fitness, Acyl-CoA gene expression, and butyrate levels in vivo, we do not know the glycan components of the diet or mucosa for each person and how the presence of specific glycans impacts butyrate-associated fitness effects in human cohorts. Relatedly, while we present accumulation of butyryl-CoA as a candidate factor mediating the inhibitory effects of butyrate on the Bacteroides, whether and how butyryl-CoA is directly toxic and the molecular mechanisms of butyrate inhibitory functions by sugar and across strains remain to be determined.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Requests for further information and resources may be directed to and will be fulfilled by the lead contact, Seth Rakoff-Nahoum (seth.rakoff-nahoum@childrens.harvard.edu).
Materials availability
Plasmids and recombinants strains generated in this study will be distributed without restriction on request.
Data and code availability
The raw RNA_Seq and Tn_Seq data from this study have been deposited in the NCBI Short-Read Archive (SRA) under the BioProject ID PRJNA640947 (BioSample IDs for RNASeq: SAMN15338436-15338444; BioSample IDs for Tn_Seq data: SAMN15338433-15338435).
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Microbial strains
Bacterial strains used in this study were listed in Key Resource Table. Bacteroidales strains were grown on brain-heart-infusion supplemented with hemin (50 mg/L) and vitamin K1 (0.25 mg/L) (BHIS) agar plates, in basal medium (BS; proteose peptone (20 g/L), yeast extract (5 g/L), NaCl (5 g/L), glucose (5 g/L), potassium phosphate dibasic (5 g/L), cysteine (0.5 g/L), hemin (50 mg/L) and vitamin K1 (0.25 mg/L)) (Pantosti et al., 1991), or in minimal media (MM; ammonium sulfate (1 g/L), sodium carbonate (1 g/L), potassium phosphate monobasic (0.9 g/L), sodium chloride (0.9 g/L), calcium chloride dihydrate (26.5 mg/L), magnesium chloride hexahydrate (2 mg/L), manganese(II) chloride tetrahydrate (1 mg/L), cobalt(II) chloride hexahydrate (1 mg/L), hemin (50 mg/L), vitamin K1 (0.25 mg/L), ferrous sulfate heptahydrate (4 mg/L), vitamin B12 (5 mg/L)) with 0.25% glucose, or the indicated sugar as carbon source (Rakoff-Nahoum et al., 2014). Cultures were grown at 37°C in an anaerobic chamber (Coy Laboratory) with 80% nitrogen, 10% CO2, and 10% hydrogen. Escherichia coli was grown in Luria-Burtani (LB) medium, or on LB agar plate. As necessary, antibiotics were added as the following concentrations: ampicillin 100 μg/mL, kanamycin 50 μg/mL, and gentamycin 200 μg/mL for E. coli, and erythromycin 5 μg/mL for Bacteroides strains. For the inducible promoter, anhydrotetracycline (aTc) was dissolved in 100% ethanol at 2 mg/mL and used at the indicated concentrations (0-25 ng/mL). Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to culture medium at the final 1 mM, or 0.4 mM concentration.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial strains | ||
| Bacteroides thetaiotaomicron 1_1_6 | BEI | HM-23 |
| Bacteroides thetaiotaomicron VPI-5482 | ATCC | ATCC 29148 |
| Bacteroides thetaiotaomicron ATCC 29741 | ATCC | ATCC 29741 |
| Bacteroides finegoldii CL09T03C10 | BEI | HM-727 |
| Bacteroides finegoldii DSM 17564 | DSMZ | DSM 17564 |
| Bacteroides xylanisolvens sp. 1_1_30 | BEI | HM-22 |
| Bacteroides xylanisolvens sp. 2_1_22 | BEI | HM-18 |
| Bacteroides xylanisolvens CL03T12C04 | BEI | HM-722 |
| Bacteroides ovatus CL02T12C04 | BEI | HM-723 |
| Bacteroides ovatus ATCC 8483 | ATCC | ATCC 8483 |
| Bacteroides ovatus CL03T12C18 | BEI | HM-724 |
| Bacteroides ovatus sp. D2 | BEI | HM-28 |
| Bacteroides ovatus 3_8_47FAA | BEI | HM-222 |
| Bacteroides caccae ATCC 43185 | ATCC | ATCC 43185 |
| Bacteroides caccae CL03T12C61 | BEI | HM-728 |
| Bacteroides nordii CL02T12C05 | BEI | HM-721 |
| Bacteroides salyersiae CL02T12C01 | BEI | HM-725 |
| Bacteroides salyersiae DSM 18765 | DSMZ | DSM 18765 |
| Bacteroides fragilis 3_1_12 | BEI | HM-20 |
| Bacteroides fragilis CL05T00C42 | BEI | HM-711 |
| Bacteroides fragilis str. S13 L11 | Lab of Laurie E. Comstock | (Coyne et al., 2016) |
| Bacteroides fragilis str. 1007-1-F #7 | Lab of Laurie E. Comstock | (Coyne et al., 2016) |
| Bacteroides fragilis NCTC 9343 | ATCC | ATCC25825 |
| Bacteroides fragilis NCTC 638R | Lab of Laurie E. Comstock | (Coyne et al., 2016) |
| Bacteroides fragilis CL03T00C08 | BEI | HM-713 |
| Bacteroides fragilis str. DS-166 | Lab of Laurie E. Comstock | (Coyne et al., 2016) |
| Bacteroides fragilis str. S36L11 | Lab of Laurie E. Comstock | (Coyne et al., 2016) |
| Bacteroides fragilis CL07T00C01 | BEI | HM-709 |
| Bacteroides fragilis sp. 2_1_16 | BEI | HM-58 |
| Bacteroides cellulosilyticus CL02T12C19 | BEI | HM-726 |
| Bacteroides oleiciplenus DSM 22535 | DSMZ | DSM 22535 |
| Bacteroides eggerthii 1_2_48FAA | BEI | HM-201 |
| Bacteroides eggerthii DSM 20697 | DSMZ | DSM 20697 |
| Bacteroides uniformis CL03T00C23 | BEI | HM-715 |
| Bacteroides uniformis ATCC 8492 | ATCC | ATCC 8492 |
| Bacteroides uniformis sp. D20 | BEI | HM-189 |
| Bacteroides dorei sp. 9_1_42FAA | BEI | HM-27 |
| Bacteroides dorei CL02T00C15 | BEI | HM-717 |
| Bacteroides dorei DSM 17855 | DSMZ | DSM 17855 |
| Bacteroides dorei CL03T12C01 | BEI | HM-718 |
| Bacteroides dorei 5_1_36/D4 | BEI | HM-29 |
| Bacteroides vulgatus ATCC 8482 | ATCC | ATCC 8482 |
| Bacteroides vulgatus CL09T03C04 | BEI | HM-720 |
| Parabacteroides goldsteinii CL02T12C30 | BEI | HM-732 |
| Parabacteroides merdae CL03T12C32 | BEI | HM-730 |
| Parabacteroides merdae ATCC 43184 | ATCC | ATCC 43184 |
| Parabacteroides merdae CL09T00C40 | BEI | HM-729 |
| Parabacteroides johnsonii CL02T12C29 | BEI | HM-731 |
| Parabacteroides distasonis sp. 3_1_19 | BEI | HM-19 |
| Parabacteroides distasonis 31_2 | BEI | HM-169 |
| Parabacteroides distasonis CL03T12C09 | BEI | HM-733 |
| Parabacteroides distasonis ATCC 8503 | ATCC | ATCC 8503 |
| Parabacteroides distasonis CL09T03C24 | BEI | HM-734 |
| Parabacteroides distasonis sp. 20_3 | BEI | HM-166 |
| Bt VIP Δ3942 | This study | N/A |
| Bv ATCC Δ1163 | This study | N/A |
| Bv ATCC Δ1163 Δ0574 | This study | N/A |
| Bv ATCC Δ1163 Δ2719 | This study | N/A |
| Bv ATCC Δ0574 | This study | N/A |
| Bv ATCC Δ2719 | This study | N/A |
| Bv ATCC Δ1163 att1:: pNBU2-erm_TetR-p1T_DP-GH023_BVU_1163 | This study | N/A |
| Bv ATCC Δ1163 Δ0574 att1:: pNBU2-erm_TetR-p1T_DP-GH023_BVU_1163 | This study | N/A |
| Bv CL09 Δ1163 | This study | N/A |
| Bv CL09 Δ2719 | This study | N/A |
| Bv CL09 Δ1163 Δ2719 | This study | N/A |
| Escherichia coli DH5a λ pir | (Pal et al., 2005) | N/A |
| Escherichia coli S17-1 λ pir | (Simon et al., 1983) | N/A |
| Escherichia coli BL21 (DE3) | Novagen | C600003 |
| Chemicals and recombinant proteins | ||
| Brain Heart Infusion | BD Bioscience | Cat# 237500 |
| Sodium formate | Sigma-Aldrich | Cat# 107603 |
| Sodium acetate | Sigma-Aldrich | Cat# S2889 |
| Sodium D-lactate | Sigma-Aldrich | Cat# 71716 |
| Sodium L-lactate | Sigma-Aldrich | Cat# 71718 |
| Sodium propionate | Sigma-Aldrich | Cat# P1880 |
| Sodium malonate dibasic | Sigma-Aldrich | Cat# 63409 |
| Sodium succinate dibasic hexahydrate | Sigma-Aldrich | Cat# S9637 |
| Sodium butyrate | Sigma-Aldrich | Cat# 303410 |
| Sodium butyrate (13C4) | Cambridge Isotope Laboratories | Cat# CLM-10426-0.1 |
| Glucose | Sigma-Aldrich | Cat# G7021 |
| Fructose | Sigma-Aldrich | Cat# F3510 |
| Mannose | Sigma-Aldrich | Cat# M6020 |
| N-Acetylglucosamine | Sigma-Aldrich | Cat# A3286 |
| Galactose | Sigma-Aldrich | Cat# G5388 |
| Arabinose | Sigma-Aldrich | Cat# A3131 |
| Xylose | Sigma-Aldrich | Cat# X3877 |
| Fucose | Sigma-Aldrich | Cat# F2252 |
| Rhamnose | Sigma-Aldrich | Cat# R3875 |
| Ribose | Sigma-Aldrich | Cat# D5251 |
| N-Acetylgalactosamine | Sigma-Aldrich | Cat# A2795 |
| 3-Sialyllactose (3-SL) | GLYCOM | Cat# GlyCare™ 3SL |
| Lacto-N-neotetraose (LNnT) | GLYCOM | Cat# GlyCare™ LNnT |
| 2-fucosyllactose (2-FL) | GLYCOM | Cat# GlyCare™ 2FL |
| Pullulan | Sigma-Aldrich | Cat# P4516 |
| Levan | Sigma-Aldrich | Cat# L8647 |
| Xylan | Sigma-Aldrich | Cat# X4252 |
| Xyloglucan (Tamarind) | Megazyme | Cat# P-XYGLN |
| Arabinan (Sugar beet) | Megazyme | Cat# P-ARAB |
| Arabinogalactan (Larch Wood) | Megazyme | Cat# P-ARGAL |
| Chondroitan sulfate | Sigma-Aldrich | Cat# R7500 |
| Inulin | Beneo | Cat# Orafti P95 |
| Pectin | Sigma-Aldrich | Cat# P9135 |
| Mannan | Sigma-Aldrich | Cat# M7504 |
| Glucomannan | Megazyme | Cat# p-GLCML |
| Isoleucine | Sigma-Aldrich | Cat# I2752 |
| Leucine | Sigma-Aldrich | Cat# L8000 |
| Valine | Sigma-Aldrich | Cat# V0500 |
| Maltose | Sigma-Aldrich | Cat# M5895 |
| Sucrose | Sigma-Aldrich | Cat# S7903 |
| Palatinose | Sigma-Aldrich | Cat# P2007 |
| Isopropyl β-D-1-thiogalactopyranoside | Sigma-Aldrich | Cat# I6758 |
| 4-chloro-phenylalanine | Sigma-Aldrich | Cat# C6506 |
| 5,5′-dithiobis (2-nitrobenzoic acid) | Sigma-Aldrich | Cat# D8130 |
| Acetyl coenzyme A | Sigma-Aldrich | Cat# A2181 |
| Propionyl coenzyme A | Sigma-Aldrich | Cat# P5397 |
| Butyryl coenzyme A | Sigma-Aldrich | Cat# B1508 |
| Succinyl coenzyme A | Sigma-Aldrich | Cat# S1129 |
| Hexanoyl coenzyme A | Sigma-Aldrich | Cat# H2012 |
| Decanoyl coenzyme A | Sigma-Aldrich | Cat# D5269 |
| Palmitoyl coenzyme A | Sigma-Aldrich | Cat# P9716 |
| BCECF, AM (2’,7’-Bis-(2 Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester) | Thermo Fisher Scientific | Cat# B1151 |
| Nigericin | Sigma-Aldrich | Cat# N7143 |
| Valinomycin | Sigma-Aldrich | Cat# V0627 |
| Propidium Iodide | Thermo Fisher Scientific | Cat# P3566 |
| BT_3942-His tag | This study | N/A |
| BVU_0767-His tag | This study | N/A |
| Antibodies | ||
| Anti-HA | Santa Cruz | Cat# sc-7392 |
| Anti-E. coli RpoA | BioLegend | Cat# 663104 |
| Critical commercial assays | ||
| SuperScript VI Reverse Transcriptase | Thermo Fisher Scientific | Cat# 18090200 |
| Fast SYBR Green Master Mix | Applied Biosystem/ Thermo Fisher Scientific | Cat# 4385617 |
| Ribo-Zero rRNA Removal Kit | Epicenter | Cat# RZH1046 |
| PureLink Genomic DNA mini kit | Thermo Fisher Scientific | Cat# K182002 |
| GeneArt Seamless Cloning and Assembly Enzyme Kit | Thermo Fisher Scientific | Cat# A14606 |
| Zymo DNA Clean and Concentrator Kits | Zymo Research | Cat# D4013 |
| ZymoBIOMICS DNA Miniprep Kit | Zymo Research | Cat# D4301 |
| NEBNext Ultra DNA Library Prep Kit | New England Biolabs | Cat# E7370 |
| AMPure XP beads | Beckman Coulter Inc. | Cat# A63880 |
| NEBNext Library Quant Kit for Illumina | New England Biolabs | Cat# E7630 |
| Platinum SuperFi PCR Master Mix | Thermo Fisher Scientific | Cat# 12358250 |
| Direct-zol RNA Miniprep Plus Kit | Zymo Research | Cat# R2072 |
| HisPur Ni-NTA Resin | Thermo Fisher Scientific | Cat# 88221 |
| Halt Protease Inhibitor Cocktail | Thermo Fisher Scientific | Cat# PI87786 |
| NuPAGE 4 to 12%, Bis-Tris Mini Protein Gel | Thermo Fisher Scientific | Cat# NP0322BOX |
| Deposited data | ||
| The raw RNA_Seq and Tn_Seq data | NCBI BioProject ID | PRJNA640947 |
| Tn-Seq_B. thetaiotaomicron VPI-5482/Glucose | NCBI BioProject ID | PRJNA640947 |
| Tn-Seq_B. vulgatus ATCC 8482/Glucose | NCBI BioProject ID | PRJNA640947 |
| Tn-Seq_Bv ΔBVU_1163/Galactose | NCBI BioProject ID | PRJNA640947 |
| sTn-Seq_B. vulgatus CL09/Galactose | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. thetaiotaomicron VPI-5482/Glucose/Acetate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. thetaiotaomicron VPI-5482/Glucose/Succinate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. thetaiotaomicron VPI-5482/Glucose/Butyrate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. thetaiotaomicron VPI-5482/Galactose | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/Glucose/Acetate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/Glucose/Succinate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/Glucose/Butyrate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/GlNAC/Butyrate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/Galactose/Butyrate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus ATCC 8482/Xylose/Butyrate | NCBI BioProject ID | PRJNA640947 |
| RNA-Seq_ B. vulgatus CL09 /Glucose/Butyrate | NCBI BioProject ID | PRJNA640947 |
| Oligonucleotides | ||
| Primers used in this study | This study | Table S1 |
| Recombinant DNA | ||
| pRK231 | (Smith et al., 1992) | N/A |
| pKNOCK-bla-ermGb | (Koropatkin et al., 2008) | N/A |
| pKNOCK-bla-ermGb_pheS* | This study | N/A |
| pSAM_BcellWH2 | (Wu et al., 2015) | N/A |
| pFD340 | (Smith et al., 1992) | N/A |
| pNBU2-bla-ermGb | (Koropatkin et al., 2008) | N/A |
| pWH2-Term6 | This study | N/A |
| pBT_3942 | This study | N/A |
| pBVU_0767 | This study | N/A |
| pBv_1163 | This study | N/A |
| pBv_1163-HA | This study | N/A |
| pBt_1163 | This study | N/A |
| pBv_3193 | This study | N/A |
| pBt_3193 | This study | N/A |
| pBt_3193-HA | This study | N/A |
| pN_1163 | This study | N/A |
| pN_3193 | This study | N/A |
| pBv_TTAG-1163 | This study | N/A |
| pBv_TTAG_down-1163 | This study | N/A |
| pBt_TTTA-1163 | This study | N/A |
| pBt_TTTA_up-1163 | This study | N/A |
| pNBU2-erm_TetR-p1T_DP-GH023 | (Lim et al., 2017) | Addgene Cat #90324 |
| pTetR_p1T_ BVU_1163 | This study | N/A |
| pET-28a | Novagen | Cat# 69864-3 |
| pET-28a_BT_3942-His | This study | N/A |
| pET-28a_BVU_0767-His | This study | N/A |
| Software and algorithms | ||
| Prism9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| MEGA | https://www.megasoftware.net/ | |
| MATLAB/ R2017b | MathWorks | https://www.mathworks.com |
| Heatmapper | (Babicki et al., 2016) | http://www.heatmapper.ca |
| DAVID 6.8 | (Huang et al., 2009) | https://david.ncifcrf.gov/ |
| Other | ||
| Anaerobic Chamber | Coy manufacturing | Vinyl Anaerobic Chamber |
| Microplate plate absorbance reader | BioTek Instruments | Synergy HTX multi-mode reader |
| QuantStudio Real-time PCR system | Applied Systems | 3 |
| Focused-ultrasonicators | Covaris | M220 |
Animal model
Six week old female germ-free C57BL/6 mice were used for gnotobiotic mouse experiments. Brigham and Women’s Hospital Massachusetts Host-Microbiome Center approved under protocol 2020N000054. Mice were housed in Class Biological Isolator in a temperature-controlled (~21°C) facility on a 12 h light/dark cycle. Mice were fed a standard chow (Laboratory Rodent Diet 5025, LabDiet, St. Louis, MO, USA) unless otherwise indicated.
METHOD DETAILS
Culture conditions
For growth assays on solid agar plates, three to five colonies from bacteria grown on BHIS plates were suspended in 100 μL of sterile phosphate buffered saline (PBS) and adjusted to OD600~0.1 using a Microplate spectrophotometer (BioTek, Synergy HTX multi-mode reader). Three μl of bacterial suspension was dotted on the MM agar containing 0.25% sugar and grown at 37°C anaerobically for the indicated days. Sugars used to supplement MM as carbon source include monosaccharides (glucose (Glc), fructose (Fru), mannose (Man), N-Acetylglucosamine (GlcNAc), galactose (Gal), arabinose (Ara), xylose (Xyl), fucose (Fuc), Rhamnose (Rha), ribose (Rib), and N-Acetylgalactosamine (GalNAc)), disaccharides (maltose (Mal), cellobiose (Cel), sucrose (Suc), and palatinose (Pal)), oligosaccharides (3-sialyllactose (3-SL), lacto-N-neotetraose (LNnT), 2-fucosyllactose (2-FL)), and polysaccharides (pullulan, levan, xylan, xyloglucan, arabinan, arabinogalactan, chondroitin sulfate, inulin, pectin, mannan, and glucomannan). All final concentration of sugars was 0.25%, except levan (0.125%). The sodium form of short chain acids formate, acetate, D- and L-lactate, propionate, malonate, succinate, and butyrate were used at 50 mM concentration. In other experiments the concentration of butyrate was titrated 50 mM down to 0.5 mM. For complex media experiments, a modified version of YCFA (Browne et al., 2016) was used: Casitone (5 g/L), yeast extract (1.25 g/L), NaHCO3 (4 g/L), cysteine (1 g/L), K2HPO4 (0.45 g/L), KH2PO4 (0.45 g/L), NaCl (0.9 g/L), MgSO4·7H2O (0.09 g/L), CaCl2 (0.09 g/L), hemin (10 mg/L), vitamin K1 (0.25 mg/L), ferrous sulfate heptahydrate (4 mg/L), Vitamin Supplement (10 mL/L; ATCC) and Trace Mineral Supplement (ATCC: 10 mL/L). For growth experiments with branched chain amino acids, 1 mM of isoleucine (Ile), leucine (Leu), valine (Val), or the mixture of Ile and Val were added to 0.25% Glc or Gal MM agar. Stock solutions of 100 mM were pH neutralized to ~7.0 with NaOH. The recombinant strains harboring plasmids expressing Acyl-CoA metabolic genes were grown in 0.25% Glc MM agar with erythromycin (5 μg/mL). For all figures except kinetics in Figure 2, day 2 images are shown. Images of bacterial growth were taken using Gel doc system (Bio-Rad) each day.
For growth in liquid MM, bacteria were cultured overnight in BS, diluted 1:10 in fresh BS, grown to mid-log (OD600~0.5), then inoculated into 0.25% sugar MM with 1:50 ratio. MM was pre-reduced in the anaerobic chamber overnight. For growth kinetics, 200 μl of bacterial culture were grown in 96-well flat bottom plates using a microplate spectrophotometer, and OD600 was measured at every 30 min for 36 h. To calculate growth rate (k), absorbance readings corresponding to exponential phase (OD600: 0.2-0.6) were fit to a log exponential growth equation (Prism 9). For growth of the recombinant strains harboring plasmids expressing Acyl-CoA transferase genes, bacteria were cultured in BS with erythromycin (5 μg/mL) overnight and inoculated to 0.25% Glc MM containing the same antibiotic with 1: 50 ratio.
Culture-seq of defined communities
Community cultivation
Quantification of bacterial strains in community cultivation (referred to as “Culture-Seq”) was performed on community cultivation with strains as indicated. Each strain was lifted from BHIS plates, adjusted to OD600~0.2 in sterile PBS, mixed equally, after which 3 μl of the bacterial suspension was dotted on 0.25% sugar MM agar with and without 50 mM butyrate and grown for 2 days. Co-culture samples were prepared in biological and technical duplicates.
Amplicon sequencing of the rpsC gene
To lyse bacteria, the dotted patches were cut from the co-culture plates, suspended in 300 μl of PBS by vertexing (3 min), followed by alkalization in 20 mM KOH at 90°C for 10 min and 70°C for 20 min, and neutralization in 20 mM HCl, 90 mM Tris-HCl and 30 mM KCl. The conserved rpsC gene was amplified with the cell lysates as template and the barcoded rpsC primers (Table S1) under PCR cycles (98°C 10 s, 60°C 10 s, 72°C 1 min with 25 cycles) using Platinum SuperFi PCR Master Mix (Thermo Fisher Scientific). For Illumina sequencing, the PCR fragments were amplified with index primers I5/I7 (Table S1) under PCR cycles (98°C 10 s, 55°C 10 s, 72°C 30 s with 10 cycles), followed by purification using Zymo DNA Clean and Concentrator Kits (Zymo Research). Libraries were pooled, normalized by qPCR using NEBNext Library Quant Kit for Illumina (New England Biolabs), and subjected to Illumina NextSeq 500 platform generating 150-nt single-end reads (Illumina Inc). Primers are listed in Table S1.
Gnotobiotic mouse experiments
Six week old female germ-free C57BL/6 mice were reared in gnotobiotic isolators. Four days prior to colonization, diets were switched from standard chow to custom glycan-free chow (Bioserv S10004) and drinking water containing either 1% inulin (Beneo) (filter sterilized) or 1% citrus pectin (Sigma) (autoclaved). Animals (n = 5/group) were then gavaged with a 4-member Bacteroides community of B. nordii CL02T12C05 (Bn CL02), B. ovatus ATCC 8483 (Bo ATCC), B. salyersiae DSM 18765 (Bs DSM), and B. caccae ATCC 43185 (Bc ATCC), each at 1x109 CFU in sterile PBS. Four days post colonization, mice were transitioned to the same dietary glycan drinking water (inulin or pectin) supplemented with 150 mM Na butyrate (Smith et al., 2013). Feces was collected daily before and after butyrate supplementation. To determine the impact of butyrate on the relative abundance in vivo of each Bacteroides member, genomic DNA was extracted from feces using ZymoBIOMICS DNA Miniprep Kit (Zymo Research), and amplicon sequencing of the rpsC gene was performed as described above. Significant differences were determined by unpaired two-tailed Student’s t test.
RNA-seq of Bacteroides
B. thetaiotaomicron VPI 5482 (Bt VPI,) B. vulgatus ATCC 8482 (Bv ATCC), and B. vulgatus CL09 (Bv CL09) were cultured overnight in BS and sub-cultured in 2 mL of MM plus the indicated sugar as described above. At early-log phase (OD600~0.25), bacteria were pulsed with acetate, succinate, butyrate (at final concentration of 50 mM), or water control for 1 h, and harvested (13,000 g, 3 min) for total RNA extraction. Following treatment with 200 μL of TRI reagent (Zymo Research) for 5 min at 50°C, bacteria were frozen in dry ice, and stored at −80°C until use. RNA was extracted using Direct-zol RNA Miniprep Plus Kit (Zymo Research) according to the manufacturer’s instructions with on-column DNase I treatment and quantified by the NanoDrop (Thermo Fisher Scientific).
RNA-Seq libraries were constructed using a modified version of the RNAtag-seq protocol (Shishkin et al., 2015) by Microbial ‘Omics Core at the Broad Institute of MIT and Harvard. Briefly, 0.5 μg of total RNA was fragmented, depleted of genomic DNA, dephosphorylated, and barcoded. rRNA was depleted by using the Ribo-Zero rRNA Removal Kit (Epicenter). After depletion of rRNA, pools of barcoded RNAs were converted to Illumina cDNA libraries (Zhu et al., 2001). cDNA libraries were sequenced on the Illumina Hiseq2500 platform to generate approximately 5 million paired-end reads per sample by Genomics Platform at the Broad Institute of MIT and Harvard.
Intracellular pH measurement
Bt VPI and Bv ATCC cultured in BS were grown in 0.25% Glc MM as described above, then at early log-phase (OD600~0.2) were pulsed with 50 mM SCFAs and water as control, and incubated for 1 h. The pH indicator BCECF-AM (Thermo Fisher Scientific) was loaded to the cultures to final 1 μM concentration for 20 min to activate in the cytoplasm. One ml of culture was pelleted (13,000 x g, 3 min), washed and resuspended in 1ml of PBS, and then fluorescence intensity of cells was measured at two excitations, 485 nm and 440 nm with fixed emission at 530 nm using a fluorimeter (Tecan). Intracellular pH was determined by the ratio (485 nm/440 nm) of emission intensities. To generate the standard curve of BCECF-AM, each bacterium grown under the Glc conditions was harvested, washed, and incubated in 50 mM potassium phosphate buffer ranging pH 4.5 to pH 9.0 with each 10 μM ionophore nigericin and valinomycin at room temperature for 5 min (Bio-protocol). The standard curve, plotted with pH and the emission ratio, was fitted with nonlinear regression (Sigmoidal, 4PL) using Prism 9 (GraphPad). Each experiment was performed 3 replicates percondition. Significant differences were determined by paired two-tailed t test.
Propidium iodide (PI) staining and flow cytometry
For PI staining experiments, Bt VPI and Bv ATCC were grown in 0.25% Glc or Gal MM as described above, then at early log-phase were pulsed with SCFAs and water as control and incubated for 1 h. Bacteria were pelleted (13,000 x g, 3 min), washed, and then incubated in PBS with 10 μg/mL PI (Sigma-Aldrich) at room temperature for 15 min. Following washing with PBS two times, cells were fixed in 1ml of 2% paraformaldehyde (Affymetrix) for flow cytometry.
Flow cytometric measurement was performed on a MoFlo XDP flow cytometer (Beckman Coulter Inc., Miami, FL, USA) equipped with a 488 nm (excitation wavelength of PI) argon laser. 50,000 cells were acquired for the analysis. Three parameters were recorded: forward scatter (FSC), related to cell size, side scatter (SSC), related to cell structure, and red fluorescence (PI), related to cell membrane integrity. FSC, SSC, and fluorescence were collected in a logarithmic scale. For gating strategy, each bacterium was grown to mid exponential phase under in Glc to prepare live cells. To prepare killed bacteria, live cells were heated at 55°C for 5 min. In addition to gating on negative controls without PI staining, the mixture of live and the heat-treated bacteria was used to create PI negative, low and high PI bacterial populations. The results of biological triplicate were shown as percentages of PI-positive subpopulation over total events. Significant differences were determined by paired two-tailed t test.
Measurement of bacterial viability
To measure the effect of butyrate on cell viability, Bv ATCC and Bt VPI were grown in 0.25% sugar MM as described for PI staining experiments. At early log-phase (OD600: ~0.25), bacteria were pulsed with 20 (Figure S3F) or 50 (Figure 3D) mM butyrate or water as control. Bacteria were collected at 0, 0.5, 1, and 5 h, diluted with PBS, and plated on BHIS agars to determine colony forming unit (CFU)/mL. Each experiment was performed with 3 replicates per condition. Significant differences were determined by paired two-tailed t test.
Acyl-CoA identification and measurement using liquid chromatography coupled with mass spectrometry (LC/MS)
For 13C butyrate tracing experiments, Bt VPI, Bv ATCC and their isogenic strains were grown in 0.25% Glc as described above. At early log-phase (OD600: ~0.25) cultures were pulsed with 10 mM 13C4 butyrate (Cambridge Isotope Laboratories) or water as control and incubated for 30 min. To determine abundance of Acyl-CoAs at steady state, bacteria were pulsed with 20 mM butyrate or water and incubated for 30 min. Five ml cultures of bacteria were harvested (4,000 rpm, 10 min) at 4°C, washed with PBS two times, and pellets stored at −80°C until further use.
For LC/MS analysis, the bacterial pellet was dissolved into 300 μL of 80% MeOH and treated with Sonifier® Cell Disruptor for 1 min. The homogenized solution was centrifuged at 4,350 rpm for 10 min, and the upper clear supernatant was filtered through a 0.2 μm PTFE filter vial. Q Exactive mass spectrometry (Thermo Fisher Scientific) with a UHPLC system (Ultimate 3000) equipped with a 150 mm C18 column (Kinetex® 2.6 μm C18 100 Å LC Column 150 × 3 mm) was used to analyze the samples. The LC/MS method used for detecting Acyl-CoAs was adopted from previous literature (Liu et al., 2015). The following LC program was used: Buffer A: water with 5 mM ammonium acetate; Buffer B: methanol. 0 min: 2% B, 1.5 min: 2% B, 3 min: 15% B, 5.5 min: 95% B, 14.5 min: 95% B, 15 min: 2% B, 20 min: 2% B. Flow speed was 0.5 mL/min. Injection volume was 2 μl. Column temperature was set at 25°C. Mass spec acquisition windows were set to 0-10 min at full MS positive mode. The following parameters were used for data collection: Resolution: 70,000, AGC target: 3e6, Maximum IT: 250 ms, scan range: 700 to 1100 m/z. The data analysis was completed using Thermo Freestyle (Version 1.6) and Qual Browser software (Version 4.3). In the run using various Acyl-CoA standards, all the Acyl-CoAs were detected as [M+H]+ formula. Therefore, we searched Acyl-CoA peaks in samples using [M+H]+ formula for each Acyl-CoA at 5 decimal and 5 ppm resolution. The peak area quantification was done using Genesis method at Freestyle software. The peak area for each sample was then normalized by cell density at OD600.
Quantitative RT-PCR (qRT-PCR)
To measure total transcript levels of CoA transferase gene, Bt VPI and Bv ATCC cultivated BS were subcultured in 0.25% sugar MM as described above, collected at early-log (OD600~0.2), mid-log (OD600~0.4), and late-log phases (OD600~0.6). For RNA extraction of the recombinant strains harboring plasmids expressing CoA transferase genes, bacteria were grown in 0.25% sugar MM with erythromycin late exponential phase (Figures 6G and 6I). After pelleting (13,000 x g, 3 min), bacteria were treatment with Trizol for 5 min, then, total RNA was extracted according to Direct-zol RNA Miniprep Plus Kit (Zymo Research) protocol.
cDNA of 200 ng RNA was synthesized using SuperScript VI (Thermo Fisher Scientific) according to the manufacturer’s instructions. Quantification of transcripts was performed by real-time PCR (QuantStudio 3) using Fast SYBR Green Master Mix (Applied Biosystems). mRNA levels of target genes were normalized to 200-fold dilution of 16S rRNA rrs gene abundance. The relative amount of each PCR product was calculated from standard curves obtained from PCRs with the same primers and serially diluted genomic DNA as templates. The BVU_1163, BT_3193, and rrs genes were detected using primer pairs 114/115, 126/127, and 110/111, respectively. The list of primers used for qRT-PCR is presented in Table S1.
Construction of Bacteroides transposon mutant library
The transposon plasmid pSAM_BcellWH2 (Wu et al., 2015) was modified by adding back the rrnB terminator sequences to the downstream region of ermG within the transposon to reduce the potential polar effect derived by the strong promoter of the ermG gene, named as pWH2-Term6. For conjugation, Bacteroides strains were grown in 20 mL of BS media to early-lag phase culture (OD600 < 0.1). The E. coli S17-1 λ pir (Simon et al., 1983) donor harboring pWH2-Term6 was grown in 1 mL of LB media to log phase. Harvested bacteria were mixed, spotted on BHIS agar plate, and incubated at 37°C less than 18 h aerobically. After pooling the conjugation reaction, Tn mutants were selected on BHIS agar supplemented with gentamicin (200 μg/mL) and erythromycin (5 μg/mL) anaerobically at 37°C for 48 h. Approximately, 100,000 colonies were pooled in LB supplemented with 25% glycerol, and stored at −80°C.
Transposon sequencing (Tn-seq) of Bacteroides Tn mutants
An aliquot of the transposon mutant bank was thawed, recovered overnight in BS (starting OD600~0.1), back diluted 1:10 to fresh BS, and grown to mid-log phase (OD600~0.5) in duplicate. This culture (an aliquot was saved as input control) was then diluted 50-fold in 2 mL of MM containing 0.25% defined sugar with and without 50 mM butyrate and grown to mid-log phase at 37°C under anaerobic conditions.
To construct Tn-seq libraries for sequencing, the collected samples were first subjected to DNA extraction using Invitrogen PureLink Genomic DNA mini kit (Thermo Fisher Scientific). An aliquot of 5 μg of DNA was sheared in 100 μL water to 300~500 bp using a M220 focused ultrasonicator (Covaris Inc). The sheared DNA was subjected to end repair, A-tailing, and ligated with prepared adapters (generated by annealing two oligos: 5¢-TACCACGACCA-NH2-3¢ and 5¢-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTGGTCGTGGTAT-3¢) using NEBNext Ultra DNA Library Prep Kit (New England Biolabs). Next, transposon junction fragments containing the insertion sites were enriched by two PCR steps using Platinum SuperFi PCR Master Mix (Thermo Fisher Scientific). In the first PCR, the fragments were amplified (98°C 10 s, 60°C 10 s, 72°C 30 s with 25 cycles) with universal primers matching the transposon and adaptor sequence (Table S1). In the second PCR, the PCR products were amplified (98°C 10 s, 60°C 10 s, 72°C 30 s with 18 cycles) with Truseq-compatible indexed primers containing specific annealing sequences (Table S1). The PCR fragments corresponding to 200~600 bp were selected using AMPure XP beads (Beckman Coulter Inc). These libraries were normalized using NEBNext Library Quant kit (New England Biolabs) and subjected to NextSeq500 High Output sequencing (Illumina Inc).
Construction of strains
To delete target loci by allelic replacement, first, we modified the suicide vector pKNOCK-bla-ermGb (Koropatkin et al., 2008) with a counter-selection system mediated by a variant phenylalanyl-tRNA synthetase (PheSA303G), lethal in the presence of 4-chlorophenylalanine (Kino et al., 2016). Briefly, the pheS gene, along with its native promoter, was PCR amplified with genomic DNA of Bacteroides fragilis as template and two primer sets (Table S1) generating the mutated pheS gene (pheS*). The PCR fragments were cloned into the MfeI site of pKNOCK-bla-ermGb using GeneArt Seamless Cloning and Assembly Enzyme Kit (Thermo Fisher Scientific), resulting in a counterselection plasmid pKNOCK-bla-ermGb_pheS*.
For deletion of BT_3942 in Bt VPI, BVU_1163, BVU_0574, or BVU_2719 in Bv ATCC, and the homolog genes of BVU_1163, or BVU_2719 in Bv CL09, approximately, 1 kb upstream and downstream flanking regions of the deletion locus were PCR-amplified, cloned into pKNOCK-bla-ermGb_pheS* digested BamHI with ClaI using GeneArt Seamless Cloning and Assembly Enzyme Kit, and transformed into E. coli DH5a λ pir (Pal et al., 2005). The plasmid DNA was transferred into Bacteroides strains by triparental mating using E. coli carrying the helper plasmid RK231 at 37°C aerobically, and merodiploid Bacteroides cells were selected on BHIS plates with gentamicin (200 μg/mL) and erythromycin (5 mg/mL) anaerobically at 37°C. Resistant colonies were restreaked on BHIS plates with erythromycin and grown anaerobically at 37°C for 2 days. For counterselection, the isolated strains were streaked on 0.25% glucose MM containing 4-chloro-phenylalanine (2 mg/mL) and incubated anaerobically for 3-4 days. The deletion of target genes was confirmed with primers corresponding to upstream and downstream flanking regions. Deletion strains and primers for the deletion constructs were listed in Key Resources Table and Table S1.
For inducible promoter-derived expression of the BVU_1163 gene, Bv ATCC isogenic strains were constructed as follows: open reading frame of BVU_1163 was amplified with primers 259/147 and genomic DNA of Bv ATCC as template and cloned into the Bacteroides species integrative plasmid pNBU2-erm_TetR-p1T_DP-GH023 containing the engineered inducible promoter(Lim et al., 2017) digested with NcoI and ClaI. Then, Bv Δ1163 and Bv Δ1163 Δ0574 strains were conjugated by E. coli carrying the integration plasmid and selected on BHIS plates with gentamicin (200 μg/mL) and erythromycin (5 μg/mL).
Construction of plasmids
Heterologous expression of Acyl-CoA thioesterases
Plasmid pBT_3942 and pBVU_0767 were constructed as follows: Open reading frames (ORF), including ~50 bp of upstream of the gene to capture native ribosomal binding site (RBS) of BT_3942 and BVU_0767, were amplified with primers 190/191 and genomic DNA of Bt VPI as template, and primers 192/193 and genomic DNA of Bv ATCC as template, respectively, using Platinum SuperFi PCR Master Mix. The PCR fragments were digested with BamHI and KpnI and cloned into the Bacteroides expression plasmid pFD340 harboring constitutive promoter digested with the same restriction sites (Smith et al., 1992).
Promoter swapping
The CoA transferase genes BVU_1163 and BT_3193 appeared to form an operon with the upstream genes BVU_1164 and BT_3193a, respectively (Figure S5C). Consistent with this, putative RNA polymerase binding sites were found upstream regions of the BVU_1164 and BT_3193a genes (Figure 6H). For promoter swapping of the CoA transferase genes, 220 bp and 200 bp upstream of the start codons of the BVU_1164 and BT_3193a genes were used for the promoter regions pBv and pBt, respectively. The ORF regions of the BVU_1163 and BT_3193 genes were designed to include their own native RBS (~50 bp upstream of the gene).
For plasmids pBv_1163 and pBt_1163, the promoter regions pBv and pBt were generated with primers 208/71 and genomic DNA of Bv ATCC as template, and primers 132/136 and genomic DNA of Bt VPI as template, respectively. ORFs of BVU_1163 were generated with primer sets 70/72 for the pBv promoter, and 135/72 for the pBt promoter and genomic DNA of Bv ATCC, respectively. The two fragments corresponding to the promoter and ORF regions were assembled into promoterless-pFD340, of which IS4351 promoter was removed by digestion with SphI and KpnI, using GeneArt Seamless Cloning and Assembly Enzyme Kit.
For plasmids pBv_3193 and pBt_3193, the promoter regions pBv and pBt were generated with primers 208/75 and genomic DNA of Bv ATCC, and primers 132/131 and genomic DNA of Bt VPI, respectively, and ORFs of BT_3193 were generated with primer sets 74/77 for the pBv promoter, and 130/77 for the pBt promoter and Bt VPI genomic DNA, respectively. The two fragments corresponding to the promoter and ORF regions were assembled into promoterless-pFD340 as described above.
For control vectors, pN_1163 and pN_3193, ORFs of BVU_1163 and BT_3193 were generated with primers 142/57 and genomic DNA of Bv ATCC, and primers 143/77 and genomic DNAof Bt VPI, respectively, and cloned into promoterless pFD340 digested with SphI and KpnI.
To examine expression level of CoA transferase proteins, plasmids pBv_1163-HA and pBt_3193-HA were constructed as follows: Plasmid pBv_1163 and pBt_3193 as template was amplified with primers 208/477 and 132/478, respectively, then the PCR fragments inserting HA tag were cloned into promoterless pFD340 digested with SphI and KpnI.
Nucleotide polymorphisms within −33 promoter region
Derivatives of plasmid pBv_1163 with nucleotide substitutions in −33 region (TTTA) of the BVU_1163 promoter were constructed with plasmid pBv_1163 as template and the following primer pairs: primers 208/214 and 213/72 for pBv_TTAG-1163 (promoter polymorphism); primers 208/212 and 211/72 for pBv_TTAG_down-1163 (polymorphism location: deletion of TAGA in the downstream region of TTTA). The PCR fragments were assembled with promoterless pFD340 digested with restriction enzymes SphI and KpnI using GeneArt Seamless Cloning and Assembly Enzyme Kit.
Derivatives of plasmid pBt_1163 with nucleotide substitutions in −33 region (TTAG) of the BT_3193 promoter were constructed with plasmid pBt_1163 as template and the following primer pairs: primers 132/216 and 215/72 for pBt_TTTA-1163 (promoter polymorphism); primers 132/324 and 323/72 for pBt_TTTA_up-1163 (polymorphism location: insertion of TTTA in the upstream region of TTAG, and replacement of TTAG with AATC). The PCR fragments were assembled with promoterless pFD340 digested with restriction enzymes SphI and KpnI using GeneArt Seamless Cloning and Assembly Enzyme Kit.
Sequence analysis confirmed that the plasmids had the expected nucleotide sequences. The list of primers used in this study is presented in Table S1.
Expression, purification, and activity of recombinant Acyl-CoA thioesterase and Acyl-CoA transferase enzymes
Acyl-CoA thioesterase BT_3942 and BVU_0767 genes, and Acyl-CoA transferase BVU_1163 gene were amplified with primers (Table S1) and genomic DNA of Bt VPI and Bv ATCC 8482 as template, respectively, and cloned into the pET-28a (Novagen) digested with NcoI and AvaI using GeneArt Seamless Cloning and Assembly Enzyme Kit. The recombinant plasmids were transformed into E. coli BL21 (DE3) (Novagen) strains, grown to OD600~0.5 in LB plus kanamycin at 37°C and induced by 1 mM of IPTG at 15°C for 4 h (BT_3942), or by 0.4 mM of IPTG at 15°C overnight (BVU_0767, BVU_1163). After harvesting (6000 g, 10 min, 4°C), cells were suspended in lysis buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1.0 mM Imidazole) with protease inhibitor (Halt Protease Inhibitor Cocktail, Thermo Fisher Scientific) and lysed by sonication. The cell extracts were spun down, filtered through a 0.45 μm filter, mixed with 2 mL of pre-washed Ni-NTA resin (HisPur Ni-NTA Resin, Thermo Fisher Scientific), and incubated on a rotator at 4°C for 1 h. The resin was loaded onto a column and washed with lysis buffer containing 25 mM Imidazole. The recombinant proteins were eluted using lysis buffer containing 250 mM Imidazole for BT_3942, or 300 mM Imidazole for BVU_0767 and BVU_1163. After concentration in a spin concentrator pre-dialysis, fractions of each protein were dialyzed overnight at 4°C in 1 L of storage buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10% glycerol) in three times. The size and purity of the recombinant proteins were verified by SDS-PAGE gel (NuPAGE 4 to 12%, Bis-Tris Mini Protein Gel, Thermo Fisher Scientific), and the concentration of each protein was measured by absorbance at 280 nm using Nanodrop (Thermo Fisher Scientific).
Acyl-CoA thioesterase activity of the recombinant proteins was determined spectrophotometrically at 412 nm with 5,5¢-dithiobis (2-nitrobenzoic acid) (DTNB) as previously reported with minor modification (Jawed et al., 2016). All reactions were carried out 200 μl of 60 mM potassium phosphate buffer (pH 7.4) containing 200 μM DTNB, 5 μM bovine serum albumin, and 100 μM of different chain length of acyl-CoAs (acetyl-CoA, propionyl-CoA, butyryl-CoA, hexanoyl-CoA, decanoyl-CoA and palmitoyl-CoA) in 96-well plate. The reaction was initiated by adding 5 μl of the recombinant enzyme at final concentration 25 nM into the mixture at 37°C. The liberation of free CoA was monitored at 412 nm for 25 min using a microplate spectrophotometer. Enzymatic assays were performed with duplicates per condition. Significant differences were determined by unpaired two-tailed t test.
Acyl-CoA transferase activity of the recombinant protein was measured by determining the concentrations of acetyl-CoA with citrate synthase assay as previously reported with minor modifications (Sato et al., 2016). Briefly, the reaction mixture (40 μl) consisted of 40 mM potassium phosphate buffer (pH 8.0), 100 mM sodium acetate, and 0.5 mM of different chain lengths of acyl-CoAs (propionyl-CoA, butyryl-CoA, succinyl-CoA, and hexanoyl-CoA). The purified enzyme was added to the reaction mixture at final concentration 1.5 μM and incubated for 5 min at 37°C. The reactions were terminated by the addition of 10 μl of 4.5% trichloroacetic acid and neutralized by adding 25 μL of 400 mM potassium phosphate buffer (pH 8.0). To determine acetyl-CoA, assay mixture (25 μl) containing 4 mM oxaloacetate, 4 mM DTNB, and 36.8 μg/mL citrate synthase was added to the reaction mixture, and incubated at 37°C for 30 min. The free CoA of the reaction mixture was measured at 412 nm using a microplate spectrophotometer. Enzymatic assays were performed with duplicates per condition.
Western blot assay of Acyl-CoA transferases
To examine expression of Acyl-CoA transferase in protein level, Bt and Bv strains harboring plasmids pBt_3193-HA and pBv_1163-HA were grown in 0.25% Glc as described above, and harvested (12,000 rpm x 5 min) at early and late log phase. Bacteria were suspended in PBS with protease inhibitor and lysed by sonication. Cell extracts (30 μg-150 μg protein) were loaded on 4 to 12% NuPAGE gel (Thermo Fisher Scientific) and then transferred to nitrocellulose membrane. Membranes were blocked with 3% skim milk solution at room temperature for 30 min. Primary antibody, mouse anti-HA (Santa Cruz) and mouse anti-E. coli RpoA (Biolegend), which cross-reacts with Bacteroides RpoA (Hecht et al., 2017), were used at 1:4000 dilution in PBST (PBS/0.05% Tween 20) with 1% skim milk. Secondary horseradish peroxidase-conjugated anti-mouse antisera (Santa Cruz) was used at 1:5,000 dilution. Blots were developed with SuperSignalWest Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific).
QUANTIFICATION AND STATISTICAL ANALYSIS
Phylogenetic tree
The phylogenic tree of Bacteroidales strains was generated (Figure 2A), based on the core genes (526 for Bacteroides and 1114 for Parabacteroides) which have 80% identity in amino acid sequence levels, using the concatenated alignment for a neighbor-joining tree in MEGA (https://www.megasoftware.net/).
Analysis of fitness effects of butyrate across strains and sugars
53 Bacteroidales strains were grown on MM agar plates under 11 monosaccharides conditions in the presence and absence of butyrate for 4 days. To calculate butyrate susceptibility across strains and sugars (Figure 2A), the images of bacterial growth over time were analyzed using a custom built MATLAB (MatWorks) script. First, the location of the focal colony was automatically detected (with manual checking and adjustment if necessary) using the CircularHough_Grd function, and the average pixel density across the colony was used as a quantitative growth measurement, normalized by the maximum pixel density in control conditions. For ease of interpretation, we then discretely binned these normalized values so that growth on a given day can vary between 0 (no growth) or 5 (pixel density equal to or above maximum value in control conditions). This process yielded two 1 × 4 vectors of discrete values that describe the growth over time of the focal strain, in the focal sugar, either with or without butyrate. Inhibition level on each day was calculated by subtracting the butyrate growth vector from the control vector (note, we set any negative values to zero, these correspond to instances where the focal strain grew more with butyrate than without). The inhibition score was defined as the sum of this inhibition vector across strains and sugars, creating a matrix that describes the level of inhibition across all conditions.
Culture-seq data analysis
Illumina raw reads were first demultiplexed using skewer v0.2.2 (Lim et al., 2017) allowing no mismatch with both primers, next quality trimmed using vsearch v2.7.1 (Rognes et al., 2016) with the setting “–fastq_filter-fastq_truncee 1,” and further trimmed to the uniform length 110 nt. The trimmed reads were dereplicated and finally used to map against a custom database of Bacteroides rpsC sequences using vsearch with the “–usearch_global” setting. Chimeras were removed using usearch v10.0.240 (Edgar, 2010).
RNA-seq data analysis
Sequencing reads from each sample in a pool were demultiplexed based on their associated barcode sequence using custom scripts. Reads were aligned to the B. thetaiotaomicron VPI 5482, B. vulgatus ATCC 8482, and B. vulgatus CL09 reference genomes using BWA (Li and Durbin, 2009) and read counts were assigned to genes and other genomic features using custom scripts. Differential expression analysis was conducted with DESeq2 (Love et al., 2014).
Pairwise comparisons were performed with log2-transformed normalized RNA-seq reads between SCFAs and water control in different sugars with biological duplicates per condition. To identify genes expression that correlated with the strength of butyrate fitness effects, expression of statistically significant genes (∣log2(fold change)∣ > 1, padj < 0.05) with 1.5 fold difference across sugars were grouped as follows: 1) Glc < GlcNAc < Gal or Xyl, 2) Glc > GlcNAc > Gal or Xyl, 3) Glc > GlcNAc = Gal = Xyl, or 4) Glc < GlcNAc = Gal = Xyl, and no gradient across 4 sugars (Glc = GlcNAc = Gal = Xyl). RNA-Seq data were listed in Table S2.
Functional annotation term and/or clustering of butyrate-responding genes was analyzed by using DAVID 6.8 (NIAID/NIH), and statistically significant functional groups were highlighted in color (pval < 0.001).
Tn-seq data analysis
To analyze the fitness of transposon mutants in the library, Illumina raw reads were subjected to the following bioinformatics pipeline. The demultiplexed reads were first trimmed using cutadapt v1.17 (Martin, 2011) to remove transposon and adaptor sequences (“-gGACTTATCATCCAACCTGT -O 17 -e 0.2” and “-aATACCACGAC -O 5 -e 0.1 -m 15”). These trimmed reads were then mapped to the corresponding Bacteroides reference genome using Bowtie v1.2.2 (Langmead et al., 2009) with the setting “-n 3 -l 28 -e 120–best.” The derived insertion tallies were subjected to comparative analysis between each pair of control and butyrate samples using Transit v2.1.0 (DeJesus et al., 2015) under the “resampling” mode with the setting “-n TTR -iN 0.05 -iC 0.05.” As each experiment was performed in biological duplicates, to combine fold change (butyrate /control) and p-values from two replicates, we used the smaller absolute value fold change and the higher p-value for each gene, which is more conservative than other methods such as Fisher’s method. As the Transit resampling method had a default p-value detection limit of 10e-4, for more significant hits that were marked as p-value = 0, we randomly assigned p-values < 10e-4 for the sake of volcano plots. Statistically significant genes were highlighted in red (∣log2(fold change)∣> 1, padj < 0.05) in volcano plots.
Tn-Seq data were listed in Table S3.
HMP2 data analysis
Functional profiles for metagenomes (MGX), metatranscriptomes (MTX) and metabolomes (MBX) were downloaded from the Inflammatory Bowel Disease Multi’omics Databases (https://www.ibdmdb.org/) (Lloyd-Price et al., 2019) on April 2019. These included 1,638 MGX, 818 MTX and 546 MBX samples, respectively, in total comprising 132 subjects (with multiple time points for each). The abundance profiles of gene families (UniRef90s) (Suzek et al., 2007) for MGX and MTX were generated by HUMAnN 2.11.0, which we previously optimized to identify and quantify genes from individual microbial species present at > 1x fold-coverage in a community sequencing sample (Franzosa et al., 2018). Gene expression profiles were analyzed as the ratios between each gene family’s MTX versus MGX abundance per sample, where undefined values (zero divided by zero) and non-finite values (non-zero divided by zero) were removed (treated as NA). Metabolomic profiles were measured using a combination of four LC-MS method (Lloyd-Price et al., 2019). The sum-normalized metabolite data (sum-normalized to 1 per sample per LC-MS method) and median-normalized metabolite counts (which is used to reduce technical sample to sample variation) were taken from the associated publication’s supporting information (Lloyd-Price et al., 2019). Both types of metabolite data were analyzed, with roughly equivalent results (Figure S6A). MGX, MTX and MBX samples were strictly matched for multi-omic association if they derived from the same subject and sampling time point (yielding 88 samples from 26 nonIBD participants: 52 samples from 14 adult participants and 36 samples from 12 child participants). Since multiple samples from the same subject were often present, we considered only the last matched MGX:MTX:MBX samples from each subject for the following correlation analysis (yielding 26 samples from 26 individuals) (Table S5). For Figure 7B, the relative abundance range for low butyrate level (minimum to median value) is 3.56 × 10−5 to 2.15 × 10~3; for high-butyrate (median to maximum value) is 2.15 × 10-3 to 9.67 × 10−3.
To explore putative associations between microbial features and metabolites in Bacteroides CoA metabolism pathway genes, we identified CoA transferase (BVU_1163) homologs in Bacteroides using a UniRef-based protein sequence catalog (Suzek et al., 2015) (UniRef release 2014_07). We first: i) identified the gene family UniRef90_R7P442 (UniRef90 cluster: > 90% amino acid identity and > 80% coverage) of BVU_1163, then ii) linked this to its corresponding UniRef50 cluster UniRef50_P52043 (requiring >50% identity and > 80% coverage), iii) summed the gene abundances of all UniRef90s in this UniRef50 cluster per sample, and finally iv) further summed the abundances of all such UniRef50 clusters (members of UniRef50_P52043) from Bacteroides species per sample. Two butyrate levels (low-butyrate and high-butyrate) were defined as being above or below the median metabolomic abundance of butyrate (across all included samples, Table S5). Within each of these two butyrate levels, we calculated the Spearman correlation between the gene abundance and gene expression contributed by BVU_1163 Bacteroides UniRef50 families. The same approach was then applied to succinate metabolite abundances as well.
BLAST alignment
Amino acid sequence alignment of Acyl-CoA thioesterase BT_3942 from Bt VPI as reference and Acyl-CoA transferase BVU_1163 from Bv ATCC as reference was performed using NCBI BLAST service against 42 Bacteroides strains (Figure S5).
Other statistical analyses
Images of bacterial growth are one representative of at least biological duplicates or triplicates. Graphpad Prism version 9 was used for all statistical analyses. For pairwise and two independent group comparison Student’s t test was used, while for multiple group comparison Two-way ANOVA were performed. Statistical significance is indicated as follows: ns > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Error bars represent the standard error of the mean. For correlation analysis of MGX:MTX:MBX samples, correction for multiple hypothesis testing was performed using a Benjamini-Hochberg false discovery rate (FDR) approach with a conservative FDR q-value < 0.05 required as a threshold for statistical significance.
Supplementary Material
Figure S1. Butyrate is a specific sugar-dependent fitness switch for the Bacteroides, related to Figure 2
(A) Bacteroides strains as indicated grown in in MM-Glc or GalNAc in the absence or presence of specific SCFAs.
(B) Correlation of growth rate and butyrate inhibition score of each given species/strain grown in different sugars. Growth rates (k) were calculated using absorbance readings corresponding to exponential phase (OD600: 0.2-0.6) fit to a log exponential growth equation (Prism 9). Correlation of k and butyrate inhibitions score (Figure 2A) was performed by Spearman correlational analysis.
(C) Bacteroides species and strains grown in MM with different sugars at the indicated concentration of butyrate.
(D and E) Bacteroides species and strains, as indicated, grown in MM with various glycans (D) and heterodimers cognate monosaccharides (E) in the absence or presence of butyrate.
Shown is a representative of biological duplicates in (A and C-E).
Figure S2. Global transcriptional regulation and impact on membrane damage and intracellular pH of short chain fatty acids on the Bacteroides, related to Figure 3
(A) RNA-seq analysis of Bt VPI (left) and Bv ATCC (right) after 1 h pulse with specific SCFAs in MM-Glc. Significantly differentially expressed genes are shown in red (∣log2(fold change)∣> 1, padj < 0.05).
(B) Intracellular pH in Bt VPI and Bv ATCC after 1 h pulse with specific SCFAs in MM-Glc. Error bars represent mean ± SEM of three biological replicates. Nonsignificant p > 0.05, *p < 0.05; paired two-tailed t test.
(C) Representative flow cytometry analysis of PI staining of Bt VPI and Bv ATCC grown in MM-Glc or Gal after 1 h pulse with and without specific SCFAs. The middle quadrant is the low PI population (% in blue), the right quadrant is the high PI population (% in green).
Figure S3. An Acyl-CoA metabolic defense to butyrate in Bacteroides, related to Figure 4
(A) Bt WT and Bt Δ3942 grown in MM with different sugars at the indicated concentration of butyrate.
(B) Bt WT, Bv WT, and their isogenic strains grown in modified YCFA complex media with glucose at indicated concentrations, in the absence or presence of butyrate.
(C) Bt WT and Bt Δ3942 grown in MM-Glc with and without specific SCFAs.
(D) Acyl-CoA transferase activity of the recombinant protein BVU_1163 with different chain lengths of acyl-CoAs as substrate.
(E) Relative abundance of 13C Acyl-CoAs in Bt and Bv grown in MM-Glc after 30 min pulse with 10 mM 13C butyrate.
(F) Colony forming units in Bt, Bv and their isogenic strains grown in MM-Glc after 30 min pulse with 20 mM butyrate and water as control.
(G) Fold difference of acyl-CoAs in Bt, Bv and their isogenic strains grown in MM-Glc after 30 min pulse with 20 mM butyrate or water.
Shown is a representative of three biological replicates in (A-C). In (D-G), error bars represent mean ± SEM of two (D and E) and three (F and G) biological replicates. Nonsignificant p > 0.05, *p < 0.05, **p < 0.01; paired two-tailed Student’s t test (F), unpaired two-tailed Student’s t test (G).
Figure S4. Acyl-CoA transferase tuning of sugar-dependent inhibitory function of butyrate, related to Figure 5
(A) Bv WT and Bv Δ1163 grown in MM-Glc or Gal in the absence or presence of butyrate.
(B) Expression of Acyl-CoA metabolic genes in Bt VPI and Bv ATCC grown in MM-Glc and MM-Gal after 30 min butyrate pulse. Presented is log2 fold difference of relative expression of target genes with and without butyrate pulse in Glc or Gal (left panel) and Gal versus Glc (right panel) based on differential gene expression of RNA-seq analysis by DESeq2. ****padj < 0.0001.
(C) Analysis of Acyl-CoAs in the butyrate-resistant Bv Δ1163 Δ0574 mutant grown in MM-Glc after 30 min pulse with 20 mM butyrate and water as control. Error bars represent mean ± SEM of three biological replicates. Nonsignificant p > 0.05; Paired two-tailed Student’s t test.
(D) Tn-seq of Bv CL09 grown in MM-Gal with and without butyrate. Genes with ∣log2(fold change)∣ > 1, and −log(pvalue) > 2 are red, with specific genes highlighted in green (Acyl-CoA metabolic genes) and blue (ilv and leu operons). See also Table S3.
(E) Bv CL09 and isogenic strains grown in Glc, Gal, and Xyl with and without butyrate.
(F) Schematic of the ilv and leu operons in Bt VPI.
Shown is a representative of three biological replicates in (A and E).
Figure S5. Species-level genetic variation in Acyl-CoA metabolic defense among the Bacteroides, related to Figure 6
(A) Protein identity of BT_3942 among the Bacteroides. * Indicates focal protein.
(B) Alignment of amino acid sequences of Acyl-CoA thioesterases of Bt VPI and Bv ATCC. Arrows indicate the predicted active sites of Acyl-CoA thioesterase.
(C) Kinetics of Acyl-CoA thioesterase activity of the recombinant proteins, BT_3942, and BVU_0767 with different chain lengths of acyl-CoAs as substrate. Error bars represent mean ± SEM of two biological duplicates.
(D) Protein identity of BVU_1164 and BVU1163 among the Bacteroides. * Indicates focal protein.
(E) Alignment of amino acid sequences of Acyl-CoA transferases of Bt VPI and Bv ATCC.
(F) western blot analysis in Bt VPI and Bv ATCC harboring plasmid expressing the Acyl-CoA transferase with HA-tag under its own promoter. Bacteria were grown in MM-Glc and harvested for lysate preparation across growth phases as indicated.
(G) Relative mRNA expression of the Acyl-CoA transferase gene BVU_1163 in Bv ATCC and Bv CL09 grown in MM-Glc across growth phases. Error bars represent mean ± SEM of three biological replicates. *p < 0.05, ***p < 0.001; unpaired two-tailed t test.
(H) Natural Bacteroides strains as indicated expressing the Acyl-CoA transferase genes BVU_1163 or BT_3193 under the low (pBt) or high (pBv) expression promoters in MM-Glc in the presence and absence of butyrate.
(I) Bv CL09 complemented with BVU_1163/ AGXZ_01713 driven under the BVU_1163/ AGXZ_01713 endogenous promoter or vector control grown in MM-Glc in the absence or presence of butyrate.
(J) Bv ATCC isogenic strains as indicated expressing the BVU_1163 gene under an inducible promoter in MM-Glc, Gal, or Xyl in the presence of butyrate. For induction, anhydrotetracycline (aTc) was used at the indicated concentration (0, 0.1, 1, 10, and 25 ng/mL).
(K) Alignment of nucleotide sequences in 200 bp upstream region of the BVU_1163 gene from Bv ATCC and Bv CL09. RNA-polymerase binding sites −33 region and −7 region are boxed.
(L) RNA-seq analysis of Bv ATCC and Bv CL09 grown in Glc with and without butyrate. In both strains, genes with −log (pvalue) > 2 are plotted and highlighted (red: Bv-specific genes; green: Bv CL09-specific genes; blue: common gene in Bv and Bv CL09). The strain-specific genes are less than 50% identity in amino acid sequences.
Shown is a representative of two (J) and three biological replicates (F, H, and I).
Figure S6. Bacteroides fitness is conditional on Acyl-CoA transferase expression and butyrate in vivo, related to Figure 7
(A) Correlation between relative DNA abundance and relative expression of the Acyl-CoA transferase BT_3193/BVU_1163 of 26 fecal samples from 26 human subjects in low versus high levels of fecal butyrate or high succinate (Benjamini-Hochberg false discovery rate (FDR) approach with a conservative FDR q-value < 0.05) as in analysis of data in Figure 7B using median-normalization rather than sum-normalization. (B) Spearman correlation to compare the HMP2 abundances of the organic acids butyrate and succinate and the BCAAs Leu, Ile, and Val in human gut samples above.
Highlights.
Butyrate inhibits gut microbiome Bacteroides
Butyrate inhibition depends on which sugar a given Bacteroides strain uses
Core genome variation in Acyl-CoA enzymes mediates butyrate defense
Each Bacteroides unique fitness landscape unpredictable by interaction of ecosystem parts
ACKNOWLEDGMENTS
We thank members of the S.R.-N. lab for helpful comments on the manuscript, Beneo (Inulin) and Glycom (HMO) for reagents, and J. Livny and the Broad MOC (assistance for RNASeq). We thank M. and B. Rakoff-O’Neill for sugar preparation and tube labelling. This work is supported by NIDDK R24DK110499 (C.H.), Keck Foundation (J.-K.W.), Mathers Foundation (J.-K.W., S.R.-N.), a NDSEG Fellowship (G.A.K.), a Sir Henry Wellcome Postdoctoral Research Fellowship (K.Z.C.), the Harvard Digestive Disease Center (P30 DK034854 to S.R.-N.), a Career Award for Medical Scientists from the Burroughs Wellcome Fund (S.R.-N.), a Pew Biomedical Scholarship (S.R.-N.), a Basil O’Connor Starter Scholar Award from the March of Dimes (S.R.-N.), and the NIH (1K08AI130392-01 and DP2GM136652 to S.R.-N.).
Footnotes
DECLARATION OF INTERESTS
J.K.W. is a member of the SAB and a shareholder of DoubleRainbow Biosciences, Galixir, and Inari Agriculture. The other authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2022.01.002.
REFERENCES
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al. ; MetaHIT Consortium (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, and Wishart DS (2016). Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44, W147–W153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcik W, Boutin RCT, Sokolowska M, and Finlay BB (2020). The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 52, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley DP, Rocha ER, and Smith CJ (2000). Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett 193, 149–154. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, and Harrison OJ (2017). Homeostatic Immunity and the Microbiota. Immunity 46, 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, and Lawley TD (2016). Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau MR, Blanton LV, DiGiulio DB, Relman DA, Lebrilla CB, Mills DA, and Gordon JI (2016). A microbial perspective of human developmental biology. Nature 535, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Roelofs KG, and Comstock LE (2016). Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements. BMC Genomics 17, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, and Macfarlane GT (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus MA, Ambadipudi C, Baker R, Sassetti C, and Ioerger TR (2015). TRANSIT–A Software Tool for Himar1 TnSeq Analysis. PLoS Comput. Biol 11, e1004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia MS, and Fischbach MA (2015). HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 349, 1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, and Relman DA (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- Elinav E, Garrett WS, Trinchieri G, and Wargo J (2019). The cancer microbiome. Nat. Rev. Cancer 19, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro A, Crook N, Gasparrini AJ, and Dantas G (2018). Multiscale Evolutionary Dynamics of Host-Associated Microbiomes. Cell 172, 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, and Sonnenburg JL (2011). Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe 10, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KR, Schluter J, Coyte KZ, and Rakoff-Nahoum S (2017). The evolution of the host microbiome as an ecosystem on a leash. Nature 548, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, Lipson KS, Knight R, Caporaso JG, Segata N, and Huttenhower C (2018). Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 15, 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblum S, Carr R, and Borenstein E (2015). Extensive strain-level copy-number variation across human gut microbiome species. Cell 160, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, Das J, Wang H, Guthmiller J, Zheng N-Y, et al. (2019). Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 178, 1313–1328.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht AL, Casterline BW, Choi VM, and Bubeck Wardenburg J (2017). A Two-Component System Regulates Bacteroides fragilis Toxin to Maintain Intestinal Homeostasis and Prevent Lethal Disease. Cell Host Microbe 22, 443–448.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, and Littman DR (2016). The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, and Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Integrative HMP (iHMP) Research Network Consortium (2019). The Integrative Human Microbiome Project. Nature 569, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, et al. (2018). A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 24, 296–307.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawed K, Mattam AJ, Fatma Z, Wajid S, Abdin MZ, and Yazdani SS (2016).Engineered Production of Short Chain Fatty Acid in Escherichia coli Using Fatty Acid Synthesis Pathway. PLoS ONE 11, e0160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser JC, and Heinrichs DE (2018). Branching Out: Alterations in Bacterial Physiology and Virulence Due to Branched-Chain Amino Acid Deprivation. MBio 9, e01188–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Wilks JC, Fitzgerald DM, Jones BD, Bondurant SS, and Slonczewski JL (2008). Rapid acid treatment of Escherichia coli: transcriptomic response and recovery. BMC Microbiol. 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino Y, Nakayama-Imaohji H, Fujita M, Tada A, Yoneda S, Murakami K, Hashimoto M, Hayashi T, Okazaki K, and Kuwahara T (2016). Counterselection employing mutated pheS for markerless genetic deletion in Bacteroides species. Anaerobe 42, 81–88. [DOI] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva-Datchary P, and Bäckhed F (2016). From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 165, 1332–1345. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk AA, Zheng D, and Elinav E (2019). Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol 17, 742–753. [DOI] [PubMed] [Google Scholar]
- Koppel N, Maini Rekdal V, and Balskus EP (2017). Chemical transformation of xenobiotics by the human gut microbiota. Science 356, eaag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Martens EC, Gordon JI, and Smith TJ (2008). Starch catabolism by a prominent human gut symbiont is directed bythe recognition of amylose helices. Structure 16, 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Cameron EA, and Martens EC (2012). How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol 10, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle A, and Sokol H (2020). Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol 17, 223–237. [DOI] [PubMed] [Google Scholar]
- Leshem A, Liwinski T, and Elinav E (2020). Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 78, 597–613. [DOI] [PubMed] [Google Scholar]
- Li H, and Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Zimmermann M, Barry NA, and Goodman AL (2017). Engineered Regulatory Systems Modulate Gene Expression of Human Commensals in the Gut. Cell 169, 547–558.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sadhukhan S, Sun S, Wagner GR, Hirschey MD, Qi L, Lin H, and Locasale JW (2015). High-Resolution Metabolomics with Acyl-CoA Profiling Reveals Widespread Remodeling in Response to Diet. Mol. Cell. Proteomics 14, 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, et al. ; IBDMDB Investigators (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, de Agüero MG, and Ganal-Vonarburg SC (2017). How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol 17, 508–517. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, and Sonnenburg JL (2011). Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal 17, 10–12. [Google Scholar]
- Mazmanian SK, Round JL, and Kasper DL (2008). A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625. [DOI] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, and Lu TK (2016). Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst. 2, 214. [DOI] [PubMed] [Google Scholar]
- Pal D, Venkova-Canova T, Srivastava P, and Chattoraj DK (2005). Multipartite regulation of rctB, the replication initiator gene of Vibrio cholerae chromosome II. J. Bacteriol 187, 7167–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG (2016). Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 352, 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantosti A, Tzianabos AO, Onderdonk AB, and Kasper DL (1991). Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect. Immun 59, 2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P, et al. (2019). Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 176, 649–662.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode ML, Beller ZW, Han ND, Cheng J, Peters SL, Terrapon N, Henrissat B, Le Gall S, Saulnier L, Hayashi DK, et al. (2019). Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 179, 59–73.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta DR, Graham DB, Subramanian S, and Xavier RJ (2019). Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell 178, 1041–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter NT, and Martens EC (2017). The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol 71, 349–369. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Coyne MJ, and Comstock LE (2014). An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol 24, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes T, Flouri T, Nichols B, Quince C, and Mahé F (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, and Garrett WS (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 16, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Yoshida Y, Nagano K, Hasegawa Y, Takebe J, and Yoshimura F (2016). Three CoA Transferases Involved in the Production of Short Chain Fatty Acids in Porphyromonas gingivalis. Front. Microbiol 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sberro H, Fremin BJ, Zlitni S, Edfors F, Greenfield N, Snyder MP, Pavlopoulos GA, Kyrpides NC, and Bhatt AS (2019). Large-Scale Analyses of Human Microbiomes Reveal Thousands of Small, Novel Genes. Cell 178, 1245–1259.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TSB, Raes J, and Bork P (2018). The Human Gut Microbiome: From Association to Modulation. Cell 172, 1198–1215. [DOI] [PubMed] [Google Scholar]
- Schwalm ND 3rd, Townsend GE 2nd, and Groisman EA (2017). Prioritization of polysaccharide utilization and control of regulator activation in Bacteroides thetaiotaomicron. Mol. Microbiol 104, 32–45. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, and Cryan JF (2019). Microbiota and the social brain. Science 366, eaar2016. [DOI] [PubMed] [Google Scholar]
- Shishkin AA, Giannoukos G, Kucukural A, Ciulla D, Busby M, Surka C, Chen J, Bhattacharyya RP, Rudy RF, Patel MM, et al. (2015). Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods 12, 323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Priefer U, and Pühler A (1983). A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nature Biotechnology 1, 784–791. [Google Scholar]
- Smith CJ, Rogers MB, and McKee ML (1992). Heterologous gene expression in Bacteroides fragilis. Plasmid 27, 141–154. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, and Garrett WS (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, and Bäckhed F (2016). Diet-microbiota interactions as moderators of human metabolism. Nature 535, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, and Sonnenburg JL (2010). Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 141, 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara MT, Dubin K, Littmann ER, Moody TU, Fontana E, Seok R, Leiner IM, Taur Y, Peled JU, van den Brink MRM, et al. (2019). Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J. Exp. Med 216, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JM, Lopatkin AJ, Lobritz MA, and Collins JJ (2019). Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 30, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek BE, Huang H, McGarvey P, Mazumder R, and Wu CH (2007). UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23, 1282–1288. [DOI] [PubMed] [Google Scholar]
- Suzek BE, Wang Y, Huang H, McGarvey PB, and Wu CH; UniProt Consortium (2015). UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 31, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ (2019). Modularity as a universal emergent property of biological traits. J. Exp. Zoolog. B Mol. Dev. Evol 332, 356–364. [DOI] [PubMed] [Google Scholar]
- Wexler AG, and Goodman AL (2017). An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol 2, 17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltereck R (1909). Weitere experimentelle Unter-suchungen uber Artveranderung, speziell uber das Wesen quantitativer Artunterschiede bei Daphniden. Verh Tsch Zool Ges, 110–172. [Google Scholar]
- Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin NW, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, et al. (2015). Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350, aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Godneva A, Bar N, Kurilshikov A, Lotan-Pompan M, Weinberger A, Fu J, Wijmenga C, Zhernakova A, and Segal E (2019). Structural variation in the gut microbiome associates with host health. Nature 568, 43–48. [DOI] [PubMed] [Google Scholar]
- Zhu YY, Machleder EM, Chenchik A, Li R, and Siebert PD (2001). Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction. Biotechniques 30, 892–897. [DOI] [PubMed] [Google Scholar]
- Ziels RM, Nobu MK, and Sousa DZ (2019). Elucidating Syntrophic Butyrate-Degrading Populations in Anaerobic Digesters Using Stable-Isotope-Informed Genome-Resolved Metagenomics. mSystems 4, e00159–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, and Goodman AL (2019). Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570, 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Ma Y, Raoult D, Kroemer G, and Gajewski TF (2018). The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 359, 1366–1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Butyrate is a specific sugar-dependent fitness switch for the Bacteroides, related to Figure 2
(A) Bacteroides strains as indicated grown in in MM-Glc or GalNAc in the absence or presence of specific SCFAs.
(B) Correlation of growth rate and butyrate inhibition score of each given species/strain grown in different sugars. Growth rates (k) were calculated using absorbance readings corresponding to exponential phase (OD600: 0.2-0.6) fit to a log exponential growth equation (Prism 9). Correlation of k and butyrate inhibitions score (Figure 2A) was performed by Spearman correlational analysis.
(C) Bacteroides species and strains grown in MM with different sugars at the indicated concentration of butyrate.
(D and E) Bacteroides species and strains, as indicated, grown in MM with various glycans (D) and heterodimers cognate monosaccharides (E) in the absence or presence of butyrate.
Shown is a representative of biological duplicates in (A and C-E).
Figure S2. Global transcriptional regulation and impact on membrane damage and intracellular pH of short chain fatty acids on the Bacteroides, related to Figure 3
(A) RNA-seq analysis of Bt VPI (left) and Bv ATCC (right) after 1 h pulse with specific SCFAs in MM-Glc. Significantly differentially expressed genes are shown in red (∣log2(fold change)∣> 1, padj < 0.05).
(B) Intracellular pH in Bt VPI and Bv ATCC after 1 h pulse with specific SCFAs in MM-Glc. Error bars represent mean ± SEM of three biological replicates. Nonsignificant p > 0.05, *p < 0.05; paired two-tailed t test.
(C) Representative flow cytometry analysis of PI staining of Bt VPI and Bv ATCC grown in MM-Glc or Gal after 1 h pulse with and without specific SCFAs. The middle quadrant is the low PI population (% in blue), the right quadrant is the high PI population (% in green).
Figure S3. An Acyl-CoA metabolic defense to butyrate in Bacteroides, related to Figure 4
(A) Bt WT and Bt Δ3942 grown in MM with different sugars at the indicated concentration of butyrate.
(B) Bt WT, Bv WT, and their isogenic strains grown in modified YCFA complex media with glucose at indicated concentrations, in the absence or presence of butyrate.
(C) Bt WT and Bt Δ3942 grown in MM-Glc with and without specific SCFAs.
(D) Acyl-CoA transferase activity of the recombinant protein BVU_1163 with different chain lengths of acyl-CoAs as substrate.
(E) Relative abundance of 13C Acyl-CoAs in Bt and Bv grown in MM-Glc after 30 min pulse with 10 mM 13C butyrate.
(F) Colony forming units in Bt, Bv and their isogenic strains grown in MM-Glc after 30 min pulse with 20 mM butyrate and water as control.
(G) Fold difference of acyl-CoAs in Bt, Bv and their isogenic strains grown in MM-Glc after 30 min pulse with 20 mM butyrate or water.
Shown is a representative of three biological replicates in (A-C). In (D-G), error bars represent mean ± SEM of two (D and E) and three (F and G) biological replicates. Nonsignificant p > 0.05, *p < 0.05, **p < 0.01; paired two-tailed Student’s t test (F), unpaired two-tailed Student’s t test (G).
Figure S4. Acyl-CoA transferase tuning of sugar-dependent inhibitory function of butyrate, related to Figure 5
(A) Bv WT and Bv Δ1163 grown in MM-Glc or Gal in the absence or presence of butyrate.
(B) Expression of Acyl-CoA metabolic genes in Bt VPI and Bv ATCC grown in MM-Glc and MM-Gal after 30 min butyrate pulse. Presented is log2 fold difference of relative expression of target genes with and without butyrate pulse in Glc or Gal (left panel) and Gal versus Glc (right panel) based on differential gene expression of RNA-seq analysis by DESeq2. ****padj < 0.0001.
(C) Analysis of Acyl-CoAs in the butyrate-resistant Bv Δ1163 Δ0574 mutant grown in MM-Glc after 30 min pulse with 20 mM butyrate and water as control. Error bars represent mean ± SEM of three biological replicates. Nonsignificant p > 0.05; Paired two-tailed Student’s t test.
(D) Tn-seq of Bv CL09 grown in MM-Gal with and without butyrate. Genes with ∣log2(fold change)∣ > 1, and −log(pvalue) > 2 are red, with specific genes highlighted in green (Acyl-CoA metabolic genes) and blue (ilv and leu operons). See also Table S3.
(E) Bv CL09 and isogenic strains grown in Glc, Gal, and Xyl with and without butyrate.
(F) Schematic of the ilv and leu operons in Bt VPI.
Shown is a representative of three biological replicates in (A and E).
Figure S5. Species-level genetic variation in Acyl-CoA metabolic defense among the Bacteroides, related to Figure 6
(A) Protein identity of BT_3942 among the Bacteroides. * Indicates focal protein.
(B) Alignment of amino acid sequences of Acyl-CoA thioesterases of Bt VPI and Bv ATCC. Arrows indicate the predicted active sites of Acyl-CoA thioesterase.
(C) Kinetics of Acyl-CoA thioesterase activity of the recombinant proteins, BT_3942, and BVU_0767 with different chain lengths of acyl-CoAs as substrate. Error bars represent mean ± SEM of two biological duplicates.
(D) Protein identity of BVU_1164 and BVU1163 among the Bacteroides. * Indicates focal protein.
(E) Alignment of amino acid sequences of Acyl-CoA transferases of Bt VPI and Bv ATCC.
(F) western blot analysis in Bt VPI and Bv ATCC harboring plasmid expressing the Acyl-CoA transferase with HA-tag under its own promoter. Bacteria were grown in MM-Glc and harvested for lysate preparation across growth phases as indicated.
(G) Relative mRNA expression of the Acyl-CoA transferase gene BVU_1163 in Bv ATCC and Bv CL09 grown in MM-Glc across growth phases. Error bars represent mean ± SEM of three biological replicates. *p < 0.05, ***p < 0.001; unpaired two-tailed t test.
(H) Natural Bacteroides strains as indicated expressing the Acyl-CoA transferase genes BVU_1163 or BT_3193 under the low (pBt) or high (pBv) expression promoters in MM-Glc in the presence and absence of butyrate.
(I) Bv CL09 complemented with BVU_1163/ AGXZ_01713 driven under the BVU_1163/ AGXZ_01713 endogenous promoter or vector control grown in MM-Glc in the absence or presence of butyrate.
(J) Bv ATCC isogenic strains as indicated expressing the BVU_1163 gene under an inducible promoter in MM-Glc, Gal, or Xyl in the presence of butyrate. For induction, anhydrotetracycline (aTc) was used at the indicated concentration (0, 0.1, 1, 10, and 25 ng/mL).
(K) Alignment of nucleotide sequences in 200 bp upstream region of the BVU_1163 gene from Bv ATCC and Bv CL09. RNA-polymerase binding sites −33 region and −7 region are boxed.
(L) RNA-seq analysis of Bv ATCC and Bv CL09 grown in Glc with and without butyrate. In both strains, genes with −log (pvalue) > 2 are plotted and highlighted (red: Bv-specific genes; green: Bv CL09-specific genes; blue: common gene in Bv and Bv CL09). The strain-specific genes are less than 50% identity in amino acid sequences.
Shown is a representative of two (J) and three biological replicates (F, H, and I).
Figure S6. Bacteroides fitness is conditional on Acyl-CoA transferase expression and butyrate in vivo, related to Figure 7
(A) Correlation between relative DNA abundance and relative expression of the Acyl-CoA transferase BT_3193/BVU_1163 of 26 fecal samples from 26 human subjects in low versus high levels of fecal butyrate or high succinate (Benjamini-Hochberg false discovery rate (FDR) approach with a conservative FDR q-value < 0.05) as in analysis of data in Figure 7B using median-normalization rather than sum-normalization. (B) Spearman correlation to compare the HMP2 abundances of the organic acids butyrate and succinate and the BCAAs Leu, Ile, and Val in human gut samples above.
Data Availability Statement
The raw RNA_Seq and Tn_Seq data from this study have been deposited in the NCBI Short-Read Archive (SRA) under the BioProject ID PRJNA640947 (BioSample IDs for RNASeq: SAMN15338436-15338444; BioSample IDs for Tn_Seq data: SAMN15338433-15338435).
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.