Abstract
Background
Identification of cognitive impairment is based traditionally on the neuropsychological tests and biomarkers that are not available widely. This study aimed to establish the association between motor function (gait speed and handgrip strength) and cognitive performance in the Mini-Mental State Examination, globally and by domains. A secondary goal was calculating a cut-off point for gait speed and handgrip strength to classify older adults as cognitively impaired.
Methods
This is a secondary analysis of SABE Colombia (Salud, Bienestar & Envejecimiento), a survey that was conducted in 2015 on health, wellbeing, and aging in Colombia. This study used linear regression models to search for an association between motor function and cognitive performance. The accuracy of motor function measurements in identifying cognitive impairment was assessed with receiver operating characteristic (ROC) curves. This study also analyzed other clinical and sociodemographical variables.
Results
Gait speed was associated with orientation (r2 = 0.16), language (r2 = 0.15), recall memory (r2 = 0.14), and counting (r2 = 0.08). Similarly, handgrip strength was associated with orientation (r2 = 0.175), language (r2 = 0.164), recall memory (r2 = 0.137), and counting (r2 = 0.08). To differentiate older adults with and without cognitive impairment, a gait speed cut-off point of 0.59 m/s had an area under the curve (AUC) of 0.629 (0.613–0.646), and a weak handgrip (strength below 17.5 kg) had an AUC of 0.653 (0.645-0.661). The cut-off points for handgrip strength and gait speed were significantly higher in male participants.
Conclusions
Gait speed and handgrip strength are similarly associated with the cognitive performance, exhibiting the most extensive association with orientation and language domains of the Mini-Mental State Examination. Gait speed and handgrip strength can easily be measured by any clinician, and they prove to be useful screening tools to detect cognitive impairment.
Keywords: gait speed, handgrip strength, cognitive impairment, biomarker, pre-clinical dementia, motor dysfunction
Introduction
Dementia has become a worldwide health priority that affects the quality of life of older adults and their caregivers (1). In Colombia, identification of cognitive impairment has been traditionally based on neuropsychological tests, imaging, and molecular biomarkers that are not widely available. This poses a major challenge in the timely diagnosis of dementia. The use of non-cognitive biomarkers is an emerging approach for early diagnosis of cognitive impairment (2) and, consequently, for setting up preventive strategies for dementia in low- and middle-income countries such as Colombia (3).
Growing evidence suggests that dementia, particularly Alzheimer's Disease (AD), is a continuum with a long pre-clinical stage that may present with early motor symptoms (4–6). In recent years, identification of the association between cognitive and motor performance suggests that these functions share neural networks in the frontal-hippocampal cortex. Impairment of this neural network can manifest as a concurrent decline of the motor and cognitive functions (7). This means motor function assessment can be a useful correlate with cognition and a promising predictor of mild cognitive impairment (MCI) and dementia (2).
In Colombia, prior reports demonstrate an association between motor dysfunction and dementia (8, 9). In addition, the 5th Canadian Consensus Conference suggested that individuals with subjective memory complaints and motor dysfunction (i.e., gait speed disturbances, and dual-task gait impairment) are prone to developing cognitive decline and should undergo close follow-up and screening (10). However, currently in the Latin America, no standardized screening protocols for cognitive impairment use motor function measurements.
Handgrip strength (HS) assessed with a hand-held dynamometer is proven as a good indicator of the whole muscular strength and wellbeing in the older adults. The HS is influenced by factors such as age, sex, socioeconomic status, and level of physical activity (11). Several reports in the literature suggest that HS might be a good predictor for the risk of cognitive decline (8), chronic diseases, depression, frailty, and dependency in instrumental activities of daily living (IADL) (12, 13).
Gait speed (GS) is another muscular function associated with cognitive function (14, 15) since it integrates motor, perceptual, and cognitive processes. Abnormalities in GS precede cognitive decline by the several years (16–18). A recent publication considered GS measurement as a novel biomarker of cognitive decline, MCI, AD, and other dementias (19).
Measurements of HS and GS are easy to perform, objective, non-invasive, low cost, widely available, and safe. Those characteristics make them acceptable, easy to generalize, and valuable in the clinical assessment of older adults. There is limited knowledge, however, about the cognitive domains predominantly related to motor performance.
This study aimed to establish the association between motor function, assessed with GS and HS, and cognitive function, assessed with the Mini-Mental State Examination (MMSE). A secondary goal was to determine a cut-off point for GS and HS and the accuracy of these motor tests in the identification of cognitive impairment. Based on preliminary reports (8, 9), it can be hypothesized that reduced motor function might be associated with global cognition in the MMSE and some specific domains, such as orientation and memory.
Materials and Methods
Study Design
This is a secondary analysis of SABE Colombia (Salud, Bienestar, & Envejecimiento) a population-based, cross-sectional study of health, wellbeing, and aging that was conducted in Colombia in 2015. The SABE Colombia included a representative sample of the Colombian population −23,694 non-institutionalized adults aged 60 years or older. The probability sampling method was clustered, multistage stratified by urban and rural areas. Methods and procedures conducted in SABE Colombia were based on those used in the international multicenter SABE study to obtain comparability, generalizability, and harmonized protocols (20) adapted to the Colombian population. The information was integrated within the general framework of the Colombian National Surveys System. Other technical details of the SABE Colombia study can be found in the official website of the Colombian Ministry of Health and Social Protection, and other independent publications (21–23).
Inclusion and Exclusion Criteria
The present analysis took a subsample of 5,381 SABE Colombia participants who were able to complete the GS and HS measurements. For the GS test, this study excluded outliers, who were participants with GS values below the percentile 1 or above percentile 99, as these could have been individuals who ran during the test or whose data were entered incorrectly by an examiner. Figure 1 shows a detailed flowchart with eligibility criteria and the selection of the subsample for this study.
Figure 1.
Flow-chart of the exclusion process before the statistical analysis. *Values below the p1 and above the p99 on the time spent wqalking 3 m were excluded from the analysis. **HS, Handgrip strenghth (only a subsample were indicated to use the an adjustable digital handgrip dynamometer).
Variables
The dependent variables assessed in this study were the cognitive variables: the MMSE and its 4 domains (orientation, recall, counting, and language). The independent variables were the HS and GS (muscular function measurements). The possible confounders were sociodemographics (age and gender), functionality measures (Barthel and Lawton scores), comorbidities (high blood pressure, diabetes, myocardial infarction, stroke, arthropathies, and mental diseases), and anthropometrics (body mass index).
Hand grip strength: The HS was measured with an adjustable digital handgrip dynamometer (Takei Scientific Instruments Co., Tokyo, Japan). An examiner instructed each participant in the use of the dynamometer and recorded in kilograms (kg) the best score for each hand. Calculated HS was the average of the left and right hands (24).
Gait speed: The GS was computed from a subtest of the Short Physical Performance Battery (SPPB), validated for the Colombian population, and applied in SABE Colombia (19). Participants were asked to walk 3 meters at their regular pace two times from a standing position. The GS was the best time of the two trials (9).
Functional status: The study assessed basic activities of daily living with the Barthel index (from 0 to 100). Lower scores indicated a functional dependency (25). The Lawton and Brody scale (from 0 to 54), scored instrumental activities of daily living, with higher scores signaling functional impairment (26, 27).
Comorbidities: The study presented the frequency of self-reported comorbidities that had been diagnosed by a physician, including high blood pressure, myocardial infarction, stroke, diabetes, arthropathies (including arthrosis, arthritis, and rheumatoid arthritis), and mental disease.
Cognitive performance: The MMSE was used to classify individuals as cognitively impaired. The MMSE has optimal psychometric characteristics, with 88.3% sensibility and 87% specificity. There is wide support for the use of the MMSE in the initial assessment and follow-up of memory, language, orientation, and visuo-constructional capacity in people with neurocognitive disorders. The MMSE total score ranges from 0 to 30, with low values indicating worse cognitive performance (28, 29). This study used an MMSE score cutoff of ≤24 to classify individuals as cognitively impaired (30, 31).
Statistical Analysis
This study performed a descriptive analysis of the subsample, given the quantitative nature of the variables. Central tendency and dispersion measures were calculated with the program R (32). The Kolmogorov-Smirnov (Lilliefors's correction) test served to determine the normal distribution of each variable.
Assessment of the Association Among Muscular Function and Cognitive Variables
The study used various linear regression models with the cognitive variables (the MMSE total score and by domains) as dependent variables, and the other variables of interest as independent variables. The p-value coefficients and McFadden's R-squared were used to test the models. For the analysis of the relationship among variables, three different regression models were tested. The first model included GS, age, and gender as the independent variables, with the cognitive variables (the MMSE total score and by domains) as dependent variables. The second model included HS, age, and gender as independent variables, and the MMSE domains as the dependent variables. The third and final model included GS and HS, comorbidities, functionality, and sociodemographic variables as independent variables, and the MMSE domains as the dependent variables. In all these models collinearity among independent variables was ruled out by calculating the Spearman correlation coefficient, where no pair of variables had a high correlation; with Rho values ranging between −0.58 and 0.36. Also, possible interactions (or confounding effects among them) were evaluated. The independent variables in the final model were selected using backward elimination.
Muscle Function for Assessment of Cognitive Impairment
To assess GS and HS performance in the identification of cognitive impairment, the authors, for each variable (GS and HS independently), applied the Youden index, identified optimal cut-off points with receiver operating characteristics (ROC) curve analyses, and calculated the areas under the curve (AUC). Considering that gender may affect motor performance, the authors defined different cutoff points stratified by gender with a post-hoc analysis, running a new ROC curve analysis for each motor function.
Results
The SABE Colombia cohort included 23,694 older adults. Cognitive impairment was identified in 4,690 individuals, for an overall prevalence of 19.79%. In the group with cognitive impairment, 62.3% (N = 2,922) were females and 37.7% (N = 1,768) were males. The median age for this group was 77 years (IQR 14), 9 years older than individuals with normal cognition (median 68, IQR 11). In addition, the body mass index (BMI) was lower in the cognitively impaired group (median 24.64 IQR 6.59 vs. median 26.39, IQR 6.23).
Table 1 summarizes the descriptive analysis of the subsample (N = 5,831) by cognitive status. Out of the 5,381 participants, 4,035 had normal cognition and 1,346 had cognitive impairment. The median age was 68 years for individuals with normal cognition and 75 years for individuals with cognitive impairment. The education median was 4 and 1 year, respectively. In the group with normal cognition, 57.62% were females and 42.38% were males. In the group with cognitive impairment, 62.78% were females and 37.22% were males. In terms of muscular function, HS and GS were lower in the group with cognitive impairment.
Table 1.
Descriptive analysis by cognitive status.
| Total (n = 5,381) | Normal cognition, n = 4,035 (74.99%) | Cognitive impairment, n = 1,346 (25.01%) | ||
|---|---|---|---|---|
| Variable | n (%) | |||
| Gender | ||||
| Female | 3,180 (59.1%) |
2,325 (57.62%) |
845 (62.78%) |
|
| Male | 2,201 (40.9%) |
1,710 (42.38%) |
501 (37.22%) |
|
| Comorbidities | ||||
| Hypertension | 3,027 | 2,184 (54.21%) |
843 (62.17%) |
|
| Myocardial infraction | 778 | 550 (13.64%) |
228 (16.79%) |
|
| Stroke | 238 | 149 (3.7%) |
89 (6.55%) |
|
| Diabetes | 897 | 657 (16.34%) |
240 (17.69%) |
|
| Arthropathies* | 1,513 | 1170 (29.05%) |
343 (25.33%) |
|
| Mental Diseases** | 485 | 326 (8.10%) |
159 (11.76%) |
|
| Median (IQR) | ||||
| Demographics | ||||
| Age (years) | 68 (10) | 75 (14) | ||
| Schooling (years) | 4 (4) | 1 (3) | ||
| Anthropometrics | ||||
| Body mass index (BMI) | 26.39 (6.23) | 24.64 (6.59) | ||
| Muscular function | ||||
| Hand grip strenght (kg)$ | 23.18 (8.84) | 18.74 (8.7) | ||
| Gait speed (m/s)$ | 0.723 (0.23) | 0.586 (0.23) | ||
| Functionality | ||||
| Lawton total | 0 (2) | 4 (10) | ||
| Barthel index | 100 (0) | 100 (5) | ||
Includes arthrosis, arthritis and rheumatoid arthritis.
Major mental disorders.
Mean (SD) t-test.
Table 2 summarizes the demographic-adjusted linear regression models to predict cognitive variables. The GS was associated with orientation (r2 = 0.16), language (r2 = 0.15), recall memory (r2 = 0.14), and counting (r2 = 0.08). Similarly, HS was associated with orientation (r2 = 0.175), language (r2 = 0.164), recall memory (r2 = 0.137), and counting (r2 = 0.08). Table 3 shows the final fully adjusted model exploring associations among motor function and cognitive variables. This model included covariates such as age, presence of mental disorder (the only comorbidity selected after backward elimination), and functionality with its interactions. The analysis revealed that 29.1% of the variability in orientation is attributed to the described model. Also, the coefficient in this model was large for language (r2 = 0.273), medium for memory recall (r2 = 0.193), and small for counting (r2 = 0.109).
Table 2.
Association between motor function and cognitive domains.
| Measurement | Mini mental state examination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Orientation | Recall | Counting | Language | Total | ||||||
| R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | |
| Gait speed* | 0.16 | <0.001 | 0.14 | <0.001 | 0.08 | <0.001 | 0.15 | <0.001 | 0.18 | <0.001 |
| Handgrip strength* | 0.175 | <0.001 | 0.137 | <0.001 | 0.08 | <0.001 | 0.164 | <0.001 | 0.19 | <0.001 |
Both measures presented significat interaction with age.
Table 3.
Association's model for cognitive variables.
| Measure | Mini mental state examination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Orientation | Recall | Counting | Language | Total | ||||||
| R2 | R2 | R2 | R2 | R2 | ||||||
| 0.291 | 0.193 | 0.109 | 0.273 | 0.303 | ||||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Gait speed | 0.66 (0.16) | <0.001 | 0.29 (0.07) | <0.001 | 0.33 (0.11) | 0.002 | 0.46 (0.13) | 0.003 | 1.73 (0.38) | <0.001 |
| Handgrip Strength | −0.14 (0.03) | <0.001 | −0.04 (0.02) | 0.03 | −0.04 (0.02) | 0.107 | −0.09 (0.3) | 0.37 | −0.3 (0.09) | <0.001 |
| Age | 0.15 (0.09) | 0.07 | 0.07 (0.04) | 0.06 | 0.14 (0.05) | 0.03 | 0.06 (0.07) | 0.14 | 0.43 (0.21) | 0.044 |
| Mental disease | −0.16 (0.12) | 0.17 | −0.16 (0.05) | 0.027 | −0.03 (0.08) | 0.74 | −0.14 (0.09) | 0.068 | −0.44 (0.28) | 0.118 |
| Barthel | 0.19 (0.07) | 0.005 | 0.08 (0.03) | 0.006 | 0.14 (0.05) | 0.004 | 0.1 (0.05) |
<0.001 | 0.51 (0.16) | 0.001 |
| Lawton | 0.3 (0.08) |
<0.001 | 0.09 (0.04) | 0.009 | −0.03 (0.06) | 0.58 | 0.11 (0.07) | <0.001 | 0.48 (0.19) | 0.017 |
| Handgrip Strength: age° | 0.002 (0.001) | <0.001 | 0.001 (0.010) | 0.047 | 0.001 (0.001) | 0.022 | 0.001 (0.001) | <0.001 | 0.005 (0.001) | <0.001 |
| Barthel: age° | −0.002 (0.001) | 0.005 | −0.001 (0.001) | 0.005 | −0.001 (0.001) | 0.004 | −0.001 (0.001) | 0.065 | −0.007 (0.002) | 0.001 |
| Lawton: age° | −0.008 (0.001) | <0.001 | −0.002 (0.001) | <0.001 | −0.001 (0.001) | 0.299 | −0.004 (0.001) | <0.001 | −0.015 (0.003) | <0.001 |
Interaction; β, Beta coefficient; SE, standard error.
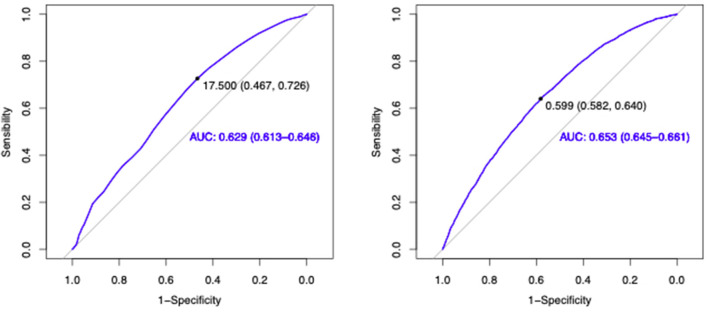
Figure 2 shows the ROC curves that evaluated GS and HS as markers of cognitive impairment. Regarding GS, a cutoff point of 0.599 m/s was identified, and the curve had an AUC of 0.653 (95% CI: 0.645–0.661). Conversely, the curve for HS had an AUC of 0.629 (95% CI: 0.613–0.646), and the cutoff point that was established was17.50 kg. Supplementary Figure 1 shows the ROC curves for HS comparing cutoff points and AUC between women and men, with cutoff points of 16.5 kg in women, and 25.5 kg in men. The GS analysis by gender revealed a cutoff point of 0.59 m/s for females and 0.74 m/s for males (Supplementary Figure 2).
Figure 2.
ROC curves. Receiver operating characteristic (ROC) curves defining cutoff points for HS (A) and GS (B) as markers for cognitive impairment. The dot represents the point with the largest AUC. Area under the curve: (A) 0.629 (CI 95%: 0.613–0.646), (B) 0.653 (CI 95%: 0.645–0.661). AUC, area under the curve; HS, handgrip strength; GS, gait speed.
Discussion
Results in this study support an association between muscular function and cognition. Motor function exhibited the largest association with the orientation domain, followed by language. Our findings complement preliminary reports showing associations among early motor function loss, dysexecutive symptoms, and impairments in the semantic memory (33).
The underlying neural mechanisms that may explain the motor-cognition relationship would be in the hippocampal place, grid, speed, and acceleration cells. Located in the entorhinal cortex, these cells play an important role in the spatial orientation and movement (34–36). In individuals without cognitive impairment, there is a reported correlation between a small volume of the left entorhinal cortex and muscular dysfunction in a dual-task gait assessment. The dysfunction consisted of gait slowing while performing a demanding cognitive task, such as counting backward, subtracting numbers, and naming animals (37). Previous reports suggest that executive functions are essential for gait control since gait requires the integration of sensory and perceptual information, a continuous updating of input, and quick adaptations of the gait pattern (38–40). Similarly, in a longitudinal study that performed a dual-task gait assessment, most of the GS variance was attributed to the level of executive attention (27.4%), and there was a link between orientation and attention (41).
Similarly, speech and language are among the most reliable markers distinguishing types of dementia that include motor dysfunction (42). Speech and language are also cognitive domains strongly associated with the supplementary motor area (SMA), a very important structure in motor execution. Alterations in the SMA and associated circuits (subcortical circuits, basal ganglia) may present clinically as alterations in language and motor performance (43). Muscle strength, specifically HS, can be an overall indicator of central nervous system integrity. Higher HS is associated with better performance on functional tasks, and it may indicate the ability to walk, rise from a chair, and hold small items such as a toothbrush or a comb (13).
Results in this study showed that for the MMSE domains, the determination coefficients (r2) are similar for GS and HS analyses. This suggests that GS and HS have a similar performance when assessing the correlation between motor function and cognitive state. It is relevant though, that this study did not find a strong correlation between GS and HS (Rho Spearman = 0.39). In addition, the AUC of GS and HS were significantly similar (0.65 and 0.63), which leads to the hypothesis that the two motor variables may be used independently to assess cognitive impairment with similar performance, especially when both variables do not present collinearity. Further studies may be needed to confirm this hypothesis. The GS has emerged as one of the motor domains strongly correlated with the incident dementia. Results in this study showed that GS and HS may be alternative parameters for the assessment of individuals at the risk of developing dementia, and also other geriatric syndromes such as frailty (44). Handgrip dynamometers are inexpensive, easily portable, non-invasive, and reliable. Their use does not require extensive training, and results are not biased by learning effects that can be seen in neuropsychological tests (13).
In line with our findings, previous reports have shown that poor physical performance is associated with cognitive decline. As well, GS and HS represent a core determinant of physical frailty and sarcopenia, both associated with cognitive impairment (45). It has recently been proposed that physical and cognitive decline can occur simultaneously and that they can share common etiologies (46). Hormonal levels and inflammatory biomarkers are thought to be implicated in cognitive dysfunction. For example, irisin myokine is expressed not only in the muscle, but also in the brain. It reduces neuroinflammation and post-ischemic oxidative stress, suggesting that this molecule may play an important role in neuroprotection and synaptic plasticity (47). Similarly, higher levels of proinflammatory cytokines, such as IL-6, were associated with greater cognitive decline and lower HS. Associations between impaired cognitive performance and poor physical performance in GS and balance suggest that abnormalities in the nervous system's processing speed might also be linked to changes in the cognitive function (13).
In this analysis, the accuracy of GS and HS as methods to identify cognitive impairment was 65 and 63 %, respectively. The cutoff point set for GS was 0.59 m/s and for HS 17.5 kg. Significant differences were seen between males and females in the HS ROC curve analysis, with cutoff points of 16.5 kg in women, and 25.5 kg in men. Similar values have been reported; in a cross-sectional study about the comprehensive geriatric assessment and GS, performed by the Chongqing Medical University, GS below 0.73 m/s had an AUC of 0.716 (48). Even so, a multivariate logistic regression reported by DeCock et al. (49) with a GS below 0.43 m/s predicted cognitive impairment with 88% accuracy. Also, a cross-sectional study using photocells and the Optogait System revealed that a gait step coefficient of variability above 3.9 s predicted the development of MCI with 85.2% accuracy (50). The model in this study did not include gait parameters such as cadence, step length, normalized speed, dual-task cost, swing time, and cycle time variability, all included in the aforementioned papers. Regarding HS, a cutoff of 20.65 kg was predicted with 71.2% accuracy functional decline in a hospitalized male cohort (51). In that cohort, more variables were included in the model, explaining its accuracy.
One of the main contributions of this study is that it proposes a non-cognitive method to identify older adults with cognitive impairment in a nationally representative sample of a middle-income country. It fills knowledge gaps in biomarkers in this field. This could, consequently, improve the prognosis of a population with access difficulties and give them an opportunity to receive specialized care. Given that there is no sufficient evidence using GS or HS to identify cognitive impairment, many recent publications suggest combining motor and cognitive measures to improve the classification of older adults at risk of dementia (52–55). So far, studies have reported the usefulness of these motor biomarkers, but there is a little evidence of which specific cognitive domains are related to the motor performance. This represents another contribution of this study, since measuring cognitive status with the MMSE allowed identification of the main cognitive domains associated with motor variables.
Around the world, and most particularly in middle-income countries, there is a pressing need to find low-cost, accurate, and accessible biomarkers to identify pre-clinical stages of dementia (56). The results of this study contribute to and enhance the opportunity for diagnosis in countries without access to expensive exams, such as Positron Emission Tomography (PET) and molecular biomarkers. This study's results also present an opportunity to establish preventive strategies based on risk assessments made with inexpensive and easy-to-apply measurements. This facilitates the access and training of health personnel, even in remote areas where populations have a high burden of disease and prevalence of dementia, but limited resources. Further research in this area may allow study findings to be generalized.
This study has some limitations. First, given that SABE is a cross-sectional study, this study could not establish causality. This demonstrates the importance of conducting longitudinal studies evaluating the predictive validity of HS and GS and standardizing the optimal cutoff for detecting individuals with impaired cognition. Second, this study did not include, in its ROC analysis, covariates such as age and schooling that may contribute to the discriminative power of the analysis. The statistic analysis did not include further covariates, since the intention when calculating cutoff points was for these to be generally applicable in a certain population. This possibility could be lost if multiple covariates (age, education, and other demographic variables) were taken into account. Even so, these covariates should be considered and included in future studies if they represent a significant difference between groups (cognitively normal and cognitively impaired).
It is also important to point out that the MMSE provides no information about executive functions, which represent a crucial cognitive domain in the evaluation of dementia in the elderly. Its deterioration is directly related to gait disturbances, as has been consistently found in the multiple studies (57, 58). Regardless, using the MMSE in this study's analysis allowed an evaluation of cognitive domains that had not been previously explored, and with which we found relationships with clinical relevance, as mentioned. Finally, the GS in the 3-meter test is not widely recommended, as results may underestimate a subject's speed (59). The authors do not consider that these biases study results, as it has been used in the previous research (9, 15). Furthermore, SABE Colombia includes the largest sample of Latin American older adults, providing good statistical power to this analysis, so long as the application of the results is carried out in populations with similar sociodemographic and anthropometric characteristics.
Conclusion
Diminished GS and HS are associated with cognitive impairment, with the largest association in orientation and language domains. The GS and HS appear to be useful screening tools that can be used by any clinician to identify cognitive impairment. Both motor function tests share similar operational characteristics and can be used independently. These easy-to-use and accessible tools may be particularly helpful in low- and middle-income countries, reducing the costs associated with a full neuropsychologic assessment, PET imaging, or biomarkers, especially in the remote areas.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/Resumen-Ejecutivo-Encuesta-SABE.pdf.
Ethics Statement
Two universities involved in developing SABE Colombia (Universidad de Caldas, protocol ID CBCS-021-14, and Universidad del Valle, protocol IDs 09-014 and O11-015) reviewed and approved the study protocol. Written informed consent was obtained from each individual before inclusion and completion of the first examination (This included permission to use secondary data and blood samples). The protocol for a secondary analysis was approved by The Human Subjects Committee at the Pontificia Universidad Javeriana (Act ID 20/2017-2017/180,FM-CIE-0459-17).
Author Contributions
EG-C provided the idea, coordinated the project, performed the statistical analysis, interpreted data, and discussed findings. FB-R performed the statistical analysis, analyzed data, and discussed findings. FR analyzed and interpreted data. IM, GG-O, and M-FA read and discussed findings. All authors read and approved the final manuscript.
Funding
This study is part of a larger project funded by Colciencias and the Ministerio de Salud y la Protección Social, the Colombian Ministry of Health and Social Protection (The SABE Study, ID 2013, no. 764). Contract of financing RC No. 829 of 2018, Colciencias – Pontificia Universidad Javeriana.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Aging Institute of the Hospital Universitario San Ignacio for its support for the Neuroscience and Aging Research Group.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.695253/full#supplementary-material
References
- 1.Alzheimer's Disease International . World Alzheimer Report 2019: Attitudes to Dementia. London: (2019). [Google Scholar]
- 2.Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, Borrie MJ, Hachinski VC, Wells J, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. (2017) 74:857–65 10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) 396:413–46. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease. Expert Rev Neurother. (2011) 11:665–76. 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beason-Held LL, Goh JO, An Y, Kraut MA, O'Brien RJ, Ferrucci L, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. (2013) 33:18008–14. 10.1523/JNEUROSCI.1402-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer's disease. Acta Neuropathol. (2015) 129:167–82. 10.1007/s00401-014-1375-y [DOI] [PubMed] [Google Scholar]
- 7.Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol Ser A Biol Sci Med Sci. (2014) 69:1415–21. 10.1093/gerona/glu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Cifuentes E, David-Pardo DG, Borda MG, Pérez-Zepeda MU, Cano CA. Two-way bridge between muscular dysfunction and cognitive impairment: secondary analysis of SABE – Bogota Study. J Frailty Aging. (2017) 6:141–3. 10.14283/jfa.2017.17 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Cifuentes E, Márquez I, Vasquez D, Aguillon D, Borda MG, Lopera F, et al. The role of gait speed in dementia: a secondary analysis from the SABE Colombia study. Dement Geriatr Cogn Disord. (2020) 49:565–72. 10.1159/000510494 [DOI] [PubMed] [Google Scholar]
- 10.Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R, et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. (2020) 16:1182–95. 10.1002/alz.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rijk JM, Roos PRKM, Deckx L, Van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. (2016) 16:5–20. 10.1111/ggi.12508 [DOI] [PubMed] [Google Scholar]
- 12.Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3-year follow-up. J Am Geriatr Soc. (2000) 48:1424–9. 10.1111/j.1532-5415.2000.tb02632.x [DOI] [PubMed] [Google Scholar]
- 13.Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline – a scoping review. Ageing Res Rev. (2017) 35:112–23. 10.1016/j.arr.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Veronese N, Stubbs B, Trevisan C, Bolzetta F, De Rui M, Solmi M, et al. What physical performance measures predict incident cognitive decline among intact older adults? A 4.4 year follow up study. Exp Gerontol. (2016) 81:110–8. 10.1016/j.exger.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Ramírez-Vélez R, Pérez-Sousa MA, Venegas-Sanabria LC, Cano-Gutierrez CA, Hernández-Quiñonez PA, Rincón-Pabón D, et al. Normative values for the short physical performance battery (SPPB) and their association with anthropometric variables in older colombian adults. The SABE Study 2015. Front Med. (2020) 7:52. 10.3389/fmed.2020.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielke M, Roberts R, Savica R, Cha R, Drubach DI, Christianson T, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. (2013) 68A:929–37. 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci. (2017) 72A:655–61. 10.1093/gerona/glw110 [DOI] [PubMed] [Google Scholar]
- 18.Buracchio T, Dodge H, Howieson D, Al E. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. (2016) 67:980–6. 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montero-Odasso M. Gait as a biomarker of cognitive impairment and dementia syndromes. Quo vadis? Eur J Neurol. (2016) 23:437–8. 10.1111/ene.12908 [DOI] [PubMed] [Google Scholar]
- 20.Albala C, Lebrão M, León Díaz E, Ham-Chande R, Hennis A, Palloni A. The Health, Well-Being, and Aging survey: methodology applied and profile of the study population. Rev Panam Salud Publica. (2005) 17:307–22. 10.1590/S1020-49892005000500003 [DOI] [PubMed] [Google Scholar]
- 21.Gomez F, Corchuelo J, Curcio CL, Calzada MT, Mendez F. SABE Colombia: survey on health, well-being, and aging in colombia - study design and protocol. Curr Gerontol Geriatr Res. (2016) 2016:7910205. 10.1155/2016/7910205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuellar, Segura CM. Documento Metodológico: Encuesta Nacional de Salud, Bienestar y Envejecimiento SABE Colombia. Ministerio de Salud y Protección Social de Colombia. Available online at: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/doc-metodologia-sabe.pdf (accessed Oct 2, 2021).
- 23.SABE Colombia 2015: Estudio Nacional de Salud Bienestar y Envejecimiento. Resumen Ejecutivo. Ministerio de Salud y Protección Social de Colombia, Colciencias. Available online at: https://www.minsalud.gov.co/sites/rid/lists/bibliotecaDigital/RIDE/VS/ED/GCFI/Resumen-ejecutivo-encuesta-SABE.pdf (accessed Oct 2, 2021).
- 24.Ramírez-Vélez R, Correa-Bautista JE, García-Hermoso A, Cano CA, Izquierdo M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle. (2019) 10:278–86. 10.1002/jcsm.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney FI, Barthel DW. Functional evaluation the barthel index. Md State Med J. (1965) 14:61–5. 10.1037/t02366-000 [DOI] [PubMed] [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 27.Graf C. The lawton instrumental activities of daily living (IADL) scale. AJN. (2008) 108:59. 10.1097/01.NAJ.0000314810.46029.74 [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 29.Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. (2016) 1:CD011145. 10.1002/14651858.CD011145.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres VL, Vila-Castelar C, Bocanegra Y, Baena A, Guzmán-Vélez E, Aguirre-Acevedo DC, et al. Normative data stratified by age and education for a Spanish neuropsychological test battery: results from the Colombian Alzheimer's prevention initiative registry. Appl Neuropsychol. (2019) 2:1–15. 10.1080/23279095.2019.1627357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arevalo-Rodriguez I, Smailagic N, Ciapponi A, Sanchez-Perez E, Giannakou A, Roquéi Figuls M, et al. Mini-Mental state examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. (2013) 2013:CD010783. 10.1002/14651858.CD010783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; (2020). [Google Scholar]
- 33.Adamo DE, Anderson T, Koochaki M, Fritz NE. Declines in grip strength may indicate early changes in cognition in healthy middle-aged adults. PLoS ONE. (2020) 15:e0232021. 10.1371/journal.pone.0232021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirtshafter HS, Wilson MA. Locomotor and hippocampal processing converge in the lateral septum. Curr Biol. (2019) 29:3177–92.e3. 10.1016/j.cub.2019.07.089 [DOI] [PubMed] [Google Scholar]
- 35.Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, et al. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat Commun. (2015) 6:1–11. 10.1038/ncomms9521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. (2008) 31:69–89. 10.1146/annurev.neuro.31.061307.090723 [DOI] [PubMed] [Google Scholar]
- 37.Sakurai R, Bartha R, Montero-Odasso M, Newman A. entorhinal cortex volume is associated with dual-task gait cost among older adults with MCI: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. (2018) 5:1–7. 10.1093/geroni/igx004.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beauchet O, Allali G, Annweiler C, Bridenbaugh S, Assal F, Kressig RW, et al. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. (2009) 55:702–6. 10.1159/000235905 [DOI] [PubMed] [Google Scholar]
- 39.Beauchet O, Annweiler C, Montero-Odasso M, Fantino B, Herrmann FR, Allali G. Gait control: a specific subdomain of executive function? J Neuroeng Rehabil. (2012) 9:12. 10.1186/1743-0003-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. (2005) 164:541–8. 10.1007/s00221-005-2280-3 [DOI] [PubMed] [Google Scholar]
- 41.MacAulay RK, Wagner MT, Szeles D, Milano NJ. improving sensitivity to detect mild cognitive impairment: cognitive load dual-task gait speed assessment. J Int Neuropsychol Soc. (2017) 23:493–501. 10.1017/S1355617717000261 [DOI] [PubMed] [Google Scholar]
- 42.Reilly J, Rodriguez AD, Lamy M, Neils-Strunjas J. Cognition, language, and clinical pathological features of non-Alzheimer's dementias: an overview. J Commun Disord. (2010) 43:438–52. 10.1016/j.jcomdis.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hertrich I, Dietrich S, Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci Biobehav Rev. (2016) 68:602–10. 10.1016/j.neubiorev.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 44.Vaz-Patto M, Bueno B, Ribeiro Ó, Teixeira L, Afonso RM. Association between handgrip strength, walking, age-related illnesses and cognitive status in a sample of Portuguese centenarians. Eur Rev Aging Phys Act. (2017) 14:9. 10.1186/s11556-017-0178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. (2019) 19:186. 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. (2007) 29:156–62. 10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y, Sumsuzzman DM, Choi J, Kang H, Lee SR, Hong Y. Molecular and functional interaction of the myokine irisin with physical exercise and Alzheimer's disease. Molecules. (2018) 23:3229. 10.3390/molecules23123229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Q, Zhang M, Deng Y, Duan J, Tu Q, Cao Y, et al. Does gait speed replace comprehensive geriatric assessment in the elderly? Int J Gerontol. (2016) 10:232–6. 10.1016/j.ijge.2016.03.010 [DOI] [Google Scholar]
- 49.De Cock A-M, Fransen E, Perkisas S, Verhoeven V, Beauchet O, Remmen R, et al. Gait characteristics under different walking conditions: association with the presence of cognitive impairment in community-dwelling older people. PLoS ONE. (2017) 12:e0178566. 10.1371/journal.pone.0178566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Párraga-Montilla JA, Pozuelo-Carrascosa DP, Carmona-Torres JM, Laredo-Aguilera JA, Cobo-Cuenca AI, Latorre-Román PÁ. Gait performance as an indicator of cognitive deficit in older people. Int J Environ Res Public Health. (2021) 18:3428. 10.3390/ijerph18073428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García-Peña C, García-Fabela LC, Gutiérrez-Robledo LM, García-González JJ, Arango-Lopera VE, Pérez-Zepeda MU. Handgrip strength predicts functional decline at discharge in hospitalized male elderly: a hospital cohort study. PLoS ONE. (2013) 8:e69849. 10.1371/journal.pone.0069849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montero-Odasso M, Speechley M, Muir-Hunter SW, Pieruccini-Faria F, Sarquis-Adamson Y, Hachinski V, et al. Dual decline in gait speed and cognition is associated with future dementia: evidence for a phenotype. Age Ageing. (2020) 49:995–1002. 10.1093/ageing/afaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mancioppi G, Fiorini L, Rovini E, Zeghari R, Gros A, Manera V, et al. Innovative motor and cognitive dual-task approaches combining upper and lower limbs may improve dementia early detection. Sci Rep. (2021) 11:7449. 10.1038/s41598-021-86579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with Mild Cognitive Impairment. The effect of working memory. BMC Geriatr. (2009) 9:41. 10.1186/1471-2318-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Åhman HB, Giedraitis V, Cedervall Y, Lennhed B, Berglund L, McKee K, et al. Dual-task performance and neurodegeneration: correlations between timed up-and-go dual-task test outcomes and Alzheimer's disease cerebrospinal fluid biomarkers. J Alzheimers Dis. (2019) 71:S75–83. 10.3233/JAD-181265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aisen PS, Cummings J, Jack CR, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: Understanding the Alzheimer's disease continuum. Alzheimers Res Ther. (2017) 9:1–10. 10.1186/s13195-017-0283-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montero-Odasso M, Hachinski V. Preludes to brain failure: executive dysfunction and gait disturbances. Neurol Sci. (2013) 35:601–4. 10.1007/s10072-013-1613-4 [DOI] [PubMed] [Google Scholar]
- 58.Sakurai R, Ishii K, Yasunaga M, Takeuchi R, Murayama Y, Sakuma N, et al. The neural substrate of gait and executive function relationship in elderly women: a PET study. Geriatr Gerontol Int. (2017) 17:1873–80. 10.1111/ggi.12982 [DOI] [PubMed] [Google Scholar]
- 59.Krumpoch S, Lindemann U, Rappl A, Becker C, Sieber CC, Freiberger E. The effect of different test protocols and walking distances on gait speed in older persons. Aging Clin Exp Res. (2020) 33:141–6. 10.1007/s40520-020-01703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/Resumen-Ejecutivo-Encuesta-SABE.pdf.