Abstract
Background
Elderly patients with glioblastoma are perceived to face a poor prognosis with perceptions surrounding older age and a relative lack of randomized data contributing. This study evaluated survival prognosticators in elderly glioblastoma patients to more accurately guide their treatment.
Methods
The records of 169 elderly (≥70 years) patients with a new diagnosis of glioblastoma who had undergone neurosurgical intervention were retrospectively examined for patient sex, age, performance status, comorbidities, MGMT promoter methylation, surgical intervention, and chemoradiation regime. The adjusted survival impact of these factors was determined using Cox proportional hazards model and used to devise a two-stage scoring system to estimate patient survival at the stage of surgical (Elderly Glioblastoma Surgical Score, EGSS) and oncological management (Elderly Glioblastoma Oncological Score, EGOS).
Results
The median overall survival (mOS) of the cohort was 28.8 weeks. Gross-total and subtotal resection were associated with improved survival compared to biopsy alone (respective mOS 65.3 and 28.1 vs 15.7 weeks, P < .001). Hypofractionated radiotherapy (40Gy in 15 fractions) with Temozolomide was noninferior to the Stupp protocol, P = .72. Exploratory subgroup analysis revealed a significant benefit of Temozolomide-based approaches in MGMT-methylated patients as well as a trend towards improved survival in MGMT-unmethylated patients. Our EGSS and EGOS scores successfully estimated survival in this retrospective cohort with 65% and 73% accuracy.
Conclusions
Where appropriate and safe, elderly glioblastoma patients may benefit from surgical resection and combined chemoradiotherapy with Temozolomide. The proposed EGSS and EGOS scores take into account important prognostic factors to help guide which patients should receive such treatment.
Keywords: chemotherapy, elderly, glioblastoma, radiotherapy, surgery
Key Points.
Elderly glioblastoma (GB) patients require individualized management decisions.
A subgroup of very fit elderly GB patients may benefit from TMZ regardless of their MGMT status.
EGSS and EGOS scores can estimate the survival of elderly GB patients.
Importance of the Study.
The optimal management of elderly patients with glioblastoma is supported by poor evidence base, which sometimes leads to a more cautious approach in both the surgical and oncological setting. The present study aims to address this uncertainty by building a prognostic scoring system based on multiple relevant variables identified through retrospective analysis of our large local patient cohort, with the goal of identifying types of patients who may benefit from more aggressive treatment with view to improving survival. As a result, the study also identifies a cohort of elderly glioblastoma patients who could benefit from more proactive treatment compared to current accepted standard of care.
Glioblastoma is the most common and aggressive primary malignant brain tumor diagnosed in approximately 2100 patients in England each year.1 With a median age at diagnosis of 55–65 years, a sizeable and growing proportion of cases occurs in the elderly patients.2,3 Compared to younger glioblastoma patients, the elderly cohort face a considerably worse prognosis with patient age and MGMT promoter methylation repeatedly reported as important survival prognosticators.1,4,5 Other prognostic factors include performance status at diagnosis, comorbidities, neurological impairment, tumor location, and size.6 Importantly, these factors also influence the probability of more aggressive surgical and oncological interventions7 and their direct impact on survival is difficult to unpick without randomized studies or multivariate adjusting. To date there are few randomized trials or multivariate analyses collectively exploring the direct impact of biological, molecular, neurosurgical, and oncological factors on the survival of elderly (≥70 years) glioblastoma patients.8 Consequently, clinicians commonly struggle with optimal management decisions further complicated by considerable comorbidities and chemoradiation sensitivity in this age group.
In this retrospective cohort study of elderly (≥70 years) patients with glioblastoma, we used multivariate Cox proportional analysis to examine the independent contribution of the following variables on patient survival: sex, age, and WHO performance status at diagnosis, MGMT promoter methylation, comorbidities, the extent of neurosurgical resection, intraoperative use of 5-aminolevulinic acid (5ALA) and (chemo)radiotherapy regime. The variables with significant independent survival effect were used to devise a two-stage scoring system to estimate survival of the elderly glioblastoma patients at the stage of neurosurgical (Elderly Glioblastoma Surgical Score, EGSS) and oncological management (Elderly Glioblastoma Oncological Score, EGOS). As a result, our study also identifies a cohort of elderly glioblastoma patients who could benefit from more proactive treatment compared to current accepted standard of care.
Methods
Study Design
Patients with a new diagnosis of isocitrate dehydrogenase (IDH)-wildtype glioblastoma aged 70 years or above who had received neurosurgical management at our tertiary center at the John Radcliffe University Hospital in Oxford, UK, between January 2013 and December 2019 were identified in the Neuropathology Database of the Oxford University Hospitals NHS Trust. All patients were discussed at the Neurooncology multidisciplinary team meeting. Dependent on patients age, performance status and MGMT promoter methylation, the chemoradiotherapy regimes were proposed by specialist Neurooncology consultants following the Thames Valley Hospital Network Protocol (Supplementary Figure 1) and agreed in discussion with the patients. All patient records were anonymized. From 188 biopsy and resection records identified, we excluded the records belonging to identical patients (4), patients with reoperations (10), patients diagnosed with IDH-mutant glioblastoma (2), or grade III astrocytoma (3). The remaining 169 patients were included in the analysis. The study and data collection was approved by the Clinical Audit Committee of the Oxford University Hospitals NHS Trust (reference: 30/04/2020-NOTTS-Plaha) and the ethical approval was issued by the Health Research Authority (IRAS Project ID – 256310).
The following data were extracted from the electronic patient records of the John Radcliffe Hospital as well as the associated district general hospitals that provided oncological treatment: patient sex, date of birth, date of symptom onset, presenting complaint, date of diagnosis (based on CT or MRI scan), tumor location, WHO performance status at diagnosis, comorbidities, histological diagnosis, MGMT promoter methylation status, date and type of neurosurgical intervention (stereotactic biopsy or resection), the extent of neurosurgical resection (subtotal versus gross-total resection based on the presence or absence of any residual enhancing tumor postoperative T1-weighted MRI imaging), intraoperative use of 5-aminolevulinic acid (5ALA), (chemo)radiotherapy regime and date of death.
Statistical Analysis
Survival was defined as the period between the date of diagnosis (based on CT/MRI scan) and the date of death; patients with unknown survival status were censored at the date of last follow-up. Kaplan-Meier survival analysis with log-rank test was used to determine survival differences between patient subpopulations.
A 2-stage scoring system was constructed to estimate the survival of elderly (≥70 years) glioblastoma patients receiving different neurosurgical (Stage 1: Elderly Glioblastoma Surgical Score, EGSS) and oncological interventions (Stage 2: Elderly Glioblastoma Oncological Score, EGOS).
To design the scoring system, factors with direct survival impact were identified using multivariate Cox proportional hazards model. Hazard radios (HRs) of factors with significant (P < .05) direct effect on survival were used to construct the EGSS and EGOS scores. Factors associated with increased risk of death (HR > 1) were assigned a score equivalent to the negative value of their HR. Factors with reduced risk of death (HR < 1) were assigned a score of 1/HR. Therefore, higher EGSS and EGOS scores were associated with improved overall survival. To simplify their clinical use, the scores were rounded to the closest multiple of 5 and divided by 5. Despite an effect on the HR-to-score ratios, a correction of +2 points was applied to the score for each factor to avoid negative values and simplify the score for clinical use.
To validate the scoring system, patients were randomly divided into a training data set and a validation data set at a ratio of 2:1 which was determined empirically. EGSS scores (range 1–8) of patients in the training set were categorized into three groups, score 1–3, 4–6, and 7–8, which maximized the log-rank statistic of pairwise comparison. Similarly, EGOS scores (range 1–12) of patients in the training set were categorized into five groups, score 1–3, 4–5, 6–8, 9–11, and 12 based on maximal log-rank statistic of pairwise comparison. Kaplan-Meier survival analysis was used to determine the median survival of each EGSS score group and the independent survival effect of each score group was evaluated using Cox proportional hazards analysis. The same method was used to determine the median survival and independent effect on survival of each EGOS score group.
The survival predictions of the EGSS and EGOS scores derived from the training set were assessed in the validation set. To evaluate accuracy, the predicted survival of each patient based on their EGSS and EGOS score was compared with the actual survival. A prediction was considered accurate when the actual survival of a patient was within the 95% CI of predicted median survival for each score group. To evaluate the discrimination ability of EGSS and EGSS score groups, their survival distributions in the validation set were compared using Kaplan-Meier survival analysis. As in the training set, the independent effect of score groups on survival in the validation set was assessed using Cox proportional hazards model using score groups as covariates. All statistical analyses were performed using IBM SPSS Statistics software. The data were analyzed using parametric tests and normality was evaluated using residual plot analysis, Saphiro–Wilk, and Kolmogorov–Smirnov tests. A two-tailed P < .05 was considered statistically significant.
Results
Three patients were still alive at the time of the analysis. The median overall survival (mOS) in our cohort of elderly (≥70 years) glioblastoma patients was 28.8 weeks (95%CI 22.6–35.1 weeks) and the average age at diagnosis was 74.6 years. Patient background characteristics and information about presenting symptoms, surgical and oncological management are summarized in Supplementary Figure 2.
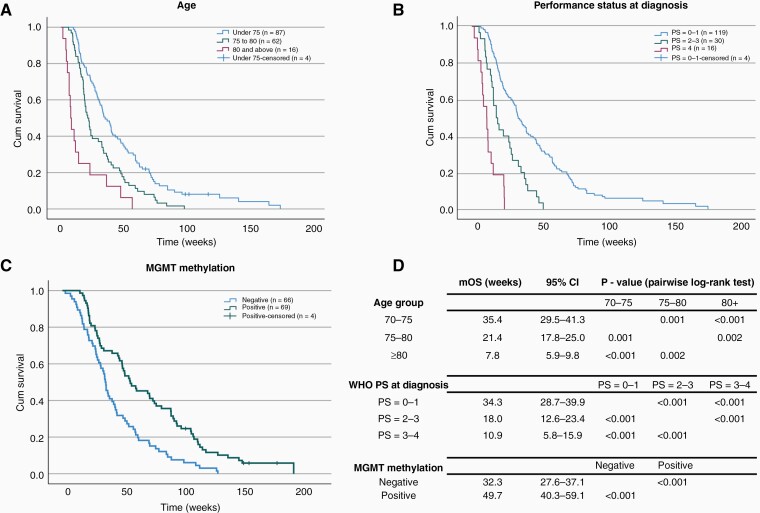
Figure 1 shows the Kaplan-Meier survival distributions of elderly glioblastoma patients stratified for age, WHO performance status, and MGMT promoter methylation. The median survival of patients aged 70–75 (mOS 35.4 weeks, 95%CI 29.5–41.3 weeks) was significantly longer in comparison to 75–80 (mOS 21.4 weeks, 95%CI 17.8–25.0 weeks) and ≥80 age groups (mOS 7.8 weeks, 95%CI 5.9–9.8 weeks, Figure 1A), P < .001. Similarly, significant improvement in mOS was observed in patients with lower performance status at diagnosis (Figure 1B) and patients with methylated MGMT promoter (Figure 1C). Patient sex and comorbidities, i.e. any medical or surgical condition requiring long term medication or surveillance, had no significant effect on mOS (Supplementary Figure 3, P > .05).
Figure 1.
Kaplan-Meier survival distributions of elderly glioblastoma patients stratified for age, WHO performance status (PS), and MGMT promoter methylation. Patients in younger age groups (A), lower PS at diagnosis (B) and methylated MGMT status (C) were observed to have significantly longer median OS compared to their respective controls. Survival distributions were compared using pairwise log-rank test (D). mOS, median overall survival; PS, WHO performance status; 95%CI, 95% confidence intervals.
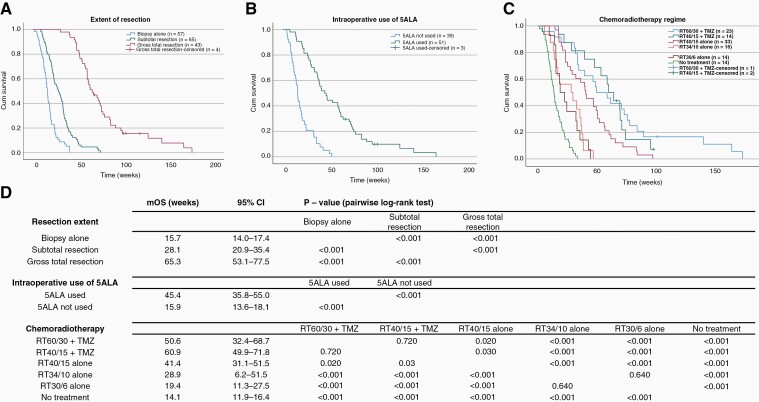
The Kaplan-Meier survival distributions for different surgical interventions are presented on Figure 2A and B. The patients with stereotactic biopsy alone (mOS 15.7 weeks, 95%CI 14.0–17.4 weeks) had significantly shorter median survival compared to patients with subtotal (mOS 28.1 weeks, 95%CI 20.9–35.4 weeks) and gross-total resection (mOS 65.3 weeks, 95%CI 53.1–77.5 weeks), P < .001 (Figure 2A). Intraoperative use of 5ALA in patients undergoing surgical resection (ie excluding patients undergoing biopsy alone) was associated with a markedly significant mOS increase from 15.9 weeks (5ALA not used) to 45.4 weeks (5ALA used, Figure 2B), P < .001.
Figure 2.
Kaplan-Meier survival distributions of elderly glioblastoma patients stratified for the extend of surgical resection, intraoperative use of 5ALA and CRT regime. Greater extent of surgical resection (A), intraoperative use of 5ALA (B), and more aggressive CRT regimes (C) were associated with significantly improved survival compared to respective controls. Survival distributions were compared using pairwise log-rank test (D). mOS, median overall survival; 95%CI, 95% confidence intervals.
Regarding oncological management (Figure 2C), there was no significant difference between patients that received Temozolomide (TMZ) in combination with standard (RT60/30+TMZ) and hypofractionated (RT40/15+TMZ) radiotherapy (P = .72). Both regimes with TMZ, however, were associated with improved survival in comparison to RT40/15 alone (P = .03). Significant difference in line with cumulative radiation dose was observed between RT40/15 alone and RT34/10 alone (P < .001) while the difference between RT34/10 and RT30/6 regimes was not statistically significant (P = .640). With the mOS of 14.1 weeks (95%CI 11.9–16.4 weeks), patients who received no chemoradiotherapy (CRT) had significantly shorter survival period compared to all other CRT regimes (P < .001). Figure 2D shows median survival of all patient subpopulations and their pairwise log-rank comparisons. Patients receiving each CRT regime were further stratified based on their MGMT methylation status (Supplementary Figure 4A and 4B). Of note, MGMT-unmethylated patients that received RT60/30+TMZ showed significantly longer survival (mOS 57.9 weeks, 95% CI 27.7–88.1 weeks) compared to MGMT-unmethylated patients who received RT40/15 alone (mOS 29.7 weeks, 95%CI 7.1–51.6 weeks), P = .002 with no excess toxicity in the RT60/30+TMZ subgroup (Supplementary Figure 4C). However it should be noted that 19 out of 29 patients in this group were aged 70–75.
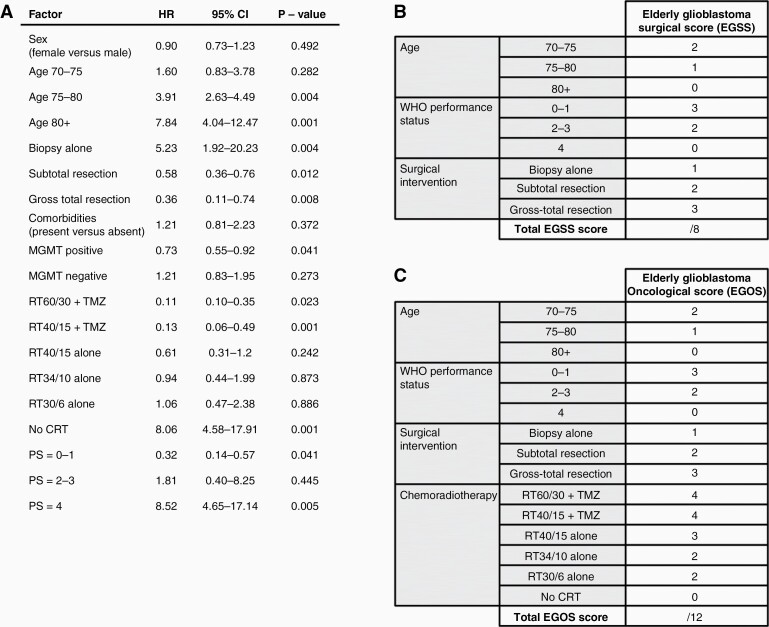
The direct survival impact of each variable was evaluated using Cox proportional hazards model (Figure 3A). Intraoperative use of 5ALA was excluded from the multivariate model due to its collinearity with gross-total resection. Adjusted for the effect of other variables, patient sex, age 70–75, comorbidities, RT40/15 alone, RT34/10 alone, RT30/6 alone, and PS = 2–3 had no direct effect on survival (P > .05). Direct survival benefit was observed for subtotal resection (adjusted hazard ratio, HR = 0.58, 95%CI 0.36–0.76), gross-total resection (HR = 0.36, 95%CI 0.11–0.74), MGMT promoter methylation (HR=0.73, 95%CI 0.55–0.92), RT60/30+TMZ (HR = 0.11, 95%CI 0.10–0.35), RT40/15+TMZ (HR = 0.13, 95%CI 0.06–0.49) and PS = 0–1 (HR = 0.32, 95% 0.14–0.57), P < 0.05. In contrast, direct negative effect on survival was demonstrated for age 75–80 (HR = 3.91, 95%CI 2.63–4.49), age >80 (HR = 7.84, 95%CI 4.04–12.47), biopsy alone (HR = 5.23, 95%CI 1.92–20.23), no CRT (HR = 8.06, 95%CI 4.58–17.91) and PS = 4 (HR = 8.52, 95%CI 4.65–17.14). Based on the HRs of variables with significant survival impact, we devised simplified Elderly Glioblastoma Surgical Score (EGSS, Figure 3B) and Elderly Glioblastoma Oncological Score (EGOS, Figure 3C) to estimate the survival of elderly glioblastoma patients at the stage of surgical and oncological management, respectively. EGOS differs from EGOS by also accounting for the effect of chemoradiotherapy while all other factors remain identical between the two scores.
Figure 3.
Elderly Glioblastoma Surgical Score (EGSS) and Elderly Glioblastoma Oncological (EGOS) based on the results of multivariate Cox proportional hazards analysis. Adjusted for the effect of other variables, Cox proportional hazards model (A) demonstrated significant direct effect of age 75–80, age 80+, biopsy alone, subtotal resection, gross-total resection, MGMT methylation status, RT60/30+TMZ, RT40/14+TMZ, no CRT, PS = 0–1 and PS = 4 on survival of elderly glioblastoma patients (P < .05). EGSS (B) and EGOS (C) survival scores were devised based on variables with significant direct survival impact identified in the Cox proportional hazards model. HR, adjusted hazard ratio; 95% CI, 95% confidence interval; PS, WHO performance status.
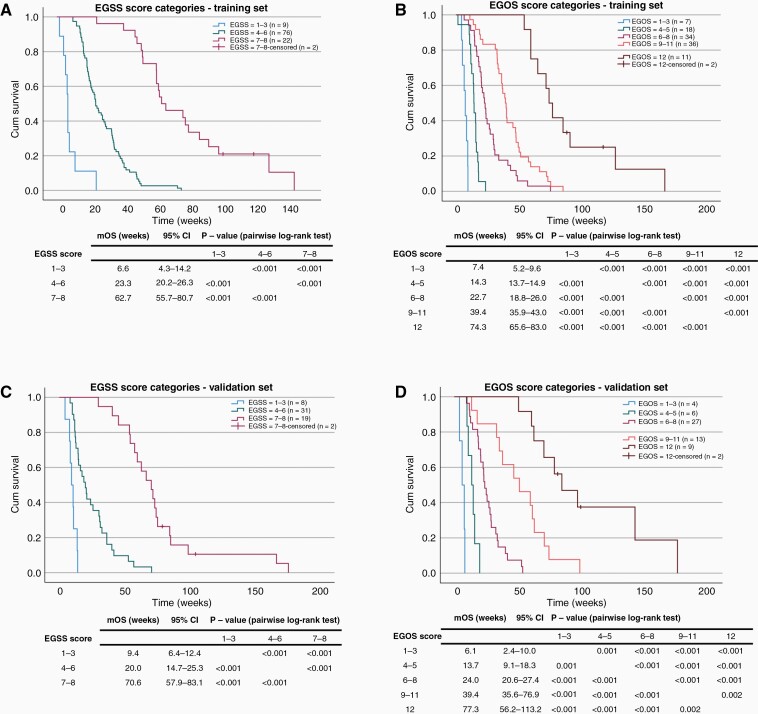
To derive and validate the survival predictions of the EGSS and EGOS scores, our patient cohort was randomly divided into a training set (n = 113, 67%) and a validation set (n = 56, 33%). There was no statistically significant difference in baseline characteristics between the two sets (Supplementary figure 5, P > 0.05). To simplify the scoring system and make it clinically convenient, the EGSS and EGOS scores of patients in the training set were grouped into categories based on maximal log-rank statistics for pairwise comparison (Supplementary Figure 6): EGSS score 1–3, 4–6, and 7–8, EGOS score 1–3, 4–5, 6–8, 9–11, and 12. Cox regression analyses using EGSS and EGOS score categories as covariates revealed that increasing EGSS and EGOS scores are associated with decreasing HRs in both the training and the validation sets (Supplementary Figure 7). The Kaplan-Meier survival curves demonstrated clear separation between the three EGSS score categories in the training (Figure 4A) and the validation set (Figure 4C). A higher EGSS score also corresponded to a longer median survival which ranged from 6.6 to 62.7 weeks in the training set and from 9.4 to 70.6 weeks in the validation set, according to the score category. Clear separation between Kaplan-Meier survival curves in the training (Figure 4B) and validation (Figure 4D) was also observed for the five EGOS score categories. An increasing EGOS score was associated with prolonged median survival ranging from 7.4 to 74.3 weeks in the training set and 6.1 to 77.3 weeks in the validation set, according to the score category.
Figure 4.
Kaplan-Meier survival distributions of EGSS and EGOS score categories are comparable between the training (A, B) and the validation (C, D) set (log-rank test, P < .05). mOS, median overall survival; 95%CI, 95% confidence intervals.
The survival predictions of EGSS and EGOS score categories were based on 95% confidence intervals of median survival in the training set (Table 1). The accuracy of EGSS and EGOS estimates in the validation set was 65% and 73%, respectively. The area under the curve of the receiver operating characteristic curve (AUC) of 0.622 for EGSS and 0.718 for EGOS (Supplementary Figure 8).
Table 1.
The Accuracy of EGSS and EGOS Survival Predictions. The predictions were based on 95% CI of median survival in the training set and their accuracy was tested in the validation set. AUC, area under the receiver operating characteristic curve.
| EGSS Score | Estimated Survival (Weeks) | EGOS Score | Estimated Survival (Weeks) |
|---|---|---|---|
| 1–3 | 4–12 | 1–3 | 5–9 |
| 4–6 | 14–34 | 4–5 | 11–18 |
| 7–8 | 51–97 | 6–8 | 20–31 |
| Accuracy | 65% | 9–11 | 34–56 |
| AUC | 0.622 | 12 | 60–95 |
| Accuracy | 73% | ||
| AUC | 0.718 |
Discussion
Prognosticators of Survival
Age.
—With longer life expectancy in the Western countries, the number of elderly glioblastoma patients continues to rise. Their management remains difficult with a concern about the poor prognosis partly resulting from their undertreatment in comparison with younger patients.9,10 This is mostly driven by the well-recognized and marked survival impact effect of age: the 12-month survival probability for glioblastoma patients ≥75 years in a large US dataset was reported as only 9.2% compared to 40.7% for patients aged 55–64.11 In line with large registry data,11,12 our study demonstrated significant survival differences between the 70–75, 75–80, and >80 age subgroups with mOS of 35.4, 21.4, and 7.8 weeks, respectively. Relative to other factors in our multivariate analysis, age was not a significant survival prognosticator in 70–75-year-olds but its direct impact on survival did became significant in the 75–80 and >80-year-old cohorts (HRs of 3.9 and 7.9, respectively, P < .05).
MGMT promoter methylation.
—The survival benefit of MGMT promoter methylation, predominantly in association with Temozolomide, has been reported by multiple randomized trials.5,13,14 In line with these findings, our results show a clear survival benefit of MGMT methylation (mOS 49.7 weeks, 95%CI 40.3–59.1 weeks) in comparison with MGMT-unmethylated status (mOS 32.3 weeks, 95%CI 27.6–37.1 weeks), P < .001. Even after adjusting for the effect of other variables, MGMT-methylated status had significant direct impact on survival (HR = 0.73, P = .041) whereas the multivariate-adjusted effect of MGMT-unmethylated status was not significant (HR = 1.21, P = .273).
Performance status.
—The impact of performance status on the survival in the elderly glioblastoma cohort is well documented.8,10 Consistent with a recursive partitioning analysis of ≥70-year-old glioblastoma patients using a large US and a French dataset,10 our results demonstrate a significant association between worse performance status and shorter survival. A recent retrospective study from Düsseldorf in Germany suggested that while elderly (≥65 years) and nonelderly (<65 years) patients with glioblastoma have comparable performance status at diagnosis, the elderly group tends to experience a more rapid physical decline with a significant performance difference detectable as early as 6 weeks post radiotherapy.15
Comorbidities.
—The survival impact of comorbidities is difficult to standardize and capture experimentally. We stratified patients into two groups based on presence or absence of significant comorbidities defined as any condition requiring regular medication or medical surveillance. Perhaps surprisingly, our data revealed no significant survival difference between the two groups even after multivariate adjusting. While chronic comorbidities near both ends of the severity spectrum clearly influence clinical decisions, it appears that the overall effect of comorbidities is less significant. This may be attributed to the short survival of elderly glioblastoma patients with other factors including age, performance status, and MGMT methylation playing a comparatively larger role.
Surgical resection.
—As for younger adults and supported by multiple studies, the standard of surgical care for elderly glioblastoma patients is maximal safe resection with preservation of neurological function.10,16–19 Despite some variability in elderly age cutoff, the superiority of resection over biopsy was demonstrated in the early Vuorinen trial,18 multiple retrospective studies16,17,20,21 and later validated in the Nordic,22 the German (Neurooncology Working Group NOA-08 trial)23 and the Perry14 trial. Mostly focusing on the oncological management, however, these trials did not report and statistically compare the mOS of patients with gross-total resection, subtotal resection, and biopsy alone. Our results demonstrate a significant survival benefit of both gross-total resection (GTR, mOS 65.3 weeks, 95%CI 53.1–77.5 weeks, HR = 0.36) and subtotal resection (STR, mOS 28.1 weeks, 95%CI 20.9–35.4 weeks, HR = 0.58) in comparison with biopsy alone (mOS 15.7 weeks, 95%CI 14.0–17.4 weeks, HR = 5.23), P < .001. Given significantly longer survival in the GTR versus STR cohort, the effect appears to be related to postoperative tumor volume, which has recently been identified as a survival prognosticator independent of age, MGMT methylation, and chemoradiation therapy.24 Similarly and albeit not stratified for age, a large meta-analysis by Brown et al. reported the superiority of GTR over STR in a population of more than 20,000 glioblastoma patients.19 The comparison of STR versus biopsy alone, however, remains more controversial with a narrower survival difference and greater variability in published literature.19 This likely results from STR encompassing a wide range of resection percentages and perhaps some overlap between biopsy and STR, particularly in the context of smaller tumors. Furthermore, the postoperative residual tumor volume has recently decreased with the availability of intraoperative MRI25 as well as widespread surgical use of 5ALA.26 The collinearity between 5ALA and GTR in our analysis suggests that intraoperative use of 5ALA largely resulted in complete resection of the contrast-enhancing tumor.
(Chemo)radiotherapy.
—After maximal safe resection and based on the landmark Stupp trial (Table 2),4,13 the mainstay of oncological management of glioblastoma in fit, younger adults (<70 years) involves 6 weeks of radiotherapy (RT) with concurrent TMZ (75 mg/m2/day) followed by maintenance TMZ (150–200 mg/m2/day × 5 days for six 28-day cycles), regardless of MGMT promoter methylation.30 Due to considerable time burden and the impression of better treatment-to-survival time, elderly patients (>70 years) are typically treated with shorter, hypofractionated regimes providing a higher daily dose but a lower cumulative dose.31 Despite some variability in elderly age cutoffs in the Roa et al.27 and Malmström et al.22 phase 3 trials, hypofractionated regimes consistently proved as effective as the standard regime (ie 60Gy in 30 fractions, RT60/30) (Table 2). Therefore, the latest European Association of Neuro-Oncology Guidelines for elderly (≥65–70 years) patients with good performance status (KPS > 70) recommend the use of hypofractionated radiation (RT40/15) with or without concurrent and adjuvant TMZ.
Table 2.
Clinical Trials and Retrospective Studies Underlying the Current Standard of Oncological Treatment of Elderly Glioblastoma Patients
| Age (Years) | PS | Radiation Dose (Gy)/Fractions | Number of Patients | Chemotherapy | MGMT Methylation | Median Survival (Months) | Study | Note |
|---|---|---|---|---|---|---|---|---|
| ≤70 | 0–2 | 60/30 | 286 | TMZ | All | 14.6 | Stupp et al., 20054,13 and Hegi et al., 20055 (Phase 3 trial) | Established adjuvant RT60/30 with concurrent and adjuvant TMZ as standard of care. Established the survival benefit of TMZ in MGMT positive patients. 170 of the 573 patients were aged 61–70 years. In this older subset of patients, mOS using the combined-modality approach compared to radiation therapy alone was similar (mOS 10.9 vs 11.8 months)13. |
| Positive | 21.7 | |||||||
| Negative | 12.7 | |||||||
| 287 | No TMZ | All | 12.1 | |||||
| Positive | 15.3 | |||||||
| Negative | 11.1 | |||||||
| ≥60 | 0–3 | 60/30 | 51 | No TMZ | 5.1 | Roa et al., 200427 (Phase 3 trial) | RT40/15 was noninferior to RT60/30, P = 0.57. | |
| 40/15 | 49 | 5.6 | ||||||
| All | 0–3a | 60/30 | 100 | No TMZ | 6.0 | Nordic Phase 3 trial22 | In patients > 70 years RT60/30 was inferior to RT34/10, P = 0.02. | |
| 34/10 | 98 | No TMZ | 7.5 | |||||
| No RT | 93 | TMZ | 8.3 | |||||
| 60–70 | 0–3a | 60/30 | 59 | No TMZ | 7.6 | |||
| 34/10 | 58 | No TMZ | 8.8 | |||||
| No RT | 51 | TMZ | 7.9 | |||||
| ≥70 | 0–3a | 60/30 | 41 | No TMZ | 5.2 | |||
| 34/10 | 40 | No TMZ | 7.0 | |||||
| No RT | 42 | TMZ | 9.0 | |||||
| ≥65 | 0–3 | 60/30 | 178 | No TMZ | All | 9.6 | NOA-08 Phase 3 trial23 | Dose-dense TMZ alone was noninferior to standard radiotherapy in elderly patients with malignant astrocytoma, P = 0.028. |
| Positive | 9.6 | |||||||
| Negative | 10.4 | |||||||
| No RT | 195 | TMZ | All | 8.6 | ||||
| Positive | Not reached | |||||||
| Negative | 7.0 | |||||||
| ≥65 | 0–2 | 40/15 | 281 | TMZ | All | 9.3 | Perry et al., 2017 Phase 3 trial14 | The addition of TMZ to RT40/15 is associated with longer survival than RT40/15 alone P < .001). The survival benefit of TMZ was greater in MGMT-methylated compared to unmethylated patients (P < .001) but a clinically meaningful survival benefit of TMZ was also detected in MGMT-unmethylated patients albeit it did not reach statistical significance(P = .055). |
| Positive | 13.5 | |||||||
| Negative | 10.0 | |||||||
| 281 | No TMZ | All | 7.6 | |||||
| Positive | 7.7 | |||||||
| Negative | 7.9 | |||||||
| ≥70 | <3 | 60/30 | 92 | No TMZ | 7.5-9.5 | McAleese et al., 2003 (retrospective case-control study)28 | Despite providing a lesser survival benefit than radical RT, hypofractionated RT is better tolerated in patients with low PS. | |
| 30/6 | 92 | No TMZ | 5.1 | |||||
| ≥65 | <3 | 50/30 | 29 | No TMZ | 10 | Bauman et al., 1994 (prospective case-control study)29 | Short course palliative RT is associated with improved survival compared to best supportive care. | |
| 30/10 | 29 | No TMZ | 6 | |||||
| No RT | 29 | No TMZ | 1 |
aChemoradiation regimes not stratified for PS. 33 patients had PS 0–1 and 9 patients PS 2–3 (of these, 7 patients were PS = 3 due to neurological impairments only.
Decisions to offer or withhold TMZ in elderly glioblastoma patients are predominantly based on the Perry et al. trial14 (Table 2), which reported a survival benefit of combined RT40/15+TMZ regime in comparison to RT40/15 alone (mOS 9.3 vs 7.6 months, P < .001). In subgroup analysis, the benefit of TMZ was most pronounced in MGMT-methylated patients (mOS 13.5 vs 7.7 months for patients with and without TMZ, respectively, P < .001). A smaller benefit was also detected in MGMT-unmethylated patients, but it did not reach statistical significance (mOS 10.0 vs 7.9 months for patients with and without TMZ, respectively, P = .055). In line with the Perry trial,14 our study validates the survival benefit of RT40/15+TMZ over RT40/15 alone in MGMT-methylated patients aged 70 years or above (mOS 60.7 vs 41.4 weeks, P < .001). Of note, we also detected a potential benefit of adding concomitant and adjuvant TMZ to RT40/15 in MGMT-unmethylated patients (54.4 vs 41.9 weeks, P = .595), yet the lack of statistical power precluded a meaningful interpretation. Given considerable clinical and near-statistical benefit demonstrated by the Perry et al. trial, the use of RT40/15+TMZ in elderly MGMT-unmethylated patients remains an area of ongoing debate with variable practice between different oncology centers.32,33 Probability ranking from a recent network meta-analysis of 5 randomized controlled trials demonstrated that combined hypofractionated radiotherapy with TMZ had the highest probability of being ranked as the best overall treatment for elderly (>60 years) glioblastoma patients.34 When adjusted for MGMT methylation, TMZ-based approaches, either as monotherapy or in combination with radiotherapy, proved superior to radiotherapy-only regimes.34
To date, there is no randomized comparison of hypofractionated chemoradiotherapy (RT40/15+TMZ) and the Stupp regime (RT60/30+TMZ).35 In this study and adjusted for age, comorbidities, performance status, surgery and MGMT promoter methylation, the standard (RT60/30+TMZ) and the hypofractionated (RT40/15+TMZ) chemoradiation regimes both significantly improved survival with HRs of 0.11 and 0.13, respectively. The difference between the two regimes was not significant (P = .72). However, a recent meta-analysis of seven retrospective studies in nearly 1000 older adults (for a younger age group than ours >65 years) demonstrated that RT40/15+TMZ regime is associated with 14 weeks shorter mOS than the standard RT60/30+TMZ regime.36 While the nature of their pooled analysis did not allow for multivariate adjustment, it suggests that until further evidence, the survival comparisons of standard and hypofractionated chemoradiation regimes should be tempered. In addition, the survival benefit of the concurrent versus the maintenance portion of TMZ remains unclear and provides scope to further optimize the tolerance and toxicity problems in the elderly patients.
In the Oxford region, elderly patients with good performance status (KPS > 70) typically receive a short course of hypofractionated (RT40/15) radiotherapy with or without TMZ, depending on their MGMT status. Given the benefit of combined RT60/30+TMZ in glioblastoma patients under 70 years of age demonstrated in the Stupp trial,4,13 a small proportion of our elderly patient in their early 70s with excellent performance status may, instead of the hypofractionated regime, receive RT60/30+TMZ regardless of their MGMT status. Extending the findings of the Stupp trial to ≥70-year-olds, our study demonstrates a greater survival benefit of RT60/30+TMZ in MGMT-methylated compared to MGMT-unmethylated patients (mOS 62.7 vs 57.6 weeks, P = .049).
Comparing the outcomes of the standard versus hypofractionated radiotherapy with TMZ, our data suggest equivalent survival of MGMT-methylated patients undergoing the standard (RT60/30+TMZ) and the hypofractionated (RT40/15+TMZ) regime (mOS 62.7 vs 60.9 weeks, P = .271). In our MGMT-unmethylated subpopulation, however, we detected a considerable survival benefit of the RT60/30+TMZ regime over RT40/15 alone (mOS 57.6 vs 29.7 weeks, P = .002). Furthermore, there is inherent patient selection bias when comparing a population who receives RT40/15+TMZ compared with the Stupp protocol, given the mandatory MGMT promoter methylation positivity required for the former, meaning that this group contains a favorable patient population. Finally, our review of adverse events revealed no excess toxicity (and no cases of grade 3 toxicity) in our MGMT-unmethylated elderly population treated with the Stupp protocol compared with RT 40/15 alone. Therefore, we propose that MGMT-unmethylated patients in their early 70s with excellent performance status should not be ruled out of consideration for RT60/30+TMZ regime on the sole basis of exceeding the arbitrary age limit of 70 years.
Elderly patients with poor performance status often better tolerate single-modality therapy, that is radiotherapy or Temozolomide alone.28,29 Subgroup analyses from randomized trials support the role of MGMT status in guiding clinical decisions between radiation and Temozolomide. In both the Nordic22 and the NOA-0823 trials, radiotherapy (standard RT60/30 and hypofractionated RT34/10 regimes) proved more effective than Temozolomide in MGMT-unmethylated patients, whereas Temozolomide was more effective in MGMT-methylated patients. Our multivariate analysis also demonstrated a significant survival benefit of hypofractionated radiation (RT40/15 alone) (mOS 41.4 weeks, HR 0.61), which was expectedly shorter than combined chemoradiation regimes with Temozolomide (mOS of 60.7 weeks for RT40/15+TMZ and 50.6 weeks with RT60/30+TMZ). The multivariate-adjusted effect of RT34/10 alone and RT30/6 alone was not significant, yet both regimes provided beneficial in comparison with no oncological treatment (mOS = 14.1 weeks).
Elderly Glioblastoma Surgical and Oncological Score
Survival estimates in elderly glioblastoma patients have historically been difficult owing to patient heterogeneity and the lack of randomized or multivariate-adjusted data that would account for a wide range of biological, histological surgical, and oncological factors. Straube et al. recently reported a survival score stratifying elderly (≥65 years) glioblastoma patients into a better (mOS 7.8 months) or poor prognosis groups (mOS 2.7 months) based on age, performance status, MGMT methylation, and surgical parameters.37 The accuracy of the score, however, has not been evaluated within their cohort or externally. Two other scores have been reported in smaller cohorts, with limited prognostic factors and not specific to the elderly glioblastoma patients.38,39 Using the results of our multivariate analysis, we devised and validated a 2-stage scoring system to estimate the survival of elderly (≥70 years) glioblastoma patients based on information available at the time of surgical and oncological management. The accuracy of the EGSS and EGOS score in the validation set was 65% and 73%, respectively. Importantly, the scoring system does not account for unbiopsied patients and for patient comorbidities. Inspection of inaccurate survival estimates in our validation cohort revealed several inaccurate estimates related to poorly compensated heart, liver, and renal failure as well as metastatic malignancies. Therefore, we recommend against the use of the EGSS and EGOS scoring system in patients with other life-limiting comorbidities. The area under the receiver operating characteristic curve was 0.622 for EGSS and 0.718 for EGOS. We recommend the score to be validated in larger registries.
Conclusion
The survival of elderly (≥70 years) patients with glioblastoma is associated with age, MGMT promoter methylation, performance status, the extent of neurosurgical resection, and adjuvant chemoradiation therapy. The multivariate-adjusted survival impact of age increases and becomes significant above the age of 75 years. By reducing residual tumor volume, gross-total and subtotal resection significantly improve survival compared to biopsy alone. In terms of adjuvant therapy, combined hypofractionated radiotherapy with concomitant and adjuvant TMZ is noninferior to the 6 week Stupp protocol. We have identified a small subset of MGMT-unmethylated patients with excellent performance status in their early 70s who may benefit from more aggressive chemoradiotherapy, with follow-up data from our region not demonstrating an excess of toxicity when this is utilized. The survival of elderly (≥70 years) glioblastoma patients at the time of surgical and oncological management can be estimated using EGSS and EGOS scores. Further studies are welcomed to further validate these predictive scores and to further investigate aggressive adjuvant therapy in the elderly MGMT-unmethylated cohort.
Limitations
Our present study has several important limitations. All data were retrospectively collected from a single tertiary neurosurgical referral center over a period of six years. In the absence of randomization, the survival outcomes may be affected by factors not accounted for in our multivariate analysis, such as tumor location, size, the morbidity of resection, and postoperative complications. The study also does not account for patients who declined the recommended surgical and oncological interventions, or who underwent palliative radiotherapy alone. Importantly, our study defined survival as the period between the date of diagnosis (based on CT/MRI scan) and the date of death, thereby limiting cross-study comparisons with other studies reporting survival from the date of randomization,14,22,27 surgery,23 or histological diagnosis.4 The average time between the first imaging diagnosis and surgery in our cohort was 4.1 weeks (95%CI 3.6–4.6 weeks). The EGSS and EGOS scores devised and validated in our cohort of 169 elderly glioblastoma patients does not account for patients with other life-limiting comorbidities and requires validation in larger studies. Given different elderly age cutoffs used in different studies and our focus on patient aged 70 years or above, the accuracy of the scores in 65- to 70-year-olds remains uncertain. Furthermore, our identification of a subpopulation of patients who may benefit from more aggressive oncological management is subject to selection bias given that in practice, only extremely fit elderly patients would have been administered prolonged chemoradiotherapy.
Supplementary Material
Acknowledgments
For their assistance with data collection, we are indebted and grateful to Dr Monika Hofer and Hannah Keyser from the Neuropathology Department of The Oxford University Hospitals, to Dr Craig Knighton and Samantha Cragg from the Northamptonshire Campus of South East Midlands Oncology Centre, and to Prof Hany Eldeeb and Shirley Manharawi from the Oncology Department at The Milton Keynes University Hospital.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. There are no financial or non-financial competing interests to declare by the authors.
Authorship statement. MJZ, PP, PW and CH contributed to study design. MJZ, PW, MN, SS and SH collected the data. MJZ and SS analyzed the data. MJZ, PW, NM, CH, PP, RS, and VA contributed to the manuscript. PP led the study.
References
- 1. Brodbelt A, Greenberg D, Winters T, et al. . Glioblastoma in England: 2007-2011. Eur J Cancer. 2015; 51(4):533–542. [DOI] [PubMed] [Google Scholar]
- 2. Dobes M, Khurana V, Shadbolt S, et al. . Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000-2008): findings of a multicenter Australian study. Surg Neurol Int. 2011; 2(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korja M, Raj R, Seppä K, et al. . Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2019; 21(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Hegi ME, Diserens A-C, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 6. Bauchet L, Zouaoui S, Darlix A, et al. . Assessment and treatment relevance in elderly glioblastoma patients. Neuro Oncol. 2014; 16(11):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008; 64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 8. Scott JG, Suh JH, Elson P, et al. . Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: a retrospective review of 206 cases. Neuro Oncol. 2011; 13(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Robles P, Cairncross G. Glioblastoma in the elderly: an age-old problem. Ann Neurol. 2008; 64(6):597–599. [DOI] [PubMed] [Google Scholar]
- 10. Scott JG, Bauchet L, Fraum TJ, et al. . Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer. 2012; 118(22):5595–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ostrom QT, Gittleman H, Farah P, et al. . CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013; 15(suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauchet L, Mathieu-Daudé H, Fabbro-Peray P, et al. . Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010; 12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 14. Perry JR, Laperriere N, O’Callaghan CJ, et al. . Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 15. Berger K, Turowski B, Felsberg J, et al. . Age-stratified clinical performance and survival of patients with IDH-wildtype glioblastoma homogeneously treated by radiotherapy with concomitant and maintenance temozolomide. J Cancer Res Clin Oncol. 2021; 147(1):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaichana KL, Garzon-Muvdi T, Parker S, et al. . Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011; 18(1):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noorbakhsh A, Tang JA, Marcus LP, et al. . Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. J Neurosurg. 2014; 120(1):31–39. [DOI] [PubMed] [Google Scholar]
- 18. Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). 2003; 145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 19. Brown TJ, Brennan MC, Li M, et al. . Association of the extent of resection with survival in glioblastoma a systematic review and meta-analysis. JAMA Oncol. 2016; 2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffermann M, Bruckmann L, Mahdy Ali K, et al. . Treatment results and outcome in elderly patients with glioblastoma multiforme - a retrospective single institution analysis. Clin Neurol Neurosurg. 2015; 128(1):60–69. [DOI] [PubMed] [Google Scholar]
- 21. Oszvald Á, Güresir E, Setzer M, et al. . Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age: clinical article. J Neurosurg. 2012; 116(2):357–364. [DOI] [PubMed] [Google Scholar]
- 22. Malmström A, Grønberg BH, Marosi C, et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012; 13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 23. Wick W, Platten M, Meisner C, et al. . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012; 13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 24. Ellingson BM, Abrey LE, Nelson SJ, et al. . Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018; 20(9):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Senft C, Bink A, Franz K, et al. . Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011; 12(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Stummer W, Pichlmeier U, Meinel T, et al. . Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 27. Roa W, Brasher PMA, Bauman G, et al. . Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004; 22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 28. McAleese JJ, Stenning SP, Ashley S, et al. . Hypofractionated radiotherapy for poor prognosis malignant glioma: matched pair survival analysis with MRC controls. Radiother Oncol. 2003; 67(2):177–182. [DOI] [PubMed] [Google Scholar]
- 29. Bauman G, Gaspar L, Fisher B, et al. . A prospective study of short-course radiotherapy in poor prognosis glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1994; 29(4):835–839. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Suo X, Ding H, et al. . Structural connectivity profile supports laterality of the salience network. Hum Brain Mapp. 2019; 40(18):5242–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wick A, Kessler T, Elia AEH, et al. . Glioblastoma in elderly patients: solid conclusions built on shifting sand? Neuro Oncol. 2018; 20(2):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alnahhas I, Alsawas M, Rayi A, et al. . Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis. Neuro-Oncology Adv. 2020; 2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nassiri F, Taslimi S, Wang JZ, et al. . Determining the optimal adjuvant therapy for improving survival in elderly patients with glioblastoma: a systematic review and network meta-analysis. Clin Cancer Res. 2020; 26(11):2664–2672. [DOI] [PubMed] [Google Scholar]
- 35. Hanna C, Lawrie TA, Rogozińska E, et al. . Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis. Cochrane Database Syst Rev. 2020; 3(3):CD013261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu VM, Kerezoudis P, Brown DA, et al. . Hypofractionated versus standard radiation therapy in combination with temozolomide for glioblastoma in the elderly: a meta-analysis. J Neurooncol. 2019; 143(2):177–185. [DOI] [PubMed] [Google Scholar]
- 37. Straube C, Kessel KA, Antoni S, et al. . A balanced score to predict survival of elderly patients newly diagnosed with glioblastoma. Radiat Oncol. 2020; 15(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phuong PC, Nam LV, Schild SE, Rades D, Khoa MT. A survival score based on symptoms and performance status for patients with high-grade gliomas receiving radiochemotherapy. In Vivo (Brooklyn). 2017; 31(4):689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rades D, Witteler J, Schild SE, et al. . A new survival score for patients receiving radiotherapy for newly diagnosed glioblastoma multiforme. Anticancer Res. 2021; 41(1):379–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.