ABSTRACT
Background
Second-generation antipsychotics (SGAs) are used to treat children for mental health disorders but in some children they cause cardiometabolic complications including weight gain and type 2 diabetes. Genetic variants can place a child at risk of developing these metabolic complications. The fat mass and obesity-associated (FTO) rs9939609 A allele has been associated with obesity and dietary energy intakes in healthy children but its relation to metabolic complications in SGA-treated children is not known.
Objectives
This study investigated the association of the FTO rs9939609 variant and SGA treatment with cardiometabolic complications and dietary intakes in children with mental health disorders.
Methods
A cross-sectional population of children (≤18 y; n = 506) with mental health disorders that were SGA-treated (n = 197) and SGA-naïve (n = 309) were recruited through the Department of Psychiatry at BC Children's Hospital. Dietary intakes were estimated using 3-d food records in a subset of children (n = 73).
Results
Genotype frequencies were not different between SGA-treated (TT genotype 42.6%, TA genotype 38.6%, AA genotype 18.8%) and SGA-naïve (TT 41.1%, TA 39.5%, AA 19.4%) children. Children with the A allele had lower BMI z-sores compared with the TT genotype (0.84 ± 1.19 compared with 1.19 ± 1.36; P = 0.005, adjusted for ethnicity). We observed an interaction between FTO genotype and SGA status on fasting glucose (P = 0.036). SGA-naïve children with the A allele had higher fasting glucose than those with the TT genotype (4.96 ± 0.35 compared with 4.81 ± 0.35 mmol/L; P = 0.001), in adjusted models (age, sex, ethnicity, and BMI z-score). This was not observed in SGA-treated children. Children with the A allele had higher daily total energy intakes compared with the TT genotype (1994 ± 619 compared with 1814 ± 484 kcal/d; P = 0.048), in adjusted models (age, sex, ethnicity, and BMI z-score); no effect of SGA-treatment was observed.
Conclusions
Our findings suggest the A allele of the FTO rs9939609 variant is associated with higher BMI in children with mental health disorders, but only in those not treated with SGAs.
Keywords: children, youth, second-generation antipsychotics, obesity, type 2 diabetes, dietary intake
Introduction
The prevalence of mental health disorders in children and youth (≤18 y of age) has been estimated to be ∼14% in Canada (1). Common mental health disorders during childhood include anxiety disorder, depressive disorders, bipolar disorder, autism spectrum disorder, and attention deficit hyperactivity disorder. Many of these children are increasingly being treated with second-generation antipsychotics (SGAs), also known as atypical antipsychotics (2–4). Treatment with SGAs is associated with metabolic complications such as rapid and excessive weight gain, elevated fasting glucose, insulin resistance, hypertension, and dyslipidemia (5, 6). A 3-fold greater risk of type 2 diabetes has been reported in children treated with antipsychotics compared with untreated controls (7–9).
Interestingly, metabolic complications develop in approximately half of SGA-treated children (10), suggesting the potential contribution of other factors such as genetic susceptibility (11). Currently, there is no way to identify SGA-treated children at risk of metabolic complications. We previously reported that the T allele of the C677T variant of the gene encoding methylenetetrahydrofolate reductase (MTHFR) (12) and the Met allele of the Val158Met variant of the gene encoding catechol-O-methyltransferase (COMT) (13) are associated with higher blood pressure and fasting blood glucose in SGA-treated, but not SGA-naïve children. However, these variants were found in only ∼25% of SGA-treated children with metabolic complications, suggesting further genetic factors and/or other environmental factors place children at risk of metabolic complications.
A genome-wide association study identified a relation between the A allele of the rs9939609 variant in the fat mass and obesity-associated gene (FTO) and higher BMI and obesity in children and adults (14). Further studies have confirmed the association between the FTO rs9939609 A allele and surrogate markers of adiposity in children, including greater weight and BMI z-scores, and total fat mass (15–17). In addition, an association between the FTO rs9939609 variant and type 2 diabetes has been reported independent of BMI in adults (18, 19), suggesting a direct relation between FTO and type 2 diabetes.
FTO encodes an RNA demethylase (20) that is highly expressed in the hypothalamus, the region of the brain that regulates appetite; it has been postulated that FTO might play a role in food intake regulation and energy homeostasis. A few published studies in children support this concept. For example, children (n = 3337; aged 8–11 y) with the AA genotype of the FTO rs9939609 variant were reported to have reduced satiety compared with those with the TT genotype (15). Another study reported that food intake was greater during a test of eating in the absence of hunger in young children (n = 131; aged 4–5 y) with the AA genotype compared with those with the TT genotype (21). Further, others have reported that overeating is more common in children (n = 190; aged 6–19 y) with the A allele compared with children with the TT genotype (17). Dietary energy intakes were higher during a test meal in children (n = 76; aged 4–10 y) with the A allele compared with those with the TT genotype (16).
We hypothesize that given the potential role of FTO in food intake regulation and energy homeostasis, the FTO rs9939609 variant can contribute to rapid weight gain and cardiometabolic complications in SGA-treated children. One study reported that the FTO rs9939609 variant was not associated with weight gain in young children (n = 181; aged 8 y) with autism spectrum disorder after 8 wk of risperidone treatment (22), but relations with other cardiometabolic risk factors and dietary energy intakes were not assessed. The objective of this study was to determine if the FTO rs9939609 variant is associated with cardiometabolic disease risk in SGA-treated and SGA-naïve children with mental health disorders. The secondary objective was to explore if the FTO rs9939609 genotype is associated with dietary energy intakes and interaction with SGA treatment.
Methods
Study design and participants
This was a cross-sectional study designed to determine the relation between the FTO rs9939609 variant, SGA treatment, and several markers of cardiometabolic health in children with mental health disorders. Participants were recruited through the Child and Adolescent Psychiatry department at British Columbia Children's Hospital between June 2008 and April 2018. The inclusion criteria were children between the ages of 6 and 18 y with a mental health diagnosis who were treated with an SGA for ≥7 d (SGA-treated) or children who were not receiving an SGA and had never received an SGA for >7 d (SGA-naïve). Children with a disorder/disease known to affect metabolism (type 1 diabetes, type 2 diabetes, polycystic ovary syndrome, Crohn disease, etc.), a diagnosed eating disorder, treatment with medications known to affect metabolism (glucocorticoids, β blocker, levothyroxine, etc.), genetic syndromes (Prader–Willi syndrome, Down syndrome, etc.), fetal alcohol spectrum disorder, and/or inborn errors of metabolism were excluded.
This research was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the University of British Columbia Clinical Research Ethics Board and the Children's and Women's Health Centre of British Columbia Research Ethics Board. Parents or legal guardians provided written informed consent, and children provided written assent where capable. Study participants were not compensated for participation in the study. Study participants were assigned a unique study number at enrolment in the study, which did not include any personal information that could identify the participant. Only this number was used on research-related information collected about the participant during the course of this study. The information on the study number and link to the study participant identification are kept in a paper file in a locked filing cabinet in the office of AMD. The study participants consented to the data being used for scientific publication. There was no direct benefit to the child for participating in this study. However, abnormal metabolic blood test results were provided to the parent/guardian of the study participant, and with permission, to their treating physician.
Demographic information was collected including sex and self-reported ethnicity. Medical records were reviewed to obtain information on the mental health disorder diagnoses (by a board-certified psychiatrist), and dose and duration of SGA treatment.
Genomic DNA extraction and genotyping
Buccal epithelial cells were collected from the participants by scraping the inner cheek with collection swabs (Puritan Medical Products). Genomic DNA was isolated using the QIAamp DNA Mini Kit (Qiagen). Genotyping of the FTO rs9939609 variant was completed using Taqman SNP Genotyping reagents and a 7500 Real-Time PCR System (Applied Biosystems).
Anthropometric assessments
Height was measured to the nearest 0.1 cm (Seca 240 Stadiometer) and weight to the nearest 0.1 kg (Tronix Scale 5002). Waist circumference was measured to the nearest 0.1 cm at the level of the umbilicus using a nonelastic flexible tape; an average of 2 measurements was used. Waist circumference z-scores were standardized for age and sex based on NHANES, cycle III (23). Elevated waist circumference was defined as ≥90th percentile for age and sex (24). BMI z-scores were standardized for age and sex based on the WHO growth charts (25); overweight was defined as a BMI z-score ≥1 SD and <2 SD for age and sex; obesity was defined as a BMI z-score ≥2 SD.
Clinical assessments
Blood pressure was assessed using a Dinamap automated monitor (PRO 100-400; GE Medical Systems). Children were seated in a chair with their back supported and feet on the floor; their right arm was supported and positioned at the heart level and fitted with an appropriately sized cuff. After 5 min of seated rest, systolic blood pressure and diastolic blood pressure were measured; an average of 3 readings was used. Blood pressure percentiles were standardized for age, sex, and height. Elevated blood pressure was defined as ≥90th percentile (age 1–13 y) or ≥120/80 mmHg (age ≥13 y) (26).
A fasting (overnight ≥8 h) blood sample was collected by venipuncture, and plasma glucose, insulin, total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride concentrations were quantified by the clinical laboratory at BC Children's Hospital. Elevated LDL cholesterol was defined by a plasma LDL cholesterol ≥3.37 mmol/L; elevated total cholesterol was defined as plasma total cholesterol ≥5.18 mmol/L; elevated plasma triglycerides was defined as plasma triglycerides ≥1.47 mmol/L (24). Insulin resistance was estimated using the updated version of the homeostatic model assessment calculator (HOMA2-IR, version 2.2.3) (27).
Dietary assessment
Three-day food records were collected on 2 weekdays and 1 weekend day; only the data from the subset of the participants (recruited between 2012 and 2018) who provided complete records were used to estimate dietary macronutrient intakes as described previously (28). Participants or their parents/guardians were instructed to record all food, drinks, and supplements consumed. The dietary information was checked for clarity, and all items consumed were confirmed by the research staff. Food records were analyzed using the Food Processor Nutrition Software (version 10.13.1.0; ESHA Research) with the Canadian Nutrient File or USDA database. Daily average total energy and macronutrient intakes were estimated.
Statistical analyses
Data distribution normality was assessed by the Kolmogorov–Smirnov test and visually assessed by histograms and Q-Q plots. All variables were normally distributed and are presented as mean ± SD. Differences in FTO rs9939609 variant genotype frequencies by SGA status were assessed using a χ2 test. Associations between FTO rs9939609 genotype (TT compared with TA/AA), cardiometabolic disease risk factors, and SGA status were determined by separate linear general models for continuous variables and logistic regression models for categorical variables. Models were adjusted for age, sex, BMI z-score, and ethnicity, as indicated. If an interaction between the FTO genotype and SGA status was observed, the effect of the FTO rs9939609 genotype was determined separately in SGA-treated and SGA-naïve groups.
In a subset of participants, the interaction and relation of the FTO rs9939609 TT genotype and SGA status on dietary total energy and macronutrient intakes were determined by separate general linear models. Models were adjusted for age, sex, BMI z-score, and ethnicity, as indicated. All statistical analyses were performed using SPSS Statistics (version 27.0; IBM Corp). A P value ≤ 0.05 was considered statistically significant.
Results
Participant characteristics
A total of 506 children were included in this analysis: 197 SGA-treated children and 309 SGA-naïve children. The demographic characteristics of the participants are presented in Table 1. SGA-treated children were younger and had a higher percentage of males compared with the SGA-naïve children. The most common primary psychiatric diagnoses were anxiety disorder, depressive disorder, and attention deficit hyperactivity disorder. Risperidone and quetiapine were the most prescribed SGAs. The median (IQR) duration of SGA treatment was 7.0 (3.0–14.8) mo. The FTO rs9939609 genotype distribution was not different between SGA-treated and SGA-naïve children (Table 1). The A allele frequency was 58.3% for all children; 57.4% in the SGA-treated children, and 58.9% in the SGA-naïve children.
TABLE 1.
Demographic characteristics of the participants1
| All children (n = 506) | SGA-naïve (n = 309) | SGA-treated (n = 197) | |
|---|---|---|---|
| Age, y | 12.87 ± 3.03 | 13.39 ± 2.74 | 12.05 ± 3.27 |
| Male sex, n (%) | 292 (57.7) | 161 (52.1) | 131 (66.5) |
| European ethnicity, n (%) | 403 (79.6) | 238 (77.0) | 165 (83.8) |
| Primary psychiatric diagnosis, n (%) | |||
| Anxiety disorder | 130 (25.7) | 79 (25.6) | 51 (25.9) |
| Depressive disorders | 103 (20.4) | 85 (27.5) | 18 (9.1) |
| ADHD | 74 (14.6) | 35 (11.3) | 39 (19.8) |
| Mood disorders | 37 (7.3) | 18 (5.8) | 19 (9.6) |
| Psychotic disorders | 32 (6.3) | 18 (5.8) | 14 (7.1) |
| Pervasive developmental disorder | 31 (6.1) | 9 (2.9) | 22 (11.2) |
| Adjustment disorders | 29 (5.7) | 29 (9.4) | 0 (0.0) |
| Disruptive behavior disorder | 9 (1.8) | 1 (0.3) | 8 (4.1) |
| Oppositional defiant disorder | 8 (1.6) | 3 (1.0) | 5 (2.5) |
| Other disorders | 53 (10.5) | 32 (10.4) | 21 (10.7) |
| Current SGA, n (%) | |||
| Risperidone | 104 (52.8) | ||
| Quetiapine | 68 (34.5) | ||
| Aripiprazole | 17 (8.6) | ||
| Olanzapine | 4 (2.0) | ||
| Ziprasidone | 2 (1.0) | ||
| Paliperidone | 1 (0.5) | ||
| Lurasidone | 1 (0.5) | ||
| FTO rs9939609 genotype, n (%) | |||
| TT | 211 (41.7) | 127 (41.1) | 84 (42.6) |
| AT | 198 (39.1) | 122 (39.5) | 76 (38.6) |
| AA | 97 (19.2) | 60 (19.4) | 37 (18.8) |
Data presented as mean ± SD, unless otherwise indicated. ADHD, attention deficit hyperactivity disorder; FTO, fat mass and obesity-associated gene; SGA, second-generation antipsychotic.
Cardiometabolic risk factors
The anthropometric and other cardiometabolic parameters are presented in Table 2. SGA-treated children had significantly higher BMI z-scores (1.30 ± 1.27 compared with 0.80 ± 1.24; P = 0.001) and a greater odds of overweight and/or obesity (≥1 SD; OR = 2.17; 95% CI: 1.19, 3.95; P = 0.012) than SGA-naïve children in adjusted models (by ethnicity). SGA-treated children had significantly higher waist circumference z-scores (0.84 ± 0.89 compared with 0.45 ± 0.98; P = 0.001) and greater odds of elevated waist circumference (≥90th percentile; OR = 1.95; 95% CI: 1.02, 3.74; P = 0.045) than SGA-naïve children in adjusted models (by ethnicity).
TABLE 2.
Cardiometabolic disease risk factors1
| All children | SGA-naïve | SGA-treated | ||||
|---|---|---|---|---|---|---|
| TT | TA/AA | TT | TA/AA | TT | TA/AA | |
| Anthropometrics | ||||||
| BMI z-score | 1.19 ± 1.36 | 0.84 ± 1.19 | 0.98 ± 1.29 | 0.66 ± 1.19 | 1.52 ± 1.41 | 1.13 ± 1.14 |
| Overweight,2n (%) | 43 (22.8) | 54 (21.6) | 26 (22.6) | 31 (20.5) | 17 (23.0) | 23 (23.2) |
| Obesity,3n (%) | 56 (28.0) | 49 (19.6) | 24 (20.9) | 22 (14.6) | 29 (39.2) | 27 (27.3) |
| Waist circumference z-score | 0.70 ± 0.98 | 0.54 ± 0.94 | 0.55 ± 0.99 | 0.37 ± 0.96 | 0.92 ± 0.94 | 0.78 ± 0.85 |
| Elevated waist circumference,4n (%) | 55 (31.4) | 57 (25.0) | 26 (25.2) | 26 (19.5) | 29 (40.3) | 31 (32.6) |
| Blood pressure | ||||||
| Systolic blood pressure percentile | 66.2 ± 27.7 | 63.0 ± 28.4 | 63.6 ± 28.3 | 62.3 ± 28.0 | 70.5 ± 26.2 | 64.2 ± 29.22 |
| Diastolic blood pressure percentile | 62.2 ± 24.1 | 57.3 ± 27.2 | 61.0 ± 24.5 | 55.8 ± 26.5 | 64.3 ± 23.3 | 59.6 ± 28.1 |
| Elevated blood pressure,5n (%) | 65 (35.1) | 88 (35.3) | 39 (33.6) | 51 (33.3) | 26 (37.7) | 37 (38.5) |
| Glucose homeostasis | ||||||
| Fasting glucose, mmol/L | 4.87 ± 0.35 | 4.96 ± 0.34 | 4.81 ± 0.35 | 4.96 ± 0.35 | 4.97 ± 0.33 | 4.97 ± 0.34 |
| Impaired fasting glucose,6n (%) | 6 (3.3) | 13 (5.4) | 2 (1.8) | 7 (5.0) | 4 (5.6) | 6 (6.1) |
| Fasting insulin, pmol/L | 57.69 ± 43.87 | 54.98 ± 37.21 | 54.37 ± 33.10 | 53.65 ± 37.94 | 62.61 ± 56.11 | 56.83 ± 36.26 |
| Elevated fasting insulin,7n (%) | 13 (7.3) | 23 (9.8) | 6 (5.6) | 10 (7.3) | 7 (9.7) | 13 (13.3) |
| HOMA2-IR | 1.08 ± 0.80 | 1.04 ± 0.69 | 1.01 ± 0.61 | 1.01 ± 0.71 | 1.20 ± 1.01 | 1.09 ± 0.67 |
| Blood lipids | ||||||
| Total cholesterol, mmol/L | 4.37 ± 0.87 | 4.22 ± 0.80 | 4.23 ± 0.77 | 4.12 ± 0.74 | 4.59 ± 0.97 | 4.37 ± 0.85 |
| Elevated total cholesterol,8n (%) | 33 (17.8) | 29 (11.4) | 11 (9.8) | 12 (8.0) | 22 (30.1) | 17 (16.2) |
| LDL cholesterol, mmol/L | 2.58 ± 0.78 | 2.45 ± 0.72 | 2.47 ± 0.67 | 2.37 ± 0.65 | 2.75 ± 0.91 | 2.56 ± 0.78 |
| Elevated LDL cholesterol,9n (%) | 25 (13.8) | 30 (12.3) | 12 (10.9) | 11 (7.8) | 13 (18.3) | 19 (18.6) |
| HDL cholesterol, mmol/L | 1.33 ± 0.35 | 1.35 ± 0.38 | 1.31 ± 0.32 | 1.34 ± 0.38 | 1.36 ± 0.40 | 1.37 ± 0.38 |
| Lowered HDL cholesterol,10n (%) | 29 (16.0) | 45 (18.4) | 19 (17.3) | 28 (19.6) | 10 (14.1) | 17 (16.7) |
| Triglycerides, mmol/L | 1.03 ± 0.52 | 0.92 ± 0.47 | 0.99 ± 0.44 | 0.93 ± 0.48 | 1.09 ± 0.62 | 0.92 ± 0.45 |
| Elevated triglycerides,11n (%) | 25 (13.4) | 33 (13.0) | 16 (14.2) | 18 (12.1) | 9 (12.3) | 15 (14.3) |
Data presented as mean ± SD, unless otherwise indicated. HOMA2-IR, updated homeostatic assessment model of insulin resistance; SGA, second-generation antipsychotic.
Overweight, BMI ≥1 SD and <2 SD for age and sex.
Obesity, BMI ≥2 SD for age and sex.
Elevated waist circumference, waist circumference ≥90th percentile for age and sex.
Elevated blood pressure, ≥90th percentile (1–13 y) or ≥120/80 mmHg (≥13 y).
Impaired fasting glucose, fasting glucose ≥5.6 mmol/L.
Elevated fasting insulin, fasting insulin ≥100 pmol/L.
Elevated total cholesterol, plasma total cholesterol ≥5.18 mmol/L.
Elevated LDL cholesterol, plasma LDL cholesterol ≥3.37 mmol/L.
Lowered HDL cholesterol, plasma HDL cholesterol <1.03 mmol/L.
Elevated triglycerides, plasma triglycerides ≥1.47 mmol/L.
Relations between the FTO genotype and SGA status on anthropometric and cardiometabolic risk factors are shown in Table 3. No interactions between the FTO genotype and SGA status on BMI z-scores or waist circumference z-scores were observed. Children with the FTO rs9939609 A allele had lower BMI z-scores compared with children with the TT genotype in a model adjusted for ethnicity (P = 0.005; Figure 1, Table 3).
TABLE 3.
Association between FTO rs9939609 genotype and SGA status with cardiometabolic risk factors1
| Interaction between FTO genotype and SGA status P value | FTO genotype | ||||
|---|---|---|---|---|---|
| FTO genotype P value | SGA status P value | SGA-naïve P value | SGA-treated P value | ||
| BMI z-score | 0.756 | 0.005 | 0.001 | ||
| Waist circumference z-score | 0.792 | 0.078 | 0.001 | ||
| Systolic blood pressure percentile | 0.516 | 0.474 | 0.853 | ||
| Diastolic blood pressure percentile | 0.540 | 0.092 | 0.323 | ||
| Fasting glucose | 0.036 | 0.001 | 0.994 | ||
| Fasting insulin | 0.420 | 0.909 | 0.055 | ||
| HOMA2-IR | 0.357 | 0.969 | 0.066 | ||
| Total cholesterol | 0.931 | 0.056 | 0.023 | ||
| LDL cholesterol | 0.934 | 0.064 | 0.111 | ||
| HDL cholesterol | 0.601 | 0.506 | 0.676 | ||
| Triglycerides | 0.141 | 0.074 | 0.242 | ||
Associations determined using general linear models adjusted by ethnicity for anthropometric and blood pressure variables, and adjusted by age, sex, ethnicity, and BMI z-score for fasting plasma concentration variables. FTO, fat mass and obesity-associated gene; HOMA2-IR, updated homeostatic assessment model of insulin resistance; SGA, second-generation antipsychotic.
FIGURE 1.
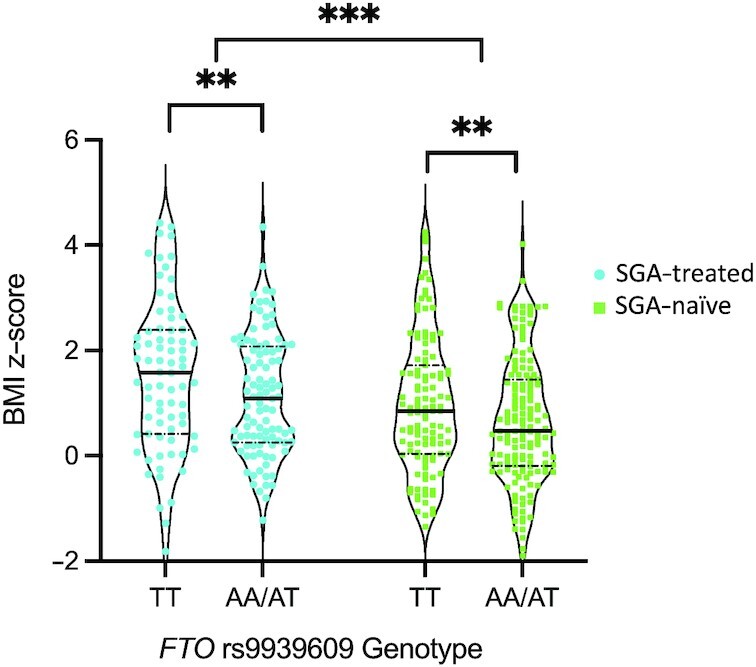
BMI z-scores in SGA-treated and SGA-naïve children by FTO rs9939609 genotype. Individual data points presented. Violin plots show the density of the data points, median (solid line), and 25th and 75th percentiles (dashed line). **Significant effect of FTO genotype, P < 0.01; ***significant effect of SGA treatment, P = 0.001. FTO, fat mass and obesity-associated gene; SGA, second-generation antipsychotic.
An interaction between the FTO rs9939609 genotype and SGA status was observed for fasting glucose concentrations (P = 0.036) in an adjusted model (age, sex, ethnicity, and BMI z-score). SGA-naïve children with the A allele had higher fasting glucose concentrations compared with children with the TT genotype (P = 0.001); this was not observed in SGA-treated children.
SGA-treated children had higher plasma total cholesterol concentrations (4.45 ± 0.91 compared with 4.16 ± 0.75 mmol/L; P = 0.023) and elevated plasma total cholesterol (≥5.18 mmol/L; OR = 3.88; 95% CI: 1.74, 8.65; P = 0.001) compared with SGA-naïve children in an adjusted model (by age, sex, ethnicity, and BMI z-score). No effect of the FTO genotype on fasting lipids was observed.
Dietary intake
Daily dietary total energy and macronutrient intakes estimated from the 3-d food records in a subset of the participants are presented in Table 4. There was no interaction between FTO rs9939609 genotype and SGA status on total energy intakes. Children with the FTO A allele consumed on average 180 kcal more than children with the FTO TT genotype in a model adjusted for age, sex, ethnicity, and BMI z-score (P = 0.048; Tables 4 and 5). No differences in total energy intakes were observed between SGA-treated and SGA-naïve children.
TABLE 4.
Daily dietary total energy and macronutrient intake1
| All children | SGA-naïve | SGA-treated | ||||
|---|---|---|---|---|---|---|
| TT | TA/AA | TT | TA/AA | TT | TA/AA | |
| (n = 30) | (n = 43) | (n = 12) | (n = 23) | (n = 18) | (n = 20) | |
| Total energy, kcal | 1814 ± 484 | 1994 ± 619 | 1597 ± 525 | 1889 ± 442 | 1959 ± 406 | 2116 ± 769 |
| Protein, g | 62.9 ± 19.7 | 73.8 ± 23.4 | 61.7 ± 22.4 | 69.3 ± 18.9 | 63.7 ± 18.3 | 79.0 ± 27.2 |
| % energy | 13.9 ± 2.29 | 15.0 ± 2.41 | 15.5 ± 2.37 | 14.7 ± 2.23 | 12.9 ± 1.57 | 15.3 ± 2.63 |
| Carbohydrates, g | 251 ± 71.5 | 269 ± 101 | 211 ± 67.5 | 247 ± 75.7 | 277 ± 62.7 | 295 ± 121 |
| % energy | 55.1 ± 5.90 | 54.0 ± 9.28 | 53.1 ± 5.60 | 52.9 ± 11.5 | 56.5 ± 5.85 | 55.2 ± 5.82 |
| Fat, g | 64.1 ± 19.4 | 70.2 ± 25.1 | 57.4 ± 20.6 | 68.6 ± 24.0 | 68.6 ± 17.8 | 72.0 ± 26.8 |
| % energy | 32.0 ± 5.76 | 31.6 ± 6.62 | 32.4 ± 5.78 | 32.3 ± 7.15 | 31.7 ± 5.90 | 30.7 ± 6.02 |
Data are 3-d average presented as mean ± SD. SGA, second-generation antipsychotic.
TABLE 5.
Associations between FTO rs9939609 genotype and SGA status with dietary energy and macronutrient intakes1
| Interaction between FTO genotype and SGA status P value | FTO genotype | ||||
|---|---|---|---|---|---|
| FTO genotype P value | SGA status P value | SGA-naïve P value | SGA-treated P value | ||
| Total energy intake (kcal) | 0.731 | 0.048 | 0.286 | ||
| Protein (% energy) | 0.003 | 0.231 | 0.004 | ||
| Carbohydrates (% energy) | 0.808 | 0.508 | 0.067 | ||
| Fat (% energy) | 0.667 | 0.889 | 0.431 | ||
Associations determined using general linear models adjusted by age, sex, ethnicity, and BMI z-score. FTO, fat mass and obesity-associated gene; SGA, second-generation antipsychotic.
An interaction between the FTO rs9939609 genotype and SGA status was observed for dietary protein intakes (as percentage of total energy, P = 0.003) in an adjusted model (by age, sex, ethnicity, and BMI z-score) (Table 5). SGA-treated children with the A allele consumed more protein (2.39% of total energy) than SGA-treated children with the TT genotype (P = 0.004, Table 5). No effect of the FTO genotype on total protein intakes was observed in SGA-naïve children. No relations between the FTO rs9939609 genotype or SGA status on carbohydrate or fat intakes were observed.
Discussion
In this study, we examined whether the FTO rs9939609 variant is associated with greater cardiometabolic complications and dietary energy intakes in SGA-treated and SGA-naïve children with mental health disorders. We postulated that given the potential role of FTO in food intake regulation and energy homeostasis (15–17, 21), children with the FTO rs9939609 A allele would be more susceptible to SGA-related cardiometabolic complications. We observed 3 main findings. First, both SGA-naïve and SGA-treated children with the FTO rs9939609 A allele had lower BMI z-scores compared with SGA-naïve and SGA-treated children with the TT genotype. This finding is in contrast to others who have reported higher BMI z-scores in children with the A allele compared with the TT genotype (15–17). Further, no interaction between the FTO rs9939609 genotype and SGA treatment on BMI z-scores was observed. Second, SGA-naïve children with the FTO rs9939609 A allele had higher fasting plasma glucose concentrations than SGA-naïve children with the TT genotype. This association between FTO genotype and fasting plasma glucose was not seen in SGA-treated children, Third, as predicted, in a subset of the children, those with the FTO rs9939609 A allele had greater dietary total energy intakes compared with children with the TT genotype. No interaction between the FTO variant and SGA treatment on dietary total energy intakes was observed. However, SGA-treated children with the A allele had higher protein intake compared with SGA-treated children with the TT genotype. This was not seen in SGA-naïve children.
The FTO rs9939609 A allele was the first identified (14), and one of the most replicated genetic variants associated with elevated BMI and obesity in children (29, 30). In contrast, our study found children with the A allele had lower BMI z-scores compared with children with the TT genotype. The mostly likely explanation for our discrepant finding is the differences in the populations that were studied. In the current study, we assessed children with a confirmed mental health disorder diagnosis, whereas the previously published studies (15–17) were conducted in children representing the general population or in otherwise healthy children with no information on mental health disorders. However, similar to what we report here, a previous study conducted in children (n = 225) with autism spectrum disorder reported no relation between risperidone-related weight gain and the FTO rs9939609 variant (31). Moreover, other studies that assessed both children and adults with mental health disorders have reported no relations between the FTO rs9939609 variant and weight gain or BMI (32–34). Taken together, this suggests that the impact of the FTO rs9939609 A allele on BMI might not be relevant in children with mental health disorders.
There are no published studies in children with mental health disorders that have interrogated the relation between the FTO rs9939609 variant and fasting glucose concentrations. Interestingly we observed an interaction between the FTO rs9939609 variant and SGA treatment on fasting glucose concentrations, with higher fasting plasma glucose concentration observed only in SGA-naïve children with the A allele. This is similar to findings of others who have also reported higher fasting glucose concentrations in those with the A allele (19, 35, 36). The lack of a relation between the A allele and fasting plasma glucose concentrations in SGA-treated children could be due to the greater effect of SGAs on glucose metabolism (11), diluting any effect of the A allele. SGAs may affect blood glucose by 2 different pathways that occur independent of each other or in concert. First, blood glucose concentrations may become elevated in SGA-treated children secondary to the development of obesity and insulin resistance. This is supported by studies that report children treated with antipsychotics have a 3-fold greater risk of type 2 diabetes compared with untreated children (7, 8). Second, SGAs may have direct effects on the insulin producing β-cells of the pancreas. We have reported that children treated with quetiapine have reduced insulin secretion (insulinogenic index) during an oral-glucose-tolerance test (37). Further, a recent study in a mouse model reported β-cell dysfunction, glucose intolerance, and impaired insulin secretion in female mice treated with aripiprazole (38).
Our finding of a positive relation between the FTO rs9939609 A allele and dietary total energy intakes is consistent with previous studies conducted in children (16, 21, 39–41) and supports the role of FTO in food intake regulation. Moreover, reduced satiety, increased food responsiveness, and overeating have been reported in children with the A allele compared with those with the TT genotype (15, 17, 42). Interestingly, we observed an association between FTO genotype and dietary energy intakes from protein only in SGA-treated children; however, the association was small and is unlikely to be clinically relevant. Discrepant findings have been reported on the relation between the FTO rs9939609 variant and macronutrient intakes. Some studies have reported no associations with macronutrient intakes (39, 41), whereas others have reported a positive association between the A allele and dietary fat intake in children (40, 43). Inconsistent findings have also been reported in adults (44). However, one study reported that the A allele was associated with lower total energy intakes and higher protein intake in adults (45).
Interestingly, children with the FTO rs9939609 A allele had lower BMI z-scores despite higher dietary energy intakes. However, this could have been impacted by the small number of children in the subset that completed dietary assessment relative to the number of children that were genotyped. Nonetheless, this observation might be due to differences in energy expenditure and/or activity between the groups. We were unable to assess energy expenditure in the current study. We previously reported that ∼59% of children with mental health disorders (SGA-treated and SGA-naïve) met physical activity guidelines of ≥60 min of activity per day (46). The most likely explanation for our findings is that the medications and/or the pathology of the mental health disorder counteract the effect of FTO on body composition and food intake regulation. The SGA medications typically target serotonin and dopamine receptors (11); it is probable that SGAs and/or mental illness affect the hypothalamus and interfere with the role of FTO in this brain region.
Although the functional effect of the FTO rs9939609 variant is still unclear, the location of the variant in an intron at the 5′ end of the gene suggests the variant could interfere with FTO transcriptional regulation rather than protein function. The main functional effect of the variant is on appetite and food intake regulation (17, 21). A study reported that FTO rs9939609 variant impacts food intake through effects on the corticolimbic activation pathway (47). An alternative explanation might relate to changes in the gene expression of adjacent genes involved in energy metabolism. It has been reported that the FTO rs9939609 variant interacts with the promotor of the iroquois-related homeobox 3 gene (IRX3) and enhances expression, indirectly affecting adipocyte function, energy metabolism, and body weight (48, 49). Recent evidence suggests increased expression of IRX3 with the FTO rs9939609 A allele in adipose tissue in children with a BMI <1.28 SD for age and sex and greater adipocyte IRX3 expression, negatively correlated with BMI compared with children with overweight and obesity (BMI ≥1.28 SD for age and sex) (50).
There are several strengths and some limitations to our study. The strengths of our study include the large sample size of children with mental health disorders (n = 506). We adjusted all the statistical analyses for relevant covariates including age, sex, ethnicity, and BMI z-score. Our study is limited because of the cross-sectional design. However, given the population of children, it is difficult to prospectively follow a large cohort of children with mental health disorders receiving SGA treatment because of high rates of attrition and medication changes during the course of their illness. There are also well-known limitations with self-reported dietary intake data, particularly related to measurement errors in estimating food portions/sizes. The possibility of response bias related to social desirability might also influence dietary information reported by the participant's parents or caregivers.
In summary, our findings suggest that the FTO rs9939609 A allele is associated with lower BMI z-scores and higher dietary energy intakes in children with mental health disorders, and with higher plasma fasting glucose concentration in SGA-naïve children. Given metabolic complications are common in children with mental health disorders, especially in those treated with SGAs, further research to understand which genetic variants can predict children at risk of developing metabolic complications is required.
Acknowledgments
We acknowledge Mikaela Barker, who assisted with collection of some of the dietary information from the children.
The authors’ responsibilities were as follows—AMD and CP: designed the research; YFN and AMH: conducted the research; AMW: analyzed data and wrote the first draft of the manuscript; AMD: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This research was supported by a grant from BC Mental Health and Substance Use Services to AMD and CP. AMW is supported by a Bertram Hoffmeister postdoctoral fellowship from BC Children's Hospital Research Institute and by Becas-Chile, Agencia Nacional de Investigación y Desarrollo. AMD is supported by an Investigator Grant from BC Children's Hospital Research Institute.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: FTO, fat mass and obesity-associated gene; IRX3, iroquois-related homeobox 3 gene; SGA, second-generation antipsychotic.
Contributor Information
Alejandra M Wiedeman, Department of Pediatrics, The University of British Columbia, Vancouver, Canada; BC Children's Hospital Research Institute, Vancouver, Canada.
Ying F Ngai, Department of Pediatrics, The University of British Columbia, Vancouver, Canada; BC Children's Hospital Research Institute, Vancouver, Canada.
Amanda M Henderson, Department of Pediatrics, The University of British Columbia, Vancouver, Canada; BC Children's Hospital Research Institute, Vancouver, Canada.
Constadina Panagiotopoulos, Department of Pediatrics, The University of British Columbia, Vancouver, Canada; BC Children's Hospital Research Institute, Vancouver, Canada.
Angela M Devlin, Email: adevlin@bcchr.ubc.ca, Department of Pediatrics, The University of British Columbia, Vancouver, Canada; BC Children's Hospital Research Institute, Vancouver, Canada.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Mental Health Commission of Canada . Making the case for investing in mental health in Canada. Ottawa: Mental Health Commission of Canada; 2013. [Google Scholar]
- 2. Sohn M, Moga D, Blumenschein K, Talbert J. National trends in off-label use of atypical antipsychotics in children and adolescents in the United States. Medicine (Baltimore). 2016;95(23):e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen W, Cepoiu-Martin M, Stang A, Duncan D, Symonds C, Cooke L, Pringsheim T. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449–55. [DOI] [PubMed] [Google Scholar]
- 4. Halfdanarson O, Zoega H, Aagaard L, Bernardo M, Brandt L, Fuste AC, Furu K, Garuoliene K, Hoffmann F, Huybrechts KFet al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol. 2017;27(10):1064–76. [DOI] [PubMed] [Google Scholar]
- 5. Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Pediatr Drugs. 2013;15(2):139–50. [DOI] [PubMed] [Google Scholar]
- 6. Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, Newcomer JW. Metabolic effects of antipsychotics on adiposity and insulin sensitivity in youths: a randomized clinical trial. JAMA Psychiatry. 2018;75(8):788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galling B, Roldán A, Nielsen RE, Nielsen J, Gerhard T, Carbon M, Stubbs B, Vancampfort D, De Hert M, Olfson Met al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73(3):247–59. [DOI] [PubMed] [Google Scholar]
- 8. Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Børglum AD, Gasse C. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. Am J Psychiatry. 2017;174(7):686–94. [DOI] [PubMed] [Google Scholar]
- 9. Panagiotopoulos C, Ronsley R, Davidson J. Increased prevalence of obesity and glucose intolerance in youth treated with second-generation antipsychotic medications. Can J Psychiatry. 2009;54(11):743–9. [DOI] [PubMed] [Google Scholar]
- 10. Wiedeman AM, Panagiotopoulos C, Devlin AM. Treatment-related weight gain and metabolic complications in children with mental health disorders: potential role for lifestyle interventions. Appl Physiol Nutr Metab. 2021;46(3):193–204. [DOI] [PubMed] [Google Scholar]
- 11. Devlin AM, Panagiotopoulos C. Metabolic side effects and pharmacogenetics of second-generation antipsychotics in children. Pharmacogenomics. 2015;16(9):981–96. [DOI] [PubMed] [Google Scholar]
- 12. Devlin AM, Ngai YF, Ronsley R, Panagiotopoulos C. Cardiometabolic risk and the MTHFR C677T variant in children treated with second-generation antipsychotics. Transl Psychiatry. 2012;2(1):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cote AT, Panagiotopoulos C, Devlin AM. Interaction between the val158met catechol-O-methyltransferase gene variant and second-generation antipsychotic treatment on blood pressure in children. Pharmacogenomics J. 2015;15(1):95–100. [DOI] [PubMed] [Google Scholar]
- 14. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NWet al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93(9):3640–3. [DOI] [PubMed] [Google Scholar]
- 16. Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359(24):2558–66. [DOI] [PubMed] [Google Scholar]
- 17. Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, Kozlosky M, Elliott C, Ranzenhofer LM, Roza CAet al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009;90(6):1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Kilpelainen TO, Liu C, Zhu J, Liu Y, Hu C, Yang Z, Zhang W, Bao W, Cha Set al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia. 2012;55(4):981–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hertel JK, Johansson S, Sonestedt E, Jonsson A, Lie RT, Platou CG, Nilsson PM, Rukh G, Midthjell K, Hveem Ket al. FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes. 2011;60(5):1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LAet al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318(5855):1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes. 2009;33(1):42–5. [DOI] [PubMed] [Google Scholar]
- 22. Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity. 2008;16(8):1961–5. [DOI] [PubMed] [Google Scholar]
- 23. Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78(6):723–9. [DOI] [PubMed] [Google Scholar]
- 24. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(09):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SKet al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 27. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2. [DOI] [PubMed] [Google Scholar]
- 28. Barker MK, Sable CM, Montgomery SE, Chow L, Green TJ, Panagiotopoulos C, Devlin AM. Diet and cardiometabolic side effects in children treated with second-generation antipsychotics. Clin Nutr ESPEN. 2018;23:205–11. [DOI] [PubMed] [Google Scholar]
- 29. Quan LL, Wang H, Tian Y, Mu X, Zhang Y, Tao K. Association of fat-mass and obesity-associated gene FTO rs9939609 polymorphism with the risk of obesity among children and adolescents: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19(4):614–23. [PubMed] [Google Scholar]
- 30. Resende CMM, Silva H, Campello CP, Ferraz LAA, de Lima ELS, Beserra MA, Muniz MTC, da Silva LMP. Polymorphisms on rs9939609 FTO and rs17782313 MC4R genes in children and adolescent obesity: a systematic review. Nutrition. 2021;91-92:111474. [DOI] [PubMed] [Google Scholar]
- 31. Nurmi EL, Spilman SL, Whelan F, Scahill LL, Aman MG, McDougle CJ, Arnold LE, Handen B, Johnson C, Sukhodolsky DGet al. Moderation of antipsychotic-induced weight gain by energy balance gene variants in the RUPP autism network risperidone studies. Transl Psychiatry. 2013;3(6):e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jassim G, Ferno J, Theisen FM, Haberhausen M, Christoforou A, Havik B, Gebhardt S, Remschmidt H, Mehler-Wex C, Hebebrand Jet al. Association study of energy homeostasis genes and antipsychotic-induced weight gain in patients with schizophrenia. Pharmacopsychiatry. 2011;44(1):15–20. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds GP, Yevtushenko OO, Gordon S, Arranz B, San L, Cooper SJ. The obesity risk gene FTO influences body mass in chronic schizophrenia but not initial antipsychotic drug-induced weight gain in first-episode patients. Int J Neuropsychopharmacol. 2013;16(6):1421–5. [DOI] [PubMed] [Google Scholar]
- 34. Zhang JP, Lencz T, Zhang RX, Nitta M, Maayan L, John M, Robinson DG, Fleischhacker WW, Kahn RS, Ophoff RAet al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42(6):1418–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saber-Ayad M, Manzoor S, El Serafi A, Mahmoud I, Hammoudeh S, Rani A, Abusnana S, Sulaiman N. The FTO rs9939609 “A” allele is associated with impaired fasting glucose and insulin resistance in Emirati population. Gene. 2019;681:93–8. [DOI] [PubMed] [Google Scholar]
- 36. Yang Y, Liu B, Xia W, Yan J, Liu HY, Hu L, Liu SM. FTO genotype and type 2 diabetes mellitus: spatial analysis and meta-analysis of 62 case-control studies from different regions. Genes. 2017;8(2):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ngai YF, Sabatini P, Nguyen D, Davidson J, Chanoine JP, Devlin AM, Lynn FC, Panagiotopoulos C. Quetiapine treatment in youth is associated with decreased insulin secretion. J Clin Psychopharmacol. 2014;34(3):359–64. [DOI] [PubMed] [Google Scholar]
- 38. Grajales D, Vazquez P, Ruiz-Rosario M, Tuduri E, Mirasierra M, Ferreira V, Hitos AB, Koller D, Zubiaur P, Cigudosa JCet al. The second-generation antipsychotic drug aripiprazole modulates the serotonergic system in pancreatic islets and induces beta cell dysfunction in female mice. Diabetologia. 2022;65(3):490–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qi Q, Downer MK, Kilpelainen TO, Taal HR, Barton SJ, Ntalla I, Standl M, Boraska V, Huikari V, Kiefte-de Jong JCet al. Dietary intake, FTO genetic variants, and adiposity: a combined analysis of over 16,000 children and adolescents. Diabetes. 2015;64(7):2467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, Davey Smith G. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88(4):971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ranzenhofer LM, Mayer LES, Davis HA, Mielke-Maday HK, McInerney H, Korn R, Gupta N, Brown AJ, Schebendach J, Tanofsky-Kraff Met al. The FTO gene and measured food intake in 5- to 10-year-old children without obesity. Obesity. 2019;27(6):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Velders FP, De Wit JE, Jansen PW, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. FTO at rs9939609, food responsiveness, emotional control and symptoms of ADHD in preschool children. PLoS One. 2012;7(11):e49131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee HJ, Kim IK, Kang JH, Ahn Y, Han BG, Lee JY, Song J. Effects of common FTO gene variants associated with BMI on dietary intake and physical activity in Koreans. Clin Chim Acta. 2010;411(21–22):1716–22. [DOI] [PubMed] [Google Scholar]
- 44. Drabsch T, Gatzemeier J, Pfadenhauer L, Hauner H, Holzapfel C. Associations between single nucleotide polymorphisms and total energy, carbohydrate, and fat intakes: a systematic review. Adv Nutr. 2018;9(4):425–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, Sonestedt E, Chu AY, Renstrom F, Lin Xet al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23(25):6961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cote AT, Devlin AM, Panagiotopoulos C. Initial screening of children treated with second-generation antipsychotics points to an association between physical activity and insulin resistance. Pediatr Exerc Sci. 2014;26(4):455–62. [DOI] [PubMed] [Google Scholar]
- 47. Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, Webb MF, De Leon MRB, Grabowski TJ, Leibel RLet al. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr. 2018;107(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, Aneas I, Credidio FL, Sobreira DR, Wasserman NFet al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran Vet al. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;373(10):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landgraf K, Scholz M, Kovacs P, Kiess W, Korner A. FTO obesity risk variants are linked to adipocyte IRX3 expression and BMI of children – relevance of FTO variants to defend body weight in lean children?. PLoS One. 2016;11(8):e0161739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.


