Abstract
Background
Uterine Artery Embolization (UAE) and Magnetic Resonance guided High Intensity Focused Ultrasound (MRgHIFU) are two noninvasive treatments for uterine leiomyoma.
Methods
This systematic review, following PRISMA guidelines, analyzed the effectiveness of two treatments by comparing percent fibroid volume shrinkage immediately after the procedure and after 3, 6, 12 and 24 months of follow-up and also assessed and compared common complications following treatment. The search utilized Science Direct, PubMed, MEDLINE, Google Scholar and BioMed Central databases, selecting manuscripts published during the period 2000 and 2020. Studies with premenopausal patients with previous treatments for uterine leiomyoma and/or with other pelvic diseases were excluded.
Results
Twenty-nine papers satisfied inclusion and exclusion criteria. Results were pooled and stratified by treatment and follow-up time. Weighted fibroid volume percent shrinkage after UAE was statistically significantly greater than MRgHIFU at 6, 12, and 24 months follow-up times. However, UAE had statistically significantly more complications, such as pain, nausea and vomiting. However, this study cannot conclude that UAE is more effective than MRgHIFU due to confounding factors.
Keywords: Magnetic resonance guided high intensity focused ultrasound, Uterine artery embolization, Uterine leiomyoma, Uterine fibroid
Introduction
Uterine leiomyoma or uterine fibroids are the most prevalent benign smooth-muscle tumors of the uterus. They are present in approximately 60% of women at reproductive age [1]. However, the real prevalence is likely substantially higher, given that some women do not present symptoms of uterine leiomyomas and thus go undiagnosed. Symptomatic leiomyomas can adversely impact women’s physical, social, and psychological functioning, as well as reduce income and work effectiveness [2]. Symptoms and signs of uterine leiomyoma depend on the type, number, size and secondary changes within the fibroid nodules. Patients with uterine leiomyoma might present with heavy menstrual bleeding, pain, significant intermittent uterine bleeding, iron-deficient anemia, pelvic pain, bowel dysfunction, urinary and pressure symptoms [3]. Uterine leiomyoma is also causally associated with 1–3% of the infertility rate and 8% of miscarriages [4]. Women with uterine leiomyoma have three times higher risk of miscarriage than women without fibroids. An estimated 5–10% of infertile women have uterine fibroids that have contributed to anatomic distortion of the uterine cavity and abnormal endometrial receptivity [5]. Additionally, uterine leiomyoma might cause complications during pregnancy, such as preterm delivery (< 37 weeks), abnormal fetal position, abnormal placentation, placental abruption, postpartum infections and postpartum bleeding [4]. Because of symptoms and complications, the presence of uterine fibroids is the primary indication for conducting a hysterectomy worldwide [6]. Thus, alternative non-invasive or minimally invasive treatments are requisite for avoiding more invasive procedures, while still effectively protecting fertility relieving clinical symptoms for women with uterine leiomyoma.
There are medical, surgical, and minimally invasive treatments for uterine leiomyoma. Asymptomatic patients might not require treatment [2]. The aim of uterine leiomyoma management is to relieve symptoms, avoid or minimize invasiveness, promote rapid recovery following treatment and preserve fertility, if necessary and dependent on the patients’ decisions. Uterine Artery Embolization (UAE) and Magnetic Resonance Guided by High Intensity Focused Ultrasound (MRgHIFU) treatments are minimally invasive procedures that reduce fibroid volume while avoiding the higher risk of uterus damage associated with more invasive procedures. Among the currently available the conservative interventional management options, UAE has the longest history and has been shown to be effective in properly selected patients [3]. Newer focused energy delivery methods are promising but need more investigation on the long-term outcomes [3]. The aim of this systematic review is to analyze and compare fibroid shrinkage following UAE and MRgHIFU treatments and to identify and compare common complications associated with these two procedures. A systematic review was conducted by Taheri et al. [7] assessing fibroid volume changes following UAE and radiofrequency ablation. However, the Taheri systematic review did not take into consideration of either the other treatments and pelvic diseases (except adenomyosis) that patients were experiencing, or the patients’ menopausal or perimenopausal status. Our systematic review evaluates and compares fibroid shrinkage following UAE and MRgHIFU treatments for premenopausal women without the presence of other treatments and other gynecological diseases to avoid confounding biases, and also evaluates and compares common complications for the two procedures.
Materials and methods
Our systematic review followed PRISMA guidelines utilizing, with searches on Science Direct, PubMed, MEDLINE, Google Scholar and BioMed Central databases to identify published journal articles on related the years 2000–2020. All original research articles, including prospective and historical cohort studies case–control studies, case reports and case series were reviewed. There were no randomized clinical trials. Each individual paper was reviewed by two out of three of the authors that conducted reviews, with any disagreements between these authors being resolved by consultation.
Published manuscripts were considered for inclusion into the systematic review if patients were women with symptomatic uterine leiomyoma who received either UAE or MRgHIFU treatments. Extracted data from the selected studies that met inclusion and exclusion criteria for the systematic review included authors, year of publication, follow-up duration, study design, interventions, participant population, characteristics of patients, size of fibroids, uterine fibroids’ shrinkage after treatment and postoperative outcomes. Outcomes of interest included fibroid volume before and after treatments, mean fibroid volume percent change, and complications following the procedures. Key phrases, including “magnetic resonance guided high intensity focused ultrasound”, “uterine artery embolization” and “uterine fibroids”, and “leiomyoma” or “uterine myomas” were utilized in title searches to identify related research publications.
Our exclusion criteria those studies where patients who have undergone or were undergoing treatments other than UAE or MRgHIFU, patients with other gynecological pathologies that were not uterine leiomyoma, redundant publications with the same data, studies including postmenopausal women, and research reporting fibroid volume changes without providing mean values. Research papers were also excluded if they were published before 2000. Only studies published in English were included. Research publications that met exclusion and inclusion criteria were eligible for inclusion into the systematic review.
Mean fibroid volume percent changes were calculated from baseline, before the procedure, to five different endpoints, of five different follow-up periods including immediately following the procedure, and after 3, 6, 12 and 24 months. A weighted mean was calculated for each follow-up period. The percentage of reported number of occurrences of each complication was calculated for all selected papers for the systematic review.
Data on selected papers were downloaded using the reference manager Mendeley, entered into Microsoft Excel and transferred for analyzed into statistical software STATA version 16.1 [8]. Fibroid volume percent change was compared utilizing a two-tailed t-test, with values of p ≤ 0.05 being considered statistically significant. The Chi square test was performed to examine statistical significance of the counts differences of complications following the procedures.
To reduce selection bias, full texts of potentially eligible articles were retrieved and independently assessed for eligibility by two reviewers out of three that conducted reviews. Any disagreement between two reviewers over the eligibility of a particular article was resolved through discussion with the third reviewer. Data extracted from selected articles included study design, population characteristics, treatment and outcomes.
Give that this was a systematic review of existing published journal articles and all data utilized was extracted from these studies, the current manuscript was exempt from human subject review, with no consent requirements. The study was conducted without funding.
Results
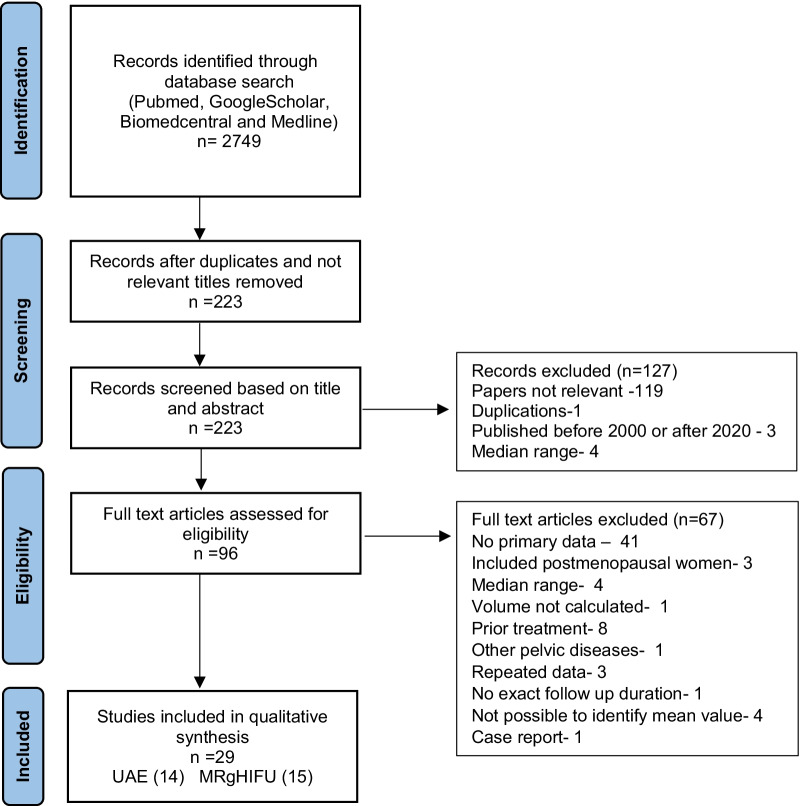
A total of 2749 papers were identified from the initial search. Out of this number, 29 papers were found eligible for inclusion in qualitative and quantitative analyses, (explained in detail in Fig. 1). Out of the 29 eligible papers, none compared UAE treatment outcomes with that of MRgHIFU. Fourteen case series papers reported UAE treatment outcomes, while 15 reported MRgHIFU treatment outcomes. The sum number of patients who were treated with UAE or with MRgHIFU treatments in the eligible studies was 1383 and 835, respectively. (Table 1).
Fig. 1.
Flowchart diagram to select eligible papers
Table 1.
Synthesis of data about general features and eligibility criteria of study population treated with UAE
| # | References | Study design | Mean age | Number of patients | Number of FB | Inclusion criteria | Exclusion criteria | Mean volume of treated FB | FB shrinkage (%) | Follow up period (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Burn et al. [9] | PRO | 39 | 18 | 32 | Age 18–53, a | – | 340 cm3 | 59 | 6 |
| 2 | Roth et al. [10] | PRO | NM | 79 | NM | – | – | 244.5 cm3 | 40 | 3 |
| 3 | Klein et al. [11] | PRO | 46 | 35 | NM | a, b | – | 209 ml | 49 | 6 |
| 4 | Zupi et al. [12] | PRO | 40 | 26 | NM | j | i, m, t | 276.8 ± 241.2 ml | 55 ± 16.9 | 6 |
| 5 | Spies et al. [13] | PRO | 43 | 100 | NM | Age 30–55, a, j | d, f, k, n | 148.7 ± 153.9 cm3 | 50.06 | 3 |
| 6 | Harman et al. [14] | PRO | 44 | 20 | 28 | – | – | 123 cm3 | 44.6 | 6 months |
| 7 | Pisco et al. [15] | RETR | 41 | 234 | NM | j, l | n | 110.5 cm3 | 60.7 | 6 |
| 8 | Naguib et al. [16] | RETR | 48 | 28 | 84 | a, b, j | d, c, g (10 cm), n, o, p | 51.6 cm3 | 52.62 | 3 |
| 73.27 | 12 | |||||||||
| 9 | Stampfl et al. [17] | PRO | 42 | 121 | NM | Age > 30, b (2 years), j | c, d, i, g, n, o, p, p, u | 137.2 ± 245.1 ml | 52.4 | 3 |
| 78.3 | 6 | |||||||||
| 91.2 | 12 | |||||||||
| 10 | Bilhim et al. [18] | PRO | 39 | 160 | NM | a | – | 201.5 cm3 | 53.1 | 3 |
| 52.95 | 6 | |||||||||
| 11 | Redecha et al. [19] | PRO | NM | 98 | NM | Age > 18, j | h | NM | 68.18 | 24 |
| 12 | Song et al. [20] | PRO | 43 | 60 | NM | a, j | d, e, i, n, p | 224.69 ml | 54.05 | 3 |
| 13 | Yoon et al. [21] | RETR | 42 | 67 | NM | a | d, i, n | 143.5 ± 135.4 cm3 | 42 ± 23.1 | 3 |
| 14 | Ukybassova et al. [22] | PRO | 43 | 337 | NM | Age > 18, j, l | c, d, f, n, o, p, q, r, s | 51.53 ± 65.53 mm3 | 9.95 | 3 |
| 32.18 | 6 | |||||||||
| 51.7 | 12 |
NM, not mentioned; FB, fibroid; PRO, prospective case series; RETR, retrospective case series
a- fibroid related disease, b- no desire for further pregnancies; c- pedunculated fibroids with more than 50% attachment; d- pregnancy; e- contraindications to MRI and Gadolinium use; f- major medical disease; g- fibroid diameter > 15 cm, h- postmenopause; i- other pelvic diseases; j- premenopause; k-lactating; l- presence of only intramural fibroids, m- subserosal fibroid, n- pelvic inflammatory disease, o- abnormal coagulation status, p- malignancy, q- abnormal endometrial biopsy results, r- abnormal PAP test, s- severe anemia, t- irregular margins and with a sonographic pattern of diffuse fibrosis, u- allergy to contrast material
The overall weighted mean age of patients treated with UAE was not possible to calculate, because not all articles reported patients’ mean age. The weighted mean age of patients who received MRgHIFU treatment was 43 (Table 2).
Table 2.
Synthesis of data about general features and eligibility criteria of study population treated with MRgHIFU
| # | References | Study design | Mean age | Number of patients | Number of FB | Inclusion criteria | Exclusion criteria | Mean volume of treated FB | Fibroid shrinkage (%) | Follow up period (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hindley et al. [23] | PRO | 45 | 109 | NM | Age > 18, b | f, g, h, i, l, m, n |
346 ± 245 cm3 (single) 294 ± 188 cm3 (multiple) |
13.5 | 6 |
| 2 | Mikami et al. [24] | RETR | 44 | 48 | NM | a, b | e, q | NM | 23 | 6 |
| 3 | Morita et al. [25] | PRO | 43 | 48 | 55 | Age > 18, b, o | d, i, n, m, s | NM | 33 ± 19 | 6 |
| 4 | Rabinovici et al. [26] | PRO | 46 | 35 | 41 | a, l | k (20 w), j (10 cm), g, h | 216 ± 223 ml | 15 ± 27 | 6 |
| 5 | Lánä́rd et al. [27] | RETR | 45 | 66 | NM | Age > 18, a, b, o | f, g, h, i, l, m, n | 255.5 ± 201.7 cm3 | 12.6 ± 16.9 | 6 |
| 9.3 ± 24.8 | 12 | |||||||||
| 6 | Zhang et al. [28] | PRO | 39 | 21 | 23 | Age > 18, a, e, o | j (10 cm), g, h, u | 77.3 ± 66.6 cm3 | 31.4 ± 29.3 | 3 |
| 7 | Funaki et al. [29] | PRO | 40 | 91 | 141 | – | f, g, h, i, n, p, s, t | NM | 33.1 | 6 |
| 38 | 12 | |||||||||
| 38.2 | 24 | |||||||||
| 8 | Kim et al. [30] | PRO | 46 | 40 | 51 | Age > 18, a, o | c, e, f, g, h, i, k | 336 ± 40.8 cm3 | 18.7 | 6 |
| 25.8 | 12 | |||||||||
| 27.6 | 24 | |||||||||
| 9 | Ruhnke et al. [31] | PRO | 47 ± 4 | 18 | 27 | Age 18–59, a, o | c, f, g, h, i, q, w | 125 ± 140 ml | 45 ± 21 | 6 |
| 10 | Thiburce et al. [32] | RETR | 44 | 36 | NM | Age > 18, o | f, j, n, r, q | 255 (190–319) cm3 | 27 | 6 |
| 11 | Tung et al. [33] | PRO | 42 | 40 | NM | – | j (10 cm), f, n | 258.1 ± 223.8 cm3 | 31.7 | 6 |
| 12 | Xu [34] | PRO | 42 | 43 | 51 | Age 18–55, a, b, o | j (12 cm), h, q | NM | 33.51 | 3 |
| 44.52 | 6 | |||||||||
| 13 | Chen et al. [35] | PRO | 45 | 107 | 130 | Age > 18, o, m < 140 kg, l | n, g, l, h | NM | 41.6 ± 22.70 | 3 |
| 50.2 ± 20.40 | 6 | |||||||||
| 14 | Jacoby et al. [36] | PRO | 44 | 13 | NM | Age > 18, a, o, b | g, l, h, u, v, j (10 cm), | 217 ± 139 | 18 | 6 |
| 15 | Keserci et al. [37] | PRO | 40 | 120 | 339 | Age > 18, a, o | n, g, s, u, h | 197.3 ± 155.7 ml | 38 ± 26 | 6 |
NM, not mentioned, FB, fibroid; PRO, prospective case series; RETR, retrospective case series
a- fibroid related disease; b- no desire for further pregnancies; c- conceive after MRgFUS; d- 3 months treatment GnRH analogue; e- bowel lies anterior to the uterus; f- abdominal scar (locate in the path of the ultrasound beam); g- pregnancy; h- contraindications to MRI and Gadolinium use; i- major medical disease; j- fibroid diameter > 15 cm, k- Uterus larger than 24 gestational weeks, l- hematocrit less than 25%; m- postmenopause; n- other pelvic diseases; o- premenopause; p-lactating; q- degeneration or calcification of fibroids; r- submucosal fibroids, s- pelvic inflammatory disease, t- abnormal coagulation status, u- malignancy, v- abnormal endometrial biopsy results, w- abnormal PAP test
The number of uterine fibroids was not reported in many studies. All papers only included patients older than 18 years of age. The most common reasons for exclusion of patients in both UAE and MRgHIFU studies included pregnancy and restriction size of leiomyoma (10 or 12 cm) or uterine volume measured gestational week (20 week of gestation). Absolute fibroid volume reduction measures were reported in all papers, but only two UAE treatment studies and five MRgHIFU studies reported percentage reductions, which always provided standard deviation or range of values. Some studies included only included measures for the volume of the dominant myoma.
Fibroid shrinkage percentages were stratified by 3, 6, 12 and 24 months follow-up times after treatment (Table 3).
Table 3.
Pooled data of fibroid volume reductions after UAE and MRgHIFU
| Variables | Follow up periods | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | 24 months | |||||||||
| UAE | MRgHIFU | P value | UAE | MRgHIFU | P value | UAE | MRgHIFU | P value | UAE | MRgHIFU | P value | |
| Weighted mean fibroid volume reduction ± SD, % | 35.59 ± 19.41 | 38.31 ± 4.29 | 0.068 | 50.57 ± 15.70 | 30.06 ± 12.76 | 0.0001 | 62.78 ± 17.10 | 25.91 ± 12.64 | 0.0001 | 68.18 | 34.96 ± 4.88 | 0.0001 |
| Minimum | 9.95 | 32.4 | 32.18 | 12.6 | 51.7 | 9.3 | 68.18 | 27.6 | ||||
| Maximum | 54.05 | 41.6 | 78.3 | 50.2 | 91.2 | 38 | 68.18 | 38.2 | ||||
| Number of papers | 8 | 3 | 8 | 14 | 3 | 3 | 1 | 2 | ||||
| Number of patients | 952 | 171 | 951 | 810 | 486 | 197 | 98 | 131 | ||||
| Number of fibroids | NPC | 204 | NPC | NPC | NPC | NPC | NPC | 192 | ||||
NPC, not possible to calculate
Eight UAE and three MRgHIFU treatment papers reported fibroid reductions after 3 months follow-up. The weighted mean difference in fibroid volume reductions between the two treatments after 3 months was not statistically significant (p = 0.068). The minimum and maximum percent fibroid volume shrinkage immediately following treatment ranged from 9.95 to 54.04% for UAE and 31.4–41.6% for MRgHIFU.
Twenty-two papers reported fibroid volume shrinkage after 6 months follow-up. These papers included eight papers following UAE treatment and 14 papers following MRgHIFU treatment. At the 6 month follow-up mark, the pooled percent fibroid volume shrinkage difference between UAE (50.57 ± 15.70%) and MRgHIFU (30.06 ± 12.76%) was statistically significant (p = 0.0001), with minimum and maximum fibroid volume shrinkages ranging from 32.18 to 78.3% for UAE, and 12.6–50.2% for MRgHIFU.
For 12 months follow-up, three papers reported fibroid volume changes for UAE and another three for MRgHIFU, showing a pooled statistically significant difference (p = 0.0001) between UAE (62.78 ± 17.10%) and MRgHIFU (25.91 ± 12.64%). The minimum and maximum percent fibroid volume reduction ranged from 51.7 to 91.2% for UAE and 9.3–38.0% for MRgHIFU. The minimum percent fibroid volume shrinkage at any followup measurement for any treatment was 9.3% for MRgHIFU.
Only one UAE treatment paper, with 98 patients, and two MRgHIFU treatment papers reported percent fibroid volume shrinkage after 24 months, showing a statistically significant difference (p = 0.0001) between pooled percent fibroid volume shrinkage between UAE (68.18%) and MRgHIFU (34.96%).
Seven papers reported complications for UAE and twelve papers for MRgHIFU. The most common reported complications were fever, pain, nausea, vomiting, anorexia, fatigue, abdominal distension, transient and permanent amenorrhea (Table 4).
Table 4.
Complications following UAE and MRgHIFU treatments
| # | Complications | UAE (# of complications/all patients, # of studies) | MRgHIFU (# of complications/all patients, %) | p value |
|---|---|---|---|---|
| 1 | Fever | 19/736, 1 study | 0/689 | < 0.0001 |
| 2 | Pain | 123/736, 4 studies | 168/689, 7 studies | 0.0004 |
| 3 | Transitory sciatic neuralgia | 0/736 | 2/689, 2 studies | 0.2336 |
| 4 | Numbness | 0/736 | 15/689, 2 studies | < 0.0001 |
| 5 | Nausea, vomiting | 56/736, 3 studies | 7/689, 2 studies | < 0.0001 |
| 6 | Anorexia | 35/736, 2 studies | 0/689 | < 0.0001 |
| 7 | Migraine | 1/736, 1 study | 0/689 | 1.0000 |
| 8 | Fatigue | 35/736, 2 studies | 0/689 | < 0.0001 |
| 9 | Discharged myoma debris | 2/736, 1 study | 0/689 | 0.5002 |
| 10 | Fibroid expulsion | 13/736, 4 studies | 0/689 | 0.0003 |
| 11 | Oligomenorrhea | 3/736,1 study | 0/689 | 0.2503 |
| 12 | Transient amenorrhea | 17/736,4 studies | 0/689 | < 0.0001 |
| 13 | Permanent amenorrhea | 8/736, 3 studies | 1/689, 1 study | 0.0394 |
| 14 | Bladder compression syndrome | 3/736, 2 studies | 1/689, 1 study | 0.6253 |
| 15 | Upper urine tract infection | 1/736, 1 study | 1/689,1 study | 1.0000 |
| 16 | Inguinal hematoma | 9/736, 3 studies | 0/689 | 0.0040 |
| 17 | Bilateral pulmonary embolism | 1/736, 1 study | 0/689 | 1.0000 |
| 18 | Abdominal distension | 34/736, 2 studies | 4/689, 1 study | < 0.0001 |
| 19 | Skin lesion | 0/736 | 61/689, 8 studies | < 0.0001 |
| 20 | Pruritic rash related to the procedure | 11/736, 2 studies | 0/689 | < 0.0010 |
| 21 | infection of the necrotic fibroid | 0/736 | 1/689, 1 study | 0.4835 |
Statistically significantly number of cases of complications of fever (p < 0.0001), anorexia (p < 0.0001), migraine (p = 1.0000), transient amenorrhea (p < 0.0001), fibroid expulsion (p = 0.0003), inguinal hematoma (p = 0.0040), fatigue (p < 0.0001) and pruritic rash (p < 0.0010) were only reported for UAE treatment, with no cases reported for MRgHIFU. Statistically significant numbers of cases of complications only reported for MRgHIFU included numbness (p < 0.0001) and skin lesions (p < 0.0001, with skin lesions defined as skin redness, edema or superficial skin burns. During management of patients, complications, such as permanent amenorrhea (p = 0.0394), and abdominal distention (p < 0.0001) were more common with patients treated with UAE. UAE-treated patients treated Patients following MRgHIFU treatment were more likely to report pain than UAE treated patients (p = 0.0004).
Discussion
Our systematic review analyzed and compared fibroid volume shrinkage and common complications following UAE and MRgHIFU procedures, two noninvasive options for the treatment of uterine leiomyoma. Effective fibroid volume shrinkage was reported following both treatments for all reported follow-up periods. Weighted mean percent fibroid reduction was higher for UAE than MRgHIFU in 6–24 months follow-up. Burn et al. 2000 reported the total disappearance of fibroids in one patient 6 months after UAE treatment. The percent fibroid volume shrinkage for UAE (68.18%) at 24 month follow-up was double that of MRgHIFU (34.96 ± 4.88%, p = 0.0001). However, Nagiub et al. [16] reported that 7 patients showed percent fibroid volume increase of 8.3% after one year of UAE treatment. Fibroid shrinkage after MRgHIFU was found to be higher than UAE after 3 months follow-up, but was not statistically significant (p = 0.068).
In addition, we found that for UAE the percent of fibroid volume shrinkage increased with follow-up time, almost doubling from 3 months follow-up (35.59%) to 24 months (68.18%). This may be explained by the gradual effect of UAE treatment, associated with progressive ischemia of the leiomyoma due to blockage of the uterine artery that supplies it. However, there is also a risk of fibroid regrowth due to collateral blood supply with ovarian arteries [38]. For MRgHIFU percent fibroid volume shrinkage was also substantial, between 25 and 40%, possibly explained by local targeting fibroid tissue with thermal ablation, consequently leading to cell death and fibroid shrinkage [39]. Further studies are needed to identify and compare the progression of fibroid shrinkage following UAE and MRgHIFU treatments.
We found that the numbers of patients complaining reporting nausea and vomiting, permanent amenorrhea and abdominal distension were greater for UAE than MRgHIFU. Whereas, the number of patients reporting pain was larger for MRgHIFU than UAE. Toor et al. 2012 reported that common complications for UAE include deep vein thrombosis, pulmonary embolism and permanent amenorrhea [40]. In our systematic review, we only found one reported UEA-treated case with bilateral pulmonary embolism (1 case/736 UEA treated patients) and none for MRgHIFU (0 cases/689 MRgHIFU treated patients). There were no reported cases with deep vein thrombosis. Commonly reported MRgHIFU complications found in our systematic review our such as skin lesion, numbness, were rare or non-existent among complications reported by UEA patients Sciatic neuralgia, and infections of the necrotic fibroid were rare or absent in our review for either MRgHIFU or UAE treated patients [41]. UAE patients reported more of the following complications than MRgHIFU patients: fever, nausea/vomiting, anorexia, fatigue, fibroid expulsion, transient and permanent amenorrhea, inguinal hematoma, abdominal distension, and pruritic rash; MRgHIFU reported more pain, numbness and skin lesions.
This is the first systematic review comparing with statistical testing the uterine fibroid volume shrinkage and procedural complications for the uterine leiomyoma noninvasive treatments UAE and MRgHIFU. Previously, Taheri et al. [7] conducted a systematic review analyzing papers with more than 20 patients with symptomatic leiomyomas after UAE, radiofrequency ablation, or ultrasound guided HIFU. They stratified the result on different follow-up times after 3, 6, 9, 12 and 26 months. This review found for fibroid shrinkage for UAE was found greater than UgHIFU after all follow-up times, but did not determine if differences found were statistically significant and did not exclude patients with prior treatments, other pelvic diseases or postmenopausal patients (which can contribute to confounding biases). The effectiveness of UAE might be reduced in postmenopausal women due to lower estrogen levels, given the estrogen-dependent pathogenesis of uterine fibroids [42]. Another previous systematic review and meta-analysis conducted by Liu et al. [43] also reported that having previous myomectomies before UAE treatments potential bias for their systematic review and meta-analysis. This systematic review and meta-analysis compared quality of life, re-intervention rate and incidence of adverse events following UAE and MRgHIFU as treatments for uterine leiomyoma. However, this study did not compare fibroid volume shrinkage between these treatments, and only seven papers were included for this analysis, in contrast to the 29 papers in our systematic review.
Our current systematic review compared, for the first time, fibroid volume shrinkage and complications, utilizing statistical testing, for the two non-invasive UAE and MRgHIFU treatments for uterine leiomyoma. There are several strengths and limitations of this study. To minimize bias, we excluded patients with previous treatments for leiomyoma, other pelvic diseases and postmenopausal women to reduce potential confounding bias. Furthermore, we included a total of 29 papers for both the qualitative and quantitative analyses. Fibroid volume shrinkage results were stratified and analyzed by different follow-up times. However, there are several limitations in this study. First, it included only studies published in English., Second, the study did not assess re-intervention rates, symptomatic improvements, quality of live, pregnancy and ovarian reserves, and cost differences between the two procedures. Third, all types of fibroids, including subserosal, submucosal, intramural and pedunculated were included in our systematic review. Further studies are needed to stratify fibroid leiomyoma nodules based on anatomic location to reduce confounding biases, especially because intramural fibroids may have higher percent fibroid volume shrinkage after UAE [42].
Conclusion
Comparing the effectiveness and safety of these two noninvasive treatments for uterine leiomyoma are essential for assuring best practices in clinical treatment of this condition. Our systematic review identified significant findings on differences between UAE and MRgHIFU by comparing fibroid volume shrinkages and post-procedural complications between treatments. The pooled weighted percent fibroid volume shrinkage for UAE treatment was statistically significantly greater than MRgHIFU at the 6, 12 and 24 months follow-up times, though both treatments showed substantial shrinkage However, UAE was more strongly statistically associated with procedural complications like fever, nausea and vomiting, anorexia, fatigue, fibroid expulsion, transient and permanent amenorrhea, inguinal hematoma, abdominal distension and pruritic rash than MRgHIFU. These findings should contribute to informing women and their physicians on making the best choice of treatment for their needs. Randomized controlled trials, are needed to further validate these findings.
Acknowledgements
We wanted to acknowledge NU for assistance and support as this systematic review is a part of the LSP granted to MY, and mentored by MT and GA.
Authors' contributions
Conceptualization: MT and GA; methodology: MT and BC; formal analysis: GA, MY; data curation: GA and MY; writing—original draft preparation: GA, MY; writing—review and editing: MT, BC, and GA; supervision: MT and GA; project administration: MT. All authors have read and approved the manuscript.
Funding
There is no funding to report.
Availability of data and material
All study materials are available pre request sent to the corresponding author via email: gulzanat.aimagambetova@nu.edu.kz.
Declarations
Ethics approval and consent to participate
Not applicable for systematic review.
Consent for publication
Not applicable for systematic review.
Competing interests
The authors have no competing interests to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR, Working Group of Society of Uterine Leiomyoma Current medical treatment of uterine fibroids. Obstet Gynecol Sci. 2018;61(2):192–201. doi: 10.5468/ogs.2018.61.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin-Tommaso SK. Non-surgical management of myomas. J Minim Invasive Gynecol. 2018;25(2):229–236. doi: 10.1016/j.jmig.2017.08.642. [DOI] [PubMed] [Google Scholar]
- 3.Vilos GA, Allaire C, Laberge PY, Leyland N. The management of uterine myomas. J Obstet Gynecol. 2015;37(2):157–178. doi: 10.1016/S1701-2163(15)30338-8. [DOI] [PubMed] [Google Scholar]
- 4.Zou M, Chen L, Wu C, Hu C, Xiong Y. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. Int J Obstet Gynecol. 2017;124(S3):30–35. doi: 10.1111/1471-0528.14742. [DOI] [PubMed] [Google Scholar]
- 5.Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin N Am. 2012;39(4):521–533. doi: 10.1016/j.ogc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Balat A, DeWilde RL, Schmeil I, Tahmasbi-Rad M, Bogdanyova S, Fathi A, Becker S. Modern myoma treatment in the last 20 years: a review of the literature. BioMed Res Int. 2018 doi: 10.1155/2018/4593875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taheri M, Galo L, Potts C, Sakhel K, Quinn SD. Nonresective treatments for uterine fibroids: a systematic review of uterine and fibroid volume reductions. Int J Hyperth. 2019;36(1):295–301. doi: 10.1080/02656736.2018. [DOI] [PubMed] [Google Scholar]
- 8.StataCorp LLC. Stata Statistical Software: Release 16. https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/#:~:text=2019.,Station%2CTX%3AStataCorpLLC.&text=StataCorp.-2017 (2019).
- 9.Burn PR, McCall JM, Chinn RJ, Vashisht A, Smith JR, Healy JC. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology. 2000;214(3):729–734. doi: 10.1148/radiology.214.3.r00fe07729. [DOI] [PubMed] [Google Scholar]
- 10.Roth AR, Spies JB, Walsh SM, Wood BJ, Gomez-Jorge J, Levy EB. Pain after uterine artery embolization for leiomyomata: can its severity be predicted and does severity predict outcome? J Vasc Interv Radiol. 2000;11(8):1047–1052. doi: 10.1016/s1051-0443(07)61337-2. [DOI] [PubMed] [Google Scholar]
- 11.Klein A, Schwartz M. Uterine artery embolization for the treatment of uterine fibroids: an outpatient procedure. Am J Obstet Gynecol. 2001;184(7):1556–1563. doi: 10.1067/mob.2001.114863. [DOI] [PubMed] [Google Scholar]
- 12.Zupi E, Pocek M, Dauri M, Marconi D, Sbracia M, Piccione E, Simonetti G. Selective uterine artery embolization in the management of uterine myomas. Fertil Steril. 2003;79(1):107–111. doi: 10.1016/S0015-0282(02)04399-6. [DOI] [PubMed] [Google Scholar]
- 13.Spies JB, Allison S, Flick P, McCullough M, Sterbis K, Cramp M, Bruno J, Jha R. Polyvinyl alcohol particles and tris-acryl gelatin microspheres for uterine artery embolization for leiomyomas: results of a randomized comparative study. J Vasc Interv Radiol. 2004;15(8):793–800. doi: 10.1097/01.RVI.0000136982.42548.5D. [DOI] [PubMed] [Google Scholar]
- 14.Harman M, ZeteroĞlu S, Arslan M, Şengül M, Etlik O. Predictive value of magnetic resonance imaging signal and contrast-enhancement characteristics on post-embolization volume reduction of uterine fibroids. Acta Radiol. 2006;47(4):427–435. doi: 10.1080/02841850600557117. [DOI] [PubMed] [Google Scholar]
- 15.Pisco JM, Bilhim T, Duarte M, Santos D. Management of uterine artery embolization for fibroids as an outpatient procedure. J Vasc Interv Radiol. 2009;20(6):730–735. doi: 10.1016/j.jvir.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Naguib NNN, Mbalisike E, Nour-Eldin NEA, Jost A, Lehnert T, Ackermann H, Vogl TJ. Leiomyoma volume changes at follow-up after uterine artery embolization: correlation with the initial leiomyoma volume and location. J Vasc Interv Radiol. 2010;21(4):490–495. doi: 10.1016/j.jvir.2009.12.388. [DOI] [PubMed] [Google Scholar]
- 17.Stampfl U, Radeleff B, Sommer C, Stampfl S, Dahlke A, Bellemann N, Kauczor HU, Richter GM. Midterm results of uterine artery embolization using narrow-size calibrated embozene microspheres. Cardiovasc Interv Radiol. 2011;34:295–305. doi: 10.1007/s00270-010-9986-8. [DOI] [PubMed] [Google Scholar]
- 18.Bilhim T, Pisco JM, Duarte M, Oliveira AG. Polyvinyl alcohol particle size for uterine artery embolization: a prospective randomized study of initial use of 350–500 μm particles versus initial use of 500–700 μm particles. J Vasc Interv Radiol. 2011;22(1):21–27. doi: 10.1016/j.jvir.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Redecha M, Mižičková M, Javorka V, Redecha M, Kurimská S, Holomáň K. Pregnancy after uterine artery embolization for the treatment of myomas: a case series. Arch Gynecol Obstet. 2013;287(1):71–76. doi: 10.1007/s00404-012-2512-2. [DOI] [PubMed] [Google Scholar]
- 20.Song YG, Jang H, Park KD, Kim MD, Kim CW. Non spherical polyvinyl alcohol versus gelatin sponge particles for uterine artery embolization for symptomatic fibroids. Minim Invasive Ther Allied Technol. 2013;22(6):364–371. doi: 10.3109/13645706.2013.826674. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JK, Han K, Kim MD, Kim GM, Kwon JH, Won JY, Lee DY. Five-year clinical outcomes of uterine artery embolization for symptomatic leiomyomas: an analysis of risk factors for reintervention. Eur J Radiol. 2018;109:83–87. doi: 10.1016/j.ejrad.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Ukybassova T, Terzic M, Dotlic J, Imankulova B, Terzic S, Shauyen F, Garzon S, Guo L, Sui L. Evaluation of uterine artery embolization on myoma shrinkage: results from a large cohort analysis. Gynecol Minim Invasive Ther. 2019;8(4):165–171. doi: 10.4103/GMIT.GMIT_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynnen K, Macdanold N, Inbar Y, Itzchak Y, Rabinovici J, Kim K, Geschwind JF, Hesley G, Gostout B, Ehrenstein T, Hengst S, Sklair-Levy M, Shushan A, Jolesz F. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. Am J Roentgenol. 2004;183(6):1713–1719. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 24.Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: early clinical experience. Radiat Med. 2008;26(4):198–205. doi: 10.1007/s11604-007-0215-6. [DOI] [PubMed] [Google Scholar]
- 25.Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids–early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):199–203. doi: 10.1016/j.ejogrb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovici J, Inbar Y, Revel A, Zalel Y, Gomori JM, Itzchak Y, Schiff E, Yagel S. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30(5):771–777. doi: 10.1002/uog.4099. [DOI] [PubMed] [Google Scholar]
- 27.Lénárd ZM, McDannold NJ, Fennessy FM, Stewart EA, Jolesz FA, Hynynen K, Tempany CM. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-imaging predictors of success 1. Radiology. 2008;249(1):187–194. doi: 10.1148/radiol.2491071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Chen WZ, Liu YJ, Hu X, Zhou K, Chen L, Peng S, Zhu H, Zou HL, Bai J, Wang ZB. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73(2):396–403. doi: 10.1016/j.ejrad.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Baik JH, Pham LD, Jacobs MA. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol. 2011;18(8):970–976. doi: 10.1016/j.acra.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhnke H, Eckey T, Bohlmann MK, Beldoch MP, Neumann A, Agic A, Hägele J, Diedrich K, Barkhausen J, Hunold P. MR-guided HIFU treatment of symptomatic uterine fibroids using novel feedback-regulated volumetric ablation: effectiveness and clinical practice. Rofo. 2013;184(10):983–991. doi: 10.1055/s-0033-1335289. [DOI] [PubMed] [Google Scholar]
- 32.Thiburce AC, Frulio N, Hocquelet A, Maire F, Salut C, Balageas P, Bouzgarrou M, Hocké C, Trillaud H. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperther. 2015;31(7):764–770. doi: 10.3109/02656736.2015.1063169. [DOI] [PubMed] [Google Scholar]
- 33.Tung SL, Chou TY, Tseng HS, Lee CM. A retrospective study of magnetic resonance-guided focused ultrasound ablation for uterine myoma in Taiwan. Taiwan J Obstet Gynecol. 2016;55(5):646–649. doi: 10.1016/j.tjog.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Fu Z, Yang L, Huang Z, Chen WZ, Wang Z. Feasibility, safety, and efficacy of accurate uterine fibroid ablation using magnetic resonance imaging-guided high-intensity focused ultrasound with shot sonication. J Ultrasound Med. 2015;34(12):2293–2303. doi: 10.7863/ultra.14.12080. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Keserci B, Bi H, Han X, Wang X, Bai W, Wang Y, Yang X, Yang J, Wei J, Seppälä M, Viitala A, Liao Q. The safety and effectiveness of volumetric magnetic resonance-guided high-intensity focused ultrasound treatment of symptomatic uterine fibroids: early clinical experience in China. J Therap Ultrasound. 2016;4(1):1–9. doi: 10.1186/s40349-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacoby VL, Kohi MP, Poder L, Jacoby A, Lager J, Schembri M, Rieke V, Grady D, Vittinghoff E, Coakley FV. PROMISe trial: a pilot, randomized, placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2016;105(3):773–780. doi: 10.1016/j.fertnstert.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Keserci B, Duc NM. Magnetic resonance imaging parameters in predicting the treatment outcome of high-intensity focused ultrasound ablation of uterine fibroids with an immediate nonperfused volume ratio of at least 90. Acad Radiol. 2018;25(10):1257–1269. doi: 10.1016/j.acra.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Abbara S, Nikolic B, Pelage J, Banovac F, Spies J. Frequency and extent of uterine perfusion via ovarian arteries observed during uterine artery embolization for leiomyomas. Women’s Imaging. 2007;188:1558–1563. doi: 10.2214/AJR.05.1383. [DOI] [PubMed] [Google Scholar]
- 39.Rueff LE, Raman SS. Clinical and technical aspects of MR-guided high intensity focused ultrasound for treatment of symptomatic uterine fibroids. Semin Interv Radiol. 2013;30(4):347–353. doi: 10.1055/s-0033-1359728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toor S, Jaberi A, Macdonald B, McInnes M, Schweitzer M, Pasteur Rasuli P. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. Am J Roentgenol. 2012;199:1153–1163. doi: 10.2214/AJR.11.8362. [DOI] [PubMed] [Google Scholar]
- 41.Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) Treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(4):1–86. [PMC free article] [PubMed] [Google Scholar]
- 42.Czuczwar P, Woźniak S, Szkodziak P, Woźniakowska E, Paszkowski M, Wrona W, Milart P, Paszkowski T, Popajewski M. Predicting the results of uterine artery embolization: correlation between initial intramural fibroid volume and percentage volume decrease. Menopause Rev. 2014;13(4):247–252. doi: 10.5114/pm.2014.45001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Wang T, Lei B. Uterine artery embolization compared with high-intensity focused ultrasound ablation for the treatment of symptomatic uterine myomas: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(2):218–227. doi: 10.1016/j.jmig.2020.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study materials are available pre request sent to the corresponding author via email: gulzanat.aimagambetova@nu.edu.kz.