Abstract
Background
This study examines the effect of prognostic patient and disease characteristics on colorectal cancer (CRC) recurrence after curative resection. We used competing risk analysis with death as a competing risk. This method provides the clinician a perspective into a patient’s actual risk of experiencing a recurrence.
Methods
A retrospective cohort study of patients diagnosed with CRC who underwent curative resection for CRC from 2003–2007 at the Royal University Hospital in Saskatoon was completed. The outcome of interest was the first CRC recurrence, either local or distant metastasis. Demographic data, tumor characteristics, adjuvant treatment and follow-up data, date of local recurrence or metastasis were recorded from the medical record. Univariate analysis was completed to look at the relationship between each of the prognostic indicators and recurrence. Multivariable modelling (subdistribution regression modelling) was done to identify the main risk factors in determining recurrence.
Results
Of 148 patients, 38 (25.7%) experienced a recurrence, 16 (10.8%) died without evidence of recurrence, and 94 (63.5%) experienced neither outcome. The median follow-up was 30.5 months (interquartile range 10.6–50). In univariable subdistribution regression, T-stage, N-stage, vascular invasion and positive margins were all predictive of cancer recurrence, with p ≤ 0.001, with subdistribution hazard ratios for T4 stage at 11.93, T3 stage at 2.46, N2 stage at 10.58, and presence of vascular invasion at 4.27. N-stage remained as the sole predictor in multivariable regression. Cumulative incidence function (CIF) of recurrence at 48 months after surgery was 15%, 27% and 90% for N1/2, N3 and N4 respectively.
Conclusion
The highest CIF of recurrence was associated with T4 stage, N2 stage, and vascular invasion. Patient’s age, tumour location, type, or histological grade were not found to have a significant effect on the success of CRC surgery in precluding a recurrence.
Keywords: Competing risk, Cumulative incidence function, Colorectal cancer, Recurrence
Background
Every year an estimated 1.4 million people worldwide are diagnosed with colorectal carcinoma (CRC) [1]. In North America, CRC is the second most common cause of cancer-related death that affects both men and women. Canada is among the countries with the highest incidence of CRC [2]. The current primary treatment for CRC is surgical resection with the intent to cure [3]. In a cohort of CRC patients with stage 1—4 disease, locoregional recurrence or metastasis was demonstrated in 26.6% of patients [4]. CRC recurrence was detected in 16.6% of patients with stage 1—3 disease, after a mean of 4.4 years, in a randomized trial comparing various degrees of follow-up [5]. In another study, 30% of CRC patients with stage 1—3 disease developed recurrence following surgery for curative intent [6].
The objectives of our study were to describe the recurrence rates of CRC after surgical resection and to determine which patient level and disease level characteristics were associated with an increased risk of recurrence in follow-up. A CRC recurrence, either local or a distant metastasis, was considered a failure to cure. The traditional Kaplan–Meier survival function results in an overestimate of the absolute likelihood of a recurrence in the presence of deaths prior to recurrence. Therefore, competing risk analysis was used in this study because it provides the clinician a perspective into a patient’s actual risk of experiencing a recurrence. The cumulative incidence function (CIF) was used to estimate the probability of a recurrence over time, treating death as a competing risk. The effects of demographic and disease characteristics as covariates on the CIF was modelled with Fine-Gray subdistribution (SD) regression.
Methods
This is a retrospective study of patients diagnosed with CRC who subsequently underwent curative resection from 2003–2007. A total of 226 medical charts from the Royal University Hospital in Saskatoon, Canada were reviewed. Patients who received pre-operative chemotherapy or radiation or demonstrated pre-operative evidence of metastatic disease were excluded. Demographics, disease characteristics, surgical approach, and adjuvant treatment were recorded. Pathology reports from the initial surgical resection were reviewed for tumor site, tumor type, histological grade of malignancy, status of margins, TNM staging, presence of vascular and perineural invasion, and degree of lymphocytic reaction. Follow-up data included time to local recurrence or metastasis, time of most recent follow-up visit, and date and cause of death. Patients not followed-up at the hospital were compared to those with follow-up, but excluded from further analysis. This study was approved by the research ethics board of the University of Saskatchewan. Informed consent was not obtained in this retrospective chart review.
Descriptive statistics were tabulated from data collected for 192 patients meeting the inclusion criteria. Characteristics of the 148 patients with follow-up and the 44 patients without were tabulated. The mean (standard deviation) and median (intra-quartile range, IQR) are presented for numeric variables, and frequency (percentage) for categorical variables. Differences between the two groups were tested with t-tests for continuous variables, and Chi-square test, or Fisher’s exact test in presence of cell sizes of 5 or fewer, for categorical variables. Further analysis was restricted to patients for whom follow-up data was available.
The outcome of interest was recurrence of CRC, either local or metastasis, with death prior to recurrence as a competing risk. Censoring time was set as most recent follow-up date. CIF curves of recurrence by each level of prognostic covariates were estimated, charted, and visually inspected. The non-parametric Gray test was employed to test for differences between pairs of CIFs [7].
The effect of covariates on the CIF was modelled using Fine-Gray SD regression [8]. SD regression allows us to estimate the relative effects of patient and disease characteristics as covariates on the risk of recurrence, while taking risk of death into account. Modeling the effect of covariates on the CIF allows one to estimate the effect of covariates on the absolute risk of the outcome over time. Subdistribution hazard ratios (SHR) obtained from the Fine‐Gray model describe the relative effect of covariates on the CIF [9]. Univariable modelling was conducted. The Wald test assessed covariate significance and SHRs with 95% CI were generated. Multivariable modelling was done using forward selection, with Akaike information criterion (AIC) and Bayesian information criterion (BIC) [10]. Plots of Schoenfeld residuals were used to evaluate the proportional hazard subdistribution assumption.
In regression analysis age was centered at 60 and divided by 10, resulting in an increase of one in the SHR for every decade over 60 years of age. Cases with missing values for covariates were excluded from any analysis involving the covariate. All testing used the traditional significance level of α = 0.05. All statistical analysis was done using the R software package, version 4.04 [11]. CIF analysis was done with package cmprsk2 version 0.0.0.9003 [12], stepwise SD regression with package crrstep version 2015–2.1 [13].
Results
Case selection
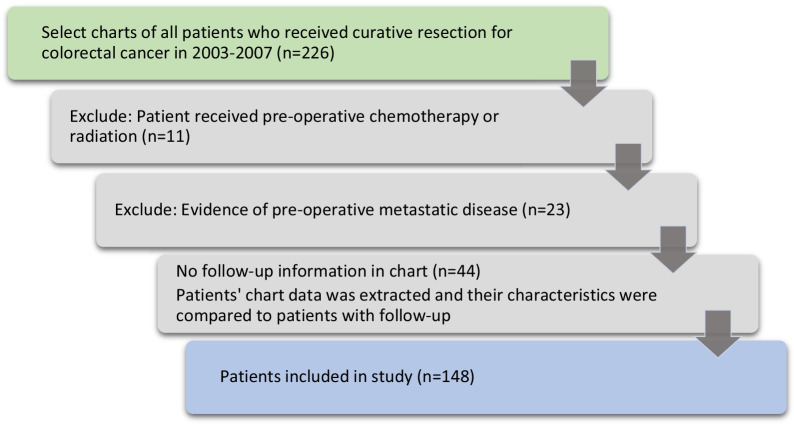
The selection process for medical charts is shown in Fig. 1. Out of 226 patient charts, 11 were excluded for pre-operative chemotherapy or radiation, and 23 for known metastatic disease prior to surgery. Of the remaining 192 charts, 44 patients lacked follow-up information. Table 1 compares characteristics of patients without follow-up to the 148 patients with follow-up visits. Disease characteristics were similar for both groups; site (p = 0.559), tumour type (p = 0.125), histology (p = 1), T-stage (p = 0.5), N-stage (p = 0.932), M-stage (p = 1), vascular invasion (p = 1), and perineural invasion (p = 0.929), as were measures of surgical quality positive margins (p = 1) and nodes examined (p = 0.319). Patients without follow-up had surgery in the earlier years of the study (p = 0.005), and were older 75.7 ± 11.1 years v. 69.2 ± 11.4 years (p = 0.001). Post-operative chemotherapy or radiation was recorded for 9.1% v. 31.1% (p = 0.003).
Fig. 1.
Selection of patient charts for inclusion in study
Table 1.
Patient, disease and treatment characteristics for 148 patients in the study and 44 patients excluded due to unavailability of follow-up
| Variable | Follow-up available | Follow-up unavailable | Follow-up vs none |
|---|---|---|---|
| No. (%) | No. (%) | p value1 | |
| All patients | 148 (100) | 44 (100) | |
| Age (years) mean ± SD | 69.2 ± 11.4 | 75.7 ± 11.1 | 0.001 |
| Median (IQR) | 71 (62, 78) | 78.5 (70, 84.2) | |
| Male | 78 (52.7) | 27 (61.4) | 0.401 |
| Year of surgery | |||
| 2003 | 27 (18.2) | 6 (13.6) | 0.005 |
| 2004 | 39 (26.4) | 9 (20.5) | |
| 2005 | 28 (18.9) | 18 (40.9) | |
| 2006 | 27 (18.2) | 10 (22.7) | |
| 2007 | 27 (18.2) | 1 (2.3) | |
| Tumour site | |||
| Colon | 59 (39.9) | 17 (39.5) | 0.559 |
| Rectum | 55 (37.2) | 13 (30.2) | |
| Sigmoid/rectosigmoid | 34 (23) | 13 (30.2) | |
| Tumour type | |||
| Adenocarcinoma NOS | 132 (89.2) | 43 (97.7) | 0.125 |
| Mucinous adenocarcinoma | 15 (1.1) | 1 (2.3) | |
| Carcinoid | 1 (.7) | 0 | |
| Histological grade | |||
| Low/well differentiated | 52 (35.2) | 15 (34.1) | 1 |
| Moderate | 78 (52.7) | 24 (54.5) | |
| High/poorly differentiated | 16 (1.8) | 5 (11.4) | |
| T-stage | |||
| T0 (in situ) | 0 | 1 (2.3) | 0.5 |
| T1 | 13 (8.8) | 2 (4.5) | |
| T2 | 35 (23.8) | 11 (25) | |
| T3 | 86 (58.5) | 26 (59.1) | |
| T4 | 13 (8.8) | 4 (9.1) | |
| N-stage | |||
| N0 | 92 (62.6) | 28 (63.6) | 0.932 |
| N1 | 35 (23.8) | 11 (25) | |
| N2 | 20 (13.6) | 5 (11.4) | |
| M-stage M1 | 3 (2) | 0 | 1 |
| Vascular invasion | 39 (27.3) | 12 (27.3) | 1 |
| Perineural invasion | 40 (27.8) | 11 (25.6) | 0.929 |
| Positive margins | 15 (10.3) | 4 (9.1) | 1 |
| Nodes examined | |||
| Mean ± SD | 15.4 ± 7.4 | 14.3 ± 5.7 | 0.319 |
| Median (IQR) | 13 (11, 19) | 14 (12, 17) | |
| Post-operative chemotherapy and/or radiation | 46 (31.1) | 4 (9.1) | 0.003 |
1 T-test for continuous variables, Pearson’s χ2 test or Fisher’s exact test for categorical variables
These 44 patients were excluded from further analysis.
Patient and disease characteristics
Table 1 describes the 148 patients in the study. Their mean age was 69.2 ± 11.4 years, with 52.5% being male. The majority of tumours, 89.2%, were adenocarcinoma type. Tumour location was colon 39.9%, rectum 37.2%, sigmoid 19.6% and rectosigmoid 3.4%. Most exhibited low grade 35.2% or moderate 52.7% histology with only 1.8% high grade. Tumour staging was T1 8.8%, T2 23.8%, T3 58.5% and T4 8.8%. Node staging showed N0 62.6%, N1 23.8% and N2 13.6%. Metastasis was discovered in 3 (2%) patients, all cases located in the omentum. Vascular invasion was noted in 27.3% and perineural invasion in 27.8%. Positive margins were realised in 10.3% of patients, and the mean number of nodes examined was 15.4 ± 7.4. Post-operative chemotherapy was given to 17.6% of patients, radiation to 2%, and both to 11.5%.
Outcomes
Table 2 shows time from curative surgery to the first CRC recurrence, or end of follow-up. CRC recurrence was diagnosed in 38 (25.7%) patients; 7 local, 26 metastasis, and 5 both. Median (IQR) time of recurrence was 19.7 (8.7, 28.2) months. Sixteen (10.8%) patients died with no indication of recurrence. Twelve deaths occurred prior to discharge from hospital, and four under follow-up. Ninety-four patients were disease-free at time of their last follow-up, with median (IQR) follow-up of 44.8 (20.9, 56.6) months.
Table 2.
Time from curative surgery to first of CRC recurrence, death or end of follow-up
| N | Time to event1 | ||
|---|---|---|---|
| Median (IQR) | Minimum–maximum | ||
| All patients | 148 | 30.5 (10.6, 50) | 0–111 |
| Diagnosed with CRC recurrence | 38 | 19.7, (8.7, 28.2) | 1.4–87.1 |
| Local recurrence | 7 | ||
| Local recurrence & metastasis | 5 | ||
| Metastasis | 26 | ||
| Died with no evidence of recurrence | 16 | ||
| Prior to hospital discharge | 12 | 7.5 (3.2, 22.2) | 0–39 |
| After discharge from hospital | 4 | 16.7 (11.7, 39.7) | 9.4–61.7 |
| Alive with no evidence of recurrence | 94 | 44.8 (20.9, 56.6) | 0.4–111 |
1All times shown in months, except for death prior to discharge from curative surgery, shown in days
A total of 12 patients died prior to hospital discharge or shortly after discharge. These patients tended to be older, mean (standard deviation) age 77.9 (9.7) vs 68.5 (11.3), t-test p = 0.006, with tumours in the colon, 75% vs 50%, chi-square test p = 0.034. Cause of death was documented in four charts: one hypoxia, two from myocardial infarction, and respiratory failure for a patient with chronic obstructive pulmonary disease. Four patients died later without any indication of recurrence. Causes of death were myocardial infarction, stroke, lymphoma, and other causes.
Figure 2 provides a graphic display of CIF for recurrence and its competing risk of death. CIF for death rises quickly at first and then stabilizes, while recurrence shows a continuous, steadily rise. At 12, 24, 36 and 48 months after surgery CIF for death is 0.09, 0.1, 0.1, 0.1, compared to 0.09, 0.18, 0.25, 0.3 for recurrence.
Fig. 2.
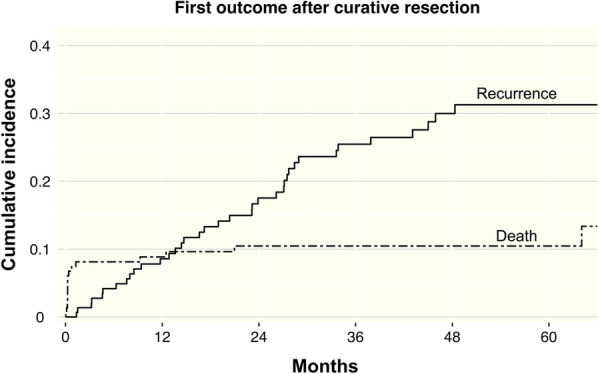
Cumulative incidence of first outcome after curative resection
CIF by patient and disease characteristics
The CIF of recurrence by patient and disease characteristics is shown in Table 3. Estimates are given at 12, 24, 36 and 48 months following curative surgery. Highest recurrence at 48 months is seen at N-stage N2 (0.90), T-stage T4 (0.71), positive margins (0.69), vascular invasion (0.64), and adjuvant chemotherapy or radiation (0.60).
Table 3.
Cumulative incidence of local/distant metastasis by potential risk factors for patients with colorectal cancer after resection surgery
| Variable | Category | Cumulative incidence (months) | |||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | ||
| All patients | 0.09 | 0.18 | 0.25 | 0.30 | |
| Age | < 60 | 0.11 | 0.23 | 0.31 | 0.37 |
| 60–74 | 0.08 | 0.16 | 0.24 | 0.31 | |
| ≥ 75 | 0.08 | 0.16 | 0.24 | 0.24 | |
| Sex | Male | 0.03 | 0.15 | 0.23 | 0.27 |
| Female | 0.16 | 0.21 | 0.29 | 0.33 | |
| Tumour location | Colon | 0.15 | 0.17 | 0.23 | 0.23 |
| Sigmoid/rectosigmoid | 0.09 | 0.29 | 0.32 | 0.39 | |
| Rectum | 0.02 | 0.10 | 0.24 | 0.31 | |
| Tumor type | Adenocarcinoma | 0.06 | 0.16 | 0.23 | 0.28 |
| Mucinous adenocarcinoma | 0.32 | 0.32 | 0.48 | 0.48 | |
| Histological grade | Low/well differentiated | 0.04 | 0.16 | 0.22 | 0.28 |
| Moderate | 0.11 | 0.20 | 0.26 | 0.30 | |
| High/poorly differentiated | 0.07 | 0.07 | 0.29 | 0.29 | |
| T stage (depth) | T1/ T2 | 0.00 | 0.07 | 0.10 | 0.14 |
| T3 | 0.07 | 0.16 | 0.28 | 0.33 | |
| T4 | 0.52 | 0.71 | 0.71 | 0.71 | |
| N stage (nodes) | N0 | 0.03 | 0.10 | 0.13 | 0.15 |
| N1 | 0.06 | 0.16 | 0.27 | 0.27 | |
| N2 | 0.35 | 0.52 | 0.73 | 0.90 | |
| Vascular invasion | Absent | 0.04 | 0.13 | 0.16 | 0.16 |
| Present/suspicious | 0.22 | 0.28 | 0.51 | 0.64 | |
| Perineural invasion | Absent | 0.07 | 0.14 | 0.22 | 0.25 |
| Present | 0.13 | 0.28 | 0.37 | 0.44 | |
| Positive margins | Uninvolved | 0.06 | 0.15 | 0.22 | 0.26 |
| One positive margin | 0.36 | 0.43 | 0.58 | 0.69 | |
| Nodes examined | < 12 | 0.08 | 0.17 | 0.20 | 0.24 |
| ≥ 12 | 0.09 | 0.18 | 0.27 | 0.31 | |
| Post-operative adjuvant therapy | None | 0.08 | 0.14 | 0.14 | 0.16 |
| Chemotherapy or radiation | 0.09 | 0.25 | 0.49 | 0.60 | |
Table 4 shows results of testing for differences between pairs of CIFs using the Gray test. Statistically significant differences were found between N-stage N2 vs N0 (p < 0.001) and N1 (p < 0.001), among the 3 groupings of T-stage; T1/ T2 vs T3 (p = 0.038), T1/ T2 vs T4 (p < 0.001), T3 vs T4 (p < 0.001), between presence vs absence of vascular invasion (p < 0.001) and perineural invasion (p = 0.001), as well as adjuvant therapy vs no further treatment (p < 0.001).
Table 4.
Gray test between pairs of cumulative incidence functions for recurrence
| Variable | Groups compared | Gray test p value |
|---|---|---|
| Age (years) | < 60 vs 60–74 | 0.858 |
| < 60 vs ≥ 75 | 0.344 | |
| 60–74 vs ≥ 75 | 0.463 | |
| Sex | Male vs female | 0.247 |
| Tumour location | Colon vs sigmoid/rectosigmoid | 0.199 |
| Colon vs rectum | 0.796 | |
| Sigmoid/rectosigmoid vs rectum | 0.142 | |
| Tumor type | Adenocarcinoma vs mucinous | 0.118 |
| Histological grade | Low/well differentiated vs moderate | 0.477 |
| Low/well differentiated vs high/poorly differentiated | 0.933 | |
| Moderate vs high/poorly differentiated | 0.793 | |
| T stage (depth) | T1/ T2 vs T3 | 0.038 |
| T1/ T2 vs T4 | < 0.001 | |
| T3 vs T4 | < 0.001 | |
| N stage (nodes) | N0 vs N1 | 0.119 |
| N0 vs N2 | < 0.001 | |
| N1 vs N2 | < 0.001 | |
| Vascular invasion | Absent vs present/suspicious | < 0.001 |
| Perineural invasion | Absent vs present | 0.076 |
| Positive margins | Uninvolved vs positive margin | 0.001 |
| Nodes examined | < 12 vs ≥ 12 | 0.852 |
| Post-operative adjuvant therapy | None vs chemotherapy and/or radiation | < 0.001 |
Modelling risk of recurrence
The Fine and Gray’s SD regression analysis was employed to model the hazard that corresponds to the CIF. The SHR for recurrence generated by the models are shown in Table 5, along with Wald test results indicating each covariate’s statistical significance. The SHR is the relative risk for a categorical covariate, defined as the ratio of subdistribution hazards for the actual group with respect to the baseline, with all other covariates being equal. If the covariate is continuous then the relative risk refers to the effect of a one unit increase in the covariate, with all other covariates being equal. In univariable modelling, SHR (95% CI) of T-stage T4 was 11.93 (3.74, 37.99), while T3 was 2.46 (1.04, 5.81) with reference T-stage T1/T2. The N-stage N2 SHR was 10.58 (5.17, 21.65) with reference to N0. Presence of vascular invasion was 4.27 (2.22, 8.21) relative to its absence, while SHR for positive margins was 3.63 (1.68, 7.84). The SHR for patients treated with adjunct chemotherapy or radiation was 3.85 (1.99, 7.46).
Table 5.
Univariable subdistribution regression for recurrence under the competing risk of death
| Variable | SHR1 (95% CI) | Wald test p value2 |
|---|---|---|
| Age, per 10-yr increase from baseline 60 years | 0.84 (0.63, 1.12) | 0.23 |
| Female | 1.45 (0.77, 2.71) | 0.249 |
| Rectum (v. colon) | 1.17 (0.52, 2.62) | 0.138 |
| Sigmoid/rectosigmoid (v. colon) | 2.01(0.94, 5.56) | – |
| Mucinous (v. adenocarcinoma) | 2.03 (0.81, 5.12) | 0.132 |
| Moderate grade (v. low grade) | 1.31 (0.636, 2.7) | 0.757 |
| High grade (v. low grade) | 1.12 (0.81, 5.12) | – |
| T3 stage v. T1/T2 stage | 2.46 (1.04, 5.81) | < 0.001 |
| T4 stage v. T1/T2 stage | 11.93 (3.74, 37.99) | – |
| N1 stage v. N0 stage | 2.02 (0.85, 4.8) | < 0.001 |
| N2 stage v. N0 stage | 10.58 (5.17, 21.65) | – |
| Vascular invasion | 4.27 (2.22, 8.21) | < 0.001 |
| Perineural invasion | 1.82 (0.95, 3.49) | 0.069 |
| Positive margins | 3.63 (1.68, 7.84) | 0.001 |
| Nodes examined (> 12) | 1.07 (0.53, 2.17) | 0.854 |
| Post-operative chemotherapy or radiation | 3.85 (1.99, 7.46) | < 0.001 |
1Subdistribution hazard ratio
2For the overall covariate
The multivariable model was fit in three stages, using the forward selection method at each stage. All a-priori determined co-variates were included at the start. The data fit was assessed with AIC, BIC, and Schoenfeld residuals. Co-variables were dropped if they did not improve model fit until the model with the best fit with the least number of co-variates remained. Figure 3 shows the CIF for recurrence by each level of N stage.
Fig. 3.
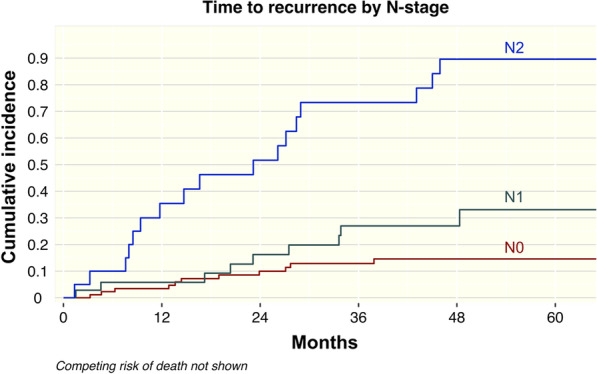
Cumulative incidence of time to recurrence by N-stage
Discussion
A total of 44 patients in the study were lost to follow-up, which is one of the limitations of the study. Most patients were not followed after discharge due to advanced age, as 19 patients were ≥ 80 years old, with a mean age of 75.7 versus 69.2. A few of the others were lost to follow-up as they were repatriated back to their own geographic region. Disease characteristics of patients without follow-up showed no marked differences from patients under follow-up. Excluding these patients is not likely to introduce noteworthy bias to the study results. CRC local recurrence (n = 7), metastatic disease (n = 26), or both (n = 5) was found in a total of 38 patients (25.7%). The median time to recurrence was found to be 19.7 months and the median follow-up time in our study was 44.8 months, a time in which most recurrences occur. Sargent et al. demonstrated in a pooled analysis of patients with colon cancer that 80% of patients who experienced a recurrence were within the first 3 years [14].
The depth of tumor invasion along with the presence and degree of lymph node metastasis and vascular invasion have long been regarded as standard prognostic factors in CRC [15]. Some of these pathological features have defined our current widely accepted American Joint Committee on Cancer (AJCC) staging system for CRC. Our study examined the CIF of CRC recurrence, local or metastatic, by patient and disease characteristics. The factors showing highest CIF of recurrence at 48 months were N2 and T4 disease, positive margins, vascular invasion, and adjuvant chemotherapy or radiation. Statistically significant differences were found between N2 vs N0 and N1, and between T stage groupings: T1/T2 vs T3, T1/T2 vs T4, and T3 vs T4, as well as the presence of vascular invasion, perineural invasion, and adjuvant therapy. Numerous studies have demonstrated through multivariate analysis that N stage is a significant independent prognostic factor in CRC-related mortality [16]. T stage also has a clear impact on overall survival and found to be an independent prognostic factor in CRC [16–19]. Ueberrueck et al. found tumor invasion beyond the muscularis propria resulted in a substantial reduction in 10-year survival [16]. While the data in our study is not novel, it confirms these factors remain at the forefront of disease prognostication.
The results of this study present statistical data to corroborate what has become increasingly clinically evident within the field of colorectal oncology in recent years. Namely, that the traditional AJCC staging system, intended to help locate individual patients within a progressive outcome prediction scale, tends to inappropriately emphasize certain risk factors for recurrence over others. For instance, any N1 status results in an AJCC stage 3a or 3b designation, depending of T-stage, whereas a T4 status, in the absence of node positive and metastatic disease, earns a maximum designation of stage 2b. This would seem to indicate that N1 status is a stronger predictor of recurrence and poor outcome than T4 status. However, treating clinicians have increasingly recognized that outcomes for T4 colorectal adenocarcinoma patients (AJCC stage 2b) are markedly worse than for T1-2N1 patients (AJCC stage 3a), as these tumors recur more frequently, both locally or distantly. The present study poignantly highlights this fact by demonstrating that T4 tumors carry the highest hazard ratio (11.93) for recurrence of all the validated risk factors, including N stage, a fact that is not consistently identified in the literature. This emphasizes the importance of advanced T stage in clinical practice when considering the benefit of adjuvant therapy for risk reduction after curative intent surgery.
Our study found vascular invasion to be one of the factors with the highest CIF of recurrence. This is consistent with other studies that have shown vascular invasion to be an important prognostic factor in survival outcomes for CRC patients [18–21]. Courtney et al. found vascular invasion to be a prognostic factor independent of T or N stage, resulting in a decreased 5-year survival in CRC patients [22]. A study by Tsai et al. examined pathological features affecting disease recurrence and overall survival following curative resection in T2-4N0M0 CRC and found only T stage and vascular invasion were significant independent prognostic factors for relapse using Cox proportional hazards analysis [23]. There are mixed reports in regards to lymphatic invasion as a significant risk factor in subsequent disease recurrence and survival. The presence of lymphatic invasion has been correlated to poorer survival time [17] and a significant prognostic factor in CRC [20, 24], whereas other studies have not found as strong a prognostic association [21]. Numerous studies have found that lymphatic invasion correlates well with lymph node status, and therefore an important predictor of disease survival [18, 24, 25]. Perineural invasion has been found to be a significant prognostic factor in both univariate [23] and multivariate analyses with poorer survival rates in patients with the presence of perineural invasion [26].
While advancement in local and systemic therapy for CRC have been made in recent years since data acquisition for the present study (i.e. radiotherapy for rectal cancer, the addition of oxaliplatin for Stage III adjuvant therapy, immunotherapy for Stage IV disease, total neoadjuvant therapy, etc.), the recurrence risks for adenocarcinoma remain consistent, a fact this paper brings to light when compared with more contemporary data sets. The data from this cohort serves as a benchmark against which more modern results can be compared. Clearly, further research is needed to identify and refine understanding of risk factors which most significantly influence CRC patient outcomes (i.e. ctDNA).
Acknowledgements
Not applicable.
Abbreviations
- CRC
Colorectal cancer
- CIF
Cumulative incidence function
- SD
Subdistribution
- IQR
Intra-quartile range
- SHR
Subdistribution hazard ratio
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
- AJCC
American Joint Committee on Cancer
Authors' contributions
Authors AES and FC contributed to the study conception and design. Data collection was performed by AES. Data analysis was performed by VM. The first draft of the manuscript as written by AES and VM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the research ethics board of the University of Saskatchewan. Informed consent was not obtained in this retrospective chart review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed]
- 2.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Carey M, Pollett W, Green J, Dicks E, Parfrey P, Yilmaz YE, Savas S. The long-term survival characteristics of a cohort of colorectal cancer patients and baseline variables associated with survival outcomes with or without time-varying effects. BMC Med. 2019 doi: 10.1186/s12916-019-1379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;331(3):263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 6.van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer. Nature Reviews Clinical Oncology 2017;14:297–315. 10.1038/nrclinonc.2016.199 [DOI] [PubMed]
- 7.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 9.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuk D, Varadhan R. Model selection in competing risks regression. Stat Med. 2013;32(18):3077–3088. doi: 10.1002/sim.5762. [DOI] [PubMed] [Google Scholar]
- 11.R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- 12.Redd R (2021) cmprsk2: cmprsk2. R package version 0.0.0.9003.
- 13.Varadhan R, Kuk D. crrstep: Stepwise Covariate Selection for the Fine & Gray Competing Risks Regression Model. R package version 2015–2.1.
- 14.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G, Grothey A, O’Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, De Gramont A. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient Data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 15.Furlan D, Carnevali IW, Bernasconi B, Sahnane N, Milani K, Cerutti R, Bertolini V, Chiaravalli AM, Bertoni F, Kwee I, Pastorino R, Carlo C. Hierarchical clustering analysis of pathologic and molecular data identifies prognostically and biologically distinct groups of colorectal carcinomas. Mod Pathol. 2011;24:126–137. doi: 10.1038/modpathol.2010.179. [DOI] [PubMed] [Google Scholar]
- 16.Ueberrueck T, Wurst C, Rauchfuβ F, Knösel T, Settmacher U, Altendorf-Hofmann A. What factors influence 10-year survival after curative resection of a colorectal carcinoma? World J Surg. 2013;37:2476–2482. doi: 10.1007/s00268-013-2138-y. [DOI] [PubMed] [Google Scholar]
- 17.Cho YB, Chun HK, Yun HR, Kim HC, Yun SH, Lee WY. Histological grade predicts survival time associated with recurrence after resection for colorectal cancer. Hepatogastroenterology. 2009;56:1335–1340. [PubMed] [Google Scholar]
- 18.Liang P, Nakada I, Hong J-W, Tabuchi T, Motohashi G, Takemura A, Nakachi T, Kasuga T, Tabuchi T. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2006;14(2):470–477. doi: 10.1245/s10434-006-9189-3. [DOI] [PubMed] [Google Scholar]
- 19.Tsai HL, Cheng KI, Lu CY, Kuo CH, Ma CJ, Wu JY, Chai CY, Hsieh JS, Wang JY. Prognostic significance of depth of invasion, vascular invasion and numbers of lymph node retrievals in combination for patients with Stage II colorectal cancer undergoing radical resection. J Surg Oncol. 2008;97:383–387. doi: 10.1002/jso.20942. [DOI] [PubMed] [Google Scholar]
- 20.Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G, Langner C. Intramural and extramural vascular invasion in colorectal cancer. Cancer. 2012;118:628–638. doi: 10.1002/cncr.26310. [DOI] [PubMed] [Google Scholar]
- 21.Fujii T, SutohT, Morita H, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H. Vascular invasion, but not lymphatic invasion, of the primary tumor is a strong prognostic factor in patients with colorectal cancer. Anticancer Res 2014;34:3147–3152. [PubMed]
- 22.Courtney ED, West NJ, Kaur C, Ho J, Kalber B, Haggar R, Finlayson C, Leicester RJ. Extramural vascular invasion is an adverse prognostic indicator of survival in patients with colorectal cancer. Colorectal Dis. 2009;11:150–156. doi: 10.1111/j.1463-1318.2008.01553.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai HL, Yeh YS, Yu FJ, Lu CY, Chen CF, Chen CW, Chang YT, Wang JY. Predicting factors of postoperative relapse in T2–4N0M0 colorectal cancer patients via harvesting a minimum of 12 lymph nodes. Int J Colorectal Dis. 2009;24:177–183. doi: 10.1007/s00384-008-0594-x. [DOI] [PubMed] [Google Scholar]
- 24.Chok KSH, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surg. 2007;31:1485–1490. doi: 10.1007/s00268-007-9089-0. [DOI] [PubMed] [Google Scholar]
- 25.Akagi Y, Adachi Y, Ohchi T, Kinugasa T, Shirouzu K. Prognostic impact of lymphatic invasion of colorectal cancer: a single-centre analysis of 1,616 patients over 24 years. Anticancer Res. 2013;33:2965–2970. [PubMed] [Google Scholar]
- 26.Fujita S, Shimoda T, Yoshimura K, Yamamoto S, Akasu T, Moriya Y. Prospective evaluation of prognostic factors in patients with colorectal cancer undergoing curative resection. J Surg Oncol. 2003;84:127–131. doi: 10.1002/jso.10308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.