Abstract
Background
Non‐specific cough is defined as non‐productive cough in the absence of identifiable respiratory disease or known aetiology. It is commonly seen in paediatric practice. These children are treated with a variety of therapies including anti‐histamines. Also, anti‐histamines are advocated as an empirical treatment in adults with chronic cough.
Objectives
To evaluate the effectiveness of anti‐histamines in treating children with prolonged non‐specific cough.
Search methods
We searched the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, OLDMEDLINE and EMBASE databases. The latest searches were performed in November 2009.
Selection criteria
All randomised controlled trials comparing anti‐histamines with a placebo or placebo‐like medication with cough as an outcome, where cough is not primarily related to an underlying respiratory disorder such as cystic fibrosis, asthma, or suppurative lung disease.
Data collection and analysis
Two review authors independently assessed study quality and extracted data.
Main results
Three included therapeutic studies had 182 randomised participants with 162 completing the trials although in one study, children with recurrent wheeze were also included. The four included safety evaluation studies randomised 3166 participants with 2862 completing the trials. Clinical heterogeneity was evident and limited data prevented combining data for meta‐analysis. The two larger therapeutic studies described significant improvement in both the intervention and the placebo/placebo‐like arms with no significant difference between the two groups. In the study with the smallest sample size, cetirizine (a second generation anti‐histamine) was significantly more efficacious than placebo in reducing chronic cough in children associated with seasonal allergic rhinitis, and the effect was seen within two weeks of therapy. In contrast three of the larger evaluation studies that enrolled children with allergic rhinitis described a non‐significant increase in cough as an adverse event. Combined data from the four safety evaluation studies revealed a non‐significant difference between groups (OR 1.47 , 95% CI 0.86, 2.49) for cough as an adverse event but the trend favoured the placebo arm.
Authors' conclusions
This review has significant limitations. However, our finding of uncertain efficacy of anti‐histamines for chronic cough are similar to that for acute cough in children. In contrast to recommendations in adults with chronic cough, anti‐histamines cannot be recommended as empirical therapy for children with chronic cough. If anti‐histamines were to be trialled in these children, current data suggest a clinical response (time to response) occurs within two weeks of therapy. However the use of anti‐histamines in children with non‐specific cough has to be balanced against the well known risk of adverse events especially in very young children.
Plain language summary
Anti‐histamines for prolonged non‐specific cough in children
Children with non‐specific cough are commonly treated with a variety of medications to treat the symptom of cough. The objective of this review was to evaluate the effectiveness of anti‐histamines in children with prolonged cough that is not related to an underlying respiratory disease, that is, non‐specific chronic cough. We included three therapeutic studies with 182 randomised participants. Two studies found that chronic cough significantly improved in both treatment and placebo groups with no difference between the two groups. One small study however described that children who had chronic cough associated with seasonal allergic rhinitis treated with cetirizine improved significantly more than children on placebo and this difference was evident by two weeks. Four studies that evaluated safety profiles included 3166 randomised participants and described a non significant increase in cough in participants who received the active medication. Despite the limitations of this review, our findings are similar to the review on anti‐histamines for acute cough which showed no good evidence for or against the use of anti‐histamines. In contrast to recommendations in adults with chronic cough, anti‐histamines cannot be recommended as empirical therapy for children with chronic cough. Further research examining the effects of this treatment using child appropriate cough outcome measures is needed.
Summary of findings
Summary of findings for the main comparison. Anti‐histamines for prolonged non‐specific cough in children.
| anti‐histamines for prolonged non‐specific cough in children | ||||||
| Patient or population: patients with prolonged non‐specific cough in children Settings: Cough reported as an adverse events in safety studies Intervention: anti‐histamines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | anti‐histamines | |||||
| Number of participants with increased cough | Study population | OR 1.47 (0.86 to 2.49) | 3090 (4 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 17 per 1000 | 25 per 1000 (15 to 41) | |||||
| Medium risk population | ||||||
| 20 per 1000 | 29 per 1000 (17 to 48) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Direction of one study favoured anti‐histamines while rest favoured placebo 2 Studies were not designed for efficacy in management of cough in children with allergic rhinitis but cough was recorded as an adverse event
Background
Cough is the most common symptom presenting to general practitioners (Britt 2002; Cherry 2003) and causes significant anxiety to parents (Cornford 1993). Worldwide the desire to reduce the impact of the symptom of cough is reflected in the billions of dollars spent on over the counter cough and cold medications. Non‐specific cough has been defined as non‐productive cough in the absence of identifiable respiratory disease or known aetiology (Chang 2001). While some children with chronic non‐specific cough have asthma, the majority do not (McKenzie 1994; Chang 1999). In adults, chronic cough is defined as cough of over eight weeks duration but the definition commonly accepted in children is that of over three to four weeks, based on the known differences between paediatric and adult cough (Chang 2005b).
The association of atopy with respiratory symptoms has been the subject of many epidemiological studies (Busquets 1996; Clough 1994; Clifford 1989; Mertsola 1991; Peat 1990). Some studies described an association (Peat 1990) but others have not when confounders were accounted for (Mertsola 1991; Clough 1994). A study also found that children with recurrent cough were no more atopic than controls (Chang 1997). Pharmaceutical allergy treatments include the use of H1 receptor antagonists widely known as anti‐histamines. Histamine is a mediator in allergic response and is secreted by mast cells, monocytes and basophils. Anti‐histamines are classed into first generation anti‐histamines (such as diphenhydramine, hydroxyzine, chlorpheniramine, brompheniramine, and clemastine) and the newer (second generation) non sedating anti‐histamines (e.g. terfenadine, astemizole, loratadine, and cetirizine) which have less central nervous system side‐effects (Meltzer 2005). The first generation anti‐histamines are not highly selective and also block muscarinic receptors and thus have some anti‐cholinergic activity (Meltzer 2005).
In adults, monotherapy with anti‐histamines is advocated as an empirical treatment for chronic cough in American and European guidelines (Morice 2004; Irwin 1998). Cochrane systematic reviews have shown that anti‐histamines are efficacious for acute cough (Schroeder 2004) and when used as combination therapy for common colds but not as monotherapy (De Sutter 2003) in adults. In contrast to adults, in children anti‐histamines either as a monotherapy or as combined therapy are not efficacious for relieving acute cough and are associated with significant adverse events (De Sutter 2003; Schroeder 2004). However, the aetiology and management of acute cough is not necessarily the same as that for chronic cough. Furthermore, some have advocated that the management of children with chronic cough should be similar to that of adults (Irwin 1998). A systematic review of the benefits or harm of anti‐histamines for chronic non specific cough or both would thus be useful to help guide clinical practice.
Objectives
To evaluate the effectiveness of anti‐histamines in treating children with prolonged non‐specific cough.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing anti‐histamines with a placebo medication with cough as an outcome, where cough is not primarily related to an underlying respiratory disorder such as cystic fibrosis, asthma, or suppurative lung disease.
Types of participants
Children with chronic (> 4 weeks) non‐specific cough (dry and non‐productive cough without any other respiratory symptom, sign or systemic illness). Exclusion criteria: cough related to mycoplasma, pertussis and chlamydia, presence of underlying cardio‐respiratory condition, current or recurrent wheeze (> 2 episodes), presence of other respiratory symptoms (productive or wet cough (Chang 2005a) , haemoptysis, dyspnoea), presence of other respiratory signs (clubbing, chest wall deformity, respiratory noises such as wheeze on auscultation and other adventitious sounds), presence of any sign of systemic illness (failure to thrive, aspiration, neurological or developmental abnormality), presence of lung function abnormality.
Types of interventions
All randomised controlled comparisons of any type of anti‐histamines. Trials only comparing two or more medications without a placebo or placebo‐like medication (i.e. medications known not to improve cough) comparison group were not included. Trials that included the use of other medications or interventions were included if all participants had equal access to such medications or interventions.
Types of outcome measures
Attempts were made to obtain data on at least one of the following outcome measures:
Primary outcomes
Proportions of participants who were not cured or not substantially improved at follow up (clinical failure).
Secondary outcomes
Proportions of participants who were not cured at follow up, Proportions of participants who not substantially improved at follow up, Mean difference in cough indices (cough diary, cough frequency, cough scores), Proportions experiencing adverse effects of the intervention, (e.g. sleepiness, school performance etc), Proportions experiencing complications e.g. requirement for medication change, etc.
It was planned that the proportions of participants who failed to improve on treatment and the mean clinical improvement will be determined using the following hierarchy of assessment measures (i.e. where two or more assessment measures are reported in the same study, the outcome measure that is listed first in the hierarchy will be used). i) Objective measurements of cough indices (cough frequency, cough receptor sensitivity). ii) Symptomatic (Quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by the patient (adult or child) iii) Symptomatic (Quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by the parents/carers. iv) Symptomatic (Likert scale, visual analogue scale, level of interference of cough, cough diary) ‐ assessed by clinicians. v) Relevant airway markers consistent with any type of inflammation.
Search methods for identification of studies
Electronic searches
The following topic search strategy was used to identify the relevant randomised controlled trials from electronic databases (seeAppendix 1 for the full search strategies):
["cough" OR "bronchitis", all as (textword) or (MeSH )] AND ["histamine H1 receptor antagonist" OR "histamine H1 antagonist" OR "anti‐histamines" OR "antazoline", OR "methapyrilene" OR "pyrilamine" OR "tripelennamine" OR "clemastine" OR "dimenhydrinate" OR "diphenhydramine", OR "doxylamine" OR "brompheniramine", OR "chlorpheniramine" OR "dimethindene", OR "pheniramine" OR "promethazine" OR "triprolidine", OR "cetirizine" OR "hydroxyzine" OR "meclizine", OR "astemizole" OR "cyproheptadine" OR "loratadine", OR "terfenadine";] AND ["child" OR "children" OR "pediatrics"; all as (textword) or (MeSH )]
Trials were identified from the following sources: 1. The Cochrane Central Register of Controlled Trials (CENTRAL), which includes the Cochrane Airways Group Specialised Trials Register. 2. MEDLINE (1966 to present). Topic search strategy combined with the RCT search filter as outlined in the Airways Group module. 3. OLD MEDLINE (1950 to 1965). Topic search strategy combined with the RCT search filter as outlined in the Airways Group module. 4. EMBASE (1980 to present).Topic search strategy combined with the RCT search filter as outlined in the Airways Group module.
The latest search was performed in November 2009.
Searching other resources
We also consulted the list of references in relevant publications, and wrote to authors of trials included in the review.
Data collection and analysis
Selection of studies
Retrieval of studies: from the title, abstract, or descriptors, two review authors (AC, MM) independently reviewed literature searches to identify potentially relevant trials for full review. Searches of bibliographies and texts were conducted to identify additional studies. From the full text using specific criteria, two review authors (AC, JP) independently selected trials for inclusion. Disagreement was resolved by consensus.
Data extraction and management
We reviewed trials that satisfied the inclusion criteria and recorded the following information: study setting, year of study, source of funding, patient recruitment details (including number of eligible participants), inclusion and exclusion criteria, other symptoms, randomisation and allocation concealment method, numbers of participants randomised, blinding (masking) of participants, care providers and outcome assessors, dose and type of intervention, duration of therapy, co‐interventions, numbers of patients not followed up, reasons for withdrawals from study protocol (clinical, side‐effects, refusal and other), details on side‐effects of therapy, and whether intention‐to‐treat analyses were possible. We extracted data on the outcomes described previously. Further information was requested from the lead or corresponding author of five studies.
Assessment of risk of bias in included studies
Two review authors (AC, MM) independently assessed studies included in the review for quality assessment. Four components of quality were assessed:
Allocation concealment. Trials were scored as: Grade A: Adequate concealment, Grade B: Unclear, Grade C: Clearly inadequate concealment. (Grade A = high quality).
Blinding. Trials were scored as: Grade A: Participant and care provider and outcome assessor blinded, Grade B: Outcome assessor blinded, Grade C: Unclear, Grade D: No blinding of outcome assessor (Grade A, B = high quality).
Reporting of participants by allocated group. Trials were scored as: Grade A: The progress of all randomised children in each group described, Grade B: Unclear or no mention of withdrawals or dropouts, Grade C: The progress of all randomised children in each group clearly not described. (Grade A = high quality).
Follow up. Trials were scored as: Grade A: Outcomes measured in > 90% (where withdrawals due to complications and side‐effects are categorised as treatment failures), Grade B: Outcomes measured in 80 to 90%, Grade C: Unclear, Grade D: Outcomes measured in < 80%. (Grade A = high quality).
While only the allocation concealment quality assessment was displayed in the meta‐analysis figures, all assessments were included in the 'Characteristics of included studies' table. Inter‐reviewer reliability for the identification of high quality studies for each component was measured by the Kappa statistic. Quality of the studies were also assessed using Revman's 'Bias tool'.
Measures of treatment effect
For the dichotomous outcome variables of each individual study, it was planned that relative and absolute risk reductions would have been calculated using a modified intention‐to‐treat analysis. This analysis assumes that children not available for outcome assessment have not improved (and probably represents a conservative estimate of effect). An initial qualitative comparison of all the individually analysed studies of all the individually analysed studies was performed to examine whether pooling of results (meta‐analysis) was reasonable. This was to take into account the differences in study populations, inclusion/exclusion criteria, interventions, outcome assessment, and estimated effect size.
Unit of analysis issues
For crossover studies, mean treatment differences would have been calculated from raw data, extracted or imputed and entered as fixed‐effect generic inverse variance (GIV) outcome, to provide summary weighted differences and 95% confidence intervals. In cross‐over trials, only data from the first arm would have been included in meta analysis if data is combined with parallel studies (Elbourne 2002).
Data synthesis
It was planned that results from studies that met the inclusion criteria and reported any of the outcomes of interest would be included in meta‐analyses. The summary weighted risk ratio and 95% confidence interval (fixed‐effect model) would have been calculated (Cochrane statistical package, RevMan version 5). Numbers needed to treat (NNT) would have been calculated from the pooled OR and its 95% CI applied to a specified baseline risk using an online calculator (Cates 2003). The cough indices would have been assumed to be normally distributed continuous variables so the mean difference in outcomes could be estimated (mean difference). If studies reported outcomes using different measurement scales, the standardised mean difference would have been estimated. Any heterogeneity between the study results would have been described and tested to determine if it reached statistical significance using a chi‐squared test. The 95% confidence interval estimated using a random‐effects model would have been included whenever there were concerns about statistical heterogeneity.
Statistical calculations for proportions were calculated using the CIA program (Gardner 1992) when these calculations were not provided in the papers.
We have presented a Summary of Findings table based on methods recommended in the Cochrane Handbook.
Subgroup analysis and investigation of heterogeneity
An a priori sub‐group analysis was planned for:
children aged less than seven years and seven years and above.
anti‐histamines as monotherapy vs combination therapy
older anti‐histamines versus new generation non sedating anti‐histamines.
Sensitivity analysis
Sensitivity analyses were planned to assess the impact of the potentially important factors on the overall outcomes: a) study quality; b) study size; c) variation in the inclusion criteria; d) differences in the medications used in the intervention and comparison groups; e) differences in outcome measures; f) analysis using random‐effects model; g) analysis by "treatment received"; h) analysis by "intention‐to‐treat"; and i) analysis by study design‐parallel and cross over studies.
Results
Description of studies
Results of the search
The Airways Group specialised register/search identified 330 potentially relevant titles in the 2005 search. After assessing the abstracts, 24 papers were retrieved and 21 potential studies were considered (see 'Characteristics of excluded studies' table). The main reason for non‐eligibility of studies for review criteria was the age of the participants (adult based studies). Two papers included small numbers of adolescents. No additional data could be obtained from authors and data specific to children could not be separated. Thus these papers were also excluded. Three studies were included (see 'Characteristics of included studies' table), two were single centre studies (Christensen 1971; Ciprandi 1997) and one was a multi centre study (van Asperen 1992) that was supported by commercial interest. All studies were published in full and all but one (Christensen 1971) were published in English. In the 2006 search, no new studies fulfilled inclusion criteria. Four papers were retrieved and three were added to the excluded studies table. In the 2007 search, five papers were retrieved and two of these were non‐therapeutic studies but were primarily on the safety of anti‐histamines (fexofenadine (Milgrom 2007) and levocetirizine (Simons 2007)); in both studies cough was reported as an outcome measure as an adverse event. In the 2008 search, two studies were identified; one (Hampel 2007) directly from the search and the second (Meltzer 2004) from the review article (Mansfield 2008) identified from the search. The 2009 search identified 33 potential abstracts. One review paper was obtained but no additional studies were included in the 2009 update.
Included studies
All studies that were therapeutic studies included cough as a major inclusion criteria but one study (van Asperen 1992) also included children with wheeze i.e. presence of chronic cough or wheeze was an inclusion criteria. One study was in very young children (age 6 to 36 months), and two other studies were in older children (mean age 8.5 (Ciprandi 1997) and 11.5 (Christensen 1971) years). In these two studies (Christensen 1971; Ciprandi 1997) children also had allergic rhinitis. Two studies utilised parallel design (Ciprandi 1997; van Asperen 1992) and one was a cross over study (Christensen 1971). The safety evaluation studies were large multi‐centre studies performed in young children (< 6 years) with allergic rhinitis (Milgrom 2007, Hampel 2007, Meltzer 2004) or atopy (Simons 2007). One study (Meltzer 2004) was however a mixture of both therapeutic (for allergy) and safety study. As cough was not an outcome in the score for the therapeutic component, the study was grouped as a 'safety evaluation study'. None of the studies utilised objective measurement of cough or quality of life measurements.
Length of intervention in the therapeutic studies varied from two weeks (Christensen 1971) to 16 weeks (van Asperen 1992). In the safety evaluation studies, the length of intervention was at least 7 days in one paper (Hampel 2007), two weeks in two studies (Milgrom 2007, Meltzer 2004) and the 18 months in the other (Simons 2007). Of the therapeutic studies, one study utilised a first generation (sedating) anti‐histamine (pimethixen) (Christensen 1971) and another utilised a second generation, non sedating anti‐histamine (cetirizine) (Ciprandi 1997). The third study (van Asperen 1992) utilised ketotifen, a unclassified anti‐histamine which also has some mast cell stabilising properties (Abelson 2004). One study compared ketotifen to placebo (van Asperen 1992), one compared cetirizine to placebo (Ciprandi 1997) and the Danish study compared a locally produced over the counter mixture to pimethixen. The safety evaluations studies utilised fexofenadine (Milgrom 2007, Hampel 2007, Meltzer 2004) and levocetirizine (Simons 2007). The sample size of the therapeutic studies were generally small (n = 20 in Ciprandi 1997), 49 participants in Reid 1989 and 113 in van Asperen 1992) compared to the safety evaluation studies. The small study did not specify whether there were equal numbers in both arms of the study (Ciprandi 1997). The inclusion criteria in three of the 4 evaluation studies were children with symptomatic allergic rhinitis (Hampel 2007, Meltzer 2004, Milgrom 2007) and in the fourth, (Simons 2007) children were atopic.
Outcome measures for all studies were subjective cough scales of varying types. Two studies had outcome assessments on multiple occasions (Ciprandi 1997; van Asperen 1992) and all provided cough as an outcome measure. Objective cough monitoring was not used in any study.
Risk of bias in included studies
For the therapeutic studies, the quality score varied with no study scoring 'high quality' in all four categories. One study (Ciprandi 1997) scored 'high quality' in three categories and two studies (Christensen 1971; van Asperen 1992) had high quality points for two categories. The reason for the difference between the three studies is because the latter two study had a withdrawal rate of > 10% of participants. None of the studies clearly described their method of randomisation and allocation concealment was unclear in all studies. Method of blinding (that is, appearance of placebo) was clearly described in one study (van Asperen 1992). Agreement between the two main review authors (AC and MM) for quality of studies was excellent; weighted kappa was 0.74 for quality assessment.
See 'Risk of bias' below. None of the studies described allocation method.
Effects of interventions
See: Table 1
The three studies with cough as inclusion criteria (that is, therapeutic studies) included 182 randomised participants with 162 completing the trials. The additional four studies that evaluated safety of the medications included 3166 randomised participants with 2862 completing the trials. The two larger therapeutic studies (Christensen 1971; van Asperen 1992) found no significant difference between treatment and placebo arms but the very small study (n = 20 in total) (Ciprandi 1997) found a significant difference between the groups. In the multicentre study (van Asperen 1992), only 13 (11%) children had chronic cough alone, the rest of the participants had cough with wheeze. The authors could not provide data specific to the cough group alone. Considering all participants, Van Asperen and colleagues reported that the "percentage of symptom free days increased significantly in both groups (P < 0.005) with placebo‐treated infants experiencing more symptom‐free days compared with the ketotifen group (P < 0.01)" (van Asperen 1992). Given the limitation of cough specific data, it was not possible to combine any data for meta‐analysis from the therapeutic studies.
In the safety evaluation studies (Milgrom 2007; Simons 2007, Meltzer 2004, Hampel 2007), cough or increased cough, was reported as an adverse event and these could be combined in a forest plot (Figure 1). Combined data from the four safety evaluation studies revealed a non‐significant difference between groups (OR 1.47 , 95% CI 0.86, 2.49) for cough as an adverse event but the trend favoured the placebo arm. These data are presented in a the Table 1. Meltzer 2004 study described significant improvement in allergy rhinitis score (cough not present in score) but with a non‐significant increase in cough as an adverse event (19 of 1110 in active arm and 6 of 700 in placebo arm) in the anti‐histamine arm compared to placebo.
1.
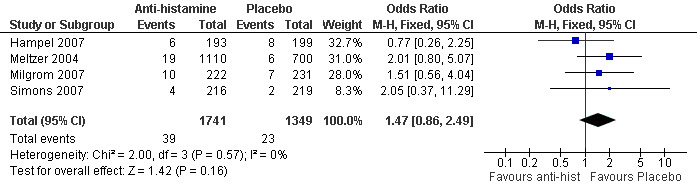
Forest plot of comparison: 1 Cough reported as an adverse events in safety studies, outcome: 1.1 Number of participants with increased cough.
Summary of specific outcomes as reported by the therapeutic studies are as follows:
Clinical failures/success
Subjective assessment of treatment as assessed by parent(s) and by investigator was not significantly different between treatment (ketotifen) and placebo (van Asperen 1992). The proportion as reported by parents to have had good/very good efficacy was 29/52 in the ketotifen arm versus 26/50 in placebo arm (observed difference between proportions = 0.04, 95% CI ‐0.16 to 0.23). Symptom free proportions were also similar between groups on pimethixen and paradryl (34/45 versus 31/45; observed difference between proportions = 0.07, 95%CI ‐0.12 to 0.25) (Christensen 1971). In contrast, the study on cetirizine reported that "cetirizine's overall therapeutic effectiveness was judged satisfactory in all patients...."; data on placebo was not provided (Ciprandi 1997). Success and failures were not relevant in the safety evaluation studies. However the study on fexofenadine reported cough increased in 10 children who received fexofenadine compared to seven who received placebo. The second safety evaluation study reported four in the levocetirizine arm had cough versus two in the placebo arm. Combining these studies, there was no significant difference between groups (OR 1.6, 95% CI 0.7 to 3.82) but the trend was reduced participants with cough in the placebo arm compared to the active arms.
Mean differences in cough scores
Despite the large difference in sample size of the studies, both studies with a longer intervention period (Ciprandi 1997; van Asperen 1992) provided the mean of the group's cough score at the various time points. However, data could not be combined in a meta‐analysis as data specific to children with chronic cough was not available in one study (van Asperen 1992). Authors of this study reported that day and night time cough scores significantly decreased with time with no difference between the treatment (ketotifen) and placebo groups (van Asperen 1992). In the cetirizine versus placebo study, cough scores were significantly less in the cetirizine group when compared to the placebo group (P = 0.01 for week two and three, P = 0.02 on week four) (Ciprandi 1997).The third study did not provide data on mean cough scores (Christensen 1971).
Time to response
In Ciprandi and colleagues' four week study, the difference between treatment and placebo arm was evident by the second week. The significant difference in cough frequency and cough intensity between the treatment and placebo arms remained at weeks two, three and four but there was no difference in the cough scores within each arm with further therapy (Ciprandi 1997). As the other two studies (Christensen 1971; van Asperen 1992) did not find a difference between groups, 'time to response' was not relevant in these studies.
Adverse events
Ciprandi and colleagues reported no adverse events (Ciprandi 1997). Van Asperen and colleagues reported more adverse events in the ketotifen group (57%) versus placebo (43%) (van Asperen 1992). Statistical calculation however showed that this difference was not significantly different (observed difference 0.14, 95% CI ‐0.05 to 0.33). Most of the events reported were minor with irritability and gastrointestinal upset being the most common events. In four children (two each in both arms of the study), adverse events lead to withdrawal of the child from the study (van Asperen 1992). In Christensen and colleagues' study, 12 children described sedation (with three being severe) during pimethixen treatment where as 13 described mild to moderate sedation during paradryl treatment (Christensen 1971).
Sensitivity analyses
Sensitivity analysis could not be performed as data could not be combined for meta‐analysis.
Discussion
This systematic review found seven studies that fulfilled the inclusion criteria, three were therapeutic studies and four safety evaluation studies. The review is limited by the lack of appropriate data; meta‐analysis could be only be performed in the safety evaluation studies. Results from the two larger therapeutic studies (van Asperen 1992; Christensen 1971) which described no beneficial effect is in contrast to the small study (Ciprandi 1997) which showed that cetirizine, a second generation anti‐histamine, was beneficial in reducing cough associated with seasonal allergic rhinitis. The combined data from the safety evaluations studies showed a non‐significant difference in number of participants with cough but the direction of effect was in favour of the placebo arm. In three of these four studies, children with allergic rhinitis were enrolled.
The three included therapeutic studies were different in several ways. Firstly, some clinical heterogeneity was present in the participants of the studies; although all studies were in children with chronic cough, the participants in one study (van Asperen 1992) were very young whereas the participants in the other studies (Christensen 1971; Ciprandi 1997) were older. Secondly, all three studies used a different type of anti‐histamine. Adult cohort data suggest that first generation anti‐histamines are more efficacious than second generation anti‐histamines in reducing chronic cough; indeed the American College of Chest Physicians guidelines recommends the use first generation anti‐histamines for adults with chronic cough (Irwin 2006). In contrast, the American Academy of Asthma, Allergy and Immunology which lists chronic cough as one of the many factors when assessing patients with allergic rhinitis (Spector 2003) recommends the use of second generation anti‐histamines as first line therapy for children with allergic rhinitis (Dykewicz 1998, Dykewicz 1998b).
Thirdly, in two therapeutic studies (Christensen 1971; Ciprandi 1997) children had allergic rhinitis and arguably anti‐histamines would be more efficacious in these children. However, interpretation of this data has be taken in the light that, although a minority of children with allergic rhinitis have co‐existent cough, the cause and effect is less clear and indeed controversial as reported in reviews (Chang 2001; Kemp 2006). Fourthly, two studies (Ciprandi 1997; van Asperen 1992) were placebo controlled and the third (Christensen 1971) was not. We included the study that compared an anti‐histamine to an OTC that consisted of ammonium acetate and ephedrine as OTCs are not efficacious for acute cough in children (Schroeder 2004; Kelly 2004). While it possible that the use of this would have reduced the demonstrable effect of the anti‐histamine in the study, it is more likely that the anti‐histamine was not efficacious; Christensen and colleagues' study (Christensen 1971) had a sample size 2.5 times that of the study (Ciprandi 1997) that found some benefit. Lastly, the studies also differed in sample size from very small (n = 20; Ciprandi 1997) to a much larger sample size (n = 113; van Asperen 1992).
Two studies reported on adverse events in both treatment and placebo groups (Christensen 1971; van Asperen 1992). When we calculated the proportions we found no significant differences between the groups. The medical literature however reports that the adverse effects of anti‐histamines, in particular first generation anti‐histamines, include sedation, paradoxic excitability, dizziness, respiratory depression, hallucinations, arrhythmia (Kelly 2004) and death (Gunn 2001). Thus the use of anti‐histamines has to be balanced with its associated adverse events. Second generation anti‐histamines have less adverse effects. Indeed in the safety evaluation studies (Simons 2007; Milgrom 2007, Meltzer 2004, Hampel 2007) cough as an adverse event occurred more frequently in the active arms then in the placebo arm although the difference was not significant.
Despite the limitations of this review, our findings are similar to that of the non substantial efficacy of anti‐histamines for acute cough in children (Schroeder 2004) as well as that for colds in children (De Sutter 2003). In contrast to recommendations in adults with chronic cough (Irwin 2006), anti‐histamines cannot be recommended as empirical therapy for children with chronic cough.
Authors' conclusions
Implications for practice.
With the lack of evidence, the routine use of anti‐histamines in treating children with non‐specific cough cannot be recommended and is arguably contra‐indicated in young children because of its side effects. If anti‐histamines are to be trialled in these children, current data suggest a clinical response (time to response) occurs within two weeks of therapy. However the use of anti‐histamines in children with non‐specific cough has to be balanced against the well known risk of adverse events especially in very young children.
Implications for research.
Randomised controlled trials of anti‐histamines to determine the effectiveness in treating children with non‐specific cough are clearly needed. Trials should be parallel studies and double blinded given the known problems in studying cough, specifically the large placebo and time period effects. Safety data should also be incorporated. Based on a single RCT, a short trial of two weeks would suffice. Outcome measures for the clinical studies on cough should be clearly defined using validated subjective data and supported by objective data if possible.
What's new
| Date | Event | Description |
|---|---|---|
| 12 November 2009 | New search has been performed | new search, SoF added |
| 24 March 2009 | Amended | Contact details changed |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 21 December 2008 | New search has been performed | Literature search re‐run. Two new studies added, conclusion unchanged |
| 30 June 2008 | Amended | Converted to new review format. |
| 4 January 2008 | New citation required and conclusions have changed | Substantive amendment |
| 1 March 2007 | New search has been performed | Two safety studies added to the review (Milgrom 2007 and Simons 2007) with 963 randomised participants. The conclusions of the review remain the same. |
Acknowledgements
We thank Toby Lasserson and Chris Cates from the Airways Group for their advice, supportive role and comments to the protocol and review. We are also very grateful to Elizabeth Arnold for performing the relevant searches and obtaining the articles, as well as for translating a Spanish paper for the original version. We are also grateful to Susan Hansen for the 2009 search. We also thank Claire Glenton for translation of the Danish article and Peter van Asperen for his correspondence.
Appendices
Appendix 1. Search strategies
| CENTRAL search | MEDLINE/OLDMEDLINE | EMBASE search |
| #1 MeSH descriptor Cough explode all trees in MeSH products #2 MeSH descriptor Bronchitis explode all trees in MeSH products #3 cough* in All Fields in all products #4 bronchiti* in All Fields in all products #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Histamine H1 Antagonists explode all trees in MeSH products #7anti‐histamine* in All Fields in all products #8 antihistamine* in All Fields in all products #9 antazoline in All Fields in all products #10 methapyrilene in All Fields in all products #11 pyrilamine in All Fields in all products #12 tripelennamine in All Fields in all products #13 clemastine in All Fields in all products #14 dimenhydrinaat in All Fields in all products #15 diphenhydramine in All Fields in all products #16 doxylamine in All Fields in all products #17 brompheniramine in All Fields in all products #18 chlorpheniramine in All Fields in all products #19 dimethindene in All Fields in all products #20 pheniramine in All Fields in all products #21 promethazine in All Fields in all products #22 triprolidine in All Fields in all products #23 cetirizine in All Fields in all products #24 hydroxyzine in All Fields in all products #25 meclizine in All Fields in all products #26 astemizole in All Fields in all product #27 cyproheptadine in All Fields in all products #28 loratadine in All Fields in all products #29 terfenadine in All Fields in all products #30 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR 18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) #31 (#5 AND #30) #32 MeSH descriptor Pediatrics explode all trees in MeSH products #33 MeSH descriptor Child explode all trees in MeSH products #34 MeSH descriptor Infant explode all trees in MeSH products #35 MeSH descriptor Adolescent explode all trees in MeSH products #36 child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat* in All Fields in all products #37 (#32 OR #33 OR #34 OR #35 OR #36) #38 (#37 AND #31) | 1. cough/ 2. exp Bronchitis/ 3. cough$.tw. 4. bronchiti$.tw. 5. or/1‐4 6. exp Histamine H1 Antagonists/ 7. anti‐histamine$.tw. 8. antihistamine$.tw. 9. antazoline.tw. 10. methapyrilene.tw. 11. pyrilamine.tw. 12. tripelennamine.tw. 13. clemastine.tw. 14. dimenhydrinaat.tw. 15. diphenhydramine.tw. 16. doxylamine.tw. 17. brompheniramine.tw. 18. chlorpheniramine.tw. 19. dimethindene.tw. 20. pheniramine.tw. 21. promethazine.tw. 22. triprolidine.tw. 23. cetirizine.tw. 24. hydroxyzine.tw. 25. meclizine.tw. 26. astemizole.tw. 27. cyproheptadine.tw. 28. loratadine.tw. 29. terfenadine.tw. 30. or/6‐29 31. exp pediatrics/ 32. exp child/ 33. exp adolescent/ 34. exp infant/ 35. (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).tw. 36. or/31‐35 37. 5 and 30 and 36 | 1. coughing/ 2. exp bronchitis/ 3. cough$.tw. 4. bronchiti$.tw. 5. or/1‐4 6. exp Histamine H1 Receptor Antagonist/ 7. anti‐histamine$.tw. 8. antihistamine$.tw. 9. antazoline.tw. 10. methapyrilene.tw. 11. pyrilamine.tw. 12. tripelennamine.tw. 13. clemastine.tw. 14. dimenhydrinaat.tw. 15. diphenhydramine.tw. 16. doxylamine.tw. 17. brompheniramine.tw. 18. chlorpheniramine.tw. 19. dimethindene.tw. 20. pheniramine.tw. 21. promethazine.tw. 22. triprolidine.tw. 23. cetirizine.tw. 24. hydroxyzine.tw. 25. meclizine.tw. 26. astemizole.tw. 27. cyproheptadine.tw. 28. loratadine.tw. 29. terfenadine.tw. 30. or/6‐29 31. exp pediatrics/ 32. Child/ 33. adolescent/ 34. Infant/ 35. (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).tw. 36. or/31‐35 37. 5 and 30 and 36 |
Data and analyses
Comparison 1. Cough reported as an adverse events in safety studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with increased cough | 4 | 3090 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.86, 2.49] |
1.1. Analysis.
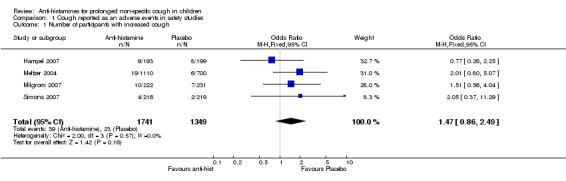
Comparison 1 Cough reported as an adverse events in safety studies, Outcome 1 Number of participants with increased cough.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Christensen 1971.
| Methods | Randomized, double‐blind, placebo controlled, cross‐over study. High quality score = 2 Grading of quality: Allocation concealment = B Blinding = A Reporting of participants by allocated group = A Follow up = B |
|
| Participants | 49 children with hay fever recruited from a hospital clinic for children with allergic diseases. Other inclusion and exclusion criteria were not described. Mean age of total group (39 boys, 10 girls) was 11.5 years (range 3 to 17). | |
| Interventions | 2 weeks each of Paradryl (ammonium acetate/ephredine, a Danish product) and pimethixen (sedating anti‐histamine). 5 ml bd for children aged 3 to 10 years and 10 ml bd for children aged 10 to 17 years. | |
| Outcomes | Parental recording of the following on 12 occasions over the 4 week study period: 1. Attacks of sneezing, nasal occlusion, nasal secretion, nasal itching; 2. Coughing and wheezing, respiratory distress, attacks of asthma; 3. Ocular itching, lacrimation; 4. Itching of the skin, skin rash; 5. Ability to sleep at night; and 6. Parental opinion of improvement and difference between trial medications. Study included assuming that Panadryl was like placebo, based on data that these do not confer any benefit for cough from other Cochrane review. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not specified |
| Allocation concealment? | Unclear risk | Not specified |
| Blinding? All outcomes | Low risk | |
Ciprandi 1997.
| Methods | Single centre, randomized, double‐blind, placebo controlled, parallel‐group study. High quality score = 3 Grading of quality: Allocation concealment = B Blinding = A Reporting of participants by allocated group = A Follow‐up = A |
|
| Participants | 20 children with cough associated with allergic rhinoconjunctivitis due to Parietaria judaica and/or grass pollen allergy for at least 2 previous seasons. Numbers in each group not given. Other inclusion criteria: skin prick or RAST positive to allergen above, symptom free outside pollen season. Exclusion: clinical asthma, spirometry FEV1 < 80%, use of topical or systemic drugs in preceding 6 weeks, specific immunotherapy before and during study, presence of other ocular or nasal disease, sensitivity to cetrizine. | |
| Interventions | Cetrizine (0.15mg/kg) daily or placebo for 4 weeks. | |
| Outcomes | Cough diary scored 4X a day on a 4‐point scale by parents, peak expiratory flow, and overall efficacy and safety (assessed by parents and trialist). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | High risk | Very small study. No declaration of support |
Hampel 2007.
| Methods | Safety and tolerability study of fexofenadine hydrochloride in children aged 6 months to 2 years. Multicenter, randomised, placebo‐controlled design in 2 studies reported together. Studies had same design.Trial sponsored by pharmaceutical company. Visit 1: enrolment and randomisation. Visit 2: Final or early withdrawal visit (day 8 or 9). Total 393 enrolled, 30 withdrew before study completion related to adverse events. High quality score = 2 Grading of quality: Allocation concealment = B Blinding = A Reporting of participants by allocated group = A Follow up = B |
|
| Participants | Children aged 6‐24 months with allergic rhinitis (AR) diagnosed my medical history, pattern or physical findings. All were either candidates for antihistamine or had tolerated antihistamine in the past. Exclusion criteria: moderate or severe asthma, short term episodes of respiratory tract infection, bronchiolitis, wheezing, and acute otitis media at enrolment; cardiac or hepatic symptoms or any condition that interfered with swallowing or absorption of oral medication. Study 1: Age (mean, SD); Female:Male (F:M) Placebo in 6‐12mo group=8.4, 1.9; 29F:35M. In 1‐2years age group=15.7, 3.3; 14F:11M Fenxofenadine in 6‐12mo group=8.8,1.6; 25F:33M. In 1‐2years age group=16.1, 32.1; 13F:14M Study 2: Age (mean, SD); Female:Male (F:M) Placebo in 6‐12mo group=10.6,0.6; 3F:2M. . In 1‐2years age group=18.7, 3.2; 26F:69M. Fenxofenadine in 6‐12mo group=10.4, 0.6; 2F:3M. . In 1‐2years age group=17.9, 3.2; 41F:62M. |
|
| Interventions | Mean treatment duration was 8 days, minimum 7 days Rx Subjects were randomised to twice‐daily fexofenadine hydrochloride, 15 or 30 mg, or placebo mixed with a standard vehicle. Dose based on age and weight: aged 1‐2 years and weighing >10.5 kg were given 30mg. 15mg given when aged >6 months and weighing =<10.5kg | |
| Outcomes | Physical examination: allergic rhinitis symptoms, vital signs, weight, ECG Recording of adverse events including cough was specifically reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Unclear risk | Supported by pharmaceutical industry |
Meltzer 2004.
| Methods | Multicentre double‐blind, randomised, placebo‐controlled, parallel‐group, 2‐wk trials. Three studies were reported within this paper. All three studies had a similar design with regard to patient selection criteria and, primary and secondary outcome measures. Following visit 1, the studies included four additional visits: visit 2, randomisation (day 1); visit 3, during double‐blind treatment (day 6–10); visit 4, end of double‐blind treatment (day15–17); and visit 5, follow‐up (day 22–24).
For inclusion at visit 1, total symptom score (TSS) (see below for score details) of 6, with two or more symptoms with a minimum score of 2 (moderate), was necessary. This was followed by completion of a single‐blind, placebo lead‐in period, the children in study were required to have a TSS score that differed in the studies. High grade score: 3 Grading: Allocation concealment= B. Blinding=A Reporting of participants by allocated group=A Follow up=A |
|
| Participants | Inclusion criteria: presence of seasonal allergic rhinitis (SAR) symptoms (see below‐outcomes) at entry and persisted after single‐blind placebo lead‐in. Children aged 6–11 years, with spring or fall SAR, and a history of SAR of approximately 1 year or more during at least one previous relevant season. Subjects were required to have a positive skin‐prick test (wheal diameter of at least 3 mm than that with diluent within 15 min of the skin prick) to at least one allergen for the current season. For one of the 3 studies, presence of an allergen‐specific immunoglobulin E (IgE) that was positive in the skin‐prick test was also required. Exclusion: patients were (but were not limited to) those with a respiratory tract nasal infection, sinusitis, or otitis media within 30 days of study entry; clinically significant cardiovascular, hepatic, neurologic, psychiatric, endocrine or other major systemic disease; or immunotherapy treatment. A total of 1810 children were included in the overall paediatric safety population (placebo, n=700; fexofenadine n=1110) |
|
| Interventions | All children were randomised to receive either fexofenadine HCl arm (15,30 or 60mg bd) or matching placebo. | |
| Outcomes | Seasonal allergic rhinitis symptom scores: sneezing; rhinorrhoea; itchy nose, palate, throat and/or ears; itchy, watery and/or red eyes; and nasal congestion. Each symptom was evaluated on a five‐point scale: 0=absent; 1=mild; 2=moderate; 3=severe;4=very severe. The total symptom score (TSS) was calculated by adding the individual symptom scores, excluding nasal congestion (maximum possible TSS=16). Seasonal allergic rhinitis symptoms were assessed daily at 19:00 (±1) hours for the previous 12‐hour period by the subject and caregiver, immediately prior to trial medication. Children (and investigators) reported any adverse events (AEs) during the study. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Unclear risk | Supported by pharmaceutical company |
Milgrom 2007.
| Methods | A safety evaluation study. Multicenter, double‐blind, randomized, placebo controlled, parallel‐group study. High quality score = 1 |
|
| Participants | Placebo group: mean age 3.6 SD 1.1; 117 boys, 123 girls. Fexofenadine group: mean age 3.6 SD 1.1; 123 boys, 99 girls. Inclusion criteria: Children aged 2 to 5 years with allergic rhinitis. Exclusion criteria: Clinically relevant abnormalities/conditions that may interfere with study (severe asthma, bronchiolitis, wheezing, acute respiratory infection, vasomotor rhinitis, acute otitis media, craniofacial abnormalities, on immunotherapy requiring dose change or frequency of dosing during study, recent vaccination (< 2 weeks), on another anti‐histamine, oral decongestion or adrenal agonists, eye drops, systemic or nasal corticosteroids, or other sinus, allergy or cold remedies, hypersensitivity to fexofenadine. |
|
| Interventions | Fexofenadine hydrochloride, 30 mg, or placebo twice daily for a 2‐week period | |
| Outcomes | Primary outcome: occurrence of adverse events. Secondary outcome: ECG, physical examination, lab tests. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | |
Simons 2007.
| Methods | A safety evaluation study. Multicentre randomized, double‐masked placebo controlled study as part of a Early Prevention of Asthma in Atopic Children Study. High quality score = 2 |
|
| Participants | 510 children in the intention‐to‐treat population: 255 (mean age 19.3 ± s.e.m. 0.3 months, 60.8% boys) in the levocetirizine treatment group, and 255 (mean age 19.4 ± s.e.m. 0.2 months, 64.3% boys) in the placebo treatment group. Inclusion criteria: aged 12 to 24 months, had atopic dermatitis, elevated specific IgE to either grass pollen or house dust mite, and a family history of allergy. Assignment to treatment was made according to pre‐selected randomization factors at baseline, including: status of sensitization to grass pollen or house dust mite, sensitization to egg, maternal history of asthma, and country of residence. Exclusion criteria: asthma or any other systemic disease; if their height or body mass were below the 5th percentile; if they had any severe neurologic or psychologic disorder requiring medical treatment; if they were known to be intolerant of levocetirizine or any other piperazine antihistamine, or to the parabens used as preservatives in H1‐antihistamine liquid formulations; if they had a personal history or sibling history of sleep apnea; or if they had renal insufficiency or any metabolic condition that might affect the elimination of levocetirizine. Regular treatment with other H1‐antihistamines was discontinued before study entry. |
|
| Interventions | Levocetirizine 0.125 mg/kg or placebo twice daily for 18 months. | |
| Outcomes | Treatment–emergent adverse events. These were described by the investigators according to primary system organ class. An adverse event was captured by spontaneous reporting on the diary cards, and also by asking the child’s caregiver. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
van Asperen 1992.
| Methods | Multicentre randomized placebo‐controlled double‐blind study. Randomisation stratified by two age groups (6 to 18 months and 19 to 36 months) and two symptom groups (cough alone and wheeze with or without cough). High quality score = 2 Grading of quality: Allocation concealment = B Blinding = A Reporting of participants by allocated group = A Follow up = B |
|
| Participants | 113 infants between 6 and 36 months of age presenting with a history of cough or wheeze for at least 50% of days over 3 or more months or both. Other inclusion criteria: nil mentioned. Exclusion criteria: presence of any serious chronic disease, other specific lung disease, previous long term use of cromoglycate, on theophylline or cromoglycate, or have used steroids for > 5 days in last 3 months. Recruited through outpatients and community advertising |
|
| Interventions | 4 week run‐in then 16 weeks of 2.5 ml twice daily of either placebo or Ketotifen (0.5 mg) syrup; this was followed by a 4 week wash‐out observation phase. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Unclear risk | Supported by pharmaceutical company |
bd: twice daily; ECG: electrocardiogram; SD: standard deviation; s.e.m: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaronson 1996 | Patients with allergic rhinitis and concomitant symptomatic mild to moderate perennial asthma. |
| Berkowitz 2002 | RCT on 488 children aged > 12 years and adults using fexofenadine/pseudoephedrine vs placebo. Data on children only could not be extracted and author did not respond to correspondence. |
| Berkowitz 2004 | RCT on 298 children aged > 12 years and adults using single dose of fexofenadine/pseudoephedrine vs placebo. Data on children only could not be extracted and author did not respond to correspondence. |
| Bone 1996 | Non RCT on allergic rhinitis with cough as an outcome. Spanish article translated. |
| Chiang 2006 | RCT on an anti‐histamine but cough not reported as a symptom |
| Ciprandi 2001 | RCT on 20 children with perennial rhino conjunctivitis or asthma or both. Authors contacted for clarification but no reply received. |
| Clemens 1997 | Randomised, double‐blind, placebo‐controlled trial of antihistamine‐decongestant combination for acute respiratory infection. |
| Conde 1995 | Comparison of Azelastine nasal spray (0.14 mg in each nostril twice a day) versus Ebastine tablets (10 mg). No placebo. |
| De Blic 2005 | RCT using levocetirizine 5 mg once‐daily in reducing seasonal allergic rhinitis (SAR) symptoms. Cough was not an outcome measure. |
| Grimfeld 2004 | Study to evaluate the efficacy and long‐term safety of loratadine in reducing the number of respiratory infections in children at 24 months, that is, not for treatment of cough. |
| Herman 1997 | RCT in 125 children comparing azelastine nasal spray 0.14 mg/nostril twice daily (0.56 mg/day) and placebo nasal spray. Cough was not an outcome measure. |
| Homnick 2007 | RCT in patients with cystic fibrosis, that is, does not fulfil criteria for non‐specific cough |
| Jaffe 1983 | RCT comparing in 217 children 'Pholcolix' and 'Actifed' Compound, in children with acute cough. |
| Kaiser 1998 | RCT designed to compare the efficacy and safety of Claritin‐D 24 Hour (once daily) with that of Claritin‐D 12 Hour (twice daily) and placebo in the treatment of patients with seasonal allergic rhinitis. Cough was not an outcome. |
| Kim 2006 | RCT examining the effect of desloratadine in reducing the symptoms of perennial allergic rhinitis. Cough was not an outcome measure. |
| Mizoguchi 2007 | Study on acute cold symptoms which included cough lasting 1 to 5 days |
| Mosges 1995 | RCT comparing 2 antihistamines nasal sprays (Levocabastine and azelastine). No placebo group. |
| Paul 2004 | RCT in 100 children with upper respiratory infections comparing dextromethorphan and diphenhydramine to placebo. Acute cough. |
| Prenner 2006 | Multicenter RCT to assess the general and cardiac safety of desloratadine syrup in children aged 6 months to less than 2 years. Cough was not an outcome measure. |
| Pujet 2002 | RCT in adults using 5‐days of an antihistaminic antitussive syrup, oxomemazine, combining a small quantity of guaifenesin, with a centrally acting antitussive, clobutinol. |
| Reid 1989 | Multicentre RCT that recruited children with recurrent cough and/or wheeze and with airway hyper‐reactivity i.e. children thus have asthma‐like disorder. |
| Shi 1996 | RCT in 170 children with cough treated with Zhenkeling oral liquor or pectoral syrup. Zhenkeling is a Chinese herbal Rx with unknown anti‐histamine properties. |
| Simons 2003 | RCT in children to document safety. No cough outcomes identified as efficacy. No reports of cough as an adverse event in either active or placebo arms. |
| Yoder 2006 | RCT in children with acute cough. |
Contributions of authors
For the protocol: Protocol was written by AC, based on previous protocols on cough in children. MM and JP reviewed protocol. For the review: AC: selection of articles from search, data extraction, data analysis and writing of review. MM: selection of articles from search, data extraction and writing of review. JP: review of articles for inclusion and writing of review.
Sources of support
Internal sources
Royal Children's Hospital Foundation, Brisbane, Australia.
External sources
National Health and Medical Research Council, Australia.
-
Queensland SmartState Clinical Fellowship, Not specified.
Support for AC
Declarations of interest
None declared.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Christensen 1971 {published data only}
- Christensen ER, Ulrich J. Palliative treatment of estival rhinitis in children. A double blind combined study of an antiamine, pimethixen, and an antihistamine, Paradryl [Palliativ behandling af rhinitis aestivalis hos børn. En dobbelt blind, sammenlignende undersøgelse imellem et antiamin, pimetiksen, og et antihistamine, Paradryl]. Nordisk Medicin 1971;86(39):1121‐4. [PubMed] [Google Scholar]
Ciprandi 1997 {published data only}
- Ciprandi G, Tosca M, Ricca V, Passalacqua G, Fregonese L, Fasce L, et al. Cetirizine treatment of allergic cough in children with pollen allergy. Allergy 1997;52(7):752‐4. [DOI] [PubMed] [Google Scholar]
Hampel 2007 {published data only}
- Hampel FC, Kittner B, Bavel JH. Safety and tolerability of fexofenadine hydrochloride, 15 and 30 mg, twice daily in children aged 6 months to 2 years with allergic rhinitis. Annals of Allergy, Asthma and Immunology 2007;Dec:549‐554. [DOI] [PubMed] [Google Scholar]
Meltzer 2004 {published data only}
- Meltzer EO, Scheinmann P, Rosado Pinto JE, Bachert C, Hedlin G, Wahn U, et al. Safety and efficacy of oral fexofenadine in children with seasonal allergic rhinitis ‐ a pooled analysis of three studies. Pediatric Allergy and Immunology 2004;15(3):253‐260. [DOI] [PubMed] [Google Scholar]
Milgrom 2007 {published data only}
- Milgrom H, Kittner B, Lanier R, Hampel FC. Safety and tolerability of fexofenadine for the treatment of allergic rhinitis in children 2 to 5 years old. Annals of Allergy, Asthma and Immunology 2007;99(4):358‐63. [DOI] [PubMed] [Google Scholar]
Simons 2007 {published data only}
- Simons FE. Safety of levocetirizine treatment in young atopic children: An 18‐month study. Pediatric Allergy and Immunology 2007;18(6):535‐42. [DOI] [PubMed] [Google Scholar]
van Asperen 1992 {published data only}
- Asperen PP, McKay KO, Mellis CM, Loh RK, Harth SC, Thong YH, et al. A multicentre randomized placebo‐controlled double‐blind study on the efficacy of Ketotifen in infants with chronic cough or wheeze. Journal of Paediatrics & Child Health 1992;28(6):442‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaronson 1996 {published data only}
- Aaronson DW. Evaluation of cetirizine in patients with allergic rhinitis and perennial asthma. Annals of Allergy, Asthma & Immunology 1996;76(5):440‐6. [DOI] [PubMed] [Google Scholar]
Berkowitz 2002 {published data only}
- Berkowitz RB, Lutz C, Weiler K, Weiler J, Moss M, Meeves S. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Annals of Allergy, Asthma & Immunology 2002;89(1):38‐45. [DOI] [PubMed] [Google Scholar]
Berkowitz 2004 {published data only}
- Berkowitz RB, McCafferty F, Lutz C, Bazelmans D, Godfrey P, Meeves S, et al. Fexofenadine HCl 60 mg/ pseudoephedrine HCl 120 mg has a 60‐minute onset of action in the treatment of seasonal allergic rhinitis symptoms, as assessed in an allergen exposure unit. Allergy and Asthma Proceedings 2004;25:335‐43. [PubMed] [Google Scholar]
Bone 1996 {published data only}
- Bone Calvo J, Botey Sala J, Caballero Gomez L, Conde Hernandez J, Gorostiza Felipe P, Maillo Del Castillo JM, et al. Long‐term safety and efficacy of Azelastine nasal spray in the treatment of children with perennial allergic rhinitis. Acta Pediatrica Espanola 1996;54(10):750‐64. [Google Scholar]
Chiang 2006 {published data only}
- Chiang Y‐C, Shyur S‐D, Chen T‐L, Huang L‐H, Wen T‐C, Lin M‐T, et al. A randomized controlled trial of cetirizine plus pseudoephedrine versus loratadine plus pseudoephedrine for perennial allergic rhinitis. Asian Pacific Journal of Allergy and Immunology 2006;24:97‐103. [PubMed] [Google Scholar]
Ciprandi 2001 {published data only}
- Ciprandi G, Tosca M, Passalacqua G, Canonica GW. Long‐term cetirizine treatment reduces allergic symptoms and drug prescriptions in children with mite allergy. Annals of Allergy, Asthma & Immunology 2001;87(3):222‐6. [DOI] [PubMed] [Google Scholar]
Clemens 1997 {published data only}
- Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine‐decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children?. Journal of Pediatrics 1997;130(3):463‐6. [DOI] [PubMed] [Google Scholar]
Conde 1995 {published data only}
- Conde Hernández J, Palma Aguilar JL, Delgado Romero J. Investigation on the efficacy and tolerance of azelastine (HCL) nasal spray versus ebastine tablets in patients with seasonal allergic rhinitis. Allergologia Et Immunopathologia 1995;23(2):51‐7. [PubMed] [Google Scholar]
De Blic 2005 {published data only}
- Blic J, Wahn U, Billard E, Alt R, Pujazon MC. Levocetirizine in children: Evidenced efficacy and safety in a 6‐week randomized seasonal allergic rhinitis trial. Pediatric Allergy & Immunology 2005;16(3):267‐75. [DOI] [PubMed] [Google Scholar]
Grimfeld 2004 {published data only}
- Grimfeld A, Holgate ST, Canonica GW, Bonini S, Borres MP, Adam D, et al. Prophylactic management of children at risk for recurrent upper respiratory infections: The Preventia I Study. Clinical & Experimental Allergy 2004;34(11):1665‐72. [DOI] [PubMed] [Google Scholar]
Herman 1997 {published data only}
- Herman D, Garay R, Gal M. A randomized double‐blind placebo controlled study of azelastine nasal spray in children with perennial rhinitis. International Journal of Pediatric Otorhinolaryngology 1997;39(1):1‐8. [DOI] [PubMed] [Google Scholar]
Homnick 2007 {published data only}
- Homnick DN, Marks JH, Rubin BK. The effect of a first‐generation antihistamine on sputum viscoelasticity in cystic fibrosis. Journal of Aerosol Medicine 2007;20(1):45‐9. [DOI] [PubMed] [Google Scholar]
Jaffe 1983 {published data only}
- Jaffe G, Grimshaw JJ. Randomized single‐blind trial in general practice comparing the efficacy and palatability of two cough linctus preparations, 'Pholcolix' and 'Actifed' compound, in children with acute cough. Current Medical Research & Opinion 1983;8(8):594‐9. [DOI] [PubMed] [Google Scholar]
Kaiser 1998 {published data only}
- Kaiser HB, Banov CH, Berkowitz RR, Bernstein DI, Bronsky EA, Georgitis JW, et al. Comparative efficacy and safety of once‐daily versus twice‐daily Loratadine‐Pseudoephedrine combinations versus placebo in seasonal allergic rhinitis. American Journal of Therapeutics 1998;5(4):245‐51. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim K, Sussman G, Hebert J, Lumry W, Lutsky B, Gates D. Desloratadine therapy for symptoms associated with perennial allergic rhinitis. Annals of Allergy, Asthma, & Immunology 2006;96(3):460‐5. [DOI] [PubMed] [Google Scholar]
Mizoguchi 2007 {published data only}
- Mizoguchi H, Wilson A, Jerdack GR, Hull JD, Goodale M, Grender JM, et al. Efficacy of a single evening dose of syrup containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulfate in subjects with multiple common cold symptoms. International Journal of Clinical Pharmacology and Therapeutics 2007;45:230‐6. [DOI] [PubMed] [Google Scholar]
Mosges 1995 {published data only}
- Mosges R, Spaeth J, Klimek L. Efficacy and tolerability of levocabastine and azelastine nasal sprays for the treatment of allergic rhinitis. Mediators of Inflammation 1995;4(Suppl 1):S11‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2004 {published data only}
- Paul IM, Yoder KE, Crowell KR, Shaffer ML, McMillan HS, Carlson LC, et al. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics 2004;114:e85‐90. [DOI] [PubMed] [Google Scholar]
Prenner 2006 {published data only}
- Prenner B, Ballona R, Bueso A, Cardona R, Kim K, Larsen L, et al. Safety of desloratadine syrup in children 6 months to younger than 2 years of age: A randomized, double‐blinded, placebo‐controlled study. Pediatric Asthma Allergy & Immunology 2006;19(2):91‐9. [Google Scholar]
Pujet 2002 {published data only}
- Pujet JC, Keddad K, Sévenier F, Jolivet‐Landreau I. Comparative study of two antitussive drugs in the treatment of acute dry cough of infectious origin (prospective, randomized, single blind study) [Etude comparative de deux antitussifs dans le traitement des toux aiguës sèches d'origine infectieuse (étude prospective, randomisée, en simple aveugle)]. Therapie 2002;57(5):457‐63. [PubMed] [Google Scholar]
Reid 1989 {published data only}
- Reid JJ. Double‐blind trial of ketotifen in childhood chronic cough and wheeze. Immunology & Allergy Practice 1989;11(4):143‐50. [Google Scholar]
Shi 1996 {published data only}
- Shi YM, Zhang YQ, Fang SQ. Clinical and experimental studies of zhenkeling oral liquor on treatment infantile cough. Chinese Journal of Integrated Traditional and Western Medicine 1996;16(7):390‐3. [PubMed] [Google Scholar]
Simons 2003 {published data only}
- Simons FE, Silas P, Portnoy JM, Catuogno J, Chapman D, Olufade AO. Safety of cetirizine in infants 6 to 11 months of age: A randomized, double‐blind, placebo‐controlled study. Journal of Allergy & Clinical Immunology 2003;111(6):1244‐8. [DOI] [PubMed] [Google Scholar]
Yoder 2006 {published data only}
- Yoder KE, Shaffer ML, Tournous SJ, Paul IM. Child assessment of dextromethorphan, diphenhydramine, and placebo for nocturnal cough due to upper respiratory infection. Clinical Pediatrics 2006;45(7):633‐40. [DOI] [PubMed] [Google Scholar]
Additional references
Abelson 2004
- Abelson MB, Ferzola NJ, McWhirter CL, Crampton HJ. Efficacy and safety of single‐ and multiple‐dose ketotifen fumarate 0.025% ophthalmic solution in a pediatric population. Pediatric Allergy & Immunology 2004;15(6):551‐7. [DOI] [PubMed] [Google Scholar]
Britt 2002
- Britt H, Miller GC, Knox S, Charles J, Valenti L, Henderson J, et al. Bettering the evaluation and care of health ‐ a study of general practice activity (AIHW Cat. No. GEP‐10). Australian Institute of Health and Welfare, 2002. [Google Scholar]
Busquets 1996
- Busquets RM, Anto JM, Sunyer J, Sancho N, Vall O. Prevalence of asthma‐related symptoms and bronchial responsiveness to exercise in children aged 13‐14 yrs in Barcelona, Spain. European Respiratory Journal 1996;9(10):2094‐8. [DOI] [PubMed] [Google Scholar]
Cates 2003 [Computer program]
- Cates C. Visual Rx. Online NNT calculator. http://www.nntonline.net/: Cates C, 2003.
Chang 1997
- Chang AB, Phelan PD, Sawyer SM, Robertson CF. Airway hyperresponsiveness and cough‐receptor sensitivity in children with recurrent cough. American Journal of Respiratory & Critical Care Medicine 1997;155(6):1935‐9. [DOI] [PubMed] [Google Scholar]
Chang 1999
- Chang AB. Cough, cough receptors, and asthma in children. Pediatric Pulmonology 1999;28(1):59‐70. [DOI] [PubMed] [Google Scholar]
Chang 2001
- Chang AB, Asher MI. A review of cough in children. Journal of Asthma 2001;38(4):299‐309. [DOI] [PubMed] [Google Scholar]
Chang 2005a
- Chang AB. Cough: are children really different to adults?. Cough 2005;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2005b
- Chang AB, Gaffney JT, Eastburn MM, Faoagali J, Cox NC, Masters IB. Cough quality in children: a comparison of subjective vs. bronchoscopic findings. Respiratory Research 2005;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherry 2003
- Cherry DK, Burt CW, Woodwell DA. National Ambulatory Medical Care Survey: 2001 summary. Advance Data 2003;337:1‐44. [PubMed] [Google Scholar]
Clifford 1989
- Clifford RD, Howell JB, Radford M, Holgate ST. Associations between respiratory symptoms, bronchial response to methacholine, and atopy in two age groups of schoolchildren. Archives of Disease in Childhood 1989;64:1133‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clough 1994
- Clough JB, Holgate ST. Episodes of respiratory morbidity in children with cough and wheeze. American Journal of Respiratory & Critical Care Medicine 1994;150:48‐53. [DOI] [PubMed] [Google Scholar]
Cornford 1993
- Cornford CS, Morgan M, Ridsdale L. Why do mothers consult when their children cough?. Family Practice 1993;10(2):193‐6. [DOI] [PubMed] [Google Scholar]
De Sutter 2003
- Sutter AIM, Lemiengre M, Campbell H, Mackinnon HF. Antihistamines for the common cold. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD001267. DOI: 10.1002/14651858.CD001267.] [DOI] [PubMed] [Google Scholar]
Dykewicz 1998
- Dykewicz MS, Fineman S, Skoner DP, Nicklas R, Lee R, Blessing‐Moore J, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Annals of Allergy, Asthma & Immunology 1998;81(5 Pt 2):478‐518. [DOI] [PubMed] [Google Scholar]
Dykewicz 1998b
- Dykewicz MS, Fineman S. Executive summary of joint task force practice parameters on diagnosis and management of rhinitis. Annals of Allergy, Asthma & Immunology 1998;81(5 Pt 2):463‐8. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Gardner 1992 [Computer program]
- Gardner SB, Winter PD, Gardner MJ. Confidence interval analysis. London: British Medical journal, 1992.
Gunn 2001
- Gunn VL, Taha SH, Liebelt EL, Serwint JR. Toxicity of over‐the‐counter cough and cold medications. Pediatrics 2001;108(3):E52. [DOI] [PubMed] [Google Scholar]
Irwin 1998
- Irwin RS, Boulet LP, Cloutier MM, Fuller R, Gold PM, Hoffstein V, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998;114(2 Suppl):133S‐181S. [DOI] [PubMed] [Google Scholar]
Irwin 2006
- Irwin RS. Diagnosis and management of cough: ACCP evidence‐based clinical practice guidelines. Chest 2006;129(1 Suppl):24S. [DOI] [PubMed] [Google Scholar]
Kelly 2004
- Kelly LF. Pediatric cough and cold preparations. Pediatrics in Review 2004;25(4):115‐23. [DOI] [PubMed] [Google Scholar]
Kemp 2006
- Kemp AS. Does post‐nasal drip cause cough in childhood?. Paediatric Respiratory Reviews 2006;7(1):31‐5. [DOI] [PubMed] [Google Scholar]
Mansfield 2008
- Mansfield LE. Fexofenadine in pediatrics: Oral tablet and suspension formulations. Expert Opinion on Pharmacotherapy 2008;9:329‐37. [DOI] [PubMed] [Google Scholar]
McKenzie 1994
- McKenzie S. Cough ‐ but is it asthma?. Archives of Disease in Childhood 1994;70:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 2005
- Meltzer EO. Evaluation of the optimal oral antihistamine for patients with allergic rhinitis. Mayo Clinic Proceedings 2005;80(9):1170‐6. [DOI] [PubMed] [Google Scholar]
Mertsola 1991
- Mertsola J, Ziegler T, Ruuskanen O, Vanto T, Koivikko A, Halonen P. Recurrent wheezy bronchitis and viral respiratory infections. Archives of Disease in Childhood 1991;66:124‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morice 2004
- Morice AH, et al. The diagnosis and management of chronic cough. European Respiratory Journal 2004;24(3):481‐92. [DOI] [PubMed] [Google Scholar]
Peat 1990
- Peat JK, Salome CM, Woolcock AJ. Longitudinal changes in atopy during a 4‐year period: relation to bronchial hyperresponsiveness and respiratory symptoms in a population sample of Australian schoolchildren. Journal of Allergy & Clinical Immunology 1990;85(1 Pt 1):65‐74. [DOI] [PubMed] [Google Scholar]
Schroeder 2004
- Schroeder K, Fahey T. Over‐the‐counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD001831. DOI: 10.1002/14651858.CD001831.pub2.] [DOI] [PubMed] [Google Scholar]
Spector 2003
- Spector SL, Nicklas RA, Chapman JA, Bernstein IL, Berger WE, Blessing‐Moore J, et al. Symptom severity assessment of allergic rhinitis: part 1. Annals of Allergy, Asthma & Immunology 2003;91(2):105‐14. [DOI] [PubMed] [Google Scholar]


