Summary
Background
Blood testosterone concentrations in women decline during the reproductive years and reach a nadir in the seventh decade, after which concentrations increase and are restored to those of reproductive-aged women early in the eighth decade. We aimed to establish the association between the concentration of testosterone in the blood and risk of major adverse cardiovascular events (MACE) and all-cause mortality in healthy older women.
Methods
SHOW was a prospective cohort substudy of the longitudinal randomised ASPREE trial. Eligible participants were women aged at least 70 years from Australia with unimpaired cognition, no previous MACE, and a life expectancy of at least 5 years. Participants who were receiving hormonal or steroid therapy were ineligible for inclusion. We measured serum concentrations of sex steroids with liquid chromatography–tandem mass spectrometry and of SHBG with immunoassay. We compared lower concentrations of sex hormones with higher concentrations using four quartiles. Primary endpoints were risk of MACE and all-cause mortality, the associations of which with sex steroid concentrations were assessed using Cox proportional hazards regression that included age, body-mass index, smoking status, alcohol consumption, diabetes, hypertension, dyslipidaemia, impaired renal function, and treatment allocation in the ASPREE trial (aspirin vs placebo). ASPREE is registered with ClinicalTrials.gov, NCT01038583.
Findings
Of the 9180 women recruited to the ASPREE trial between March 10, 2010, and Dec 31 2014, 6358 participants provided sufficient biobank samples at baseline and 5535 were included in the final analysis. Median age at entry was 74·0 years (IQR 71·7–77·7). During a median 4·4 years of follow-up (24 553 person-years), 144 (2·6%) women had a first MACE (incidence 5·9 per 1000 person-years). During a median 4·6 years of follow-up (3·8–5·6), 200 women died (7·9 per 1000 person-years). In the fully adjusted models, higher concentrations of testosterone were associated with a lower incidence of MACE (quartile 4 vs quartile 1: hazard ratio 0·57 [95% CI 0·36–0·91]; p=0·02), as were higher concentrations of DHEA (quartile 4 vs quartile 1: 0·61 [0·38–0·97]; p=0·04). For oestrone, a lower risk of MACE was seen for concentrations in quartile 2 only, compared with quartile 1 (0·55 [0·33–0·92]; p=0·02). In fully adjusted models, no association was seen between SHBG and MACE, or between any hormone or SHBG and all-cause mortality.
Interpretation
Blood concentrations of testosterone and DHEA above the lowest quartile in older women were associated with a reduced risk of a first-ever MACE. Given that the physiological effects of DHEA are mediated through its steroid metabolites, if the current findings were to be replicated, trials investigating testosterone therapy for the primary prevention of ischaemic cardiovascular disease events in older women would be warranted.
Funding
The National Health and Medical Research Council of Australia, US National Institute on Aging, the Victorian Cancer Agency, the Commonwealth Scientific and Industrial Research Organisation, and Monash University.
Introduction
Oestrogen has been implicated as protective against cardiovascular disease in women.1 Counterintuitively, testosterone, which is present in women in higher serum concentrations than oestrogen across the lifespan, might influence risk of cardiovascular disease in postmenopausal women.2 Blood testosterone concentrations decrease in the order of 25% across women’s reproductive years,3 followed by a more gradual age-related reduction, with no acute change at natural menopause.4,5 A nadir in serum testosterone appears to occur in women aged approximately 62 years, after which serum concentrations of testosterone increase.4,6
We previously measured serum testosterone in women of reproductive age (aged 18–39 years) and in older women (aged ≥70 years) using liquid chromatography–tandem mass spectrometry (LC–MS/MS), which provides precision at low blood concentrations of sex hormones, and showed that the median concentration of serum testosterone in women aged 70–79 years was the same as that in premenopausal women.3,7 Additionally, we observed that serum concentrations of testosterone had a tendency to increase with age in women aged from approximately 70 years onwards.6,8 Dehydroepiandrosterone (DHEA), predominantly made by the adrenal glands, is the main precursor for testosterone and oestrogen production in postmenopausal women, with oestrone being the predominant oestrogen after menopause.9 Compared with testosterone, blood concentrations of DHEA and DHEA sulphate, the circulating reservoir of DHEA, decrease almost linearly with age and continue to decline after age 70 years.4,7,10 The increase in concentrations of serum testosterone in older women suggests that having a high concentration of circulating testosterone either offers a survival advantage or is a marker of longevity.
It has been proposed that low serum testosterone in women is detrimental to cardiovascular health,11 given that testosterone has favourable effects on vasomotor tone, endothelial function, and peripheral vascular resistance at physiological concentrations.12 While data pertaining to sex steroids and risk of ischaemic cardiovascular disease in older women (aged ≥70 years) are scant, studies in women younger than 70 years have provided conflicting results. Most studies have reported that low endogenous concentrations of testosterone were associated with an increased risk of ischaemic cardiac events13–15 and of ischaemic stroke, as evidenced by an increased thickness of the intima and media layers of the carotid artery and by atherosclerosis in women younger than 70 years14,16,17 and in those aged approximately 70 years.13,15,18 Two further studies in women younger than 70 years reported that a high endogenous concentration of testosterone was associated with an increased risk of ischaemic heart disease and death.19,20 By contrast, other studies have found no significant associations between endogenous testosterone and mortality from cardiovascular disease.21,22 Apart from the KORA-F4 cohort study by Schederecker and colleagues,22 all of these studies reported that testosterone concentrations were measured by immunoassays, which can have poor sensitivity and precision for detecting the low concentrations of testosterone seen in women, therefore rendering these findings unreliable.23,24 Furthermore, previous studies have been limited by the reporting of deaths many years after blood samples were collected for sex steroid measurement,13,19,22 as well as by the use of convenience samples and selected samples.13,14,21,22 It is unknown whether DHEA is associated with ischaemic cardiovascular disease and all-cause mortality in women because most studies have only measured DHEA sulphate, of which many reported no association between this steroid and cardiovascular disease events.25–27
Sex hormone-binding globulin (SHBG), which binds testosterone with high affinity, is not only a crucial determinant of the proportion of testosterone, which circulates unbound in plasma, but low SHBG is also an independent risk factor for insulin resistance, type 2 diabetes, and adverse lipid profile in young women and women at midlife, even when other factors are taken into account.28,29,30 Hence, the associations between SHBG and ischaemic cardiovascular disease merit further consideration.
The Sex Hormones in Older Women (SHOW) study was designed to establish the association between the concentration of the main circulating androgen in the blood, testosterone, and risk of major adverse cardiovascular events (MACE) and all-cause mortality in community-dwelling women aged 70 years and older. We hypothesised that low serum concentrations of testosterone were associated with an increased risk of MACE and all-cause mortality in women.
Methods
Study design and participants
The SHOW study was designed as a cohort substudy of the longitudinal randomised ASPREE trial and is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies.31 The ASPREE trial recruited 16 703 participants from Australia aged 70 years and older between March 10, 2010, and Dec 31, 2014, of whom 9180 (55%) participants were female. The study design and recruitment procedures for ASPREE have been published in detail elsewhere.32,33 In brief, study participants were from the southern Australian states of Victoria, South Australia, New South Wales, Tasmania, and the Australian Capital Territory, and were recruited through partnerships with over 2500 primary care practitioners.
People were excluded from the ASPREE trial if they had a life expectancy of less than 5 years, a history of cardiovascular events (including cerebrovascular), a disability (ie, severe difficulty or inability to perform any of the six basic activities of daily living34), dementia or a score of less than 78 on Modified Mini-Mental State Examination,35 a high risk of bleeding or other contraindication to aspirin, clinically significant anaemia, or uncontrolled elevated blood pressure (systolic blood pressure ≥180 mm Hg, diastolic blood pressure ≥105 mm Hg, or both). Participants were excluded from this analysis if they were receiving sex hormones, anti-oestrogens, anti-androgens, or systemic glucocorticoids.
The SHOW substudy of the ASPREE trial was approved by the Monash Human Research Ethics Committee (CF16/10–2016000001) and the Alfred Hospital Human Research Ethics Committee (616/15). The ASPREE trial was also approved by the ethics committee at each participating centre. Participants contributing biological specimens to the ASPREE Healthy Ageing Biobank provided written informed consent. Study centres were randomly audited; completeness and accuracy of the data was monitored by a data quality committee and an independent data and safety monitoring board monitored safety.
Procedures
Non-fasting blood samples were obtained at, or within, 12 months of participant recruitment, and plasma aliquots were stored under nitrogen vapour. Sex steroids were measured by LC–MS/MS in a single plasma sample at the ANZAC Research Institute (Sydney, NSW, Australia).7 Testosterone, DHEA, and oestrone were quantified within a single run without derivatisation, as previously described.7,36,37 Deuterated isotopes were d3-testosterone, d2-DHEA, and d4-oestrone. All steroid standards and internal standards were from the National Measurement Institute (Sydney, NSW, Australia), except for those for d4-oestrone (Steraloids; Newport, RI, USA) and oestrone (Cerilliant; Round Rock, TX, USA). The LC-MS/MS was performed on an API-5000 triple-quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA), equipped with an atmospheric pressure photoionisation source that operated in both positive and negative ion modes. Over three quality control concentrations for measurements, the limit of detection was 0·035 nmol/L for testosterone, 0·070 nmol/L for DHEA, and 3·700 pmol/L for oestrone; the limit of quantification was 0·09 nmol/L for testosterone, 0·17 nmol/L for DHEA, and 11·00 pmol/L for oestrone; the within-run range of the coefficient of variation was 2·0% for testosterone, 3·0–6·0% for DHEA, and 4·7% for oestrone; and the between-run range of the coefficient of variation was 3·9–6·5% for testosterone, 8·0–12·0% for DHEA, and 4·6–7·5% for oestrone.38 SHBG, the main transport protein for sex steroids in the blood, was measured in batches by automated immunoassay (Roche Diagnostics; North Ryde, NSW, Australia) with a coefficient of variation of 1·0–2·0%.
Outcomes
Cardiovascular disease was a prespecified secondary endpoint of the ASPREE trial and included fatal coronary heart disease, non-fatal myocardial infarction, fatal or non-fatal stroke, and fatal or non-fatal hospitalisation for heart failure.32,39 The present analysis is of MACE likely to be due to ischaemia or thrombosis.39 MACE comprised fatal coronary heart disease (but not death from heart failure, which can be due to multiple non-ischaemic causes), non-fatal myocardial infarction, and fatal or non-fatal ischaemic stroke.39 The definitions of each of these outcomes were provided in the ASPREE primary outcome paper.39 All such events were formally adjudicated by expert committees using source documents that included clinical notes, hospitalisation records, and imaging studies (including CT or MRI scans).32 The underlying cause of death was also adjudicated using clinical documentation, death certificate information, and autopsy findings, as previously described.40
Statistical analysis
Categorical data are expressed as percentages. Continuous data are expressed as mean (SD) for normal distributions and median (IQR) or, for hormone concentrations, median (interdecile range [IDR]) for skewed distributions.
For any samples with a hormone concentration below the limits of detection, the concentration was estimated by dividing limits of detection by √2 because this formula has been shown to be minimally biased in overcoming left censoring.41 Such method was applied to measurements for of oestrone (n=88), testosterone (n=126), and DHEA (n=57). To assess the association between serum concentrations of sex hormones and the risk of MACE and all-cause mortality, we used Cox proportional hazards regression analysis. We compared lower concentrations of sex hormones with higher concentrations using four quartiles. We used both graphical displays and testing to examine the proportional hazards assumption. For the proportional hazards assumption, a regression model of scaled Schoenfeld residuals against time was assessed for zero slope and all p values were found to be greater than 0·1, indicating the proportional hazards assumptions for MACE and all-cause mortality were satisfactory. Unadjusted Kaplan-Meier failure curves were used to illustrate the association between sex hormones and the risk of MACE and all-cause mortality. Nelson-Aalen cumulative hazard estimates were used to show event risk. Cox proportional hazards regression models were adjusted for risk factors for cardiovascular disease—namely, age, body-mass index, smoking, and alcohol consumption (ie, current, former, or never), diabetes, hypertension, dyslipidaemia, and impaired renal function, along with treatment allocation in the ASPREE trial (ie, aspirin or placebo). As per the statistical analysis plan for the ASPREE trial, diabetes was defined as the participant self-reporting the condition, having a fasting plasma glucose concentration of at least 126 mg/dL (≥7 mmol/L), or receiving treatment for diabetes at baseline.42 Hypertension was defined as the participant receiving treatment for high blood pressure or having a blood pressure of above 140/90 mm Hg at study entry. Dyslipidaemia was defined as the participant taking cholesterol-lowering medication or having a serum cholesterol of at least 212·0 mg/dL (≥5·0 mmol/L) or LDL concentration of above 160·0 mg/dL (>4·1 mmol/L).42 Impaired renal function was defined as the participant having an estimated glomerular filtration rate of less than 60 mL/min per 1·73 m2. All statistical tests were two-sided and a p value of less than 0·05 was considered to be significant. All statistical analyses were performed using Stata (version 16.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
6392 (69·6%) of the 9180 Australian women recruited onto the ASPREE trial provided biobank samples for the SHOW study, of whom 6358 (99·5%) provided sufficient samples from which baseline serum concentrations of sex hormones and SHBG could be measured (figure 1). Compared with participants who provided a blood sample, those who did not provide a sample were slightly older (median age 75·0 years [IQR 72·4–79·1] vs 73·9 years [71·7–77·5]; p<0·001) and more likely to have hypertension (2145 [76·1%] of 2822 participants vs 4670 [73·4%] of 6358 participants; p=0·01). After excluding 823 women who were taking sex hormones, anti-oestrogens, anti-androgens, or systemic glucocorticoids, 5535 women were included in the final analysis (table 1). At study entry, the included 5535 women were aged 70·0–94·8 years, with a median age of 74·0 years (IQR 71·7–77·7). Most participant characteristics were similar across all testosterone quartiles; however, compared with the other quartiles, more women in in the lowest quartile (quartile 1) had diabetes (10·4% vs 6·5–7·4%) and more women in the highest quartile (quartile 4) were current smokers (3·5% vs 2·0–3·2%).
Figure 1:
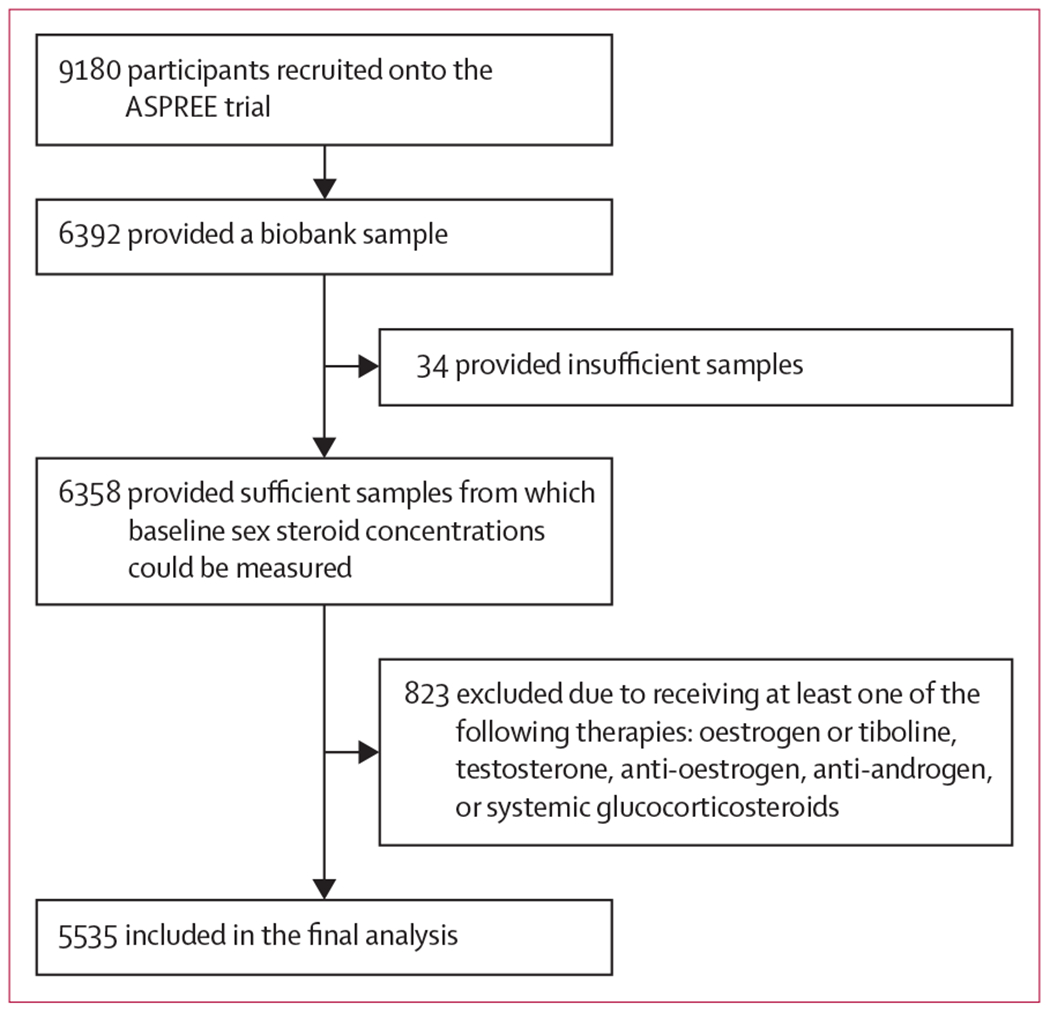
Study profile
Table 1:
Baseline characteristics of study participants overall and by testosterone quartile
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| Number of participants | 5535 (100·0%) | 1641 (29·6%) | 1104 (20·0%) | 1497 (27·0%) | 1293 (23·4%) |
|
| |||||
| Age, years | 74·0 (71·7–77·7) | 73·9 (71·6–77·4) | 73·9 (71·7–77·8) | 73·7 (71·5–77·2) | 74·5 (72·0–78·1) |
|
| |||||
| Age distribution, years | |||||
| 70–74 | 2781 (50·2%) | 847 (51·6%) | 557 (50·5%) | 792 (52·9%) | 585 (45·2%) |
| 75–79 | 1745 (31·5%) | 501 (30·5%) | 351 (31·8%) | 442 (29·5%) | 451 (34·9%) |
| 80–84 | 760 (13·7%) | 220 (13·4%) | 143 (13·0%) | 205 (13·7%) | 192 (14·8%) |
| ≥85 | 249 (4·5%) | 73 (4·4%) | 53 (4·8%) | 58 (3·9%) | 65 (5·0%) |
|
| |||||
| Weight, kg* | 69·8 (61·9–79·2) | 69·0 (61·8–79·0) | 69·6 (62·0–79·0) | 70·4 (61·6–79·6) | 69·8 (62·2–79·3) |
|
| |||||
| Height, cm† | 159·0 (155·0–163·0) | 159·0 (155·0–163·0) | 159·0 (155·0–164·0) | 160·0 (155·0–163·0) | 159·0 (155·0–163·0) |
|
| |||||
| Body-mass index, kg/m2‡ | |||||
| <18·5 | 44 (0·8%) | 15 (0·9%) | 9 (0·8%) | 11 (0·7%) | 9 (0·7%) |
| 18·5–24·9 | 1560 (28·2%) | 479 (29·2%) | 296 (26·8%) | 429 (28·7%) | 356 (27·5%) |
| 25·0–29·9 | 2187 (39·5%) | 635 (38·7%) | 461 (41·8%) | 575 (38·4%) | 516 (40·0%) |
| ≥30·0 | 1715 (31·0%) | 504 (30·7%) | 332 (30·1%) | 477 (31·9%) | 402 (31·1%) |
|
| |||||
| Ethnicity | |||||
| European ancestry | 5470 (98·8%) | 1624 (99·0%) | 1091 (98·8%) | 1475 (98·5%) | 1280 (99·0%) |
| Other§ | 65 (1·2%) | 17 (1·0%) | 13 (1·2%) | 22 (1·5%) | 13 (1·0%) |
|
| |||||
| Blood pressure, mm Hg | |||||
| Systolic | 137·0 (126·0–149·0) | 136·0 (126·0–149·0) | 138·0 (127·0–150·0) | 138·0 (127·0–150·0) | 137·0 (127·0–149·0) |
| Diastolic | 76·0 (70·0–83·0) | 75·0 (69·0–82·0) | 76·0 (70·0–84·0) | 77·0 (70·0–84·0) | 76·0 (70·0–84·0) |
|
| |||||
| Smoking status | |||||
| Current | 156 (2·8%) | 33 (2·0%) | 35 (3·2%) | 43 (2·9%) | 45 (3·5%) |
| Former | 1739 (31·4%) | 524 (31·9%) | 353 (32·0%) | 470 (31·4%) | 392 (30·3%) |
| Never | 3640 (65·8%) | 1084 (66·1%) | 716 (64·9%) | 984 (65·7%) | 856 (66·2%) |
|
| |||||
| Alcohol consumption | |||||
| Current | 4130 (74·6%) | 1254 (76·4%) | 830 (75·2%) | 1116 (74·5%) | 930 (71·9%) |
| Former | 216 (3·9%) | 67 (4·1%) | 38 (3·4%) | 66 (4·4%) | 45 (3·5%) |
| Never | 1189 (21·5%) | 320 (19·5%) | 236 (21·4%) | 315 (21·0%) | 318 (24·6%) |
|
| |||||
| Cardiovascular disease risk factors | |||||
| Diabetes | 440 (7·9%) | 170 (10·4%) | 82 (7·4%) | 98 (6·5%) | 90 (7·0%) |
| Hypertension | 4055 (73·3%) | 1174 (71·5%) | 816 (73·9%) | 1108 (74·0%) | 957 (74·0%) |
| Dyslipidaemia | 4268 (77·1%) | 1266 (77·1%) | 849 (76·9%) | 1151 (76·9%) | 1002 (77·5%) |
| Impaired renal function¶ | 1007 (18·8%) | 311 (19·0%) | 190 (17·2%) | 245 (16·4%) | 261 (20·2%) |
Data are n (%) or median (IQR).
Data available for 5517 participants.
Data available for 5521 participants.
Data available for 5506 participants.
Other includes 32 Asian participants and seven Aboriginal or Torres Strait Islander participants.
Data available for 5382 participants.
Over a median 4·4 years (IQR 3·5–5·5) of follow-up (24 553 person-years), 144 (2·6%) of the 5535 women had a first-ever MACE, with an incidence rate of 5·9 events per 1000 person-years. These events included a non-fatal myocardial infarction in 57 (39·6%) women, a non-fatal ischaemic stroke in 66 (45·8%) women, and both in one woman (0·7%). 20 (13·9%) women had a fatal MACE. Six (4·2%) of the 144 women who had a first-ever MACE died of other causes during follow-up, including one women who had previously had a non-fatal myocardial infarction and five women who had previously had a non-fatal ischaemic stroke.
With the lowest quartile (quartile 1) as the reference, the risk of MACE and all-cause mortality by quartile of each hormone and SHBG were examined with and without adjustment for risk factors for cardiovascular disease (table 2). After adjusting for age, body-mass index, hypertension, dyslipidaemia, diabetes, impaired renal function, smoking status, alcohol consumption, and treatment allocation, serum testosterone concentrations in quartiles 3 and 4 were associated with a significantly lower incidence of a first-ever MACE than were concentrations in quartile 1. Although a similarly low risk was seen for women with a testosterone concentration in quartile 2, this risk did not reach statistical significance (p=0·09). Compared with quartile 1, all higher quartiles of DHEA were associated with a significantly lower risk of MACE in the fully adjusted models. For oestrone, a significantly lower risk of MACE was only seen for women with a concentration in quartile 2, compared with those with a concentration in quartile 1. SHBG was not associated with risk of MACE.
Table 2:
Associations between serum concentrations of sex steroids and SHBG and risk of MACE and all-cause mortality
| Median blood concentration (IDR) | Number of samples | MACE (n=144) |
All-cause mortality (n=200) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis |
Adjusted analysis |
Unadjusted analysis |
Adjusted analysis |
|||||||
| HR (95% CI) | p value* | HR (95% CI) | p value† | HR (95% CI) | p value* | HR (95% CI) | p value† | |||
| Oestrone | ||||||||||
|
| ||||||||||
| Quartile 1 | 96·2 pmol/L (48·1–122·1)‡ | 1405 (25·4%) | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Quartile 2 | 155·3 pmol/L (133·1–177·5)‡ | 1425 (25·7%) | 0·62 (0·38–1·02) | 0·061 | 0·55 (0·33–0·92) | 0·022 | 1·27 (0·83–1·93) | 0·27 | 1·28 (0·84–1·96) | 0·26 |
| Quartile 3 | 218·2 pmol/L (188·6–258·9)‡ | 1426 (25·8%) | 1·01 (0·68–1·60) | 0·86 | 0·92 (0·60–1·43) | 0·72 | 1·30 (0·86–1·98) | 0·21 | 1·25 (0·82–1·91) | 0·30 |
| Quartile 4 | 336·6 pmol/L (284·8–484·5)‡ | 1279 (23·1%) | 0·91 (0·58–1·44) | 0·70 | 0·69 (0·43–1·11) | 0·12 | 1·46 (0·96–2·21) | 0·074 | 1·31 (0·85–2·01) | 0·22 |
|
| ||||||||||
| Testosterone | ||||||||||
|
| ||||||||||
| Quartile 1 | 0·17 nmol/L (0·10–0·24)§ | 1641 (29·6%) | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Quartile 2 | 0·31 nmol/L (0·28–0·35)§ | 1104 (20·0%) | 0·70 (0·44–1·12) | 0·13 | 0·67 (0·42–1·07) | 0·094 | 0·90 (0·60–1·36) | 0·62 | 0·87 (0·56–1·29) | 0·44 |
| Quartile 3 | 0·45 nmol/L (0·38–0·55)§ | 1497 (27·0%) | 0·66 (0·43–1·01) | 0·06 | 0·64 (0·41–0·99) | 0·045 | 1·02 (0·71–1·47) | 0·92 | 1·02 (0·70–1·47) | 0·93 |
| Quartile 4 | 0·79 nmol/L (0·59–1·98)§ | 1293 (23·4%) | 0·65 (0·42–1·02) | 0·064 | 0·57 (0·36–0·91) | 0·018 | 0·94 (0·64–1·39) | 0·77 | 0·83 (0·56–1·23) | 0·36 |
|
| ||||||||||
| DHEA | ||||||||||
|
| ||||||||||
| Quartile 1 | 1·11 nmol/L (0·52–1·53)¶ | 1307 (23·6%) | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Quartile 2 | 2·08 nmol/L (1·70–2·46)¶ | 1420 (25·7%) | 0·57 (0·37–0·89) | 0·012 | 0·60 (0·38–0·94) | 0·026 | 1·11 (0·76–1·62) | 0·58 | 1·35 (0·92–1·98) | 0·13 |
| Quartile 3 | 3·19 nmol/L (2·70–3·81)¶ | 1422 (25·7%) | 0·49 (0·31–0·78) | 0·002 | 0·57 (0·36–0·90) | 0·017 | 0·73 (0·48–1·10) | 0·14 | 0·93 (0·61–1·42) | 0·73 |
| Quartile 4 | 5·58 nmol/L (4·19–8·60)¶ | 1386 (25·0%) | 0·52 (0·33–0·81) | 0·004 | 0·61 (0·38–0·97) | 0·037 | 0·78 (0.52–1·18) | 0·24 | 1·08 (0·71–1·64) | 0·72 |
|
| ||||||||||
| SHBG | ||||||||||
|
| ||||||||||
| Quartile 1 | 25·10 nmol/L (17·70–29·80) | 1383 (25·0%) | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Quartile 2 | 36·20 nmol/L (32·00–40·60) | 1423 (25·8%) | 0·92 (0·58–1·46) | 0·73 | 0·82 (0·51–1·33) | 0·43 | 1·09 (0·72–1·67) | 0·67 | 0·98 (0·63–1·51) | 0·92 |
| Quartile 3 | 47·40 nmol/L (42·60–53·20) | 1409 (25·5%) | 0.96 (0·61–1·52) | 0·87 | 0·85 (0·52–1·38) | 0·52 | 1·09 (0·71–1·66) | 0·70 | 0·88 (0·56–1·37) | 0·57 |
| Quartile 4 | 65·80 nmol/L (56·80–91·20) | 1309 (23·7%) | 1·05 (0·66–1·66) | 0·83 | 0·89 (0·54–1·48) | 0·65 | 1·76 (1·19–2·60) | 0·004 | 1·32 (1·10–2·04) | 0·20 |
SHBG=sex hormone-binding globulin. MACE=major adverse cardiovascular events. IDR=interdecile range. HR=hazard ratio. DHEA=dehydroepiandrosterone.
Separate univariable models fitted for each sex steroid.
Models for each hormone were all adjusted for age, body-mass index, smoking status, alcohol consumption, diabetes, dyslipidaemia, hypertension, impaired renal function, and treatment allocation (aspirin vs placebo). Quartiles did not include exactly 25% of the observations for each hormone because for some hormones, especially oestrone and testosterone, many women shared the same value.
To convert to pg/mL, divide by 3·699.
To convert to ng/dL, divide by 0·0347.
To convert to mg/L, divide by 3·467.
The cumulative hazards of MACE by quartile of testosterone concentration suggested the highest risk of events to be in women with testosterone concentrations in the lowest quartile (quartile 1), which appeared to emerge after year 2 of follow-up and be significant by year 3 (HR 0·53, 95% CI 0·34–0·82; p=0·005; figure 2A). Divergence of the curve for the lowest quartile of DHEA appeared earlier, with quartile 1 significantly different by year 3 (0·52, 0·33–0·81; p=0·004; figure 2B). The curves for quartile 1 for both testosterone and DHEA continued to rise more rapidly than those for the other quartiles, suggesting a favourable effect of testosterone and DHEA concentrations above quartile 1 for each hormone. The cumulative hazards of MACE did not differ between the oestrone quartiles (figure 2C).
Figure 2: Cumulative hazard estimates for major adverse cardiovascular events by quartiles of sex steroid concentrations in the blood.
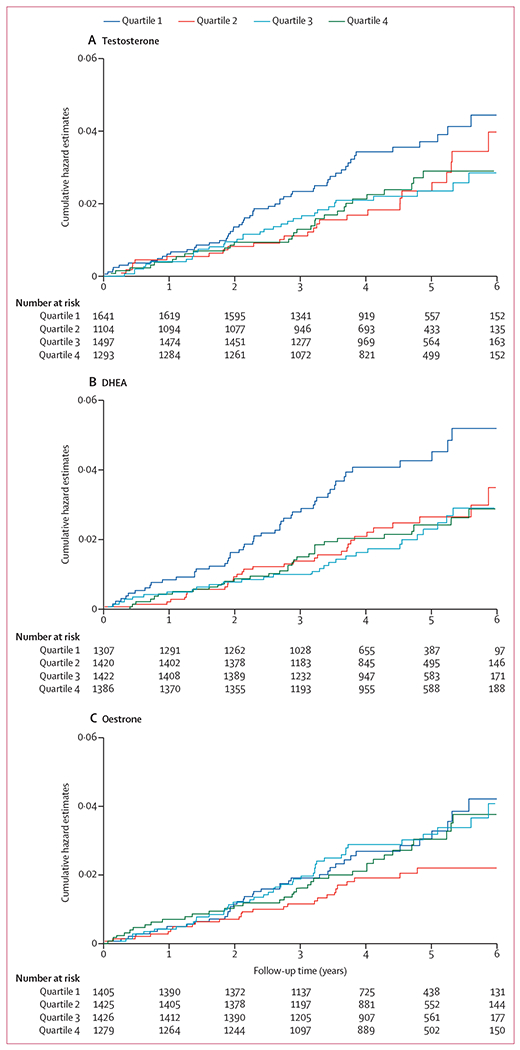
Cumulative hazard estimates provided for testosterone (A), DHEA (B), and oestrone (C). DHEA=dehydroepiandrosterone.
Over a median 4·6 years (IQR 3·8–5·6) of follow-up (25 295 person-years), 200 (3·6%) of the 5535 women died, an incidence rate of 7·9 deaths per 1000 person-years. Of these deaths, 37 were adjudicated as being cardiovascular events, including 20 fatal MACE, 108 were due to cancer, 12 were due to major haemorrhage (including haemorrhagic stroke), 42 were due to other causes, and one was of unknown cause. In both the unadjusted and adjusted models, none of the serum concentrations of sex steroids or SHGB were associated with all-cause mortality (table 2).
Discussion
This study showed that, among healthy women aged 70 years and older, blood concentrations of testosterone and DHEA above the lowest quartile were associated with nearly half the risk of a MACE, independent of traditional risk factors for cardiovascular disease. A consistent association between oestrone, the main circulating oestrogen after menopause, and MACE was not seen, despite DHEA being the precursor for both oestrone and testosterone production.43 The apparent protective effects of testosterone and DHEA appeared to emerge early, with the higher three quartiles (quartiles 2–4) for each hormone tracking together. This overall pattern suggests that, although concentrations of testosterone and DHEA above quartile 1 were associated with a reduced risk of MACE, a concentration of either testosterone or DHEA above quartile 2 conveyed no additional benefit.
Our findings dispel the previous assumption that a high concentration of testosterone in the blood is associated with an increased risk of cardiovascular disease in older women. In support of our findings, Meun and colleagues44 observed that, in postmenopausal women with a mean age of 70 years, risk of cardiovascular disease did not differ between individuals with a serum testosterone concentration in the highest quartile and those with a concentration in the two middle quartiles. The outcomes for women with testosterone in the lowest quartile were not reported.44 Although other studies have reported that postmenopausal women with low serum testosterone were at increased risk of cardiovascular disease, methodological limitations, including the use of immunoassays for testosterone measurement, have rendered their findings to be less robust.13,14 Consistent with our findings, the KORA-F4 cohort study by Schederecker and colleagues22 showed that testosterone measured by LC–MS/MS was not significantly associated with all-cause mortality in women aged 45–82 years. To our knowledge, the SHOW study is the first to demonstrate a significant association between a relatively low concentration of DHEA in the blood and an increased risk of ischaemic cardiovascular disease events, with an earlier study reporting no association between baseline serum DHEA concentrations and cardiovascular events over 12 years in younger women (mean age 64·9 years).20
DHEA is an adrenal prohormone with little, or no, intrinsic oestrogenic or androgenic activity.25,45 Instead, the biological effects of DHEA are mediated through its metabolism via androstenedione to testosterone and oestrone. DHEA sulphate, formed from DHEA in the adrenal glands, is the most abundant steroid in women’s blood.46 Concentrations of DHEA and DHEA sulphate decrease, almost linearly, with age.4 Low DHEA concentrations might reflect poor overall health; therefore, such concentrations could be a consequence rather than a cause of disease.47 However, despite the fact that production of testosterone and oestrone in postmenopausal women is dependent on DHEA as a substrate (even in older women), DHEA sulphate circulates in micromolar concentrations, an order of magnitude higher than bioactive sex steroids, which provides an adequate reservoir for peripheral tissue availability of DHEA and, hence, oestrone and testosterone.46 Therefore, production of testosterone and oestrone in postmenopausal women does not seem to be limited by substrate but is dependent on the capacity of peripheral tissues to convert DHEA into these active hormones.48,49 Consequently, in women aged 70 years and older, although serum DHEA concentrations are about half of those observed in premenopausal women, blood testosterone concentrations are restored to, and maintained at, the concentrations seen in premenopausal women. When measured by LC’MS/MS, the median concentration of testosterone across the menstrual cycle in women aged 18–39 years is 0·34 nmol/L (IQR 0·04–1·01).3
We did not include oestradiol concentration in our analysis. Although 5447 (98·4%) of the women in our study had measurable oestrone concentrations, 66·1% of participants had oestradiol concentrations below our assay’s limit of detection.50 Besides oestrone being the main circulating oestrogen after the menopause, we have previously shown that blood oestrone concentration is a robust surrogate for blood oestradiol in older postmenopausal women.50 The association between an increased rate of accumulation of MACE over time and low serum concentrations, as observed for both testosterone and DHEA, was not seen in women with a low serum oestrone concentration. This finding, taken together with evidence that increasing oestrone with oestrogen replacement therapy does not protect against MACE in older postmenopausal women,51,52 suggests that the apparent protective effect of DHEA in this age group of women is likely to be mediated by testosterone.
The associations between MACE and serum concentrations of testosterone and DHEA were independent of known risk factors for cardiovascular disease, suggesting that the effects of high concentrations of these sex steroids on MACE risk is mediated through other pathways. Supporting this hypothesis, treatment of postmenopausal women younger than 70 years with transdermal testosterone, at a dose that increases blood testosterone concentrations to the high end of the physiological range for premenopausal women, has neutral effects on serum lipids concentrations and carbohydrate metabolism in women.53 Other protective effects of testosterone might be operative. Lower endogenous testosterone concentrations in women have been associated with poorer vascular endothelial function after the menopause.54 Exogenous testosterone enhances endothelium-dependent and endothelium-independent brachial artery vasodilation in postmenopausal women.55 Additionally, when administered as a single dose, exogenous testosterone caused an acute drop in systolic blood pressure, in the order of 10 mm Hg.56 These effects have been shown to be independent of aromatisation of testosterone to oestradiol.57
Animal models have provided some insight into effects of testosterone in the context of myocardial ischaemia. Studies of myocardial ischaemia–reperfusion in female aromatase-knockout mice, which have a complete depletion of oestrogen but an elevated concentration of blood testosterone, as well as in oophorectomised mice, have shown that testosterone protected the myocardium from ischaemic injury.58,59
Only one placebo-controlled trial of testosterone therapy in women with cardiovascular disease has been reported. In this pilot study of women with severe heart failure and a mean age of 68 years, participants randomly allocated to receive transdermal testosterone had significant improvements in the 6-min walk test and in oxygen consumption, compared with those who received placebo.60 A safety study of transdermal testosterone versus placebo in postmenopausal women at increased risk of cardiovascular disease, which applied a novel adaptive design to optimise study power, accrued over 7000 women-years of data.61 Although the safety monitoring committee recommended continuation of this study after six unmasked reviews, the study was halted because of insufficient funds. Specifically, trials of testosterone to prevent ischaemic events in healthy women have not been reported.
Low SHBG concentration has been identified as an independent risk factor for insulin resistance, type 2 diabetes, and dyslipidaemia in young women and in women at midlife.62–64 Despite diabetes being a strong risk factor for cardiovascular disease in women, we found no association between SHBG concentrations and MACE. Our findings are consistent with that of Zhao and colleagues,20 who reported no association between SHBG concentration and incident cardiovascular disease in women with a mean age of 65 years. The proportion of women with diabetes in that study20 was greater than in our cohort, suggesting that our findings are not simply reflecting a slightly lower prevalence of diabetes in our study sample than is seen in the general population.
Strengths of this study include the large sample of relatively healthy women aged 70 years or older, rigorous screening at baseline, measurement of sex steroids by the gold standard method of LC–MS/MS, and formal adjudication of all MACE endpoints. Given that our cohort comprised healthy volunteers, our study sample is not representative of all older women in the general population. However, only including women with no previous MACE and a life expectancy of at least 5 years enhanced our capacity to explore the associations between blood concentrations of sex steroids and SHBG and the risk of MACE in the absence of extreme comorbidity. Although our analysis is based on a single measurement of serum concentrations of sex steroids, through samples taken 3 years apart, we have previously shown that, in women aged 70 years and older, testosterone concentrations (on average) increased only modestly with advancing age, with no change in DHEA.8 Thus, a single measurement provided a sound basis for our analysis.
One limitation of our study is that, being observational in nature, our study cannot ascertain conclusively whether the associations between blood testosterone and DHEA concentrations and risk of MACE were causative effects. Another limitation is that participants in our study were primarily of European ancestry, consistent with the older Australian population.65 Therefore, our findings should not be generalised to women of other ancestries.
In conclusion, in healthy women from Australia aged 70 years and older, having blood testosterone and DHEA concentrations above the lowest quartile appears to be cardioprotective. Replication of these findings would justify the consideration of trials investigating testosterone therapy for the primary prevention of ischaemic cardiovascular disease events in older women with low circulating concentrations of testosterone.
Research in context.
Evidence before this study
We searched PubMed with no language restrictions for articles published from date of inception to Sept 1, 2021, using the search terms “testosterone”, “DHEA”, “postmenopausal women”, “cardiovascular disease”, “stroke”, and “mortality”. Most studies that have investigated associations between circulating concentrations of testosterone and cardiovascular disease and all-cause mortality in postmenopausal women suggested that lower concentrations of testosterone were associated with a greater risk of cardiovascular disease. However, previous studies had considerable limitations—namely, testosterone concentrations were not measured with sufficiently sensitive methods required to precisely identify the low blood testosterone concentrations seen in women, compared with men.
Added value of this study
To our knowledge, this study constitutes the largest, prospective, longitudinal study of women aged 70 years and older, which measured sex hormones with liquid chromatography–tandem mass spectrometry and adjudicated major adverse cardiovascular events and all-cause mortality. The findings establish the importance of androgens in older women in relation to cardiovascular health, challenging the previous assumption that androgens exert deleterious cardiovascular effects in older women.
Implications of all the available evidence
These findings suggest that relatively low blood concentrations of testosterone and DHEA are physiologically disadvantageous in terms of risk of major adverse cardiovascular events. However, further studies of testosterone in older women are needed to replicate our findings and to establish whether testosterone supplementation would provide benefits that outweigh any associated risks in postmenopausal women.
Acknowledgments
The ASPREE trial was supported by the US National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant numbers U01AG029824 and U19AG062682); the National Health and Medical Research Council of Australia (NHMRC; grant numbers 34047 and 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia). The ASPREE Healthy Ageing Biobank was funded by the CSIRO (flagship grant), the National Cancer Institute (grant number U01 AG029824), and Monash University. This analysis of sex hormones was funded by an NHMRC Project Grant (number 1105305). SRD is an NHMRC Australian Senior Principal Research Fellow (grant number 1135843) and CMR is an NHMRC Principal Research Fellow (Grant 1136372). The funding bodies had no role in study design, collection, analysis, or interpretation of the data, or the writing of this report.
Footnotes
Declarations of interests
SRD reports honoraria from Besins Healthcare, Mayne Pharma, Pfizer Australia, BioFemme, Lawley Pharmaceuticals, Southern Star Research, and Que Oncology. SRD also reports serving on advisory boards for Mayne Pharma, Astellas Pharmaceuticals, Roche Diagnostics, Theramex, and Abbott Pharmaceuticals, and is an institutional investigator for Que Oncology and Ovoca Bio. DJH has received institutional grant funding (but no personal income) for investigator-initiated clinical testosterone pharmacology studies (Lawley, Besins Healthcare) and has provided expert testimony to anti-doping and professional standards tribunals and testosterone litigation. MRN has served on a Novartis advisory board. AMT reports serving on advisory boards or receiving honoraria for lectures from Amgen, Bayer, Boehringer-Ingelheim, Merck, Novartis, and Pfizer and as a data safety monitoring board member for Medicines Group and Novartis. All other authors declare no competing interests.
Data sharing statement
After deidentification (ie, text, tables, figures, and supplementary material), individual participant data will be made available. On application, meta-data and a data dictionary will be made available to others. The ASPREE study protocol is available on the ASPREE website. The ASPREE trial statistical analysis plan is published.66 On request, a copy of the clinical trial consent form can be made available. Data will be available on publication of this Article. Requests for data access will be via the ASPREE Principal Investigators with details for applications provided through SHOW substudy data on sex hormones can be requested through this system with approval by the corresponding author. Data will be made available to investigators whose proposed use of the data, registered as a project through the ASPREE Access Management Site, has been approved by a review committee. Access will be through a secure web-based data portal (the ASPREE Safe Haven system), based at Monash University (Monash, VIC, Australia).
References
- 1.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 2005; 308: 1583–87. [DOI] [PubMed] [Google Scholar]
- 2.Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med 2014. [DOI] [PubMed] [Google Scholar]
- 3.Skiba MA, Bell RJ, Islam RM, Handelsman DJ, Desai R, Davis SR. Androgens during the reproductive years, what’s normal for women? J Clin Endocrinol Metab 2019; 104: 5382–92. [DOI] [PubMed] [Google Scholar]
- 4.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005; 90: 3847–53. [DOI] [PubMed] [Google Scholar]
- 5.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 2000; 85: 2832–38. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Ratcliffe SJ, Bhasin S, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab 2007; 92: 509–16. [DOI] [PubMed] [Google Scholar]
- 7.Davis SR, Bell RJ, Robinson PJ, et al. Testosterone and estrone increase from the age of 70 years; findings from the Sex Hormones in Older Women Study. J Clin Endocrinol Metab 2019;104: 6291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam RM, Bell RJ, Handelsman DJ, et al. Longitudinal changes over three years in sex steroid hormone levels in women aged 70 years and over. Clin Endocrinol (Oxf) 2021; 94: 443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids 1998; 63: 322–28. [DOI] [PubMed] [Google Scholar]
- 10.Labrie F, Bélanger A, Cusan L, Gomez J-L, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 1997; 82: 2396–402. [DOI] [PubMed] [Google Scholar]
- 11.Spoletini I, Vitale C, Pelliccia F, Fossati C, Rosano GM. Androgens and cardiovascular disease in postmenopausal women: a systematic review. Climacteric 2014; 17: 625–34. [DOI] [PubMed] [Google Scholar]
- 12.Jones RD, Hugh Jones T, Channer KS. The influence of testosterone upon vascular reactivity. Eur J Endocrinol 2004; 151: 29–37. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab 2010; 95: 740–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sievers C, Klotsche J, Pieper L, et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur J Endocrinol 2010; 163: 699–708. [DOI] [PubMed] [Google Scholar]
- 15.Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM. Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab 2010; 95: 1889–97. [DOI] [PubMed] [Google Scholar]
- 16.Bernini GP, Sgro’ M, Moretti A, et al. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 1999; 84: 2008–12. [DOI] [PubMed] [Google Scholar]
- 17.Golden SH, Maguire A, Ding J, et al. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol 2002; 155: 437–45. [DOI] [PubMed] [Google Scholar]
- 18.Debing E, Peeters E, Duquet W, Poppe K, Velkeniers B, Van den Brande P. Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy. Eur J Endocrinol 2007; 156: 687–93. [DOI] [PubMed] [Google Scholar]
- 19.Benn M, Voss SS, Holmegard HN, Jensen GB, Tybjærg-Hansen A, Nordestgaard BG. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol 2015; 35: 471–77. [DOI] [PubMed] [Google Scholar]
- 20.Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 2018; 71: 2555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A, Gerstein HC, Lee SF, et al. Testosterone and sex hormone-binding globulin in dysglycemic women at high cardiovascular risk: a report from the Outcome Reduction with an Initial Glargine Intervention trial. Diab Vasc Dis Res 2021; 18: 14791641211002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schederecker F, Cecil A, Prehn C, et al. Sex hormone-binding globulin, androgens and mortality: the KORA-F4 cohort study. Endocr Connect 2020; 9: 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab 2010; 95: 4542–48. [DOI] [PubMed] [Google Scholar]
- 24.Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem 2003; 49: 1381–95. [DOI] [PubMed] [Google Scholar]
- 25.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies Eur J Endocrinol 2004; 151: 1–14. [DOI] [PubMed] [Google Scholar]
- 26.Jia X, Sun C, Tang O, et al. Plasma dehydroepiandrosterone sulfate and cardiovascular disease risk in older men and women J Clin Endocrinol Metab 2020; 105: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shufelt C, Bretsky P, Almeida CM, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health--National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab 2010;95: 4985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009; 361: 1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis SR, Robinson PJ, Moufarege A, Bell RJ. The contribution of SHBG to the variation in HOMA-IR is not dependent on endogenous oestrogen or androgen levels in postmenopausal women. Clin Endocrinol (Oxf) 2012; 77: 541–47 [DOI] [PubMed] [Google Scholar]
- 30.Worsley R, Robinson PJ, Bell RJ, Moufarege A, Davis SR. Endogenous estrogen and androgen levels are not independent predictors of lipid levels in postmenopausal women. Menopause 2013; 20: 640–45. [DOI] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–49. [DOI] [PubMed] [Google Scholar]
- 32.Group AI. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013; 36: 555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeil JJ, Woods RL, Nelson MR, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci 2017; 72: 1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976; 6: 493–508. [DOI] [PubMed] [Google Scholar]
- 35.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48: 314–18. [PubMed] [Google Scholar]
- 36.Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta 2009; 409: 78–84. [DOI] [PubMed] [Google Scholar]
- 37.Keski-Rahkonen P, Desai R, Jimenez M, Harwood DT,Handelsman DJ. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem 2015; 87: 7180–86. [DOI] [PubMed] [Google Scholar]
- 38.Hsu B, Cumming RG, Hirani V, et al. Temporal trend in androgen status and androgen-sensitive outcomes in older men. J Clin Endocrinol Metab 2016; 101: 1836–46. [DOI] [PubMed] [Google Scholar]
- 39.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly N Engl J Med 2018; 379: 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018;379: 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handelsman DJ, Ly LP. An accurate substitution method to minimize left censoring bias in serum steroid measurements. Endocrinology 2019; 160: 2395–400. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe R, Murray AM, Woods RL, et al. The aspirin in reducing events in the elderly trial: statistical analysis plan. Int J Stroke 2018; 13: 335–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labrie F All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol 2015; 145: 133–38. [DOI] [PubMed] [Google Scholar]
- 44.Meun C, Franco OH, Dhana K, et al. High androgens in postmenopausal women and the risk for atherosclerosis and cardiovascular disease: the Rotterdam Study. J Clin Endocrinol Metab 2018; 103: 1622–30. [DOI] [PubMed] [Google Scholar]
- 45.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab 2011; 96: 1642–53. [DOI] [PubMed] [Google Scholar]
- 46.Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 1986; 15: 213–28. [DOI] [PubMed] [Google Scholar]
- 47.Ohlsson C, Vandenput L, Tivesten A. DHEA and mortality: what is the nature of the association? J Steroid Biochem Mol Biol 2015;145: 248–53. [DOI] [PubMed] [Google Scholar]
- 48.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA The regulation of steroid action by sulfation and desulfation. Endocr Rev 2015; 36: 526–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005; 26: 171–202. [DOI] [PubMed] [Google Scholar]
- 50.Davis SR, Martinez-Garcia A, Robinson PJ, et al. Estrone is a strong predictor of circulating estradiol in women age 70 years and older. J Clin Endocrinol Metab 2020; 105: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291: 1701–12. [DOI] [PubMed] [Google Scholar]
- 52.Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015; 3: CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam RM, Bell RJ, Green S, Page MJ, Davis SR. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol 2019; 7: 754–66. [DOI] [PubMed] [Google Scholar]
- 54.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F,Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis 2007; 18: 9–13. [DOI] [PubMed] [Google Scholar]
- 55.Worboys S, Kotsopoulos D, Teede H, McGrath BP, Davis SR. Parental testosterone improves endothelium-dependent and independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 2001; 86: 158–61. [DOI] [PubMed] [Google Scholar]
- 56.Davison S, Thipphawong J, Blanchard J, et al. Pharmacokinetics and acute safety of inhaled testosterone in postmenopausal women. J Clin Pharmacol 2005; 45: 177–84. [DOI] [PubMed] [Google Scholar]
- 57.Navarro-Dorado J, Orensanz LM, Recio P, et al. Mechanisms involved in testosterone-induced vasodilatation in pig prostatic small arteries. Life Sci 2008; 83: 569–73. [DOI] [PubMed] [Google Scholar]
- 58.Bell JR, Mellor KM, Wollermann AC, et al. Aromatase deficiency confers paradoxical postischemic cardioprotection. Endocrinology 2011; 152: 4937–47. [DOI] [PubMed] [Google Scholar]
- 59.Fu L, Liu Y, Wang J, et al. Cardioprotection by low-dose of estrogen and testosterone at the physiological ratio on ovariectomized rats during ischemia/reperfusion injury. J Cardiovasc Pharmacol 2017; 70: 87–93. [DOI] [PubMed] [Google Scholar]
- 60.Iellamo F, Volterrani M, Caminiti G, et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol 2010; 56: 1310–16. [DOI] [PubMed] [Google Scholar]
- 61.Snabes MC, Berry SD, Berry DA, Zborowski JD, White WB. Low cardiovascular event rate in postmenopausal women with increased cardiac risk: initial findings from the ongoing blinded Libigel (testosterone gel) cardiovascular safety study. 92nd Annual Meeting of the Endocrine Society; June 19-22, 2010. (abstr P2-419). [Google Scholar]
- 62.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009; 361: 1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis SR, Robinson PJ, Moufarege A, Bell RJ. The contribution of SHBG to the variation in HOMA-IR is not dependent on endogenous oestrogen or androgen levels in postmenopausal women. Clin Endocrinol (Oxf) 2012; 77: 541–47 [DOI] [PubMed] [Google Scholar]
- 64.Worsley R, Robinson PJ, Bell RJ, Moufarege A, Davis SR. Endogenous estrogen and androgen levels are not independent predictors of lipid levels in postmenopausal women. Menopause 2013; 20: 640–45. [DOI] [PubMed] [Google Scholar]
- 65.Australian Bureau of Statistics. Reflecting a nation: stories from the 2011 Census, 2012-2013. https://www.abs.gov.au/ausstats/abs@.nsf/lookup/2071.0main+features902012-2013 (accessed June 29, 2021).
- 66.Wolfe R, Murray AM, Woods RL, et al. The aspirin in reducing events in the elderly trial: statistical analysis plan. Int J Stroke 2018; 13: 335–38 [DOI] [PMC free article] [PubMed] [Google Scholar]


