Abstract
Recent advances in our understanding of racial disparities in prostate cancer (PCa) incidence and mortality that disproportionately affect African American (AA) men have provided important insights into the psychosocial, socioeconomic, environmental, and molecular contributors. There is, however, limited mechanistic knowledge of how the interplay between these determinants influences prostate tumor aggressiveness in AA men and other men of African ancestry. Growing evidence indicates that chronic psychosocial stress in AA populations leads to sustained glucocorticoid signaling through the glucocorticoid receptor (GR), with negative physiological and pathological consequences. Compelling evidence indicates that treatment of castration-resistant prostate cancer (CRPC) with anti-androgen therapy activates GR signaling. This enhanced GR signaling bypasses androgen receptor (AR) signaling and transcriptionally activates both AR-target genes and GR-target genes, resulting in increased prostate tumor resistance to anti-androgen therapy, chemotherapy, and radiotherapy. Given its enhanced signaling in AA men, GR—together with specific genetic drivers—may promote CRPC progression and exacerbate tumor aggressiveness in this population, potentially contributing to PCa mortality disparities. Ongoing and future CRPC clinical trials that combine standard of care therapies with GR modulators should assess racial differences in therapy response and clinical outcomes in order to improve PCa health disparities that continue to exist for AA men.
Keywords: African American, African ancestry, clinical trials, glucocorticoid receptor, glucocorticoid signaling, health disparities, prostate cancer, psychosocial stress
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer and the second leading cause of cancer mortality in men in the United States (U.S.) (Siegel et al., 2020). Approximately 191,930 men will be diagnosed with PCa and 33,330 will die from this malignancy in the U.S. in 2020 (Siegel et al., 2020). African-American (AA) men have the highest rates of PCa incidence and mortality compared to men of other races and ethnicities in the U.S. (Siegel et al., 2020). These disparities have also been reported in other populations of men with African ancestry (Petersen et al., 2019; Rebbeck, 2017). At time of diagnosis, AA men and African men show a greater frequency of high-risk prostate tumors compared to men from other racial groups, ultimately resulting in increased mortality (Cuevas et al., 2019; Petersen et al., 2019; Rebbeck, 2017; Woods-Burnham et al., 2018a). Emerging evidence supports the notion that these disparities stem from the interplay between multiple factors including socioeconomic status (SES), environment, and biology (Abdalla et al., 1999; Chornokur et al., 2011; DeSantis et al., 2019; Moul et al., 1995).
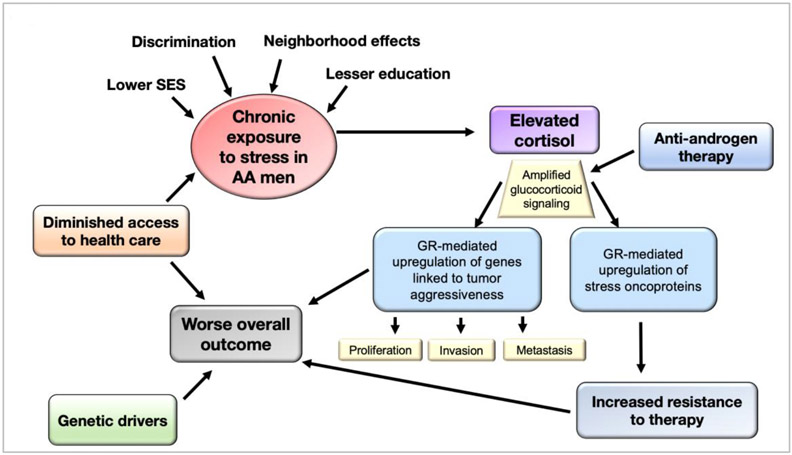
An emerging area in the field of PCa health disparities research is the contribution of psychosocial stress or socioenvironmental adversity to an increased risk of tumor aggressiveness, particularly in AA men (Cuevas et al., 2019; Kantor et al., 2019; Woods-Burnham et al., 2018a). In this review we discuss recent studies linking psychosocial stress with increased glucocorticoid signaling in AA populations. We also discuss emerging evidence pointing to glucocorticoid signaling through the glucocorticoid receptor (GR) as critical for PCa progression into the therapy-resistant advanced stage. We propose a model (Fig. 1) that integrates psychosocial stress, glucocorticoid signaling, and PCa progression in the context of PCa health disparities. In this model, cumulative exposure to psychosocial stressors (e.g. discrimination, negative neighborhood effects, low SES, limited access to health care) contributes to sustained elevated levels of cortisol resulting in amplified GR signaling. This amplified GR signaling is further increased by anti-androgen therapy during PCa treatment, leading to the activation of molecular mechanisms associated with PCa aggressiveness and therapy resistance.
Fig. 1. Psychosocial stress, glucocorticoid signaling, and prostate cancer progression in the context of health disparities.
Chronic or cumulative exposure of AA men to psychosocial stressors (e.g. discrimination, negative neighborhood effects, low SES, limited access to health care) contributes to sustained elevated levels of cortisol resulting in amplified GR signaling leading to the activation of molecular mechanisms associated with prostate tumor aggressiveness and therapy resistance. AA, African American; GR, glucocorticoid receptor; SES, socioeconomic status.
Prostate cancer health disparities
AA men are more likely to be diagnosed and die from PCa than European American (EA) men (Siegel et al., 2020). While it is estimated that 1 in 9 EA men will be diagnosed with PCa in their lifetime, the estimation rate in AA men is 1 in 7 (DeSantis et al., 2019). We recently reported that 1 in 3 AA men had elevated circulating PSA in a random sample of 414 adult AA men from the community (Woods-Burnham et al., 2018b). The biological characteristics of prostate tumors are exaggerated in AA men compared to EA men at time of diagnosis, including higher PSA levels, higher Gleason scores, differential anatomical localization of the tumors, and advanced tumor stage (Abdalla et al., 1999; Chornokur et al., 2011; Moul et al., 1995). Moreover, AA men show a higher rate of errors at the time of biopsy, leading to under-detection of higher grade disease at the time of diagnosis, which may compromise their outcomes (Sanchez-Ortiz et al., 2006; Sundi et al., 2013).
Multifactorial causes of PCa health disparities.
The causes of these racial disparities are complex and include the interplay between multiple factors such as SES, biological and genetic determinants, stress, diet, lifestyle, and access to healthcare (DeRouen et al., 2018; DeSantis et al., 2019; Deshmukh et al., 2017; Kelly et al., 2017; Kinlock et al., 2016; Krok-Schoen et al., 2017; Mahal et al., 2017; Singh and Jemal, 2017; Tsodikov et al., 2017; Weprin et al., 2019). Low SES directly affects diet, lifestyle, and access to healthcare, and therefore contributes significantly to cancer health disparities (Bach et al., 2002; Benjamins et al., 2016; DeSantis et al., 2019; Ward et al., 2004). AAs have been reported to suffer disparities for many cancer risk factors, such as lower dietary quality, greater rates of obesity, lower rates of physical activity, higher rates of exposure to endocrine disruptive chemicals, and higher prevalence of night-shift work (Wang and Chen, 2011).
Income inequalities also contribute to increased exposure to risk factors such as barriers to high-quality cancer prevention, early detection, and cutting-edge treatment options (Bach et al., 2002; Benjamins et al., 2016; DeSantis et al., 2019; Ward et al., 2004). The level of education affects potential income, and AAs have lower percentages of college degrees and higher rates of poverty than EAs (DeSantis et al., 2019). Less education leading to lower income also affects neighborhood placement, and low SES neighborhoods are more likely to be targeted by marketing that promotes behaviors known to increase cancer risk (DeSantis et al., 2016). Lower SES also translates to worse overall cancer survival rates for several reasons that include limited access to high-quality health care (Bach et al., 2002; Shavers and Brown, 2002; Ward et al., 2008; Zeng et al., 2015).
Worse overall survival is also influenced by the fact that AAs are more likely to be diagnosed with PCa at advanced tumor stage, which limits treatment options and reduces their efficacy (Arace et al., 2020; DeSantis et al., 2016). AA men also experience lower PCa screening rates compared to non-AA men (Misra-Hebert et al., 2017). We recently reported that in a sample of 264 AA/Black men over 45 years old living in the U.S. who met the American Cancer Society criteria for screening, only 49.6% had ever been screened and only 29.2% had a PSA test within the last year, consistent with other reports of lower rates of screening among AA men (Roberts et al., 2018). In another study of 414 AA/Black men (mean age 48.9 years) living in the U.S., we found that less than half (45.2%) of the participants had discussed PCa screening with their physicians, and detected higher-than-normal PSA values in 29.1% of the men who had not discussed PCa screening (Woods-Burnham et al., 2018b).
Even when provided the same PCa treatment as EA men, AA men are more likely to experience delay in treatment administration and suffer greater postoperative complications (Schmid et al., 2016). In addition, AAs are less likely to enroll in clinical trials, preventing them from exposure to cutting-edge treatment options (Murthy et al., 2004; Wallace et al., 2011). Co-morbidities affecting delivery of optimal treatment, including obesity, diabetes, and hypertension, are higher in AAs and may exacerbate PCa mortality (Braithwaite et al., 2009; Tammemagi et al., 2005; Yancik et al., 1998). The current COVID-19 pandemic has clearly exposed how these co-morbidities, combined with an adverse and stressful host-environment, have rendered AA populations more vulnerable during the pandemic (Holmes et al., 2020).
Molecular determinants of prostate cancer disparities.
Although studies from the U.S. Veteran Administration health care system suggest that PCa health disparities can be attenuated with better access to health care (Daskivich et al., 2015; Riviere et al., 2020), other studies indicate that these disparities persist even after controlling for SES, clinical setting, and access to care (Du et al., 2011; Kish et al., 2014; Nettey et al., 2018). This suggests that molecular or biological determinants may also contribute to these disparities (Bhardwaj et al., 2017; Singh et al., 2017). Genomic differences between AA and EA men with PCa hint that genetic mediators may drive PCa health disparities (Batai et al., 2016; Gusev et al., 2016; Han et al., 2015; Hoffman et al., 2001; Powell et al., 2013; Rand et al., 2016; Reams et al., 2009; Wallace et al., 2008; Wang et al., 2017). This is supported by the identification of PCa susceptibility loci for AA men in both linkage studies and genome-wide association studies (GWAS) (Gudmundsson et al., 2007; Kote-Jarai et al., 2011; Yeager et al., 2007). Interestingly, genetic variation on the chromosome 8q24 region, where the c-MYC gene is located, has been consistently associated with PCa risk, and ethnic specific mutations and haplotypes have been reported in African populations (Chung et al., 2014; Darst et al., 2020).
In addition to inherited genomic factors linked to PCa, there are also genetic alterations that are associated with PCa risk (Rebbeck, 2017). Recent genomic studies on human prostate tumors have identified multiple oncogenic drivers of PCa development. These include chromosomal translocations resulting in the generation of a fusion between the TMPRSS2 (a transmembrane serine protease) and ERG (a member of the ETS transcription factor family) genes, as well as mutations or alterations in genes associated with phosphoinositide 3-kinase(PI3K)-AKT signaling, WNT/β-catenin pathway, transcription and epigenetic regulation (e.g. ETS, FOXA1, KMT2C/D, SWI/SNF complex members), ubiquitination (e.g. SPOP and CUL3), DNA repair (e.g. BRCA2, ATM, CDKT2), tumor suppression (e.g. TP53, PTEN), RAS-MAPK signaling, and AR signaling (Armenia et al., 2018; Banerjee et al., 2018; Frank et al., 2018; Warner et al., 2019). The frequencies of mutations and alterations in these genetic drivers vary according to disease stage.
The TMPRSS2:ERG fusion has been found at lower frequencies in AA men (~28%) and Black men from Africa (~13%), compared to EA men (49%) (Blackburn et al., 2019). AA and EA men also have significant differences in ERG expression (Yamoah et al., 2015). The prognostic values of TMPRSS2:ERG fusion and ERG expression are not clear, but the relationship with PCa risk factors differs by TMPRSS2:ERG translocation status (Ahearn et al., 2016; Netto, 2013). In addition, the association between obesity and worse PCa outcome has been found in men harboring the TMPRSS2:ERG (Pettersson et al., 2013). By default, AA men, who have greater rates of obesity than EA men (Rebbeck, 2017), harboring the TMPRSS2:ERG translocation may have a poorer PCa prognosis than EA men. Interestingly, exome and whole-genome sequencing of AA prostate tumors revealed loss of function mutations in ERF, an ETS transcriptional repressor, lower frequency of ERG fusions, PIK3CA mutations and PTEN deletions, as well as increased frequency of SPOP mutations and expression of long non-coding RNAs (lncRNAs), compared to EA PCa (Huang et al., 2017; Jaratlerdsiri et al., 2018; Yuan et al., 2020). Another recent study showed that TP53 mutations, mutations in the DNA repair gene BRCA2, and deletions in CDKN1B (cyclin-dependent kinase inhibitor B1) are associated with increased risk of metastasis among AA men with PCa (Petrovics et al., 2019). However, another study showed that alterations in DNA repair genes, including BRCA1/2 and ATM, are less likely to be detected in AA patients with PCa compared to EA patients (Sartor et al., 2020).
Gene expression profiling of PCa tumors have also revealed differences in tumor immunobiology between AA and EA men (Wallace et al., 2008). For instance, genes associated with autoimmunity and inflammation, particularly those clustering in immune response, stress response, cytokine signaling, and chemotaxis pathways are differentially upregulated in AA prostate tumors (Wallace et al., 2008). A recent study that integrated the genomic and transcriptomic landscape between AA PCa and EA PCa revealed an enrichment of highly expressed differentially expressed genes (DEGs) for immune-related pathways in AA men, compared to increased enrichment for PTEN/PI3K signaling in EA men (Yuan et al., 2020). Metastasis-promoting genes are also more highly expressed in AA prostate tumors, including autocrine mobility factor receptor, chemokine receptor 4, and matrix metalloproteinase 9 (Wallace et al., 2008). Inflammation associated genes such as IL6, IL8, IL1B, CXCR4, and FASN were also found to be significantly expressed at higher levels in prostate tumors from AA compared to EA men (Powell et al., 2013). The expression of many of these genes have been associated with diet and lifestyle, higher Gleason scores, androgen receptor (AR) signaling, aggressive PCa tumors, and metastasis (Dubrovska et al., 2012; Finley et al., 2009; Jia et al., 2004; Nguyen et al., 2010; Powell et al., 2013; Yang et al., 2004). Consistent with the notion of differential expression of immune function-related genes between AA and EA men with PCa, our group reported race-related differences in serum autoantibody responses to specific tumor-associated antigens in PCa patients (Sanchez et al., 2016).
The RNA splicing landscape has also been explored as a potential biological determinant of PCa health disparities (Olender and Lee, 2019; Wang et al., 2017). A genome-wide analysis of differential splicing (DS) events in racially diverse prostate tumors revealed hundreds of DS events that were unique to AA PCa and affected specific splice variants of several oncogenes such as PIK3CD, FGFR3, TSC2, and RASGRP2 (Olender and Lee, 2019). Validation studies showed that ectopic overexpression of a short splice variant of PIK3CD, enriched in AA tumors, enhanced the aggressive properties of PCa cells compared to the corresponding variant enriched in EA tumors. These results suggested that differential RNA splicing may contribute to increased tumor aggressiveness in AA PCa and could be exploited for developmental therapeutics in aggressive PCa.
Given that androgens drive PCa etiology and disease progression prior to metastatic CRPC (mCRPC), several studies have also explored racial differences in androgen production and AR signaling (Bosland and Mahmoud, 2011; Karakas et al., 2017; Massengill et al., 2003; Schatzl et al., 2003). These studies have shown that AA men have higher testosterone and active 5-alpha reductase levels than EA men, resulting in enhanced conversion of testosterone to the more potent DHT (Kheirandish and Chinegwundoh, 2011; Ross et al., 1992). The differential expression of epithelial and stromal AR in PCa tissue is also emerging as a possible driver of castration resistance in patients receiving ADT (Karakas et al., 2017), and there is evidence that while nuclear AR levels are increased in AA PCa patients compared to EA patients, stromal levels are decreased (Li et al., 2008; Singh et al., 2014). In addition, AA PCa patients have higher frequency of germline and somatic AR mutations and their tumors show increased expression of specific AR target genes associated with tumor aggressive properties compared to those of EA PCa men (Gaston et al., 2003; Jemal et al., 2006; Karakas et al., 2017).
African Americans and psychosocial stress
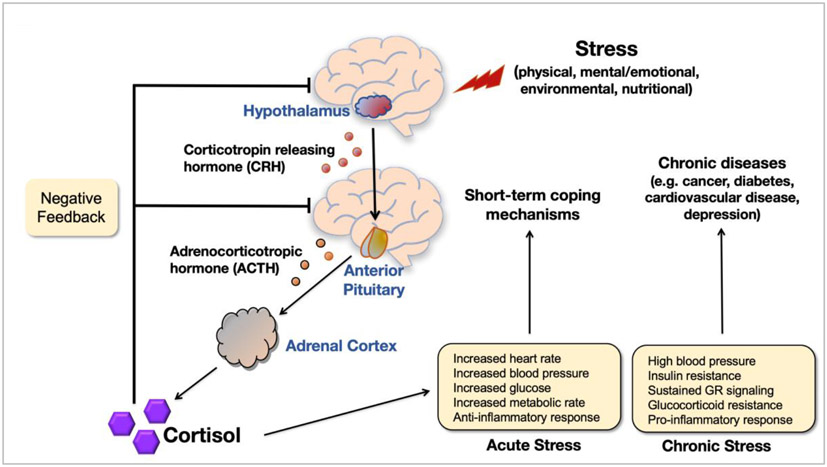
AAs are exposed to more cumulative lifetime stressors than other racial/ethnic groups, which detrimentally alters psychological and physical health (Cohen et al., 2006; Young et al., 1991; Zannas et al., 2015). The elevated levels of stress among AAs can be due, among other factors, to social isolation, racial discrimination, perceived discrimination, and segregation (Cacioppo and Hawkley, 2003; Cuevas et al., 2019; Williams and Collins, 2001). Chronic stress leading to dysregulation of endogenous cortisol production via the hypothalamic-pituitary-adrenocortical (HPA) axis can enhance risk for metabolic disorders and cancer (Cohen et al., 2006; Steptoe et al., 2000; Vedhara et al., 1999; Zannas et al., 2015). As illustrated in Fig. 2, when the HPA axis is activated, neurons in the paraventricular nucleus of the hypothalamus are triggered to release corticotropin-releasing hormone (CRH) and arginine vasopressin, which stimulate the production and secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, resulting in the synthesis and secretion of the steroid hormone cortisol, an endogenous glucocorticoid, from the adrenal cortex (Joseph and Whirledge, 2017; Stephens and Wand, 2012). A classical endocrine negative feedback loop inhibits further release of CRH and ACTH in response to rising levels of cortisol, thus maintaining a physiological homeostasis under normal conditions (Joseph and Whirledge, 2017). In addition, the HPA axis tightly regulates glucose metabolism, cardiovascular function, cell proliferation and survival, growth, cognition and behavior, immune function, and reproduction directly through cortisol production (Joseph and Whirledge, 2017). Cortisol is secreted diurnally, peaking early in the morning when blood glucose levels are at the lowest and tapering throughout the day (Cohen et al., 2006). This diurnal rhythm is altered in response to chronically stressful situations (Adam and Gunnar, 2001; Cohen et al., 2006).
Fig. 2. Physiological response to stress.
Cortisol is secreted from the adrenal cortex in response to acute stress. A classical endocrine negative feedback loop inhibits further release of CRH and ACTH in response to rising levels of cortisol to maintain a physiological homeostasis under normal conditions. Chronic stress leading to dysregulation of endogenous cortisol production via the HPA axis can enhance risk for metabolic disorders and cancer. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; GR, glucocorticoid receptor; HPA, hypothalamic-pituitary-adrenocortical.
Chronic exposure to stressors is increased in individuals of low SES, and there is a positive association between low SES and stress in AA men (Cohen et al., 2006; Williams, 2003). Low SES is defined by lower income, education, or occupational status, and often results in increased exposure to environmental stressors leading to stress-related dysregulation of physiological systems and increased risk for disease (Adler et al., 1994; Cohen et al., 2006; McEwen, 1998). However, even in AAs who have high SES, racial disparities in health persist, suggesting that there are alternative sources of stress other than SES (Farmer and Ferraro, 2005). For example, a poor lipid profile characterized by high triglycerides, LDL cholesterol, and total cholesterol, as well as lower HDL cholesterol, actually increases in AAs as education increases (Knox et al., 1996).
National data reveals that strikingly high levels of racial inequality in SES exist in the U.S., having changed little over time, and that AAs continue to suffer from disproportionately lower SES (Williams et al., 2010), which has been shown to influence emotions and behaviors that alter cortisol levels (Adler et al., 1994). Lower SES is also associated with greater perceived stress, depressive symptoms, negative affect, weak social networks and support, and sleep deprivation (Cohen et al., 2006), factors linked to greater cortisol responses (Leproult et al., 1997; Polk et al., 2005; Pruessner et al., 2003; Seeman et al., 2001). However, whether lower SES by itself is associated with increased cortisol responses among AAs remains to be unambiguously established. A major study by Cohen and colleagues explored whether SES-associated dysregulation of cortisol diurnal rhythm is independent of race and occurs equally in AAs and EAs (Cohen et al., 2006). This study reported that both lower SES (education and income) and being Black were associated with higher evening levels of cortisol (Cohen et al., 2006). These higher cortisol levels in AAs were associated with poorer health practices (e.g. smoking), higher levels of depressive symptoms, poorer social networks and supports, and feelings of helplessness (Cohen et al., 2006). This dysregulation has negative long-term repercussions for the health of AAs (Williams et al., 2010; Williams and Sternthal, 2010; Zannas et al., 2015).
Employed AAs are also more likely to be exposed to carcinogens and occupational hazards compared to other racial groups with matched education and job experience (Kaufman et al., 1997; Williams and Sternthal, 2010). Compounding these realities, AAs have less purchasing power, as the costs of goods and services are highest in predominantly AA communities (Kaufman et al., 1997; Williams and Sternthal, 2010). Outside the workplace, AAs are exposed to daily stressors within neighborhoods that are highly segregated (Williams et al., 2010), the effects of which negatively impact the SES and health of AA residents (Schulz et al., 2002; Williams and Collins, 2001). For instance, optimal health is jeopardized in economically-disadvantaged, segregated neighborhoods as nutrition suffers in the presence of higher cost, lower quality, and decreased availability of healthy foods (Schulz et al., 2002; Williams and Collins, 2001). Similarly, physical activity is reduced in the absence of suitable recreational facilities amid safety concerns (Schulz et al., 2002; Williams and Collins, 2001). Exposure to environmental toxins and poor-quality living conditions also exist in neighborhoods accustomed to institutional neglect and disinvestment (Schulz et al., 2002; Williams and Collins, 2001). Adding to these cumulative stressful events are frequent experiences of discrimination and incarceration among AAs, which are associated with psychological distress and adverse physical health effects, including high incidence of chronic conditions such as hypertension, obesity, diabetes, substance abuse, and cancer (Byrd, 2012; Krieger et al., 2011).
Combined, these stressors are directly linked to elevated risk of illness and death among AAs, and greatly contribute to existing racial disparities in health (Acevedo-Garcia et al., 2003; Williams and Collins, 2001). For example, AA men ages 57-85 may have worse metabolic outcomes than their EA counterparts due to chronic inflammation arising from cumulative, multi-dimensional stress experienced over their lives (Das, 2013). Interestingly, a study based in Argentina of men ages 45-70 reported that uncontrollable stressful life events were inversely correlated with PSA among men with low cortisol, but positively correlated with PSA among men with high cortisol, suggesting that such events may be related to prostatic tumorigenic processes in men with high cortisol (Gidron et al., 2011).
Glucocorticoid receptor signaling
GR biology.
The cellular and pharmacological actions of cortisol and other glucocorticoids are mediated by GR, although low levels of glucocorticoids can also stimulate the mineralocorticoid receptor (MR) (Gomez-Sanchez and Gomez-Sanchez, 2014; Joseph and Whirledge, 2017). GR is ubiquitously expressed throughout the human body, and signaling through this receptor regulates metabolism, growth, development, cardiovascular homeostasis, and cognition (Biddie et al., 2012; Oakley and Cidlowski, 2013). Synthetic glucocorticoids such as prednisone and dexamethasone have long been integral components of treatment regimens for inflammatory and autoimmune diseases as well as certain cancers (Vandewalle et al., 2018). As a member of the nuclear receptor family (which also includes receptors for estrogen, progesterone, and androgen), GR has both genomic (regulating gene transcription by binding to glucocorticoid response elements, GREs, in promoter regions) and non-genomic (modulating the function of intracellular kinases, including c-Src) effects (Oakley and Cidlowski, 2013).
In the absence of ligand binding, GR resides in the cytoplasm as part of a large multi-protein complex including chaperone heat shock proteins hsp90, hsp70, and p23 as well as immunophilins of the FK506 family including FK506-binding protein (FKBP) 51 and FKBP52 (Grad and Picard, 2007; Joseph and Whirledge, 2017; Pratt and Toft, 1997). Upon ligand binding, a conformational change occurs releasing GR from the chaperone proteins and promoting its translocation into the nucleus where it exerts transcriptional regulation functions (Joseph and Whirledge, 2017). This nuclear translocation is promoted by signaling through the semaphorins Sema4D/Sema3C upon binding to their receptor PlexinB1 (Williamson et al., 2019). GR-mediated transcriptional control also extends to sequestration of other transcription factors (including NFkB and AP-1) to inhibit pro-inflammatory gene expression as well as tethering transcription factors to facilitate gene transcription (Oakley and Cidlowski, 2011; Vandevyver et al., 2014)
While most cells express GR, its genetic structure is highly complex, and tissue-specific control of GR signaling is conferred, to a large extent, by the type and level of GR expressed (Ito et al., 2006). The gene encoding GR, NR3C1 (nuclear receptor subfamily 3 group C member 1), is composed of 9 exons; however, the major transcriptional start site is within exon 2 and only exons 2 through 9 encode protein (Ito et al., 2006; Vandevyver et al., 2014). Exon 2 encodes the amino (N)-terminal modulatory domain, exons 3 and 4 encode the DNA-binding domain, exon 5 codes for a hinge region, and exons 6-9 encode the carboxy (C)-terminal ligand binding domain (Kadmiel and Cidlowski, 2013). The 13 currently-identified variants of exon 1, with 9 possible promoter regions, form the 5′-untranslated region (UTR) and are thought to confer tissue specificity of GR expression (Turner and Muller, 2005).
Alternative splicing of GR mRNA yields GRα, GRβ, GRγ, GR-A, and GR-P. GRα is expressed at higher levels in most cells, including cancer cells, than the other GR forms (Biddie et al., 2012; Vandevyver et al., 2014). GRβ is a closely-related variant to GRα, differing by ~35 amino acids within the ligand binding domain (Fig. 3). In contrast to GRα, GRβ does not bind glucocorticoids and is constitutively located in the nucleus (Biddie et al., 2012; Kassel and Herrlich, 2007; Mata-Greenwood et al., 2015). Although it does not directly mediate transcription, GRβ has been shown to regulate gene expression through dominant-negative effects on GRα via heterodimer formation and also by epigenetically modifying chromatin structure through interactions with histone deacetylases (Nicolaides et al., 2010; Oakley and Cidlowski, 2013). GRγ, which makes up about 10% of total GR expression in most cells, is formed through alternative splicing between exons 3 and 4, with the incorporation of a single additional arginine nucleotide into the DNA binding domain (Morgan et al., 2016). This minor structural difference, while not affecting DNA binding affinity or total GR occupancy of target genes, still confers different sequence specificity and target gene control (through variable allosteric signal interpretation) compared to GRα (Morgan et al., 2016). Other notable aspects of GRγ are a delayed ligand-induced nuclear import (unlike beta, resides in the cytoplasmic region) as well as a pro-cellular respiration function within mitochondria (Morgan et al., 2016). The GR-A and GR-P isoforms are formed through removal of large portions of the ligand binding domain (exons 5-7 and exons 8-9, respectively) (Oakley and Cidlowski, 2013). Comparatively less is known concerning these isoforms, but evidence suggests a ligand-independent ability to modulate GRα function (Oakley and Cidlowski, 2013; Vandevyver et al., 2014).
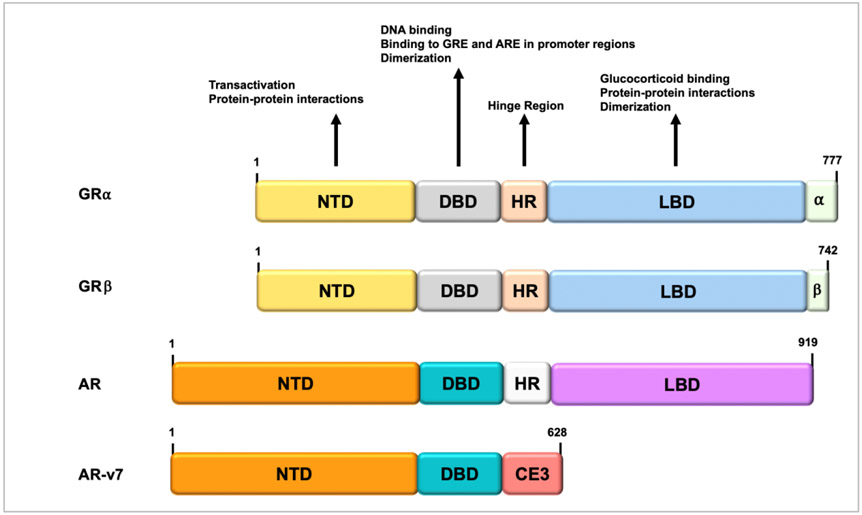
Fig. 3. NR3C1 (GR) domain structure.
The domain structure of GRα; is composed of an N-terminal modulatory domain, a DNA-binding domain, a hinge region, a ligand-binding domain, and a C-terminal ligand binding domain. Regions involved in transcriptional activation, dimerization, glucocorticoid binding, and DNA binding are indicated. The nuclear localization signal is located within the flexible hinge region. For comparison, the domain structures of GRβ and AR and its splice variant AR-v7 are included. Note the structural similarities between these transcription factors. AR, androgen receptor, ARE, androgen response element; CE3, cryptic exon 3; DBD, DNA-binding domain; DNA, deoxyribonucleic acid; GR, glucocorticoid receptor; GRE, glucocorticoid response element; LBD, ligand-binding domain; NTD, N-terminal domain.
A further layer of complexity in GR expression is introduced by 8 alternate translation initiation sites within exon 2, yielding a number of additional isoforms (Oakley and Cidlowski, 2013; Vandevyver et al., 2014). These have been identified for GRα and are predicted to exist for each of the other splice variants GRβ, GRγ, GR-A, and GR-P (Oakley and Cidlowski, 2013; Vandevyver et al., 2014). Furthermore, post-translational modifications of GR also regulate the receptor’s function in target cells (Vandevyver et al., 2014). Kinase activity (including MAPK, CDK, CK2, GSK-3β) at serine residues within the N-terminal domain occurs following glucocorticoid exposure (Oakley and Cidlowski, 2013; Vandevyver et al., 2014). These phosphorylation events may result in cytoplasmic sequestration (Ser-203, −226, −404), degradation, or enhanced transcriptional activity (−211) (Oakley and Cidlowski, 2013; Vandevyver et al., 2014). Other GR modifications include the addition of a ubiquitin moiety at lysine 419, which targets GR for proteasomal degradation; sumoylation of lysines 277, 293, and 703, which modulate GR interactions with transcriptional co-regulators; and acetylation of lysines 494 and 495, which inhibit GR binding (and suppression) to NFkB, thus regulating GR’s anti-inflammatory function (Oakley and Cidlowski, 2013; Vandevyver et al., 2014).
In addition to modulating the level and form of GR expression, cells and tissues may also regulate immediate ligand availability (Oakley and Cidlowski, 2013). At the cellular level, the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) catalyzes the inactivation of cortisol to cortisone, while the opposing type 1 enzyme (11β-HSD1) reverses the reaction, promoting cortisol production (Oakley and Cidlowski, 2013). Thus, the relative activities of these enzymes within a cell confer control over the local availability of cortisol. Of note, most synthetic glucocorticoids are not inactivated by 11β-HSD2 and their activities are preserved despite cellular upregulation of 11β-HSD2 (Oakley and Cidlowski, 2013).
As GRα is the biologically relevant isoform, all references henceforth to GR will imply GRα (Mata-Greenwood et al., 2015). GR encompasses three functional domains including an amino-terminal transactivation domain, a central DNA-binding domain, and a carboxy-terminal ligand-binding domain (Joseph and Whirledge, 2017). There is a flexible hinge region that contains a nuclear localization signal between the DNA-binding domain and the ligand-binding domain (Fig. 3). It is within this flexible hinge region that genomic interactions occur (Joseph and Whirledge, 2017). In the nucleus, GR homodimers bind to GR response elements (GRE) within promoter regions of target genes (Luisi et al., 1991). The consensus GRE sequences are comprised of two hexameric half-sites separated by a spacer of three nucleotides (e.g., AGAACAnnnTGTTCT) (Strahle et al., 1987). Once GR homodimers bind to GREs, chromatin is remodeled, co-regulators are recruited, and GR-induced transcription is initiated (Joseph and Whirledge, 2017). In addition to activation of GR-target genes, GR may also negatively repress genes (Surjit et al., 2011). This occurs when GR binds to GREs with consensus sequence CTCC(n)0-2GGAGA and co-repressors are recruited (Surjit et al., 2011). GR also mediates gene transcription via interactions with other transcription factors (Joseph and Whirledge, 2017).
GR signaling in AAs.
GR signaling is triggered by a variety of physiological and environmental factors (Joseph and Whirledge, 2017). Chronic stress resulting in sustained elevated glucocorticoid exposure throughout a lifetime has negative physiological consequences (Cohen et al., 2006; Joseph and Whirledge, 2017; Williams et al., 2010; Zannas et al., 2015). Constant GRE binding induces local lasting changes in DNA methylation, shaping subsequent responses to stressors and glucocorticoids (Klengel et al., 2013; Thomassin et al., 2001; Wiench et al., 2011a; Wiench et al., 2011b; Zannas and West, 2014). It is therefore plausible that chronic stress confers cumulative effects on DNA methylation sites with long-term epigenetic ramifications (Zannas et al., 2015). Profound changes in DNA methylation are associated with aging-related diseases (Bjornsson et al., 2008; Christensen et al., 2009; Hernandez et al., 2011; Heyn et al., 2012; Horvath, 2013; Horvath et al., 2012; Rakyan et al., 2010). Because of this, several DNA methylation-based predictors of aging have been recently developed (Bocklandt et al., 2011; Hannum et al., 2013; Horvath, 2013; Weidner et al., 2014). For example, a composite predictor comprised of 353 Cytosine-phosphate-Guanosine sites (CpGs) across the genome was shown to strongly correlate with chronological age across multiple human tissues (Horvath, 2013). Several studies have used this predictor to calculate accelerated epigenetic aging, defined as the difference between DNA-methylation-predicted age and chronological age (Boks et al., 2015; Horvath, 2015; Marioni et al., 2015a; Marioni et al., 2015b). This accelerated epigenetic aging has been associated with cancer, obesity, PTSD, physical and cognitive decline, all-cause mortality, lower SES, and cumulative lifetime stress (Boks et al., 2015; Horvath, 2013; Marioni et al., 2015a; Zannas et al., 2015).
Cumulative lifetime stress has been associated with accelerated epigenetic aging in AAs (Zannas et al., 2015). This is attributed to altered GR signaling marked by an increased number of epigenetic clock CpGs located within functional GREs, dynamic methylation changes following exposure to dexamethasone, and dynamic regulation by genes with enriched association for aging-related diseases which neighbored these CpGs (Zannas et al., 2015). These results support a model of stress-induced accelerated epigenetic aging mediated by the lasting effects of chronic stressor exposure and aberrant glucocorticoid signaling on the epigenome (Zannas et al., 2015).
AAs also appear to have amplified GR signaling and increased glucocorticoid resistance (Frazier et al., 2010). This was reported in a study that explored the role of body weight and body composition in insulin resistance among participants who were treated with placebo or 4 mg dexamethasone (Frazier et al., 2010). Results revealed that AAs were significantly more hyperinsulinemic after dexamethasone treatment than EAs, indicated by higher peak insulin and postprandial insulin (Frazier et al., 2010). AAs were also found to be more insulin resistant as determined by fasting insulin and homeostatic model assessment (Frazier et al., 2010). This hyperinsulinemia and increased insulin resistance in AAs was independent of body weight or composition (body mass index, percent body fat, waist circumference), suggesting that amplified GR signaling was more prevalent in the AA study participants (Frazier et al., 2010). In another study, AAs showed increased activity in pro-inflammatory pathways and GR signaling, compared to EAs, which was linked to exposure to discrimination (Thames et al., 2019).
Taken together, the studies discussed above provide support for our model (Fig. 1) which links chronic exposure to stressors (low SES, discrimination, neighborhood effects, lesser education status) to elevated cortisol and amplified GR signaling. This amplified GR signaling could be exacerbated during the current COVID-19 pandemic, given the chronic psychosocial stress disproportionately affecting minority populations, particularly AAs, during this pandemic (Holmes et al., 2020). The implications of this increased GR signaling for PCa progression and resistance to therapy is discussed below.
GR signaling in PCa.
GR has recently emerged as a major driver of PCa progression and resistance to AR-signaling inhibitor (ARSI) therapy, chemotherapy, and radiotherapy (Arora et al., 2013; Beer et al., 2017; Chen et al., 2019; Claessens et al., 2017; Kroon et al., 2016; Li et al., 2017; Montgomery et al., 2014; Narayanan et al., 2016; Puhr et al., 2018; Sartor et al., 2014). A seminal study by Sawyers and colleagues identified GR overexpression as a common feature of ARSI-resistant PCa tumors using pre-clinical models and confirmed in patient samples (Arora et al., 2013). GR overexpression in ARSI-resistant PCa cells and tissues have since been validated in cellular models of resistance and patient biospecimens (Isikbay et al., 2014; Li et al., 2017; Puhr et al., 2018). Growth factors produced in prostate stroma regulate glandular epithelial proliferation and differentiation, and steroid hormones including glucocorticoids are important modulators of stromal-epithelial cell signaling interactions in the prostate (Hidalgo et al., 2011; Taylor and Risbridger, 2008). GR-mediated transcriptional activity has been found to be altered in carcinoma-associated stroma and confers cell-specific effects with the potential to induce antiandrogen therapy resistance (Hidalgo et al., 2011; Zhao et al., 2014).
Significant structural similarities (Fig. 3) and transcriptomic overlap between AR and GR accounts for GR-mediated bypass of AR blockade (Sahu et al., 2013). In addition, there is overlap in the transcription protein interactome of both nuclear receptors (Lempiainen et al., 2017). Induction of GR expression is also accompanied in ARSI-resistant tissues by the loss of 11B-HSD2, resulting in increased stability of intratumoral cortisol (Li et al., 2017). The implications of these initial findings have sparked great interest, as PCa patients are routinely administered synthetic glucocorticoids (e.g. prednisone and dexamethasone) alongside ARSI and taxane chemotherapy for palliative purposes (Chi et al., 2017; Collins et al., 2007; Narayanan et al., 2016; Tannock et al., 1989). Furthermore, the mechanisms underlying ARSI-resistance in PCa and their contribution to disease progression had not been fully previously elucidated, and these findings paved the way for research on the role of GR in these processes (Narayanan et al., 2016).
While anti-androgen therapy is highly effective in producing an initial period of PCa regression, mCRPC eventually develops for many patients, characterized by rapidly rising PSA levels, even though circulating testosterone levels are in the typical castration range (<50 ng/dl) (Chen et al., 2004; Feldman and Feldman, 2001; Lamont and Tindall, 2011; Schrecengost and Knudsen, 2013; Sharifi, 2013). This means that AR-target genes are operating in the absence of androgen to stimulate PCa cell survival, growth, and PSA secretion (Narayanan et al., 2016). To understand the prospect of GR bypassing the AR signaling pathway and directly activating AR-target genes (Fig. 4), the similarities between AR and GR must be considered. AR and GR belong to the same intracellular receptor family of transcriptional regulators, and the DNA binding domains of AR and GR are highly conserved with an 80% match in amino acid sequence (Mangelsdorf et al., 1995; Narayanan et al., 2016; Rundlett and Miesfeld, 1995). As shown in Fig. 3 the domain structures of GR and AR are very similar. Like GREs, AR response elements (AREs) in the promoter regions of AR-target genes are composed of a 15 base pair binding sequence comprised of two hexamer half-sites and separated by a 3 base pair spacer (Bolton et al., 2007). This similarity allows GR to interact with AREs and alter the expression of AR-target genes in the absence of androgens (Arora et al., 2013). While that study identified 52 common overlapping genes out of 105 AR signature genes and 121 GR signature genes, several canonical AR-target genes were found to be regulated by GR, including PCa key genes KLK3 (encoding PSA) and TMPRSS2 (involved in fusions with ERG in a race-related manner) (Arora et al., 2013). Also, GR expression is normally repressed in PCa cells in the presence of AR, however this study demonstrated that AR blockade removes this GR inhibition and stimulates GR amplification (Arora et al., 2013). Intriguingly, the TMPRSS2 protein is a key receptor used by SARS-Cov-2 virus to infect host cells, which has prompted recent speculation that race-related and gender-related differences in AR signaling may explain in part the racial and gender variations in COVID-19 deaths and that anti-androgen agents combined with TMPRSS2 inhibitors could potentially decrease disease severity (McCoy et al., 2020).
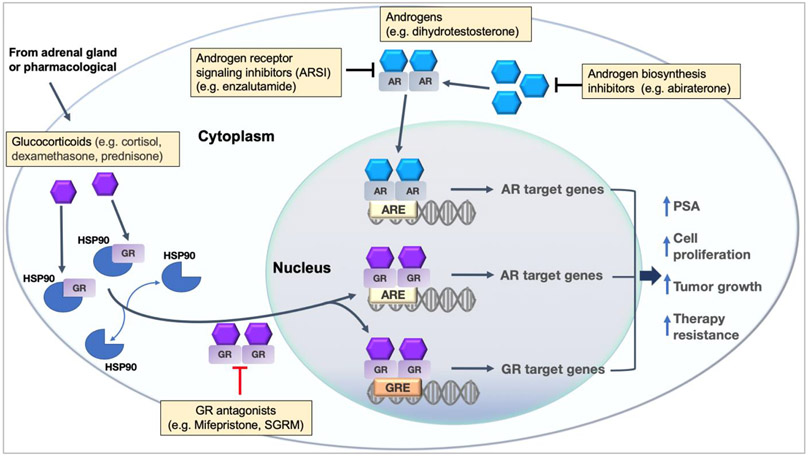
Fig. 4. The interplay between GR and AR in the context of PCa.
AR and GR belong to the same intracellular receptor family of transcriptional regulators and their DNA binding domains are highly conserved. GREs and AREs in the promoter regions of GR-and AR-target genes are also similar and are composed of a 15 base pair binding sequence with 2 hexamer half-sites separated by a 3 base pair spacer. GR expression is normally repressed in PCa cells in the presence of AR. However, an AR blockade removes GR inhibition and stimulates GR amplification allowing GR to interact with AREs and alter the expression of AR-target genes. This complex AR-GR interplay creates a major clinical dilemma as the effects of glucocorticoids can be beneficial and/or harmful to patients.
Treatment options for prostate cancer patients
The interplay between GR and AR in the context of PCa treatment presents a major clinical dilemma because the effects of glucocorticoids are both beneficial and harmful to patients (Arora et al., 2013; Claessens et al., 2017; Montgomery et al., 2014; Narayanan et al., 2016; Puhr et al., 2018; Sartor et al., 2014). AR signaling has been traditionally considered as the key actionable driver of PCa recurrence and progression (Banerjee et al., 2018). Because of this, targeting androgen biosynthesis and AR has been a standard of care for PCa treatment for over seven decades (Huggins and Hodges, 1972). Huggins and colleagues made the seminal observation that both surgical castration and estrogen administration resulted in regression of PCa metastasis (Huggins and Hodges, 1972), which led to the development of androgen deprivation therapy (ADT). Both agonists and antagonists of luteinizing hormone-releasing hormone (LHRH) are typically used as first-line ADT in patients with hormone-sensitive PCa to decrease endogenous testosterone production through the hypothalamic-pituitary-testicular (HPT) axis. There are also first-line anti-androgens that bind to AR and inhibit its activity including flutamide, bicalutamide, and nilutamide (Boccon-Gibod et al., 1997; Kolvenbag and Nash, 1999; Todd et al., 2005).
While ADT with first-line anti-androgens is initially successful in most PCa patients who choose this option after biochemical recurrence, resistance to this therapy is inevitable, occurring within 18-24 months (Asmane et al., 2011). Prostatic epithelial cells demonstrate great plasticity in response to ADT, giving rise to a highly heterogeneous co-existence of AR-positive and AR-negative cells (Banerjee et al., 2018). A relatively short time after development of ADT-resistance, the patient enters a disease stage referred to as mCRPC, which typically has a 5-year survival rate of 30% (Scher et al., 2004; Thoreson et al., 2014). Therapeutic options for patients with mCRPC include ARSI, immunotherapy, radiation therapy with radium-223, and taxane-based chemotherapy with docetaxel (DTX) or cabazitaxel (CBZ) plus the glucocorticoids dexamethasone or prednisone (Arlen and Gulley, 2005; Corn et al., 2017; Gilbert and Parker, 2005). Second-line, next-generation ARSIs such as enzalutamide and apalutamide have been developed during the past decade for PCa patients who have failed LHRH agonists/antagonists or other first-line anti-androgens (Banerjee et al., 2018). Another clinically relevant ARSI, abiraterone, is an inhibitor of androgen biosynthesis that acts by blocking the activity of cytochrome P450 17 alpha-hydroxylase (CYP17), a key enzyme that is essential for the generation of the androgen precursor dehydroepiandrosterone (DHEA) (Rehman and Rosenberg, 2012).
DTX and/or CBZ can extend patient survival by a few months, but chemoresistance develops, curtailing the beneficial effects of these taxane drugs (Arlen and Gulley, 2005; Corn et al., 2017; Gilbert and Parker, 2005). Although not all PCa patients that fail anti-androgen therapy undergo chemotherapy, results from the recent clinical trials CHAARTED and STAMPEDE showed that administering DTX together with anti-androgen therapy in newly diagnosed mCRPC patients provides a dramatic increase in overall survival advantage compared to ADT alone (James et al., 2016; Kyriakopoulos et al., 2018). Other recent trials combining emerging therapies or modifying the sequence of available therapies have yielded promising results for the treatment of mCRPC (Ku et al., 2019; Schmid and Omlin, 2020).
Because of the tumor heterogeneity observed within and among PCa patients, developing effective therapies for this malignancy remains challenging (Ku et al., 2019; Maitland et al., 2019). Despite the spectrum of therapeutic options for mCRPC which provide increased overall survival benefits with improved quality of life, this advanced stage of the disease still remains incurable due to the development of therapy resistance. A promising emerging therapy for mCRPC based on prostate specific membrane antigen (PSMA) theranostics, which combines positron emission tomography (PET) imaging with tumor targeting, is bringing hope to the diagnosis and treatment of men with advanced PCa (Kratochwil et al., 2019). PSMA is a transmembrane glycoprotein that is highly expressed in PCa, particularly in high grade tumors or mCRPC, with minimal or no expression in normal tissues (Silver et al., 1997). Currently, a PSMA specific ligand labeled with a positron emitter is a desirable diagnostic tool for detecting metastatic disease at much lower PSA levels than conventional imaging. Patients with high PSMA tumor expression can then undergo targeted radioligand therapy using the same PSMA ligand labeled with a beta emitter (e.g. 177lutetium) or an alpha emitter (e.g. 225actinium) to selectively destroy the cancer cells without damaging normal tissues (Baum and Kulkarni, 2012). The implementation of PSMA theranostics in clinical practice has the potential to revolutionize mCRPC treatment and improve patient outcomes. Prospective randomized clinical trials are currently underway worldwide to validate PSMA theranostics compared to available standard of care therapies for mCRPC. The optimal timing for administering this therapy relative to the sequence of other therapies has yet to be defined.
Glucocorticoids in PCa treatment
The synthetic glucocorticoids prednisone and dexamethasone are routinely used therapeutically and have much higher potency than cortisol in activating GR. For example, 4 mg of prednisone and 0.75 mg of dexamethasone provide the physiological equivalent of 20 mg of cortisol.(Narayanan et al., 2016) Prednisone is clinically used in doses of 5-10 mg once or twice per day and dexamethasone is used in doses at 0.75-1 mg once or twice per day (Narayanan et al., 2016). When co-administered with taxane chemotherapy for PCa, their potent anti-inflammatory properties counteract pain, nausea, lack of appetite, fatigue, hypersensitivity, and fluid retention (de Bono et al., 2010; Dorff and Crawford, 2013; Lafeuille et al., 2013; Schwartz, 2012; Tannock et al., 2004).
While the benefits of glucocorticoid co-administration to PCa patients have been established, there is evidence that increased GR signaling could also be detrimental to these patients (Arora et al., 2013; Claessens et al., 2017; Isikbay et al., 2014; Kroon et al., 2016; Li et al., 2017; Montgomery et al., 2014; Narayanan et al., 2016; Puhr et al., 2018; Sartor et al., 2014). One study by Puhr et al. found that GR expression is initially reduced in primary PCa tissue, but is restored in metastatic lesions (Puhr et al., 2018). This group also found that genetic or pharmacological inhibition of GR impaired the proliferation and 3D spheroid-forming capabilities of PCa cell lines (Puhr et al., 2018). Additionally, these investigators also reported that GR levels increase in DTX-resistant PCa cell lines and in tissues from patients who have been treated with DTX, and that patients who relapse with biochemical recurrence and have high GR levels experience shortened progression-free survival (Puhr et al., 2018). There are also reports that PCa patients enrolled in clinical trials have worse overall survival outcomes when receiving glucocorticoids compared to patients not receiving glucocorticoids (Montgomery et al., 2014; Montgomery et al., 2015; Narayanan et al., 2016). This trend was observed in the AFFIRM phase 3 clinical trial evaluating the use of enzalutamide as well as in the COU-AA-301 phase 3 clinical trial in which patients were randomized to prednisone plus abiraterone after failing taxane chemotherapy (de Bono et al., 2011; Narayanan et al., 2016). On the other hand, recent results from the SWITCH trial showed that in selected clinically stable mCRPC patients with limited disease progression, the combination of abiraterone with dexamethasone provided a benefit, measurable by PSA decline and disease stabilization, to patients with normal AR status but not patients with AR aberrations (Romero-Laorden et al., 2018). One limitation of this study, however, was the lack of a molecular analysis that included predictive biomarkers such as the AR-v7 splicing variant (Fig. 3), also implicated in both ARSI and taxane resistance, and GR expression or signaling (Romero-Laorden et al., 2018).
Our group recently demonstrated the ability of liganded GR to upregulate the expression of clusterin (CLU) and lens epithelium-derived growth factor of 75 kD (LEDGF/p75), two stress oncoproteins previously established as key contributors to therapy resistance in various cancer types, in racially diverse preclinical PCa cellular models (Chun, 2014; Djeu and Wei, 2009; Huang et al., 2007; July et al., 2002; Koltai, 2014; Matsumoto et al., 2013; Mediavilla-Varela et al., 2009; Rios-Colon et al., 2017; Woods-Burnham et al., 2018a). This upregulation could be reversed by blocking GR signaling with the steroidal antagonist mifepristone (RU-486) or GR knockdown (Woods-Burnham et al., 2018a). The particular observation of high endogenous CLU expression in the AA PCa cell line MDA-PCa-2b suggests that downstream effects of GR signaling such as the upregulation of genes associated with therapy-resistance may be exaggerated in AA PCa patients, and is consistent with the emerging notion that GR signaling is enhanced in the AA population (Frazier et al., 2010; Woods-Burnham et al., 2018a; Zannas et al., 2015). Our recent study also revealed higher median values of GR in AA prostate tissues compared to EA prostate tissues using the Taylor and Wallace datasets within the Oncomine database, supporting the premise that AA men with PCa may have enhanced intratumoral GR signaling (Woods-Burnham et al., 2018a).
Regardless of whether GR drives resistance to ARSI by activating AR-target genes only or also by activating an independent transcriptome that drives therapy resistance, it is becoming very clear that GR plays a major role in the progression of mCRPC. There remains an urgent need, however, to further elucidate genes driven by GR signaling that are specifically associated with ARSI-resistance while also identifying precise genes that have been linked to taxane chemotherapy. This is critical to our understanding of mechanisms by which GR may contribute to therapy resistance, supporting the development of novel therapeutic strategies. Furthermore, given that AA men suffer from disproportionate PCa incidence and mortality as well as an enhanced physiological response to glucocorticoids, additional studies are warranted to fully elucidate the interplay between GR signaling and PCa tumor aggressiveness specifically in this racial/ethnic group.
Given the emerging role of GR signaling in PCa progression, we propose that cumulative psychosocial stress leading to chronically elevated cortisol levels, increased GR expression, and sustained GR signaling in AA men over time could predispose them to develop aggressive PCa tumors as well as prime them towards poor response to conventional treatments (Fig. 1).
Therapeutic GR Modulators.
The emerging contribution of GR signaling to PCa therapy resistance has led to increasing efforts to therapeutically target this signaling as a potential treatment for mCRPC. A phase I/II clinical trial (NCT02012296) is currently ongoing to determine if combination therapy with enzalutamide and mifepristone, a GR antagonist that delayed mCRPC in pre-clinical models, extends the time to PSA progression. Because complete antagonism of GR may introduce adverse systemic consequences, highly selective GR modulators (SGRMs) that target this receptor in specific tissues are currently under development and are being evaluated in pre-clinical models of mCRPC (Hunt et al., 2018; Kach et al., 2017; Nguyen et al., 2017). The specificity of these new generation modulators are often dependent upon their superior selectivity for GR over other steroid receptors, which is a characteristic of mifepristone (Baulieu, 1991; Hunt et al., 2018; Kach et al., 2017; Meijer et al., 2018; Nguyen et al., 2017). For example, the SGRM CORT118335 has a high GR affinity with only modest MR affinity (Atucha et al., 2015; Hunt et al., 2012; Nguyen et al., 2017). This and another SGRM, CORT108297, showed ability to block GR transcriptional activity and slow CRPC progression in pre-clinical models, and unlike mifepristone did not affect AR signaling (Kach et al., 2017). Another SGRM, CORT125134, was shown to reverse the effects of prednisone without binding to progesterone receptor (PR) and is well tolerated in humans. However, it should be noted that the overwhelming majority of study participants were EA males and the study did not take into account potential racial or ethnic differences in GR signaling (Hunt et al., 2018). An additional determinant of the ability of GR modulators to bind to specific tissues is the presence or lack of GR co-regulators that may either serve as coactivators or corepressors (Hunt et al., 2018; Lonard and O'Malley, 2012; Meijer et al., 2018; Nguyen et al., 2017). GREs in different genes depend on particular sets of coactivators (Lachize et al., 2009; Meijer et al., 2018; Zalachoras et al., 2016). Selective GR modulators that act via GREs may differ in their ability to induce interactions with other transcription factors that bind DNA in the vicinity of the GREs (Meijer et al., 2018).
The ability to treat a specific disease independently from all the other GR-dependent effects would be considered a “game changer” in medicine (De Bosscher et al., 2016; Meijer et al., 2018). To that end, SGRMs are being introduced in clinical trials for PCa patients. A current trial seeks to establish the recommended dose, safety, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of the specific GR antagonist ORIC-101 in combination with enzalutamide in mCRPC patients (NCT04033328) (Multani, 2019). A similar trial is underway to examine the same primary outcomes using a different GR antagonist, CORT125281 (NCT03437941) (Shepherd, 2018). Given the potential role of GR in promoting DTX resistance, future pre-clinical studies and clinical trials evaluating selective GR modulation in combination with taxane drugs for patients with mCRPC are warranted.
Conclusions and future perspectives
Emerging data support the notion that PCa health disparities are influenced by the interplay between socioeconomic, psychosocial, health care, and biological/genetic factors. Chronic and amplified GR signaling triggered by cumulative psychosocial stress may negatively influence health outcomes in AA men by promoting the activation of molecular pathways that contribute to PCa progression (Fig. 1). This enhanced GR signaling, combined with diminished access to health care and genetic drivers of PCa that increase risk of aggressive disease in AA men may promote a more aggressive tumor phenotype, including the possibility of increased resistance to standard therapies (Fig. 1). However, it remains to be determined if mCRPC therapies involving glucocorticoids such as dexamethasone or prednisone produce lower benefits in AA patients compared to EA patients. Likewise, it remains to be determined if AA men with mCRPC may benefit more from combinatorial therapies involving GR antagonists such as mifepristone and SGRMs compared to EA patients. Critical for addressing these issues is the recruitment and retention of large numbers of AA PCa patients to current and future clinical trials examining the contribution of glucocorticoids or GR antagonists to overall patient survival. It would therefore be of great interest to determine if there are racial differences in the outcomes of the SWITCH trial (abiraterone plus dexamethasone), the NCT02012296 trial (enzalutamide plus mifepristone), the NCT03437941 trial (enzalutamide plus the GR antagonist CORT125281), and the NCT0403328 trial (enzalutamide plus the GR antagonist ORIC-101).
As our understanding of the contribution of GR signaling to PCa progression and therapy resistance increases, clinicians and researchers must consider carefully the clinical implications of standard and upcoming treatments for PCa that modulate GR function in AA men. It is probable that ongoing clinical trials may reveal race-related differential benefits, or harms, of modulating GR function for mCRPC treatment. This would necessitate clinical and socio-behavioral scientists working together to measure psychosocial indicators linked to increased GR signaling in AA PCa patients. Socio-behavioral scientists and clinicians could also implement novel community- and clinic-based interventions to reduce chronic stress, which may result in attenuated GR signaling and, potentially, better outcomes for AA men, who are most at risk of developing aggressive PCa. Finally, preventive policy interventions targeting upstream determinants of SES including education, housing, urban planning, community development, employment, and income enhancements should be considered to ameliorate the discriminatory contributors to chronic psychosocial stress experienced by African American men.
Acknowledgements
The authors thank Dr. Marino De Leon, Director of the LLUH Center for Health Disparities and Molecular Medicine, for his support of this work. We also thank the members of this center and many other colleagues for stimulating discussions leading to the writing of this comprehensive review paper.
Funding Sources
The authors acknowledge research support from the National Institutes of Health (NIH) grants R21CA226654-01A1 (C.A.C) and P20MD006988-Project 2 (C.A.C.), the Loma Linda University Health (LLUH) Center for Health Disparities and Molecular Medicine (C.A.C. and S.M) and the LLUH Schools of Medicine (C.A.C, H.C.R., F.G.A), Behavioral Health (S.M.), and Nursing (L.R.R). L.W.B., S.R.M., and E.S.S.H. were supported by NIH grant R25GM060507 and the LLU-NIH Initiative for Maximizing Student Development (IMSD) graduate training program. E.S.S.H. is currently supported by NIH grant R21CA226654-01A1S1. L.W.B. is currently supported by NIH grant T32 CA186895.
Footnotes
Conflict of interest
The authors declare that no competing or conflict of interests exist. The funders had no role in study design, writing of the manuscript, or decision to publish.
REFERENCES
- Abdalla I, Ray P, Vaida F, and Vijayakumar S (1999). Racial differences in prostate-specific antigen levels and prostate-specific antigen densities in patients with prostate cancer. Am J Clin Oncol 22, 537–541. [DOI] [PubMed] [Google Scholar]
- Acevedo-Garcia D, Lochner KA, Osypuk TL, and Subramanian SV (2003). Future directions in residential segregation and health research: a multilevel approach. Am J Public Health 93, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, and Gunnar MR (2001). Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology 26, 189–208. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, and Syme SL (1994). Socioeconomic status and health. The challenge of the gradient. Am Psychol 49, 15–24. [DOI] [PubMed] [Google Scholar]
- Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, Flicks JL, Wilson KM, Rider JR, Sesso HD, et al. (2016). A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. Journal of the National Cancer Institute 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arace J, Flores V, Monaghan T, Robins D, Karanikolas N, Winer A, and Weiss J (2020). Rates of clinically significant prostate cancer in African Americans increased significantly following the 2012 US Preventative Services Task Force recommendation against prostate specific antigen screening: A Single Institution Retrospective Study. Int J Clin Pract 74, e13447. [DOI] [PubMed] [Google Scholar]
- Arlen PM, and Gulley JL (2005). Docetaxel-based regimens, the standard of care for metastatic androgen-insensitive prostate cancer. Future Oncol 1, 19–22. [DOI] [PubMed] [Google Scholar]
- Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, Chatila WK, Chakravarty D, Han GC, Coleman I, et al. (2018). The long tail of oncogenic drivers in prostate cancer. Nat Genet 50, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, et al. (2013). Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmane I, Ceraline J, Duclos B, Rob L, Litique V, Barthelemy P, Bergerat JP, Dufour P, and Kurtz JE (2011). New strategies for medical management of castration-resistant prostate cancer. Oncology 80, 1–11. [DOI] [PubMed] [Google Scholar]
- Atucha E, Zalachoras I, van den Heuvel JK, van Weert LT, Melchers D, Mol IM, Belanoff JK, Houtman R, Hunt H, Roozendaal B, et al. (2015). A Mixed Glucocorticoid/Mineralocorticoid Selective Modulator With Dominant Antagonism in the Male Rat Brain. Endocrinology 156, 4105–4114. [DOI] [PubMed] [Google Scholar]
- Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, and Begg CB (2002). Survival of blacks and whites after a cancer diagnosis. Jama 287, 2106–2113. [DOI] [PubMed] [Google Scholar]
- Banerjee PP, Banerjee S, Brown TR, and Zirkin BR (2018). Androgen action in prostate function and disease. Am J Clin Exp Urol 6, 62–77. [PMC free article] [PubMed] [Google Scholar]
- Batai K, Murphy AB, Nonn L, and Kittles RA (2016). Vitamin D and Immune Response: Implications for Prostate Cancer in African Americans. Front Immunol 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE (1991). The antisteroid RU486: its cellular and molecular mode of action. Trends Endocrinol Metab 2, 233–239. [DOI] [PubMed] [Google Scholar]
- Baum RP, and Kulkarni HR (2012). THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy - The Bad Berka Experience. Theranostics 2, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Hotte SJ, Saad F, Alekseev B, Matveev V, Flechon A, Gravis G, Joly F, Chi KN, Malik Z, et al. (2017). Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. The Lancet Oncology 18, 1532–1542. [DOI] [PubMed] [Google Scholar]
- Benjamins MR, Hunt BR, Raleigh SM, Hirschtick JL, and Hughes MM (2016). Racial Disparities in Prostate Cancer Mortality in the 50 Largest US Cities. Cancer Epidemiol 44, 125–131. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Srivastava SK, Khan MA, Prajapati VK, Singh S, Carter JE, and Singh AP (2017). Racial disparities in prostate cancer: a molecular perspective. Front Biosci (Landmark Ed) 22, 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddie SC, Conway-Campbell BL, and Lightman SL (2012). Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology (Oxford, England) 51, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Ekstrom TJ, Harris TB, et al. (2008). Intra-individual change over time in DNA methylation with familial clustering. Jama 299, 2877–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn J, Vecchiarelli S, Heyer EE, Patrick SM, Lyons RJ, Jaratlerdsiri W, van Zyl S, Bornman MSR, Mercer TR, and Hayes VM (2019). TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: A focus on Africa. Prostate 79, 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccon-Gibod L, Fournier G, Bottet P, Marechal JM, Guiter J, Rischman P, Hubert J, Soret JY, Mangin P, Mallo C, et al. (1997). Flutamide versus orchidectomy in the treatment of metastatic prostate carcinoma. Eur Urol 32, 391–395; discussion 395-396. [PubMed] [Google Scholar]
- Bocklandt S, Lin W, Sehl ME, Sanchez FJ, Sinsheimer JS, Horvath S, and Vilain E (2011). Epigenetic predictor of age. PLoS One 6, e14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, van Mierlo HC, Rutten BP, Radstake TR, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JC, et al. (2015). Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 51, 506–512. [DOI] [PubMed] [Google Scholar]
- Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, and Yamamoto KR (2007). Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes & development 21, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosland MC, and Mahmoud AM (2011). Hormones and prostate carcinogenesis: Androgens and estrogens. Journal of carcinogenesis 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, West DW, Satariano WA, Liebman M, and Esserman L (2009). Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer 124, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Byrd DR (2012). Race/ethnicity and self-reported levels of discrimination and psychological distress, California, 2005. Preventing Chronic Disease 9, E156–E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, and Hawkley LC (2003). Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med 46, S39–52. [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, and Sawyers CL (2004). Molecular determinants of resistance to antiandrogen therapy. Nat Med 10, 33–39. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen F, Ren Y, Weng G, Keng PC, Chen Y, and Lee SO (2019). Glucocorticoid receptor upregulation increases radioresistance and triggers androgen independence of prostate cancer. Prostate 79, 1386–1398. [DOI] [PubMed] [Google Scholar]
- Chi KN, Higano CS, Blumenstein B, Ferrero JM, Reeves J, Feyerabend S, Gravis G, Merseburger AS, Stenzl A, Bergman AM, et al. (2017). Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol 18, 473–485. [DOI] [PubMed] [Google Scholar]
- Chornokur G, Dalton K, Borysova ME, and Kumar NB (2011). Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. The Prostate 71, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, Nelson HH, Karagas MR, Padbury JF, Bueno R, et al. (2009). Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS genetics 5, e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YJ (2014). Knockdown of clusterin expression increases the in vitro sensitivity of human prostate cancer cells to paclitaxel. J Toxicol Environ Health A 77, 1443–1450. [DOI] [PubMed] [Google Scholar]
- Chung CC, Hsing AW, Edward Y, Biritwum R, Tettey Y, Adjei A, Cook MB, De Marzo A, Netto G, Tay E, et al. (2014). A comprehensive resequence-analysis of 250 kb region of 8q24.21 in men of African ancestry. The Prostate 74, 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens F, Joniau S, and Helsen C (2017). Comparing the rules of engagement of androgen and glucocorticoid receptors. Cell Mol Life Sci 74, 2217–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, and Seeman T (2006). Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med 68, 41–50. [DOI] [PubMed] [Google Scholar]
- Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, Birtle A, Palmer S, and Riemsma R (2007). A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer. Health Technol Assess 11, iii-iv, xv-xviii, 1–179. [DOI] [PubMed] [Google Scholar]
- Corn PG, Agarwal N, Araujo JC, and Sonpavde G (2017). Taxane-based Combination Therapies for Metastatic Prostate Cancer. European urology focus. [DOI] [PubMed] [Google Scholar]
- Cuevas AG, Trudel-Fitzgerald C, Cofie L, Zaitsu M, Allen J, and Williams DR (2019). Placing prostate cancer disparities within a psychosocial context: challenges and opportunities for future research. Cancer Causes Control 30, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst BF, Wan P, Sheng X, Bensen JT, Ingles SA, Rybicki BA, Nemesure B, John EM, Fowke JH, Stevens VL, et al. (2020). A Germline Variant at 8q24 Contributes to Familial Clustering of Prostate Cancer in Men of African Ancestry. Eur Urol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A (2013). How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life. Social Science & Medicine 77, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskivich TJ, Kwan L, Dash A, and Litwin MS (2015). Racial parity in tumor burden, treatment choice and survival outcomes in men with prostate cancer in the VA healthcare system. Prostate Cancer Prostatic Dis 18, 104–109. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr., Saad F, et al. (2011). Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364, 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al. (2010). Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Beck IM, Ratman D, Berghe WV, and Libert C (2016). Activation of the Glucocorticoid Receptor in Acute Inflammation: the SEDIGRAM Concept. Trends Pharmacol Sci 37, 4–16. [DOI] [PubMed] [Google Scholar]
- DeRouen MC, Schupp CW, Koo J, Yang J, Hertz A, Shariff-Marco S, Cockburn M, Nelson DO, Ingles SA, John EM, et al. (2018). Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol 53, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Miller KD, Goding Sauer A, Jemal A, and Siegel RL (2019). Cancer statistics for African Americans, 2019. CA Cancer J Clin 69, 211–233. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, and Jemal A (2016). Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 66, 290–308. [DOI] [PubMed] [Google Scholar]
- Deshmukh SK, Azim S, Ahmad A, Zubair H, Tyagi N, Srivastava SK, Bhardwaj A, Singh S, Rocconi RP, and Singh AP (2017). Biological basis of cancer health disparities: resources and challenges for research. Am J Cancer Res 7, 1–12. [PMC free article] [PubMed] [Google Scholar]
- Djeu JY, and Wei S (2009). Clusterin and chemoresistance. Adv Cancer Res 105, 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorff TB, and Crawford ED (2013). Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol 24, 31–38. [DOI] [PubMed] [Google Scholar]
- Du XL, Lin CC, Johnson NJ, and Altekruse S (2011). Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979-2003. Cancer 117, 3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, Yan F, Wang Y, Bouchez LC, Kularatne SA, et al. (2012). CXCR4 expression in prostate cancer progenitor cells. PLoS One 7, e31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MM, and Ferraro KF (2005). Are racial disparities in health conditional on socioeconomic status? Soc Sci Med 60, 191–204. [DOI] [PubMed] [Google Scholar]
- Feldman BJ, and Feldman D (2001). The development of androgen-independent prostate cancer. Nat Rev Cancer 1, 34–45. [DOI] [PubMed] [Google Scholar]
- Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, Zaldivar F, Santos R, Tyson DR, and Ornstein DK (2009). Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol 182, 1621–1627. [DOI] [PubMed] [Google Scholar]
- Frank S, Nelson P, and Vasioukhin V (2018). Recent advances in prostate cancer research: large-scale genomic analyses reveal novel driver mutations and DNA repair defects. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier B, Hsiao CW, Deuster P, and Poth M (2010). African Americans and Caucasian Americans: differences in glucocorticoid-induced insulin resistance. Horm Metab Res 42, 887–891. [DOI] [PubMed] [Google Scholar]
- Gaston KE, Kim D, Singh S, Ford OH 3rd, and Mohler JL (2003). Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol 170, 990–993. [DOI] [PubMed] [Google Scholar]
- Gidron Y, Fabre B, Grosman H, Nolazco C, Mesch V, Mazza O, and Berg G (2011). Life events, cortisol and levels of prostate specific antigen: a story of synergism. Psychoneuroendocrinology 36, 874–880. [DOI] [PubMed] [Google Scholar]
- Gilbert DC, and Parker C (2005). Docetaxel for the treatment of prostate cancer. Future Oncol 1, 307–314. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez E, and Gomez-Sanchez CE (2014). The multifaceted mineralocorticoid receptor. Compr Physiol 4, 965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad I, and Picard D (2007). The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 275, 2–12. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, et al. (2007). Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39, 631–637. [DOI] [PubMed] [Google Scholar]
- Gusev A, Shi H, Kichaev G, Pomerantz M, Li F, Long HW, Ingles SA, Kittles RA, Strom SS, Rybicki BA, et al. (2016). Atlas of prostate cancer heritability in European and African-American men pinpoints tissue-specific regulation. Nat Commun 7, 10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Signorello LB, Strom SS, Kittles RA, Rybicki BA, Stanford JL, Goodman PJ, Berndt SI, Carpten J, Casey G, et al. (2015). Generalizability of established prostate cancer risk variants in men of African ancestry. Int J Cancer 136, 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, et al. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular cell 49, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, van der Brug M, Chong S, Moore M, Longo DL, Cookson MR, Traynor BJ, et al. (2011). Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet 20, 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, Diez J, Sanchez-Mut JV, Setien F, Carmona FJ, et al. (2012). Distinct DNA methylomes of newborns and centenarians. Proceedings of the National Academy of Sciences of the United States of America 109, 10522–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo AA, Montecinos VP, Paredes R, Godoy AS, McNerney EM, Tovar H, Pantoja D, Johnson C, Trump D, and Onate SA (2011). Biochemical characterization of nuclear receptors for vitamin D3 and glucocorticoids in prostate stroma cell microenvironment. Biochem Biophys Res Commun 412, 13–19. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, and Potosky AL (2001). Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst 93, 388–395. [DOI] [PubMed] [Google Scholar]
- Holmes L Jr., Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, Chinacherem C, Comeaux C, Pelaez L, Okundaye O, et al. (2020). Black-White Risk Differentials in COVID-19 (SARS-COV2) Transmission, Mortality and Case Fatality in the United States: Translational Epidemiologic Perspective and Challenges. Int J Environ Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S (2013). DNA methylation age of human tissues and cell types. Genome Biol 14, R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S (2015). Erratum to: DNA methylation age of human tissues and cell types. Genome Biol 76, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, van den Berg LH, and Ophoff RA (2012). Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol 13, R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FW, Mosquera JM, Garofalo A, Oh C, Baco M, Amin-Mansour A, Rabasha B, Bahl S, Mullane SA, Robinson BD, et al. (2017). Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function ERF Mutations. Cancer Discov 7, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Myklebust LM, Kjarland E, Gjertsen BT, Pendino F, Bruserud O, Doskeland SO, and Lillehaug JR (2007). LEDGF/p75 has increased expression in blasts from chemotherapy-resistant human acute myelogenic leukemia patients and protects leukemia cells from apoptosis in vitro. Molecular cancer 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins C, and Hodges CV (1972). Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 22, 232–240. [DOI] [PubMed] [Google Scholar]
- Hunt H, Donaldson K, Strem M, Zann V, Leung P, Sweet S, Connor A, Combs D, and Belanoff J (2018). Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin Pharmacol Drug Dev 7, 408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt HJ, Ray NC, Hynd G, Sutton J, Sajad M, O'Connor E, Ahmed S, Lockey P, Daly S, Buckley G, et al. (2012). Discovery of a novel non-steroidal GR antagonist with in vivo efficacy in the olanzapine-induced weight gain model in the rat. Bioorg Med Chem Lett 22, 7376–7380. [DOI] [PubMed] [Google Scholar]
- Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, Conzen SD, and Szmulewitz RZ (2014). Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer 5, 72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Chung KF, and Adcock IM (2006). Update on glucocorticoid action and resistance. J Allergy Clin Immunol 117, 522–543. [DOI] [PubMed] [Google Scholar]
- James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, Collins S, Daunton A, McLaren D, O'Sullivan J, et al. (2016). Clinical Outcomes and Survival Following Treatment of Metastatic Castrate-Refractory Prostate Cancer With Docetaxel Alone or With Strontium-89, Zoledronic Acid, or Both: The TRAPEZE Randomized Clinical Trial. JAMA Oncol 2, 493–499. [DOI] [PubMed] [Google Scholar]
- Jaratlerdsiri W, Chan EKF, Gong T, Petersen DC, Kalsbeek AMF, Venter PA, Stricker PD, Bornman MSR, and Hayes VM (2018). Whole-Genome Sequencing Reveals Elevated Tumor Mutational Burden and Initiating Driver Mutations in African Men with Treatment-Naive, High-Risk Prostate Cancer. Cancer Res 78, 6736–6746. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, and Thun MJ (2006). Cancer statistics, 2006. CA: a cancer journal for clinicians 56, 106–130. [DOI] [PubMed] [Google Scholar]
- Jia L, Choong CS, Ricciardelli C, Kim J, Tilley WD, and Coetzee GA (2004). Androgen receptor signaling: mechanism of interleukin-6 inhibition. Cancer research 64, 2619–2626. [DOI] [PubMed] [Google Scholar]
- Joseph DN, and Whirledge S (2017). Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- July LV, Akbari M, Zellweger T, Jones EC, Goldenberg SL, and Gleave ME (2002). Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate 50, 179–188. [DOI] [PubMed] [Google Scholar]
- Kach J, Long TM, Selman P, Tonsing-Carter EY, Bacalao MA, Lastra RR, de Wet L, Comiskey S, Gillard M, VanOpstall C, et al. (2017). Selective Glucocorticoid Receptor Modulators (SGRMs) Delay Castrate-Resistant Prostate Cancer Growth. Molecular cancer therapeutics 16, 1680–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadmiel M, and Cidlowski JA (2013). Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 34, 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor ED, Haneuse S, Valdimarsdottir UA, Williams DR, Signorello LB, and Rider JR (2019). Socioenvironmental adversity and risk of prostate cancer in non-Hispanic black and white men. Cancer Causes Control 30, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas C, Wang C, Deng F, Huang H, Wang D, and Lee P (2017). Molecular mechanisms involving prostate cancer racial disparity. Am J Clin Exp Urol 5, 34–48. [PMC free article] [PubMed] [Google Scholar]
- Kassel O, and Herrlich P (2007). Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol 275, 13–29. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, Cooper RS, and McGee DL (1997). Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology 8, 621–628. [PubMed] [Google Scholar]
- Kelly SP, Rosenberg PS, Anderson WF, Andreotti G, Younes N, Cleary SD, and Cook MB (2017). Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. Eur Urol 71, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish P, and Chinegwundoh F (2011). Ethnic differences in prostate cancer. Br J Cancer 105, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock BL, Thorpe RJ Jr., Howard DL, Bowie JV, Ross LE, Fakunle DO, and LaVeist TA (2016). Racial Disparity in Time Between First Diagnosis and Initial Treatment of Prostate Cancer. Cancer Control 23, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish JK, Yu M, Percy-Laurry A, and Altekruse SF (2014). Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. Journal of the National Cancer Institute Monographs 2014, 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, et al. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SS, Jacobs DR Jr., Chesney MA, Raczynski J, and McCreath H (1996). Psychosocial factors and plasma lipids in black and white young adults: the Coronary Artery Risk Development in Young Adults Study data. Psychosom Med 58, 365–373. [DOI] [PubMed] [Google Scholar]
- Koltai T (2014). Clusterin: a key player in cancer chemoresistance and its inhibition. Onco Targets Ther 7, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolvenbag GJ, and Nash A (1999). Bicalutamide dosages used in the treatment of prostate cancer. Prostate 39, 47–53. [DOI] [PubMed] [Google Scholar]
- Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, Campa D, Riboli E, Key T, Gronberg H, et al. (2011). Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 43, 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil C, Fendler WP, Eiber M, Baum R, Bozkurt MF, Czernin J, Delgado Bolton RC, Ezziddin S, Forrer F, Hicks RJ, et al. (2019). EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging 46, 2536–2544. [DOI] [PubMed] [Google Scholar]
- Krieger N, Kosheleva A, Waterman PD, Chen JT, and Koenen K (2011). Racial discrimination, psychological distress, and self-rated health among US-born and foreign-born Black Americans. American Journal of Public Health 101, 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krok-Schoen JL, Fisher JL, Baltic RD, and Paskett ED (2017). White-Black Differences in Cancer Incidence, Stage at Diagnosis, and Survival Among Older Adults. Journal of aging and health, 898264317696777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon J, Puhr M, Buijs JT, van der Horst G, Hemmer DM, Marijt KA, Hwang MS, Masood M, Grimm S, Storm G, et al. (2016). Glucocorticoid receptor antagonism reverts docetaxel resistance in human prostate cancer. Endocr Relat Cancer 23, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SY, Gleave ME, and Beltran H (2019). Towards precision oncology in advanced prostate cancer. Nat Rev Urol 16, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, Shevrin DH, Dreicer R, Hussain M, Eisenberger M, et al. (2018). Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, and Meijer OC (2009). Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America 106, 8038–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeuille MH, Gravel J, Grittner A, Lefebvre P, Ellis L, and McKenzie RS (2013). Real-World Corticosteroid Utilization Patterns in Patients with Metastatic Castration-Resistant Prostate Cancer in 2 Large US Administrative Claims Databases. Am Health Drug Benefits 6, 307–316. [PMC free article] [PubMed] [Google Scholar]
- Lamont KR, and Tindall DJ (2011). Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol 25, 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempiainen JK, Niskanen EA, Vuoti KM, Lampinen RE, Goos H, Varjosalo M, and Palvimo JJ (2017). Agonist-specific Protein Interactomes of Glucocorticoid and Androgen Receptor as Revealed by Proximity Mapping. Mol Cell Proteomics 16, 1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, and Van Cauter E (1997). Sleep loss results in an elevation of cortisol levels the next evening. Sleep 20, 865–870. [PubMed] [Google Scholar]
- Li J, Alyamani M, Zhang A, Chang KH, Berk M, Li Z, Zhu Z, Petro M, Magi-Galluzzi C, Taplin ME, et al. (2017). Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li CX, Ye H, Chen F, Melamed J, Peng Y, Liu J, Wang Z, Tsou HC, Wei J, et al. (2008). Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med 12, 2790–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, and O'Malley BW (2012). Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol 8, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, and Sigler PB (1991). Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 352, 497–505. [DOI] [PubMed] [Google Scholar]
- Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, Hu JC, Sweeney CJ, Yu JB, Feng EY, et al. (2017). Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol 28, 1098–1104. [DOI] [PubMed] [Google Scholar]
- Maitland NJ, Frame FM, Rane JK, Erb HH, Packer JR, Archer LK, and Pellacani D (2019). Resolution of Cellular Heterogeneity in Human Prostate Cancers: Implications for Diagnosis and Treatment. Adv Exp Med Biol 1164, 207–224. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. (1995). The nuclear receptor superfamily: the second decade. Cell 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, et al. (2015a). DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, et al. (2015b). The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. International journal of epidemiology 44, 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, Lance R, Foley J, Sexton W, Kusuda L, et al. (2003). Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol 169, 1670–1675. [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E, Jackson PN, Pearce WJ, and Zhang L (2015). Endothelial glucocorticoid receptor promoter methylation according to dexamethasone sensitivity. J Mol Endocrinol 55, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Yamamoto Y, Shiota M, Kuruma H, Beraldi E, Matsuyama H, Zoubeidi A, and Gleave M (2013). Cotargeting Androgen Receptor and Clusterin Delays Castrate-Resistant Prostate Cancer Progression by Inhibiting Adaptive Stress Response and AR Stability. Cancer research 73, 5206–5217. [DOI] [PubMed] [Google Scholar]
- McCoy J, Wambier CG, Vano-Galvan S, Shapiro J, Sinclair R, Ramos PM, Washenik K, Andrade M, Herrera S, and Goren A (2020). Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J Cosmet Dermatol 19, 1542–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. N Engl J Med 338, 171–179. [DOI] [PubMed] [Google Scholar]
- Mediavilla-Varela M, Pacheco FJ, Almaguel F, Perez J, Sahakian E, Daniels TR, Leoh LS, Padilla A, Wall NR, Lilly MB, et al. (2009). Docetaxel-induced prostate cancer cell death involves concomitant activation of caspase and lysosomal pathways and is attenuated by LEDGF/p75. Molecular cancer 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, Koorneef LL, and Kroon J (2018). Glucocorticoid receptor modulators. Ann Endocrinol (Paris) 79, 107–111. [DOI] [PubMed] [Google Scholar]
- Misra-Hebert AD, Hu B, Klein EA, Stephenson A, Taksler GB, Kattan MW, and Rothberg MB (2017). Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 120, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery B, Cheng HH, Drechsler J, and Mostaghel EA (2014). Glucocorticoids and prostate cancer treatment: friend or foe? Asian J Androl 16, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery B, Kheoh T, Molina A, Li J, Bellmunt J, Tran N, Loriot Y, Efstathiou E, Ryan CJ, Scher HI, et al. (2015). Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: exploratory analysis from COU-AA-301. Eur Urol 67, 866–873. [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Poolman TM, Williamson AJ, Wang Z, Clark NR, Ma'ayan A, Whetton AD, Brass A, Matthews LC, and Ray DW (2016). Glucocorticoid receptor isoforms direct distinct mitochondrial programs to regulate ATP production. Sci Rep 6, 26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul JW, Sesterhenn IA, Connelly RR, Douglas T, Srivastava S, Mostofi FK, and McLeod DG (1995). Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. Jama 274, 1277–1281. [PubMed] [Google Scholar]
- Multani PS (2019). Study of ORIC-101 in Combination with Enzalutamide. [Google Scholar]
- Murthy VH, Krumholz HM, and Gross CP (2004). Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama 291, 2720–2726. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Srinivas S, and Feldman D (2016). Androgen-glucocorticoid interactions in the era of novel prostate cancer therapy. Nature reviews Urology 13, 47–60. [DOI] [PubMed] [Google Scholar]
- Nettey OS, Walker AJ, Keeter MK, Singal A, Nugooru A, Martin IK, Ruden M, Gogana P, Dixon MA, Osuma T, et al. (2018). Self-reported Black race predicts significant prostate cancer independent of clinical setting and clinical and socioeconomic risk factors. Urol Oncol 36, 501 e501–501 e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto GJ (2013). Clinical applications of recent molecular advances in urologic malignancies: no longer chasing a "mirage"? Advances in anatomic pathology 20, 175–203. [DOI] [PubMed] [Google Scholar]
- Nguyen ET, Streicher J, Berman S, Caldwell JL, Ghisays V, Estrada CM, Wulsin AC, and Solomon MB (2017). A mixed glucocorticoid/mineralocorticoid receptor modulator dampens endocrine and hippocampal stress responsivity in male rats. Physiol Behav 178, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PL, Ma J, Chavarro JE, Freedman ML, Lis R, Fedele G, Fiore C, Qiu W, Fiorentino M, Finn S, et al. (2010). Fatty acid synthase polymorphisms, tumor expression, body mass index, prostate cancer risk, and survival. J Clin Oncol 28, 3958–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, and Charmandari E (2010). The human glucocorticoid receptor: molecular basis of biologic function. Steroids 75, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, and Cidlowski JA (2011). Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286, 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, and Cidlowski JA (2013). The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 132, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olender J, and Lee NH (2019). Role of Alternative Splicing in Prostate Cancer Aggressiveness and Drug Resistance in African Americans. Adv Exp Med Biol 1164, 119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DC, Jaratlerdsiri W, van Wyk A, Chan EKF, Fernandez P, Lyons RJ, Mutambirw SBA, van der Merwe A, Venter PA, Bates W, et al. (2019). African KhoeSan ancestry linked to high-risk prostate cancer. BMC Med Genomics 12, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovics G, Price DK, Lou H, Chen Y, Garland L, Bass S, Jones K, Kohaar I, Ali A, Ravindranath L, et al. (2019). Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis 22, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, Finn S, Graff RE, Penney KL, Rider JR, et al. (2013). Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. Journal of the National Cancer Institute 105, 1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, and Kirschbaum C (2005). State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology 30, 261–272. [DOI] [PubMed] [Google Scholar]
- Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, Herawi M, Everson R, Giroux CN, Schwartz AG, et al. (2013). Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev 22, 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, and Toft DO (1997). Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18, 306–360. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, and Lupien SJ (2003). Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med 65, 92–99. [DOI] [PubMed] [Google Scholar]
- Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, Kroon J, Leo A, Heidegger I, Eder I, et al. (2018). The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 927–938. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Whittaker P, McCann OT, Finer S, Valdes AM, et al. (2010). Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome research 20, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand KA, Rohland N, Tandon A, Stram A, Sheng X, Do R, Pasaniuc B, Allen A, Quinque D, Mallick S, et al. (2016). Whole-exome sequencing of over 4100 men of African ancestry and prostate cancer risk. Hum Mol Genet 25, 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reams RR, Agrawal D, Davis MB, Yoder S, Odedina FT, Kumar N, Higginbotham JM, Akinremi T, Suther S, and Soliman KF (2009). Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infect Agent Cancer 4 Suppl 1, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck TR (2017). Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol 27, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Y, and Rosenberg JE (2012). Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther 6, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Colon L, Cajigas-Du Ross CK, Basu A, Elix C, Alicea-Polanco I, Sanchez TW, Radhakrishnan V, Chen CS, and Casiano CA (2017). Targeting the stress oncoprotein LEDGF/p75 to sensitize chemoresistant prostate cancer cells to taxanes. Oncotarget 8, 24915–24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere P, Luterstein E, Kumar A, Vitzthum LK, Deka R, Sarkar RR, Bryant AK, Bruggeman A, Einck JP, Murphy JD, et al. (2020). Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. [DOI] [PubMed] [Google Scholar]
- Roberts LR, Wilson CM, Stiel L, Casiano CA, and Montgomery SB (2018). Prostate Cancer Screening among High-Risk Black Men. J Nurse Pract 14, 677–682 e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Laorden N, Lozano R, Jayaram A, Lopez-Campos F, Saez MI, Montesa A, Gutierrez-Pecharoman A, Villatoro R, Herrera B, Correa R, et al. (2018). Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study). Br J Cancer 119, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, and Henderson BE (1992). 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 339, 887–889. [DOI] [PubMed] [Google Scholar]
- Rundlett SE, and Miesfeld RL (1995). Quantitative differences in androgen and glucocorticoid receptor DNA binding properties contribute to receptor-selective transcriptional regulation. Mol Cell Endocrinol 109, 1–10. [DOI] [PubMed] [Google Scholar]
- Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, and Janne OA (2013). FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res 73, 1570–1580. [DOI] [PubMed] [Google Scholar]
- Sanchez TW, Zhang G, Li J, Dai L, Mirshahidi S, Wall NR, Yates C, Wilson C, Montgomery S, Zhang JY, et al. (2016). Immunoseroproteomic Profiling in African American Men with Prostate Cancer: Evidence for an Autoantibody Response to Glycolysis and Plasminogen-Associated Proteins. Mol Cell Proteomics 15, 3564–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ortiz RF, Troncoso P, Babaian RJ, Lloreta J, Johnston DA, and Pettaway CA (2006). African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer 107, 75–82. [DOI] [PubMed] [Google Scholar]
- Sartor O, Parker CC, and de Bono J (2014). Reappraisal of glucocorticoids in castrate-resistant prostate cancer. Asian J Androl 16, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O, Yang S, Ledet E, Moses M, and Nicolosi P (2020). Inherited DNA-repair gene mutations in African American men with prostate cancer. Oncotarget 11, 440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzl G, Madersbacher S, Haitel A, Gsur A, Preyer M, Haidinger G, Gassner C, Ochsner M, and Marberger M (2003). Associations of serum testosterone with microvessel density, androgen receptor density and androgen receptor gene polymorphism in prostate cancer. J Urol 169, 1312–1315. [DOI] [PubMed] [Google Scholar]
- Scher HI, Buchanan G, Gerald W, Butler LM, and Tilley WD (2004). Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 11, 459–476. [DOI] [PubMed] [Google Scholar]
- Schmid M, Meyer CP, Reznor G, Choueiri TK, Hanske J, Sammon JD, Abdollah F, Chun FK, Kibel AS, Tucker-Seeley RD, et al. (2016). Racial Differences in the Surgical Care of Medicare Beneficiaries With Localized Prostate Cancer. JAMA Oncol 2, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, and Omlin A (2020). Progress in therapy across the spectrum of advanced prostate cancer. Nat Rev Urol 17, 71–72. [DOI] [PubMed] [Google Scholar]
- Schrecengost R, and Knudsen KE (2013). Molecular pathogenesis and progression of prostate cancer. Semin Oncol 40, 244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz AJ, Williams DR, Israel BA, and Lempert LB (2002). Racial and spatial relations as fundamental determinants of health in Detroit. The Milbank quarterly 80, 677–707, iv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JR (2012). Dexamethasone premedication for prophylaxis of taxane toxicities: can the doses be reduced when paclitaxel or docetaxel are given weekly? J Oncol Pharm Pract 18, 250–256. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, and Berkman L (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 20, 243–255. [DOI] [PubMed] [Google Scholar]
- Sharifi N (2013). Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology 154, 4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers VL, and Brown ML (2002). Racial and ethnic disparities in the receipt of cancer treatment. Journal of the National Cancer Institute 94, 334–357. [DOI] [PubMed] [Google Scholar]
- Shepherd S (2018). Study to Evaluate CORT125281 in Combination with Enzalutamide in Patients with mCRPC. [Google Scholar]
- Siegel RL, Miller KD, and Jemal A (2020). Cancer statistics, 2020. CA Cancer J Clin 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Silver DA, Pellicer I, Fair WR, Heston WD, and Cordon-Cardo C (1997). Prostate-specific membrane antigen expression in normal and malignant human tissues. Clinical cancer research : an official journal of the American Association for Cancer Research 3, 81–85. [PubMed] [Google Scholar]
- Singh GK, and Jemal A (2017). Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017, 2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Jha R, Melamed J, Shapiro E, Hayward SW, and Lee P (2014). Stromal androgen receptor in prostate development and cancer. Am J Pathol 184, 2598–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Lillard JW Jr., and Singh R (2017). Molecular basis for prostate cancer racial disparities. Front Biosci (Landmark Ed) 22, 428–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, and Wand G (2012). Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34, 468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Cropley M, Griffith J, and Kirschbaum C (2000). Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosom Med 62, 286–292. [DOI] [PubMed] [Google Scholar]
- Strahle U, Klock G, and Schutz G (1987). A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proceedings of the National Academy of Sciences of the United States of America 84, 7871–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, and Schaeffer EM (2013). African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31, 2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, and Chambon P (2011). Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145, 224–241. [DOI] [PubMed] [Google Scholar]
- Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, and Nathanson D (2005). Comorbidity and survival disparities among black and white patients with breast cancer. Jama 294, 1765–1772. [DOI] [PubMed] [Google Scholar]
- Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, and Rider W (1989). Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 7, 590–597. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. (2004). Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351, 1502–1512. [DOI] [PubMed] [Google Scholar]
- Taylor RA, and Risbridger GP (2008). Prostatic tumor stroma: a key player in cancer progression. Curr Cancer Drug Targets 8, 490–497. [DOI] [PubMed] [Google Scholar]
- Thames AD, Irwin MR, Breen EC, and Cole SW (2019). Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology 106, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinas ML, and Grange T (2001). Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J 20, 1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson GR, Gayed BA, Chung PH, and Raj GV (2014). Emerging therapies in castration resistant prostate cancer. Can J Urol 21, 98–105. [PubMed] [Google Scholar]
- Todd NF, Lieberman R, Gulley JL, Dahut W, and Arlen PM (2005). Prolonged response to nilutamide in a patient with stage D0.5 prostate cancer who previously failed androgen deprivation therapy. Am J Ther 12, 172–174. [DOI] [PubMed] [Google Scholar]
- Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, de Koning HJ, and Etzioni R (2017). Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 123, 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, and Muller CP (2005). Structure of the glucocorticoid receptor (NR3C1) gene 5' untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol 35, 283–292. [DOI] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, and Libert C (2014). Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev 35, 671–693. [DOI] [PubMed] [Google Scholar]
- Vandewalle J, Luypaert A, De Bosscher K, and Libert C (2018). Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab 29, 42–54. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, and Shanks NM (1999). Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet 353, 627–631. [DOI] [PubMed] [Google Scholar]
- Wallace TA, Martin DN, and Ambs S (2011). Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis 32, 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, and Ambs S (2008). Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer research 68, 927–936. [DOI] [PubMed] [Google Scholar]
- Wang BD, Ceniccola K, Hwang S, Andrawis R, Horvath A, Freedman JA, Olender J, Knapp S, Ching T, Garmire L, et al. (2017). Alternative splicing promotes tumour aggressiveness and drug resistance in African American prostate cancer. Nat Commun 8, 15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, and Chen X (2011). How much of racial/ethnic disparities in dietary intakes, exercise, and weight status can be explained by nutrition- and health-related psychosocial factors and socioeconomic status among US adults? J Am Diet Assoc 111, 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, Siegel R, Stewart A, and Jemal A (2008). Association of insurance with cancer care utilization and outcomes. CA: a cancer journal for clinicians 58, 9–31. [DOI] [PubMed] [Google Scholar]
- Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, and Thun M (2004). Cancer disparities by race/ethnicity and socioeconomic status. CA: a cancer journal for clinicians 54, 78–93. [DOI] [PubMed] [Google Scholar]
- Warner EW, Yip SM, Chi KN, and Wyatt AW (2019). DNA repair defects in prostate cancer: impact for screening, prognostication and treatment. BJU Int 123, 769–776. [DOI] [PubMed] [Google Scholar]
- Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, Bauerschlag DO, Jockel KH, Erbel R, Muhleisen TW, et al. (2014). Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol 15, R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weprin SA, Parker DC, Jones JD, Kaplan JR, Giusto LL, Mydlo JH, Yu SS, Lee DI, Eun DD, and Reese AC (2019). Association of Low Socioeconomic Status With Adverse Prostate Cancer Pathology Among African American Men Who Underwent Radical Prostatectomy. Clin Genitourin Cancer 17, e1054–e1059. [DOI] [PubMed] [Google Scholar]
- Wiench M, John S, Baek S, Johnson TA, Sung MH, Escobar T, Simmons CA, Pearce KH, Biddie SC, Sabo PJ, et al. (2011a). DNA methylation status predicts cell type-specific enhancer activity. EMBO J 30, 3028–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiench M, Miranda TB, and Hager GL (2011b). Control of nuclear receptor function by local chromatin structure. FEBS J 278, 2211–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (2003). The health of men: structured inequalities and opportunities. Am J Public Health 93, 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, and Collins C (2001). Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports (Washington, DC : 1974) 116, 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, and Collins C (2010). Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 1186, 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, and Sternthal M (2010). Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav 51 Suppl, S15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Garg R, and Wells CM (2019). PlexinB1 Promotes Nuclear Translocation of the Glucocorticoid Receptor. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods-Burnham L, Cajigas-Du Ross CK, Love A, Basu A, Sanchez-Hernandez ES, Martinez SR, Ortiz-Hernandez GL, Stiel L, Duran AM, Wilson C, et al. (2018a). Glucocorticoids induce stress oncoproteins associated with therapy-resistance in African American and European American prostate cancer cells. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods-Burnham L, Stiel L, Wilson C, Montgomery S, Duran AM, Ruckle HR, Thompson RA, De Leon M, and Casiano CA (2018b). Physician Consultations, Prostate Cancer Knowledge, and PSA Screening of African American Men in the Era of Shared Decision-Making. Am J Mens Health, 1557988318763673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah K, Johnson MH, Choeurng V, Faisal FA, Yousefi K, Haddad Z, Ross AE, Alshalafa M, Den R, Lal P, et al. (2015). Novel Biomarker Signature That May Predict Aggressive Disease in African American Men With Prostate Cancer. J Clin Oncol 33, 2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, and Yates JW (1998). Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer 82, 2123–2134. [PubMed] [Google Scholar]
- Yang L, Yeh SD, Xie S, Altuwaijri S, Ni J, Hu YC, Chen YT, Bao BY, Su CH, and Chang C (2004). Androgen suppresses PML protein expression in prostate cancer CWR22R cells. Biochem Biophys Res Commun 314, 69–75. [DOI] [PubMed] [Google Scholar]
- Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, et al. (2007). Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39, 645–649. [DOI] [PubMed] [Google Scholar]
- Young RF, Waller JB Jr., and Kahana E (1991). Racial and socioeconomic aspects of myocardial infarction recovery: studying confounds. Am J Prev Med 7, 438–444. [PubMed] [Google Scholar]
- Yuan J, Kensler KH, Hu Z, Zhang Y, Zhang T, Jiang J, Xu M, Pan Y, Long M, Montone KT, et al. (2020). Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet 16, e1008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I, Verhoeve SL, Toonen LJ, van Weert LT, van Vlodrop AM, Mol IM, Meelis W, de Kloet ER, and Meijer OC (2016). Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol Psychiatry 21, 1733–1739. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Roh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, et al. (2015). Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, and West AE (2014). Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 264, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Wen W, Morgans AK, Pao W, Shu XO, and Zheng W (2015). Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol 1, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Choi JP, Jaehne M, Gao YR, Desai R, Tuckermann J, Zhou H, Handelsman DJ, and Simanainen U (2014). Glucocorticoid receptor in prostate epithelia is not required for corticosteroid-induced epithelial hyperproliferation in the mouse prostate. Prostate 74, 1068–1078. [DOI] [PubMed] [Google Scholar]