Abstract
Conventional ureases possess dinuclear nickel active sites that are oxygen-stable and require a set of accessory proteins for metallocenter biosynthesis. By contrast, oxygen-labile ureases have active sites containing dual ferrous ions and lack a requirement for maturation proteins. The structures of the two types of urease are remarkably similar, with an active site architecture that includes two imidazoles and a carboxylate ligand coordinated to one metal, two imidazoles coordinated to the second metal, and a metal-bridging carbamylated lysine ligand. The electronic spectrum of the diferric form of the enzyme resembles that of methemerythrin. Resonance Raman spectroscopic analyses confirm the presence of a μ-oxo ligand and indicate the presence of one or more terminal solvent ligands.
Keywords: Urease, Dinuclear iron, Resonance Raman spectroscopy
1. Importance of urease
The enzyme urease hydrolyzes urea to release ammonium ion and carbamate, which then spontaneously decomposes to form bicarbonate and a second molecule of ammonium ion (Eq. 1). This activity is widely distributed in plants and also is found in selected bacteria, archaea, yeasts, filamentous fungi, and algae [1]. In plants, urease plays important roles during germination and senescence, and it allows crop plants to incorporate nitrogen from urea-based fertilizers [2]. Some microorganisms also utilize urease for nitrogen assimilation, but the microbial enzyme additionally is associated with detrimental medical consequences [1]. For example, ureolytic pathogens of the urinary tract are responsible for pyelonephritis, inflammation of the kidney, 10–15% of urinary stones, and catheter encrustations [3–5]. Furthermore, peptic ulcers, gastritis, and some stomach cancers arise due to colonization of the stomach lining by Helicobacter pylori, a pathogen containing high levels of urease [6–8]. The agricultural and medical importance of urease has led to extensive efforts to characterize this enzyme.
 |
Eq. 1 |
To set the stage for the focus of this article, we first survey selected properties of the archetype nickel-containing urease in Section 2. Section 3 then describes the surprising discovery and distinct properties of an iron-containing form of these enzymes, including extensive Raman studies to define the coordination environment of the metallocenter.
2. The nickel-dependent urease paradigm
In 1975, Bert Zerner and colleagues established that jack bean urease, an oxygen-stable enzyme, possesses two atoms of nickel ion per active site [9]. Subsequent studies by many laboratories revealed that ureases from a vast array of other organisms also require nickel for activity.
2.1. Structure of nickel-containing ureases
The first crystal structure reported for a nickel-containing urease was that from Klebsiella aerogenes [10]. This bacterial enzyme contains three types of subunits (11.1-kDa UreA, 11.7-kDa UreB, and 60.3-kDa UreC) that align with portions of the ~90-kDa single subunit jack bean urease sequence. The K. aerogenes subunits assemble into a trimer of trimers, (UreABC)3, containing three dinuclear nickel active sites (Fig. 1). The two metal ions are separated by 3.6 Å and bridged by a carbamylated lysine residue (K217), Ni-1 is coordinated by two histidine residues (H246 and H272), and Ni-2 is coordinated by two histidine residues (H134 and H136) plus an aspartic acid residue (D360). Each metal has a bound water and a third water/hydroxide molecule bridges the two nickel ions, thus providing 5-coordinate and 6-coordinate geometries for the dinuclear site. Two additional histidine residues (H219 and H320) reside at the active site and assist in catalysis. Structures of nickel-containing ureases were later reported for enzymes from Sporosarcina pasteurii [11], H. pylori [12, 13], Yersinia enterocolitica [14], jack bean [15], and pigeon pea [16]. The S. pasteurii protein architecture is identical to that for K. aerogenes. The H. pylori protein possesses only two subunits (its UreA corresponds to a fusion of the two small subunits of K. aerogenes and its large subunit is named UreB) that assemble into a [(UreAB)3]4 spherical dodecahedron with twelve active sites. Y. enterocolitica urease has three types of subunit like K. aerogenes, but it also assembles into a spherical [UreABC)3]4 macromolecule. Finally, the single-subunit plant ureases associate into hexamers made up of back-to-back trimers. Importantly, the di-nickel active sites of all of these enzymes have identical components, although a flap covering this region occupies distinct conformations in the different structures.
Fig. 1.
Structure of a nickel-dependent urease. Two protein views of the urease from K. aerogenes (PDB 1FWJ) are shown in cartoon mode with each UreABC unit depicted in three shades of blue, maroon, or yellow, and the nickel atoms illustrated as green spheres. The expanded view of the active site depicts the side chain ligands as blue sticks, two nearby histidine residues as cyan sticks, and metal-coordinated water/hydroxide molecules as red spheres. Ni-1 is to the upper right and Ni-2 is to the lower left.
2.2. Catalytic mechanism of the archetype nickel-containing urease
The reaction mechanism of nickel-dependent urease has been intensively investigated by multiple experimental and computational approaches (summarized in [17, 18]), but several nuanced aspects of this process continue to be debated. A simplified urease mechanism is illustrated in Fig. 2. In the first step, the two terminal water molecules are displaced as the carbonyl oxygen of urea coordinates to Ni-1, with stabilization provided by H219, and one of its amide nitrogen atoms coordinates to Ni-2. In the second step, the μ-hydroxo group attacks the urea carbonyl carbon (shown here by the simultaneous cleavage of the Ni-2-to-OH bond) to yield a tetrahedral intermediate. Finally, the tetrahedral species decomposes with H320 serving as a general acid, yielding ammonia and carbamic acid. Proton exchange subsequently produces ammonium ion and carbamate, which decomposes. Notably, the protein flap covering the di-nickel active site must open for urea binding, close for catalysis to occur, and open again for product dissociation. Of particular interest, the crystal structure of urea-bound, fluoride-inhibited S. pasteurii urease is consistent with the proposed urea-bound intermediate in the mechanism [19]; in that structure, the inhibitory fluoride ion substitutes for the bridging water molecule.
Fig. 2.
A simplified reaction mechanism of nickel-dependent urease. Urea is proposed to bind with an amine coordinated to Ni-2 and its carbonyl oxygen atom coordinated to Ni-1, stabilized by a nearby histidine residue. The bridging hydroxide attacks the carbonyl carbon atom to form a transient tetrahedral species that decomposes to products with the assistance of a nearby histidine functioning as a general acid. The residue numbering shown is for the enzyme from K. aerogenes.
2.3. Urease nickel metallocenter assembly
The dinuclear nickel site of urease is synthesized in bacteria by a sophisticated process that requires the action of four accessory proteins typically encoded adjacent to the urease structural genes [20–22]. For example, ureDABCEFG of K. aerogenes encodes the three-subunit urease and four proteins (UreD, UreE, UreF, and UreG) needed to obtain mature enzyme [23, 24]. Similarly, ureABIEFGH of H. pylori codes for the two-subunit enzyme, a proton-gated urea channel (UreI) [25], and the same four maturation components (note: Helicobacter UreH is homologous to UreD of other bacteria) [26, 27]. Three analogous accessory proteins (though not a homolog of UreE) are found in eukaryotes and encoded separately from the structural genes [28]. The accessory proteins participate in nickel metallocenter assembly by a process requiring transient formation of multiple protein complexes (Fig. 3). UreE serves as a metallochaperone that delivers nickel to UreG. Nickel-bound UreG subsequently associates into a complex with the scaffold proteins UreF and UreD/H, and the heterotrimeric complex interacts with the urease apoprotein. GTP hydrolysis by UreG is suggested to release nickel into a molecular tunnel that passes through UreF and UreD to access the nascent metallocenter site in the large subunit of urease. Dissociation of the accessory proteins then produces the active nickel-containing urease holoenzyme [20, 29]. As indicated below, this highly orchestrated process for assembling the urease nickel metallocenter differs from the situation for biosynthesis of iron-containing ureases.
Fig. 3.
Nickel-containing urease maturation pathway. (a) Homodimeric UreE with bound nickel (from Sporosarcina pasteurii, PDB 4L3K). (b) Homodimeric UreG with bound nickel and GMPPNP (from Klebsiella pneumoniae, PDB 5XKT). (c) (UreF·UreD)2 complex (from Helicobacter pylori, PDB 3SF5). (d) GDP-bound (UreG·UreF·UreD)2 complex (from H. pylori, PDB 4HI0). (e) (UreA·UreB·UreC)3 urease apoprotein (from Klebsiella aerogenes, PDB 1KRA). (f) Model of the complex of (UreG·UreF·UreD/H) bound via the UreD/H component to UreB of the urease apoprotein. (g) Urease holoenzyme generated after GTP hydrolysis by UreG, release of nickel into a molecular tunnel, transfer through UreF and UreD, delivery to the nascent active site in UreC, and dissociation of the accessory proteins. Proteins are depicted in distinct colors using cartoon mode. Nickel atoms are shown as green spheres, the nickel-binding sites are indicated by the red circles, and the nickel transfer tunnel from UreG to the nascent active site is shown by a red arrow. Nucleotides are illustrated in stick mode with red carbon atoms.
3. Overturning the paradigm: iron-containing ureases
Despite the wealth of urease-related studies published over the past several decades, it was only in 2008 that hints appeared in the literature regarding an alternative to the nickel-dependent form of the enzyme. The following subsections describe findings that uncovered the first iron-dependent urease, summarize the evidence that such enzymes exist in other microorganisms, examine the structure of the best characterized iron urease, compare the iron urease metallocenter to that of other diiron proteins, and highlight the novel properties of its unique metallocenter with an emphasis on resonance Raman spectroscopy.
3.1. Discovery of an iron-containing UreA2B2 urease in Helicobacter mustelae
Several critical observations made during investigation of the ferret pathogen, Helicobacter mustelae, laid the foundation for overturning the paradigm of urease as a nickel-dependent enzyme [30]. This bacterium possesses both a ureABIEFGH gene cluster, like the H. pylori counterpart, and a second copy of the structural genes, denoted ureA2B2. Of keen interest, these two sets of genes are inversely regulated by nickel and iron. Thus, nickel positively regulates the gene cluster encoding the traditional urease using a transcriptional regulator encoded by nikR, whereas ureA2B2 is only expressed in nickel-restricted medium and exhibits greater expression when the cells are supplemented with iron. Gene deletion analysis demonstrated that UreAB and UreA2B2 both exhibit activity within intact cells. In addition, this work showed that cellular UreA2B2 activity does not depend on the production of UreG. Significantly, the UreA2B2 activity was lost when the cells were lysed.
Following up on these seminal genetic/regulation studies, UreA2B2 was purified and characterized from H. mustelae cells (containing ureB and nikR deletions to enhance ureA2B2 expression) and from recombinant Escherichia coli cells [31]. Anaerobic purification of UreA2B2 from H. mustelae yielded enzyme containing 1.1 Fe per heterodimer, lacking nickel ion, and with activity of 14 U mg−1 (i.e. 14 μmol per min per mg of protein) and a Km of 1.6 mM. As expected, the aerobically purified protein was inactive; however, subsequent anaerobic treatment with sodium dithionite restored 76% of the activity (10.7 U mg−1) to the sample. Metal analyses of the aerobically purified sample revealed approximately 2 Fe per UreA2B2 unit, with no nickel detected. Heterologous production of H. mustelae UreA2B2 in E. coli and purification using aerobic conditions yielded similarly inactive protein containing about 1 Fe per heterodimer that was also partially activated (to 9.8 U mg−1) by dithionite reduction. Incubation of UreA2B2 apoprotein with nickel or zinc in the presence of bicarbonate (needed to carbamylate the active site lysine residue) failed to generate any activity [32]. For comparison, the traditional nickel-containing urease UreAB was isolated both from wild-type H. mustelae cells and from H. mustelae cells containing deletions in nikR and ureB2 [31]. The two samples of UreAB urease were oxygen stable, much more active that UreA2B2 (390 and 840 U mg−1 and Km of 1.7 and 2.3, respectively) and possessed 0.6 and 1.1 Ni per heterodimer, respectively. These studies revealed that H. mustelae UreA2B2 is an oxygen-labile ferrous ion-dependent urease with significantly depressed activity compared to the standard nickel-containing enzyme.
3.2. Iron-containing ureases in other organisms
Several other microorganisms possess dual urease gene clusters, although the additional ureA2B2-encoded proteins have not been purified and their metal contents remain unknown. Bacterial gastric pathogens of cats or dogs, big cats, and dolphins or whales (Helicobacter acinonychis, Helicobacter felis, and Helicobacter cetorum, respectively) have genomic sequences analogous to the two sets of urease genes in H. mustelae. UreB2 of H. acinonychis is produced only when nickel is absent, as seen in the case for H. mustelae [30]. In H. felis, deletion of ureB2 reduced the cellular urease activity level, consistent with the gene cluster being functional; however, deletion of just ureB yielded H. felis cells that lacked urease activity, indicating that UreA2B2 was inactive under the conditions tested [33]. H. cetorum UreA2B2 was proposed to be an iron-containing urease based on sequence data [34], but it has not been directly substantiated. Of interest, all of the Helicobacter species described above are isolated from carnivores, which have diets deficient in nickel (a metal that is mainly found in vegetarian diets) and rich in iron. Thus, metal accessibility may have led to the evolution of the iron-dependent form of the protein [30]. Additional dual urease gene clusters have been identified in other species, including Brucella suis and Brucella abortus [35, 36], but in these microorganisms both clusters include genes encoding accessory proteins in addition to the enzyme subunits.
Iron-containing ureases also have been generated and studied using genes for the nickel-dependent enzyme of two other species. First, the apoprotein form of K. aerogenes urease was isolated and subjected to anaerobic incubation in buffer containing bicarbonate and ferrous ions; the resulting protein was weakly active (8–9 U/mg), oxygen sensitive, and contained about 0.3 Fe per UreABC [31, 32]. By contrast, when K. aerogenes apoprotein was activated using bicarbonate and nickel ions in the same buffer [37, 38], much greater oxygen-stable activity was observed and a greater metal content was obtained (approximately 400 U mg−1 and 2 Ni per UreABC). This form of the enzyme is still much less active than nickel-containing K. aerogenes urease purified from cells as the holoprotein (nearly 2,500 U mg−1 with 2 Ni per UreABC). The second example involves an acid-stable urease from Bacillus paralicheniformis produced by expressing the ureABCEFGDH gene cluster (ureH is a metal permease) in E. coli [39]. Recombinant cells grown in medium supplemented with iron exhibited moderate activity and metal content (54.6 U mg−1 with 0.3 Fe per UreABC unit). When the same cells were grown with nickel supplementation, the authors observed much greater activity and metal content (603 U mg−1 and 1.9 Ni/UreABC), indicating this form was physiologically relevant. Surprisingly, the iron-containing form of B. paralicheniformis urease was stable to oxygen. These results demonstrate that nickel-dependent ureases can, under the right conditions, become partially active with iron incorporated at their active sites. While this observation may suggest mechanistic similarities between the two types of urease, the situation remains unclear. As already noted, efforts to activate H. mustelae UreA2B2 apoprotein with nickel and bicarbonate failed to generate an active species [32].
3.3. Structure of an iron-dependent urease
H. mustelae UreA2B2 urease was purified aerobically from E. coli and the sitting drop procedure was used to generate flat, diamond-shaped crystals. The crystal structure of the diferric protein was solved by molecular replacement to a resolution of 3.0 Å after refinement [31]. Three UreA2B2 units form a triangle and four triangles associate into a hollow dodecameric sphere (Fig. 4), equivalent in architecture to the H. pylori protein. The residues serving as ligands to the dinuclear metal site are present in the same geometry as for the nickel-dependent enzymes, as are two other nearby histidine residues. The electron density was consistent with a solvent molecule bridging the iron atoms (separated by ~3.4 Å), but the low resolution precluded visualization of additional water molecules. Some additional density was located near Fe-1 and tentatively assigned to acetate from the mother liquor.
Fig. 4.
Structure of UreA2B2 urease from Helicobacter mustelae. Two cartoon views are shown for the [(UreA2B2)3]4 H. mustelae protein (PDB 3QGA) with three UreA2B2 pairs colored in different shades of blue, green, and yellow, whereas other pairs are gray. The iron atoms are indicated by brown spheres. Also depicted is a close-up view of the diferric active site with the metal-coordinating residues as blue sticks, two nearby residues as cyan sticks, and a metal-bridging oxygen atom as a red sphere. The lower portion of the figure depicts a portion of the 568-residue H. mustelae UreB2 sequence and compares it with the corresponding region of the 567-residue K. aerogenes UreC. The metallocenter ligands and two active site histidine residues are highlighted in cyan and other identities are shaded in gray.
To identify residues unique to iron-dependent ureases, the conserved amino acids among Helicobacter strains were compared to UreC sequences from other bacteria. The unique features were mapped onto the H. mustelae UreA2B2 structure and revealed a cluster of residues encircling the entrance to the active site. Notably, this region would be expected to interact with the accessory proteins that deliver the correct metal during apoprotein activation. To date, no accessory proteins specific to iron delivery have been identified.
3.4. Structural comparison to other diiron sites
The diiron center of H. mustelae UreA2B2, bound by four histidine and two carboxylate side chains (including a μ−1,3-O2CR metal-bridging carbamylated lysine), is distinct from the metallocenters of other diiron proteins, although similarities in the structures are apparent (Fig. 5). Nine examples from this large protein family [40–42] illustrate the general features of these diverse active sites. The dinuclear site of Phascolopsis gouldii hemerythrin (Hr), an oxygen-carrying protein of a benthic marine worm, in its diferric state (met-Hr) (Fig. 5a) is coordinated by five histidine and two carboxylate residues (both as bridging μ−1,3-O2CR ligands) and binds a metal-bridging solvent molecule along with a terminally-bound ligand (chloride in this particular structure) on one iron [43]. Five histidines and two carboxylates also coordinate the diferric site of the thioester hydrolase glyoxalase II from Salmonella typhimurium, although in this case one μ−1,1-O2CR carboxylate bridges the metals (as does a solvent) and the other exhibits bidentate coordination to a single iron atom (Fig. 5b) [44]. The diferric site of the cyclic-di-GMP phosphodiesterase from Bdellovibrio bacteriovorus is coordinated by four histidines, one asparagine, and two carboxylates (including one μ−1,3-O2CR ligand, Fig. 5c) [45]. Again, a solvent bridges the metals. Three other examples of diiron metallocenters coordinated by four histidine and two carboxylate residues (and a metal-bridging solvent molecule) include human myo-inositol oxygenase (Fig. 5d) [46], a phosphorylcholine esterase domain of Streptococcus pneumoniae virulence factor choline-binding protein E (with an additional asparaginyl ligand, Fig. 5e) [47], and an uncharacterized protein with a β-lactamase-like fold from Vibrio cholerae (Fig. 5f). The oxygenase and uncharacterized protein each have one μ−1,3-O2CR carboxylate, whereas the esterase has a μ−1,1-O2CR ligand. Other representatives illustrated have three or fewer histidine ligands to diiron centers. A β-hydroxylase used during chloramphenicol biosynthesis in Streptomyces venezuelae, denoted CmlA (Fig. 5g), is coordinated by three histidine and three carboxylate side chains including one μ−1,1-O2CR ligand. This metallocenter also binds a bridging solvent molecule and, in the structure shown, has a coordinating acetate molecule [48]. The diferric metallocenter of methane monooxygenase (MMO, Fig. 5h) from Methylococcus capsulatus (Bath) is coordinated by only two histidine residues and four carboxylates (one as a μ−1,3-O2CR ligand), and additionally has one terminal and two bridging bound solvent molecules [49]. Finally, the ferroxidase site of bullfrog M ferritin (Fig. 5i), designed to only transiently bind and oxidize the metals before they access the interior of the spherical iron storage protein, uses one histidine and three carboxylate residues (one as a μ−1,3-O2CR ligand) to bind the metallocenter that also coordinates a bridging and two terminal water molecules [50]. As illustrated by these examples, one must be cautious when attempting to discern the function of a protein containing a diiron metallocenter based on analysis of the metal coordination environment. Selected proteins with such sites bind or activate oxygen, whereas others catalyze hydrolysis reactions using a variety of substrate types. Nevertheless, the wealth of knowledge available from studies of such proteins is likely to provide insights into the properties of the urease dinuclear site. Notably, none of the other described diiron proteins require accessory proteins for their metallocenter biosynthesis [42]; such self-assembly also appears to occur in iron urease.
Fig. 5.
Structures of selected other protein diiron centers and urea-bound synthetic complexes. (a) Phascolopsis gouldii methemerythrin with bound chloride ion (PDB 1I4Y). (b) Salmonella typhimurium glyoxalase II (PDB 2QED), (c) Bdellovibrio bacteriovorus cyclic-di-GMP phosphodiesterase with bound phosphate (PDB 3TM8). (d) Human myo-inositol oxygenase in complex with myo-inosose-1 (PDB 2IBN). (e) Streptococcus pneumoniae phosphocholine esterase domain of virulence factor choline-binding protein E with bound phosphorylcholine (PDB 1WRA) (f) Uncharacterized metalloprotein with a β-lactamase fold from Vibrio cholera (PDB 3BV6). (g) Streptomyces venezuelae CmlA (β-hydroxylase for chloramphenicol biosynthesis) (PDB 4JO0). (h) Methylococcus capsulatus (Bath) methane monooxygenase (PDB 1MTY). (i) Bullfrog M ferritin ferroxidase center (PDB 3RBC). (j) [FeIII2(μ-oxo)( μ-OC(NH2)NH)(TPA)2]3+, where TPA is tris(2-pyridylmethyl)amine. (k) [Fe2(μ-oxo)(μ-OC(NH2)NH)(BPMEN)2]3+, where BPMEN is N,N’-dimethyl-N,N’-bis(2-pyridylmethyl)ethane-1,2-diamine.
The metallocenter of the iron-dependent urease in its oxidized state also can be compared to the structures of an extensive number of dinuclear iron chemical models [51–53]. Rather than attempt to summarize the vast array of such complexes, this section will focus on two examples with bound urea. Addition of urea to an aqueous acetonitrile solution containing [FeIII2(μ-oxo)(TPA)2(OH)(H2O)](ClO4)3, where TPA is tris(2-pyridylmethyl)amine, leads to the release of two water molecules and yields [FeIII2(μ-oxo)(μ-OC(NH2)NH)(TPA)2]3+ (Fig. 5e) [54]. The bidentate binding mode of urea in this complex is remarkably similar to that observed for urea-bound, fluoride-inhibited nickel urease [19] and, except for the distinct metal speciation and redox state, mimics an intermediate of the proposed enzyme mechanism (Fig. 2). A similar urea-bound diiron complex was crystallized using the tetradentate ligand BPMEN, N,N’-dimethyl-N,N’-bis(2-pyridylmethyl)ethane-1,2-diamine (Fig. 5f) [55]. The [Fe2(μ-oxo)(μ-OC(NH2)NH)(BPMEN)2](ClO4)3 complex formed from [Fe2(μ-oxo)(μ-OH)(BPMEN)2](ClO4)3 also exhibits an N,O-bridging deprotonated urea ligand. UV-visible spectroscopy was used to monitor the opening and expansion of the FeIII(μ-oxo)(μ-OH)FeIII core, resulting in diminishment of a prominent transition at 555 nm as it converted to the product complex with transitions at 437, 460, 509, 541, and 677 nm (1306, 1145, 509, 541, and 123 M−1 cm−1, respectively). No urea hydrolysis was detected using this complex.
3.5. Properties of the UreA2B2 metallocenter
3.5.1. Inhibitors that coordinate the metallocenter
The metallocenter of anaerobically purified UreA2B2 urease from H. mustelae is stable when treated with 1 mM EDTA, similar to the stability observed for nickel-dependent ureases. Indeed, the UreA2B2 urease activity was boosted two-fold by incubating with this metal chelator, suggesting it removes contaminating inhibitory metal ions [31]. By contrast, UreA2B2 progress curves containing 2 mM acetohydroxamic acid showed a reduction in activity by over 50% (Fig. 6a) [31]. This compound is known to be a slow-binding competitive inhibitor of nickel-dependent ureases [56]. Crystal structures have been reported for the acetohydroxamic acid-inhibited form of nickel-containing ureases from K. aerogenes, S. pasteurii, and H. pylori [12, 57, 58], allowing one to predict the likely binding geometry for the UreA2B2 cluster (Fig. 6c). The carbonyl group of this inhibitor coordinates Ni-1 and the hydroxide group of the hydroxylamine moiety is located between the two metals by displacement of the bridging solvent. Analogous progress curve analysis with 2 mM phenylphosphorodiamidate nearly eliminated UreA2B2 urease activity (Fig. 6a), demonstrating that this compound binds more tightly to the enzyme. The nickel-containing urease from jack bean also is potently inhibited by this compound, which was shown to be a slow substrate for that enzyme [59]. A crystal structure of the nickel-containing urease from S. pasteurii treated with phenylphosphorodiamidate (Fig. 6d) revealed the hydrolysis product, diamidophosphate, bound with an oxygen atom at the bridging solvent position, another oxygen atom coordinated to Ni-1, and an amine group coordinated to Ni-2 [11]; this structure closely mimics an intermediate state during urea hydrolysis (Fig. 2). Boric acid also inhibits H. mustelae iron urease (Fig. 6b). This compound is a competitive inhibitor of nickel ureases [60], and was shown by crystallography to coordinate the dinuclear nickel site by using two of its hydroxyl groups to displace the terminal water molecules bound to each nickel atom, while leaving the bridging solvent molecule in place (Fig. 6e) [61].
Fig. 6.
Inhibitors that coordinate the metallocenter of H. mustelae iron-containing urease. (a) The activity of UreA2B2 (measured by reacting timed aliquots with a colorimetric reagent specific for the product ammonia thus generating absorbance at 625 nm) was examined at 37 °C using 25 mM urea in 50 mM HEPES buffer (pH 7.8) (•), with added 2 mM acetohydroxamic acid (■), or with added 2 mM phenylphosphorodiamide (▲). (b) A similar experiment was carried out with ~10-fold greater amount of enzyme, 30 mM urea, and 0 (♦), 0.1 (■), 0.3 (▲), 1 (◊), 3 (□), 10 (Δ) mM boric acid. Inhibited structures of UreA2B2 are not available, but they may be similar to those for nickel-containing ureases inhibited by (c) acetohydroxamic acid (PDB 4UBP), (d) diamidophosphate derived from hydrolysis of phenylphosphorodiamide (PDB 3UBP), and (e) boric acid (PDB 1S3T).
3.5.2. Electronic absorption properties
The UV-visible spectrum of inactive, aerobically-purified UreA2B2 exhibits transitions near 320 and 380 nm (~4100 and ~2000 M−1 cm−1), as well as a weak broad feature at 500 nm (Fig. 7a) [31]. The addition of dithionite to the inactive oxidized UreA2B2 protein under anaerobic conditions leads to a slow bleaching of the chromophore concomitant with the development of active enzyme [31] (Fig. 7b). Subsequent exposure to oxygen restores the original signal. These transitions are reminiscent of the spectra for met-Hr or its monomeric analog myomet-Hr; e.g., P. gouldii myomet-Hr has transitions at 324 and 366 nm (6600 and 6400 M−1 cm−1) along with a weak band near 600 nm [62], a Hr-like domain in a chemotaxis protein (DcrH) from Desulfovibrio vulgaris (Hildenborough) has maxima at 322 and 380 nm (~6500 and ~4700 M−1 cm−1) and weaker features at ~480 and ~620 nm [63], and M. capsulatus (Bath) Hr showed major absorption peaks at 327 and 376 nm (4431 and 3715 M−1 cm−1) and a weak feature at 492 nm [64]. The UreA2B2 spectrum was not affected by varying the pH from 7.4 to 9.4 or by adjusting the buffer to contain 20 mM urea [32]. Unlike the dramatic spectral changes reported upon adding azide or thiocyanate to Hr [65], these additives had no effect on the UreA2B2 spectrum [32]. Indeed, the H. mustelae iron-containing urease metallocenter resembled the I119E-engineered D. vulgaris DcrH protein, with five histidine and three carboxylate metal ligands, by exhibiting poor binding to such exogenous ligands [66]. The Hr absorption has been attributed to a μ-oxo bridge connecting the two Fe(III) atoms in this oxygen-carrying protein [67, 68].
Fig. 7.
Electronic absorption spectra of H. mustelae iron-containing urease. (a) Inactive UreA2B2 protein (100 μM) was purified aerobically from recombinant E. coli cells expressing H. mustelae ureA2B2 and its spectrum was obtained in buffer containing 200 mM Tris-HCl (pH 7.4). (b) Reduction of the same sample under anaerobic conditions using 0.5 mM dithionite with 10 min timepoints. Copyright (2011) National Academy of Sciences.
3.5.3. Electron paramagnetic resonance (EPR) studies
Neither the oxidized nor the reduced H. mustelae UreA2B2 protein exhibited an EPR signal using the standard perpendicular mode. These results are consistent with antiferromagnetic coupling between two Fe(III) centers in the oxidized protein to yield an S = 0 state. The lack of signal in the reduced sample indicates that both metals are Fe(II) in the active enzyme.
Analogous diferric (met) and diferrous (deoxy) states have been characterized in several Hrs [67]. In contrast to the situation with UreA2B2, the mixed-valent and semi-met states can be generated by dithionite treatment of the Hr proteins from P. gouldii and Thermiste zostericola [69–71]. Also, the mixed-valence state has been characterized for some other diiron proteins such as myo-inositol oxygenase [46].
3.6. Resonance Raman spectroscopy of iron urease
We previously reported that the resonance Raman spectrum of oxidized H. mustelae UreA2B2 obtained with 413.1 nm excitation revealed an intense vibration at 500 cm−1 [32]. This mode was downshifted to ~495 cm−1 when using 363.8 nm excitation, for which the spectrum was invariant between pH 7.4 and pH 9.4. This prominent feature coincided well with the symmetric stretching mode (vs) of Fe(III)-O-Fe(III) sites in several proteins with a μ-oxo bridge; e.g., 506–511 cm−1 for P. gouldii met-Hr, 492–506 cm−1 for P. gouldii and Thermiste dyscritum hydroxomet-Hr, 503 cm−1 for M. capsulatus met-Hr, and 500 cm−1 and 504 cm−1 for the wild-type and I119E variant of DcrH [64, 66, 68, 72–74].
3.6.1. Diferric bridging structure
The structural origin of the 500 cm−1 vibration can be examined by substituting stable isotopes of oxygen and hydrogen. The initial isotopic study of diferric UreA2B2 found no evidence for 16O/18O exchange with bulk water (λex = 413 nm) [32]. Follow-up Raman measurements described here were conducted in a similar manner using excitation at 406.7 nm. Small vibrational changes were detected upon incubation in H216O vs. H218O without affecting the frequency of the main 500 cm−1 mode. While reduction of metal ions (using 5 mM Na2S2O4) is expected to facilitate oxygen exchange by weakening the Fe-O bonds and by promoting protonation of the oxygen atom, oxidation of UreA2B2 by molecular oxygen is not a physiological reaction and its mechanism is not known. Chemical oxidation of UreA2B2 with 3 mM [Fe(CN)6]3+ under anaerobic conditions provides a more controlled method for obtaining the diferric enzyme after incubation of the diferrous state in isotopically-labelled medium. Indeed, chemical oxidation of UreA2B2 in H218O resulted in the downshift of the main mode to 486 cm−1 from 501 cm−1 observed for the sample oxidized in H216O (Fig. 8, top). Both isotopes were exchanged into H216O-containing buffer prior to measurement to select for the vibrations of oxygen atom(s) that are non-exchangeable in the diferric state. The contribution of ligands that remain exchangeable in diferric UreA2B2 was probed by identical re-oxidation in H218O followed by exchange into H216O or H218O buffer (Fig. 8, bottom). The post-oxidation bulk water substitution resulted in a smaller and slightly different isotopic difference pattern, although the main peak and trough frequencies were identical to those observed for the pre-oxidation substitution. Noticeably, the same pattern was observed previously upon bulk water substitution using normal abundance UreA2B2 with UV excitation. The similarity of the bulk water isotopic shift observed with the 16O- [32] and 18O- (here) non-exchangeable isotopomers argues that the isotopic shifts illustrated in the top and bottom of Fig. 8 originate from distinct oxygen atoms, likely from one or more bridging atoms and one or more terminal ligands, respectively.
Fig. 8.
Effects of isotopic substitution on the Raman spectra of diferric UreA2B2 upon redox cycling in isotopic water. Top: Absolute spectra of UreA2B2 oxidized in H216O (a, blue) or H218O (b, red) buffers. The oxidant was removed by exchange into H216O-containing buffer for both samples. Redox cycling allows for exchange of the bridging ligand with the bulk water oxygen isotope present during the oxidation, as shown by the isotopic difference spectrum (a – b, black). Bottom: Absolute spectra of UreA2B2 oxidized in H218O buffer and subsequently aerobically exchanged into H216O (a, blue) or H218O (b, red) buffers. The difference spectrum (c – d, black) reveals additional oxygen vibrations from terminal ligands around 500 cm−1 that remain exchangeable in the diferric state. Samples were reduced using sodium dithionite and anaerobically oxidized using [Fe(CN)6]3−. The excitation was at 406.7 nm. Asterisks indicate the plasma lines.
The frequency and the 16 cm−1 isotopic shift of a 500 cm−1 mode are consistent with several types of oxygenic ligands. Depending on the complex geometry, it can arise from the vs of a singly bridged (Fe2(μ-O)) or doubly bridged (Fe2(μ-O)2 or Fe2(μ-O)(μ-OH)) diferric center [75]. It can also arise from the vFe-O stretching mode of a terminal solvent molecule. The distinguishing vibrational signature of bis-μ-oxo complexes cannot be probed for using the 16O18O mixed oxygen isotope, as was done for O2-dependent enzymes [76, 77], since UreA2B2 does not incorporate atoms from 18O2 into the cluster [32]. Isotope exchange upon redox cycling of UreA2B2 in the medium containing an equimolar mixture of H216O and H218O would be expected to yield either a 1:2:1 distribution for the 16O2:16O18O:18O2 isotopomers of a Fe2-(μ-O)2 complex or a 1:1 distribution for the 16O:18O isotopomers of a Fe2-μ-O complex. Only the 16O18O isotopomer is expected to yield a new vibration around 492 cm−1 whereas all other isotopomers will be vibrationally identical to those obtained upon exchange in H216O or H218O separately (500 cm−1 and 484 cm−1, respectively), including terminal ligands. Such exchange in UreA2B2 follows the pattern expected for Fe2-μ-O complex, as shown in Fig. 9 (a, top). The exchange in the mixed water isotope medium in the absence of urea yielded two modes at 500 cm−1 and 484 cm−1, each with 50% intensity of that observed for single isotope-derived isotopomers. Quantitative analysis conclusively shows that no new frequencies appear after H216O:H218O exchange (Fig. 9b, top), ruling out a bis-μ-oxo structure for diferric UreA2B2. The addition of urea causes an approximate 10 cm−1 downshift of the corresponding oxygen vibrations of 16O-only and 18O-only isotopomers to 491 cm−1 and 474 cm−1, respectively (Fig. 9a, bottom). Corresponding vibrations in the 16O:18O isotopomer also shift down and remain split. Quantitative comparison of the mixed and homogenous isotopomers confirm that the single oxygen atom is retained the cluster in the presence of urea (Fig. 9b, bottom), despite structural perturbations.
Fig. 9.
Identity of the bridging oxygen ligands in diferric UreA2B2. (a) Absolute spectra of diferric UreA2B2 anaerobically oxidized in H216O medium (black), H218O medium (blue), or medium containing a stoichiometric mixture of H216O and H218O (red). The top spectra were obtained without any further amendments, whereas the bottom spectra are for the same samples to which 50 mM urea was added and allowed to incubate for at least 30 min. (b) Analysis of the isotopic composition of the bridging ligands. Isotopic differences between the 16O-only and 18O-only isotopomers from panel (a) show a downshift of the oxygen vibration upon addition of urea (black lines). The differences between the 16O:18O mixed oxygen isotopomer and the average of the separate 16O- and 18O-isotopomers (red lines) show that no new vibrations appear when both 16O and 18O isotopes are present simultaneously, indicative of a single bridging oxygen atom. The excitation was at 406.7 nm. Asterisks indicate the plasma lines.
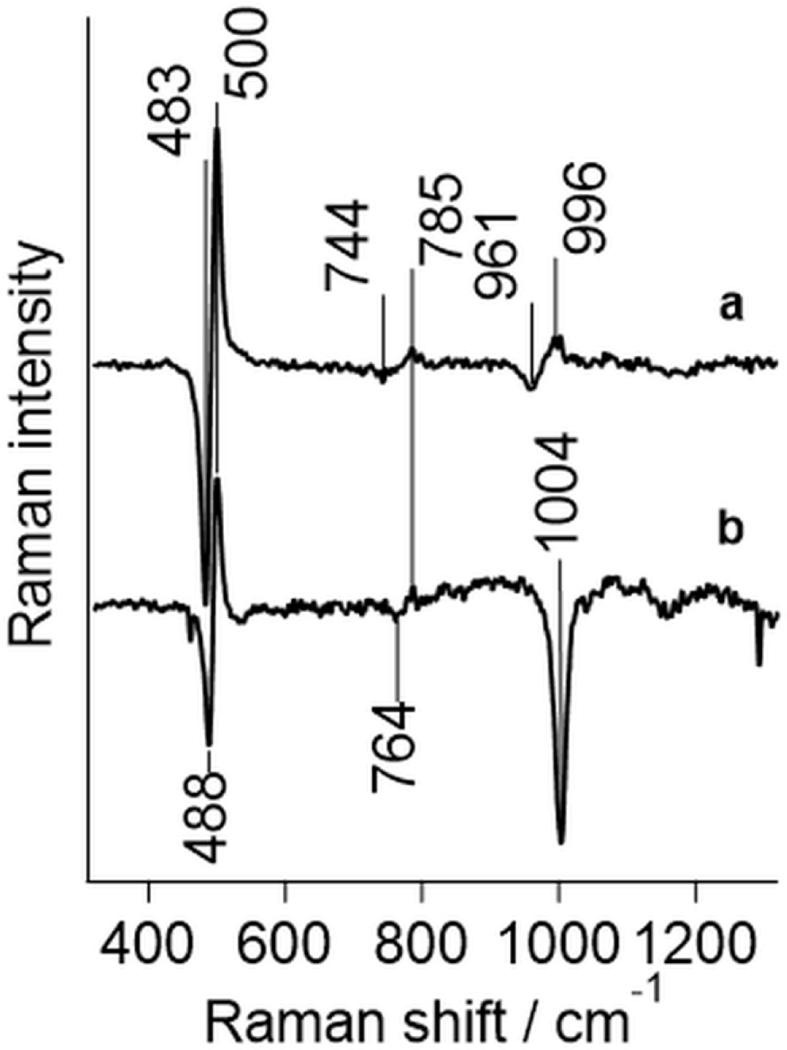
Bridged dinuclear clusters exhibit symmetrical (vs, < 650 cm−1) and asymmetrical (vas, >650 cm−1) oxygen isotope-sensitive stretching modes while terminal ligands typically exhibit only the vFeO stretching mode. A weak vibration at 785 cm−1 in the Raman spectrum of UreA2B2 (Fig. 10) is sensitive to both 18O substitution (Δv = −41 cm−1) and urea binding (Δv = −21 cm−1). This vibration was also observed with greater relative intensity upon UV excitation [32]. 18O sensitivity of the 785 cm−1 mode shown here supports an assignment to vas of a Fe(III)-O(H)-Fe(III) center with the corresponding vs at 500 cm-1. The UreA2B2 Fe-O-Fe angle of ~130° obtained for vs or vas modes using the Sanders-Loehr correlation [78, 79] were in a good agreement with each other and are consistent with the crystal structure. This bond geometry is common for protein diferric clusters containing a single bridging oxygen atom. Corresponding model complexes tend to exhibit larger angles, while proteins with a bis-μ-oxo cluster show smaller angles. This geometry is comparable to that of Ni-OH-Ni clusters in K. aerogenes or S. pasteurii ureases at 121° and 119°, respectively [11, 57].
Fig. 10.
High frequency vibrational modes in diferric UreA2B2. (a) The 16O vs 18O bridging oxygen isotopic difference in re-oxidized sample is compared to (b) downshifts induced by the addition of 60 mM urea to the normal abundance UreA2B2. In addition to the symmetrical stretching modes near 500 cm−1, an asymmetrical stretching mode (785 cm−1) and the overtone of the symmetrical stretching mode (996 cm−1) are visible. The negative band at 1004 cm−1 in (b) is due to the added urea. The excitation was at 406.7 nm.
The resonance Raman enhancement of the vs mode in the near-UV and violet spectral region is attributed to charge-transfer (CT) transitions from π orbitals of the μ-O bridge to FeIII dxy orbitals. The Raman excitation profiles of the vs mode in several Fe-O-Fe model complexes show maxima at λ ≥ 400 nm and are distinctly shifted towards lower energy from the corresponding absorption maxima. While the excitation profiles have not been quantified for UreA2B2 using an internal standard, the current results indicate that the corresponding CT in UreA2B2 appears at higher energy than in the model complexes and better aligns with oxy-Hr. This assignment is supported by the reported sensitivity of the excitation profile of vs in Hr to exogenous ligands [78].
Another 18O-sensitive vibration in UreA2B2 at 996/961 cm−1 is assigned to the vs,0→2 overtone of the fundamental vs,0→1 vibration at 500/483 cm-1. Changes in vs,0→2 upon urea binding are masked by the vs(CN) of the latter compound. [80] The relative intensity of vs,0→2 vs vs,0→1 in UreA2B2 is similar to the previously reported overtone progression reported for [Fe2O(O2CCH3)2(HB(pz)3)2] [81]. An estimated average anharmonicity constant for 16O and 18O isotopomers (X11 ≈ 2.3 cm−1) and the ratio X11/w1 (≈ 4.5×10−3) of UreA2B2 are larger than values observed in model complexes [81], as expected for the sterically-constrained protein environment, although the lack of higher overtones in the Raman spectra of UreA2B2 does not allow for precise evaluation.
The relative intensities of vas and vs (Ias/Is) provide additional insight into the ligand structure of the UreA2B2 diferric cluster. Spiro and colleagues reported that the B2 symmetry of vas in [Fe2O(O2CCH3)2(HB(pz)3)2] results in a very weak fundamental Raman mode in favor of the IR intensity [81]. The correlation between the Ias/Is and the cluster symmetry is supported by many examples of proteins and model compounds [82]. Diferric model complexes with single and multiple bridges exhibit Ias/Is of < 0.2 and <0.1, respectively, while higher ratios are found in proteins (0.1 – 0.3) [78, 83]. In cases of extreme asymmetry the Ias/Is ratio can greatly exceed 1, such as reported for [N5FeOFeX3]+ (X= Cl, Br), mixed-valence FeII-O-FeIII models, or heme/nonheme diiron nitric oxide reductase [82, 84, 85]. Studies on small molecule binding to Hr typically show small increase of Ias/Is in the presence of an external ligand, further supporting the notion that the relative intensity of vas correlates with the differences in the ligand environment between the two metal atoms [78]. Raman studies on oxo-bridged diferric proteins typically focus on the vs region <700 cm−1, however several studies published since the original Sanders-Loehr analysis reported the vas spectral region can be used for comparison with UreA2B2. The Raman spectra (λex = 561 nm) of the chloramphenicol biosynthesis enzyme CmlIox indicate comparable intensities of these two modes, so vas is detectable despite vibrational overlap with other vibrations [86]. A prominent vas was reported for a stearoyl-CoA Δ9 desaturase (Δ9D) (λex = 351 nm, close to λex = 364 nm used for UreA2B2 earlier) in agreement with the low sensitivity of Ias/Is to the excitation wavelength [78, 87]. D. vulgaris rubrerythrin (Rbr) shows Ias/Is > 0.5 (λex = 407 nm) [88]. The μ-oxo bridge of oxy-Hr shows vas at nearly half the intensity of vs (λex = 364 nm) [89]. Similar intensity ratios were reported for oxy-Hrs from Lingula unguis and Siphonosoma cumanense [90] along with D. vulgaris Rbr (λex = 407 nm) [88]. The diferric ribonucleotide reductase (RNR) R2 subunit shows one of the lowest Ias/Is = 0.20 known for proteins [78]. This value may be an overestimate considering the likely superposition between vas and a protein mode at 756 cm−1 in the radical-free form of the protein (λex = 406 nm) [91]. The same study reported that vas was undetectable in the native form of the enzyme, also hinting at a low Ias/Is.
Notably, the Ias/Is = 0.05 observed for UreA2B2 makes this the lowest known intensity of the vas mode among diferric proteins. By extension of the aforementioned correlation with cluster symmetry, such a low Ias indicates a high degree of symmetry in the ligand environment of the two Fe(III) in the protein. Of interest, this symmetry is not disrupted upon the binding of urea, despite vibrational changes noted in the cluster. The high degree of cluster symmetry in UreA2B2 is consistent with the ligation of each metal ion by two imidazole residues as shown by crystallography [31]. The coordination of D361 to one Fe necessitates coordination of at least one terminal solvent oxygen atom to the other Fe to maintain symmetry. The presence of at least one such ligand is supported by the weak Raman changes observed in UreA2B2 upon bulk water substitution [32]. The low intensity of the terminal water vibration(s) relative to vs—despite a very close match in their frequencies—indicate that the terminal ligand is oriented out of the Fe-O-Fe plane, reducing resonance enhancement by an Fe→μ-O CT transition. Such a geometry is consistent with the presence of H247 opposite to the μ-O-Fe bond in the crystal structure of UreA2B2. While the binding of solvent molecules to one or both metals of diferric clusters is typical, the appearance of their vibrations in the resonance Raman spectra is less common due to cluster symmetry and selection rules. The prominent Raman vFe-OH stretching mode of a terminal ligand at 565 cm−1 was reported previously for P. gouldii hydroxomet-Hr (λex = 364 nm) and a corresponding δFe-O-D bending mode in deuterated solvent was implicated in Fermi resonance with the vs mode of the μ-O bridge [72, 73]. Both frequencies are typical of terminal Fe-OH ligands and are substantially higher than the ~500 cm−1 vibrations detected in UreA2B2 upon bulk water substitution without redox cycling [32], suggesting that the diiron cluster in the latter state is coordinated by terminal water rather hydroxide.
3.6.2. Protonation state of the diferric bridging ligand
The Sanders-Loehr geometric correlation is generally valid for clusters containing single- and double-oxo bridging ligands that can be either protonated or deprotonated, and even dinuclear clusters containing other metals [92]. Therefore, frequencies alone are not sufficient to establish ligand protonation states. Probing the protonation states of terminal solvent ligands by resonance Raman spectroscopy typically is straightforward. The presence of covalent protons with natural Fe-O-H angles results in a substantial downshift of the vFe-OH(2) stretching mode upon deuteration (ΔH/D < −15 cm−1). The sensitivity of the vFe-OH mode to deuteration decreases as the Fe-O-H/D angle approaches 90° [93]. Hydrogen bonding to the terminal oxygen results in a much smaller upshift in the vFe-O mode upon deuteration (ΔH/D ≈ +1–5 cm−1) that arises from small differences in the hydrogen bonding distances between two isotopomers [94].
Interpretation of deuteration sensitivity of Raman spectra in diiron clusters is more complex, in part due to the steric constraints imposed upon the geometry of a proton by the Fe-O-Fe cluster and its ligands. Fortuitously, the lack of 16O/18O exchange of the bridging oxygen atom in UreA2B2 allows one to accentuate Fe-O-Fe vibrations with high selectivity by comparing 18O – 16O isotopic difference spectra for samples in H2O versus 2H2O (Fig. 11). Such analysis revealed no detectable changes in the frequency of this vibration in diferric UreA2B2. In the presence of urea, the frequency of the vs mode remained similarly insensitive to deuteration (not shown). This and several other observations support a deprotonated state of the Fe-μ(O)-Fe cluster in UreA2B2.
Fig. 11.
Pronation state of the bridging oxygen in diferric UreA2B2. UreA2B2 samples were re-oxidized in H216O and H218O media to exchange the bridging oxygen atom. The 16O vs. 18O isotope difference spectra were obtained in the absence of urea using protonated (top) or deuterated (bottom) medium after buffer exchange. The absence of a detectable shift in the frequency of the bridging mode between H2O and 2H2O signifies the lack of exchangeable protons associated with the bridging oxygen. The excitation was at 406.7 nm.
Oxygen atoms in bent diferric clusters with strong π/σ superexchange pathways have increased electron density due to weaker in-plane π-bonding interactions with metal ions. This situation leads to increased proton affinities of the μ-O atom [95]. However, most mono-bridged clusters in diferric proteins with FeIII-O-FeIII angles of 120–130° possess deprotonated μ-O bridges, including Hr, Rbr, RNR, purple acid phosphatase, and MMO. Several observations argue that the μ-O bridge in diferric UreA2B2 also is deprotonated. The vs Raman vibration was observed in UreA2B2 with high intensity upon excitation at 364 nm and 407 nm, where both wavelengths are close to the prominent near-UV optical absorption band (Fig. 7). The pronounced near-UV optical absorption is characteristic of Fe-O-Fe clusters, while protonated Fe-μ(OH)-Fe clusters lack this optical signature [96]. Furthermore, Raman spectra of both urea-free and urea-bound states of UreA2B2 showed no detectable ΔH/D, although the absence of H/D sensitivity alone may be difficult to interpret unambiguously, as illustrated below.
Comparative Raman studies revealed differences in H/D sensitivity in the diferric forms of RNR subunit R2 from different organisms. E. coli RNR in both active and radical-free forms showed a small upshift of the vs mode in D2O, which was attributed to hydrogen bonding between the μ-O atom and a base other than terminal Fe-OH(2) [91]. In contrast, the active form of mouse RNR showed a ΔH/D ≈ −5 cm−1, which is substantially smaller than expected based on the reduced mass of an oscillator [97]. Such an anomalously small downshift was attributed to either vibrational coupling between vs,Fe-O-Fe and vFe-OH(2) or to hydrogen bonding between the μ-O bridge and a terminal Fe-OH(2).
Differences in hydrogen bonding interactions with the μ-O atom in RND were also proposed to play a role in its different exchangeabilities with the bulk solvent, and they control the fate of the bridging ligand upon reduction. Data accumulated in many studies show that the role and manifestation of hydrogen bonding may be more complex. In addition to the noted changes in E. coli RNR, values of ΔH/D ≈ +2–4 cm−1 were reported for vs of Fe(III)2-μ-O in oxy-Hr [73] and Rbr [88]. A negative ΔH/D ≈ −5 cm−1 was also observed in the E238A variant of E. coli RNR [98] and in CmlI [86]. A third group of clusters with ΔH/D ≈ 0 cm−1 includes met-Hr [73], Bacillus cereus RNR R2 [99], CmlA [100], Δ9D [87], and now UreA2B2 (with and without urea). The lack of H/D sensitivity is generally attributed to a lack of hydrogen bonding to the μ-O atom. However, the differences in the susceptibility of the μ-O atom to 16O/18O isotope exchange with the bulk solvent suggest that in some cases the lack of H/D sensitivity may be a result of two competing hydrogen bonding interactions yielding a net H/D insensitive vs mode.
3.6.3. The role of hydrogen bonding in 16O/18O exchange of the bridging ligand
The differences in the rate of 16O/18O isotopic exchange with bulk solvent between RNRs from various organisms was attributed to the differences in hydrogen bonding to the μ-O atom, with stronger hydrogen bonding hindering isotopic exchange in the diferric state and stabilizing its protonation in the mixed valence FeII-μ(OH)-FeIII states [97, 101–103]. By extension, one would expect very strong hydrogen bonding in UreA2B2, for which no exchange was detected after tens of h in H218O. However, this situation should also result in a large ΔH/D of vs which was not observed. Quantitative reports of the 16O/18O isotope exchange in other diferric proteins for comparison with UreA2B2 are limited, but qualitative results indicate dissociation between H/D sensitivity and susceptibility of the Fe(III)2-μ-O clusters to 16O/18O isotope exchange. For example, ligand-free met-Hr shows no evidence of 16O/18O exchange while B. cereus RNR [99] and CmlA [100] show 16O/18O exchange on the min time scale. None of these proteins exhibit detectable ΔH/D. An 16O/18O exchange on the min time scale was observed in proteins with a positive ΔH/D ≈ +5 cm−1 (Δ9D, RNR, and Rbr), as well as with negative ΔH/D ≈ −7 cm−1 (CmlI) [86]. N3−, OCN−, and CN− complexes of Hr show facile 16O/18O exchange while SCN− and F− complexes of the same protein do not [104]. The covalency of the bridge in these complexes (and similar Fe-O-Fe angles) as well as the diversity in their deuteration sensitivities illustrate that the lability of the bridging ligand is more nuanced than previously thought and likely cannot be attributed to the presence of a particular type or geometry of the hydrogen bonding interaction. The presence of Fe-OH(2) terminal ligands is also insufficient to enable the exchange. The currently available data suggest that the susceptibility of the μ-O bridge to isotope exchange depends on the availability of non-ligated solvent molecules in the active site, as proposed for the Mn2 clusters [105]. More quantitative studies are needed to test such a mechanism in diferric proteins.
3.6.4. Effects of substrate and inhibitors
The mechanism of the urea-induced structural perturbations in UreA2B2 was further examined by comparing the effects of binding substrate and inhibitors. After confirming the downshift of vs from 500 cm−1 to 492 cm−1 in the presence of urea by Raman (Fig. 12a, top and middle), the UreA2B2 sample was dialyzed overnight against substrate-free buffer. Repeating the Raman measurement showed the characteristic spectrum of as-isolated diferric protein (Fig. 12a, bottom), demonstrating that the urea-induced structural perturbations are completely reversible. The urea-induced downshift is not due to chaotropic effects as indicated by the absence of detectable vibrational changes when the sample (Fig. 12b, top) was treated with guanidinium chloride (Fig. 12b, middle) at the same concentration as examined for urea (Fig. 12b, bottom). The compound acetohydroxamic acid, a known inhibitor of UreA2B2 urease, also had no effect on the resonance Raman spectrum of the protein (Fig. 12c, top and middle). In contrast, the potent UreA2B2 urease inhibitor phenylphosphorodiamide (Fig. 6A) lowered the frequency of the vs mode to 491 cm−1, which is similar to the effect of urea. The phenylphosphorodiamide-induced downshift also was reversed upon removal of the inhibitor (not shown). With its dual amine functionalities, phenylphosphorodiamide and its hydrolysis product diamidophosphate closely mimic urea, perhaps accounting for the similarities in their effects on the Raman spectrum.
Fig. 12.
Sensitivity of the bridging mode in UreA2B2 to ligands. (a) Addition of 20 mM urea to as-isolated UreA2B2 (top) causes a downshift of the bridging mode to 492 cm−1 (middle). This downshift was fully reversed when the sample was dialyzed overnight against urea-free buffer and re-measured (bottom). (b) The spectrum of the as-isolated UreA2B2 (top) is indistinguishable from UreA2B2 in the presence of 30 mM guanidinium-Cl, a chaotropic agent (middle), in contrast to the effect of 30 mM urea (bottom). (c) The bridging oxygen vibration of UreA2B2 (top) is not affected by addition of 20 mM acetohydroxamic acid (middle), whereas 20 mM phenylphosphorodiamide (bottom) leads to a downshift comparable to that induced by urea (a). The excitation was at 406.7 nm. Asterisks indicate the plasma lines.
Both the vs and vas modes show downshifts in the presence of urea, indicating that its effect is not limited to the Fe-O-Fe angle of the cluster, in which case opposing changes in vs and vas are expected. The magnitude of the shift of the vs mode (−8 cm−1) indicates substantial structural perturbations. For comparison, N3− binding to DcrH Hr or CN− to P. gouldi Hr causes < 2 cm−1 changes while OH− binding causes a 6 cm−1 downshift of in the vs mode (when binding via trans conformation) versus the corresponding met-Hr [66, 73]. Raman measurements using 18O and 15N isotopomers of urea so far did not yield evidence of direct coordination by either urea atom to FeIII, thus localizing structural perturbations to the secondary coordination shell. The simultaneous downshift of both vs and vas upon urea binding strongly argues against changes in the Fe-O-Fe angle, also making substantial changes in the Fe-Fe distance less likely. An insignificant change in the Ias/Is indicates that the symmetry of the cluster is maintained in the presence of urea. Thus, a change in coordination of one FeIII would likely necessitate a symmetrical change in the other metal. Additional Raman studies on the exchangeable terminal ligands are needed to carefully evaluate such a possibility. While protonation or substantial changes in hydrogen bonding upon urea binding could cause weakening of the FeIII2-μ-O bond, the lack of ΔH/D and 16O/18O exchangeability collectively argue against hydrogen bonding to the μ-O bridge in UreA2B2. The absence of stabilizing interactions would favor the loss of the bridging atom upon protonation in the catalytic diferrous state.
4. Conclusions and questions remaining
In addition to the extensively investigated and well characterized nickel ureases found in plants and selected microorganisms, certain Helicobacter strains associated with carnivores possess a physiologically-relevant, oxygen-labile, di-ferrous urease. The structure of the diiron enzyme is remarkably similar to that of nickel-containing ureases, with identical residues functioning as ligands to and surrounding the disparate metallocenters. Despite this correspondence, no activity was obtained when attempting to incorporate nickel into the apoprotein form of the diiron enzyme. The low resolution of the UreA2B2 protein from H. mustelae prevented the precise identification of the numbers and protonation states of metallocenter-associated water molecules; however, a bridging solvent molecule appears to be present. UV-visible spectroscopy of the diferric enzyme revealed similarities to met-Hr, and detailed resonance Raman spectroscopic investigation confirmed the presence of a non-protonated μ-oxo bridge between the metals. The lack of vibrational changes upon H/D substitution suggests the absence of hydrogen bonding to the bridging oxygen. The available Raman data support high symmetry of the ligand environment between the two metal ions. Considering the metal-bound residues identified by crystallography, it implies that a single terminal water molecule is bound to the cluster with its Fe-OH2 oriented out of the Fe-O-Fe plane. The resilience of the bridging atom to 16O/18O isotopic exchange suggests that there are no additional water molecules in the immediate vicinity of the diferric cluster. The differences in nucleophilicity of a μ-oxo group bridging two ferric ions versus the μ-hydroxo group bridging two nickel (and probably two ferrous) ions account for the lack of activity of the oxidized diiron enzyme and may relate to the lower activity of the ferrous enzyme compared to the nickel-containing enzyme.
Many open questions remain to be answered about this form of the enzyme. Are iron-dependent ureases present in other organisms? Why does the diiron enzyme exhibit such poor specific activity (<1%) compared to the di-nickel enzyme? Does the cell use any accessory proteins to deliver iron or otherwise assist in the biosynthesis of the iron-containing enzyme? What is the precise coordination geometry of each metal ion in the oxidized form? More importantly, what is the coordination geometry for the two iron sites in the reduced, active enzyme? What is the mechanism of urea hydrolysis catalyzed by the iron enzyme and how does it compare to that of the paradigmatic nickel urease? We hope that these questions in the context of this summary of current knowledge on the unique iron-containing enzyme will stimulate further studies.
Highlights.
In contrast to the dinuclear nickel paradigm, some ureases contain iron active sites
Iron-containing ureases are active in their di-ferrous state and are inactivated by oxygen
Nickel ureases acquire low levels of activity when substituted by iron, but iron ureases are inactive with nickel
Electronic and resonance Raman spectra identify a μ-oxo bridging ligand in the oxidized state of iron-containing urease
Acknowledgments
The authors thank Eric Bergeron for assistance with resonance Raman data collection. We thank the National Institutes of Health for financial support (GM096132 to D.A.P. and DK45686 to R.P.H.)
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mobley HLT, Hausinger RP, Microbiol. Rev, 53 (1989) 85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Witte C-P, Plant Sci., 180 (2011) 431–438. [DOI] [PubMed] [Google Scholar]
- [3].Flannigan R, Choi WH, Chew B, Lange D, Nat. Rev. Urol, 11 (2014) 333–341. [DOI] [PubMed] [Google Scholar]
- [4].Nielubowicz GR, Mobley HLT, Nat. Rev. Urol, 7 (2010) 430–441. [DOI] [PubMed] [Google Scholar]
- [5].Norsworthy AN, Pearson MM, Trends Microbiol., 25 (2017) 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Atherton JC, Annu. Rev. Pathol, 1 (2006) 63–96. [DOI] [PubMed] [Google Scholar]
- [7].Kusters JG, Van Vliet AHM, Kuipers EJ, Clin. Microbiol. Rev, 19 (2006) 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wroblewski LE, Peek RM Jr., Wilson KT, Clin. Microbiol. Rev, 23 (2010) 713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dixon NE, Gazzola C, Blakeley RL, Zerner B, J. Am. Chem. Soc, 97 (1975) 4131–4133. [DOI] [PubMed] [Google Scholar]
- [10].Jabri E, Carr MB, Hausinger RP, Karplus PA, Science, 268 (1995) 998–1004. [PubMed] [Google Scholar]
- [11].Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S, Structure, 7 (1999) 205–216. [DOI] [PubMed] [Google Scholar]
- [12].Ha N-C, Oh S-T, Sung JY, Cha KA, Lee MH, Oh B-H, Nature Struct. Biol, 8 (2001) 505–509. [DOI] [PubMed] [Google Scholar]
- [13].Cunha ES, Chen X, Sanz-Gaitero M, Mills DJ, Luecke H, Nat Commun, 12 (2021) 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Righetto RD, Anton L, Adaixo R, Jakob RP, Zivanov J, Mahi M-A, Ringler P, Schwede T, Maier T, Stahlberg H, Nat Commun, 11 (2020) 5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Balasubramanian A, Ponnuraj K, J. Molec. Biol, 400 (2010) 274–283. [DOI] [PubMed] [Google Scholar]
- [16].Balasubramanian A, Durairajpandian V, Elumalai S, Mathivanan N, Munirajan AK, Ponnuraj K, Int. J. Biol. Macromolecules, 58C (2013) 301–309. [DOI] [PubMed] [Google Scholar]
- [17].Mazzei L, Musiani F, Ciurli S, J. Biol. Inorg. Chem, 25 (2020) 829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mazzei L, Musiani F, Ciurli S, J. Biol. Inorg. Chem, 26 (2021) 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mazzei L, Cianci M, Benini S, Ciurli S, Angew. Chem. Int. Ed, 58 (2019) 7415–7419. [DOI] [PubMed] [Google Scholar]
- [20].Farrugia MA, Macomber L, Hausinger RP, J. Biol. Chem, 288 (2013) 13178–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hausinger RP, Urease activation, in: Johnson MK, Scott RA(Eds.) Metalloprotein Active Site Assembly, Wiley & Sons, 2017, pp. 251–260. [Google Scholar]
- [22].Mobley HLT, Island MD, Hausinger RP, Microbiol. Rev, 59 (1995) 451–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mulrooney SB, Hausinger RP, J. Bacteriol, 172 (1990) 5837–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee MH, Mulrooney SB, Renner MJ, Markowicz Y, Hausinger RP, J. Bacteriol, 174 (1992) 43244330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weeks DL, Eskandari S, Scott DR, Sachs G, Science, 287 (2000) 482–485. [DOI] [PubMed] [Google Scholar]
- [26].Labigne A, Cussac V, Courcoux P, J. Bacteriol, 173 (1991) 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cussac V, Ferrero RL, Labigne A, J. Bacteriol, 174 (1992) 2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Myrach T, Zhu A, Witte C-P, J. Biol. Chem, 292 (2017) 14556–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eschweiler JD, Farrugia MA, Hausinger RP, Ruotolo BT, Structure, 26 (2018) 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stoof J, Breijer S, Pot RGJ, van der Neut D, Kuipers EJ, van Vliet AHM, Environ. Microbiol, 10 (2008) 2586–2597. [DOI] [PubMed] [Google Scholar]
- [31].Carter EL, Tronrud DE, Taber SR, Karplus PA, Hausinger RP, Proc. Natl. Acad. Sci. USA, 108 (2011) 13095–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carter EL, Proshlyakov DA, Hausinger RP, J. Inorg. Biochem, 111 (2012) 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pot RGJ, Stoof J, Nuijten PJM, de Haan LAM, Loeffen P, Kuipers EJ, Van Vliet AHM, Kusters JG, FEMS Immunol. Med. Microbiol, 50 (2007) 273–279. [DOI] [PubMed] [Google Scholar]
- [34].Kersulyte D, Rossi M, Berg DE, PLoS One, 8 (2013) e83177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bandara AB, Contreras A, Contreras-Rodriguez A, Martins AM, Dobrean V, Poff-Reichow S, Rajasekaran P, Sriranganathan N, Schurig GG, Boyle SM, BMC Microbiol., 7 (2007) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sangari FJ, Seoane A, Rodríguez MC, Agüero J, Lobo JMG, Inf. Immun, 75 (2007) 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park I-S, Hausinger RP, Science, 267 (1995) 1156–1158. [DOI] [PubMed] [Google Scholar]
- [38].Park I-S, Hausinger RP, Biochemistry, 35 (1996) 5345–5352. [DOI] [PubMed] [Google Scholar]
- [39].Liu Q, Chen Y, Yuan M, Du G, Chen J, Kang Z, Appl. Environ. Microbiol, 83 (2017) e01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Trehoux A, Mahy J-P, Avenier F, Coord. Chem. Rev, 322 (2016) 142–158. [Google Scholar]
- [41].Komor AJ, Jasniewski AJ, Que L Jr., Lipscomb JD, Nat. Prod. Rep, 35 (2018) 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Caldas Nogueira ML, Pastore AJ, Davidson VL, Arch. Biochem. Biophys, 705 (2021) 108917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Farmer CS, Kurtz DM Jr., Liu Z-J, Wang BC, Rose J, Ai J, Sanders-Loehr J, J. Biol. Inorg. Chem, 6 (2001) 418–429. [DOI] [PubMed] [Google Scholar]
- [44].Campos-Bermudez VA, Leite NR, Krog R, Costa-Filho AJ, Soncini FC, Oliva G, Vila AJ, Biochemistry, 46 (2007) 11069–11079. [DOI] [PubMed] [Google Scholar]
- [45].Lovering AL, Capeness MJ, Lambert C, Hobley L, Sockett RE, mBio, 2 (2011) e00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thorsell A-G, Persson C, Voevodskaya N, Busam RD, Hammarström M, Gräslund S, Gräslund A, Hallberg BM, J. Biol. Chem, 283 (2008) 15200–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garau G, Lemaire D, Vernet T, Dideberg O, Di Guilmi AM, J. Biol. Chem, 280 (2005) 28591–28600. [DOI] [PubMed] [Google Scholar]
- [48].Makris TM, Knoot CJ, Wilmot CM, Lipscomb JD, Biochemistry, 52 (2013) 6662–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rosenzweig AC, Brandstetter H, Whittington DA, Nordland P, Lippard SJ, Frederick CA, Proteins, 29 (1997) 141–152. [PubMed] [Google Scholar]
- [50].Bertini I, Lalli D, Mangani S, Pozzi C, Rosa C, Theil EC, Turano P, J. Am. Chem. Soc, 134 (2012) 6169–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jasniewski AJ, Que L Jr., Chem. Rev, 118 (2018) 2554–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schenk G, Mitic N, Gahan LR, Ollis DL, McGeary RP, Guddat LW, Acc. Chem. Res, 45 (2012) 1593–1603. [DOI] [PubMed] [Google Scholar]
- [53].Du Bois J, Mizoguchi TJ, Lippard SJ, Coord. Chem. Rev, 200–202 (2000) 443–485. [Google Scholar]
- [54].Kryatov SV, Nazarenko AY, Robinson PD, Rybak-Akimova EV, Chem. Commun, (2000) 921–922. [Google Scholar]
- [55].Taktak S, Kryatov SV, Rybak-Akimova EV, Inorg. Chem, 43 (2004) 7196–7209. [DOI] [PubMed] [Google Scholar]
- [56].Todd MJ, Hausinger RP, J. Biol. Chem, 264 (1989) 15835–15842. [PubMed] [Google Scholar]
- [57].Pearson MA, Michel LO, Hausinger RP, Karplus PA, Biochemistry, 36 (1997) 8164–8172. [DOI] [PubMed] [Google Scholar]
- [58].Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S, J. Biol. Inorg. Chem, 5 (2000) 110–118. [DOI] [PubMed] [Google Scholar]
- [59].Andrews RK, Dexter A, Blakeley RL, Zerner B, J. Am. Chem. Soc, 108 (1986) 7124–7125. [Google Scholar]
- [60].Brietenbach JM, Hausinger RP, Biochem. J, 250 (1988) 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Benini S, Rypneiwski WR, Wilson KS, Mangani S, Ciurli S, J. Am. Chem. Soc, 126 (2004) 3714–3715. [DOI] [PubMed] [Google Scholar]
- [62].Zhang J-H, Kurtz DM Jr., Xia Y-M, Debrunner P, Biochim. Biophys. Acta, 1122 (1992) 293–298. [DOI] [PubMed] [Google Scholar]
- [63].Xiong J, Kurtz DM Jr., Ai J, Sanders-Loehr J, Biochemistry, 39 (2000) 5117–5125. [DOI] [PubMed] [Google Scholar]
- [64].Kao W-C, Wang VC-C, Huang Y-C, Yu SS-F, Chang T-C, Chan SI, J. Inorg. Biochem, 102 (2008) 1607–1614. [DOI] [PubMed] [Google Scholar]
- [65].Garbett K, Darnall DW, Klotz IM, Williams RJP, Arch. Biochem. Biophys, 103 (1969) 419–434. [DOI] [PubMed] [Google Scholar]
- [66].Okamoto Y, Onoda A, Sugimoto H, Takano Y, Hirota S, Kurtz DM Jr., Shiro Y, Hayashi T, Inorg. Chem, 52 (2013) 13014–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kurtz DM Jr., Shriver DF, Klotz IM, Coord. Chem. Rev, 24 (1977) 145–178. [Google Scholar]
- [68].Freier SM, Duff LL, Shriver DF, Klotz IM, Arch. Biochem. Biophys, 205 (1980) 449–463. [DOI] [PubMed] [Google Scholar]
- [69].Harrington PC, DeWaal DJA, Wilkins RG, Arch. Biochem. Biophys, 191 (1978) 444–451. [DOI] [PubMed] [Google Scholar]
- [70].Babcock LM, Bradic Z, Harrington PC, Wilkins RG, Yoneda GS, J. Am. Chem. Soc, 102 (1980) 2849–2850. [Google Scholar]
- [71].Armstrong FA, Harrington PC, Wilkins RG, J. Inorg. Biochem, 18 (1983) 83–91. [Google Scholar]
- [72].McCallum JD, Shiemke AK, Sanders-Loehr J, Biochemistry, 23 (1984) 2819–2825. [DOI] [PubMed] [Google Scholar]
- [73].Shiemke AK, Loehr TM, Sanders-Loehr J, J. Am. Chem. Soc, 108 (1986) 2437–2443. [DOI] [PubMed] [Google Scholar]
- [74].Xiong J, Phillips RS, Kurtz DM Jr., Jin S, Ai J, Sanders-Loehr J, Biochemistry, 39 (2000) 8526–8536. [DOI] [PubMed] [Google Scholar]
- [75].Que L Jr., Tolman WB, Angew. Chem. Int. Ed, 41 (2002) 1114–1137. [DOI] [PubMed] [Google Scholar]
- [76].Grzyska PK, Appelman EH, Hausinger RP, Proshlyakov DA, Proc. Natl. Acad. Sci. USA, 107 (2010) 3982–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Banerjee R, Proshlyakov Y, Lipscomb JD, Proshlyakov DA, Nature, 518 (2015) 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sanders-Loehr J, Wheeler WD, Shiemke AK, Averill BA, Loehr TM, J. Am. Chem. Soc, 111 (1989) 8084–8093. [Google Scholar]
- [79].Zheng H, Zang Y, Dong Y, Young VG Jr., Que L Jr., J. Am. Chem. Soc, 121 (1999) 2226–2235. [Google Scholar]
- [80].Keuleers R, Desseyn HO, Rousseau B, Van Alsenoy C, J. Phys. Chem. A, 103 (1999) 4621–4630. [Google Scholar]
- [81].Czernuszewicz RS, Sheats JE, Spiro TG, Inorg. Chem, 26 (1987) 2063–2067. [Google Scholar]
- [82].Gomez-Romero P, Witten EH, Reiff WM, Backes G, Sanders-Loehr J, Jameson GB, J. Am. Chem. Soc, 111 (1989) 9039–9047. [Google Scholar]
- [83].Que L Jr., True AE, Dinuclear iron- and manganese-oxo sites in biology, in: Lippard SJ (Ed.) Progress in Inorganic Chemistry, John Wiley & Sons, New York, 1990, pp. 97–200. [Google Scholar]
- [84].Cohen JD, Payne S, Hagen KS, Sanders-Loehr J, J. Am. Chem. Soc, 110 (1997) 2960–2961. [Google Scholar]
- [85].Moënne-Loccoz P, Richter O-MH, Huang H.-w., Wasser IM, Ghiladi RA, Karlin KD, de Vries S, J. Am. Chem. Soc, 122 (2000) 9344–9345. [Google Scholar]
- [86].Jasniewski AJ, Komor AJ, Lipscomb JD, Que L Jr., J. Am. Chem. Soc, 139 (2017) 10472–10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Fox BG, Shanklin J, Ai J, Loehr TM, Sanders-Loehr J, Biochemistry, 33 (1994) 12776–12786. [DOI] [PubMed] [Google Scholar]
- [88].Dave BC, Czernuszewicz RS, Prickril BC, Kurtz DM Jr., Biochemistry, 33 (1994) 3572–3576. [DOI] [PubMed] [Google Scholar]
- [89].Shiemke AK, Loehr TM, Sanders-Loehr J, J. Am. Chem. Soc, 106 (1984) 4951–4956. [DOI] [PubMed] [Google Scholar]
- [90].Kaminaka S, Takizawa H, Handa T, Kihara H, Kitagawa T, Biochemistry, 31 (1992) 6997–7002. [DOI] [PubMed] [Google Scholar]
- [91].Sjöberg B-M, Sanders-Loehr J, Loehr TM, Biochemistry, 26 (1987) 4242–4247. [DOI] [PubMed] [Google Scholar]
- [92].Spaeth AD, Gagnon NL, Dhar D, Yee GM, Tolman WB, J. Am. Chem. Soc, 139 (2017) 4477–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ogo S, Yamahara R, Roach M, Suenobu T, Aki M, Ogura T, Kitagawa T, Masuda H, Fukuzumi S, Watanabe Y, Inorg. Chem, 41 (2002) 5513–5520. [DOI] [PubMed] [Google Scholar]
- [94].Proshlyakov DA, Ogura T, Shinzawa-Itoh K, Yoshikawa S, Appelman EH, Kitagawa T, J. Biol. Chem, 269 (1994) 29385–29388. [PubMed] [Google Scholar]
- [95].Brown CA, Remar GJ, Musselman RL, Solomon EI, Inorg. Chem, 34 (1995) 688–717. [Google Scholar]
- [96].Kurtz DM Jr., Chem. Rev, 90 (1990) 585–606. [Google Scholar]
- [97].Hanson MA, Schmidt PP, Strand KR, Gräslund A, Solomon EI, Andersson KK, J. Am. Chem. Soc, 121 (1999) 6755–6756. [Google Scholar]
- [98].Persson BO, Karlsson M, Climent I, Ling JS, Loehr JS, Sahlin M, Sjobert BM, J. Biol. Inorg. Chem, 1 (1996) 247–256. [Google Scholar]
- [99].Tomter AB, Zoppellaro g., Bell CB III, Barra AL, Anderson NH, Solomon EI, Andersson KK, PLoS One, 7 (2012) e33436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Vu VV, Makris TM, Lipscomb JD, Que L Jr., J. Am. Chem. Soc, 133 (2011) 6938–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Galli C, Atta M, Andersson KK, Gräslund A, Brudvig GW, J. Am. Chem. Soc, 117 (1995) 740–746. [Google Scholar]
- [102].Smoukov SK, Davydov RM, Doan PE, Sturgeon B, Kung IY, Hoffman BM, Kurtz DM Jr., Biochemistry, 42 (2003) 6201–6208. [DOI] [PubMed] [Google Scholar]
- [103].Sjöberg B-M, Loehr TM, Sanders-Loehr J, Biochemistry, 21 (1982) 96–102. [DOI] [PubMed] [Google Scholar]
- [104].Duff LL, Klippenstein GL, Shriver DF, Klotz IM, Proc. Natl. Acad. Sci. USA, 78 (1981) 4138–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tagore R, Crabtree RH, Brudvig GW, Inorg. Chem, 46 (2007) 2193–2203. [DOI] [PubMed] [Google Scholar]